 Open Access Article
Open Access ArticleCopper-based single-atom nanozymes: from fundamental insights to biomedical applications
Dong
Peng
 a,
Shanshan
Huang
a,
Shanshan
Huang
 a,
Mingming
Que
a,
Mingming
Que
 a,
Xiulong
Deng
a,
Xiulong
Deng
 a,
Zhonggao
Zhou
a,
Zhonggao
Zhou
 *a and
Hongdeng
Qiu
*a and
Hongdeng
Qiu
 *b
*b
aCollege of Chemistry and Materials Science, Gannan Normal University, Ganzhou 341000, China
bKey Laboratory of Rare Earths, Ganjiang Innovation Academy, Chinese Academy of Sciences, Ganzhou 341119, China
First published on 22nd December 2025
Abstract
Copper-based single-atom nanozymes (Cu SAzymes) have emerged as a revolutionary class of enzyme mimics, distinguished by their maximized atom utilization efficiency, well-defined active sites, and superior catalytic performance. This review provides a comprehensive overview of the latest advances in Cu SAzymes, systematically outlining their diverse enzyme-like activities, the atomic-level strategies for modulating their catalytic behavior, and their burgeoning applications in biosensing, antibacterial and anticancer therapy, and anti-inflammatory treatment. We emphasize the critical structure-activity relationships that govern their multi-enzyme capabilities and highlight innovative designs that enable synergistic and stimulus-responsive therapies. By establishing a framework that links atomic structure to enzyme-mimicking function and, ultimately, to advanced therapeutic utility, this review not only summarizes the current state of the art but also outlines prevailing challenges and future directions, underscoring the significant potential of Cu SAzymes to drive interdisciplinary innovations in nanobiotechnology and precision medicine.
1. Introduction
Nanozymes, as a class of enzyme-mimicking nanomaterials, have emerged as compelling alternatives to natural enzymes, overcoming inherent limitations such as low stability, high cost, and difficulty in recycling.1–3 Since the seminal discovery of the intrinsic peroxidase (POD)-like activity of Fe3O4 nanoparticles, the field has witnessed an explosive growth, evolving from simple nanoparticle-based mimics to sophisticated nanostructures with tailored functionalities.4 Among these advancements, single-atom nanozymes (SAzymes), which feature atomically dispersed metal active sites anchored on solid supports, have emerged as a new generation of artificial enzymes that combine high catalytic efficiency with exceptional structural stability.5,6 By maximizing atom utilization efficiency and offering well-defined coordination environments, SAzymes bridge the gap between natural enzymes and conventional nanozymes, demonstrating superior activity and selectivity in diverse catalytic reactions.7–9 Since the concept was introduced in 2019, SAzymes have rapidly evolved into a vibrant research frontier, with applications spanning biomedical therapy, biosensing, and environmental catalysis.10Among various metal-based SAzymes, copper-based single-atom nanozymes (Cu SAzymes) exhibit distinctive advantages owing to their versatile enzyme-mimicking capabilities and unique physicochemical properties.11–15 Cu SAzymes can mimic a broad spectrum of natural enzymes, including POD, oxidase (OXD), superoxide dismutase (SOD), and catalase (CAT), often within a single material system.16–19 Their catalytic behavior is highly tunable through coordination engineering, enabling precise control over substrate specificity and reaction kinetics. Beyond single-atom systems, copper-based molecular catalysts and nanocomposites represent complementary approaches to enzyme mimicry.20–22 The molecular catalysts typically offer a clearer definition of the active site and mechanism, but may face challenges in terms of physiological stability; while Cu SAzymes utilize a robust metal-support coordination to exhibit good durability in biological environments, their active site accessibility and catalytic turnover rate can be affected by the solid support. Additionally, their size determines different application modes; small molecules facilitate diffusion and penetration, while nanozymes can achieve targeting and multifunctional integration. The essential biological role of copper in redox homeostasis and metalloenzyme function makes Cu SAzymes particularly suitable for biomedical applications, such as antibacterial activity, anticancer therapy, and inflammatory regulation.
Despite rapid progress, current research on Cu SAzymes remains largely fragmented, often concentrating on isolated enzyme activities or particular applications, which has left significant knowledge gaps. Broad nanozyme reviews have extensively summarized the catalytic mechanisms, classification, and biomedical applications of diverse nanozymes across metal oxides, metal nanoparticles, carbon-based nanostructures, and MOFs, but they typically discuss copper systems only briefly as part of larger material categories.14,23–27 Similarly, recent general SAzyme reviews emphasize the conceptual evolution, synthesis strategies, and universal coordination-activity relationships of single-atom catalysts, yet they seldom provide an in-depth analysis of copper-based SAzymes as an independent subclass.28–33 A few copper-focused nanozyme reviews have emerged, but they mainly describe Cu-based nanoparticle or cluster systems, lacking an atomically resolved discussion of single-atom Cu coordination environments and their multi-enzyme behaviors.34,35 A comprehensive review that classifies their multi-enzyme activities, elucidates the structure-function relationships at the atomic level, and explores their potential in combined therapeutic strategies is therefore urgently needed.
This review aims to provide a comprehensive and up-to-date overview of the rational design, catalytic mechanisms, and emerging applications of Cu SAzymes, which is structured around a unifying framework that connects atomic-level engineering to macro-level biomedical efficacy (Fig. 1). Firstly, we systematically classified and rationalized the multi-enzyme activities of Cu SAzymes under a coherent mechanistic umbrella, linking specific coordination environments to their catalytic performance. Then, we elucidated the structure–activity relationships at the atomic scale, demonstrating how deliberate engineering of the copper center's coordination sphere and the supporting matrix dictates selectivity, activity, and stimulus-responsiveness. Furthermore, we integrated these fundamental insights into innovative therapeutic applications (including antibacterial therapy, anticancer treatment, biosensing, and anti-inflammatory interventions), showcasing how the tunable multi-enzyme properties of Cu SAzymes enable synergistic and smart therapeutic strategies. Finally, we outline current challenges and prospects, including clinical translation, biocompatibility optimization, and intelligent therapeutic systems. Together, this review provides an integrative and design-oriented roadmap that advances the understanding and engineering of Cu SAzymes beyond what has been presented in existing reviews, providing a roadmap for the rational design of next-generation-specific SAzymes.
 | ||
| Fig. 1 A schematic overview of the enzyme-like properties, modulation strategies, and major applications of Cu SAzymes. | ||
2. Diverse enzyme-like activities of Cu SAzymes
Cu SAzymes have distinguished themselves in the nanozyme landscape not merely by replicating individual enzyme functions, but by exhibiting a broad and tunable catalytic repertoire. This versatility stems from their atomically dispersed copper centers, whose coordination environment can be precisely engineered to mimic a spectrum of natural enzymes, including POD, CAT, OXD, and SOD, and even more specialized oxidoreductases like laccase, galactose oxidase (GAO), glutathione oxidase (GSHOx), L-cysteine oxidase (LCO), and NADH oxidase (NOx). This chapter systematically illustrates the catalytic processes and mechanisms for different enzyme-like activities.2.1 POD-like activity
Cu SAzymes have emerged as highly efficient POD mimics owing to their atomically dispersed active centers. Compared with natural horseradish peroxidase (HRP), Cu SAzymes not only maximize metal utilization but also provide flexible regulation over hydrogen peroxide (H2O2) activation, enabling the controlled generation of reactive oxygen species (ROS).The POD-like catalysis of Cu SAzymes generally follows a Fenton-like process. H2O2 molecules adsorb onto the Cu active site, where electron transfer between the Cu center and its coordination ligands weakens the O–O bond. Cleavage of this bond yields hydroxyl radicals (˙OH) or surface-bound *OH intermediates that oxidize chromogenic substrates such as 3,3′,5,5′-tetramethylbenzidine (TMB), o-phenylenediamine (OPD), or 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS). Evidence for this pathway has been confirmed through ESR spectroscopy, fluorescence probes, and density functional theory (DFT) studies, which consistently show that unsaturated Cu sites lower the energy barrier for O–O bond dissociation (Fig. 2A and B).36–38 Kinetic analyses highlight the superior performance of Cu SAzymes compared with nanoparticle-based catalysts. For example, Cu-N4/S-rGO exhibits kcat/Km values nearly 2500 times greater than pristine rGO (Fig. 2C),39 while Cu-SAZs encapsulated in platelet membranes achieve Michaelis constant (Km) and maximum reaction rate (Vmax) values comparable to natural PODs.40
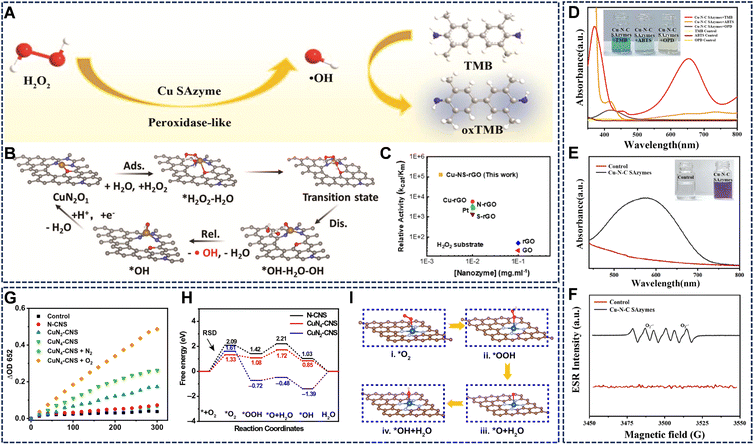 | ||
| Fig. 2 (A and B) The POD-like catalytic mechanism of Cu SAzymes. Reproduced with permission from ref. 36 and 37 Copyright (2023) Wiley-VCH. (C) The outstanding catalytic performance of Cu-N4/S-rGO. Reproduced with permission from ref. 39 Copyright (2024) Elsevier. (D) ABTS, TMB, and OPD catalyzed by Cu-N-C. Reproduced with permission from ref. 41 Copyright (2022) American Chemical Society. (E) UV-vis spectra of Cu-N-C in the NBT reaction system and (F) ESR spectra of O2˙− produced by the DMPO-Cu-N-C. Reproduced with permission from ref. 41 Copyright (2022) American Chemical Society. (G) OXD-like activity of N-CNS, CuN2-CNS, and CuN4-CNS. Reproduced with permission from ref. 42 Copyright (2023) Science and Technology Review Publishing House. (H) OXD-like free energy diagram and (I) schematic representation of the catalytic reaction pathway. Reproduced with permission from ref. 42 Copyright (2023) Science and Technology Review Publishing House. | ||
2.2 OXD-like activities
Cu SAzymes exhibit remarkable OXD-like activity, enabling the catalytic oxidation of various substrates without the need for H2O2. This section delves into the general OXD-like activity of Cu SAzymes and their specific activities toward distinct target substrates.The OXD-like activity of Cu SAzymes is closely related to their electronic structure and local coordination environment. For instance, Cu-N4 configurations exhibit a superior catalytic performance compared to Cu-N2 sites, as evidenced by lower O2 reduction rates (Fig. 2G).42 DFT calculations have revealed that the Cu-N4 coordination structure possesses an optimal O2 adsorption energy and a low reaction barrier for catalytic oxidation, outperforming Cu-N2 sites in both adsorption and electron-transfer efficiency (Fig. 2H and I). The catalytic process involves the reversible redox cycling of Cu between Cu(I) and Cu(II) oxidation states, resembling the catalytic behavior of natural copper-containing oxidases. The N-doped carbon support facilitates charge delocalization and provides strong π-d orbital coupling between Cu centers and the carbon framework, thus promoting efficient electron transfer to O2 and facilitating ROS formation.
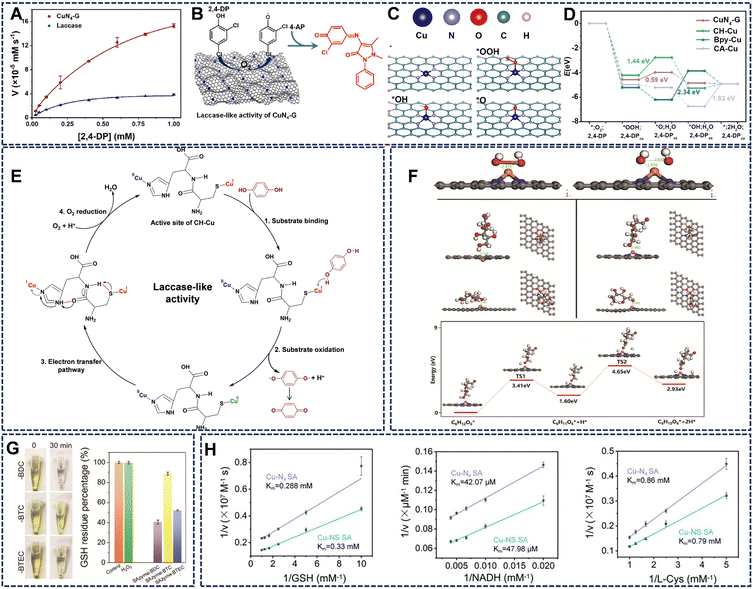 | ||
| Fig. 3 (A and B) CuN4-G exhibits outstanding catalytic performance in the oxidation reaction of phenolic substrates and the reaction of 2,4-DP and 4-AP catalyzed by CuN4-G. (C and D) The intermediate structures of surface O species on the CuN4-G, and the free energy diagram of 2,4-DP oxidation reaction pathways on the surface of four catalytic models. Reproduced with permission from ref. 43 Copyright (2023) Elsevier. (E) Schematic of possible catalytic mechanism involving the CH-Cu nanozymes (black dotted lines indicate through-space interactions and red dotted lines indicate H-bonds). Reproduced with permission from ref. 44 Copyright (2022) Elsevier. (F) DFT calculations were employed to predict and evaluate POD-like and GAO-like reactions. Reproduced with permission from ref. 45 Copyright (2024) Wiley-VCH. (G) Cu SAzyme exhibits both GSHOx and GPx activities, demonstrating optimal performance. Reproduced with permission from ref. 46 Copyright (2024) Wiley-VCH. (H) Comparative kinetic studies indicate that Cu-N3S1 exhibits superior substrate affinity and electron transfer properties. Reproduced with permission from ref. 19 Copyright (2023) Wiley-VCH. | ||
The catalytic mechanism of laccase-mimicking copper nanozymes involves the oxidation of phenolic compounds coupled with the four-electron reduction of O2 to water, bypassing the generation of H2O2. DFT calculations indicate that the electron coupling effect in the Cu-N4 structure facilitates rapid electron transfer and enhances electrical conductivity, leading to a lower energy barrier in the rate-determining step (Fig. 3C and D).43 In the case of the cysteine (Cys)–histidine (His) dipeptide coordinated Cu(I)/Cu(II) (CH-Cu), the proposed mechanism involves substrate oxidation near Cu+ sites, electron transfer via the Cys–His pathway, and O2 reduction at Cu2+ sites, closely resembling the electron transfer pathway in natural laccase (Fig. 3E).44 This biomimetic approach ensures efficient catalytic cycles without the instability associated with natural enzymes.
In summary, the OXD-like activities of Cu SAzymes span general substrate oxidation and highly specific reactions. Continued exploration of these activities will further unlock their potential in biotechnology and medicine.
2.3 CAT-like activity
Cu SAzymes have emerged as potent CAT mimics, efficiently catalyzing the decomposition of H2O2 into O2 and water (H2O). Compared to natural CAT, Cu SAzymes offer enhanced stability, tunable coordination structures, and responsiveness to external stimuli, making them versatile O2-generating catalysts.42,47–50 The CAT-like reaction pathway initiates with H2O2 adsorption at isolated copper centers, followed by O–O bond weakening and subsequent O2 release. Spectroscopic analyses and DFT calculations highlight the coordination environment as the determinant of CAT-like performance.Systems such as CuN4-CNS can generate substantial amounts of O2 rapidly, rivaling the performance of classical CAT mimics like Pt/C (Fig. 4A).42 This O2-generation capability is crucial for modulating hypoxic environments, particularly in tumor therapy. Furthermore, the CAT-like activity can be integrated with other functions. For example, L-Arg@Cu-SAzymes not only possessed CAT-like activity to generate O2 but also could efficiently load L-arginine to mediate NO release, triggering subsequent reactive nitrogen species generation (Fig. 4B).51
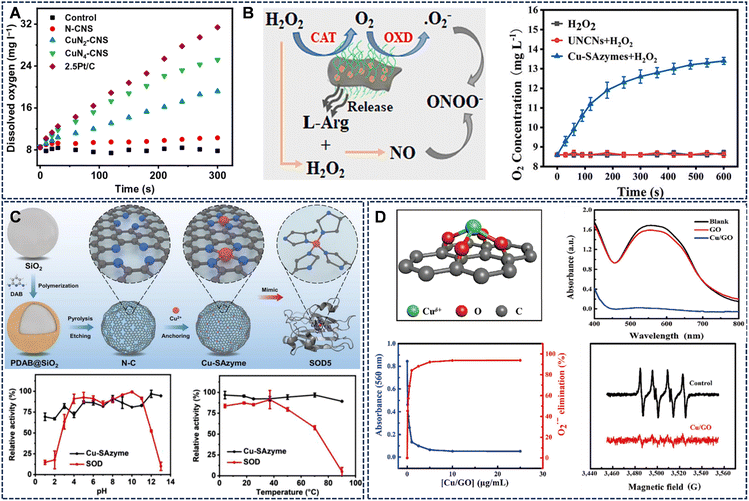 | ||
| Fig. 4 (A) CuN4-CNS rivals the O2 evolution capacity of the classic CAT simulant Pt/C. Reproduced with permission from ref. 42 Copyright (2023) Science and Technology Review Publishing House. (B) L-Arg@Cu-SAzymes not only possessed CAT- and OXD-like activities to generate cytotoxic O2˙−, but also can efficiently load L-arginine to mediate NO release and trigger subsequent ONOO generation. Reproduced with permission from ref. 51 Copyright (2023) Elsevier. (C) Synthesis schematic illustration of Cu-SAzyme and its potent and stable SOD-like enzyme activity across a broad pH range (1–13) and temperature range (4–90 °C). Reproduced with permission from ref. 52 Copyright (2022) John Wiley and Sons. (D) The possible coordination structure of Cu/GO SACs material and its excellent SOD-like properties. Reproduced with permission from ref. 53 Copyright (2022) Springer Nature. | ||
2.4 SOD-like activity
SOD-like activity is a critical feature of Cu SAzymes, enabling them to catalyze the dismutation of O2˙− into H2O2 and O2, thereby intercepting ROS and mitigating oxidative damage. While POD- and CAT-like activities have been extensively studied, reports on SOD-like catalysis remain limited due to challenges in selectively converting O2˙− while maintaining catalytic stability.For instance, a de novo-designed Cu-N4 SAzyme successfully reproduced the electronic and structural features of Cu-SOD5, exhibiting strong and stable O2˙− dismutation activity across a wide pH range (1–13) and temperature range (4–90 °C), surpassing the stability of natural SOD (Fig. 4C).52 Notably, graphene oxide (GO)-supported Cu single-atom catalysts (Cu/GO SACs) exhibit exceptional specificity for O2˙− dismutation without engaging in Fenton-like or POD side reactions (Fig. 4D).53 This selectivity arises from atomic-level coordination sites, which create an electronic environment favoring O2˙− binding and redox cycling. ESR spectra and nitroblue tetrazolium (NBT) inhibition assays confirm efficient O2˙− elimination and concentration-dependent SOD-like responses.
2.5 Multi-enzyme-like activities
Unlike natural enzymes that typically exhibit singular catalytic specificity evolved for precise biological functions, Cu SAzymes demonstrate remarkable multi-enzyme mimicking capabilities that enable complex cascade reactions reminiscent of natural enzymatic systems. This unique characteristic stems from their tunable atomic coordination environments and versatile redox chemistry, allowing simultaneous or switchable expression of POD-, CAT-, OXD-, and SOD-like activities under physiological conditions.The multi-enzyme functionality enables sophisticated therapeutic applications through synergistic effects. For example, CuN4-CNS SAzyme exhibited triple enzyme-like activities (POD-, CAT-, and OXD-like), facilitating parallel and cascaded reactions for ROS generation. Particularly noteworthy is its exceptional CAT-like activity that decomposes H2O2 into O2, subsequently utilized by OXD-like activity to generate O2˙−, creating self-supplying catalytic cycles in hypoxic environments. In another work, Cu-N,O/C nanozymes exhibit concurrent POD-like activity for ˙OH generation, CAT-like activity for O2 production, and OXD-like activity for O2˙− formation, effectively creating ROS storms in tumor microenvironments.54 This coordinated action addresses multiple therapeutic barriers simultaneously: CAT-like activity alleviates hypoxia, while POD- and OXD-like activities synergize to enhance oxidative stress.
In summary, the multi-enzyme capabilities of Cu SAzymes represent a paradigm shift in artificial enzyme design, moving beyond single-activity mimicry to integrated catalytic systems. Through precise coordination engineering, these nanomaterials achieve sophisticated reaction control that addresses complex biological challenges, positioning them as powerful tools for next-generation biomedical applications.
3. Strategies for modulating the catalytic activity
The catalytic properties of Cu SAzymes are intricately linked to the specific coordination environment of the copper centers, and the relationship between these parameters and the enzyme-like functions is summarized in Table 1. These diverse enzyme-like activities of Cu SAzymes can be precisely programmed and dynamically regulated through rational design. This chapter systematically delineates the primary strategies for modulating catalytic performance, progressing from static structural engineering at the atomic level to dynamic control via external physical fields.| Nanozyme | Active center | Supporting materials | Enzyme-like activity | Substrates | K m (mM) | V max (M min−1) | k cat (min−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| CuSACO | Cu-C3 | Graphene | POD-like | H2O2 | 4.87 | 7.5 × 10−4 | 0.96 | 48 |
| CAT-like | H2O2 | 12.48 | 1.2 × 10−3 | 1.54 | ||||
| OXD-like | TMB | 0.13 | 1.74 × 10−6 | 1.54 | ||||
| CuN3-SAzyme | Cu-N3 | 2D carbon nanosheets | POD-like | H2O2 | 65.8 | 8.76 × 10−5 | 9.41 | 55 |
| TMB | 1.61 | 9.84 × 10−5 | 10.57 | |||||
| CuN4-SAzyme | Cu-N4 | H2O2 | 752 | 4.80 × 10−6 | 1.27 | |||
| TMB | 14.2 | 7.8 × 10−6 | 2.06 | |||||
| Cu-CNNDs | Cu-N3 | Atomic-thick C3N4 | POD-like | H2O2 | 1.26 | 4.61 × 10−6 | 17.0 | 36 |
| TMB | 0.186 | 3.61 × 10−6 | 13.25 | |||||
| CuN4-CNS | Cu-N4 | N-doped carbon nanosheets | POD-like | H2O2 | 5.63 | 8.28 × 10−5 | N.D. | 42 |
| TMB | 0.22 | 5.7 × 10−6 | N.D. | |||||
| OXD-like | TMB | 0.57 | 3.9 × 10−6 | N.D. | ||||
| Cu SAs/CN | Cu-N4 | g-C3N4 | APX-like | AsA | 0.01 | 1.15 × 10−5 | 2.67 | 56 |
| Cu-N/S-C | Cu-N1S2 | N-doped carbon | CAT-like | H2O2 | 13.32 | 1.86 × 10−4 | 311.4 | 18 |
| POD-like | H2O2 | 15.41 | 8.08 × 10−5 | 13.8 | ||||
| Cu-N-C | Cu-N3 | CAT-like | H2O2 | 183.9 | 1.72 × 10−4 | 66.0 | ||
| POD-like | H2O2 | 22.60 | 4.42 × 10−5 | 6.6 | ||||
| Cu-NS-rGO | Cu-N4S | rGO | POD-like | H2O2 | 3.122 | N.D. | N.D. | 39 |
| TMB | 0.078 | N.D. | N.D. | |||||
| Cu-NS@UK@POx | Cu-N3S1 | N-doped carbon | POD-like | H2O2 | 0.45 | 9.96 × 10−6 | 0.66 | 19 |
| NOx-like | NADH | 0.048 | 1.81 × 10−5 | 11.98 | ||||
| GSHOx-like | GSH | 0.33 | 5.71 × 10−5 | 37.8 | ||||
| LCO-like | L-Cys | 0.79 | 9.20 × 10−5 | 69.6 | ||||
| Au@CuBCats | Cu-N2O1 | N-doped carbon | POD-like | H2O2 | 1.789 | 3.4 × 10−5 | N.D. | 37 |
| Cu-N/O | Cu-N2O2 | N-doped carbon | POD-like | H2O2 | 2.61 | 1.24 × 10−4 | 134.4 | 16 |
| TMB | 1.24 | 1.31 × 10−4 | 100.8 | |||||
| F@D-CHTP SN-MF | Cu-O2 | Cu-HTP MOFs | POD-like | TMB | 3.47 | 8.4 × 10−6 | N.D. | 57 |
| ac-Cu-D | Cu-O2 | N-doped carbon | POD-like | H2O2 | 14.7 | 1.07 × 10−5 | N.D. | 58 |
| CuN-CDs | Cu-O4 | Lignosulfonate | OXD-like | TMB | 0.17 | 4.9 × 10−6 | N.D. | 59 |
| POD-like | TMB | 0.18 | 2.41 × 10−5 | N.D. |
3.1 Role of nitrogen coordination number
The nitrogen coordination number in Cu SAzymes is a fundamental structural parameter that dictates the geometric configuration and electronic structure of the copper center, thereby exerting a profound influence on the type and efficiency of enzyme-mimicking reactions. By precisely tailoring the first coordination sphere (e.g., Cu-N2, Cu-N3, Cu-N4), the dynamic evolution and stability of the active site under operational conditions are equally critical for sustained therapeutic efficacy.60In general, a lower coordination number often creates an unsaturated and electron-rich metal center, which favors the adsorption and activation of substrates. This is particularly beneficial for POD-like activity. For instance, Wu et al. demonstrated that Cu-N3 configurations consistently demonstrate superior POD-like performance compared to their Cu-N4 counterparts.55 As highlighted in Fig. 5A, the CuN3-SAzyme exhibits a specific POD activity of 11.33 U mg−1, significantly surpassing that of Cu-N4-based nanozymes (2.08 U mg−1). Kinetic analyses further corroborate this advantage, with CuN3-SAZyme showing a lower Km of 0.658 mM for H2O2, indicating stronger substrate affinity. DFT calculations suggested that most of the Cu d-states in CuN3-SAzyme lie within an energy range 2 eV below the Fermi level, whereas the vast majority of Cu d-states in CuN4-Sazyme lie outside this range. Furthermore, the single Cu atom on CuN3-SAzyme protrudes more than 2 Å above the graphene plane, which facilitates the binding with H2O2 compared with the in-plane Cu atom on CuN4-SAzyme.
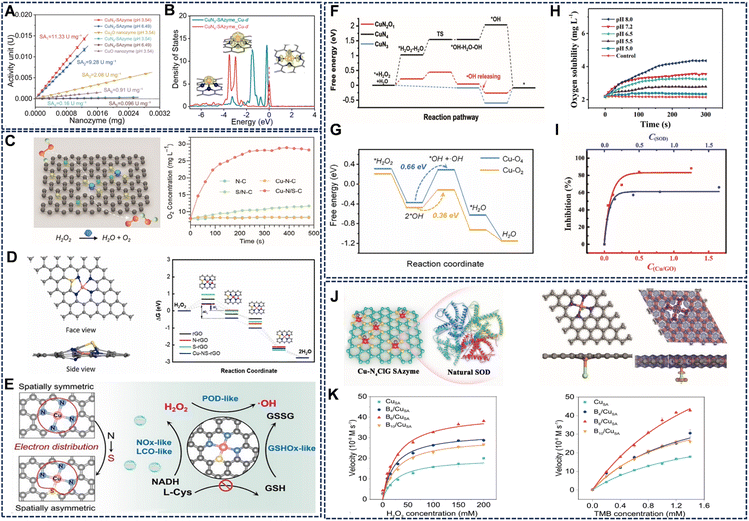 | ||
| Fig. 5 (A) The POD activity of Cu-N3-SAzyme is significantly higher than that of Cu-N4-Sazyme, and (B) calculated density of states for the d-orbitals of Cu single atom on CuN3-SAzyme and CuN4-SAzyme. Reproduced with permission from ref. 55 Copyright (2024) Springer Nature. (C) The catalytic activity of the Cu-N/S-C system was enhanced by 65.2-fold compared to the Cu-N3 site. Reproduced with permission from ref. 18 Copyright (2023) Wiley-VCH. (D) Structural simulation of Cu-N4S active site-containing Cu-NS-rGO and outstanding POD-like catalytic activity. Reproduced with permission from ref. 39 Copyright (2024) Elsevier. (E) The introduction of S endows the Cu-N3S1 active site with strong electron transfer ability, which enhances the multi-enzyme activities of Cu-NS SA. Reproduced with permission from ref. 19 Copyright (2023) Wiley-VCH. (F) Free energy diagram for the reaction process on CuN2O1, CuN4, and CuN3 sites. Reproduced with permission from ref. 37 Copyright (2023) Wiley-VCH. (G) Corresponding free energy diagram for the POD-like reaction on Cu-O4 and Cu-O2 catalytic sites. Reproduced with permission from ref. 57 Copyright (2024) Elsevier. (H) PH-responsive POD-like behaviour. Reproduced with permission from ref. 58 Copyright (2024) Wiley-VCH. (I) The relationship between inhibition of NBT reduction by O2˙−, and concentration of Cu/GO SACs and natural SOD enzyme. Reproduced with permission from ref. 53 Copyright (2022) Springer Nature. (J) Schematic illustrations of Cu-N4ClG SAzyme synthesis and the DFT calculation-rooted energy diagrams of the SOD-like catalytic reaction process. Reproduced with permission from ref. 63 Copyright (2022) Wiley-VCH. (K) Response of CuSA, B4/CuSA, B6/CuSA and B10/CuSA to varying concentrations of H2O2 and TMB. Reproduced with permission from ref. 64 Copyright (2025) American Chemical Society. | ||
In contrast, tetracoordinated Cu-N4 centers, with their more symmetric and stable coordination geometry, often support a broader spectrum of enzyme-like activities and demonstrate higher performance in certain oxidation reactions. For example, Bai et al. compared CuN2-CNS and CuN4-CNS SAzymes, showing that increasing the coordination number from 2 to 4 enhances multi-enzyme performance (Fig. 5B).42 Specifically, in OXD-like catalysis, the CuN4-CNS SAzyme outperforms the CuN2-CNS analogue, achieving a lower Km (0.57 mM) for TMB, as detailed in Table 1. This is due to the optimal O2 adsorption energy and facilitated electron transfer provided by the Cu-N4 structure. Furthermore, the Cu-N4 configuration is highly effective for mimicking antioxidant enzymes like ascorbate peroxidase (APX), with Cu SAs/CN nanozymes exhibiting a Km for ascorbic acid as low as 0.01 mM, rivaling natural enzymes.56
In summary, the nitrogen coordination number serves as a powerful knob to tune catalytic specificity. Lower coordinations (e.g., Cu-N3) are preferred for maximizing POD-like activity due to unsaturation and high substrate affinity, while Cu-N4 sites are often superior for multi-enzyme activities and certain OXD-like reactions, offering a balanced electronic structure that accommodates diverse catalytic cycles.
3.2 Effect of heteroatom doping
Beyond the nitrogen coordination number, introducing heteroatoms (e.g., S, O, Cl, B) into the first coordination sphere of copper is a sophisticated strategy to break coordination symmetry, tailor the electronic structure, and thereby dramatically enhance catalytic performance. This approach allows for fine-tuning that surpasses the capabilities of pure Cu-Nx sites.In conclusion, heteroatom doping is a versatile and powerful tool for engineering the catalytic properties of Cu SAzymes. By carefully selecting the dopant atom and its position within the coordination sphere, it is possible to selectively enhance specific enzyme-like activities, introduce multi-functionality, and achieve unprecedented levels of catalytic selectivity for advanced biomedical applications.
3.3 Support structures and defects
The support material of Cu-SAzymes is not merely an inert scaffold but also plays an active role in stabilizing single copper atoms and modulating the catalytic performance through its structure, composition, and defects.Furthermore, the electronic structure and coordination environment provided by different supports critically determine the catalytic behavior of Cu SAzymes. For instance, a comparative theoretical-experimental investigation demonstrated that Cu single atoms dispersed on g-C3N5 form a well-defined Cu-N coordination environment, in which the unique pentazine-based structure strengthens Cu anchoring and facilitates charge redistribution between the metal center and the support (Fig. 6A and B).60 This optimized electron density around Cu not only lowers the energy barrier of the rate-determining step in laccase-like reactions but also enhances substrate adsorption and electron-transfer efficiency. In contrast, supports such as g-C3N4 or C3N6 provide relatively weaker metal-support interactions, resulting in less favorable electronic modulation and inferior catalytic performance. The strengthened Cu-support interaction within g-C3N5 also suppresses atom aggregation, maintaining atomic dispersion during catalytic cycles and ensuring significantly higher laccase-like activity compared with Cu nanoparticles on the same substrate. These findings underline that tailoring the nitrogen configuration and electronic structure of carbon nitride supports is an effective strategy to boost Cu-based single-atom enzyme-mimicking activity.
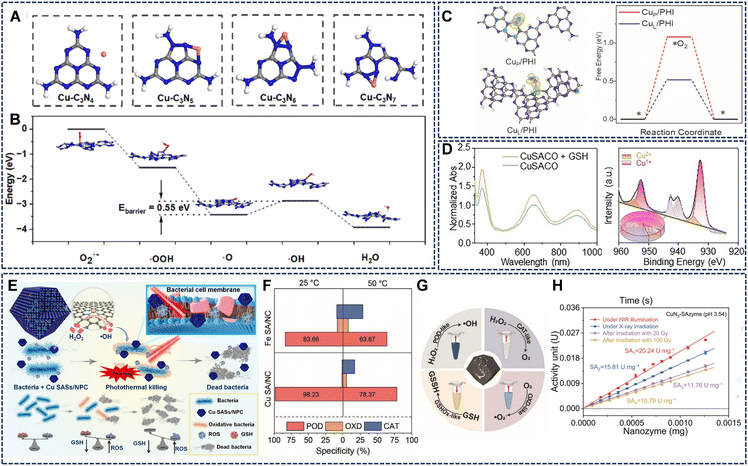 | ||
| Fig. 6 (A) Schematic structure of g-C3N4, g-C3N5, C3N6, and C3N7 with Cu atoms, respectively. (B) Schematic calculation of the pathway of laccase activity of Cu SAs loaded on C3N5. Reproduced with permission from ref. 60 Copyright (2025) the Royal Society of Chemistry. (C) The charge density difference of the CuL/PHI and CuP/PHI with the adsorption of O2 and DFT calculation of free energy of O2 adsorption (ΔG*O2). Reproduced with permission from ref. 67 Copyright (2023) Wiley-VCH. (D) The dynamic interconversion between Cu+ and Cu2+ driven by GSH. Reproduced with permission from ref. 48 Copyright (2024) Wiley-VCH. (E) Cu SASs/NPC with GSH-depleting performance were successfully synthesized for photothermal-catalytic therapy against bacteria. Reproduced with permission from ref. 71 Copyright (2021) Elsevier. (F) Photothermal effect-induced specific switching of enzyme activity in Cu SA/NC nanoenzymes. Reproduced with permission from ref. 47 Copyright (2023) Wiley-VCH. (G) Schematic diagram of the catalytic reaction of the multiple enzyme-like activities of Cu-NC. Reproduced with permission from ref. 72 Copyright (2023) Wiley-VCH. (H) CuN3-SAzyme exhibits significantly enhanced POD-like activity under X-ray irradiation. Reproduced with permission from ref. 55 Copyright (2024) Springer Nature. | ||
In conclusion, conductive or π-conjugated supports (e.g., graphene, carbon nitride) promote electron transfer, enhancing overall catalytic kinetics. Atomic dispersion stability and support interactions determine long-term durability and catalytic reproducibility, with strong metal-support coordination preventing Cu migration and maintaining single-atom integrity under physiological conditions.
3.4 Copper valence state and loading
The copper valence state and its loading amount are two interdependent parameters that directly influence the redox activity and the density of active sites.3.5 Dynamic regulation by external stimuli
Beyond static structural design, the catalytic activity of Cu SAzymes can be regulated dynamically and spatiotemporally by external physical stimuli, enabling on-demand therapy with high precision.In summary, the catalytic performance of Cu SAzymes can be hierarchically modulated through an integrated design strategy that bridges static atomic architecture with dynamic responsiveness. The foundational approach involves atomic-level coordination environment (type/number of ligands, support) engineering to define the intrinsic activity and selectivity, establishing the core structure-activity relationship. This is synergistically enhanced by optimizing the copper valence state and loading amount, which can further optimize the electron transfer capability and the density of active sites, respectively, thereby amplifying the catalytic efficiency predefined by the atomic structure. Ultimately, external physical stimuli (light, electric fields, or radiation) can be coupled with the pre-designed nanozymes to achieve dynamic, spatiotemporal control over the catalytic activity, enabling stimulus-responsive on/off or enhanced functions in complex biological environments. This progressive optimization design logic provides a clear roadmap for the rational development of smart, next-generation nanozymes for precision biomedical applications.
4. Biosensing applications
Leveraging the tunable enzyme-like activities, high catalytic efficiency, and structural robustness, Cu SAzymes have become powerful tools in analytical chemistry. The integration into various sensing platforms has enabled the detection of a diverse range of analytes, from small molecules and ions to enzymes and entire cells. The following sections summarize these advances, categorized by detection modality, with a focus on application paradigms and performance metrics.4.1 Colorimetric sensing
Colorimetric sensing, owing to its simplicity, low cost, and visual readout, is a dominant application for Cu SAzymes.73,74 Their OXD- and POD-like activities are particularly exploited to generate color changes in the presence of target analytes.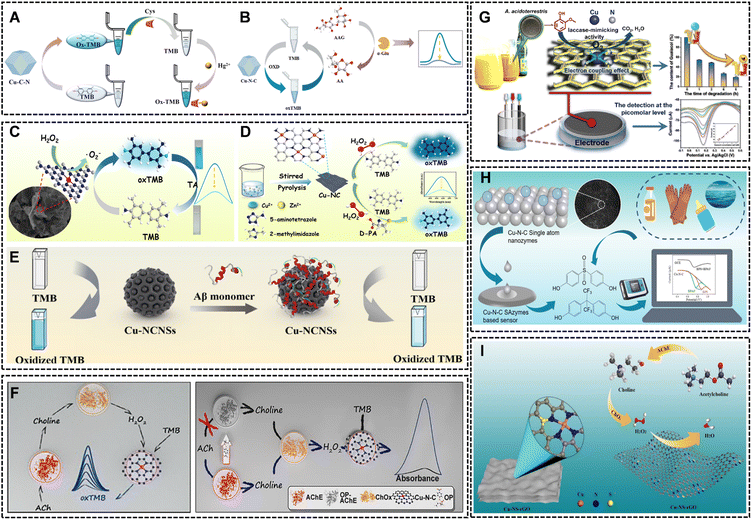 | ||
| Fig. 7 (A) Illustration of the detection of Hg2+ ions based on SACu-C-N nanozyme with OXD-like activity. Reproduced with permission from ref. 75 Copyright (2024) Elsevier. (B) The sensing principle of the Cu-N-C SAzyme-based colorimetric sensor. Reproduced with permission from ref. 76 Copyright (2024) Elsevier. (C) Schematic diagram of TA colorimetric detection principle. Reproduced with permission from ref. 69 Copyright (2023) Elsevier. (D) Schematic diagram of D-PA colorimetric detection principle. Reproduced with permission from ref. 78 Copyright (2024) Elsevier. (E) Illustration of the Cu-NCNSs-based colorimetric sensing platform for Aβ monomer. Reproduced with permission from ref. 79 Copyright (2024) Springer-Verlag GmbH. (F) Schematic illustration of the ACC system for ACh and OP detection. Reproduced with permission from ref. 81 Copyright (2020) American Chemical Society. (G) Highly selective detection of guaiacol using CuN4-G. Reproduced with permission from ref. 43 Copyright (2023) Elsevier. (H) Rapid detection of BPA, BPS, and BPAF via double-electron, double-proton electrochemical oxidation at Cu-Nx sites. Reproduced with permission from ref. 82 Copyright (2024) Elsevier. (I) Schematic of Cu-NS-rGO nanozyme application in choline (Ch) and acetylcholine (ACh) detection. Reproduced with permission from ref. 39 Copyright (2024) Elsevier. | ||
Obviously, the strategies based on TMB or ABTS oxidation offer the supreme advantages of simplicity and low cost, making them ideal for point-of-care testing (POCT) and field deployable kits (e.g., lateral flow assays). However, the sensitivity is often lower than electrochemical or fluorescence methods, and they can be susceptible to interference from colored samples or ambient light.
4.2 Electrochemical sensing
Electrochemical sensors benefit from the direct electron transfer capabilities and high catalytic efficiency of Cu SAzymes, offering rapid, sensitive, and quantitative analysis.The laccase-like activity of CuN4-G was exploited for the highly selective and sensitive detection of guaiacol, achieving a remarkably low LOD of 1.2 pM (Fig. 7G).43 The stability and efficiency of nanozyme allowed for a wide logarithmic linear range, showcasing its potential for monitoring food spoilage. The superior catalytic capacity of Cu-N-C SAzymes has also been applied for the direct electro-oxidation of environmental pollutants, which enabled the simultaneous rapid detection of bisphenols (BPA, BPS, BPAF) via a two-electron, two-proton process at the isolated Cu-Nx sites (Fig. 7H).82 The well-resolved redox peaks and sensitive quantification of BPS in the 1–50 µM range (LOD = 50 nM), highlighting their potential for environmental monitoring. Furthermore, the high POD-like activity of Cu-NS-rGO was integrated into an enzymatic cascade for neurotransmitter detection.39 In the presence of choline oxidase and acetylcholinesterase, the nanozyme electrocatalytically reduced the generated H2O2, enabling ultrasensitive detection of choline and acetylcholine with detection limits in the low nanomolar range (Fig. 7I).
The electrochemical methods possess extremely high sensitivity and a wide linear range, making them suitable for the detection of trace analytes in complex biological fluids. However, the need for specialized electrode systems and portable potentiometers currently restricts their application outside centralized laboratories. The integration with microelectronic devices will be the key in the future.
4.3 Luminescence-based sensing
The integration of catalytic activity with luminescent signal readouts has opened avenues for highly sensitive and versatile sensing platforms.For example, Liu et al. proposed a Cu-activated carbon dot@SiO2 nanozyme combined POD-like activity with room-temperature phosphorescence (RTP).83 The detection of glyphosate was based on its chelation with Cu2+ ions, which simultaneously inhibited the catalytic reaction (colorimetric mode) and restored the RTP emission (luminescent mode), providing complementary detection channels with high sensitivity (Fig. 8A). Similarly, the robust catalytic activity of Cu SAs@CN was leveraged to enhance the electrochemiluminescence (ECL) of the luminol-H2O2 system. Combining with a catalytic hairpin assembly (CHA) and CRISPR-Cas12a amplification strategy, this platform achieved an ultra-low detection limit of 1.09 fM for miRNA-622 in serum, highlighting its potential for clinical diagnostics (Fig. 8B).84
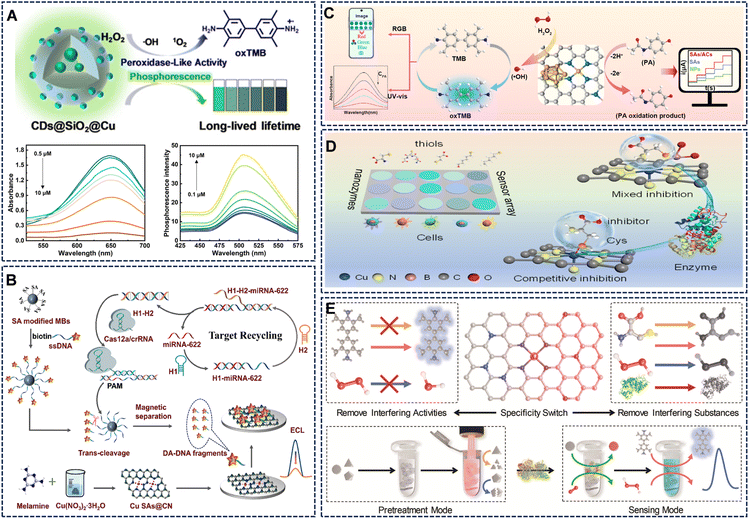 | ||
| Fig. 8 (A) CDs@SiO2@Cu that integrates neutral-pH POD-like activity and RTP. Reproduced with permission from ref. 83 Copyright (2025) The Royal Society of Chemistry. (B) Design principles of a novel ECL biosensor for miRNA-622 Detection. Reproduced with permission from ref. 84 Copyright (2024) American Chemical Society. (C) Schematic illustration of the electrochemical-colorimetric dual-mode detection mechanism of PA. Reproduced with permission from ref. 85 Copyright (2025) Elsevier. (D) Schematic diagram of pathological lesion recognition for various cell types and colorimetric response patterns of the sensor array for five cell types. Reproduced with permission from ref. 64 Copyright (2025) American Chemical Society. (E) Schematic diagram of light-thermal switchable Cu SA/NC for anti-interference detection. Reproduced with permission from ref. 47 Copyright (2023) Wiley-VCH. | ||
4.4 Multimodal and smart sensors
Systems that combine color determination, photothermal, and electrochemical signals represent a powerful trend towards self-validating and highly reliable detection. It can effectively overcome the limitations of any single mode, enhance the credibility of the results, and increase the adaptability to different sample matrices. Cu SAzymes have been engineered into multimodal and stimulus-responsive systems for wide applications.5. Antibacterial therapy
Nanozyme-based antibacterial strategies offer a potent alternative to conventional antibiotics, particularly in the fight against drug-resistant bacteria and stubborn biofilms. While the fundamental enzyme-like activities (e.g., POD-like, OXD-like) of Cu SAzymes are common across different applications, their deployment in antibacterial therapy addresses distinct biological targets and challenges compared to those in cancer treatment. In this case, the primary goal is to directly destroy the structure of prokaryotic cells and combat resistant biofilms, which is completely different from the targets for eukaryotic tumor cells. This section will therefore focus on Cu SAzyme designs and mechanisms specifically engineered to exploit bacterial vulnerabilities.5.1 Direct catalytic bactericidal effects
The intrinsic enzyme-like activities of Cu SAzymes can be directly harnessed to generate a flux of ROS at the infection site. Notably, the bacterial cell membrane, rich in unsaturated lipids, is particularly susceptible to oxidative damage by radicals like ˙OH. The exceptional POD-like activity of atomically thin Cu-CNNDs was leveraged to create durable antibacterial coatings on cotton fabrics.36 The ultrasmall size of the nanozymes facilitated close contact with microbes, enabling sustained intracellular ˙OH generation. This resulted in >99% bactericidal and fungicidal efficiency against E. coli, S. aureus, and R. solani, demonstrating potential for medical textiles (Fig. 9A). Cu-N-C nanozymes with combined intrinsic POD- and OXD-like activities were employed to simultaneously generate ˙OH and O2˙−, achieving efficient bacterial killing and biofilm disruption.41 This catalytic activity was further enhanced under LED light, promoting rapid wound healing in murine infection models (Fig. 9B). The study on MN4 (M = Cu, Co) structured SAzymes demonstrated that the CuN4 configuration exhibits superior POD-, CAT-, SOD-, and NADPH oxidase (NOX)-like multi-enzymatic activities. When combined with low levels of H2O2, CuN4 showed an impressive 82.2% antibacterial efficiency against E. Coli by significantly enhancing intracellular ROS accumulation.86 Furthermore, the design of carbon dot-based Cu nanozymes (Cu-CDs) with POD-like and OXD-like activities has been reported to effectively kill bacteria through ROS generation, while their inherent antioxidant (SOD-like) activity can also help modulate oxidative stress in the wound microenvironment, presenting a dual-function strategy for infected wound therapy.87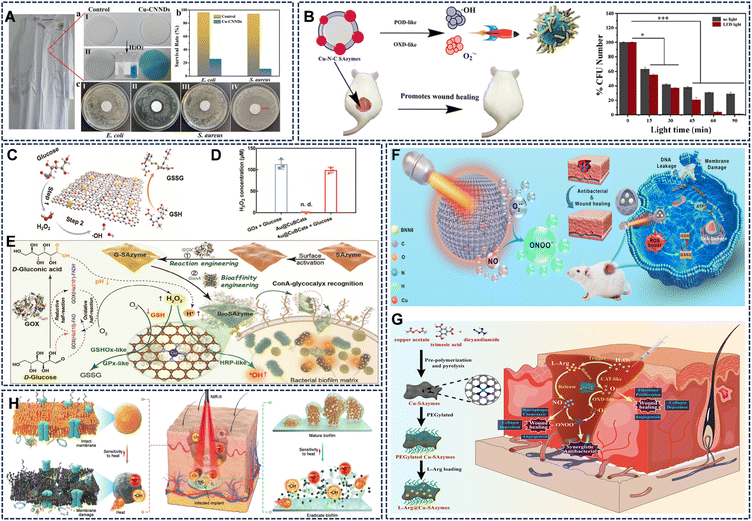 | ||
| Fig. 9 (A) Atomic-layer-thin Cu-CNNDs exhibit outstanding broad-spectrum antibacterial properties under various conditions. Reproduced with permission from ref. 36 Copyright (2023) Wiley-VCH. (B) Cu-N-C can generate ˙OH and O2˙− in oxidase-catalyzed reactions, exhibiting excellent antibacterial effects that are further enhanced through photocatalytic action. Reproduced with permission from ref. 41 Copyright (2022) American Chemical Society. (C and D) Schematic illustration of Au@CuBCats as a dual-enzyme-mimetic platform for initiating cascade catalysis and GSH depletion, and the exceptional ability of Au@CuBCats to oxidize glucose into H2O2. Reproduced with permission from ref. 37 Copyright (2023) Wiley-VCH. (E) Schematic illustration of the synthesis and function of the BioSAzyme reactor. Reproduced with permission from ref. 46 Copyright (2024) Wiley-VCH. (F) Schematic illustration of the anti-infection application of Cu HSAz@BNN6. Reproduced with permission from ref. 68 Copyright (2025) Wiley-VCH. (G) Schematic illustration of the main mode of action for L-Arg@Cu-SAzymes-based synergistic nanocatalytic therapy for MDR bacterial-infected wounds. Reproduced with permission from ref. 51 Copyright (2023) Elsevier. (H) antibacterial therapies from superficial to deep tissue infections. Reproduced with permission from ref. 42 Copyright (2023) Science and Technology Review Publishing House. | ||
Thus, the direct catalytic killing strategy is most suitable for surface prevention and prophylaxis, such as on biomedical implants, textiles, or wound dressings. However, its efficacy may be limited against established, dense biofilms or in deep-seated infections due to poor penetration and potential depletion of local substrate (e.g., H2O2).
5.2. Cascade catalysis for enhanced efficacy
Cascade reactions, which mimic metabolic pathways in cells, can significantly amplify antibacterial efficacy by producing high local concentrations of ROS and depleting protective antioxidants. For example, Fan et al. developed a dual-enzyme-mimetic bionanocatalyst (Au@CuBCats) integrating GOx-mimetic Au nanoparticles with POD-mimetic Cu-N2O1 single-atom sites (Fig. 9C).37 This system initiated a two-step cascade: glucose oxidation to generate H2O2, followed by its conversion into highly toxic ˙OH (Fig. 9D). This simultaneous nutrient depletion and ROS storm effectively overwhelmed bacterial defenses, showing great efficacy against multidrug-resistant bacteria in diabetic ulcer models. To address the challenge of biofilms, Huang et al. designed a protein-directed single-atom bionanozyme (BioSAzyme) combining a Cu SAzyme with natural GOx and the targeting molecule concanavalin A (ConA) (Fig. 9E).46 ConA provided specific binding to the biofilm glycocalyx. The GOx then generated a localized, acidic environment rich in H2O2, which was utilized by the multi-enzyme active Cu-N4 sites to produce ˙OH and deplete the GSH in the biofilm. This multi-pronged attack effectively disrupted the extracellular polymeric matrix and eradicated biofilms of pathogenic E. coli and MRSA.This approach performs particularly well in environments where there are high levels of endogenous substrates, such as diabetic wounds with high concentrations of glucose. By self-supplying H2O2 and converting it to toxic ˙OH, it creates a powerful, localized oxidative burst. However, the treating efficiency relies on a specific supply of substrates, which may vary depending on the location of the infection.
5.3. Stimulus-responsive and synergistic systems
Spatiotemporal control over catalytic activity, achieved through external stimuli like light, allows for on-demand, localized treatment with enhanced precision and reduced off-target effects.For example, A hollow Cu SAzyme (Cu HSAz) responsive to NIR-II light was designed to co-deliver a nitric oxide (NO) donor (BNN6) (Fig. 9F).68 Upon irradiation, the photothermal effect triggered NO release, which reacted with the O2˙− (generated by the OXD-like activity) to form a high density of peroxynitrite (ONOO−). This powerful oxidant disrupted bacterial membranes, depleted energy and antioxidants, and caused DNA fragmentation, leading to the complete eradication of MRSA-infected wounds. Qiu et al. engineered an L-arginine-loaded Cu SAzyme (L-Arg@Cu-SAzyme) to execute a sophisticated ROS/RNS cascade (Fig. 9G).51 The CAT- and OXD-like activity of Cu SAzyme first generated O2 and O2˙−, while the tumor microenvironment triggered NO release from L-arginine. The subsequent coupling of NO and O2˙− produced ONOO−, creating a powerful oxidative/nitrosative stress that dismantled biofilms and accelerated the healing of MRSA-infected wounds, promoting anti-inflammatory responses, angiogenesis, and collagen deposition. Wu et al. further stimulated the GOx-like activity as well as the POD-like activity with light irradiation, which is responsible for ROS generation by a cascade reaction to eradicate Gram-positive and Gram-negative multidrug-resistant bacteria.88
These stimulus-responsive and synergistic therapeutic approaches represent the most advanced paradigm for precision treatment of complex and deep-seated infections. The spatiotemporal control minimizes off-target damage to healthy tissue. The integration of multiple killing mechanisms (ROS, RNS, cuproptosis, photothermal) addresses heterogeneity and resistance. However, the complexity of design and the need for external stimulus restrict the wide-scale application.
5.4. Integration of physical and chemical modalities
Combining the catalytic activity of Cu SAzymes with physical energy conversion, such as photothermal effects, creates a powerful synergistic approach to overcome multidrug resistance. Bai et al. designed an ultrathin 2D CuN4-CNS nanozyme to combine triple enzyme-mimetic activities (POD, CAT, OXD) with high NIR-II photothermal conversion efficiency (Fig. 9H).42 The system continuously generated ˙OH and O2˙−, while the localized photothermal heating (∼48 °C) accelerated ROS production and disrupted membrane integrity. This synergy enabled the eradication of both planktonic bacteria and entrenched biofilms on implants, effectively treating both superficial and deep-tissue infections. Similarly, Cu SASs/NPC nanozymes with Cu-N4 centers and an exceptional 82.8% photothermal conversion efficiency achieved >99% killing of E. coli and MRSA in vitro under 808 nm irradiation, and significantly accelerated wound healing in vivo through the combined action of ROS, GSH depletion, and hyperthermia.71Overall, the choice of strategy should be guided by the infection profile. The direct catalytic coating strategies are ideal for surface protection, and cascade catalysis systems can provide self-enhanced killing effects for substrate-rich local infections. For deep, persistent, or biofilm-associated infections that require precise, potent, and multi-mechanistic intervention, stimulus-responsive synergistic systems are the most promising approach. Future designs should increasingly focus on environmentally adaptive nanozymes that can dynamically switch or combine these modalities according to the infection microenvironment (such as pH, enzyme, or glutathione levels).
6. Anticancer therapy
In contrast to the direct killing objectives in antibacterial therapy, Cu SAzymes-based anticancer strategies must navigate the complex and immunosuppressive TME. The therapeutic focus shifts to regulating specific programmed cell death pathways in eukaryotic cells and reshaping the tumor microenvironment to achieve sustained immune monitoring.89 This section delineates the major strategic paradigms in which Cu SAzymes are employed to achieve enhanced antitumor efficacy.6.1 Synergistic catalytic cascade and cell death pathways
Moving beyond simple ROS generation, Cu SAzymes can be designed to orchestrate complex intracellular cascades that activate specific programmed cell death pathways, such as cuproptosis and ferroptosis, for a more potent and mechanistically diverse attack.90 Specifically, cuproptosis is a novel form of regulated cell death driven by intracellular copper accumulation, leading to toxic aggregation of lipoylated proteins in the mitochondria.91 Cu SAzymes can release Cu2+ ions in the reducing TME, which directly disrupt mitochondrial metabolism and induce cuproptosis.For instance, Liu et al. developed a defective-copper-based MOF single-site nanozyme (F@D-CHTP SN) that exposed highly active sites that simultaneously generate intense ROS and release Cu2+ ions upon degradation (Fig. 10A).57 This dual action effectively triggered a combination of cuproptosis and ferroptosis. Furthermore, the co-delivery of a STING agonist and a VEGFR inhibitor via this platform remodeled the tumor vasculature and immunosuppressive TME, leading to enhanced T-cell infiltration and potent antitumor immunity. Similarly, Zhang et al. designed a Cu SAzyme embedded in disulfide-bridged dendritic mesoporous organosilica (CDPh), which can release the atomically dispersed Cu-O2/Cu-O4 sites under high-GSH conditions of the TME (Fig. 10B).58 This initiated ROS production and GSH depletion. The synergistic effect was amplified by the co-delivered phloretin, which inhibited glucose uptake. This metabolic interference collaborated with the catalytic activity to induce DLAT-dependent cuproptosis, achieving nearly complete tumor suppression with minimal side effects. In another work, Cu(I)-anchored C3N4 nanofibers (Cu-CN) served as a bifunctional catalyst, mimicking both glutathione oxidase and peroxidase activities (Fig. 10C).70 This enabled a self-cascade process: oxidizing GSH to produce H2O2, which was subsequently converted into highly toxic ˙OH. The resulting collapse of the redox balance induced extensive lipid peroxidation, effectively activating ferroptosis/apoptosis pathways and achieving strong tumor growth inhibition.
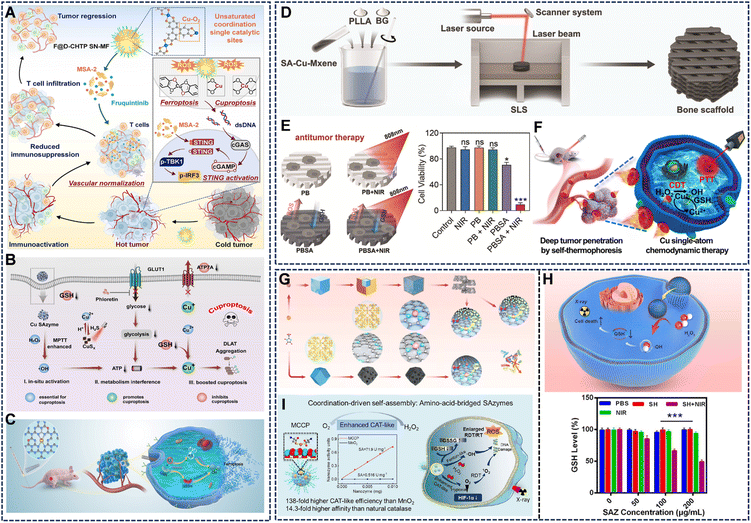 | ||
| Fig. 10 (A) Schematic illustration of tumor therapy mechanism mediated by F@D-CHTP SN-MF. Reproduced with permission from ref. 57 Copyright (2024) Elsevier. (B) Schematic illustration of the antitumor performance of CDPh in CT26 tumor cells. Reproduced with permission from ref. 58 Copyright (2024) Wiley-VCH. (C) The Cu-CN, as a bifunctional enzyme, mimics tumor cascade catalytic therapy. Reproduced with permission from ref. 70 Copyright (2023) Elsevier. (D) Synthetic procedure of PLLA/BG/SA-Cu-MXene composite bone scaffolds. Reproduced with permission from ref. 65 Copyright (2024) Wiley-VCH. (E) Under NIR irradiation, this system generates mild hyperthermia to enhance antitumor efficacy. Reproduced with permission from ref. 65 Copyright (2024) Wiley-VCH. (F) NIR light-driven active movement for enhanced penetration of in vivo tumors by self-thermophoretic diffusion, and subsequent CDT and PTT combined therapy. Reproduced with permission from ref. 92 Copyright (2023) American Chemical Society. (G) Schematic diagram of the synthesis strategy of CuN3-SAzyme and CuN4-SAzyme. Reproduced with permission from ref. 55 Copyright (2024) Springer Nature. (H) NIR-triggered release promotes ˙OH generation and efficiently reduces intracellular GSH levels. Reproduced with permission from ref. 40 Copyright (2024) IOP Publishing Ltd. (I) Schematic illustration of the structure and electron transfer process of MCCP with enhanced CAT-like activity for increasing O2 generation. Reproduced with permission from ref. 66 Copyright (2023) American Chemical Society. | ||
This synergistic catalytic cascade strategy relies solely on excessive H2O2 and an acidic environment, which exhibits advantages in its tumor selectivity and low requirement for external intervention. However, the therapeutic effectiveness is often limited by the insufficient and heterogeneous endogenous H2O2 in many solid tumors, resulting in incomplete therapy.
6.2 External energy-based synergistic therapy
The integration of external energy sources, such as light and radiation, with Cu SAzymes enables spatiotemporal control over therapy, enhancing tumor ablation while overcoming common therapeutic barriers like hypoxia.Introducing external triggers (such as NIR, ultrasound, and self-powered electric field) enables spatial and temporal control as well as on-demand activation, thereby significantly enhancing the catalytic activity beyond the natural limits within the body. This strategy is highly effective but requires tumor accessibility to the stimulus and adds complexity to treatment protocols.
6.3 Synergy with immunotherapy
Cu SAzymes can act as immunomodulatory agents by inducing immunogenic cell death (ICD) and reversing the immunosuppressive TME, thereby bridging localized tumor destruction with systemic antitumor immunity.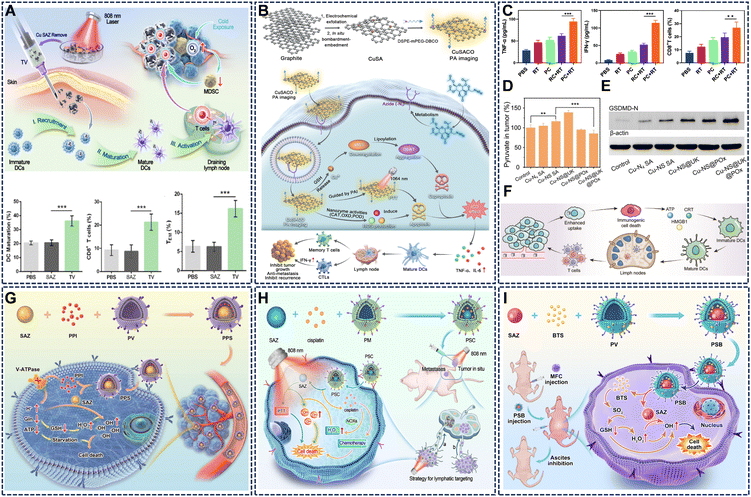 | ||
| Fig. 11 (A) The ability to best promote dendritic cell maturation and sustained immune competence. Reproduced with permission from ref. 93 Copyright (2025) American Chemical Society. (B) CuSACO efficiently targets tumors through a bioorthogonal click reaction with azide groups on cell surfaces via a membrane turnover mechanism. Reproduced with permission from ref. 48 Copyright (2024) Wiley-VCH. (C) Characteristics of tumor-infiltrating lymphocytes were analyzed across treatment groups to evaluate the feasibility of PC-enhanced radiotherapy for activating immunotherapy. Reproduced with permission from ref. 94 Copyright (2024) Dove Medical Press Ltd. (D) Pyruvate levels in tumor tissue cells were significantly downregulated in the Cu-NS@UK@POx group. Reproduced with permission from ref. 19. Copyright (2024) Wiley-VCH. (E) Cu-NS SA, with its stronger enzyme activities, can more effectively trigger pyroptosis than Cu-N4 SA. Reproduced with permission from ref. 19 Copyright (2024) Wiley-VCH. (F) Schematic diagram of the construction and antitumor immunotherapy mechanism of Cu SA-DOX@COD. Reproduced with permission from ref. 49 Copyright (2024) Wiley-VCH. (G) Schematic illustration of a biomimetic SAzyme system for self-enhanced NCT. Reproduced with permission from ref. 95 Copyright (2022) Springer Nature. (H) Schematic illustration of biomimetic SAzyme synergistic chemotherapy against gastric cancer lymphatic metastasis. Reproduced with permission from ref. 96 Copyright (2025) Wiley-VCH. (I) Schematic diagram showing the biomimetic SAzyme system for efficient inhibition of gastric cancer ascites via SO2 gas-enhanced nanocatalytic cancer therapy. Reproduced with permission from ref. 97 Copyright (2023) Elsevier. | ||
6.4 Targeted delivery systems
Precise delivery to tumor tissues is crucial for maximizing therapeutic efficacy and minimizing systemic toxicity. Biomimetic strategies have been widely adopted to improve the targeting capability of Cu SAzymes.Overall, these approaches exhibit great potential in anticancer therapy. The selection of suitable strategies should be matched with the tumor profiling. For superficial or manageable tumors with moderate oxidative stress, external stimulation often yields significant results. However, for deep, heterogeneous, and resistant tumors, a combined approach is indispensable. Future development lies in the “closed-loop” intelligent system, in which Cu SAzyme not only performs treatment but also senses specific TME biomarkers (such as pH, GSH, and adenosine triphosphate), and autonomously switches or combines its catalytic mode as needed, thereby achieving truly personalized and dynamic treatment.
7. Anti-inflammatory therapy
Inflammation is often driven by a cascade of reactive oxygen and nitrogen species (RONS). Cu SAzymes, functioning as robust catalytic scavengers, can intervene at various stages of this process. Their therapeutic strategies progress from the targeted elimination of specific RONS to the sequential disruption of the entire oxidative stress cascade, offering solutions for a spectrum of inflammatory conditions.The O2˙− is a primary ROS that initiates inflammatory signaling and can damage cellular components. The strategic design of Cu SAzymes as highly specific SOD mimics allows for the interception of inflammation at its earliest stage. For instance, Lu et al. engineered Cu/graphene oxide SAzymes (Cu/GO SACs) for exceptional specificity, mimicking SOD activity without engaging in pro-oxidant side reactions (Fig. 12A).53 This precise targeting enabled the safe and effective removal of O2˙−, as demonstrated in models of cigarette smoke-induced oxidative stress, positioning them as a viable option for preventing airway inflammation. For treating severe systemic inflammation like sepsis, Cu-N4 SAzymes were developed as highly stable and efficient SOD mimics (Fig. 12B).52 In a murine sepsis model, these nanozymes effectively neutralized O2˙−, leading to a significant reduction in systemic pro-inflammatory cytokine levels (TNF-α and IL-6), alleviation of multi-organ injury, and a marked improvement in survival rates. For these single-enzyme mimic systems, the therapy efficiencies are usually straightforward and modular. However, inflammatory cascades often involve multiple, interdependent RONS; neutralizing one may not be sufficient to halt the entire oxidative stress cascade.
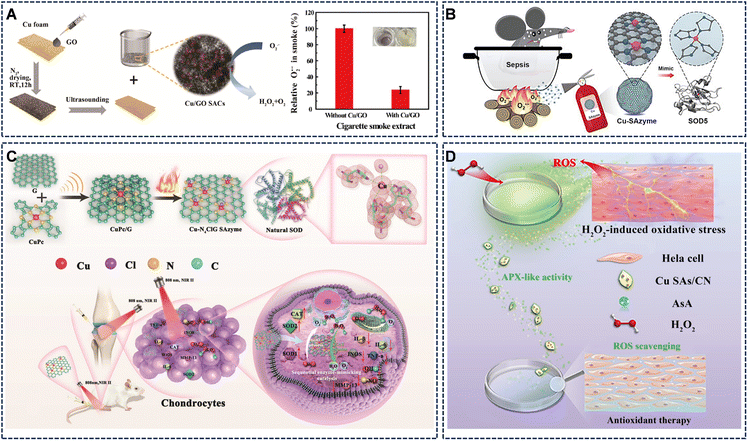 | ||
| Fig. 12 (A) Schematic diagram of the synthesis of Cu/GO SACs and the SOD-mimetic process, along with their high efficiency in scavenging O2˙−. Reproduced with permission from ref. 53 Copyright (2022) Springer Nature. (B) This nanozyme efficiently scavenges O2˙−, inhibits TNF-α and IL-6 secretion, and effectively reduces mortality. Reproduced with permission from ref. 52 Copyright (2022) John Wiley and Sons. (C) Chematic illustrations on Cu-N4ClG SAzyme synthesis and the principles of biomimetic SOD and CAT for ROS scavenging. Reproduced with permission from ref. 63 Copyright (2022) Wiley-VCH. (D) Cu SAs/CN, owing to their inherent high-efficiency nanozyme activity mimicking APX and excellent biocompatibility, have been employed to effectively shield cells treated with H2O2in vitro from oxidative damage. Reproduced with permission from ref. 56 Copyright (2021) Wiley-VCH. | ||
In chronic inflammatory diseases, multiple RONS are present and interconnected. Advanced Cu SAzymes can be designed to perform multi-step catalytic cascades, mimicking the body's natural antioxidant enzyme systems for more comprehensive protection. A sophisticated nanozyme (Cu-N4ClG) was constructed to execute sequential SOD- and CAT-like activities (Fig. 12C).63 This design enabled the continuous elimination of O2˙− and the subsequent decomposition of the resulting H2O2 into harmless O2. In osteoarthritis models, this multi-level ROS scavenging significantly decreased oxidative stress in chondrocytes, reduced cell apoptosis, and slowed cartilage degradation. The activity could be further enhanced by near-infrared photothermal stimulation, offering a synergistic strategy for managing chronic joint diseases. Systems mimicking SOD/CAT-like activities sequentially dismantle the ROS network. This broad-spectrum, “firewall” approach is more robust for chronic or complex inflammatory diseases where multiple RONS coexist and perpetuate inflammation. Their superior efficacy comes at the cost of a more intricate design.
The most complex strategies are no longer merely about eliminating reactive oxygen species. Instead, they can actively re-adjust the inflammatory microenvironment and promote tissue repair. This is an ideal strategy for inflammatory diseases accompanied by tissue damage. Cu SAzymes can be tailored to function inside cells, bolstering the endogenous antioxidant defense system. Chen et al. further developed Cu single atoms supported on graphitic carbon nitride (Cu SAs/CN) with intensive APX-like activity (Fig. 12D).56 By utilizing intracellular ascorbate to reduce H2O2 to water, these nanozymes effectively shielded cells from H2O2-induced oxidative stress and apoptosis in vitro, highlighting their potential for mitigating inflammation at the cellular level and preventing oxidative damage.
8. Summary and perspectives
Cu SAzymes have emerged as a transformative platform in artificial enzyme technology, characterized by their atomically dispersed copper active sites that enable high catalytic efficiency, tunable activities, and multi-enzyme mimicry. Through rational design of the local coordination environment, Cu SAzymes can simulate diverse natural enzymes, including POD, OXD, CAT, and SOD. As comprehensively discussed in this review, these properties have facilitated broad biomedical applications, ranging from sensitive biosensing and antibacterial therapy to anticancer treatments and anti-inflammatory interventions via ROS scavenging. By bridging atomic-level precision with biomedical functionality, Cu SAzymes exemplify the potential of nanozyme technology to advance precision medicine.Despite the promising progress, current research on Cu SAzymes remains largely fragmented between fundamental material discovery and proof-of-concept biological demonstrations. A critical gap exists between their impressive in vitro performance and the stringent requirements for clinical translation. Three major challenges must be urgently addressed:
(1) Long-term biosafety and pharmacological uncertainties: the clinical application of Cu SAzymes is severely hindered by an incomplete understanding of their in vivo behavior. While cuproptosis is a leveraged mechanism for therapy, uncontrolled Cu(I)/(II) release poses a significant risk of off-target toxicity to copper-sensitive organs. The pharmacological profile of Cu SAzymes starkly contrasts with that of FDA-approved copper-based agents, which are used under strict, protocol-defined pharmacokinetic control. Comprehensive toxicology studies establishing maximum tolerated doses are scarce. Furthermore, interactions with the immune system and dominant clearance routes are poorly characterized, directly impacting bioavailability, therapeutic window, and potential for chronic inflammation. The metabolic fate of these inorganic-organic hybrids is largely unknown, raising unanswered questions about potential accumulation, degradation products, and long-term tissue responses.
(2) Manufacturing and characterization reproducibility: the catalytic performance of Cu SAzymes is extremely sensitive to the atomic coordination structure. Minor changes in synthesis parameters can lead to differences in active sites between batches, thereby making the catalytic activity unpredictable and non-reproducible. The lack of standardized protocols for synthesis, purification, and activity benchmarking hinders comparative analysis and reliable scale-up.
(3) Activity regulation and selectivity in complex biological environments: while multi-enzyme activity is an advantage, it is still challenging to temporally and spatially control specific activities in dynamic and mutually interfering TME or infection sites. Overcoming substrate competition, achieving tumor-selective activation over healthy tissues, and ensuring sufficient catalytic efficiency under physiological conditions are all difficult problems.
Looking ahead, subsequent research must shift from a purely activity-based approach to a comprehensive, translation-oriented model. To bridge the gap between the laboratory and clinical settings, a series of collaborative efforts should be conducted:
(1) Safety design and clearable architectures: priority should be given to engineering biodegradable or readily clearable Cu SAzymes. The strategies that can be adopted include designing ultra-small sizes (<6 nm) for efficient renal clearance, incorporating responsive linkers that trigger disintegration into non-toxic components after treatment, or using endogenous biomolecules for in situ synthesis and subsequent metabolic clearance. At the same time, strict long-term toxicology studies in accordance with Good Laboratory Practice (GLP) standards must be conducted.
(2) Standardization and scalable manufacturing: this field requires the establishment of standardized protocols for the synthesis of Cu SAzymes, physicochemical characterization (especially atomic coordination verification), and enzymatic kinetic assay. The development of robust, scalable, and green synthetic methods is crucial for repeatable large-scale production.
(3) Precision and intelligent catalysis: the next generation of designs must integrate multiple targeting and activation strategies to maximize therapeutic specificity and minimize side effects. This includes conjugating active targeting ligands for cell recognition and designing “switches” (pH values, redox, enzymes) that respond to the disease microenvironment. The goal is to strictly limit catalytic activity to the pathological site, thereby constructing a “closed-loop” therapeutic system that can autonomously respond to biomarkers.
(4) Synergistic platforms and beyond conventional therapy: the intrinsic multi-enzyme activity of Cu SAzymes is ideal for constructing cascade catalytic systems. Their integration with other modalities (such as photothermal agents, immunomodulators, sonosensitizers, or ferroptosis inducers) can generate potent synergistic effects, overcoming the resistance of single therapy. Furthermore, the antioxidant (SOD/CAT-like) properties should be explored beyond cancer, in areas such as neurodegenerative diseases, rheumatoid arthritis, or ischemia-reperfusion injury, where regulating oxidative stress is crucial.
In conclusion, Cu SAzymes are promising in nanotechnology-based solutions for global health challenges. While their atomic precision offers unprecedented opportunities to simulate and even surpass natural enzymes, moving them from the laboratory to clinical application requires focused efforts to address fundamental translational challenges, such as safety, reproducibility, and controllability. By adopting a multidisciplinary approach that integrates materials science, pharmacology, and clinical insights, Cu SAzymes is poised to transform from an intriguing laboratory spectacle into a reliable next-generation therapeutic and diagnostic drug.
Author contributions
All of the authors contributed to the manuscript preparation. D. P., M. Q., and S. H. conceived the outline of the manuscript. D. P. and S. H. wrote the original draft of the manuscript. Z. Z., X. D., and H. Q. discussed and helped revise the manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
No primary research results, software, or code have been included, and no new data were generated or analyzed as part of this review. Data available on request from the authors.Acknowledgements
The work was supported by the National Natural Science Foundation of China (22405059, 22261002, 22365004), and the Natural Science Foundation of Jiangxi Province (20242BAB20097, 20232ACB203016).References
- W. Wu, L. Huang, E. Wang and S. Dong, Chem. Sci., 2020, 11, 9741–9756 RSC.
- V. G. Panferov, X. Zhang, K.-Y. Wong, J. H. Lee and J. Liu, Angew. Chem., Int. Ed., 2025, e202512409 Search PubMed.
- X. Li, Z. Wang, J. He, H. Al-Mashriqi, J. Chen and H. Qiu, Chem. Sci., 2025, 16, 29–42 RSC.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- Q. Shi, T. Yu, R. Wu and J. Liu, ACS Appl. Mater. Interfaces, 2021, 13, 60815–60836 CrossRef CAS PubMed.
- S. Cai, W. Zhang and R. Yang, Nano Res., 2023, 16, 13056–13076 CrossRef CAS.
- Y. Xu, C. Dong, X. Liu, D. Wei, Z. Chen, H. Zhou, J.-b. Li, Y. Yang and W. Tan, Small, 2025, e05750 CrossRef CAS.
- J. Shen, J. Chen, Y. Qian, X. Wang, D. Wang, H. Pan and Y. Wang, Adv. Mater., 2024, 36, 2313406 CrossRef CAS PubMed.
- C. Peng, R. Pang, J. Li and E. Wang, Adv. Mater., 2024, 36, 2211724 CrossRef CAS.
- L. Huang, J. Chen, L. Gan, J. Wang and S. Dong, Sci. Adv., 2019, 5, eaav5490 CrossRef CAS PubMed.
- X. Yu, Y. Wang, J. Zhang, J. Liu, A. Wang and L. Ding, Adv. Healthcare Mater., 2024, 13, 2302023 CrossRef CAS PubMed.
- R. Yan, Y. Li and N. Gu, ACS Appl. Nano Mater., 2025, 8, 15419–15440 CrossRef CAS.
- Y. Li, X. Hu and H. Deng, Colloids Surf., B, 2025, 256, 115032 Search PubMed.
- C. Deng, Z. Ye, C. J. Zheng, H. Cheng and J. Ge, Nanoscale, 2025, 17, 14103–14117 RSC.
- J. Zhao, F. Han, C. Cheng, H. Wang, G. Zhao, P. Jia, N. Zhang, Y. Wang, J. Zhang and Q. Wei, Microchem. J., 2024, 207, 111731 CrossRef CAS.
- Q. Wu, G. Zheng, L. Li and L. Wang, Adv. Funct. Mater., 2025, 2422588 CrossRef CAS.
- X. Lu, S. Gao, H. Lin, L. Yu, Y. Han, P. Zhu, W. Bao, H. Yao, Y. Chen and J. Shi, Adv. Mater., 2020, 32, 2002246 CrossRef CAS PubMed.
- B. Xu, S. Li, A. Han, Y. Zhou, M. Sun, H. Yang, L. Zheng, R. Shi and H. Liu, Adv. Mater., 2024, 36, 2312024 CrossRef CAS PubMed.
- R. Niu, Y. Liu, B. Xu, R. Deng, S. Zhou, Y. Cao, W. Li, H. Zhang, H. Zheng, S. Song, Y. Wang and H. Zhang, Adv. Mater., 2024, 36, 2312124 CrossRef CAS.
- Z. H. Zhang, H. Xue, Y. Xiong, Y. T. Geng, A. C. Panayi, S. Knoedler, G. D. Dai, M. A. Shahbazi, B. B. Mi and G. H. Liu, Theranostics, 2024, 14, 547–570 CrossRef CAS.
- S. Han, L. Xu, Y. Fang and S. Dong, Chem. Commun., 2024, 60, 12738–12741 RSC.
- Y. He, Y. Chen, X. Yang, C. Song, X. Shen, S. Ma, J. Sun, F. Gao and L. Wang, Chem. Commun., 2025, 61, 7871–7874 RSC.
- R. Zhang, X. Yan, L. Gao and K. Fan, Nat. Commun., 2025, 16, 6817 CrossRef CAS.
- Y. Wang, Y. Wang, L. Y. S. Lee and K.-Y. Wong, Nanoscale, 2023, 15, 18173–18183 RSC.
- J. Li, X. Cai, P. Jiang, H. Wang, S. Zhang, T. Sun, C. Chen and K. Fan, Adv. Mater., 2024, 36, 2307337 CrossRef CAS.
- S.-S. Li, A.-J. Wang, P.-X. Yuan, L.-P. Mei, L. Zhang and J.-J. Feng, Chem. Sci., 2022, 13, 5505–5530 RSC.
- D. Peng, M. Que, S. Huang, S. Wei, N. Wang, Q. He, Z. Zhou and H. Qiu, Trends Anal. Chem., 2026, 194, 118530 CrossRef CAS.
- H. Goel, I. Rana, K. Jain, K. R. Ranjan and V. Mishra, J. Mater. Chem. B, 2024, 12, 10466–10489 RSC.
- J. Pei, R. Zhao, X. Mu, J. Wang, C. Liu and X.-D. Zhang, Biomater. Sci.-UK, 2020, 8, 6428–6441 RSC.
- F. Meng, P. Zhu, L. Yang, L. Xia and H. Liu, Chem. Eng. J., 2023, 452, 139411 Search PubMed.
- Z. Lyu, J. Zhou, S. Ding, D. Du, J. Wang, Y. Liu and Y. Lin, Trends Anal. Chem., 2023, 168, 117280 Search PubMed.
- C.-N. Zhu, X. Chen, Y.-Q. Xu, F. Wang, D.-Y. Zheng, C. Liu, X.-H. Zhang, Y. Yi and D.-B. Cheng, ACS Biomater. Sci. Eng., 2024, 10, 7352–7371 CrossRef CAS PubMed.
- S. Zhang, W. Ruan and J. Guan, Food Chem., 2024, 456, 140094 Search PubMed.
- Q. Wei, Y. Pan, Z. Zhang, S. Yan and Z. Li, Chem. Eng. J., 2024, 483, 149040 CrossRef CAS.
- Q. Han, X. Chen, K. Li, H. Huang and Y. Li, Trends Environ. Anal. Chem., 2025, 48, e00281 Search PubMed.
- X. Dai, H. Liu, B. Cai, Y. Liu, K. Song, J. Chen, S.-Q. Ni, L. Kong and J. Zhan, Small, 2023, 19, 2303901 CrossRef CAS PubMed.
- X. Fan, Y. Gao, F. Yang, J. L. Low, L. Wang, B. Paulus, Y. Wang, A. Trampuz, C. Cheng and R. Haag, Adv. Funct. Mater., 2023, 33, 2301986 CrossRef CAS.
- X. Wang, Q. Chen, Y. Zhu, K. Wang, Y. Chang, X. Wu, W. Bao, T. Cao, H. Chen, Y. Zhang and H. Qin, Signal Transduction Targeted Ther., 2023, 8, 277 CrossRef CAS.
- P. G. Le, X. A. Le, H. S. Duong, S. H. Jung, T. Kim and M. I. Kim, Biosens. Bioelectron., 2024, 255, 116259 Search PubMed.
- Y. Zhong, X. Li, P. Qi, C. Sun and Z. Wang, Nanotechnology, 2024, 35, 135102 CrossRef CAS.
- J. Zhu, Q. Li, X. Li, X. Wu, T. Yuan and Y. Yang, Langmuir, 2022, 38, 6860–6870 CrossRef CAS.
- J. Bai, Y. Feng, W. Li, Z. Cheng, J. M. Rosenholm, H. Yang, G. Pan, H. Zhang and D. Geng, Research, 2023, 6, 0031 CrossRef CAS.
- X. Niu, L. Wu, F. Wu, J. Guan and H. Wang, Biosens. Bioelectron., 2023, 238, 115606 CrossRef CAS PubMed.
- J. Wang, R. Huang, W. Qi, R. Su, B. P. Binks and Z. He, Appl. Catal., B, 2019, 254, 452–462 Search PubMed.
- E. M. Hamed, L. He, V. Rai, S. Hu and S. F. Y. Li, Small, 2024, 20, 2405986 CrossRef CAS.
- L. Huang, H. Pu and D.-W. Sun, Small, 2024, 20, 2407747 CrossRef CAS PubMed.
- Y. Wu, Y. Tang, W. Xu, R. Su, Y. Qin, L. Jiao, H. Wang, X. Cui, L. Zheng, C. Wang, L. Hu, W. Gu, D. Du, Y. Lin and C. Zhu, Small, 2023, 19, 2302929 CrossRef CAS.
- L. Wu, H. Lin, X. Cao, Q. Tong, F. Yang, Y. Miao, D. Ye and Q. Fan, Angew. Chem., Int. Ed., 2024, 63, e202405937 CrossRef CAS.
- B. Xu, R. Niu, R. Deng, Y. Tang, C. Wang and Y. Wang, Adv. Funct. Mater., 2024, 34, 2405265 CrossRef CAS.
- S. Dai, L. Jiang, L. Liu, Z. Su, L. Yao, P. Yang and N. Huang, Regen. Biomater., 2024, 11, rbae119 CrossRef PubMed.
- X. Qiu, L. Zhuang, J. Yuan, H. Wang, X. Dong, S. He, S. Guan, Z. Chang, P. Bao and J. Colloid Interf, Sci, 2023, 652, 1712–1725 CAS.
- J. Yang, R. Zhang, H. Zhao, H. Qi, J. Li, J.-F. Li, X. Zhou, A. Wang, K. Fan, X. Yan and T. Zhang, Exploration, 2022, 2, 20210267 CrossRef CAS PubMed.
- M. Lu, J. Wang, G. Ren, F. Qin, Z. Zhao, K. Li, W. Chen and Y. Lin, Nano Res., 2022, 15, 8804–8809 CrossRef CAS.
- H. Zhou, H. Peng, Y. Lian, T. Sun, C. Su and Z. Wang, Nano Res., 2025, 18, 94907292 CrossRef.
- J. Wu, X. Zhu, Q. Li, Q. Fu, B. Wang, B. Li, S. Wang, Q. Chang, H. Xiang, C. Ye, Q. Li, L. Huang, Y. Liang, D. Wang, Y. Zhao and Y. Li, Nat. Commun., 2024, 15, 6174 CrossRef CAS.
- Y. Chen, H. Zou, B. Yan, X. Wu, W. Cao, Y. Qian, L. Zheng and G. Yang, Adv. Sci., 2022, 9, 2103977 CrossRef CAS.
- Y. Liu, H. Zhao, R. Niu, B. Zhang, B. T. G. Lim, S. Song, Y. Wang, H. Zhang and Y. Zhao, Chem, 2025, 11, 102297 CAS.
- W. Zhang, M. Wang, B. Liu, H. Chen, J. Tan, Q. Meng, J. Li, B. Ding, P. a. Ma and J. Lin, Angew. Chem., Int. Ed., 2024, 63, e202402397 CrossRef CAS.
- Y. Pan, Y. Zhang, D. Sun, L. Kong, Y. Liu, D. Yu, K. Song, S. Ni, W. Jiang and J. Zhan, Adv. Funct. Mater., 2025, 2509879 CrossRef CAS.
- Y. Gao, B. Pan, Y. Wang and Z. Zhu, Chem. Commun., 2025, 61, 7466–7469 RSC.
- M. Yuan, K. Han, H. Yang, L. Mi, C. Huang, X. Hu and F. He, Small, 2024, 20, 2401756 CrossRef CAS.
- Y. Pan, Y. Zhang, D. Sun, L. Kong, Y. Liu, D. Yu, K. Song, S. Ni, W. Jiang and J. Zhan, Adv. Funct. Mater., 2025, 2509879 CrossRef CAS.
- J. Zhong, X. Yang, S. Gao, J. Luo, J. Xiang, G. Li, Y. Liang, L. Tang, C. Qian, J. Zhou, L. Zheng, K. Zhang and J. Zhao, Adv. Funct. Mater., 2023, 33, 2209399 CrossRef CAS.
- L. Hu, L. Jiao, C. Chen, X. Jia, X. Li, D. Yan, Y. Zhai and X. Lu, Anal. Chem., 2025, 97, 1767–1774 CrossRef CAS PubMed.
- Z. Yan, X. Wu, W. Tan, J. Yan, J. Zhou, S. Chen, J. Miao, J. Cheng, C. Shuai and Y. Deng, Adv. Healthcare Mater., 2024, 13, 2304595 CrossRef CAS PubMed.
- J. Zhou, D. Xu, G. Tian, Q. He, X. Zhang, J. Liao, L. Mei, L. Chen, L. Gao, L. Zhao, G. Yang, W. Yin, G. Nie and Y. Zhao, J. Am. Chem. Soc., 2023, 145, 4279–4293 CrossRef CAS.
- H. Ou, Y. Qian, L. Yuan, H. Li, L. Zhang, S. Chen, M. Zhou, G. Yang, D. Wang and Y. Wang, Adv. Mater., 2023, 35, 2305077 CrossRef CAS.
- W. Tang, Y. Fang, L. Xie, W. Cai, G. Liu, Y. Lu, Y. Guan, W. Xue, J. Zhao and S. Yu, Adv. Healthcare Mater., 2025, 2405009 Search PubMed.
- X. Xie, X. Chen, Y. Wang, M. Zhang, Y. Fan and X. Yang, Talanta, 2023, 257, 124387 CrossRef CAS.
- H. Liu, B. Yu, J. Shi, X. Peng, W. Zhou, K. Wang, X. Zhang and H. Wang, Chem. Eng. J., 2024, 480, 148273 CrossRef CAS.
- X. Wang, Q. Shi, Z. Zha, D. Zhu, L. Zheng, L. Shi, X. Wei, L. Lian, K. Wu and L. Cheng, Bioact. Mater., 2021, 6, 4389–4401 CAS.
- S. Zhong, C. Xiong, Y. Zhao, S. Yao, Q. Hu, S. Wang, Q. Zhao and L. Li, Adv. Funct. Mater., 2023, 33, 2305625 Search PubMed.
- J. Chen, Y. Liu, Z. Long, Y. Li and H. Qiu, Chin. Chem. Lett., 2024, 35, 109463 Search PubMed.
- K. An, X. Li, J. Chen, S. Zhang, J. Xiao, Q. Wang and H. Qiu, Anal. Methods-UK, 2024, 16, 3551–3561 RSC.
- J. Ge, Y. Yuan, H. Yang, R. Deng, Z. Li and Y. Yang, Mater. Today Chem., 2024, 37, 102037 Search PubMed.
- Y. Jiang, Z. Chen, Y. Yuan, L. Tian, C. Dong, W. Shen, J. Wei, S. Wang, Y. Yang and J. Ge, Mater. Today Chem., 2024, 41, 102327 CrossRef CAS.
- X. Liu, F. Wu, X. Zheng, H. Liu, F. Ren, J. Sun, H. Ding, R. Yang and L. Jin, ACS Appl. Nano Mater., 2023, 6, 10303–10311 Search PubMed.
- X. Yang, X. Xie, L. Jiang, Y. Fan, C. Zhang and Y. Wang, Talanta, 2025, 283, 127131 CrossRef CAS PubMed.
- Y. Wei, Q. Bai, X. Ning, X. Bai, J. Lv and M. Li, Anal. Bioanal. Chem., 2025, 417, 1081–1092 CrossRef CAS PubMed.
- L. Nie, H. Zhang, W. Kong, R.-M. Kong, E.-S. Zhang, J. Li, Y. Zhao and F. Qu, Anal. Chem., 2024, 96, 13158–13165 CrossRef CAS PubMed.
- Y. Wu, J. Wu, L. Jiao, W. Xu, H. Wang, X. Wei, W. Gu, G. Ren, N. Zhang, Q. Zhang, L. Huang, L. Gu and C. Zhu, Anal. Chem., 2020, 92, 3373–3379 Search PubMed.
- M. Zhang, G. Wang, J. Chen and X. Lu, Anal. Chim. Acta, 2024, 1307, 342628 CrossRef CAS.
- M.-X. Liu, W.-Y. Liu, H.-Y. Wang and Y.-L. Yu, Chem. Commun., 2025, 61, 4812–4815 RSC.
- R. Liu, C. Li, L. Zhu, S. Wang, D. Liu, L. Xie, S. Ge and J. Yu, Anal. Chem., 2024, 96, 12838–12845 CrossRef CAS.
- H. Liao, J. Li, F. Wang, Y. Chen, W. Deng, B. Li, J. Liu, D. Qian and G. I. N. Waterhouse, Biosens. Bioelectron., 2025, 280, 117454 CrossRef CAS PubMed.
- Z. Wan, Q. Liu, Y. Zhe, J. Li, D. Ding, S. Liu, H. Wang, H. Qiao, J. Yang, S. Zhang and X. Mu, Biomater. Sci.-UK, 2025, 13, 1033–1044 RSC.
- M. Yu, P. Li, J. Li, X. Chen, Z. Hu, Y. Wang, J. Zeng, F. Han, X. Gong, B. Li and X. Xing, Adv. Healthcare Mater., 2025, 14, 2403201 CrossRef CAS PubMed.
- F. Wu, Y. Wang, Y. Li, L. Shi, L. Yuan, Y. Ren, H. C. van der Mei and Y. Liu, ACS Nano, 2025, 19, 10816–10828 Search PubMed.
- Y. Wu, Y. Li, G. Lv and W. Bu, Chem. Sci., 2022, 13, 2202–2217 Search PubMed.
- X. Cai, Q. Zheng, Y. Wu, X. Cheng and C. Zhu, Chin. Chem. Lett., 2025, 111670 Search PubMed.
- L. Mao, J. Lu, X. Wen, Z. Song, C. Sun, Y. Zhao, F. Huang, S. Chen, D. Jiang, W. Che, C. Zhong, C. Yu, K. Li, X. Lu and J. Shi, Chem. Soc. Rev., 2025, 54, 6282–6334 RSC.
- Y. Xing, J. Xiu, M. Zhou, T. Xu, M. Zhang, H. Li, X. Li, X. Du, T. Ma and X. Zhang, ACS Nano, 2023, 17, 6789–6799 CrossRef CAS.
- J. Ye, H. Wang, J. Zheng, S. Ning, D. Zhu, J. Shi and R. Shi, ACS Appl. Mater. Interfaces, 2025, 17, 11752–11763 CrossRef CAS.
- C. Chen, C. Nandi, Q. Yan, L. Meng, W. Chaoyan, X. Conghua and H. Yu, Int. J. Nanomed., 2024, 19, 403–414 CrossRef CAS PubMed.
- D. Zhu, R. Ling, H. Chen, M. Lyu, H. Qian, K. Wu, G. Li and X. Wang, Nano Res., 2022, 15, 7320–7328 CrossRef CAS.
- X. Luo, Y. Zhou, K. Rao, J. Xiang, S. Ning, D. Zhu, G. Li and H. Chen, Small, 2025, 21, 2411576 CrossRef CAS.
- T. Chen, X. Luo, L. Zhu, J. Xiang, C. Fang, D. Zhu, G. Li and Y. Duo, Chem. Eng. J., 2023, 467, 143386 CrossRef CAS.
- C. Guo, Y. Zhou, X. Zhang, J. Shi, Y. Zu, L. Qi, J. Zhao, Q. Fan, Y. Chen and H. Zhou, Chem. Commun., 2025, 61, 18096–18099 RSC.
| This journal is © The Royal Society of Chemistry 2026 |
