 Open Access Article
Open Access ArticleConfining chromophores by rigidification of polymer conformation for room-temperature phosphorescence hydrogels
Weihao
Feng
ab,
Muqing
Si
 ab,
Kuangzheng
Cao
c,
Wei
Lu
ab,
Kuangzheng
Cao
c,
Wei
Lu
 *ab,
Xiaoye
Zhang
*ab and
Tao
Chen
*ab,
Xiaoye
Zhang
*ab and
Tao
Chen
 *ab
*ab
aState Key Laboratory of Advanced Marine Materials, Zhejiang Key Laboratory of Extreme-environmental Material Surfaces and Interfaces, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Ningbo 315201, China. E-mail: luwei@nimte.ac.cn; zhangxiaoye@nimte.ac.cn; tao.chen@nimte.ac.cn
bSchool of Chemical Sciences, University of Chinese Academy of Sciences, 19A Yuquan Road, Beijing 100049, China
cDepartment of Digital Technologies, Hainan Bielefeld University of Applied Sciences, Danzhou 571700, China
First published on 23rd December 2025
Abstract
Organic room-temperature phosphorescence (RTP) materials have played an important part in several emerging photonic applications, such as flexible electronics, bioimaging, and information anti-counterfeiting encryption, due to their large Stokes shift and long lifetime. However, RTP emission of polymer materials requires restriction of a rigid chemical environment, which greatly limits their applications in the dry state, and they usually have poor stretchability. Polymeric RTP hydrogels offer excellent soft, wet features and tunable mechanical properties, providing an ideal solution to address this challenge. Various preparation strategies have been developed to achieve RTP emission by confining chromophores through polymer conformation rigidification, such as physical doping, chemical grafting, and supramolecular polymerization. This review aims to systematically summarize recent progress in this young (but flourishing) research area. Subsequently, the application fields of polymeric RTP hydrogels are reviewed briefly. The current challenges and future outlooks of this field are discussed to attract new interest and inspire more efforts.
1. Introduction
Organic room-temperature phosphorescence (RTP) materials are photoluminescent materials with long emission lifetimes.1–6 RTP emission can persist after the excitation light stops. Due to a large Stokes shift and long lifetime, RTP materials play an important part in many emerging photonic applications, such as flexible electronics,7–10 bioimaging,11–14 and information anti-counterfeiting encryption.15–18 RTP generation requires electrons to overcome spin prohibition and realize the intersystem crossing of singlet and triplet states. However, triplet excitons are readily quenched by oxygen or return to the ground state through non-radiative transitions.19,20 Therefore, in order to achieve RTP emission, strategies such as host–guest interactions,21,22 metal–organic frameworks (MOFs),23,24 and crystals25,26 are commonly employed to confine the chromophores in a dry and rigid chemical environment. These approaches reduce the non-radiative decay of the triplet state caused by the movement and collision of phosphorescent molecules and the quenching of triplet excitons by oxygen, and achieve RTP emission. Therefore, most traditional RTP materials often have poor biocompatibility and flexibility, which greatly limits their promotion in practical applications.27,28 The development of flexible and soft RTP materials has attracted great interest.Polymeric hydrogels, which usually exist as highly water-swollen quasi-solids and combine many advantages of solution and solid states, have excellent biocompatibility and tunable mechanical properties.29–34 Combining the superior properties of RTP materials with hydrogels to construct polymeric RTP hydrogels provides an ideal solution to the challenges stated above. Polymeric RTP hydrogels exhibit outstanding flexibility and processability, which enable them to adapt to complex surfaces for applications in wearable devices, flexible sensors and soft robots. Meanwhile, the hydrogel network can respond to external stimuli (e.g., pH, temperature, and specific molecules), resulting in changes in volume or conformation, thereby modulating RTP emission. This property provides a new strategy for anti-counterfeiting and information encryption. In addition, compared with many traditional RTP materials with poor biocompatibility and difficulty in aqueous dispersion, polymeric RTP hydrogels have broad application prospects in bioimaging. Consequently, polymeric RTP hydrogels have numerous irreplaceable advantages over conventional rigid RTP materials. However, polymeric RTP hydrogels are highly sensitive to environmental conditions and readily quenched. The swelling of polymer networks and hydration effect of molecular fragments inevitably enhance polymer chain flexibility, resulting in a significant increase in fast non-radiative decay (knr) or oxygen quenching (kq).35 The core idea to solve the problems stated above is to provide a rigid microenvironment for chromophores in hydrogels. “Rigid microenvironment” refers to the rigidification of the chemical environment around chromophores, including molecular-scale (e.g., hydrogen bonds, host–guest interactions, and coordination bonds) or larger-scale (e.g., crystallization and phase separation) interactions. Construction of a rigid microenvironment can stabilize triplet excitons, inhibit non-radiative transitions, and thereby achieve RTP emission.36–43
Research on polymeric RTP hydrogels is in its infancy, but several excellent studies have been reported. A review has summarized RTP hydrogels from the perspective of chromophore types,44 but a review examining RTP hydrogels from the standpoint of preparation strategies is lacking. This review aims to systematically outline the construction of polymer RTP hydrogels and their RTP properties. Polymeric RTP hydrogels are primarily classified into three fabrication strategies: physical doping, chemical grafting and supramolecular polymerization of hydrogels (Scheme 1). Subsequently, a brief introduction is provided to the various potential applications of polymeric RTP hydrogels, including three-dimensional (3D) printing, bioimaging and anti-counterfeiting encryption. Finally, future challenges and prospects are discussed. This review is likely to attract widespread attention from interdisciplinary researchers, providing theoretical guidance and innovative insights for the design and application of next-generation smart materials.
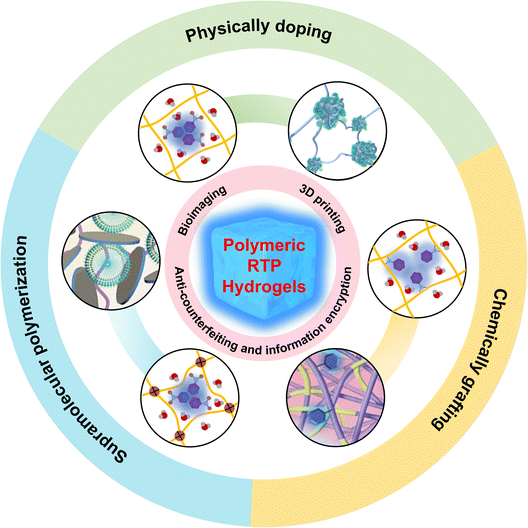 | ||
| Scheme 1 Synthetic strategy of polymeric RTP hydrogels and their applications in numerous frontier fields. | ||
2. Mechanistic insights
2.1 Fundamental mechanism of RTP
RTP arises from a process in which molecules, upon excitation, undergo intersystem crossing (ISC) to reach the triplet state, and then slowly radiate light.45 The key challenge is that excitons in the delicate triplet state are highly prone to losing energy through non-radiative transitions, such as vibrational and rotational motions, at room temperature, leading to phosphorescence quenching. Therefore, suppressing non-radiative transitions is essential for achieving efficient RTP. The question then becomes: how can we create such a rigid microenvironment in soft, water-rich hydrogels?2.2 Crucial role of rigid microenvironments in RTP
Hydrogels are macroscopically soft, but their crosslinked network structure at the microscopic level provides effective spatial confinement, or a rigid microenvironment, which is a decisive factor in suppressing non-radiative transitions and regulating RTP lifetime (τ). The crosslinking density of the polymer network influences the RTP intensity of the hydrogel. Studies have shown that, as the polymerization time increases, the RTP intensity gradually improves.46 The water content is also an important factor influencing RTP performance. Compared with polymer networks with high water content, those with low water content have more densely packed polymer chains, blocking oxygen pathways, and significantly enhancing RTP. Furthermore, introducing rigid segments is a key method to improve RTP. For example, the crystallization within a polyvinyl alcohol (PVA) gel network increases rigidity, reducing the mobility of chromophore molecules and enhancing RTP performance.47 Incorporation of rigid nanoparticles as multifunctional crosslinking points improves mechanical properties but also creates more restricted “rigid nanodomains” that help stabilize triplet excitons (Fig. 1).48 | ||
| Fig. 1 Mechanism of action of polymeric RTP hydrogels. Schematic Jablonski illustration depicts luminescence mechanisms in (a) soft networks and (b) rigid microenvironments. | ||
In addition to the adjustment of the polymer network, the interactions between the polymer matrix and chromophore affect RTP performance. For instance, hydrogen bonds between molecules and side-chain amides can limit the mobility of the chromophore but may also alter its electronic structure, thereby facilitating ISC. The spatial confinement of chromophore molecules in an ultra-entangled polymer network, induced by hydrogen bonding, results in ultralong RTP. Moreover, some dynamic crosslinked hydrogels may exhibit reversible changes in RTP performance in response to external stimuli. In summary, this strategy of creating a “rigid microenvironment within a soft matrix” establishes the conditions necessary to stabilize triplet excitons within the macroscopic softness of hydrogels. This forms the foundation for achieving long-lived, high-intensity RTP.
3. Construction strategy of polymeric RTP hydrogels
3.1 Physical doping of chromophores into polymeric hydrogels
Physical doping is the simplest and most direct method for creating polymeric RTP hydrogels. However, phosphorescent chromophores are highly sensitive to the abundant water and dissolved oxygen in hydrogels, readily leading to non-radiative transitions and quenching of triplet excitons. Therefore, chromophores are often protected by encapsulating them within nanoparticles or by utilizing their interactions with polymer matrices.The formation of stable nanoparticles through assembly represents an effective method for preparing RTP hydrogels. In 2022, He et al. proposed a strategy for the preparation of polymeric RTP hydrogels by boric acid (BA)-doped silicon nanoparticles (BSiNPs). (3-Aminopropyl)trimethoxysilane (APTMS) was reacted with trisodium citrate under microwave irradiation to produce silicon-based nanoparticles (SiNPs), which exhibited blue fluorescence emission. BAs were subsequently doped into the SiNPs system. Glassy-state BSiNPs were formed after a high-temperature and pressure reaction (Fig. 2a).48 The encapsulated glass-state BSiNPs could stabilize triplet excitons and reduce non-radiative transitions. RTP properties were effectively enhanced. As shown in Fig. 2b, BSiNPs were uniformly dispersed in polyvinyl alcohol (PVA) hydrogels. They exhibited ultralong afterglow up to 20 s (Fig. 2c).
Carbon nanodots (CNDs) enable long-lived phosphorescence. In 2024, Liu et al. developed a strategy to achieve phosphorescence emission in hydrogels by encapsulating CNDs within a silica shell, thereby shielding them from water and oxygen. Polymeric RTP hydrogels were then fabricated through in situ polymerization with acrylic acid (AA), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond-modified β-cyclodextrin (β-CD-DA) and adamantane (Ad-DA) (Fig. 3a).49 As shown in Fig. 3b, CNDs@silica were uniformly dispersed in the hydrogels. CNDs@silica provided long-lived green phosphorescence, and the polymeric RTP hydrogels exhibited an ultralong lifetime of up to 1261 ms (Fig. 3c). β-CD-DA and Ad-DA confer >30-times the stretchability to hydrogels through host–guest interactions (Fig. 3d). Furthermore, the hydrogel was able to efficiently transfer energy to the fluorescence dyes rhodamine B and eosin Y through efficient TS-FRET, resulting in long-lived red and yellow delayed fluorescence (Fig. 3a).
C bond-modified β-cyclodextrin (β-CD-DA) and adamantane (Ad-DA) (Fig. 3a).49 As shown in Fig. 3b, CNDs@silica were uniformly dispersed in the hydrogels. CNDs@silica provided long-lived green phosphorescence, and the polymeric RTP hydrogels exhibited an ultralong lifetime of up to 1261 ms (Fig. 3c). β-CD-DA and Ad-DA confer >30-times the stretchability to hydrogels through host–guest interactions (Fig. 3d). Furthermore, the hydrogel was able to efficiently transfer energy to the fluorescence dyes rhodamine B and eosin Y through efficient TS-FRET, resulting in long-lived red and yellow delayed fluorescence (Fig. 3a).
Beyond encapsulating phosphorescent chromophores to shield them from water and oxygen, leveraging physical interactions between chromophores and polymer matrices offers a more universal and direct approach to forming physically doped RTP hydrogels. For example, the hydrogen bonding between chromophores and polymeric chains can effectively achieve RTP emission. In 2025, Wang et al. developed a polymeric RTP hydrogel construction method based on strong hydrogen bonding interactions between nonconventional chromophores (NCCs) and polymeric chains (Fig. 4a).50 Biuret (BIU), polyvinyl alcohol (PVA), and polymaleic acid (PMAc) were dissolved in a glycerol/water mixed solvent and formed organohydrogels (PVA/BIU/PMAc) through freeze–thawing. The strong hydrogen bonds between NCCs and polymer chains suppress non-radiative transitions and promote the aggregation of NCCs, thereby stiffening molecular conformations and forming spatial conjugation. RTP hydrogels exhibited an RTP lifetime of up to 782.8 ms (Fig. 4b). Furthermore, the hydrogels demonstrated significant excitation-dependent RTP emission. Remarkably, the RTP emission wavelength of the hydrogels varied with the change in the excitation wavelength (Fig. 4c).
By forming hydrogen bond interactions between the polymer chains and chromophores, not only can polymeric RTP hydrogels be constructed, but the hydrogels are also endowed with excellent mechanical properties. In 2024, Chen et al. developed high-strength polymeric RTP hydrogels (W-hydrogels) by the in situ polymerization of acrylamide (AAm) in the presence of delignified wood. This strategy aimed to form molecular clusters through hydrogen bond interactions between lignin and acrylamide (Fig. 4d).51 Such enhanced interactions facilitate the confinement of lignin and trigger the radiative migration of triplet excitons, thus enabling RTP emission. The hydrogels exhibited RTP emission at ∼490 nm and gave out obvious green luminescence after removing UV light (Fig. 4e). As a result of the molecular interactions between the components of delignified wood and polyacrylamide (PAAm), the W-hydrogels exhibited extremely high tensile strength. Moreover, the tensile strength of W-hydrogel treated with ethanol increased significantly, and the entire process was reversible (Fig. 4f). The reversibility of this process enables the RPT hydrogel material to be applied in a wide range of scenarios.
These chromophores interact with polymer chains to form a super-entangled state, enabling the hydrogel to effectively achieve phosphorescent emission and super-stretch properties. In 2025, Chen et al. incorporated 4-diphenylboronic acid@β-Cyclodextrin (4-BB@β-CD) into a poly(2-(acryloxy)ethyl trimethylammonium chloride) (PAETC) network to prepare an ultra-stretchable polymer RTP hydrogel. Under water-limiting conditions, the PAETC chain forms a hyper-entangled structure and synergistically densifies the 4-BB@β-CD aggregates through supramolecular confinement, effectively suppressing molecular vibrations and stabilizing triplet states (Fig. 5a).52 Impressively, the hydrogels exhibit intense RTP emission at ∼500 nm and ultralong lifetime up to 970.7 ms under room conditions (Fig. 5b and c). Crucially, the entanglement-dominated physical network free of static chemical crosslinking enables continuing chain disentanglement during stretching for efficient energy dissipation, achieving excellent uniaxial/biaxial (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000%/10
000%/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000%) stretchability (Fig. 5d and e). Notably, these PAETC/4-BB@β-CD hydrogels maintained bright and long-lived RTP under various strains (Fig. 5e).
000%) stretchability (Fig. 5d and e). Notably, these PAETC/4-BB@β-CD hydrogels maintained bright and long-lived RTP under various strains (Fig. 5e).
All these impressive recent advancements suggest that excellent and multifunctional RTP properties can be imparted to hydrogel materials through simple physical doping as well as material design.
3.2 Chemical grafting of chromophores into polymeric hydrogels
Physical doping is simple, but the problem of phosphorescent small-molecule leakage significantly limits the long-term stability and practical applications of polymeric RTP hydrogels. To fundamentally address this problem, researchers have covalently grafted chromophores onto the three-dimensional network of hydrogels. Due to the stability of covalent bonds, grafting chromophores onto polymer chains enhances the stability of luminescence intensity and lifetime.51 Interactions within polymeric networks provide a highly rigid microenvironment for the phosphorescence of chromophores, effectively stabilizing triplet excitons and suppressing non-radiative transitions, ultimately facilitating the construction of polymer RTP hydrogels.For example, formation of a rigid microenvironment through host–guest interactions can enable the preparation of polymeric RTP hydrogels by grafting host and guest molecules onto the polymeric main chain. In 2014, Tian et al. grafted β-cyclodextrin (β-CD) and α-bromonaphthalene (α-BrNp) onto the polymeric main chain (Fig. 6a).53 The host–guest recognition between β-CD and α-BrNp enabled hydrogel formation without the need for additional crosslinking. The rigid microenvironment provided by the host–guest interactions achieved RTP emission and exhibited a lifetime of 0.56 ms. Simultaneously, the hydrogels exhibited excellent self-healing properties (Fig. 6b). Similarly, in 2016, they achieved RTP emission in hydrogels with a lifetime of 0.32 ms by using different host molecule γ-cyclodextrin (γ-CD) and guest molecule 4-bromo-1,8-naphthalic anhydride (BrNpA) (Fig. 6c).54
Designing the molecular structure and multi-functionalization of phosphorescent molecules can enable the construction of a rigid microenvironment in hydrogels. In 2024, He et al. developed a novel multifunctional molecule based on benzothiadiazole-based dialkene (BTD-HEA), which serves as a phosphorescence emitter, initiator, and crosslinker in polymeric RTP hydrogels. BTD-HEA exhibits high triplet exciton generation and a dialkene structure, enabling it to effectively initiate the polymerization of acrylamide-based monomers while acting as a site to form a crosslinked network structure (Fig. 7a).46 Additionally, the distinct fluorescent-phosphorescent colorimetric characteristics of the hydrogel offer a more sensitive approach for visually monitoring polymerization (Fig. 7b). RTP intensity and rheological tests demonstrate that the crosslinking degree of the hydrogel network increases with polymerization time (Fig. 7c and d). Consequently, the thermal motion of BTD-HEA was restricted, and non-radiative transitions were suppressed, leading to changes in RTP emission. By utilizing BTD-HEA as a photoinitiator, various shapes of polyhydroxyethyl acrylate (PHEA) hydrogels can be prepared through 3D printing. Moreover, the hydrogel retains a purple afterglow even after the cessation of 365-nm UV excitation (Fig. 7e). Although purple afterglow is uncommon, its long-lasting luminescence characteristics confirm its RTP emission.
Formation of a phase-separated structure in hydrogels can also achieve the construction of a rigid microenvironment. In 2024, Lü et al. induced in situ phase separation in hydrogels via acid treatment, thereby preparing polymeric RTP hydrogels. Initially, the researchers prepared hydrogels by copolymerizing AAm and N-(4-(9H-carbazole-9-yl)phenyl)-2-acrylamide chromophore (CzPA). The hydrogels were then immersed in aqueous hydrochloric acid to hydrolyze the amide groups. The interaction between amide and carboxylic acid groups formed dense hydrogen bonds, leading to phase separation. This process resulted in the formation of a tight network structure that restricted the motion of chromophores and suppressed non-radiative transitions (Fig. 7f).55 The hydrogels displayed excellent stability in water, showing minimal change over 30 days, with consistent RTP intensity and afterglow (Fig. 7g and h). Mechanical properties play a very important part in expanding the application range of polymeric RTP hydrogels and extending the service life of materials. However, the emission of RTP requires the suppression of non-radiative transitions, which is contradictory to the softness, high water content, and stretchable properties of hydrogels. Therefore, it is necessary to construct a local rigid microenvironment in the hydrogels. Construction of rigid microenvironments does not compromise the overall mechanical properties of the hydrogel, so polymeric RTP hydrogels have the advantage of widely adjustable mechanical properties. In addition to excellent RTP properties, polymeric RTP hydrogels have excellent and widely tunable mechanical properties. In a series of hydrogels with different H-treatment times, the hydrogel treated for 6 h exhibits a fracture strain of nearly 1000%, while the hydrogel treated for 14 h shows a fracture stress of >11 MPa.
In addition to introducing typical chromophores, RTP emission can be achieved by the aggregation and confinement of electron-rich groups, such as carbonyl groups. In 2024, Wu et al. proposed a strategy for the preparation of polymeric RTP hydrogels through polymerization-induced crystallization (Fig. 8a).56 This strategy exploited the solubility difference of crown ethers (CEs) in monomer and polymer states. During polymerization, the solubility of the CEs sharply decreases, leading to the formation of large spherulites that compress and confine the polyacrylamide hydrogel matrix. The approach leads to the formation and aggregation of carbonyl group clusters, thereby achieving RTP emission. The hydrogels exhibit excellent stretchability and resilience, with visible afterglow (Fig. 8b).
Building on the research findings stated above, in 2024, Wu et al. achieved control of spherulite structure in hydrogels by modulating the hydrogel network stiffness. The stiffness of the PAAm network was precisely adjusted by regulating the concentrations of photoinitiator and chemical crosslinking agents, as well as the intensity of UV light. Regular spherulites are formed in relatively stiff hydrogels, whereas banded spherulites with twisted crystal fibers are obtained in hydrogels (Fig. 8c).57 The structure of the spherulites can be directly observed through polarized optical microscopy (Fig. 8d and f). The formation of twisted crystalline fibers was linked to the dynamic changes in crystallization pressure and network impedance. Hydrogels with regular spherulites exhibited stronger fluorescence and phosphorescence compared with those with banded spherulites. Surprisingly, the hydrogels with banded spherulites exhibited circularly polarized luminescence (CPL) (Fig. 8e and g). This luminescence arises from the aggregation of clusters constrained by the crystal, while the twisted crystalline fibers give the achiral luminescent clusters CPL. In addition to crystallization-induced carbonyl groups clustering in polymers, the aggregation of carbonyl groups can also be achieved through salt-induced aggregation. In 2024, Zhao et al. proposed a new strategy for constructing polymeric RTP hydrogels via salt-induced aggregation. AA and diethylenetriamine (DETA) were copolymerized to prepare a polymer network, which was then immersed in a sodium bromide solution.58 The salt treatment promotes hydrophobic aggregation in the hydrogel network. The resulting dense structure enabled the clustering of carboxyl groups and oxygen atoms, suppressing non-radiative transitions and thereby enabling RTP emission.
However, despite this impressive progress, the development of polymeric RTP hydrogels is largely lagging, partially because of the tedious synthetic procedures. More efforts are suggested to explore new and simple synthetic methods or the employment of well-organized supramolecular interactions to construct powerful polymeric RTP hydrogels.
3.3 Polymeric RTP hydrogels via supramolecular polymerization
Polymeric RTP hydrogels can also be constructed through the spontaneous self-assembly process of supramolecular polymerization of phosphorescence molecules and copolymer monomer molecules. The rigid microenvironment in hydrogels can be constructed through the process of supramolecular self-assembly, enabling RTP emission. Supramolecular polymerization is usually driven by electrostatic interactions, hydrogen bonds, and dipole–dipole interactions. Compared with physically doped polymeric hydrogels and chemically crosslinked polymeric hydrogel systems, supramolecular interactions exhibit high dynamism and reversibility, showing great application potential.Electrostatic interactions are typical supramolecular interactions between ions with opposite charges (e.g., cations such as quaternary ammonium groups or protonated amino groups and anions such as carboxylate or sulfate groups). Supramolecular interaction sites are formed by the interaction between chromophores and a group with the opposite charge, and the polymeric RTP hydrogel can be prepared by supramolecular polymerization/crosslinking based on electrostatic interaction. For example, in 2021, Garain et al. synthesized a simple heavy atom-substituted cationic phthalimide derivative (CPthBr). After that, the researchers used an organic–inorganic supramolecular scaffolding strategy to form supramolecular action sites through the electrostatic interactions between CPthBr and negatively charged LAPONITE® (LP) clays (Fig. 9a).59 As LP was continuously added to the aqueous solution of CPthBr, an RTP emission peak ∼487 nm was continuously enhanced (Fig. 9b). The phosphorescence lifetime was 1.62 ms (Fig. 9c). At higher LP concentrations, the LP scaffold promoted the orderly arrangement of molecules through electrostatic interactions to form unique self-standing supramolecular RTP hydrogels. Efficient triplet-to-singlet FÖrster resonance energy transfer and delayed fluorescence were achieved by doping Sulforhodamine G (SRG) and Sulforhodamine101 (SR101) dyes into CPthBr-LP hydrogels. Yellow and orange RTP hydrogels can be produced with average lifetimes of 162 µs and 55 µs, respectively (Fig. 9d).
Hydrogen bonds are also important supramolecular interactions for constructing supramolecular polymeric RTP hydrogels. Usually, a single hydrogen bond is too weak to stabilize supramolecular materials, but the synergistic effect of hydrogen bonds and other supramolecular interactions is conducive to the preparation of supramolecular polymeric hydrogels. For example, in 2024, Dai et al. developed near-infrared (NIR) RTP supramolecular hydrogels based on amphiphilic bromonaphthalimide pyridinium derivative (G), LP nanosheets and PAAm. Initially, G molecules can self-assemble into positively charged spherical nanoparticles without RTP properties. Driven by electrostatic interactions, these nanoparticles can be co-assembled with negatively charged LP to form supramolecular RTP hydrogels (Fig. 9e).60 This hydrogel exhibits red RTP phosphorescence at 586 nm with a lifetime of 4.03 ms. Furthermore, the physical crosslinking network is enhanced by introducing PAAm into the supramolecular RTP hydrogel system. The abundant hydrogen bonding intensifies RTP intensity and improves lifetime to 4.20 ms (Fig. 9f and g). In particular, RTP emission can be achieved at a higher temperature (363 K) with a lifetime of 2.46 ms (Fig. 9h and i).
Similarly, in 2025, Dai et al. prepared supramolecular polymeric RTP hydrogels based on electrostatic interactions and hydrogen bonding synergy through green one-pot copolymerization of monoalkene-functionalized iodoisoquinolinium derivatives (G′), LP, and AAm monomers in aqueous solution (Fig. 9j).61 This approach circumvents the multi-step assembly procedures employed in previous studies, thereby facilitating the construction of supramolecular polymeric RTP hydrogels.
Dipole–dipole interactions play an important part in supramolecular polymerization. By rationally designing the polar groups in molecules, the molecules can be induced to self-assemble into nanoscale structural units and then be polymerized into hydrogels. Formation of these supramolecular polymeric hydrogels typically relies on dipole–dipole interactions, but also on other types of supramolecular interactions. For example, in 2023, Xiao et al. designed supramolecular polymeric RTP hydrogels prepared by simply heating/mixing and cooling hexamethyl cucurbit[5]uril (HmeQ[5]) and 1,4-diaminobenzene (DB).62 HmeQ[5] has two ports surrounded by carbonyl groups. The dipole–dipole interaction and hydrogen bonding between HmeQ[5] and the amino group of protonated DB played an important part in supramolecular polymerization. Scanning electron microscopy (SEM) showed the morphology of the freeze-dried hydrogel, exhibiting a fiber network structure. The incorporation of chromophores induces stimulated RTP emission in the ordered fiber structure of the supramolecular hydrogel, effectively suppressing non-radiative transitions. This effect can be proved by confocal laser scanning microscopy. Meanwhile, due to the temperature sensitivity of the supramolecular interactions, a sol-to-gel transition was observed when the hydrogels were cooled to 25 °C, while a gel-to-sol transition was observed at 75 °C.
4. Applications of polymeric RTP hydrogels
Polymeric RTP hydrogels, as a new generation of intelligent materials, hold immense application potential due to their outstanding biocompatibility, flexible and stretchable mechanical properties, as well as a three-dimensional porous network.63–68 In terms of optical performance, their unique long-term stability, tunability, and stimulus-responsive luminescence characteristics make them highly promising for applications in 3D printing, bioimaging, and complex information encryption technologies. This section will briefly summarize the application progress of RTP hydrogels in these fields.4.1 3D printing
Polymeric RTP hydrogels have the advantages of long lifetime, non-interference background and easy preparation into 3D shapes.69,70 Polymeric RTP hydrogels have been processed into 3D shapes with distinct luminescence (Fig. 10a).51 Subsequently, these hydrogels were fabricated into micron-sized hydrogel threads and applied to the suture of biological tissues (Fig. 10b and c). However, hydrogels prepared using the template method exhibit low precision, making it challenging to fabricate polymeric RTP hydrogels with complex 3D macroscopic structures (e.g., porous scaffolds). 3D printing technology offers advantages in forming complex structures and achieving high precision. Photo-initiated polymerization and rheological properties enable the 3D printing of polymeric RTP hydrogels (Fig. 10d).46 This fabrication process has achieved micron-scale precision and produced structures with outstanding mechanical performance. Importantly, the printed lattice structures maintained their completeness even after multiple repeated compression cycles (Fig. 10e).4.2 Bioimaging
The core challenge restricting the sensitivity and accuracy of bioimaging is how to accurately extract the target signal from the complex and auto-fluorescent biological background.71–75 Certain polymeric RTP hydrogels have ultralong emission lifetimes of milliseconds or even seconds. Researchers can use time-resolved imaging technology to collect long-lived RTP signals after the complete decay of short-lived background fluorescence, thereby achieving ultra-high signal-to-noise ratio imaging. For example, an NIR RTP supramolecular hydrogel was developed based on amphiphilic bromonaphthalimide pyridinium derivatives, nanosheets (LP), AAm and heptamethine cyanine. The hydrogel exhibits low cytotoxicity and good biocompatibility. The NIR emission signal of the RTP hydrogel in HeLa cells was observed by laser scanning microscopy, revealing distinct cellular contours (Fig. 11a).60 In addition, a NIR RTP hydrogel constructed through supramolecular polymerization using monoalkene-functionalized iodoisoquinolinium derivatives (G′), LP, rhodamine 800 ((Rh800)) and AAm monomers can also achieve similar effects (Fig. 11b).614.3 Anti-counterfeiting and information encryption
Tunable luminescent performance and the stimulus responsiveness of polymeric RTP hydrogels provide significant application value in anti-counterfeiting and information encryption.76–79Wu and colleagues used the quenching effect of halogen ions on polymeric RTP hydrogels and a fluorescein solution to make hydrogels display different information when the UV lamp was on and off, achieving information encryption. The strategy involves printing the real information “OFF” onto the hydrogel with KI solution. Then, the word “OPEN” is printed onto the hydrogel with fluorescein solution to cover and encrypt the word “OFF”. Upon irradiation under a UV lamp, the area printed with fluorescein had bright-yellow fluorescence, showing the word “OPEN”. Upon removal of the UV lamp, the KI printed area revealed the true information “OFF” due to the difference in phosphorescence intensity between the quenched area and unquenched area (Fig. 12a).56
Polymeric RTP hydrogels with excitation wavelength-dependent luminescence provide more possibilities for information encryption. Wang and colleagues obtained a series of excitation wavelength-dependent RTP hydrogels by changing the acid component in the hydrogel (PVA/BIU/PMAc) to alginic acid (Alg), poly(itaconic acid) (PITAc) or poly(maleic acid-co-vinyl pyrrolidone) (PMVPAc). The information code was constructed by hydrogel squares with different compositions, which can display various patterns under different wavelengths of excitation. Additionally, several types of hydrogels were used to form the letters “Y,” “O,” “U,” and “R.”, respectively. Under excitation of light at 254, 312 or 400 nm, all patterns showed the word “YOUR”. However, after turning off the light sources at these respective wavelengths, the patterns of “YOU”, “OUR” and “OR” could be observed separately (Fig. 12b).50
Furthermore, Lü et al. replaced the grafted chromophores in the polymeric RTP hydrogel (H-CzPA-co-PAM) with N-1-pyrenyl-2-propenamide (PyPA) to achieve regulation of afterglow color and duration. Also, a conceptual camouflage skin of marine animals was proposed. As shown in Fig. 12c, different polymeric RTP hydrogels were used as multiple functional skins of different deep-sea fishes. After removing the 365 nm UV excitation, the blue lure of the anglerfish attracts other fish (e.g., eyelight fish, marked in red) due to the phototactic of most deep-sea fish preying. The blue lure can also be used for communication between identical species. After removing the 365 nm UV excitation for 2 s, the blue lure was intelligently observed due to the difference in the afterglow time of different polymeric RTP hydrogels, achieving an underwater dynamic temporal information display.55
5. Summary and outlook
Polymeric RTP hydrogels are highly sensitive to environmental conditions, particularly water and oxygen, which lead to rapid quenching. The solution to these challenges lies in providing a rigid microenvironment for chromophores in hydrogels to inhibit non-radiative transitions, thereby achieving RTP emission. This review systematically summarizes the construction strategies for polymeric RTP hydrogels: physical doping, chemical grafting, and supramolecular polymerization. Furthermore, the applications of RTP hydrogels in 3D printing, bioimaging, and anti-counterfeiting technology are outlined. Undoubtedly, RTP hydrogels have made significant progress in achieving multifunctionality and stable afterglow, primarily through innovative molecular design and structural design of polymeric hydrogel networks. However, six critical shortcomings remain to be addressed. First, luminous efficiency is lower than that of traditional rigid RTP materials, and the RTP lifetime is relatively short. Second, there are difficulties in the transition from small-scale laboratory preparation to industrial production. Third, RTP performance varies at different positions during 3D printing. Fourth, the design of multi-stimuli-responsive polymeric RTP hydrogels. Fifth, UV excitation is commonly required to achieve RTP emission. Sixth, achieving full-spectrum RTP luminescence remains challenging, especially NIR phosphorescence.Given these challenges, research should focus on four main areas. First, it is necessary to improve the performance of polymeric RTP hydrogels by developing new chromophores and innovative polymer structure designs. Thus, on the premise of ensuring the advantages of flexibility, efforts should aim to endow polymeric RTP hydrogels with higher luminous efficiency, longer RTP lifetime, and wider spectral emission range. Advances in high-performance RTP hydrogels will drive technological innovations in anti-counterfeiting and information encryption.
Second, the synthetic routes of efficient organic RTP molecules are usually complex, involving multiple reaction steps, with relatively low yields. Gram-scale preparation is feasible, but challenges arise when scaling up to kilogram- or ton-scale production. The key indicators, such as the RTP properties of the final product, may fluctuate between batches, making it difficult to meet the stringent stability requirements for industrial products. The same problem also occurs in 3D printing due to the stability of the prepared RTP polymer ink and the layer-by-layer accumulation process involved in 3D printing. This leads to inconsistent RTP properties in different positions of 3D-printed products, as well as poor stability between batches. Whether it is large-scale production or 3D printing, the future breakthrough direction lies in the deep integration of molecular design, material engineering and process innovation.
Third, the hydrogel network can respond to external stimuli (e.g., pH, temperature, and specific molecules), resulting in changes in volume or conformation, and then manipulating RTP emission provides new opportunities for the design of multi-stimulus-response polymeric RTP hydrogels. The advantage of multi-stimulus-response polymeric RTP hydrogels is that they break the linear mode of “single stimulus–single response”, and then turn to the networked mode of “multi-input–intelligent processing–multidimensional output”. Thus, “intelligent” materials that can “sense” complex environments and make readable responses are constructed. This provides a new strategy for applications in advanced sensing, information anti-counterfeiting, and other fields.
Fourth, most traditional RTP materials require high-energy UV light excitation, which possesses strong penetration and energy, potentially damaging biological tissues. In contrast, visible light-excited RTP is not affected by these problems, offering vast applications in cutting-edge fields such as bioimaging and intelligent sensing. Owing to the deeper tissue penetration and lower biological background interference of NIR phosphorescence, the development of efficient NIR RTP hydrogels will be a key step in achieving deep, high-resolution in vivo imaging, which is extremely challenging but valuable. In addition, there have been relatively few biocompatibility tests conducted on the application of polymeric RTP hydrogels in bioimaging. Related studies on bioimaging should involve comprehensive assessment of biocompatibility, including cytotoxicity and blood compatibility, to ensure that imaging does not cause damage to the organism. In addition, the biodistribution and metabolic pathways of polymeric RTP hydrogels should be studied by in vivo imaging systems, and the systemic distribution, organ enrichment and clearance time of polymeric RTP hydrogels (or their degradation products) after injection or implantation should be dynamically monitored to assess their safety in bioimaging applications.
In conclusion, research on polymeric RTP hydrogels has moved from basic material synthesis to the development of function-oriented intelligent devices. It provides innovative solutions to solve challenging problems in biomedicine and information technology.
Author contributions
Tao Chen supervised the manuscript. Wei Lu and Xiaoye Zhang revised the manuscript. Weihao Feng wrote the manuscript. Muqing Si and Kuangzheng Cao contributed to manuscript preparation. All authors revised and finalized the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Acknowledgements
This work was supported by Natural Science Foundation of China (22322508), and Zhejiang Provincial Natural Science Foundation of China (LR23E030001).References
- Z. Li, X. Ji, H. Xie and B. Z. Tang, Adv. Mater., 2021, 33, 2100021 CrossRef CAS PubMed.
- S. Cai, Z. Sun, H. Wang, X. Yao, H. Ma, W. Jia, S. Wang, Z. Li, H. Shi, Z. An, Y. Ishida, T. Aida and W. Huang, J. Am. Chem. Soc., 2021, 143, 16256–16263 CrossRef CAS PubMed.
- X. Ma, J. Wang and H. Tian, Acc. Chem. Res., 2019, 52, 738–748 Search PubMed.
- J. Guo, C. Yang and Y. Zhao, Acc. Chem. Res., 2022, 55, 1160–1170 Search PubMed.
- L. Li, J. Zhou, J. Han, D. Liu, M. Qi, J. Xu, G. Yin and T. Chen, Nat. Commun., 2024, 15, 3846 Search PubMed.
- H. Sun, Q. Zhang, L. Meng, Z. Wang, Y. Fan, M. Mayor, M. Pan and C.-Y. Su, Chem. Sci., 2024, 15, 8905–8912 Search PubMed.
- H. Shi, W. Yao, W. Ye, H. Ma, W. Huang and Z. An, Acc. Chem. Res., 2022, 55, 3445–3459 CrossRef CAS.
- K. Benson, A. Ghimire, A. Pattammattel and C. V. Kumar, Adv. Funct. Mater., 2017, 27, 1702955 CrossRef.
- X. Yu, A. A. Ryadun, D. I. Pavlov, T. Y. Guselnikova, A. S. Potapov and V. P. Fedin, Adv. Mater., 2024, 36, 2311939 CrossRef CAS.
- Z. Zhang, L. Qian, B. Zhang, C. Ma and G. Zhang, Angew. Chem., Int. Ed., 2024, 63, e202410335 Search PubMed.
- Q. Dang, Y. Jiang, J. Wang, J. Wang, Q. Zhang, M. Zhang, S. Luo, Y. Xie, K. Pu, Q. Li and Z. Li, Adv. Mater., 2020, 32, 2006752 CrossRef.
- Y. Fan, S. Liu, M. Wu, L. Xiao, Y. Fan, M. Han, K. Chang, Y. Zhang, X. Zhen, Q. Li and Z. Li, Adv. Mater., 2022, 34, 2201280 CrossRef CAS.
- M. Cui, P. Dai, J. Ding, M. Li, R. Sun, X. Jiang, M. Wu, X. Pang, M. Liu, Q. Zhao, B. Song and Y. He, Angew. Chem., Int. Ed., 2022, 61, e202200172 Search PubMed.
- F. Xiao, H. Gao, Y. Lei, W. Dai, M. Liu, X. Zheng, Z. Cai, X. Huang, H. Wu and D. Ding, Nat. Commun., 2022, 13, 186 CrossRef CAS PubMed.
- M. Yao, W. Wei, W. Qiao, Y. Zhang, X. Zhou, Z. Li, H. Peng and X. Xie, Adv. Mater., 2025, 2414894 CrossRef CAS.
- H. Yang, S. Li, J. Zheng, G. Chen, W. Wang, Y. Miao, N. Zhu, Y. Cong and J. Fu, Adv. Mater., 2023, 35, 2301300 CrossRef CAS.
- H. Zhang, B. Zhang, C. Cai, K. Zhang, Y. Wang, Y. Wang, Y. Yang, Y. Wu, X. Ba and R. Hoogenboom, Nat. Commun., 2024, 15, 2055 CrossRef CAS.
- W. Luo, L. Chen, G. Yin, C. Yue, S. Xie, J. Zhou, W. Feng, Y. Nie, H. Qiu, F. Li, S. Cai, Y. Li, Z. Cai and T. Chen, Angew. Chem., Int. Ed., 2025, 64, e202423650 CrossRef CAS PubMed.
- Kenry, C. Chen and B. Liu, Nat. Commun., 2019, 10, 2111 CrossRef CAS.
- C. Chen, Z. Chi, K. Chong, A. S. Batsanov, Z. Yang, Z. Mao, Z. Yang and B. Liu, Nat. Mater., 2021, 20, 175–180 CrossRef CAS PubMed.
- X.-Y. Dai, M. Huo and Y. Liu, Nat. Rev. Chem., 2023, 7, 854–874 CrossRef CAS PubMed.
- D. Li, Z. Liu, M. Fang, J. Yang, B. Z. Tang and Z. Li, ACS Nano, 2023, 17, 12895–12902 CrossRef CAS.
- M. Roucan, M. Kielmann, S. J. Connon, S. S. R. Bernhard and M. O. Senge, Chem. Commun., 2018, 54, 26–29 RSC.
- Q. Yu, Z. Deng, R. Chen, J. Zhang, R. T. K. Kwok, J. W. Y. Lam, J. Sun and B. Z. Tang, J. Am. Chem. Soc., 2025, 147, 10530–10539 CrossRef CAS PubMed.
- S. Garain, S. N. Ansari, A. A. Kongasseri, B. Chandra Garain, S. K. Pati and S. J. George, Chem. Sci., 2022, 13, 10011 RSC.
- Y. Deng, P. Chen, X. Yang, N. Li, S. Ma, Z. Huang and S. Lü, Adv. Mater., 2025, 37, e14693 Search PubMed.
- F. Nie and D. Yan, Angew. Chem., Int. Ed., 2023, 62, e202302751 CrossRef CAS.
- B. Zhou, Z. Qi and D. Yan, Angew. Chem., Int. Ed., 2022, 61, e202208735 Search PubMed.
- Y. N. Ye, K. Cui, W. Hong, X. Li, C. Yu, D. Hourdet, T. Nakajima, T. Kurokawa and J. P. Gong, Proc. Natl. Acad. Sci. U. S. A, 2021, 118, e2014694118 Search PubMed.
- W. Lu, M. Si, X. Le and T. Chen, Acc. Chem. Res., 2022, 55, 2291–2303 Search PubMed.
- M. Hua, S. Wu, Y. Ma, Y. Zhao, Z. Chen, I. Frenkel, J. Strzalka, H. Zhou, X. Zhu and X. He, Nature, 2021, 590, 594–599 CrossRef CAS.
- L. Chen, Z. Jin, W. Feng, L. Sun, H. Xu and C. Wang, Science, 2024, 383, 1455–1461 CrossRef CAS PubMed.
- G. Zhang, J. Steck, J. Kim, C. H. Ahn and Z. Suo, Sci. Adv., 2023, 9, eadh7742 CrossRef CAS.
- W. Li, X. Wang, Z. Liu, X. Zou, Z. Shen, D. Liu, L. Li, Y. Guo and F. Yan, Nat. Mater., 2024, 23, 131–138 CrossRef CAS PubMed.
- X. Yao, J. Wang, D. Jiao, Z. Huang, O. Mhirsi, F. Lossada, L. Chen, B. Haehnle, A. J. C. Kuehne, X. Ma, H. Tian and A. Walther, Adv. Mater., 2021, 33, 2005973 CrossRef CAS.
- C. Zheng, S. Tao, X. Zhao, C. Kang and B. Yang, Angew. Chem., Int. Ed., 2024, 63, e202408516 CrossRef CAS PubMed.
- J. Xiao, J. Deng, X. Wang, H. Ho, C. Bai, Y. Bai and H. Wang, Small, 2024, 20, 2405615 CrossRef CAS.
- Y. Zhang, Y. Su, H. Wu, Z. Wang, C. Wang, Y. Zheng, X. Zheng, L. Gao, Q. Zhou, Y. Yang, X. Chen, C. Yang and Y. Zhao, J. Am. Chem. Soc., 2021, 143, 13675–13685 Search PubMed.
- S. Tang, S. Jiang, K. Wang, Y. Zhang, L. Yi, J. Hou, L. Qu, Y. Zhao and C. Yang, Adv. Mater., 2025, 37, 2416397 Search PubMed.
- Z. Man, Z. Lv, Z. Xu, J. He, Q. Liao, Y. Yang, J. Yao and H. Fu, J. Am. Chem. Soc., 2023, 145, 13392–13399 CrossRef CAS PubMed.
- J. Li, L. Zhang, C. Wu, Z. Huang, S. Li, H. Zhang, Q. Yang, Z. Mao, S. Luo, C. Liu, G. Shi and B. Xu, Angew. Chem., Int. Ed., 2023, 62, e202217284 CrossRef CAS.
- T. Zhu, S. Zheng, T. Yang and W. Z. Yuan, J. Phys. Chem. Lett., 2023, 14, 6451–6458 CrossRef CAS PubMed.
- M. Qi, J. Huang, J. Wei, J. Zhou, D. Liu, L. Li, W. Luo, G. Yin and T. Chen, Angew. Chem., Int. Ed., 2025, 64, e202501054 Search PubMed.
- X. Huang, Y. Zhang, G. Li, T. Wang, P. Sun, J. Shi, B. Tong, J. Zhi, Z. Li, Z. Cai and Y. Dong, ChemPhotoChem, 2024, 9, e202400315 CrossRef.
- M. Fang, J. Yang and Z. Li, Prog. Mater. Sci., 2022, 125, 100914 Search PubMed.
- Y. Cao, D. Wang, Y. Zhang, G. Li, C. Gao, W. Li, X. Chen, X. Chen, P. Sun, Y. Dong, Z. Cai and Z. He, Angew. Chem., Int. Ed., 2024, 63, e202401331 Search PubMed.
- J. Deng, H. Liu, D. Liu, L. Yu, Y. Bai, W. Xie, T. Li, C. Wang, Y. Lian and H. Wang, Adv. Funct. Mater., 2024, 34, 2308420 CrossRef CAS.
- X. Jiang, M. Wu, L. Zhang, J. Wang, M. Cui, J. Wang, X. Pang, B. Song and Y. He, Anal. Chem., 2022, 94, 7264–7271 CrossRef CAS PubMed.
- Y. Sun, L. Jiang, Y. Chen and Y. Liu, Chin. Chem. Lett., 2024, 35, 108644 Search PubMed.
- J. Deng, D. Liu, H. Liu, L. Yu, Y. Bai, J. Xiao and H. Wang, Adv. Funct. Mater., 2024, 34, 2408821 CrossRef CAS.
- R. Liu, H. Guo, S. Liu, J. Li, S. Li, T. D. James and Z. Chen, Nat. Commun., 2024, 15, 10588 Search PubMed.
- W. Feng, F. Li, Z. Jiang, C. Yue, G. Yin, N. Zhu, K. Zhang, T. Chen and W. Lu, Angew. Chem., Int. Ed., 2025, 64, e202505192 Search PubMed.
- H. Chen, X. Ma, S. Wu and H. Tian, Angew. Chem., Int. Ed., 2014, 53, 14149–14152 Search PubMed.
- H. Chen, L. Xu, X. Ma and H. Tian, Polym. Chem., 2016, 7, 3989–3992 Search PubMed.
- P. Chen, H. Qie, X. Yang, S. Ma, Z. Wang, N. Li, Y. Deng, F. Bian and S. Lü, Adv. Funct. Mater., 2025, 35, 2416430 Search PubMed.
- H. Ju, H. Zhang, L. X. Hou, M. Zuo, M. Du, F. Huang, Q. Zheng and Z. L. Wu, J. Am. Chem. Soc., 2023, 145, 3763–3773 CrossRef CAS PubMed.
- Z. Zhang, H. Ju, H. Zhang, Z. Wang, M. Du, H. Li, F. Huang, Q. Zheng and Z. L. Wu, Adv. Mater., 2025, 37, 2505444 Search PubMed.
- S. Yu, J. Zhang, X. Chen, X. Wu, X. Zhao, Z. Zhu, J. Zhang, Y. Zuo and C. Zhao, Adv. Opt. Mater., 2024, 12, 2303330 Search PubMed.
- S. Garain, B. C. Garain, M. Eswaramoorthy, S. K. Pati and S. J. George, Angew. Chem., Int. Ed., 2021, 60, 19720–19724 CrossRef CAS.
- Q. Song, Z. Liu, J. Li, Y. Sun, Y. Ge and X. Dai, Adv. Mater., 2024, 36, 2409983 CrossRef CAS PubMed.
- Q. Song, Z. Liu, X. Bai, Y. Zhang, S. Yang, Y. Ge and X. Dai, Adv. Opt. Mater., 2025, 13, 2500624 CrossRef CAS.
- X. Sun, H. Yang, W. Zhang, S. Zhang, J. Hu, M. Liu, X. Zeng, Q. Li, C. Redshaw, Z. Tao and X. Xiao, ACS Appl. Mater. Interfaces, 2023, 15, 4668–4676 Search PubMed.
- W. Zhao, Z. He and B. Z. Tang, Nat. Rev. Mater., 2020, 5, 869–885 Search PubMed.
- G. Baryshnikov, B. Minaev and H. Ågren, Chem. Rev., 2017, 117, 6500–6537 CrossRef CAS PubMed.
- H. Sun, M. He, G. V. Baryshnikov, B. Wu, R. R. Valiev, S. Shen, M. Zhang, X. Xu, Z. Li, G. Liu, H. Ågren and L. Zhu, Angew. Chem., Int. Ed., 2024, 63, e202318159 CrossRef CAS PubMed.
- G. Yin, W. Lu, J. Huang, R. Li, D. Liu, L. Li, R. Zhou, G. Huo and T. Chen, Aggregate, 2023, 4, e344 CrossRef CAS.
- Z. Xie, P. Sun, Z. Wang, H. Li, L. Yu, D. Sun, M. Chen, Y. Bi, X. Xin and J. Hao, Angew. Chem., Int. Ed., 2020, 59, 9922–9927 CrossRef CAS PubMed.
- J. Chen, F. Lin, D. Guo, T. Tang, Y. Miao, Y. Wu, W. Zhai, H. Huang, Z. Chi, Y. Chen and Z. Yang, Adv. Mater., 2024, 36, 2409642 CrossRef CAS PubMed.
- H. Sun, Y. Xiao, Y. He, X. Wei, J. Zou, Y. Luo, Y. Wu, J. Zhao, V. K.-M. Au and T. Yu, Chem. Sci., 2025, 16, 5299–5309 RSC.
- P. Wu, P. Li, M. Chen, J. Rao, G. Chen, J. Bian, B. Lue and F. Peng, Adv. Mater., 2024, 36, 2402666 CrossRef CAS.
- J. Zhu, L. Luo, M. Gu, Y. Chen, L. Wang, M. Li, X. Chen and X. Peng, CCS Chem., 2025, 7, 1552–1566 CrossRef CAS.
- J. Yuan, H. Yang, W. Huang, S. Liu, H. Zhang, X. Zhang and X. Peng, Chem. Soc. Rev., 2025, 54, 341–366 RSC.
- T. Xiong, Y. Chen, Q. Peng, M. Li, S. Lu, X. Chen, J. Fan, L. Wang and X. Peng, J. Am. Chem. Soc., 2024, 146, 24158–24166 CrossRef CAS.
- H. Gao, T. Zhang, Y. Lei, D. Jiao, B. Yu, W. Z. Yuan, J. Ji, Q. Jin and D. Ding, Angew. Chem., Int. Ed., 2024, 63, e202406651 Search PubMed.
- L. Li, D. Liu, J. Zhou, M. Qi, G. Yin and T. Chen, Mater. Horiz., 2024, 11, 5895–5913 Search PubMed.
- Y. Shen, X. Le, Y. Wu and T. Chen, Chem. Soc. Rev., 2024, 53, 606–623 RSC.
- J. Xiang, Y. Shi, J. Jiang, J. Yan, S. Xiao, H. Lin and T. Yi, Adv. Opt. Mater., 2024, 12, 2302671 CrossRef CAS.
- J. Wei, Y. Xiao, J. Luo, Z. He, J. Chen, Q. Peng and D. Kuang, Chem. Sci., 2025, 16, 7239–7248 RSC.
- J. Xue, Z. Qi, D. Yan, G. Yang and Y. Wang, Angew. Chem., Int. Ed., 2025, 64, e202501951 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |











