Open Access Article
Open Access ArticleCross-scale understanding of cascade electrocatalysis for carbon and nitrogen utilization
Shiping
Li
a,
Youyu
Pang
ab,
Hongmei
Li
a,
Rui
Yang
c,
Zhaoyu
Jin
 c,
Guihua
Yu
c,
Guihua
Yu
 *d and
Panpan
Li
*d and
Panpan
Li
 *a
*a
aCollege of Materials Science and Engineering, Sichuan University, Chengdu, 610065, China. E-mail: panpanli@scu.edu.cn
bCollege of Materials Science and Engineering, Chongqing Jiaotong University, Chongqing, 400074, China
cInstitute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu 611731, China
dMaterials Science and Engineering Program, Walker Department of Mechanical Engineering, The University of Texas at Austin, Austin, Texas 78712, USA. E-mail: ghyu@austin.utexas.edu
First published on 10th December 2025
Abstract
Cascade electrocatalysis is increasingly being recognized as a promising approach for achieving efficient and selective conversion of carbon and nitrogen resources, in response to critical challenges in energy sustainability and environmental management. Compared to conventional single-site systems, cascade pathways are designed to enable spatial and temporal coordination of multiple reaction steps, through which the generation, migration, and transformation of key intermediates can be precisely controlled. In this review, a multiscale perspective is provided, spanning atomic, sub-nanoscale, nanoscale, and macroscopic dimensions. Recent progress is summarized in several representative strategies, including the use of multiple active sites for relay catalysis, the engineering of crystal facets and heterointerfaces, the application of nanoconfinement, and the implementation of tandem reactor systems. Particular emphasis is placed on their applications in CO2 reduction, nitrate reduction, and urea electrosynthesis. Moreover, state-of-the-art in situ characterization techniques are highlighted, through which dynamic insights into catalyst evolution and intermediate behaviour have been obtained. Finally, opportunities are outlined for future development, where rational catalyst design, integrated system construction, and data-driven optimization are expected to further advance cascade electrocatalysis for sustainable chemical transformations.
Introduction
Carbon and nitrogen cycles are fundamental processes that govern Earth's climate regulation and resource metabolism.1–3 However, intensified human activities have profoundly disturbed the equilibrium of these two cycles. In the case of the nitrogen cycle, although the traditional Haber–Bosch process can effectively reduce nitrogen to synthesize ammonia, its high temperature and pressure requirements not only result in enormous energy consumption but also cause severe environmental impacts.4,5 In contrast, electrochemical approaches, which can achieve the reaction under mild conditions and directly utilize renewable electricity, are considered as more sustainable alternatives. Nevertheless, electrochemical nitrogen reduction suffers from low efficiency due to the strong N≡![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond strength (945 kJ mol−1) and resultant high reaction overpotentials.6–8 Nitrate, with its lower N–O bond dissociation energy, is more readily reduced electrochemically and is viewed as a promising alternative nitrogen source for ammonia synthesis.9,10 Similarly, the carbon cycle plays a crucial role in addressing climate change and global warming, but excessive CO2 emissions have intensified the greenhouse effect, creating an urgent demand for carbon capture and conversion technologies. CO2 electroreduction, as a potential carbon resource utilization method, has emerged as a research focus.11,12 However, both CO2 and NO3− electroreduction reactions involve complex multistep reaction pathways and face challenges such as slow reaction kinetics, poor selectivity, and high energy barriers.13 Due to thermodynamic and kinetic limitations, traditional single-step electrochemical reduction processes lack sufficient control over key intermediates, thereby leading to low utilization rates and poor product selectivity, which severely hampers the efficient and directional conversion of carbon and nitrogen resources.
N bond strength (945 kJ mol−1) and resultant high reaction overpotentials.6–8 Nitrate, with its lower N–O bond dissociation energy, is more readily reduced electrochemically and is viewed as a promising alternative nitrogen source for ammonia synthesis.9,10 Similarly, the carbon cycle plays a crucial role in addressing climate change and global warming, but excessive CO2 emissions have intensified the greenhouse effect, creating an urgent demand for carbon capture and conversion technologies. CO2 electroreduction, as a potential carbon resource utilization method, has emerged as a research focus.11,12 However, both CO2 and NO3− electroreduction reactions involve complex multistep reaction pathways and face challenges such as slow reaction kinetics, poor selectivity, and high energy barriers.13 Due to thermodynamic and kinetic limitations, traditional single-step electrochemical reduction processes lack sufficient control over key intermediates, thereby leading to low utilization rates and poor product selectivity, which severely hampers the efficient and directional conversion of carbon and nitrogen resources.
Cascade electrocatalysis, a strategy that converts reactants through multiple reaction steps, provides a new approach to address carbon and nitrogen resource utilization challenges. Cascade electrocatalysis refers to the integration of multiple electrocatalytic reaction steps in space and time, where different active sites sequentially transform intermediates, thus overcoming the thermodynamic and kinetic constraints of a single reaction step.14,15 In microbes, the conversion of NO3− to NH3 is a cascade reaction: nitrate reductase reduces NO3− to NO2−, which is then further reduced to NH3 by nitrite reductase or nitrogenase.16–18 Inspired by this, researchers have started applying cascade electrocatalysis to carbon and nitrogen resource utilization. Cascade electrocatalysis allows multiple electrocatalytic reactions to occur in series on a single catalyst surface or within a reaction system, not only overcoming the thermodynamic and selectivity limitations in nitrogen and carbon conversion but also significantly improving reaction efficiency and product selectivity.19,20 Current research on cascade electrocatalysis primarily focuses on studies at a single scale, such as investigating single-atom catalysis mechanisms at the atomic scale. While such studies help to elucidate the reaction mechanisms of individual active sites, they face numerous limitations, such as limited compatibility of single active sites and hindered interfacial mass transfer. To address these limitations, cross-scale cooperative effects have drawn increasing attention. In cascade catalytic systems, structural features and reaction behaviors at different scales are closely coupled. At the atomic scale, active sites govern the adsorption and transformation pathways of key intermediates. At the nanoscale, interfacial and confinement structures regulate intermediate migration, short-range spillover, and the local reaction microenvironment. At the reactor scale, mass-transfer rates, electrolyte distribution, and pH collectively dictate overall reaction kinetics and efficiency21,22 Integrating these multiscale strategies enables precise control over the spatiotemporal distribution of reaction intermediates, thereby establishing an efficient and selective cascade pathway for complex multistep reactions. However, this cross-scale research also faces challenges, including the limitations of multiscale modeling and characterization techniques, the complexity of reaction mechanisms, and the challenges of material design. Through a deeper understanding and optimization of cascade electrocatalysis mechanisms at multiple scales, future advancements could offer viable solutions to the global energy crisis and environmental pollution issues.
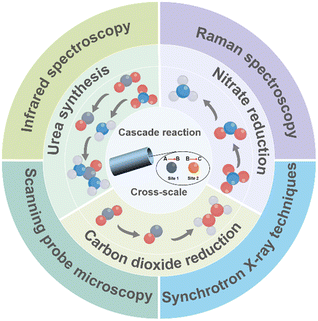
This review provides a comprehensive assessment of the mechanisms and applications of cascade electrocatalysis from a multiscale perspective (Fig. 1). It begins by elucidating the reaction mechanisms underlying cascade electrocatalysis, encompassing atomic, sub-nanoscale, nanometer, and macroscopic scales. The review then highlights in situ characterization techniques employed to study cascade electrocatalysis. Subsequently, it summarizes key advancements in cascade electrocatalytic strategies, including multiple active sites, crystal facets and heterointerfaces, nanoconfinement and tandem reactors with a particular focus on their application in the utilization of carbon and nitrogen resources, such as nitrate reduction reaction (NO3RR), carbon dioxide reduction reaction (CO2RR), and urea synthesis. Finally, the challenges facing the field of cascade electrocatalysis are discussed, along with a forward-looking perspective on its future prospects, aiming to promote the development of cascade electrocatalysis in the field of electrochemical technologies.
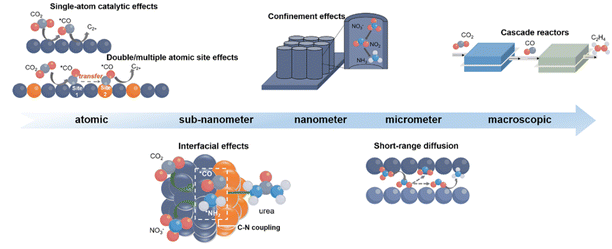
Cross-scale strategies for cascade electrocatalysis
Cascade electrocatalytic reactions, involving sequential transformations, hold immense potential for efficient resource conversion. However, their mechanistic complexity remains a formidable challenge. At the atomic scale, catalytic behaviour is dictated by single-atom effects and synergistic interactions between multi-atom sites. At the nanoscale, catalytic performance relies on the interplay between surface structure and reactant dynamics. Given the diversity of intermediates and their varied migration pathways, single-scale analyses are insufficient to capture the full mechanistic landscape. Therefore, a comprehensive multiscale approach is indispensable for elucidating the formation, transformation, and coupling of intermediates, thereby laying a robust theoretical foundation for cascade electrocatalysis. This section systematically explores the mechanisms at the atomic, sub-nanoscale, nanoscale, and macroscopic levels (Fig. 2), highlighting the distinct contributions of each to cascade processes.Atomic scale: single-atom and dual/multi-atom site interactions
Cascade electrocatalysis achieves high efficiency through consecutive reactions, with atomic-scale insights proving pivotal for optimizing both selectivity and conversion. At this scale, single-atom and dual- and multi-atom site effects govern catalytic performance. Single-atom catalysts (SACs) utilize isolated metal atoms as active centers, exploiting their distinct electronic structures and maximizing atomic utilization to enhance reaction kinetics and selectivity.23 In the NO3RR, SACs provide well-defined active sites and efficient electron transfer pathways, thereby lowering energy barriers and accelerating reaction rates.24–27 Li et al.28 developed a copper-based single-atom gel (Cu SAGs) catalyst that selectively adsorbs and activates nitrite intermediates at single-atom sites during the cascade NO3RR. This configuration effectively suppresses the competing hydrogen evolution reaction (HER), enabling highly selective NO3− to NH3 conversion under neutral conditions. This work demonstrates the synergistic integration of pulse electrolysis and three-dimensional (3D) structured SAGs, facilitating highly efficient NO3− to NH3 conversion via tandem catalysis of otherwise unfavorable intermediates.Beyond single-atom effects, dual- and multi-atom catalysts leverage cooperative interactions between adjacent metal centers to modulate intermediate adsorption and migration, thereby enhancing both activity and selectivity.29–31 In the CO2RR and NO3RR, such bimetallic sites can establish energetically favorable tandem pathways.32 For instance, Xie et al.33 reported a MOF-supported Pd–Cu bimetallic catalyst that promotes CO2 to C2+ conversion through a tandem mechanism: Cu facilitates *CO adsorption and C–C coupling, while Pd stabilizes *CH2CHO intermediates, achieving a C2+ faradaic efficiency (FE) of 81.9%. Similarly, Yan et al.34 designed a Cu–Ni bimetallic MOF catalyst where Cu reduces NO3− to NO2−, and Ni subsequently converts NO2− to NH3. Although SACs exhibit high atom utilization and selectivity, their isolated nature limits the cooperative interactions crucial for multistep transformations.35 In contrast, dual- and multi-atom configurations enable spatially adjacent, functionally complementary active centers that facilitate sequential intermediate conversion and minimize diffusion losses.36,37
Sub-nanoscale: interfacial effects
At the sub-nanoscale, interfacial effects arising from charge redistribution and atomic coordination at boundaries between distinct catalytic phases play a pivotal role in intermediate stability, charge transfer kinetics, and reaction selectivity.38 These interfacial regions can significantly influence reaction pathways by modulating local electronic structures and adsorption energetics.39 Metal–metal interfaces often exhibit catalytic behavior that differs markedly from that of their individual components. As demonstrated by Zhang et al.,40 a well-designed Cu/Ag interface promotes asymmetric CO–CHO coupling: Ag domains generate *CO intermediates, while the Cu/Ag interface concurrently suppresses the HER, favors *CO hydrogenation to *CHO, and enhances C2H4 formation. Similarly, metal–support interactions can substantially tune electronic states and surface reactivity. Xu et al.41 developed a MOF-derived CuPd/N-doped carbon nanoarrays catalyst, wherein interfacial charge polarization between the metal sites and carbon support facilitates an efficient cascade pathway for the NO3RR to NH3.Nanoscale: confinement effects
Confinement effects in nanostructured catalysts create spatially restricted environments that enhance reactant-site interaction frequency and minimize diffusion distances.42,43 This spatial confinement accelerates reaction kinetics while suppressing side reactions, thereby improving selectivity.44 For instance, Qiu et al.45 validated this concept in a nanoconfinement-based cascade denitrification system, utilizing pulsed photocatalysis to mediate chlorine spillover within TiO2 nanotubes integrated with CuOx. In this confined environment, cathodically generated NH4+ and photogenerated chlorine radicals (˙Cl) are localized in close proximity. The spillover of ˙Cl facilitates the selective oxidation of NH4+ to N2. This approach demonstrates highly efficient treatment of nitrate-contaminated wastewater and holds great promise for the removal of complex nitrate pollutants from water bodies in future applications.Macroscopic scale: reactor cascading
At the macroscopic scale, cascade electrocatalysis can be engineered through reactor-level integration, where sequential reactions are spatially separated into interconnected electrolyzers. In these systems, the first cell produces intermediates that are subsequently converted in downstream reactors under optimized conditions of potential, pH, and electrolyte composition.46 This modular approach enables precise reaction control and enhanced product selectivity. Ozden et al.47 developed a two-step CO2 to C2H4 system that integrates a solid oxide electrolyzer cell (for CO2 to CO conversion) with a membrane electrode assembly (for CO to C2H4 reduction). This configuration minimizes CO2 loss to carbonate formation and reduces total energy consumption by nearly 48%, achieving efficient ethylene synthesis. Moreover, hybrid cascade systems can further integrate electrochemical, thermocatalytic, or biocatalytic processes. For example, Xia et al.48 presented a tandem electrothermal-catalytic system that efficiently utilizes cathodic CO and anodic O2 products in the CO2RR, converting arylboronic acids and amines into valuable organic amides within a tandem thermocatalytic reactor. This study demonstrates the feasibility of a tandem electrothermal-catalytic strategy for achieving CO2 conversion and amide synthesis. It offers a novel pathway for comprehensively exploiting CO2 electrolysis. In addition, Han et al.49 presented a spatially decoupled electrobiological system that first utilizes tannic acid-Cu2+ metal-phenolic networks to electrocatalytically convert CO2 into ethanol. Subsequently, engineered Escherichia coli ferment the ethanol into itaconic acid, isopropanol, and polyhydroxybutyrate, achieving efficient conversion of CO2 into high-value chemicals.Advanced in situ techniques for probing cascade reactions
In cascade electrocatalytic reactions, the intricate and interdependent nature of successive reaction steps demands precise coordination among active sites. Achieving a comprehensive understanding of both the underlying reaction mechanisms and the catalyst's performance under working conditions is therefore essential. In situ characterization techniques serve as powerful, real-time analytical tools, enabling the direct observation of reaction dynamics. These techniques provide direct insights into the evolution of catalyst surface microstructures and reaction processes, thereby furnishing critical experimental evidence that can inform the optimization of catalytic performance and the design of advanced catalysts. This section highlights several widely utilized in situ techniques and underscores their pivotal contributions to advancing cascade electrocatalysis research.Synchrotron X-ray techniques
Synchrotron X-ray techniques, particularly X-ray absorption spectroscopy (XAS), have found extensive application in elucidating the electronic structure, oxidation states, and local coordination environments of catalysts (Fig. 3a). Specifically, in situ extended X-ray absorption fine structure (EXAFS) analysis enables the tracking of variations in the coordination environments of absorbing atoms under reaction conditions, thus facilitating the determination of atomic coordination states. Concurrently, in situ X-ray absorption near-edge structure (XANES) offers insights into potential-dependent chemical state changes.50–52 In cascade electrocatalysis, XAS is instrumental in monitoring valence state evolution within tandem catalysts across multiple reaction stages, thereby providing mechanistic insights for each step.53 For instance, Lou et al.54 employed XAS to quantitatively analyze the atomic structure of a Janus Cu@Ni catalyst (Fig. 3b and c). EXAFS confirmed the distinct Cu- and Ni-enriched domains, while XANES, in conjunction with XPS, revealed electron transfer from Ni to Cu. These findings corroborated a tandem mechanism, wherein NO3− adsorption and activation occur on Cu sites, while H species generated on Ni sites migrate to the interface, facilitating N–O bond cleavage. Such studies elucidate the intrinsic nature of multi-site synergy and cascade reaction mechanisms at the electronic level, advancing our understanding of the coupling between charge transfer and reaction pathways in catalytic processes.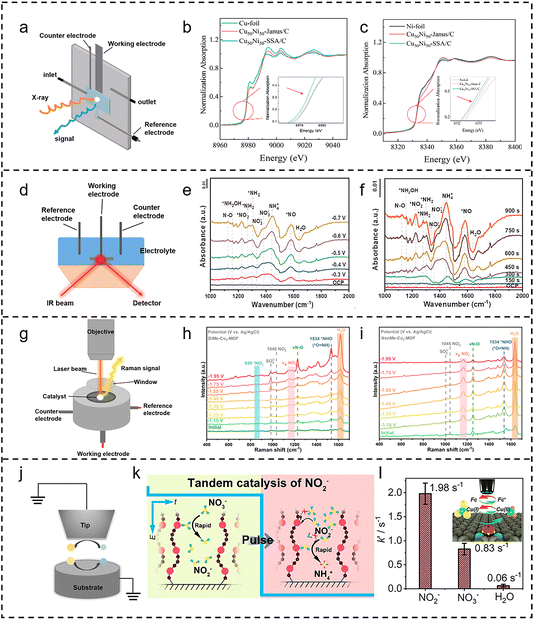 | ||
| Fig. 3 In situ characterization techniques. (a) Schematic diagram of in situ XAS. (b) Cu K-edge and (c) Ni K-edge XANES spectra of Cu50Ni50-Janus/C, Cu50Ni50-SSA/C, and reference samples. Reproduced with permission.54 Copyright 2024, American Chemical Society. (d) Schematic diagram of in situ FTIR spectroscopy. In situ FTIR spectra of Cu5/Mo0.6WO3 (e) from OCP to −0.7 V vs. RHE, and (f) from OCP (0 s) to 900 s at −0.7 V vs. RHE. Reproduced with permission.55 Copyright 2025, Wiley-VCH. (g) Schematic diagram of in situ Raman spectroscopy. In situ Raman spectra of (h) DiMe-Cu3-MOF and (i) NonMe-Cu3-MOF at various applied potentials in 50 mM KNO3 and 0.5 M K2SO4 electrolytes. Reproduced with permission.56 Copyright 2025, Royal Society of Chemistry. (j) Schematic diagram of in situ SECM. (k) Schematic diagram of the NO3RR via pulse electrolysis cascade catalysis. (l) Kinetic rate constants of NO2−, NO3−, and H2O adsorption on Cu SAGs determined with SI-SECM. Reproduced with permission.28 Copyright 2023, American Chemical Society. | ||
Infrared spectroscopy
Fourier-transform infrared spectroscopy (FTIR) detects molecular vibrations via infrared absorption at characteristic frequencies, offering real-time, molecular-level insights into the chemical species involved in electrocatalytic processes.57,58Fig. 3d illustrates a schematic of the FTIR setup. Within cascade electrocatalytic systems, this technique allows for the direct monitoring of adsorbed reactants, intermediates, and products on electrode surfaces, facilitating the identification of intermediate species within the cascade pathway and their transformation processes. When integrated with complementary techniques, FTIR can effectively track the formation, conversion, and consumption of key intermediates, offering crucial evidence for understanding reaction kinetics, electron transfer, and molecular interactions.59 Dai et al.55 utilized FTIR to confirm the cascade mechanism of a Cu/Mo-WO3 catalyst, dynamically capturing the spectral evolution of key intermediates. The depletion of certain peaks and the emergence of new ones provided real-time evidence of NO3− reduction to NO2− and subsequent conversion to NH3, validating the proposed stepwise mechanism (Fig. 3e and f). Such real-time spectral tracking under operando conditions underscores the power of FTIR in unraveling complex cascade reaction pathways.Raman spectroscopy
Raman spectroscopy, which integrates laser excitation with electrochemical measurements, provides valuable information on chemical composition and molecular structure by detecting characteristic shifts in scattered light (Fig. 3g).60,61 This technique offers real-time tracking of intermediates, enabling the precise tracking of pathway changes in complex cascade reactions.62 Such molecular-level insight is essential for correlating structural dynamics with the optimization of catalytic performance. For instance, Fu et al.56 utilized Raman spectroscopy to monitor the characteristic peaks of the NHO intermediate and dynamic Cu–N bond variations on DiMe-Cu3-MOF and NonMe-Cu3-MOF surfaces (Fig. 3h and i). Based on the Raman results, the *NHO intermediate was detected on both DiMe-Cu3-MOF and NonMe-Cu3-MOF, whereas the *NOH intermediate was not detected, indicating that NO3− is reduced to NH3via the NHO pathway. Combined with DFT calculations, the results revealed a 0.32 eV reduction in the energy barrier for *NO2 to *NO2H conversion through hydrogen bonding, thus optimizing the cascade pathway toward highly selective and industrially relevant NO3RR to NH3 conversion.Scanning probe microscopy
Scanning electrochemical microscopy (SECM) is a high-resolution in situ electrochemical imaging technique that enables the study of local surface reactivity with micrometer spatial and millisecond-scale temporal resolution (Fig. 3j).63,64 Using micro- or nanoelectrode probes, SECM quantifies current responses between the probe and the substrate, allowing real-time mapping of localized reaction activity, kinetics, and intermediate distribution.65 In cascade electrocatalysis, SECM is particularly useful for visualizing how intermediates form and dissipate at different active sites. For example, in NO3RR and CO2RR, SECM has been employed to track key intermediates such as NO2− and CO, elucidating reaction coupling between spatially separated active centers.66–68 He et al.69 utilized the surface generation-tip collection (SG-TC) mode to detect NO2− and NH3 formed on a Cu/Co(OH)2 model catalyst during NO3− electroreduction. NH3 generation significantly increased at the Cu-Co(OH)2 interface, accompanied by a marked decrease in NO2− production. These results confirmed that NO2− preferentially forms on Cu sites and subsequently diffuses to adjacent Co(OH)2 sites, where it undergoes further reduction to NH3via a tandem process. In another study, Li et al.28 employed surface interrogation scanning electrochemical microscopy (SI-SECM) to demonstrate that a Cu SAG catalyst exhibited a substantially higher adsorption affinity for NO2− than for NO3− or H2O (Fig. 3k and l). This finding established a potential-dependent cascade mechanism, where intermediate accumulation was observed at low potentials followed by efficient conversion to NH3 at high potentials, enabling highly efficient NO3RR.In addition to these techniques, in situ electron microscopy, such as liquid-cell transmission electron microscopy (TEM), is emerging as a powerful approach for visualizing catalyst structural evolution during electrochemical reactions.70–72 While its application to cascade electrocatalysis remains limited, it holds significant promise. However, current in situ characterization methods still face notable limitations. Their temporal and spatial resolutions are often insufficient to fully capture the short-lived intermediates and transient changes at nanoscale active sites during cascade reactions, and it remains challenging to simultaneously resolve the dynamic evolution of catalyst structures and reaction intermediates. Given the strong complementarity among different in situ techniques, a comprehensive understanding of cascade electrocatalytic mechanisms requires the combined use of multiple in situ methods to compensate for individual weaknesses and to investigate the reaction from distinct temporal and spatial perspectives. For example, coupling synchrotron-based X-ray methods with in situ vibrational spectroscopy enables the concurrent tracking of catalyst structural evolution and the formation and consumption of intermediates. Moreover, advancing in situ techniques with higher temporal and spatial resolution, such as ultra-high-resolution SECM, will provide powerful tools for probing mechanistic pathways in greater detail.
Recent progress on cascade electrocatalysis
Carbon and nitrogen resource utilization is a pivotal link in advancing sustainable energy and green chemistry.73–75 However, typical CO2RR, NO3RR, and their coupled reactions often involve multi-electron/proton transfers and the dynamic migration of various reaction intermediates. This presents a series of challenges, including sluggish reaction kinetics, complex competitive pathways, and low product selectivity.22,76–84 Traditional single-step electrocatalysis often suffers from intermediate accumulation, low energy efficiency, and limited product selectivity in these reactions. In contrast, cascade electrocatalysis, where multiple reaction steps are spatially and temporally integrated, offers a new avenue for efficient carbon–nitrogen resource utilization by enabling effective intermediate generation, transport, and transformation. This section discusses recent advancements in cascade electrocatalytic systems targeting carbon and nitrogen resource conversion.Relay catalysis with multiple active sites
The strategy of relay catalysis with multiple active sites leverages the different selectivities of various sites for specific reaction steps, decoupling elementary steps within complex reaction networks in space. This approach utilizes the cooperative effect between sites to integrate each reaction step in a relay fashion, optimizing the functional complementarity and energy matching between active sites, thereby significantly enhancing overall reaction efficiency and product selectivity.85,86 In the typical multi-electron and multi-proton transfer process of the NO3RR, researchers have successfully achieved precise regulation of reaction pathways and directional transformation of intermediates by rationally designing multiple active site catalysts, significantly improving catalytic performance and opening new avenues for the development of efficient NO3RR processes.87–92 For instance, Qin et al.93 designed an atomically precise Ag30Pd4 bimetallic nanocluster, where Ag sites preferentially adsorb NO3− and efficiently convert it into NO2− intermediates. Subsequently, the NO2− intermediate migrates directionally to the adjacent Pd sites for deep reduction to the final product NH3. This study decoupled the NO3RR steps and allocated them to Ag and Pd sites, achieving high FE for NH3 production (>90%). Furthermore, external field control can effectively enhance the relay catalysis of the NO3RR via multiple active sites. For instance, Liu et al.94 designed a Ni-modified copper oxide single-atom alloy (Ni1Cu SAAO) catalyst, which achieved efficient NO3RR to NH3via a thermally-coupled electrocatalytic strategy. The cascade reaction mechanism, shown in Fig. 4a, involved heating (60–80 °C), which significantly promoted the hydrolysis of Ni single-atom sites to generate active hydrogen. These active hydrogens were relayed to neighboring Cu sites, accelerating the hydrogenation of NOx intermediates (e.g., NO2) adsorbed on the Cu surface, thereby overcoming the kinetic barriers of NOx hydrogenation and the competition with the HER. This catalyst exhibited optimized NH3 production performance at elevated temperatures, with a FE of approximately 80% and a rate of 9.70 mg h−1 cm−2 (Fig. 4b).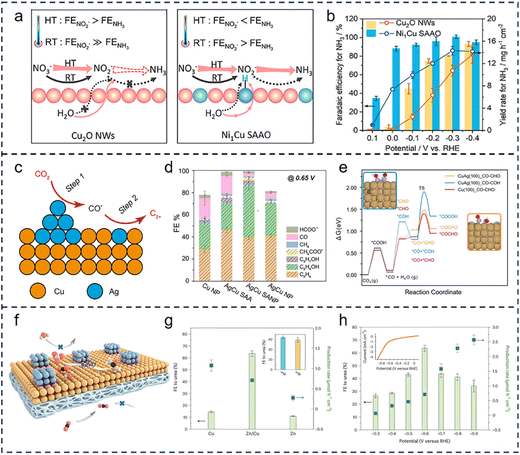 | ||
| Fig. 4 Relay catalysis with multiple active sites. (a) Schematic diagram of the electrothermal synergistic effect of Cu2O NWs and Ni1Cu SAAO in the NO3RR process. (b) FE and yield of NH3 for Cu2O NWs and Ni1Cu SAAO at different applied potentials. Reproduced with permission.94 Copyright 2024, American Chemical Society. (c) Scheme of the cascade catalysis mechanism over AgCu SANP. (d) FE results of Cu NP, AgCu SAA, AgCu SANP, and Ag NP catalysts toward the CO2RR at −0.65 V. (e) Comparison of the C–C coupling activation barrier for the *CHO (on CuAg and pure Cu) and *COH intermediates (CuAg). Reproduced with permission.96 Copyright 2023, Springer Nature. (f) Mixed catalyst design where one component (blue) lowers the activation energy for C–N bond formation, and the second component (orange) reduces the activation energy for protonation steps required for urea formation. (g) Zn/Cu hybrid catalyst and its single-component counterparts for urea FE. (h) FE and yield of urea on the Zn-0.5/Cu hybrid catalyst at different applied potentials. Reproduced with permission.1 Copyright 2023, Springer Nature. | ||
In addition, relay catalysis with multiple active sites can significantly promote key processes such as C–C coupling and C–N coupling, enabling the efficient synthesis of high-value multi-carbon or nitrogen-containing compounds.95 Du et al.96 reported an Ag–Cu bimetallic catalytic system that demonstrated exceptional performance in the CO2RR. In this system, Ag nanoparticles efficiently reduced CO2 to CO, while the Ag sites induced compression strain in the Cu lattice, enhancing the adsorption energy of the *CO intermediate on the Cu sites and significantly promoting C–C coupling kinetics, thereby achieving a high FE for multi-carbon products (94%) (Fig. 4c–e). Similarly, Luo et al.1 designed a Zn/Cu bimetallic catalyst that further extended the application of tandem catalysis in C–N coupling reactions. As shown in Fig. 4f, the Zn sites preferentially stabilized the key C–N coupling intermediate CO2NO3, while the Cu sites relayed the reduction of the COOHNH2 protonation energy barrier. This strategy successfully optimized the reaction pathway and suppressed side reactions, achieving 75% selectivity in simulated wastewater containing 1000 ppm NO3− (Fig. 4g and h). In summary, multi-active-site catalysts can sequentially activate different reactants and intermediates based on the selective specificity of different sites toward reaction steps. This enables them to regulate the energy barriers and pathways of key steps, thereby achieving tandem catalysis for complex reactions. However, cascade strategies based on multiple active sites still face limitations. These include insufficient microenvironmental compatibility between sites, restricted migration of reaction intermediates, and the structural complexity of catalysts, which complicates mechanistic analysis and undermines stability.97 Integrating multiscale approaches can help align individual steps more effectively and thereby improve the overall efficiency and stability of cascade reactions. Table 1 summarizes the catalytic processes of various multiple active site catalysts. This strategy provides important theoretical and experimental paradigms for the design of high-performance cascade catalytic systems and the high-value conversion of carbon and nitrogen cycles.
| Catalyst | Mechanism | Electrolyte | Potential | FE |
|---|---|---|---|---|
| Cu2O–CoPO-2 (ref. 88) | Cu2O: NO3− → NO2− | 0.1 M NO3− + 1.0 M KOH | −0.37 V vs. RHE | 95% |
| CoPO: H2O → *H | ||||
| Ru–Ni nanosheets (ref. 89) | Ru: NO3− → *HONO | 0.1 M K2SO4 + 0.002 M KNO3 | −0.8 V vs. RHE | 96% |
| Ni: *HONO + *H → NH3 | ||||
| TTA–TPH–CuCo (ref. 98) | Cu: NO3− → NO2− | 0.5 M K2SO4 + 0.3 M KNO3 | −0.75 V vs. RHE | 92% |
| Co: NO2− → NH3 | ||||
| Ag30Pd4 nanocluster (ref. 93) | Ag: NO3− → NO2− | 1.0 M NaOH + 1500 ppm NO3−–N | −0.6 V vs. RHE | 90% |
| Pd: NO2− → NH3 | ||||
| Ni1Cu single-atom alloy oxide nanowires (ref. 94) | Ni: H2O → *H | 1.0 M KOH + 0.1 M KNO3 | + 0.1 V vs. RHE | 80% |
| Cu: *NOx + *H → NH3 | ||||
| Mo1Fe1Pd dual single-atom alloy (ref. 99) | Mo: NO3− → NO2− | 1.0 M KNO3 + 1.0 M KOH | −0.7 V vs. RHE | 94% |
| Fe: NO2− → NH3 | ||||
| AgCu single-atom and nanoparticle (ref. 96) | Ag NP: CO2 → CO | 1.0 M KOH | −0.65 V vs. RHE | 94% |
| AgCu SAA: C–C coupling | ||||
| 0.2% CoCu single-atom alloy (ref. 100) | Co: CO2 → CO | 1.0 M KHCO3 | −1.01 V vs. RHE | 51% |
| Cu: C–C coupling | ||||
| Cu3N–Ag nanocubes (ref. 97) | Ag: CO2 → CO | 1.0 M KOH | −0.7 V vs. RHE | 50% |
| Cu: CO → *CHO → C2H4 | ||||
| CuO/Ni single atoms (ref. 101) | Ni: CO2 → CO | 1.0 M KOH | −0.892 V vs. RHE | 81% |
| Cu: CO → C2+ | ||||
| CoCu composite (ref. 102) | Co: CO2 → CO | 0.1 M KHCO3 | −0.8 V vs. RHE | 71% |
| Cu: CO → C2H5OH | ||||
| Mo1Cu single-atom alloy (ref. 103) | Mo: H2O → *H | 1.0 M KOH | −1.7 V vs. RHE | 86% |
| Cu0: CO2 → CO | ||||
| Cuδ+: C–C coupling | ||||
| Zn/Cu hybrid catalyst (ref. 1) | Zn: stabilize CO2NO3 | 0.1 M KHCO3 + 500 ppm NO3−–N | −0.6 V vs. RHE | 75% |
| Cu: lower the protonation barrier of COOHNH2 | ||||
| Zn1/In2O3−x (ref. 83) | In-OV: CO2 → *CO | 0.1 M KHCO3 + 0.1 M KNO3 | −0.7 V vs. RHE | 56% |
| Zn1-OV: NO3− → *NH2 | ||||
| Fe1/MoS2 (ref. 104) | Fe1–S3: *CO2NO2 → * CO2NH2 | 0.1 M KHCO3 + 0.1 M KNO3 | −0.5 V vs. RHE | 55% |
| MoS2: *CO2NH2 → *COOHNH2 | ||||
| Mo–PCN-222(Co) (ref. 105) | Co: CO2 → *CO | 0.1 M KHCO3 + 50 mM KNO3 | −0.4 V vs. RHE | 34% |
| Mo: NO3− → *NH2 | ||||
| CuPd1Rh1 diatomic alloy (ref. 106) | Pd1–Cu: *CO2NO2 → * CO2NH | 0.1 M KHCO3 + 0.1 M KNO3 | −0.5 V vs. RHE | 72% |
| Rh1–Cu: *CO2NH2 → *COOHNH2 | ||||
| CuSn composite (ref. 107) | Cu: CO2 → *CO | 0.1 M KNO3 | −0.7 V vs. RHE | 79% |
| Sn: NO3− → *NH2 |
Crystal facet and heterointerface coupling
The crystal facet and heterointerface cascade catalysis enhances the overall catalytic efficiency by constructing functionally complementary active regions, which collaboratively guide the orientation and coupling of reaction pathways. This synergy optimizes the multiple step reaction energy barriers and the mass transfer of intermediates, ultimately improving catalytic performance. The facet-based cascade strategy involves tuning the exposed crystal facets of the catalyst, leveraging the distinct catalytic properties arising from variations in atomic arrangement, coordination, and electronic structure across different facets. These properties enable the selective catalysis of specific reaction steps.108–110 Fu et al.111 proposed a crystal facet cascade catalysis strategy, where the Cu(100) and Cu(111) facets cooperatively perform tandem catalysis (Fig. 5a). The Cu(100) facet preferentially adsorbs NO3− and efficiently reduces them to NO2−, while the NO2− intermediate migrates to the adjacent Cu(111) facet for the hydrogenation step. This catalyst achieves a current density of 665 mA cm−2 for NH3 production and a rate of 1.41 mmol h−1 cm−2 in a flow cell under −0.59 V vs. RHE (Fig. 5b), significantly enhancing the NO3RR to NH3 performance.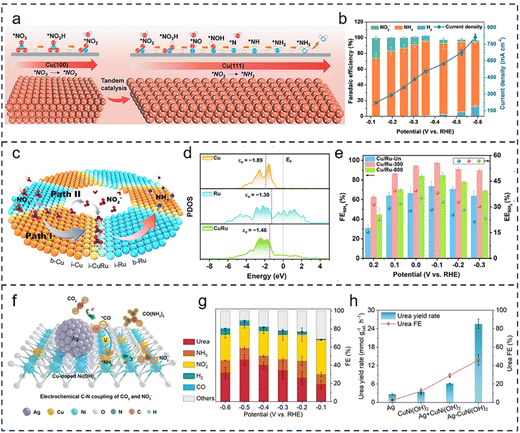 | ||
| Fig. 5 Crystal facet and heterointerface coupling in cascade catalysis. (a) Synergistic effects between Cu(100) and Cu(111) crystal facets. (b) Total current density and FE of different products on Cu nanosheets. Reproduced with permission.111 Copyright 2023, Wiley-VCH. (c) Schematic illustration of the proposed mechanism for NO3− to NH3 conversion on the Cu/Ru HHNS. (d) Projected density of states (PDOS) for b-Cu, b-Ru, and i-CuRu. (e) FENH3 and EENH3 of Cu/Ru-Un, Cu/Ru-300, and Cu/Ru-800 across a potential range of 0.2 to −0.3 VRHE. Reproduced with permission.112 Copyright 2025, American Chemical Society. (f) Schematic diagram of CO2 and NO3− co-reduction to urea on the Ag-CuNi(OH)2 electrocatalyst. (g) FE of possible products from the Ag-CuNi(OH)2 composite material. (h) Comparison of urea yield and FE between Ag NPs, CuNi(OH)2 NSs, their physical mixture, and Ag-CuNi(OH)2. Reproduced with permission.113 Copyright 2024, Wiley-VCH. | ||
In contrast, the heterointerface cascade strategy capitalizes on the unique local atomic and electronic environments created at the interfaces of different materials due to lattice mismatch, electronic interactions, and other factors. These environments facilitate the effective adsorption and activation of reactants and intermediates.114,115 For example, Chen et al.112 demonstrated a Cu/Ru heterointerface shell–core nanocavity catalyst that enabled efficient cascade electroreduction of NO3− to NH3, significantly enhancing the reaction rate and selectivity (Fig. 5c–e). NO3− is first adsorbed and reduced to NO2− at the Cu site, after which NO2− desorbs and accumulates in the nanocavity. The accumulated NO2− is rapidly transferred to the Cu/Ru heterointerface, where it undergoes further reduction to NH3. The Cu/Ru heterointerface facilitates electron transfer from Cu to Ru, improving the adsorption and activation of reaction intermediates at the interface, thus enhancing the utilization of NO2− intermediates. Moreover, the heterointerface cascade strategy can also be employed to regulate the generation and conversion of active hydrogen intermediates, facilitating C–N coupling reactions for the synthesis of complex carbon–nitrogen compounds. For instance, Ye et al.113 designed an Ag-CuNi(OH)2 heterostructure that efficiently catalyzed urea electrosynthesis. As shown in Fig. 5f, at the Ag site of the Ag-CuNi(OH)2 catalyst, CO2 is reduced to the critical *CO intermediate, while at the CuNi(OH)2 site, NO3− is reduced to the *NH2 intermediate. These intermediates spontaneously couple at the adjacent heterointerface to form urea, while the interface also optimizes the H2O splitting process to provide active hydrogen atoms. The Ag-CuNi(OH)2 composite material achieved an impressive urea production rate of 25.6 mmol g−1 h−1 and a FE of 46% (Fig. 5g and h). The cascade catalysis via crystal facets and heterointerfaces allows precise control over reaction pathways and intermediate transformations, substantially enhancing the overall efficiency and selectivity of cascade reactions. This approach offers valuable insights into the efficient use of carbon- and nitrogen-based resources and the sustainable synthesis of green chemicals. Although this strategy holds potential for enhancing cascade reaction efficiency, its practical implementation remains constrained. Differences in adsorption selectivity between crystal facets and interfacial charge redistribution can impede the transfer of intermediates across interfaces. In addition, interfacial reconstruction or fluctuations in the local environment during operation may disrupt the stability of the cascade process.116 Employing multiscale strategies can reinforce interfacial stability, strengthen the coupling between reaction steps, and improve system scalability, offering new pathways for practical deployment. Table 2 summarizes the catalytic processes of crystal facet and heterointerface-based cascade catalysts.
| Catalyst | Mechanism | Electrolyte | Potential | FE |
|---|---|---|---|---|
| Cu nanosheets (ref. 111) | Cu(100): NO3− → NO2− | 1.0 M KOH + 0.2 M KNO3 | −0.59 V vs. RHE | 88% |
| Cu(111): NO2− → NH3 | ||||
| Cu/Ru hollow heteronanostructure (ref. 112) | Cu: NO3− → NO2− | 0.1 M KNO3 + 0.1 M KOH | −0.1 V vs. RHE | 97% |
| Cu/Ru: NO2− → NH3 | ||||
| 4H/fcc Au–Cu heterostructures (ref. 117) | 4H/fcc Au: NO3− → NO2− | 1.0 M KOH + 1.0 M KNO3 | −0.6 V vs. RHE | 98% |
| 4H/fcc Cu: NO2− → NH3 | ||||
| CuO@CeO2−x heterostructured nanotubes (ref. 118) | Amorphous CuO: NO3− → NO2− | 0.1 M KOH + 0.01 M KNO3 | −0.4 V vs. RHE | 96% |
| Cu–Ce: NO2− → NH3 | ||||
| c-Co3O4/a-CuO heterostructure (ref. 119) | a-CuO: NO3− → *NO2 | 0.2 M K2SO4 + 0.1 M NO3− | −0.8 V vs. RHE | 90% |
| c-Co3O4: *NO2 → NH3 | ||||
| Ni@Cu2−xSe nanoarrays (ref. 120) | Cu2−xSe: NO3− → NO2− | 1.0 M KOH + 0.1 M KNO3 | −0.4 V vs. RHE | 91% |
| Interface: modulating *H | ||||
| Ni: H2O → *H | ||||
| CoO–CuOx heterostructure (ref. 121) | CuOx: NO3− → *NO2 | 0.5 M Na2SO4 + 200 ppm NaNO3–N | −0.5 V vs. RHE | 92% |
| Interface: promoting the transfer of electrons | ||||
| CoO: *NO2 → NH3 | ||||
| Ag–Cu heterostructure (ref. 116) | Ag: NO3− → NO2− | 0.1 M KOH + 0.1 M KNO3 | −0.5 V vs. RHE | >80% |
| Interface: facilitating the spillover of intermediates | ||||
| Cu: NO2− → NH3 | ||||
| SnO2/Cu6Sn5/CuO nanocatalysts (ref. 122) | Cu6Sn5/SnO2: CO2 → *CO2− | 0.5 M NaHCO3 | −0.95 V vs. RHE | 90% |
| Cu6Sn5/CuO: H2O → *H | ||||
| Ag/Cu self-evolution tandem catalysts (ref. 123) | Ag: CO2 → CO | 1.0 M KOH | −1.8 V vs. RHE | 89% |
| Interface: enhancing the adsorption of intermediates | ||||
| Cu: CO → C2+ | ||||
| Au–Cu heterostructure (ref. 124) | AuCu: CO2 → CO | 0.1 M KHCO3 | −0.75 V vs. RHE | — |
| Cu: CO → C2+ | ||||
| Au/Cu heterostructure (ref. 125) | Au (110): CO2 → *CO | 0.1 M KHCO3 | −0.75 V vs. RHE | — |
| Cu(110): CO → C2+ | ||||
| Cu2O nanoparticle (ref. 126) | (100): CO2 → *CO; C–C coupling | 0.2 M KI | −1.3 V vs. RHE | 57% |
| (111): C2H4 overflow | ||||
| Ag–Cu Janus nanostructures (ref. 127) | Ag domains: CO2 → CO | 0.1 M KHCO3 | −1.2 V vs. RHE | 72% |
| Cu domains: CO → C2+ | ||||
| Ag–Cu biphasic heterostructure (ref. 128) | Ag: CO2 → CO | 0.1 M KHCO3 | −1.05 V vs. RHE | 44% |
| Interface: promoting the transport of intermediates | ||||
| Cu: CO → C2+ | ||||
| Cu/Cu2O heterostructures (ref. 129) | Cu0: NO3− → *NH2; C–N coupling | 0.5 M KNO3; 0.2 M PA | −0.6 V vs. RHE | 75% |
| Interface: promoting the transport of intermediates | ||||
| Cu+: *CH3CNHCOO− → *CH3CHNH2COO− | ||||
| Ag–Cu composite (ref. 130) | Ag: CO2 → CO | 3.0 M KOH | −1.0 V vs. RHE | 80% |
| Interface: promoting the transport of intermediates | ||||
| Cu: CO → C2+ |
Nanoconfinement
Nanoconfinement involves constructing nanoreactors or confined spaces that impose spatial constraints on reactants and intermediates, effectively orchestrating multistep reactions within a limited volume. Confined cascade catalysis capitalizes on these spatial constraints to enhance intermediate retention and proximity, leading to higher reaction rates and selectivity.131–134 For example, Li et al.135 designed a trifunctional nanoreactor system with a 1D geometry, open mesoporous channels, and synergistic active sites for the highly efficient electrocatalytic NO3RR to NH3 (Fig. 6a and b). The system uses one-dimensional mesoporous carbon as a support, creating a unique microenvironment that favors NO3− transport and adsorption while restricting the diffusion of NOx intermediates. Simultaneously, the iron single-atom sites (Fe–N4) and iron nanoclusters (Fe4) embedded within the support synergistically control the electronic structure to dominate the reduction of NO3− and the oxidation of H2O, respectively. This system demonstrates excellent NO3RR performance under both alkaline and neutral conditions. Qiu et al.45 confined the intermediate product (NH4+) and the active chlorine species (˙Cl) within localized regions using TiO2 nanotubes (Fig. 6c and d). Leveraging the spillover effect of ˙Cl, they achieved the highly efficient and directed conversion of NH4+ to N2. Furthermore, by precisely controlling the structure of the nanoreactor, the confinement effect can be enhanced, promoting the formation, diffusion, and subsequent utilization of reaction intermediates. For instance, Lu et al.136 synthesized a series of Ag@Cu2O cascade nanoreactors with tunable shell thicknesses, where the Cu2O shell thickness was precisely controlled to achieve spatial confinement of the CO intermediate (Fig. 6e and f). In this system, the Ag core efficiently generates the CO intermediate, which, upon escaping, is confined within the porous Cu2O shell, significantly increasing the local CO concentration and thus promoting C–C coupling to form C2+ products. This study not only proposes an effective strategy for fine-tuning the local CO concentration through precise control of the Cu2O shell thickness but also provides important theoretical insights into the confinement catalysis mechanisms within cascade nanoreactors.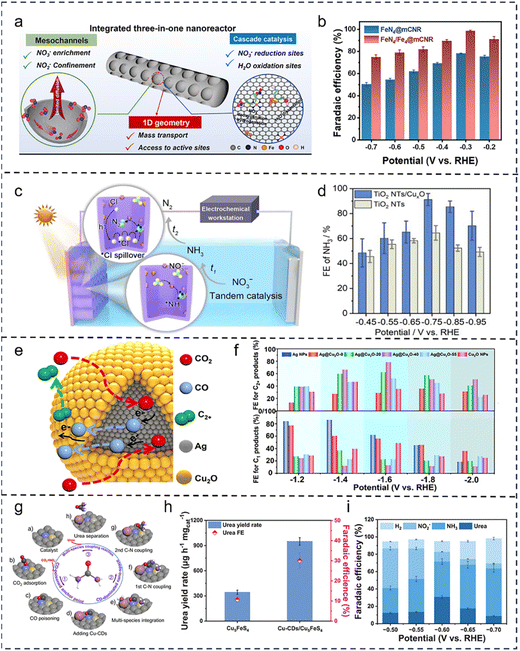 | ||
| Fig. 6 Nanoconfinement in cascade catalysis. (a) Schematic illustration of the three-in-one nanoreactor design to accelerate NO3RR to NH3 synthesis. (b) FENH3 of FeN4@mCNR and FeN4/Fe4@mCNR at varied potentials. Reproduced with permission.135 Copyright 2025, American Chemical Society. (c) Schematic illustration of NO3−-to-N2 process via the pulsed photoelectrolysis-mediated reactive chlorine spillover process. (d) FE of NH3 for TiO2 NTs and TiO2 NTs/CuOx. Reproduced with permission.45 Copyright 2025, Elsevier B.V. (e) Schematic illustration of the confinement catalysis mechanism in Ag@Cu2O cascade nanoreactors. (f) FE for C2+ and C1 products at various applied potentials. Reproduced with permission.136 Copyright 2024, American Chemical Society. (g) The Cu-CDs/Cu5FeS4 multisite nanoreactor for stepwise multiple species coupling to synthesize urea within the CDs-dominated nano-space via utilizing the CO poisoning effect. (h) Urea yield rate and FE of Cu5FeS4 and Cu-CDs/Cu5FeS4. (i) FE of urea, NH3, NO2−, and H2 of Cu-CDs/Cu5FeS4 in the H-cell. Reproduced with permission.137 Copyright 2025, Wiley-VCH. | ||
Moreover, confined cascade catalysis can effectively control the accumulation behavior of toxic intermediates, converting them into beneficial factors and enabling complex reactions involving multiple species.138 For example, Zhang et al.137 designed a nanoreactor composed of copper-carbon dots (Cu-CDs) and chalcopyrite (Cu5FeS4) for the efficient synthesis of urea (Fig. 6g–i). In this system, Cu-CDs provide confined nano-spaces that stabilize *CO intermediates, preventing CO poisoning of the Fe sites. Meanwhile, two structurally distinct Cu sites facilitate the activation and transformation of nitrogen-containing species, driving the continuous C–N coupling steps and ultimately enabling efficient urea synthesis. This system achieved a urea yield of 1131.84 µg h−1 mgcat−1 with a selectivity of 42%, significantly enhancing the thermodynamic and kinetic advantages of multiple site synergy and multiple species integration in urea synthesis. These examples demonstrate that nanoscale confinement can markedly influence cascade catalysis by spatially constraining and locally enriching reactive intermediates, thereby promoting desired cascade pathways while suppressing side reactions. Designing catalysts with intrinsic nanoscale confinement or incorporating external confined architectures, such as porous frameworks or core–shell structures, has thus become a key strategy for enhancing the performance of cascade electrocatalytic systems. Nevertheless, confined cascade strategies still present notable limitations. Excessive confinement may suppress intermediate diffusion and poison active sites. Moreover, precisely constructing confined architectures with appropriate spatial dimensions, pore connectivity, and optimized interfacial chemistry remains challenging.139 Combining confinement engineering with multiscale approaches enables the coordinated optimization of intermediate transport, interfacial mass transfer, and overall system stability. Table 3 summarizes the performance data for relevant confined cascade catalytic systems.
| Catalyst | Mechanism | Electrolyte | Potential | FE |
|---|---|---|---|---|
| FeN4/Fe4 mesoporous carbon nanorod (ref. 135) | Fe–N4: NO3− → NO2− | 0.1 M NO3− + 1.0 M KOH | −0.3 V vs. RHE | 92% |
| NO2− confinement | ||||
| Fe4: H2O → *H | ||||
| CuFe@CuNi composite (ref. 140) | CuFe LDH: NO3− → NO2− | 0.1 M Na2SO4 + 50 ppm NO3−–N | −1.4 V vs. Ag/AgCl | 100% |
| NO2− confinement | ||||
| CuNi LDH: NO2− → NH3 | ||||
| (CoPc-NH2 + NiPc-OCH3)/carbon nanotubes (ref. 141) | NiPc-OCH3: CO2 → CO | 0.1 M KHCO3 | −0.98 V vs. RHE | 50% |
| CO confinement | ||||
| NiPc-OCH3: CO → CH3OH | ||||
| Ni sodalite topology/NC (ref. 142) | NiN3: CO2 → CO | 0.5 M KHCO3 | −0.72 V vs. RHE | 62% |
| CO confinement | ||||
| Cu NPs: CO → C2H4 | ||||
| Ag@Cu2O cascade nanoreactor (ref. 136) | Ag core: CO2 → CO | 0.1 M KHCO3 | −1.6 V vs. RHE | 78% |
| CO confinement | ||||
| Cu2O shell: CO → C2+ | ||||
| CoPc/carbon nanotubes (ref. 44) | CoPc-out: CO2 → CO | 0.1 M KHCO3 | −0.96 V vs. RHE | 41% |
| CO confinement | ||||
| CoPc-in: CO → CH3OH | ||||
| Ag@Cu2O-x nanoreactors (ref. 143) | Ag core: CO2 → CO | 1.0 M KOH | −1.2 V vs. RHE | 74% |
| CO confinement | ||||
| Cu2O shell: CO → C2+ | ||||
| Ag–Cu nanoporous bimetallic catalyst (ref. 144) | Ag: CO2 → CO | 0.2 M KHCO3 | −1.2 V vs. RHE | 73% |
| CO confinement | ||||
| Cu: CO → C2+ | ||||
| Cu-CDs/Cu5FeS4 nanoreactor (ref. 137) | Fe: CO2 → CO | 0.1 M KNO3 | −0.35 V vs. RHE | 42% |
| C and N intermediate confinement | ||||
| Cu: NO3− → *NH2 | ||||
| CuCo@N-doped microporous hollow carbon spheres (ref. 145) | Co: CO2 → CO | 0.05 M KNO3 + 0.1 M KHCO3 | −0.55 V vs. RHE | 32% |
| C and N intermediate confinement | ||||
| Cu: NO3− → *NH2 |
Tandem reactors
The strategy of tandem reactors enables the sequential integration of different reaction steps across multiple reactors, where the differential conditions between reactors (e.g., pH, potential, and temperature) can be finely tuned to optimize the reaction environment. This approach enhances both the rate of target product formation and its selectivity.46,146 For instance, Fig. 7a illustrates a cascaded electrolyzer system in which the reduction of CO2 to CO and the subsequent reduction of CO to C2+ are coupled in different electrolyzers.147 By creating tailored catalytic environments in each electrolyzer, this strategy significantly accelerates the production rate of C2+ products (Fig. 7b). Chi et al.148 reported a dual electrolyzer cascade system: the first electrolyzer operates under alkaline conditions to electrochemically reduce CO2 to ethanol, while the second electrolyzer, under acidic conditions, electrooxidizes ethanol and condenses it into the high-value six-carbon compound 1,1-diethoxyethane (DEE). This system achieves a highly efficient and selective conversion from CO2 to multi-carbon products. The cascading reactor design not only greatly enhances product selectivity but also leads to efficient energy utilization and resource conversion. | ||
| Fig. 7 Tandem reactors. (a) Schematic diagram of the tandem CO2 electrolyzer. (b) Comparison of C2H4 yield between the tandem electrolyzer cascade structure and a single electrolyzer. Reproduced with permission.147 Copyright 2023, Springer Nature. (c) Tandem electrochemical-thermochemical reactors for CO2 conversion to C3 oxygenate products. (d) CO2-based selectivity of products for 5 cm2 Cu/C, oxide-derived Cu/C, and Cu/reinforced C cathodes at −220 mA cm−2 in combination with 160 and 200 °C hydroformylation temperatures. Reproduced with permission.149 Copyright 2022, American Chemical Society. (e) Hybrid electrocatalytic-bioelectrocatalytic cascade integrated on a gas diffusion electrode for the CO2RR under selective formation of methanol. (f) FE for the CO2RR performed with a GDE constituted of Ag-Bi2O3 nanofibers on a hydrophobic carbon paper. Reproduced with permission.150 Copyright 2025, Wiley-VCH. | ||
Additionally, electrochemical reactors can be coupled with thermocatalytic and biocatalytic reactors, forming hybrid cascaded systems. This strategy effectively overcomes the limitations of single electrochemical transformations, improving reaction efficiency and enabling the synthesis of more complex, high-value products, thereby significantly enhancing the economic viability of the entire conversion process. For example, Chen et al.149 proposed a cascaded electrochemical-thermochemical strategy in which C2H4 reacts with CO and H2 to produce propanol and propionaldehyde (Fig. 7c and d). In this approach, CO2 is first electrochemically reduced to C2H4, CO, and H2, followed by a thermocatalytic hydrogenation–formylation reaction that converts these intermediates into C3 oxygenated compounds. This cascading method achieves approximately 18% selectivity for C3 products. Wang et al.150 proposed a system that integrates electrochemical and biocatalytic processes on a gas diffusion electrode for the CO2RR (Fig. 7e and f). Under neutral pH conditions, Ag-Bi2O3 selectively reduces gaseous CO2 to carbonates, which are then further reduced to formaldehyde and methanol by formaldehyde dehydrogenase and alcohol dehydrogenase, respectively. This hybrid reactor cascade not only enhances reaction efficiency but also improves the overall selectivity and sustainability of the system. In summary, the tandem reactor strategy enhances the efficiency and selectivity of complex reactions at the macroscale by optimizing environmental conditions within each reactor and precisely controlling product distribution. Although tandem reactors can optimize reaction environments and regulate product distribution, diffusion limitations in intermediate transport between reactors, system operational complexity, and coupling interference between reaction conditions at different stages still constrain their precise control and efficiency enhancement for multistep reactions.151 Therefore, integrating multiscale strategies to construct an integrated catalytic system featuring active sites, nanoconfinement, interfacial mass transfer, and reactor series connection enables efficient intermediate transport, thereby enhancing the overall efficiency of cascade reactions. Table 4 summarizes the catalytic performance of tandem reactors.
| Cascade system | Catalyst | Mechanism | Electrolyte | Reaction conditions |
|---|---|---|---|---|
| Electrochemical–electrochemical | Ni–N–C; Cu (ref. 147) | Cell-1: CO2 → CO | Cell-1: 0.1 M KHCO3 | Cell-1: 200 mA cm−2 |
| Cell-2: CO → C2+ | Cell-2: 1.0 M KHCO3 | Cell-2: 700 mA cm−2 | ||
| Graphite flakes (ref. 148) | Cell-1: CO2 → ethanol | Cell-1: 1.0 M KOH | Cell-1: −1.8 V vs. SCE | |
| Cell-2: ethanol → DEE | Cell-2: 0.5 M H2SO4 ethanol solution | Cell-2: 20 mA cm−2 | ||
| 3D single-atom Ni; Cu2O (ref. 152) | Cell-1: CO2 → CO | Cell-1: 0.5 M KHCO3 | Cell-1: 140 mA cm−2 | |
| Cell-2: CO → n-propanol | Cell-2: 1.0 M KOH | Cell-2: 42.5 mA cm−2 | ||
| Solid-oxide electrolysis cell; membrane electrode assembly (ref. 47) | Cell-1: CO2 → CO | Cell-1: 30 wt% aqueous ethanolamine | Cell-1: 815 mA cm−2 | |
| Cell-2: CO → C2H4 | Cell-2: 3.0 M KOH | Cell-2: 150 mA cm−2 | ||
| Ni–N–C; Cu nanoparticle (ref. 153) | Cell-1: CO2 → CO | Cell-1: 0.5 M K2SO4 + H2SO4 | Cell-1: 5 A | |
| Cell-2: CO → C2+ | Cell-2: 0.5 M KOH | Cell-2: 4 A | ||
| Ni–Cu; Cu–Al (ref. 154) | Cell-1: CO2 → CO | Cell-1: 1.0 M KOH | Cell-1: 400 mA cm−2 | |
| Cell-2: CO → C2+ | Cell-2: 1.0 M KOH | Cell-2: 300 mA cm−2 | ||
| Electrochemical–thermochemical | Cu; Rh1Co3/MCM-41 (ref. 149) | Cell-1: CO2 → C2H4 | Cell-1: 50 mM KHCO3 | Cell-1: 220 mA cm−2 |
| Cell-2: C2H4 → C3 | Cell-2: — | Cell-2: 160, 200 °C | ||
| Pd/C; FeCo (ref. 155) | Cell-1: CO2 → CO | Cell-1: 50 mM KHCO3 | Cell-1: 150 mA cm−2 | |
| H2O → H2 | Cell-2: — | Cell-2: 450 °C | ||
| Cell-2: CO2, H2 → CNF | ||||
| Ag-hexamethylenetetramine/Ni-hydroxide carbonate; Pd(II) (ref. 48) | Cell-1: CO2 → CO | Cell-1: 0.5 M KHCO3 | Cell-1: −0.8 V vs. RHE | |
| H2O → H2 | Cell-2: — | Cell-2: 120 °C | ||
| Cell-2: CO2, O2 → organic amides | ||||
| Rh/SmxCe1−xO2−δ (ref. 156) | Cell-1: H2O → H2 | Cell-1: acceptor-doped LaGaO3 | Cell-1: 300–500 mA cm−2 | |
| Cell-2: CO2 + CH4 → CO + H2 | Cell-2: — | Cell-2: 800 °C | ||
| Membrane electrode assembly; Ga/ZSM-5/P (ref. 157) | Cell-1: CO2 → C2H4 | Cell-1: 0.5 M KHCO3 | Cell-1: 200 mA cm−2 | |
| Cell-2: C2H4 → BTEX | Cell-2: — | Cell-2: 600 °C | ||
| Electrochemical-biocatalytic | Ag-Bi2O3; dehydrogenase (ref. 150) | Cell-1: CO2 → formate | Cell-1: 0.1 M phosphate | Neutral PH |
| Cell-2: formate → formaldehyde → methanol | Cell-2: — | 25–400 mA cm−2 | ||
| MPN@deCOP@Ag-Cu2O; engineered Escherichia coli (ref. 49) | Cell-1: CO2 → ethanol | Cell-1: 1.0 M KOH | Cell-1: 900 mA cm−2 | |
| Cell-2: ethanol → itaconic acid, isopropanol, and PHB | Cell-2: — | Cell-2: ADH, ALDH | ||
| Cu–Ag; Cupriavidus necator (ref. 158) | Cell-1: CO2 → C2 oxygenates | Cell-1: 0.1 M KH2PO4 | Cell-1: 300 mA cm−2 | |
| Cell-2: C2 oxygenates → PHB | Cell-2: — | Cell-2: pH 7.0 | ||
| C/Ag/carbon GDE (ref. 159) | Cell-1: CO2 → CO | Cell-1: 0.05 M H2SO4 + 3.0 M KCl | Cell-1: 100 mA | |
| Cell-2: CO → MCFAs | Cell-2: — | Cell-2: 35 °C, pH 6.2 | ||
| Cu/Cu2O (ref. 160) | Cell-1: CO2 → formate | Cell-1: 0.5 M KHCO3 | Cell-1: 50 mA | |
| Cell-2: formate → SCP | Cell-2: — | Cell-2: 37 °C |
Conclusion and outlook
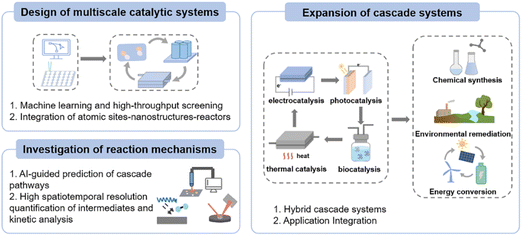
Based on the above, cascade electrocatalysis provides a novel pathway for the efficient conversion of carbon and nitrogen resources through atomic-scale catalyst design, nanoscale structural engineering, and macroscopic reactor integration. This review systematically summarizes the latest advancements in this field, delving into the crucial role of the synergistic effects between single-atom/multi-atom sites at the atomic scale, interfacial and confinement effects at the sub-nanoscale and nanoscale, and reactor design at the macroscopic level in enhancing reaction activity and selectivity. Through multiscale cooperative effects, cascade electrocatalysis not only effectively reduces the energy barriers of reactions but also significantly improves reaction selectivity, especially in complex multistep reaction systems, demonstrating unique advantages. Despite substantial progress across various applications, cascade electrocatalysis continues to face several key challenges. Limited compatibility among different active sites constrains the sequential coupling required for efficient cascade pathways. The coexistence of multiple competing reaction routes further complicates selective control over the desired pathway. Moreover, the short lifetimes and low concentrations of critical intermediates hinder real-time detection and impede mechanistic elucidation. Nevertheless, with advances in cross-scale design strategies and in situ characterization techniques featuring high temporal and spatial resolution, cascade electrocatalysis is poised to offer broad opportunities for future applications (Fig. 8).Design of multiscale catalytic systems
Cascade reactions involve multiple elementary steps and diverse intermediates, with overall efficiency constrained by the synergistic interplay of active site spatial distribution, mass transport processes, and electron transfer kinetics. Therefore, constructing catalysts with a multiscale framework is pivotal for enhancing the performance of cascade reactions. Future research will focus on the deep integration of artificial intelligence with experimental methods, employing machine learning models to predict and optimize the structural features of cascade catalysts. High-throughput synthesis and characterization platforms will be employed for rapid screening, thus establishing a closed-loop research and development cycle encompassing computational design, high-throughput fabrication and testing, and machine learning-driven optimization. The ultimate goal is to construct an integrated catalytic system spanning from atom-level active sites and nanoscale mass transport structures to reactor-scale engineering.Investigation of reaction mechanisms
In cascade electrocatalysis, the dynamic evolution of complex intermediates and multiple reaction pathways presents significant challenges for real-time monitoring and mechanistic analysis. Future advancements may leverage artificial intelligence to predict possible reaction pathways and combine SI-SECM with in situ spectroscopic techniques such as infrared, Raman, and X-ray absorption spectroscopy to track the dynamic evolution of different active sites and the generation and consumption of intermediates in real time. This integrated approach will comprehensively reveal the intrinsic mechanisms and kinetic processes underlying cascade reactions.Expansion of cascade systems
In single-mode electrocatalysis, driving complex multistep cascade reactions is often limited by low energy efficiency. Future developments will focus on hybrid catalytic systems that couple multiple energy fields, such as electrochemical-photonic, electrochemical-thermal, and electrochemical-biological coupling. By rationally designing and optimizing these multi-energy input, multi-reaction integrated systems, this approach should overcome the constraints of traditional single-mode catalysis, substantially expanding the scope of cascade catalysis for chemical synthesis, environmental remediation, and energy conversion. Such advances may enable higher selectivity, efficiency, and sustainability in complex molecule construction, deep pollutant transformation, and renewable energy utilization.In summary, cascade electrocatalysis has been extensively explored for carbon–nitrogen resource utilization. With continued development in multiscale catalyst design, reaction mechanism investigation, and the integration of cascade systems, cascade electrocatalysis holds great promise for achieving more efficient resource conversion, thereby advancing the sustainable development of energy and the environment.
Author contributions
Writing – original draft, S. Li.; visualization, H. Li., R. Y., Y. P.; writing – review & editing, Z. J., P. Li.; funding acquisition, Z. J., P. Li.; supervision, P. Li, G. Y.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
P. L. acknowledges funding support from the National Natural Science Foundation of China (22476141), the China Postdoctoral Science Foundation (2025M771235), and the Open Fund of Sichuan Engineering Technology Research Center for High Salt wastewater Treatment and Resource Utilization, Sichuan University of Science and Engineering (SCGCY-2403). G. Y. acknowledges the funding support from the Welch Foundation Award F-1861.Notes and references
- Y. Luo, K. Xie, P. Ou, C. Lavallais, T. Peng, Z. Chen, Z. Zhang, N. Wang, X.-Y. Li, I. Grigioni, B. Liu, D. Sinton, J. B. Dunn and E. H. Sargent, Nat. Catal., 2023, 6, 939–948 CrossRef CAS.
- E. T. A. Mitchard, Nature, 2018, 559, 527–534 CrossRef CAS PubMed.
- P. H. van Langevelde, I. Katsounaros and M. T. M. Koper, Joule, 2021, 5, 290–294 CrossRef.
- C. Smith, A. K. Hill and L. Torrente-Murciano, Energy Environ. Sci., 2020, 13, 331–344 Search PubMed.
- W. Chen, X. Yang, Z. Chen, Z. Ou, J. Hu, Y. Xu, Y. Li, X. Ren, S. Ye, J. Qiu, J. Liu and Q. Zhang, Adv. Funct. Mater., 2023, 33, 2300512 CrossRef CAS.
- P. Li, Z. Jin, Z. Fang and G. Yu, Energy Environ. Sci., 2021, 14, 3522–3531 RSC.
- S. Kuang, T. Xiao, H. Chi, J. Liu, C. Mu, H. Liu, S. Wang, Y. Yu, T. J. Meyer, S. Zhang and X. Ma, Angew. Chem., Int. Ed., 2024, 63, e202316772 CrossRef CAS PubMed.
- Y. Xiong, Y. Wang, J. Zhou, F. Liu, F. Hao and Z. Fan, Adv. Mater., 2024, 36, 2304021 CrossRef CAS.
- Y. Liu, K. Liu, P. Wang, Z. Jin and P. Li, Carbon Neutrality, 2023, 2, 14 CrossRef CAS.
- M. Yi, H. Li, M. Xie, P. Li, Z. Jin and G. Yu, Sci. China: Chem., 2025, 68, 2322–2342 CrossRef CAS.
- T. Galimova, M. Ram, D. Bogdanov, M. Fasihi, A. Gulagi, S. Khalili and C. Breyer, Renewable Sustainable Energy Rev., 2023, 183, 113420 CrossRef CAS.
- W. Zhang, B. Jia, X. Liu and T. Ma, SmartMat, 2022, 3, 5–34 CrossRef CAS.
- S. Guo, T. Asset and P. Atanassov, ACS Catal., 2021, 11, 5172–5188 CAS.
- Y.-B. Huang, J. Liang, X.-S. Wang and R. Cao, Chem. Soc. Rev., 2017, 46, 126–157 RSC.
- Y. Zhu, X. Cui, H. Liu, Z. Guo, Y. Dang, Z. Fan, Z. Zhang and W. Hu, Nano Res., 2021, 14, 4471–4486 CrossRef CAS.
- M. Duca, J. R. Weeks, J. G. Fedor, J. H. Weiner and K. A. Vincent, ChemElectroChem, 2015, 2, 1086–1089 CrossRef CAS.
- R. D. Milton and S. D. Minteer, ChemPlusChem, 2017, 82, 513–521 CrossRef CAS.
- C. Coelho and M. J. Romao, Protein Sci., 2015, 24, 1901–1911 CrossRef CAS.
- J. M. Carceller, K. S. Arias, M. J. Climent, S. Iborra and A. Corma, Chem. Soc. Rev., 2024, 53, 7875–7938 Search PubMed.
- C. Zhang, Q. Wang, Z. Li, H. Liu, L. Zhong, J. Liu, Z. Wang, R. Wu, P. Song, W. J. Chen, Z. Qi, C. Yan, L. Song, Q. Yan and C. Lv, Angew. Chem., Int. Ed., 2025, 64, e202502957 Search PubMed.
- X. Gao, C. Zhu and L. Zhang, Chem Catal., 2025, 5, 101544 CAS.
- T. Yan, X. Chen, L. Kumari, J. Lin, M. Li, Q. Fan, H. Chi, T. J. Meyer, S. Zhang and X. Ma, Chem. Rev., 2023, 123, 10530–10583 CrossRef CAS.
- C. Liu, B. Qiao and T. Zhang, JACS Au, 2024, 4, 4129–4140 CrossRef CAS PubMed.
- H. Yin, F. Dong, H. Su, Z. Zhuang, Y. Wang, D. Wang, Y. Peng and J. Li, ACS Nano, 2023, 17, 25614–25624 Search PubMed.
- X. F. Cheng, J. H. He, H. Q. Ji, H. Y. Zhang, Q. Cao, W. J. Sun, C. L. Yan and J. M. Lu, Adv. Mater., 2022, 34, e2205767 Search PubMed.
- Y. Wang, P. Zhu, R. Wang, K. C. Matthews, M. Xie, M. Wang, C. Qiu, Y. Liu, H. Zhou, J. H. Warner, Y. Liu, H. Wang and G. Yu, ACS Nano, 2024, 18, 26751–26758 CrossRef CAS.
- Z. Li, Y. Xie, Y. Han, H. Wang, J. Gao, L. Chen, L. Xu, L. Lu, Y. Zhao, E. Wang and G. Li, Nano Res. Energy, 2025, 4, e9120169 Search PubMed.
- P. Li, R. Li, Y. Liu, M. Xie, Z. Jin and G. Yu, J. Am. Chem. Soc., 2023, 145, 6471–6479 Search PubMed.
- C. Wang, Z. Lv, X. Feng, W. Yang and B. Wang, Adv. Energy Mater., 2024, 14, 2400160 CrossRef CAS.
- J. Xiang, P. Wang, P. Li, M. Zhou, G. Yu and Z. Jin, Angew. Chem., Int. Ed., 2025, 64, e202500644 CrossRef CAS PubMed.
- S. Zhu, K. Liu, Z. Feng, H. Jiang and J. Lin, Nano Res. Energy, 2025, 4, e9120188 CrossRef.
- Y. Hu, J. Liu, W. Luo, J. Dong, C. Lee, N. Zhang, M. Chen, Y. Xu, D. Wu, M. Zhang, Q. Zhu, E. Hu, D. Geng, L. Zhong and Q. Yan, Chem. Sci., 2024, 15, 8204–8215 RSC.
- G. Xie, W. Guo, Z. Fang, Z. Duan, X. Lang, D. Liu, G. Mei, Y. Zhai, X. Sun and X. Lu, Angew. Chem., Int. Ed., 2024, 63, e202412568 CrossRef CAS PubMed.
- J. Yan, J. Li, P. Liu, H. Huang and W. Song, Green Chem., 2023, 25, 8645–8651 RSC.
- Y. Wang, H. Su, Y. He, L. Li, S. Zhu, H. Shen, P. Xie, X. Fu, G. Zhou, C. Feng, D. Zhao, F. Xiao, X. Zhu, Y. Zeng, M. Shao, S. Chen, G. Wu, J. Zeng and C. Wang, Chem. Rev., 2020, 120, 12217–12314 CrossRef CAS PubMed.
- K. Liu, J. Li, Y. Liu, M. Wang and H. Cui, J. Energy Chem., 2023, 79, 515–534 Search PubMed.
- K. Liu, Z. Sun, X. Peng, X. Liu, X. Zhang, B. Zhou, K. Yu, Z. Chen, Q. Zhou, F. Zhang, Y. Wang, X. Gao, W. Chen and P. Chen, Nat. Commun., 2025, 16, 2167 Search PubMed.
- Y. Cheng, Q. Li, M. I. B. Salaman, C. Wei, Q. Wang, X. Ma, B. Liu and A. B. Wong, J. Am. Chem. Soc., 2025, 147, 12438–12448 CrossRef CAS.
- M. Melchionna, P. Fornasiero, M. Prato and M. Bonchio, Energy Environ. Sci., 2021, 14, 5816–5833 RSC.
- Y. Zhang, F. Chen, X. Hao, Y. Liu, W. Wu, X. Zhang, Z. Zang, H. Dong, W. Wang, F. Lu, Z. Lu, H. Liu, H. Liu, F. Luo and Y. Cheng, Appl. Catal., B, 2024, 344, 123666 CrossRef CAS.
- Y. Xu, K. Shi, T. Ren, H. Yu, K. Deng, X. Wang, Z. Wang, H. Wang and L. Wang, Small, 2022, 18, 2203335 CrossRef CAS.
- H. Liu, L. An, P. Wang, C. Yu, J. Zhang, H. Shin, B. Peng, J. Li, M. Li, H. An, J. Yu, Y. Chen, P. Wang, K.-S. Lee, K. Lalit, Z. Liu, O. K. Farha, W. Huang, J. Z. Liu, L. Qi, K. Xie and E. H. Sargent, Nat. Commun., 2025, 16, 6185 CrossRef CAS.
- C. Bie, J. Yang, X. Zeng, Z. Wang, X. Sun, Z. Yang, J. Yu and X. Zhang, Small, 2025, 21, e2411184 Search PubMed.
- G. Shi, W. Zhang, Y. Kang, J. Zhao, T. Lu, C. Yang, M. Chang, Y. Shen, X. Gao, J. Wu, Y.-F. Li, K. Cao and L. Zhang, Nat. Commun., 2025, 16, 7359 Search PubMed.
- W. Qiu, P. Wang, Z. Jin, W. Liu, P. Li and Z. Pan, Appl. Catal., B, 2025, 367, 125102 CrossRef CAS.
- M. Wang, W. Fang, D. Zhu, C. Xia, W. Guo and B. Y. Xia, Chin. J. Catal., 2025, 69, 1–16 CrossRef.
- A. Ozden, Y. Wang, F. Li, M. Luo, J. Sisler, A. Thevenon, A. Rosas-Hernández, T. Burdyny, Y. Lum, H. Yadegari, T. Agapie, J. C. Peters, E. H. Sargent and D. Sinton, Joule, 2021, 5, 706–719 CrossRef CAS.
- G. Mei, Y. Lu, X. Yang, S. Chen, X. Yang, L. M. Yang, C. Tang, Y. Sun, B. Y. Xia and B. You, Angew. Chem., Int. Ed., 2024, 63, e202314708 CrossRef CAS.
- L. Han, Y. Li, X. Wang, Z. Yao, Z. Chen, J. p. Li, T. Tan, S. Y. Lee and Y. Lv, Adv. Energy Mater., 2025, 15, e03056 CrossRef CAS.
- Y. Liu, X. Su, J. Ding, J. Zhou, Z. Liu, X. Wei, H. B. Yang and B. Liu, Chem. Soc. Rev., 2024, 53, 11850–11887 RSC.
- S. K. Beaumont, Phys. Chem. Chem. Phys., 2020, 22, 18747–18756 RSC.
- Z. Jin, R. Guan, X. Li, D. Yuan and P. Li, Chin. Chem. Lett., 2025, 36, 110506 CrossRef CAS.
- Q. Xu, J. A. Zamora Zeledon, B. O. Joensen, L. Trotochaud, A. Sartori, L. M. Kaas, A. B. Moss, M. Mirolo, L. Mairena, S. Huynh, S. Garg, S. Helveg, I. Chorkendorff, S. Zhao, B. Seger and J. Drnec, Nat. Nanotechnol., 2025, 20, 889–896 CrossRef CAS PubMed.
- Y.-Y. Lou, Q.-Z. Zheng, S.-Y. Zhou, J.-Y. Fang, O. Akdim, X.-Y. Ding, R. Oh, G.-S. Park, X. Huang and S.-G. Sun, ACS Catal., 2024, 14, 5098–5108 CrossRef CAS.
- Y. Dai, S. Li, X. Li, K. Liu, Y. Guo, H. Li and B. Jiang, Adv. Funct. Mater., 2025, 35, 2420282 CrossRef CAS.
- X.-X. Fu, H. Guo, D.-H. Si, H.-J. Zhu, Y.-Y. Lan, Y.-B. Huang and R. Cao, Chem. Sci., 2025, 16, 13503–13513 Search PubMed.
- J. Ding, L. Liu, J. Zhang, Y. Liu, H. Xu, Z. Shen, H. B. Yang, X. Feng, Y. Huang and B. Liu, J. Am. Chem. Soc., 2025, 147, 9601–9609 CrossRef CAS.
- W. Shen, Y. Ye, Q. Xia and P. Xi, EES Catal., 2025, 3, 10–31 RSC.
- K. Chen, F. Wang, X. Lu, Y. Li and K. Chu, ACS Catal., 2023, 13, 9550–9557 CrossRef CAS.
- A. Prajapati, C. Hahn, I. M. Weidinger, Y. Shi, Y. Lee, A. N. Alexandrova, D. Thompson, S. R. Bare, S. Chen, S. Yan and N. Kornienko, Nat. Commun., 2025, 16, 2593 CrossRef CAS PubMed.
- J. Song, Z.-X. Qian, J. Yang, X.-M. Lin, Q. Xu and J.-F. Li, ACS Energy Lett., 2024, 9, 4414–4440 CrossRef CAS.
- Z. Yao, C. Lin, J. Gao, J. Feng, M. Li, W. Yan, W. Zhang and H. Wang, Appl. Catal., B, 2025, 378, 125550 CrossRef CAS.
- J. Xu, R. Chen, J. Song, S. Liu, Y. Shen and Y. Zhang, Chem. Sci., 2025, 16, 9564–9576 RSC.
- H. Li, Y. Guo and Z. Jin, Carbon Neutrality, 2023, 2, 22 CrossRef CAS.
- L. He, Z. Cai, C. Wang, L. Yang, W. Yong and Z. Jin, Adv. Funct. Mater., 2025, e14592 CrossRef.
- Z. Jin, Anal. Chem., 2023, 95, 6477–6489 CrossRef CAS.
- P. Wang, P. Li, Z. Pan, K. Liu, M. Xie, L. Zhou, M. Zhou, G. Yu and Z. Jin, Nat. Commun., 2025, 16, 5581 CrossRef.
- R. Yang, L. Yang, W. Yong, P. Li, M. Zhou and Z. Jin, Chem. Sci., 2025, 16, 18482–18495 RSC.
- W. He, J. Zhang, S. Dieckhöfer, S. Varhade, A. C. Brix, A. Lielpetere, S. Seisel, J. R. C. Junqueira and W. Schuhmann, Nat. Commun., 2022, 13, 1129 CrossRef CAS PubMed.
- L. Xiao, G. Wang, X. Huang, S. Zhou, R. Zhou, Y. Jiang, S. Liu, G. Li, H. Zheng, S.-G. Sun and H.-G. Liao, Appl. Catal., B, 2022, 307, 121164 CrossRef CAS.
- Q. Zhang, Z. Song, X. Sun, Y. Liu, J. Wan, S. B. Betzler, Q. Zheng, J. Shangguan, K. C. Bustillo, P. Ercius, P. Narang, Y. Huang and H. Zheng, Nature, 2024, 630, 643–647 CrossRef CAS PubMed.
- T.-H. Shen, R. Girod, J. Vavra and V. Tileli, J. Electrochem. Soc., 2023, 170, 056502 CrossRef CAS.
- Y. Yao, Z. Sun, T. Li, Z. Zhao, Z. Li, X. Lu, Y. Wan, Y. Fan and Z. Chen, ACS Nano, 2025, 19, 18947–18975 CrossRef CAS PubMed.
- X. Peng, L. Zeng, D. Wang, Z. Liu, Y. Li, Z. Li, B. Yang, L. Lei, L. Dai and Y. Hou, Chem. Soc. Rev., 2023, 52, 2193–2237 RSC.
- Y. Zhou, G. Xing, C. Sun, Z. Chen, S. Li, R. Yang, M. Chen, P. Zhang, C. Feng, A. Abudula and G. Guan, Adv. Funct. Mater., 2025, e15635 CrossRef.
- W. Qiu, Y. Liu, M. Xie, Z. Jin, P. Li and G. Yu, EES Catal., 2024, 2, 202–219 RSC.
- X. Liang, H. Zhu, X. Yang, S. Xue, Z. Liang, X. Ren, A. Liu and G. Wu, Small Struct., 2022, 4, 2200202 CrossRef.
- T. Yang, M. Kuang and J. Yang, Nano Res., 2023, 16, 8670–8683 CrossRef CAS.
- J. Li, Z. Wang, C. McCallum, Y. Xu, F. Li, Y. Wang, C. M. Gabardo, C.-T. Dinh, T.-T. Zhuang, L. Wang, J. Y. Howe, Y. Ren, E. H. Sargent and D. Sinton, Nat. Catal., 2019, 2, 1124–1131 CrossRef CAS.
- X. Kong, J. Zhao, J. Ke, C. Wang, S. Li, R. Si, B. Liu, J. Zeng and Z. Geng, Nano Lett., 2022, 22, 3801–3808 CrossRef CAS PubMed.
- L. Lv, H. Tan, Y. Kong, B. Tang, Q. Ji, Y. Liu, C. Wang, Z. Zhuang, H. Wang, M. Ge, M. Fan, D. Wang and W. Yan, Angew. Chem., Int. Ed., 2024, 63, e202401943 CrossRef CAS PubMed.
- Y. Wan, M. Zheng, W. Yan, J. Zhang and R. Lv, Adv. Energy Mater., 2024, 14, 2303588 Search PubMed.
- Y. Zhang, Z. Li, K. Chen, X. Yang, H. Zhang, X. Liu and K. Chu, Adv. Energy Mater., 2024, 14, 2402309 CrossRef CAS.
- J. M. Moore and A. R. Fout, Chem. Sci., 2025, 16, 840–845 RSC.
- S. Tian, R. Wu, H. Liu, C. Yan, Z. Qi, P. Song, W. J. Chen, L. Song, Z. Wang and C. Lv, Angew. Chem., Int. Ed., 2025, 64, e202510665 CrossRef CAS PubMed.
- J. Liu, Y. Xu, R. Duan, M. Zhang, Y. Hu, M. Chen, B. Han, J. Dong, C. Lee, L. S. R. Kumara, O. Seo, J. Tseng, T. Watanabe, Z. Liu, Q. Zhu, J. Xu, M.-F. Ng, D. Wu and Q. Yan, Nat. Commun., 2025, 16, 3595 CrossRef CAS.
- Z. Wu, Y. Song, H. Guo, F. Xie, Y. Cong, M. Kuang and J. Yang, Interdiscip. Mater., 2024, 3, 245–269 CAS.
- B. Kang, B. Xu, Z. Chen, F. Li and Y. Wang, Appl. Catal., B, 2025, 360, 124528 CrossRef CAS.
- X. You, J. Xu, Z. Zhuang, J. Xia, S. Wang, H. Wei, Y. Li, Y. Cai, H. Xiang and B. Yu, Nano Res., 2024, 17, 4815–4824 Search PubMed.
- M. Xie, S. Tang, Z. Li, M. Wang, Z. Jin, P. Li, X. Zhan, H. Zhou and G. Yu, J. Am. Chem. Soc., 2023, 145, 13957–13967 CrossRef CAS PubMed.
- Z. Liu, C. Xing, Y. Shan, M. Ma, S. Wu, R. Ge, Q. Xue and J. Tian, Chem. Sci., 2025, 16, 7010–7017 Search PubMed.
- C.-H. Shen, Y. Zhao, H. N. Nam, L. Zhu, Q. M. Phung, V. Austen, M. Kim, D. Jiang, X. Wei, T. Yokoshima, C.-W. Kung and Y. Yamauchi, Chem. Sci., 2025, 16, 7026–7038 RSC.
- L. Qin, F. Sun, Z. Gong, G. Ma, Y. Chen, Q. Tang, L. Qiao, R. Wang, Z.-Q. Liu and Z. Tang, ACS Nano, 2023, 17, 12747–12758 CrossRef CAS PubMed.
- K. Liu, H. Li, M. Xie, P. Wang, Z. Jin, Y. Liu, M. Zhou, P. Li and G. Yu, J. Am. Chem. Soc., 2024, 146, 7779–7790 CrossRef CAS.
- Z. Jin, K. Liu, Z. Pan, X. Shan, F. Cai, D. Yang, P. Li, G. Yu and M. Zhou, J. Am. Chem. Soc., 2025 DOI:10.1021/jacs.5c15278.
- C. Du, J. P. Mills, A. G. Yohannes, W. Wei, L. Wang, S. Lu, J.-X. Lian, M. Wang, T. Guo, X. Wang, H. Zhou, C.-J. Sun, J. Z. Wen, B. Kendall, M. Couillard, H. Guo, Z. Tan, S. Siahrostami and Y. A. Wu, Nat. Commun., 2023, 14, 6142 CrossRef CAS PubMed.
- J. Li, Y. Chen, B. Yao, W. Yang, X. Cui, H. Liu, S. Dai, S. Xi, Z. Sun, W. Chen, Y. Qin, J. Wang, Q. He, C. Ling, D. Wang and Z. Zhang, J. Am. Chem. Soc., 2024, 146, 5693–5701 CrossRef CAS PubMed.
- J. Zhong, H. Duan, M. Cai, Y. Zhu, Z. Wang, X. Li, Z. Zhang, W. Qu, K. Zhang, D. Han, D. Cheng, Y. Shen, M. Xie, E. Cortes and D. Zhang, Angew. Chem., Int. Ed., 2025, 64, e202507956 CrossRef CAS.
- W. Ye, Y. Yao, X. Wei, M. Xu, S. Zhao, W. Wang, G. Jia, F. Dai, P. Gao, X. Lu, X. Li, B. Xi, N. Wang and S. Xiong, Angew. Chem., Int. Ed., 2025, 64, e202509303 CrossRef CAS PubMed.
- B. Kim, Y. C. Tan, Y. Ryu, K. Jang, H. G. Abbas, T. Kang, H. Choi, K.-S. Lee, S. Park, W. Kim, P.-P. Choi, S. Ringe and J. Oh, ACS Energy Lett., 2023, 8, 3356–3364 CrossRef CAS.
- Y. Zhang, P. Li, C. Zhao, G. Zhou, F. Zhou, Q. Zhang, C. Su and Y. Wu, Sci. Bull., 2022, 67, 1679–1687 Search PubMed.
- S. A. Chala, R. Liu, E. O. Oseghe, S. T. Clausing, C. Kampf, J. Bansmann, A. H. Clark, Y. Zhou, I. Lieberwirth, J. Biskupek, U. Kaiser and C. Streb, ACS Catal., 2024, 14, 15553–15564 CrossRef CAS.
- C. Jin, Y. Lin, Y. Wang, J. Shi, R. Li, Y. Liu, Z. Yue, K. Leng, Y. Zhao, Y. Wang, X. Han, Y. Qu and J. Bai, Adv. Mater., 2025, 37, 2412658 CrossRef CAS.
- W. Du, Z. Sun, S. Shang, K. Chen, X. Yang and K. Chu, ACS Nano, 2024, 18, 27718–27726 CrossRef CAS PubMed.
- Y. Gao, J. Wang, M. Sun, Y. Jing, L. Chen, Z. Liang, Y. Yang, C. Zhang, J. Yao and X. Wang, Angew. Chem., Int. Ed., 2024, 63, e202402215 CrossRef CAS PubMed.
- K. Chen, D. Ma, Y. Zhang, F. Wang, X. Yang, X. Wang, H. Zhang, X. Liu, R. Bao and K. Chu, Adv. Mater., 2024, 36, e2402160 CrossRef PubMed.
- X. Wu, Y. Chen, B. Tang, Q. Yan, D. Wu, H. Zhou, H. Wang, H. Zhang, D. He, H. Li, J. Zeng, L. Lu, S. Yang and T. Ma, Nat. Commun., 2025, 16, 8785 CrossRef.
- J. Lim, C.-Y. Liu, J. Park, Y.-H. Liu, T. P. Senftle, S. W. Lee and M. C. Hatzell, ACS Catal., 2021, 11, 7568–7577 CrossRef CAS.
- Z. Li, M. Zheng, C. Yan, D. Yang, R. Yang, C. Zhang, H. Liu, P. Song, C. Yin, Z. Qi, D. Liu, X. Zhou, L. Song, C. Lv and G. Yu, Nat. Commun., 2025, 16, 8940 CrossRef CAS PubMed.
- S. Bhowmick, A. Adalder, A. Maiti, S. Kapse, R. Thapa, S. Mondal and U. K. Ghorai, Chem. Sci., 2025, 16, 4806–4814 RSC.
- Y. Fu, S. Wang, Y. Wang, P. Wei, J. Shao, T. Liu, G. Wang and X. Bao, Angew. Chem., Int. Ed., 2023, 62, e202303327 CrossRef CAS.
- S. Chen, Z. Zhu, K. Song, H. Zhang, D. Luo, T. Cao, Y. Zou, C. Liu, L. Gan, D. Zhang, Y. Han and J. Huang, J. Am. Chem. Soc., 2025, 147, 36494–36507 CrossRef CAS PubMed.
- W. Ye, Y. Zhang, L. Chen, F. Wu, Y. Yao, W. Wang, G. Zhu, G. Jia, Z. Bai, S. Dou, P. Gao, N. Wang and G. Wang, Angew. Chem., Int. Ed., 2024, 63, e202410105 Search PubMed.
- Z. Li, Z. Shi, Y. Ou, L. Zhong, C. Yan, C. Zhang, K. Song, H. Liu, D. Liu, P. Song, C. Yin, Z. Qi, L. Song and C. Lv, Angew. Chem., Int. Ed., 2025, 64, e202510287 Search PubMed.
- C. Zhang, Q. Zhou, Z. Li, C. Yan, H. Liu, D. Liu, L. Song, Q. Yan and C. Lv, Angew. Chem., Int. Ed., 2025, 64, e202507869 Search PubMed.
- X. Gao, C. Zhu, C. Yang, G. Shi, Q. Xu and L. Zhang, ACS Catal., 2024, 14, 17871–17878 Search PubMed.
- Y. Ma, L. Guo, L. Chang, W. Guo, T. Zhou, F. Hao, W. Su, J. Zhou, G. Wang, M. Shao, J. Yu, J. Yin, Y. Wang, F. Liu, A. Zhang, K. Qian, J. Wang, X. Zhang, W. Zhou, S. Chu, C. Ling, L. Gan, Z. Guo and Z. Fan, Nat. Commun., 2025, 16, 7632 CrossRef CAS PubMed.
- Z. Li, Q. Wang, L. Zhong, C. Yan, Z. Shi, Y. Ou, Y. Shang, C. Zhang, S. Tian, H. Liu, D. Liu, P. Song, Z. Qi, L. Song and C. Lv, Mater. Today, 2025, 85, 49–59 Search PubMed.
- Y. Chen, X. Liu, S. Li, J. Li, M. Fan and S. Shu, Adv. Funct. Mater., 2025, e21409 CrossRef.
- Y. Dang, R. Zhang, S. Ren, S. Pitchaimuthu, S. Liu, R. Xing and X. Yang, Chem. Eng. J., 2025, 520, 166370 CrossRef CAS.
- Y. Tang, S. Liu, C. Guo, Y. Liu and Z. Tang, Sustainable Energy Fuels, 2023, 7, 5039–5045 Search PubMed.
- Y. Shi, Y. Wang, J. Yu, Y. Chen, C. Fang, D. Jiang, Q. Zhang, L. Gu, X. Yu, X. Li, H. Liu and W. Zhou, Adv. Energy Mater., 2023, 13, 2203506 CrossRef CAS.
- Z. Zhang, X. Ma, Y. Song, X. Yang, Q. Fang, Y. Yamauchi and J. Tang, Angew. Chem., Int. Ed., 2025, 64, e202511704 CrossRef CAS.
- C. Zhu, L. Zhou, Z. Zhang, C. Yang, G. Shi, S. Zhao, H. Gu, J. Wu, X. Gao, Y. Li, K. Liu, S. Dai and L. Zhang, Chem, 2022, 8, 3288–3301 Search PubMed.
- C. Zhu, Z. Zhang, R. Qiao, C. Yang, S. Zhao, G. Shi, X. Gao, H. Gu, K. Liu and L. Zhang, J. Phys. Chem. C, 2023, 127, 3470–3477 CrossRef CAS.
- Z.-x. Yang, X. Wen, L.-j. Gao, J. Zhang, R.-p. Wei, X.-m. Pan and G.-m. Xiao, Catal. Commun., 2023, 174, 106595 Search PubMed.
- Y. Ma, J. Yu, M. Sun, B. Chen, X. Zhou, C. Ye, Z. Guan, W. Guo, G. Wang, S. Lu, D. Xia, Y. Wang, Z. He, L. Zheng, Q. Yun, L. Wang, J. Zhou, P. Lu, J. Yin, Y. Zhao, Z. Luo, L. Zhai, L. Liao, Z. Zhu, R. Ye, Y. Chen, Y. Lu, S. Xi, B. Huang, C. S. Lee and Z. Fan, Adv. Mater., 2022, 34, e2110607 Search PubMed.
- X. Gao, Y. Jiang, J. Liu, G. Shi, C. Yang, Q. Xu, Y. Yun, Y. Shen, M. Chang, C. Zhu, T. Lu, Y. Wang, G. Du, S. Li, S. Dai and L. Zhang, Nat. Commun., 2024, 15, 10331 Search PubMed.
- J. Tan, Q. Shen, X. Jin, M. Wang, B. A, W. Chen, Z. Wei, L. Zhang and J. Shi, J. Am. Chem. Soc., 2025, 147, 23635–23642 Search PubMed.
- Z. Cai, N. Cao, F. Zhang, X. Lv, K. Wang, Y. He, Y. Shi, H. Bin Wu and P. Xie, Appl. Catal., B, 2023, 325, 122310 CrossRef CAS.
- Y. Chen, X. Y. Li, Z. Chen, A. Ozden, J. E. Huang, P. Ou, J. Dong, J. Zhang, C. Tian, B. H. Lee, X. Wang, S. Liu, Q. Qu, S. Wang, Y. Xu, R. K. Miao, Y. Zhao, Y. Liu, C. Qiu, J. Abed, H. Liu, H. Shin, D. Wang, Y. Li, D. Sinton and E. H. Sargent, Nat. Nanotechnol., 2024, 19, 311–318 CrossRef CAS PubMed.
- Y. Yu, D. Wang, Y. Hong, T. Zhang, C. Liu, J. Chen, G. Qin and S. Li, Chem. Commun., 2022, 58, 11163–11166 RSC.
- C. Lv, C. Lee, L. Zhong, H. Liu, J. Liu, L. Yang, C. Yan, W. Yu, H. H. Hng, Z. Qi, L. Song, S. Li, K. P. Loh, Q. Yan and G. Yu, ACS Nano, 2022, 16, 8213–8222 CrossRef CAS PubMed.
- R. Qi, Q. Jiang, L. Deng, X. Yu, B. Shi, M. Zhong, Y. Wang and X. Lu, Chem. Sci., 2025, 16, 378–385 Search PubMed.
- M. Li, C. Qi, J. Xu, R. Zou, L. Wang, W. Jiang, Y. Fan, P. Qiu and W. Luo, ACS Nano, 2025, 19, 11309–11322 Search PubMed.
- Y. Lu, H. Li, H. Sun, J. Zhao, Y. Zhang, Y. Wang, C. Zhu, D. Gao, Y. Tuo, J. Zeng, D. Chen and Z. Yan, ACS Catal., 2024, 14, 14744–14753 CrossRef CAS.
- D. Zhang, D. Jiang, B. Mao, Y. Liu, Q. Chen, H. Li, L. Xing, H. Huang, W. Zhang, W. Shi and Z. Kang, Angew. Chem., Int. Ed., 2025, 64, e202511259 CrossRef CAS.
- W. Huang, W. Luo, J. Liu, B.-E. Jia, C. Lee, J. Dong, L. Yang, B. Liu and Q. Yan, ACS Nano, 2024, 18, 20258–20267 CrossRef CAS PubMed.
- L. Chen, M. Li and J.-N. Zhang, Nano Res., 2024, 17, 7880–7899 Search PubMed.
- B. Zhang, Y. Hou, S. Wang, S. Zhou, Z. Li, Y. Hou, L. Lei and B. Yang, Appl. Catal., B, 2025, 379, 125727 Search PubMed.
- J. Li, Q. Zhu, A. Chang, S. Cheon, Y. Gao, B. Shang, H. Li, C. L. Rooney, L. Ren, Z. Jiang, Y. Liang, Z. Feng, S. Yang, L. Robert Baker and H. Wang, Nat. Nanotechnol., 2025, 20, 515–522 CrossRef CAS PubMed.
- J. Chen, D. Wang, X. Yang, W. Cui, X. Sang, Z. Zhao, L. Wang, Z. Li, B. Yang, L. Lei, J. Zheng, L. Dai and Y. Hou, Angew. Chem., Int. Ed., 2023, 62, e202215406 CrossRef CAS PubMed.
- F. Yu, X. Liu, L. Liao, G. Xia and H. Wang, Small, 2023, 19, e2301558 Search PubMed.
- H. Pan, J. Wu, X. Lu, Y. Li, Q. Zhang, J. Ling, Y. Wang and B. Min, ACS Appl. Energy Mater., 2025, 8, 8299–8310 CrossRef CAS.
- X. Yu, S. Zeng, R. Li, X. Dong, Q. Cao, L. Li and X. Guo, Chem. Eng. J., 2025, 519, 165559 Search PubMed.
- M. Liu, X. Lu, R. He, Y. Zhou, Y. Liu, L. Chen, X. Suo, J. Hou, Q. Ke, Z. Chen, P. Feng and C. Wan, Chem. Eng. J., 2025, 523, 168293 CrossRef CAS.
- T. Möller, M. Filippi, S. Brückner, W. Ju and P. Strasser, Nat. Commun., 2023, 14, 5680 Search PubMed.
- H. Chi, Z. Liang, S. Kuang, Y. Jin, T. Xiao, J. Lin, D. Han, L. Wang, S. Zhang and X. Ma, J. Am. Chem. Soc., 2025, 147, 32640–32648 Search PubMed.
- A. N. Biswas, Z. Xie, R. Xia, S. Overa, F. Jiao and J. G. Chen, ACS Energy Lett., 2022, 7, 2904–2910 Search PubMed.
- P. Wang, X. Wang, S. Chandra, A. Lielpetere, T. Quast, F. Conzuelo and W. Schuhmann, Angew. Chem., Int. Ed., 2025, 64, e202422882 CrossRef CAS.
- J. Feng, A. Badreldin and Y. Li, Energy Fuels, 2025, 39, 21175–21225 CrossRef CAS.
- G. Wu, Y. Song, Q. Zheng, C. Long, T. Fan, Z. Yang, X. Huang, Q. Li, Y. Sun, L. Zuo, S. Lei and Z. Tang, Adv. Energy Mater., 2022, 12, 2202054 CrossRef CAS.
- H. Li, H. Li, P. Wei, Y. Wang, Y. Zang, D. Gao, G. Wang and X. Bao, Energy Environ. Sci., 2023, 16, 1502–1510 RSC.
- J. Weidner, C. N. Tchassem, D. Das, R. Zerdoumi, G. Lu, X. Wang, M. Muhler, N. Sikdar and W. Schuhmann, ChemElectroChem, 2025, 12, e202400664 CrossRef CAS.
- Z. Xie, E. Huang, S. Garg, S. Hwang, P. Liu and J. G. Chen, Nat. Catal., 2024, 7, 98–109 CrossRef CAS.
- H. Lv, X. Dong, R. Li, C. Zeng, X. Zhang, Y. Song, H. Liu, J. Shao, N. Ta, Q. Zhao, Q. Fu, J. Xiao, G. Wang and X. Bao, Nat. Chem., 2025, 17, 695–702 CrossRef CAS PubMed.
- S. Garg, Z. Xie, A. X. Lam and J. G. Chen, ACS Energy Lett., 2024, 9, 2990–2996 CrossRef CAS.
- G. Lee, H. J. Jo, J. Choi, M. F. Guzman, Y. Shan, H. K. D. Le, J. Feijoo, N. Soland, D. S. Clark and P. Yang, Proc. Natl. Acad. Sci. U. S. A., 2025, 122, e2512565122 CrossRef CAS.
- Y. Pu, Y. Wang, G. Wu, X. Wu, Y. Lu, Y. Yu, N. Chu, X. He, D. Li, R. J. Zeng and Y. Jiang, Environ. Sci. Technol., 2024, 58, 7445–7456 CrossRef CAS.
- H. Cui, W. Liu, C. Ma, P. Shiri, Z. Zhu, H. Jiang, D. Li and L. Zhang, Appl. Catal., B, 2024, 350, 123946 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |