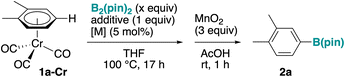Open Access Article
Open Access ArticleTransient π-coordination enables nucleophilic borylation of simple arenes
Yuichiro
Mutoh
 *a,
Relam
Khalaf
ab,
Sobi
Asako
*a,
Relam
Khalaf
ab,
Sobi
Asako
 a and
Laurean
Ilies
a and
Laurean
Ilies
 *a
*a
aRIKEN Center for Sustainable Resource Science, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan. E-mail: yuichiro.mutoh@riken.jp; laurean.ilies@riken.jp
bDepartment of Chemistry, Graduate School of Science and Engineering, Saitama University, Shimo-okubo, Sakura-ku, Saitama 338-8570, Japan
First published on 4th November 2025
Abstract
Borylation of an arene typically proceeds via transition-metal-catalyzed C–H activation. We report here that transient π-coordination to chromium activates 1 equiv. of a simple arene towards nucleophilic borylation with B2(pin)2 in the presence of an additive such as K2CO3 or KF. The transient activation of the substrate is achieved via in situ ligand exchange with a naphthalene chromium complex, followed by borylation of the resulting arene chromium complex. Electron-rich and sterically hindered arenes, challenging substrates for transition-metal-catalyzed C–H borylation, react well under these conditions. Mechanistic studies support an anionic mechanism, where a borate species undergoes nucleophilic addition to the arene chromium complex, followed by hydride migration.
Introduction
Functionalization of the C–H bond in an arene enables rapid construction of molecular complexity.1–3 For these reactions to proceed efficiently, specialized substrates such as arenes bearing a directing group,4 and/or complex catalysts are often required.5 π-Coordination of a metal fragment is known to decrease the electron density of an arene and has been often used in organic synthesis,6–11 but applications to the C–H functionalization of arenes remain scarce.12,13 Recently, Larossa and others showed that palladium-catalyzed C–H arylation of arenes with aryl halides can be enhanced by π-coordination of the arene substrate to chromium or ruthenium (Fig. 1B).14–25 However, these reactions require the synthesis and isolation of the arene metal complex as a starting material, typically using an excess amount of arene; to the best of our knowledge, there is only one example of generation of an arene chromium complex through arene exchange, followed by one-pot palladium/silver-catalyzed C–H arylation.20 We report here that 1 equiv. of a simple arene can be borylated with B2(pin)2 in the presence of a base such as K2CO3 or KF, upon transient activation through in situ arene exchange with a π-arene chromium complex precursor (Fig. 1C). Mechanistic studies suggest that the reaction proceeds through nucleophilic attack of a borate species to the arene activated by π-coordination, followed by hydride migration.Results and discussion
Reaction development: a model study
Transition-metal-catalyzed borylation of an arene is one of the most widely utilized C–H functionalization reactions,26–29 because it proceeds without the need of directing groups, and the boroester group can be easily converted to valuable functionality (Fig. 1A).30 Iridium has been the mostly used catalyst to date,31–33 and because oxidative addition is typically the turnover-limiting step, the reactions proceed the best for electron-deficient arenes. We envisioned that transient π-coordination of an arene to a metal fragment such as tricarbonylchromium through arene exchange with an appropriate precursor would decrease its electron density and enable efficient borylation, even for electron-rich substrates (Fig. 1C).To achieve this scenario, we first studied a model reaction of a xylene chromium complex (1a-Cr) with B2(pin)2 (Table 1). We initially investigated several catalysts often used for C–H borylation such as iridium24,31–33 or cobalt,34,35 but a control experiment showed that in the presence of an additive such as K2CO3, the reaction proceeds well without the need of the catalyst (entry 1). This result is intriguing, because borylation of an arene without the requirement of a transition metal catalyst has been achieved to date36 by using electrophilic borylating reagents,37,38 frustrated Lewis pairs,39 deprotonative40 or photochemical conditions.41 A brief screening of additives (entries 1–7) revealed a puzzling trend: K2CO3 and Na2CO3 were the most efficient, while Li2CO3, KF and KOMe also gave moderate yields (entries 3, 4, and 7). CsF and KOt-Bu were largely ineffective (entries 5 and 6). Notably, in the absence of an additive, the reaction did not proceed at all (entry 8). However, despite numerous efforts, the yield could not be further improved by the choice of the additive. Moreover, we also encountered reproducibility issues, with the yields fluctuating in the 40–80% range. We wondered if a small amount of chromium, which may leak from complex 1a-Cr during the course of the reaction could affect the result, and we added a catalytic amount of [Cr(acac)3] to find that the reaction proceeded reproducibly with a high yield (92% yield as determined by GC, entry 7), when 2 equiv. of borylating reagent was used. The reaction proceeded cleanly, and recovery of the starting material accounted for the rest of the material balance. Product 2a was obtained after decomplexation using MnO2/AcOH;14,15,17,20 for sensitive compounds (2m in Fig. 3), we used LED (450 nm) irradiation42 for decomplexation. Monitoring the reaction mixture before decomplexation by GC and GC/MS confirmed the formation of 2a-Cr (Fig. S1). The additive is important even in the presence of [Cr(acac)3], and in its absence the reaction did not proceed (entry 11). According to our hypothesis that π-coordination is the key enabler for this reactivity, when we conducted the reaction using 1a instead of 1a-Cr as the substrate, the product did not form at all (entry 12). Changing the chromium source to [CrCl3] gave a similarly high yield (entry 13). At the moment, we do not have an explanation for the effect of the added chromium on the reaction of chromium complexes 1-Cr. The reaction also proceeded well in its absence (entries 1 and 2), but sometimes less reproducibly; as described below (Table 2 and Fig. 2), the in situ activation of arenes 1 with a chromium complex precursor does not require additional chromium.
| Entry | x | Additive | [M] | 2a (%) | 1a (%) |
|---|---|---|---|---|---|
| a The yields were determined using GC in the presence of tridecane as an internal standard. b nd = Not detected. c 1a was used instead of 1a-Cr as the substrate. | |||||
| 1 | 1 | K2CO3 | None | 68 | 28 |
| 2 | 1 | Na2CO3 | None | 62 | 33 |
| 3 | 1 | Li2CO3 | None | 38 | 61 |
| 4 | 1 | KF | None | 47 | 49 |
| 5 | 1 | CsF | None | 5 | 85 |
| 6 | 1 | KOt-Bu | None | 3 | 79 |
| 7 | 1 | KOMe | None | 37 | 53 |
| 8 | 1 | None | None | ndb | >99 |
| 9 | 2 | K2CO3 | None | 77 | 27 |
| 10 | 2 | K2CO3 | [Cr(acac)3] | 92 | 8 |
| 11 | 2 | None | [Cr(acac)3] | <1 | >99 |
| 12c | 2 | K2CO3 | [Cr(acac)3] | ndb | >99 |
| 13 | 2 | K2CO3 | [CrCl3] | 83 | 11 |
| Entry | L | Additive | [M] | 2a (%) | L−B(pin)a (%) | 1a (%) |
|---|---|---|---|---|---|---|
| a The yields were determined using GC in the presence of tridecane as an internal standard. b NP = naphthalene. c Yield of a diborylated product. d With 5 equiv. of 1a. e 1,5-DMN = 1,5-dimethylnaphthalene. f 2,6-DMN = 2,6-dimethylnaphthalene. g TMTACH = 1,3,5-trimethyl-1,3,5-triazacyclohexane. h nd = Not detected. i Py = pyridine. j Under static vacuum. k With 2 equiv. of KF. | ||||||
| 1 | NPb | K2CO3 | None | 48 | 5 | 50 |
| 2 | NP | KF | None | 67 | 5 | 34 |
| 3 | NP | KF | [Cr(acac)3] | 65 (1)c | 4 | 28 |
| 4d | NP | KF | None | 44 | 4 | 98 |
| 5 | 1,5-DMNe | KF | None | 58 | 9 | 39 |
| 6 | 2,6-DMNf | KF | None | 63 | 4 | 20 |
| 7 | Pyrene | KF | None | 63 | 3 | 30 |
| 8 | TMTACHg | KF | None | 4 | ndh | >99 |
| 9 | 3Pyi | KF | None | ndh | 10 | >99 |
| 10j | 3CO | KFk | None | 4 | ndh | 98 |
Preliminary scope of the model reaction
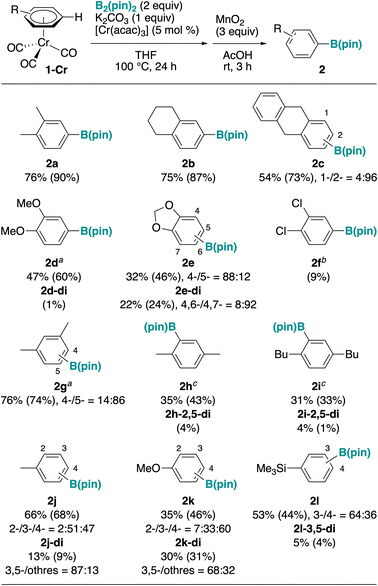
Under the optimized conditions (Table 1, entry 10), we briefly investigated the reaction of several arene chromium complexes (Fig. 2). Ortho-dialkyl (1a–1c) and ortho-dialkoxyarenes (1d, 1e) reacted with good yields. Interestingly, the reaction of 1d and 1e proceeded with different site selectivity, possibly due to steric control in the reaction of the bulkier 1d, as opposite to the ortho-directing effect in the reaction of the smaller 1e, as also observed for iridium catalysis.43 A small amount of diborylated product was also observed in some cases, and its amount increased in the case of 1e. Meta-xylene (1g) reacted well, selectively at the least hindered site; however, the borylation proceeded also at the sterically hindered C–H ortho to the methyl group (selectivity 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 86), whereas iridium-catalyzed borylation is typically sensitive to sterics. Accordingly, sterically demanding para-disubstituted alkylbenzenes (1h and 1i) reacted with moderate yields upon prolonged reaction time. Monosubstituted electron-rich arenes such as toluene (1j), anisole (1k), and phenyltrimethylsilane (1l) reacted well, but the product was obtained as a mixture of regioisomers, and diborylation also proceeded. A chromium complex of electron-deficient 1,2-dichlorobenzene (1f) reacted poorly, probably because of its lower stability under the reaction conditions.
86), whereas iridium-catalyzed borylation is typically sensitive to sterics. Accordingly, sterically demanding para-disubstituted alkylbenzenes (1h and 1i) reacted with moderate yields upon prolonged reaction time. Monosubstituted electron-rich arenes such as toluene (1j), anisole (1k), and phenyltrimethylsilane (1l) reacted well, but the product was obtained as a mixture of regioisomers, and diborylation also proceeded. A chromium complex of electron-deficient 1,2-dichlorobenzene (1f) reacted poorly, probably because of its lower stability under the reaction conditions.
Activation of an arene through transient π-coordination
Next, we investigated the reaction of a stoichiometric amount of xylene (1a) with B2(pin)2 in the presence of a chromium complex that can undergo arene exchange with 1a (Table 2). In the presence of a naphthalene (NP) chromium complex, known to undergo fast arene exchange,20,44–46 and using K2CO3 as an additive, 1a reacted with B2(pin)2 in THF at 100 °C to produce 2a in 48% yield after oxidative demetallation, together with a small amount of borylated naphthalene, and recovery of the substrate in 50% (entry 1). Changing the additive to KF slightly increased the yield (entry 2). In contrast to the reaction of chromium complex 1a-Cr, a catalytic amount of [Cr(acac)3] did not affect the reaction (entry 3), reinforcing that its presence is not essential for the reaction to proceed. Increasing the amount of substrate did not improve the yield (entry 4). Under the optimal reaction conditions at entry 2, we studied several chromium complex precursors (entries 5–9). To prevent the unproductive borylation of the naphthalene ligand, we used dimethylnaphthalene complexes (entries 5 and 6), but significant improvement was not achieved. A pyrene complex gave a similar result (entry 7). A triamine chromium complex47 could also be used, albeit with a decreased yield (entry 8). A trispyridine complex did not promote the reaction at all (entry 9). Commercially available [Cr(CO)6] was a less efficient precursor and gave the product in 4% yield, together with recovery of the starting material (entry 10).Reaction scope for the borylation of arenes (1) in situ activated by π-coordination
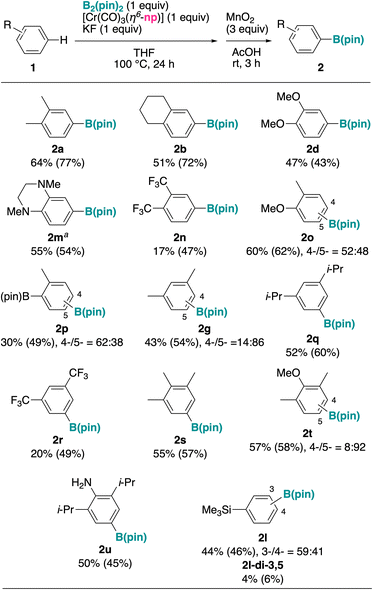
Under the reaction conditions in Table 2, entry 2, we investigated the reaction of several arene substrates (Fig. 3). To avoid regioselectivity issues and multiborylation, we mainly investigated the reaction of di- and trisubstituted arenes. As expected, the reactivity trend paralleled that of chromium complexes 1-Cr (Fig. 2), and alkylarenes and electron-rich arenes reacted with moderate yields, slightly lower than those for the corresponding 1-Cr substrates. In most of the cases, recovery of the starting material accounted for the remaining of the material balance, and a small amount of borylated naphthalene also formed (similarly with the reaction profile of the model substrate 1a in Table 2, entry 1). Ortho-Dialkylbenzenes 1a and 1b were borylated at the less sterically hindered position with good yields. Dimethoxybenzene 1d and diaminobenzene (1m), electron-rich substrates challenging for iridium-catalyzed borylation,48 reacted well. The reaction of an unsymmetric ortho-disubstituted arene (1o) gave an essentially 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of regioisomers. Trisubstituted arenes (1s–u) also reacted well. Notably, ortho-disustituted aniline (1u) reacted without the need of N-protection. A monosubstituted arene such as phenyltrimethylsilane (1l) reacted well, but a mixture of regioisomers and a small amount of diborylated product was obtained. Using the in situ exchange procedure, electron-deficient arenes such as 2n and 2r also reacted with moderate yields.
1 mixture of regioisomers. Trisubstituted arenes (1s–u) also reacted well. Notably, ortho-disustituted aniline (1u) reacted without the need of N-protection. A monosubstituted arene such as phenyltrimethylsilane (1l) reacted well, but a mixture of regioisomers and a small amount of diborylated product was obtained. Using the in situ exchange procedure, electron-deficient arenes such as 2n and 2r also reacted with moderate yields.
Borylation followed by functionalization
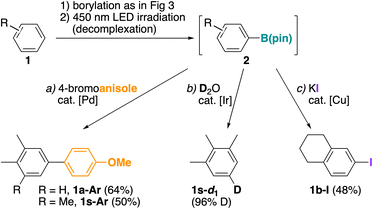
To demonstrate the synthetic utility of π-coordination-enabled borylation, we carried out several representative transformations of boroester 2 (Fig. 4). Suzuki–Miyaura cross coupling of 2a and 2s with 4-bromoanisole afforded the corresponding biaryls 1a-Ar and 1s-Ar, the latter known to exhibit a relatively large Stokes shift.49 Iridium-catalyzed deuteration50,51 of 2s afforded 1s-d1 selectively labeled at C5. Likewise, copper-mediated iodination52 selectively occurred at the aromatic carbon–boron bond to give the aryl iodide 1b-I, which is typically obtained via a Sandmeyer reaction from the corresponding aniline precursor, method that requires multiple synthetic steps.Preliminary mechanistic studies
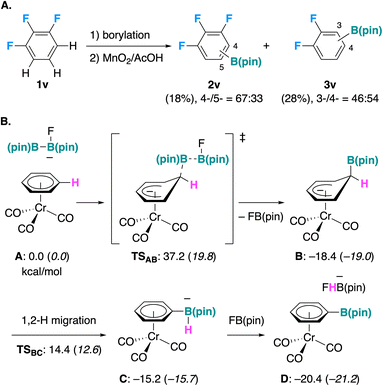
As shown in Table 1, entry 1, the borylation of a chromium arene complex proceeds without the need of a transition metal catalyst, even if the influence of trace amounts of chromium leaked into solution cannot be completely ruled out. To get insights into the mechanism of this reaction, we performed experimental and theoretical preliminary studies. Radical intermediates or single-electron transfer steps are likely not involved, because the reaction was not inhibited by scavengers such as 9,10-dihydroanthracene (1c-Cr, Fig. 3) or 1,4-bis(trifluoromethyl)benzene (Section 4.1 in SI),53–55 and 1,5-hydrogen transfer was not observed when a complex of 1,4-dibutylbenzene (1i-Cr) was used as the substrate (Fig. 2).The necessary presence of base (Table 1, entries 8 and 11) suggests the involvement of an anionic species. Activation of B2(pin)2 by a base to generate a nucleophilic borate species is well documented in the literature.56,57 We could not find examples of the attack of such anionic borate species to simple arenes in the literature, but the nucleophilic addition of strong organometallic compounds to arene chromium complexes is known.58 Also, recent reports on the attack of an Al(I) anion to simple arenes59–61 suggest that a similar reactivity of borate species is plausible. We reacted trifluorobenzene 1v under the optimized reaction conditions, and we obtained a mixture of C–H borylated products 2v and nucleophilic aromatic substitution products 3v (Fig. 5A),62 as was also observed for the alumination reaction.60 Moreover, we also performed a KIE study for two parallel reactions, to find a primary KIE of 2.4, similar to the value observed for the alumination reaction (1.7).60
Based on the considerations above, and a very recent example of nucleophilic aromatic substitution of hydrogen in arenes π-coordinated to ruthenium,63 we propose the mechanism depicted in Fig. 5B, which was also probed by DFT calculations.64 A base such as fluoride first activates B2(pin)2 as a borate species, which then attacks the arene coordinated to chromium (A) with concomitant C–B bond formation and B–B bond cleavage, releasing F(Bpin). Note that coordination of arene to a [Cr(CO)3] fragment is well known to enhance the nucleophilic attack of organometallic reagents for example.58 The resulting intermediate (B) then rearomatizes through 1,2-hydride shift from carbon to boron to afford an arylboronate product (C, then D).
Conclusions
We found that a stoichiometric amount of a simple arene can be transiently activated by π-coordination to chromium via in situ ligand exchange with a naphthalene chromium complex, to enable nucleophilic C–H borylation with B2(pin)2 in the presence of a base such as K2CO3 or KF. Electron-rich and sterically biased arenes, challenging substrates for transition-metal-catalyzed borylation, reacted well using the π-coordination strategy.Author contributions
Y. M.: formal analysis, funding acquisition, investigation, methodology, resources, supervision, validation, visualization, writing – original draft, review and editing. R. K.: formal analysis, investigation, validation. S. A.: formal analysis, methodology, visualization, writing – original draft, review and editing. L. I.: conceptualization, funding acquisition, investigation, project administration, supervision, visualization, writing – original draft, review and editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data for the manuscript “Transient π-Coordination Enables Nucleophilic Borylation of Simple Arenes” have been included as part of the supplementary information (SI). Supplementary information: experimental procedures, characterization data, compound data, computational data, and copies of NMR charts. See DOI: https://doi.org/10.1039/d5sc08107f.Acknowledgements
This work was supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (B) No. JP25K01777 (L. I.), Grant-in-Aid for Transformative Research Areas No. JP24H01101 (Digi-TOS) (L. I.), JSPS KAKENHI Grant Number JP22K05104 (Y. M.), and RIKEN Incentive Research Project grant (Y. M.). Computational time provided by the Research Center for Computational Science, Okazaki, Japan (Projects: 24-IMS-C061, 25-IMS-C063) and the RIKEN HOKUSAI BigWaterfall are gratefully acknowledged. We thank Dr Zhaomin Hou and Dr Masanori Takimoto (RIKEN) for generously allowing us to use the mass spectrometer.Notes and references
-
G. Dyker, in Handbook of C–H Transformations, ed. G. Dyker, Wiley-VCH, Weinheim, 2005 Search PubMed
.
-
P. H. Dixneuf and H. Doucet, in Topics in Organometallic Chemistry: C–H Bond Activation and Catalytic Functionalization, ed. P. H. Dixneuf and H. Doucet, Springer, Berlin, 2016 Search PubMed
.
-
J.-Q. Yu, in Catalytic Transformations via C–H Activation, Science of Synthesis, ed. J.-Q. Yu, Thieme, Stuttgart, vols 1–2, 2016 Search PubMed
.
- S. Murai, F. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda and N. Chatani, Efficient Catalytic Addition of Aromatic Carbon-Hydrogen Bonds to Olefins, Nature, 1993, 366, 529–531 CrossRef CAS
.
- S. Rej, A. Das and N. Chatani, Strategic Evolution in Transition Metal-Catalyzed Directed C–H Bond Activation and Future Directions, Coord. Chem. Rev., 2021, 431, 213683 CrossRef CAS
.
-
E. P. Kündig, Topics in Organometallic Chemistry: Transition Metal Arene π-Complexes in Organic Synthesis and Catalysis, Springer-Verlag, Berlin Heidelberg, 2004 Search PubMed
.
- M. Rosillo, G. Domínguez and J. Pérez-Castells, Chromium Arene Complexes in Organic Synthesis, Chem. Soc. Rev., 2007, 36, 1589–1604 RSC
.
- S. Takemoto and H. Matsuzaka, Recent Topics on Catalytic Transformations of Aromatic Molecules via η6-Arene Transition Metal Complexes, Tetrahedron Lett., 2018, 59, 697–703 CrossRef CAS
.
- J. W. Walton and L. A. Wilkinson, π-Coordinated Arene Metal Complexes and Catalysis, Organomet. Chem., 2019, 42, 125–171 CAS
.
- L. J. Williams, Y. Bhonoah, L. A. Wilkinson and J. W. Walton, As Nice as π: Aromatic Reactions Activated by π-Coordination to Transition Metals, Chem.–Eur. J., 2020, 27, 3650–3660 CrossRef
.
- K. Chen and H. Shi, Nucleophilic Aromatic Substitution of Halobenzenes and Phenols with Catalysis by Arenophilic π Acids, Acc. Chem. Res., 2024, 57, 2194–2206 CrossRef CAS
.
- F. Hu and M. Szostak, Pd-Catalyzed C–H Activation: Expanding the Portfolio of Metal-Catalyzed Functionalization of Unreactive C–H Bonds by Arene–Chromium π-Complexation, ChemCatChem, 2015, 7, 1061–1063 CrossRef CAS
.
- Y. Mutoh and L. Ilies, Activation of Strong Bonds Enabled by Arene π-Coordination, Synthesis, 2025, 57, 2783–2792 CrossRef CAS
.
- P. Ricci, K. Krämer, X. C. Cambeiro and I. Larrosa, Arene–Metal π-Complexation as a Traceless Reactivity Enhancer for C–H Arylation, J. Am. Chem. Soc., 2013, 135, 13258–13261 CrossRef CAS
.
- P. Ricci, K. Krämer and I. Larrosa, Tuning Reactivity and Site Selectivity of Simple Arenes in C–H Activation: Ortho-Arylation of Anisoles via Arene–Metal π-Complexation, J. Am. Chem. Soc., 2014, 136, 18082–18086 CrossRef CAS PubMed
.
- D. Whitaker, J. Burés and I. Larrosa, Ag(I)-Catalyzed C–H Activation: The Role of the Ag(I) Salt in Pd/Ag-Mediated C–H Arylation of Electron-Deficient Arenes, J. Am. Chem. Soc., 2016, 138, 8384–8387 CrossRef CAS PubMed
.
- D. Whitaker, M. Batuecas, P. Ricci and I. Larrosa, A Direct Arylation-Cyclisation Reaction for the Construction of Medium-Sized Rings, Chem.–Eur. J., 2017, 23, 12763–12766 CrossRef CAS
.
- L. A. Wilkinson, J. A. Pike and J. W. Walton, C–H Activation of π-Arene Ruthenium Complexes, Organometallics, 2017, 36, 4376–4381 CrossRef CAS
.
- M. Batuecas, J. Luo, I. Gergelitsová, K. Krämer, D. Whitaker, I. J. Vitorica-Yrezabal and I. Larrosa, Catalytic Asymmetric C–H Arylation of (η6-Arene)Chromium Complexes: Facile Access to Planar-Chiral Phosphines, ACS Catal., 2019, 9, 5268–5278 CrossRef CAS
.
- A. Panigrahi, D. Whitaker, I. J. Vitorica-Yrezabal and I. Larrosa, Ag/Pd Cocatalyzed Direct Arylation of Fluoroarene Derivatives with Aryl Bromides, ACS Catal., 2020, 10, 2100–2107 CrossRef CAS PubMed
.
- M. J. Fuchter, D. K. Judge, M. Weimar and A. J. P. White, Stoichiometric C–H Arylation of Tricarbonyl(Arene)Chromium Complexes Bearing Pyridine Directing Groups, Dalton Trans., 2013, 42, 5615–5618 RSC
.
- E. M. D'Amato, C. N. Neumann and T. Ritter, Selective Aromatic C–H Hydroxylation Enabled by η6-Coordination to Iridium(III), Organometallics, 2015, 34, 4626–4631 CrossRef PubMed
.
- C. Odena, M. Perez-Jimenez, R. Lin and P. J. Chirik, Exploring the Site Selectivity of Arene Oxidation in (η5-C5Me5)Ir(η6-arene)2 Complexes, Organometallics, 2024, 43, 3067 CrossRef CAS
.
- W.-Q. Wu, Y. Lin, Y. Li and H. Shi, Catalytic Dehydrogenative (3 + 2) Cycloaddition of Alkylbenzenes via π-Coordination, J. Am. Chem. Soc., 2023, 145, 9464–9470 CrossRef CAS PubMed
.
- While this manuscript was in preparation, an example of iridium-catalyzed borylation of chromium arene complexes was reported: A. Mandal, C. Maurer, C. Plett, K. R. K. Chandramohan, R. Fleischer, G. Schnakenburg, S. Grimme and A. Bunescu, Selective C–H Borylation of Polyaromatic Compounds Enabled by Metal-Arene π-Complexation, J. Am. Chem. Soc., 2025, 147, 15281–15293 CrossRef CAS PubMed
.
- I. A. I. Mkhalid, J. H. Barnard, T. B. Marder, J. B. Murphy and J. F. Hartwig, C–H Activation for the Construction of C–B Bonds, Chem. Rev., 2010, 110, 890–931 CrossRef CAS
.
- J. F. Hartwig, Borylation and Silylation of C–H Bonds: A Platform for Diverse C–H Bond Functionalizations, Acc. Chem. Res., 2012, 45, 864–873 CrossRef CAS PubMed
.
- L. Xu, G. Wang, S. Zhang, H. Wang, L. Wang, L. Liu, J. Jiao and P. Li, Recent Advances in Catalytic C–H Borylation Reactions, Tetrahedron, 2017, 73, 7123–7157 CrossRef CAS
.
- R. Bisht, C. Haldar, M. M. M. Hassan, M. E. Hoque, J. Chaturvedi and B. Chattopadhyay, Metal-Catalysed C–H Bond Activation and Borylation, Chem. Soc. Rev., 2022, 51, 5042–5100 RSC
.
-
D. G. Hall, Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, Wiley-VCH, Weinheim, 2005 Search PubMed
.
- T. Ishiyama, J. Takagi, K. Ishida, N. Miyaura, N. R. Anastasi and J. F. Hartwig, Mild Iridium-Catalyzed Borylation of Arenes. High Turnover Numbers, Room Temperature Reactions, and Isolation of a Potential Intermediate, J. Am. Chem. Soc., 2002, 124, 390–391 CrossRef CAS PubMed
.
- T. Ishiyama, J. Takagi, J. F. Hartwig and N. Miyaura, A Stoichiometric Aromatic C–H Borylation Catalyzed by Iridium(I)/2,2′-Bipyridine Complexes at Room Temperature, Angew. Chem., Int. Ed., 2002, 41, 3056–3058 CrossRef CAS PubMed
.
- J. Y. Cho, M. K. Tse, D. Holmes, R. E. Maleczka Jr. and M. R. Smith III, Remarkably Selective Iridium Catalysts for the Elaboration of Aromatic C–H Bonds, Science, 2002, 295, 305–308 CrossRef CAS PubMed
.
- J. V. Obligacion, P. J. Semproni and P. J. Chirik, Cobalt-Catalyzed C–H Borylation, J. Am. Chem. Soc., 2014, 136, 4133–4136 CrossRef CAS PubMed
.
- N. G. Léonard, M. J. Bezdek and P. J. Chirik, Cobalt-Catalyzed C(sp2)–H Borylation with an Air-Stable, Readily Prepared Terpyridine Cobalt(II) Bis(acetate) Precatalyst, Organometallics, 2017, 36, 142–150 CrossRef
.
- H. Zhang and L. Wang, Metal-Free Catalytic Aromatic C–H Borylation, Synlett, 2020, 31, 1857–1861 CrossRef CAS
.
- J. Lv, X. Chen, X.-S. Xue, B. Zhao, Y. Liang, M. Wang, L. Jin, Y. Yuan, Y. Han, Y. Zhao, Y. Lu, J. Zhao, W.-Y. Sun, K. N. Houk and Z. Shi, Metal-Free Directed sp2-C–H Borylation, Nature, 2019, 575, 336–341 CrossRef CAS PubMed
.
- L. Britton, A. D. Bage, S. L. McOnie and S. P. Thomas, Multifaceted Hidden Catalysis Revealed by Mechanistic Analysis of FeBr3-Catalysed Arene Borylation, Angew. Chem., Int. Ed., 2025, 64, e202423929 CrossRef CAS PubMed
.
- M.-A. Légaré, M.-A. Courtemanche, E. Rochette and F.-G. Fontaine, Metal-Free Catalytic C–H Bond Activation and Borylation of Heteroarenes, Science, 2015, 349, 513–516 CrossRef
.
- X. Liu, D. Li, G. Li, M. Wei, S. Yuan, M. Yang, L. He, S. Chen, Z. Li, L. Gao, G. Wang and S. Li, Organometallic-type Reactivity of Stable Organoboronates for Selective (Hetero)arene C−H/C-halogen Borylation and Beyond, Nat. Commun., 2025, 16, 5458 CrossRef CAS PubMed
.
- A. M. Mfuh, V. T. Nguyen, B. Chhetri, J. E. Burch, J. D. Doyle, V. N. Nesterov, H. A. Arman and O. V. Larionov, Additive- and Metal-Free, Predictably 1,2- and 1,3-Regioselective, Photoinduced Dual C−H/C−X Borylation of Haloarenes, J. Am. Chem. Soc., 2016, 138, 8408–8411 CrossRef CAS
.
- K. Li, W. Wu, Y. Lin and H. Shi, Asymmetric hydrogenation of 1,1-diarylethylenes and benzophenones through a relay strategy, Nat. Commun., 2023, 14, 2170 CrossRef CAS PubMed
.
- B. A. Vanchura II, S. M. Preshlock, P. C. Roosen, V. A. Kallepalli, R. J. Staples, R. E. Maleczka Jr., D. A. Singleton and M. R. Smith III, Electronic Effects in Iridium C–H Borylations: Insights from Unencumbered Substrates and Variation of Boryl Ligand Substituents, Chem. Commun., 2010, 46, 7724–7726 RSC
.
- E. P. Kündig, C. Perret, S. Spichiger and G. Bernardinelli, Naphthalene Complexes V. Arene Exchange Reactions in Naphthalenechromium Complexes, J. Organomet. Chem., 1985, 286, 183–200 CrossRef
.
- J. A. S. Howell, N. F. Ashford, D. T. Dixon, J. C. Kola, T. A. Albright and S. K. Kang, The Arene-Exchange Reaction in Naphthalene- and Pyrene Chromium Tricarbonyl, Organometallics, 1991, 10, 1852–1864 CrossRef CAS
.
- M. Wang, W. Zeng, L. Chen, Y. Yuan and W. Li, Umpolung-Enabled Divergent Dearomative Carbonylations, Angew. Chem., Int. Ed., 2024, 63, e202403917 CrossRef CAS PubMed
.
- N. L. Armanasco, M. V. Baker, M. R. North, B. W. Skelton and A. H. White, Chromium(0) Tricarbonyl Complexes of 1,3,5-Triazacyclohexanes, J. Chem. Soc., Dalton Trans., 1997, 3, 1363–1368 RSC
.
- Y. Jin, B. Ramadoss, S. Asako and L. Ilies, Noncovalent Interaction with a Spirobipyridine Ligand Enables Efficient Iridium-Catalyzed C–H Activation, Nat. Commun., 2024, 15, 2886 CrossRef CAS PubMed
.
- X. Liu, Y. Zhang, B. Li, L. N. Zakharov, M. Vasiliu, D. A. Dixon and S.-Y. Liu, A Modular Synthetic Approach to Monocyclic 1,4-Azaborines, Angew. Chem., Int. Ed., 2016, 55, 8333–8337 CrossRef CAS
.
- V. A. Kallepalli, K. A. Gore, F. Shi, L. Sanchez, G. A. Chotana, S. L. Miller, R. E. Maleczka and M. R. Smith, Harnessing C–H Borylation/Deborylation for Selective Deuteration, Synthesis of Boronate Esters, and Late Stage Functionalization, J. Org. Chem., 2015, 80, 8341–8353 CrossRef CAS PubMed
.
- R. Oeschger, B. Su, I. Yu, C. Ehinger, E. Romero, S. He and J. Hartwig, Diverse functionalization of strong alkyl C–H bonds by undirected borylation, Science, 2020, 368, 736–741 CrossRef CAS PubMed
.
- B. M. Partridge and J. F. Hartwig, Sterically Controlled Iodination of Arenes via Iridium-Catalyzed C–H Borylation, Org. Lett., 2013, 15, 140–143 CrossRef CAS PubMed
.
- T. Niwa, H. Ochiai, Y. Watanabe and T. Hosoya, Ni/Cu-Catalyzed Defluoroborylation of Fluoroarenes for Diverse C–F Bond Functionalizations, J. Am. Chem. Soc., 2015, 137, 14313–14318 CrossRef CAS PubMed
.
- T. Niwa, H. Ochiai and T. Hosoya, Copper-Catalyzed Ipso-Borylation of Fluoroarenes, ACS Catal., 2017, 7, 4535–4541 CrossRef CAS
.
- Y.-M. Tian, X.-N. Guo, I. Krummenacher, Z. Wu, J. Nitsch, H. Braunschweig, U. Radius and T. B. Marder, Visible-Light-Induced Ni-Catalyzed Radical Borylation of Chloroarenes, J. Am. Chem. Soc., 2020, 142, 18231–18242 CrossRef CAS PubMed
.
- J. J. Carbó and E. Fernández, Alkoxide Activation of tetra-Alkoxy Diboron Reagents in C–B Bond Formation: a Decade of Unpredictable Reactivity, Chem. Commun., 2021, 57, 11935–11947 RSC
.
- Z. Liu, G. K. Kole, Y. P. Budiman, Y. Tian, A. Friedrich, X. Luo, S. A. Westcott, U. Radius and T. B. Marder, Transition Metal Catalyst-Free, Base-Promoted 1,2-Additions of Polyfluorophenylboronates to Aldehydes and Ketones, Angew. Chem., Int. Ed., 2021, 60, 16529–16538 CrossRef CAS
.
- For example:
(a) M. F. Semmelhack and G. Clark, Meta-Substituted Aromatics by Carbanion Attack on π-Anisole and π-Toluenechromium Tricarbonyl, J. Am. Chem. Soc., 1977, 99, 1675–1676 CrossRef CAS
; (b) R. Bigler and V. K. Aggarwal, ortho-Directing Chromium Arene Complexes as Efficient Mediators for Enantiospecific C(sp2)–C(sp3) Cross-Coupling Reactions, Angew. Chem., Int. Ed., 2018, 57, 1082–1086 CrossRef CAS PubMed
.
- J. Hicks, P. Vasko, A. Heilmann, J. M. Goicoechea and S. Aldridge, Arene C−H Activation at Aluminium(I): meta Selectivity Driven by the Electronics of SNAr Chemistry, Angew. Chem., Int. Ed., 2020, 59, 20376–20380 CrossRef CAS PubMed
.
- S. Kurumada, K. Sugita, R. Nakano and M. Yamashita, A Meta-Selective C−H Alumination of Mono-Substituted Benzene by Using An Alkyl-Substituted Al Anion through Hydride-Eliminating SNAr Reaction, Angew. Chem., Int. Ed., 2020, 59, 20381–20384 CrossRef CAS PubMed
.
- J. J. Cabrera-Trujillo and I. Fernández, Factors Controlling the Aluminum(I)-Meta-Selective C−H Activation in Arenes, Chem.–Eur. J., 2021, 27, 12422–12429 CrossRef CAS PubMed
.
- The formation of 3v-4 having a boryl group at the C4 position can be explained by a second borylation of 2v-5 and 3v-3 followed by protodeborylation. See the SI for details.
- S. Melnikov, D. Hwang, B. Park, M. Lutz, M.-H. Baik and D. L. J. Broere, Ruthenium-mediated Nucleophilic Aromatic Substitution of Hydrogen in Benzene, Chem. Sci., 2025, 16, 13422–13434 RSC
.
- However, we cannot completely exclude alternative mechanisms such as C–H borylation by a low-valent chromium species at the moment; Y. Mutoh, R. Khalaf, S. Asako and L. Ilies, Transient π-Coordination Enables Nucleophilic Borylation of Simple Arenes, ChemRxiv, 2025, preprint, DOI:10.26434/chemrxiv-2025-bszc1-v3.
| This journal is © The Royal Society of Chemistry 2026 |