Open Access Article
Open Access ArticleLow-valent cobalt catalyzed direct dehydroxylative cross-coupling of benzyl alcohols with aryl-chlorides
Prashant S.
Shinde
 a,
Valmik S.
Shinde
a,
Valmik S.
Shinde
 *ab and
Magnus
Rueping
*ab and
Magnus
Rueping
 *a
*a
aKAUST Catalysis Center (KCC), King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia. E-mail: magnus.rueping@kaust.edu.sa
bMedicinal and Process Chemistry Division, CSIR-Central Drug Research Institute, Lucknow 226031, Uttar Pradesh, India
First published on 12th November 2025
Abstract
The direct functionalization of alcohols via C–O bond cleavage is a synthetically valuable but challenging transformation. In this work, we report a reductive C(sp3)–C(sp2) cross-coupling reaction between benzyl alcohols and a broad range of aryl chlorides. The success of this transformation is attributed to the development of low-coordinate cobalt/bipyridine complexes, which enable the selective conversion of benzyl alcohols into the corresponding diarylmethanes, while minimizing undesired homocoupling of either the benzyl alcohol or aryl chlorides.
Alcohols are among the most abundant and widely distributed functional groups in natural products and bioactive molecules and they also serve as important industrial feedstock chemicals.1–3 One of the most common functional group interconversions in synthetic chemistry involves the conversion of alcohols to halides and pseudohalides. However, these reactions often require significant time and resources for synthesis, purification and waste management, creating a bottleneck in the efficient production of pharmaceutical compound libraries derived from alcohols.4–6 Therefore, developing a general and mild approach to functionalize alcohols has received attention7–24 and their conversion to alkyl halides,25 sulfonate esters,26–31 alkyl acetates,32–35 pivalates,36,37 methyl ethers,38 chloroformates,39 redox-activated intermediates,40,41 and other derivatives42–45 has become a crucial strategy to achieve alkylative cross-coupling reactions.
The generation of alkyl radicals through dehydroxylation of alcohols under mild conditions is strategically appealing as alcohols can be directly used as alkylating reagents without the pre-conversion to more activated derivatives.46–57 However, the strong C–O bond dissociation energy (BDE ≈ 95 kcal mol−1) and the high redox potentials of unactivated alcohols make the radical cleavage of the hydroxyl group challenging, often requiring harsh reaction conditions and posing significant difficulties.58–60 Thus far, Barton-deoxygenation for alkyl C–O bond fragmentation represents the most widely used protocol that effectively generates alkyl radicals by homolytic scission of C(sp3)–O bonds under photo- or radical-initiator-induced conditions.61,62
More recently, alkyl oxalates have been developed as an alternative to the Barton deoxygenation.63–66 Building on these pioneering studies, several methods have been developed for the deoxygenative activation of alcohols using oxalate esters, enabling the generation of open-shell species for various C–C bond-forming reactions.67–70
Shu67,71–74 and Gong's75–78 research group independently reported the functionalization of oxalate esters, which were prepared either separately or in situ using dialkyl oxalates and stoichiometric amounts of zinc or manganese powder as reducing agents. More recently, this transformation has been accomplished with highly reactive organo-magnesium, lithium, or zinc reagents. However, the use of pyrophoric metal sources in stoichiometric amounts and the need for well-defined dialkylzinc reagents can constitute drawbacks of these methods.79–82 Consequently, there is growing interest in developing alternative strategies that employ more stable and readily available coupling partners. Such approaches offer an attractive pathway for generating benzyl radicals directly from benzyl alcohol, a more accessible and benign precursor.83–85 In this context, metal-catalyzed deoxygenative coupling of alcohols with organohalides or pseudohalides has emerged as a direct and efficient method for synthesizing deoxygenative coupling products, such as diarylmethanes, structural motifs that are prevalent in a wide range of bioactive compounds, pharmaceuticals, and functional organic materials.86–90
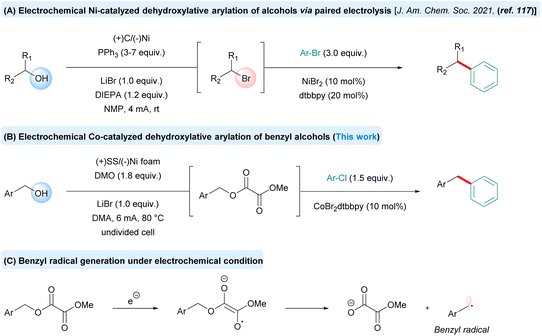
In the current study, we envisioned in situ electrochemically generated methyl-oxalate as an activating group, which was pioneered by Utley and co-workers.91–94 Consistent with their findings, it was observed that methyl oxalate effectively facilitates C–O bond activation.95 Over the past decade, synthetic electrochemistry has emerged as a powerful and sustainable platform for redox reactions, transforming a broad array of classical transformations into greener and more efficient processes.96–116 A notable example is the recent work by Li and co-workers, who developed a paired electrolysis protocol that merges the anodic Appel reaction with a nickel-catalyzed cathodic cross-electrophile coupling in an undivided electrochemical cell. This method enables the direct arylation of readily available alcohols with aryl bromides, providing a streamlined and environmentally friendly approach to C–C bond formation (Scheme 1). In addition to its broad substrate scope and excellent functional group compatibility, this method offers a significant advantage by avoiding the use of stoichiometric and hazardous halogenating agents such as CBr4 or Br2, commonly employed in the Appel reaction. However, the protocol still requires the use of 3–7 equivalents of triphenylphosphine (PPh3), which not only increases material cost but also leads to the formation of byproducts and may complicate purification, reducing overall efficiency.117
We sought to develop a complementary approach that enables efficient catalysis for C(sp2)–C(sp3) bond formation under electrochemical conditions. However, the implementation of our proposed electrolysis strategy presented two key challenges. First, it was essential to match the intrinsic redox properties of the paired reaction partners to ensure selective and efficient electron transfer. Second, successful reaction execution required careful adjustment of the rates of the anodic oxidation, cathodic reduction, and the metal-catalyzed cross-coupling cycle. These interdependent processes had to be finely balanced to avoid unproductive pathways and achieve high overall efficiency.
To initiate our study, we began by evaluating earth-abundant transition metal catalysts, such nickel and cobalt with 4-methoxy benzyl alcohol (1a) and methyl 4-chlorobenzoate (2a) as the standard coupling partners (Table 1).
| Entry | Variation from standard conditionsa,b | 3a (%) | 3a′ (%) | 3a″ (%) |
|---|---|---|---|---|
| a Standard conditions: 1a (0.4 mmol), 2a (0.6 mmol), CoBr2dtbbpy (10 mol%), DMO (1.8 equiv.), LiBr (1.0 equiv.), DMA, (4.0 mL), constant current i = 6 mA, 80 °C, 15 h. b GC-FID yield using dodecane as the internal standard. c Isolated yield. ND = not detected. | ||||
| 1 | Standard conditions | 90c | Trace | Trace |
| 2 | NiBr2dtbbpy (10 mol%) | 78c | 18 | Trace |
| 3 | CoBr2bpy (10 mol%) | 84c | 10 | Trace |
| 4 | CoCl2(PPh3)2 (10 mol%) | 76 | Trace | 18 |
| 5 | (+)Fe/(−)Ni foam | 70 | 21 | 09 |
| 6 | (+)Zn/(−)Ni foam | 77 | 10 | 12 |
| 7 | (+)RVC/(−)Ni foam, Et3N (3.0 equiv.) | ND | ND | 24 |
| 8 | (+)RVC/(−)Ni foam, TMEDA (3.0 equiv.) | ND | ND | 15 |
| 9 | ACN as solvent | 46 | 21 | 24 |
| 10 | DMF as solvent | 77c | 20 | 15 |
| 11 | NMP as solvent | 72 | 15 | ND |
| 12 | w/o CoBr2dtbbpy | ND | ND | 45c |
| 13 | w/o DMO | ND | 70 | 10 |
| 14 | w/o electricity | ND | ND | ND |
| 15 | Reaction at rt | ND | 78c | 10 |
The reaction was carried out in an undivided electrochemical cell equipped with a stainless-steel anode and a nickel foam cathode, affording the desired product 3a in 40–45% yield, along with minor side products 3a′ and 3a″. Subsequent efforts focused on optimizing the reaction parameters to improve efficiency and selectivity. Key variables investigated included the nature of the electrodes (both anodes and cathodes), electrolyte composition, current density, solvent system, and the choice of metal complexes for promoting the deoxygenative reductive cross-coupling reaction118,119 (see the SI for details, S3–S5). After extensive efforts, the desired product was obtained in 90% isolated yield by using 10 mol% CoBr2(dtbbpy) as the catalyst, 1.8 equiv. of dimethyl oxalate (DMO) as the activator, LiBr as the electrolyte, in DMA solvent (entry 1, Table 1). The use of a nickel catalyst slightly diminished the yield of the diaryl methane product with the formation of a homodimerized side product (entry 2, Table 1). Using different cobalt catalysts led to lower product yields along with some side reactions [3a′ & 3a″] (entries 3 and 4, Table 1). Altering the anode or cathode resulted in decreased yields (entries 5 and 6). The use of a non-sacrificial anode along with a sacrificial donor such as Et3N and TMEDA yielded no product (entries 7 and 8). The reaction was less efficient in solvents such as ACN, DMF and NMP (entries 9–11). Finally, the control experiments demonstrated that all reaction parameters, including the cobalt catalyst, DMO, and electricity are essential for accomplishing this transformation (entries 12–15).
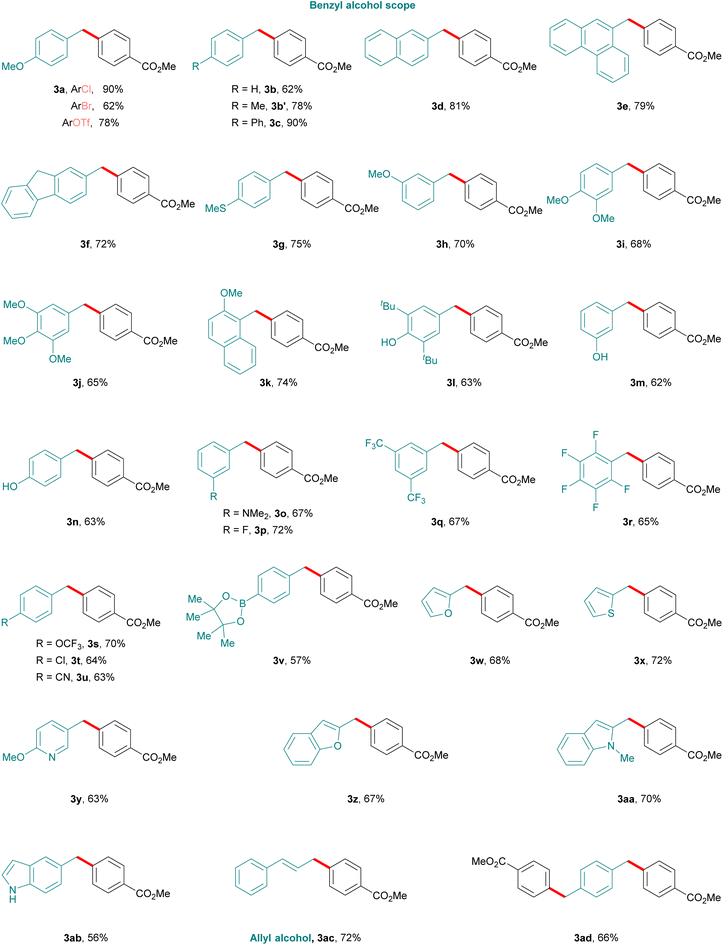
Following the successful optimization, we evaluated the scope and generality of the developed protocol. As shown in Fig. 1 and 2, a series of electronically and sterically diverse benzyl alcohols and aryl chlorides participated in this newly developed cobalta-electrocatalyzed protocol, and the corresponding diaryl methane products were isolated in good to excellent yields. Notably, aryl chlorides as reaction partners provided better yields as compared to aryl bromides and aryl triflates due to homocoupling of both reaction partners (3aFig. 1). Reaction with primary alcohols bearing biphenyl, naphthyl, phenanthrene and fluorene substituents proceeds with good efficiency (3c–3f). Benzyl alcohols with methylthio (3g), m-methoxy (3h), di- and trimethoxy (3i–3j) reacted equally well under the reaction conditions. Importantly, free phenolic hydroxyl groups at various positions as well as the dimethylamino group were also well tolerated. The chemoselectivity of the transformation was found to be good as illustrated by the tolerance of functional groups including fluoro (3p, 3r), trifluoromethyl (3q), trifluoromethoxyl (3s) and cyano (3u) moieties. Furthermore, reactive functional groups such as fluoro or chloro substituents or boronic esters were tolerated, providing opportunities for further late-stage functionalization (3r, 3s, 3t, and 3v). In addition, the reaction also proceeds with good efficiency using heterocyclic benzyl alcohol substrates containing furan, thiophene, pyridinyl, benzofuran, and indole motifs (3w–3ab). Notably, cinnamyl alcohol as a substrate also provided 1,1′-(1-propene-1,3-diyl)dibenzene (3ac) in 72% yield. 1,4-benzenedimethanol also underwent the reaction, affording the desired product in moderate yield (3ad).
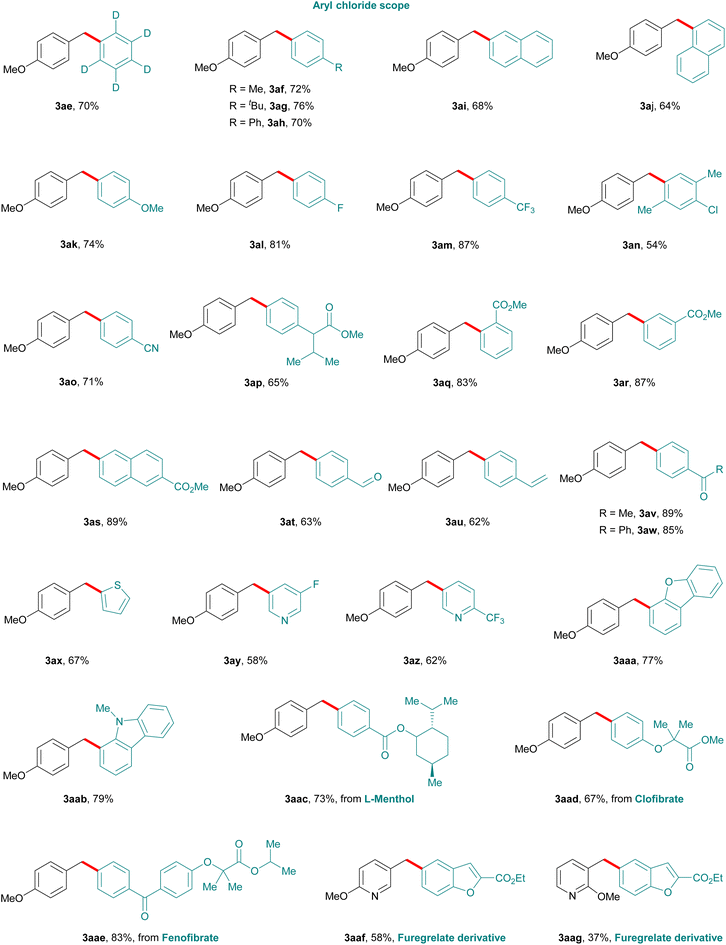
We next turned our attention to evaluate the scope of aryl chlorides (2), using 4-methoxy benzyl alcohol (1a) as a model substrate (Fig. 2). As anticipated a variety of aryl chlorides having various steric and electronic substituents at different positions of the aryl ring were well tolerated under the standard reaction conditions. Substituents such as deuterium (3ae), methyl (3af), tBu (3ag), biphenyl (3ah), naphthyl (3ai and 3aj), and methoxy (3ak) afforded diarylmethanes in good to excellent yields. Halo-substituted aryl halides such as fluoro (3al) and trifluoromethyl (3m) derivatives, resulted in 81% and 87% yields respectively. Of note, the method also allowed the formation of monosubstituted chloro-diaryl methane (3an) from a 1,4-dichloroarene substrate which can be used as a handle for further functionalization.
Furthermore, substituents such as cyano (3ao), esters (3ap–3as), aldehyde (3at), alkene (3au), and aliphatic and aromatic ketones (3av and 3aw), also proved efficient. In addition, heteroaromatic chlorides with groups such as thiophene (3ax), pyridine (3ay and 3az), dibenzofuran (3aaa), carbazole (3aab) provided the corresponding products in good to moderate yields. Notably, the current methodology is suitable for late-stage functionalization as chloro compounds derived from L-menthol, clofibrate, fenofibrate and furegrelates reacted smoothly, affording the corresponding products (3aac–3aag) in good to moderate yields.
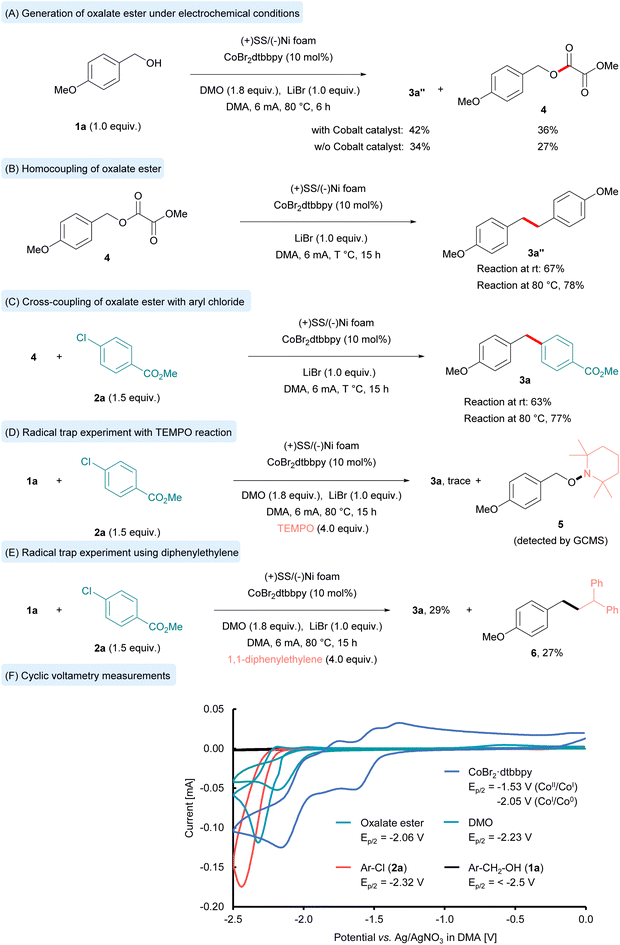
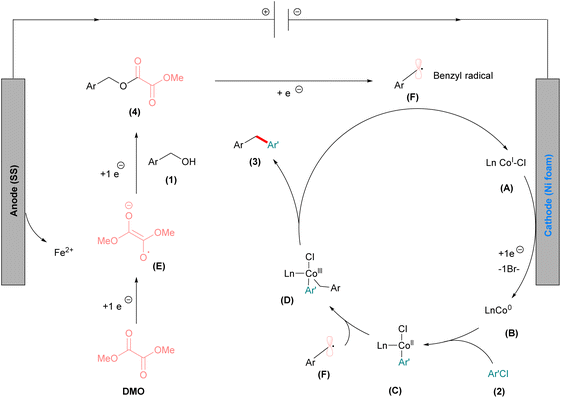
To gain insights into the reaction mechanism, a series of control experiments were conducted (Fig. 3). First, the formation of the oxalate ester intermediate was confirmed under the optimized reaction conditions. The conversion of 4-methoxybenzyl alcohol (1a) to oxalate ester (4) was sluggish at room temperature but proceeded efficiently at 80 °C. In the presence of the cobalt catalyst, the reaction afforded oxalate ester (4) in 36% yield and the deoxygenative homocoupled product (3a″) in 42% yield. In contrast, in the absence of cobalt, the oxalate ester (4) was obtained in 27% yield and 3a″ in 34% yield (Fig. 3, equation (A)). The enhanced formation of the oxalate ester in the presence of cobalt suggests that the cobalt catalyst facilitates transesterification. In another experiment the use of separately prepared oxalate ester (4) under standard reaction conditions led to the exclusive formation of deoxygenative homocoupled product (3a″) (Fig. 3, equation (B)). The reductive cross coupling reaction of the oxalate ester with aryl chlorides under standard reaction conditions afforded the cross-coupled diaryl methane compound (3a) in 77% yield (Fig. 3, equation (C)). Upon adding an excess amount of the radical scavenger TEMPO (2,2,6,6-tetramethylpiperidine-N-oxy), only a trace amount of product (3a) was detected (Fig. 3, equation (D)). Finally, the reaction with 1,1-diphenylethylene afforded the benzyl radical addition product (6) in 27% isolated yield (Fig. 3, equation (E)). These experiments indicate that a benzyl radical intermediate is involved in the reaction pathway, and the benzyl alcohol serves as the radical precursor via oxalate ester formation for the cross-coupling reaction. Notably, when 4-methoxybenzyl chloride was employed instead of 4-methoxybenzyl alcohol (1a) with methyl 4-chlorobenzoate (2a), only a trace amount of the cross-coupled product was obtained (see detailed control experiments in the SI, S-7). We subsequently conducted cyclic voltammetry (CV) measurements in DMA, as illustrated in Fig. 3 (equation (F)). The reduction potential of 4-methoxybenzyl alcohol (1a) was ascertained to be below −2.5 V vs. Ag/AgNO3, rendering its reduction unfeasible under the present protocol. Conversely, the reduction potentials of methyl 4-chlorobenzoate (2a), DMO, and oxalate ester were −2.32 V, −2.23 V, and −2.06 V vs. Ag/AgNO3, respectively. These findings suggest that DMO is initially reduced at the cathode, leading to the in situ formation of the oxalate ester. This oxalate ester can subsequently undergo further reduction at the cathode, yielding the benzylic radical. We also determined the reduction potential of the cobalt catalyst (CoBr2·dtbbpy), observing two reduction peaks. These peaks correspond to the CoII/CoI reduction (−1.53 V vs. Ag/AgNO3) and the CoI/Co0 reduction (−2.05 V vs. Ag/AgNO3). Given that the reduction of CoI to Co0 is more facile than that of DMO and aryl chloride (2a), we postulate that Co0 is produced during the electrochemical reaction. This suggests that aryl chloride more readily undergoes oxidative addition with Co0 than direct reduction.
Based on the gathered experimental evidence and literature reports72,75,93,120–122 we proposed a reaction mechanism that accounts for the formation of the observed product (Fig. 4). Initially, the pre-catalyst CoBr2dtbbpy undergoes cathodic reduction to form the catalytically competent low valent Co0 species (B) via two electron transfer processes. The low valent cobalt species then undergoes oxidative addition with aryl chloride (2) that leads to formation of a CoII intermediate (C). In parallel, under electrochemical conditions, DMO undergoes single electron reduction to afford the radical anion intermediate (E). The subsequent proton exchange process yields the alcohol anion and the trans-esterification with benzyl alcohol (1) leads to the formation of the oxalate ester (4) intermediate. The oxalate ester (4) immediately undergoes one electron reduction, generating the reactive benzyl radical (F). The generated benzyl radical (F) now participates in the catalytic cycle, resulting in the formation of the CoIII intermediate (D). Finally intermediate (D) undergoes reductive elimination to afford the diarylmethane (3) along with CoI species that again enters the next catalytic cycle. The alternative pathway of benzyl radical addition to the Co0 complex (B) to afford the CoI intermediate followed by oxidative addition of aryl chloride to generate the CoIII intermediate (C) cannot be completely ruled out.
In conclusion, we present the first example of in situ generated O-benzyl oxalate ester pronucleophiles derived from abundant benzyl alcohols in C(sp3)–C(sp2) cross-coupling reactions by the strategic merger of electrochemistry with cobalt catalysis. The method relies on the electrochemical generation of benzyl radicals via selective cleavage of the oxalate ester, employing dimethyl oxalate, a low-molecular-weight, inexpensive, and commercially available reagent as an effective activator under mild electrochemical conditions. A key feature of this protocol is its ability to selectively suppress competing radical dimerization pathways, leveraging the inherent chemoselectivity of the electrocatalytic cobalt catalyst. Notably, the method circumvents the use of stoichiometric metal reductants such as Zn or Mn, or costly reagents like hydrosilanes, which are often employed in reductive cross-coupling reactions. By utilizing alcohols as unconventional radical precursors, this approach opens a new avenue for C (sp3)–C (sp2) bond-forming chemistry, expanding the synthetic utility of readily available alcohols beyond their traditional use in sustainable and modular C–C bond construction.
Author contributions
P. S. S., V. S. S., and M. R. conceived and designed the experiments. P. S. S. and V. S. S. conducted the experiments, analyzed the data and wrote the manuscript, while M. R. supervised the project and manuscript preparation.Conflicts of interest
The authors declare no competing financial interests.Data availability
Experimental procedures and characterization of the new compounds are available in the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5sc05919d.Acknowledgements
This work was financially supported by the King Abdullah University of Science and Technology (KAUST), Saudi Arabia, Office of Sponsored Research (URF/1/4405 and URF/1/5246).Notes and references
- P. Ertl and T. Schuhmann, A Systematic Cheminformatics Analysis of Functional Groups Occurring in Natural Products, J. Nat. Prod., 2019, 82, 1258–1263 CrossRef CAS PubMed.
- P. Ertl, E. Altmann and J. M. McKenna, The most common functional groups in bioactive molecules and how their popularity has evolved over time, J. Med. Chem., 2020, 63, 8408–8418 CrossRef CAS PubMed.
- T. Henkel, R. M. Brunne, H. Müller and F. Reichel, Statistical investigation into the structural complementarity of natural products and synthetic compounds, Angew. Chem., Int. Ed., 1999, 38, 643–647 CrossRef CAS PubMed.
- J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Analysis of the reactions used for the preparation of drug candidate molecules, Org. Biomol. Chem., 2006, 4, 2337–2347 RSC.
- S. D. Roughley and A. M. Jordan, The medicinal chemist's toolbox: an analysis of reactions used in the pursuit of drug candidates, J. Med. Chem., 2011, 54, 3451–3479 CrossRef CAS.
- N. Schneider, D. M. Lowe, R. A. Sayle, M. A. Tarselli and G. A. Landrum, Big data from pharmaceutical patents: a computational analysis of medicinal chemists' bread and butter, J. Med. Chem., 2016, 59, 4385–4402 CrossRef CAS PubMed.
- S.-Y. Zhang, F.-M. Zhang and Y.-Q. Tu, Direct Sp3 α-C–H activation and functionalization of alcohol and ether, Chem. Soc. Rev., 2011, 40, 1937–1949 RSC.
- J. M. Ketcham, I. Shin, T. P. Montgomery and M. J. Krische, Catalytic Enantioselective C-H Functionalization of Alcohols by Redox-Triggered Carbonyl Addition: Borrowing Hydrogen, Returning Carbon, Angew. Chem., Int. Ed., 2014, 53, 9142–9150 CrossRef CAS PubMed.
- E. J. Tollefson, L. E. Hanna and E. R. Jarvo, Stereospecific nickel-catalyzed cross-coupling reactions of benzylic ethers and esters, Acc. Chem. Res., 2015, 48, 2344–2353 CrossRef CAS PubMed.
- E. Bisz and M. Szostak, Iron-Catalyzed C− O Bond Activation: Opportunity for Sustainable Catalysis, ChemSusChem, 2017, 10, 3964–3981 CrossRef CAS PubMed.
- I. Borthakur, A. Sau and S. Kundu, Cobalt-catalyzed dehydrogenative functionalization of alcohols: Progress and future prospect, Coord. Chem. Rev., 2022, 451, 214257 CrossRef CAS.
- R. Kuwano and Y. Kondo, Palladium-catalyzed benzylation of active methine compounds without additional base: Remarkable effect of 1, 5-cyclooctadiene, Org. Lett., 2004, 6, 3545–3547 CrossRef CAS PubMed.
- B. Su, Z.-C. Cao and Z.-J. Shi, Exploration of earth-abundant transition metals (Fe, Co, and Ni) as catalysts in unreactive chemical bond activations, Acc. Chem. Res., 2015, 48, 886–896 CrossRef CAS PubMed.
- B.-T. Guan, S.-K. Xiang, B.-Q. Wang, Z.-P. Sun, Y. Wang, K.-Q. Zhao and Z.-J. Shi, Direct benzylic alkylation via Ni-catalyzed selective benzylic sp3 C− O activation, J. Am. Chem. Soc., 2008, 130, 3268–3269 CrossRef CAS PubMed.
- L. Niu, J. Liu, X.-A. Liang, S. Wang and A. Lei, Visible light-induced direct α C–H functionalization of alcohols, Nat. Commun., 2019, 10, 467 CrossRef PubMed.
- B. L. Taylor, E. C. Swift, J. D. Waetzig and E. R. Jarvo, Stereospecific nickel-catalyzed cross-coupling reactions of alkyl ethers: Enantioselective synthesis of diarylethanes, J. Am. Chem. Soc., 2011, 133, 389–391 CrossRef CAS.
- D.-G. Yu, X. Wang, R.-Y. Zhu, S. Luo, X.-B. Zhang, B.-Q. Wang, L. Wang and Z.-J. Shi, Direct arylation/alkylation/magnesiation of benzyl alcohols in the presence of Grignard reagents via Ni-, Fe-, or Co-catalyzed sp3 C–O bond activation, J. Am. Chem. Soc., 2012, 134, 14638–14641 CrossRef CAS.
- A. J. Oelke, J. Sun and G. C. Fu, Nickel-catalyzed enantioselective cross-couplings of racemic secondary electrophiles that bear an oxygen leaving group, J. Am. Chem. Soc., 2012, 134, 2966–2969 CrossRef CAS.
- L. R. Jefferies and S. P. Cook, Iron-catalyzed arene alkylation reactions with unactivated secondary alcohols, Org. Lett., 2014, 16, 2026–2029 CrossRef CAS PubMed.
- H.-B. Wu, X.-T. Ma and S.-K. Tian, Palladium-catalyzed stereospecific cross-coupling of enantioenriched allylic alcohols with boronic acids, Chem. Commun., 2014, 50, 219–221 RSC.
- Z.-C. Cao, D.-G. Yu, R.-Y. Zhu, J.-B. Wei and Z.-J. Shi, Direct cross-coupling of benzyl alcohols to construct diarylmethanes via palladium catalysis, Chem. Commun., 2015, 51, 2683–2686 RSC.
- R. Martin-Montero, T. Krolikowski, C. Zarate, R. Manzano and R. Martin, Stereospecific nickel-catalyzed borylation of secondary benzyl pivalates, Synlett, 2017, 28, 2604–2608 CrossRef CAS.
- S. Akkarasamiyo, J. Margalef and J. S. Samec, Nickel-Catalyzed Suzuki-Miyaura Cross-Coupling Reaction of Naphthyl and Quinolyl Alcohols with Boronic Acids, Org. Lett., 2019, 21, 4782–4787 CrossRef CAS PubMed.
- P. T. Marcyk, L. R. Jefferies, D. I. AbuSalim, M. Pink, M. H. Baik and S. P. Cook, Stereoinversion of unactivated alcohols by tethered sulfonamides, Angew. Chem., Int. Ed., 2019, 58, 1727–1731 CrossRef CAS PubMed.
- Q. Lin, G. Ma and H. Gong, Ni-catalyzed formal cross-electrophile coupling of alcohols with aryl halides, ACS Catal., 2021, 11, 14102–14109 CrossRef CAS.
- G. A. Molander, K. M. Traister and B. T. O'Neill, Engaging nonaromatic, heterocyclic tosylates in reductive cross-coupling with aryl and heteroaryl bromides, J. Org. Chem., 2015, 80, 2907–2911 CrossRef CAS PubMed.
- L. K. Ackerman, L. L. Anka-Lufford, M. Naodovic and D. J. Weix, Cobalt co-catalysis for cross-electrophile coupling: diarylmethanes from benzyl mesylates and aryl halides, Chem. Sci., 2015, 6, 1115–1119 RSC.
- W. Liu, X. Yang, Y. Gao and C.-J. Li, Simple and efficient generation of aryl radicals from aryl triflates: synthesis of aryl boronates and aryl iodides at room temperature, J. Am. Chem. Soc., 2017, 139, 8621–8627 CrossRef CAS PubMed.
- J. Wang, J. Zhao and H. Gong, Nickel-catalyzed methylation of aryl halides/tosylates with methyl tosylate, Chem. Commun., 2017, 53, 10180–10183 RSC.
- B. A. Vara, N. R. Patel and G. A. Molander, O-benzyl xanthate esters under Ni/photoredox dual catalysis: selective radical generation and Csp3–Csp2 cross-coupling, ACS Catal., 2017, 7, 3955–3959 CrossRef CAS PubMed.
- M. Y. Ibrahim, G. R. Cumming, R. Gonzalez de Vega, P. Garcia-Losada, O. de Frutos, C. O. Kappe and D. Cantillo, Electrochemical nickel-catalyzed C (sp3)–C (sp3) cross-coupling of alkyl halides with alkyl tosylates, J. Am. Chem. Soc., 2023, 145, 17023–17028 CrossRef CAS.
- L. L. Anka-Lufford, M. R. Prinsell and D. J. Weix, Selective cross-coupling of organic halides with allylic acetates, J. Org. Chem., 2012, 77, 9989–10000 CrossRef CAS.
- X. Cui, S. Wang, Y. Zhang, W. Deng, Q. Qian and H. Gong, Nickel-catalyzed reductive allylation of aryl bromides with allylic acetates, Org. Biomol. Chem., 2013, 11, 3094–3097 RSC.
- H. Chen and M. Rueping, Facile, general allylation of unactivated alkyl halides via electrochemically enabled radical-polar crossover, Chem. Sci., 2025, 16, 6317–6324 RSC.
- H. Chen, C. Zhai, C. Zhu and M. Rueping, Allylgermane synthesis via facile and general nickela-electrocatalyzed electrophile coupling, Chem Catal., 2025, 5, 101257 CAS.
- M. O. Konev, L. E. Hanna and E. R. Jarvo, Intra- and Intermolecular Nickel-Catalyzed Reductive Cross-Electrophile Coupling Reactions of Benzylic Esters with Aryl Halides, Angew. Chem., Int. Ed., 2016, 55, 6730–6733 CrossRef CAS.
- X. Tao, Y. Chen, J. Guo, X. Wang and H. Gong, Preparation of α-amino acids via Ni-catalyzed reductive vinylation and arylation of α-pivaloyloxy glycine, Chem. Sci., 2021, 12, 220–226 RSC.
- K. M. Arendt and A. G. Doyle, Dialkyl Ether Formation by Nickel-Catalyzed Cross-Coupling of Acetals and Aryl Iodides, Angew. Chem., Int. Ed., 2015, 54, 9876–9880 CrossRef CAS.
- Y. Pan, Y. Gong, Y. Song, W. Tong and H. Gong, Deoxygenative cross-electrophile coupling of benzyl chloroformates with aryl iodides, Org. Biomol. Chem., 2019, 17, 4230–4233 RSC.
- Y. Wei, B. Ben-Zvi and T. Diao, Diastereoselective Synthesis of Aryl C-Glycosides from Glycosyl Esters via C-O Bond Homolysis, Angew. Chem., Int. Ed., 2021, 60, 9433–9438 CrossRef CAS.
- J. Xu, Y. Liu, Q. Wang, X. Tao, S. Ni, W. Zhang, L. Yu, Y. Pan and Y. Wang, Electrochemical deoxygenative amination of stabilized alkyl radicals from activated alcohols, Nat. Commun., 2024, 15, 6116 CrossRef CAS PubMed.
- L.-L. Zhang, Y.-Z. Gao, S.-H. Cai, H. Yu, S.-J. Shen, Q. Ping and Z.-P. Yang, Ni-catalyzed enantioconvergent deoxygenative reductive cross-coupling of unactivated alkyl alcohols and aryl bromides, Nat. Commun., 2024, 15, 2733 CrossRef CAS PubMed.
- W. L. Lyon and D. W. MacMillan, Expedient access to underexplored chemical space: deoxygenative C(sp3)–C(sp3) cross-coupling, J. Am. Chem. Soc., 2023, 145, 7736–7742 CrossRef CAS PubMed.
- Q. Cai, I. M. McWhinnie, N. W. Dow, A. Y. Chan and D. W. MacMillan, Engaging alkenes in metallaphotoredox: a triple catalytic, radical sorting approach to olefin-alcohol cross-coupling, J. Am. Chem. Soc., 2024, 146, 12300–12309 CrossRef CAS PubMed.
- Z. Li, L. Wang, T. Zeng, Z. Huang, S. Song, X. Qi and J. Zhu, Cross-Double Deoxygenative Carbon–Carbon Bond Formation between Benzyl Benzoates and Allylic Alcohols, ACS Catal., 2024, 14, 9496–9504 CrossRef CAS.
- D.-H. Lee, K.-H. Kwon and C. S. Yi, Selective catalytic C–H alkylation of alkenes with alcohols, Science, 2011, 333, 1613–1616 CrossRef CAS PubMed.
- J. Cornella, C. Zarate and R. Martin, Metal-catalyzed activation of ethers via C–O bond cleavage: a new strategy for molecular diversity, Chem. Soc. Rev., 2014, 43, 8081–8097 RSC.
- J. Jin and D. W. MacMillan, Alcohols as alkylating agents in heteroarene C–H functionalization, Nature, 2015, 525, 87–90 CrossRef CAS PubMed.
- T. Suga and Y. Ukaji, Nickel-catalyzed cross-electrophile coupling between benzyl alcohols and aryl halides assisted by titanium co-reductant, Org. Lett., 2018, 20, 7846–7850 CrossRef CAS PubMed.
- Y. Gao, Z. Wu, L. Yu, Y. Wang and Y. Pan, Alkyl Carbazates for Electrochemical Deoxygenative Functionalization of Heteroarenes, Angew. Chem., Int. Ed., 2020, 59, 10859–10863 CrossRef CAS PubMed.
- X. Pang and X.-Z. Shu, Titanium: a unique metal for radical dehydroxylative functionalization of alcohols, Synlett, 2021, 32, 1269–1274 CrossRef CAS.
- A. Cai, W. Yan and W. Liu, Aryl radical activation of C–O bonds: copper-catalyzed deoxygenative difluoromethylation of alcohols, J. Am. Chem. Soc., 2021, 143, 9952–9960 CrossRef CAS PubMed.
- H.-M. Guo and X. Wu, Selective deoxygenative alkylation of alcohols via photocatalytic domino radical fragmentations, Nat. Commun., 2021, 12, 5365 CrossRef CAS.
- B. K. Chi, J. K. Widness, M. M. Gilbert, D. C. Salgueiro, K. J. Garcia and D. J. Weix, In-situ bromination enables formal cross-electrophile coupling of alcohols with aryl and alkenyl halides, ACS Catal., 2021, 12, 580–586 CrossRef PubMed.
- W. Guan, Y. Chang and S. Lin, Electrochemically driven deoxygenative borylation of alcohols and carbonyl compounds, J. Am. Chem. Soc., 2023, 145, 16966–16972 CrossRef CAS.
- A. Cook, P. St. Onge and S. G. Newman, Deoxygenative Suzuki–Miyaura arylation of tertiary alcohols through silyl ethers, Nat. Synth., 2023, 2, 663–669 CrossRef CAS.
- T. Mandal, S. Mallick, M. Islam and S. De Sarkar, Alcohols as Alkyl Synthons Enabled by Photoredox-Catalyzed Deoxygenative Activation, ACS Catal., 2024, 14, 13451–13496 CrossRef CAS.
- J. B. Pedley, Thermochemical data of organic compounds, Springer Science & Business Media, 2012 Search PubMed.
- V. B. Oyeyemi, J. A. Keith and E. A. Carter, Trends in bond dissociation energies of alcohols and aldehydes computed with multireference averaged coupled-pair functional theory, J. Phys. Chem. A, 2014, 118, 3039–3050 CrossRef CAS PubMed.
- H. G. Roth, N. A. Romero and D. A. Nicewicz, Experimental and calculated electrochemical potentials of common organic molecules for applications to single-electron redox chemistry, Synlett, 2016, 27, 714–723 CAS.
- S. W. McCombie, W. B. Motherwell and M. J. Tozer, in Organic Reactions, ed. S. E. Denmark, Wiley, Hoboken, New Jersey, 2012, vol. 77, pp. 161–591 Search PubMed.
- J. M. Herrmann and B. König, Reductive deoxygenation of alcohols: catalytic methods beyond Barton–McCombie deoxygenation, Eur. J. Org Chem., 2013, 2013, 7017–7027 CrossRef CAS.
- G. L. Lackner, K. W. Quasdorf and L. E. Overman, Direct construction of quaternary carbons from tertiary alcohols via photoredox-catalyzed fragmentation of tert-alkyl N-phthalimidoyl oxalates, J. Am. Chem. Soc., 2013, 135, 15342–15345 CrossRef CAS PubMed.
- C. C. Nawrat, C. R. Jamison, Y. Slutskyy, D. W. MacMillan and L. E. Overman, Oxalates as activating groups for alcohols in visible light photoredox catalysis: formation of quaternary centers by redox-neutral fragment coupling, J. Am. Chem. Soc., 2015, 137, 11270–11273 CrossRef CAS PubMed.
- C. R. Jamison and L. E. Overman, Fragment coupling with tertiary radicals generated by visible-light photocatalysis, Acc. Chem. Res., 2016, 49, 1578–1586 CrossRef CAS PubMed.
- X. Zhang and D. W. MacMillan, Alcohols as latent coupling fragments for metallaphotoredox catalysis: sp3–sp2 cross-coupling of oxalates with aryl halides, J. Am. Chem. Soc., 2016, 138, 13862–13865 CrossRef CAS PubMed.
- X.-B. Yan, C.-L. Li, W.-J. Jin, P. Guo and X.-Z. Shu, Reductive coupling of benzyl oxalates with highly functionalized alkyl bromides by nickel catalysis, Chem. Sci., 2018, 9, 4529–4534 RSC.
- F. W. Friese and A. Studer, Deoxygenative borylation of secondary and tertiary alcohols, Angew. Chem., Int. Ed., 2019, 58, 9561–9564 CrossRef CAS PubMed.
- S. G. E. Amos, D. Cavalli, F. Le Vaillant and J. Waser, Direct Photoexcitation of Ethynylbenziodoxolones: An Alternative to Photocatalysis for Alkynylation Reactions, Angew. Chem., Int. Ed., 2021, 60, 23827–23834 CrossRef CAS PubMed.
- Y. Chen, F. Wang, B.-X. Liu, W.-D. Rao and S.-Y. Wang, A Ni (ii)-catalyzed reductive cross-coupling reaction of oxalates and thiosulfonates/selenosulfonates, Org. Chem. Front., 2022, 9, 731–736 RSC.
- P. Guo, K. Wang, W.-J. Jin, H. Xie, L. Qi, X.-Y. Liu and X.-Z. Shu, Dynamic kinetic cross-electrophile arylation of benzyl alcohols by nickel catalysis, J. Am. Chem. Soc., 2020, 143, 513–523 CrossRef PubMed.
- W.-Y. Ma, G.-Y. Han, S. Kang, X. Pang, X.-Y. Liu and X.-Z. Shu, Cobalt-catalyzed enantiospecific dynamic kinetic cross-electrophile vinylation of allylic alcohols with vinyl triflates, J. Am. Chem. Soc., 2021, 143, 15930–15935 CrossRef CAS PubMed.
- M.-X. You, P.-F. Su, Z.-H. She and X.-Z. Shu, Nickel-catalyzed cross-electrophile C-Ge coupling of benzyl pivalates and chlorogermanes, Sci. China:Chem., 2023, 66, 3562–3566 CrossRef CAS.
- X. Peng, J. Huang, G.-Y. Han and X.-Z. Shu, Nickel-catalyzed dynamic kinetic cross-electrophile coupling of benzylic alcohols and alkenyl triflates, Org. Chem. Front., 2024, 11, 94–99 RSC.
- Y. Ye, H. Chen, J. L. Sessler and H. Gong, Zn-mediated fragmentation of tertiary alkyl oxalates enabling formation of alkylated and arylated quaternary carbon centers, J. Am. Chem. Soc., 2018, 141, 820–824 CrossRef PubMed.
- M. Gao, D. Sun and H. Gong, Ni-catalyzed reductive C–O bond arylation of oxalates derived from α-hydroxy esters with aryl halides, Org. Lett., 2019, 21, 1645–1648 CrossRef CAS PubMed.
- H. Chen, Y. Ye, W. Tong, J. Fang and H. Gong, Formation of allylated quaternary carbon centers via C–O/C–O bond fragmentation of oxalates and allyl carbonates, Chem. Commun., 2020, 56, 454–457 RSC.
- Y. Ye, H. Chen, K. Yao and H. Gong, Iron-catalyzed reductive vinylation of tertiary alkyl oxalates with activated vinyl halides, Org. Lett., 2020, 22, 2070–2075 CrossRef CAS PubMed.
- M. Amatore and C. Gosmini, Synthesis of functionalised diarylmethanes via a cobalt-catalysed cross-coupling of arylzinc species with benzyl chlorides, Chem. Commun., 2008, 5019–5021 RSC.
- A. D. Benischke, I. Knoll, A. Rérat, C. Gosmini and P. Knochel, A practical cobalt-catalyzed cross-coupling of benzylic zinc reagents with aryl and heteroaryl bromides or chlorides, Chem. Commun., 2016, 52, 3171–3174 RSC.
- X. Shao, Y. Zheng, V. Ramadoss, L. Tian and Y. Wang, Recent advances in P III-assisted deoxygenative reactions under photochemical or electrochemical conditions, Org. Biomol. Chem., 2020, 18, 5994–6005 RSC.
- Y. Wang, J. Xu, Y. Pan and Y. Wang, Recent advances in electrochemical deoxygenation reactions of organic compounds, Org. Biomol. Chem., 2023, 21, 1121–1133 RSC.
- A. Flaherty, A. Trunkfield and W. Barton, Palladium-catalyzed cross-coupling of B-benzyl-9-borabicyclo [3.3. 1] nonane to furnish methylene-linked biaryls, Org. Lett., 2005, 7, 4975–4978 CrossRef CAS PubMed.
- L. S. Chupak, J. P. Wolkowski and Y. A. Chantigny, Palladium-catalyzed cross-coupling reactions of benzyl indium reagents with aryl iodides, J. Org. Chem., 2009, 74, 1388–1390 CrossRef CAS PubMed.
- J. C. Tellis, D. N. Primer and G. A. Molander, Single-electron transmetalation in organoboron cross-coupling by photoredox/nickel dual catalysis, Science, 2014, 345, 433–436 CrossRef CAS PubMed.
- Y.-Q. Long, X.-H. Jiang, R. Dayam, T. Sanchez, R. Shoemaker, S. Sei and N. Neamati, Rational design and synthesis of novel dimeric diketoacid-containing inhibitors of HIV-1 integrase: implication for binding to two metal ions on the active site of integrase, J. Med. Chem., 2004, 47, 2561–2573 CrossRef CAS PubMed.
- S. Messaoudi, A. Hamze, O. Provot, B. Tréguier, J. Rodrigo De Losada, J. Bignon, J. M. Liu, J. Wdzieczak-Bakala, S. Thoret and J. Dubois, Discovery of isoerianin analogues as promising anticancer agents, ChemMedChem, 2011, 6, 488–497 CrossRef CAS PubMed.
- D. Ameen and T. J. Snape, Chiral 1, 1-diaryl compounds as important pharmacophores, MedChemComm, 2013, 4, 893–907 RSC.
- S. Mondal and G. Panda, Synthetic methodologies of achiral diarylmethanols, diaryl and triarylmethanes (TRAMs) and medicinal properties of diaryl and triarylmethanes-an overview, RSC Adv., 2014, 4, 28317–28358 RSC.
- T. Jia, P. Cao and J. Liao, Enantioselective synthesis of gem-diarylalkanes by transition metal-catalyzed asymmetric arylations (TMCAAr), Chem. Sci., 2018, 9, 546–559 RSC.
- J. H. Utley and S. Ramesh, Electroorganic reactions. Part 58. Revisiting the cleavage of oxalate ester radical-anions, Arkivoc, 2003, 12, 18–26 Search PubMed.
- D. W. Sopher and J. H. Utley, Alkene formation in the cathodic reduction of oxalates, J. Chem. Soc. Chem. Commun., 1981, 134–136 RSC.
- N.-u. Islam, D. W. Sopher and J. H. Utley, Electro-organic reactions: Part 27. The mechanism of cathodic cleavage of activated esters; oxalates, squarates and oxamates, Tetrahedron, 1987, 43, 959–970 CrossRef CAS.
- D. W. Sopher and J. H. Utley, Electro-organic reactions. part 28. preparative applications of the oxalate cathodic cleavage reaction including one-pot conversions of aldehydes and ketones, Tetrahedron, 1987, 43, 2741–2748 CrossRef.
- P. Villo, A. Shatskiy, M. D. Kärkäs and H. Lundberg, Electrosynthetic C− O bond activation in alcohols and alcohol derivatives, Angew. Chem., Int. Ed., 2023, 62, e202211952 CrossRef CAS PubMed.
- E. J. Horn, B. R. Rosen and P. S. Baran, Synthetic organic electrochemistry: an enabling and innately sustainable method, ACS Cent. Sci., 2016, 2, 302–308 CrossRef CAS PubMed.
- H. Wang, X. Gao, Z. Lv, T. Abdelilah and A. Lei, Recent advances in oxidative R1-H/R2-H cross-coupling with hydrogen evolution via photo-/electrochemistry: focus review, Chem. Rev., 2019, 119, 6769–6787 CrossRef CAS PubMed.
- D. Pollok and S. R. Waldvogel, Electro-organic synthesis–a 21 st century technique, Chem. Sci., 2020, 11, 12386–12400 RSC.
- R. D. Little, A perspective on organic electrochemistry, J. Org. Chem., 2020, 85, 13375–13390 CrossRef CAS PubMed.
- Y. Kawamata and P. S. Baran, Electrosynthesis: Sustainability is not enough, Joule, 2020, 4, 701–704 CrossRef.
- T. H. Meyer, I. Choi, C. Tian and L. Ackermann, Powering the future: how can electrochemistry make a difference in organic synthesis?, Chem, 2020, 6, 2484–2496 CAS.
- P. Gandeepan, L. H. Finger, T. H. Meyer and L. Ackermann, 3d metallaelectrocatalysis for resource economical syntheses, Chem. Soc. Rev., 2020, 49, 4254–4272 RSC.
- C. Zhu, N. W. Ang, T. H. Meyer, Y. Qiu and L. Ackermann, Organic electrochemistry: molecular syntheses with potential, ACS Cent. Sci., 2021, 7, 415–431 CrossRef CAS PubMed.
- C. A. Malapit, M. B. Prater, J. R. Cabrera-Pardo, M. Li, T. D. Pham, T. P. McFadden, S. Blank and S. D. Minteer, Advances on the merger of electrochemistry and transition metal catalysis for organic synthesis, Chem. Rev., 2021, 122, 3180–3218 CrossRef PubMed.
- N. Li, R. Sitdikov, A. P. Kale, J. Steverlynck, B. Li and M. Rueping, A review of recent advances in electrochemical and photoelectrochemical late-stage functionalization classified by anodic oxidation, cathodic reduction, and paired electrolysis, Beilstein J. Org. Chem., 2024, 20, 2500–2566 CrossRef CAS PubMed.
- H. Chen, C. Zhai, H. Zhang, C. Zhu and M. Rueping, Switchable electrochemical pathways for the selective C(sp3)-Ge bond formation, Nat. Commun., 2025, 16, 7247 CrossRef CAS PubMed.
- H. Chen and M. Rueping, Alkynyl-Germanium Architectures Through Electrochemical Low-Valent Iron-Catalyzed Conjunctive Coupling, Angew. Chem., Int. Ed., 2025, e202516109 CAS.
- M. Ghosh, V. S. Shinde and M. Rueping, A review of asymmetric synthetic organic electrochemistry and electrocatalysis: concepts, applications, recent developments and future directions, Beilstein J. Org. Chem., 2019, 15, 2710–2746 CrossRef CAS PubMed.
- G. S. Kumar, A. Peshkov, A. Brzozowska, P. Nikolaienko, C. Zhu and M. Rueping, Nickel-Catalyzed Chain-Walking Cross-Electrophile Coupling of Alkyl and Aryl Halides and Olefin Hydroarylation Enabled by Electrochemical Reduction, Angew. Chem., Int. Ed., 2020, 59, 6513–6519 CrossRef CAS PubMed.
- C. Zhu, A. P. Kale, H. Yue and M. Rueping, Redox-neutral cross-coupling amination with weak N-nucleophiles: arylation of anilines, sulfonamides, sulfoximines, carbamates, and imines via nickelaelectrocatalysis, JACS Au, 2021, 1, 1057–1065 CrossRef CAS PubMed.
- C. Zhu, H. Yue and M. Rueping, Nickel catalyzed multicomponent stereodivergent synthesis of olefins enabled by electrochemistry, photocatalysis and photo-electrochemistry, Nat. Commun., 2022, 13, 3240 CrossRef CAS PubMed.
- H. Chen, C. Zhu, H. Yue and M. Rueping, Carbon–Germanium Bond Formation via Low-Valent Cobalt-Catalyzed Cross-Electrophile Coupling, ACS Catal., 2023, 13, 6773–6780 CrossRef CAS.
- H. Chen, C. Zhu, H. Yue and M. Rueping, Group 14 Elements Hetero-Difunctionalizations via Nickel-Catalyzed Electroreductive Cross-Coupling, Angew. Chem., Int. Ed., 2023, 62, e202306498 CrossRef CAS PubMed.
- G. S. Kumar, C. Zhu, R. Kancherla, P. S. Shinde and M. Rueping, Metal Cations from Sacrificial Anodes Act as a Lewis Acid Co-Catalyst in Electrochemical Cross-Coupling of Aryl Bromides and Aziridines, ACS Catal., 2023, 13, 8813–8820 CrossRef CAS.
- C. Zhu, H. Chen, H. Yue and M. Rueping, Electrochemical chemo-and regioselective arylalkylation, dialkylation and hydro (deutero) alkylation of 1, 3-enynes, Nat. Synth., 2023, 2, 1068–1081 CrossRef CAS.
- P. S. Shinde, V. S. Shinde and M. Rueping, Electrochemical low valent cobalt-catalyzed addition of aryl and vinyl chlorides to α-ketoamides via C–Cl bond activation, Chem. Commun., 2024, 60, 3826–3829 RSC.
- Z. Li, W. Sun, X. Wang, L. Li, Y. Zhang and C. Li, Electrochemically enabled, nickel-catalyzed dehydroxylative cross-coupling of alcohols with aryl halides, J. Am. Chem. Soc., 2021, 143, 3536–3543 CrossRef CAS PubMed.
- X. Pang, P.-F. Su and X.-Z. Shu, Reductive cross-coupling of unreactive electrophiles, Acc. Chem. Res., 2022, 55, 2491–2509 CrossRef CAS PubMed.
- Y. Liu, P. Li, Y. Wang and Y. Qiu, Electroreductive cross-electrophile coupling (eXEC) reactions, Angew. Chem., Int. Ed., 2023, 135, e202306679 CrossRef.
- P. S. Shinde, V. S. Shinde, C. Zhu and M. Rueping, Electrochemical Cobalt-Catalyzed Cross-Electrophile Coupling of Alcohols and Trifluoroalkenes via Simultaneous C–F and C–O Bond Cleavage, ACS Catal., 2025, 15, 17198–17205 CrossRef CAS.
- Y. Yan, W. Xiong, S. Li, Z. Wang, T. Kang, G. Li, G. Song, J. Dong and D. Xue, Nickel-Catalyzed O-Alkylisourea-Enabled Electrochemical Radical C(sp3)− C(sp2) Cross-Coupling, Angew. Chem., Int. Ed., 2025, e202516173 CAS.
- C. Dorval, M. Tricoire, J.-M. Begouin, V. Gandon and C. Gosmini, Cobalt-catalyzed C(sp2)–CN bond activation: cross-electrophile coupling for biaryl formation and mechanistic insight, ACS Catal., 2020, 10, 12819–12827 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |