Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tandem mechanochemical engineering yields highly crystalline metal–organic frameworks
Zhuorigebatu
Tegudeer
 and
Wen-Yang
Gao
and
Wen-Yang
Gao
 *
*
Department of Chemistry and Biochemistry, Nanoscale & Quantum Phenomena Institute, Ohio University, Athens, Ohio 45701, USA. E-mail: gaow@ohio.edu
First published on 29th December 2025
Abstract
The formation of highly crystalline metal–organic frameworks (MOFs) relies on reversible metal–ligand (M–L) bond formation under conditions that enable defect annealing. While solvothermal synthesis remains the most common method for producing crystalline MOFs, mechanochemical synthesis is emerging as a greener alternative. However, the solid-state nature of mechanochemical reactions—even when assisted by catalytic amounts of liquid additives—limits molecular mobility, thereby impeding defect annealing and crystallization. This work introduces a tandem mechanochemical engineering strategy to achieve highly crystalline MOFs by incorporating a second class of reversible bond formation—imine condensation—alongside traditional M–L coordination. The critical role of cooperative dynamics between M–L and imine reversible bonds is highlighted by systematic investigations using similar high-connectivity ligand analogues featuring irreversible covalent linkages (e.g., ether, amide, or alkyne), which fail to produce quality crystalline MOF phases under mechanochemical conditions. The synergistic effect of dual reversible bonds addresses sluggish reaction kinetics inherent to solid-state processes, enhances crystallization kinetics, and enables the efficient mechanochemical synthesis of MOFs with improved crystallinity under ambient conditions, particularly for frameworks constructed from high-connectivity ligands.
Introduction
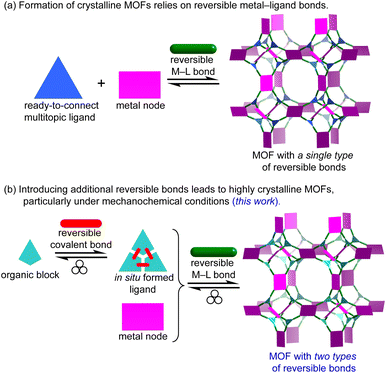
The formation of highly crystalline metal–organic frameworks (MOFs) hinges on reversible metal–ligand (M–L) dative bond formation between ready-to-connect organic ligands and metal ions under appropriate synthetic conditions (Fig. 1a).1–4 Traditionally, solvothermal methods dominate MOF synthesis, where reactants are dissolved in excess organic solvents and incubated at elevated temperatures over extended periods.5–7 These conditions allow the system to reach thermodynamic equilibrium, enabling defect annealing and promoting the growth of well-ordered crystalline phases.As a more sustainable alternative, mechanochemical synthesis has emerged, using mechanical energy to drive MOF assembly under solvent-free conditions or in the presence of only catalytic amounts of solvents.8–19 However, the (near) solid-state nature of mechanochemistry limits molecular mobility and typically reduces the reversibility of M–L bonds. Our previous studies have shown that ligand exchange kinetics significantly impact the crystallinity of mechanochemically synthesized MOFs, and that relatively inert metals, coupled with low M–L bond reversibility, often yield only small crystallites.20,21 This constraint—the decreased reversibility of M–L bonds under solid-state conditions—poses a particular challenge for incorporating bulky or inherently sluggish ligands that require dynamic chemical environments to assemble into ordered MOF structures. This explains why multitopic ligands with 2 to 4 connection points have been mechanochemically integrated into MOFs,22–40 but ligands with higher connectivity are rarely encountered in mechanochemistry.41 Herein, we propose tandem mechanochemical engineering as a new synthetic strategy to overcome this challenge and enable the efficient incorporation of bulky or less mobile ligands (e.g., high-connectivity multitopic ligands) into MOFs under mechanochemical conditions (Fig. 1b). Our earlier study showed that imines can be directly introduced into MOFs by mechanochemistry.42 In contrast, the present work departs from that approach to tackle the sluggish reaction kinetics inherent to solid-state processes during crystalline phase formation. By introducing a second type of reversible bonds, namely a dynamic covalent imine bond, alongside the M–L dative bond, this tandem approach enables the one-pot mechanochemical synthesis of highly crystalline MOFs from high-connectivity ligands. Remarkably, it also reduces reaction time by approximately 100-fold (e.g., from typical 1.5 hours to 56 seconds or less). The use of multiple reversible interactions mitigates the limited molecular mobility inherent to solventless conditions and significantly expands the scope of mechanochemical synthesis to a broader family of MOFs with diverse topologies.
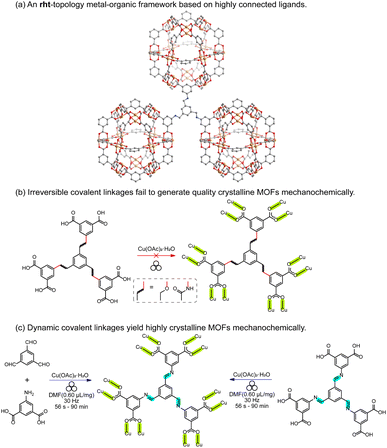
As a proof-of-concept, we selected rht-topology MOFs (Fig. 2a), which are typically constructed from highly connected hexacarboxylic ligands and metal paddlewheel dimers (e.g., Cu2(OOC)4).43–50 The (3,24)-connected rht-topology network represents a canonical example of reticular chemistry, employing supermolecular building blocks—specifically, 24-connected cuboctahedra composed of 24 isophthalate moieties and 12 dinuclear copper paddlewheel units.51 These frameworks exemplify best practices in MOF reticular design, achieving record-high surface areas and free pore volumes through ligand expansion while avoiding framework interpenetration.52–54 However, to the best of our knowledge, no rht-topology MOFs has been synthesized mechanochemically. While the fast kinetics of Cu–O bond formation have enabled the mechanochemical assembly of copper paddlewheel-based MOFs26,29,55,56 (e.g., HKUST-1,57 MOF-14,58 and MOF-505 (ref. 59)) using tricarboxylic or tetracarboxylic ligands, the incorporation of hexacarboxylic ligands remain a significant challenge under mechanochemical conditions. This difficulty is tentatively attributed to the high lattice energy barrier that must be overcome during the annealing of M–L defects involving metal nodes and high-connectivity ligands.
Results and discussion
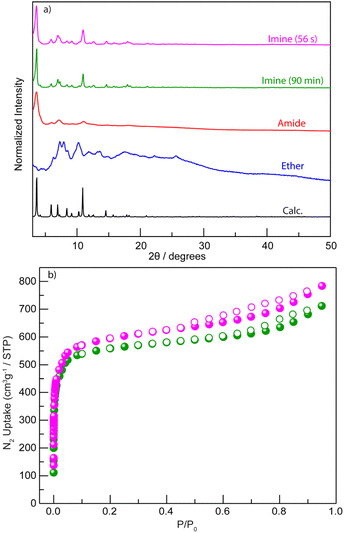
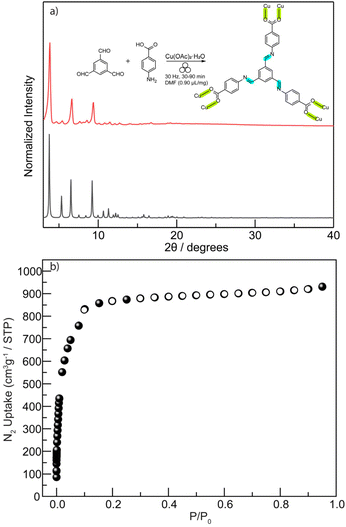
Two hexacarboxylic ligands featuring ether and amide linkages (Fig. 2b)—both previously known to yield rht-topology MOFs via solvothermal synthesis47,60,61—were extensively attempted for mechanochemical assembly under various milling conditions. However, powder X-ray diffraction (PXRD, Table S1 and Fig. 3a and S1) reveal poorly crystalline phases for the ether-linked ligand. The amide-linked ligand produced relatively broad PXRD patterns matching the calculated rht structure under certain conditions (Table S2 and Fig. 3a and S2). Nevertheless, N2 adsorption isotherms at 77 K (Fig. S3) showed significantly reduced Brunauer–Emmett–Teller (BET) surface areas compared to those obtained from solvothermal synthesis (215–1069 m2 g−1vs. 3160 m2 g−1).61 The parameter scope of our mechanochemical trials included variation in the type and amount of liquid additive (e.g., N,N-dimethylformamide, DMF), milling time, and milling frequency. Moreover, we also tested high-temperature ball-milling conditions62—blowing 80 °C and 120 °C hot air onto the stainless-steel milling jars–for reactions involving both the amide- and ether-linked ligands, in order to evaluate how elevated temperature influences reagent mobility in the solid state and the resulting crystallinity during the MOF assembly. However, no improvement in the PXRD patterns (Fig. S4) was observed, and reflections corresponding to elemental Cu appeared instead. These unsuccessful attempts underscore the challenge of incorporating high-connectivity ligands into crystalline MOFs under mechanochemical conditions, likely due to the reduced reversibility of M–L bond formation in the (near) solid environment and other potential deleterious reactions observed for Cu(II).In contrast to the previously tested irreversible covalent linkages (e.g., ether and amide), we propose that incorporating reversible or dynamic covalent linkage (e.g., imine) into highly connected ligands can mitigate the irreversibility of M–L interactions by effectively reducing the number of ligand connectivity during the assembly process (Fig. 2c). This enhanced reversibility is expected to facilitate the mechanochemical crystallization of MOFs from otherwise sluggish, highly connected ligands. Therefore, we initiated the mechanochemical synthesis of an rht-topology MOF by milling trimesaldehyde, 5-aminoisophthalic acid, and Cu(OAc)2·H2O in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in the presence of DMF (η = 0.60 µL mg−1, Table S3 and Fig. S5) using a stainless-steel milling cup and two stainless steel balls. The reaction mixture was milled at 30 Hz for 90 min. The resultant blue solids were washed with acetone and collected by centrifugation. PXRD analysis (Fig. 3a) confirmed the phase purity of the obtained imine-derived rht-topolgy MOF, based on the excellent agreement between the experimental patterns and the simulated ones (calculated from the amide-linked framework). Additionally, we synthesized the imine-based hexacarboxylic ligand under standard solution-phase condensation conditions catalyzed by acetic acid (see details in SI) and used it for the mechanochemical synthesis of the above rht-MOF. The isolated imine ligand was confirmed by 1H NMR (Fig. S6), high-resolution mass spectrometry (HRMS), and infrared spectroscopy (Fig. S7). The mechanochemical MOF product obtained from this pre-synthesized imine ligand was identified by PXRD (Fig. S8) as the same phase as the rht-MOF prepared via the one-pot cascade reaction. This observation is consistent with previous findings that mechanochemical synthesis can deliver crystalline imine-based covalent organic frameworks63–67 and MOFs,42 and further demonstrates the imine formation and cleavage remain reversible under the applied mechanochemical conditions. In contrast, similar mechanochemical reactions employing the hexacarboxylic ligands with irreversible covalent ether or amide linkages failed to yield the targeted high-quality crystalline MOFs. This comparison underscores the effectiveness of our tandem mechanochemical engineering strategy, where the introduction of an additional class of reversible bonds significantly improves the crystallization of MOFs under mechanochemical conditions.
3 in the presence of DMF (η = 0.60 µL mg−1, Table S3 and Fig. S5) using a stainless-steel milling cup and two stainless steel balls. The reaction mixture was milled at 30 Hz for 90 min. The resultant blue solids were washed with acetone and collected by centrifugation. PXRD analysis (Fig. 3a) confirmed the phase purity of the obtained imine-derived rht-topolgy MOF, based on the excellent agreement between the experimental patterns and the simulated ones (calculated from the amide-linked framework). Additionally, we synthesized the imine-based hexacarboxylic ligand under standard solution-phase condensation conditions catalyzed by acetic acid (see details in SI) and used it for the mechanochemical synthesis of the above rht-MOF. The isolated imine ligand was confirmed by 1H NMR (Fig. S6), high-resolution mass spectrometry (HRMS), and infrared spectroscopy (Fig. S7). The mechanochemical MOF product obtained from this pre-synthesized imine ligand was identified by PXRD (Fig. S8) as the same phase as the rht-MOF prepared via the one-pot cascade reaction. This observation is consistent with previous findings that mechanochemical synthesis can deliver crystalline imine-based covalent organic frameworks63–67 and MOFs,42 and further demonstrates the imine formation and cleavage remain reversible under the applied mechanochemical conditions. In contrast, similar mechanochemical reactions employing the hexacarboxylic ligands with irreversible covalent ether or amide linkages failed to yield the targeted high-quality crystalline MOFs. This comparison underscores the effectiveness of our tandem mechanochemical engineering strategy, where the introduction of an additional class of reversible bonds significantly improves the crystallization of MOFs under mechanochemical conditions.
Moreover, due to the hydrolytic instability of the imine motif,68,69 imine-based complexes and MOFs are well-suited for direct mechanochemical synthesis from aldehyde and primary amine precursors, along with the metal source.42,70–72 We attempted a series of solvothermal reactions using the pre-synthesized imine-based hexacarboxylic acid ligand (see details in SI, Table S4, and Fig. S9) to access this rht-MOF. However, the yields were very low, or the reactions were unsuccessful. Consistent with the lability of the imine linkages, HRMS analysis of reaction solutions indicated that the ligand decomposed back into its aldehyde and amine precursors, particularly in water-rich environment. In contrast, the tandem mechanochemical approach not only avoids the decomposition pathways that often occur under solvothermal conditions, but also enables the formation of the first imine-based rht-MOF among its various analogues, eliminating the need for the tedious pre-synthesis of imine ligands.
Additional characterizations of the imine-based rht-MOF obtained by tandem mechanochemical synthesis include IR spectroscopy, thermogravimetric analysis (TGA), and N2 adsorption analysis at 77 K. The IR spectrum (Fig. S10) shows that a strong peak emerged at 1371 cm−1, attributed to the coordinated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch, validating the formation of the desired MOF. The TGA analysis (Fig. S11) reveals that the obtained imine-based rht-topology MOF exhibits thermal stability comparable to other known rht-MOFs,60,61 which is stable up to 280 °C or above. Thus, the imine-based rht-MOF was activated by heating at 60 °C under high vacuum for 18 h prior to gas adsorption measurements. The permanent porosity of the imine-based rht-MOF was characterized by N2 adsorption isotherms at 77 K (Fig. 3b), which provided a BET surface area of 2238 m2 g−1 (P/P0 = 0.007–0.03). This value is somewhat lower than that of its amide analogue (3160 m2 g−1), likely due to the entrapment of molecular fragments from incomplete reactions within the cavities, which partially occupy the pore volume. Nevertheless, the BET surface area of the mechanochemically synthesized imine-based rht-MOF remains higher than that of the solvothermally obtained samples (Fig. S12), which exhibit BET surface area values ranging from 1244 to 1265 m2 g−1 under the same activation conditions.
O stretch, validating the formation of the desired MOF. The TGA analysis (Fig. S11) reveals that the obtained imine-based rht-topology MOF exhibits thermal stability comparable to other known rht-MOFs,60,61 which is stable up to 280 °C or above. Thus, the imine-based rht-MOF was activated by heating at 60 °C under high vacuum for 18 h prior to gas adsorption measurements. The permanent porosity of the imine-based rht-MOF was characterized by N2 adsorption isotherms at 77 K (Fig. 3b), which provided a BET surface area of 2238 m2 g−1 (P/P0 = 0.007–0.03). This value is somewhat lower than that of its amide analogue (3160 m2 g−1), likely due to the entrapment of molecular fragments from incomplete reactions within the cavities, which partially occupy the pore volume. Nevertheless, the BET surface area of the mechanochemically synthesized imine-based rht-MOF remains higher than that of the solvothermally obtained samples (Fig. S12), which exhibit BET surface area values ranging from 1244 to 1265 m2 g−1 under the same activation conditions.
Furthermore, to evaluate how the second type of reversible bond influences reaction time, we systematically examined the minimum milling duration required to synthesize the imine-based rht-MOF (Table S5). Whereas many mechanochemical studies employ ∼90 minutes milling times, well-defined PXRD patterns were observed in as little as 56 seconds (Fig. 3a), with all precursor-related PXRD peaks disappearing (Fig. S13). Remarkably, even after only 28 seconds, the MOF phase already dominates, with no apparent signatures of precursors. This rapid reaction yields materials of comparable quality to those produced at 90 min, as confirmed by N2 adsorption isotherms at 77 K (Fig. 3b). The BET surface area calculated from the 56 seconds product is 2278 m2 g−1 (P/P0 = 0.007–0.03), even slightly higher than that of the 90 min sample. This remarkably short reaction time highlights the critical role of dynamic imine bond in facilitating rapid MOF crystallization under mechanochemical conditions.
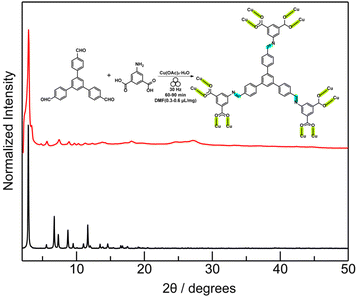
Tandem mechanochemical engineering also enables us to synthesize an expanded analogue of the imine-based rht-MOF by replacing trimesaldehyde with 1,3,5-tris(4-formylphenyl)benzene under similar milling conditions. The obtained dark green-colored crystalline solids were explored (Table S6; Fig. S14) and confirmed by PXRD (Fig. 4), consistent with the pattern calculated from an amide-linked MOF analogue.73 The reaction progress was monitored by IR spectroscopy (Fig. S15), illustrating the appearance of coordinated carbonyl stretch at 1372 cm−1. TGA (Fig. S16) and N2 adsorption measurements (Fig. S17) provide additional characterization data on the mechanochemically obtained expanded rht-topology MOF. N2 adsorption isotherms at 77 K, which provided a calculated BET surface area of 976 m2 g−1 (P/P0 = 0.007–0.03). The decreased surface area value of the mechanochemically obtained large-pore MOF compared to that of the calculated one remains common,21,29,55 tentatively due to molecular fragments trapped in the expanded cavities. Control experiments to synthesize this expanded imine-linked rht-MOF under mechanochemical and solvothermal using the preformed imine-based expanded hexacarboxylic ligand were also attempted (Fig. S18–S21 and Table S7), which are consistent with observations in the last study.
In addition to the rht-topology MOFs, we also applied tandem mechanochemical engineering to an imine-based pto-topology MOF (Fig. 5a), isostructural to MOF-14.58 Its direct mechanochemical synthesis was achieved by milling trimesaldehyde, 4-aminobenzoic acid, and Cu(OAc)2·H2O in a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 with the addition of DMF (η = 0.90 µL mg−1, Fig. S22) for 60 min (Fig. S23 and Table S8). The harvested blue solids were characterized by a suite of solid-state characterizations, including PXRD, IR, TGA, and N2 adsorption analysis. The PXRD pattern (Fig. 5a) matches well with that of an amide-based pto-MOF indicating an isostructural lattice. The IR spectrum (Fig. S24) confirms the coordinated C
3 with the addition of DMF (η = 0.90 µL mg−1, Fig. S22) for 60 min (Fig. S23 and Table S8). The harvested blue solids were characterized by a suite of solid-state characterizations, including PXRD, IR, TGA, and N2 adsorption analysis. The PXRD pattern (Fig. 5a) matches well with that of an amide-based pto-MOF indicating an isostructural lattice. The IR spectrum (Fig. S24) confirms the coordinated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch observed at 1400 cm−1 in the imine-linked pto-MOF illustrates the formation of dative bonds. In addition, the disappearance of N–H stretches between 3473 cm−1 and 3364 cm−1 from the primary amine precursor highlights its conversion to imine bonds. The TGA plot (Fig. S25) shows its thermal stability until 280 °C, comparable to the previous imine-based rht-MOF. Notably, while this imine-based pto-MOF can be synthesized mechanochemically using the preformed imine-based tricarboxylic ligand (Fig. S26–28), direct solvothermal synthesis fails (see details in SI and Table S9), largely due to the ready collapse of the imine ligand.
O stretch observed at 1400 cm−1 in the imine-linked pto-MOF illustrates the formation of dative bonds. In addition, the disappearance of N–H stretches between 3473 cm−1 and 3364 cm−1 from the primary amine precursor highlights its conversion to imine bonds. The TGA plot (Fig. S25) shows its thermal stability until 280 °C, comparable to the previous imine-based rht-MOF. Notably, while this imine-based pto-MOF can be synthesized mechanochemically using the preformed imine-based tricarboxylic ligand (Fig. S26–28), direct solvothermal synthesis fails (see details in SI and Table S9), largely due to the ready collapse of the imine ligand.
Though MOF-14 allows for its direct mechanochemical synthesis using 1,3,5-tris(4-carboxylphenyl)benzene (H3btb) and Cu(OAc)2·H2O,29,55 we have attempted a number of milling conditions using other extended tricarboxylic ligands featuring irreversible covalent linkages (e.g., amide and alkyne) and not been able to generate any crystalline MOF phases (Tables S10–S11 and Fig. S29–S32). This is another highlight that tandem mechanochemical engineering streamlines the incorporation of bulky or sluggish ligands into MOFs. In addition, the solvothermally obtained MOF-14 analogues with the amide74,75 and alkyne76–79 linkages readily collapse upon solvent removal (even activated by supercritical CO2) and thus were not characterized by N2 adsorption analysis in the literature. In contrast, our mechanochemically built imine-based pto-MOF still demonstrates its permanent high porosity based on N2 adsorption analysis at 77 K (Fig. 5b). The BET surface area was calculated to be 3136 m2 g−1 (P/P0 = 0.007–0.03), representing the highest value reported to date for mechanochemically prepared porous materials.
The developed tandem mechanochemical engineering approach integrates two types of reversible bond formation processes into one single mechanochemical step, provides an alternative to bypass high lattice energy associated with multitopic ligands required to anneal defects, and incorporates hydrolytically unstable imine motifs into extended MOFs. The introduction of reversible dynamic covalent bonds coupled with the reversible M–L bond overcomes the sluggish reaction kinetics typically associated with solid-state reactions and expands the applicability of mechanochemical MOF synthesis. The byproduct of the reaction between carboxylic ligands and copper acetate is acetic acid, which is a known catalyst for imine condensation. Thus, the dative bond formation releases acetic acid, which accelerates the kinetics of imine bond formation and breakage. This process creates an autocatalytic cycle that promotes defect annealing and ultimately yields high-quality crystalline MOF phases. Fig. 6 presents a schematic energy diagram comparing the developed tandem mechanochemical engineering with traditional solvothermal methods. Incorporating high-connectivity ligands into crystalline MOF lattices typically requires high activation energy during crystal nucleation and growth, which is usually achievable only under solvothermal conditions. In contrast, introducing a second type of reversible bond in the mechanochemical synthesis segments the otherwise high activation barrier for bulky ligand assembly into a series of imine-mediated steps with lower individual barriers. This stepwise alternative pathway facilitates smoother kinetic transitions, accelerates crystallization, promotes defect annealing, and eventually drives the system toward the thermodynamically favored crystalline phases.
Conclusions
In conclusion, we report a powerful synthetic strategy—tandem mechanochemical engineering—to access MOFs that are challenging under conventional mechanochemical conditions. The incorporation of a second type of reversible dynamic bonds (e.g., imine) coupled with the M–L dative bonds yields highly crystalline MOFs in solid-state reactions, which also decreases reaction time to as little as 56 seconds. The synergy of M–L and imine reversible bonds promotes crystallization kinetics and facilitates efficient mechanochemical synthesis of MOFs with improved crystallinity under ambient conditions, especially those based on high-connectivity ligands. This strategy offers a general and efficient pathway for constructing well-ordered frameworks via solvent-free routes, while streamlining in situ ligand synthesis and MOF formation in a one-pot process.Author contributions
WG and ZT conceived and designed the project. ZT carried out the materials synthesis and characterization as well as performed data analysis. Both authors discussed the results and wrote the manuscript.Conflicts of interest
The authors declare the following competing financial interest(s): a provisional patent application has been filed based on the results reported in this manuscript. This patent application is held by Ohio University. The authors have no other relevant financial interests to disclose.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: details of synthesis and characterization using powder X-ray diffraction, infrared spectroscopy, N2 adsorption isotherms, thermogravimetric analysis, and others. See DOI: https://doi.org/10.1039/d5sc07662e.Acknowledgements
This material is based upon work supported by the National Science Foundation under Grant No. [2345469]. Exploratory studies of the mechanochemistry were supported by the start-up funds of Ohio University.References
- J. Han, X. He, J. Liu, R. Ming, M. Lin, H. Li, X. Zhou and H. Deng, Determining factors in the growth of MOF single crystals unveiled by in situ interface imaging, Chem, 2022, 8, 1637–1657 CAS.
- A. A. Ezazi, W.-Y. Gao and D. C. Powers, Leveraging Exchange Kinetics for the Synthesis of Atomically Precise Porous Catalysts, ChemCatChem, 2021, 13, 2117–2131 Search PubMed.
- W. Lu, Z. Wei, Z.-Y. Gu, T.-F. Liu, J. Park, J. Park, J. Tian, M. Zhang, Q. Zhang, T. Gentle Iii, M. Bosch and H.-C. Zhou, Tuning the structure and function of metal–organic frameworks via linker design, Chem. Soc. Rev., 2014, 43, 5561–5593 Search PubMed.
- H. Ghasempour, K.-Y. Wang, J. A. Powell, F. ZareKarizi, X.-L. Lv, A. Morsali and H.-C. Zhou, Metal–organic frameworks based on multicarboxylate linkers, Coord. Chem. Rev., 2021, 426, 213542 Search PubMed.
- N. Stock and S. Biswas, Synthesis of metal-organic frameworks (MOFs): routes to various MOF topologies, morphologies, and composites, Chem. Rev., 2012, 112, 933–969 Search PubMed.
- M. Safaei, M. M. Foroughi, N. Ebrahimpoor, S. Jahani, A. Omidi and M. Khatami, A review on metal-organic frameworks: Synthesis and applications, Trends Anal. Chem., 2019, 118, 401–425 CrossRef CAS.
- V. F. Yusuf, N. I. Malek and S. K. Kailasa, Review on Metal-Organic Framework Classification, Synthetic Approaches, and Influencing Factors: Applications in Energy, Drug Delivery, and Wastewater Treatment, ACS Omega, 2022, 7, 44507–44531 CrossRef CAS.
- J. M. Marrett, F. Effaty, X. Ottenwaelder and T. Friščić, Mechanochemistry for Metal-Organic Frameworks and Covalent-Organic Frameworks (MOFs, COFs): Methods, Materials, and Mechanisms, Adv. Mater., 2025, 2418707 Search PubMed.
- T. Stolar, L. Batzdorf, S. Lukin, D. Žilić, C. Motillo, T. Friščić, F. Emmerling, I. Halasz and K. Užarević, In Situ Monitoring of the Mechanosynthesis of the Archetypal Metal-Organic Framework HKUST-1: Effect of Liquid Additives on the Milling Reactivity, Inorg. Chem., 2017, 56, 6599–6608 Search PubMed.
- T. Plant-Collins, Z. Tegudeer, D. V. Athapaththu, H. Ringle, J. Chen and W.-Y. Gao, Beyond Solution Chemistry: Mechanochemistry Enables Clustered Defects in Metal–Organic Frameworks, Inorg. Chem., 2025, 64, 17436–17447 CrossRef CAS.
- T. Stolar and K. Užarević, Mechanochemistry: an efficient and versatile toolbox for synthesis, transformation, and functionalization of porous metal-organic frameworks, CrystEngComm, 2020, 22, 4511–4525 RSC.
- D. Chen, J. Zhao, P. Zhang and S. Dai, Mechanochemical synthesis of metal–organic frameworks, Polyhedron, 2019, 162, 59–64 CrossRef CAS.
- T. Friščić, C. Mottillo and H. M. Titi, Mechanochemistry for Synthesis, Angew. Chem., Int. Ed., 2020, 59(3), 1018–1029 CrossRef.
- F. Afshariazar and A. Morsali, The unique opportunities of mechanosynthesis in green and scalable fabrication of metal–organic frameworks, J. Mater. Chem. A, 2022, 10, 15332–15369 RSC.
- C.-A. Tao and J.-F. Wang, Synthesis of Metal Organic Frameworks by Ball-Milling, Crystals, 2020, 11, 15 CrossRef.
- H. M. Titi, J.-L. Do, A. J. Howarth, K. Nagapudi and T. Friščić, Simple, scalable mechanosynthesis of metal-organic frameworks using liquid-assisted resonant acoustic mixing (LA-RAM), Chem. Sci., 2020, 11, 7578–7584 RSC.
- O. Barreda, G. R. Lorzing and E. D. Bloch, Mechanochemical synthesis of two-dimensional metal-organic frameworks, Powder Diffr., 2019, 34, 119–123 CrossRef CAS.
- S. Głowniak, B. Szczęśniak, J. Choma and M. Jaroniec, Mechanochemistry: Toward green synthesis of metal–organic frameworks, Mater. Today, 2021, 46, 109–124 CrossRef.
- O. Barreda, G. A. Taggart, C. A. Rowland, G. P. A. Yap and E. D. Bloch, Mechanochemical Synthesis of Porous Molecular Assemblies, Chem. Mater., 2018, 30, 3975–3978 CrossRef CAS.
- F. E. Salvador, Z. Tegudeer, H. Locke and W.-Y. Gao, Facile mechanochemical synthesis of MIL-53 and its isoreticular analogues with a glance at reaction reversibility, Dalton Trans., 2024, 53, 4406–4411 RSC.
- Z. Tegudeer, J. Moon, J. Wright, M. Das, G. Rubasinghege, W. Xu and W.-Y. Gao, Generic and facile mechanochemical access to versatile lattice-confined Pd(II)-based heterometallic sites, Chem. Sci., 2024, 15, 10126–10134 RSC.
- A. Pichon, A. Lazuen-Garay and S. L. James, Solvent-free synthesis of a microporous metal-organic framework, CrystEngComm, 2006, 8, 211–214 Search PubMed.
- Z. Wang, Z. Li, M. Ng and P. J. Milner, Rapid mechanochemical synthesis of metal–organic frameworks using exogenous organic base, Dalton Trans., 2020, 49, 16238–16244 RSC.
- E. Y. Chen, R. M. Mandel and P. J. Milner, Evaluating solvothermal and mechanochemical routes towards the metal–organic framework Mg2(m-dobdc), CrystEngComm, 2022, 24, 7292–7297 RSC.
- W. Yuan, J. O'Connor and S. L. James, Mechanochemical synthesis of homo- and hetero-rare-earth(iii) metal–organic frameworks by ball milling, CrystEngComm, 2010, 12, 3515–3517 RSC.
- W. Yuan, A. L. Garay, A. Pichon, R. Clowes, C. D. Wood, A. I. Cooper and S. L. James, Study of the mechanochemical formation and resulting properties of an archetypal MOF: Cu3(BTC)2 (BTC = 1,3,5-benzenetricarboxylate), CrystEngComm, 2010, 12, 4063–4065 Search PubMed.
- P. J. Beldon, L. Fábián, R. S. Stein, A. Thirumurugan, A. K. Cheetham and T. Friščić, Rapid room-temperature synthesis of zeolitic imidazolate frameworks by using mechanochemistry, Angew. Chem., Int. Ed., 2010, 49, 9640–9643 CrossRef CAS.
- T. Friščić and L. Fábián, Mechanochemical conversion of a metal oxide into coordination polymers and porous frameworks using liquid-assisted grinding (LAG), CrystEngComm, 2009, 11, 743–745 RSC.
- M. Klimakow, P. Klobes, A. F. Thünemann, K. Rademann and F. Emmerling, Mechanochemical Synthesis of Metal−Organic Frameworks: A Fast and Facile Approach toward Quantitative Yields and High Specific Surface Areas, Chem. Mater., 2010, 22, 5216–5221 Search PubMed.
- B. Karadeniz, D. Žilić, I. Huskić, L. S. Germann, A. M. Fidelli, S. Muratović, I. Lončarić, M. Etter, R. E. Dinnebier, D. Barišić, N. Cindro, T. Islamoglu, O. K. Farha, T. Friščić and K. Užarević, Controlling the Polymorphism and Topology Transformation in Porphyrinic Zirconium Metal-Organic Frameworks via Mechanochemistry, J. Am. Chem. Soc., 2019, 141, 19214–19220 CrossRef CAS.
- B. Karadeniz, A. J. Howarth, T. Stolar, T. Islamoglu, I. Dejanović, M. Tireli, M. C. Wasson, S.-Y. Moon, O. K. Farha, T. Friščić and K. Užarević, Benign by Design: Green and Scalable Synthesis of Zirconium UiO-Metal–Organic Frameworks by Water-Assisted Mechanochemistry, ACS Sustain. Chem. Eng., 2018, 6, 15841–15849 CrossRef CAS.
- S. Darwish, S.-Q. Wang, D. M. Croker, G. M. Walker and M. J. Zaworotko, Comparison of Mechanochemistry vs. Solution Methods for Synthesis of 4,4′-Bipyridine-Based Coordination Polymers, ACS Sustain. Chem. Eng., 2019, 7, 19505–19512 CrossRef CAS.
- K. Užarević, T. C. Wang, S.-Y. Moon, A. M. Fidelli, J. T. Hupp, O. K. Farha and T. Friščić, Mechanochemical and solvent-free assembly of zirconium-based metal-organic frameworks, Chem. Commun., 2016, 52, 2133–2136 RSC.
- D. Prochowicz, K. Sokołowski, I. Justyniak, A. Kornowicz, D. Fairen-Jimenez, T. Friščić and J. Lewiński, A mechanochemical strategy for IRMOF assembly based on pre-designed oxo-zinc precursors, Chem. Commun., 2015, 51, 4032–4035 RSC.
- Y. Chen, J. Xiao, D. Lv, T. Huang, F. Xu, X. Sun, H. Xi, Q. Xia and Z. Li, Highly efficient mechanochemical synthesis of an indium based metal-organic framework with excellent water stability, Chem. Eng. Sci., 2017, 158, 539–544 Search PubMed.
- A. M. Fidelli, B. Karadeniz, A. J. Howarth, I. Huskić, L. S. Germann, I. Halasz, M. Etter, S.-Y. Moon, R. E. Dinnebier, V. Stilinović, O. K. Farha, T. Friščić and K. Užarević, Green and rapid mechanosynthesis of high-porosity NU- and UiO-type metal-organic frameworks, Chem. Commun., 2018, 54, 6999–7002 Search PubMed.
- D. Prochowicz, J. Nawrocki, M. Terlecki, W. Marynowski and J. Lewiński, Facile Mechanosynthesis of the Archetypal Zn-Based Metal-Organic Frameworks, Inorg. Chem., 2018, 57, 13437–13442 Search PubMed.
- I. Brekalo, W. Yuan, C. Mottillo, Y. Lu, Y. Zhang, J. Casaban, K. T. Holman, S. L. James, F. Duarte, P. A. Williams, K. D. M. Harris and T. Friščić, Manometric real-time studies of the mechanochemical synthesis of zeolitic imidazolate frameworks, Chem. Sci., 2020, 11, 2141–2147 Search PubMed.
- I. Brekalo, K. Lisac, J. R. Ramirez, P. Pongrac, A. Puškarić, S. Valić, Y. Xu, M. Ferguson, J. M. Marrett, M. Arhangelskis, T. Friščić and K. T. Holman, Mechanochemical Solid Form Screening of Zeolitic Imidazolate Frameworks Using Structure-Directing Liquid Additives, J. Am. Chem. Soc., 2025, 147, 27413–27430 CrossRef CAS.
- S.-Q. Wang, S. Darwish and M. J. Zaworotko, The impact of solution vs. slurry vs. mechanochemical syntheses upon the sorption performance of a 2D switching coordination network, Inorg. Chem. Front., 2023, 10, 3821–3827 RSC.
- W.-Y. Gao, A. Sur, C.-H. Wang, G. R. Lorzing, A. M. Antonio, G. A. Taggart, A. A. Ezazi, N. Bhuvanesh, E. D. Bloch and D. C. Powers, Atomically Precise Crystalline Materials Based on Kinetically Inert Metal Ions via Reticular Mechanopolymerization, Angew. Chem., Int. Ed., 2020, 59, 10878–10883 CrossRef CAS.
- Z. Tegudeer, L. C. Davenport, M. E. Kordesch and W.-Y. Gao, Harnessing Mechanochemistry for Direct Synthesis of Imine-Based Metal–Organic Frameworks, J. Am. Chem. Soc., 2025, 147, 13522–13530 CrossRef CAS PubMed.
- F. Nouar, J. F. Eubank, T. Bousquet, L. Wojtas, M. J. Zaworotko and M. Eddaoudi, Supermolecular Building Blocks (SBBs) for the Design and Synthesis of Highly Porous Metal-Organic Frameworks, J. Am. Chem. Soc., 2008, 130, 1833–1835 CrossRef CAS PubMed.
- R. Luebke, J. F. Eubank, A. J. Cairns, Y. Belmabkhout, L. Wojtas and M. Eddaoudi, The unique rht-MOF platform, ideal for pinpointing the functionalization and CO2 adsorption relationship, Chem. Commun., 2012, 48, 1455–1457 RSC.
- D. Yuan, D. Zhao, D. Sun and H.-C. Zhou, An Isoreticular Series of Metal–Organic Frameworks with Dendritic Hexacarboxylate Ligands and Exceptionally High Gas-Uptake Capacity, Angew. Chem., Int. Ed., 2010, 49, 5357–5361 Search PubMed.
- D. Zhao, D. Yuan, D. Sun and H.-C. Zhou, Stabilization of Metal−Organic Frameworks with High Surface Areas by the Incorporation of Mesocavities with Microwindows, J. Am. Chem. Soc., 2009, 131, 9186–9188 Search PubMed.
- Y. Zou, M. Park, S. Hong and M. S. Lah, A designed metal–organic framework based on a metal–organic polyhedron, Chem. Commun., 2008, 2340–2342 RSC.
- S. Hong, M. Oh, M. Park, J. W. Yoon, J.-S. Chang and M. S. Lah, Large H2 storage capacity of a new polyhedron-based metal–organic framework with high thermal and hygroscopic stability, Chem. Commun., 2009, 5397–5399 RSC.
- Y. Yan, X. Lin, S. Yang, A. J. Blake, A. Dailly, N. R. Champness, P. Hubberstey and M. Schröder, Exceptionally high H2 storage by a metal–organic polyhedral framework, Chem. Commun., 2009, 1025–1027 RSC.
- W.-Y. Gao, R. Cai, T. Pham, K. A. Forrest, A. Hogan, P. Nugent, K. Williams, L. Wojtas, R. Luebke, Ł. J. Weseliński, M. J. Zaworotko, B. Space, Y.-S. Chen, M. Eddaoudi, X. Shi and S. Ma, Remote Stabilization of Copper Paddlewheel Based Molecular Building Blocks in Metal–Organic Frameworks, Chem. Mater., 2015, 27, 2144–2151 Search PubMed.
- V. Guillerm, D. Kim, J. F. Eubank, R. Luebke, X. Liu, K. Adil, M. S. Lah and M. Eddaoudi, A supermolecular building approach for the design and construction of metal-organic frameworks, Chem. Soc. Rev., 2014, 43, 6141–6172 Search PubMed.
- H. Jiang, D. Alezi and M. Eddaoudi, A reticular chemistry guide for the design of periodic solids, Nat. Rev. Mater., 2021, 6, 466–487 CrossRef CAS.
- V. Guillerm and M. Eddaoudi, The Importance of Highly Connected Building Units in Reticular Chemistry: Thoughtful Design of Metal-Organic Frameworks, Acc. Chem. Res., 2021, 54, 3298–3312 Search PubMed.
- M. O'Keeffe and O. M. Yaghi, Deconstructing the crystal structures of metal-organic frameworks and related materials into their underlying nets, Chem. Rev., 2012, 112, 675–702 CrossRef.
- M. Klimakow, P. Klobes, K. Rademann and F. Emmerling, Characterization of mechanochemically synthesized MOFs, Microporous Mesoporous Mater., 2012, 154, 113–118 CrossRef CAS.
- Y. Chen, H. Wu, Z. Liu, X. Sun, Q. Xia and Z. Li, Liquid-Assisted Mechanochemical Synthesis of Copper Based MOF-505 for the Separation of CO2 over CH4 or N2, Ind. Eng. Chem. Res., 2018, 57, 703–709 CrossRef CAS.
- S. S.-Y. Chui, S. M.-F. Lo, J. P. H. Charmant, A. G. Orpen and I. D. Williams, A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3]n, Science, 1999, 283, 1148–1150 CrossRef CAS PubMed.
- B. Chen, M. Eddaoudi, S. T. Hyde, M. O'Keeffe and O. M. Yaghi, Interwoven metal-organic framework on a periodic minimal surface with extra-large pores, Science, 2001, 291, 1021–1023 CrossRef CAS PubMed.
- B. Chen, N. W. Ockwig, A. R. Millward, D. S. Contreras and O. M. Yaghi, High H2 adsorption in a microporous metal-organic framework with open metal sites, Angew. Chem., Int. Ed., 2005, 44, 4745–4749 CrossRef CAS.
- J. F. Eubank, F. Nouar, R. Luebke, A. J. Cairns, L. Wojtas, M. Alkordi, T. Bousquet, M. R. Hight, J. Eckert, J. P. Embs, P. A. Georgiev and M. Eddaoudi, On Demand: The Singular rht Net, an Ideal Blueprint for the Construction of a Metal–Organic Framework (MOF) Platform, Angew. Chem., Int. Ed., 2012, 51, 10099–10103 CrossRef CAS PubMed.
- B. Zheng, J. Bai, J. Duan, L. Wojtas and M. J. Zaworotko, Enhanced CO2 binding affinity of a high-uptake rht-type metal-organic framework decorated with acylamide groups, J. Am. Chem. Soc., 2011, 133, 748–751 CrossRef CAS.
- T. Seo, N. Toyoshima, K. Kubota and H. Ito, Tackling Solubility Issues in Organic Synthesis: Solid-State Cross-Coupling of Insoluble Aryl Halides, J. Am. Chem. Soc., 2021, 143, 6165–6175 CrossRef CAS.
- N. Brown, Z. Alsudairy, R. Behera, F. Akram, K. Chen, K. Smith-Petty, B. Motley, S. Williams, W. Huang, C. Ingram and X. Li, Green mechanochemical synthesis of imine-linked covalent organic frameworks for high iodine capture, Green Chem., 2023, 25, 6287–6296 RSC.
- H. Pan, N. Wang and G.-W. Wang, Mechanochemically synthesized covalent organic frameworks as catalysts for the Suzuki–Miyaura coupling reaction, Chem. Commun., 2025, 61, 8184–8187 RSC.
- H. Chen, D. Feng, F. Wei, F. Guo and A. K. Cheetham, Hydrogen-Bond-Regulated Mechanochemical Synthesis of Covalent Organic Frameworks: Cocrystal Precursor Strategy for Confined Assembly, Angew. Chem., Int. Ed., 2025, 64, e202415454 CrossRef CAS PubMed.
- S. T. Emmerling, L. S. Germann, P. A. Julien, I. Moudrakovski, M. Etter, T. Friščić, R. E. Dinnebier and B. V. Lotsch, In situ monitoring of mechanochemical covalent organic framework formation reveals templating effect of liquid additive, Chem, 2021, 7, 1639–1652 Search PubMed.
- G. Das, D. Balaji Shinde, S. Kandambeth, B. P. Biswal and R. Banerjee, Mechanosynthesis of imine, β-ketoenamine, and hydrogen-bonded imine-linked covalent organic frameworks using liquid-assisted grinding, Chem. Commun., 2014, 50, 12615–12618 Search PubMed.
- E. H. Cordes and W. P. Jencks, On the Mechanism of Schiff Base Formation and Hydrolysis, J. Am. Chem. Soc., 1962, 84, 832–837 CrossRef CAS.
- R. W. Layer, The Chemistry of Imines, Chem. Rev., 1963, 63, 489–510 CrossRef CAS.
- K. Wu, W. Zhao, L. Huang, W. T. Zeng, Q. Zhu, H. B. Wang, Q. H. Wang, X. Shi, H. Li, W. Lu, G. H. Ning, D. Zhao and D. Li, Aqueous-Phase Synthesis of Cyclic Trinuclear Cluster-Based Metal-Organic Frameworks, J. Am. Chem. Soc., 2025, 147, 13711–13720 Search PubMed.
- M. Ferguson, N. Giri, X. Huang, D. Apperley and S. L. James, One-pot two-step mechanochemical synthesis: ligand and complex preparation without isolating intermediates, Green Chem., 2014, 16, 1374–1382 RSC.
- X. Li, J. Wang, F. Xue, Y. Wu, H. Xu, T. Yi and Q. Li, An Imine-Linked Metal–Organic Framework as a Reactive Oxygen Species Generator, Angew. Chem., Int. Ed., 2021, 60, 2534–2540 CrossRef CAS PubMed.
- B. Zheng, Z. Yang, J. Bai, Y. Li and S. Li, High and selective CO2 capture by two mesoporous acylamide-functionalized rht-type metal–organic frameworks, Chem. Commun., 2012, 48, 7025–7027 RSC.
- L. Rajput, D. Kim and M. S. Lah, Conformational control of ligands to create a finite metal–organic cluster and an extended metal–organic framework, CrystEngComm, 2013, 15, 259–264 Search PubMed.
- W. Zeng, G. Wang, B. Zheng, Z. Wang and J. Bai, A porous amide-functionalized pto-type MOF exhibiting selective capture and separation of cationic MB dye, J. Coord. Chem., 2021, 74, 241–251 CrossRef CAS.
- P. Müller, R. Grünker, V. Bon, M. Pfeffermann, I. Senkovska, M. S. Weiss, X. Feng and S. Kaskel, Topological control of 3,4-connected frameworks based on the Cu2-paddle-wheel node: tbo or pto, and why?, CrystEngComm, 2016, 18, 8164–8171 RSC.
- N. Zhu, M. J. Lennox, G. Tobin, L. Goodman, T. Duren and W. Schmitt, Hetero-Epitaxial Approach by Using Labile Coordination Sites to Prepare Catenated Metal-Organic Frameworks with High Surface Areas, Chem.–Eur. J., 2014, 20, 3595–3599 CrossRef CAS.
- N. Zhu, M. J. Lennox, T. Düren and W. Schmitt, Polymorphism of metal-organic frameworks: direct comparison of structures and theoretical N2-uptake of topological pto- and tbo-isomers, Chem. Commun., 2014, 50, 4207–4210 Search PubMed.
- N. Zhu, D. Sensharma, P. Wix, M. J. Lennox, T. Düren, W. Y. Wong and W. Schmitt, Framework Isomerism: Highly Augmented Copper(II)-Paddlewheel-Based MOF with Unusual (3,4)-Net Topology, Eur. J. Inorg. Chem., 2015, 2016, 1939–1943 CrossRef.
| This journal is © The Royal Society of Chemistry 2026 |