Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The bismuth phosphanides Bi(PR2)3: sources of phosphanyl radicals, P-inversion, and reversible olefin insertion
Sascha Reith a,
Kai Oberdorfa,
Sebstián Martíneza,
Jann B. Landgraf
a,
Kai Oberdorfa,
Sebstián Martíneza,
Jann B. Landgraf b,
Alena Ahrensa,
Felix Jakobia,
Xiulan Xiea and
Crispin Lichtenberg
b,
Alena Ahrensa,
Felix Jakobia,
Xiulan Xiea and
Crispin Lichtenberg *a
*a
aDepartment of Chemistry, Philipps-University Marburg, Hans-Meerwein-Str. 4, D-35032 Marburg (D), Germany. E-mail: crispin.lichtenberg@chemie.uni-marburg.de
bDepartment of Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, D-97074 Würzburg (D), Germany
First published on 8th January 2026
Abstract
Understanding and controlling radical reactions for selective transformations under mild conditions remains one of the key challenges in synthetic chemistry. The detailed investigation of previously inaccessible structural motifs can grant important insights, provide new stimuli, and offer innovative strategies for further developments in the field. In this respect, the controlled release of simple phosphanyl radicals [PR2]˙ from metal precursors is significantly underdeveloped to date. Here we report the synthesis, isolation, and full characterization of a series of homoleptic parent bismuth phosphanides [Bi(PRR’)3] (R, R’ = alkyl). The ability of these compounds to release phosphanyl radicals under mild conditions is demonstrated, revealing an unprecedented radical pathway for the inversion of phosphorus atoms, enabling ethylene activation, and facilitating reversible olefin insertion into Bi–P bonds.
Introduction
The exploration of simple, homoleptic complexes forms the basis for the profound understanding of principal classes of compounds. Such fundamental studies grant important insights into the intrinsic properties and reactivity patterns of the structural motif under investigation and can often be extrapolated to its behavior in more complex molecular frameworks. Among the various functional groups known in coordination chemistry, phosphanides, i.e. compounds featuring an M–PR2 bond (M = metal atom, R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H, alkyl, aryl) open up unusual pathways in CH activation and small molecule activation and play key roles in stoichiometric and catalyzed P–P and P–C bond formation as well as olefin hydrogenation reactions.1–12 In addition, unexpected selectivities in insertion reactions and unusual spectroscopic features such as intermolecular through-space spin–spin coupling have been reported.13,14 Key questions in the understanding of the M–PR2 structural motif center around the potential M–P multiple bond character,15–18 the inversion of the phosphorus atom,18–21 and potential radical character of the PR2 ligand in the coordination sphere of a transition metal.22 The design of reactive metal phosphanide complexes [M]–PR2 is an intriguing, but yet underdeveloped strategy for the release of reactive phosphanyl radicals under mild conditions to be utilized in selective intermolecular reactions.23–27 In this context, bismuth compounds appear as very promising candidates due to their typically low homolytic Bi–X bond dissociation energies (e.g.: X = C, N, O, Bi).28–40
H, alkyl, aryl) open up unusual pathways in CH activation and small molecule activation and play key roles in stoichiometric and catalyzed P–P and P–C bond formation as well as olefin hydrogenation reactions.1–12 In addition, unexpected selectivities in insertion reactions and unusual spectroscopic features such as intermolecular through-space spin–spin coupling have been reported.13,14 Key questions in the understanding of the M–PR2 structural motif center around the potential M–P multiple bond character,15–18 the inversion of the phosphorus atom,18–21 and potential radical character of the PR2 ligand in the coordination sphere of a transition metal.22 The design of reactive metal phosphanide complexes [M]–PR2 is an intriguing, but yet underdeveloped strategy for the release of reactive phosphanyl radicals under mild conditions to be utilized in selective intermolecular reactions.23–27 In this context, bismuth compounds appear as very promising candidates due to their typically low homolytic Bi–X bond dissociation energies (e.g.: X = C, N, O, Bi).28–40
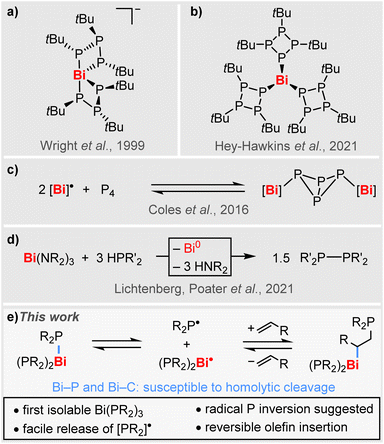
However, compounds featuring Bi–P bonds are rare and investigations have mostly been focused on the synthesis and structure elucidation of heteroleptic species.41–51 Well-defined homoleptic bismuth phosphanides have so far been limited to a very small number of special cases. For instance, the serendipitously generated complex anion [Bi(P3tBu3)2]− could be structurally characterized (Scheme 1a), but its high sensitivity precluded a satisfactory spectroscopic characterization.52 More recently, the neutral species [Bi(P4tBu3)3] with its unusual phosphacyclic ligand motif has been reported (Scheme 1b), revealing a pronounced photosensitivity,53 while selective reactivity patterns remain to be explored. In a broader context, the reversible addition of isolable Bi(II) radical species to P4 has been reported on a single instance and selective P–P bond formation from suggested [Bi]–PR2 intermediates has recently been observed (Scheme 1c and d).30,41 However, there are no examples of simple homoleptic bismuth phosphanides Bi(PR2)3, which could act as isolable sources of phosphanyl radicals [PR2]˙ (R = alkyl).
Here we report the synthesis, isolation, and full characterization of the first simple homoleptic bismuth phospanides, [Bi(PR2)3], uncovering the facile release of phosphanyl radicals [PR2]˙, suggesting an unprecedented mechanism for phosphorus inversion, and demonstrating the reversible insertion of unactivated α-olefins into Bi–P bonds involving BiC/BiP homolysis (Scheme 1e).
Results and discussion
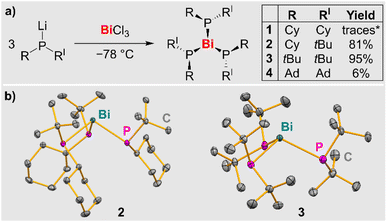
Straightforward access to the first simple bismuth phosphanides Bi(PR2)3 was granted via salt elimination strategies (R = alkyl; Scheme 2a). Balancing dispersion interactions and steric congestion proved to be a decisive factor in order to suppress thermal decomposition pathways in this class of compounds and to sufficiently stabilize the target species. Hints at the accessibility of compounds Bi(PR2)3 could be obtained through single-crystal XRD data on Bi(PCy2)3 (1), which eluded a detailed characterization in solution due to its thermal instability (vide infra and SI). By increasing the steric bulk of the substituents at the phosphorus atom, compounds Bi(PtBuCy)3 (2), Bi(PtBu2)3 (3), and Bi(PAd2)3 (4) could be isolated, representing the first examples of simple homoleptic bismuth phosphanides that could be characterized in detail (Cy = cyclohexyl, Ad = adamantyl). Multiple recrystallization steps were required to obtain compound 4 in pure form leading to low isolated yields, but facile, high-yielding multi-gram syntheses could be realized for compounds 2 and 3, respectively. Compounds 2–4 were obtained as intensely colored orange (2) to deep-red (3, 4) solids. Aryl groups at the phosphorus atom increased the lability of the targeted homoleptic bismuth phosphanides (SI).Crystallographic characterization of compounds 2 and 3
Single-crystal XRD analysis of 2 and 3 (triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) confirmed the expected trigonal pyramidal coordination geometry around the central atom (Scheme 2b). The molecular structures show an apparent C3 axis which runs through the Bi atom and is orthogonal to the plane defined by the three phosphorus atoms. For each phosphorus atom, one of the organic substituents points towards the bismuth atom, while the other one points away from it. In the case of compound 2, it is important to note that each phosphorus atom represents a stereocenter, i.e. in the solid state, a racemic mixture of the (R,R,R)- and the (S,S,S)-enantiomer is realized. The bond angles in tri-coordinate bismuth(III) compounds commonly approach 90°, because the 6 s(Bi) orbital tends not to contribute significantly to bond formation in these compounds due to hybridization defects.54,55 In compound 2, this situation is reflected by an angle sum of 295.6° around bismuth. In contrast, the higher steric pressure of the tBu groups in 3 (vs. Cy groups in 2) induces a remarkably large angle sum of 316.9° around the central atom. This is also reflected by the Bi–P bond lengths in 2 and 3, which range from 2.6484(7) to 2.7011(9) Å and are on average 0.04 Å longer for the bulkier species 3. In the case of compound 1, data from single-crystal X-ray diffraction experiments could be obtained, but suffered from poor quality due to higher twinning of the crystal. Thus, a discussion of the bonding parameters is not possible, but the connectivity is definite (for details, see SI).
) confirmed the expected trigonal pyramidal coordination geometry around the central atom (Scheme 2b). The molecular structures show an apparent C3 axis which runs through the Bi atom and is orthogonal to the plane defined by the three phosphorus atoms. For each phosphorus atom, one of the organic substituents points towards the bismuth atom, while the other one points away from it. In the case of compound 2, it is important to note that each phosphorus atom represents a stereocenter, i.e. in the solid state, a racemic mixture of the (R,R,R)- and the (S,S,S)-enantiomer is realized. The bond angles in tri-coordinate bismuth(III) compounds commonly approach 90°, because the 6 s(Bi) orbital tends not to contribute significantly to bond formation in these compounds due to hybridization defects.54,55 In compound 2, this situation is reflected by an angle sum of 295.6° around bismuth. In contrast, the higher steric pressure of the tBu groups in 3 (vs. Cy groups in 2) induces a remarkably large angle sum of 316.9° around the central atom. This is also reflected by the Bi–P bond lengths in 2 and 3, which range from 2.6484(7) to 2.7011(9) Å and are on average 0.04 Å longer for the bulkier species 3. In the case of compound 1, data from single-crystal X-ray diffraction experiments could be obtained, but suffered from poor quality due to higher twinning of the crystal. Thus, a discussion of the bonding parameters is not possible, but the connectivity is definite (for details, see SI).
NMR spectroscopic analysis
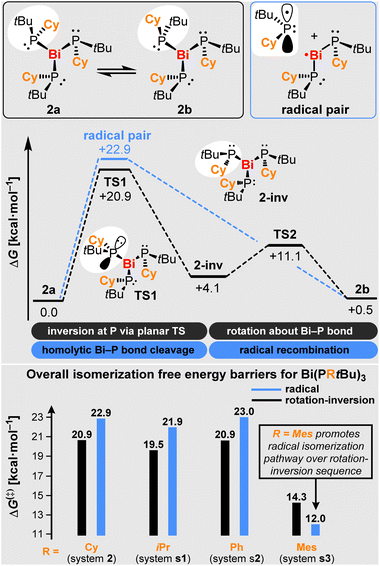
NMR spectroscopic investigations of compounds 2–4 in solution indicate the formation of typical molecular complexes without significant intermolecular interactions. The 31P NMR spectroscopic chemical shifts cover a relatively broad range of 42.4–86.4 ppm. For compounds 3 and 4 with their symmetrically substituted phosphorus atoms, one set of signals is observed in the solution NMR spectra, indicating that the bonding situation that has been found for 3 in the solid state is preserved in solution. In contrast, two sets of signals are observed for compound 2 with its unsymmetrically substituted phosphorus atoms. In the 31P NMR spectrum, one singlet is ascribed to compound 2a, which resembles the bonding situation found in the solid state (Scheme 3, top left). The second set of signals consists of three resonances of identical intensity, with 2JPP coupling constants being barely resolved. This set of signals is ascribed to the diastereomer 2b, which can formally be obtained from 2a by the inversion of one phosphorus center followed by a 180° rotation about the respective Bi–P bond. Note that 2b shows three chemically inequivalent phosphorus atoms, since the C3 symmetry element that is present in 2a is no longer present in 2b due to the inversion of the configuration at one P atom. Variable temperature NMR spectroscopic analysis in the range of −80 to +80 °C reveal that the relative intensities of the resonances ascribed to 2a and 2b are reversibly shifted from 2a:2b = 1.00/0.93 at −80 °C via 2a:2b = 1.00/1.09 at +25 °C to 2a:2b = 1.00/1.21 at +80 °C (concomitant decomposition is observed at elevated temperatures, but does not interfere with these analyses; vide infra). This translates into thermodynamic parameters of ΔG(293 K) = −0.027 kcal mol−1, ΔH = +0.50 kcal mol−1, and ΔS = +1.8 cal mol−1 for the isomerization process 2a⇌2b, as determined from a van't Hoff plot (SI). Dynamic exchange between 2a and 2b was further supported by 31P–31P EXSY NMR spectroscopic experiments (SI). From a mechanistic point of view, two classical scenarios have been encountered for the inversion of phosphorus centers:56,57 i) a planar transition state in the absence of electron-withdrawing substituents (with barriers commonly ranging from ca. 30–40 kcal mol−1 for trialkyl phosphanes58–60) and ii) a T-shaped transition state in the presence of electron-withdrawing groups at the phosphorus atom (with a calculated barrier of 53.8 kcal mol−1 for PF3).61,62Computational studies
In order to gain insights into the isomerization mechanism, we performed computational studies. In a first approach, the thermodynamic stability of relevant isomers of 2 was evaluated. Our study shows that isomers 2a and 2b (Scheme 3) present similar thermodynamic stabilities (ΔGb−a = +0.5 kcal mol−1), which agrees with the experimental detection of two isomers of 2. Isomers 2c and 2d represent complexes with different relative orientations of the tBu and Cy groups (see SI) and are predicted to be less stable, presenting ΔG values that are > 8.0 kcal mol−1 higher than that of 2a, and are therefore unlikely to be detected experimentally. Next, we focused on investigating the possible isomerization pathways for the interconversion of 2a and 2b. A sequence of inversion-rotation via TS1 and TS2, was found to be the most plausible reaction pathway, which accounts for an overall free energy barrier of +20.9 kcal mol−1 (Scheme 3; for correlation with experimental data from an Eyring plot see SI). Notably, a radical isomerization pathway involving homolytic Bi–P bond dissociation and re-association, presents a barrier that is only 2.0 kcal mol−1 higher in energy than the former pathway, suggesting that both mechanisms could be competing, especially at elevated temperature due to the positive entropy term in the barrierless homolytic bond splitting.63 A crossing point for the two pathways was predicted at a temperature of 334 K based on DFT calculations. In order to evaluate the impact of the substituents at the phosphorus atoms on the isomerization process, the analogous systems Bi(PtBuR)3 were studied computationally (R = iPr (s1), Ph (s2), Mes (s3); Scheme 3 (bottom) and SI). Our results show that an inversion-rotation sequence is slightly preferred for s1 and s2. Remarkably, s3 is predicted to favor isomerization via the newly proposed radical pathway, even at ambient temperature.EPR spectroscopic investigations
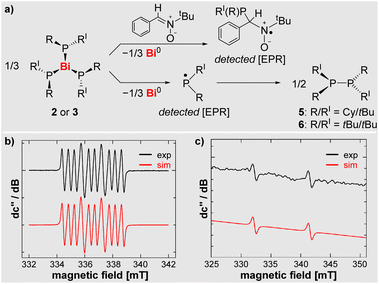
In view of the low calculated Bi–P bond dissociation energy in compounds 2 (22.9 kcal mol−1) and 3 (18.3 kcal mol−1), these compounds were investigated by EPR spectroscopy. Indeed, resonances of the free phosphanyl radicals, (PCytBu)˙ and (PtBu2)˙, could be detected, when monitoring toluene solutions of compounds 2 and 3 at elevated temperatures of 95 °C and 70 °C, respectively, in the cavity of the EPR spectrometer (Scheme 4, for details see SI). To the best of our knowledge, this is the first example of the direct detection of simple dialkylphosphanyl radicals in typical wet-chemical approaches without the use of stabilization strategies such as chelation or excessive bulk.64,65 The spectroscopic signature of reactive [PRtBu]˙ radicals reveals large coupling constants of a(31P) = 9.31 mT. This is in congruency with data reported for an isolable sterically protected dialkylphosphanyl radical [P(C(SiMe3)2CH2)2]• (a(31 P) = 9.07 mT).64When adding the spin trap PBN (phenyl-N-t-butylnitrone) to solutions of 2 and 3, the corresponding radicals could readily be detected at ambient temperature (Scheme 4 and SI).
The facile entrance into radical chemistry provided by compounds 2 and 3 motivated the closer investigation of their solution behavior. In benzene solution at 25 °C, these compounds selectively form the corresponding diphosphanes CytBuP–PtBuCy (5) and (tBu)2P–P(tBu)2 (6) along with Bi0, leading to half-life times of 15.3 d and 9.8 d for compounds 2 and 3, respectively. At elevated temperatures of 110 °C (for 2) and 65 °C (for 3) or upon irradiation of the reaction mixture with an LED (λ = 365 nm), near-quantitative yields are obtained in 15 min (for 5 and 6).
H-atom abstractions reactions
In order to evaluate the reactivity of 2 and 3 towards external substrates, reactions with a series of hydrocarbon-based H-atom donors were performed. While H-atom transfer to the phosphorus atom to give phosphanes HPR2 was feasible in both cases, it was more effective for compound 3, the formation of diphosphanes 5 or 6 being the competing transformation (Scheme 5 and SI). For compound 3, H-atom transfer proved to be the preferred reaction pathway for substrates with a C–H bond dissociation free energy of up to 75.7 kcal mol−1. Thus, balancing the steric bulk of the substituents at the phosphorus atoms appears to be crucial: a sufficient bulk is essential to grant access to these compounds (cf. compound 1 vs. compound 2), but further increasing the steric bulk in small increments quickly enhances the reactivity due to sterically promoted Bi–P bond homolysis (cf. compound 2 vs. 3).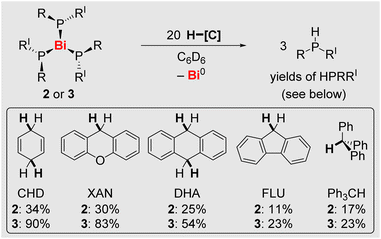 | ||
| Scheme 5 Reactions of 2 and 3 with H-atom donors (that require the scission of a C–H bond) to the corresponding phosphanes. Yields are given after 43 h reaction time (for details see SI). | ||
Reactivity as a phosphanyl radical precursor
The diphospane R2P–PR2 (R = NDippCH2) has been reported to readily insert CS2 into its P–P bond via a radical pathway, involving [PR2]˙.66 In contrast, compounds (alkyl)2P–P(alkyl)2 do not readily release phosphanyl radicals [P(alkyl)2]˙ under moderate conditions, as confirmed by EPR spectroscopic experiments with isolated CytBuP–PtBuCy (5) and (tBu)2P–P(tBu)2 (6) (SI). In view of the ability of 3 to release phosphanyl radicals, its reactivity towards the potential phosphanyl radical scavenger CS2 was probed (Scheme 6). Indeed, the addition of CS2 (2.2 equiv.) to a solution of 3 led to an immediate color change from dark red via dark violet to smaragd green, along with the precipitation of a dark solid (presumably Bi0). After workup, compound 7 was isolated as a dark green solid in 92% yield and fully characterized (SI). This demonstrates the ability of 3 to transfer phosphanyl radicals to external substrates.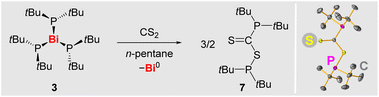 | ||
| Scheme 6 Left: reaction of 3 with CS2 to give 7. Right: molecular structure of 7 in the solid state as determined by single-crystal X-ray diffraction analysis (for details see SI). | ||
Reactivity towards olefins
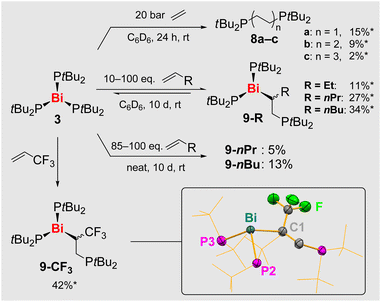
With compound 3 as a promising candidate, we conducted initial reactivity studies towards olefins including ethylene, a benchmark substrate in the field.67–69 Reactions of 3 with ethylene yielded mixtures of compounds tBu2P(C2H4)nPtBu2 (8a–c) suggesting that up to three consecutive olefin insertion reactions into Bi–P/Bi–C bonds as well as reductive elimination processes can take place (Scheme 7).70–72 Reactions with non-activated terminal alkenes H2C = CHR (R = Et, nPr, nBu) were monitored by 31P NMR spectroscopy, showing one new set of resonances each, which was ascribed to the mono-insertion products (tBu2P)2Bi–(C2H3R)PtBu2 (9-R, Scheme 7). These reactions were strongly impeded by the exclusion of ambient light.Irradiation of the reaction mixtures with LEDs (λ = 365–525 nm) increased the rates of reactions, but even in the most promising cases (λ = 525 nm), the selectivity towards the insertion product was only improved at early stages of the reaction (32% spectroscopic yield of 9-nBu after 2 h), when significant amounts of unreacted and inseparable 3 were still present (for details see SI).
In stoichiometric reactions of 3 with 1-pentene and 1-hexene under ambient light, only partial conversion of the starting material 3 was observed, which was accompanied by its slow degradation to give the diphosphane 6 (vide supra). This led to the initial hypothesis of an equilibrium reaction, which could be confirmed: reacting 3 with neat 1-pentene or 1-hexene followed by a tedious work-up to remove the side-product 6 led to the isolation of yellow powders of 9-nPr and 9-nBu, which were pure by 31P NMR spectroscopy, when using the respective olefin as the solvent. When other solvents were applied, mixtures of 3, 6, and 9-R were obtained, i.e. the starting material 3 is partially regenerated when there is no excess olefin present in solution. In good agreement with these findings, compounds 9-R proved to be also vacuum-sensitive, hampering a detailed characterization of these compounds to date. Furthermore, a crossover experiment was performed: dissolving 9-nBu in 1-pentene led to its slow conversion to 9-nPr (and vice versa), confirming the reversible insertion of α-olefins into Bi–P bonds (Scheme 8).
 | ||
| Scheme 8 Crossover experiment between 8-nBu and 1-pentene (H2C = CHnPr), demonstrating the reversibility of olefin insertion with Bi(PtBu2)3 via Bi–P and Bi–C bond cleavage/formation. | ||
When it comes to the utilization of reactive radical intermediates for synthetic purposes, the reversibility of homolytic bond dissociation reactions can be crucial. For literature-known, carbon-centered radicals generated by reversible Bi–C homolysis, this has led to unprecedented catalytic applications in olefin polymerization, C–N coupling, and radical cyclo-isomerization.29,31,32,71,72 The key steps in such synthetic applications have been focused on the reversible homolytic Bi–X bond dissociation of one type of Bi–X bond (typically a Bi–C bond). Here we show that the peerless release of [PR2]˙ radicals under mild reaction conditions (tied to reversible Bi–P bond cleavage/formation) can be combined with reversible Bi–C bond formation, an important step towards greater functional group diversity in controlled radical reactions.
A more robust olefin insertion product 9-CF3 could be obtained from the reaction of 3 with electron-deficient 3,3,3-trifluoropropene (Scheme 7, bottom). In contrast to the other insertion products 9-R, compound 9-CF3 did not release the previously inserted olefin, when dissolved in common organic solvents such as benzene, which can be ascribed to the more polar nature of the Bi–C bond in 9-CF with its electron-withdrawing CF3 group at the bismuth-bound carbon atom. 31P NMR spectroscopy indicated the presence of only one phosphorus compound 9-CF3 besides traces (<2%) of diphosphane 6. However, the 1H NMR spectrum showed additional broadened resonances in the aliphatic region, which was ascribed to lower oligomers of 3,3,3-trifluoropropene. This was in agreement with an oily residue that co-precipitated with crystalline 9-CF3 and could not be separated due to similar solubility properties. The oligomeric nature of this side product was further confirmed by elemental analysis and high-resolution mass spectrometry, the latter also supporting the formation of 9-CF3. Despite the difficulties in isolating 9-CF3 in pure form, its molecular structure in the solid state could unambiguously be identified by single-crystal X-ray diffraction analyses (monoclinic space group P21/n; Z = 4; Scheme 7 bottom). Compound 9-CF3 forms a typical mononuclear species with a trigonal pyramidal coordination geometry around the bismuth atom (angle sum around Bi, 307°). Notably, the introduction of the secondary alkyl group as a bismuth-bound ligand increases the pyramidalization of the central atom (as compared to 3), with two large (P1–Bi–P2/C1, 105.2–106.8°) and one small angle (P2–Bi–C1, 95.7°) around the central atom. The newly formed Bi–C bond (2.38 Å) is exceptionally long compared to Bi–C bonds in more traditional motifs,46,47,73–76 which was ascribed to steric congestion around the central atom and the presence of the electron-withdrawing CF3 group at the α-C-atom.
Conclusions
In summary we present the synthesis, isolation, and characterization of the first series of parent dialkyl bismuth phosphanides Bi(PRR’)3. This new class of compounds shows facile release of simple phosphanyl radicals [P(alkyl)2]˙. This enables the inversion of the bismuth-bound P atoms through a radical dissociation/re-association mechanism, adding a peerless alternative to the two well-established pathways for P-inversion via trigonal planar or T-shaped transition states. Reactivity studies on the title compounds Bi(PRR’)3 show that in the absence of external substrates, selective near-quantitative radical P–P coupling reactions dominate. The radical reactivity can be extended to external substrates: the radical transfer on CS2 and the insertion of ethylene and unactivated α-olefins into Bi–P bonds is facilitated, the latter proceeding in a reversible manner. This combines reversible Bi–P homolysis with reversible Bi–C homolysis for the first time, opening up perspectives for dual mode controlled radical reactions. It is anticipated that these fundamental investigations of a new class of compounds will stimulate research into selective stoichiometric and catalyzed radical reactions of bismuth phosphanide structural motifs [Bi]–PR2 embedded in supporting ligand scaffolds. Research along these lines is currently being pursued in our laboratories.Author contributions
The synthesis and the analysis of the compounds were conducted by SR and KO with the support of JBL, AA and FJ. X-Ray diffraction analyses were conducted by SR and KO. EPR analyses were performed and interpreted by CL. XX carried out EXSYX 31P–31P NMR experiments. DFT-calculations were conducted by SM and CL. The manuscript was drafted by SR and CL and was finalized with contributions from all authors.Conflicts of interest
There are no conflicts to declare.Data availability
Data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental, analytical, and theoretical details. See DOI: https://doi.org/10.1039/d5sc07240a.CCDC 2451991–2451995 (1, 2, 3, 5, 9-CF3) and 2502129 (7) contain the supplementary crystallographic data for this paper.77a–f
Acknowledgements
The authors thank Dr K. Radacki for helpful discussions about crystallographic problems. Funding by the Deutsche Forschungsgemeinschaft (DFG, grant number LI2860/5-1) and the LOEWE program (LOEWE/4b//519/05/01.002(0002)/85) is gratefully acknowledged. This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement No. 946184).References
- A. K. Hickey, S. B. Muñoz, S. A. Lutz, M. Pink, C.-H. Chen and J. M. Smith, Arrested α-hydride migration activates a phosphido ligand for C-H insertion, Chem. Commun., 2016, 53, 412–415 RSC.
- S. P. Vilanova, I. Del Rosal, M. L. Tarlton, L. Maron and J. R. Walensky, Functionalization of Carbon Monoxide and tert-Butyl Nitrile by Intramolecular Proton Transfer in a Bis(Phosphido) Thorium Complex, Angew. Chem., Int. Ed., 2018, 57, 16748–16753 CrossRef CAS PubMed.
- M. L. Tarlton, I. Del Rosal, S. P. Vilanova, S. P. Kelley, L. Maron and J. R. Walensky, Comparative Insertion Reactivity of CO, CO2tBuCN, and tBuNC into Thorium– and Uranium–Phosphorus Bonds, Organometallics, 2020, 39, 2152–2161 CrossRef CAS.
- A. M. Geer, Á. L. Serrano, B. de Bruin, M. A. Ciriano and C. Tejel, Terminal phosphanido rhodium complexes mediating catalytic P-P and P-C bond formation, Angew. Chem., Int. Ed., 2015, 54, 472–475 CrossRef CAS.
- A. K. King, A. Buchard, M. F. Mahon and R. L. Webster, Facile, Catalytic Dehydrocoupling of Phosphines Using β-Diketiminate Iron(II) Complexes, Chem.–Eur. J., 2015, 21, 15960–15963 CrossRef CAS.
- T. M. Horsley Downie, J. W. Hall, T. P. Collier Finn, D. J. Liptrot, J. P. Lowe, M. F. Mahon, C. L. McMullin and M. K. Whittlesey, The first ring-expanded NHC-copper(i) phosphides as catalysts in the highly selective hydrophosphination of isocyanates, Chem. Commun., 2020, 56, 13359–13362 Search PubMed.
- J. Yuan, H. Hu and C. Cui, N-Heterocyclic Carbene-Ytterbium Amide as a Recyclable Homogeneous Precatalyst for Hydrophosphination of Alkenes and Alkynes, Chem.–Eur. J., 2016, 22, 5778–5785 CrossRef CAS PubMed.
- K. Kaniewska, A. Dragulescu-Andrasi, Ł. Ponikiewski, J. Pikies, S. A. Stoian and R. Grubba, Syntheses, Structures and Reactivity of Terminal Phosphido Complexes of Iron(II) Supported by a β-Diketiminato Ligand, Eur. J. Inorg. Chem., 2018, 2018, 4298–4308 Search PubMed.
- A. T. Normand, C. G. Daniliuc, B. Wibbeling, G. Kehr, P. Le Gendre and G. Erker, Phosphido- and Amidozirconocene Cation-Based Frustrated Lewis Pair Chemistry, J. Am. Chem. Soc., 2015, 137, 10796–10808 Search PubMed.
- M. E. Garner, B. F. Parker, S. Hohloch, R. G. Bergman and J. Arnold, Thorium Metallacycle Facilitates Catalytic Alkyne Hydrophosphination, J. Am. Chem. Soc., 2017, 139, 12935–12938 Search PubMed.
- M. T. Whitelaw, S. Banerjee, A. R. Kennedy, A. van Teijlingen, T. Tuttle and R. E. Mulvey, Catalytic hydrophosphination of alkynes using structurally diverse sodium diphenylphosphide donor complexes, Cell Rep. Phys. Sci., 2022, 3, 100942 CrossRef CAS.
- R. J. Schwamm, J. R. Fulton, M. P. Coles and C. M. Fitchett, Hydrophosphination-type reactivity promoted by bismuth phosphanides: scope and limitations, Dalton Trans., 2017, 46, 2068–2071 RSC.
- L. M. Harris, E. C. Y. Tam, S. J. W. Cummins, M. P. Coles and J. R. Fulton, The Reactivity of Germanium Phosphanides with Chalcogens, Inorg. Chem., 2017, 56, 3087–3094 Search PubMed.
- J. Arras, K. Eichele, B. Maryasin, H. Schubert, C. Ochsenfeld and L. Wesemann, Intermolecular 119Sn,31P Through-Space Spin-Spin Coupling in a Solid Bivalent Tin Phosphido Complex, Inorg. Chem., 2016, 55, 4669–4675 CrossRef CAS.
- L. D. Hutchins, R. T. Paine and C. F. Campana, Structure and bonding in a phosphenium ion-metal complex, CH3NCH2CH2N(CH3)PMo(η5-C5H5)(CO)2. An example of a molybdenum-phosphorus multiple bond, J. Am. Chem. Soc., 1980, 102, 4521–4523 Search PubMed.
- P. J. Domaille, B. M. Foxman, T. J. McNeese and S. S. Wreford, Preparation and molecular structure of TaH[P(C6H5)2]2[(CH3)2PC2H4P(CH3)2]2, a metal hydride of the type MHL2(bidentate phosphine)2n+ having a pentagonal-bipyramidal structure, J. Am. Chem. Soc., 1980, 102, 4114–4120 Search PubMed.
- D. S. Bohle, T. C. Jones, C. E. F. Rickard and W. R. Roper, Reactivity patterns associated with pyramidal and planar phosphido-ligand geometries; synthesis and structure of [Os(PHPh)Cl(CO)2(PPh3)2] and of [Os{PH(OMe)Ph}(CO)2(PPh3)2], J. Chem. Soc., Chem. Commun., 1984, 865 RSC.
- K. Izod, P. Evans and P. G. Waddell, A Fully Phosphane-Substituted Disilene, Angew. Chem., Int. Ed., 2017, 56, 5593–5597 CrossRef CAS.
- E. C. Y. Tam, N. A. Maynard, D. C. Apperley, J. D. Smith, M. P. Coles and J. R. Fulton, Group 14 metal terminal phosphides: correlating structure with |J(MP), Inorg. Chem., 2012, 51, 9403–9415 Search PubMed.
- M. S. Winston and J. E. Bercaw, A Novel Bis(phosphido)pyridine [PNP]2− Pincer Ligand and Its Potassium and Bis(dimethylamido)zirconium(IV) Complexes, Organometallics, 2010, 29, 6408–6416 CrossRef CAS.
- M. D. Fryzuk, K. Joshi, R. K. Chadha and S. J. Rettig, Thermal and photochemical transformations of organoiridium phosphide complexes. Mechanistic studies on carbon-phosphorus bond formation to generate cyclometalated hydride complexes by alpha.-hydride abstraction, J. Am. Chem. Soc., 1991, 113, 8724–8736 Search PubMed.
- J. Abbenseth, D. Delony, M. C. Neben, C. Würtele, B. de Bruin and S. Schneider, Interconversion of Phosphinyl Radical and Phosphinidene Complexes by Proton Coupled Electron Transfer, Angew. Chem., Int. Ed., 2019, 58, 6338–6341 Search PubMed.
- J.-P. Bezombes, P. B. Hitchcock, M. F. Lappert and J. E. Nycz, Synthesis and P-P cleavage reactions of P(X)X’2; X-ray structures of CoP(X)X’(CO)3 and P4P(X)X’2 [X = N(SiMe3)2, X’= NPri2], Dalton Trans., 2004, 499–501 RSC.
- B. Wittwer, K. N. McCabe, D. Leitner, M. Seidl, L. Maron and S. Hohloch, Light-induced splitting of P–C bonds in a lanthanum(iii) hemiphosphinal complex, Inorg. Chem. Front., 2024, 11, 4158–4166 Search PubMed.
- B. Das, A. Makol and S. Kundu, Phosphorus radicals and radical ions, Dalton Trans., 2022, 51, 12404–12426 RSC.
- S. Marque and P. Tordo, in New aspects in phosphorus chemistry, ed. J.-P. Majoral and M. Alajarín, Springer, Berlin, 2005, pp. 43–76 Search PubMed.
- C. D. Martin, M. Soleilhavoup and G. Bertrand, Carbene-Stabilized Main Group Radicals and Radical Ions, Chem. Sci., 2013, 4, 3020–3030 RSC.
- D. P. Mukhopadhyay, D. Schleier, S. Wirsing, J. Ramler, D. Kaiser, E. Reusch, P. Hemberger, T. Preitschopf, I. Krummenacher, B. Engels, I. Fischer and C. Lichtenberg, Methylbismuth: an organometallic bismuthinidene biradical, Chem. Sci., 2020, 11, 7562–7568 RSC.
- J. Ramler, I. Krummenacher and C. Lichtenberg, Bismuth Compounds in Radical Catalysis: Transition Metal Bismuthanes Facilitate Thermally Induced Cycloisomerizations, Angew. Chem., Int. Ed., 2019, 58, 12924–12929 CrossRef CAS.
- K. Oberdorf, A. Hanft, J. Ramler, I. Krummenacher, F. M. Bickelhaupt, J. Poater and C. Lichtenberg, Bismuth Amides Mediate Facile and Highly Selective Pn-Pn Radical-Coupling Reactions (Pn=N, P, As), Angew. Chem., Int. Ed., 2021, 60, 6441–6445 CrossRef CAS.
- M. Mato, D. Spinnato, M. Leutzsch, H. W. Moon, E. J. Reijerse and J. Cornella, Bismuth radical catalysis in the activation and coupling of redox-active electrophiles, Nat. Chem., 2023, 15, 1138–1145 Search PubMed.
- S. Martínez, M. A. Junghanns, T. Dunaj and C. Lichtenberg, Bismuth Radical Catalysis: Thermally Induced Intramolecular C(sp3)-C(sp) Cyclization of Unactivated Alkyl Iodides and Alkynes, ACS Catal., 2025, 15, 14976–14982 CrossRef.
- S. Martínez and C. Lichtenberg, Bismuth-Centered Radical Species: Access and Applications in -Organic Synthesis, Synlett, 2024, 35, 1530–1539 Search PubMed.
- M. Mato and J. Cornella, Bismuth in Radical Chemistry and Catalysis, Angew. Chem., Int. Ed., 2024, 63, e202315046 Search PubMed.
- X. Yang, E. J. Reijerse, K. Bhattacharyya, M. Leutzsch, M. Kochius, N. Nöthling, J. Busch, A. Schnegg, A. A. Auer and J. Cornella, Radical Activation of N-H and O-H Bonds at Bismuth(II), J. Am. Chem. Soc., 2022, 144, 16535–16544 CrossRef CAS PubMed.
- S. Ishida, F. Hirakawa, K. Furukawa, K. Yoza and T. Iwamoto, Persistent antimony- and bismuth-centered radicals in solution, Angew. Chem., Int. Ed., 2014, 53, 11172–11176 CrossRef CAS PubMed.
- C. Ganesamoorthy, C. Helling, C. Wölper, W. Frank, E. Bill, G. E. Cutsail and S. Schulz, From stable Sb- and Bi-centered radicals to a compound with a Ga=Sb double bond, Nat. Commun., 2018, 9, 87 CrossRef.
- C. Helling and S. Schulz, Long-Lived Radicals of the Heavier Group 15 Elements Arsenic, Antimony, and Bismuth, Eur. J. Inorg. Chem., 2020, 2020, 3209–3221 CrossRef CAS.
- C. Lichtenberg, in Scott (Hg.) 2020 – Encyclopedia of inorganic and bioinorganic, 2020, pp. 1–12 Search PubMed.
- G. E. Cutsail, Applications of electron paramagnetic resonance spectroscopy to heavy main-group radicals, Dalton Trans., 2020, 49, 12128–12135 Search PubMed.
- R. J. Schwamm, M. Lein, M. P. Coles and C. M. Fitchett, Bi-P Bond Homolysis as a Route to Reduced Bismuth Compounds and Reversible Activation of P4, Angew. Chem., Int. Ed., 2016, 55, 14798–14801 CrossRef CAS.
- A. Hinz, A. Schulz and A. Villinger, Synthesis of Heavy Cyclodipnictadiphosphanes ClE(µ-P-Ter)2 E = P, As, Sb, or Bi; Ter = 2,6-bis(2,4,6-trimethylphenyl)phenyl, Inorg. Chem., 2016, 55, 3692–3699 CrossRef CAS.
- T. Dunaj, M. Egorycheva, A. Arebi, K. Dollberg and C. von Hänisch, 2,6-Di-iso-propylphenyl substituted Bismuth Halide and Interpnictogen Compounds, Z. Anorg. Allg. Chem., 2023, 649, e202300004 CrossRef CAS.
- T. Dunaj and C. von Hänisch, Heavy Chains: Synthesis, Reactivity and Decomposition of Interpnictogen Chains with Terminal Diaryl Bismuth Fragments, Chem.–Eur. J., 2022, 28, e202202932 CrossRef CAS PubMed.
- C. Ritter, B. Ringler, F. Dankert, M. Conrad, F. Kraus and C. von Hänisch, Synthesis and crystal structures of novel tertiary butyl substituted (pseudo-)halogen bismuthanes, Dalton Trans., 2019, 48, 5253–5262 RSC.
- T. Dunaj, K. Dollberg, C. Ritter, F. Dankert and C. Hänisch, 2,6-Diisopropylphenyl-Substituted Bismuth Compounds: Synthesis, Structure, and Reactivity, Eur. J. Inorg. Chem., 2021, 2021, 870–878 Search PubMed.
- T. Dunaj, K. Dollberg and C. von Hänisch, Binary interpnictogen compounds bearing diaryl bismuth fragments bound to all lighter pnictogens, Dalton Trans., 2022, 51, 7551–7560 RSC.
- C. Ritter, F. Weigend and C. von Hänisch, Synthesis of a Molecule with Five Different Adjacent Pnictogens, Chem.–Eur. J., 2020, 26, 8536–8540 Search PubMed.
- S. Traut, A. P. Hähnel and C. von Hänisch, Dichloro organosilicon bismuthanes as precursors for rare compounds with a bismuth-pnictogen or bismuth-tellurium bond, Dalton Trans., 2011, 40, 1365–1371 RSC.
- C. von Hänisch and D. Nikolova, Synthesis and Characterisation of Molecular Bismuth Phosphorus Compounds Containing Bi 2 Units with Bi–Bi Single and Double Bonds, Eur. J. Inorg. Chem., 2006, 2006, 4770–4773 CrossRef.
- C. von Hänisch and S. Stahl, Synthesis of macrocyclic aluminum-phosphorus and gallium-phosphorus compounds, Angew. Chem., Int. Ed., 2006, 45, 2302–2305 CrossRef.
- M. A. Beswick, N. Choi, A. D. Hopkins, Y. G. Lawson, M. McPartlin, A. Rothenberger, D. Stalke, A. E. H. Wheatley and D. S. Wright, The First Bismuth Phosphide Complex: [Li(thf)4]+[{(tBuP)3}2Bi], Angew. Chem., Int. Ed., 1999, 38, 3053–3055 Search PubMed.
- V. J. Eilrich, T. Grell, P. Lönnecke and E. Hey-Hawkins, Facile synthesis of cyclo-(P4tBu3)-containing oligo- and pnictaphosphanes, Dalton Trans., 2021, 50, 14144–14155 RSC.
- M. Kaupp, in Chemical bonding across the periodic table, ed. G. Frenking, S. Shaik and S. S. Shaik, WILEY-VCH, Weinheim, 1st edn, 2014, pp. 1–24 Search PubMed.
- W. Kutzelnigg, Chemical Bonding in Higher Main Group Elements, Angew Chem. Int. Ed. Engl., 1984, 23, 272–295 CrossRef.
- K. D. Reichl, D. H. Ess and A. T. Radosevich, Catalyzing pyramidal inversion: configurational lability of P-stereogenic phosphines via single electron oxidation, J. Am. Chem. Soc., 2013, 135, 9354–9357 CrossRef CAS.
- J. Popp, S. Hanf and E. Hey-Hawkins, Unusual Racemization of Tertiary P-Chiral Ferrocenyl Phosphines, Chem.–Eur. J., 2020, 26, 5765–5769 CrossRef CAS.
- C. D. Montgomery, Factors Affecting Energy Barriers for Pyramidal Inversion in Amines and Phosphines: A Computational Chemistry Lab Exercise, J. Chem. Educ., 2013, 90, 661–664 CrossRef CAS.
- C. Klmel, C. Ochsenfeld and R. Ahlrichs, Anab initio investigation of structure and inversion barrier of triisopropylamine and related amines and phosphines, Theor. Chim. Acta, 1992, 82, 271–284 CrossRef.
- R. D. Baechler and K. Mislow, Effect of structure on the rate of pyramidal inversion of acyclic phosphines, J. Am. Chem. Soc., 1970, 92, 3090–3093 CrossRef.
- D. A. Dixon and A. J. Arduengo, Periodic trends in the edge and vertex inversion barriers for tricoordinate pnicogen hydrides and fluorides, J. Am. Chem. Soc., 1987, 109, 338–341 CrossRef CAS.
- R. D. Baechler and K. Mislow, Note that low inversion barriers have been reported for phosphanes with one EMe3 substituent (E = Si, Ge, Sn), J. Am. Chem. Soc., 1971, 93, 773–774 CrossRef.
- Note that Eyring theory (where ΔG‡ is strongly temperature-sensitive due to the temperature-weighting of the ΔH‡ term) does not apply to the barrierless homolytic bond dissociation. In the case of the barrierless homolytic bond dissociation, the temperature-sensitivity of the ΔG term stems from the temperature-weighting of the ΔS term according to the Gibbs-Helmholtz equation.
- S. Ishida, F. Hirakawa and T. Iwamoto, A stable dialkylphosphinyl radical, J. Am. Chem. Soc., 2011, 133, 12968–12971 CrossRef CAS.
- S. L. Hinchley, C. A. Morrison, D. W. H. Rankin, C. L. B. Macdonald, R. J. Wiacek, A. H. Cowley, M. F. Lappert, G. Gundersen, J. A. C. Clyburne and P. P. Power, Persistent phosphinyl radicals from a bulky diphosphine: an example of a molecular jack-in-the-box, Chem. Commun., 2000, 2045–2046 RSC.
- N. A. Giffin, A. D. Hendsbee and J. D. Masuda, Reactions of a persistent phosphinyl radical/diphosphine with heteroallenes, Dalton Trans., 2016, 45, 12636–12638 RSC.
- K. Oberdorf and C. Lichtenberg, Small molecule activation by well-defined compounds of heavy p-block elements, Chem. Commun., 2023, 59, 8043–8058 RSC.
- K. L. Mears, G.-A. Nguyen, B. Ruiz, A. Lehmann, J. Nelson, J. C. Fettinger, H. M. Tuononen and P. P. Power, Hydrobismuthation: Insertion of Unsaturated Hydrocarbons into the Heaviest Main Group Element Bond to Hydrogen, J. Am. Chem. Soc., 2024, 146, 19–23 CrossRef CAS.
- S. Satheesh, K. Oberdorf, L. Roeck, A. Fetoh, F. M. Bickelhaupt, J. Poater and C. Lichtenberg, Bismuth Meets Olefins: Ethylene Activation and Reversible Alkene Insertion into Bi–N Bonds, Angew. Chem., Int. Ed., 2025, e202505434 CAS.
- For examples of the insertion of activated olefins into Bi–C bonds, see ref. 49 and 50.
- S. Yamago, E. Kayahara, M. Kotani, B. Ray, Y. Kwak, A. Goto and T. Fukuda, Highly controlled living radical polymerization through dual activation of organobismuthines, Angew. Chem., Int. Ed., 2007, 46, 1304–1306 CrossRef CAS.
- C. Lichtenberg, F. Pan, T. P. Spaniol, U. Englert and J. Okuda, The bis(allyl)bismuth cation: a reagent for direct allyl transfer by Lewis acid activation and controlled radical polymerization, Angew. Chem., Int. Ed., 2012, 51, 13011–13015 CrossRef CAS.
- D. M. Hawley and G. Ferguson, The stereochemistry of some organic derivatives of Group VB elements. The crystal and molecular structure of triphenylbismuth, J. Chem. Soc. Inorg. Phys. Theor., 1968, 2059–2063 Search PubMed.
- C. A. Roller, B. Doler, B. G. Steller, R. Saf and R. C. Fischer, A Distibene with Extremely Long Sb=Sb Distance and Related Heavier Dipnictenes from Salt-Free Metathesis Reactions, Eur. J. Inorg. Chem., 2024, 27, DOI:10.1002/ejic.202300586.
- S. Solyntjes, J. Bader, B. Neumann, H.-G. Stammler, N. Ignat’ev and B. Hoge, Pentafluoroethyl Bismuth Compounds, Chem.–Eur. J., 2017, 23, 1557–1567 CrossRef CAS.
- S. Schulz, A. Kuczkowski, D. Bläser, C. Wölper, G. Jansen and R. Haack, Solid-State Structures of Trialkylbismuthines BiR3 (R = Me, i-Pr), Organometallics, 2013, 32, 5445–5450 CrossRef CAS.
- (a) CCDC 2451991: Experimental Crystal Structure Determination, 2026, DOI:10.5517/ccdc.csd.cc2n9hhz; (b) CCDC 2451992: Experimental Crystal Structure Determination, 2026, DOI:10.5517/ccdc.csd.cc2n9hj0; (c) CCDC 2451993: Experimental Crystal Structure Determination, 2026, DOI:10.5517/ccdc.csd.cc2n9hk1; (d) CCDC 2451994: Experimental Crystal Structure Determination, 2026, DOI:10.5517/ccdc.csd.cc2n9hl2; (e) CCDC 2451995: Experimental Crystal Structure Determination, 2026, DOI:10.5517/ccdc.csd.cc2n9hm3; (f) CCDC 2502129: Experimental Crystal Structure Determination, 2026, DOI:10.5517/ccdc.csd.cc2pznv6.
| This journal is © The Royal Society of Chemistry 2026 |