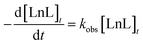Open Access Article
Open Access ArticleLanthanide complexes with acetophenone-based push-pull antennas as efficient MRI and two-photon microscopy imaging probes
Baptiste
Chartier
ab,
Luke
Marchetti
c,
Lamiaa M. A.
Ali
 d,
Dina
Akl
e,
Guillaume
Micouin
e,
Akos
Banyasz
d,
Dina
Akl
e,
Guillaume
Micouin
e,
Akos
Banyasz
 e,
Sandra
Même
e,
Sandra
Même
 c,
Didier
Boturyn
c,
Didier
Boturyn
 b,
Sule
Erbek
fg,
Véronique
Martel-Frachet
b,
Sule
Erbek
fg,
Véronique
Martel-Frachet
 fg,
Alexei
Grichine
fg,
Alexei
Grichine
 f,
Olivier
Maury
f,
Olivier
Maury
 e,
Magali
Gary-Bobo
e,
Magali
Gary-Bobo
 *d,
Célia S.
Bonnet
*d,
Célia S.
Bonnet
 *c and
Olivier
Sénèque
*c and
Olivier
Sénèque
 *a
*a
aUniv. Grenoble Alpes, CNRS, CEA, IRIG, LCBM (UMR 5249), F-38000 Grenoble, France. E-mail: olivier.seneque@univ-grenoble-alpes.fr
bUniv. Grenoble Alpes, CNRS, DCM (UMR 5250), F-38000 Grenoble, France
cCentre de Biophysique Moléculaire, CNRS (UPR 4301), Université d′Orléans, F-45041 Orléans, France. E-mail: celia.bonnet@cnrs.fr
dIBMM, Univ Montpellier, CNRS, ENSCM, F-34000 Montpellier, France. E-mail: magali.garybobo@umontpellier.fr
eUniv Lyon, ENS de Lyon, CNRS UMR 5182, Laboratoire de Chimie, Lyon F-69342, France
fUniv. Grenoble Alpes, INSERM U1209, CNRS UMR 5309, Institute for Advanced Biosciences, F-38000 Grenoble, France
gEPHE, PSL Research University, 4-14 Rue Ferrus, 75014 Paris, France
First published on 18th December 2025
Abstract
Lanthanide(III) (Ln3+) complexes possess unique magnetic and optical properties that make them ideal candidates for the development of multimodal MRI and optical probes. However, the requirements for developing effective MRI and optical probes are difficult to meet within a single ligand. Here, we propose the use of a DOTA-type ligand equipped with a π-extended acetophenone moiety that (i) serves as a coordinating moiety to form stable Ln3+ complexes, (ii) acts as a 2P-excitable push–pull antenna to sensitize Eu3+ luminescence, and (iii) displays a carboxylate handle offering high water solubility and enabling conjugation to biomolecules for targeting purposes. We show that the Ln3+ complexes obtained are kinetically inert and thermodynamically stable. The Gd3+ complex exhibits positive in vivo characteristics with good contrast in all organs and rapid renal clearance, while the corresponding Eu3+ complex has excellent one-photon and two-photon (2P) absorption properties enabling high-quality in vivo 2P microscopy imaging of zebrafish embryos or in vitro imaging of living cells when conjugated to a cell-penetrating peptide.
Introduction
Over the past number of decades, lanthanide complexes have been implemented with great success in various modalities in the field of biological imaging. Luminescent lanthanide complexes possess many advantageous properties for optical techniques, such as narrow emission bands at fixed wavelengths that are specific to each lanthanide, long luminescence lifetimes, and resistance to photobleaching.1,2 Indeed, there are numerous examples of luminescent lanthanide complexes, such as those containing Eu3+, Tb3+ or Yb3+, being exploited for not only cellular imaging3–8 but also for detection of various analytes.3,9–12 Magnetic resonance imaging (MRI) is an imaging technique, endowed with unlimited tissue depth penetration and excellent spatiotemporal resolution. The exploitation of Gd3+-based contrast agents (CAs) in MRI has allowed for improved diagnostic accuracy13 and having the option to incorporate targeting moieties into the CA14 or render it “responsive”,12,15,16 enabling the selective detection of various biologically relevant species – including but not limited to cations,17–19 extracellular proteins,20 and neurotransmitters.21These two imaging modalities are not without their own drawbacks, however. While luminescence techniques display high sensitivity, they suffer from low macroscopic resolution and often require the integration of a cell-internalization vector, such as a peptide, to penetrate into living cells. Meanwhile, MRI has high macroscopic resolution but low sensitivity, requiring high concentrations of CAs to be detected. In recent years, the development of imaging probes active in both modalities has seen increased traction.22–31 By incorporating complementary imaging modalities within a single molecular design, one could achieve non-invasive, real-time monitoring with high sensitivity and resolution, effectively overcoming the limitations of individual imaging modalities.24,27,28 This would also have the benefit of complementary imaging at different scales, enabling monitoring of cellular processes and the anatomical context in real time.
Lanthanide complexes are particularly well-suited for these applications because of their different optical and magnetic properties across the series while still maintaining similar coordination properties and chemical reactivities. However, the development of pairs of lanthanide complexes relying on the same ligand, but displaying both MRI and luminescent activities is not without its own set of hurdles, either. MRI CAs require at least one inner-sphere coordinated water molecule to the Gd3+-center for efficient reduction of the T1 relaxation time of water protons. This can be seen as incompatible with luminescent complexes which usually display a saturated coordination sphere, as directly coordinated water molecules quench lanthanide luminescence due to non-radiative deactivation.
Moreover, luminescent lanthanide complexes suffer from low molar absorption coefficients with values of about 1 to 5 M−1 cm−1, due to the Laporte rule that forbids the f–f transition. Nevertheless, this can be bypassed by the well-documented ‘antenna effect’, whereby a chromophore is implemented close to the lanthanide.2,12,32 This chromophore should efficiently absorb light, typically in the ultraviolet (UV) range, followed by subsequent energy transfer to the lanthanide, thus allowing for the sensitization of the lanthanide and resulting luminescence emission. It is important to note that the use of UV light to excite the antenna is problematic, as UV light has not only been proven to be cytotoxic, but can also be absorbed and scattered by biological tissues. An alternative to this excitation pathway is the use of a two-photon (2P) absorption process that allows for the simultaneous absorption of two photons, with half the energy required for a normal excitation with one photon.33–36 This method allows for a bathochromic shift of the excitation wavelength, moving from the UV to the red or near infrared (NIR) region, which is known to be less toxic and less scattered by biological tissues. Unlike MRI, one of the main drawbacks of luminescence is its low tissue penetration. 2P excitation in the NIR region allows deeper tissue penetration, thus compensating for the limitation of fluorescence imaging.37,38
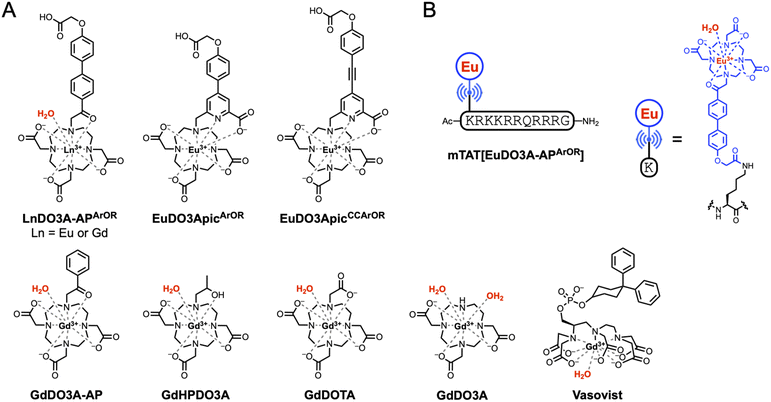
We have recently developed 2P excitable Ln3+ probes that were used successfully for 2P microscopy of live cells.39–43 These probes are based on a DO3Apic ligand that saturates the Ln3+ coordination sphere, precluding their use as MRI CAs. With the aim to design an octadentate Ln3+ chelator that leaves a single water molecule in the coordination sphere of the Ln3+ and features a 2P absorbing antenna, we identified DO3A-AP in the literature. This ligand was first reported to bind Eu3+ and sensitize its luminescence through the acetophenone antenna.44 EuDO3A-AP is mono-hydrated and shows a Eu3+ luminescence quantum yield of 0.058 upon excitation in the acetophenone absorption band at 265 nm. The absorption could be red-shifted by introducing electron-rich methoxy44 or triazole45 groups in the para-position of the acetophenone or by substituting the phenyl-ketone by other aryl-ketones (aryl = naphthyl, carbazolyl or phenantrenyl).46 The relaxometric properties of GdDO3A-AP and related derivatives with hydroxy substituents were recently described.47,48 However, the in vivo MRI (with Gd3+ as Ln3+) or in vitro/in vivo microscopy imaging (with Eu3+) capacities of the LnDO3A-AP system were never explored.
Based on this, we wanted to push the advantage and propose for the first time a monohydrated Ln3+ complex for both high resolution 2P microscopy, with Eu3+ as Ln3+, and MRI, with Gd3+. We present here DO3A-APArOR (Fig. 1), a ligand derived from DO3A-AP bearing an antenna with strong push–pull capabilities providing 2P absorption properties and a reactive handle for the facile incorporation of a cell-penetrating peptide. The coordination sphere remains unsaturated, allowing for one water molecule in the inner-sphere, providing the opportunity for its use as an MRI contrast agent. The photophysical properties of the Eu3+ complex were investigated, alongside its 2P excitation properties, and compared to those of the previously described non-hydrated picolinate analogues EuDO3ApicArOR and EuDO3ApicCCArOR.39,43 Despite its coordinated water molecule, EuDO3A-APArOR shows good luminescence properties. The relaxometric properties of the Gd3+ complex were characterized by variable temperature 17O NMR experiments and nuclear magnetic relaxation dispersion. The complex also displays very high kinetic inertness. These positive features allow for the in vivo use of the Gd3+ complex as an efficient MRI contrast agent and the Eu3+ complex as a luminescent probe for 2P microscopy in zebrafish. A cell-penetrating peptide, mTAT, was conjugated to the antenna which allowed for internalization of the complex to the cytosol of live HeLa cells, visualized by 2P microscopy. Therefore, this versatile design based on a Ln3+ complex allows in vivo MRI in mice and 2P microscopy on zebrafish and high quality cell imaging by 2P microscopy.
Results and discussion
Synthesis of the ligand and its lanthanide complexes
The synthetic pathway for the preparation of the acetophenone-based Ln3+ complexes is depicted in Fig. 2A. The synthesis starts with the alkylation of DO3A(tBu)31 (ref. 49) with 2-bromo-1-(4-iodophenyl)ethan-1-one 2 in MeCN to give compound 3 in 94% yield. A Miyaura–Suzuki coupling between 3 and boronic ester 4 (ref. 43) in DMF with polymer-bound Pd(PPh3)4 as a catalyst affords the tBu-protected ligand 5 in 56% yield. After acidolysis of the tBu esters in a TFA/DCM mixture, metalation with Eu3+ or Gd3+ salt in water (pH 7) followed by HPLC purification and freeze-drying gives complexes LnDO3A-APArOR in 80–90% yield. Note that these compounds are sensitive to basic conditions, which can cause N-dealkylation of the acetophenone arm. The detailed synthetic procedures and NMR, HPLC and mass spectrometry characterization of these compounds are given in the SI.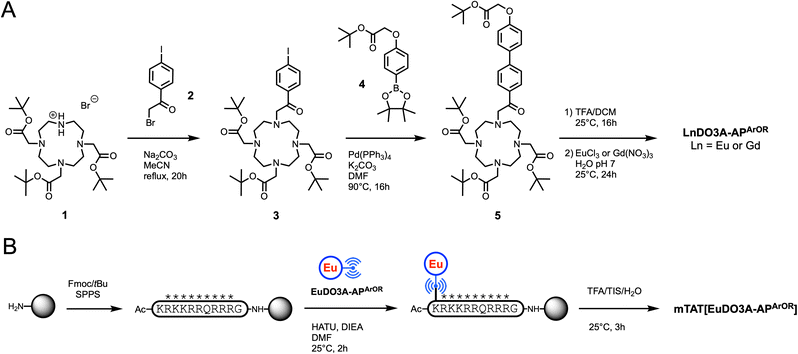 | ||
| Fig. 2 Synthetic pathway for (A) EuDO3A-APArOR and GdDO3A-APArOR and (B) mTAT[EuDO3A-APArOR]. In (B), * denotes standard side chain protecting groups. | ||
Photophysical properties
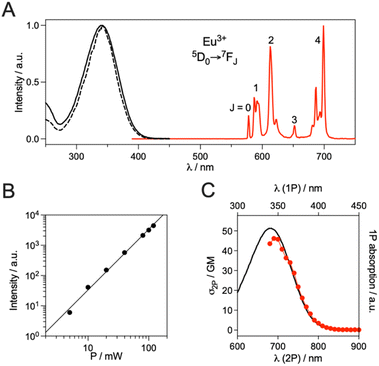
The photophysical properties of the EuDO3A-APArOR complex were investigated in phosphate-buffered saline (PBS, pH 7.4). The absorption spectrum of EuDO3A-APArOR shows a broad structureless band in the UV region with a maximum at 342 nm (λmax) extending up to 403 nm (λcut-off), which is assigned to an intra-ligand charge transfer transition (ILCT) within the antenna from the anisole donor to the coordinated carbonyl acceptor moieties (Fig. 3). This absorption is red-shifted by ca. 35 nm and 10 nm (Fig. S8) compared to the picolinate analogues EuDO3ApicArOR and EuDO3ApicCCArOR (Fig. 1) with alkoxy-phenyl-picolinate and alkoxy-phenyl-ethynyl-picolinate antennas, respectively.39,43 The molar absorption coefficient of EuDO3A-APArOR was determined to be 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 at λmax (Fig. S9) similar to EuDO3ApicArOR (21
000 M−1 cm−1 at λmax (Fig. S9) similar to EuDO3ApicArOR (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1)43 and EuDO3ApicCCArOR (21
000 M−1 cm−1)43 and EuDO3ApicCCArOR (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1).39 Upon excitation into the ILCT band at 340 nm, the Eu3+ emission is observed with 5D0 → 7FJ transitions (J = 0, 1, 2, 3 and 4) at 580, 595, 615, 650 and 700 nm, respectively. The excitation spectrum (λem = 615 nm) matches well with the absorption spectrum, confirming that the alkoxy-phenyl-acetophenone antenna sensitizes Eu3+ luminescence. The Eu3+ luminescence decay could be perfectly fitted with a mono-exponential giving a lifetime value of 0.53 (2) ms (Fig. S10), which is about half that of the picolinate analogues EuDO3ApicArOR and EuDO3ApicCCArOR (1.1 ms) with a saturated Eu3+ coordination sphere (hydration number q = 0).39,43 The lifetime remains unchanged upon degassing the solution. In PBS prepared with D2O, the Eu3+ decay lifetime was 1.49 ms. According to Parker's equation, a hydration number q of 1.2 (2) was calculated from the lifetimes in H2O and D2O,50 indicating that a single water molecule stands in the coordination sphere of Eu3+. This is in agreement with the literature on Ln3+ complexes of DO3A-AP ligands.44–48 The Eu3+ emission quantum yield, ΦEu, is 0.075 (Fig. S11), again almost half that of the picolinate analogues (0.14 and 0.17 for EuDO3ApicArOR and EuDO3ApicCCArOR, respectively).39,43 The energy of the alkoxy-phenyl-acetophenone antenna triplet state, T1, is 21
000 M−1 cm−1).39 Upon excitation into the ILCT band at 340 nm, the Eu3+ emission is observed with 5D0 → 7FJ transitions (J = 0, 1, 2, 3 and 4) at 580, 595, 615, 650 and 700 nm, respectively. The excitation spectrum (λem = 615 nm) matches well with the absorption spectrum, confirming that the alkoxy-phenyl-acetophenone antenna sensitizes Eu3+ luminescence. The Eu3+ luminescence decay could be perfectly fitted with a mono-exponential giving a lifetime value of 0.53 (2) ms (Fig. S10), which is about half that of the picolinate analogues EuDO3ApicArOR and EuDO3ApicCCArOR (1.1 ms) with a saturated Eu3+ coordination sphere (hydration number q = 0).39,43 The lifetime remains unchanged upon degassing the solution. In PBS prepared with D2O, the Eu3+ decay lifetime was 1.49 ms. According to Parker's equation, a hydration number q of 1.2 (2) was calculated from the lifetimes in H2O and D2O,50 indicating that a single water molecule stands in the coordination sphere of Eu3+. This is in agreement with the literature on Ln3+ complexes of DO3A-AP ligands.44–48 The Eu3+ emission quantum yield, ΦEu, is 0.075 (Fig. S11), again almost half that of the picolinate analogues (0.14 and 0.17 for EuDO3ApicArOR and EuDO3ApicCCArOR, respectively).39,43 The energy of the alkoxy-phenyl-acetophenone antenna triplet state, T1, is 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 cm−1 (determined from the onset of the phosphorescence emission of GdDO3A-APArOR at 77 K), which is 2000 cm−1 below that of the picolinate analogue (Fig. S12).43 However, the antenna T1 state remains high enough to prevent efficient back energy transfer from the Eu3+ 5D0 excited state. In the end, EuDO3A-APArOR is half as bright as its picolinate analogue because of the presence of one Eu3+-bound water molecule.
100 cm−1 (determined from the onset of the phosphorescence emission of GdDO3A-APArOR at 77 K), which is 2000 cm−1 below that of the picolinate analogue (Fig. S12).43 However, the antenna T1 state remains high enough to prevent efficient back energy transfer from the Eu3+ 5D0 excited state. In the end, EuDO3A-APArOR is half as bright as its picolinate analogue because of the presence of one Eu3+-bound water molecule.
The 2P absorption properties of EuDO3A-APArOR were determined by the two-photon excited fluorescence method. First, the quadratic dependence of the emitted Eu3+ intensity on laser power was checked under excitation at 700 nm with a Ti:sapphire laser (Fig. 3B). Then, the 2P absorption spectrum was measured. It matches nicely the wavelength-doubled 1P absorption spectrum (Fig. 3C). At 720 nm, the wavelength used in 2P microscopy experiments (vide infra), the 2P absorption cross-section, σ2P, is 36 GM (1 GM = 10−50 cm4 s photon−1), similar to that of the alkoxy-phenyl-ethynyl-picolinate antenna (35 GM)51 and ca. 10 times higher than that measured for an Eu3+ complex with a methoxy-phenyl-picolinamide antenna.42 At 720 nm, the 2P brightness, B2P = σ2P × ΦEu, of EuDO3A-APArOR is 2.7 GM.
Relaxometric characterization of GdDO3A-APArOR
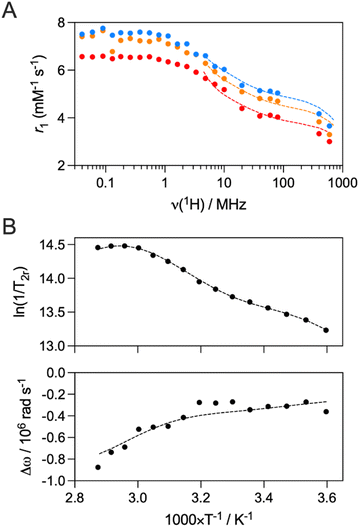
Since EuDO3A-APArOR has a hydration number q of 1, its Gd3+ analogue appears to be a good candidate for MRI imaging. In order to characterize the efficacy of GdDO3A-APArOR and relate this to the microscopic parameters that govern the efficacy (relaxivity) of the complex, nuclear magnetic relaxation dispersion (NMRD) profiles, in combination with variable temperature 17O NMR experiments, were recorded. The NMRD profiles were recorded within the range of 0.01–600 MHz, at three different temperatures: 25, 37, and 50 °C (Fig. 4A). As temperature increased, the r1 values decreased, indicating that the rotational correlation time is the limiting factor in the relaxivity, which is expected with a small molecular complex. The relaxivity at 20 MHz, 25 °C is 5.4 mM−1 s−1, consistent with a monohydrated complex of this size and aligns well with the values found under the same conditions for GdHPDO3A (4.6 mM−1 s−1)52 and GdDO3A-AP (5.1 mM−1 s−1).47The reduced transverse relaxation rates (1/T2r) were measured as a function of temperature to determine the water exchange rate, kex, while the reduced chemical shifts give access to the number of coordinated water molecules on the Gd3+ complex. The 17O-reduced transverse relaxation rates increase (up to ca. 333 K), followed by a plateau (Fig. 4B). The shape of the curve indicates that there are at least two isomers present in solution, most likely the SAP and TSAP coordination isomers which display greatly different water exchange rates, as seen with GdHPDO3A,52 GdDOTA bisamide derivatives53,54 or GdDO3A-AP.47 Thusly, a 1H NMR study was performed (700 MHz at 288, 298, and 318 K) to determine the ratio between the SAP and TSAP isomers of EuDO3A-APArOR in solution (Fig. S14). The SAP isomer is found to be the main isomer as already reported for the majority of DOTA-based complexes.55 The ratio of SAP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TSAP is found to be 80
TSAP is found to be 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, which is consistent with complexes reported in the literature with a similar coordination sphere.46,47 Importantly, this ratio is constant over the range of temperature studied. The reduced chemical shifts measured are consistent with a monohydrated complex in solution, in accordance with the luminescence measurements on the Eu3+ complex.
20, which is consistent with complexes reported in the literature with a similar coordination sphere.46,47 Importantly, this ratio is constant over the range of temperature studied. The reduced chemical shifts measured are consistent with a monohydrated complex in solution, in accordance with the luminescence measurements on the Eu3+ complex.
The NMRD profiles, transverse 17O relaxation rates, and 17O chemical shifts were fitted simultaneously using Solomon-Bloembergen and Morgan (SBM) theory, while considering the relative populations of the two isomers in solution, to yield the microscopic parameters that govern the proton relaxivity of the complex (Tables 1 and S1). The NMRD profiles were fitted in the range 4–600 MHz, where the effect of the electron spin relaxation, which is not well described by the SBM theory alone, is negligible, and the SBM approach gives reliable information on dynamic processes like the water exchange rate and rotational correlation time for small complexes.56,57
Under the reasonable assumption that the rotational correlation time (τR) is the same for both SAP and TSAP species, a value of 134 ps is found. This value is higher than that of GdHPDO3A (65 ps) and GdDO3A-AP (100 ps), which is consistent with the larger size of GdDO3A-APArOR. The fitting of the data yields kex = 0.49 × 106 s−1 for the SAP isomer and kex = 18 × 106 s−1 for the TSAP isomer. This is a well-known phenomenon, whereby the increased steric hindrance around the Gd3+-center of the TSAP isomer results in a faster water exchange rate. It was previously observed that the introduction of a ketone pendant arm resulted in slow water exchange rates.47,58 In this case, the exchange rates of the SAP and TSAP isomers are even slower than those of GdDO3A-AP. This could be explained by the electronic effects of the benzene on the acetophenone, which decreases the electronic density on the ketone; therefore the steric crowding around Gd3+. In such complexes with positive activation entropy (dissociatively activated mechanism), this results in a lower water exchange rate.59
Due to the presence of the hydrophobic biphenyl moiety, the complex could be prone to aggregation processes. Paramagnetic Relaxation Enhancements (PRE) measurements were performed at 60 MHz and 25 °C as a function of concentration (Fig. S15). A linear trend is observed, which demonstrates no aggregation of the complex in the concentration range studied (ca. 80 µM–8 mM).
The incorporation of a lipophilic group into a Gd3+-based contrast agent is a well-established method to promote non-covalent interactions between the CA and human serum albumin (HSA). This interaction has several advantages, such as increased relaxivity of the CA, mainly due to the increased rotational correlation time of the chelate, and a prolonged vascular retention time. Considering that biphenyl moieties have been leveraged multiple times to this effect, we considered that the biphenyl antenna present in DO3A-APArOR could possibly interact with HSA in this manner. Thus, the relaxivity of GdDO3A-APArOR in the presence of 0.6 mM HSA was measured at 60 MHz, 25 °C. An increase of ca. 110% in relaxivity was observed in the presence of HSA (r1 = 11.4 mM−1 s−1vs. r1 = 5.38 mM−1 s−1 in HEPES buffer).
To further evaluate the potential of this complex as a contrast agent, T1-weighted phantom images were recorded at 7 T, where the relaxivity of GdDO3A-APArOR was measured in mouse blood serum and compared to that of GdDOTA, a Gd3+-based CA used clinically (Fig. 5). While GdDO3A-APArOR did not retain its enhanced relaxivity at higher field strengths, it did display a similar signal intensity (3.89 × 104) to the clinically used GdDOTA (3.97 × 104) at 7 T, 25 °C (0.3 mM in mouse serum).
Stability
Given the in vitro potential of the system both in terms of luminescence and relaxivity, we wanted to assess its in vivo applicability. However, it is of prime importance to check the thermodynamic stability and kinetic inertness of the system prior to in vivo use. Macrocyclic lanthanide complexes based on DOTA or DOTA-monoamide systems are known to display high thermodynamic stability and kinetic inertness.60 In this case, one ketone function is coordinating the Ln3+. Therefore, we first investigated the thermodynamic stability and kinetic inertness of EuDO3-APArOR by competitions with DTPA at pH 7.4. EuDO3A-APArOR was mixed with DTPA (1, 10 and 100 eq.) at pH 7.4 in PBS and the Eu3+ emission intensity was monitored after 24 h. At 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 EuDO3A-APArOR/DTPA ratios, the Eu3+ emission intensity remained unchanged (Fig S16). A slight decrease in Eu3+ intensity was observed for a 1
10 EuDO3A-APArOR/DTPA ratios, the Eu3+ emission intensity remained unchanged (Fig S16). A slight decrease in Eu3+ intensity was observed for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ratio. This is indicative of a high thermodynamic stability or kinetic inertness. Under more challenging conditions to assess the kinetic inertness and compare to other macrocyclic complexes, EuDO3-APArOR was dissolved in HCl 1 M. The emission spectrum is the same as that at pH 7.4 (Fig. S17A), indicating that the coordination sphere of the emissive Eu3+ species is not altered by the low pH. Under these conditions, the complex fully dissociates (Fig. S17B). Given the very high proton concentration, the dissociation follows a pseudo-first order kinetics, and the dissociation rate is proportional to the total concentration of the complex [LnL]t, with kobs being the pseudo-first order rate constant:
100 ratio. This is indicative of a high thermodynamic stability or kinetic inertness. Under more challenging conditions to assess the kinetic inertness and compare to other macrocyclic complexes, EuDO3-APArOR was dissolved in HCl 1 M. The emission spectrum is the same as that at pH 7.4 (Fig. S17A), indicating that the coordination sphere of the emissive Eu3+ species is not altered by the low pH. Under these conditions, the complex fully dissociates (Fig. S17B). Given the very high proton concentration, the dissociation follows a pseudo-first order kinetics, and the dissociation rate is proportional to the total concentration of the complex [LnL]t, with kobs being the pseudo-first order rate constant:The dissociation half-life and kobs values determined in 1 M HCl are presented in Table 2. The kobs is one order of magnitude higher than that of GdDOTA,61 but two to three orders of magnitude lower than that of the other macrocyclic compounds (GdDO3A62 or EuDO3Apic63). Importantly, it is one order of magnitude lower than that of commercially available GdHPDO3A.64 The half-life in 1 M HCl is 5.3 h, which demonstrates the strong inertness of EuDO3-APArOR.
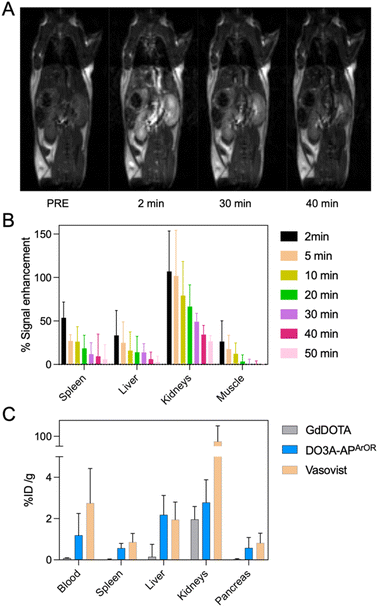
In vivo MRI biodistribution in mice
Encouraged by the in vitro results and the very high inertness of the complex, an in vivo MRI study was then carried out, where five healthy mice were injected with GdDO3A-APArOR (100 µmol kg−1). After intravenous administration of the contrast agent, an immediate increase in signal intensity was observed, with the signal enhancement peaking two minutes after injection for all organs (Fig. 6). The kidneys experienced the greatest signal enhancement over other organs. This indicates that the CA is cleared via the kidneys, with a negligible portion via the liver, which is expected for a small molecular complex. The clearance is fast as the signal enhancement in the main organs (liver, spleen and muscle) is negligible 50 min post-injection. Ex vivo biodistribution was also studied 1 h post injection and the Gd3+ content of various organs was measured ex vivo by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) (Fig. 6). Less than 3% of the injected dose per organ gram is found, showing a good elimination of the contrast agent after one hour. Interestingly, the Gd content one hour post injection is intermediate to that found for GdDOTA and Vasovist,65 a blood-pool agent. This is consistent with the in vitro results showing some HSA binding and a certainly longer circulation time than GdDOTA, but shorter than Vasovist. The excellent stability and inertness of GdDO3A-APArOR and successful MRI experiments prompted us to evaluate the potential of the Eu3+ analogue EuDO3A-APArOR for in vivo 2P microscopy imaging in zebrafish embryos.In vivo 2P microscopy of zebrafish embryos
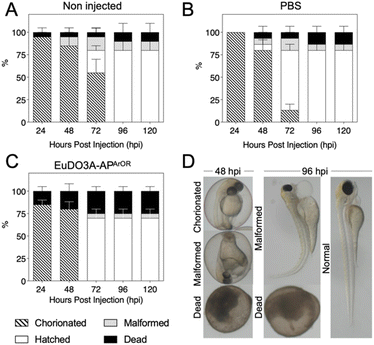
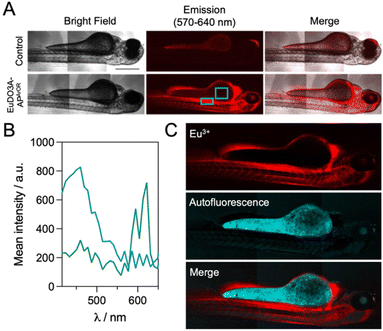
The use of zebrafish (Danio rerio) embryos is increasingly recognized as an alternative to testing on small mammals.66,67 Although imaging of zebrafish embryos with fluorescent probes is classical,68 to our knowledge, examples of 2P zebrafish imaging with Ln3+ probes are scarce.7,69 First, the toxicity of EuDO3A-APArOR in zebrafish embryos was studied at the single cell stage (see the SI for details). The development of embryos was observed at different time points (24, 48, 72, 96 and 120 h) post-injection. The viability was based on the morphology and the presence of heartbeat. Malformation including pericardial oedema, yolk sac oedema, spinal curvature and tail malformation was recorded. Dead embryos were removed immediately. The results shown in Fig. 7 demonstrate the mild toxicity of EuDO3A-APArORin vivo (Fig. 7c). At 72 hpi (hours post-injection), an increase in the death rate of the EuDO3A-APArOR injected group (25%) compared to the non-injected group (5%) was observed. Furthermore, an increase in the hatching rate in the PBS and EuDO3A-APArOR injected groups (∼70%) compared to the non-injected group (25%) was recorded. EuDO3A-APArOR did not induce higher morphological abnormalities than the non-injected or PBS injected group. The toxicity profile of EuDO3A-APArOR remains constant from 72 hpi until 120 hpi.Then, 2P microscopy imaging of zebrafish embryos was performed under 720 nm excitation with spectral detection for 3 h after intravenous injection of EuDO3A-APArOR (see the SI for details). As shown in Fig. 8A, the luminescence signal was collected with a 570–640 bp filter and a long integration time of 131 µs per pixel. The emission was detected in the heart, intersegmental vessels, caudal vein, caudal artery, primary head sinus and the inner optic circle. In contrast, in the control (no Eu3+ probe injected), the luminescence was detected mainly in the yolk, which usually shows high autofluorescence. In injected embryos, spectral detection (Fig. 8B) allowed for the discrimination of the broad autofluorescence signal with a maximum at ca. 460 nm and the Eu3+ emission with characteristic peaks at 580 nm and 620 nm. As expected, the latter was not detected in the control. Linear unmixing of the autofluorescence and Eu3+ signals is shown in Fig. 8C. EuDO3A-APArOR is clearly distributed in the cardiovascular circulatory system. Finally, the viability of embryos injected with EuDO3A-APArOR was 80% after 3 days post-injection, confirming its low toxicity. Therefore, EuDO3A-APArOR is suitable to provide high-quality 2P images in zebrafish.
Preparation and characterization of the TAT conjugate
The in vivo applicability of the complex is well established in both MRI and luminescence to probe the extracellular environment. However, for bioimaging applications, it can be interesting to specifically vectorize the probe, for example, towards cancer cells or to make it cell-permeable in order to probe the intracellular environment and obtain complementary information. This is generally achieved by coupling the probe to a peptide or a protein that provides recognition properties. Here, we choose to demonstrate the applicability of EuDO3A-APArOR for 2P microscopy at the cell level by conjugating it to the well-known TAT cell penetrating peptide.41,42,70 The preparation of TAT conjugates with LnDO3Apic complexes, which were described previously, relies on the coupling of a protected macrocyclic ligand to the peptide on resin, followed by (i) acidic cleavage from the resin and deprotection (side chains and ligand), (ii) HPLC purification and (iii) metalation with Ln3+. Here we opted for a more straightforward strategy, directly coupling the Ln3+ complex to the peptide on resin (Fig. 2B). The peptide was elongated on Rink Amide resin using solid phase peptide synthesis following classical procedures for the Fmoc/tBu strategy. The N-terminal lysine was orthogonally protected with an alloc group. After selective removal of this group on resin using Pd0, EuDO3A-APArOR was coupled to the peptide via its carboxylate handle using HATU/DIEA activation for 2 h. After resin cleavage and protecting group removal using TFA/H2O/TIS, the conjugate was purified by HPLC and freeze-dried. The Eu3+ complex resisted the TFA treatment and the acidic HPLC conditions (H2O/MeCN mixture with 0.1% TFA, pH ca. 2) and no Eu3+ release was observed, further validating the high inertness of the complex. Proper conjugation through the carboxylate handle on the electron donating group of the antenna was confirmed by mass spectrometry and Eu3+ luminescence properties.The spectroscopic properties of mTAT[EuDO3A-APArOR] are very similar to those of the parent Eu3+ complex (Fig. S13), with an identical absorption band, the same Eu3+ emission spectrum, which attests that the conjugation has not altered the Eu3+ coordination sphere, and identical Eu3+ luminescence lifetime (0.50 (2) ms) and quantum yield (ΦEu = 0.077) within the error margin. All this indicates that conjugation to the TAT peptide has no influence on the emission properties of EuDO3A-APArOR.
In vitro 2P microscopy on living HeLa cells
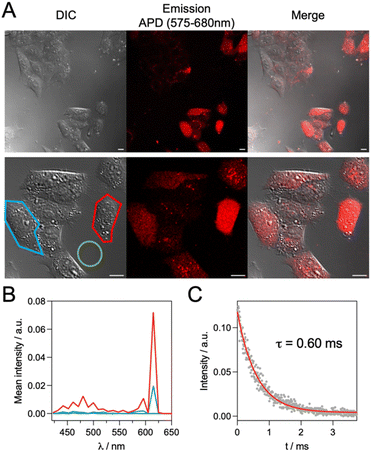
In vitro imaging was attempted with live HeLa cells incubated with a mixture of mTAT[EuDO3A-APArOR] and dFFLIPTAT, a dimeric cell penetrating peptide (sequence: (CFFLIPRKKRRQRRRG)2, dimerized via a disulfide bond) that helps TAT monomers such as mTAT[LnL] probes to enter live cells.41,42,71 Before conducting 2P microscopy on live HeLa cells, the cytotoxicity of the conjugate in co-incubation with dFFLIPTAT (1.5 µM) was examined using the MTT assay (Fig. S18). After 48 h of cell proliferation, no cytotoxicity was observed when the conjugate was incubated at 5 µM and 10 µM.For microscopy experiments, HeLa cells were incubated with mTAT[EuDO3A-APArOR] (5 µM) and dFFLIPTAT (1.5 µM) for 1 h before washing and imaging on a 2P microscope under 720 nm excitation using an APD (avalanche photodiode) for sensitive detection and a 580–680 nm bandpass filter to collect Eu3+ emission Fig. 9A. The DIC (differential interference contrast) image shows HeLa cells with a phenotype of living cells in agreement with the MTT assay. The luminescence channel shows stained cells with diffuse red emission within the whole cell, typical of successful cytosolic delivery of the probe.40,42 The cell-to-cell intensity heterogeneity is a common feature of probes based on cell penetrating peptides.40,42,72,73 In order to confirm that the luminescence channel collects Eu3+ emission, a spectral image was recorded using the PMT array of the 2P microscope. Fig. 9B shows the mean emission spectra of cells, recorded over the area outlined in red and blue in the DIC image. For the 5D0 → 7FJ, J = 1 and 2, emission bands of Eu3+ emission are unambiguously observed between 575 and 630 nm. The Eu3+ luminescence lifetime was measured in cells using the TSLIM method (Fig. 9C).74 A value of 0.57 ± 0.03 ms (average over 15 measurements in three distinct cells) was found, similar to the one recorded in solution. This was surprising because with other Eu3+ or Tb3+ probes featuring chelators that saturate the Ln3+ coordination sphere, we have always measured lifetimes ca. 30% shorter in cells than in the cuvette.40,75 This shorter lifetime was proposed to be the consequence of additional de-excitation pathways in the cell via protein co-factors. In the present case, the higher lifetime suggests a change in the average hydration number of Eu3+, i.e. a partial replacement of the Eu3+-bound H2O by coordinating amino acid side chains of a protein or small molecule. Note that under similar incubation conditions (1 h, 5 µM probe), the non-conjugated complex EuDO3A-APArOR is not detected within cells. Addition of DMSO (up to 5%) in the incubation medium to permeabilize membranes did not improve uptake. This demonstrates the value of conjugating the probe to a cell penetrating peptide. To sum up, despite its Eu3+-bound water molecule, EuDO3A-APArOR conjugated to the TAT cell penetrating peptide allows 2P microscopy of live cells and gives high-quality images of live cells.
Conclusions
In this article, we have demonstrated that adding a π-conjugated extension to the acetophenone moiety of ligand DO3A-AP provides a luminescent Eu3+ complex, EuDO3A-APArOR, with excellent 2P absorption properties compared to its picolinate analogue. Despite an Eu3+-bound water molecule, which quenches Eu3+ emission and lowers luminescence properties, this complex has sufficiently good luminescence properties to make it suitable for in vivo 2P imaging of zebrafish and live cell 2P imaging. Compared to the picolinate analogue, the water molecule bound to Ln3+ proves to be an advantage, allowing MRI imaging in vivo with the Gd analogue, GdDO3A-APArOR. This complex is therefore able to probe the extracellular environment in vivo both in MRI, with Gd3+, and 2P microscopy imaging, with Eu3+. Several DO3A- or DOTA-based ligand systems were already described to elaborate by simple Ln3+ exchange on both luminescent Eu3+ or MRI-active Gd3+ probes, with predictable MRI properties due to the DO3A/DOTA scaffold.26–29 However, they were used in luminescence microscopy imaging with UV excitation. The system described here integrates a push–pull antenna that permits 2P excitation in the NIR region, which is less damaging and allows better tissue penetration. The strong 2P absorption properties of this system provided for instance excellent quality microscopy images of zebrafish embryos. Finally, another advantage of this system is its pending carboxylate, which allows straightforward conjugation of LnDO3A-APArOR complexes to biomolecules such as peptides. This has been exemplified here with a TAT conjugate, a cell-penetrating peptide, allowing high-quality living cell microscopy images to be obtained following 2P absorption despite the presence of the water molecule on Ln3+. Therefore, LnDO3A-APArOR complexes have great potential and versatility for the safe development of efficient agents for MRI and 2P microscopy imaging.Ethical statement
All animal experiments were carried out in accordance with the guidelines for animal experiments and under permission number 54201, from the French “Ministère de l’Enseignement Supérieur, de la Recherche et de l’Innovation”.Author contributions
Conceptualization: O. S., C. S. B., O. M., and M. G.-B.; investigation: B. C., L. M., L. M. A. A., D. A., G. M., S. M., S. E., V. M.-F., A. G., and O. S.; validation: O. S., C. S. B., M. G.-B., O. M., A. B., S. M. A. G., V. M.-F., and D. B.; writing (original draft): B. C., L. M., L. M. A. A., O. S., C. S. B., and M. G.-B.; writing (review & editing): all authors. visualization: O. S., C. S. B., B. C., L. M., and L. M. A. A.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: procedures for the synthesis of new compounds, 1P and 2P spectroscopy (absorption and luminescence), relaxometry, stability and inertness assays by luminescence, protocols for MRI experiments, toxicity assays and 2P microscopy of zebrafish embryos, cell culture, toxicity assays and 2P microscopy. See DOI: https://doi.org/10.1039/d5sc06902e.Acknowledgements
Agnès Pallier is acknowledged for performing ICP experiments. The authors thank the MO2VING platform for spectroscopic and MRI experiments. For zebrafish embryo imaging, the authors acknowledge the imaging facility MRI, a member of the France-BioImaging national infrastructure, supported by the French National Research Agency (ANR-10-INBS-04, “Investments for the future”) and also Nicolas Cubedo and Mireille Rossel from the aquatic model facility ZEFIX from MMDN/LPHI/CRBM/IGF. Cell 2P microscopy imaging experiments were done on the Microcell core facility of the Institute for Advanced Biosciences (UGA – Inserm U1209 CNRS 5309). This facility belongs to the IBISA-ISdV platform, a member of the national infrastructure France-BioImaging supported by the French National Research Agency (ANR-10-INBS-04). The authors acknowledge the Agence Nationale de la Recherche [LANTEN project (ANR-21-CE29-0018) and the Zinc-Espionage project (ANR-22-CE44-0041)], the Labex ARCANE and CBH-EUR-GS (ANR-17-EURE-0003) and the CEA FOCUS Biomarqueurs for financial support.References
- J.-C. G. Bünzli and S. V. Eliseeva, in Lanthanide Luminescence, ed P. Hänninen and H. Härmä, Springer Berlin Heidelberg, 2011, pp. 1–45 Search PubMed.
- J.-C. G. Bünzli, On the design of highly luminescent lanthanide complexes, Coord. Chem. Rev., 2015, 293, 19–47 CrossRef.
- C. Alexander, Z. Guo, P. B. Glover, S. Faulkner and Z. Pikramenou, Luminescent Lanthanides in Biorelated Applications: From Molecules to Nanoparticles and Diagnostic Probes to Therapeutics, Chem. Rev., 2025, 125, 2269–2370 CrossRef CAS PubMed.
- M. Wang, Y. Kitagawa and Y. Hasegawa, Current Development of Lanthanide Complexes for Biomedical Applications, Chem.-Asian J., 2024, 19, e202400038 CrossRef CAS PubMed.
- E. Mathieu, A. Sipos, E. Demeyere, D. Phipps, D. Sakaveli and K. E. Borbas, Lanthanide-based tools for the investigation of cellular environments, Chem. Commun., 2018, 54, 10021–10035 RSC.
- G.-Q. Jin, Y. Ning, J.-X. Geng, Z.-F. Jiang, Y. Wang and J.-L. Zhang, Joining the journey to near infrared (NIR) imaging: the emerging role of lanthanides in the designing of molecular probes, Inorg. Chem. Front., 2020, 7, 289–299 Search PubMed.
- N. Hamon, A. Roux, M. Beyler, J.-C. Mulatier, C. Andraud, C. Nguyen, M. Maynadier, N. Bettache, A. Duperray, A. Grichine, S. Brasselet, M. Gary-Bobo, O. Maury and R. Tripier, Pyclen-Based Ln(III) Complexes as Highly Luminescent Bioprobes for In Vitro and In Vivo One- and Two-Photon Bioimaging Applications, J. Am. Chem. Soc., 2020, 142, 10184–10197 Search PubMed.
- R. Sánchez-Fernández, I. Obregon-Gomez, A. Sarmiento, M. E. Vázquez and E. Pazos, Luminescent lanthanide metallopeptides for biomolecule sensing and cellular imaging, Chem. Commun., 2024, 60, 12650–12661 RSC.
- D. Parker, J. D. Fradgley and K.-L. Wong, The design of responsive luminescent lanthanide probes and sensors, Chem. Soc. Rev., 2021, 50, 8193–8213 Search PubMed.
- M. Sy, A. Nonat, N. Hildebrandt and L. J. Charbonnière, Lanthanide-based luminescence biolabelling, Chem. Commun., 2016, 52, 5080–5095 RSC.
- J. M. Zwier, H. Bazin, L. Lamarque and G. Mathis, Luminescent Lanthanide Cryptates: from the Bench to the Bedside, Inorg. Chem., 2014, 53, 1854–1866 CrossRef CAS.
- M. C. Heffern, L. M. Matosziuk and T. J. Meade, Lanthanide Probes for Bioresponsive Imaging, Chem. Rev., 2014, 114, 4496–4539 CrossRef CAS PubMed.
- J. Wahsner, E. M. Gale, A. Rodríguez-Rodríguez and P. Caravan, Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers, Chem. Rev., 2019, 119, 957–1057 CrossRef CAS PubMed.
- P. Caravan, D. Esteban-Gómez, A. Rodríguez-Rodríguez and C. Platas-Iglesias, Water exchange in lanthanide complexes for MRI applications. Lessons learned over the last 25 years, Dalton Trans., 2019, 48, 11161–11180 RSC.
- P. Yue, T. Nagendraraj, G. Wang, Z. Jin and G. Angelovski, The role of responsive MRI probes in the past and the future of molecular imaging, Chem. Sci., 2024, 15, 20122–20154 RSC.
- M. Liu, J. Gao, Y. Zhang, X. Zhou, Y. Wang, L. Wu, Z. Tian and J.-H. Tang, Recent advances in bioresponsive macrocyclic gadolinium(III) complexes for MR imaging and therapy, Dalton Trans., 2025, 54, 6741–6777 RSC.
- M. V. C. Jordan, S.-T. Lo, S. Chen, C. Preihs, S. Chirayil, S. Zhang, P. Kapur, W.-H. Li, L. M. D. De Leon-Rodriguez, A. J. M. Lubag, N. M. Rofsky and A. D. Sherry, Zinc-sensitive MRI contrast agent detects differential release of Zn(II) ions from the healthy vs. malignant mouse prostate, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E5464–E5471 CrossRef.
- M. Isaac, A. Pallier, F. Szeremeta, P.-A. Bayle, L. Barantin, C. S. Bonnet and O. Sénèque, MRI and luminescence detection of Zn2+ with a lanthanide complex–zinc finger peptide conjugate, Chem. Commun., 2018, 54, 7350–7353 RSC.
- M. Sanadar, K. Zimmeter, H. Martin, A. Pallier, B. Vileno, P. Faller, A. Sour and C. S. Bonnet, Exploring Bioinspired Ln3+ Complexes for Cu2+ Detection: Design and Efficacy as MRI Contrast Agents, Eur. J. Inorg. Chem., 2025, 28, e202500049 Search PubMed.
- C. Moreau, T. Lukačević, A. Pallier, J. Sobilo, S. Aci-Sèche, N. Garnier, S. Même, É. Tóth and S. Lacerda, Peptide-Conjugated MRI Probe Targeted to Netrin-1, a Novel Metastatic Breast Cancer Biomarker, Bioconjugate Chem., 2024, 35, 265–275 CrossRef CAS.
- F. Oukhatar, S. Même, W. Même, F. Szeremeta, N. K. Logothetis, G. Angelovski and É. Tóth, MRI Sensing of Neurotransmitters with a Crown Ether Appended Gd3+ Complex, ACS Chem. Neurosci., 2015, 6, 219–225 CrossRef CAS PubMed.
- C. S. Bonnet and É. Tóth, Towards highly efficient, intelligent and bimodal imaging probes: Novel approaches provided by lanthanide coordination chemistry, C. R. Chim., 2010, 13, 700–714 CrossRef CAS.
- C. S. Bonnet, F. Buron, F. Caille, C. M. Shade, B. Drahos, L. Pellegatti, J. Zhang, S. Villette, L. Helm, C. Pichon, F. Suzenet, S. Petoud and E. Toth, Pyridine-Based Lanthanide Complexes Combining MRI and NIR Luminescence Activities, Chem.–Eur. J., 2012, 18, 1419–1431 Search PubMed.
- B. Song, J. Jiang, H. Yan, S. Huang and J. Yuan, A tumor-targetable probe based on europium(III)/gadolinium(III) complex-conjugated transferrin for dual-modal time-gated luminescence and magnetic resonance imaging of cancerous cells in vitro and in vivo, J. Mater. Chem. B, 2023, 11, 4346–4353 Search PubMed.
- S. Laurent, L. Vander Elst, C. Galaup, N. Leygue, S. Boutry, C. Picard and R. N. Muller, Bifunctional Gd(III) and Tb(III) chelates based on a pyridine–bis(iminodiacetate) platform, suitable optical probes and contrast agents for magnetic resonance imaging, Contrast Media Mol. Imaging, 2014, 9, 300–312 CrossRef CAS PubMed.
- J. He, C. S. Bonnet, S. V. Eliseeva, S. Lacerda, T. Chauvin, P. Retailleau, F. Szeremeta, B. Badet, S. Petoud, É. Tóth and P. Durand, Prototypes of Lanthanide(III) Agents Responsive to Enzymatic Activities in Three Complementary Imaging Modalities: Visible/Near-Infrared Luminescence, PARACEST-, and T1-MRI, J. Am. Chem. Soc., 2016, 138, 2913–2916 CrossRef CAS.
- Y. Zhang, X. Ma, H.-F. Chau, W. Thor, L. Jiang, S. Zha, W.-Y. Fok, H.-N. Mak, J. Zhang, J. Cai, C.-F. Ng, H. Li, D. Parker, L. Li, G.-L. Law and K.-L. Wong, Lanthanide–Cyclen–Camptothecin Nanocomposites for Cancer Theranostics Guided by Near-Infrared and Magnetic Resonance Imaging, ACS Appl. Nano Mater., 2021, 4, 271–278 CrossRef CAS.
- T.-L. Cheung, L. K. B. Tam, W.-S. Tam, L. Zhang, H.-Y. Kai, W. Thor, Y. Wu, P.-L. Lam, Y.-H. Yeung, C. Xie, H.-F. Chau, W.-S. Lo, T. Zhang and K.-L. Wong, Facile Peptide Macrocyclization and Multifunctionalization via Cyclen Installation, Small Methods, 2024, e2400006 CrossRef.
- R. Jouclas, S. Laine, S. V. Eliseeva, J. Mandel, F. Szeremeta, P. Retailleau, J. He, J.-F. Gallard, A. Pallier, C. S. Bonnet, S. Petoud, P. Durand and É. Tóth, Lanthanide-Based Probes for Imaging Detection of Enzyme Activities by NIR Luminescence, T1- and ParaCEST MRI, Angew. Chem., Int. Ed., 2024, 63, e202317728 CrossRef CAS.
- C. A. Foster, D. Sneddon, L. Hacker, E. T. Sarson, M. Robertson, D. Sokolova, L. A. W. Martin, M. F. Allen, A. Khrapichev, K. A. Vincent, E. M. Hammond, S. J. Conway and S. Faulkner, LnDOTA Releasing Probes for Luminescence and Magnetic Resonance Imaging, Inorg. Chem., 2025, 64, 6640–6647 CrossRef CAS.
- B. Woolley, Y. Wu, L. Xiong, H.-F. Chau, J. Zhang, G.-L. Law, K.-L. Wong and N. J. Long, Lanthanide–tetrazine probes for bio-imaging and click chemistry, Chem. Sci., 2025, 16, 3588–3597 RSC.
- S. I. Weissman, Intramolecular Energy Transfer: The Fluorescence of Complexes of Europium, J. Chem. Phys., 1942, 10, 214–217 CrossRef CAS.
- M. Pawlicki, H. A. Collins, R. G. Denning and H. L. Anderson, Two-Photon Absorption and the Design of Two-Photon Dyes, Angew. Chem., Int. Ed., 2009, 48, 3244–3266 Search PubMed.
- G. Piszczek, B. P. Maliwal, I. Gryczynski, J. Dattelbaum and J. R. Lakowicz, Multiphoton ligand-enhanced excitation of lanthanides, J. Fluoresc., 2001, 11, 101–107 CrossRef CAS PubMed.
- M. H. V. Werts, N. Nerambourg, D. Pélégry, Y. L. Grand and M. Blanchard-Desce, Action cross sections of two-photon excited luminescence of some Eu(III) and Tb(III) complexes, Photochem. Photobiol. Sci., 2005, 4, 531–538 Search PubMed.
- A. Picot, A. D'Aleo, P. L. Baldeck, A. Grichine, A. Duperray, C. Andraud and O. Maury, Long-lived two-photon excited luminescence of water-soluble europium complex: Applications in biological imaging using two-photon scanning microscopy, J. Am. Chem. Soc., 2008, 130, 1532–1533 CrossRef CAS PubMed.
- A. D’Aléo, A. Bourdolle, S. Brustlein, T. Fauquier, A. Grichine, A. Duperray, P. L. Baldeck, C. Andraud, S. Brasselet and O. Maury, Ytterbium-Based Bioprobes for Near-Infrared Two-Photon Scanning Laser Microscopy Imaging, Angew. Chem., Int. Ed., 2012, 51, 6622–6625 CrossRef PubMed.
- R. Lengacher, K. E. Martin, D. Śmiłowicz, H. Esseln, P. Lotlikar, A. Grichine, O. Maury and E. Boros, Targeted, Molecular Europium(III) Probes Enable Luminescence-Guided Surgery and 1 Photon Post-Surgical Luminescence Microscopy of Solid Tumors, J. Am. Chem. Soc., 2023, 145, 24358–24366 CrossRef CAS.
- J.-H. Choi, G. Fremy, T. Charnay, N. Fayad, J. Pécaut, S. Erbek, N. Hildebrandt, V. Martel-Frachet, A. Grichine and O. Sénèque, Luminescent Peptide/Lanthanide(III) Complex Conjugates with Push–Pull Antennas: Application to One- and Two-Photon Microscopy Imaging, Inorg. Chem., 2022, 61, 20674–20689 Search PubMed.
- K. P. Malikidogo, T. Charnay, D. Ndiaye, J.-H. Choi, L. Bridou, B. Chartier, S. Erbek, G. Micouin, A. Banyasz, O. Maury, V. Martel-Frachet, A. Grichine and O. Sénèque, Efficient cytosolic delivery of luminescent lanthanide bioprobes in live cells for two-photon microscopy, Chem. Sci., 2024, 15, 9694–9702 RSC.
- J.-H. Choi, A. Nhari, T. Charnay, B. Chartier, L. Bridou, G. Micouin, O. Maury, A. Banyasz, S. Erbek, A. Grichine, V. Martel-Frachet, F. Thomas, J. K. Molloy and O. Sénèque, Carbazole-Based Eu3+ Complexes for Two-Photon Microscopy Imaging of Live Cells, Inorg. Chem., 2025, 64, 2006–2019 Search PubMed.
- B. Chartier, A. Grichine, L. Bridou, A. Nhari, G. Micouin, A. Banyasz, D. Boturyn, J. K. Molloy, S. Erbek, V. Martel-Frachet, O. Maury and O. Sénèque, Amido/alkoxy–aryl–aryl–picolinate push–pull antennas for two-photon sensitization of Eu3+ luminescence, Inorg. Chem. Front., 2025, 12, 3313–3323 RSC.
- L. Bridou, L. Collobert, K. P. Malikidogo, S. R. Kiraev, M. Hojorat, N. Hamon, A. T. Bui, F. Riobé, A. Banyasz, M. Beyler, R. Tripier, O. Sénèque and O. Maury, Exploring Photophysical Properties and Sensitivity to Oxygen in Anisolyl-Picolinate Antenna Conjugated to Azamacrocycles, Inorg. Chem., 2025, 64, 13635–13646 CrossRef CAS PubMed.
- A. Beeby, L. M. Bushby, D. Maffeo and J. a. G. Williams, Intramolecular sensitisation of lanthanide(III) luminescence by acetophenone-containing ligands: the critical effect of para-substituents and solvent, J. Chem. Soc.-Dalton Trans., 2002, 48–54 Search PubMed.
- M. Tropiano, A. M. Kenwright and S. Faulkner, Lanthanide Complexes of Azidophenacyl-DO3A as New Synthons for Click Chemistry and the Synthesis of Heterometallic Lanthanide Arrays, Chem.–Eur. J., 2015, 21, 5697–5699 Search PubMed.
- J. D. Routledge, M. W. Jones, S. Faulkner and M. Tropiano, Kinetically Stable Lanthanide Complexes Displaying Exceptionally High Quantum Yields upon Long-Wavelength Excitation: Synthesis, Photophysical Properties, and Solution Speciation, Inorg. Chem., 2015, 54, 3337–3345 CrossRef CAS PubMed.
- L. Leone, D. Esteban-Gómez, C. Platas-Iglesias, M. Milanesio and L. Tei, Accelerating water exchange in GdIII–DO3A-derivatives by favouring the dissociative mechanism through hydrogen bonding, Chem. Commun., 2019, 55, 513–516 Search PubMed.
- L. Leone, S. Camorali, A. Freire-García, C. Platas-Iglesias, D. E. Gomez and L. Tei, Scrutinising the role of intramolecular hydrogen bonding in water exchange dynamics of Gd(III) complexes, Dalton Trans., 2021, 50, 5506–5518 RSC.
- B. Jagadish, G. L. Brickert-Albrecht, G. S. Nichol, E. A. Mash and N. Raghunand, On the synthesis of 1,4,7-tris(tert-butoxycarbonylmethyl)-1,4,7,10-tetraazacyclododecane, Tetrahedron Lett., 2011, 52, 2058–2061 CrossRef CAS PubMed.
- A. Beeby, I. M. Clarkson, R. S. Dickins, S. Faulkner, D. Parker, L. Royle, A. S. de Sousa, J. A. G. Williams and M. Woods, Non-radiative deactivation of the excited states of europium, terbium and ytterbium complexes by proximate energy-matched OH, NH and CH oscillators: an improved luminescence method for establishing solution hydration states, J. Chem. Soc., Perkin Trans., 1999, 2, 493–504 Search PubMed.
- S. Mizzoni, S. Ruggieri, A. Sickinger, F. Riobé, L. Guy, M. Roux, G. Micouin, A. Banyasz, O. Maury, B. Baguenard, A. Bensalah-Ledoux, S. Guy, A. Grichine, X.-N. Nguyen, A. Cimarelli, M. Sanadar, A. Melchior and F. Piccinelli, Circularly polarized activity from two photon excitable europium and samarium chiral bioprobes, J. Mater. Chem. C, 2023, 11, 4188–4202 RSC.
- D. Delli Castelli, M. C. Caligara, M. Botta, E. Terreno and S. Aime, Combined High Resolution NMR and 1H and 17O Relaxometric Study Sheds Light on the Solution Structure and Dynamics of the Lanthanide(III) Complexes of HPDO3A, Inorg. Chem., 2013, 52, 7130–7138 CrossRef CAS PubMed.
- S. Zhang, Z. Kovacs, S. Burgess, S. Aime, E. Terreno and A. D. Sherry, DOTA-bis(amide)lanthanide Complexes: NMR Evidence for Differences in Water-Molecule Exchange Rates for Coordination Isomers, Chem.–Eur. J., 2001, 7, 288–296 CrossRef CAS.
- A. Pagoto, R. Stefania, F. Garello, F. Arena, G. Digilio, S. Aime and E. Terreno, Paramagnetic Phospholipid-Based Micelles Targeting VCAM-1 Receptors for MRI Visualization of Inflammation, Bioconjugate Chem., 2016, 27, 1921–1930 CrossRef CAS.
- S. Aime, M. Botta, Z. Garda, B. E. Kucera, G. Tircso, V. G. Young and M. Woods, Properties, Solution State Behavior, and Crystal Structures of Chelates of DOTMA, Inorg. Chem., 2011, 50, 7955–7965 Search PubMed.
- P. H. Fries and E. Belorizky, Electronic relaxation of paramagnetic metal ions and NMR relaxivity in solution: Critical analysis of various approaches and application to a Gd(III)-based contrast agent, J. Chem. Phys., 2005, 123, 124510 Search PubMed.
- P. Miéville, H. Jaccard, F. Reviriego, R. Tripier and L. Helm, Synthesis, complexation and NMR relaxation properties of Gd3+ complexes of Mes(DO3A)3, Dalton Trans., 2011, 40, 4260–4267 RSC.
- K. N. Green, S. Viswanathan, F. A. Rojas-Quijano, Z. Kovacs and A. D. Sherry, Europium(III) DOTA-Derivatives Having Ketone Donor Pendant Arms Display Dramatically Slower Water Exchange, Inorg. Chem., 2011, 50, 1648–1655 CrossRef CAS PubMed.
- É. Tóth, L. Helm and A. Merbach, in The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, ed A. Merbach, L. Helm and É. Tóth, John Wiley & Sons, Ltd, 2013, pp. 25–81 Search PubMed.
- E. Brücher, G. Tircsó, Z. Baranyai, Z. Kovács and A. D. Sherry, in The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, John Wiley and Sons, 2013, pp. 157–208 Search PubMed.
- Z. Baranyai, Z. Pálinkás, F. Uggeri, A. Maiocchi, S. Aime and E. Brücher, Dissociation Kinetics of Open-Chain and Macrocyclic Gadolinium(III)-Aminopolycarboxylate Complexes Related to Magnetic Resonance Imaging: Catalytic Effect of Endogenous Ligands, Chem.–Eur. J., 2012, 18, 16426–16435 CrossRef CAS.
- A. Takács, R. Napolitano, M. Purgel, A. C. Bényei, L. Zékány, E. Brücher, I. Tóth, Z. Baranyai and S. Aime, Solution Structures, Stabilities, Kinetics, and Dynamics of DO3A and DO3A–Sulphonamide Complexes, Inorg. Chem., 2014, 53, 2858–2872 CrossRef PubMed.
- M. Regueiro-Figueroa, B. Bensenane, E. Ruscsak, D. Esteban-Gomez, L. J. Charbonniere, G. Tircso, I. Toth, A. de Blas, T. Rodriguez-Blas and C. Platas-Iglesias, Lanthanide dota-like Complexes Containing a Picolinate Pendant: Structural Entry for the Design of Ln(III)-Based Luminescent Probes, Inorg. Chem., 2011, 50, 4125–4141 CrossRef CAS.
- É. Tóth, R. Király, J. Platzek, B. Radüchel and E. Brücher, Equilibrium and kinetic studies on complexes of 10-[2,3-dihydroxy-(1-hydroxymethyl)-propyl]-1,4,7,10-tetraazacyclododecane-1,4,7-triacetate, Inorganica Chim. Acta, 1996, 249, 191–199 CrossRef.
- R. B. Lauffer, D. J. Parmelee, S. U. Dunham, H. S. Ouellet, R. P. Dolan, S. Witte, T. J. McMurry and R. C. Walovitch, MS-325: albumin-targeted contrast agent for MR angiography, Radiology, 1998, 207, 529–538 CrossRef CAS.
- B. Bauer, A. Mally and D. Liedtke, Zebrafish Embryos and Larvae as Alternative Animal Models for Toxicity Testing, Int.J. Mol. Sci., 2021, 22, 13417 CrossRef CAS PubMed.
- T.-Y. Choi, T.-I. Choi, Y.-R. Lee, S.-K. Choe and C.-H. Kim, Zebrafish as an animal model for biomedical research, Exp. Mol. Med., 2021, 53, 310–317 Search PubMed.
- S.-K. Ko, X. Chen, J. Yoon and I. Shin, Zebrafish as a good vertebrate model for molecular imaging using fluorescent probes, Chem. Soc. Rev., 2011, 40, 2120–2130 Search PubMed.
- J. Zheng, Q. Zhan, L. Jiang, D. Xing, T. Zhang and K.-L. Wong, A bioorthogonal time-resolved luminogenic probe for metabolic labelling and imaging of glycans, Inorg. Chem. Front., 2020, 7, 4062–4069 RSC.
- E. Vivès, P. Brodin and B. Lebleu, A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus, J. Biol. Chem., 1997, 272, 16010–16017 CrossRef PubMed.
- J. Allen and J.-P. Pellois, Hydrophobicity is a key determinant in the activity of arginine-rich cell penetrating peptides, Sci. Rep., 2022, 12, 15981 CrossRef CAS PubMed.
- A. Erazo-Oliveras, K. Najjar, L. Dayani, T.-Y. Wang, G. A. Johnson and J.-P. Pellois, Protein delivery into live cells by incubation with an endosomolytic agent, Nat. Methods, 2014, 11, 861–867 Search PubMed.
- M. Serulla, P. Anees, A. Hallaj, E. Trofimenko, T. Kalia, Y. Krishnan and C. Widmann, Plasma membrane depolarization reveals endosomal escape incapacity of cell-penetrating peptides, Eur. J. Pharm. Biopharm., 2023, 184, 116–124 CrossRef CAS PubMed.
- A. Grichine, A. Haefele, S. Pascal, A. Duperray, R. Michel, C. Andraud and O. Maury, Millisecond lifetime imaging with a europium complex using a commercial confocal microscope under one or two-photon excitation, Chem. Sci., 2014, 5, 3475–3485 RSC.
- B. Chartier, N. Hamon, D. Akl, A. Sickinger, L. Corne, G. Micouin, A. Banyasz, O. Maury, S. Erbek, V. Martel-Frachet, A. Grichine, M. Beyler, O. Sénèque and R. Tripier, Clickable Pyclen-Based Luminescent Lanthanide Complexes: Application to Two-Photon Microscopy, Chem.–Eur. J., 2025, 31, e02044 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |