Open Access Article
Open Access ArticleSpinel-type Al4C3 attainable above 7 GPa and more high-pressure phases of Al4C3
Mitchell
Falgoust
and
Peter
Kroll
 *
*
Department of Chemistry and Biochemistry, The University of Texas at Arlington, 700 Planetarium Place, Arlington, Texas 76019, USA. E-mail: pkroll@uta.edu
First published on 4th December 2025
Abstract
We predict that Al4C3 adopts a cubic, anti-spinel-type structure (Al4C3-II) between 7 and 33 GPa, peaking in stability relative to other Al4C3 structures at 26 GPa. At ambient pressure, Al4C3-II is mechanically robust, with a bulk modulus of 160 GPa and a Vickers hardness of around 30 GPa. Beyond Al4C3-II, we identify three additional post-spinel phases appearing in the Al4C3 phase diagram, including an anti-Th3P4-type at 140 GPa. The clear enthalpy differences under pressure leave little doubt that the known trigonal R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m ground state of Al4C3 will undergo multiple phase transitions. The accessible pressure window for spinel-type Al4C3-II is easily accessible in both laser-heated diamond anvil cell and large-volume multi-anvil cell experiments. We therefore encourage experimental exploration of the Al–C system at high pressure.
m ground state of Al4C3 will undergo multiple phase transitions. The accessible pressure window for spinel-type Al4C3-II is easily accessible in both laser-heated diamond anvil cell and large-volume multi-anvil cell experiments. We therefore encourage experimental exploration of the Al–C system at high pressure.
Introduction
High-pressure chemistry is a rich field in materials research, and advanced synthesis techniques have delivered a variety of new compounds in recent times.1–4 A notable research method is the laser heating-diamond anvil cell (LH-DAC) used in conjunction with in situ X-ray diffraction (XRD).5–7 Modern LH-DAC equipment can explore temperatures and pressures above ∼5000 K and 300 GPa,8–10 and enables dynamic compression rates of several 100 GPa s−1.11–13 Larger volumes of material can be synthesized using Multi-Anvil-Cells (MAC) at pressures up to 50 GPa.14Computational methods are now a standard tool for characterizing synthesized materials and are essential for exploring the phase space and advancing experimental research at high pressure.15,16 For example, the spinel-type γ-Si3N4 was achieved at 15 GPa and 2000 K through a collaborative effort of computation and experiment.17 Recently, after more than three decades of effort, a crystal phase of C3N4 was synthesized at high pressure (>100 GPa).18–21
Hitherto, the only known polymorph of Al4C3 is the trigonal (R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) ground state structure.22–24 The high-pressure behavior of Al4C3 was explored up to 6 GPa at 300 K.25 Exploration at higher temperatures occurred only up to 8 GPa, but resulted in an incongruent thermal decomposition of Al4C3.26,27 Recently, M4C3 with anti-Th3P4 type structures have been synthesized for Dy4C3 at 19 GPa and for Sc4C3 at 10 GPa.28,29 Despite its simplicity, Al4C3 appears to have been overlooked,30,31 and our own efforts had been communicated but not published. However, its composition suggests it may display a similar rich high-pressure chemistry as Si3N4.32–38
m) ground state structure.22–24 The high-pressure behavior of Al4C3 was explored up to 6 GPa at 300 K.25 Exploration at higher temperatures occurred only up to 8 GPa, but resulted in an incongruent thermal decomposition of Al4C3.26,27 Recently, M4C3 with anti-Th3P4 type structures have been synthesized for Dy4C3 at 19 GPa and for Sc4C3 at 10 GPa.28,29 Despite its simplicity, Al4C3 appears to have been overlooked,30,31 and our own efforts had been communicated but not published. However, its composition suggests it may display a similar rich high-pressure chemistry as Si3N4.32–38
Results and discussion
While computing several hundred polymorphs with composition A4X3 at different pressures, we ultimately identified four high-pressure modifications of Al4C3, indexed with Roman numerals (II, III, IV, and V), that surpass the trigonal ground-state modification Al4C3-I. In sequence, these are an anti-spinel type, an anti-CaFe2O4-like orthorhombic modification, an anti-CaFe2O4-type, and an anti-Th3P4 type. Polyhedral structure depictions are shown in Fig. 1. | ||
| Fig. 1 The five phases of Al4C3 studied. Space groups and (average) coordination numbers (CN) of Al are indicated. Black spheres represent carbon; aluminum is depicted in colored polyhedra. | ||
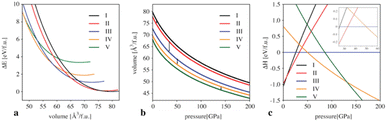
Ground state energies, lattice parameters, volumes, and bulk moduli of the structures are given in Table 1. The energy–volume, ΔE–V, diagram is shown in Fig. 2a, the corresponding pressure–volume diagram in Fig. 2b, and the enthalpy–pressure, ΔH–p, diagram is presented in Fig. 2c. Accordingly, the ground state modification will transform into the denser (anti-) spinel type of Al4C3-II at 7 GPa. This is a relatively low-pressure process that can be attained with various experimental equipment, including large-volume presses. The largest enthalpy difference of Al4C3-II to another phase, hence the maximum driving force for its formation, about 0.2 eV/Al4C3, is attained at 26 GPa. At 33 GPa, cubic Al4C3-II will be superseded by an orthorhombic structure that is related to the (anti-) CaFe2O4-type. This Al4C3-III remains favored up to 50 GPa, at which point it will transform into an (anti-) CaFe2O4-type. Finally, at pressures above 140 GPa, an (anti-) Th3P4-type of Al4C3-V will be the most favorable structure. This final structure resembles the high-pressure phases of Sc4C3 and Dy4C3, attained at 10 and 19 GPa, respectively.28,29 Hence, the larger trivalent cations of Sc and Dy attain the (anti-) Th3P4-type at much lower pressures than the smaller Al3+ – a trend commonly observed in high-pressure chemistry of elements and compounds, including sesquioxides and sesquisulfide.39–41 As expected, the densities of the Al4C3 polymorphs increase from phase I to V. Since the bulk moduli of the polymorphs are comparable, the initial slopes of the pressure–volume graphs (Fig. 2b) are very similar. That they remain so indicates a similar pressure dependence of the compressibility of the polymorphs. The average coordination number of Al4C3 polymorphs increases from phase I to V—except for the transition from the ground-state to the spinel-type structure (Fig. 1). This progression of phases thus reflects a systematic densification and coordination enhancement under pressure. The sequence of phase transformations, including transition pressure pt and volume change at pt, is summarized by
| Energy (eV/Al4C3) | Lattice parameters (Å) | Volume (Å3/Al4C3) | Bulk modulus (GPa) | |
|---|---|---|---|---|
| I | −62.89 | a = 3.33 | 79.64 | 172 |
| c = 24.88 | ||||
| II | −62.81 | a = 8.53 | 77.65 | 176 |
| III | −61.83 | a = 9.24 | 72.56 | 170 |
| b = 3.12 | ||||
| c = 10.08 | ||||
| IV | −61.02 | a = 8.79 | 69.61 | 171 |
| b = 3.03 | ||||
| c = 10.45 | ||||
| V | −59.56 | a = 6.48 | 68.00 | — |
At ambient pressure, the energy difference between the (anti-) spinel-type of Al4C3-II and the ground state modification Al4C3-I is only 80 meV/Al4C3. LDA calculations even place Al4C3-II below Al4C3-I by 23 meV/Al4C3, although both structures are built up by AlC4-tetrahedra only, and, thus, the number of nearest atoms (coordination number) of Al is identical.
The elastic constants of Al4C3 polymorphs (I–V) computed at zero pressure are shown in Table 2. Based on stability criteria,42,43 the structures of Al4C3-I–IV are mechanically stable at ambient pressure. Thus, the high-pressure phases may be recoverable. While Al4C3-V is mechanically stable at 140 GPa, it becomes unstable below ∼30 GPa. A possible distortion along a Bain strain path may transform it into the spinel-type Al4C3-II.33 The elastic constants can be used to compute the aggregate moduli, particularly the elastic shear modulus G. We obtain 123, 148, 70, and 110, for Al4C3-I–IV, respectively. Using the formula of Chen,44 we estimate the Vickers hardness of Al4C3-I to 22 GPa and that of spinel-type Al4C3-II to 30 GPa. All polymorphs of Al4C3 presented here are semi-conductors, with band gaps of 1.6, 1.4, 1.9, 2.2, and 2.5 eV (GGA values) for Al4C3-I–V, respectively.
| I | II | III | IV | V | |
|---|---|---|---|---|---|
| C11 | 347 | 342 | 306 | 351 | 110 |
| C22 | 347 | 342 | 424 | 510 | 110 |
| C33 | 397 | 342 | 455 | 407 | 110 |
| C44 | 111 | 168 | 17 | 65 | −966 |
| C55 | 116 | 168 | 17 | 53 | −966 |
| C66 | 116 | 168 | 151 | 141 | −966 |
| C12 | 124 | 94 | 65 | 76 | 134 |
| C13 | 55 | 94 | 78 | 44 | 134 |
| C14 | 14 | 0 | 0 | 0 | 0 |
| C23 | 55 | 94 | 47 | 44 | 134 |
Computational method
We started our search for potential high-pressure modifications of Al4C3 by screening models that we previously considered for Si3N4.45 The results were confirmed by evolutionary algorithms using the USPEX code,46–48 with a slight modification to Al4C3-III. All structures were computed using density functional theory (DFT), implemented with the Vienna Ab initio Simulations Package (VASP).49,50 We employed the projector augmented wave (PAW) formalism, along with the strongly constrained and approximately normed (SCAN) functional, for electron correlation and exchange.51–53 A plane wave energy cutoff of 500 eV was applied, and fine grids sampled the Brillouin zone with spacings of 0.030–0.040 Å−1.54 With these parameters, forces, and energies converged to within 5 meV Å−1 and 0.1 meV per atom, respectively. Throughout the work, we approximate Gibbs energy differences by enthalpy differences, hence ΔG ≈ ΔH. Potential contributions from defects, non-stoichiometry, or surface effects during growth may be factors that alter the thermodynamic balance through configurational or vibrational entropy. We assume that if these effects occur, they affect all structures similarly. Remaining entropy differences are, in general, much smaller in comparison to the much larger variation of ΔH within a few GPa of pressure. Justification for this common approach stems from calculations of the phase boundary between the β- and γ-phase of Si3N4, with γ-Si3N4 adopting the spinel-structure,55 and from the prediction of a synthesis of Hf3N4 with Th3P4-structure from the elements.56Conclusions
We predict a cubic (anti-) spinel-type of Al4C3 succeeding the known trigonal ground state modification at higher pressures. While Al4C3-II is 0.08 meV/Al4C3 above the ground state at ambient pressure, it becomes thermodynamically favored between 7 and 33 GPa with a maximum enthalpy difference (hence, driving force) of 0.2 eV f.u.−1 at 26 GPa. Al4C3-II is also stable against decomposition into the elements, by 1.8 and 1.7 eV/Al4C3 at ambient pressure and 30 GPa, respectively. The reported energy differences are well above the “uncertainty” of DFT calculations for enthalpies of formation differences, about 10 meV per atom.57 The proposed stability range of spinel-type Al4C3 is accessible with large-volume presses58 or LH-DAC.5 However, high temperatures may be required to facilitate the transition. Previous experiments examined pressures up to 8 GPa and maintained temperatures below 2500 K.26,27 Al4C3-II is mechanically stable at ambient pressure, with a bulk modulus of 160 GPa comparable to that of Fe3C or ThC.59,60 The hardness of Al4C3-II will be substantially higher than that of the known modification, we estimate Hv ≈ 30 GPa for Al4C3-II.Beyond Al4C3-II, the phase diagram of Al4C3 features three post-spinel modifications. Several other materials also exhibit post-spinel modifications, including CaFe2O4, ZnGa2O4, and CdCr2Se4.61–66 For Si3N4, such phases were also proposed,45,61 but ultimately a pernitride SiN2 with N24−-units emerged. Motivated by this analogy, we explored a series of Al–C compounds with C2-dimers in various oxidation states (C22−, C24−, and C26−), but none yielded a thermodynamically stable polymorph. In addition to Al4C3-I to Al4C3-V, we also identified several candidate structures of Al4C3 that, at certain pressures, approach the stability of the thermodynamically most favorable structure shown here. These include types related to SrPb2O4, P4S3, and further variants of the CaFe2O4-type. While our calculations neglect possible contributions from defects, non-stoichiometry, or surface effects – factors that could alter the thermodynamic balance through configurational or vibrational entropy – it is evident that Al4C3 will undergo pressure-induced phase transitions. We therefore encourage experimental efforts to synthesize the predicted Al4C3-II modification and advance the high-pressure chemistry of metal carbides.
Author contributions
Mitchell Falgoust: writing – review & editing, writing – original draft, visualization, methodology, investigation, formal analysis, data curation. Peter Kroll: writing – review & editing, writing – original draft, visualization, supervision, resources, project administration, methodology, investigation, funding acquisition, formal analysis, data curation, conceptualization.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting the article is available from the corresponding author upon reasonable request.Acknowledgements
This work used Stampede3 at Texas Advanced Computing Center (TACC) through allocation DMR190103 from the Advanced Cyberinfrastructure Coordination Ecosystem: Services & Support (ACCESS) program, which is supported by National Science Foundation grants #2138259, #2138286, #2138307, #2137603, and #2138296. Additional computational work was made possible by the High-Performance Computing facilities at the University of Texas at Arlington. MF was supported through a Graduate Dean Research Assistance fellowship.References
- J. V. Badding, High-Pressure Synthesis, Characterization, and Tuning of Solid State Materials, Annu. Rev. Mater. Res., 1998, 28, 631–658 CrossRef CAS.
- Y. Fei and M. J. Walter, Static and Dynamic High Pressure Mineral Physics, Cambridge University Press, Cambridge, 2022 Search PubMed.
- W. Zhao, J. Zhang, Z. Sun, G. Xiao, H. Zheng, K. Li, M.-R. Li and B. Zou, Chemical Synthesis Driven by High Pressure, CCS Chem., 2025, 7, 1250–1271 CrossRef CAS.
- S. Guo, Y. Zhang, K. Bu, Y. Zhan and X. Lü, High-Pressure Chemistry of Functional Materials, Chem. Commun., 2025, 61, 1773–1789 RSC.
- A. Jayaraman, Diamond Anvil Cell and High-Pressure Physical Investigations, Rev. Mod. Phys., 1983, 55, 65 CrossRef CAS.
- W. A. Bassett, Diamond Anvil Cell, 50th Birthday, High Pressure Res., 2009, 29, Cp5–Cp186 CrossRef.
- M. E. Alabdulkarim, W. D. Maxwell, V. Thapliyal and J. L. Maxwell, A Comprehensive Review of High-Pressure Laser-Induced Materials Processing, Part I: Laser-Heated Diamond Anvil Cells, J. Manuf. Mater. Process., 2022, 6, 111 Search PubMed.
- L. R. Benedetti and P. Loubeyre, Temperature Gradients, Wavelength-Dependent Emissivity, and Accuracy of High and Very-High Temperatures Measured in the Laser-Heated Diamond Cell, High Pressure Res., 2004, 24, 423–445 CrossRef CAS.
- S. Tateno, K. Hirose, Y. Ohishi and Y. Tatsumi, The Structure of Iron in Earth's Inner Core, Science, 2010, 330, 359–361 CrossRef CAS PubMed.
- N. Dubrovinskaia, et al., Terapascal Static Pressure Generation with Ultrahigh Yield Strength Nanodiamond, Sci. Adv., 2016, 2, e1600341 CrossRef PubMed.
- M. Ricks, A. E. Gleason, F. Miozzi, H. Yang, S. Chariton, V. B. Prakapenka, S. Sinogeikin, R. L. Sandberg, W. L. Mao and S. Pandolfi, Phase Transition Kinetics Revealed by in Situ X-Ray Diffraction in Laser-Heated Dynamic Diamond Anvil Cells, Phys. Rev. Res., 2024, 6 DOI:10.1103/PhysRevResearch.6.0133316.
- L. Q. Huston, L. Miyagi, R. J. Husband, K. Glazyrin, C. Kiessner, M. Wendt, H. P. Liermann and B. T. Sturtevant, New Dynamic Diamond Anvil Cell for Time-Resolved Radial X-Ray Diffraction, Rev. Sci. Instrum., 2024, 95, 043904 CrossRef CAS PubMed.
- W. J. Evans, C. S. Yoo, G. W. Lee, H. Cynn, M. J. Lipp and K. Visbeck, Dynamic Diamond Anvil Cell (Ddac): A Novel Device for Studying the Dynamic-Pressure Properties of Materials, Rev. Sci. Instrum., 2007, 78, 073904 CrossRef PubMed.
- J.-a. Xu and H.-k. Mao, Moissanite: A Window for High-Pressure Experiments, Science, 2000, 290, 783–785 CrossRef CAS PubMed.
- N. P. Salke, et al., Prediction and Synthesis of Dysprosium Hydride Phases at High Pressure, Inorg. Chem., 2020, 59, 5303–5312 CrossRef CAS PubMed.
- P. V. Marshall, S. D. Thiel, E. E. Cote, R. Hrubiak, M. L. Whitaker, Y. Meng and J. P. S. Walsh, Combined First-Principles and Experimental Investigation into the Reactivity of Codeposited Chromium-Carbon under Pressure, ACS Mater. Au, 2024, 4, 393–402 CrossRef CAS PubMed.
- A. Zerr, G. Miehe, G. Serghiou, M. Schwarz, E. Kroke, R. Riedel, H. Fuess, P. Kroll and R. Boehler, Synthesis of Cubic Silicon Nitride, Nature, 1999, 400, 340–342 CrossRef CAS.
- A. Y. Liu and M. L. Cohen, Prediction of New Low Compressibility Solids, Science, 1989, 245, 841–842 CrossRef CAS PubMed.
- P. V. Zinin, L. C. Ming, S. K. Sharma, S. M. Hong, Y. Xie, T. Irifune and T. Shinmei, Synthesis of New Cubic C3N4 and Diamond-Like BC3 Phases under High Pressure and High Temperature, in Joint 21st AIRAPT and 45th EHPRG International Conference on High Pressure Science and Technology, 2008, vol. 121 Search PubMed.
- D. Laniel, et al., Synthesis of Ultra-Incompressible and Recoverable Carbon Nitrides Featuring CN4 Tetrahedra, Adv. Mater., 2024, 36, 2308030 CrossRef CAS PubMed.
- D. Laniel, et al., High-Pressure Synthesis of Op28-C3N4 Recoverable to Ambient Conditions, Adv. Funct. Mater., 2025, 35, 2416892 CrossRef CAS.
- M. von Stackelberg and E. Schorrenberg, The Structure of Aluminium Carbide Al4C3, Z. Phys. Chem. B, 1934, 27, 37 Search PubMed.
- J. H. Cox and L. M. Pidgeon, The X-Ray Diffraction Patterns of Aluminum Carbide Al4C3 and Aluminum Oxycarbide Al4O4C, Can. J. Chem., 1963, 41, 1414–1416 CrossRef CAS.
- T. M. Gesing and W. Jeitschko, The Crystal Structure and Chemical Properties of U2Al3C4 and Structure Refinement of Al4C3, Z. Naturforsch., B: J. Chem. Sci., 1995, 50, 196–200 CrossRef CAS.
- V. L. Solozhenko and O. O. Kurakevych, Equation of State of Aluminum Carbide Al4C3, Solid State Commun., 2005, 133, 385–388 CrossRef CAS.
- J. C. Schuster, A Reinvestigation of the Thermal Decomposition of Aluminum Carbide and the Constitution of the Al-C System, J. Phase Equilib., 1991, 12, 546–549 CrossRef CAS.
- V. Turkevich, A. Garan, O. Kulik and I. Petrusha, Phase Diagram and Diamond Synthesis in the Aluminum–Carbon System at a Pressure of 8 GPa, Innovative Superhard Materials and Sustainable Coatings for Advanced Manufacturing, 2005, pp. 335–343 Search PubMed.
- E. A. Juarez-Arellano, et al., Formation of Scandium Carbides and Scandium Oxycarbide from the Elements at High-(P, T) Conditions, J. Solid State Chem., 2010, 183, 975–983 CrossRef CAS.
- F. I. Akbar, et al., High-Pressure Synthesis of Dysprosium Carbides, Front. Chem., 2023, 11 DOI:10.3389/fchem.2023.1210081.
- L. Sun, Y. Gao, K. Yoshida, T. Yano and W. Wang, Prediction on Structural, Mechanical and Thermal Properties of Al4SiC4, Al4C3 and 4H-SiC under High Pressure by First-Principles Calculation, Mod. Phys. Lett. B, 2017, 31, 1750080 CrossRef CAS.
- A. Pisch, A. Pasturel, G. Deffrennes, O. Dezellus, P. Benigni and G. Mikaelian, Investigation of the Thermodynamic Properties of Al4C3: A Combined DFT and DSC Study, Comput. Mater. Sci., 2020, 171, 109100 CrossRef CAS.
- E. Kroke, High-Pressure Syntheses of Novel Binary Nitrogen Compounds of Main Group Elements, Angew. Chem., Int. Ed., 2002, 41, 77–82 CrossRef CAS PubMed.
- P. Kroll, Pathways to Metastable Nitride Structures, J. Solid State Chem., 2003, 176, 530–537 CrossRef CAS.
- P. Kroll and M. Milko, Theoretical Investigation of the Solid State Reaction of Silicon Nitride and Silicon Dioxide Forming Silicon Oxynitride (Si2N2O) under Pressure, Z. Anorg. Allg. Chem., 2003, 629, 1737–1750 CrossRef CAS.
- E. Horvath-Bordon, R. Riedel, A. Zerr, P. F. McMillan, G. Auffermann, Y. Prots, W. Bronger, R. Kniep and P. Kroll, High-Pressure Chemistry of Nitride-Based Materials, Chem. Soc. Rev., 2006, 35, 987–1014 RSC.
- B. H. Yu and D. Chen, Phase Transition Characters and Thermodynamics Modeling of the Newly-Discovered Wii- and Post-Spinel Si3N4 Polymorphs: A First-Principles Investigation, Acta Metall. Sin. (Engl. Lett.), 2013, 26, 131–136 CrossRef CAS.
- L. Cui, M. Hu, Q. Q. Wang, B. Xu, D. L. Yu, Z. Y. Liu and J. L. He, Prediction of Novel Hard Phases of Si3N4: First-Principles Calculations, J. Solid State Chem., 2015, 228, 20–26 CrossRef CAS.
- Q. H. Wu, Z. T. Huo, C. Chen, X. Q. Li, Z. Wang, C. J. Wang, L. J. Zhang, Y. F. Gao, M. Xiong and K. M. Pan, Prediction of Four Si3N4 Compounds by First-Principles Calculations, AIP Adv., 2023, 13 DOI:10.1063/5.0130194.
- A. Mujica, A. Rubio, A. Muñoz and R. J. Needs, High-Pressure Phases of Group-IV, III–V, and II–VI Compounds, Rev. Mod. Phys., 2003, 75, 863–912 CrossRef CAS.
- L. Liu and W. A. Bassett, Elements, Oxides, Silicates: High Pressure Phases with Implications for the Earth's Interior, Oxford University Press, New York, 1986, p. 250 Search PubMed.
- F. J. Manjón and D. Errandonea, Pressure-Induced Structural Phase Transitions in Materials and Earth Sciences, Phys. Status Solidi B, 2009, 246, 9–31 CrossRef.
- J. F. Nye, Physical Properties of Crystals: Their Representation by Tensors and Matrices, Oxford University Press, 1985 Search PubMed.
- F. Mouhat and F.-X. Coudert, Necessary and Sufficient Elastic Stability Conditions in Various Crystal Systems, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 224104 CrossRef.
- X.-Q. Chen, H. Niu, D. Li and Y. Li, Modeling Hardness of Polycrystalline Materials and Bulk Metallic Glasses, Intermetallics, 2011, 19, 1275–1281 CrossRef CAS.
- P. Kroll and J. von Appen, Post-Spinel Phases of Silicon Nitride, Phys. Status Solidi B, 2001, 226, R6–R7 CrossRef CAS.
- A. R. Oganov and C. W. Glass, Crystal Structure Prediction Using Ab Initio Evolutionary Techniques: Principles and Applications, J. Chem. Phys., 2006, 124, 244704 CrossRef PubMed.
- A. R. Oganov, A. O. Lyakhov and M. Valle, How Evolutionary Crystal Structure Prediction Works—and Why, Acc. Chem. Res., 2011, 44, 227–237 CrossRef CAS PubMed.
- A. O. Lyakhov, A. R. Oganov, H. T. Stokes and Q. Zhu, New Developments in Evolutionary Structure Prediction Algorithm USPEX, Comput. Phys. Commun., 2013, 184, 1172–1182 CrossRef CAS.
- P. Hohenberg and W. Kohn, Inhomogeneous Electron Gas, Phys. Rev., 1964, 136, B864–B871 CrossRef.
- W. Kohn and L. J. Sham, Self-Consistent Equations Including Exchange and Correlation Effects, Phys. Rev., 1965, 140, A1133–A1138 CrossRef.
- G. Kresse and J. Furthmüller, Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- G. Kresse and D. Joubert, From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- J. Sun, A. Ruzsinszky and J. P. Perdew, Strongly Constrained and Appropriately Normed Semilocal Density Functional, Phys. Rev. Lett., 2015, 115, 036402 CrossRef PubMed.
- H. J. Monkhorst and J. D. Pack, Special Points for Brillouin-Zone Integrations, Phys. Rev. B, 1976, 13, 5188–5192 CrossRef.
- A. Togo and P. Kroll, First-Principles Lattice Dynamics Calculations of the Phase Boundary between β-Si3N4 and γ-Si3N4 at Elevated Temperatures and Pressures, J. Comput. Chem., 2008, 29, 2255–2259 CrossRef CAS PubMed.
- P. Kroll, Hafnium Nitride with Thorium Phosphide Structure: Physical Properties and an Assessment of the Hf-N, Zr-N, and Ti-N Phase Diagrams at High Pressures and Temperatures, Phys. Rev. Lett., 2003, 90, 125501 CrossRef PubMed.
- A. Wang, R. Kingsbury, M. McDermott, M. Horton, A. Jain, S. P. Ong, S. Dwaraknath and K. A. Persson, A Framework for Quantifying Uncertainty in DFT Energy Corrections, Sci. Rep., 2021, 11, 15496 CrossRef CAS PubMed.
- L. G. Khvostantsev and V. N. Slesarev, Large-Volume High-Pressure Devices for Physical Investigations, Phys.-Usp., 2008, 51, 1099–1104 CrossRef.
- M. Umemoto and H. Ohtsuka, Mechanical Properties of Cementite, ISIJ Int., 2022, 62, 1313–1333 CrossRef CAS.
- C. Yu, et al., Structural Phase Transition of ThC under High Pressure, Sci. Rep., 2017, 7, 96 CrossRef PubMed.
- A. Zerr, A New High-Pressure Delta-Phase of Si3N4, Phys. Status Solidi B, 2001, 227, R4–R6 CrossRef CAS.
- W. Xu, G. Hearne, S. Layek, D. Levy, M. Pasternak, G. K. Rozenberg and E. Greenberg, Interplay between Structural and Magnetic-Electronic Responses of FeAl2O4 to a Megabar: Site Inversion and Spin Crossover, Phys. Rev. B, 2018, 97, 085120 CrossRef CAS.
- M. Sahu, Pressure-induced volume collapse and metallization in inverse spinel Co2TiO4, J. Phys.: Condens. Matter., 2025, 37, 345402 CrossRef CAS PubMed.
- I. Efthimiopoulos, Z. T. Y. Liu, M. Kucway, S. V. Khare, P. Sarin, V. Tsurkan, A. Loidl and Y. Wang, Pressure-Induced Phase Transitions in the CdCr2Se4 Spinel, Phys. Rev. B, 2016, 94, 174106 CrossRef.
- D. Errandonea, R. S. Kumar, Y. Ma and C. Y. Tu, High-Pressure Structural Stability of Spinel-Type ZnGa2O4: A Synchrotron X-Ray Diffraction Study, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 024103 CrossRef.
- T. Yamanaka, A. Uchida and Y. Nakamoto, Structural Transition of Post-Spinel Phases CaMn2O4, CaFe2O4, and CaTi2O4 under High Pressures up to 80 GPa, Am. Mineral., 2008, 93, 1874–1881 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |