Open Access Article
Open Access ArticleBoraindenes as versatile precursors to benzannulated boron heterocycles
Nele
Wieprecht
ab,
Ivo
Krummenacher
 ab,
Leonie
Wüst
ab,
Maximilian
Michel
ab,
Marco
Neder
ab,
Andreas
Häfner
ab,
Jordan
Karg
ab,
Leonie
Wüst
ab,
Maximilian
Michel
ab,
Marco
Neder
ab,
Andreas
Häfner
ab,
Jordan
Karg
 ab and
Holger
Braunschweig
ab and
Holger
Braunschweig
 *ab
*ab
aInstitute for Inorganic Chemistry, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany. E-mail: h.braunschweig@uni-wuerzburg.de
bInstitute for Sustainable Chemistry & Catalysis with Boron, Julius-Maximilians-Universität Würzburg, Am Hubland, 97074 Würzburg, Germany
First published on 2nd December 2025
Abstract
Five-membered boroles and their doubly benzannulated 9-borafluorene derivatives are established precursors for synthesizing various boron-doped heterocycles of broad applied interest. Extending this chemistry, we show that a series of 1-aryl-substituted boraindenes – the mono-benzannulated analogues of boroles – can similarly generate diverse boracycles via insertion of various substrates into their five-membered BC4 ring. Insertion of chalcogens (O, S, Se), alkynes and an aryl azo compound yields boraindene-derived products that are distinguished from earlier examples by their unique ring-fusion patterns, thereby broadening the structural diversity within this class of boron heterocycles. Notably, these transformations provide access to unprecedented boron–sulfur and boron–selenium naphthalene analogues, while azobenzene insertion into the boraindene framework yields two distinct diazaborepin isomers.
Introduction
Boroles are a distinct class of five-membered boron heterocycles in which π-conjugation between a three-coordinate boron center and a butadienediyl carbon framework generates an antiaromatic system.1 This unique electronic structure, characterized by a narrow HOMO–LUMO gap, coupled with strong Lewis acidity and electron-accepting capabilities, renders boroles attractive building blocks in materials chemistry.2 These advantageous properties can be further tuned through functionalization of ring substituents or incorporation into extended π systems, strategies that simultaneously mitigate the inherent instability and high reactivity of the borole core.1,2Beyond their fascinating electronic and optical features, boroles serve as versatile synthetic precursors for diverse aromatic and non-aromatic boron ring structures.3 For instance, they readily undergo ring expansion reactions with unsaturated substrates, efficiently generating larger boron-containing heterocycles such as six-membered 1,2-azaborinines (boron-nitrogen analogues of benzene)4 and seven-membered borepins5 – structural motifs with promise for optoelectronic applications.2,4,5 While boroles (A) and their bis-benzannulated derivatives, 9-borafluorenes (C), have been thoroughly explored for such transformations, the mono-benzannulated 1-boraindene analogues (B) remain largely overlooked (Fig. 1).
 | ||
| Fig. 1 Representative examples of borole frameworks with increasing degrees of benzannulation, from monocyclic boroles (A) over bicyclic 1-boraindenes (B) to tricyclic 9-borafluorene systems (C). | ||
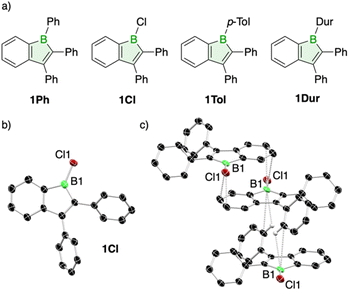
The first reported derivative, a 1-chloro-substituted boraindene synthesized by Kaufmann via pyrolysis in 1987, was too reactive for isolation but exhibited characteristic borole insertion reactivity in both alkyne trapping reactions and self-dimerization.6 Decades later, the Piers group exploited the unique reactivity of a perfluorinated boraindene in the activation of both H–H and H–Si bonds, thereby demonstrating its versatility in σ-bond activation chemistry.7–9 To further develop the chemistry of the boraindene system, we recently reported the synthesis of 1,2,3-triphenyl-1-boraindene (1Ph, Fig. 2) and demonstrated how its distinct molecular framework imparts unique Lewis acidic, optical, electronic and antiaromatic characteristics.10 Initial reactivity studies with organic azides revealed facile nitrogen atom insertion to form 2,1-azaboranaphthalenes, the benzannulated analogues of 1,2-azaborinines.10
Inspired by these findings and the broader synthetic potential of 1-boraindenes as precursors to benzannulated boron heterocycles, we now report three new derivatives bearing chloro, p-tolyl, and duryl substituents on the boron atom, and expand our reactivity studies to encompass chalcogens, alkynes, and azobenzene. These investigations furnished the first boron–sulfur and boron–selenium naphthalene analogues, along with benzannulated borepin derivatives from alkyne reactions, revealing a pronounced preference of these 1-boraindenes for endocyclic B–C insertion over cycloaddition with alkynes. Reactions with azobenzene unveiled a sterically-governed pathway, affording distinct diazaborepin isomers depending on the boron substituent. These findings establish fundamental reactivity patterns of 1-boraindenes and highlight their versatility as platforms for the synthesis of diverse boron heterocycles, offering new routes to both known and previously inaccessible derivatives.
Results and discussion
Preparation of new 1-boraindene derivatives
Our work extends 1-boraindene chemistry through the synthesis of three new derivatives (Fig. 2). In a similar manner to the previously reported 1,2,3-triphenyl-1-boraindene (1Ph),10 both the 1-p-tolyl (1Tol) and 1-chloro derivatives (1Cl) were prepared efficiently via tin-boron exchange between 1,1-dimethyl-2,3-diphenyl-1-stannaindene8 and the corresponding dibromoborane RBBr2 (R = p-tolyl)11 or BCl3, with yields above 76%. The 1-duryl derivative 1Dur, not accessible via this route, was obtained from 1Cl by reaction with duryl lithium in 79% yield. The distinct nature of the duryl derivative is reflected in its 11B NMR signal at 74.8 ppm, which is significantly downfield-shifted compared to those of the other derivatives, clustered in a narrow range between 65.0 and 65.5 ppm. This marked difference is most likely attributable to the steric demand of the duryl group, which disrupts π conjugation between boron and the exocyclic substituent. X-ray crystallographic analysis supports this conclusion, revealing that the duryl group adopts an orientation close to perpendicular (69.9°) relative to the boraindene ring, whereas the phenyl group in 1Ph is considerably less twisted (32.6°). The p-tolyl derivative 1Tol could not be included in the comparison, as all attempts to obtain single crystals were unsuccessful. The B–Cl bond length in 1Cl (1.747(5) Å) falls within the expected range, as do the remaining structural parameters of this haloborole derivative (Fig. 2).12–14 Crystal packing analysis reveals weak intermolecular interactions between the boron atom and the π system of neighboring benzene rings, with the shortest B⋯C contact at 3.453(7) Å. Additionally, the Lewis-acidic boron atom engages in an interaction with the C–H bond of a neighboring exocyclic phenyl group (CH⋯B 2.928 Å), leading to a stacked molecular arrangement (Fig. 2). Notably, comparable intermolecular boron contacts are also present in the crystal structure of 9-chloro-9-borafluorene.12In contrast to the less sterically protected 1-chloro-1-boraindene reported by Kaufmann,6 which bears only hydrogen substituents, and the monocyclic 1-chloro-2,3,4,5-tetraphenylborole,13,151Cl is remarkably stable both in solution and the solid state, showing no signs of decomposition or dimerization at room temperature and elevated temperatures. A related benzannulated derivative of 1Cl, 9-chloro-9-borafluorene, also exhibits striking stability, as evidenced by its ability to be purified by distillation at 90 °C.12
To evaluate the effect of the different boron substituents on Lewis acidity, we employed the Gutmann–Beckett NMR method in benzene solution.16 Adducts with one equivalent of triethylphosphine oxide (OPEt3) formed readily for 1Cl and 1Tol, resulting in 31P NMR signals at 77.6 and 75.4 ppm, respectively. In contrast, the duryl derivative 1Dur forms only a weak adduct at room temperature, with an averaged 31P NMR resonance at 49.9 ppm, likely due to steric shielding of the Lewis-acidic boron center by the ortho methyl groups. This value increases with decreasing temperature, and at −80 °C in toluene-d8 solution the 31P NMR signal shifts to 73.2 ppm, indicating complete adduct formation. Analysis of the resulting acceptor numbers (AN) demonstrates that chloroboraindene 1Cl is the strongest Lewis acid (AN = 80.8) in the series, followed by 1Ph (AN = 76.9) and 1Tol (AN = 76.1), which display progressively lower Lewis acidity. The AN value for 1Dur (AN = 71.1) cannot be directly compared to the others, as it was obtained under different conditions (−80 °C in toluene vs. room temperature in benzene). Nonetheless, it aligns with the expected trend of decreasing Lewis acidity corresponding to the weaker π-accepting ability of more electron-rich aryl substituents.
Building on the well-established tendency of boroles to undergo functionalization reactions that relieve the destabilizing 4π-electron system and drive the formation of larger heterocycles, we investigated the reactivity of the 1-aryl derivatives toward chalcogens, alkynes, and azobenzene. These transformations effected ring expansion and produced new benzannulated boron heterocycles.
Chalcogen insertions
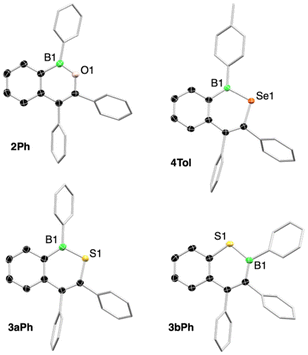
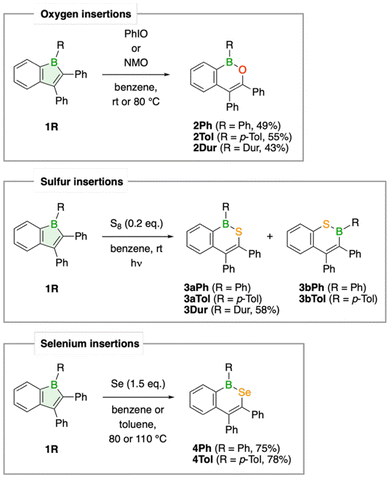
By inserting chalcogens into boraindenes, we aimed to access aromatic boron-chalcogen naphthalene analogues, fundamental structural motifs that had previously been reported only for the lightest chalcogen, oxygen.17,18Treatment of the 1-boraindenes 1Ph, 1Tol, and 1Dur with either iodosobenzene or N-methylmorpholine N-oxide afforded the corresponding oxygen-atom-expanded ring systems as white solids in yields ranging from 43 to 55% (Scheme 1). The transformation to 1,2-B,O-naphthalenes is readily observed by decolorization of the red boraindene solution and confirmed by the more shielded 11B NMR resonances, resulting from the π-donating effect of the oxygen atom. Compounds 2Ph, 2Tol, and 2Dur exhibit 11B NMR chemical shifts tightly grouped between 42.0 and 44.2 ppm, consistent with related structures by Martin, Wagner and Dong.18–22 The structures of all three compounds have been determined by X-ray diffraction analysis, confirming their naphthalene-like framework. The B–O bond lengths (1.377(2)–1.383(3) Å) are consistent with those reported for a related B,O-containing naphthalene derivative prepared by an alternative synthetic route.18 As observed in the boraindene 1Dur, the duryl substituent in 2Dur is twisted nearly perpendicular (80.5°) to the plane of the bicyclic core due to steric hindrance, whereas the other aryl boron substituents show a lesser degree of twisting, namely 45.9° for 2Tol and 46.7° for 2Ph (Fig. 3).
 | ||
| Scheme 1 Synthesis of B,E-naphthalenes via chalcogen atom (E = O, S, Se) insertion into 1-aryl-1-boraindenes 1R (R = Ph, p-Tol, Dur). | ||
The sulfur analogues, 3R, were obtained by irradiating 1R (R = Ph, p-Tol, Dur) in benzene with elemental sulfur for 16 h to 3 days, depending on the aryl substituent (Scheme 1). The 11B NMR resonances of the pale-yellow derivatives 3Ph, 3Tol, and 3Dur appear between 56.6 and 59.4 ppm and fall within the expected range for such compounds.23–25 A closer analysis by NMR spectroscopy revealed that the insertion reaction for the phenyl and p-tolyl derivatives proceeds non-selectively, producing regioisomeric products 3bR alongside the expected insertion products 3aR (R = Ph, p-Tol). The 1,2-B,S isomers 3aR, in which the sulfur atom inserts into the endocyclic B–C bond opposite the benzo substituent, predominate in both cases. In contrast, the sterically more demanding duryl derivative 1Dur converts cleanly to the expected sulfur insertion product, the 1,2-B,S isomer 3Dur, which was isolated as a pale-yellow solid in 58% yield. While only an isomeric mixture of isomers 3aTol and 3bTol could be isolated for the p-tolyl derivative, the major 1,2-B,S isomer 3aPh for the phenyl derivative could be separated in analytically pure form in 15% yield. Assignment and characterization of the different regioisomers were achieved by X-ray crystallographic analysis (Fig. 3).
The isomers can also be readily distinguished by characteristic through-space interactions between the boron-bound aryl group and neighboring protons, as observed in the 2D 1H–1H NOESY experiment (see SI for details). Both isomers of the phenyl derivative could be structurally characterized, whereas only isomer 3bTol of the p-tolyl derivative was accessible by single-crystal X-ray diffraction. The B–S bond lengths in the structures are essentially identical, ranging from 1.788(4) to 1.791(2) Å, and reflect significant π-delocalization comparable to that found in BSC4 rings exhibiting aromatic character.23,24
Incorporation of the heavier chalcogen selenium into 1R to generate boron-selenium-containing naphthalene analogues were achieved by reaction of 1Ph and 1Tol with elemental selenium (Scheme 1). When a benzene solution of 1Ph was heated with a slight excess of red selenium (1.5 equiv) at 80 °C for three days, the solution gradually changed color from red to orange, and a new 11B NMR resonance appeared at 63.6 ppm, consistent with formation of the insertion product 4Ph. Formation of the p-tolyl derivative 4Tol did not proceed under the same conditions, requiring heating at 110 °C in toluene, whereas no formation of the duryl derivative 4Dur was observed even at 120 °C. Unlike the sulfur insertion reactions, these selenium insertions are selective, producing exclusively a single product. Compound 4Tol exhibits a 11B NMR signal at 64.0 ppm. Good isolated yields of 75% (4Ph) and 78% (4Tol) were achieved for both insertion products. The 77Se NMR signals were observed at 479 (4Ph) and 475 ppm (4Tol), respectively, in good agreement with the value reported for a boron–selenium-containing phenanthrene analogue (δ(77Se) = 494 ppm).23 Compound 4Tol was further characterized by single-crystal X-ray diffraction. Suitable crystals were obtained by layering a saturated toluene solution of 4Tol with pentane at −30 °C. Structural parameters of the planar BSeC4 unit closely resemble those of the B,Se-phenanthrene derivative, with a B–Se bond length of 1.915(4) Å and a small C–Se–B bond angle of 101.4(1) ° at the selenium atom (Fig. 3).23
The observed trend of decreased shielding of the 11B NMR resonances down the chalcogen group, exemplified by resonances at 43.5, 57.3, and 63.6 ppm for 2Ph, 3Ph and 4Ph, respectively, can be explained by the progressively weaker π-donation from the lone pairs of the heavier chalcogen atoms to the empty boron p orbital. Related studies of 9-borafluorene derivatives have shown a similar 11B NMR chemical shift progression across the chalcogen series.23 The aromaticity of the boracycles, evaluated by nucleus-independent chemical shifts (NICS),26 indicates comparable aromatic character among the derivatives: 2Ph (NICSzz(−1/1) = −7.05), 3Ph (NICSzz(−1/1) = −9.30), and 4Ph (NICSzz(−1/1) = −7.87). While shielding effects from the heavier chalcogens are expected to influence NICS values, a comparison of the sulfur derivative 3Ph with its non-annulated pentaphenyl-1,2-thiaborinine analogue (NICS(1)zz = −12.75 ppm)24 and benzannulated 10-phenyl-10,9-borathiaphenanthrene analogue (NICS(−1/1)zz = −2.84 ppm)23 reveals that aromaticity in the boracycle decreases with increasing benzannulation, consistent with expected trends. Moreover, the differences in aromaticity between the 1,2- and 2,1-B,E isomers (E = O, S, Se) are minimal, which is consistent with their only slight differences in thermodynamic stability. For example, the 1,2-B,S isomer, observed in the sulfur insertion reaction of 1Ph, was found to be more stable than its 1,2-counterpart by merely +0.47 kcal mol−1 (see SI for details). To gain richer spatial insight into the conjugation and aromaticity of the derivatives, we analyzed the anisotropy of the current (induced) density (ACID, Fig. 4).27 Visualizations of the current density reveal diatropic (aromatic) ring currents that become progressively disrupted with heavier chalcogen substitution, indicating a gradual loss of aromaticity in contrast to the trends suggested by the NICS values (Fig. 4). The selenium derivative exhibits discontinuous induced current loops that are interrupted at the chalcogen position, reflecting a loss of aromatic stabilization.
Alkyne insertions
We further explored the reaction of boraindenes 1Ph and 1Tol with alkynes, a classic and characteristic reactivity pattern of boroles, documented for non-, mono-, and bis-benzannulated derivatives.6,28–39 The reaction can proceed through multiple pathways, producing diverse products such as seven-membered borepins and bicyclic boranorbornadienes.Both the phenyl- and p-tolyl-substituted boraindenes were found to react with diphenylacetylene at room temperature to give the borepins 5PhPh and 5TolPh in good isolated yields of 83% and 82%, respectively (Scheme 2). Monitoring the reaction indicated completion over 4 days, with no observable intermediates. The products, obtained as white solids, exhibit 11B NMR signals at 72.1 ppm (5PhPh) and 71.5 ppm (5TolPh), consistent with the expected range for tricoordinate boron atoms in borepins.33,36,37,40 Similar reactivity was observed with 2-butyne (dimethylacetylene), which afforded the corresponding borepins 5PhMe (84% yield, δ(11B) = 68.3 ppm) and 5TolMe (81%, δ(11B) = 69.2 ppm).
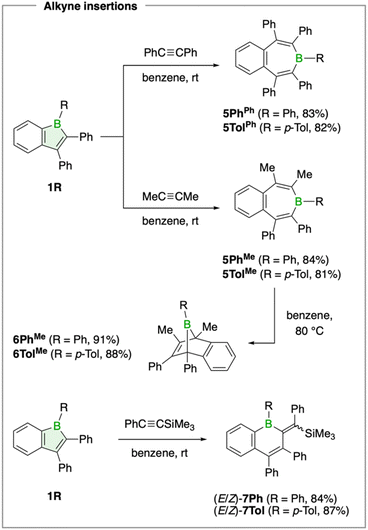 | ||
| Scheme 2 Products obtained from the reaction of aryl-substituted 1-boraindenes 1Ph and 1Tol with symmetrical and unsymmetrical alkynes. | ||
The seven-membered rings in all four borepin structures, as determined by X-ray diffraction analysis, adopt nonplanar boat conformations that disrupt delocalization within the ring, consistent with previous observations for aryl-substituted borepins.30,33,37 The carbon–carbon bond lengths within the ring further indicate minimal π conjugation, showing values characteristic of localized single and double bonds.
Next, we investigated the thermal stability of the borepins to determine whether alternative isomeric forms are accessible. The aryl-substituted borepins 5PhPh and 5TolPh remained unchanged, whereas the methyl derivatives 5PhMe and 5TolMe gradually converted at 80 °C over 4 days into new species exhibiting 11B NMR resonances at −5.2 and −4.4 ppm (Scheme 2). The pronounced upfield shifts are consistent with the formation of bicyclic 7-boranorbornadiene structures, stabilized by intramolecular interaction between the electron-deficient boron atom and the non-conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond.29–32,38 Single-crystal X-ray diffraction analysis of crystals obtained by slow evaporation of saturated pentane solutions at −30 °C confirmed the proposed structures, which correspond to the formal [4 + 2] Diels–Alder cycloaddition product of the corresponding boraindenes with 2-butyne. Compound 6PhMe crystallizes with two molecules in the unit cell, each exhibiting different geometric parameters, which can be attributed to two distinct orientations of the phenyl substituent on the bridgehead boron atom (Fig. 5). While both BPh units are markedly tilted toward the non-annulated double bond, in one conformation the phenyl group is oriented roughly orthogonally to the axis defined by the carbon atoms of the C
C double bond.29–32,38 Single-crystal X-ray diffraction analysis of crystals obtained by slow evaporation of saturated pentane solutions at −30 °C confirmed the proposed structures, which correspond to the formal [4 + 2] Diels–Alder cycloaddition product of the corresponding boraindenes with 2-butyne. Compound 6PhMe crystallizes with two molecules in the unit cell, each exhibiting different geometric parameters, which can be attributed to two distinct orientations of the phenyl substituent on the bridgehead boron atom (Fig. 5). While both BPh units are markedly tilted toward the non-annulated double bond, in one conformation the phenyl group is oriented roughly orthogonally to the axis defined by the carbon atoms of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond, facilitating optimal overlap of the vacant boron p orbital and the π system. Despite the enhanced overlap, the C
C double bond, facilitating optimal overlap of the vacant boron p orbital and the π system. Despite the enhanced overlap, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond length in this conformation (1.388(3) Å) is not statistically different from that in the alternate conformation (1.373(3) Å), where the phenyl group is twisted relative to the double bond. The bridgehead boron bond angles are nearly identical in both conformations (97.6(2)° and 97.4(2)°), with values typical for these strained systems.30–32,38 The crystal structure of 6TolMe reveals a single conformation in the unit cell, with the p-tolyl group oriented approximately perpendicular to the axis defined by the double bond and markedly tilted toward it. The overall structural parameters closely match those of 6PhMe in the same conformation.
C bond length in this conformation (1.388(3) Å) is not statistically different from that in the alternate conformation (1.373(3) Å), where the phenyl group is twisted relative to the double bond. The bridgehead boron bond angles are nearly identical in both conformations (97.6(2)° and 97.4(2)°), with values typical for these strained systems.30–32,38 The crystal structure of 6TolMe reveals a single conformation in the unit cell, with the p-tolyl group oriented approximately perpendicular to the axis defined by the double bond and markedly tilted toward it. The overall structural parameters closely match those of 6PhMe in the same conformation.
The reactions of boraindenes with the methyl-substituted alkynes establish a clear order of thermodynamic stability among the isomeric products: at room temperature, the reaction proceeds via insertion to form seven-membered borepins, while upon heating, thermal isomerization occurs, yielding the bicyclic boranorbornadienes 6PhMe and 6TolMe as the thermodynamically favored species. This two-step pathway, consisting of insertion followed by rearrangement, offers an alternative to the direct [4 + 2] cycloaddition of the borole with the alkyne that forms the boranorbornadiene. We have proposed a similar competitive insertion mechanism for pentaphenylborole,36 where boranorbornadiene formation was long assumed to proceed via a Diels–Alder pathway.28 Although an insertion pathway is clearly favored for the boraindene–alkyne combinations reported here and in Kaufmann's previous study,6 cycloaddition reactivity of boroles with alkynes has been unequivocally demonstrated elsewhere.30 These findings highlight that both steric and electronic effects of the substituents on the alkyne and the borole govern the reaction pathway and ultimately determine the relative stability of the products, which can also interconvert.
The pronounced dependence on the nature of the substituents is further illustrated by the reaction of boraindenes 1Ph and 1Tol with the unsymmetrical alkyne 1-phenyl-2-trimethylsilylacetylene. Treatment of these boraindenes with one equivalent of the alkyne afforded an additional, distinct insertion product (Scheme 2). This transformation was accompanied by a gradual color change of the solution from red to orange over 3 days, along with new 11B NMR signals at 71.4 ppm (7Ph) and 69.9 ppm (7Tol), respectively. The resulting yellow solids were isolated in yields of 84% (7Ph) and 87% yield (7Tol), respectively. Single-crystal X-ray diffraction revealed the ring expansion of the borole unit to form a boracyclohexadiene, a structural motif previously established in borole insertion chemistry (Fig. 5).30,34 For 7Ph, both the E and Z isomers, arising from different configurations of substituents across the exocyclic double bond on the six-membered boracycle, were observed. These correspond to isomers in which the silyl and boryl groups are either cis or trans to each other, respectively. 7Tol crystallized exclusively in the E conformation (Fig. 5). Overall, the structural parameters confirm the assignment of 7R (R = Ph, p-Tol) as boron-containing dihydronaphthalene analogues. The two geometric isomers of 7Ph and 7Tol are readily distinguished by NMR spectroscopy. The observed isomeric ratio is approximately 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 7Ph and 5
1 for 7Ph and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for 7Tol, both favoring the E isomer in solution. The divergent insertion pathway, involving a one-carbon ring expansion of the borole unit to a boracyclohexadiene, is likely facilitated by a facile silyl migration that occurs after the initial adduct formation between the alkyne and the boron atom.41
2 for 7Tol, both favoring the E isomer in solution. The divergent insertion pathway, involving a one-carbon ring expansion of the borole unit to a boracyclohexadiene, is likely facilitated by a facile silyl migration that occurs after the initial adduct formation between the alkyne and the boron atom.41
Azobenzene insertion
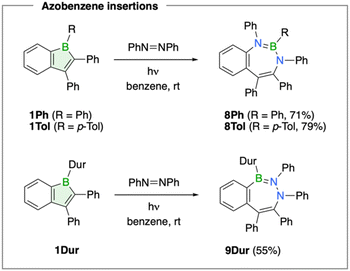
Drawing on established reactivity patterns known from monocyclic boroles, we examined the reaction of boraindenes 1Ph, 1Tol, and 1Dur with azobenzene.42 In prior studies, photolytic reaction of pentaphenylborole with this aryl azo compound was shown to produce seven-membered 1,3,2-diazaborepin products,42 BN analogues of azepines.43When the boraindenes 1Ph and 1Tol were irradiated with UV light from a mercury lamp in the presence of one equivalent of azobenzene for 16 h at room temperature, selective formation of new products was observed, exhibiting 11B NMR resonances at 32.7 ppm (8Ph) and 32.1 ppm (8Tol), respectively (Scheme 3). 11B NMR spectroscopic monitoring indicated that the reaction proceeds without observable intermediates. The boron chemical shifts of these products align closely with that reported for a related fully phenyl-substituted 1,3,2-diazaborepin derivative (δ(11B) = 31.6 ppm).42 The formation of the benzannulated BN2C4 ring as the reaction product was confirmed by single-crystal X-ray diffraction analysis. Due to structural disorder in 8Ph, bond parameters are discussed only for the p-tolyl derivative 8Tol (Fig. 6). Analysis of 8Tol reveals a nonplanar, boat-like conformation of the boracycle featuring two distinct B–N bonds characteristic of a σ bond (1.473(3) Å) and a dative π bond (1.409(3) Å).44 The nitrogen atom not involved in π bonding exhibits slight pyramidalization, with an angular sum of 353.3(3)°. Overall, the structural parameters of 8Tol are closely comparable to those reported for the perphenylated derivative by the Martin group.42
Interestingly, the reaction of the duryl derivative 1Dur with azobenzene under similar conditions led to a different outcome, as indicated by the significantly downfield-shifted 11B NMR signal at 46.3 ppm for the isolated product 9Dur (55% yield, Scheme 3). X-ray diffraction analysis revealed a seven-membered BN2C4 boracycle with an intact N–N bond, formed by the formal insertion of azobenzene into the endocyclic B–C bond opposite the benzo group (Fig. 6). The boracycle adopts a more twisted boat conformation compared to those observed for 8Ph and 8Tol. The two phenyl groups across the N–N single bond (1.421(3) and 1.433(2) Å) are mutually trans, with C–N–N–C endocyclic torsion angles of 98.3(2) and 106.7(2)° for the two independent molecules in the unit cell. The B–N bond lengths of 1.419(2) and 1.420(2) Å are characteristic of significant double bond character.44
Although seven-membered 1,3,2-diazaborepin products such as 8Ph and 8Tol have been previously reported from the reaction of pentaphenylborole with azo compounds,42 the direct insertion of an azo group into the borole unit of 1Dur to form 9Dur represents an unprecedented transformation – one analogous to alkyne insertion that results in formal 1,2-carboboration of the unsaturated N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond. Thus, depending on the steric demand of the aryl boron substituent, the photochemical reaction of boraindenes with azobenzene affords two distinct isomeric forms of B,N-azepine. Notably, 9Dur, featuring a B–N–N connectivity within the ring, represents an unprecedented structural motif among diazaborepins.
N bond. Thus, depending on the steric demand of the aryl boron substituent, the photochemical reaction of boraindenes with azobenzene affords two distinct isomeric forms of B,N-azepine. Notably, 9Dur, featuring a B–N–N connectivity within the ring, represents an unprecedented structural motif among diazaborepins.
Conclusions
In summary, we have expanded the range of known boraindene derivatives by synthesizing 1-p-tolyl, 1-duryl, and 1-chloro variants, all sharing the characteristic 2,3-diphenyl-substituted backbone. Insertion of chalcogen atoms into the boraindenes afforded B,O-, B,S-, and B,Se-naphthalenes, thereby adding previously unknown sulfur and selenium derivatives to this family. The reactivity of the boraindenes with various alkynes under thermal conditions demonstrated a preference for an insertion pathway, initially yielding stable borepin derivatives. At elevated temperatures, the dimethyl-substituted borepins derived from 2-butyne rearrange into bicyclic boranorbornadienes, which are the formal [4 + 2] cycloaddition products of boraindenes and alkynes and represent the thermodynamically favored species. Reaction with 1-phenyl-2-trimethylsilylacetylene induced one-carbon insertion and ring expansion to form a boracyclohexadiene ring featuring an exocyclic double bond, a structural motif previously reported in monocyclic borole derivatives. Lastly, the aryl-substituted boraindenes reacted with diazobenzene to form diazaborepins (or BN-azepines), with the isomer outcome influenced by the steric bulk of the 1-aryl substituent. Notably, the 1-duryl derivative undergoes a formal 1,2-carboboration through insertion of the azo group, resembling the mechanism observed in borepin formation from alkynes. These reactions establish boraindenes as versatile precursors to heteroatom-doped polycyclic compounds, offering synthetic access to scaffolds that complement those derived from boroles and 9-borafluorenes. Future studies will further exploit their potential and elucidate differences between monocyclic boroles and tricyclic 9-borafluorene analogues.Author contributions
N. W.: conceptualization, methodology, investigation. I. K.: writing-original draft preparation, visualization. L. W.: formal analysis. M. M: formal analysis. M. N.: formal analysis. A. H.: formal analysis. J. K.: investigation. H. B.: supervision.Conflicts of interest
There are no conflicts to declare.Data availability
CCDC 2499789–2499810 (1Cl, 1Dur, 2Dur, 2Ph, 2Tol, 3Dur, 3aPh, 3bPh, 3bTol, 4Tol, 5PhMe, 5PhPh, 5TolMe, 5TolPh, 6PhMe, 6TolMe, E-7Ph, Z-7Ph, E-7Tol, 8Ph, 8Tol, and 9Dur). contain the supplementary crystallographic data for this paper.45a–vThe data supporting the findings of this study are available in the supplementary information (SI) of this article. Supplementary information is available. See DOI: https://doi.org/10.1039/d5sc08526h.
Acknowledgements
We thank the Julius-Maximilians-Universität Würzburg and the Deutsche Forschungsgemeinschaft (DFG grant 466754611) for financial support of this work.Notes and references
- For general borole reviews, see: (a) J. J. Eisch, Adv. Organomet. Chem., 1996, 39, 355–391 CrossRef CAS; (b) A. Steffen, R. M. Ward, W. D. Jones and T. B. Marder, Coord. Chem. Rev., 2010, 254, 1950–1976 CrossRef CAS; (c) H. Braunschweig and T. Kupfer, Chem. Commun., 2011, 47, 10903–10914 RSC; (d) H. Braunschweig, I. Krummenacher and J. Wahler, Adv. Organomet. Chem., 2013, 61, 1–53 CrossRef CAS; (e) B. Su and R. Kinjo, Synthesis, 2017, 49, 2985–3034 CrossRef CAS; (f) C. Hong, J. Baltazar and J. D. Tovar, Eur. J. Org Chem., 2022, e202101343 CrossRef CAS.
- (a) F. Jäkle, Chem. Rev., 2010, 110, 3985–4022 Search PubMed; (b) S. Kervyn, C. Aurisicchio and D. Bonifazi, in Functional Supramolecular Architectures, ed. P. Samorì and F. Cacialli, Wiley-VCH, Weinheim, 2011, ch. 8, pp. 233–280 Search PubMed; (c) A. Escande and M. J. Ingleson, Chem. Commun., 2015, 51, 6257–6274 RSC; (d) Y. Ren and F. Jäkle, Dalton Trans., 2016, 45, 13996–14007 Search PubMed; (e) L. Ji, S. Griesbeck and T. B. Marder, Chem. Sci., 2017, 8, 846–863 RSC; (f) W. Zhang, B. Zhang, D. Yu and G. He, Sci. Bull., 2017, 62, 899–900 Search PubMed; (g) A. Wakamiya, in Main Group Strategies Towards Functional Hybrid Materials, ed. T. Baumgartner and F. Jäkle, John Wiley & Sons Ltd, Hoboken (New Jersey), 2018, vol. 1, pp. 1–26 Search PubMed; (h) E. v. Grotthuss, A. John, T. Kaese and M. Wagner, Asian J. Org. Chem., 2018, 7, 37–53 CrossRef; (i) J. He, F. Rauch, M. Finze and T. B. Marder, Chem. Sci., 2021, 12, 128–147 RSC.
- (a) J. H. Barnard, S. Yruegas, K. Huang and C. D. Martin, Chem. Commun., 2016, 52, 9985–9991 RSC; (b) Y. Su and R. Kinjo, Chem. Soc. Rev., 2019, 48, 3613–3659 RSC; (c) X. Su, T. A. Bartholome, J. R. Tidwell, A. Pujol, S. Yruegas, J. J. Martinez and C. D. Martin, Chem. Rev., 2021, 121, 4147–4192 CrossRef CAS PubMed; (d) X. Li, Y.-M. Chen and Z.-G. Xu, Org. Chem. Front., 2025, 12, 3521–3533 RSC.
- (a) M. J. D. Bosdet and W. E. Piers, Can. J. Chem., 2009, 87, 8–29 CrossRef; (b) P. G. Campbell, A. J. V. Marwitz and S.-Y. Liu, Angew. Chem., Int. Ed., 2012, 51, 6074–6092 CrossRef CAS PubMed; (c) G. Bélanger-Chabot, H. Braunschweig and D. K. Roy, Eur. J. Inorg. Chem., 2017, 4353–4368 CrossRef; (d) Z. X. Giustra and S.-Y. Liu, J. Am. Chem. Soc., 2018, 140, 1184–1194 CrossRef CAS PubMed.
- (a) R. E. Messersmith and J. D. Tovar, J. Phys. Org. Chem., 2015, 28, 378–387 CrossRef CAS; (b) L. Wang, J. Ma, E. Si and Z. Duan, Synthesis, 2021, 53, 623–635 CrossRef CAS.
- W. Schacht and D. Kaufmann, Angew. Chem., Int. Ed. Engl., 1987, 26, 665–666 CrossRef.
- A. Y. Houghton, V. A. Karttunen, W. E. Piers and H. M. Tuononen, Chem. Commun., 2014, 50, 1295–1298 RSC.
- A. Y. Houghton, Doctoral Thesis, University of Calgary, 2014 Search PubMed.
- A. Y. Houghton, J. Hurmalainen, A. Mansikkamäki, W. E. Piers and H. M. Tuononen, Nat. Chem., 2014, 6, 983–988 CrossRef CAS PubMed.
- N. Wieprecht, I. Krummenacher, L. Wüst, M. Michel, S. Fuchs, S. Nees, M. Härterich and H. Braunschweig, Chem. Sci., 2024, 15, 12496–12501 RSC.
- W. Haubold, J. Herdtle, W. Gollinger and W. Einholz, J. Organomet. Chem., 1986, 315, 1–8 CrossRef CAS.
- S. Biswas, I. M. Oppel and H. F. Bettinger, Inorg. Chem., 2010, 49, 4499–4505 CrossRef CAS PubMed.
- H. Braunschweig, C.-W. Chiu, J. Wahler, K. Radacki and T. Kupfer, Chem. Eur. J., 2010, 16, 12229–12233 CrossRef CAS PubMed.
- (a) T. Heitkemper, L. Naß and C. P. Sindlinger, Dalton Trans., 2020, 49, 2706–2714 RSC; (b) J. Sarcevic, T. Heitkemper and C. P. Sindlinger, Chem. Commun., 2022, 58, 246–249 RSC.
- H. Braunschweig and T. Kupfer, Chem. Commun., 2008, 4487–4489 RSC.
- (a) V. Gutmann, Coord. Chem. Rev., 1976, 18, 225–255 CrossRef CAS; (b) M. A. Beckett, G. C. Strickland, J. R. Holland and K. Sukumar Varma, Polymer, 1996, 37, 4629–4631 CrossRef CAS; (c) A. E. Ashley, T. J. Herrington, G. G. Wildgoose, H. Zaher, A. L. Thompson, N. H. Rees, T. Krämer and D. O'Hare, J. Am. Chem. Soc., 2011, 133, 14727–14740 CrossRef CAS PubMed; (d) P. Erdmann and L. Greb, Angew. Chem., Int. Ed., 2022, 61, e202114550 CrossRef CAS PubMed.
- (a) M. J. S. Dewar and R. Dietz, Tetrahedron Lett., 1959, 1, 21–23 CrossRef; (b) M. J. S. Dewar and R. Dietz, J. Chem. Soc., 1960, 1344–1347 RSC; (c) V. L. Arcus, L. Main and B. K. Nicholson, J. Organomet. Chem., 1993, 460, 139–147 CrossRef CAS; (d) C. Körner, P. Starkov and T. D. Sheppard, J. Am. Chem. Soc., 2010, 132, 5968–5969 CrossRef PubMed; (e) R. Guo, K.-N. Li, B. Liu, H.-J. Zhu, Y.-M. Fan and L.-Z. Gong, Chem. Commun., 2014, 50, 5451–5454 RSC; (f) L. Benhamou, D. W. Walker, D.-K. Bučar, A. E. Aliev and T. D. Sheppard, Org. Biomol. Chem., 2016, 14, 8039–8043 RSC; (g) H. Saito, S. Otsuka, K. Nogi and H. Yorimitsu, J. Am. Chem. Soc., 2016, 138, 15315–15318 CrossRef CAS PubMed; (h) S. Tsuchiya, H. Saito, K. Nogi and H. Yorimitsu, Org. Lett., 2017, 19, 5557–5560 CrossRef CAS PubMed.
- O. Ouadoudi, T. Kaehler, M. Bolte, H.-W. Lerner and M. Wagner, Chem. Sci., 2021, 12, 5898–5909 RSC.
- S. Yruegas, D. C. Patterson and C. D. Martin, Chem. Commun., 2016, 52, 6658–6661 RSC.
- M. O. Akram, J. R. Tidwell, J. L. Dutton, D. J. D. Wilson, A. Molino and C. D. Martin, Inorg. Chem., 2022, 61, 9595–9604 CrossRef CAS PubMed.
- A. Budanow, E. v. Grotthuss, M. Bolte, M. Wagner and H.-W. Lerner, Tetrahedron, 2016, 72, 1477–1484 CrossRef CAS.
- Y. Ge, Q. Zhu, Y. Zhu and G. Dong, Nat. Chem., 2025 DOI:10.1038/s41557-025-01971-0.
- T. Bischof, N. Wieprecht, S. Fuchs, L. Endres, I. Krummenacher, M. Michel, C. Mihm, H. Braunschweig and M. Finze, Inorg. Chem., 2023, 62, 21329–21335 CrossRef CAS PubMed.
- S. Yruegas and C. D. Martin, Chem. Eur. J., 2016, 22, 18358–18361 CrossRef CAS PubMed.
- A. D. Rohr, M. M. Banaszak Holl, J. W. Kampf and A. J. Ashe III, Organometallics, 2011, 30, 3698–3700 CrossRef CAS.
- (a) P. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao and N. J. R. v. E. Hommes, J. Am. Chem. Soc., 1996, 118, 6317–6318 CrossRef CAS PubMed; (b) A. C. Tsipis, Phys. Chem. Chem. Phys., 2009, 11, 8244–8261 RSC.
- (a) R. Herges and D. Geuenich, J. Phys. Chem. A, 2001, 105, 3214–3220 CrossRef CAS; (b) D. Geuenich, K. Hess, F. Köhler and R. Herges, Chem. Rev., 2005, 105, 3758–3772 CrossRef CAS PubMed.
- (a) J. J. Eisch, N. K. Hota and S. Kozima, J. Am. Chem. Soc., 1969, 91, 4575–4577 CrossRef CAS; (b) J. J. Eisch and J. E. Galle, J. Am. Chem. Soc., 1975, 97, 4436–4437 CrossRef CAS; (c) J. J. Eisch, Adv. Organomet. Chem., 1977, 16, 67–109 CrossRef CAS; (d) J. J. Eisch and J. E. Galle, J. Organomet. Chem., 1977, 127, C9–C13 CrossRef CAS; (e) J. J. Eisch, J. E. Galle, B. Shafii and A. L. Rheingold, Organometallics, 1990, 9, 2342–2349 CrossRef CAS; (f) J. J. Eisch, Adv. Organomet. Chem., 1996, 39, 355–388 CrossRef CAS.
- P. J. Fagan, E. G. Burns and J. C. Calabrese, J. Am. Chem. Soc., 1988, 110, 2979–2981 CrossRef CAS.
- C. Fan, W. E. Piers, M. Parvez and R. McDonald, Organometallics, 2010, 29, 5132–5139 CrossRef CAS.
- H. Braunschweig, J. Maier, K. Radacki and J. Wahler, Organometallics, 2013, 32, 6353–6359 CrossRef CAS.
- (a) F. Ge, G. Kehr, C. G. Daniliuc and G. Erker, J. Am. Chem. Soc., 2014, 136, 68–71 CrossRef CAS PubMed; (b) F. Ge, X. Tao, C. G. Daniliuc, G. Kehr and G. Erker, Angew. Chem., Int. Ed., 2018, 57, 14570–14574 CrossRef CAS PubMed.
- Y. Shoji, N. Tanaka, S. Muranaka, N. Shigeno, H. Sugiyama, K. Takenouchi, F. Hajjaj and T. Fukushima, Nat. Comm., 2016, 7, 12704 CrossRef PubMed.
- Z. Wang, Y. Zhou, J.-X. Zhang, I. Krummenacher, H. Braunschweig and Z. Lin, Chem. Eur. J., 2018, 24, 9612–9621 CrossRef CAS PubMed.
- H. Kelch, S. Kachel, J. Wahler, M. A. Celik, A. Stoy, I. Krummenacher, T. Kramer, K. Radacki and H. Braunschweig, Chem. Eur. J., 2018, 24, 15387–15391 CrossRef CAS PubMed.
- F. Lindl, X. Guo, I. Krummenacher, F. Rauch, A. Rempel, V. Paprocki, T. Dellermann, T. E. Stennett, A. Lamprecht, T. Brückner, K. Radacki, G. Bélanger-Chabot, T. B. Marder, Z. Lin and H. Braunschweig, Chem. Eur. J., 2021, 27, 11226–11233 CrossRef CAS PubMed.
- T. Bischof, L. Beßler, I. Krummenacher, L. Erhard, H. Braunschweig and M. Finze, Chem. Eur. J., 2023, 29, e202300210 CrossRef CAS PubMed.
- C. Zhang, R. J. Gilliard Jr. and C. C. Cummins, Chem. Sci., 2024, 15, 17873–17880 RSC.
- P. H. R. Oliveira, M. O. Rodrigues, C. D. G. Da Silva, J. L. Bohlen, M. Arrowsmith, A. Jayaraman, L. Lubczyk, F. Fantuzzi, E. N. da Silva Júnior and H. Braunschweig, Angew. Chem., Int. Ed., 2025, e202423391 CAS.
- For additional examples of 11B NMR shifts of borepins, see: (a) L. G. Mercier, W. E. Piers and M. Parvez, Angew. Chem., Int. Ed., 2009, 48, 6108–6111 CrossRef CAS PubMed; (b) K. Schickedanz, J. Radtke, M. Bolte, H.-W. Lerner and M. Wagner, J. Am. Chem. Soc., 2017, 139, 2842–2851 CrossRef CAS PubMed.
- M. Kira and T. Iwamoto, in The Chemistry of Organic Silicon Compounds, ed. Z. Rappoport and Y. Apeloig, Wiley & Sons Ltd, Weinheim, 2001, vol. 3, ch. 16, pp. 854–948 Search PubMed.
- V. A. K. Adiraju and C. D. Martin, Chem. Eur. J., 2017, 23, 11437–11444 CrossRef CAS PubMed.
- H. Lv, K. Xiang and D.-T. Yang, Eur. J. Org Chem., 2022, e202201208 CrossRef CAS.
- K.-A. Østby, G. Gundersen, A. Haaland and H. Nöth, Dalton Trans., 2005, 2284–2291 RSC.
- (a) CCDC 2499789: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc12kl18; (b) CCDC 2499790: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7d8; (c) CCDC 2499791: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7f9; (d) CCDC 2499792: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7gb; (e) CCDC 2499793: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7hc; (f) CCDC 2499794: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7jd; (g) CCDC 2499795: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7kf; (h) CCDC 2499796: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7lg; (i) CCDC 2499797: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7mh; (j) CCDC 2499798: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7nj; (k) CCDC 2499799: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7pk; (l) CCDC 2499800: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7ql; (m) CCDC 2499801: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7rm; (n) CCDC 2499802: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7sn; (o) CCDC 2499803: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7tp; (p) CCDC 2499804: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7vq; (q) CCDC 2499805: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7wr; (r) CCDC 2499806: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7xs; (s) CCDC 2499807: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7yt; (t) CCDC 2499808: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px7zv; (u) CCDC 2499809: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px80x; (v) CCDC 2499810: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2px81y.
| This journal is © The Royal Society of Chemistry 2026 |