 Open Access Article
Open Access ArticlepH-dependent redox hydrogen pump for electrochemical direct air capture of CO2
Xiangyu Zhangab,
Yun Zhao *a,
Yangkai Hanab,
Zhiwei Renab,
Tao Weiab,
Riyang Huangab and
Zhigang Shao
*a,
Yangkai Hanab,
Zhiwei Renab,
Tao Weiab,
Riyang Huangab and
Zhigang Shao *a
*a
aFuel Cell System and Engineering Laboratory, Key Laboratory of Fuel Cells & Hybrid Power Sources, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China. E-mail: yunzhao@dicp.ac.cn; zhgshao@dicp.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 16th January 2026
Abstract
Redox-mediated electrochemical DAC offers a promising, energy-efficient alternative to conventional thermal methods but faces challenges with the oxidative degradation of organic redox mediators in air. While multi-electrolyzer systems mitigate oxygen exposure and solvent loss, the use of common pH-independent redox limits further voltage reductions. To overcome the voltage limitation of pH-independent mediators in electrochemical DAC, this study introduces a new electrochemical DAC strategy utilizing a pH-dependent redox mediator, anthraquinone-2,7-disulfonic acid salt (AQDS), within a dual-electrolyzer system coupled with a hydrogen pump. The pH-dependent redox potential of AQDS lowers the electrolyzer voltage, enabling an actual energy consumption of 257 kJ mol−1 CO2.
1 Introduction
Rising atmospheric concentrations of carbon dioxide (CO2), a major greenhouse gas, intensify its trapping of long-wave radiation, driving global warming and climate change.1–3 Direct Air Capture (DAC) is a vital decarbonization strategy that removes low-concentration CO2 directly from the atmosphere, offering key advantages over flue gas capture such as flexible scalability and minimal geographical constraints.4,5 Conventional DAC technologies, typically utilizing solid sorbents (e.g., metal–organic frameworks, solid amines, alkali-based sorbents) or alkaline solutions, suffer from significant energy demands during sorbent/solution regeneration and potential secondary pollution.6–10 In contrast, electrochemical CO2 capture technologies provide benefits including low energy consumption, simple equipment, and adaptable system size.11 As electrification advances, electrochemically driven CO2 capture operating under ambient conditions emerges as a highly attractive solution.The fundamental principle of electrochemical DAC involves modulating sorbent CO2 affinity electrochemically to enable reversible CO2 adsorption/desorption.11 Recent studies indicate that incorporating redox-active organic molecules can significantly reduce the energy required for electrochemical CO2 capture and release.12–14 These strategies primarily utilize two classes of redox-active molecules: (1) proton-responsive molecules, which facilitate CO2 capture under alkaline conditions or release under acidic conditions by inducing pH shifts through water dissociation;13–15 and (2) direct CO2-binding molecules: in their reduced state, these molecules react directly with CO2, and release the captured CO2 upon oxidation.16,17 However, the reduced forms of these organics, often containing amino or hydroxyl groups, are susceptible to O2-induced oxidative degradation.14,18 Furthermore, the volatility of the organic solvents/co-solvents employed complicates operation through solvent loss.16,19
To mitigate O2 exposure and solvent evaporation, multi-electrolyzer designs have been adopted and proven effective for both CO2 capture and reactive capture processes.20,21 These systems leverage the inherent O2 tolerance of inorganic CO2 absorbents while utilizing organic redox mediators to maintain high reaction rates. Nonetheless, a key limitation persists: the pH-independent redox potentials of commonly used mediators restrict the achievable minimization of electrolyzer voltage, whose theoretical voltage is defined as the potential difference between the hydrogen oxidation reaction (HOR) and hydrogen evolution reaction (HER). One pathway to overcome this constraint is by selecting mediators with pH-dependent redox potentials. Quinone compounds represent promising pH-dependent redox with favorable electrochemical properties,22 yet their application in multi-electrolyzer DAC systems remains unexplored.
Herein, we propose a DAC strategy employing a pH-dependent redox within a dual-electrolyzer system coupled with a hydrogen pump. This configuration utilizes HER to generate OH− for CO2 capture and the HOR to produce H+ for CO2 release. We selected anthraquinone-2,7-disulfonic acid salt (AQDS) as the redox mediator due to its high reversibility, solubility, and low cost. Crucially, its pH-dependent redox potential enables electrolyte pH modulation, significantly reducing electrolyzer voltage. Using this approach, we achieved direct CO2 capture from ambient air with an actual energy consumption of 257 kJ mol−1 CO2 at 40 mA cm−2.
2 Experimental
2.1 Electrochemical measurements
All electrochemical measurements were conducted at ambient conditions. No iR compensation was applied in the electrochemical tests.Cyclic voltammetry (CV) was performed using a Gamry Interface 1010E electrochemical workstation. In all three-electrode CV configurations, a glassy carbon electrode (GCE) with a diameter of 3.0 mm was employed as the working electrode. A saturated Ag/AgCl electrode, pre-soaked in a saturated KCl solution, served as the reference electrode, while a platinum wire (0.5 mm × 37 mm) was used as the counter electrode. The scan rate was set at 50 mV s−1.
Linear sweep voltammetry (LSV) was conducted using the same workstation in a single-cell configuration. A saturated Ag/AgCl electrode, similarly pre-soaked in saturated KCl solution, was used as the reference electrode. A platinum-coated nickel felt (1 × 1 cm2) served as the working electrode, and a 3.0 mm diameter glassy carbon electrode was used as the counter electrode. The LSV was performed at a scan rate of 1 mV s−1. The electrolyte consisted of 50 mL of saturated Li2CO3 aqueous solution.
Electrochemical impedance spectroscopy (EIS) was also performed using the Gamry Interface 1010E system. Measurements were carried out in the frequency range of 10 kHz to 1 Hz with an amplitude of 0.5 A.
2.2 Ultraviolet-visible (UV-vis) absorption spectroscopy
Ultraviolet-visible (UV-vis) spectra were recorded using a SHIMADZU UV-2550 spectrophotometer. A 2 M LiCl aqueous solution was used as the reference solvent.2.3 Scanning electron microscopy (SEM)
The morphology and microstructure of the carbon felt were characterized using a field-emission scanning electron microscope (FE-SEM, JSM-IT300LA).2.4 Electrolytic cell configuration
A custom three-chamber electrolytic cell with an active area of 5.0 cm2 was assembled for dual-mode operation (Mode I and Mode II). In Mode I configuration, hydrophobic carbon paper was used in the anode chamber. A Nafion 211 membrane, coated with Pt/C catalyst, separated the anode chamber from the central chamber. A piece of carbon felt was placed in the central chamber and served as a shared electrode. In Mode II configuration, a Nafion 115 membrane separated the cathode chamber from the central chamber. A platinum-coated nickel felt electrode was installed in the cathode chamber. Rubber gaskets ensured liquid-tight sealing throughout the assembly.At the beginning of each experiment, the Mode II cathode chamber was filled with 20 mL of post-capture solution, while the neutralization reservoir was charged with 20 mL of an AQDS/LiCl aqueous. Prior to applying voltage, the central chamber was purged with N2 gas for 15 minutes. Both solutions were introduced into their respective chambers from the bottom inlet and circulated back into their reservoirs via the top outlet of the cell.
2.5 Calculation of energy consumption and faradaic efficiency
Due to the continuous variation of cell voltage during the electrolyzer operation, the operating energy consumption is calculated using eqn (E1) via computer processing.
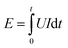 | (E1) |
Faradaic efficiency is calculated as the ratio between the actual and theoretical masses of Li2CO3 produced. Based on the reaction eqn (E2)–(E6), each mole of AQDS theoretically yields one mole of Li2CO3.
In Mode I:
| Anode: H2 − 2e− → 2H+ | (E2) |
| Cathode: AQDS + 2e− + 2H+ → AQDSH2 | (E3) |
In Mode II:
Anode:
| AQDSH2 − 2e− + Li2CO3 → AQDS + CO2(g) + H2O + 2Li+ | (E4) |
| Cathode: 2H2O + 2e− → 2OH− + H2 | (E5) |
In DAC
| 2Li+ + 2OH− + CO2 → Li2CO3(s) + H2O | (E6) |
Therefore, the faradaic efficiency is determined according to eqn (E7)
 | (E7) |
3 Results and discussion
3.1 Concept and validation of an AQDS-mediated hydrogen pump for DAC cell voltage reduction
To prevent AQDS deactivation by oxygen, we developed an electrochemical strategy coupling a redox pair with a hydrogen pump (Fig. 1). This system simultaneously leverages both the O2-tolerance of inorganic CO2 sorbents and exploits the pH-dependent redox potential of the organic mediator.The system operates alternately in Mode I and Mode II, utilizing a carbon felt electrode in the central compartment that functions as the cathode during Mode I and as the anode during Mode II. Both electrolyzers employ cation exchange membranes (CEMs) to enable cation migration from anode to cathode (Fig. 1).
In Mode I, the HOR at the anode generates H+ ions, which migrate through the CEM and react with AQDS at the cathode to form AQDSH2. Following Mode I, Li2CO3—formed by atmospheric CO2 absorption into LiOH solution in Mode II—is introduced, establishing an alkaline environment.
In Mode II, AQDSH2 undergoes oxidation at the anode. The elevated pH (from Li2CO3 addition) lowers the AQDS redox potential, reducing the required electrolyzer voltage. Concurrently, generated H+ protonates carbonate ions (CO32−) to release CO2, while Li+ migrates to the cathode. HER at the cathode then produces LiOH and H2, enabling H2 recycling to the Mode I anode and the LiOH reuse for CO2 capture.
The theoretical total voltage for Mode I and Mode II operation varies with redox selection (Fig. 2a). Redox couples like I2/I− (ref. 21) and BPPV2+/BPPV+ (ref. 20) exhibit pH-independent potential; consequently, their combined voltage equals the thermodynamic HOR/HER potential difference (EHOR–EHER). For example, using BPPV2+/BPPV+, Mode I requires a voltage (ΔE4) equivalent to the potential gap between HOR and BPPV reduction, while Mode II requires a voltage (ΔE3) spanning BPPV oxidation to HER. The sum ΔE3 + ΔE4 thus equals EHOR–EHER. In contrast, AQDS/AQDSH2 possesses a pH-dependent redox potential. During Mode I (acidic conditions), its more positive potential narrows the gap to HOR (ΔE6 < ΔE4). During Mode II (alkaline conditions), its more negative potential approaches HER (ΔE5 < ΔE3). This pH-triggered potential shift yields a lower total voltage (ΔE5 + ΔE6), which is strictly less than EHOR–EHER.
To validate this concept, CV measurements of AQDS under varying pH conditions confirm significant potential shifts (Fig. 2c). In 0.2 M HCl (simulating Mode I), AQDS exhibits a redox potential of +0.009 V vs. Ag/AgCl. Under 0.2 M Li2CO3 (simulating Mode II), the potential cathodically shifts to −0.467 V vs. Ag/AgCl—a 476 mV difference. These results demonstrate that acidic and alkaline conditions profoundly modulate the AQDS redox potential, directly enabling the electrolyzer voltage reduction mechanism proposed in Fig. 2a.
3.2 Electrolytic cell performance analysis
We first assessed the performance of each electrolyzer operating at 40 mA cm−2, circulating 20 mL of 0.10 M AQDS solution through the middle chamber at 20 mL min−1. As shown in Fig. 3b, Mode I commenced at a cell voltage of 0.243 V, while Mode II started at 0.624 V. As electrolysis approached completion in both modes, a pronounced increase indicated near-complete consumption of AQDS reactant during reduction (Mode I) and oxidation (Mode II).Under the experimental conditions, the HOR potential is 0 V vs. SHE, while LSV measurements show a HER onset potential of the −0.715 V vs. SHE in saturated lithium carbonate solution (EHOR–EHER = 0.715 V). The theoretical voltages for Mode I and Mode II using the I2/I− and BPPV2+/BPPV+ redox couples is therefore 0.715 V.
However, due to the lowered AQDS redox potential from the lithium carbonate addition, the theoretical sum for Mode I and II is expected to be below 0.715 V. Experimentally, at 10 mA cm−2, the sum of the initial onset voltages is indeed lower than 0.715 V (Fig. 3a). Conversely, at higher current densities (40, 70, and 100 mA cm−2), this sum exceeds 0.715 V, attributed to increased ohmic losses. To evaluate these ohmic losses in the Mode I and II electrolyzers, EIS was conducted as shown in Fig. 3c.
UV-vis absorption spectroscopy confirmed AQDS reduction during Mode I operation (Fig. 3d). Oxidized AQDS exhibits a strong peak at 325 nm (attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups) and a broad, weak peak near 482 nm, potentially arising from Li+ complexation with C
O groups) and a broad, weak peak near 482 nm, potentially arising from Li+ complexation with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O oxygen due to the similar ionic radii of Li+ and H+. Upon reduction, a new peak emerges at 391 nm, characteristic of O–H groups in AQDSH2. The persistence of the 325 nm peak is likely due to incomplete reduction or sample dilution effects during measurements. The disappearance of the 482 nm peak is attributed to decreased solution pH upon AQDS reduction, where increased H+ concentration outcompetes Li+ for complexation sites. Furthermore, a visible color change from red to green in the middle chamber during Mode I operation directly confirmed the redu ction of AQDS.
O oxygen due to the similar ionic radii of Li+ and H+. Upon reduction, a new peak emerges at 391 nm, characteristic of O–H groups in AQDSH2. The persistence of the 325 nm peak is likely due to incomplete reduction or sample dilution effects during measurements. The disappearance of the 482 nm peak is attributed to decreased solution pH upon AQDS reduction, where increased H+ concentration outcompetes Li+ for complexation sites. Furthermore, a visible color change from red to green in the middle chamber during Mode I operation directly confirmed the redu ction of AQDS.
To evaluate the DAC potential, the Mode II-regenerated LiOH solution was exposed to ambient laboratory air (CO2 concentration: 504.2 ± 8.4 ppm) at 200 sccm for ∼23 hours (Fig. 3e). The initial catholyte pH in Mode II was 11.09 ± 0.57; after electrochemical regeneration, it increased to 13.16 ± 0.07, confirming strong alkalinity. During DAC, the pH decreased from 13.16 ± 0.07 to 11.09 ± 0.57, consistent with OH− conversion to CO32− upon CO2 absorption. Centrifugation of the CO2-loaded solution produced 78.6 ± 8.2 mg of solid Li2CO3, corresponding to a CO2 capture faradaic efficiency of 67%.
Theoretically, Mode I and Mode II should operate for equivalent durations. Experimentally, however, Mode II electrolysis completed 495 seconds faster than Mode I (Fig. 3b). Gas evolution during Mode II operation and solid precipitates observed in post-operation are attributed to partial reaction of reduced AQDS species with CO2, forming a precipitate. Cyclic voltammograms confirmed significantly reduced AQDS. Cyclic voltammograms confirmed significantly reduced AQDS redox reversibility under CO2 versus N2 (Fig. 3f). The underlying mechanism involves dissociation of AQDSH2 into H+ and AQDS2−; the H+ reacts with Li2CO3 to liberate CO2, which then reacts with AQDS2− to form the AQDS(CO2)22− precipitate. This process, described by reactions (1)–(3) in Fig. 4, aligns with literature reports.12
 | ||
| Fig. 4 Reaction equations between AQDSH2 and CO2. The liberated H+ then reacts with Li2CO3 to release CO2, which subsequently combines with AQDS2− to form the AQDS(CO2)22− precipitate. | ||
3.3 Comparison of energy consumption
The energy consumption for CO2 capture varies across different systems. For systems where by-products are generated, such as in water electrolysis-based CO2 capture,23 the total system energy consumption must be subtracted by the Gibbs free energy of generating the by-products (e.g., hydrogen) to obtain the net energy consumption for CO2 capture. In this work, under Mode II, the hydrogen generated is consumed by Mode I, resulting in net zero hydrogen production and no other by-products. Consequently, the total system energy consumption corresponds directly to the energy required for CO2 capture. The minimum theoretical energy input for CO2 capture via this AQDS-mediated process is 173 kJ mol−1 CO2, calculated under ideal conditions assuming 100% molecular utilization and no side reactions between reduced AQDS and CO2. However, under actual operating conditions (40 mA cm−2), unavoidable side reactions with CO2 cause precipitate formation, increasing the practical energy demand to 257 kJ mol−1 CO2 for the coupled electrolyzer-hydrogen pump system. This corresponds to a practical faradaic efficiency of 67%. Table 1 benchmarks this system against other redox-active DAC molecules, revealing that the our ideal energy consumption is notably lower than previously reported values. We also compare the energy consumption of several common DAC methods. It is evident that the multi-chamber system can significantly reduce operational energy consumption. The comparison further demonstrates that the energy consumption of the this work remains at a relatively low level within this field.| Type | Mechanism | CO2 separation work inputs (kJ per mol CO2) | Current density (mA cm−2) | Ref. |
|---|---|---|---|---|
| a In the table indicates the faradaic efficiency under ideal conditions (100% molecular utilization). | ||||
| Multi-electrolyzer system (using redox active molecules) | This work | 173a | 40 | — |
| 257 | ||||
| BPPV alternating electrocatalysis | 202a | 40 | 20 | |
| 220 | ||||
| Iodide alternating electrocatalysis | 238a | 40 | 21 | |
| 264 | ||||
| Multi-electrolyzer system (no redox active molecules) | Porous solid-electrolyte reactor | 299 | 3 | 24 |
| Ferricyanide coupled DOC using BPMED | 155 | 3.3 | 25 | |
| DAC using bipolar membrane electrodialysis (BPMED) | 462 | 8.6 | 26 | |
| Single-electrolyzer systerm | Traditional alkaline sorbent regeneration | 458 | 10 | 27 |
| Fuel cell concentrator | 809 | 20 | 28 | |
| Alkaline sorbent regeneration through anion exchange resin | 374 | 5 | 29 | |
3.4 Electrolytic cell cycling and analysis
We conducted five consecutive Mode I/II cycles at 40 mA cm−2 (Fig. 5a). A sharp voltage increase occurred during the second Mode II cycle, attributed to precipitate formation from AQDS-CO2 reactions depositing onto the carbon felt. This deposit blocked electroactive sites and impeded redox kinetics, as confirmed by post-operation disassembly. After cleaning the carbon felt, normal performance resumed in the third cycle. To assess long-term effect, cycles four and five proceeded without cleaning, leading to rapid performance decay. Filtering the central compartment solution post-cycling collected visible precipitate, confirming in situ precipitate formation (Fig. 5b). SEM analysis further substantiated the degradation mechanism: cycled carbon fibers showed extensive coating by solid deposits (Fig. 5d), contrasting sharply with the pristine pre-cycling structure (Fig. 5c). Collectively, these results demonstrate that electrode fouling by precipitates drives the observed electrochemical degradation.The precipitated molecules can be regenerated into AQDSH2 by the addition of hydrochloric acid. Upon evaporation of the solvent under ambient air conditions, the AQDS molecules can be recovered for reuse. However, this process involves additional costs and energy consumption. The deactivation of AQDS molecules leading to electrode passivation is a challenge specific to AQDS molecules, and quinone compounds in general, rather than an inherent flaw in the overall concept. This issue is addressable and can be effectively mitigated through future molecular design strategies. Therefore, future efforts should focus on developing advanced redox-active molecules that satisfy three key criteria: (i) stability across a wide pH range, (ii) a CO2-resistant reduced form, and (iii) a pH-tunable redox potential, to enhance the performance and durability of the electrochemical DAC systems.
4 Conclusions
In summary, we developed an electrochemical direct air capture system leveraging oxygen-tolerant hydroxide capture solutions and pH-dependent redox mediators to achieve low-energy CO2 capture. At 40 mA cm−2, the ideal energy consumption is 173 kJ mol−1 CO2 (100% molecular utilization). In practical operation, however, side reactions between reduced AQDS and CO2, cause molecular deactivation and precipitate formation, increasing energy demand to 257 kJ mol−1 CO2 and compromising cycling stability. Consequently, molecular design of advanced redox-active compounds emerges as the critical pathway to improve robustness and long-term performance in this DAC approach.Author contributions
Xiangyu Zhang: writing – original draft, methodology, investigation, data curation, conceptualization. Yun Zhao: writing – review & editing, supervision, funding acquisition, conceptualization. Yangkai Han: investigation, data curation. Zhiwei Ren: investigation. Tao Wei: investigation. Riyang Huang: data curation. Zhigang Shao: supervision.Conflicts of interest
The authors declare no conflicts of interest.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Supplementary information (SI) is available. See DOI: https://doi.org/10.1039/d5ra09310d.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 22279134) and LiaoNing Revitalization Talents Program (No. XLYC2203150).Notes and references
- J.-L. Dufresne, V. Eymet, C. Crevoisier and J.-Y. Grandpeix, J. Clim., 2020, 33, 3827–3844 Search PubMed.
- K. Hu, G. Huang, P. Huang, Y. Kosaka and S. P. Xie, Nat. Geosci., 2021, 14, 377–382 CrossRef CAS.
- A. Mikhaylov, N. Moiseev, K. Aleshin and T. Burkhardt, J. Entrep., Sustain. Issues, 2020, 7, 2897–2913 Search PubMed.
- G. Li and J. Yao, Engineering, 2024, 5, 1298–1336 CrossRef.
- J. Wang, R. Fu, S. Wen, P. Ning, M. H. Helal, M. A. Salem, B. B. Xu, Z. M. El-Bahy, M. Huang, Z. Guo, L. Huang and Q. Wang, Adv. Compos. Hybrid Mater., 2022, 5, 2721–2759 CrossRef CAS.
- V. Chanal, S. Humpage and M. Millinger, Environ. Res. Lett., 2025, 20, 044047 Search PubMed.
- B. Francesco, G. Claudia, M. Fabrizio and P. Maurizio, ACS Sustainable Chem. Eng., 2020, 8, 14013–14021 CrossRef.
- L. Qiu, N. Mokhtarinori, H. Liu, D. e. Jiang, Z. Yang and S. Dai, Mater. Today Energy, 2025, 47, 101740 CrossRef CAS.
- C. Radu, Chem. Sci., 2021, 12, 12518–12528 RSC.
- Q. Sun, J. Xiong, H. Gao, T. Sema, W. Olson and Z. Liang, Green Chem., 2023, 25, 4647–4655 Search PubMed.
- K. Sun, M. Tebyetekerwa, H. Zhang, X. Zeng, Z. Wang, Z. Xu, T. E. Rufford and X. Zhang, Adv. Energy Mater., 2024, 14, 2400625 CrossRef CAS.
- Y. Jing, K. Amini, D. Xi, S. Jin, A. M. Alfaraidi, E. F. Kerr, R. G. Gordon and M. J. Aziz, ACS Energy Lett., 2024, 9, 3526–3535 Search PubMed.
- L. Luo, L. Hou, Y. Liu, K. Wu, Y. Zhu, H. Lu and B. Liang, Energy Fuels, 2021, 35, 12260–12269 Search PubMed.
- S. Pang, S. Jin, F. Yang, M. Alberts, L. Li, D. Xi, R. G. Gordon, P. Wang, M. J. Aziz and Y. Ji, Nat. Energy, 2023, 8, 1126–1136 CrossRef CAS.
- H. Seo, M. P. Nitzsche and T. A. Hatton, Acc. Chem. Res., 2023, 56, 3153–3164 CrossRef CAS PubMed.
- Y. Guo, M. Massen-Hane, G. Endy and T. A. Hatton, Adv. Mater., 2024, 36, 2407567 Search PubMed.
- X. Li, X. Zhao, Y. Liu, T. A. Hatton and Y. Liu, Nat. Energy, 2022, 7, 1065–1075 Search PubMed.
- Y. Liu, H.-Z. Ye, K. M. Diederichsen, T. Van Voorhis and T. A. Hatton, Nat. Commun., 2020, 11, 2278 Search PubMed.
- M. Abdinejad, H. Seo, M. E. L. M. Hane and T. A. Hatton, Angew. Chem., Int. Ed. Engl., 2024, 63, e202412229 Search PubMed.
- S. Liu, J. Zhang, F. Li, J. P. Edwards, Y. C. Xiao, D. Kim, P. Papangelakis, J. Kim, D. Elder, P. De Luna, M. Fan, G. Lee, R. K. Miao, T. Ghosh, Y. Yan, Y. Chen, Y. Zhao, Z. Guo, C. Tian, P. Li, Y. Xu, E. H. Sargent and D. Sinton, Energy Environ. Sci., 2024, 17, 1266–1278 RSC.
- Y. Xu, S. Liu, J. P. Edwards, Y. C. Xiao, Y. Zhao, R. K. Miao, M. Fan, Y. Chen, J. E. Huang, E. H. Sargent and D. Sinton, Joule, 2023, 7, 2107–2117 Search PubMed.
- T. Liu, Y. Wang, Y. Wu, W. Jiang, Y. Deng, Q. Li, C. Lan, Z. Zhao, L. Zhu, D. Yang, T. Noël and H. Xie, Nat. Commun., 2024, 15, 10920 CrossRef PubMed.
- A. Prajapati, R. Sartape, T. Rojas, N. K. Dandu, P. Dhakal, A. S. Thorat, J. Xie, I. Bessa, M. T. Galante, M. H. S. Andrade, R. T. Somich, M. V. Rebouças, G. T. Hutras, N. Diniz, A. T. Ngo, J. Shah and M. R. Singh, Energy Environ. Sci., 2022, 15, 680–692 RSC.
- P. Zhu, Z.-Y. Wu, A. Elgazzar, C. Dong, T.-U. Wi, F.-Y. Chen, Y. Xia, Y. Feng, M. Shakouri, J. Y. Kim, Z. Fang, T. A. Hatton and H. Wang, Nature, 2023, 618, 959–966 CrossRef CAS PubMed.
- I. A. Digdaya, I. Sullivan, M. Lin, L. Han, W.-H. Cheng, H. A. Atwater and C. Xiang, Nat. Commun., 2020, 11, 4412 CrossRef CAS.
- M. D. Eisaman, K. Parajuly, A. Tuganov, C. Eldershaw, N. Chang and K. A. Littau, Energy Environ. Sci., 2012, 5, 7346–7352 RSC.
- S. Stucki, A. Schuler and M. Constantinescu, Int. J. Hydrogen Energy, 1995, 20, 653–663 CrossRef CAS.
- S. Matz, B. P. Setzler, C. M. Weiss, L. Shi, S. Gottesfeld and Y. Yan, J. Electrochem. Soc., 2021, 168, 014501 CrossRef CAS.
- Q. Shu, M. Haug, M. Tedesco, P. Kuntke and H. V. M. Hamelers, Environ. Sci. Technol., 2022, 56, 11559–11566 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |




