Open Access Article
Open Access ArticleAn overview on emerging green organic corrosion inhibitors: sustainable solution for oil and gas industrial applications
Nikhil Kumar
 *ad,
Pranav Anunay
b,
Sunil Kumar
cd,
Lalit Kumar Meena
*ad,
Pranav Anunay
b,
Sunil Kumar
cd,
Lalit Kumar Meena
 ad and
Dharmender Singh
ad and
Dharmender Singh
 e
e
aAdvanced Materials & Corrosion (AMC) Division, CSIR-National Metallurgical Laboratory (NML), Jamshedpur, Jharkhand-831007, India. E-mail: kumar.nikhil@nml.res.in
bDepartment of Metallurgical and Material Engineering, Indian Institute of Technology Kanpur, Uttar Pradesh – 208016, India
cMaterials Engineering (MTE) Division, CSIR-National Metallurgical Laboratory (NML), Jamshedpur, Jharkhand-831007, India
dAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad, Uttar Pradesh-201002, India
eChemical Division, National Test House (NTH-NR), Ghaziabad, Uttar Pradesh-201002, India
First published on 19th January 2026
Abstract
Corrosion remains a significant challenge across various industrial sectors, resulting in substantial economic losses, environmental hazards, and safety risks. Traditional corrosion inhibitors, though effective, often pose toxicity and environmental concerns, prompting the need for sustainable alternatives. The demand for eco-friendly and sustainable chemical species is increasing as industries seek to replace conventional toxic substances. This review emphasizes organic inhibitors derived from natural sources, such as amino acids, biopolymers, plant extracts, and environmentally benign synthetic compounds. Additionally, compounds synthesized through ultrasound and microwave irradiation, polyethylene glycols, ionic liquids, and multicomponent reactions are also considered environmentally friendly potential candidates to inhibit corrosion. The chemicals or compounds synthesized using water, ionic liquids, and supercritical carbon dioxide are also green corrosion inhibitors (CIs). Industrial processes such as oil extraction and gas transportation, where equipment are exposed to highly acidic environments, face significant corrosion challenges. Therefore, organic, green, eco-friendly, and cost-effective CIs are the primary demands of industry, researchers, and society. The development of new efficient green CIs can lead to the current research towards a sustainable pathway for future generations. In this context, researchers are continually working to develop more efficient CIs with the least hazardous environmental effects. The mechanisms of action, structural characteristics, and adsorption behaviour of OCIs are discussed in detail, along with their applicability in corrosive environments, such as oil wells, pipelines, and marine systems. This review provides a comprehensive overview of corrosion fundamentals, different classes of OCIs, key factors responsible for corrosion inhibition, mechanistic pathways, molecular modelling, and artificial neural networks for determining the rate of corrosion, followed by a future prospective. This review aims to support ongoing research efforts towards the sustainable corrosion control, offering valuable insights for scientists, engineers, and industries striving for greener and more cost-effective corrosion mitigation strategies.
Introduction
Corrosion in offshore platforms, oil wells, pipelines, and other infrastructure is a significant problem that has existed for a long time. It accounts for billions of dollars in annual production losses and repairs in oil and gas sectors as well as the loss of valuable lives.1,2 Further, the oil and gas industrial processes employ hazardous gases and acidic solutions, which are extremely corrosive and aggressive. Acids can cause the evolution of hydrogen gas (H2), which further results in hydrogen embrittlement and failures of metallic structures due to corrosion. One such industrial procedure is acid pickling, which is used to clean metal plates, wires, pipes, and machinery such as heat exchangers, cooling systems, and boilers of rust and surface contaminants. Many acids are frequently employed for this function, including HCl, H2SO4, H3PO4, HNO3, H3NSO3, C6H8O7, and HF acids.3,4 Descaling is another industrial process that is utilized to remove hard-to-remove metal oxides from the surface, such as high-temperature scale and rust, by mechanical or chemical means, and is often followed by pickling. Oil well acidification involves injecting extremely concentrated acidic solutions down a well through metallic pipes to increase the oil productivity by dissolving all acid-soluble components within underground rock formations or removing material at the wellbore face. A conventional method, namely well-acidizing, is employed to increase the productivity of pipelines. It involves dissolving rock formations like calcite, limestone, and dolomite by introducing acids under high pressure into their pore spaces. This process aims to increase the size of current flow channels and develop new ones, which will improve the flow of water or natural resources to the wellbore. Acidizing is often combined with matrix acidizing procedures and hydraulic fracturing. Acids are also used to remove scale from newly drilled wells and to repair drilling mud damage. Since the tubular materials and equipment used in the acidizing process are exposed to temperatures up to 200 °C, as well as chemicals like H2S and CO2, effective corrosion prevention is required. The characteristics of the groundwater reservoir determine the choice of acids applied in acidizing. Acids like HCl, HF, acetic acid, formic acid and their combination with other substances are often utilised. For instance, HF is suitable for sandstone deposits whereas HCl is regularly used for carbonate-based reserves. Mud acid, a solution of HCl and HF, is sometimes used in particular applications. The type, concentration, temperature, fluid velocity, and formation material of the acid are responsible factors for its reactivity. Heterocyclic compounds are utilised as CIs to reduce corrosion of the metallic tubes used in such operations. To reduce the adverse effects of acids and safeguard the metallic structures engaged, CIs must be carefully chosen and used.4–6 The combination of two dissimilar metals used in pipelines leads to different forms of corrosion, including uniform corrosion, localised corrosion, internal corrosion, stress corrosion cracking, and galvanic corrosion, which occur in the petroleum sector. CIs must thus be used to reduce the corrosive effects, assuring the safety and efficiency of the acidizing process. Although regular chemical treatments are required for corrosion control, carbon steels (CS) are the most often used type of steel in the petroleum sector due to their affordable cost. Alternatives that are more resistant to corrosion can be used, such as austenitic or duplex stainless steels, although they are costlier and prone to chloride corrosion. Since API N80 CS is a steel that is often used for downhole tubular, flow lines and pipelines, the majority of research on CIs has been conducted on this material. At temperatures exceeding 93.3 °C, the corrosion resistance of various steel types might vary by up to 35%.5 Different type of industrial applications of CIs are portrayed in Fig. 1.A large number of organic and inorganic reagents like ammonia, thiourea, and heterocyclic compounds containing oxygen (O), nitrogen (N), sulphur (S), or phosphorus (P) are utilized in the HCl pickling of mild steel and carbon steel. Similarly, sulphuric acid pickling uses organic amines, quaternary ammonium salts, urea derivatives, and other substances to fight corrosion. The development of CIs is difficult as nitric acid pickling is extremely oxidizing, yet substances like hydrazine, Na2S, NH4SCN, thiourea, and others are utilized. Heterocyclic substances such as triazole, quaternary amines, benzotriazole, derivatives of urea, polyvinylpyrrolidone, imidazoline, and polyethyleneimine are needed for phosphoric acid pickling.3–7 The research and development (R&D) fraternity across industries and academia has been continuously putting efforts into minimizing the economic and environmental impact of corrosion through various innovative solutions. Green inhibitors are commonly referred to as environmentally friendly corrosion inhibitors (CIs), which prevent corrosion of metals and alloys while reducing their detrimental effect on the environment. The current demand is to replace traditional CIs, which are associated with hazardous environmental impact, with a newer class of non-toxic CIs. More precisely, inorganic CIs incorporate either heavy metals or other hazardous substances, which pose a danger to humans and the ecological system. Besides direct influence, these compounds may leak into the environment during usage or disposal, resulting in soil contamination or water body pollution.8 Additionally, inorganic inhibitors may not offer appropriate multipurpose protection in various corrosive conditions or for range of metals and alloys since they are developed for specific types of corrosion or metal/alloy substrates. This inherent specificity or lack of multipurpose protection limits their use in particular fields or environments where a broader spectrum of corrosion protection is necessary. Stability problems may also affect the performance of inorganic inhibitors, as few substances are likely to degrade or decompose over time, reducing their effectiveness and necessitating regular reapplication. Such limitations pose a significant concerns for long-term applications like infrastructure or offshore industries that require long-term corrosion preventive solution. In addition, inorganic inhibitors could also face compatibility issues with other type of coatings, paints, or protective layers, which can diminish the overall performance, impair adhesion, or trigger adverse chemical interactions that accelerate corrosion.1,2,8
To ensure that CIs are effective and suitable for a range of applications, various criteria must be considered while choosing them for corrosion protection. It should be inexpensive i.e. economically feasible, easy to obtain, and readily available. CIs should be regulated by environmental laws to support sustainable practices and be in agreement with environmental protection and conservation. Additionally, the CIs should preferably be made from renewable resources, which will lessen dependence on scarce resources and ensure a continuous supply of inhibitors. Researchers and enterprises may use CIs that offer efficient protection while reducing environmental impact and support sustainable practices by taking these considerations into account.7,9 A variety of CIs are used to prevent corrosion of metals and alloys; scavenging inhibitors are one kind and it works by eliminating aggressive chemicals from the corrosive medium by trapping or reacting with volatile gaseous substances to capture them or inhibit oxygen-induced corrosion. In packaging for storage or display, scavenger inhibitors are frequently utilized. Another type is interface inhibitor which prevents rusting by establishing a barrier at the metal–environment contact point. This interface inhibitor can be divided into two groups: liquid phase inhibitors and vapour phase inhibitors. Furthermore, vapour phase CIs are employed for the prevention of small atmospheric corrosion particularly in enclosed situations by establishing a protective environment by lightly saturating wrapping paper inside a closed container. The most often used kind of CIs are liquids, which may be further divided into anodic, cathodic or mixed types depending on the type of reaction. Overall, these innumerable CIs either remove aggressive materials, create protective layers, or modify electrochemical processes at the metal surface to effectively prevent corrosion.3,7 The individual corrosion scenario and the intended level of protection must be taken into account when choosing an inhibitor. This review demonstrates basic corrosion and its types, background and current scenario of OCIs, factors affecting corrosion, mechanistic pathway, molecular modelling, artificial neural network to determine the rate of corrosion and future applications of organic CIs especially in oil well and pipelines.
Corrosion in oil and gas industries
In the oil and gas sector, corrosion is a major problem because it may result in the deterioration of pipelines, infrastructure, and equipment, posing a risk to public safety, causing environmental harm, and resulting in financial losses. The infrastructure of oil and gas industries is under constant threat due to several types of corrosion, such as uniform corrosion and different types of localized corrosion. Uniform corrosion is the most predictable type of corrosion that happens evenly throughout the metal surface, and it is controllable with protective coatings and routine maintenance. On the other hand, localised corrosion comprises galvanic, pitting, crevice, inter-grannular, filiform corrosion and stress corrosion cracking. Pitting corrosion is recognized by the formation of small and deep holes on the metal surface, which are often challenging to detect until severe damage has occurred. In small areas where the corrosive environment becomes trapped, crevice corrosion happens due to the formation of differential aeration or concentration cells. The filiform corrosion typically occurs on painted or coated surfaces and results from inadequate surface preparation. Further, galvanic corrosion occurs when two dissimilar metals are electrically coupled in a conductive electrolyte, causing the more anodic (less noble) metal to undergo accelerated dissolution relative to the cathodic counterpart. The inter-grannular corrosion (IGC) is another form of corrosion that preferentially occurs along the grain boundaries of metals/alloys and can crumble a structure without causing much weight loss. In the oil and gas sector, crack initiation and propagation can occur in corrosive environments under the influence of sustained tensile stresses, a phenomenon referred to as Stress Corrosion Cracking (SCC).1,2 At many places, this cracking phenomenon involves a hydrogen sulphide gas environment called sulphide stress cracking (SSC), and if somewhere hydrogen atoms seep into the metal, creating internal fractures called hydrogen-induced cracking (HIC). In some cases, the gas pipelines may contain fungus, bacteria, or algae, which can lead to a particular type of corrosion, i.e., microbiologically influenced corrosion (MIC). Also, the high velocities of corrosive fluids in pipelines may vastly accelerate the rate of material loss, and this phenomenon is known as erosion corrosion (EC) or flow assisted/accelerated corrosion (FAC). Further, the corrosion mechanisms and various forms of corrosion are discussed in the forthcoming section.Basic definition & classification of corrosion
The corrosion is a natural degradation process of metals/alloys resulting from chemical and eletcrochemical interactions with the surrounding environment. This progressive phenomenon gradually compromises the physicochemical and structural properties of the metallic components, often leading to severe consequences if left uncontrolled. Metals are particularly suscptible to corrosion, which typically occurs upon exposure to certain aggressive species oxygen, sulphur, chlorides, and other chemicals in presence of water.10 The interactions between metal and it's surrounding can produce corrosion products i.e. oxides, hydroxides or salts-that are thermodynamically more stable than the parent metal. The chemical reactions take places at cathode and anode in different kind of corrosions are displayed in Fig. 2. The type of corrosion that occurs relies on the factors such as the metallurgical charcteristics of componenets, environmental chemistry and the external forces. Having a comprehensive understanding of the corrosion causes and mechanisms facilitates the creation of efficient defences against this expensive and natural process. As reported in literature, corrosion can appear in various ways, comprising galvanic corrosion, uniform corrosion, pitting corrosion, crevice corrosion, intergranular corrosion and many others as discussed below. | ||
| Fig. 2 Pictorial representation of relevant chemical equations to corrosion investigation, reproduced from ref. 11 with permission from [Elsevier] [React. Funct. Polym., 2015, 95, 25–45] copyright 2015. | ||
If the corrosion is not controlled or stopped, the metal deterioration continues and may result into component failure ultimately. Thus, corrosion control is very much crucial and should be achieved carefully through viable means. The corrosion control can achieved by various techniques, like as the usage of corrosion-resistant materials, protective coatings, cathodic protection, and appropriate design and maintenance procedures. The various protection methodologies are discussed briefly below.
Corrosion protection methodologies
To date, several corrosion mitigation methodologies have been systematically adopted to protect metals/alloys with particular emphasis on pipelines in the oil, gas, and related industrial sectors. The appropriate choice of more corrosion-resistant alloys (nickel, titanium-based alloys, and stainless steel) is an important protection method owing to their superior resistance to harsh conditions encountered in the oil and gas industries. It is also important to make sure that different materials used in combination do not promote galvanic corrosion, which necessitates the careful selection of components with compatible electrochemical properties. Another widely employed corrosion protection approach involves the application of surface coatings, such as polymer linings, paints, and epoxy-based systems, which serve as protective barriers between the metal substrate and the corrosive environment.3,7 These coatings enhance durability by minimizing direct chemical interaction and improve resistance to abrasion, chemical attack, and moisture ingress, thereby extending the service life of critical infrastructure such as pipelines and process equipment.Further, the metallic coatings, such as galvanizing (Zn coatings) and aluminizing (Al coatings), proved efficient in protecting the underlying material, where a sacrificial layer (Zn/Al) corrodes preferentially and cathodically safeguards steel structures.12 Another important and most widely used corrosion protection method is cathodic protection, where the structural component that needs to be safeguarded is made cathode of the electrochemical system either by connecting to more anodic/reactive materials (in case of iron/steel, sacrificial anode; zinc, aluminium, or magnesium) or by supplying additional electrons to the system from an external DC power source thus hindered anodic reaction and reduced corrosion of the structure. Another corrosion protection technique is anodic protection, where an external electrical current is applied to the metallic structure to uphold it at sufficiently high anodic potentials (passivation zone), where a stable, passive, and protective oxide layer forms onto the surface. This technique is less popular than cathodic protection in the oil and gas industries, because it is restricted mainly to active-passive types metals/alloys and applied in some specialised circumstances, e.g. safeguarding the storage tanks that contain corrosive acids. An important methodology to control corrosion processes is adding chemicals to the corrosive environment, which are chemicals named as CIs (amines, phosphates, chromates or molybdates).13 These inhibitors develop a protective or barrier layer through a very thin coating and decrease the corrosiveness of the contiguous environment or hinder the electrochemical process of corrosion. The advantages and different types of inhibitors are as follows:
Usage of corrosion inhibitors
Several reasons offer the usage of appropriate CIs in terms of industrial suitability. CIs are economically cost-effective chemicals when considering the possible costs of maintenance, equipment failure and downtime. CIs are very easy to apply in any industrial process as it does not require any significant changes to existing systems and may be implemented simply. CIs are very flexible towards their surrounding environment, it may be acidic, oil, gas, aqueous. They also provide extra shielding to metal and alloys when combined with other well-known corrosion inhibition techniques like cathodic protection and coatings. Another important factor regarding CIs is the suitable place where it works. CIs are extensively used in boiler water systems, water injection systems and cooling water systems to stop the internal corrosion of pipelines and equipment. Inhibitors are the most recommended candidates employ to shield the tubing and well casing from H2S and CO2 in wells of oil and gas production.14,15 Furthermore, pipelines to carrying natural gas, crude oil and processed fluids are packed with inhibitors to stopover internal corrosion triggered by impurities and corrosive agents of fluid. Even, when machine or equipment is being shutdown, inhibitors are used due to the presence of stagnant fluids and surrounding environment which can accelerates corrosion. Inhibitors provide an alternate kind of protection in such circumstances when it is difficult to apply some protective coatings.Types of corrosion inhibitors
Numerous types of CIs are reported in literature with their applicability in various industrial sectors. Anodic inhibitors, which lowers the anodic reaction by producing a protective oxide layer on the metal sites through oxidation. Molybdates, nitrates, chromates and phosphates are well known examples and often used in aquatic situations where metals are susceptible to anodic corrosion such as cooling water systems. Cathodic inhibitors are also a well-recognized class of inhibitors, which also slow down the cathodic process, to create a protective layer on the cathodic regions via precipitation or by lowering the availability of reducible species such as oxygen. Examples include calcium bicarbonate, polyphosphates, and zinc salts. These inhibitors are widely used in boiler water treatment, cooling systems and other places where cathodic reactions must be regulated. Mixed type inhibitors works by affecting both the cathodic and anodic processes through creating a protective layer over the whole metal surface. Most common examples are phosphates, amine and nitro compounds based organic inhibitors, which are employed in various oil production activities as well as in cooling systems. Volatile CIs include mixtures of nitrite, phosphate and amine compounds utilized to prevent corrosion in metal parts during transportation and storage in enclosed systems such as pipelines, boilers and packaging. A popular class of inhibitor is organic inhibitors, which are renowned to make a hydrophobic (water-resistant) layer on the substrate metal surface and stops corrosive species from penetrating the metal. Some industrially active organic inhibitors are amines, thiols, imidazolines and certain surfactants and vastly incorporated as additives in water treatments, protective coatings and oil and gas production.16 Similarly, another class named inorganic inhibitors work effectively by forming a protective oxide film or passive coating on the metal surface to stops the corrosion. Chromates, silicates, molybdates, phosphates are few examples which frequently utilised as paint and coating additives, industrial water treatment systems and cooling systems. In addition, oxygen scavengers remove dissolved oxygen from the surrounding environment, thereby reducing the risk of oxygen-induced corrosion. Hydrazine, ascorbic acid and sulphites are well-known oxygen scavengers often applied in boiler water treatment systems.17 Another class of inhibitors is film-forming inhibitors, which works well by making a thin protective film that blocks corrosive species to come on the surface of metal, usually by chemical reaction or adsorption. Phosphate esters, fatty amines and polymers are utilised as film-forming inhibitors in pipelines carrying oil and gas, water treatment systems and producing wells. Last ten year and country wise classification of OCIs are portrayed in Fig. 4.Background of organic corrosion inhibitors
Organic compounds are recognized as potential CIs for different kind of metal and their alloys for last three decades (thirty years). A plethora of research has been done to explore the corrosion efficiency/anti-corrosion potential of different organic compounds and molecules. In this context, I. E. Uwah et al. reported an inhibitive action of ethanol extracts from leaves (LV), bark (BK) and roots (RT) of Nauclea latifolia on mild steel corrosion in H2SO4 solutions at 30–60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.6 A series of different fatty acid triazoles was developed by another research group (Quraishi and Jamal) and also evaluated them in HCl as CIs for cold-rolled mild steel (CRMS) and N-80 steel. Importantly, these substances recognized as highly safe for environment, show minimal toxicity and worked well as mixed-type inhibitors.18 Additionally, Quraishi and Jamal also reported an eco-friendly and economical 4-salicylideneamino-3-hydrazino-5-mercapto-1,2,4-triazolefor CRMS and N-80 steel. It demonstrated mixed-type inhibition using propargyl alcohol as the standard CI and does not release any harmful vapours.19 A class of oxadiazoles and dianils has been investigated as CIs for MS and CRMS. These substances have shown strong inhibition, with different inhibitory methods and performances depending on the substance.20 Yildirim and Cetin synthesized acetamide, isoxazolidine, and isoxazoline derivatives and found them to be very effective CIs for cold rolled low carbon steel. However, their practical use in oil-field applications may be limited due to the use of acetone and abrasive cleaning.21 Other researchers also synthesized various compounds such as mercapto-triazoles, isoxazolidines, 3,5-bis(n-pyridyl)-4-amino-1,2,4-triazoles, 3,5-bis(2-thienyl)-1,3,4-thiadiazole, gemini surfactants, thiosemicarbazides, quinoline derivatives, imidazolines, thiocarbohydrazides and tested their corrosion inhibition efficiency for different metals and acid environments. On the contrary, naturally occurring plants-based Henna extract and its key constituents such as α-D-glucose, lawsone, gallic acid, and tannic acid have been studied as CIs. They act as mixed-type inhibitors and can prevent corrosion, but their efficiency decreases with increasing temperature.22 Justiciagendarussa plant extract contains components such as friedelin,β-sitosterol, lupenol, ortho-substituted aromatic amines, phenolic dimmers, and flavonoids. It also acts as aninhibitor of mixed-type, but its corrosion inhibition effectiveness decreases at higher temperatures due to compound decomposition.23 Three important amino acids (glycine, alanine, and leucine) have also been investigated as efficient CIs at certain concentrations. However, at lower concentrations, they may promote corrosion, potentially due to complexation with iron (Fe).24 A detailed literature review was carried out for already reported OCIs (Table 1, Appendix A), inhibitor source with protective metals (Table 2, Appendix B). For additional details please refer to the Appendix.
°C.6 A series of different fatty acid triazoles was developed by another research group (Quraishi and Jamal) and also evaluated them in HCl as CIs for cold-rolled mild steel (CRMS) and N-80 steel. Importantly, these substances recognized as highly safe for environment, show minimal toxicity and worked well as mixed-type inhibitors.18 Additionally, Quraishi and Jamal also reported an eco-friendly and economical 4-salicylideneamino-3-hydrazino-5-mercapto-1,2,4-triazolefor CRMS and N-80 steel. It demonstrated mixed-type inhibition using propargyl alcohol as the standard CI and does not release any harmful vapours.19 A class of oxadiazoles and dianils has been investigated as CIs for MS and CRMS. These substances have shown strong inhibition, with different inhibitory methods and performances depending on the substance.20 Yildirim and Cetin synthesized acetamide, isoxazolidine, and isoxazoline derivatives and found them to be very effective CIs for cold rolled low carbon steel. However, their practical use in oil-field applications may be limited due to the use of acetone and abrasive cleaning.21 Other researchers also synthesized various compounds such as mercapto-triazoles, isoxazolidines, 3,5-bis(n-pyridyl)-4-amino-1,2,4-triazoles, 3,5-bis(2-thienyl)-1,3,4-thiadiazole, gemini surfactants, thiosemicarbazides, quinoline derivatives, imidazolines, thiocarbohydrazides and tested their corrosion inhibition efficiency for different metals and acid environments. On the contrary, naturally occurring plants-based Henna extract and its key constituents such as α-D-glucose, lawsone, gallic acid, and tannic acid have been studied as CIs. They act as mixed-type inhibitors and can prevent corrosion, but their efficiency decreases with increasing temperature.22 Justiciagendarussa plant extract contains components such as friedelin,β-sitosterol, lupenol, ortho-substituted aromatic amines, phenolic dimmers, and flavonoids. It also acts as aninhibitor of mixed-type, but its corrosion inhibition effectiveness decreases at higher temperatures due to compound decomposition.23 Three important amino acids (glycine, alanine, and leucine) have also been investigated as efficient CIs at certain concentrations. However, at lower concentrations, they may promote corrosion, potentially due to complexation with iron (Fe).24 A detailed literature review was carried out for already reported OCIs (Table 1, Appendix A), inhibitor source with protective metals (Table 2, Appendix B). For additional details please refer to the Appendix.
Different classes of OCIs
 | ||
| Fig. 5 The basic chemical structure of biopolymers (cellulose and starch) which utilized as corrosion inhibitors, reproduced from ref. 25 with permission from [MDPI] [Polymers (Basel), 2017, 9, 523] copyright 2017. | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups, prevent corrosion. Alkaloids are a different class of well-known plant extracts that are utilised as CIs. Alkaloids have aromatic structures and nitrogen-containing heterocyclic rings that offer good inhibitory characteristics. Inhibiting corrosion processes and blocking the passage of hostile species like oxygen and water, they make a stable complex with the metal surface. A subclass of polyphenols called flavonoids has also demonstrated incredible promise as a CI. Strong adsorption onto the metal surface is made possible by the complex aromatic ring structures of these molecules, which contain a variety of functional groups including hydroxyl, methoxy, and carbonyl. They can also prevent some well-known forms of corrosion (pitting corrosion and stress corrosion cracking), in accumulation to acting as anodic/cathodic inhibitors.
O) groups, prevent corrosion. Alkaloids are a different class of well-known plant extracts that are utilised as CIs. Alkaloids have aromatic structures and nitrogen-containing heterocyclic rings that offer good inhibitory characteristics. Inhibiting corrosion processes and blocking the passage of hostile species like oxygen and water, they make a stable complex with the metal surface. A subclass of polyphenols called flavonoids has also demonstrated incredible promise as a CI. Strong adsorption onto the metal surface is made possible by the complex aromatic ring structures of these molecules, which contain a variety of functional groups including hydroxyl, methoxy, and carbonyl. They can also prevent some well-known forms of corrosion (pitting corrosion and stress corrosion cracking), in accumulation to acting as anodic/cathodic inhibitors.Through experimental techniques together with weight loss test, electrochemical measurements, and surface analysis methods like scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS), the inhibitive capabilities of these plant extracts have been investigated and validated. Plant extracts can be applied directly to the metal surface as coatings to prevent corrosion or combined with other protective agents to increase their potency. To generate hybrid materials that provide prolonged and regulated corrosion protection, plant extracts can also be added to polymer coatings or nano-composites. The stability and solubility of plant extracts in the targeted environment must be taken into account. Under certain circumstances, some plant extracts may deteriorate quickly, reducing their long-term inhibitive power. As a result, research is still being done to improve their functionality and create formulations that are appropriate for certain uses. Overall, the use of plant extracts as efficient and environmentally benign CIs has shown significant potential.28 The usage of these natural chemicals in corrosion prevention for diverse sectors and applications is anticipated to acquire greater momentum as the globe looks for sustainable and environmentally friendly alternatives. A schematic diagram for the process used to make plant extracts as CIs is demonstrated in Fig. 6.
 | ||
| Fig. 6 The laboratory process for the formation of corrosion inhibitors from plants extract, reproduced from ref. 5 with permission from [RSC] [Mater. Adv., 2021, 2, 3806–3850] copyright 2021. | ||
Nonsteroidal anti-inflammatory drugs (NSAIDs), for example, and other pharmaceuticals and medications that include aromatic rings have also demonstrated inhibitive characteristics. Stable adsorption layers can emerge as a result of interactions between the pi-electrons (Π electrons) of aromatic rings and surfaces of metal substrates. On metals like steel and aluminium, the ability to reduce corrosion by substances like ibuprofen and naproxen has been investigated. Some drug molecules feature functional groups, as carboxylate (–COOH) and hydroxyl (–OH) groups, that allow them to chemisorb or physisorb on the metal surface.30 A medication having carboxyl groups that has demonstrated inhibitory efficacy against aluminium and carbon steel is aspirin (acetylsalicylic acid). The responsible functional groups for corrosion inhibition in a medicine is portrayed in Fig. 7.
 | ||
| Fig. 7 The responsible functional groups of imidazole family as corrosion inhibitors, reproduced from ref. 29 with permission from [Springer] [BMC Chem., 2019, 13, 136] copyright 2019. | ||
CIs can also be found in inorganic medicines and substances. For instance, the ability of substances containing phosphorus, nitrogen, and sulphur atoms, such as phosphates, amines, and thiols, to suppress corrosion on metals like iron and copper has been studied. Chemical medications inhibitive methods include adsorption phenomenon onto the metal surface, resulting in the creation of a barrier that stops corrosive species alike oxygen, water molecule, and ions from reaching to the metal. Anodic, cathodic, or mixed inhibitory effects may be responsible for the inhibition process, liable on the particular drug and metallic system,31,32 which is significant to highlight that while some chemical medications may have qualities that reduce corrosion, their usage as specific CIs are not common for a variety of reasons. First off, the dosage needed to effectively prevent against corrosion may go over safe therapeutic limits. To guarantee long-term efficacy as CIs, it is also necessary to properly analyse the medications stability and compatibility in various settings and applications. In conclusion, due to their distinct chemical compositions and interactions with metal surfaces, chemical medications and medicines have shown potential as CIs. To maximise their inhibitory effects and determine their viability in certain corrosion prevention applications, more study is required.
 | ||
| Fig. 8 Representation of ionic liquids as corrosion inhibitors, reproduced from ref. 34 with permission from [Elsevier] [J. Mol. Liq., 2021, 321, 114484] copyright 2021. | ||
 | ||
| Fig. 10 Chemical substance synthesized by MCRs utilized as corrosion inhibitor, reproduced from ref. 38 with permission from [Springer] [Sci. Rep., 2017, 7, 44432] copyright 2017. | ||
Additionally, scalability and cost-effectiveness are advantages of ultrasonic and microwave-assisted synthesis. These methods are appropriate for industrial applications since they are easily modified for mass production. Cost reductions in the production process are also a result of faster reaction times and higher yields. In conclusion, potent instruments for producing OCIs include ultrasonic and microwave irradiations. They are useful in the synthesis of various inhibitor structures with enhanced characteristics because of their capacity to increase reaction rates, selectivity, and scalability.39 Researchers may definitely anticipate more progress and advancements in the designing of OCIs for more effective and long-lasting corrosion resistance as research on these approaches continues.
In line with the ideals of green chemistry, the usage of green solvents as CIs promotes more environmentally friendly and long-lasting corrosion prevention techniques. By using environmentally friendly solvents, businesses may lessen their impact on the environment and cut back on the production of hazardous waste. It is crucial to remember that depending on the exact application and environment, green solvents may or may not work as CIs. To assure their effectiveness and compatibility with various metal surfaces and corrosion conditions, thorough testing and assessment are required. In conclusion, green solvents provide a more ecologically responsible and long-lasting method of corrosion inhibition. They are appealing substitutes for traditional solvents due to their low toxicity, biodegradability, and renewable nature.40 Industries may support a greener, more sustainable future while successfully protecting their metal assets from corrosion damage by implementing green solvents in corrosion protection.
Factors responsible for inhibition
An OCIs molecular framework is an essential variable in determining how efficient it is. Amino (–NH2), hydroxy (–OH), and carboxylate (–COOH) groups, among other functional groups, improve the inhibitor capacity to adsorb upon the metallic surface. These functional groups interact chemically with the metal to create chemical reactions that result in the development of a barrier. The inhibitor species or molecules connect with the metal surface by chemical bonding or weak contact, depending on the adsorption method, which might be physical or chemical.1,2 A graphic representation of all the responsible key factors for the performance of any CI (Fig. 12). | ||
| Fig. 13 Representation of types of adsorption and their strength for corrosion inhibitors, reproduced from ref. 41 with permission from [Springer] [Sci. Rep., 2021, 11, 3771] copyright 2021. | ||
 | ||
| Fig. 14 Representation of concentration effecting efficiency of corrosion inhibitors, reproduced from ref. 42 with permission from [Elsevier] [J. Colloid Interface Sci., 2017, 502, 134–145] copyright 2017. | ||
 | ||
| Fig. 15 Schematic illustration of inhibition film formation and sustainability at the ZE surface with surface over potentials ranging from 0 to +75 Mv, reproduced from ref. 43 with permission from [RSC] [Mol. Syst. Des. Eng., 2024, 9, 29–45] copyright 2024. | ||
 | ||
| Fig. 16 Representation of synergism effect for corrosion inhibition efficiency, reproduced from ref. 44 with permission from [RSC] [RSC Adv., 2020, 10, 15163–15170] copyright 2020. | ||
Several other factors responsible for influencing the performance of CIs includes: metal or alloy type with their chemical composition i.e. one CI works efficiently on steel but the same might not work well for aluminium due to variations in surface characteristics and chemical reactivity. Surface condition of metal substrates is an important factor i.e. the inhibitor's ability to adsorb and, in turn, its ability to provide protection can be impacted by the presence of oxides, pollutants, or surface roughness. The presence of an aggressive ion in surroundings may also affect the performance of CIs particularly in chlorides, sulphates and saline conditions. The concentration of CIs including optimal dosage and intrinsic efficiency of inhibitor are vital parameters to measure the activeness of inhibitor. The stability and solubility of inhibitor in the media definitely decides the efficiency of any CIs. In addition, the interaction with other chemical species and competition for active adsorption sites may influence the performance of the CIs. Mass transfer and flow dynamics including flow rate may allow CIs to build up and offer more protection to metal substrates.
Inhibition efficiency validation
The corrosion is a thermodynamically driven process which progresses through chemical and electrochemical reactions between metals/alloys with surrounding environments. However, thermodynamics alone may not help to understand the kinetics of the process at which it occurs. Thus, different techniques have been employed to elucidate the corrosion mechanism, kinetics and efficacy of inhibitor in various environments. The gravimetric analysis/weight loss measurements are commonly and most widely utilized to define the efficiency of organic corrosion inhibitors due to ease of experimentation and no additional requirement of costly equipment. Further, some modern electrochemical techniques such as potentiodynamic polarization, electrochemical impedance spectroscopy (EIS) and electrochemical noise analysis, are also being employed to define the efficacy of the inhibitors and to divulge the protection mechanisms involved. These modern techniques are also proved equally comparative to the conventional weight loss measurements to define the efficacy of the inhibitors in the laboratory conditions in accelerated manner. Despite the theoretical differences among these methodologies, the results exhibit a consistent trend in magnitude, suggesting that they are acceptable and can substantiate the efficacy of corrosion inhibition as discussed below:Conventional immersion exposures (CIE)
As mentioned above, the corrosion performance is largely monitored through the gravimetric analysis/weight loss method, which entails immersing/exposing the metal/alloy specimens in a simulated environment for a predetermined time duration. Following this exposure, the sample is retrieved, and the change in its weight before and after the exposure is measured45 (Table 3, Appendix C). This method is fundamental, reliable, and exact for determining the rate of metal corrosion. The metal sample is initially subjected to a pretreatment process prior to the experiment, which involves grinding with emery paper, followed by cleaning with double distilled water, degreasing with acetone, and finally drying. The specimen utilised for the measurement is weighed on a balance with a sensitivity of ±0.01 mg. It is also noted in the methodology that after the metal sample is immersed in various test solutions at a specific temperature (avoiding temperature influence) for a specific duration with and without different quantities of inhibitors. Following the execution of the experiment, the sample was subsequently washed, dried, and weighed. The inhibitor concentration for weight loss and the electrochemical study was measured in mg L−1. More often, the experiments are conducted with triplicate set of specimens as advised in the standard ASTM-G31 and the averaged values is taken into account.46,47 Surface coverage (θ), inhibition efficiency (IE, %), and corrosion rate (CR) are determined using the following equations:
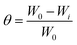 | (1) |
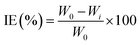 | (2) |
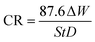 | (3) |
As mentioned previously, the weight loss method is very much reliable and advantageous in determing the efficiency of organic inhibitors (by eqn (2)). Mahdi et al. examined the corrosion resistance of mild steel in 1 M HCl solutions, both with and without the inhibitor 2,2′-(1,4-phenylenebis(methanylylidene)) bis(N-(3-methoxyphenyl) hydrazinecarbothioamide) (PMBMH), at various temperatures (30, 40, 50, and 60 °C) using the weight loss method.52 The weight change and corrosion rate derived for mild steel with varying quantities of corrosion inhibitors, found to indicate that the IE increases while the CR values decrease with increasing inhibitor concentrations irrespective of the exposure temperature as shown in Fig. 17. Further, the corrosion rate reported to increase and inhibition efficiency found to decrease with increasing temperature from 30 °C to 60 °C at all the inhibitor concentrations. The temperature-dependent reduction in IE is believed to result from diminished physical adsorption of the inhibitor on the mild steel surface, caused by an increase in hydrogen gas evolution with rising temperatures, leading to decreased physical attachment and elevated corrosion rates as compared to lower temperatures. However, the effect of temperature increase on IE is more pronounced at lower inhibitor concentrations as compared to higher concentrations where IE remained ≥ 90% at all temperatures (Fig. 17). The efficiency of inhibitors at higher concentrations across varying temperatures demonstrated consistency, which thought to be attributed to minimal or slight disturbance in adsorption and chemisorption occurance independently or in conjunction with physisorption.
 | ||
| Fig. 17 Plot showing the trend in mild steel corrosion rate (CR, left side) and inhibitor efficiency (IE, right side) with increase in PMBMH concentration in HCl solution at different temperatures. Reproduced from ref. 52 with permission from [MDPI] [Molecules, 2022, 27, 4857] copyright 2022. | ||
Electrochemical techniques
Corrosion inhibitors are known to control the metal corrosion through slowing down or hindering the anodic and/or cathodic electrochemical reactions occurring during the process. The corrosion inhibition is accomplished by suppressing the rate of the cathodic reduction and/or obstructing the anodic oxidative dissolution of metal.53 The use of cathodic inhibitors for corrosion inhibition entails multiple processes. Initially, these inhibitors elevate the overpotential of the cathodic reaction, thereby difficulty in the occurrence of corrosion and reduced rate of corrosion. Cathodic inhibitors obstruct the cathodic process via deposition at cathodic sites, either through the creation of insoluble compounds or by enhancing the metal's liability to hydrogen, thus, reduced corrosion due to low or non-availability of cathodic reactants. Cathodic inhibitors serve as a barrier, impeding electron passage and thereby diminishing the total corrosion rate. Anodic protection occurs when a corrosion inhibitor adsorbs onto the surface, creating an oxide layer on the metal surface. This protective film serves as a barrier, obstructing the anodic process. Anodic inhibitors which contains non-oxidizing ions, require external oxygen for safeguarding the metals against corrosion, whereas oxidising ions containing inhibitors can safeguard metals without the need for external oxygen. Further, there are certain inhibitors which supress both anodic and cathodic reactions to control the corrosion, known as mixed type of inhibitors. As, the inhibitors control the corrosion through electrochemical mechanisms, the electrochemical techniques are good tool to understand the type of inhibitors and subsequent mechanisms involved in the process.Open circuit potential (OCP)
The open circuit potential (OCP), often reffered to as the self-corrosion potential (Ecorr), represents the corrosion potential difference between the working electrode and reference electrode in the absence of current, is a crucial indicator of the corrosion or passivation state of the working electrode surface.53 The OCP measurement at zero current and the stabilized values reflect the equilibrium between anodic (metal dissolution) and cathodic (reduction) reactions. An inhibitor either doesn't alter much or pushes this equilibrium towards a more noble (positive) or active (negative) potential side depending upon its nature of protection. The organic inhibitors which form a protective barrier (passivation) via adsorbing onto the metal surface and blocks the corrosive species to reach the metal/solution interface, thereby shifting the OCP to more positive side and reduce the corrosion rates. Further, a stable OCP indicates the uniform protective film formation over the surface while a fluctuating OCP suggests the unstable film formation onto the surface. The large positive stable OCP values are often regarded as log-lasting protection while a sudden drop towards more negative values is considered as inhibitor depletion or protective film failure during the corrosion process. In addition, sometime a negative shift in OCP is observed but the inhibitor film protects the metal well, these inhibitors are named as cathodic inhibitors. The mixed type inhibitors cause a smaller shift in OCP but supresses both the anodic and cathodic reactions but need to be validated with other electrochemical techniques (PDP) for their mechanisms. Thus, the OCP measurement, can offer valuable insight into the type of inhibitor based on the potential shift obtained with the incorporation of the inhibitor into the process (Table 3, Appendix C). Dehghani et al. monitored the OCP of mild steel specimens in 1 M HCl solution with varying concentration of Ziziphora leaves as an inhibitor before conducting the EIS and PDP experiments.54 The OCP values reported to drop toward negative (active) potential side up to 20 minutes and later on became relatively stable both in unhibited or inhibited solutions (Fig. 18). The stabilisation is attributed to the formation of a stable iron oxide/hydroxide coating on mild steel in the absence of inhibitors, while in the case of inhibited HCl solutions, it is due to the complete adsorption of inhibitor molecules (Fig. 18). Evidence showed that increasing the concentration of Ziziphora leaves extract in the electrolyte led to lower OCP values towards more negative side but a complete stabilization, which means that the molecules of Ziziphora leaves extract were able to adsorb well and cover a lot of iron surfaces. Though the potentials were observed towards negative side but the shift remained <82 mV, thus the inhibiter can be called as a mixed type inhibitor. | ||
| Fig. 18 The open circuit potential (OCP) variation with immersion time for mild steel exposed in uninhibited and inhibited 1 M HCl solution (Inhibitor; different quantities of Ziziphora leaves extract); inset shows the enlarged view of region ‘A’ marked in red colour, reproduce from ref. 54, with permission from [Elsevier] [Constr. Build. Mater., 2020, 245, 118464] copyright 2020. | ||
Electrochemical impedance spectroscopy (EIS)
This technique is often being deployed to obtain the additional details on corrosion mechanisms during the interaction of inhibitor and metals surface (Table 3, Appendix C). EIS is used to determine the material's resistance and capacitance characteristics in absence and presence of inhibitor by applying small alternating current (AC) perturbations. The EIS spectra is obtained through varying frequency in certain range at open circuit potential (OCP). The output of EIS is plotted in form of Bode and Nyquist plots, where impedance and phase angle is plotted on y-axis against frequency at x-axis, and imaginary part of impedance is plotted on y-axis against real part of impedance at axis, respectively. The spectra is fitted with a suitable electrochemical equivalent circuit (EEC) to obtain polarization/charge transfer resistance (Rct) and double layer capacitance (Cdl). Also, the technique is utilized to determine the corrosion rates accurately in accelerated manner non-destructively. The inhibition efficiency is calculated through the equation mentioned below by utilizing the resistance data:
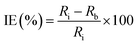 | (4) |
When the concentration of the inhibitor is increased, the value of Rct increases and the value of Cdl decreases if the inhibitor is well adsorbed.55 As previously mentioned, typically, the data obtained from instruments to create two forms of graphical representations that enhance the understanding of the corrosion inhibition process. To elucidate the graphical representation, an examples is taken for the EIS data derived from literature. The graphical representation comprises Fig. 19, Nyquist and Bode plots, pertaining to the two different inhibitors ethyl 2-(4-phenyl-1H-1,2,3-triazol-1-yl) acetate [Tria-CO2Et], and 2-(4-phenyl-1H-1,2,3-triazol-1-yl) acetohydrazide [Tria-CONHNH2] inhibitors utilised for the mitigation of MS corrosion.56 These plots shown a depressed semicircle, non-ideal with their center, which is related to different physical phenomena such as surface heterogeneity. EIS measurements revealed that both inhibitors exhibited a high inhibition performance, achieving an inhibition efficiency of 95.3% for [Tria-CO2Et] and 95.0% for [Tria-CONHNH2] with a concentration of 1.0 × 10−3 M. The Bode plot showed that the impedance value is increased with increasing amount of inhibitor in the HCl media and derived highest for 1.0 × 10−3 M levels of inhibitor addition. Also, the phase angle values were found to be shifted towards higher values with the presence of inhibitor. This indicated that the inhibitor has covered the metallic surface fully as indicated by the increased phase angle values (approaching towards −90° i.e. much closer to capacitive behaviour thereby much uniform surface). Further, the capacitive loop diameter revealed the presence of a thin layer of inhibitor on metal surface. Further, Yousif et al. utilized the EIS technique to study the inhibition mechanism of newly synthesized (S,E)-2-((1-(4-minophenyl)ethylidene)amino) (3-(4-hydroxyphenyl)propanoic acid), “AEPA”, and (2S,5R,6R)-3,3-dimethyl-7-oxo-6-propionamido-4-thia-1-azabicyclo[3.2.0] heptane-2-carboxylic acid, “DOCA”, for carbon steel in 1 M HCl solution.57 The fitted Bode and Nyqist plots obtained for various concentration of AEPA and DOCA are illustrated in Fig. 20a–d, respectively. The EIS data was fitted to the double time constant electrochemical equivalent circuit (EEC) as shown in inset of Fig. 20a and b. The EEC is comprised of circuit includes the solution resistance (Rs), film resistance (Rf), charge transfer resistance (Rct), constant phase element of the adsorbed film (CPEfilm) and double layer (CPEdl) with phase shifts n1 and n2, respectively.
 | ||
| Fig. 19 Nyquist and Bode plots for mild steel in 1.0 M HCl with and without various [Tria-CO2Et] and [Tria-CONHNH2] concentrations, reproduced from ref. 56 with permission from [RSC] [RSC Adv., 2021, 11, 4147–4162] copyright 2021. | ||
 | ||
| Fig. 20 Nyquist plots, Bode and phase angle plots for steel in 1.0 M HCl solution without and with different concentrations of AEPA (a and c), and DOCA (b and d) compounds at 30 °C, reproduced from ref. 57, with permission from [RSC] [RSC Adv., 2025, 15, 28666–28688] copyright 2025. | ||
The imperefect dippresed semi-cricle shape of all the curves revealed the similar type of corrosion mechanism over the entire frequency range which is charge transfer mechanism. Further, the imperfection in the shape was correlated to the surface roughness factor of steel specimens. The diameter of the semi-circles was reported to increase with increasing the concentration of AEPA and DOCA in the solution. This increase was attributed to the increase in charge transfer resistance value and inhibitor efficiency, suggesting that the efficacy of the inhibitor may be directly proportional to the concentration of inhibitors. An increase in charge transfer resistance suggested that a greater number of inhibitor species were adsorbed onto the surface as concentration rise. The adsorption created a film onto the surface thereby increase in the resistance (Rf) as concentration increased. This can be ascribed to the enhancement of local dielectric/insulation around the surface due to the augmented adsorption of the inhibitor. This adsorption of the inhibitor displaces water molecules and chloride ions from the surface, thereby reducing active corrosion sites and decreasing the corrosion rate. The ‘n’ values are often related to the electrolyte and metallic interface homoginity or hetroginity. The values of n1 and n2 in inhibited solution were determined much higher as compared to the blank solutions, which indicated a reduction in surface roughness due to the adsorption of inhibitor on to the surface, thereby reduced corrosion rates. The value of ‘n’ rises with an increase in inhibitor concentration, indicating that a superior anti-corrosion effect is achieved with higher inhibitor concentrations. This shows that the EIS is an effective tool to divulge the inhibition mechanism and efficacy of the inhibitors in an electrolyte.
Potentiodynamic polarization (PDP)
PDP is another healthy tool to study the corrosion mechanism and inhibitor efficacy in accelerated manner in the laboratory. This is carried out with the help of a potentiostat/Galvanostat and standard three electrode electrochemical cell comprising of working, counter and reference electrode. The specimens are scanned in a defined potential range at predetermined rates to obtain the cathodic and anodic current densities. The current response as a function of applied potential reveals several alterations, including the shape of polarisation curves and the positioning of anodic or cathodic current densities relative to the curve in absence of GCI, which can be utilised to assess the efficacy of GCIs in corrosion inhibition. Further, the kinetics of anodic and cathodic reactions can be comprehended by polarisation curves. Also, the PDP enables the measurement of corrosion kinetics and employed to determine the electrochemical parameters such as corrosion potential (Ecorr), corrosion current density (icorr), cathodic Tafel slope (βc), anodic Tafel slope (βa), and percentage of inhibition (IE, %) as a function of inhibitor concentration derived from the curves. The Tafel slopes (βa) and (βc) will be ascertained based on the mechanism of the process, which may involve inhibition through cathodic, anodic, or both types of reactions, while the Ecorr value will indicate the nature of the inhibitor employed.58,59 If the curve on the graph shifts towards a lower current density, it indicates a decrease in the corrosion rate.60
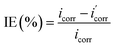 | (5) |
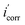 are the corrosion current density in absence and presence of the inhibitor in the medium, respectively.
are the corrosion current density in absence and presence of the inhibitor in the medium, respectively.
The Tafel polarisation curves illustrated in Fig. 21 have been documented in the literature, where AEPA and DOCA were employed to mitigate the corrosion rate of carbon steel in an acidic environment.57
 | ||
| Fig. 21 Potentiodynamic polarization curves for the corrosion of carbon steel in 1.0 M HCl in absence and presence of different concentrations of AEPA (a) and DOCA (b) compounds at 30 °C, reproduced from ref. 57, with permission from [RSC] [RSC Adv., 2025, 15, 28666–28688] copyright 2025. | ||
To study the mechanism of dissolution process of carbon steel, potentiodynamic polarization measurement were performed. Several parameters such as anodic and cathodic slopes (βa and βc in mV dec−1), corrosion current density (in mA cm−2), corrosion potential (in mV vs. SCE) and the inhibition efficiency (ηPDP)(%) were calculated by analysis of PDP curves. A drop in current density was observed in the polarization curves after the addition of both the inhibitors. The anodic and cathodic curves were marked to suppressed towards less current density values and Ecorr values were found to shift towards more negative side with incorporation of inhibitors. Also, the Ecorr values were shifted to beyond 85 mV in both the cases thus inhibitors can be considered cathodic type. Further, inhibition efficiency was increased linearly on carbon steel surface as concentration of the inhibitors (AEPA and DOCA molecules) increased in the solution.
Corrosion protection mechnanisms
The efficacy of inhibitor is closely linked to the adsorption tendency of the inhibitor molecules onto the metal surface. Thus, it is imperative to understand the adsorption isotherm involved to get the better insight of the corrosion prevention mechanisms. As, the corrosion progresses through the galvanic/electrochemical cell formation onto the metallic surface. Thus, efficient inhibitor prevent the corrosion by disruptin the interaction between various components in an corrosion cell such as elimination of local anode, cathode, or hindering the path of an electrolyte necessary for electron transport by effectively adsorbing onto the surface. Adsorption is influenced by various factors, including charge, ionic species, solvent adsorption, electrochemical potential at the solvent–solution interface, characteristics of the metal surface, reaction temperature, electronic properties, and the presence of electron-donating or electron-repelling groups of derivatives. Determining the degree of surface coverage by the inhibitor (θ) and assessing the adsorption isotherms through various mathematical models are crucial steps in comprehending corrosion mechanisms. The values of θ can be accurately derived using the gravimetric method of weight loss as mentioned in eqn (1).There are various adsorption isotherms mentioned in the literature which are the key factors to describe the interaction between sample and inhibitor (Table 4, Appendix D). Adsorption is validated if surface coverage data conforms to any isotherm. The graphical depiction of the isotherm should yield a linear representation, and the regression coefficient must equal one. The intercept of the adsorption isotherm provides the value of the adsorption equilibrium constant (Kads), which is subsequently utilised to characterise the free energy value.61 The universal representation of all isotherms is shown below:
| f (θ,X)exp(−2aθ) = KadsC | (6) |
ΔGads0 = RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kads Csolvent Kads Csolvent
| (7) |
 | ||
| Fig. 22 Various adsorption isotherm parameters determined for adsorption of Ziziphora leaves extract to mild steel in 1 M HCl solution with different concentrations (a) Langmuir, (b) Frumkin, (c) Temkin and (d) Freundlich adsorption isotherms, reproduce from ref. 54, with permission from [Elsevier] [Constr. Build. Mater., 2020, 245, 118464] copyright 2020. | ||
This adsorption was further confirmed by FT-IR and UV-Vis spectrophotometry characterization techniques. The FT-IR and UV-Vis spectra are portrayed in Fig. 23. The FT-IR elucidated the presence of polar functional groups and heteroatoms in the adsorption of inhibitor compounds onto the iron surface. The vibrations of O–H (3397 cm−1), C–H (2930 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1707 cm−1), C–O (1601 cm−1), C–C (1410 cm−1), and CH3 (1260 cm−1) were identified in the structure of Ziziphora leaves extract, which may facilitate the adsorption of extract molecules on the MS surface, thereby enhancing their anti-corrosion efficacy against corrosion. The extract of Ziziphora leaves contains several heteroatoms (e.g., O) that can be readily protonated and adsorbed by electrostatic interactions. The UV-Vis spectrum illustrated two distinct peaks prior to MS immersion in HCl (Fig. 21b). The initial peak at 225 nm indicates a π–π* transition, signifying the absorption of the C
O (1707 cm−1), C–O (1601 cm−1), C–C (1410 cm−1), and CH3 (1260 cm−1) were identified in the structure of Ziziphora leaves extract, which may facilitate the adsorption of extract molecules on the MS surface, thereby enhancing their anti-corrosion efficacy against corrosion. The extract of Ziziphora leaves contains several heteroatoms (e.g., O) that can be readily protonated and adsorbed by electrostatic interactions. The UV-Vis spectrum illustrated two distinct peaks prior to MS immersion in HCl (Fig. 21b). The initial peak at 225 nm indicates a π–π* transition, signifying the absorption of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond (aromatic). The second peak, centred at 331 nm, corresponds to the n–π* transition, demonstrating the absorption capacity of C
C bond (aromatic). The second peak, centred at 331 nm, corresponds to the n–π* transition, demonstrating the absorption capacity of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. After the immersion of MS substrate, the π–π* and n–π* transition peaks shifted to 221 and 326 nm, respectively. These differences indicated the sequential interaction of C
O groups. After the immersion of MS substrate, the π–π* and n–π* transition peaks shifted to 221 and 326 nm, respectively. These differences indicated the sequential interaction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds with iron cations, thereby successful adsorption on to the surface.
O bonds with iron cations, thereby successful adsorption on to the surface.
 | ||
| Fig. 23 (a) FT-IR and (b) UV-Vis spectra of Ziziphora leaves extract powder with and without MS substrate, reproduce from ref. 54, with permission from [Elsevier] [Constr. Build. Mater., 2020, 245, 118464] copyright 2020. | ||
Numerous studies have illustrated that the adsorption of inhibitor molecules results into a protective film formations containing specific chemical orientations, thereby no or very minimal corrosion of underlying substrate. The development of a protective film on metal surfaces is correlated with open circuit (OC) or self-corrosion potential of the metals/alloys. The moment open circuit potential becomes relatively stable, indicated the full surface coverage and development of the protective layer onto the substrate.66,67 Several surface characterization techniques including X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), atomic force microscopy (AFM) were used to characterize these organic thin films which forms on to the surface of the carbon-steel while immersed in inhibitor containing mediums. Song et al.68 examined the production of protective films on metal surfaces utilising XPS technique. According to the XPS spectra in Fig. 24a, the protective layer was mostly made up of iron oxide, hydroxyl oxide, and organic compounds that contained carbon. The XPS analysis led them to conclude that an organic coating with carbon formed on the steel surface, indicating that the plant extract may improve the steel sample's corrosion resistance. Simovic et al.69 examined the effectiveness of green corrosion inhibitors derived from black pine (Pinus nigra) oil for carbon steel. The contact angle measurements were utilized to look at the hydrophilic properties of inhibited and non-inhibited carbon steel samples according to ASTM standards [ASTM, D7490–13 standard test method for measurement of the surface tension of solid coatings, substrates and pigments using contact angle measurements, B ASTM Stand. 6 (2013) 1–5]. Fig. 24b shows the results of the contact angle test on metal that was in inhibitors for 1 and 4 hours and carbon steel that was in 1 M HCl for one hour. The higher contact angle of the inhibited sample compared to the non-inhibited one shows that there is an organic inhibitor barrier coating on the metal surface. The data also demonstrated that the hydrophobicity of the inhibited samples got better with time, showing that an inhibitor layer works as a shield against harsh conditions. They infered that the creation of a hydrophobic layer of inhibitors on the metallic surface.
 | ||
| Fig. 24 (a) XPS spectra of steels with plant extract added to the corrosive solution (1): wide-scan spectrum; (2) Fe 2p spectrum, (3) O 1s spectrum and (4) C 1s spectrum, reproduced from ref. 68, with permission from [Elsevier] [J. Ind. Eng. Chem., 2022, 111, 464–479] copyright 2022 and (b) Carbon steel contact angle measurements after being immersed in (1) 1 M HCl for 1 h; (2) 1 M HCl with 50 ppm PN for 1 h; (3) 1 M HCl with 50 ppm PN for 4 h, reproduced from ref. 69, with permission from [MDPI] [Metals, 2023, 13, 508] copyright 2023. | ||
Qiang et al.70 examined the uninhibited and inhibited steel specimens for the developed surface films under SEM and AFM. They saw that the exposed steel surface had a lot of damage and corrosion in strong acidic environment as shown in Fig. 25. As the temperature increased, the specimen surface become more rougher due to increased attack Fig. 26. Furthermore, the surfaces exposed to the plant extract incorporated acidic media at all evaluated temperatures had far smoother morphologies than those of the uninhibited surfaces. The effect of temperature over time during immersion has a significant influence on the corrosion inhibition effectiveness of plant-based green inhibitors in comparison to conventional inhibitors. As the temperature fluctuates, the corrosion rate goes up because of the increased randomness in molecules movement around the metallic surface, which could make the inhibitor layer less stable. Because they effectively stick to metal surfaces, green inhibitors that contain bioactive chemicals with functional groups like –OH, –COOH, and –NH2 can withstand higher temperatures. Conventional inhibitors, which often use synthetic chemicals, may not work as well at higher temperatures because they break down faster. Research has demonstrated that organic inhibitors sustain a consistent adsorption mechanism during prolonged immersion across many temperatures, indicating their exceptional resilience and environmental compatibility. The ability to withstand temperature-induced deterioration illustrates the potential of green inhibitors for long-term industrial applications. Additional comparative research emphasises the necessity of exploring sustainable alternatives to enhance efficiency and environmental compatibility.
 | ||
| Fig. 25 FE-SEM micrographs of the X70 steel specimens immersed in 1 M HCl solution (a–c) without and (d–f) with 200 mg L−1 GLE for 4 h at different temperatures, reproduced from ref. 70 with permission from [Elsevier] [Corros. Sci., 2018, 133, 6–16] copyright 2018. | ||
 | ||
| Fig. 26 3D AFM images of the X70 steel specimens immersed in 1 M HCl solution without GLE for 0.5 h at (a) 298 K, (b) 308 K, and (c) 318 K; 3D AFM images of the X70 steel specimens immersed in 1 M HCl solution with 200 mg L−1 GLE for 0.5 h at (d) 298 K, (e) 308 K, and (f) 318 K, reproduced from ref. 70, with permission from [Elsevier] [Corros. Sci., 2018, 133, 6–16] copyright 2018. | ||
Interaction of inhibitor molecules with metallic surface: theoretical approaches
 | ||
| Fig. 27 A mechanistic pathway of corrosion inhibitors through different kind of interactions, reproduced from ref. 72 with permission from [RSC] [RSC Adv., 2025, 15, 12342–12363] copyright 2025. | ||
A recent work73 has clearly demonstrated the interaction of water molecules with the iron surface is significantly blocked in the presence of a protective layer formed by various organic compounds namely: anthracene (ANC), anthrone (AEN), xanthene (XEN), xanthone (XAN), and xanthione (XION) as inhibitors. In first step, water molecules gradually adsorb onto the coated iron surface as depicted by the reactive molecular dynamic simulations. With the progression of simulation, water molecules entered into the weakly adsorbed inhibitor coating, leading to desorption of loosely bound molecules into the water. However, inhibitor molecules strongly chemisorbed remained intact, continuing to provide long-standing protection. Fig. 28a shows the stable and atomically thin inhibitor film persists on the surface, effectively reducing water-induced corrosion. The above observation is supported by radial density distribution function, g(χ), for iron (with and without coated) and oxygen of water molecule gFe–O(χ) as quantitatively given in Fig. 28b. It is found that the height of first peak of gFe–O(χ) found maximum for Fe (without coated) at χ = 1.85 Å and minimum for Fe coated with XION at χ = 1.92 Å, which is specifying that oxygen atom able to interact with Fe (without coated) freely and forming various kinds of iron oxide compounds. From these simulation results, we can conclude that the XION corrosion inhibitor molecule successfully restricts and prevent the oxygen (associated with water) to interact with iron surface.74
 | ||
| Fig. 28 (a) Snapshots of water interaction with the corrosion inhibitor-coated iron during reactive molecular dynamics simulation. The zoomed-in views show inhibitor layer at the iron–water interface. (b) The graph of the radial distribution function, gFe–O(χ), between the oxygen atom of water molecules and the coated iron surface. Blue, red, green, yellow and gray color atom depicts carbon, oxygen, hydrogen, sulfur and iron atoms, reproduced from ref. 74 with permission from [Elsevier] [Surfaces and Interfaces, 2025, 74, 107647] copyright 2025. | ||
Numerous computational techniques depending on length and time scale, were established to evaluate the protective efficiency of CIs (as illustrated in Fig. 29). The quantum level examines the electronic interaction of the chemical corrosive compounds with the metallic substrate during the adsorption and desorption processes. The atomic level simulation provides information about the bond dissociation and formation, diffusion, or movement of atoms/molecules during the corrosion process. Kinetic Monte Carlo simulations study the microscale corrosion processes. The mesoscale evaluates the microstructural evolution during the corrosion process. The macro-scale, such as FEM, examines the component mechanical properties and surface degradation of materials after corrosion. These multi-scale computational approaches consistently indicate that OCIs exert their protective effects primarily through adsorption onto the metal surface at the metal–electrolyte interface.
 | ||
| Fig. 29 Multi-scale simulation strategies for corrosion-related investigation. The x- and y-axis of flowchart shows the length and time scale respectively, reproduced from ref. 76 with permission from [Elsevier] [Prog. Mater. Sci., 2025, 148, 101359] copyright 2025. | ||
Molecular modelling, such as DFT and RMD simulations, has emerged as a very efficient tool to carry out mathematical modelling and simulation for the selection of corrosion inhibitor molecule and their inhibition mechanism.77,78 These realistic models demonstrated six key features:
(a) Electronic properties of isolated CIs,
(b) Interaction of the CI with the surface of the substrate,
(c) Adsorption model,
(d) The effect of both anodic and cathodic zones on the surface,
(e) Type of solvents,
(f) Electrodes potential effects.
Concerning about feature (a), polarizability, lone-pair electrons, dipolar moment, HOMO–LUMO energy etc., may influence the binding ability of the CI to the surface. Feature (b) will provide information about the robustness of the interaction between inhibitor and surface. Feature (c) is also significant for the manipulation of surface–inhibitor interactions and its wettability. Feature (d) can play a vital role in modelling of CIs since the different polarity affect the inhibitor–surface interaction. Feature (e) will study how the inhibitor dislocates the solvent molecules from the metallic surface to be adsorbed on it. Feature (f) allows for system polarization: metallic interface, inhibitor, and the solvent.
Electronic properties of isolated inhibitors
The relationship between the hardness parameter and the energy gap is established numerically. It is reported that the CI molecules with a larger energy gap (i.e., higher hardness) are less chemically reactive, while those with a smaller gap (i.e., lower hardness) exhibit higher reactivity. Consequently, softer molecules are generally more effective as CIs, due to their greater tendency to interact with the metal surface. Hard molecules attain a high HOMO–LUMO gap, whereas soft molecules have a small energy gap, and thus soft base chemical species-based inhibitors are the most effective for corrosion inhibition of metallic substrates.
In recent years, first-principles calculations based on quantum mechanical principles used to describe the behaviour of electrons, atoms, ions, and molecules have proven to be effective tools in studying corrosion inhibition mechanisms. Several notable applications have emerged from this approach. For instance, Chiter and colleagues84 conducted density functional theory (DFT) simulations to explore how 2-mercaptobenzothiazole (MBTH, in its thione form) interacts with Cu surfaces coated by Cu2O oxide layers. As illustrated in Fig. 30a, their results revealed that MBTH adsorbs nearly flat on the surface with a slight tilt. The calculated adsorption energy was approximately 3.40 eV mol−1. The sulphur atom of MBTH forms a bond with a copper atom at a distance of about 2.17 Å, and two carbon atoms from the cyclic aromatic ring contribute to the surface interaction. Their findings suggest that MBTH can effectively replace surface-bound water and hydroxyl groups, thereby forming a stable protective film over the oxidized copper surface in aqueous conditions.
 | ||
| Fig. 30 Notable atomistic simulation work related to adsorption of corrosion inhibitor over the metallic surface: (a) snapshot and adsorption energy of the most stable configuration of 2-mercaptobenzothiazole on Cu and Cu2O surface, reproduced from ref. 84 with permission from [IOP Science] [J. Electrochem. Soc., 2020, 167, 161506] copyright 2025, (b) densities of states of three inhibitors before (upper panel) and after (lower panel) the adsorption on Fe(110) surfaces, reproduced from ref. 85 with permission from [Elsevier] [Appl. Surf. Sci., 2017, 406, 301–306] copyright 2017, (c) two-dimensional cross-section of the charge density differences at critical bonding locations for Co adsorption systems, reproduced from ref. 86 with permission from [Elsevier] [Colloids Surfaces A Physicochem. Eng. Asp., 2020, 605, 125392] copyright 2020 and (d) equilibrium adsorption configurations and the corresponding charge density differences for 1-phenoxybenzene and 1,4-diphenoxybenzene moieties on the Fe(110) plane, reproduced from ref. 87 with permission from [Elsevier] [Corros. Sci., 2019, 146, 134–151] copyright 2019. | ||
Guo and co-workers85 conducted a thorough research on the adsorption characteristics of three common heterocyclic compounds: N-containing pyrrole, O-based furan, and S-containing thiophenone, on Fe(110) surfaces. As shown in Fig. 30b, adsorption of these molecules onto the iron surface leads to a slight broadening of their molecular density of states (DOS) peaks, which is attributed to orbital hybridization between the inhibitor and the metal substrate. This interaction also results in a shift of the DOS profiles for both the inhibitors and the iron surface toward lower energy levels. Their findings support an established empirical trend in efficiency of corrosion inhibition related to heteroatoms, indicating the order of effectiveness as O < N < S. Zang et al.86 explored the performance of corrosion inhibition of TT-LYK molecules in the context of chemical–mechanical polishing (CMP) of cobalt barrier by employing the CASTEP module within the Materials Studio software. Their simulation results demonstrated that TT-LYK can adsorb onto the cobalt surface in two distinct configurations: vertically, involving coordination through the N9 and N10 atoms and the formation of O17–H⋯Co hydrogen bonds; and parallelly, where both the benzene and triazole rings lie flat against the surface. As depicted in Fig. 30c, the charge density difference maps reveal significant electronic redistribution at the interface regions of charge depletion are shown in blue, while areas of charge accumulation appear in red confirming strong interaction between the inhibitor and the cobalt surface. To reduce computational cost, Murmu et al.87 employed Density Functional Tight Binding (DFTB) simulations to study the adsorption behavior of two specific inhibitor segments on the Fe(110) surface. As illustrated in Fig. 30d, the charge density difference maps highlight regions of electron accumulation and depletion, suggesting a stronger donor–acceptor interaction for 1,4-diphenoxybenzene compared to 1-phenoxybenzene. These computational insights align well with the experimental observations, supporting the higher inhibition efficiency of the former molecule.
Prediction of corrosion rate using artificial neural networks (ANNs)
ANNs are computational models stimulated by the functioning of the human brain, containing of interconnected processing units called neurons. Each ANN is defined by three vital elements: behavior of individual nodes, network architecture, and learning algorithm. The behavior of a node is determined by factors such as the number of inward and outward signals, the weights allocated to these connections, and the activation function used to process the weighted inputs. The architecture defines how the nodes are arranged and linked together, while the learning rule defines how connection weights are adjusted during training. A simplified representation of a neural network, shown in Fig. 31, shows how each artificial neuron obtains multiple input signals modified by a weight, which can be either positive or negative. These inputs are shared and passed through an activation function to yield the neuron's output. The activation function, often a sigmoid function due to its bounded, smooth, and differentiable nature, determines the final output. | ||
| Fig. 31 Artificial Neural Network (ANN) model with linear and sigmoidal functions to predict environmental parameters. | ||
Typically, ANNs are organized into three main layers: (a) the input layer, (b) one or more hidden layers, and (c) the output layer. A most commonly used model is the multilayer perceptron (MLP), which is a feed-forward network that uses backpropagation for learning, as depicted in Fig. 31. Input data are usually scaled to a range of 0 to 1 to ensure efficient learning and to avoid overwhelming the hidden neurons. In supervised learning, the network adjusts the weights based on the variance of predicted and actual outputs by propagating the error backwards through the network, refining the weights iteratively to minimize the error. Since the early 1990s, ANNs have been widely applied to predict corrosion inhibition performance. For example, Rosen and Silverman88 combined ANNs with expert systems to predict various types of corrosion based on Tafel curve parameters. Their model used input features such as passivation potential, passive current density, pitting potential etc. to assess the likelihood of general, crevice, and pitting corrosion. In another study, a quantitative structure–activity relationship (QSAR) model was developed to assess the inhibition efficiency of 17 α-amino acids against copper corrosion in acidic medias.89 Molecular descriptors derived from DFT and MC simulations including global hardness (ω), LUMO energy (ELUMO), electron-donating capacity (DN), and total negative charge (TNC) were used as inputs for both multiple linear regression (MLR) and ANN models. The ANN model outperformed MLR in predictive accuracy and was further used to design and evaluate 10 new molecular derivatives. Additionally, the corrosion inhibition performance of 31 aromatic hydrazide and Schiff base molecules was modelled using ANNs. Input variables included a range of quantum chemical parameters such as EHOMO, ELUMO, total energy (TE), dipole moment (µ), molar volume (MV), and partition coefficient (P). The ANN used was a feed-forward backpropagation model implemented in MATLAB (version 7), which achieved a great correlation coefficient (R = 0.968), indicating excellent predictive performance compared to traditional methods. Given the inherently complex and nonlinear nature of corrosion processes, conventional analytical methods often fall short in capturing the full spectrum of variables involved. ANN models, with their powerful learning and generalization capabilities, have emerged as effective tools for modelling, predicting, and understanding corrosion behaviour across diverse materials and environmental conditions.
In summary, Fig. 32 provides an overview of the classification, mechanisms, and applications of OCIs. The type of corrosion is classify based on its inherent nature and properties. The mechanism involves adsorption, protective layer formation, charge on the metal–surface, interaction between water molecule and inhibitor, different types of electrochemical reactions of inhibitor. These are the possible mechanistic pathways to inhibit any metallic substrate. These inhibitors are extensively used in automobile sectors, oil and gas industries, food industries, water treatments, paint industries and many more.
 | ||
| Fig. 32 Overall summary of the classification, mechanism and applications of organic corrosion inhibitors. | ||
Corrosion inhibitors and environmental regulations
Relative cost of corrosion inhibitors
The global CIs market size was projected 8.82 million USD in 2024 and is predicted to increase from 9.19 million USD in 2025 to approximately 13.34 million USD by 2034.1,2 The economic cost of corrosion may be assessed directly from the operation, application and maintenance of corrosion resistant technologies such as protective coatings, corrosion-resistant materials, corrosion inhibitors, cathodic/anodic protection and corrosion inspection including monitoring tools; or indirectly from the loss of productivity, compensation for casualties and environmental pollution, and any other cost that is not directly incurred within that industry. The cost analysis of any process is a prominent parameter before its implementation towards society. There are two important points in a cost analysis (1) it should be a short term expenses and (2) long term savings. In terms of short-term expenses, although the initial cost of using traditional inhibitors may be lower, there may be unanticipated expenses related to environmental harm, regulatory compliance, and potential liabilities. The consideration of long-term savings by lessening environmental impact, enhancing worker safety, and adhering to increasingly stringent environmental requirements, organic inhibitors – while perhaps costlier initially – can save money overall. Additionally, the reputation of an industry might be improved and the chance of regulatory fines decreased.Conclusions and prospects
In conclusion, a viable approach to addressing the problems initiated by corrosion is the development and installation of green CIs for industrial applications. Traditional CIs may be ineffective in a variety of corrosive circumstances and sometimes affect both the environment and the health of human beings. Green inhibitors provide an eco-friendly and sustainable alternative, such as biopolymers made from renewable resources, plant extracts, and chemical medicines from natural source and exhibit a number of desired qualities, such as affordability, little environmental harm, and compatibility with sustainable practices. Biopolymers like chitosan and cellulose, which may create protective barriers at the metal-environment interface, have demonstrated significant corrosion prevention effectiveness. With their electron-rich centres and high solubility, plant extracts are an attractive alternative, and the complex molecular structures of chemical medicines produced from natural sources provide these products with remarkable anti-corrosion properties. Industries can efficiently prevent corrosion in metals and alloys while minimising their environmental impact by considering these factors and using the appropriate green inhibitors. The development of more effective and long-lasting CIs might result from more research and development in this area, which would enhance industrial operations overall and save priceless assets.The next generation organic biodegradable corrosion inhibitors are the prime necessity. As academics, industries, research and development continue to investigate and expand the development of OCIs, the future of these materials is quite promising. Metal assets and structures are protected by OCIs, whose future application are influenced by a number of events and trends include sustainable solutions, customizations, new delivery methods, advances testing with modelling and next generation applications as per the specific requirement of the industry:
• Green and sustainable solution: due to environmental concerns, there is a significant shift in focus toward eco-friendly, sustainable inhibitors. Under the basic principles of green chemistry, more products are produced from bio-based, renewable resources that are less harmful and have a lesser environmental impact.
• Industry-specific solutions: future development will be highly tailored because it has been established an approach, which fits all industries, is ineffective. We will see inhibitors designed especially to address the particular difficulties faced by sectors including oil and gas, maritime engineering, and aerospace.
• The potential of nanotechnology: the performance and efficiency of inhibitors will be enhanced by nanotechnology. Stronger, more durable corrosion protection can be obtained by combining nanoparticles and nanocomposites, as well as by developing hybrid materials that combine organic inhibitors with materials like polymers.
• Intelligent, self-healing solutions: in future, whole world will look for inhibitors that have the ability to respond to its environment and even heal itself. These “smart” inhibitors, which will be possible by advances in materials science, would significantly increase the lifespan of metal structures by automatically adapting to corrosive conditions and repairing any damage to their protective layer.90
• Smarter delivery systems: the usage guidelines and procedure for any inhibitor matters just as much as its make-up. The inhibitor will be released gradually and under control, recognitions to new delivery techniques including encapsulation and nanocarriers. This translates to less frequent reapplication and more extended protection.
• Improved prediction through modelling & AI: gaining a better grasp of the molecular mechanisms underlying inhibitors through sophisticated computer modelling and simulations. This will expedite development and increase accuracy by enabling researchers to forecast an inhibitor's activity in particular conditions.
To put it briefly, the future seems promising. The next generation of OCIs will better safeguard our essential metal infrastructure by embracing sustainability, personalization, and state-of-the-art technology, guaranteeing a more robust and sustainable future for present and upcoming generations.
Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Appendices
Appendix A
| Plant or source | Metal | Corrosive environment | Corr. inhibition efficiency/% | Inhibitor conc. | Year | Ref |
|---|---|---|---|---|---|---|
| Henna leaves | MS | 1.0 M HCl | 90.34 @ 25 °C | 1.2 g L−1 | 2000 | 91 |
| Olive dry leaves | CS | 2.0 M HCl | 93 @ 25 °C | 0.9 g L−1 | 2007 | 92 |
| Streptomycin | MS | 1.0 M HCl | 88.5 @ 35 °C | 0.5 g L−1 | 2009 | 93 |
| Aspidosperma album bark | C38 steel | 1.0 M HCl | 90 @ 25 °C | 0.1 g L−1 | 2011 | 94 |
| Palicoureaguianensis leaves | C38 steel | 1.0 M HCl | 89 @ 25 °C | 0.1 g L−1 | 2011 | 95 |
| Schinopsislorentzii tree powder | Low CS | 1.0 M HCl | 66 | 0.2 g L−1 | 2012 | 96 |
| Piper guineense seeds | MS | 0.5 M H2SO4 | 97.7 | 0.9 g L−1 | 2012 | 97 |
| Propolis | CS | NaCl (10 ppm) & Al2(SO4)3 (35 ppm) | 92.7 @ 30 °C | 0.6 g L−1 | 2013 | 98 |
| Neolamarckiacadamba bark | MS | 1.0 M HCl | 91 @ 30 °C | 0.005 g L−1 | 2013 | 99 |
| 11% w/v extract of ethanolicpropolis | MS | 3.5% NaCl | –N.A.– | 13 gm | 2014 | 100 |
| Citrus peel | Al | 0.5 to 2.0 M HCl | 91 @ 10 °C | 8.0 g L−1 | 2015 | 101 |
| Pectin from apples | X60 steel | 0.5 M HCl | 78.7 @ 25 °C | 1.0 g L−1 | 2015 | 102 |
| Ascorbic acid | MS | H2SO4 @ pH 4 | 71.5 @ 20 °C | 10 mol L−1 | 2015 | 103 |
| Retamamonosperma (L.) Boiss. Seeds | CS | 1.0 M HCl | 94.42 @ 30 °C | 0.4 g L−1 | 2015 | 104 |
| Watermelon seeds | MS | 1.0 M HCl | 86.08 | 2.0 g L−1 | 2015 | 105 |
| Cryptostegia grandiflora leaves | MS | 1.0 M H2SO4 | 83.54 @ 30 °C | 0.5 g L−1 | 2016 | 106 |
| Citrus peel | MS | 1.0 M HCl | 94.2 @ 45 °C | 2.0 g L−1 | 2016 | 107 |
| Pectin from tomato | X60 steel | 0.5 M HCl | 75.4 @ 25 °C | 0.5 g L−1 pectin & 5 mM CeO2 | 2016 | 108 |
| Salvia hispanica seeds | Bronze | Simul. soln. of acid rain | 95 | 0.4 g L−1 | 2017 | 109 |
| Grains of Peganumharmala | Al alloy | 1.0 M HCl | 91.78 @ 25 °C | 0.025 g L−1 | 2017 | 110 |
| Bark of Rhizophoraapiculata | MS | 1.0 M HCl | 57.9 | 0.1 g L−1 | 2017 | 111 |
| Scenedemusalge | MS | 1.0 M HCl | 95.1 | 0.035 g L−1 | 2018 | 112 |
| Solid waste: | MS | 1.0 M HCl | 69.60, 68.41 and 58.15 @ 25 °C | 10% v/v | 2018 | 113 |
| Banana leaves | ||||||
| Sugarcane | ||||||
| Watermelon | ||||||
| Shrimps shell waste | CS | 1.0 M HCl | 88.50 @ 25 °C | 10 M | 2018 | 114 |
| Cladodes of Opuntia Ficus indica | Steel | 1.0 M HCl | 94 @ 35 °C | 1.0 g L−1 | 2020 | 115 |
| Tomato peel waste | Sn | 2% NaCl + 1% acetic acid + 0.5% citric acid | 72.98 @ 25 °C | 4.0 g L−1 | 2020 | 116 |
| Piper guineense seeds | MS | 1.0 M HCl | 88.4 | 1.0 g L−1 | 2021 | 117 |
| Opuntia Dillenii seed oil | Iron | Acid rain | 98.37 ± 0.08 | 1.0 g L−1 | 2021 | 118 |
| Thevetia peruviana flower | MS | 1.0 M HCl | 91.2 | 0.2 g L−1 | 2021 | 119 |
| Paederia Foetida leaves | MS | 1.0 M HCl | 73.77 | — | 2021 | 120 |
| Rhoeo discolor leaves | MS | 0.5 M HCl | 87.72 @ 35 °C | 2.0 g L−1 | 2022 | 121 |
| Ocimum basilicum seed | MS | 1.0 M HCl | 95.12 | 1.0 g L−1 | 2022 | 122 |
| Azadirachta indica leaves | MS | 5.0 M HCl | 89.25 | 4.0 g L−1 | 2022 | 123 |
| Turnip peel extract | Copper | 3.5 wt% NaCl | 92 @ 25 °C | 20% v/v | 2022 | 124 |
| Dysphania ambrosioides | MS | 1.0 M HCl | 81.69 @ 30 °C | 1.5 g L−1 | 2022 | 125 |
| Artemisia argyi leaves | CS | 1.0 M HCl | 97.1 @ 25 °C | 0.5 g L−1 | 2022 | 126 |
| Jatropha curcas | MS | 3.5% NaCl | 90.26 | 4.0 g L−1 | 2022 | 127 |
| Hibiscus sabdariffa (Roselle) | 81.32 | 1.48 g L−1 | ||||
| Colocasia esculenta | (SS)-410 | 0.5 M H2SO4 | 93 | 0.5 g L−1 | 2023 | 128 |
| Verbena officinalis | CS | 0.5 M H2SO4 | 91.1 | 1.0 g L−1 | 2024 | 129 |
| Citrullus colocynthis | CS | 1.0 M HCl | 91.8 @ 30 °C | 0.5 g L−1 | 2024 | 130 |
| Mahonia nepalensis | MS | 1.0 M H2SO4 | 87.86 | 0.005 g L−1 | 2024 | 131 |
| Photinia leaves | CS | 1.0 M HCl | 97.82 | 1.0 g L−1 | 2025 | 132 |
| Aleppo pine fibers | MS | 1.0 M HCl | 84.3 | 1.0 g L−1 | 2025 | 133 |
Appendix B
| Inhibitor source | Metal | Year | Ref |
|---|---|---|---|
| Natural honey | Cu | 2000 | 134 |
| Aqueous extract of Rosmarinus officinalis | Al–Mg alloy | 2000 | 135 |
| Opuntia extract | Al | 2003 | 136 |
| Extract of khillah (Ammivisnaga) seeds | SX 316 steel | 2006 | 137 |
| Prosopis—cineraria (khejari) | Al | 2006 | 138 |
| Carica papaya leaves extract | Mild steel | 2007 | 139 |
| Hibiscus sabdariffa (Calyx extract) | Steel | 2008 | 140 |
| Musa sapientumpeels (banana peels) | Mild steel | 2008 | 141 |
| Saccharides—mannose and fructose | Al and Zn | 2008 | 142 |
| Citric acid | Al | 2008 | 143 |
| Lawsonia extract (henna) | C-steel, Ni, Zn | 2009 | 144 |
| Neem leaves extract (Azadirachtaindica) | Mild steel | 2010 | 145 |
| Mango/orange peels | Steel | 2010 | 146 |
| Vanillin | Al | 2010 | 147 |
| Emblica officinalis | Steel | 2010 | 148 |
| Gum exudates | Mild steel | 2012 | 149 |
| Vernonia amygdalina (bitter leaf) | Al | 2012 | 150 |
| Garcinia kola seed | Mild steel | 2013 | 73 |
| Aloe leaves | Steel | 2013 | 151 |
| Rosemary | Steel | 2014 | 152 |
| Terminalia bellerica | Steel | 2014 | 153 |
| Tobacco plant extract and its parts | Al, steel | 2014 | 154 |
| Pomegranate juice and peels | Steel | 2015 | 155 |
| Amino acids & aspartic acid | Al | 2016 | 156 |
| Tamarind | Steel | 2018 | 157 |
| Tea leaves | Steel | 2018 | 158 |
| Rutin and quercetin | Al | 2018 | 159 |
| Eucalyptus oil | Steel | 2019 | 160 |
| Saponin | Al | 2019 | 161 |
| Acacia concianna | Al | 2020 | 162 |
| Benzoic acid | Fe, Al | 2020 | 163 |
| CeCl3 and mercaptobenzothiazole (MBT) | Al | 2020 | 164 |
| Tannin beetroot | Al | 2022 | 165 |
| Acacia catechu | Mild steel | 2022 | 166 |
| Artabotrys odoratissimus | Mild steel | 2022 | 167 |
| Pichia sp. biofilm | Mild steel | 2022 | 168 |
| Camellia chrysantha flower | Steel | 2023 | 169 |
| Datura stramonium plant | Mild steel | 2023 | 170 |
| Feverfew root | Carbon steel | 2023 | 171 |
| Walnut husk | Carbon steel | 2024 | 172 |
| Chlorella vulgaris | Steel | 2024 | 173 |
| Artemisia argyi | Steel | 2025 | 174 |
| Chrysanthemum indicum | |||
| Centipeda minima | |||
| Corynocarpus laevigatus | Mild steel | 2025 | 175 |
| Helianthus tuberosus L. | Al | 2025 | 176 |
| Poria cocos | |||
| Peganum harmala | Iron | 2025 | 177 |
| Potentilla erecta | Carbon steel | 2025 | 178 |
| Vicia faba | Mild steel | 2025 | 179 |
Appendix C
| Technique | Outcomes | Significance | Reference |
|---|---|---|---|
| Conventional immersion exposures | • If corrosion rate decreases with the increase in inhibitor concentration | • Excellent adsorption along with high surface coverage | 46, 62 and 180 |
| • Inhibition efficacy decreases with the increase in temperature | • Increased desorption rate and possibility of inhibitor film disintegration higher temperatures | ||
| • Inhibition efficiency increases with addition of multiple compounds | • Synergism | ||
| Electrochemical impedance spectroscopy | • Increase in polarization resistance | • Formation of a protective layer thereby reduced charge transfer at metal–electrolyte interface | 181–186 |
| • Decrease in double layer capacitance | • Reduced dielectric constant due to increased thickness of the electric double layer | ||
| • Nyquist plots semicircle diameter increases with increase in inhibitor concentration | • Decrease in corrosion rate | ||
| • Phase angle in the bode plot for inhibited samples greater than uninhibited samples | • Formation of inhibitor's film (protective) on metal surface | ||
| Potentiodynamic polarization | • Deviation in Ecorr values | • Change in Ecorr compare to uninhibited surface | 180 and 182 |
| • A decrease in the icorr | >85mv; anodic or cathodic inhibitor. <85mv; mixed type inhibitor | ||
| • Alteration in the cathodic and anodic Tafel slope towards lower values due to inhibitor addition | • Excellent protection & formation of a protective film | ||
| • Mixed type inhibitor; retarding both cathodic and anodic reactions |
Appendix D
| Isotherms | Equation | Plot | Reference |
|---|---|---|---|
| Freundlich | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = log θ = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kads + n Kads + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C C |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ vs. log θ vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C C |
187 |
| Langmuir | Cθ−1 = Kads−1 + C | Cθ−1 vs. C | 188 |
| Temkin | Kads C = efθ | θ vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C C |
186 |
| Frumkin | B C e2ae = θ (1 − θ)−1 | θ vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C C |
189 |
| Flory–Huggins | log (Cθ−1) = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kads + x log (1 − θ) Kads + x log (1 − θ) |
log (Cθ−1) vs. log (1 − θ) | 184 |
| El-Awady | log {θ (1 − θ)−1} = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K + Y K + Y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C C |
log {θ (1 − θ)−1} vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C C |
190 |
Appendix E
| Parameters | Significance | Reference |
|---|---|---|
| ΔGads0 | Insight on the adsorption process spontaneousity | 64 |
| Type of adsorption | ||
| ΔGads0 ≤ −20kj/mol; physisorption | ||
| ΔGads0 ≤ −40 KJ mol; chemisorption | ||
| −20 kj mol−1 ≤ ΔGads0 ≤ −40kjmol−1; both physical and chemical adsorption | ||
| ΔHads0 | ΔHads0 > 0; endothermic adsorption process | |
| ΔHads0 < 0; exothermic adsorption process | ||
| ΔSads0 | ΔSads0 > 0; adsorption is supported by randomness increase | |
| ΔSads0 < 0; adsorption is supported by randomness decrease as reactant converted to activated complex | ||
| Ea | The presence of an inhibitor leads to an elevation in activation energy (Ea), indicating physical adsorption |
Appendix F
| Concentration (ppm) | Immersion time (h) | Kads (M−1) | ΔGads0 (kJ mol−1) |
|---|---|---|---|
| 200 | 0.5 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 666 666 |
−35.9 |
| 2.5 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 666 666 |
−35.9 | |
| 5.0 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 461 461 |
−35.7 | |
| 400 | 0.5 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 166 166 |
−33.6 |
| 2.5 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
−34.7 | |
| 5.0 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 730 730 |
−34.0 | |
| 600 | 0.5 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 484 484 |
−33.5 |
| 2.5 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 851 851 |
−34.0 | |
| 5.0 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
−33.7 | |
| 800 | 0.5 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 375 375 |
−33.6 |
| 2.5 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607 607 |
−34.0 | |
| 5.0 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 375 375 |
−33.6 |
Acknowledgements
The corresponding author is grateful to Director, CSIR-NML for his support and encouragement through a research grant OLP-0452 project.References
- C. Verma, D. S. Chauhan, R. Aslam, P. Banerjee, J. Aslam, T. W. Quadri, S. Zehra, D. K. Verma, M. A. Quraishi, S. Dubey, A. AlFantazi and T. Rasheed, Green Chem., 2024, 26, 4270–4357 RSC.
- S. Zamindar, S. Mandal, M. Murmu and P. Banerjee, Mater. Adv., 2024, 5, 4563–4600 RSC.
- C. Yin, M. Kong, J. Zhang, Y. Wang, Q. Ma, Q. Chen and H. Liu, ACS Omega, 2020, 5, 2620–2629 CrossRef CAS.
- L. T. Popoola, Corros. Rev., 2019, 37, 71–102 CrossRef CAS.
- C. Verma, E. E. Ebenso, M. A. Quraishi and C. M. Hussain, Mater. Adv., 2021, 2, 3806–3850 RSC.
- I. E. Uwah, P. C. Okafor and V. E. Ebiekpe, Arab. J. Chem., 2013, 6, 285–293 CrossRef CAS.
- N. Hossain, M. Aminul Islam and M. Asaduzzaman Chowdhury, Results Chem., 2023, 5, 100883 Search PubMed.
- B. R. Fazal, T. Becker, B. Kinsella and K. Lepkova, npj Mater. Degrad., 2022, 6, 5 CrossRef CAS.
- A. Zakeri, E. Bahmani and A. S. R. Aghdam, Corros. Commun., 2022, 5, 25–38 CrossRef.
- S. Almi, Z. Rais, S. Seridi, R. Benakcha, K. Almi, R. Hadjeb, H. Menasra and F. Adjal, New J. Chem., 2025, 49, 5639–5664 Search PubMed.
- B. D. B. Tiu and R. C. Advincula, React. Funct. Polym., 2015, 95, 25–45 CrossRef CAS.
- D. Li, Z. Zhang, B. Sukhbat, X. Wang, X. Zhang, J. Yan, J. Zhang, Q. Zhang, Y. Li, H. Wang and Y. Yan, RSC Appl. Polym., 2025, 3, 532–548 Search PubMed.
- M. A. Ahmed, S. Amin and A. A. Mohamed, RSC Adv., 2024, 14, 31877–31920 RSC.
- Y. Liu, F. Chen, Z. Wang, H. Ma and Y.-C. Wu, Mater. Today Commun., 2025, 46, 112759 CrossRef CAS.
- M. Jiang, X. Liu, Y. Xu, S. Li, X. Liu, X. Niu, L. Lu, X. Sun, Z. Xie, Z. Wang and S. Cui, ACS Sustain. Chem. Eng., 2025, 13, 2411–2428 CrossRef CAS.
- M. Li, F. Liu, F. Mou, Z. Guo, W. Wang, Y. Zeng, L. B. Sim and B. Chen, ACS Omega, 2025, 10, 24628–24641 CrossRef PubMed.
- A. Omari Alaoui, W. Elfalleh, B. Hammouti, A. Titi, M. Messali, S. Kaya, B. EL IBrahimi and F. El-Hajjaji, RSC Adv., 2025, 15, 12645–12652 RSC.
- M. A. Quraishi and D. Jamal, J. Am. Oil Chem. Soc., 2000, 77, 1107–1111 CrossRef CAS.
- M. Quraishi and D. Jamal, Mater. Chem. Phys., 2001, 68, 283–287 CrossRef CAS.
- M. A. Quraishi and D. Jamal, Mater. Chem. Phys., 2001, 71, 202–205 CrossRef CAS.
- A. Yıldırım and M. Çetin, Corros. Sci., 2008, 50, 155–165 CrossRef.
- A. Ostovari, S. M. Hoseinieh, M. Peikari, S. R. Shadizadeh and S. J. Hashemi, Corros. Sci., 2009, 51, 1935–1949 CrossRef CAS.
- A. K. Satapathy, G. Gunasekaran, S. C. Sahoo, K. Amit and P. V. Rodrigues, Corros. Sci., 2009, 51, 2848–2856 CrossRef CAS.
- H. Ashassi-Sorkhabi, M. Majidi and K. Seyyedi, Appl. Surf. Sci., 2004, 225, 176–185 CrossRef CAS.
- H. Nakajima, P. Dijkstra and K. Loos, Polymers, 2017, 9, 523 CrossRef.
- J. Hidalgo, L. Hidalgo, C. D. Serrano Aguiar, D. B. García Madroñero, I. Galambos, J. E. Vilasó-Cadre, I. A. Reyes-Domínguez, A. M. V. Brânzanic, N. Ignat and G. L. Turdean, Langmuir, 2025, 41, 9406–9421 CrossRef CAS PubMed.
- A. Rizi, A. Sedik, A. Acidi, K. O. Rachedi, H. Ferkous, M. Berredjem, A. Delimi, A. Abdennouri, M. ALAM, B. Ernst and Y. Benguerba, ACS Omega, 2023, 8, 47224–47238 Search PubMed.
- J. Lopez de Lacalle, D. Minudri, M. L. Picchio, A. Gallastegui, D. Mantione, M. Forsyth and D. Mecerreyes, RSC Sustain., 2024, 2, 1809–1818 RSC.
- M. Marinescu, BMC Chem., 2019, 13, 136 CrossRef CAS.
- G. Gece, Corros. Sci., 2011, 53, 3873–3898 CrossRef CAS.
- M. Abdallah, K. A. Soliman, M. Alfakeer, H. Hawsawi, A. M. Al-bonayan, S. S. Al-Juaid, S. Abd El Wanees and M. S. Motawea, ACS Omega, 2023, 8, 34516–34533 CrossRef CAS PubMed.
- S. Abd El Maksoud, A. E. A. Fouda and H. Badawy, Sci. Rep., 2024, 14, 9052 Search PubMed.
- R. A. El-Nagar, M. I. Nessim, N. A. Khalil and S. I. Elewa, Sci. Rep., 2024, 14, 11484 CrossRef CAS.
- C. Verma, S. H. Alrefaee, M. A. Quraishi, E. E. Ebenso and C. M. Hussain, J. Mol. Liq., 2021, 321, 114484 CrossRef CAS.
- Q. Wang, Y. Sang, J. Yang and H. Liu, Polymers, 2025, 17, 422 CrossRef CAS.
- S. Kumar, D. Sharma, P. Yadav and M. Yadav, Ind. Eng. Chem. Res., 2013, 52, 14019–14029 CrossRef CAS.
- N. F. H. Mahmoud and A. El-Sewedy, J. Chem., 2018, 2018, 1–9 CrossRef.
- C. Verma, M. A. Quraishi, K. Kluza, M. Makowska-Janusik, L. O. Olasunkanmi and E. E. Ebenso, Sci. Rep., 2017, 7, 44432 Search PubMed.
- S. KM, B. M. Praveen and B. K. Devendra, Results Surf. Interfaces, 2024, 16, 100258 CrossRef.
- K. Shalabi, H. M. Abd El-Lateef, M. M. Hammouda and A. A. Abdelhamid, ACS Omega, 2024, 9, 18932–18945 Search PubMed.
- R. Oukhrib, Y. Abdellaoui, A. Berisha, H. Abou Oualid, J. Halili, K. Jusufi, M. Ait El Had, H. Bourzi, S. El Issami, F. A. Asmary, V. S. Parmar and C. Len, Sci. Rep., 2021, 11, 3771 Search PubMed.
- G. Khan, W. J. Basirun, S. N. Kazi, P. Ahmed, L. Magaji, S. M. Ahmed, G. M. Khan, M. A. Rehman and A. B. B. M. Badry, J. Colloid Interface Sci., 2017, 502, 134–145 CrossRef CAS PubMed.
- Q. Deng, J. M. Castillo-Robles, E. de Freitas Martins, P. Ordejón, J.-N. Gorges, P. Eiden, X.-B. Chen, P. Keil and I. Cole, Mol. Syst. Des. Eng., 2024, 9, 29–45 RSC.
- J. Tan, L. Guo, H. Yang, F. Zhang and Y. El Bakri, RSC Adv., 2020, 10, 15163–15170 RSC.
- I. B. Obot, E. E. Ebenso and M. M. Kabanda, J. Environ. Chem. Eng., 2013, 1, 431–439 CrossRef CAS.
- S. Bashir, A. Thakur, H. Lgaz, I. M. Chung and A. Kumar, Surf. Interfaces, 2020, 20, 100542 Search PubMed.
- A. K. Singh, S. Mohapatra and B. Pani, J. Ind. Eng. Chem., 2016, 33, 288–297 CrossRef CAS.
- S. Bashir, V. Sharma, H. Lgaz, I.-M. Chung, A. Singh and A. Kumar, J. Mol. Liq., 2018, 263, 454–462 CrossRef CAS.
- A. Biswas, S. Pal and G. Udayabhanu, J. Adhes. Sci. Technol., 2017, 31, 2468–2489 Search PubMed.
- N. A. Negm, N. G. Kandile, E. A. Badr and M. A. Mohammed, Corros. Sci., 2012, 65, 94–103 CrossRef CAS.
- E. A Badr, H. H. H. Hefni, S. H. Shafek and S. M. Shaban, Int. J. Biol. Macromol., 2020, 157, 187–201 Search PubMed.
- B. S. Mahdi, M. K. Abbass, M. K. Mohsin, W. K. Al-azzawi, M. M. Hanoon, M. H. H. Al-kaabi, L. M. Shaker, A. A. Al-amiery, W. N. R. W. Isahak, A. A. H. Kadhum and M. S. Takriff, Molecules, 2022, 27, 4857 Search PubMed.
- N. Leslie and J. Mauzeroll, in Encyclopedia of Solid-Liquid Interfaces, Elsevier, 2024, pp. 461–478 Search PubMed.
- A. Dehghani, G. Bahlakeh, B. Ramezanzadeh and M. Ramezanzadeh, Constr. Build. Mater., 2020, 245, 118464 CrossRef CAS.
- S. Bashir, H. Lgaz, I. I. I. M. Chung and A. Kumar, Chem. Pap., 2019, 73, 2255–2264 CrossRef CAS.
- A. Nahle, R. Salim, F. El Hajjaji, M. R. Aouad, M. Messali, E. Ech-chihbi, B. Hammouti and M. Taleb, RSC Adv., 2021, 11, 4147–4162 RSC.
- Q. A. Yousif, M. A. Bedair, A. M. Abuelela, A. Ansari, S. S. Malhotra and Z. Fadel, RSC Adv., 2025, 15, 28666–28688 Search PubMed.
- H. Tristijanto, M. N. Ilman and P. Tri Iswanto, Egypt. J. Pet., 2020, 29(2), 155–162 CrossRef.
- P. Mourya, S. Banerjee and M. M. Singh, Corros. Sci., 2014, 85, 352–363 Search PubMed.
- B. P. Markhali, R. Naderi, M. Mahdavian, M. Sayebani and S. Y. Arman, Corros. Sci., 2013, 75, 269–279 CrossRef CAS.
- O. A. Akinbulumo, O. J. Odejobi and E. L. Odekanle, Results Mater., 2020, 5, 100074 CrossRef.
- S. A. Umoren, U. M. Eduok and E. E. Oguzie, Port. Electrochim. Acta, 2007, 26, 533–546 CrossRef.
- H. Lgaz, K. Subrahmanya Bhat, R. Salghi, Shubhalaxmi, S. Jodeh, M. Algarra, B. Hammouti, I. H. Ali and A. Essamri, J. Mol. Liq., 2017, 238, 71–83 Search PubMed.
- S. Sharma and A. Kumar, J. Mol. Liq., 2021, 322, 114862 CrossRef CAS.
- A. Dehghani, G. Bahlakeh, B. Ramezanzadeh and M. Ramezanzadeh, J. Taiwan Inst. Chem. Eng., 2019, 102, 349–377 Search PubMed.
- B. Tan, B. Xiang, S. Zhang, Y. Qiang, L. Xu, S. Chen and J. He, J. Colloid Interface Sci., 2021, 582, 918–931 Search PubMed.
- A. Dehghani, G. Bahlakeh, B. Ramezanzadeh and M. Ramezanzadeh, J. Mol. Liq., 2019, 277, 895–911 CrossRef CAS.
- Z. Song, H. Cai, Q. Liu, L. Jiang and H. Chu, J. Ind. Eng. Chem., 2022, 111, 464–479 CrossRef CAS.
- A. R. Simović, B. N. Grgur, J. Novaković, P. Janaćković and J. Bajat, Metals, 2023, 13, 508 CrossRef.
- Y. Qiang, S. Zhang, B. Tan and S. Chen, Corros. Sci., 2018, 133, 6–16 CrossRef CAS.
- J. Zhang, F. Yang, Y. Dai, Y. Liu, Y. Yu and S. Wang, Langmuir, 2023, 39, 18043–18051 CrossRef CAS PubMed.
- S. Y. Mosavian, Z. Hamidi, N. Sabbaghi, M. Shahabi, M. Noroozifar, M. A. Karimi Zarchi and H. Raissi, Polym. Bull., 2023, 80, 7569–7598 CrossRef CAS.
- A. I. Ikeuba, P. C. Okafor, U. J. Ekpe and E. E. Ebenso, Int. J. Electrochem. Sci., 2013, 8, 7455–7467 Search PubMed.
- S. Kumar, N. Kumar, L. K. Meena, A. Kumar, R. Kumar, V. S. Yadav and L. M. Pandey, Surf. Interfaces, 2025, 74, 107647 CrossRef CAS.
- M. A. V. Devanathan and B. V. K. S. R. A. Tilak, Chem. Rev., 1965, 65, 635–684 Search PubMed.
- T. Wenga, D. D. Macdonald and W. Ma, Prog. Mater. Sci., 2025, 148, 101359 Search PubMed.
- P. Liu, Q. Xu, Q. Zhang, Y. Huang, Y. Liu, H. Li, R. Zhang and G. Lei, J. Adhes. Sci. Technol., 2024, 38, 1563–1584 Search PubMed.
- E. E. Ebenso, C. Verma, L. O. Olasunkanmi, E. D. Akpan, D. K. Verma, H. Lgaz, L. Guo, S. Kaya and M. A. Quraishi, Phys. Chem. Chem. Phys., 2021, 23, 19987–20027 RSC.
- M. E. Foster and B. M. Wong, J. Chem. Theory Comput., 2012, 8, 2682–2687 Search PubMed.
- L. N. Anderson, M. B. Oviedo and B. M. Wong, J. Chem. Theory Comput., 2017, 13, 1656–1666 CrossRef CAS PubMed.
- R. S. Bhatta, G. Pellicane and M. Tsige, Comput. Theor. Chem., 2015, 1070, 14–20 CrossRef CAS.
- A. Nakata, Y. Imamura, T. Otsuka and H. Nakai, J. Chem. Phys., 2006, 124, 094105 CrossRef.
- I. B. Obot, D. D. Macdonald and Z. M. Gasem, Corros. Sci., 2015, 99, 1–30 Search PubMed.
- F. Chiter, D. Costa, V. Maurice and P. Marcus, J. Electrochem. Soc., 2020, 167, 161506 Search PubMed.
- L. Guo, I. B. Obot, X. Zheng, X. Shen, Y. Qiang, S. Kaya and C. Kaya, Appl. Surf. Sci., 2017, 406, 301–306 CrossRef CAS.
- X. Zhang, G. Pan, L. Hu, H. Wang and C. Wang, Colloids Surf., A, 2020, 605, 125392 CrossRef CAS.
- M. Murmu, S. K. Saha, N. C. Murmu and P. Banerjee, Corros. Sci., 2019, 146, 134–151 Search PubMed.
- E. M. Rosen and D. C. Silverman, Corrosion, 1992, 48, 734–745 CrossRef CAS.
- B. El Ibrahimi, A. Jmiai, K. El Mouaden, R. Oukhrib, A. Soumoue, S. El Issami and L. Bazzi, J. King Saud Univ., Sci., 2020, 32, 163–171 CrossRef.
- H. Zhao, X. Zhang, L. Ji, H. Hu and Q. Li, Corros. Sci., 2014, 83, 261–271 CrossRef CAS.
- H. Al-Sehaibani, Materwiss. Werksttech., 2000, 31, 1060–1063 CrossRef CAS.
- A. Y. El-Etre, J. Colloid Interface Sci., 2007, 314, 578–583 CrossRef CAS PubMed.
- S. K. Shukla, A. K. Singh, I. Ahamad and M. A. Quraishi, Mater. Lett., 2009, 63, 819–822 CrossRef CAS.
- C. R. M. Faustin, M. Lebrini and F. Robert, Int. J. Electrochem. Sci., 2011, 6, 4095–4113 CrossRef.
- F. R. M. Lebrini and C. Roos, Int. J. Electrochem. Sci., 2011, 6, 847–859 CrossRef.
- H. Gerengi and H. I. Sahin, Ind. Eng. Chem. Res., 2012, 51, 780–787 CrossRef.
- E. E. Oguzie, C. B. Adindu, C. K. Enenebeaku, C. E. Ogukwe, M. A. Chidiebere and K. L. Oguzie, J. Phys. Chem. C, 2012, 116, 13603–13615 Search PubMed.
- S. F. A and H. B. A, African J. Pure Appl. Chem., 2013, 7, 350–359 CrossRef.
- P. B. Raja, A. K. Qureshi, A. Abdul Rahim, H. Osman and K. Awang, Corros. Sci., 2013, 69, 292–301 CrossRef CAS.
- K. Agarwal, J. Mater. Sci. Surf. Eng., 2014, 1, 44–48 Search PubMed.
- N. Chaubey, V. K. Singh and M. A. Quraishi, Int. J. Ind. Chem., 2015, 6, 317–328 CrossRef CAS.
- S. A. Umoren, I. B. Obot, A. Madhankumar and Z. M. Gasem, Carbohydr. Polym., 2015, 124, 280–291 CrossRef CAS.
- M. A. Chidiebere, E. E. Oguzie, L. Liu, Y. Li and F. Wang, J. Ind. Eng. Chem., 2015, 26, 182–192 CrossRef CAS.
- N. El Hamdani, R. Fdil, M. Tourabi, C. Jama and F. Bentiss, Appl. Surf. Sci., 2015, 357, 1294–1305 CrossRef CAS.
- N. A. Odewunmi, S. A. Umoren and Z. M. Gasem, J. Environ. Chem. Eng., 2015, 3, 286–296 Search PubMed.
- M. Prabakaran, S.-H. Kim, V. Hemapriya and I.-M. Chung, J. Ind. Eng. Chem., 2016, 37, 47–56 CrossRef CAS.
- L. M. P. Dolabella, J. G. Oliveira, V. Lins, T. Matencio and W. L. Vasconcelos, J. Coatings Technol. Res., 2016, 13, 543–555 CrossRef.
- A. N. Grassino, J. Halambek, S. Djaković, S. Rimac Brnčić, M. Dent and Z. Grabarić, Food Hydrocoll., 2016, 52, 265–274 Search PubMed.
- A. K. Larios-Galvez, J. Porcayo-Calderon, V. M. Salinas-Bravo, J. G. Chacon-Nava, J. G. Gonzalez-Rodriguez and L. Martinez-Gomez, Anti-Corros. Methods Mater., 2017, 64, 654–663 CrossRef CAS.
- D. Amar, J. Chem. Pharm. Res., 2017, 9, 311–318 Search PubMed.
- M. A. Asaad, M. Ismail, P. B. Raja and N. H. A. Khalid, Surf. Rev. Lett., 2017, 24, 1850013 CrossRef CAS.
- A. Khanra, M. Srivastava, M. P. Rai and R. Prakash, ACS Omega, 2018, 3, 12369–12382 CrossRef CAS PubMed.
- S. Marzorati, L. Verotta and S. Trasatti, Molecules, 2018, 24, 48 CrossRef PubMed.
- A. A. Farag, A. S. Ismail and M. A. Migahed, Egypt. J. Pet., 2018, 27, 1187–1194 CrossRef.
- R. Boulmerka and S. Abderrahmane, Bull. Mater. Sci., 2020, 43, 242 Search PubMed.
- J. Halambek, I. Cindrić and A. Ninčević Grassino, Carbohydr. Polym., 2020, 234, 115940 Search PubMed.
- D. I. Njoku, C. N. Njoku, H. Lgaz, P. C. Okafor, E. E. Oguzie and Y. Li, J. Mol. Liq., 2021, 330, 115619 CrossRef CAS.
- M. Rehioui, S. Abbout, B. Benzidia, H. Hammouch, H. Erramli, N. A. Daoud, N. Badrane and N. Hajjaji, Heliyon, 2021, 7, e06674 CrossRef CAS PubMed.
- J. Haque, C. Verma, V. Srivastava and W. B. W. Nik, Sustain. Chem. Pharm., 2021, 19, 100354 CrossRef CAS.
- N. Hossain, M. A. Chowdhury, A. K. M. P. Iqbal, M. S. Islam, N. Y. Sheikh Omar and A. Z. A. Saifullah, Curr. Res. Green Sustain. Chem., 2021, 4, 100191 CrossRef CAS.
- N. Gayakwad, V. Patil and B. M. Rao, Mater. Today Proc., 2022, 49, 536–541 CrossRef CAS.
- Y. Fernine, R. Salim, N. Arrousse, R. Haldhar, F. El Hajjaji, S.-C. Kim, M. Ebn Touhami and M. Taleb, J. Mol. Liq., 2022, 355, 118867 CrossRef CAS.
- H. Kumar, V. Yadav and A. Kumari, J. Phys. Chem. Solids, 2022, 165, 110690 CrossRef CAS.
- M. H. Fekri, F. Omidali, M. M. Alemnezhad and A. Ghaffarinejad, Mater. Chem. Phys., 2022, 286, 126150 CrossRef CAS.
- W. Daoudi, A. El Aatiaoui, N. Falil, M. Azzouzi, A. Berisha, L. O. Olasunkanmi, O. Dagdag, E. E. Ebenso, M. Koudad, A. Aouinti, M. Loutou and A. Oussaid, J. Mol. Liq., 2022, 363, 119839 Search PubMed.
- Q. Wang, L. Liu, Q. Zhang, X. Wu, H. Zheng, P. Gao, G. Zeng, Z. Yan, Y. Sun, Z. Li and X. Li, Sustain. Chem. Pharm., 2022, 27, 100710 CrossRef CAS.
- M. A. A. Abdul Aziz, E. Hamzah and M. Selamat, Mater. Today Proc., 2022, 51, 1344–1349 CrossRef CAS.
- R. Singh, D. Prasad, Z. Safi, N. Wazzan and L. Guo, J. Bio- Tribo-Corrosion, 2023, 9, 76 CrossRef.
- A. E.-A. S. Fouda, A. F. Molouk, M. F. Atia, A. El-Hossiany and M. S. Almahdy, Sci. Rep., 2024, 14, 16112 Search PubMed.
- M. Tabyaoui, M. Tourabi, H. Zarrok, C. Jama, F. Benhiba, A. Zarrouk and F. Bentiss, Environ. Sci. Pollut. Res., 2024, 31, 43757–43780 CrossRef CAS.
- A. Kumari Das, S. Neupane, K. Kumar Nayak, S. Shrestha, N. Karki, D. Kumar Gupta and A. Prasad Yadav, Results Chem., 2024, 12, 101866 CrossRef CAS.
- H. Mei, B. Zhou, S. Hu, C. Wu and H. Cen, J. Adhes. Sci. Technol., 2025, 39, 2384–2405 Search PubMed.
- E. M. Saoudi Hassani, R. Salim, E. Ech-chihbi, H. Zejli, I. Mehdaoui, R. Mahmoud, Y. A. Younous, G. A. Shazly, A. Aqdas, A. Taleb, M. Taleb and Z. Rais, Sci. Rep., 2025, 15, 12164 Search PubMed.
- A. Y. El-Etre and M. Abdallah, Corros. Sci., 2000, 42, 731–738 Search PubMed.
- M. Kliškić, J. Radošević, S. Gudić and V. Katalinić, J. Appl. Electrochem., 2000, 30, 823–830 CrossRef.
- A. Y. El-Etre, Corros. Sci., 2003, 45, 2485–2495 Search PubMed.
- A. Y. El-Etre, Appl. Surf. Sci., 2006, 252, 8521–8525 CrossRef CAS.
- M. K. Sharma, S. Kumar, R. Ratnani and S. P. Mathur, Bull. Electrochem., 2006, 22, 69–73 Search PubMed.
- P. C. Okafor and E. E. Ebenso, Pigment Resin Technol., 2007, 36, 134–140 CrossRef CAS.
- E. E. Oguzie, Port. Electrochim. Acta, 2007, 26, 303–314 Search PubMed.
- E. E. Eddy and N. O. Ebenso, African J. Pure Appl. Chem., 2008, 2, 46–54 Search PubMed.
- P. B. Raja and M. G. Sethuraman, Mater. Lett., 2008, 62, 113–116 CrossRef CAS.
- R. Solmaz, G. Kardaş, B. Yazıcı and M. Erbil, Corros. Eng., Sci. Technol., 2008, 43, 186–191 CrossRef CAS.
- M. A. S. Rajendran, R. B. Devi, B. S. Devi, K. Rajam and J. Jeyasundari, Zašt. Mater., 2009, 50, 77–84 Search PubMed.
- P. C. Okafor, E. E. Ebenso and U. J. Ekpe, Int. J. Electrochem. Sci., 2010, 5, 978–993 Search PubMed.
- J. C. da Rocha, J. A. da Cunha Ponciano Gomes and E. D'Elia, Corros. Sci., 2010, 52, 2341–2348 Search PubMed.
- R. Rosliza, A. Nora’aini and W. B. Wan Nik, J. Appl. Electrochem., 2010, 40, 833–840 CrossRef CAS.
- V. G. V. R. Saratha, E-J. Chem., 2010, 7, 677–684 Search PubMed.
- M. A. Abu-Dalo, A. A. Othman and N. A. F. Al-Rawashdeh, Int. J. Electrochem. Sci., 2012, 7, 9303–9324 Search PubMed.
- F. A. Ayeni, I. A. Madugu, P. Sukop, A. P. Ihom, O. O. Alabi, R. Okara and M. Abdulwahab, J. Miner. Mater. Charact. Eng., 2012, 11, 667–670 Search PubMed.
- R. N. V. Sribharathy, S. Rajendran and P. Rengan, Eur. Chem. Bull., 2013, 2, 471–476 Search PubMed.
- M. A. Velázquez-González, J. G. Gonzalez-Rodriguez, M. G. Valladares-Cisneros and I. A. Hermoso-Diaz, Am. J. Anal. Chem., 2014, 5, 55–64 CrossRef.
- E. E. Oguzie, M. A. Chidiebere, K. L. Oguzie, C. B. Adindu and H. Momoh-Yahaya, Chem. Eng. Commun., 2014, 201, 790–803 CrossRef CAS.
- A. S. Fouda, G. Y. Elewady, K. Shalabi and S. Habouba, Int. J. Adv. Res., 2014, 2, 817–832 CAS.
- H. Ashassi-Sorkhabi, S. Mirzaee, T. Rostamikia and R. Bagheri, Int. J. Corros., 2015, 2015, 1–6 CrossRef.
- M. A. Migahed, S. M. Rashwan, M. M. Kamel and R. E. Habib, J. Mol. Liq., 2016, 224, 849–858 CrossRef CAS.
- N. B. Iroha and A. O. James, J. Chem. Soc. Niger., 2018, 43, 510–517 Search PubMed.
- A. B. Hamdan, Suryanto and F. I. Haider, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 290, 012086 Search PubMed.
- D. Sukul, A. Pal, S. K. Saha, S. Satpati, U. Adhikari and P. Banerjee, Phys. Chem. Chem. Phys., 2018, 20, 6562–6574 Search PubMed.
- G. Fekkar, F. Yousfi, H. Elmsellem, M. Aiboudi, M. Ramdani, I. Abdеl-Rahman, B. Hammouti and L. Bouyazza, Int. J. Corros. Scale Inhib., 2020, 9, 446–459 CAS.
- A. I. Ikeuba and P. C. Okafor, Pigment Resin Technol., 2019, 48, 57–64 CrossRef CAS.
- R. Haldhar, D. Prasad and N. Bhardwaj, Arab. J. Sci. Eng., 2020, 45, 131–141 Search PubMed.
- M. Alahiane, R. Oukhrib, Y. A. Albrimi, H. A. Oualid, H. Bourzi, R. A. Akbour, A. Assabbane, A. Nahlé and M. Hamdani, RSC Adv., 2020, 10, 41137–41153 RSC.
- A. C. Balaskas, M. Curioni and G. E. Thompson, J. Electroanal. Chem., 2020, 863, 114081 CrossRef CAS.
- A. Kumar, J. Kumar and Nishtha, Mater. Today Proc., 2022, 64, 141–146 CrossRef CAS.
- R. Karki, A. K. Bajgai, N. Khadka, O. Thapa, T. Mukhiya, H. B. Oli and D. P. Bhattarai, Electrochem, 2022, 3, 668–687 Search PubMed.
- M. R. Rathod, R. L. Minagalavar and S. K. Rajappa, J. Indian Chem. Soc., 2022, 99, 100445 CrossRef CAS.
- B. Tamilselvi, D. S. Bhuvaneshwari, S. Padmavathy and P. B. Raja, J. Mol. Liq., 2022, 359, 119359 CrossRef CAS.
- J. Dai and X. An, Int. J. Electrochem. Sci., 2023, 18, 100080 CrossRef.
- K. Hjouji, E. Ech-chihbi, I. Atemni, M. Ouakki, T. Ainane, M. Taleb and Z. Rais, Sustain. Chem. Pharm., 2023, 34, 101170 CrossRef CAS.
- Z. Zhou, X. Min, S. Wan, J. Liu, B. Liao and X. Guo, Results Eng., 2023, 17, 100971 CrossRef CAS.
- M. Akbari Shahmirzadi and M. Azadi, Heliyon, 2024, 10, e29962 CrossRef CAS PubMed.
- E. Almanza, L. Del Carmen Gutierrez Pua, Y. Pineda, W. Rozo, M. Marquez and A. Fonseca, Heliyon, 2024, 10, e39717 CrossRef CAS.
- T.-S. Chu, W.-J. Mai, H.-Z. Li, B.-X. Wei, Y.-Q. Xu and B.-K. Liao, Int. J. Mol. Sci., 2025, 26, 561 CrossRef CAS PubMed.
- A. I. Obike, K. S. Eze, I. Abdel-Rahman, A. I. Ikeuba, I. K. Nwokolo and C. Aghalibe, Curr. Res. Green Sustain. Chem., 2025, 10, 100447 CrossRef CAS.
- R. Ao, J. Liu, X. Ren, Y. Liu and Y. Jia, J. Alloys Compd., 2025, 1010, 177977 CrossRef CAS.
- H. Hamidi, F. Shojaei, H. Eslami, R. Aliabadi, M. Pourfath and M. Vaez-Zadeh, Langmuir, 2025, 41, 3249–3258 CrossRef CAS PubMed.
- H. M. Hassan, Int. J. Electrochem. Sci., 2025, 20, 101028 CrossRef CAS.
- S. Ben Dlala, M. Haj Romdhane, S. Lakard, B. Lakard, D. Le Cerf, H. Majdoub, N. Jaffrezic-Renault and H. Ben Halima, Int. J. Biol. Macromol., 2025, 310, 143149 CrossRef CAS.
- Z. Bensouda, E. H. El Assiri, M. Sfaira, M. Ebn Touhami, A. Farah and B. Hammouti, J. Bio- Tribo-Corrosion, 2019, 5, 84 CrossRef.
- G. A. Swetha, H. P. Sachin, A. M. Guruprasad, B. M. Prasanna and J. Fail, Anal. Prev., 2019, 19, 1113–1126 CrossRef.
- A. M. Guruprasad, H. P. Sachin, G. A. Swetha and B. M. Prasanna, Int. J. Ind. Chem., 2019, 10, 17–30 CrossRef CAS.
- X. Luo, S. Zhang and L. Guo, Int. J. Electrochem. Sci., 2014, 9, 7309–7324 CrossRef.
- A. Singh, K. R. Ansari and M. A. Quraishi, Int. J. Hydrogen Energy, 2020, 45, 25398–25408 Search PubMed.
- S. John and A. Joseph, Mater. Chem. Phys., 2012, 133, 1083–1091 CrossRef CAS.
- P. Dohare, D. S. Chauhan, A. A. Sorour and M. A. Quraishi, Mater. Discov., 2017, 9, 30–41 CrossRef.
- E. E. Oguzie, Corros. Sci., 2007, 49, 1527–1539 CrossRef CAS.
- A. Singh, N. Soni, Y. Deyuan and A. Kumar, Results Phys., 2019, 13, 102116 CrossRef.
- A. A. El-Awady, B. A. Abd-El-Nabey and S. G. Aziz, J. Electrochem. Soc., 1992, 139, 2149–2154 Search PubMed.
- I. B. Obot, N. O. Obi-Egbedi and S. A. Umoren, Int. J. Electrochem. Sci., 2009, 4, 863–877 Search PubMed.
| This journal is © The Royal Society of Chemistry 2026 |