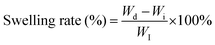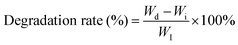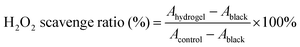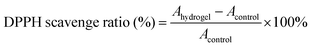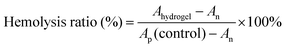Self-healing photothermal antibacterial hydrogels constructed through multiple dynamic chemical bonds
Jingrui
Chang
,
Xinyu
Wang
,
Xuejiao
Ma
and
Bo
Lu
 *
*
School of Chemistry, Department of Chemical Engineering and Life Sciences, Wuhan University of Technology, Wuhan, 430070, China. E-mail: lvb@whut.edu.cn; Tel: 086-027-87749300
First published on 11th November 2025
Abstract
Hydrogels, with their highly hydrophilic, three-dimensional polymer network structure, offer great potential as antimicrobial biomedical materials. However the overuse of antibiotics has led to drug-resistant bacteria, highlighting the need for multifunctional biomaterials that do not rely on antibiotics to combat infections. In this study, a multifunctional photothermal antimicrobial hydrogel (PHDF hydrogel) was synthesized using a one-pot method from polyvinyl alcohol, borax, dopamine-grafted hyaluronic acid, and ferric chloride. The hydrogel's self-healing properties were achieved through the formation of borate bonds between polyvinyl alcohol and borax, metal–ligand bonds between dopamine and Fe3+, and hydrogen bonds between macromolecules, prolonging its action time. The catechol–Fe3+ complex demonstrated outstanding photothermal antibacterial performance, achieving approximately 99% antibacterial efficacy against Staphylococcus aureus and Escherichia coli upon exposure to near-infrared light. In addition, the hydrogel has adjustable rheological properties, antioxidant properties, tissue adhesion, injectability and good hemocompatibility and cytocompatibility, making it a promising antimicrobial material.
Introduction
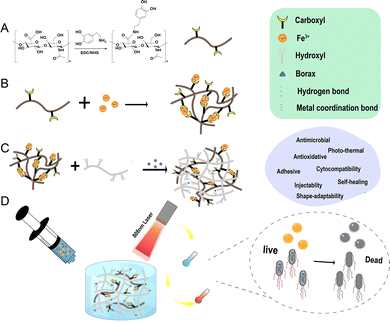
Microbial infections due to drug-resistant pathogens following surgery or trauma are a major challenge to global health, significantly delaying wound healing and threatening lives,1 leading to a significant increase in healthcare costs, and posing a significant threat to people's normal lives.2 Antibiotics as a common treatment have been shown to be insufficient in isolation to deal with the problem of bacterial resistance, and the misuse of antibiotics is the main reason for the emergence of drug-resistant strains of bacteria.3,4 Therefore, there is an urgent need to develop biosafe antimicrobial materials with low toxicity, high efficiency, and drug resistance to effectively prevent bacterial infections and fulfill the needs in the medical field. Hydrogels, exhibiting highly hydrophilic three-dimensional polymer network structures with good biocompatibility, a porous structure for drug loading, suitable physicochemical and mechanical properties similar to extracellular matrices (ECMs), and physical barrier functions,5,6 have been widely used in wound dressings,7,8 tissue scaffolds,9,10 and bio-coatings in medicine.11However, conventional hydrogels are prone to degradation and accidental fracture due to their inherent mechanical properties,12,13 and intrinsic tiny cracks are often difficult to detect and repair, causing damage to the integrity and mechanical properties of the gel network, shortening its service life,14 and possibly triggering safety hazards such as drug or microbial infiltration, while the destruction of the physical barrier increases the risk of bacterial infection.15,16 Therefore, there is a need to endow hydrogels with self-healing abilities so that they can spontaneously repair after being damaged under dynamic physiological environments or mechanical stresses, restoring the integrity of their physical barriers and loading functions to ensure long-term antimicrobial effectiveness and integrity.17–19 The borate bond, which is a type of dynamic reversible covalent bond, confers good self-healing properties to hydrogels.20,21 Poly(vinyl alcohol) (PVA) is a synthetic polymer with excellent biocompatibility, and its molecular chain is rich in polyhydroxyl structures,22–24 allowing it to form a dynamic network through borate ester bonds when cross-linked with borax solution.25 On the other hand, metal–ligand bonds are another commonly used dynamic interaction (reversible non-covalent bonds),26 and a coordination system consisting of Fe3+ and catechol groups is particularly common. The metal–ligand bond between catechol and Fe3+ can effectively dissipate mechanical energy and thus significantly enhance the self-healing ability of the material.27,28 Based on this, by introducing both metal-ion coordination bonds (e.g., Fe3+-catechol) and borate bonds (e.g., the PVA-borax system) into the hydrogel network and through the synergistic effect of the two dynamic bonds, hydrogels with excellent self-repairing properties can be designed.
Hydrogels have emerged as a kind of highly promising antimicrobial material, owing to their exceptional biocompatibility, tunable physicochemical properties, and high water content.29–31 However, their antimicrobial strategy is still facing significant challenges. Loading antibiotic drugs into hydrogels can easily lead to an initial sudden release or a late release of drugs, which would have an insufficient impact in terms of long-lasting antimicrobial effects,32,33 and may exacerbate the problem of bacterial resistance. The introduction of metal ions or their nanoparticles can confer broad-spectrum antimicrobial properties,34,35 but their distribution in vivo is often uneven, which may lead to potential challenges in terms of their effectiveness and safety.36 The use of quaternized or other polymers with intrinsic antimicrobial activities to construct hydrogels often results in challenges of either insufficient antimicrobial performance or excessive cytotoxicity. In the face of the above problems, photothermal therapy (PTT), as an emerging antimicrobial strategy, utilizes photothermal reagents to physically sterilize bacteria by converting laser light energy into thermal energy,37,38 which has become a powerful antimicrobial method in the biomedical field due to its high efficiency and rapid response without inducing bacterial resistance and local rapid antimicrobial advantages.39,40 The integration of photo-thermal conversion materials into the self-healing hydrogel network ensures its photo-thermal stability and avoids the problem of uneven distribution of photo-thermal materials due to structural damage, thus realizing efficient and long-lasting antimicrobial activity. Among them, catechol–Fe3+ chelates have good photothermal properties.41 Hyaluronic acid (HA) is an unbranched anionic polysaccharide that naturally occurs in the human body.42,43 Hyaluronic acid (HA), a key constituent of the extracellular matrix, exhibits outstanding characteristics, including non-immunogenicity, biocompatibility, and biodegradability, and is therefore often used as a backbone material for hydrogels.44–46 Dopamine (DA) is a natural substance secreted by mussels with high adhesion, metal coordination ability, and antioxidant properties.47–49 Grafting dopamine onto hyaluronic acid increases the adhesion of hydrogels and has a photothermal effect through coordination bonds with iron ions.
Based on this, a smart antimicrobial hydrogel (PHDF hydrogel) with excellent self-healing properties and an efficient NIR photo-thermal response was designed in this study (Scheme 1). It was synthesized by a one-pot method using poly(vinyl alcohol), borax, dopamine grafted to hyaluronic acid, and trivalent ferric ions. The gel can realize rapid self-repair through the borate bond formed by polyvinyl alcohol and borax, and the metal–ligand bond between dopamine and Fe3+, while dopamine complexed with Fe3+ has an excellent photothermal effect. We systematically investigated its rheological properties, adhesion, shape adaptation, injection properties, self-healing properties, photothermal effect and stability, in vitro antimicrobial properties, and evaluated its hemocompatibility and cytocompatibility. The results show that the hydrogel combines excellent self-healing ability, strong photothermal antimicrobial activity and good biocompatibility, and is a potential multifunctional antimicrobial biomaterial.
Experimental
Materials
Polyvinyl alcohol (PVA, 1799 type, M.W. ca. 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–124
000–124![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and borax (AR, 99.5% purity) were sourced from Sinopharm Chemical Reagent Co., Ltd. N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC·HCl), N-hydroxysuccinimide (NHS), and dopamine hydrochloride were bought from Macklin (Shanghai, China). Hyaluronic acid (HA, MW 50 K Da) was purchased from Bloomage Biotechnology Co., Ltd. Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS), cell counting Kit-8 (CCK-8), streptomycin, penicillin, and Live/dead fluorescent kit (Calcein AM/PI) were obtained from Thermo Fisher Scientific.
000) and borax (AR, 99.5% purity) were sourced from Sinopharm Chemical Reagent Co., Ltd. N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC·HCl), N-hydroxysuccinimide (NHS), and dopamine hydrochloride were bought from Macklin (Shanghai, China). Hyaluronic acid (HA, MW 50 K Da) was purchased from Bloomage Biotechnology Co., Ltd. Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), phosphate-buffered saline (PBS), cell counting Kit-8 (CCK-8), streptomycin, penicillin, and Live/dead fluorescent kit (Calcein AM/PI) were obtained from Thermo Fisher Scientific.
Synthesis and characterization of HA-DA
HA-DA was synthesized by amidation reaction between the carboxyl groups of HA and the amine groups of dopamine hydrochloride. Briefly, HA (1 g) was dissolved in 100 mL of distilled water. After purging with argon gas, EDC (575 mg) and NHS (345 mg) were added in the dark for 30 min, DA (569 mg) was added into this solution, and the mixed solution was stirred overnight, The mixture was subsequently dialyzed using a dialysis bag (3000 Da cutoff) in deionized water (pH 5–6) for 2 days and in distilled water for 1 day, with frequent changes of distilled water to remove impurities. Finally, the HA-DA was obtained by freeze-drying. The chemical structure of HA-DA was identified by attenuated total reflectance Fourier transform infrared spectroscopy and 1H-nuclear magnetic resonance (NMR). The catechol substitution ratio in HA-DA was quantified by measuring the absorbance at 280 nm using UV-vis spectroscopy, referencing a standard curve of dopamine.Preparation of hydrogels
Polyvinyl alcohol (PVA), borax, hydroxyapatite-doped alginate (HA-DA), and ferric chloride (FeCl3) were individually dissolved in distilled water to prepare PVA solution (15% fully transparent, dissolved at 90 °C), borax solution (5%), HD solution (1%) and FeCl3 solution (0.6%). Subsequently, the FeCl3 and HA-DA solutions were mixed to form the HD/Fe3+ mixture. The PVA solution was used in equal volume to the HD/Fe3+ mixture, and the resulting solution was stirred for 1 hour to achieve a homogeneous mixture. Finally, the borax solution was slowly added to the PVA/HA-DA/Fe3+ mixture, yielding a hydrogel named PHDF.Additionally, PVA and borax solutions were mixed to obtain a hydrogel named PB. Similarly, a hydrogel containing PVA, borax, and hydroxyapatite (HA) was named PBHA, while a hydrogel consisting of PVA, borax, and HA-DA was named PBHD.
SEM observation
The hydrogels PBHD and PHDF were freeze-dried and then rapidly fractured under liquid nitrogen conditions. After gold sputter coating on the fractured surface, the hydrogel's internal pore structure was analyzed through scanning electron microscopy (SEM), and corresponding images were acquired.Rheological properties of the hydrogels
The rheological behavior of the hydrogels was studied using the frequency scan mode on a rheometer (TA), with all samples having a diameter of 20 mm and a thickness of 1 mm. Time sweep tests were performed under both constant strain (1 Hz) and constant strain (1%) to investigate the rheological properties of the different hydrogels. Subsequently, the storage modulus (G′) and loss modulus (G″) of the hydrogels were determined within the frequency range of 0.1–10 Hz under constant strain (1%). The critical strain point of the hydrogels was determined using strain amplitude sweep experiments.Swelling behavior of the hydrogels
The hydrogels were dried to obtain the initial weight (Wi), and then hydrogels were subsequently soaked in distilled water at 37 °C. Once equilibrium was achieved, they were removed and the surface water was gently blotted with filter paper, and the weights of the hydrogels were recorded as Wd. The swelling rate of the hydrogel was calculated as follows:where Wi and Wd are the weights of the hydrogel initially and after swelling equilibrium.
The degradation behavior of hydrogels
The degradation experiment was evaluated for the hydrogels (500 mg) in PBS solution at 37 °C. The remaining hydrogel samples were collected and weighed, and the surface water was removed at the specific time point. The degradation rate of the hydrogel was calculated as follows:where Wd is the weight of the original hydrogel, and Wi is the amount of hydrogel remaining at different time points.
Injectable properties of PHDF hydrogels
The prepared hydrogel sample was loaded into syringes, and the injection process was recorded with both video and photos. The viscosity and shear-thinning behavior of the hydrogels were measured using a rheometer, and their shear-thinning properties were quantitatively analyzed.Shape-adaptive test of PHDF hydrogels
The prepared hydrogel was put into different molds, and its compatibility with the molds was assessed. After the hydrogel had formed, it was carefully removed from the mold for photographic documentation and further examination.Tissue adhesion strength
The tissue adhesion strength was evaluated by lap shear tests. Fresh porcine skin was cut into 10 mm × 30 mm and then put in PBS (7.4) for 2 h. The sample hydrogels (10 mm × 10 mm) were placed between two pieces of porcine skin. The electronic universal testing machine was used to perform the pigskin lap shear test at a tensile rate of 5 mm min−1 while the pigskin was adhered. Finally, the maximum tensile strength was regarded as the measured maximum load by the self-adhesion area.Self-healing test of PHDF hydrogels
Visual self-healing properties were demonstrated using two cut pieces of hydrogels; the two cut parts were placed together and allowed to heal after 10 minutes. Macroscopic images of the original hydrogel and the healed hydrogel were taken.Quantitative detection of the hydrogel's self-healing properties was carried out using a rheometer (TA). Time sweep tests were conducted at 37 °C with a frequency of 1 Hz (γ = 1%, with a 100 s interval), gradually increasing to large strain (γ = 100%, with a 100 s interval), and repeated for 3 cycles.
Antioxidant assay
The ROS-scavenging ability of the hydrogel was evaluated in a previous study. KI solution (60 mmol) was used to assess their ability to scavenge H2O2. 500 mg hydrogel was prepared in a H2O2 solution (5 mL, 0.2 mmol), and in distilled water as the blank group (500 mL distilled water was used instead of hydrogel as the control group). All the groups were put in the dark, 2 mL of the supernatant was collected at different time points, then the KI solution (2 mL) and hydrochloric acid solution (2 mL) were added for 5 min. The absorption of each group's supernatant at 341.5 nm was recorded. The H2O2 scavenging ability of the hydrogel was calculated as follows:Hydroxyl radical scavenging experiment: the hydroxyl radical scavenging ability was assessed using a Fenton reaction and methylene blue (MB) solution. The color change of the MB solution from blue to dark blue indicated the generation of hydroxyl radicals. Hydroxyl radicals were produced by the reaction of hydrogen peroxide (H2O2) with ferrous chloride tetrahydrate (FeCl2·4H2O). In the experimental group, 0.5 g of the sample was added to this solution, while in the control group, 0.5 g of water was added. The blank group only contained deionized water. After incubation for a specified period, the MB solution was added to all three groups. The supernatant was then collected, and the UV absorbance spectra were recorded between 400 and 800 nm. Photographs were taken to document the color changes.
DPPH radical scavenging experiment: the antioxidant activity of the hydrogel was tested using the DPPH assay. 4 mg of DPPH was dispersed in 100 mL of ethanol solution. In the experimental group, 0.1 g of hydrogel was placed into 3 mL of DPPH solution. In the control group, 0.1 g of water was used instead of the hydrogel. Both groups were incubated in the dark for 1 hour, after which the UV absorbance at 517 nm was measured, and photographs were taken to record the color changes.
The DPPH scavenging ability of the hydrogel was calculated as follows:
Photothermal performance tests
The photothermal properties of the hydrogel were assessed using near-infrared (NIR) light with a wavelength of 808 nm and an intensity of 1.5 W cm−2. The different types of hydrogels were irradiated for 5 minutes in a tube under this light source. Furthermore, the photothermal conversion efficiency of PHDF hydrogels was evaluated at different optical densities (0.5 W cm−2, 1 W cm−2, 1.5 W cm−2, and 2 W cm−2). Real-time thermal images were captured every 30 seconds using an infrared thermal imaging camera. Additionally, a photothermal cycle test was conducted, the NIR laser system was turned off after 5 minutes of irradiation, and waited for the surface temperature of the hydrogel to drop to room temperature. The temperature change of the hydrogel during this process was recorded, and the above process was repeated 3 times.Evaluation of antibacterial activity and NIR-assisted antibacterial activity
Escherichia coli and Staphylococcus aureus were used to assess the antimicrobial activity of the hydrogels. The evaluation was carried out by optical density (OD) assay, the agar solution dispersion method, and the coated plate method. The hydrogels were placed in 48-well plates, and 1 × 106 CFU mL−1 of both bacterial solutions were added to each well and incubated with the hydrogels for 12 hours. After incubation, 1 mL of medium was added to resuspend the bacteria, and 300 µL of supernatant was added to 3 mL of medium, and incubation was continued for 18 hours. Then, the absorbance value at 600 nm was measured and recorded, and the test was repeated three times for each group of samples. After the experiment, the bacterial solution was diluted and spread on agar plates, and the number of bacteria was observed and photographed for recording after incubation for 18 hours in an incubator at 37 °C.The circle of inhibition experiment was performed by agar diffusion method, taking 1 × 108 CFU mL−1 of both bacterial solutions and spreading them evenly on the agar plate. The prepared PHDF hydrogel was placed in the center of the Petri dish and incubated in a thermostat (37 °C) for 24 hours. The diameter of the ring of inhibition was measured and recorded and photographed. The experiment was repeated three times for each group of bacterial samples.
Testing the photothermal antimicrobial properties of the PHDF hydrogel. The hydrogel was placed in a 96-well plate and 100 µL of bacterial suspension containing 1 × 106 CFU mL−1 was added dropwise to the surface of the hydrogel. The hydrogel was irradiated using an 808 nm infrared exciter for 0, 1, 5 and 10 min. After irradiation, medium was added to resuspend the bacteria and 300 µL of supernatant was added to 3 mL of medium and incubated for 18 hours. The absorbance values at 600 nm were recorded and measured. The test was repeated three times for each set of samples. After the experiments, the bacterial solution was diluted and spread on agar plates and incubated in an incubator at 37 °C for 18 h. The bacterial counts were observed and photographed for recording. The photothermal antibacterial experiment was repeated three times for each group.
In vitro hemolytic test and cytotoxicity assessment
Hemolytic test: after collecting blood samples, centrifuge at 3000 rpm for 5 minutes in centrifuge tubes. Discard the supernatant and retain the blood cell pellet and wash three times with PBS buffer solution. Discard the supernatant again, then dilute the blood cells with PBS buffer solution to a 5% concentration suspension for later use. Incubate the hydrogel (100 µL) with 1 mL of PBS at 37 °C for 1 hour. At the same time, take 1 mL of deionized water as the positive control, denoted as Ap, and 1 mL of PBS buffer solution as the negative control, denoted as An. After incubation, remove the hydrogel and centrifuge the supernatant at 3000 rpm for 5 minutes, then take photographs. Measure the absorbance of the supernatant at 545 nm using a microplate reader, and calculate the hemolysis rate using the formula:The cytotoxicity of the hydrogel was assessed using L929 mouse fibroblast cells using the extract method. L929 cells were maintained in a 37 °C incubator with 5% CO2. The complete culture medium consisted of DMEM, penicillin–streptomycin solution, and fetal bovine serum. The hydrogel was first immersed in alcohol for 10 minutes, followed by washing twice with sterile PBS, and then sterilized under UV light for 30 minutes. The hydrogel was completely immersed in the culture medium (0.1 g mL−1) for 24 hours, after which the medium was collected as the extract. L929 cells (5 × 104) were inoculated in 96-well plates with four replicates for each group. After 24 hours of cell attachment, the hydrogel extracts were added to the cells for co-culture for 24 and 48 hours. Cell viability was then assessed using the CCK-8 assay. The specific procedure was as follows: after 24 and 48 hours of incubation with the extracts, cells were washed twice with PBS to remove any residual medium. Next, 100 µL of 10%CCK-8 medium was added and the cells were further cultured for 45 minutes. The absorbance at 450 nm was measured using a microplate reader. Each experimental group was performed in triplicate.
Cell viability and death staining. L929 cells (5000 cells per well) were seeded in a 96-well plate and cultured in a 37 °C incubator with 5% CO2. After 24 hours of cell attachment, the material extracts were added. The control group received only culture medium, while the experimental group received the hydrogel extracts. After 24 and 48 hours of incubation, cells were stained with Calcein AM/PI reagent under dark conditions. The stained cells were observed under an inverted fluorescence microscope. Live cells were stained green, while dead cells were stained red.
Statistical analysis
At least three replications for each of the aforementioned studies were performed. The experimental data were presented as mean ± standard deviation (SD) values and the statistical significance of the differences was determined with the student's test: * p < 0.05, ** p < 0.01, *** p < 0.001.Results and discussion
Preparation and physicochemical characterization of hydrogels
HA-DA was synthesized from dopamine and hyaluronic acid by amidation reaction with the aim of introducing the catechol moiety into hyaluronic acid. The synthesized catechol endocompounds were identified by infrared and UV-visible spectroscopy. Fig. 1A shows that HA-DA exhibits a distinct absorption peak at 280 nm, corresponding to the UV absorption peak of dopamine, while hyaluronic acid has no absorption peak from 225 to 300 nm, which proves that the dopamine was successfully grafted onto hyaluronic acid. From the infrared spectra (Fig. S1), HA-DA had a stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of the benzene ring skeleton at 1513 cm−1, and the amide bond peak at 1739 cm−1 confirmed the reaction of the amino group (C
C double bond of the benzene ring skeleton at 1513 cm−1, and the amide bond peak at 1739 cm−1 confirmed the reaction of the amino group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) of dopamine with the carboxyl group of hyaluronic acid. Additionally, HA-DA was identified as a functionalized catechol via1H-NMR spectroscopy (Fig. S1), where the peak at δ 6.7–7.0 ppm corresponds to the characteristic dopamine peak, representing the coupled protons at the ortho and meta positions of the aromatic ring.50 PHDF hydrogels had benzene ring characteristic absorption peaks at about 1540 cm−1. After ferric ions are loaded into the hydrogel, the hydroxyl peak of dopamine moves to a low wave number in the range of 3000–3600 cm−1, and the peak deformation is narrow, and the successful formation of boronic ester bonds in the cross-linked hydrogel is evidenced by the presence of two distinct peaks at 1336 cm−1 (B–O) and 1081 cm−1 (C–O–B). After that, the grafting rate of dopamine onto hyaluronic acid was 12% calculated using the UV absorption standard curve of dopamine at 280 nm and the absorbance value of HA-DA, which was 0.22 mg mg−1. The hydrogel was mainly composed of poly(vinyl alcohol), borax, dopamine grafted hyaluronic acid and iron ions. In this system, poly(vinyl alcohol) forms a reversible borate bond with borax, while dopamine interacts with iron ions to form a metal–ligand bond, and the coordination of iron ions with dopamine imparts a black appearance to the gel (Fig. 1D). In order to elucidate the chemical structure of the hydrogel PHDF, high-resolution XPS spectra were measured, and the hydrogel was analyzed from the full spectrum to contain the elements C, O, B, and Fe (Fig. 1B). A split-peak fit to XPS, C1S was performed, and the signal peak at 282 eV was C–C, and the C
N) of dopamine with the carboxyl group of hyaluronic acid. Additionally, HA-DA was identified as a functionalized catechol via1H-NMR spectroscopy (Fig. S1), where the peak at δ 6.7–7.0 ppm corresponds to the characteristic dopamine peak, representing the coupled protons at the ortho and meta positions of the aromatic ring.50 PHDF hydrogels had benzene ring characteristic absorption peaks at about 1540 cm−1. After ferric ions are loaded into the hydrogel, the hydroxyl peak of dopamine moves to a low wave number in the range of 3000–3600 cm−1, and the peak deformation is narrow, and the successful formation of boronic ester bonds in the cross-linked hydrogel is evidenced by the presence of two distinct peaks at 1336 cm−1 (B–O) and 1081 cm−1 (C–O–B). After that, the grafting rate of dopamine onto hyaluronic acid was 12% calculated using the UV absorption standard curve of dopamine at 280 nm and the absorbance value of HA-DA, which was 0.22 mg mg−1. The hydrogel was mainly composed of poly(vinyl alcohol), borax, dopamine grafted hyaluronic acid and iron ions. In this system, poly(vinyl alcohol) forms a reversible borate bond with borax, while dopamine interacts with iron ions to form a metal–ligand bond, and the coordination of iron ions with dopamine imparts a black appearance to the gel (Fig. 1D). In order to elucidate the chemical structure of the hydrogel PHDF, high-resolution XPS spectra were measured, and the hydrogel was analyzed from the full spectrum to contain the elements C, O, B, and Fe (Fig. 1B). A split-peak fit to XPS, C1S was performed, and the signal peak at 282 eV was C–C, and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O signal peak at 286 eV was derived from the introduction of the catechol group in dopamine into the structure of the hydrogel, and a split-peak fit was further performed to O1S, B1S, and a split-peak fit to B1 for split peak fitting, two signals at 194 eV and 188 eV can be obtained from B1s, and a distinct absorption peak at 528 eV can be obtained from O1s, both of which confirm the formation of the borate bond (Fig. S2).
O signal peak at 286 eV was derived from the introduction of the catechol group in dopamine into the structure of the hydrogel, and a split-peak fit was further performed to O1S, B1S, and a split-peak fit to B1 for split peak fitting, two signals at 194 eV and 188 eV can be obtained from B1s, and a distinct absorption peak at 528 eV can be obtained from O1s, both of which confirm the formation of the borate bond (Fig. S2).
As illustrated in Fig. 1D, the hydrogels underwent a phase transition from sol to gel with the addition of borax, leading to a significant change in their properties. The stability of the PHDF hydrogel was investigated by the rheological behavior test. Through the dynamic frequency scanning test, the G′ of the hydrogel was consistently higher than the G″ from the low frequency to the high frequency range, which confirmed that the sample was in a stable gel state, exhibiting gelatinous properties (Fig. 1C).
The porous network structure of the hydrogel is formed by cross-linking of polymers, which provides the hydrogel with excellent water absorption and retention capabilities, ensuring the maintenance of a moist environment, and the three-dimensional porous structure mimics the extracellular matrix. Fig. 1E shows that the lyophilized PHDF hydrogel had an obvious three-dimensional network under the scanning electron microscope, and its pore size was in the range of 3.11 ± 1.34 um, but the pore size of the PBHD hydrogel was in the range of 8.50 ± 0.31 um (Fig. S3). The energy storage modulus (G′) and loss modulus (G″) of a series of hydrogels were recorded using a rheometer and the G′ of the PHDF hydrogel was 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 951.6 pa higher than that of the G′ of the PBHD hydrogel (9911.01 pa), it was the addition of Fe3+ that led to an increase in the cross-linking density of the hydrogel, which in turn led to the pore diameter of the hydrogel becoming smaller and an increase in the G′ of the hydrogel (Fig. 2A).
951.6 pa higher than that of the G′ of the PBHD hydrogel (9911.01 pa), it was the addition of Fe3+ that led to an increase in the cross-linking density of the hydrogel, which in turn led to the pore diameter of the hydrogel becoming smaller and an increase in the G′ of the hydrogel (Fig. 2A).
Swelling and degradation properties of hydrogels
Hydrogels should ideally exhibit controlled swelling behavior to maintain a moist microenvironment while absorbing excess exudate. The swelling rates of the PB, PBHA, PBHD, and PHDF were 1321.07%, 856.42%, 1027.05%, and 911.88% (Fig. 2B). In the PB hydrogel with a simple mixture of poly(vinyl alcohol)-borax, the low density of cross-linking can result in a large amount of water being absorbed, after adding hyaluronic acid in the system, the crosslinking density of the hydrogel network is increased, and grafting dopamine onto hyaluronic acid, the hydrophilic component of the gel network, increased the swelling rate. However, the addition of Fe3+ and catechol-metal coordination led to a more tightly connected hydrogel network, leading to a reduction in the swelling rate. The degradation rate is a critical parameter for evaluating hydrogels used in biomedicine. All hydrogels showed gradual degradation over time, maintaining stability for more than 14 days. 31.63% of the PBHD hydrogel remained after 14 days. However, the network of the hydrogel became more compact after the addition of Fe3+ and 46.46% of PHDF hydrogel remained (Fig. 2C).Adhesion, shape-adaptive, and injectable properties of hydrogels
Numerous studies have shown that dopamine is an important component of adhesion proteins secreted by marine mussel-like organisms.51 By observing the illustration, it can be found that the hydrogel can flexibly adhere to human fingers and wrists, etc. (Fig. S4), and will not be easily dislodged during the experimental process. In order to quantitatively evaluate the adhesion properties of the hydrogels, the laboratory measured the maximum shear stress by pig skin shear test (Fig. 2F). Hyaluronic acid-containing hydrogel (PBHA) and dopamine-modified hydrogel (PHDF) with added iron ions were compared in the experiment. The results showed that the tissue adhesive strengths of PBHA and PHDF hydrogels to pig skin were 0.9627 Kpa and 2.923 Kpa, respectively, and there was a significant difference between them (Fig. 2D). Analyzing from the perspective of the adhesion mechanism (Fig. 2E), the chelating coordination of iron ions with catechol promotes the formation of hydrogen bonds between the hydrogel and the sulfhydryl, amino, or hydroxyl groups in the tissues, which significantly improves the adhesion properties of the hydrogel.We tested the shape adaptability of PHDF hydrogels by placing them in different shapes of molds, and it can be clearly seen that the hydrogels completely filled the molds and maintained their original shapes after removing the molds (Fig. 2G), demonstrating excellent plasticity and excellent shape-adaptive performances. Through rheological testing, with the increase of shear rate, the viscosity of the hydrogel decreases significantly, and then the gel enters a relatively viscous and flowable state, which can be injected smoothly through the syringe (Fig. 2H). From the macroscopic observation, the hydrogel can be extruded and used for writing after being placed in the syringe, thus proving its good injectability (Fig. 2I). The excellent adhesion, shape adaptability, and injectability of the hydrogel make it highly versatile, allowing it to conform to irregular surfaces and be applied in various biomedical fields for effective coverage and treatment.
Self-healing properties of the hydrogel
Traditional hydrogels are susceptible to damage, which can result in fracture when used in external environments. In contrast, self-healing hydrogels can have an extended service life by dynamically reconfiguring their three-dimensional network structure through interactions among polymer chains.46,52 The rheological test of the PHDF hydrogel showed the solid-like elasticity of the hydrogel by strain-dependent oscillatory measurements, after reaching the strain point of 3.06%, the G′ of hydrogel is equal to G″, the hydrogel was in the middle between a solid and liquid state, and the network structure of the hydrogel was destroyed after exceeding this critical strain (Fig. 3A). The self-healing properties of the hydrogel were tested by scanning the hydrogel in stage strain mode, choosing 1% as low strain and 100% as large strain, and the G′ and G″ of the hydrogel had no noticeable change after three low and high strain cycles, which indicated a good self-healing performance (Fig. 3B). On the macroscopic level, the fractured PHDF hydrogel can be seen in the figure to heal itself after ten minutes of contact under non-stimulated conditions, and it did not fracture after slight stretching. The mechanism of self-healing of the PHDF hydrogel can be seen in the figure to be a combination of borate bonds, metal–ligand bonds, and hydrogen bonds (Fig. 3C), and the physicochemical double-crosslinked hydrogel was constructed through a combination of physical cross-linking and chemical cross-linking, which showed excellent self-healing properties.ROS scavenging ability evaluation
Reactive oxygen species (ROS), including hydroxyl radicals, hydrogen peroxide, and nitrogen radicals, are typically generated during the inflammatory phase and can contribute to oxidative stress.53 These ROS can oxidize DNA, proteins, lipids, and other biomolecules, resulting in cellular damage.54 Grafting of dopamine onto the hyaluronic acid structure, the catechol structure of dopamine can scavenge reactive oxygen species. It is evident that the color of the solution in groups PB and PBHA remained consistent with the purple color of the control DPPH solution. In contrast, the color of the solutions in groups PBHD and PHDF faded to yellow after co-incubation with the DPPH solution (Fig. S5). The nitrogen radical scavenging rates of the PB, PBHA, PBHD, and PHDF hydrogels were 2.10%, 5.42%, 71.41%, and 60.03% (Fig. 3D), respectively. The clear nitrogen radical scavenging efficiency of PBHD increased greatly due to the presence of dopamine in PBHD, whereas the scavenging rate was slightly decreased by the addition of trivalent iron ions, which should be attributed to the formation of chelating complexes between the iron ions and dopamine, and a decrease in the content of dopamine. Meanwhile, the ROS scavenging efficiency of the hydrogels was tested by measuring the hydrogen peroxide scavenging rate. PB, PBHA, PBHD, and PHDF hydrogels were co-incubated with hydrogen peroxide solution, and the hydrogen peroxide content was tested at different time points. And with the increase of time, the hydrogen peroxide content of the PB, PBHA group was slightly decreased, and the hydrogen peroxide content of the PBHD, PHDF group was significantly decreased. After 24 hours of co-incubation with PHDF hydrogel, the hydrogen peroxide content was only 40.22% (Fig. S5). In order to assess the hydroxyl radical (˙OH) scavenging ability of the hydrogels, a Fenton reaction was used in this study, in which the generation of ˙OH relies on the reaction of H2O2 with Fe2+.55 The change in˙OH concentration was monitored by the change in absorbance of the MB solution. In the absence of ˙OH generation, the solution showed a blue color after the addition of MB; after the addition of H2O2 and Fe2+, the generation of ˙OH led to the discoloration of the MB solution.15 The color changes in the control and hydrogel incubation groups were compared by taking photographs of the MB solution at different periods. The results showed that after co-incubation with PHDF hydrogel for different times (5 min, 30 min, 1 h), the color of the solution always remained blue, while the color of the control group gradually faded to light yellow with time (Fig. S5). For the ˙OH solution after co-incubation, the absorbance curves were further analyzed. In the 500–600 nm wavelength range, a distinct UV absorption peak was observed at 664 nm for the MB solution. Upon the addition of hydrogel, the absorbance of the solution slightly decreased, approaching that of the blank MB solution (Fig. 3E). In contrast, the absorbance value of the control group significantly diminished. As the incubation time increased, the absorbance of the solution gradually declined, albeit with minimal change. This could be attributed to the gradual reduction in the hydroxyl radical (˙OH) scavenging capacity of the hydrogel over time. These experimental findings indicate that the hydrogel effectively lowers the concentration of reactive oxygen species (ROS).Photothermal effect
In this study, an 808 nm near-infrared (NIR) laser was used as an excitation light source, and the PHDF hydrogel samples were irradiated by lasers of different powers. Four different laser powers (0.5 W cm−2, 1 W cm−2, 1.5 W cm−2, and 2 W cm−2) were used in the experiment, and each irradiation process lasted for 5 min (Fig. 4B). The experimental results showed that when the laser power was set at 0.5 W cm−2 and 1 W cm−2, the hydrogel temperature increased by less than 10 °C, which was insufficient for effective photothermal sterilization. However, at a power of 2 W cm−2, the temperature exceeded 40 °C, potentially causing damage. Therefore, selecting a power of 1.5 W cm−2 resulted in a temperature increase of about 25 °C, which effectively killed bacteria without damaging surrounding tissues. After irradiating different types of hydrogels for 5 min with the near-infrared laser lamp with the power of 1.5 W cm−2, it is found that the temperatures of the PB hydrogel, the PBHA hydrogel, and the PBHD hydrogel are almost unchanged and that the temperature of the PHDF hydrogel increases to 54.4 °C, which is a very good result (Fig. 4A). The temperature of the PHDF hydrogel was determined using a thermal imager for five minutes with 30s (Fig. 4D), which visually proved that the PHDF hydrogel had a good photothermal effect; the reason should be that the catechol-ferric ion chelates exhibit strong and stable near-infrared (NIR) responsive photothermal conversion capabilities, which absorbed the light energy and converted it into heat energy. The NIR excitation is a controllable behavior for thermal response regulation, which can be adjusted using different irradiation times and excitation light power to raise the temperature of the hydrogel. Meanwhile, the photothermal stability of the hydrogel was evaluated, and the temperature change was small after five cycles of irradiation, which showed an excellent photothermal effect (Fig. 4C).In vitro antibacterial activity
Bacterial infection has become a challenge for the World Health Organization. Currently, antibiotics are predominantly loaded into dressings to treat infected wounds in clinical practice, however, the misuse of antibiotics has led to the emergence of multidrug-resistant bacteria and a decrease in antimicrobial efficiency. The most common bacterial species implicated are Escherichia coli and Staphylococcus aureus, which were selected to assess the antimicrobial activity of the hydrogel. The bacterial suspensions were co-cultured with the hydrogel for 12 hours, and the optical density (OD) at 600 nm was measured to evaluate the antimicrobial efficacy. After co-culturing with PB and PBHA hydrogels, the survival rates of both bacterial species were approximately 100%, whereas E. coli survival was 88.64% and 89.38% and S. aureus survival was 79.57% and 71.06% after co-culturing with PBHD and PHDF hydrogels, respectively (Fig. 4E and F). The antimicrobial properties of the four hydrogels were visualized using the test of an agar plate coating experiment (Fig. 4G). The mean diameter of the circle of inhibition of the PHDF hydrogel was 1.11 cm (E. coli) and 1.37 cm (S. aureus) when measured individually (Fig. 4H and I). The inhibitory activity of PHDF was related to the catechol group and Fe3+. Photothermal antimicrobial therapy (PTT) exhibits a potent bactericidal effect by activating a photosensitizer, which generates a localized photothermal effect upon laser irradiation. This results in localized heating that effectively eradicates surrounding bacteria. PTT demonstrates strong selectivity for bacteria, causing minimal damage to surrounding healthy tissues. Additionally, it does not contribute to the development of bacterial resistance, thereby circumventing the issues associated with the prolonged use of antibiotics. Therefore, the antimicrobial effect of the PHDF hydrogel was tested after 0 min, 1 min, 5 min, and 10 min of irradiation with an 808 nm laser, and the bactericidal efficiency against Staphylococcus aureus and Escherichia coli reached 99% only after 5 min of light (Fig. 4J, K), and the pictures of agar-coated plates also showed its antimicrobial effect (Fig. 4L), while the antimicrobial time was reduced. Therefore, this PHDF hydrogel is expected to have a rapid antibacterial impact due to its photothermal properties.In vitro hemocompatibility and cell cytotoxicity
The hydrogel, when utilized for biomedical applications, must exhibit excellent hemocompatibility and cytocompatibility. To evaluate the hemocompatibility of each hydrogel, hemolysis tests were conducted. Results showed no significant hemolysis occurred in any hydrogel group, with the supernatant remaining clear (Fig. 5A). Data in Fig. 5A indicate that the hemolysis ratios of all hydrogels were under 5%, meeting international standards and demonstrating good hemocompatibility. The cytotoxicity of the hydrogel was assessed using both the CCK8 kit and Live/Dead fluorescence staining methods to assess the influence of the hydrogel (PB, PBHA, PBHD, PHDF) extracts on the L929 cells cultured for 24 h and 48 h. Each group of hydrogel extracts was co-incubated for 24 h and the cell viability was: 113.98%, 100.07%, 94.63%, and 86.68%. After co-incubation for 48 h, the cell viabilities were: 97.08%, 96.65%, 97.20%, and 91.33%, respectively (Fig. 5B and C). The cell survival rates of all the sample groups were above 80% demonstrating good cytocompatibility. Poly(vinyl alcohol) (PVA) and hyaluronic acid (HA) are well-established biocompatible polymers, and as a result, both PB and PBHA matrix hydrogels demonstrated superior cytocompatibility, while the addition of iron ions to the PHDF hydrogel may adversely affect the L929 cells, and its fine compatibility is reduced. The cell morphology was assessed using the Live/Dead fluorescence staining method, where viable cells exhibited green fluorescence and dead cells displayed red fluorescence. After 24 and 48 hours of co-culture, cells in all experimental groups maintained a shuttle-shaped morphology and exhibited strong green fluorescence, while the red fluorescence (dead cells) was almost invisible, indicating high cell viability (Fig. 5D). These observations confirm that the hydrogel materials in each group exhibited excellent cytocompatibility, highlighting their potential for use in biomedical applications.Conclusions
In this study, a photothermal antimicrobial hydrogel was prepared from borate bonds of polyvinyl alcohol and borax, and a catechol structure of dopamine-grafted hyaluronic acid with metal–ligand bonding of iron for bacterial infection treatment. The borate bonds, metal coordination bonds, and hydrogen bonds give the hydrogel excellent self-healing properties, shape adaptability, and injectability. The introduction of dopamine-grafted hyaluronic acid into the hydrogel provided adhesion and antioxidant properties, while the catechol-iron ion chelate endowed the material with excellent photothermal effects and stability, which demonstrated highly effective antimicrobial effects against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) by photothermal therapy (PTT). More importantly, the presence of hyaluronic acid due to polyvinyl alcohol gives this multifunctional hydrogel good cytocompatibility. The drawback is that there is no further validation of the treatment of bacterial infections in complex physiological situations through animal models. However, it provides a design idea and experimental basis for the development of smart light-responsive hydrogels, which can be used as promising antimicrobial materials in the biomedical field.Author contributions
Jingrui Chang: conceptualization, conducting the investigation, developing the methodology, performing formal analysis, and writing the original draft. Xinyu Wang: conducting formal analysis, curating data, and developing the methodology. Xuejiao Ma: supervision and validation. Bo Lu: conceptualization, resources, funding acquisition, supervision, project administration, and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
All the data supporting this article have been included in the main text and the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5qm00525f.Acknowledgements
Financial support was provided by the National Natural Science Foundation of China (51773162 and 21204071).Notes and references
- A. Vaishnav, G. Gurukiran, O. Ighodaro and V. Kandi, Radiological and Imaging Evidence in the Diagnosis and Management of Microbial Infections: An Update, Cureus, 2023, 15, e48756–e48756 Search PubMed.
- F. Mancinetti, A. Marinelli, V. Boccardi and P. Mecocci, Challenges of infectious diseases in older adults: From immunosenescence and inflammaging through antibiotic resistance to management strategies, Mech. Ageing Dev., 2024, 222, 111998 CrossRef CAS.
- A. Baran, A. Kwiatkowska and L. Potocki, Antibiotics and Bacterial Resistance—A Short Story of an Endless Arms Race, Int. J. Mol. Sci., 2023, 24, 5777 CrossRef CAS.
- S. K. Ahmed, S. Hussein, K. Qurbani, R. H. Ibrahim, A. Fareeq, K. A. Mahmood and M. G. Mohamed, Antimicrobial resistance: Impacts, challenges, and future prospects, J. Med. Surg. Public Health, 2024, 2, 100081 CrossRef.
- F. Farjadian, S. Mirkiani, P. Ghasemiyeh, H. R. Kafshboran, S. Mehdi-Alamdarlou, A. Raeisi, R. Esfandiarinejad, S. Soleymani, G. Goshtasbi, N. Firouzabadi, S. Mohammadi-Samani, M. H. Morowvat and M. Doroudian, Smart nanogels as promising platform for delivery of drug, gene, and vaccine; therapeutic applications and active targeting mechanism, Eur. Polym. J., 2024, 219, 113400 CrossRef CAS.
- B. Gajurel, K. B. Tamang, D. Das and R. Adhikari, Advances in synthetic strategies and applications of polymeric hydrogels, Polym. Eng. Sci., 2025, 65, 2803–2840 CrossRef CAS.
- Y. Yang, J. Zhang, S. Wu, Y. Deng, S. Wang, L. Xie, X. Li and L. Yang, Exosome/antimicrobial peptide laden hydrogel wound dressings promote scarless wound healing through miR-21-5p-mediated multiple functions, Biomaterials, 2024, 308, 122558 CrossRef CAS.
- H. Zhao, Y. Wu, Y. Xie, Y. Li, C. Chen, C. Li, F. Yang, D. Zhang, Y. Wang and J. Yuan, Hydrogel dressings for diabetic foot ulcer: A systematic review and meta-analysis, Diabetes, Obes. Metab., 2024, 26, 2305–2317 CrossRef.
- X. Guo, J. Li, Y. Wu and L. Xu, Recent advancements in hydrogels as novel tissue engineering scaffolds for dental pulp regeneration, Int. J. Biol. Macromol., 2024, 264, 130708 CrossRef CAS.
- P. Wu, C. Xu, X. Zou, K. Yang, Y. Xu, X. Li, X. Li, Z. Wang and Z. Luo, Capacitive-Coupling-Responsive Hydrogel Scaffolds Offering Wireless In Situ Electrical Stimulation Promotes Nerve Regeneration, Adv. Mater., 2024, 36, e2310483 CrossRef PubMed.
- C. Wei, S. Xing, Y. Li, M. Koosha, S. Wang, H. Chen, Y. Zhai, L. Wang, X. Yang and R. Fakhrullin, Gelatin/carboxymethyl chitosan/aloe juice hydrogels with skin-like endurance and quick recovery: Preparation, characterization, and properties, Int. J. Biol. Macromol., 2024, 261, 129720 CrossRef CAS.
- X. Li and J. P. Gong, Design principles for strong and tough hydrogels, Nat. Rev. Mater., 2024, 9, 380–398 CrossRef CAS.
- X. Yan, H. Huang, A. M. Bakry, W. Wu, X. Liu and F. Liu, Advances in enhancing the mechanical properties of biopolymer hydrogels via multi-strategic approaches, Int. J. Biol. Macromol., 2024, 272, 132583 CrossRef CAS.
- J. m Jin, C. c Sun, K. y Xu, X. Sun, L. Cao and L. Liu, Multifunctional self-healing peptide hydrogel for wound healing, Int. J. Biol. Macromol., 2024, 261, 129734 CrossRef CAS.
- Z. J. Shao, T. Y. Yin, J. B. Jiang, Y. He, T. Xiang and S. Zhou, Wound microenvironment self-adaptive hydrogel with efficient angiogenesis for promoting diabetic wound healing, Bioact. Mater., 2023, 20, 561–573 CAS.
- L. l Deng, B. x Wang, W. y Li, Z. l Han, S. Chen and H. Wang, Bacterial cellulose reinforced chitosan-based hydrogel with highly efficient self-healing and enhanced antibacterial activity for wound healing, Int. J. Biol. Macromol., 2022, 217, 77–87 CrossRef CAS PubMed.
- S. Khattak, I. Ullah, H. Xie, X.-D. Tao, H.-T. Xu and J. Shen, Self-healing hydrogels as injectable implants: Advances in translational wound healing, Coord. Chem. Rev., 2024, 509, 215790 CrossRef CAS.
- Z. Deng, H. Wang, P. X. Ma and B. Guo, Self-healing conductive hydrogels: preparation, properties and applications, Nanoscale, 2020, 12, 1224–1246 RSC.
- S. Mehrnoosh Hasan, H. Afra and A. Majid, Advances in self-healing hydrogels to repair tissue defects, Polym. Bull., 2022, 80, 1155–1177 Search PubMed.
- M. Y. Shan, X. Chen, X. Y. Zhang, S. Zhang, L. Zhang, J. Chen, X. Wang and X. Liu, Injectable conductive hydrogel with self-healing, motion monitoring, and bacteria theranostics for bioelectronic wound dressing, Adv. Healthcare Mater., 2024, 13, e2303876 CrossRef.
- C. x Zhang, H. y Lu and X. Wang, Transient polymer hydrogels based on dynamic covalent borate ester bonds, Chin. J. Chem., 2022, 40, 2794–2800 CrossRef CAS.
- Y. Chen, J. Li, J. Lu, M. Ding and Y. Chen, Synthesis and properties of Poly(vinyl alcohol) hydrogels with high strength and toughness, Polym. Test., 2022, 108, 107516 CrossRef CAS.
- X. Liang, H.-J. Zhong, H. Ding, B. Yu, X. Ma, X. Liu, C.-M. Chong and J. He, Polyvinyl Alcohol (PVA)-Based Hydrogels: Recent Progress in Fabrication, Properties, and Multifunctional Applications, Polymers, 2024, 16, 2755 CrossRef CAS PubMed.
- M. Wang, J. Bai, K. Shao, W. Tang, X. Zhao, D. Lin, S. Huang, C. Chen, Z. Ding and J. Ye, Poly(vinyl alcohol) Hydrogels: The Old and New Functional Materials, Int. J. Polym. Sci., 2021, 2021, 2225426 Search PubMed.
- H. Zhu, J. Zeng, S. Jiang, S. Huang, Z. Xie, Y. Huang, S. Lin and B. Wang, Poly(vinyl alcohol)/borax/modified silver nanowire composite hydrogel with self-healing, high conductivity, and electromagnetic interference shielding properties, J. Alloys Compd., 2025, 1011, 178437 CrossRef CAS.
- J. L. Obeso, M. T. Huxley, C. Leyva, J. Gabriel Flores, N. Martín-Guaregua, M. Viniegra, J. Aguilar-Pliego and J. Antonio, de los Reyes, I. A. Ibarra and R. A. Peralta, The role of dynamic metal-ligand bonds in metal-organic framework chemistry, Coord. Chem. Rev., 2023, 496, 215403 CrossRef CAS.
- M. Wu, L. Han, B. Yan and H. Zeng, Self-healing hydrogels based on reversible noncovalent and dynamic covalent interactions: A short review, SMAT, 2023, 2, 100045 Search PubMed.
- I. Condò, S. M. Giannitelli, D. Lo Presti, B. Cortese and O. Ursini, Overview of Dynamic Bond Based Hydrogels for Reversible Adhesion Processes, Gels, 2024, 10, 442 CrossRef.
- D. Fan, R. Xie, X. Liu, H. Li, Z. Luo, Y. Li, F. Chen and W. Zeng, A peptide-based pH-sensitive antibacterial hydrogel for healing drug-resistant biofilm-infected diabetic wounds, J. Mater. Chem. B, 2024, 12, 5525–5534 RSC.
- J. Guo, L. Yao, X. Wang, R. Song, B. Yang, D. Jin, J. Guo and G. Wu, Dual-Responsive Antibacterial Hydrogel Patch for Chronic-Infected Wound Healing, Biomacro, 2024, 25, 7283–7297 CrossRef CAS.
- Y. Yang, Y. Ma, H. Wang, C. Li, C. Li, R. Zhang, S. Zhong, W. He and X. Cui, Chitosan-based hydrogel dressings with antibacterial and antioxidant for wound healing, Int. J. Biol. Macromol., 2024, 280, 135939 CrossRef CAS PubMed.
- F. Yang, Y. Ge, Y. Zhang, Z. Cui, S. Lin, W. Ni, Z. Sun, D. Shen, J. Zhu, L. Liu, S. Zhao, N. Huang, F. Sun, Y. Lu, S. Shi and J. Li, NIR-Activated Hydrogel with Dual-Enhanced Antibiotic Effectiveness for Thorough Elimination of Antibiotic-Resistant Bacteria, ACS Appl. Mater. Interfaces, 2025, 17, 2952–2965 CrossRef CAS.
- S. Abri, H. Durr, H. A. Barton, K. Adkins-Travis, L. P. Shriver, D. D. Pukale, J. A. Fulton and N. D. Leipzig, Chitosan-based multifunctional oxygenating antibiotic hydrogel dressings for managing chronic infection in diabetic wounds, ACS Biomater. Sci. Eng., 2024, 12, 3458–3470 RSC.
- J. Chen, X. Xing, D. Liu, L. Gao, Y. Liu, Y. Wang and H. Cheng, Copper nanoparticles incorporated visible light-curing chitosan-based hydrogel membrane for enhancement of bone repair, J. Mech. Behav. Biomed. Mater., 2024, 158, 106674 CrossRef CAS PubMed.
- X. Wei, C. Liu, Z. Li, Z. Gu, J. Yang and K. Luo, Chitosan-based hydrogel dressings for diabetic wound healing via promoting M2 macrophage-polarization, Carbohydr. Polym., 2024, 331, 121873 CrossRef CAS.
- L. P. Qiao, Y. P. Liang, J. Y. Chen, Y. Huang, S. A. Alsareii, A. M. Alamri, F. A. Harraz and B. Guo, Antibacterial conductive self-healing hydrogel wound dressing with dual dynamic bonds promotes infected wound healing, Bioact. Mater., 2023, 30, 129–141 CAS.
- L. He, D. Di, X. Chu, X. Liu, Z. Wang, J. Lu, S. Wang and Q. Zhao, Photothermal antibacterial materials to promote wound healing, J. Controlled Release, 2023, 363, 180–200 CrossRef CAS PubMed.
- J. Cao, Z. Song, T. Du and X. Du, Antimicrobial materials based on photothermal action and their application in wound treatment, Int. J. Burns Trauma, 2024, 12, tkae046 CrossRef.
- H. Gao, H. Li, S. Shao, L. Tan, Y. Wang, D. Li, W. Zhang, T. Zhu, G. Liu and X. Meng, Self-enhanced PTX@HSA-HA loaded functionalized injectable hydrogel for effective local chemo-photothermal therapy in breast cancer, Carbohydr. Polym., 2024, 345, 122569 CrossRef CAS PubMed.
- S. Zhou, X. Zhang, W. Ni, Y. He, M. Li, C. Wang, Y. Bai, H. Zhang and M. Yao, An Immune-Regulating Polysaccharide Hybrid Hydrogel with Mild Photothermal Effect and Anti-Inflammatory for Accelerating Infected Wound Healing, Adv. Healthcare Mater., 2024, 13, e2400003 CrossRef.
- S. Guo, M. Yao, D. Zhang, Y. He, R. Chang, Y. Ren and F. Guan, One-Step Synthesis of Multifunctional Chitosan Hydrogel for Full-Thickness Wound Closure and Healing, Adv. Healthcare Mater., 2022, 11, e2101808 CrossRef.
- W. Chang, L. Chen and K. Chen, The bioengineering application of hyaluronic acid in tissue regeneration and repair, Int. J. Biol. Macromol., 2024, 270, 132454 CrossRef CAS.
- A. Zhang, J. Huang, Y. Liu, H. Gong, F. Guan, W. Li, F. Han and Y. Wang, Hyaluronic acid application strategies for plant bioactive component delivery: A review, Int. J. Biol. Macromol., 2024, 282, 137129 CrossRef CAS PubMed.
- A. Chhillar and A. Jaiswal, Hyaluronic Acid-Based Self-Healing Hydrogels for Diabetic Wound Healing, Adv. Healthcare Mater., 2025, 14, e2404255 CrossRef PubMed.
- W. Duan, X. Jin, Y. Zhao, S. Martin-Saldaña, S. Li, L. Qiao, L. Shao, B. Zhu, S. Hu, F. Li, L. Feng, Y. Ma, B. Du, L. Zhang and Y. Bu, Engineering injectable hyaluronic acid-based adhesive hydrogels with anchored PRP to pattern the micro-environment to accelerate diabetic wound healing, Carbohydr. Polym., 2024, 337, 122146 CrossRef CAS.
- N. Yuan, K. Shao, S. Huang and C. Chen, Chitosan, alginate, hyaluronic acid and other novel multifunctional hydrogel dressings for wound healing: A review, Int. J. Biol. Macromol., 2023, 240, 124321 CrossRef CAS.
- I. R. Ausri, S. Sadeghzadeh, S. Biswas, H. Zheng, P. GhavamiNejad, M. D. T. Huynh, F. Keyvani, E. Shirzadi, F. A. Rahman, J. Quadrilatero, A. GhavamiNejad and M. Poudineh, Multifunctional Dopamine-Based Hydrogel Microneedle Electrode for Continuous Ketone Sensing, Adv. Mater., 2024, 36, e2402009 CrossRef.
- Y. Wang, Y. Zhang, Y. P. Yang, M. Y. Jin, S. Huang, Z. M. Zhuang, T. Zhang, L. L. Cao, X. Y. Lin, J. Chen, Y. Z. Du, J. Chen and W. Q. Tan, Versatile dopamine-functionalized hyaluronic acid-recombinant human collagen hydrogel promoting diabetic wound healing via inflammation control and vascularization tissue regeneration, Bioact. Mater., 2024, 35, 330–345 CAS.
- M. Zeng, J. Ding, Y. Tian, Y. Zhang, X. Liu, Z. Chen, J. Sun, C. Wu, H. Yin, D. Wei and H. Fan, Dopamine-integrated all-hydrogel multi-electrode arrays for neural activity recording, Mater. Horiz., 2024, 11, 6423–6434 RSC.
- C. Hwang, M.-H. Choi, H.-E. Kim, S.-H. Jeong and J.-U. Park, Reactive oxygen species-generating hydrogel platform for enhanced antibacterial therapy, NPG Asia Mater., 2022, 14, 72 CrossRef CAS.
- H. Zhang, J. Wang, H. Hu and L. Ma, A highly transparent dopamine-copolymerized hydrogel with enhanced ROS-scavenging and tissue-adhesive properties for chronic diabetic wounds, Acta Biomater., 2025, 198, 161–173 CrossRef CAS.
- Y. Wang, X. Fang, S. Li, H. Pan and J. Sun, Complexation of Sulfonate-Containing Polyurethane and Polyacrylic Acid Enables Fabrication of Self-Healing Hydrogel Membranes with High Mechanical Strength and Excellent Elasticity, ACS Appl. Mater. Interfaces, 2023, 15, 25082–25090 CrossRef CAS PubMed.
- J. Liu, X. Han, T. Zhang, K. Tian, Z. Li and F. Luo, Reactive oxygen species (ROS) scavenging biomaterials for anti-inflammatory diseases: from mechanism to therapy, J. Hematol. Oncol., 2023, 16, 116 CrossRef CAS PubMed.
- P. Wang, W. C. Liu, C. Han, S. Wang, M. Y. Bai and C. P. Song, Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development, J. Integr. Plant Biol., 2024, 66, 330–367 CrossRef CAS.
- D. Meyerstein, Re-examining Fenton and Fenton-like reactions, Nat. Rev. Chem., 2021, 5, 595–597 CrossRef CAS.
| This journal is © the Partner Organisations 2026 |