Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A crystalline chiral phosphide for the synthesis of the first P-stereogenic P(III) fluoride: a stable ligand for the Rh-catalyzed asymmetric arylation of isatins
Laila Al Baridi,
Giorgio Parla,
Alberto Herrera*,
Frank W. Heinemann and
Romano Dorta *
*
Department Chemie und Pharmazie, Anorganische und Allgemeine Chemie, Friedrich–Alexander–Universität Erlangen–Nürnberg, Egerlandstraße 1, 91058 Erlangen, Germany. E-mail: romano.dorta@fau.de
First published on 18th December 2025
Abstract
Stable P-stereogenic P(III) fluorides of the type *PR1R2F have long resisted isolation, despite their great potential as ligands in asymmetric catalysis. We report the synthesis of a crystalline, chiral lithium alkene-phosphide that undergoes rapid, enantiospecific fluorination with N-fluorobenzenesulfonimide with retention of configuration to yield the corresponding fluorophosphinamide–alkene hybrid ligand in >99% ee. The ligand is configurationally stable up to 100 °C and forms a Rh(I) complex that catalyzes the base- and water-free asymmetric arylation of isatins to biologically important 3-hydroxyoxindoles with up to 99.5% ee.
Introduction
Despite the fact that Burg and Brendel 66 years ago reported the synthesis of the first organo-fluorophosphine, namely (CF3)2PF,1 the use of P(III) fluorides as ligands in coordination chemistry is, apart from PF3,2 scarcely described.3 Applications in catalysis are even rarer,4 and asymmetric versions have not yet been reported, due to the lack of effective synthetic methods towards optically pure fluorophosphines. Chiral phosphines are the ligands of choice for many transition-metal-catalyzed asymmetric reactions in industry and academia.5 Of special interest are P-stereogenic phosphines, which place chirality in proximity of the metal center. This concept gained industrial maturity with Monsanto's L-Dopa process in the late 1970's, for which Knowles was awarded the Nobel prize.6 However, the synthesis of enantiopure P-stereogenic compounds is notoriously difficult7 and a topic of high relevance to asymmetric catalysis.8 In particular, the stereoselective installation of a P–F bond in P-stereogenic phosphine ligands has remained elusive so far and is of prime interest because it would allow to introduce strong steric and electronic differentiation on the P-donor and considerably widen the diversity of chiral ligand design.9 Even though the P–F bond is polar and possesses a significant strength of 545 kJ mol−1,10 applications of fluorophosphines in catalysis have been hampered by their high propensity towards redox disproportionation.4In a recent evolution of the ‘privileged ligand’ 111 we found the planar chirality in the diastereomers (pS,R)-2 and (pR,R)-2 to completely overwhelm the axial chirality of the potent binol auxiliary in the enantioselective Hayashi-Miyaura reaction.12 Some years ago, we explored the possibility to introduce the promising P-chiral tert-butylmethylphosphine function13 to such systems by isolating the stereochemically stable ligand rac-3.14 Having taken inspiration from the seminal reports on P-stereogenic P(III)-fluorides by Wild,15 Pringle,4b Puckette,4e and others,16 we disclose here a perfectly stereoselective P–F bond forming protocol that allowed us to isolate the first enantiopure P-stereogenic P(III) fluoride, its Rh(I) complex, and use in catalytic asymmetric C–C bond formation.
Results and discussion
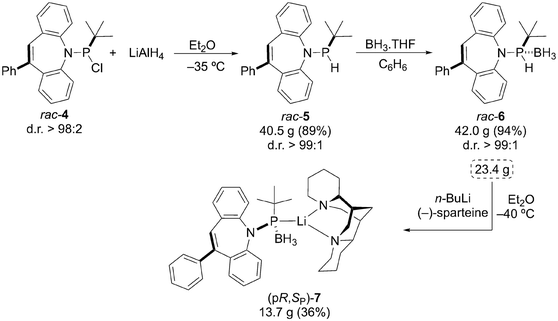
We opted for Livinghouse's protocol for the synthesis of optically pure P-stereogenic phosphines via chiral phosphide intermediates obtained by enantioselective deprotonation of secondary phosphine-boranes, which then are quenched with organic electrophiles.17 In our case, the diastereomerically pure, BH3-protected, secondary phosphinamide rac-6 (Scheme 1) is prepared by reducing diastereomerically enriched (d.r. > 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) chlorophosphine rac-4 with LiAlH4 to almost diastereopure rac-5 followed by protection with BH3·THF. Deprotonation of 23.4 g of rac-6 with the n-BuLi/(−)-sparteine mixture in Et2O at −40 °C, yields 13.7 g of phosphide (pR,SP)-7. Non-decoupled NMR spectra of the 31P, 11B, and 7Li nuclei display multiplets centered at 96.5, 31.6, and 0.8 ppm, respectively. The molecular mass for 7, estimated by DOSY-NMR (585 g mol−1) corresponds to a monomer (MW = 612 g mol−1). Single crystals of 7 grow from 1,2-difluorobenzene/Et2O and XRD analysis confirms its absolute configuration and monomeric structure featuring a P–Li bond (see Fig. 1) contrasting Livinghouse's chiral phosphide, in which the borane moiety bridges the Li-sparteine complex.18 Unlike Livinghouse's dynamically resolving system, we think that in our case the BuLi/sparteine deprotonation enables resolution of the lithium phosphide sparteine complex by diastereoselective crystallization19 from cold Et2O solutions, which might explain the modest yields of (pR,SP)-7. The (pS,RP)-antipode is accessible by using (+)-sparteine (see the SI for details).
2) chlorophosphine rac-4 with LiAlH4 to almost diastereopure rac-5 followed by protection with BH3·THF. Deprotonation of 23.4 g of rac-6 with the n-BuLi/(−)-sparteine mixture in Et2O at −40 °C, yields 13.7 g of phosphide (pR,SP)-7. Non-decoupled NMR spectra of the 31P, 11B, and 7Li nuclei display multiplets centered at 96.5, 31.6, and 0.8 ppm, respectively. The molecular mass for 7, estimated by DOSY-NMR (585 g mol−1) corresponds to a monomer (MW = 612 g mol−1). Single crystals of 7 grow from 1,2-difluorobenzene/Et2O and XRD analysis confirms its absolute configuration and monomeric structure featuring a P–Li bond (see Fig. 1) contrasting Livinghouse's chiral phosphide, in which the borane moiety bridges the Li-sparteine complex.18 Unlike Livinghouse's dynamically resolving system, we think that in our case the BuLi/sparteine deprotonation enables resolution of the lithium phosphide sparteine complex by diastereoselective crystallization19 from cold Et2O solutions, which might explain the modest yields of (pR,SP)-7. The (pS,RP)-antipode is accessible by using (+)-sparteine (see the SI for details).
With optically pure phosphide (pR,SP)-7 in hand we first validated its utility as a stereospecific nucleophile for the synthesis of our well-understood P-alkene rac-3 since earlier attempts of stereospecific C–N bond formation between lithium phenyldibenzoazepinate20 and enantiopure (R)-(Me)(tBu)PBr(BH3) (Imamoto's method)21 only afforded rac-3 albeit in diasteromerically pure form. Gratifyingly, methyl iodide reacts smoothly with (pR,SP)-7 to produce the protected phosphinamide (pR,RP)-8 in 99% ee (Scheme 2), which is deprotected to (pR,RP)-3 by DABCO. Its precise stereochemistry is established by the crystal structure of the Rh(I) complex 11 (see below and Fig. S1).
Likewise, phosphide (pR,SP)-7 reacts with N-fluorobenzenesulfonimide22 with retention of configuration at phosphorous to the BH3-protected diastereo- and enantiopure amido-t-butyl fluorophosphine (pR,RP)-9 (Scheme 2).23 P–F bond formation is evident in 31P{1H} and non-decoupled 19F NMR spectra, which show a doublet of multiplets and a doublet of quartets centered at 155.9 (JPF ≈ 1050 Hz) and −109.4 ppm (JFP ≈ 1050 Hz, JFH = 16.1 Hz), respectively (Fig. S26). The 1H NMR spectrum shows a doublet at 1.09 ppm and broad multiplets between 0.45–0.26 ppm corresponding to the tBu and BH3 moieties. Enantiopurity was confirmed by chiral HPLC (Fig. S41 in the SI). Fig. 2 shows the crystal structure of (pR,RP)-9, which confirms the formation of the P–F bond (dP–F = 1.585(10) Å), the absolute configuration of the P-atom, and the planar chirality of the dibenzoazepine ring, which are (pR,RP). Importantly, expensive (−)-sparteine can be recycled quantitatively. The basicity of the P-donor in (pR,RP)-9 seems lower than in (pR,RP)-3,24 because removal of the BH3 moiety from (pR,RP)-9 is achieved with NEt3, instead of DABCO affording diastereo- and enantiopure free fluorophosphinamide (pR,RP)-10 in excellent yields. To make sure deprotection did not erode enantiopurity, (pR,RP)-10 was re-protected with BH3·THF giving back (pR,RP)-9 in > 99% optical purity. 31P and 19F NMR spectra show new doublets at 176.7 ppm and −132.3 ppm, respectively, with JPF = 970.6 Hz. In the 13C NMR spectrum, the quaternary carbon and the methyl groups of the tBu moiety, appear at 35 ppm as a doublet of doublets at 35.0 (JCP = 25.3 Hz, JCF = 12.1 Hz) and 25.4 ppm (JCP = 19.5 Hz, JCF = 1.7 Hz), respectively. The crystal structure confirms the unaltered configuration in (pR,RP)-10 and shows significant elongation of both the P–F (to 1.6286(10) Å) and P–N bonds compared with (pR,RP)-9 (Fig. 2). Deprotected (pR,RP)-9 is surprisingly robust: It is air-stable in the solid state and withstands boiling chloroform and toluene solutions without showing signs of decomposition or epimerization.
(pR,RP)-3 and (pR,RP)-10 both react with [RhCl(COE)2]2 (COE = cyclooctene) to form the respective P-alkene ligated dinuclear complexes (pR,RP)-11 (see Fig. S1 in the SI for its crystal structure) and (pR,RP)-1225 according to eqn (1). The 31P{1H} spectrum of (pR,RP)-12 shows the formation of a single isomer with a doublet of doublets centered at 231.5 ppm (JPF = 1066 Hz, JPRh = 249.6 Hz), and the non-decoupled 19F spectrum exhibits a doublet of doublets at −104.8 ppm (JF–P = 1065 Hz, JF–Rh = 16.4 Hz). The alkene–C–H resonates at relatively low frequency as a singlet at 5.70 ppm, indicating alkene coordination. (pR,RP)-12 crystallizes as red blocks from benzene solution, and its crystal structure in Fig. 3 confirms the bidentate coordination of the ligands in an anti-fashion to the Rh2Cl4 butterfly core, which spans an angle of 99° between the square coordination planes around the Rh atoms. The P–F bond is shorter than in the free ligand and is comparable to the P–F bond in borane complex 8. The P–F bond in complex (pR,RP)-12 is significantly shorter than the P–Me bond in complex (pR,RP)-11, measuring 1.58 vs. 1.82 Å, respectively (1.63 vs. 1.82 Å in the respective free ligands). Including the H-atoms of the methyl substituent an even larger difference in the respective van-der-Waals volumes is expected. Fluorine substitution at the P-donor also shortens the Rh–P bond in complex (pR,RP)-12 (2.132(4) Å) compared to (pR,RP)-11 (2.1622(9) Å); a small but statistically significant difference.
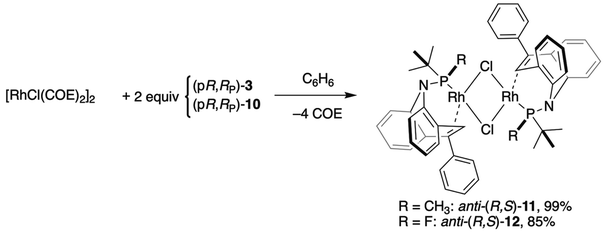 | (1) |
The P–F ligand (pR,RP)-10 was then benchmarked against ligands (pR,RP)-2 and (pR,RP)-3 of identical planar chirality in the base-free arylation of isatins with sodium tetraarylborates26 to biologically important 3-aryl-3-hydroxyoxindoles (Table 1).27 The arylation of benzyl-protected isatin 14a with NaBPh4, is catalyzed by (pR,RP)-12 bearing the P–F ligand affords 16aa quantitatively in 86% ee, whereas the previously reported cationic complex [Rh((pR,R)-2)2][BF4]12a and (pR,RP)-11 bearing ligands (pR,RP)-2 and (pR,RP)-3, respectively, give conversions of <10%. Only with the electron-poor isatin 14b do these catalysts afford relevant quantities of 16ba. For this product, catalyst (pR,RP)-11 exhibits good enantioselectivity compared with the much more active but less selective (pR,RP)-12. The sense of induction of the ligands with the Me- and the F-substituted P-donors is identical.28 In situ generation of the cationic catalyst [Rh((pR,R)-10)2][NTf] pushes the ee of the protected dimethyl hydroxyoxindole 16ca up to 96%. Surprisingly, catalyst (pR,RP)-12 works even better with unprotected NH isatins29 at reduced catalyst loadings. Electron-donating substituents at RI para to the NH function appear to favor enantioselectivity affording hydroxyoxindoles of very high enantiomeric purity (compounds 16fa, 16ha and 16ia), while N-protection and the use of tetra-p-tolylborate 15b significantly erode enantioselectivity.
Conclusions
We report a significant advance in the long-standing synthetic challenge of preparing a stereochemically stable P-stereogenic fluorophosphines of the type PR1R2F. This was achieved via enantiospecific electrophilic fluorination of the crystalline alkene-phosphide (pR,SP)-7, yielding the configurationally stable fluorophosphinamide (pR,RP)-10 in gram quantities. This compound introduces a novel donor motif for chiral ligand design and functions as a bidentate ligand in the Rh(I) complex (pR,R)-12. In rhodium-catalyzed, water- and base-free arylations of isatins using NaBAr4 nucleophiles, (pR,RP)-10 performs favorably compared to benchmark planar-chiral ligands 2 and 3, particularly in the arylation of unprotected NH-isatins. This transformation marks the first application of a fluorophosphinamide in asymmetric catalysis. Notably in this context, the P(t-Bu)F synthon outperforms the generally effective P*(t-Bu)(Me) analog.13 Furthermore, the crystalline phosphide (pR,SP)-7 provides a versatile platform for accessing new classes of P-stereogenic P-alkene hybrid ligands, the exploration of which is currently underway.Conflicts of interest
The authors declare no conflicts of interest.Data availability
Synthetic procedures, X-ray crystallographic data, NMR spectra, and HPLC traces for this study are available as Supplementary Information. See DOI: https://doi.org/10.1039/d5qi01994j.CCDC 2390089–2390093 contain the supplementary crystallographic data for this paper.30a–e
Acknowledgements
We thank Ms Antigone Roth for carrying out the elemental analyses, Mr Shao Kai Lu for assistance in up-scaling the synthesis of (pR,SP)-7, and Mr Jochen Schmidt for measuring NMR spectra. Financial support by Friedrich–Alexander University is gratefully acknowledged.References
- A. B. Burg and G. Brendel, Fluorocarbon-Phosphinoborines and Related Chemistry, J. Am. Chem. Soc., 1958, 80, 3198–3202 CrossRef CAS.
- For reviews, see: (a) T. Kruck and M. Höfler, Synthesis of Tetrakis(trimethoxyphosphine)nickel(0), Angew. Chem., Int. Ed. Engl., 1967, 6, 563 CrossRef CAS; (b) J. F. Nixon and J. R. Swain, Trifluorophosphine Complexes of the Platinum Metals, Platinum Metals Rev., 1975, 19, 22–29 CrossRef CAS; (c) H.-R. Jaw and J. I. Zink, Angular-overlap interpretation of σ and π bonding of phosphorus trifluoride and phosphorus trichloride in platinum PtCl3L complexes, Inorg. Chem., 1988, 27, 3421–3424 CrossRef CAS.
- For crystallographically characterized complexes of RPF2, see: (a) J. R. Goerlich, J.-V. Weiss, P. G. Jones and R. Schmutzler, Phosphorus, Sulfur Silicon Relat. Elem., 1992, 66, 223–243 CrossRef CAS; (b) F. Delgado Calvo, V. Mirabello, M. Caporali, W. Oberhauser, K. Raltchev, K. Karaghiosoff and M. Peruzzini, Dalton Trans., 2016, 45, 2284 RSC. For crystallographically characterized complexes of R2PF, see: (c) W. S. Sheldrick and O. Stelzer, Preparation, crystal and molecular structure of trans-dibromobis-[di(t-butyl)fluorophosphine]nickel(II), J. Chem. Soc., Dalton Trans., 1973, 926 RSC; (d) L. Heuer and D. Schomburg, t,Bu2PF as a ligand in tri-osmium clusters, J. Organomet. Chem., 1995, 495, 53–59 CrossRef CAS.
- For a recent review see: (a) A. Miles-Hobbs, P. G. Pringle, J. D. Woollins and D. Good, Monofluorophos–Metal Complexes: Ripe for Future Discoveries in Homogeneous Catalysis, Molecules, 2024, 29, 2368–2395 CrossRef CAS PubMed. For applications in alkene hydroformylation, see: (b) N. Fey, M. Garland, J. P. Hopewell, C. L. McMullin, S. Mastroianni, A. Guy Orpen and P. G. Pringle, Stable fluorophosphines: predicted and realized ligands for catalysis, Angew. Chem., Int. Ed., 2012, 51, 118–122 CrossRef CAS PubMed. Notable patents assigned to Eastman Chemical Company are: (c) T. A. Puckette and G. E. Struck, Hydroformylation process using phosphite-metal catalyst system, US5840647, 1998; (d) T. A. Puckette, Hydroformylation process for the preparation of glycolaldehyde, US7301054B1, 2007; (e) T. A. Puckette, Amido-Fluorophosphite Compounds and Catalysts, US8492593B2, 2013; A very recent application in cross-coupling is: (f) F. Flecken, A. Neyyathala, T. Grell and S. Hanf, A Bench-stable Fluorophosphine Nickel(0) Complex and Its Catalytic Application, Angew. Chem., Int. Ed., 2025, 64, e202506271 CrossRef CAS PubMed.
- R. Noyori, in Asymmetric Catalysis in Organic Synthesis, Wiley, 1994 Search PubMed; Asymmetric catalysis on Industrial Scale, ed. H.-U. Blaser and H.-J. Federsel, Wiley-VCH, 2010 Search PubMed.
- (a) W. S. Knowles, Asymmetric hydrogenation, Acc. Chem. Res., 1983, 16, 106–112 CrossRef CAS; (b) W. S. Knowles, Asymmetric hydrogenations (Nobel Lecture), Angew. Chem., Int. Ed., 2002, 41, 1998–2007 CrossRef CAS . For a new synthetic route to DIPAMP, see: Z. S. Han, N. Goyal, M. A. Herbage, J. D. Sieber, B. Qu, Y. Xu, Z. Li, J. T. Reeves, J.-N. Desrosiers, S. Ma, N. Grinberg, H. Lee, H. P. R. Mangunuru, Y. Zhang, D. Krishnamurthy, B. Z. Lu, J. J. Song, G. Wang and C. H. Senanayake, Efficient asymmetric synthesis of p-chiral phosphine oxides via properly designed and activated benzoxazaphosphinine-2-oxide agents, J. Am. Chem. Soc., 2013, 135, 2474–2477 Search PubMed ; For P-stereogenic ligands used in industry post DIPAMP, see: ; (c) T. Imamoto, T. Oshiki, T. Onozawa, T. Kusumoto and K. Sato, P-chiral bis(trialkylphosphine) ligands and their use in highly enantioselective hydrogenation reactions, J. Am. Chem. Soc., 1998, 120, 1635–1636 CrossRef CAS; (d) T. Imamoto, K. Sugita and K. Yoshida, An air-stable p-chiral phosphine ligand for highly enantioselective transition-metal-catalyzed reactions, J. Am. Chem. Soc., 2005, 127, 11934–11935 CrossRef CAS PubMed; (e) K. Tamura, M. Sugiya, K. Yoshida, A. Yanagisawa and T. Imamoto, Enantiopure 1,2-Bis(tert-butylmethylphosphino)benzene as a Highly Efficient Ligand in Rhodium-Catalyzed Asymmetric Hydrogenation, Org. Lett., 2010, 12, 4400–4403 CrossRef CAS PubMed; (f) T. Imamoto, K. Tamura, Z. Zhang, Y. Horiuchi, M. Sugiya, K. Yoshida, A. Yanagisawa and I. D. Gridnev, Rigid P-Chiral Phosphine Ligands with tert-Butylmethylphosphino Groups for Rhodium-Catalyzed Asymmetric Hydrogenation of Functionalized Alkenes, J. Am. Chem. Soc., 2012, 134, 11934–11935 CrossRef PubMed.
- For reviews of stoichiometric and catalytic methods, see: (a) M. Dutartre, J. Bayardon and S. Jugé, Applications and stereoselective syntheses of P-chirogenic phosphorus compounds, Chem. Soc. Rev., 2016, 45, 5771–5794 RSC. Selected reports on catalytic routes to P-chiral compounds are: (b) X. Ye, L. Penga, X. Baoa, C.-H. Tan and H. Wang, Recent developments in highly efficient construction of P-stereogenic centers, Green Synth. Catal., 2021, 2, 6–18 Search PubMed; (c) D. Glueck, Catalytic Asymmetric Synthesis of P-Stereogenic Phosphines: Beyond Precious Metals, Synlett, 2021, 31, 875–884 CrossRef; C. Wang, Y.-H. Dai, Z. Wang, B. Lu, W. Cao, J. Zhao, G. Mei, Q. Yang, J. Guo and W.-L. Duan, Nickel-Catalyzed Enantioselective Alkylation of Primary Phosphines, J. Am. Chem. Soc., 2021, 143, 5685–5690 Search PubMed; (d) X. Gu, X. Mo, W.-J. Bai, P. Xie, W. Hu and J. Jiang, Catalytic Asymmetric P−H Insertion Reactions, J. Am. Chem. Soc., 2023, 145, 20031–20040 CrossRef CAS PubMed; (e) M. Formica, T. Rogova, H. Shi, N. Sahara, B. Ferko, A. J. M. Farley, K. E. Christensen, F. Duarte, K. Yamazaki and D. J. Dixon, Catalytic enantioselective nucleophilic desymmetrization of phosphonate esters, Nat. Chem., 2023, 15, 714–721 CrossRef CAS PubMed; (f) O. Berger and J. L. Montchamp, A general strategy for the synthesis of P-stereogenic compounds, Angew. Chem., Int. Ed., 2013, 52, 11377–11380 CrossRef CAS PubMed.
- Excellent reviews are: (a) A. Grabulosa, P-stereogenic Ligands in Enantioselective Catalysis, RSC Catalysis Series, RSC Publishing, 2011 Search PubMed; (b) G. Xu, C. H. Senanayake and W. Tang, P-chiral phosphorus ligands based on a 2,3-dihydrobenzo[d][1,3]oxaphosphole motif for asymmetric catalysis, Acc. Chem. Res., 2019, 52, 1101–1112 CrossRef CAS PubMed; (c) P. Rojo, A. Riera and X. Verdaguer, Bulky P-stereogenic ligands. A success story in asymmetric catalysis, Coord. Chem. Rev., 2023, 489, 215192 CrossRef CAS; (d) T. Imamoto, P-Stereogenic Phosphorus Ligands in Asymmetric Catalysis, Chem. Rev., 2024, 124, 8657–8739 CrossRef CAS PubMed.
- Chiral Ligands: Evolution of Ligand Libraries for Asymmetric Catalysis, ed. M. Diéguez, Taylor & Francis Group, 1st edn, 2021 Search PubMed.
- D. J. Grant, M. H. Matus, J. R. Switzer, D. A. Dixon, J. S. Francisco and K. O. Christe, Bond, dissociation energies in second-row compounds, J. Phys. Chem. A, 2008, 112, 3145–3156 CrossRef CAS PubMed.
- (a) C. Defieber, M. A. Ariger, P. Moriel and E. M. Carreira, Iridium-Catalyzed Synthesis of Primary Allylic Amines from Allylic Alcohols: Sulfamic Acid as Ammonia Equivalent, Angew. Chem., Int. Ed., 2007, 47, 3139–3143 CrossRef PubMed; (b) A. Briceño and R. Dorta, cis-Dichloridobis{[(S)-N-(3,5-dioxa-4-phosphacyclohepta[2,1-a;3,4-a’]-dinaphthalen-4-yl]dibenz[b,f]azepin-κP}palladium(II) deuterochloroform disolvate, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, 63, m1718–m1719 CrossRef; (c) R. Mariz, A. Briceño, R. Dorta and R. Dorta, Chiral Dibenzazepine-Based P-Alkene Ligands and Their Rhodium Complexes: Catalytic Asymmetric 1,4 Additions to Enones, Organometallics, 2008, 27, 6605–6613 CrossRef CAS; (d) E. Drinkel, A. Briceño, R. Dorta and R. Dorta, Hemilabile P-Alkene Ligands in Chiral Rhodium and Copper Complexes: Catalytic Asymmetric 1,4 Additions to Enones. 2, Organometallics, 2010, 29, 2503–2514 CrossRef CAS. For an optimized synthesis of 1, see: (e) A. Herrera, A. Linden, F. Heinemann, R.-C. Brachvogel, M. von Delius and R. Dorta, Optimized Syntheses of Optically Pure P-Alkene Ligands; Crystal Structures of a Pair of P-Stereogenic Diastereomers, Synthesis, 2016, 48, 1117–1123 CrossRef CAS.
- (a) L. Leinauer, G. Parla, J. Messelbeger, A. Herrera, F. W. Heinemann, J. Langer, I. Chuchelkin, A. Grasruck, S. Frieß, A. Chelouan, K. Gavrilov and R. Dorta, Evolution of a ‘privileged’ P-alkene ligand: added planar chirality beats BINOL axial chirality in catalytic asymmetric C–C bond formation, Chem. Commun., 2023, 59, 14451–14454 RSC. Energy barriers for the flipping of the dibenzazepine moiety in compounds such as 2 and rac-3 have been calculated to exceed 30 kcal mol−1: (b) J. C. Calderón, A. Herrera, F. W. Heinemann, J. Langer, A. Linden, A. Chelouan, A. Grasruck, R. Añez, T. Clark and R. Dorta, Stereochemical stability of planar-chiral benzazepine tricyclics: inversion energies of P- and S-alkene ligands, J. Org. Chem., 2023, 88, 16144–16154 CrossRef PubMed.
- E. Salomó, S. Orgué, A. Antoni Riera and X. Verdaguer, Efficient Preparation of (S)- and (R)-tert-Butylmethylphosphine–Borane: A Novel Entry to Important P-Stereogenic Ligands, Synthesis, 2016, 48, A–E Search PubMed , and references cited therein.
- rac-3 is synthesized by methylation of the diastereopure chloride precursor rac-4 (shown in Scheme 1) with MeLi: A. Herrera, A. Grasruck, F. W. Heinemann, A. Scheurer, A. Chelouan, S. Frieß, F. Seidel and R. Dorta, Developing P-stereogenic, planar-chiral P-alkene ligands: monodentate, bidentate, and double agostic coordination modes on Ru(II), Organometallics, 2017, 36, 714–720 CrossRef CAS.
- M. Pabel, A. C. Willis and S. B. Wild, First resolution of a free fluorophosphine chiral at phosphorus. Resolution and reactions of free and coordinated (±)-fluorophenylisopropylphosphine, Inorg. Chem., 1996, 35, 1244–1249 CrossRef CAS PubMed.
- (a) M.-L. Y. Riu, R. L. Jones, W. J. Transue, P. Müller and C. C. Cummins, Isolation of an elusive phosphatetrahedrane, Sci. Adv., 2020, 6, eaaz3168 CrossRef CAS PubMed; (b) P. M. Miura-Akagi, T. W. Chapp, W. Y. Yoshida, G. P. A. Yap, A. L. Rheingold, R. P. Hughes and M. F. Cain, Synthesis and structure of P-halogenated benzazaphospholes and their reactivity toward Pt(0) sources, Organometallics, 2023, 42, 672–688 CrossRef CAS.
- (a) B. Wolfe and T. Livinghouse, A direct synthesis of P-chiral phosphine-boranes via dynamic resolution of lithiated racemic tert-butylphenylphosphine-borane with (-)-sparteine, J. Am. Chem. Soc., 1998, 120, 5116–5117 CrossRef CAS.
- G. Müller and J. Brand, Mono(borane)phosphides as ligands to lithium and aluminum, Organometallics, 2003, 22, 1463–1467 CrossRef.
- For an in-depth study of crystallization-induced dynamic resolution of a tertiary phosphine with an inexpensive chiral amine, see: M. Y. Kuzu, A. Schmidt and C. Strohmann, Enantioselective Synthesis of Phosphine Boranes via Crystallization-Induced Dynamic Resolution of Lithiated Intermediate by Understanding the Underlying Epimerization Process, Angew. Chem., Int. Ed., 2024, 63, e202319665 CrossRef CAS PubMed.
- B. Freitag, H. Elsen, J. Pahl, G. Ballmann, A. Herrera, R. Dorta and S. Harder, s-Block Metal Dibenzoazepinate Complexes: Evidence for Mg−Alkene Encapsulation, Organometallics, 2017, 36, 1860–1866 CrossRef CAS.
- T. Imamoto, Y. Saitoh, A. Koide, T. Ogura and K. Yoshida, Synthesis and Enantioselectivity of P-Chiral Phosphine Ligands with Alkynyl Groups, Angew. Chem., Int. Ed., 2007, 46, 8636–8639 CrossRef CAS PubMed.
- (a) E. Differding and H. Ofner, N-fluorobenzenesulfonimide: a practical reagent for electrophilic fluorinations, Synlett, 1991, 187–189 CrossRef CAS; (b) E. Differding, R. O. Duthaler, A. Krieger, G. M. Rüegg and C. Schmit, Electrophilic fluorinations with N-fluorobenzenesulfonimide: convenient access to α-fluoro- and α,α-difluorophosphonates, Synlett, 1991, 395–396 CrossRef CAS.
- As pointed out by a reviewer, the planar-chiral phenyl dibenzoazepine ring in phosphide (pR,SP)-7 might play a role in the stereoselective addition of the electrophiles.
- The basicity of fluorophosphines resembles that of phosphites and phosphoramidites.
- Please note that the stereochemical descriptors in these complexes have been maintained (according to the CIP rules they should read SP).
- For a pioneering report, see: (a) R. Shintani, Y. Tsutsumi, M. Nagaosa, T. Nishimura and T. Hayashi, J. Am. Chem. Soc., 2009, 131, 13588–13589 CrossRef CAS PubMed. For non-asymmetric additions of tetraarylborates to aldehydes and enones, see: (b) R. A. Batey, A. N. Thadani and D. V. Smil, Org. Lett., 1999, 1, 1683–1686 CrossRef CAS , and to isatin in the presence of base, see: ; (c) C. Marques and A. Burke, Enantioselective Rhodium(I)-Catalyzed Additions of Arylboronic Acids to N-1,2,3-Triazole-Isatin Derivatives: Accessing N-(1,2,3-Triazolmethyl)-3-hydroxy-3-aryloxindoles, ChemCatChem, 2016, 8, 3518–3526 CrossRef.
- For a reviews, see: (a) K. Xie, A. Li, B. R. Konga, Z. C. Chen, W. Dua and Y. C. Chen, Recent Advances in Asymmetric Addition Reactions to Isatins, Synthesis, 2024, 56, A–P Search PubMed; (b) B. Yu, H. Xing, D.-Q. Yu and H.-M. Liu, Catalytic asymmetric synthesis of biologically important 3-hydroxyoxindoles: an update, Beilstein J. Org. Chem., 2016, 12, 1000–1039 CrossRef CAS PubMed.
- Literature with unambiguous assignations of the absolute stereochemistry of 3-hydroxy-3-aryloxindoles is quite scarce(see SI). The determination of their absolute stereochemistry, along with DFT-modelling of enantio-determining steps, are the subjects of ongoing investigations in our laboratory.
- (a) J. Gui, G. Chen, P. Cao and J. Liao, Rh(I)-catalyzed asymmetric addition of arylboronic acids to NH isatins, Tetrahedron: Asymmetry, 2012, 23, 554–563 CrossRef CAS; (b) X. Feng, Y. Nie, L. Zhang, J. Yang and H. Du, Rh(I)-catalyzed asymmetric 1,2-additions of arylboronic acids to isatins with chiral sulfur−alkene hybrid ligands, Tetrahedron Lett., 2014, 55, 4581–4584 CrossRef CAS; (c) N. Saleh, C. Besnard and J. Lacour, Concave P–Stereogenic Phosphorodiamidite Ligands for Enantioselective Rh(I) Catalysis, Org. Lett., 2024, 26, 2202–2206 CrossRef CAS PubMed.
- (a) CCDC 2390089: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2l72nl; (b) CCDC 2390090: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2l72pm; (c) CCDC 2390091: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2l72qn; (d) CCDC 2390092: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2l72rp; (e) CCDC 2390093: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2l72sq.
| This journal is © the Partner Organisations 2026 |