Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unsupported gallatabenzene via rare-earth-metal pentadienyl complexes
Jakob Lebon,
Manfred Manßen ,
Cäcilia Maichle-Mössmer,
Peter Sirsch
,
Cäcilia Maichle-Mössmer,
Peter Sirsch * and
Reiner Anwander
* and
Reiner Anwander *
*
Institut für Anorganische Chemie, Eberhard Karls Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen, Germany. E-mail: reiner.anwander@uni-tuebingen.de
First published on 3rd December 2025
Abstract
The treatment of rare-earth-metal aluminata/gallata complexes with the Lewis acids AlMe3 and ECl3 (E = Al, Ga) gives access to new group 13 metallacyclic fragments. The reaction of the mixed yttrocene (C5Me5)Y(μ-Me)(AlC5H3Me-1-tBu2-3,5) with AlMe3 produced the bis(heteroaluminato) complex (C5Me5)Y[(3,5-tBu2C5H3AlMe2)(AlMe3)]. The reaction of (C5Me5)Y(μ-Me)(AlC5H3Me-1-tBu2-3,5) with AlCl3 gave an organoaluminium entity with a central chair conformation of alternating aluminium and carbon atoms. Alternatively, the new Al3 entity can be interpreted as a monoanionic aluminatabenzene moiety [(1-Me-3,5-tBu2-C5H3Al)(THF)], which is stabilized by the cationic dialuminium fragment [3,5-tBu2-C5H3(AlMe)2]. The donor (THF) free Ga3 homologue was obtained from bis(gallata) “open” lutocene (1-Me-3,5-tBu2C5H3Ga)(μ-Me)Lu(1-Me-3,5-tBu2C5H3Ga) and GaCl3. The reaction of the bis(gallata) lutetium sandwich complex with KOtBu gave the potassium coordination polymer [K(1-Me-3,5-tBu2C5H3Ga)(THF)]n, which can be converted to the separated ion pair [(1-Me-3,5-tBu2C5H3Ga)][K([2.2.2]crypt)] by addition of the [2.2.2]cryptand. The isolated compounds were analyzed by 1H, 13C, 89Y, and variable temperature 1H NMR spectroscopy, SC-XRD, IR spectroscopy, and elemental analysis. The bonding of the unsupported gallatabenzene moiety was further investigated by DFT calculations.
Introduction
The chemistry of 5- and 6-membered heterocycles of the heavier group 13 and 14 elements – and more specifically the analysis of their aromatic behaviour – is an ongoing topic in main group chemistry.1 For the group 14 elements silicon and germanium, both the dianionic heterocyclopentadienediides and the neutral sila- and germabenzenes were first synthesized several decades ago and feature well-understood structural motifs (Chart 1).2–6 In the case of the heavier group 13 elements, the variety of 5- and 6-membered heterocycles is far more limited.7–14 While alumoles and galloles and their dianionic structures have been investigated for a longer period (Chart 1),7–10 the advances in the chemistry of the monoanionic heterobenzene congeners have remained modest.11–14 Even though work in the field of group 13 element heterobenzenes has been highlighted by the gallatabenzene manganese complex by Ashe in 1995,11 it was further exploited only 20 years later by Yamashita.12–14 The Yamashita group described a series of transition metal heterobenzene complexes, including the first ion-separated lithium aluminata (A)- and gallatabenzenes (B). Focus was also put on the extent of aromatic bonding in such monoanionic metallacycles.11–14 In our previous work, we utilized a di-tBu-substituted pentadienyl ligand (dtbp, C5H3tBu2-3,5)15 to access rare-earth metal aluminata- and gallatabenzene complexes, including the (mixed) sandwich methyl complexes (C5Me5)Y(μ-Me)(AlC5H3Me-1-tBu2-3,5) and (1-Me-3,5-tBu2C5H3Ga)(μ-Me)Lu(1-Me-3,5-tBu2C5H3Ga).16,17 While the formation of such heterobenzene-type ligands at small- to medium-sized rare-earth metals (Lu, Y) was easily achieved, a conceivable salt-metathesis route utilizing K(2,4-dtbp) and compounds EClMe2 (E = Al, Ga) to access unsupported metallacycles has been unsuccessful so far. Here, we describe that an ion-separated potassium gallatabenzene can be ultimately obtained by betting on the oxophilicity of rare-earth metals. | ||
| Chart 1 Milestones in the development of group 13 heterocyclopentadienediides7,10 and group 13/14 heterobenzenes (Tbt = 2,4,6-tris[bis(trimethylsilyl)methyl]phenyl).2,3,12,13 | ||
Results and discussion
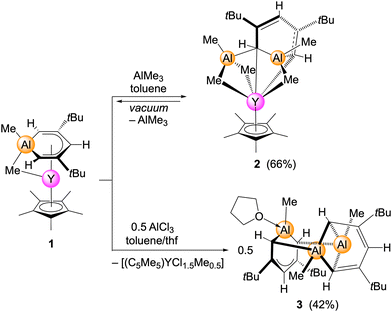
Reactivity of (C5Me5)Y(μ-Me)(AlC5H3Me-1-tBu2-3,5) (1) towards AlMe3 and AlCl3
Previous derivatization reactions of the half-open sandwich complexes (1-Me-3,5-tBu2-C5H3E)(μ-Me)Y(2,4-dtbp) (E = Al, Ga) revealed that the coordination capability of the 2,4-dtbp ligand is significantly enhanced via cyclometallation.18 For example, the open pentadienyl ligand is easily displaced by pentamethylcyclopentadienyl to afford the mixed yttrocene (C5Me5)Y(μ-Me)(AlC5H3Me-1-tBu2-3,5) (1).17 It was also revealed that the coordination strength of the aluminata/gallatabenzene ligands is additionally promoted by a strong interaction of the ring group 13 element with the methyl group bridging to the rare-earth-metal centre.16,17 The bridging methyl group could neither be cleaved by donor molecules nor be abstracted via the use of trityl borate [CPh3][B(C6F5)4].16,17 We assumed that a reactivity study involving competitive Lewis acids might give further insights into the stability of the [Y(μ-Me)(AlC5H3Me-1-tBu2-3,5)] fragment. Therefore, the C5Me5-supported aluminatabenzene complex 1 was treated with the strong Lewis acids AlMe3 and AlCl3. The AlMe3 reaction resulted in heteroaluminate formation across the dianionic aluminatabenzene ligand and the formation of the trimetallic complex (C5Me5)Y[(3,5-tBu2C5H3AlMe2)(AlMe3)] (2, Scheme 1). Although complex 2 can be readily reproduced and isolated as single crystals, it decomposes under vacuum to precursor 1 and AlMe3, impeding its isolation as a solid bulk material.As revealed by a single-crystal X-ray diffraction (SC-XRD) analysis, the pentadienyl backbone of compound 2 displays conjugated localized double bonds (C2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C3 and C4
C3 and C4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C5). Overall, the molecular structure of 2 is reminiscent of the half-sandwich bis(aluminate) (C5Me5)Y(AlMe4)2,19 referring to the quite symmetric coordination of the AlR4 fragments to the yttrium centre (Fig. 1/top). The Y⋯Al distances (2.7999(6) and 2.8233(5) Å) in 2 are shorter than those in (C5Me5)Y(AlMe4)2 (2.9257(7) and 3.1112(7) Å), while the Y–C distances (2.5144(17) and 2.8095(17) Å) are in the range of those detected for (C5Me5)Y(AlMe4)2 (2.549(3)–2.655(3) Å).19
C5). Overall, the molecular structure of 2 is reminiscent of the half-sandwich bis(aluminate) (C5Me5)Y(AlMe4)2,19 referring to the quite symmetric coordination of the AlR4 fragments to the yttrium centre (Fig. 1/top). The Y⋯Al distances (2.7999(6) and 2.8233(5) Å) in 2 are shorter than those in (C5Me5)Y(AlMe4)2 (2.9257(7) and 3.1112(7) Å), while the Y–C distances (2.5144(17) and 2.8095(17) Å) are in the range of those detected for (C5Me5)Y(AlMe4)2 (2.549(3)–2.655(3) Å).19
In contrast, the tetracyclic Al3 species [(1-Me-3,5-tBu2-C5H3Al)(THF)][3,5-tBu2-C5H3(AlMe)2] (3), resulting from the AlCl3 reaction, was isolated as thermally stable crystals. The formation of 3 seems mainly driven by the co-formation of two equiv. of the mostly chlorinated half-sandwich species ([C5Me5)YCl1.5Me0.5], which could be separated via extraction of 3 with n-pentane and verified from the residue as the metathesis products (C5Me5)2YCl(THF) and [(C5Me5)YCl1.5Me0.5] via proton NMR spectroscopy and SC-XRD.20 Compound 3 features a multi-metallacyclic structure (Fig. 1). One cycle comprises the atoms Al3–C1–C2–C3–C4–C5 forming a 6-membered monoanionic fragment. Compared to precursor 1, this 6-membered ring displays minor deviations of the C–C distances (1.407(4)–1.428(4) Å) and shorter Al–Cring distances (1.961(3) and 1.980(3) Å) (1: C–C 1.376(3)–1.446(3); Al–Cring 2.016(3) and 2.019(3) Å). The second and third cycles incorporate a second pentadienyl backbone, expanding the formal 6-membered cycle by another AlMe fragment, in a lid-like structure. This structural motif can be interpreted as a pentadienyl stabilized-“bisalumocyclobutane” cation. The second cycle, which involves the atoms μ-Al1–C19–C18–C17–C16–C15–μ-Al2, displays more localized, but conjugated double bonds with C–C interatomic distances ranging from 1.389(4) Å to 1.427(4) Å. The high-valent bonding situation of carbon atom C15 led to the conclusion that compound 3 is a zwitterionic compound composed of a monoanionic aluminatabenzene moiety (Ib, Scheme 2) and an Al2 cationic fragment (Ic, Scheme 2). The third cycle, featuring the “bisalumolcyclobutane” moiety Al1–C19–Al2–C15, displays a hitherto unknown organoaluminium structural pattern, with common Al–C distances (2.027(3)–2.042(3) Å). Furthermore, the Al1–Al2 distance of 2.6810(14) Å does not indicate any metal–metal interaction. The aluminium atoms of the “bisalumolcyclobutane” moiety connect to the ortho-C atoms of the aluminatabenzene moiety with usual Al–C distances (2.072(3) and 2.071(3) Å).
For comparison, fused-ring tricyclic organoaluminium species featuring a 4-membered Al–C–Al–C were obtained from hydroalumination of di(tert-butyl)butadiyne with dimethylaluminium hydride.21,22 Furthermore, similar 4-membered metallacycles were also detected in a heptacyclic species originating from the reaction of EX3 (E = Al and Ga) with the trilithium compound 1,3-[PhMe2Si–C(Li)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(H)]2C6H3Li.23 Fully separated organoaluminium ion pairs include the structurally characterized compounds [Me2Al(15-crown-5)][Me2AlCl2] and [tBu2Al(tmeda)][tBu2AlBr2].24,25
C(H)]2C6H3Li.23 Fully separated organoaluminium ion pairs include the structurally characterized compounds [Me2Al(15-crown-5)][Me2AlCl2] and [tBu2Al(tmeda)][tBu2AlBr2].24,25
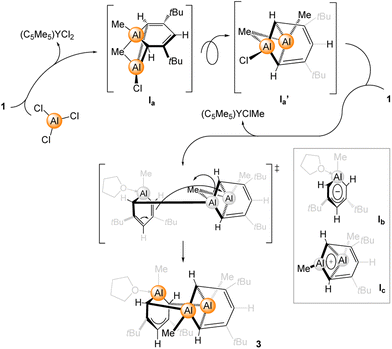
Since we were able to isolate the side product [(C5Me5)YCl1.5Me0.5] and could revert to complex 2 for initiating the reaction step/path, the following reaction mechanism for the formation of compound 3 can be proposed (Scheme 2). Accordingly, complex 1 readily reacts with AlCl3 forming [(C5Me5)YCl2] and intermediate Ia. By changing the perspective of Ia to Ia′, it is apparent that the displacement of the remaining chlorido by another equivalent of 1 can take place, with the cationic part being already in place. Thus, via exchange of the chlorido against the monoanionic aluminatabenzene ligand and elimination of [(C5Me5)YClMe], a formal anion (Ib)–cation (Ic) combination may lead to the isolation of 3. To further prove the overall ionic nature of 3, its fragmentation pattern was investigated by mass spectrometry. By using mild ionization methods at ambient temperature, the dominant peak obtained via the electron ionization method was identified as the cationic fragment Ic. Other smaller signals could be referred to as compound 3 and 3-THF-CH3.
Reactivity of bis(gallatabenzene) lutetocene (1-Me-3,5-tBu2-C5H3Ga)(μ-Me)Lu(1-Me-3,5-tBu2-C5H3Ga) (4) towards GaCl3 and KOtBu
Since gallium forms more covalent Ga–C bonds, the respective abstraction of a gallatabenzene moiety from a rare-earth-metal complex should increase the feasibility of ion fragmentation in solution. Therefore, we examined the corresponding reactivity of the previously published and fully characterized bis(gallatabenzene) lutetium sandwich complex (1-Me-3,5-tBu2-C5H3Ga)(μ-Me)Lu(1-Me-3,5-tBu2-C5H3Ga) (4, Scheme 3).17 Gratifyingly, the GaCl3 reaction gave compound [(1-Me-3,5-tBu2-C5H3Ga)(1-Me)2-3,5-tBu2-C5H3(μ-Ga)2] (5) as the THF free heavier homologue of compound 3. Due to the presence of two gallatabenzene moieties in 4, compared to the single aluminatabenzene moiety in precursor 1, the formation of 5 requires only 1 equiv. of GaCl3. Comparing both SC-XRD structures of 3 and 5, no significant difference is detected in the overall bonding situation. However, the Ga–C and C–C ring distances in compound 5 differ more than expected (Fig. 2). The anionic 6-membered ring moiety involving Ga3–C1–C2–C3–C4–C5 revealed comparatively shorter C–C distances (1.395(6) and 1.399(6) for C2–C3 and C3–C4, respectively), indicating a higher degree of conjugation and aromaticity. This is also reflected in the Ga–C distances (1.946(4) and 1.953(4) Å), which are shorter than the Al–C distances in compound 2. The Ga–Cring distances are comparable to those in the heteroadamantane cluster [(GaEt)6(CEt)4] (1.965(4)–1.992(7) Å), obtained via hydrogallation of propyne with Me2GaH,26 and in Yamashita's lithium gallatabenzene [1-Mes-2,6-Si(iPr3)2C5H3Ga]Li(dme) (1.939(3) and 1.941(3) Å).13 The metallacycle comprising Ga1–C15–C16–C17–C18–C19 features allylic C–C bonding in the pentadienyl backbone, instead of two conjugated double bonds, with three almost equal C–C distances (1.432(5), 1.401(5) and 1.412(5) Å).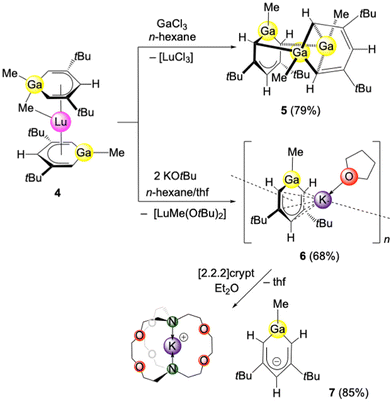 | ||
| Scheme 3 Metathetical synthesis of Ga3 compound 5 and polymeric potassium salt 6, which can be converted to ion-separated complex 7 via addition of cryptand. | ||
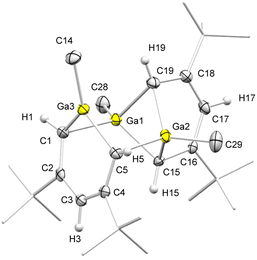 | ||
| Fig. 2 Crystal structure of 5, with ellipsoids set at 50%. Methyl hydrogen atoms have been omitted for clarity. For selected interatomic distances, see the SI. | ||
Exploiting the highly oxophilic character of rare-earth metals, gallatabenzene displacement from complex 4 was also achieved by treatment with KOtBu. Accordingly, the potassium salt [(1-Me-3,5-tBu2-C5H3Ga)K(thf)]n (6) could be accessed in good yield (Scheme 3, Fig. 3/top). Coordination polymer 6 allows a more detailed view of the bonding situation and potential aromaticity of the gallatabenzene moiety. The gallatabenzene coordination to the potassium entails only a minor distortion of the gallium atom out of the ring plane (0.01 Å). Enhanced participation of the Ga–C bond in the conjugated ring system is further revealed by shorter Ga–C ring distances of 1.895(5) and 1.893(5) Å. For comparison, the terminal Ga–Cmethyl distance measures 1.963 Å. The C–C ring distances range from 1.395(6) to 1.424(6) Å.
The 1H NMR spectrum of compound 6 in thf-d8 displays the hydrogen atoms at the C1/C5 (ortho) and C3 (para) ring positions at lower fields (6.63/5.74 ppm) compared to precursor 4, corroborating an enhanced aromatic character of the gallatabenzene, despite the coordination of potassium. The K–Cring distances in 6 are in the range from 3.095(4) to 3.323(5) Å and the K⋯Ga distance amounts to 3.4458(11) Å. For further comparison, Yamashita's lithium gallatabenzene [1-Mes-2,6-Si(iPr3)2C5H3Ga]Li(dme) exhibits C–C ring and Li–Cring distances ranging from 1.396(4) to 1.408(5) Å and from 2.301(7) to 2.702(7) Å, respectively (Li⋯Ga, 2.931(6) Å).13 The meta- and para-ring protons of the lithium gallatabenzene [1-Mes-2,6-Si(iPr3)2C5H3Ga]Li(dme) were observed at 8.23 and 6.01 ppm (C6D6), respectively.13
To further investigate the feasibility of a metal-unsupported “free” monoanionic gallatabenzene and the effect of potassium coordination on the structure of the 6-membered metallacycle, compound 6 was reacted with [2.2.2]cryptand. As anticipated, potassium encapsulation yielded the separated ion pair [(1-Me-3,5-tBu2-C5H3Ga)][K([2.2.2]crypt)] (7) (Scheme 3 and Fig. 3/bottom). However, any significant differences in the interatomic distances in complexes 6 and 7 were not observed. The 1H NMR spectra revealed slightly distinct chemical shifts for the ring protons. Accordingly, the ring protons of 6 (6.63/5.74 ppm versus 6.41/5.57 ppm in 7) indicate a higher degree of deshielding caused by the coordinated potassium. The NMR spectra in C6D6 of ion-separated gallatabenzene [1-Mes-2,6-Si(iPr3)2C5H3Ga][Li(dme)3] (B, Chart 1) were found to be identical to those of gallatabenzene [1-Mes-2,6-Si(iPr3)2C5H3Ga]Li(dme).13 This was interpreted in a way that in solution dme dissociates from B to give [1-Mes-2,6-Si(iPr3)2C5H3Ga]Li(dme). Similarly, germanylbenzenylpotassium derivatives [(1-tBu-C5H4Ge)K]n (layer structure) and [1-tBu-C5H4Ge][K([2.2.2]crypt)] revealed very similar 1H NMR chemical shifts in thf-d8 (Δδ ≈ 0.2 ppm), in agreement with ion separation and a fully solvated potassium cation in both solutions.27 The 13C resonances of the C1/C5 (ortho), C2/C4 (meta) and C3 (para) ring positions of gallatabenzene [1-Mes-2,6-Si(iPr3)2C5H3Ga]Li(dme) in C6D6 were detected at 129.5, 148.2 and 103.0 ppm, respectively.13 Complexes 6 and 7 revealed the respective 13C NMR chemical shifts at 97.0/161.7/121.2 ppm and 97.2/159.0/119.7 ppm, respectively. The germanylbenzenyl anion in the proposed ion-separated [1-tBu-C5H4Ge][K(thf)x] displayed the 13C resonances of the ring carbon atoms at 203.7 (C1/ortho), 130.1 (C2/meta), 112.9 (C3/para), 128.4 (C4/meta) and 171.2 (C1/ortho),27 which slightly shifted upfield compared to its neutral germabenzene precursor (cf., Chart 1).
At this point, a more detailed comparison of the monoanionic gallatabenzene moiety of 7 to Yamashita's unsupported monoanionic gallatabenzene B (Chart 1) seems expedient. Both compounds clearly differ with respect to synthesis and ring substitution. Briefly, gallatabenzene B was obtained from an aluminacyclohexadiene with GaCl3 involving metal exchange and subsequent reaction of the gallacyclohexadiene with mesityllithium and finally DME.13 The aluminacyclohexadiene was accessed from a bis(tri-isopropysilyl)diyne by subsequent treatment with HAliBu2, nBu2SnCl2 and pyridine (for structural drawings, see Chart 1).12 Consequently, gallatabenzene B displays an ortho silyl substitution, while ours bears tert-butyl substituents in the meta position (Fig. 4).
 | ||
| Fig. 4 Comparison of the interatomic Ga–C and C–C ring distances of the two known unsupported gallatabenzenes 7 (left, this work) and B (right).13 Ring carbon atoms are numbered 1/5 (ortho), 2/4 (meta) and 3 (para). | ||
As shown in Fig. 4, the crystal structures of 7 and B reveal slightly different bond lengths within the ring moieties. For 7, they seem to indicate a strengthening of the Ga–C bonds, relative to B, and a shortening of the bonds between the carbon atoms in ortho and meta positions, at the cost of the C(meta)–C(para) bonds, which are elongated.
DFT calculations
To gain more insight into the nature of the bonding within these substituted gallatabenzene moieties, we carried out DFT calculations at the B3LYP/6-311++G(d,p) level of theory,28 followed by a natural bond orbital (NBO) analysis.29 In a previous study,17b the bonding in a DFT model system for a nonsubstituted monoanionic gallatabenzene was analysed and found to be very similar to the one reported earlier by Yamashita for B.13 Accordingly, the bonding in the heterocycle is characterized by polar Ga–C σ bonds and a partial delocalization of π electron density from the ring carbon atoms into the vacant p orbital on Ga. Here, we wanted to investigate in more detail, whether the alkyl and silyl substituents in both systems also affect the bonding within the gallatabenzene ring, as suggested by the respective X-ray structures (see above).Interestingly, in the crystal structure of 7, the tert-butyl substituents are oriented in different ways, where one C–Me bond in each case is almost aligned with the ring plane, but both are pointing in opposite directions (see Fig. 3). A DFT-optimized geometry for 7 confirmed this arrangement and also revealed an asymmetric bonding within the ring moiety: interatomic distances on the left- and right-hand sides of the ring differed slightly, within a range of 0.01–0.02 Å (for details, see the SI). In the crystal structure of 7, this is not clearly discernible, due to experimental noise and a minor disorder for one of the substituents. The small differences in bond length can be attributed to the unequal conformation of the substituents. This is corroborated by a model system, where both substituents are related by mirror symmetry, and consequently, all respective distances are equal (for details, see the SI). While at first sight these observations might seem insignificant, they help rationalize the aforementioned bond differences between 7 and B, which are in a similar range (see Fig. 4). Accordingly, hyperconjugative interactions involving the alkyl and silyl substituents, respectively, might be the cause for these differences.
To identify hyperconjugation, the NBO approach was shown to be ideally suited.30 Against this background, we reoptimized the DFT geometry of a model system for the gallatabenzene moiety of B and analysed the bonding in both 7 and B using NBO methods. A range of geminal and vicinal hyperconjugative interactions within the heterocycle could be identified for B, some of them also involving the silyl substituents. Two of the latter are displayed in Fig. 5 (for further details, see the SI). They represent the transfer of electron density from a Ga–C σ bond and a C–C π bond, respectively, to anti-bonding Si–C σ* orbitals, therefore contributing to the weakening and elongation of the involved bonds.
 | ||
| Fig. 5 Selected hyperconjugative interactions in B, representing the transfer of electron density from a Ga–C σ bond (left) and a C–C π bond (right) to antibonding Si–C σ* orbitals. | ||
For 7, a different set of hyperconjugative interactions was found. They were smaller in number and, in general, somewhat weaker than those for B. Additionally, the geometries of two other model systems were optimized, in which the silyl and alkyl substituents, respectively, were moved from the ortho to the meta position or vice versa (for details, see the SI). The results indicated that the position of the substitution is at least as important for the bond lengths in the heterocycle, as the nature of the substituent. It needs to be kept in mind, however, that for all these cases the differences in bond lengths are comparatively small.
Finally, we were also interested in the actual number of π electrons in the heterocycles of 7 and B, as alkyl and silyl substituents should exhibit distinct electron-donating and -withdrawing properties, through their hyperconjugative interactions. The results indicate, however, that there are only minor differences: for 7, the NBO analysis revealed a total of 5.96 electrons in orbitals contributing to the π density of the heterocycle, compared to 5.89 electrons for B. Nucleus-independent chemical shift (NICS)31 calculations for 7 produced NICS(0) and NICS(1) values of −4.8 and −5.9, respectively. These numbers differ slightly from the ones reported for B (−2.4 and −4.4),13 but suggest a similar aromatic character for both systems.
Conclusions
Reactions of simple Lewis acids such as ECl3 (E = Al and Ga) with rare-earth-metal (Ln) supported aluminata/gallatabenzene complexes give access to new E3 multicyclic species. These compounds contain the known anionic heterobenzene-type moiety and hitherto unknown pentadienyl stabilized “bisheterocyclobutane” cations, as suggested by mass spectroscopy. Metathetical Ln → metal gallatabenzene transfer is also feasible by exploiting the oxophilic character of the rare-earth element. Applying this strategy, the potassium coordination polymer [(1-Me-3,5-tBu2-C5H3Ga)K(THF)]n could be obtained from potassium tert-butoxide. Coordinative displacement of the potassium cation with [2.2.2]cryptand generates the separated ion pair [(1-Me-3,5-tBu2-C5H3Ga)][K([2.2.2]crypt)]. For the unsupported monoanionic gallatabenzene, DFT calculations in combination with a natural bond analysis revealed that a range of hyperconjugative interactions are at play, which help explain the slightly different bonding within the heterocycle, compared to the related silyl-substituted [1-Mes-2,6-Si(iPr3)2-C5H3Ga][Li(dme)3]. Therefore, the observed structural differences should not be over-interpreted in terms of a notable change in aromaticity. In line with this interpretation, the different nature of the substituents did not afford notable differences in the π electron density for these two systems.Author contributions
JL: synthesis and characterization of compounds and writing and editing – original draft; MM: mass spectrometry of compound 3; CM-M: crystallography and editing – original draft; PS: DFT calculations, and writing and editing – original draft, and funding acquisition; RA: conceptualization, supervision, writing and editing – original draft, project administration, and funding acquisition.Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available in the supplementary information (SI). Supplementary information: supporting figures, detailed crystallographic data, spectroscopic data (NMR and IR), and analytical details. See DOI: https://doi.org/10.1039/d5qi02023a.CCDC 2491667–2491671 contain the supplementary crystallographic data for this paper.32a–e
Acknowledgements
We acknowledge support from the State of Baden-Württemberg through bwHPC and the German Research Foundation (DFG) through grant no INST 40/575-1 FUGG (JUSTUS 2 cluster).References
- For reviews, see: (a) K. K. Baldridge, O. Uzan and J. M. L. Martin, The silabenzenes: structure, properties, and aromaticity, Organometallics, 2000, 19, 1477–1487 Search PubMed; (b) N. Tokitoh, New Progress in the Chemistry of Stable Metallaaromatic Compounds of Heavier Group 14 Elements, Acc. Chem. Res., 2004, 37, 86–94 CrossRef CAS PubMed; (c) P. Cui and Y. Chen, Boratabenzene rare-earth metal complexes, Coord. Chem. Rev., 2016, 314, 2–13 CrossRef CAS; (d) W. Ma, C. Yu, T. Chen, L. Xu, W.-X. Zhang and Z. Xi, Metallacyclopentadienes: synthesis, structure and reactivity, Chem. Soc. Rev., 2017, 46, 1160–1192 RSC; (e) M. Saito, Transition-metal complexes featuring dianionic heavy group 14 element aromatic ligands, Acc. Chem. Res., 2018, 51, 160–169 CrossRef CAS PubMed; (f) J. Wei, W.-X. Zhang and Z. Xi, The aromatic dianion metalloles, Chem. Sci., 2018, 9, 560–568 RSC; (g) Z. Dong, L. Albers and T. Müller, Trialkylsilyl-Substituted Silole and Germole Dianions as Precursors for Unusual Silicon and Germanium Compounds, Acc. Chem. Res., 2020, 53, 532–543 Search PubMed; (h) K. Ota and R. Kinjo, Heavier element-containing aromatics of [4n + 2]-electron systems, Chem. Soc. Rev., 2021, 50, 10594–10673 RSC; (i) D. Schädle and R. Anwander, in Comprehensive Organometallic Chemistry IV, ed. G. Parkin, K. Meyer and D. O'Hare, Elsevier, Kidlington, UK, 2022, vol. 4, pp. 1–28 Search PubMed; (j) X. Sun and P. W. Roesky, Group 14 metallole dianions as η5-coordinating ligands, Inorg. Chem. Front., 2023, 10, 5509–5516 RSC.
- (a) K. Wakita, N. Tokitoh, R. Okazaki and S. Nagase, Synthesis and Properties of an Overcrowded Silabenzene Stable at Ambient Temperature, Angew. Chem., Int. Ed., 2000, 39, 634–636 CrossRef CAS; (b) K. Wakita, N. Tokitoh, R. Okazaki, N. Takagi and S. Nagase, Crystal Structure of a Stable Silabenzene and Its Photochemical Isomerization into the Corresponding Silabenzvalene, J. Am. Chem. Soc., 2000, 122, 5648–5649 CrossRef CAS.
- (a) N. Nakata, N. Takeda and N. Tokitoh, Synthesis and Properties of the First Stable Germabenzene, J. Am. Chem. Soc., 2002, 124, 6914–6920 CrossRef CAS PubMed; (b) N. Tokitoh, N. Nakata, A. Shinohara, N. Takeda and T. Sasamori, Coordination Chemistry of a Kinetically Stabilized Germabenzene: Syntheses and Properties of Stable η6-Germabenzene Complexes Coordinated to Transition Metals, Chem. – Eur. J., 2007, 13, 1856–1862 CrossRef CAS PubMed.
- (a) R. Haga, M. Saito and M. Yoshioka, Reversible Redox Behavior between Stannole Dianion and Distannole-1,2-Dianion, J. Am. Chem. Soc., 2006, 128, 4934–4935 CrossRef CAS PubMed; (b) Y. Mizuhata, T. Sasamori, N. Takeda and N. Tokitoh, A Stable Neutral Stannaaromatic Compound: Synthesis, Structure and Complexation of a Kinetically Stabilized 2-Stannanaphthalene, J. Am. Chem. Soc., 2006, 128, 1050–1051 CrossRef CAS PubMed; (c) C. Kaiya, K. Suzuki and M. Yamashita, A Monomeric Stannabenzene: Synthesis, Structure, and Electronic Properties, Angew. Chem., Int. Ed., 2019, 58, 7749–7752 CrossRef CAS.
- (a) M. Saito, M. Skaguchi, T. Tajima, K. Ishimura, S. Nagase and M. Hada, Dilithiobplumbole: A Lead-Bearing Aromatic Cyclopentadienyl Analog, Science, 2010, 328, 339–342 CrossRef CAS PubMed; (b) M. Saito, T. Akiba, M. Kaneko, T. Kawamura, M. Abe, M. Hada and M. Minoura, Synthesis, Structure, and Reactivity of Lewis Base Stabilized Plumbacyclopentadienylidenes, Chem. – Eur. J., 2013, 19, 16946–16953 CrossRef CAS PubMed.
- (a) C. Krüger, J. C. Sekutowski, H. Hoberg and R. Krause-Göing, (Pentaphenyl)aluminacyclopentadiene as a complexing ligand. The molecular structure of (pentaphenyl)aluminacyclopentadiene and its complex with 1,5-cyclooctadienenickel, J. Organomet. Chem., 1977, 141, 141–148 CrossRef; (b) H. Hoberg, R. Krause-Göing, C. Krüger and J. C. Sekutowski, Novel Organonickel Compounds from (Pentaphenyl)aluminacyclopentadiene, Angew. Chem., Int. Ed. Engl., 1977, 16, 183–184 CrossRef; (c) H. Hoberg and R. Krause-Göing, Darstellung und Eigenschaften von (Pentaphenyl)aluminacyclopentadiene, J. Organomet. Chem., 1977, 127, C29–C31 CrossRef CAS.
- (a) T. Agou, T. Wasano, P. Jin, S. Nagase and N. Tokitoh, Syntheses and Structures of an “Alumole” and Its Dianion, Angew. Chem., Int. Ed., 2013, 52, 10031–10034 CrossRef CAS PubMed; (b) T. Wasano, T. Agou, T. Sasamori and N. Tokitoh, Synthesis, structure and reactivity of a 1-bromoalumole, Chem. Commun., 2014, 50, 8148–8150 RSC.
- (a) A. Decken, F. P. Gabbai and A. H. Cowley, The Benzannelation Approach to Novel Gallium and Indium Heterocycles, Inorg. Chem., 1995, 34, 3853–3854 CrossRef CAS; (b) A. H. Cowley, D. S. Brown, A. Decken and S. Kamepalli, , Novel dimeric ring systems containing gallium, Chem. Commun., 1996, 2425–2426 RSC.
- P. Wei, X.-W. Li and G. H. Robinson, [Ga2Cl4(dioxane)2]x: molecular structure and reactivity of a polymeric gallium(II) halide containing two five-coordinate gallium atoms about a Ga–Ga bond, Chem. Commun., 1999, 1287–1288 RSC.
- T. Agou, T. Wasano, T. Sasamori and N. Tokitoh, Syntheses and structures of a stable gallole free of Lewis base coordination and its dianion, J. Phys. Org. Chem., 2015, 28, 104–107 CrossRef CAS.
- A. J. Ashe III, S. Al-Ahmad and J. W. Kampf, Aromatic Gallium Heterocycles: Synthesis of the First Gallatabenzene, Angew. Chem., Int. Ed. Engl., 1995, 34, 1357–1359 CrossRef.
- (a) T. Nakamura, K. Suzuki and M. Yamashita, An Anionic Aluminabenzene Bearing Aromatic and Ambiphilic Contributions, J. Am. Chem. Soc., 2014, 136, 9276–9279 CrossRef CAS PubMed; (b) T. Nakamura, K. Suzuki and M. Yamashita, Aluminabenzene–Zirconium Complexes: Intramolecular Coordination of Chloride to Aluminum, Organometallics, 2015, 34, 813–816 CrossRef CAS; (c) T. Nakamura, K. Suzuki and M. Yamashita, Aluminabenzene–Rh and –Ir Complexes: Synthesis, Structure, and Application toward Catalytic C–H Borylation, J. Am. Chem. Soc., 2017, 139, 17763–17766 CrossRef CAS PubMed; (d) T. Nakamura, K. Suzuki and M. Yamashita, Zwitterionic Aluminabenzene-Alkylzirconium Complex Having Half-Zirconocene Structure: Synthesis and Application for an Additive-Free Ethylene Polymerization, Chem. Commun., 2018, 54, 4180–4183 RSC.
- T. Nakamura, K. Suzuki and M. Yamashita, An Isolable Anionic Gallabenzene: Synthesis and Characterization, Organometallics, 2015, 34, 1806–1808 CrossRef CAS.
- T. Nakamura, K. Suzuki and M. Yamashita, Anionic Indabenzene: synthesis and characterization, Chem. Commun., 2017, 53, 13260–13263 RSC.
- J. Raeder, M. Reiners, R. Baumgarten, K. Münster, D. Baabe, M. Freytag, P. G. Jones and M. D. Walter, Synthesis and molecular structure of pentadienyl complexes of the rare-earth metals, Dalton Trans., 2018, 47, 14468–14482 RSC.
- (a) D. Barisic, D. Schneider, C. Maichle-Mössmer and R. Anwander, Formation and Reactivity of an Aluminabenzene Ligand at Pentadienyl-Supported Rare-Earth Metals, Angew. Chem., Int. Ed., 2019, 58, 1515–1518 CrossRef CAS PubMed; (b) D. Barisic, D. A. Buschmann, D. Schneider, C. Maichle-Mössmer and R. Anwander, Rare-Earth Metal Pentadienyl Half-Sandwich and Sandwich Tetramethylaluminates – Synthesis, Structure, Reactivity, and Performance in Isoprene Polymerization, Chem. – Eur. J., 2019, 25, 4821–4832 CrossRef CAS PubMed; (c) D. Barisic, J. Lebon, C. Maichle-Mössmer and R. Anwander, Pentadienyl migration and abstraction in yttrium aluminabenzene complexes including a single-component catalyst for isoprene polymerization, Chem. Commun., 2019, 7089–7092 RSC.
- (a) J. Lebon, D. Barisic, C. Maichle-Mössmer and R. Anwander, Yttrium Complexes with Group 13 Heterobenzene-type Ligands, Chem. – Eur. J., 2023, 29, e202302846 CrossRef CAS PubMed; (b) J. Lebon, M. Beauvois, C. Maichle-Mössmer, P. Sirsch and R. Anwander, Gallatabenzene Ligands Emerging from Open Lutetocene and Yttrocene Methyl Complexes, Chem. – Eur. J., 2024, 30, e202403472 CrossRef CAS PubMed.
- Other examples of pentadienyl cyclometallation: (a) M. S. Kralik, J. P. Hutchinson and R. D. Ernst, New Carbon–Carbon Bond-Forming Reaction of Carbon Monoxide: Remarkable Trialkylation of a Carbonyl Ligand in a Molybdenum Pentadienyl Complex, J. Am. Chem. Soc., 1985, 107, 8296–8297 CrossRef CAS; (b) M. S. Kralik, A. L. Rheingold and R. D. Ernst, (Pentadienyl)molybdenum Carbonyl Chemistry: Conversion of a Pentadienyl Ligand to a Coordinated Metallabenzene Complex, Organometallics, 1987, 6, 2612–2614 CrossRef CAS; (c) J. R. Bleeke, Y.-F. Xie, W.-J. Peng and M. Chiang, Metallabenzene: Synthesis, Structure, and Spectroscopy of a 1-Irida-3,5-dimethylbenzene Complex, J. Am. Chem. Soc., 1989, 111, 4118–4120 CrossRef CAS; (d) J. R. Bleeke and W.-J. Peng, Synthesis, Structure, and Spectroscopy of Iridacyclohexadiene Complexes, Organometallics, 1987, 6, 1576–1578 CrossRef CAS; (e) J. R. Bleeke, Metallabenzene Chemistry, Acc. Chem. Res., 1991, 24, 271–277 CrossRef CAS; (f) M. S. Kralik, L. Stahl, A. M. Arif, C. E. Strouse and R. D. Ernst, Open and Half-Open Manganocene Chemistry: More Associated Salts, Organometallics, 1992, 11, 3617–3621 CrossRef CAS; (g) U. Effertz, U. Englert, F. Podewils, A. Salzer, T. Wagner and M. Kaupp, Reaction of Pentadienyl Complexes with Metal Carbonyls: Synthetic, Structural, and Theoretical Studies of Metallabenzene π-Complexes, Organometallics, 2003, 22, 264–274 CrossRef CAS; (h) U. Bertling, U. Englert and A. Salzer, From Triple-Decker to Metallabenzene: A New Generation of Sandwich Complexes, Angew. Chem., Int. Ed. Engl., 1994, 33, 1003–1004 CrossRef; (i) U. Englert, F. Podewils, I. Schiffers and A. Salzer, The First Homoleptic Metallabenzene Sandwich Complex, Angew. Chem., Int. Ed., 1998, 37, 2134–2136 CrossRef CAS.
- M. H. Dietrich, K. W. Törnroos, E. Herdtweck and R. Anwander, Tetramethylaluminate and Tetramethylgallate Coordination in Rare-Earth Metal Half-Sandwich and Metallocene Complexes, Organometallics, 2009, 28, 6739–6749 CrossRef.
- (a) W. J. Evans, J. W. Grate, K. R. Levan, I. Bloom, T. T. Peterson, R. J. Doedens, H. Zhang and J. L. Atwood, Synthesis and X-ray Crystal Structure of Bis(pentamethylcyclopentadienyl) Lanthanide and Yttrium Halide Complexes, Inorg. Chem., 1986, 25, 3614–3619 CrossRef CAS; (b) W. J. Evans, T. T. Peterson, M. D. Rausch, W. E. Hunter, H. Zhang and J. L. Atwood, Synthesis and X-ray Crystallographic Characterization of an Asymmetric Organoyttrium Halide Dimer: (C5Me5)2Y(μ-Cl)YCl(C5Me5)2, Organometallics, 1985, 3, 554–559 CrossRef.
- W. Uhl, Hydroalumination and hydrogallation of alkynes: New insights into the course of well-known reactions, Coord. Chem. Rev., 2008, 252, 1540–1563 CrossRef CAS.
- W. Uhl, A. Vinogradov and S. Grimme, C–H Bond Activation by Hyperconjugation with Al–C Bonds and by Chelating Coordination of the Hydride Ion, J. Am. Chem. Soc., 2007, 129, 11259–11264 CrossRef CAS PubMed.
- A. Brand, A. Hepp, E.-U. Würthwin and W. Uhl, Tridentate Ligand with Three Carbanions as Donor Atoms: Formation of Dinuclear, Heptacyclic Complexes of Boron, Aluminum, or Gallium with B–C–B, Al–C–Al, or Ga–C–Ga Three-Center–Two-Electron Bonds, Organometallics, 2020, 59, 5558–5563 CAS.
- S. G. Bott, A. Alvanipour, S. D. Morley, D. A. Atwood, C. M. Means, A. W. Coleman and J. L. Atwood, Stabilization of the [AlMe2]+ Cation by Crown Ethers, Angew. Chem., Int Ed. Engl., 1987, 26, 485–486 CrossRef.
- W. Uhl, J. Wagner, D. Fenske and G. Baum, N,N,N′,N′-tetramethylethylenediamin-di(tert-butyl)aluminium-Kationen – Molekülstruktur des [(Me3C)2Al·TMEDA]+[(Me3C)2Al Br2]−, Z. Anorg. Allg. Chem., 1992, 612, 25–34 CrossRef CAS.
- W. Uhl, L. Cuypers, B. Neumüller and F. Weller, Hydrogallation of Alkynes: Syntheses of Carbon–Gallium Cages Possessing Heteroadamantane Structures, Organometallics, 2002, 21, 2365–2368 CrossRef CAS.
- Y. Mizuhata, S. Fujimori, T. Sasamori and N. Tokitoh, Germabenzenylpotassium: A Germanium Analogue of a Phenyl Anion, Angew. Chem., Int. Ed., 2017, 56, 4588–4592 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT, 2019 Search PubMed.
- C. R. Landis and F. Weinhold, in The Chemical Bond: Fundamental Aspects of Chemical Bonding Weinheim, ed. G. Frenking and S. Shaik, Wiley-VCH, Germany, 2014, pp. 91–120 Search PubMed.
- V. Pophristic and L. Goodman, Hyperconjugation not steric repusion leads to staggered structure of ethane, Nature, 2001, 411, 565–568 CrossRef CAS PubMed.
- Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta and P. v. R. Schleyer, Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion, Chem. Rev., 2005, 105, 3842–3888 CrossRef CAS PubMed.
- (a) CCDC 2491667: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pmsch; (b) CCDC 2491668: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pmsdj; (c) CCDC 2491669: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pmsfk; (d) CCDC 2491670: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pmsgl; (e) CCDC 2491671: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pmshm.
| This journal is © the Partner Organisations 2026 |