Open Access Article
Open Access Article4D printing innovations and the embracing of additive manufacturing transformations
Debarupa Dutta
Chakraborty
a,
Prithviraj
Chakraborty
 *a,
Subhasish
Pramanik
b,
Manali
Dutta
b,
Arpan
Sen
ac,
Sudarshana
Borah
a,
Pallab
Kumar Nath
a and
Sabnam
Nargis
a
*a,
Subhasish
Pramanik
b,
Manali
Dutta
b,
Arpan
Sen
ac,
Sudarshana
Borah
a,
Pallab
Kumar Nath
a and
Sabnam
Nargis
a
aRoyal School of Pharmacy, The Assam Royal Global University, Betkuchi, Guwahati, Assam-781035, India. E-mail: prithvirajchakraborty.pc@gmail.com
bMata Gujri College of Pharmacy, Mata Gujri University, Kishanganj, Bihar-855107, India
cDmbH Institute of Medical Science, Dadpur, West Bengal 712305, India
First published on 28th October 2025
Abstract
The transition from 3D to 4D printing has revolutionized additive manufacturing by introducing dynamic shape-changing capabilities. The limitations of 3D printing have led to the development of 4D printing, which uses ultraviolet light to deposit materials layer-by-layer, creating customizable soft fabric structures that can transform over time in response to external stimuli. The stimuli can be physical, chemical, or biological. Predetermined interaction mechanisms and mathematical modelling, facilitated by tools such as CAD and FEEA, play crucial roles in orchestrating these shape-shifting behaviours. 4D printing has applications in the medical, manufacturing, and educational sectors, with applications extending to adaptive medical implants and devices. Research on 4D printing focuses on various shape alterations, with promising transformative effects on manufacturing processes, medical interventions, and educational tools. As 4D printing progresses, it has the potential to revolutionize industries and provide innovative solutions to complex challenges. The interplay between stimuli and responsive materials, guided by advanced modelling techniques, opens new avenues for unprecedented development. The shift from 3D to 4D printing signifies a paradigm change in additive manufacturing, offering a glimpse into the future, where products dynamically adapt to their environment and user needs.
1. Introduction
For more than 30 years, three-dimensional (3D) printing technology has made countless contributions to the additive manufacturing industry and many other arenas, such as product design, biotechnology, and medicine.1 Researchers are still discovering new models, methods, materials, unique designs, and the applications of 3D printing. This revolution has led to four-dimensional (4D) printing.In 3D printing, a two-dimensional (2D) object is printed layer-by-layer in the printer to create a 3D structure from bottom to top until a 3D volume2 is created. However, conventional 3D printing cannot fulfil the demands of dynamic applications like self-folding packing, adaptive wind turbines, and soft grippers for surgery.3
4D printing depends on stereolithography, where materials are laid down layer-by-layer with the help of ultraviolet light during the printing process. This is an accurate and rapid process for customizing soft-fabric structures. 4D printing is a technology in which a 3D-printed structure is transformed to have a targeted shape or property by an external stimulus. Stimuli fall into one of these three categories: physical stimuli (e.g., temperature, moisture, light, UV light, magnetic energy, and electricity), chemical Stimuli (e.g., oxidants, reductants, ionic strength, and pH level), and biological energy (e.g., presence of glucose and enzymes).4 Different types of 4D printing bases are listed in Fig. 1.5
Not all structures undergo the desired change during exposure to a stimulus. A predetermined interaction mechanism is required to schedule the sequence of shape-shifting behaviours that occur when the stimulus is active for a sufficient period. Mathematical modelling is required to plan out the appropriate time and sequence of stimuli to affect the stimulus-responsive component with the help of geometric programming, such as computer-aided design (CAD) and finite-element-analysis (FEA).6 Additive manufacturing methods, such as three-dimensional (3D) and four-dimensional (4D) printing, are used to create novel products. Time, an additional dimension in the case of 4D printing, is the only difference.7 Recent research on 4D printing focuses on different types of shape-changing properties of 4D structures, like bending, twisting, stretching, and folding.8
4D printing technology impacts different sectors, such as medicine, manufacturing, and education. In healthcare, 4D printing technology fulfils the necessity of developing medical implants and devices. With the help of smart materials, 4D medical implants introduced into the human body can change their shape according to certain requirements over time. These smart materials have the capability to self-deform.9
2. History of 4D printing
Research into 4D printing has been extensive because of its enormous potential.10 Modern commercial printed materials are primarily designed to be durable, elastic, transparent, colorful, or recyclable, among other properties, to fulfill various end uses.11 However, more can be done to increase the utility of printed components, particularly given the rapid advancement of intelligent and active materials research. Several studies have focused on various aspects of the 4D printing process, from the materials used in the printing procedures to the development of the technology itself. This research focuses on the development of 4D printing and the various strategies used by the pioneers of this field12 (Fig. 2).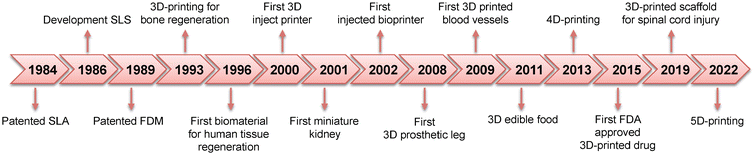 | ||
| Fig. 2 History and milestones of AM in the biomedical field; adapted without modification from ref. 15, copyright MDPI. | ||
The concept of 4D printing, in which time is the fourth dimension, was introduced. The innovative approach of 4D printing over 3D printing has attracted much interest from scientists and engineers throughout the field. The printed items are no longer static, which is crucial for 4D printing. It can change over time in a pre-programmed manner and is sometimes accompanied by functional evolution. Skylar Tibbits was the first to introduce 4D printing. Tibbits used one such technique, materials composed of sections that expanded at various rates.13 Tibbits showed how an initially static printed item can change over time. Shape-programming of non-electric materials was accomplished with this technique, where water was used as an activating agent. Jerri Qi's study on shape programming was a turning point for thermally sensitive materials. These materials can be shaped into the desired three-dimensional form by virtue of their shape memory effect, which manifests at the optimal temperature (Fig. 3).14
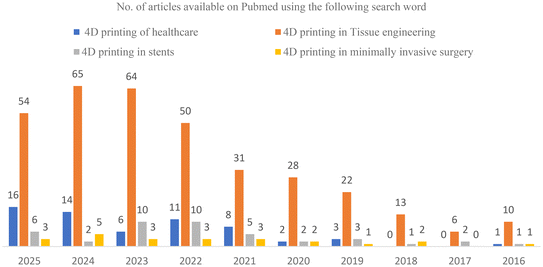 | ||
| Fig. 3 Total number of published papers on 4D printing in different areas of application from 2016 to 2025, compiled from the PubMed database (as of 27 August 2025). | ||
3. The key differences between 4D printing & 3D printing
The rapid development of 3D printing has paved the way for the emergence of 4D printing (Table 1). 4D printing involves the creation of objects using multi-material components that can change in response to external stimuli. Both 3D and 4D printing processes utilize additive manufacturing to create new products.16| Sl. no. | Characteristics | 3D printing | 4D printing |
|---|---|---|---|
| 1 | Build process | Layer-by-layer, from bottom to top, 3D printing replicates a 2D structure. | The evolution of 3D printing is 4D printing. |
| 2 | Material used | Nanomaterials, biomaterials, ceramics, metals, or thermoplastics. | Intelligent, multi-material, and self-assembling materials to create an object that alters its shape after being manufactured. |
| 3 | Design | 3D digital information (scanning, drawing). | 3D digital information for change (deformation). |
| 4 | Key features | Static elements, tailored design, decreased time and expenses. | Active elements, responsive to stimuli, self-repairing, and adaptable to the patient's circumstances. |
| 5 | Printer | 3D printer (ex. stereolithography apparatus, material extrusion, and selective laser sintering). | Smart 3D printer (ex. modified nozzle, binder, and laser multi-material 3D printer). |
| 6 | Shape flexibility | No flexibility, characterized by rigidity. The object shapes are altered during this procedure. | With time and temperature changes, the object's form changes. |
| 7 | Programming of material | Do not use any programmable or advanced material. | Use programmable and advanced materials that provide a variety of functions. |
| 8 | Application | Medicine, engineering, dentistry, automotive, robotics, fashion, aerospace, defense, jewellery, toys, bio/medical devices, etc. | Dynamically changing configuration for all applications by 3D printing. |
| 9 | Future potential | Improved accuracy in medical production. | Innovative, customized healthcare options, tissue repairing. |
| 10 | Examples | Personalized implants, surgical models. | Drug-delivery systems that adapt to patient needs, prosthetics that can repair themselves and bio-implants that work with body tissues. |
The only difference in 4D printing is the time factor. Time is the extra dimension that makes 4D printing possible. 3D printing requires time for the final structure formation, healing, and cooling. In contrast, 4D-printed parts begin to act only after exposure to external energy.
The most common materials used by 3D printers are nylon, ABS plastic, resin, wax, and polycarbonate. Meanwhile, 4D printing technology uses smart materials, which are multimaterials with properties that can be transformed by external energy. Smart materials include piezoelectric, electrostrictive, magnetostrictive, thermoelectric, and shape memory alloys, like Cu–Al–Ni, Ni–Ti, and Cu–Au–Zn.
After material selection, hardware plays a crucial role in 3D printing. Various 3D printing machines are available for home and production use, each using different technologies.17 For instance, Stratasys’ Connex multi-material 3D printer allows the integration of multiple material properties into a single structure using water as an external activation factor. In addition, the RoVa4D Full-colour Blender 3D printer from ORD Solutions allows reasonably priced desktop printing of full colours using many materials.
Advancements in the printing industry have led to the development of new software tools that go beyond traditional modelling software. There is a demand for software capable of incorporating bioprinting, multi-material printing, 4D printing, and electronic printing processes. Some notable software includes Project Cyborg from Autodesk, ANVAS software from Mosaic Manufacturing, Foundry from MIT's Computer Science and Artificial Intelligence Lab, and Monolith multi-material voxel software. Advanced modelling software is required for 4D printing compared to that used for 3D printing.
While an object is being printed, the process often remains unmonitored, allowing objects to be built overnight without human interference. 4D printing processes are becoming even simpler than 3D printing technology, and simple structures can be transformed into complex, large functional structures with the help of an external activating agent. Self-assembly structures sense and physically react to the surrounding environment without human involvement. The potential of 4D printing technology could lead to a massive shift in the design and manufacturing of objects and structures in the future.
4. Laws of 4D printing
F. Momeni and J. Ni established three fundamental laws that govern the shape-changing behavior of all 4D printed structures.18 These laws enhance our understanding of the physics underlying the shape-changing capabilities of these structures. These are outlined below.4.1. First law
This law states that all shape-changing behaviours, such as coiling, curling, twisting, and bending, of multi-material 4D structures result from the relative expansion between active and passive materials.4.2. Second law
This law identifies four physical factors that contribute to the shape-changing ability of all multimaterial 4D structures: mass diffusion, thermal expansion, molecular transformation, and organic growth.These elements result in relative growth between active and passive materials, leading to shape changes in response to a stimulus. Changes in mass owing to the absorption or adsorption of stimuli (such as water or ions) can result in shape deformation. Mass changes can also occur in response to electrical, thermal, chemical, or light stimuli. Thermal expansion can deform structures through temperature changes that affect atomic and molecular distances. Similar deformations can occur in response to electrical, light, and UV stimuli, as these can alter the temperature.
4.3. Third law
The third law of 4D printing states that the time-dependent shape-morphing behaviour of nearly all multi-material 4D printed structures is governed by two types of time constants. These constants can vary based on the stimulus and materials used. A mathematical bi-exponential equation for representing the fourth dimension was established for potential use in 4D structures.5. 4D printers
Researchers have initially focused on examining traditional commercial 3D printers. However, it has been discovered that when using smart materials to print particular objects, the printing material can clump together in standard machines. To address this issue, some research teams have designed specialised 4D printers. For example, Choi et al. developed a printer featuring a coated extrusion nozzle designed explicitly for printing smart materials, such as thermal polyurethane (TPU), using material extrusion 3D printing.19 The longer nozzle was coated with polytetrafluoroethylene to reduce friction, and the printer included a heating bed to prevent nozzle clogging by maintaining proper heat circulation. Another example is a 4D printer created by Ge et al., which is an adaptation of micro-SLA printers capable of producing structures with a resolution of up to 1 micron.20 This printer is not compatible with regular SLA printers because it employs photocurable smart materials, which are significantly more energy-intensive than regular acrylate-based polymers.6. 4D printing methods
Additive manufacturing (AM) can be categorized into three main types based on the base material used: solid-based, powder-based, and liquid-based AM (Fig. 4). Solid-based AM encompasses various methods, including laminated object manufacturing (LOM), fused deposition modeling (FDM), wire and arc additive manufacturing (WAAM), and electron beam freeform fabrication (EBF3). Powder-based additive manufacturing includes techniques such as selective laser sintering (SLS), electron beam melting (EBM), selective laser melting (SLM), and laser metal deposition (LMD). Liquid-based methods primarily consist of material jetting (MJ) and vat-based printing techniques, which include stereolithography (SLA) and digital light processing (DLP).21 Three significant 4D printing methods are discussed here: SLA, FDM and SLS (Table 2). | ||
| Fig. 4 Classification of AM methods based on the base material used, including solid-based, powder-based, and liquid-based materials; adapted from ref. 22, copyright MDPI. | ||
| Category | Fusion deposition method (FDM) | Selective laser sintering (SLS) | Stereolithography (SLA) |
|---|---|---|---|
| Operational principal | Material extrusion | Laser sintering | UV curing |
| Resolution (layer thickness) | 50–400 microns | 50–150 microns | 25–100 microns |
| Printing (speed) | Fast (simple designs) | Slow (due to cooling/powder handling) | Medium (depends on resin curing) |
| Heat source | Heated nozzle | High-power laser | UV laser |
| Accuracy | Moderate | High | Very high |
| Surface finish | Rough texture | Powdery texture | Smooth, glossy finish |
| Design complexity | Simpler geometries | Excellent (supports not required) | High (supports needed) |
| Advantages | Rapid printing method, low cost for producing parts, a broad range of materials is necessary | Functional components, greater design flexibility, no need for support structures | Capable of producing fine-detailed parts, precision-engineered results, suitable for diverse applications |
| Disadvantages | Lower surface quality, requires support structures | Uneven surface texture, long process time | Cost maintenance, restricted material options |
| Polymer | Polylactic acid, polypropylene, polyvinyl alcohol, acrylonitrile butadiene styrene | Polymethyl methacrylate, polyethylene terephthalate | Polycaprolactone, polyethylene glycol, trimethylolpropane carbonate, poly tetrahydrofuran ether |
6.1. Stereolithography (SLA)
Ge et al. introduced a novel printing method that utilizes high-resolution projection.23 This method allows for modifications of material properties, benefiting from its high-resolution projection. Stereolithography is applicable in producing electronic devices, jewelry, fashion items, and biomedical applications. The process involves creating a layer-by-layer structure through the solidification of a photo-curable hydrogel. A UV laser is used to scan the hydrogel, which results in the formation of covalent bonds between polymer chains22 (Fig. 5). The optimization of the 4D printing process can be achieved by controlling factors such as the power of the UV laser, scan speed, exposure time, wavelength, and spot size. | ||
| Fig. 5 Diagram illustrating the SLA 3D printing process, along with a sample print; adapted from ref. 24, copyright MDPI. | ||
6.2. Fusion deposition method (FDM)
The core objective of the fused deposition modeling is to enable shape memory or shape shifting via a heating mechanism.25 During the fusion deposition process, the material undergoes thermal and mechanical training. Key printing parameters that influence the process include speed, temperature, and deposition path (Fig. 6). Additionally, layer orientation and thickness are critical design parameters that allow for self-folding and self-twisting features.26 The difference in thermal expansion coefficients is responsible for the shape-shifting action. During fabrication, internal stress develops within the printed material due to rapid heating and cooling cycles. Advanced 4D bio-printing is achieved using bio-composites, aiming to enhance strength and self-shaping, making 4D bio-printing beneficial for engineering and biomedical applications.27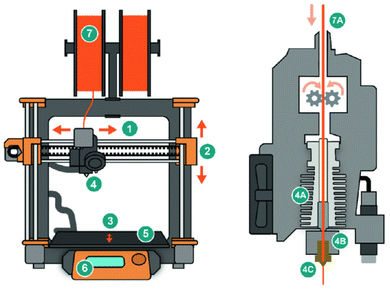 | ||
| Fig. 6 Schematic of the fused deposition modeling (FDM) 3D printing technology illustrated in two parts. The left image displays the overall structure of a typical FDM printer, with the following parts labeled: (1) x-axis motor, (2) y-axis motor, (3) z-axis motor, (4) hot nozzle, (5) printing bed, (6) controller display board, and (7) filaments. The right image provides a closer view of the printing nozzle, where the components are labeled as follows: (7A) feed filament, (4A) heating wires, (4B) hot end, and (4C) extruded materials; adapted without modification from ref. 24, copyright MDPI. | ||
6.3. Selective laser sintering (SLS)
Support materials are not required for the manufacture of these components.28 SLS is a 3D printing technique that uses laser energy to selectively heat and fuse powder particles into a solid object. An SLS system includes a spreading platform, powder bed, and laser system (which includes the laser and scanner)29 (Fig. 7). The powder is evenly spread onto the building platform and processed in layers, with each layer created by predetermined laser scanning elements called vectors. The laser fuses the material at temperatures below its melting point. After each layer is fused, the powder bed lowers, and a new layer is added. This process repeats until the object is fully constructed. Once completed, the object cools in the printer and is extracted from the loose powder.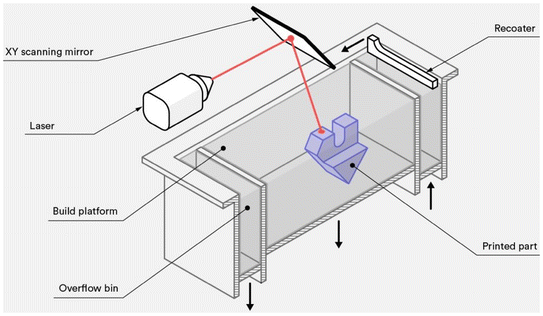 | ||
| Fig. 7 Diagram of selective laser sintering (SLS) 3D printers; adapted without modification from ref. 24, copyright MDPI. | ||
7. The choice of materials for 4D printing
More accurate and flexible material placement has been made possible by the recent advancements in multi-material 3D printing, which are essential for 4D printing.30 Plastic, metal, and ceramic materials are frequently used in 3D printing to create 3D objects. Unfortunately, these materials are unsuitable for 4D printing due to their lack of responsiveness to external stimuli. As a result, selecting the right materials is crucial for 4D printing. In 4D printing, two major types of smart materials are used: hydrogels that expand when in contact with water or other solvents and shape memory polymers (SMPs) that respond to external stimuli, like temperature, pH, magnetic field or UV radiation (Table 3).31| Function | AM method | Description | Material | Application |
|---|---|---|---|---|
| Cell shapes | Advanced structured materials | Honeycomb, tetra chiral and hexachiral cells, etc. | Polymers, resins, metallic powders, etc. | Automotive, aerospace, biomedical, art, etc. |
| Auxetic shapes | Cells that shrink or expand along two directions | Polymers, resins, Ti6Al4V powder | Stents, implants | |
| Specific patterns | Reproduce the principal directions of the stress | ABS, PLA | Biomedical | |
| Topological optimization | Distribution of material(s) according to the objective functions | Polymers, resins, metallic powders, etc. | New lighter structure | |
| Shape memory | Responsive materials | Multi-materials change shape with external stimuli | NiTi, UV-responsive materials, ceramics, monomers | Aerospace, defence, biomedical, textiles |
| Self-assembly | Automated folding or molecule aggregations | PCL, TPU, PLA copolymers and nanoparticles | Biomedical | |
| Self-actuating | Automated actuation by external stimuli | Piezoelectric materials, carbon nanostructures | Actuators, sensors, touch screen | |
| Self-evolving | Activation when exposed to water | Viscoelastic ink, acrylamide monomer, nanoclay, glucose, NFC | Robotic behavior | |
| Self-sensing | Automated detection of external stimuli | PLA and graphene, piezoelectric materials, carbon nanostructures | Biomedical, robotics |
7.1. Shape memory materials (SMMs)
Shape memory materials can revert to their original shape when stimuli are applied, a phenomenon known as the shape memory effect (SME). Smart materials with the SME can return to their original shape. Researchers have become interested in the transformable features of materials that show SME because these properties allow the building of a structure that can be activated after printing.32 Shape memory materials are a class of materials that display the smart memory effect, which are divided into shape memory alloys (SMA), shape memory polymers (SMP), shape memory hybrids (SMH), shape memory ceramics (SMC), and shape memory gels (SMG).3Shape-changing materials respond to stimuli, morphing temporarily and returning to their original shape when the stimuli are removed. According to Zhou, the transition is often restricted to simple changes, such as stretching or shrinking, but inhomogeneous expansion can lead to surface topography changes, like buckling, folding, and bending. Shape memory polymers (SMPs) are popular for 4D printing (Table 4). They have a wide range of glass transition temperatures, allowing their stiffness to be tailored. In contrast to SMAs, which can achieve only about 7%–8% of plastic strain, SMPs can exhibit a form recovery property up to 400% of strain. SMPs are advantageous due to their simple manufacturing methods, low costs, and great recovery. When exposed to stimuli, they can revert to their preprogrammed shape from a deformed configuration. SMPs exhibit high elastic deformation, low density, biodegradability and biocompatibility for medical applications.
| Property | SMP | SMA |
|---|---|---|
| Density (g cm−3) | 0.9–1.2 | 6–8 |
| Extent of deformation | Up to 800% | <8% |
| Required stress for deformation (MPa) | 1–3 | 50–200 |
| Stress generated upon recovery (MPa) | 1–3 | 150–300 |
| Transition temperature (°C) | −10 to 100 | −10 to 100 |
| Recovery time | >1 s | <1 s |
| Processing condition | <200 °C; low pressure | >1000 °C; high pressure |
7.2. Shape memory effect (SME)
The main characteristic of shape memory materials (SMMs) is the ability to recover their programmed shape from a temporary shape. Applying the stimulus causes what is referred to as the shape memory effect. SMMs require a programming process to deform the material into a temporary shape, followed by a shape recovery process triggered by the right stimulus.34 The rate of shape change from a temporary shape depends on the material's responsiveness and the physical design of the geometrical part.The shape memory material's network elasticity determines the “memory” of one or more shapes. The majority of SMMs have a one-way shape memory effect, while some have a two-way shape memory effect. Eujin et al. explain the one-way shape memory effect as the process where the SMP returns from its temporary shape to the original permanent shape under an applied stimulus.35 SMP, with a two-way shape memory effect, can remember two different shapes when exposed to stimuli. The material can change from a temporary shape back to its permanent shape, and this change is reversible.
The two-way SME can be found in liquid crystalline elastomers and photo-actuated deformation polymers. Chen et al. successfully demonstrated the two-way shape memory behavior using a polymer laminate prepared from a 1.0 mm-thick active layer of PHAG5000 polyurethane-based shape memory with a 1.0 mm-thick substrate of PBAG600-based polyurethane.36 The effect was observed by bending upon heating from 25 to 60 °C and reverse bending upon cooling from 60 to 25 °C.
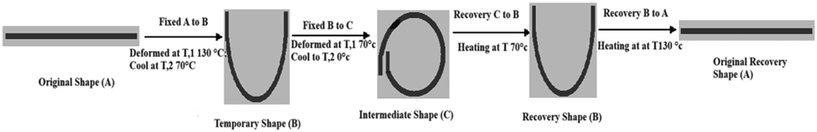
Erkeçoğlu explained that the main difference between one-way and three-way shape memory effects is that the three-way shape memory effect has one intermediate shape between its original and temporary shapes.37 A “multiple shape memory effect” occurs when there is more than one intermediate shape. This effect can be achieved either by heating a programmed shape memory polymer to temperatures above its glass transition and then its melting transition or by combining multiple two-way shape memory polymers with different glass transition temperatures.38Fig. 8 illustrates the three-way shape memory effect with two distinct thermal transition temperatures: Tlow,1 (70 °C) and Tlow,2 (0 °C). These temperatures are attributed to the presence of two separate crystalline domains in the original shape. Li offered various methods for managing triple-shape memory effects, including blending, grafting, and blocking of copolymers, SMP hybrids, or other polymer laminates.39
7.3. Shape memory polymer types
SMPs are gaining interest because of their dynamic behavior stimulated by external stimuli, which helps them change their shape over time in 4D printing. Different types of stimuli, including temperature, moisture, electricity, light, and magnetic fields, are reviewed (Table 5).| Stimulation method | Material type | Theory | Key properties | Advantages | Disadvantages | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Water/humidity/pH | Hydrogel | Swelling/shrinkage | Swell or shrink in response to water or pH changes | Clean/convenient | Slow response | Tissue engineering, drug delivery | 60 and 61 |
| Temperature | Shape Memory Polymers (SMPs), Liquid Crystal Elastomers (LCEs) | Internal stress inequality | Can “remember” and return to original shape after deformation/exhibit large reversible shape changes due to molecular alignment (LCE) | Controlled adjustable | Slow response, complicated | Biomedical devices, aerospace components, soft robotics, sensors | 62–64 |
| Light | Photo-responsive polymers | Photo-thermal effect | Undergo structural changes when exposed to specific wavelengths | High-resolution/remote control | Complicated | Optical switches, smart textiles | 65–68 |
| Electric field | Electro-responsive materials | Electro-thermal effect | Fast | Operating inconvenience | Adaptive structures, deployable systems | 69 and 70 | |
| Magnetic field | Magneto-responsive materials | Magnetic drive | Change shape or orientation under magnetic influence | Remote control | Operating inconvenience | Actuators, remote-controlled devices | 71–73 |
| Cell traction force (CTF) | Actin binding and interaction | Biological compatibility | Cell traction force is small and hard to control, high design requirement | 74 and 75 |
Wang et al. developed a phenomenological model and introduced the theory of phase evolution to characterize the glass transition behavior of SMPs.42 In a typical shape memory cycle, the SMP sample first changes from its initial shape at a temperature above its transition temperature and then cools to a lower temperature while adhering to external limitations. The stress–strain relationship iterative formats are presented for phase generation and vanishing processes, providing closed-form analytical solutions for shape memory behaviour and effective assistance in 4D printing design using shape memory materials. The model shows promise for expansion into other soft materials with similar phase-evolution characteristics.
Mulakkal et al. created and tested a cellulose-hydrogel composite to demonstrate its suitability for printing responsive structures using four-dimensional printing techniques. The primary emphasis was on a cellulose-hydrogel composite ink for additive manufacturing.45 When cellulose pulp fibres were combined with a carboxymethyl cellulose hydrocolloid, an ink with a high total cellulose content and good fibre dispersion inside the hydrogel matrix was produced. Villar et al. used two picoliters of water droplets combined with a lipid interface at two different osmotic pressures. When the osmotic pressure was high, the droplets expanded, and when it was low, they contracted until the osmotic pressure balanced.46 However, hydrogels have a significant structural drawback, making them weak and extremely brittle.
In some cases, researchers wait for hydrogels to dry and shrink over an extended period. One solution to this problem is the use of a secondary polymeric network called an interpenetrating polymer network (IPN) hydrogel, which is formed by crosslinking hydrogel polymers.47 Highly flexible and biodegradable hydrogels with cellular structures are developed by crosslinking sodium alginate with PEG (Fig. 9).48
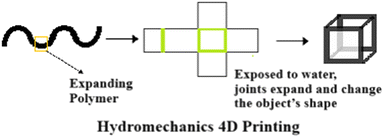 | ||
| Fig. 9 Hydromechanical 4D printing.49 | ||
Kuksenok et al. employed light differently as a catalyst for deformation.51 Light is a typical stimulus that controls polymers through remote induction. A trigger made of various wavelengths of light may alter the polymer form. Since light does not harm cells by raising the material's temperature or causing other physiological changes, this stimulation is appropriate for use in biomedicine and in vivo drug administration.
For instance, Luo et al. verified that near-infrared rays (NIR) caused shape deformation in an alginate/polydopamine (PDA)-based scaffold. The photothermal agent and room-temperature folding rate of the FDA-approved alginic acid scaffold are both compromised by dehydration.52 It has the potential to convert the absorbed light into heat, thereby accelerating the dehydration and deformation of the alginic acid scaffold. The intensity and duration of light exposure can be used to modulate the bending of the alginic acid/PDA bilayer. Light is helpful because it can enable high-resolution control in terms of location and time as an external stimulus meant to alter the colour of printed items.
Jeong et al. showed how to create SMPs in many colors using a 4D printer.53 They accomplished a remote drive using light based on colour-dependent selective light absorption and multicolour SMP composite heating. Under red lighting, the thermomechanical programming structure curves into an n-shape.
Okuzaki et al. created a tiny origami robot utilizing the polypyrrole membrane.55 The feet of this robot have a unique design that makes it less resistant to forward motion. In an electric field, the voltage moves the head forward by absorbing moisture, and the tail rises when the voltage is reduced owing to desorption.
In Mohr et al.'s study, Fe2O3 nanoparticle-filled thermoplastic SMP composites were magnetically induced. Heating in an alternating magnetic field can induce the form recovery of SMP composites.57 Using ferromagnetic nanoparticles combined with AAM-carbomer ink in a Petri dish, Cheng et al. created a magnetic hydrogel octopus that could be remotely controlled using a magnetic field and could move freely.58 The limitation on the print size results from the requirement that it be light enough to be affected by the magnetic field. Bodaghi et al. introduced a novel concept for creating bi-stable magnetorheological elastomer (MRE)-based electroactive composite actuators using 4D printing technology. The researchers combined MRE composites with 4D-printed conductive shape memory polymers to develop a functional, lightweight, and bistable composite actuator with programmable magnetic patterns. The actuator is composed of silicone resins loaded with strontium ferrite magnetic particles and a thin conductive carbon black polylactic acid (CPLA) core, which is 4D printed and embedded in the composite.59Table 5 and Fig. 10 describe some of the stimuli and their application for 4D printing.
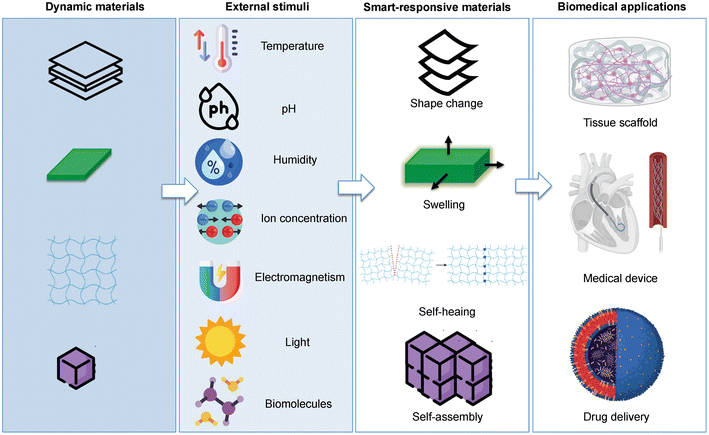 | ||
| Fig. 10 Schematic depicting the various types of stimuli and the corresponding responses in smart materials, including shape alteration, swelling, self-assembly, and self-repair, highlighting their potential applications in the biomedical sector; adapted without modification from ref. 15, copyright MDPI. | ||
8. Smart design
Apart from utilizing smart materials, one way to create 4D-printed structures is by manipulating the product's design. By adjusting the design and orientation of smart materials, it is feasible to induce specific physical changes in the final product under certain conditions. This was demonstrated by Ge et al., who designed a structure with SMP fibers oriented in a specific manner.20 When exposed to heat, the printed structure underwent complex shape transformations with curvatures that varied based on the initial orientation of the fibres. Therefore, the combination of smart materials and smart design in 4D printing enables the creation of structures with intricate physical changes.76 By employing both smart materials and thoughtful design, 4D printing can produce structures that are too complex to be achieved using only 3D printing. Additionally, beginning with a simple morphology in the initial design stage enhances the speed and cost efficiency of the process, while also improving the logistics of storage of such objects. In addition, many of the problems that might arise with 3D printing, such as feedstock blockage and structural collapse before solidification, can be circumvented if 2D structures are printed first. When necessary, the structure can take on the required 3D form to achieve its intended function.9. Mathematical modeling of 4D printing
Mathematical modelling plays a crucial role in the advancement of 4D printing technology. This allows researchers to design and optimise complex self-assembling structures. By integrating traditional constitutive models with emerging machine learning techniques, we can enhance our understanding of the behaviour of 4D-printed materials. The 4D printing process relies on mathematical models to achieve various objectives. These include predicting how the shape will change over time, developing theoretical models to avoid collisions during the self-assembly process, and reducing the need for repeated experiments.By using mathematics and theoretical models, researchers can predict the final shape more accurately, significantly reducing the number of test experiments required. The key components of the theoretical model for 4D printing include the final desired shape, material structure, material properties, and stimulus properties. By using Gladman et al.'s categorization, 4D printing mathematics can be divided into the forward problem and the inverse problem.77 The forward problem involves determining the final desired shape given the material structures, material properties, and stimulus properties. In contrast, the inverse problem entails determining the material structure, print paths, and nozzle sizes given the final desired shape, material properties, and stimulus properties.
Mathematical modelling is crucial for predicting the behavior of 4D-printed structures. There is increasing interest in utilising machine learning-based modelling approaches. These innovative techniques aim to establish structure–property relationships and design-shape transformations of 4D bioprinted constructs.78
Additionally, Wang et al. studied the mathematics of the single-loop foldable 8R (revolute joint) with multiple modes, which is related to folding.79 Their work provides valuable mathematical tools for addressing forward and inverse problems in 4D printing processes.
Research was conducted by Purushottam Suryavanshi et al. 2025![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 on novel shape memory-responsive cellulosic composites (RCC) designed for 4D printing, offering self-initiated, reversible shape transformation. By combining experimental, theoretical, and computational approaches, the study refines the material performance for sophisticated biomedical and pharmaceutical uses. This investigation presents RCC composites uniquely blending starch and AffnisolTM. RCC-based filaments were used to print single-layer strips using fused deposition modeling 3D printing technology, demonstrating reversible, contactless shape changes in response to swelling and heat. The programming phase involves swelling and heating the composite strip, followed by shape recovery through heating. Shape deformation during self-activated programming was estimated using experimental, theoretical, and computational methods. The study tested varying thicknesses (1.5, 2.0, and 2.5 mm) and temperatures (25 °C and 37 °C) to confirm the model's effectiveness in predicting the bending curvature. The model showed less than 13.96% discrepancy between the theoretical and experimental modeling, with lower thicknesses achieving less than 2.0% difference. These RCC materials demonstrated potential for reversible 4D printing and aligned with the adopted methodologies for predicting the bending curvature. This study introduces new composite materials for 4D applications, with models for predicting the bending curvature.80
80 on novel shape memory-responsive cellulosic composites (RCC) designed for 4D printing, offering self-initiated, reversible shape transformation. By combining experimental, theoretical, and computational approaches, the study refines the material performance for sophisticated biomedical and pharmaceutical uses. This investigation presents RCC composites uniquely blending starch and AffnisolTM. RCC-based filaments were used to print single-layer strips using fused deposition modeling 3D printing technology, demonstrating reversible, contactless shape changes in response to swelling and heat. The programming phase involves swelling and heating the composite strip, followed by shape recovery through heating. Shape deformation during self-activated programming was estimated using experimental, theoretical, and computational methods. The study tested varying thicknesses (1.5, 2.0, and 2.5 mm) and temperatures (25 °C and 37 °C) to confirm the model's effectiveness in predicting the bending curvature. The model showed less than 13.96% discrepancy between the theoretical and experimental modeling, with lower thicknesses achieving less than 2.0% difference. These RCC materials demonstrated potential for reversible 4D printing and aligned with the adopted methodologies for predicting the bending curvature. This study introduces new composite materials for 4D applications, with models for predicting the bending curvature.80
10. Application of 4D printing in healthcare
The integration of 4D printing in healthcare has transformed the design and functionality of medical devices. This technology enables materials to maintain a fixed shape, transform and adapt in response to specific biological stimuli over time. As a result, it creates new possibilities for medical applications where adaptability, self-repair, and real-time environmental responses significantly enhance patient care.16These advancements utilise smart materials that react to changes in temperature, humidity, pH, or mechanical stress. This enables the development of patient-specific solutions that can improve the efficiency and longevity of medical devices. With these innovations, 4D printing has the potential to revolutionise healthcare, offering solutions that are more adaptive, efficient, and personalised than ever before.
10.1. Tissue engineering
4D printing is an emerging technology. 4D bioprinting combines the 3D bioprinting process with the time factor, allowing printed medical models to change their shape and functionality. 4D bioprinting requires the realisation of dynamic operations to produce complex and dynamic tissues in vitro. The possibility of creating biomimetic blood vessels in vitro has undergone a substantial shift due to recent techniques (e.g., 4D printing) to understand blood vessel structure.81 For example, self-folding polymers can be used to create artificial blood vessels; when exposed to water, these polymers encapsulate various cells and form multilayered tube-like structures. By taking advantage of the self-organization ability of cells, 4D printing may be used to produce vasculature.82 Vascularization is a crucial problem in tissue engineering that must be solved since there is an immediate need for functioning tissues.83,84 A promising solution to this problem is 4D bioprinting. The cells mixed with the hydrogels can be bioprinted layer-by-layer to form cylinder-shaped structures imitating the vasculature.85 Vascular cells can then quickly achieve maturity after stimulation by maturation factors, leading to vascularisation. Biocompatibility and biodegradability are essential to prevent the body from rejecting the materials, and mechanical strength is also necessary to support cell growth.86 Although this method has been used with both synthetic and natural polymers, with several reported results, the complexity involved, such as growing organs in a lab, requires the use of different fabrication processes.An alternative approach is the cell traction force, which generates vasculature through residual stress and elastic modulus. A different technique allows for self-folding by a tangential tension produced by the cells on the extracellular matrix components (EMC) or underlying layer.87 Through the cell traction force, the 2D patterned microplates roll up in the diagonal direction. By adjusting the angle of the diagonal lines, the diameter of the tubes can be adjusted. Bovine carotid artery endothelial cells (EC) and regular HUVECs have been employed to create cylindrical tubes resembling vascular structures.88
Recent studies have demonstrated the shape memory scaffolds’ potential for use in the minimally invasive delivery of functioning tissues.89 Shape-memory scaffolds can regulate the transmission process remotely and accurately using bioelectronics and biodegradation machines.90 Different studies have shown that bioscaffolds can fully recover to their initial state at the normal human body temperature and have a high capacity for cell adhesion and development. Miao et al. employed this technique to fix a novel renewable soybean oil epoxy acrylate on a biomaterial to stimulate the development of bone marrow mesenchymal stem cells.91 To potentially repair peripheral nerve injury, a programmable nerve-guiding conduit was created using stereolithographic 4D bioprinting. Some stimuli, such as high temperatures and extreme pH, should be avoided during tissue engineering when living cells are involved.92 The use of 4D printing for tissue engineering is currently in the proof-of-concept study stage, and there is still a long way to go before this method is widely used in clinical practice.
10.2 Stent
The medical process known as angiography involves inserting the proper materials into the arteries to identify any blocked spots.93 Using the body heat of the patient as a stimulus, 4D printing can create stents that can expand and assume the desired shape. During angioplasty, guidewires are used to open blocked arteries. Guidewires direct this medium, which is fed through a catheter, to its intended location. Initially, stainless-steel guidewires were used in this procedure. However, directing these guidewires inside the human body is challenging because of their propensity for distortion and bending. These days, many nitinol guidewires are employed for this purpose because of their great flexibility and good kink resistance.94 The exceptional flexibility and elasticity of nitinol enable it to move without deformation through various intricate pathways in blood arteries. Nitinol is biocompatible because it forms a passive titanium oxide coating that shields it from corrosion and reduces its susceptibility to chemical breakdown.95The primary function of a stent is to sustain a hollow structure. For instance, stents unblock or widen arteries affected by coronary artery disease. In the past, stents had to be surgically implanted into patients’ bodies after being manufactured, considerably increasing the safety risk. Scaffolds are now being produced using smaller stimuli-responsive materials thanks to the development of 4D bioprinting.96 The danger of surgery is significantly reduced after transplantation because the stent will naturally conform to the right size and shape with the right stimulation. Ionov et al. created a self-folding stent with a hollow structure using 4D printing and a hydrogel, which has a minimum diameter of 20 μm. The polymer may experience a reversible shape change when the Ca2+ ion concentration changes.97 The cell survival rate of stents manufactured from these biocompatible hydrogels is comparatively high.
There are various steps in the fabrication of stents due to their complex and patient-specific geometries.98 Using 4D printing, customised stents can be fabricated quickly. To further reduce the surgical invasion of the implantation site, the stent can be “shape memory” printed at the final diameter and then “programmed” to a lower diameter for easier and more precise insertion. The stent will shrink to its original diameter at body temperature after being implanted.99 For use in minimally invasive surgeries, Ge et al. created a shape memory stent that was printed in a high resolution.40 First, the temporary shape of the stent can be sustained at a small diameter. The diameter of the blood vessel can be increased by implanting the stent, and the stent can transform back into its original form when heated. Liao et al. used 4D bioprinting to create an adaptable structure.100 When the temperature changes, the structure can expand and shrink. Vascular stenosis is typically treated using stents. The trachea is a more common intraluminal feature. The presence of the illness causes the trachea to narrow or collapse. Cohn et al. used shape memory thermosetting polymers to create the heat-driven lumen device.101 The device can be transformed into a tracheal stent as the temperature increases. The outline of the SMP structure can be minimised by custom design, thereby reducing the harm to the human body.
The advancement of 4D-printed smart stents holds significant promise, especially in the realm of vascular structures, where their ability to adapt can greatly enhance therapeutic outcomes. By employing shape memory alloys or hydrogel-based composites that react to temperature changes, these stents can be engineered to fit the vessel's dimensions more accurately once they are in place. This level of adaptability allows for improved patient-specific customization during stent placement and the optimization of mechanical force exerted on the vessel wall, thereby minimizing the risk of further injury or excessive tissue damage. Additionally, smart stents that can adjust to other biological factors, such as variations in pH levels due to localized inflammation or tissue healing, could reduce the necessity for additional procedures or interventions. 4D-printed smart stents can improve patient outcomes through personalized medicine customized to individual conditions. This approach is expected to reduce stent failures and enhance treatment effectiveness for cardiovascular and vascular conditions.
By employing shape memory alloys or hydrogel-based composites that react to temperature changes, these stents can be engineered to fit the vessel's dimensions more accurately once they are in place. This level of adaptability allows for improved patient-specific customization during stent placement and the optimization of mechanical force exerted on the vessel wall, thereby minimizing the risk of further injury or excessive tissue damage. Additionally, smart stents that can adjust to other biological factors, such as variations in pH levels due to localized inflammation or tissue healing, could also reduce the necessity for additional procedures or interventions. 4D-printed smart stents can improve patient outcomes through personalized medicine customized to individual conditions. This approach is expected to reduce stent failures and enhance treatment effectiveness for cardiovascular and vascular conditions (Kantaros A. et al., 2023![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 76).
76).
4D bioprinting has enabled the creation of smart stents in a novel manner. The need for 4D bioprinted stents in the medical field will increase in the future. Improvements in biocompatibility and adjustments to human biological characteristics remain challenging in this area.
10.3. Microfluidics
Microfluidics has numerous uses in biomolecular, biodefence, cell analysis, high-throughput screening, DNA chip technology, diagnosis, and treatment.102,103 Low power consumption, low cost, and unrestricted miniaturisation are required for the operation and management of microfluidic devices. Some micro and smart components can fulfil these requirements. As a result, the use of soft and stimulus-responsive hydrogels in microfluidic devices represents a promising future development.104 It can precisely control the fluid by changing its shape in response to external stimuli. For instance, D'eramo et al. developed 2 μm horizontally resolved microfluidic actuators using poly(N-isopropyl acrylamide) hydrogel columns.105 Elevating the hydrogel system temperature above the lower critical solution temperature can create up to 7800 microcages in 0.6 s. To regulate microfluidics, Beebe et al. created a pH-responsive smart hydrogel actuator.106 They created several pH-responsive hydrogel forms using photolithography, and the reaction time required was less than 10 s. According to their chemical components, each hydrogel gate reacts to a certain pH value and then automatically identifies the fluid. By in situ γ-irradiation, Zhu et al. created poly(N-isopropyl acrylamide)/graphene oxide (PNIPAM/GO) nanocomposite hydrogels that are photothermally sensitive and exhibit excellent photothermal characteristics.107 Using a near-infrared (NIR) laser, the phase transitions can be remotely managed, and through laser exposure or non-exposure, they can be fully reversed.10.4. Drug delivery
With characteristics like excellent biocompatibility and flexibility, hydrogels are often employed in biomedical applications.108 It is still quite difficult to accurately release medicine at lesion sites. The release of medications at the site of action based on changes in the environment and under controlled circumstances is referred to as the “ideal drug-delivery system”. Researchers are currently aiming to achieve this. Structures that can regulate the rate and localisation of drug release can be produced via 4D printing. Hongyan et al. developed a self-folding polymeric bilayer hydrogel for drug encapsulation and therapeutic release. One layer is the pH-sensitive swelling layer, which is made of crosslinked poly(methacrylic acid) (PMAA), and the other is the mucoadhesive layer (non-swelling layer), which is made of poly(hydroxyethyl methacrylate) (PHEMA). The hydrogel can self-fold into mucus due to the swelling of PMAA, and it also facilitates mucoadhesion due to the presence of PHEMA.109 The PHEMA layer helps minimise drug leakage in the intestine, which acts as a diffusion wall. Using a bilayer structure, acid orange 8 and bovine serum albumin were encapsulated in a hydrogel for unidirectional drug release in the mucosal epithelium. A novel technique was put out by Dai et al., which employed thermally sensitive double network hydrogel (pluronic F127 diacrylic macromolecule and poly(lactide-co-glycolide))-based shape memory when exposed to near-infrared light.110By integrating graphene oxide (GO) into the hydrogels, the shape memory characteristics were improved, and the mechanical properties were improved by the inclusion of PLGA, which acted as a second network. The folded hydrogel must be exposed to NIR light for 300 s to completely transform back into its original shape. The key element affecting the drug release rate is the surface area created by the change in the shape of the structure. Consequently, the surface area decreases, and the drug release rate decreases when the temporary form is altered. Larush et al. developed a drug-release device using Digital Light Processing (DLP) technology, which releases medications in response to pH and shape-dependent swelling.111 3D printing technology improves conventional solid dosage forms by enabling control over drug release through adjustments in pH and surface area. The responsiveness of the printed object plays a significant role in drug release and can potentially change the systemic pH to facilitate drug release at a particular site in the gastrointestinal tract. Based on 4D bioprinting, Akbari et al. created a directly activated drug-delivery system. They began by printing various porous sensors, primarily composed of alginate fibres and pH-responsive substances. Alginate fibers that had been loaded with gentamicin were used to print the drug-eluting stent concurrently.112 The procedure for working is as follows: if the sensor detects a shift in pH, the drug-eluting stent may deliver the medication to the site of the shift, eliminating any microbes. Researchers must have a particular knowledge base in medicinal chemistry, pathology, and bioengineering technology to create an effective drug-delivery system.
The transition points of SME in thermoresponsive materials can be adjusted to match physiological temperatures for targeted drug release. Porous polymers, with their lightweight and extensive surface area, serve as effective drug carriers. Moisture-responsive polymers activated by bodily fluids offer another research direction. Hydrogels can encapsulate drugs, antibodies, and biological elements for drug delivery.113 Vehse et al. developed PEGDA scaffolds using microstereolithography, incorporating acetylsalicylic acid before printing.114 The drug remained stable under UV light, but the polymer chain network was disrupted, reducing the structure's compressive strength. Gioumouxouzis et al.12 identified PLA, PVA, and polyacrylics as suitable biocompatible shape-memory polymers for drug-delivery systems.115
10.5. Splints
For biomedical devices, splints are a crucial component because the device's fit on the subject is necessary for the device's proper functioning. Traditional biomedical devices, especially when large, are often unable to adapt to a subject's growth over time. 4D printing can accommodate shape changes based on the subject's growth. In several infant patients with tracheobronchomegaly (TBM), Morrison et al. successfully implanted 4D-printed external airway splints during surgery.116 TBM is a life-threatening disorder that causes excessive collapse of the patient's airway during breathing. The splint is designed to fit the expansion of the airway, preventing uncontrolled compression. Miao et al. 4D-printed (using stereolithography) a nerve guidance conduit (NGC) that performed a variety of responsibilities for the regeneration of nerve tissue, utilising a natural photocrosslinking monomer (soybean oil epoxidised acrylate, SOEA). Due to the NGC's shape memory property, it is easy to readjust (through thermal stimulation) it during implantation.9110.6. Minimal invasive surgeries
4D printed structures are desirable for personalised medical devices because of their shape-memory characteristics. Printed surgical implants would enable minimally invasive procedures and speed up patient recovery because they can be deformed into a small temporary shape before being implanted into the body to function in an unreachable area.117 4D printed structures can be locally activated by a patient's body fluid, pH, or temperature or triggered by external stimuli (e.g. magnetic power or electric charge). Kashyap et al. printed radio-opaque and porous semi-crystalline thermoplastic shape memory polyurethane (SMPU) for use in endovascular embolization (EE).118 EE is an invasive surgical procedure for treating abnormal blood vessels in the brain and other body parts by blocking blood flow.119To realize the full potential of the technique, more research into the biomedical uses of porous SMP-printed scaffolds is necessary.11 Porous SMP foams have better advantages in this industry due to their larger volumetric extension, lightweight, and higher surface area compared to conventional solid SMPs. According to Miao et al., indirect doping materials can be used to design thermo-responsive SMPs that react to the physiological temperature (≈37 °C).91 Yang et al. invented a surgical procedure for improved conduit implantation that involves momentarily opening and closing the initially closed conduit.97
Implementing 4D printing may reduce the risk of difficult surgeries because minimally invasive implantable devices have smaller incisions and faster recovery times. The overall improvement in operation and surgical complications would enhance the patient experience in general.
10.7. AI-based 4D printing
AI-driven 4D printing presents an innovative solution to the complex challenges associated with modeling and controlling 4D-printed robots. As these robots become increasingly sophisticated and capable, the demand for advanced tools to manage their complexity grows. AI techniques have emerged as promising strategies to tackle these challenges.120AI technologies are employed to train models using labeled data to predict robot performance and enable these robots to develop optimal control strategies through interaction with their environments (Fig. 11). The challenges inherent in 4D printing include highly nonlinear and time-variant dynamics, morphing hysteresis, and the multi-domain nature of 4D-printed robots.
 | ||
| Fig. 11 Analysis of forward and inverse challenges in 3D/4D printing with machine learning and artificial intelligence. | ||
Supervised learning, a branch of AI, is utilized to model the intricate dynamics of 4D printing, thereby reducing the need for extensive physics analyses.15 Reinforcement learning is particularly well-suited for closed-loop control tasks, facilitating autonomous policy training and online learning to accommodate time-variant properties. Additionally, some research focuses on leveraging AI to fine-tune parameters in controller development.
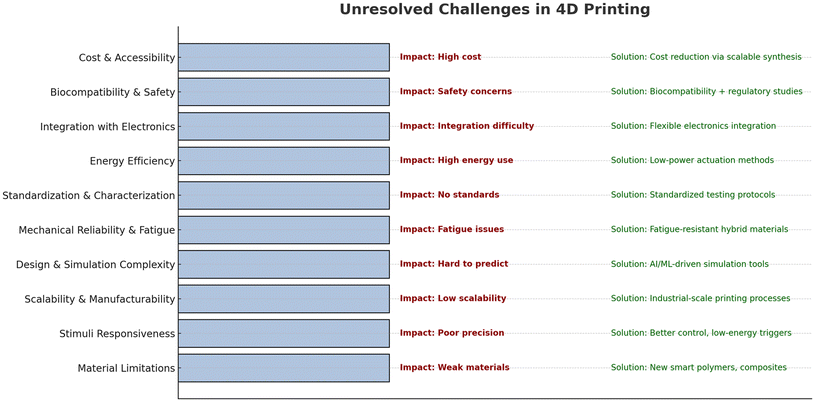
11. Challenges of 4D printing
The future of 4D printing is promising, but there are challenges related to materials, design, and technology. One significant challenge is the limited availability of suitable AM printing technologies for 4D printing. The current technologies have drawbacks, such as high equipment costs and limited material choices. Additional research is needed to address these challenges and improve the performance of 4D printing (Fig. 12).121Innovative structures must have the ability to dynamically adjust to the environment and the ability to sense and respond to environmental variations.122 For example, soft robots require membranes that can deform before the actuator operates, but current design and 3D-printing technologies do not directly support this. More research on smart structure design is needed to address these challenges and advance 4D printing, which has the potential to revolutionize production rates in many industries.
Some of the problems with 4D printing include a lack of material for certain uses, delayed actuation, and inaccurate control during the intermediate deformation stages. To further enhance the adaptability and practicality of 4D printing, future research should investigate dependable supplementary approaches for applying stimuli and comprehending the effects of structural patterns and restorations.123,124
4D printing has many problems, such as its inability to handle complicated things, the lack of printers that can print with several materials, high cost, long printing time, and limited dependability. Stimuli-responsive materials are restricted to specific environmental conditions, leading to varied response times and limited size and accuracy in the spatial manipulation of 4D-printed structures. Therefore, new, easily tunable materials must be developed for better adaptability to complex microenvironments.
The challenges of advanced simulation and topological transformation in manufacturing, as well as material constraints, are addressed using 4D-printing software. Chung et al. examined various 4D-printing software. They analysed the limitations of six software programs designed to fully accommodate the key stages of the 4D printing process.125 These software solutions are designed to perform specific functions at each stage of the 4D printing process, collectively providing all the necessary operations for 4D printing. Commercial software and mathematical models are currently used, but further development is needed to cater to all responsive materials for 4D-printing applications. Additionally, hardware and software development must consider ease of operation and multitasking features.
To advance 4D printing innovation, rational computer design of stimuli-responsive processes is essential for fabricating complex objects in self-transformation, robotics, and bioengineering applications.126 Minimal activation of mechanical deformation occurs in stimuli-responsive materials through heat and swelling. Cui et al. emphasized the integration of nanomaterials into stimuli-responsive polymers to achieve dynamic and remote-controlled shape transformations.127
It is also crucial to evaluate and address the ethical dimensions of 4D printing in healthcare, particularly with regard to personalized medicine. Dynamic, adaptive technologies require robust informed consent procedures, ensuring that patients fully understand potential risks and long-term effects. Furthermore, equitable access to these advanced medical solutions must be prioritized, with policies designed to promote both affordability and widespread availability.
By developing new standards, fostering cross-disciplinary collaboration, and implementing pilot programs, regulatory bodies and stakeholders can create practical pathways for the safe integration of 4D-printed medical devices in clinical practice, ultimately benefiting patients through increased innovation and customization.
12. Future prospective of 4D printing
Future work will focus on realising 4D-printed parts that eliminate the need for mechanical load. New 4D-printing software is required for different types of 4D-printing techniques. There is a need to develop highly customizable materials that undergo appropriate deformation in response to various external stimuli.128,129 The form of printed components is one area where 4D printing trends may shift as the technology evolves. The use of SMPs raises the bar for what can be done to make current 3D printers compliant. The reversible shape-changing effect of a multi-material layered composite has been investigated recently. The materials used in this composite exhibit diverse responses to stimuli, enabling the composite to transition between stable and rigid forms without mechanical stress. The design includes a hydrogel, elastomers, and a layer of thermoresponsive SMP.Bingcong Jian et al. discussed the future applications of two-photon polymerisation (TPP)-based 4D printing. TPP can be utilised in biomedical microrobots, and future applications may involve more advanced targeted drug-delivery systems capable of navigating complex biological environments to release medications with unprecedented precision. The ability to create intricate 3D structures at the micro-and nanoscale could lead to significant advancements in tissue engineering, potentially allowing for the development of complex, functional tissue structures at the cellular level. Additionally, the shape-changing capabilities of 4D-printed structures could be further developed to create self-repairing materials on the micro and nanoscale.130
Other novel works involve parts capable of self-assembly and disassembly, such as a multi-polymer 3D-printed trestle design with four equal, active composite strips connected to the centre. SMPs come in various types, each with distinct transformation temperatures, moduli, biocompatibility, and quality, making them suitable for diverse applications. The development of new materials can lead to innovations in printing technologies, deformation behaviours, and constitutive models.131 The additive manufacturing industry is still growing, and the ongoing exploration of new materials and techniques has led to significant advancements in 4D printing.132 This new technology provides feasible manufacturing processes for small-scale structures.
4D-printed products have potential applications in the human body, such as wearable sensors, artificial muscles, and implantable biomedical devices.133–135 The 4D printing market has witnessed the development of new eco-friendly products and partnerships that promote efficient resource use and sustainable consumption. Future research should explore the generation, storage, and use of passive, abundant energy sources to activate shape memory or stimuli-responsive materials.
Regulatory challenges present substantial hurdles to the advancement and adoption of 4D-printed medical devices, largely because existing approval frameworks do not account for the unique qualities of these technologies. Unlike conventional devices, which are static and unchanging after placement, 4D-printed devices are engineered with materials that can actively transform in response to environmental cues. For example, material transformation properties refer to the ability of certain materials to change shape, structure, or function when stimulated by factors, such as temperature, pH levels, or moisture, within the body. These dynamic characteristics mean that a 4D-printed stent—such as one designed to expand in response to body heat—does not behave like a traditional stent. Its adaptive nature complicates standard safety assessments, which typically rely on predictable, fixed device performance.
Current regulatory systems, including the FDA and European Medicines Agency (EMA), are tailored to evaluate static devices and lack protocols for assessing devices whose form and function may shift after implantation. This gap introduces complexity in determining the long-term safety and efficacy of 4D-printed devices. Regulatory bodies must therefore develop new standards that specifically address the unique behaviors of materials used in 4D printing. This includes creating testing protocols that account for material transformation properties, ensuring that devices remain safe and effective as they adapt over time within the human body.
The approval process is further complicated by limited long-term data for these innovative materials and by the unpredictable ways they may interact with biological systems. For instance, the performance of a temperature-responsive stent may vary depending on individual patient physiology, making it challenging to establish universal safety benchmarks. The personalized nature of 4D-printed devices also raises issues for standardization and mass approval, as each device may be tailored to the needs of a specific patient.
To address these challenges, regulatory agencies should consider launching pilot programs that enable controlled clinical testing of adaptive devices. These programs would generate valuable data on device performance and inform future approval processes. Additionally, agencies could form interdisciplinary committees composed of engineers, clinicians, regulatory experts, and patient advocates. These groups would be tasked with drafting preliminary guidelines and standards for the safe approval and monitoring of 4D-printed medical devices.
13. Conclusion
With the progression of additive manufacturing technologies and the expansion of our understanding of smart materials, accessible stimuli, mathematical modelling, and geometric programming, researchers have begun to investigate the potential of 4D printing as a new technology. This article offers a general introduction to 4D printing, touching on topics such as technology, design, and materials that should be considered when creating 4D-printing components. The use of smart materials for 4D printing has opened up new potential applications for this technology. Additional research is necessary before 4D printing can be considered a commercially viable option. One example is the requirement for computer-aided design software to integrate multi-scale physics simulations with the ability to visualise smart material attributes. The repeatability and appropriateness of the spacing between the voxels that represent the materials depend on the reliability of the fabrication equipment. Finally, new metrology equipment or other forms of measurement may need to be developed to ensure part quality. Further research is needed to ascertain commercial viability, including on computer-aided design, fabrication systems, and metrology equipment.Conflicts of interest
All authors declare that they have no conflict of interest.Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. Thus, no informed consent is applicable in this work.Abbreviations
| 1D | 1-Dimension |
| 2D | 2-Dimension |
| 3D | 3-Dimension |
| 4D | 4-Dimension |
| AM | Additive manufacturing |
| CAD | Computer-aided design |
| EA | Finite element analysis |
| ABS plastic | Acrylonitrile butadiene styrene |
| TPU | Thermal polyurethane |
| SLA | Stereolithography |
| LOM | Laminated object manufacturing |
| WAAM | Wire and arc additive manufacturing |
| FDM | Fused deposition modeling |
| SLS | Selective laser sintering |
| SLM | Selective laser melting |
| LMD | Laser metal deposition |
| PLA | Polylactic acid |
| Ti6Al4V powder | Titanium aluminum vanadium |
| NiTi | Nickel titanium |
| PCL | Poly(ε-caprolactone) |
| NFC | Nuclear fuel complex |
| SMA | Shape memory alloys |
| SMP | Shape memory polymers |
| SMH | Shape memory hybrids |
| SMC | Shape memory ceramics |
| SMG | Shape memory gels |
| SMMs | Shape memory materials |
| SME | Shape memory effect |
| IPN | Interpenetrating polymer network |
| PEG | Polyethylene glycol |
| NIR | Near infrared rays |
| PDA | Polydopamine |
| EMC | Extracellular matrix components |
| HUVEC | Human umbilical vein endothelial cells |
| PMAA | Poly(methacrylic acid) |
| PHEMA | Poly(hydroxyethyl methacrylate) |
| DLP | Digital light processing |
| TBM | Tracheobronchomegaly |
| SMPU | Shape memory polyurethane |
| EE | Endovascular embolization |
| NGC | Nerve guidance conduit |
| SOEA | Soybean oil epoxidized acrylate |
| MRE | Magnetorheological elastomer |
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.References
- B. C. Gross, J. L. Erkal, S. Y. Lockwood, C. Chen and D. M. Spence, Anal. Chem., 2014, 86, 3240–3253 CrossRef PubMed.
- J. Norman, R. D. Madurawe, C. M. V. Moore, M. A. Khan and A. Khairuzzaman, Adv. Drug Delivery Rev., 2017 DOI:10.1016/j.addr.2016.03.001.
- Z. Zhang, K. G. Demir and G. X. Gu, Int. J. Smart Nano Mater., 2019, 10, 205–224 CrossRef.
- E. Pei and G. H. Loh, Prog. Addit. Manuf., 2018 DOI:10.1007/s40964-018-0047-1.
- W. Zhou, Z. Qiao, E. N. Zare, J. Huang, X. Zheng, X. Sun, M. Shao, H. Wang, X. Wang, D. Chen, J. Zheng, S. Fang, Y. M. Li, X. Zhang, L. Yang, P. Makvandi and A. Wu, J. Med. Chem., 2020 DOI:10.1021/acs.jmedchem.9b02115.
- T. A. Campbell, S. Tibbits and B. Garrett, Sci. Am., 2014, 311, 60–65 CrossRef PubMed.
- A. Roy, M. S. Hossain, A. Bhowmick, N. Nizhum, S. K. Mondal and T. Saha, Pharmacologyonline, 2020, 3, 292–302 Search PubMed.
- J. Choi, O. C. Kwon, W. Jo, H. J. Lee and M. W. Moon, 3D Print. Addit. Manuf., 2015 DOI:10.1089/3dp.2015.0039.
- M. Javaid and A. Haleem, Clin. Epidemiol. Global Health, 2019, 7, 317–321 CrossRef.
- S. Joshi, K. Rawat, C. Karunakaran, V. Rajamohan, A. T. Mathew, K. Koziol, V. K. Thakur and A. S. S. Balan, Appl. Mater. Today, 2020, 18 DOI:10.1016/j.apmt.2019.100490.
- J. J. Wu, L. M. Huang, Q. Zhao and T. Xie, Chin. J. Polym. Sci., 2018 DOI:10.1007/s10118-018-2089-8.
- S. Ramesh, S. K. Reddy, C. Usha, N. K. Naulakha, C. R. Adithyakumar and M. L. K. Reddy, in IOP Conference Series: Materials Science and Engineering, Institute of Physics Publishing, 2018, vol. 376.
- S. Tibbits, Archit. Des., 2014, 84, 116–121 Search PubMed.
- K. Yu, A. Ritchie, Y. Mao, M. L. Dunn and H. J. Qi, in Procedia IUTAM, Elsevier, 2015, vol. 12, pp. 193–203 Search PubMed.
- R. Pugliese and S. Regondi, Polymers (Basel)., 2022, 14(14), 2794 CrossRef PubMed.
- A. Kantaros, F. I. T. Petrescu and T. Ganetsos, Biomimetics, 2025, 10, 125 CrossRef PubMed.
- D. Gurung, Technological comparison of 3D and 4D printing : Analytical study, Arcada University of Applied Sciences, 2017 Search PubMed.
- F. Momeni and J. Ni, Engineering, 2020, 6, 1035–1055 CrossRef.
- J. Choi, O.-C. Kwon, W. Jo, H. J. Lee and M.-W. Moon, 3D Print. Addit. Manuf., 2015, 2, 159–167 CrossRef.
- Q. Ge, A. H. Sakhaei, H. Lee, C. K. Dunn, N. X. Fang and M. L. Dunn, Sci. Rep., 2016, 6, 31110 CrossRef PubMed.
- X. Wang, J. Liu, Y. Zhang, P. M. Kristiansen, A. Islam, M. Gilchrist and N. Zhang, Taylor and Francis Ltd., 2023, DOI:10.1080/17452759.2023.2248101, preprint.
- A. Kafle, E. Luis, R. Silwal, H. M. Pan, P. L. Shrestha and A. K. Bastola, Polymers (Basel)., 2021 DOI:10.3390/polym13183101.
- Q. Ge, C. K. Dunn, H. J. Qi and M. L. Dunn, Smart Mater. Struct., 2014, 23 DOI:10.1088/0964-1726/23/9/094007.
- L. Zhou, J. Miller, J. Vezza, M. Mayster, M. Raffay, Q. Justice, Z. Al Tamimi, G. Hansotte, L. D. Sunkara and J. Bernat, Sensors (Basel)., 2024, 24, 2668 CrossRef PubMed.
- G. F. Hu, A. R. Damanpack, M. Bodaghi and W. H. Liao, Smart Mater. Struct., 2017, 26 DOI:10.1088/1361-665X/aa95ec.
- M. Y. Razzaq, J. Gonzalez-Gutierrez, G. Mertz, D. Ruch, D. F. Schmidt and S. Westermann, Appl. Sci., 2022, DOI:10.3390/app12157880.
- D. Saritha and D. Boyina, in Materials Today: Proceedings, Elsevier Ltd, 2021, vol. 46, pp. 692–695 Search PubMed.
- A. Baig, R. Barse, A. Paryekar and V. Jagtap, Intell. Pharm., 2024 DOI:10.1016/j.ipha.2024.05.008.
- N. A. Charoo, S. F. B. Ali, E. M. Mohamed, M. A. Kuttolamadom, T. Ozkan, M. A. Khan and Z. Rahman, Drug Dev. Ind. Pharm., 2020 DOI:10.1080/03639045.2020.1764027.
- M. Vaezi, S. Chianrabutra, B. Mellor and S. Yang, Virtual Phys. Prototyping, 2013, 8, 19–50 CrossRef.
- J. Firth, S. Gaisford and A. W. Basit, in 3D Printing of Pharmaceuticals, ed. A. W. Basit and S. Gaisford, Springer International Publishing, Cham, 2018, pp. 153–162 Search PubMed.
- D.-G. Shin, T.-H. Kim and D.-E. Kim, Int. J. Precis. Eng. Manuf.-Green Technol., 2017, 4, 349–357 CrossRef.
- C. Liu, H. Qin and P. T. Mather, J. Mater. Chem., 2007, 17, 1543–1558 RSC.
- K. Mirasadi, D. Rahmatabadi, I. Ghasemi, M. Khodaei, M. Baniassadi and M. Baghani, Macromol. Mater. Eng., 2024, 309(6) DOI:10.1002/mame.202400038.
- E. Pei and G. H. Loh, Prog. Addit. Manuf., 2018 DOI:10.1007/s40964-018-0047-1.
- S. Chen, J. Hu, H. Zhuo and Y. Zhu, Mater. Lett., 2008, 62, 4088–4090 CrossRef CAS.
- S. Erkeçoğlu, A. D. Sezer and S. Bucak, Smart Drug Delivery Syst., 2016, 80, 1–29 Search PubMed.
- I. Bellin, S. Kelch, R. Langer and A. Lendlein, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 18043–18047 CrossRef CAS.
- X. Li, Y. Zhu, Y. Dong, M. Liu, Q. Ni and Y. Fu, J. Nanomater., 2015, 2015, 2 Search PubMed.
- Q. Ge, A. H. Sakhaei, H. Lee, C. K. Dunn, N. X. Fang and M. L. Dunn, Sci. Rep., 2016, 6, 31110 CrossRef.
- M. D. Hager, S. Bode, C. Weber and U. S. Schubert, Prog. Polym. Sci., 2015, 49–50, 3–33 CrossRef CAS.
- F. Wang, C. Yuan, D. Wang, D. W. Rosen and Q. Ge, Smart Mater. Struct., 2020, 29, 055016 CrossRef CAS.
- Y. S. Zhang, K. Yue, J. Aleman, K. Mollazadeh-Moghaddam, S. M. Bakht, J. Yang, W. Jia, V. Dell'Erba, P. Assawes, S. R. Shin, M. R. Dokmeci, R. Oklu and A. Khademhosseini, Ann. Biomed. Eng., 2017, 45, 148–163 CrossRef.
- A. S. Gladman, E. A. Matsumoto, R. G. Nuzzo, L. Mahadevan and J. A. Lewis, Nat. Mater., 2016, 15, 413–418 CrossRef.
- M. C. Mulakkal, R. S. Trask, V. P. Ting and A. M. Seddon, Mater. Des., 2018, 160, 108–118 CrossRef.
- G. Villar, A. D. Graham and H. Bayley, Science, 2013, 340, 48–52 CrossRef.
- J.-Y. Sun, X. Zhao, W. R. K. Illeperuma, O. Chaudhuri, K. H. Oh, D. J. Mooney, J. J. Vlassak and Z. Suo, Nature, 2012, 489, 133–136 CrossRef.
- S. Hong, D. Sycks, H. F. Chan, S. Lin, G. P. Lopez, F. Guilak, K. W. Leong and X. Zhao, Adv. Mater., 2015, 27, 4035–4040 CrossRef.
- X. Kuang, D. J. Roach, J. Wu, C. M. Hamel, Z. Ding, T. Wang, M. L. Dunn and H. J. Qi, Adv. Funct. Mater., 2019, 29, 1805290 CrossRef.
- Y. Liu, B. Shaw, M. D. Dickey and J. Genzer, Sci. Adv., 2023, 3, e1602417 CrossRef PubMed.
- O. Kuksenok and A. C. Balazs, Mater. Horiz., 2016, 3, 53–62 RSC.
- X. Wan, L. Luo, Y. Liu and J. Leng, Adv. Sci., 2020, 7, 2001000 CrossRef.
- H. Y. Jeong, B. H. Woo, N. Kim and Y. C. Jun, Sci. Rep., 2020, 10, 6258 CrossRef.
- A. Miriyev, K. Stack and H. Lipson, Nat. Commun., 2017, 8, 596 CrossRef.
- H. Okuzaki, T. Kuwabara, K. Funasaka and T. Saido, Adv. Funct. Mater., 2013, 23, 4400–4407 CrossRef.
- J. C. Breger, C. Yoon, R. Xiao, H. R. Kwag, M. O. Wang, J. P. Fisher, T. D. Nguyen and D. H. Gracias, ACS Appl. Mater. Interfaces, 2015, 7, 3398–3405 CrossRef.
- R. Mohr, K. Kratz, T. Weigel, M. Lucka-Gabor, M. Moneke and A. Lendlein, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 3540–3545 CrossRef.
- T. Li, G. Li, Y. Liang, T. Cheng, J. Dai, X. Yang, B. Liu, Z. Zeng, Z. Huang, Y. Luo, T. Xie and W. Yang, Sci. Adv., 2023, 3, e1602045 CrossRef.
- M. L. Dezaki and M. Bodaghi, Sens. Actuators, A, 2023, 349 DOI:10.1016/j.sna.2022.114063.
- L. T. de Haan, J. M. N. Verjans, D. J. Broer, C. W. M. Bastiaansen and A. P. H. J. Schenning, J. Am. Chem. Soc., 2014, 136, 10585–10588 CrossRef PubMed.
- M. Dai, O. T. Picot, J. M. N. Verjans, L. T. de Haan, A. P. H. J. Schenning, T. Peijs and C. W. M. Bastiaansen, ACS Appl. Mater. Interfaces, 2013, 5, 4945–4950 CrossRef.
- S. Dutta and D. Cohn, J. Mater. Chem. B, 2017, 5, 9514–9521 RSC.
- G. Stoychev, N. Puretskiy and L. Ionov, Soft Matter, 2011, 7, 3277–3279 RSC.
- L. Klouda and A. G. Mikos, Eur. J. Pharm. Biopharm., 2008, 68, 34–45 CrossRef.
- F. D. Jochum and P. Theato, Chem. Soc. Rev., 2013, 42, 7468–7483 RSC.
- X. Jiang, C. A. Lavender, J. W. Woodcock and B. Zhao, Macromolecules, 2008, 41, 2632–2643 CrossRef.
- I. Roppolo, A. Chiappone, A. Angelini, S. Stassi, F. Frascella, C. F. Pirri, C. Ricciardi and E. Descrovi, Mater. Horiz., 2017, 4, 396–401 RSC.
- H. Yang, W. R. Leow, T. Wang, J. Wang, J. Yu, K. He, D. Qi, C. Wan and X. Chen, Adv. Mater., 2017, 29, 1701627 CrossRef.
- B. Guo, Y. Sun, A. Finne-Wistrand, K. Mustafa and A.-C. Albertsson, Acta Biomater., 2012, 8, 144–153 CrossRef.
- S. Sayyar, M. Bjorninen, S. Haimi, S. Miettinen, K. Gilmore, D. Grijpma and G. Wallace, ACS Appl. Mater. Interfaces, 2016, 8, 31916–31925 CrossRef.
- P. Zhu, W. Yang, R. Wang, S. Gao, B. Li and Q. Li, ACS Appl. Mater. Interfaces, 2018, 10, 36435–36442 CrossRef.
- M. Boncheva, S. A. Andreev, L. Mahadevan, A. Winkleman, D. R. Reichman, M. G. Prentiss, S. Whitesides and G. M. Whitesides, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 3924–3929 CrossRef PubMed.
- M. Zrínyi, L. Barsi and A. Büki, J. Chem. Phys., 1996, 104, 8750–8756 CrossRef.
- J. H. C. Wang and J.-S. Lin, Biomech. Model. Mechanobiol., 2007, 6, 361–371 CrossRef PubMed.
- K. Kuribayashi-Shigetomi, H. Onoe and S. Takeuchi, in 2012 IEEE 25th International Conference on Micro Electro Mechanical Systems (MEMS), IEEE, 2012, pp. 72–75.
- A. Kantaros, T. Ganetsos and D. Piromalis, J. Mechatron. Rob., 2023, 7, 1–14 CrossRef.
- A. S. Gladman, E. A. Matsumoto, R. G. Nuzzo, L. Mahadevan and J. A. Lewis, Nat. Mater., 2016, 15, 413–418 CrossRef.
- E. Yarali, M. J. Mirzaali, A. Ghalayaniesfahani, A. Accardo, P. J. Diaz-Payno and A. A. Zadpoor, Adv. Mater., 2024 DOI:10.1002/adma.202402301.
- J. Wang, G. Bai and X. Kong, in New Trends in Mechanism and Machine Science: Theory and Industrial Applications, Springer, 2017, pp. 503–510 Search PubMed.
- P. Suryavanshi, S. Lekurwale, P. Kumar, S. K. Dwivedy and S. Banerjee, Rapid Prototyping J., 2025, 31, 1053–1064 CrossRef.
- S. Shakibania, L. Ghazanfari, M. Raeeszadeh-Sarmazdeh and M. Khakbiz, Drug Dev. Ind. Pharm., 2021 DOI:10.1080/03639045.2020.1862179.
- Z. X. Khoo, J. E. M. Teoh, Y. Liu, C. K. Chua, S. Yang, J. An, K. F. Leong and W. Y. Yeong, Virtual Phys. Prototyping, 2015, 10, 103–122 CrossRef.
- J. Rouwkema, N. C. Rivron and C. A. van Blitterswijk, Trends Biotechnol., 2008, 26, 434–441 CrossRef PubMed.
- R. K. Jain, P. Au, J. Tam, D. G. Duda and D. Fukumura, Nat. Biotechnol., 2005, 23, 821–823 CrossRef PubMed.
- C. Norotte, F. S. Marga, L. E. Niklason and G. Forgacs, Biomaterials, 2009, 30, 5910–5917 CrossRef.
- W. L. Ng, S. Wang, W. Y. Yeong and M. W. Naing, Trends Biotechnol, 2016 DOI:10.1016/j.tibtech.2016.04.006.
- K. Kuribayashi-Shigetomi, H. Onoe and S. Takeuchi, PLoS One, 2012, 7, e51085 CrossRef PubMed.
- S. Hong, S.-J. Song, J. Y. Lee, H. Jang, J. Choi, K. Sun and Y. Park, J. Biosci. Bioeng., 2013, 116, 224–230 CrossRef PubMed.
- A. Sadraei and S. M. Naghib, Polym. Rev., 2025, 65, 104–168 CrossRef.
- V. A. B. Quiñones, H. Zhu, A. A. Solovev, Y. Mei and D. H. Gracias, Adv. Biosyst., 2018, 2, 1800230 CrossRef.
- S. Miao, W. Zhu, N. J. Castro, M. Nowicki, X. Zhou, H. Cui, J. P. Fisher and L. G. Zhang, Sci. Rep., 2016, 6, 27226 CrossRef PubMed.
- S. Miao, H. Cui, M. Nowicki, L. Xia, X. Zhou, S.-J. Lee, W. Zhu, K. Sarkar, Z. Zhang and L. G. Zhang, Adv. Biosyst., 2018, 2, 1800101 CrossRef PubMed.
- S. Joshi, K. Rawat, C. Karunakaran, V. Rajamohan, A. T. Mathew, K. Koziol, V. K. Thakur and A. S. S. Balan, Appl. Mater. Today, 2020, 18 DOI:10.1016/j.apmt.2019.100490.
- N. B. Morgan, Mater. Sci. Eng., A, 2004, 378, 16–23 CrossRef.
- T. Duerig, A. Pelton and D. Stöckel, Mater. Sci. Eng., A, 1999, 273–275, 149–160 CrossRef.
- H. Chu, W. Yang, L. Sun, S. Cai, R. Yang, W. Liang, H. Yu and L. Liu, Micromachines, 2020 DOI:10.3390/MI11090796.
- Q. Yang, B. Gao and F. Xu, Biotechnol. J., 2020 DOI:10.1002/biot.201900086.
- A. W. Martinez and E. L. Chaikof, WIREs Nanomed. Nanobiotechnol., 2011, 3, 256–268 CrossRef CAS.
- Z. Zhang, K. G. Demir and G. X. Gu, Int. J. Smart Nano Mater., 2019, 10, 205–224 CrossRef.
- M. Bodaghi, A. R. Damanpack and W. H. Liao, Smart Mater. Struct., 2016, 25, 105034 CrossRef.
- M. Zarek, N. Mansour, S. Shapira and D. Cohn, Macromol. Rapid Commun., 2017, 38, 1600628 CrossRef.
- G. M. Whitesides, Nature, 2006, 442, 368–373 CrossRef CAS.
- A. Sontheimer-Phelps, B. A. Hassell and D. E. Ingber, Nat. Rev. Cancer, 2019, 19, 65–81 CrossRef CAS PubMed.
- M. López-Valdeolivas, D. Liu, D. J. Broer and C. Sánchez-Somolinos, Macromol. Rapid Commun., 2018, 39(5) DOI:10.1002/marc.201700710.
- L. D'Eramo, B. Chollet, M. Leman, E. Martwong, M. Li, H. Geisler, J. Dupire, M. Kerdraon, C. Vergne, F. Monti, Y. Tran and P. Tabeling, Microsyst. Nanoeng., 2018, 4, 17069 CrossRef.
- D. J. Beebe, G. A. Mensing and G. M. Walker, Annu. Rev. Biomed. Eng., 2002, 4, 261–286 CrossRef CAS.
- C.-H. Zhu, Y. Lu, J. Peng, J.-F. Chen and S.-H. Yu, Adv. Funct. Mater., 2012, 22, 4017–4022 CrossRef CAS.
- R. Edri, I. Gal, N. Noor, T. Harel, S. Fleischer, N. Adadi, O. Green, D. Shabat, L. Heller, A. Shapira, I. Gat-Viks, D. Peer and T. Dvir, Adv. Mater., 2019, 31, 1803895 CrossRef.
- H. He, J. Guan and J. L. Lee, J. Controlled Release, 2006, 110, 339–346 CrossRef CAS PubMed.
- W. Dai, H. Guo, B. Gao, M. Ruan, L. Xu, J. Wu, T. B. Kirk, J. Xu, D. Ma and W. Xue, Chem. Eng. J., 2019, 356, 934–949 CrossRef CAS.
- L. Larush, I. Kaner, A. Fluksman, A. Tamsut, A. A. Pawar, P. Lesnovski, O. Benny and S. Magdassi, J. 3D Print. Med., 2017, 1, 219–229 CrossRef.
- B. Mirani, E. Pagan, B. Currie, M. A. Siddiqui, R. Hosseinzadeh, P. Mostafalu, Y. S. Zhang, A. Ghahary and M. Akbari, Adv. Healthcare Mater., 2017, 6, 1700718 CrossRef.
- P. Kumar, P. Suryavanshi, S. K. Dwivedy and S. Banerjee, J. Mol. Liq., 2024, 410 DOI:10.1016/j.molliq.2024.125553.
- M. Vehse, S. Petersen, K. Sternberg, K. Schmitz and H. Seitz, Macromol. Symp., 2014, 346, 43–47 CrossRef CAS.
- C. I. Gioumouxouzis, O. L. Katsamenis, N. Bouropoulos and D. G. Fatouros, J. Drug Delivery Sci. Technol., 2017, 40, 164–171 CrossRef CAS.
- R. J. Morrison, S. J. Hollister, M. F. Niedner, M. G. Mahani, A. H. Park, D. K. Mehta, R. G. Ohye and G. E. Green, Sci. Transl. Med., 2015, 7, 285ra64–285ra64 Search PubMed.
- J. E. M. Teoh, J. An, C. K. Chua, M. Lv, V. Krishnasamy and Y. Liu, Virtual Phys. Prototyping, 2017, 12, 61–68 CrossRef.
- D. Kashyap, P. K. Kumar and S. Kanagaraj, Addit. Manuf., 2018, 24, 687–695 CAS.
- J. Thornton, Z. Dovey, A. Alazzaz, M. Misra, V. A. Aletich, G. M. Debrun, J. I. Ausman and F. T. Charbel, Surg. Neurol., 2000, 54(5), 352–360 CrossRef CAS.
- M. Bodaghi, L. Wang, F. Zhang, Y. Liu, J. Leng, R. Xing, M. D. Dickey, S. Vanaei, M. Elahinia, S. V. Hoa, D. Zhang, K. Winands, T. Gries, S. Zaman, H. Soleimanzadeh, T. B. Palmić, J. Slavič, Y. Tadesse, Q. Ji, C. Zhao, L. Feng, K. Ahmed, M. N. I. Shiblee, L. Zeenat, F. Pati, L. Ionov, A. Chinnakorn, W. Nuansing, A. M. Sousa, J. Henriques, A. P. Piedade, E. Blasco, H. Li, B. Jian, Q. Ge, F. Demoly, H. J. Qi, J.-C. André, M. Nafea, Y.-F. Fu, B. Rolfe, Y. Tao, G. Wang and A. Zolfagharian, Smart Mater. Struct., 2024, 33 DOI:10.1088/1361-665x/ad5c22.
- Z. Zhang, K. G. Demir and G. X. Gu, Int. J. Smart Nano Mater., 2019, 10, 205–224 CrossRef.
- G. K. Tairidis, G. Foutsitzi and G. E. Stavroulakis, Approximation and Optimization: Algorithms, Complexity and Applications, 2019, pp. 185–217 Search PubMed.
- F. Momeni and J. Ni, Engineering, 2020, 6, 1035–1055 CrossRef.
- Y. S. Lui, W. T. Sow, L. P. Tan, Y. Wu, Y. Lai and H. Li, Acta Biomater., 2019, 92, 19–36 CrossRef CAS.
- S. Chung, S. E. Song and Y. T. Cho, Int. J. Precis. Eng. Manuf.-Green Technol., 2017, 4, 359–371 CrossRef.
- C. Sánchez-Somolinos, Mechanically responsive materials for soft robotics, 2020, pp. 347–362 Search PubMed.
- H. Cui, S. Miao, T. Esworthy, S. Lee, X. Zhou, S. Y. Hann, T. J. Webster, B. T. Harris and L. G. Zhang, Nano Res., 2019, 12, 1381–1388 CrossRef PubMed.
- Y.-H. Lin, T.-Y. Chuang, W.-H. Chiang, I.-W. P. Chen, K. Wang, M.-Y. Shie and Y.-W. Chen, Mater. Sci. Eng., C, 2019, 104, 109887 CrossRef CAS.
- I. Apsite, A. Biswas, Y. Li and L. Ionov, Adv. Funct. Mater., 2020, 30, 1908028 CrossRef CAS.
- B. Jian, H. Li, X. He, R. Wang, H. Y. Yang and Q. Ge, Int. J. Extrem. Manuf., 2024 DOI:10.1088/2631-7990/acfc03.
- J. Li, Z. Liang, T. Li, Q. Wan, X. Huang and Q. Kan, Int. J. Smart Nano Mater., 2025 DOI:10.1080/19475411.2025.2458833.
- M.-Y. Shie, Y.-F. Shen, S. D. Astuti, A. K.-X. Lee, S.-H. Lin, N. L. B. Dwijaksara and Y.-W. Chen, Polymers, 2019, 11, 1864 CrossRef CAS PubMed.
- Q. Ge, H. J. Qi and M. L. Dunn, Appl. Phys. Lett, 2013, 103 DOI:10.1063/1.4819837.
- D. J. Roach, C. M. Hamel, C. K. Dunn, M. V. Johnson, X. Kuang and H. J. Qi, Addit. Manuf., 2019, 29, 100819 Search PubMed.
- J. Ni, H. Ling, S. Zhang, Z. Wang, Z. Peng, C. Benyshek, R. Zan, A. K. Miri, Z. Li and X. Zhang, Mater. Today Bio, 2019, 3, 100024 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |