Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advancements in nano-based drug delivery systems for therapeutics: a comprehensive review
Joicy
John

Physics Department, Kuwait College of Science and Technology, Al-Asimah, 13133, Kuwait. E-mail: j.john@kcst.edu.kw
First published on 16th October 2025
Abstract
In contemporary medicine, nano-based drug delivery systems (NDDS) have become a ground-breaking strategy, offering notable improvements in the regulated and targeted release of medicinal drugs. These systems use nanotechnology to reduce adverse effects, increase therapeutic efficacy, and improve medication absorption. In order to achieve certain drug release patterns and improve patient outcomes, recent developments have focused on the creation and optimization of nanoparticles, liposomes, dendrimers, and micelles, among other materials. With an emphasis on advancements in materials, formulation techniques, and targeting mechanisms, this paper reviews current developments in NDDS. In addition to their challenges, such as toxicity, scaling issues, and regulatory barriers, the potential applications of NDDS in the future, such as gene therapy, customized medicine, and several drug delivery systems, are also reviewed. The development of nanotechnology for drug delivery has enormous potential to transform treatment paradigms in a number of therapeutic domains, such as infectious diseases, cancer, and chronic illnesses.
1 Introduction
Natural ingredients have been used in healing since ancient times. However, many of them are still failing clinical trial stages. Among the causes are significant difficulties associated with the use of large materials in the delivery of medications, such as in vivo instability, lack of target-specific delivery, poor absorption, low solubility and bioavailability, and likely harmful consequences of drugs. Thus, the use of novel drug delivery methods that target certain medications may be a solution to these pressing problems.1 Drug delivery methods have seen a revolution thanks to nanoparticles (NPs), which provide previously unheard of chances to enhance the potency, targeting, and controlled release of therapeutic agents. The pharmaceutical and medical sciences have seen a dramatic change with the discovery of nanoparticles as drug delivery vehicles. These particles, which are usually between 1 and 1000 nanometers in size, provide unmatched benefits in drug delivery systems, such as improved stability, targeted distribution, and controlled release.2 Nanoparticles have several advantages over conventional drug delivery techniques because of their distinct size, large surface area, and capacity to alter their chemical and physical characteristics. With their diameters ranging from 1 to 100 nanometers, NPs can be designed to encapsulate a broad range of medications, from biologics to small compounds, allowing precise control over drug release kinetics, increased bioavailability, and better therapeutic results.3 The precision provided by nanoparticle-based technologies makes it easier to create treatments that are suitable for particular diseases, reducing adverse effects and improving therapeutic results. Therefore, the constraints of conventional drug administration techniques may be addressed by nanoparticle drug delivery systems, particularly for complicated diseases such as cancer, viral infections, and genetic disorders.In the field of medication delivery, nanoparticles may be engineered to target certain tissues or cells, minimizing adverse effects and improving treatment selectivity.
This is especially true for targeted antibacterial medicines, gene delivery, and cancer therapy. Moreover, the incorporation of multifunctional nanoparticles presents the possibility of concurrent diagnosis and treatment, an approach called theranostics, making them indispensable instruments in customized medicine.4 Novel nano-based drug delivery systems (NDDS) seek to maximize medication absorption, reduce side effects, and improve therapeutic efficacy by using the special qualities of nanoscale materials. In order to improve patient outcomes, this review explores the latest advancements in the production and optimization of materials including nanoparticles, liposomes, dendrimers, and micelles. The problems that still exist in this area, such as toxicity, scalability, and regulatory barriers, are also discussed. The fascinating prospective applications of NDDS, such as gene therapy, tailored medication, and multidrug delivery systems, are also examined. The ongoing development of nanotechnology has the potential to completely alter therapeutic approaches in a number of therapeutic domains, such as cancer, infectious diseases, and chronic diseases. By incorporating a new dimension, such as a synthesis of recent clinical trials and regulatory approvals that highlight translational progress, this work offers an updated and thorough overview of nano-based drug delivery systems. This review provides a more current and forward-looking picture of the changing NDDS landscape by fusing developments in materials science with clinical validation.
2 Types of nano-based drug delivery systems
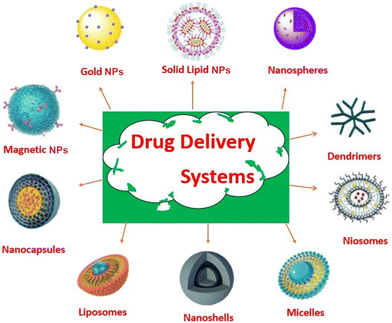
The term “NDDS” refers to a broad range of nanomaterials that have been created to enable the controlled and targeted release of medications. Due to their variations in composition, structure, and mode of action, these materials can be used in a variety of therapeutic contexts. Table 1 lists different types of nano-based drug delivery systems, along with their descriptions, advantages, challenges, and applications. Fig. 1 illustrates drug delivery systems for the diagnosis of various diseases.| Types of nano-based drug delivery systems | Description | Advantages | Challenges | Applications | Ref. |
|---|---|---|---|---|---|
| Nanoparticles | Solid, submicron-sized particles (1–1000 nm) used for drug encapsulation | High drug loading capacity, controlled release | Potential cytotoxicity and long-term safety concerns | Cancer therapy, gene therapy, protein delivery | 34 and 35 |
| Liposomes | Spherical vesicles composed of lipid bilayers that encapsulate drugs | Biocompatible, reduced drug toxicity, improved bioavailability | Drug leakage during storage or in vivo stability issues (fusion, oxidation of lipids) | Anticancer drugs, vaccines, antifungal drugs | 36 and 37 |
| Polymeric micelles | Amphiphilic copolymers that form nanometer-scale spherical structures in aqueous environments | Enhanced solubility of hydrophobic drugs, controlled release | Low drug loading for some hydrophobic drugs, instability upon dilution in blood | Chemotherapy, protein/peptide delivery | 38 and 39 |
| Dendrimers | Branched, tree-like macromolecules with functional surface groups for drug loading | High surface area, well-defined structure, controlled release | Cytotoxicity due to high surface charge (especially cationic), high cost of synthesis and purification | Gene delivery, drug targeting, imaging | 40 and 41 |
| Solid lipid nanoparticles (SLNs) | Lipid-based nanoparticles that are solid at room temperature and encapsulate drugs | Biodegradable, improved stability, controlled release | Low drug loading capacity (compared to polymers), aggregation tendency | Drug delivery, cosmetics, controlled release formulations | 42 and 43 |
| Nanoemulsions | Nano-sized emulsions (typically 20–200 nm) consisting of oil, water, and surfactants | High drug solubilization, enhanced bioavailability | Sensitivity to temperature and pH changes, limited drug loading capacity for hydrophilic drugs | Hydrophobic drug delivery, vaccines | 44 and 45 |
| Polymeric nanoparticles | Nanoparticles made of biocompatible polymers, which can load drugs and deliver them in a controlled manner | Versatile, biodegradability, tunable release profiles | Drug leakage or burst release, reduced circulation time due to clearance by the RES | Controlled release of chemotherapeutic agents, antibiotics | 46 and 47 |
| Nanocapsules | Nanoparticles with a core–shell structure where the drug is encapsulated inside a polymer shell | Protection of drugs from degradation, targeted delivery | Limited drug loading capacity for certain drugs, regulatory hurdles due to the lack of standardization | Targeted delivery | 48 and 49 |
| Gold nanoparticles | Metal nanoparticles (typically 1–100 nm) that are used to carry drugs or for targeted therapies | Easy functionalization, high surface area, targeting ability | Long-term accumulation and poor biodegradability, regulatory and safety concerns for clinical use | Cancer treatment, imaging, gene delivery | 50 and 51 |
| Carbon nanotubes | Cylindrical nanostructures made of carbon atoms, which can load drugs and penetrate cell membranes | High drug loading, stability, targeted drug delivery | Biocompatibility and toxicity concerns (especially pulmonary and hepatic toxicity), risk of accumulation in organs | Drug delivery, gene delivery, cancer therapy | 52 and 53 |
2.1 Nanoparticles
Nanoparticles are among the most widely studied types of NDDS. They can be composed of various materials, including lipids, polymers, metals, and ceramics, and can range in size from 1 to 1000 nanometers. Due to their small size, nanoparticles can easily penetrate biological barriers, such as the blood–brain barrier or tumor vasculature, and deliver drugs to targeted areas with high specificity.5 Moreover, nanoparticles can be engineered to release their payload in response to external stimuli, such as pH, temperature, or enzyme activity, enabling controlled release over time.2.2 Liposomes
A potential class of drug delivery methods, liposomes improve the safety and therapeutic effectiveness of a variety of pharmacological drugs. The Greek words “lipos”, which means fat, and “soma”, which means body, are the roots of the word “liposome”. This term refers to the body's structural elements, particularly phospholipid molecules.6 A mesomorphic structure composed of lipid, phospholipid, and water molecules is referred to as a liposome. To increase the effectiveness of medications, nutraceuticals, and other bioactive substances, liposomes primarily include phospholipid molecules that entrap and release lipid soluble, amphiphilic materials as well as water-soluble components in a regulated way.7 A liposome is a spherical vesicle that closely mimics cell membranes in structure and is made up of one or more phospholipid bilayers.8 Liposomes have become effective drug delivery vehicles due to their capacity to encapsulate hydrophilic or lipophilic medications.9 They are very helpful in the delivery of nucleic acids, antibiotics, and chemotherapeutic drugs. By halting premature medication release, liposomes can increase bioavailability, decrease toxicity, and improve drug solubility.10 Using the increased permeability and retention effect, liposome based methods efficiently deliver therapeutic molecules to the locations of the disease, improving treatment efficacy.11 By precisely directing molecules to affected cells or tissues, this particular administration reduces side effects and improves the effectiveness of treatment.12 PEGylation, or the addition of polyethylene glycol (PEG) chains to liposomes, was introduced in the 1990s and greatly enhanced the pharmacokinetics and biocompatibility of these liposomes. PEGylation and other surface changes can help lengthen the period of circulation of liposomes in the bloodstream, facilitating more effective drug delivery to the intended location. In liposomal formulations, polyethylene glycol, chitosan, and polydopamine are the most often utilized polymers.13 Liposomes’ efficacy in treating cancer has prompted much research, and their unique properties such as their high entrapment efficiency of active ingredients, accessibility, and scalability in production make them intriguing drug delivery systems (DDS). The quick release of the active ingredient and the capacity to alter its surface, however, restrict their use. Research into liposomal formulations for mRNA vaccines, such the Pfizer-BioNTech and Moderna vaccines, which use lipid nanoparticles (like liposomes) to transport mRNA, was pushed in 2020 by the COVID-19 pandemic. Lipid-based vesicles known as “fusogenic liposomes” are made to fuse with biological membranes in order to transport their contents straight into a target cell or organelle. Under specific circumstances (such as pH changes, the presence of particular ions, or temperature shifts), these liposomes can fuse with cell membranes because they usually contain particular lipid compositions or alterations. Fusogenic liposomes are extensively studied and used in gene therapy, vaccine development, and medication delivery systems. Fusogenic liposomes are useful instruments in pharmaceutical and medical research because they provide a regulated and focused way to distribute medicinal compounds.2.3 Solid lipid nanoparticles (SLNs)
Solid lipid nanoparticles (SLNs) are lipid nanoparticles made with a solid matrix. These are created by using solid lipids to create oil-in-water nanoemulsions. Early in the 1990s, the first SLN generations were established.14 Cheap raw ingredients, the use of physiological lipids, the avoidance of organic solvents, the simplicity of scale-up, good biocompatibility, increased bioavailability, protection of susceptible molds from environmental threats, and controlled drug release are some of the advantages of SLNs.15 Recently, SLNs loaded with ciprofloxacin (CIP) with potent antibacterial activity were created by ultrasonic melt emulsification.16 SLNs, which are made of solid lipids at body temperature, are more stable than liposomes, while still retaining their drug release and biocompatibility properties. Although their manufacturing scalability and surface modification for targeted delivery are still being studied, they offer great promise for controlled drug release over long periods of time. In comparison with a bromocriptine (BCR) solution, pharmacokinetic research showed that improved bromocriptine-loaded solid lipid nanoparticle (BCR-SLN) and bromocriptine-loaded nanostructured lipid carrier (BCR-NLC) formulations increase the drug's bioavailability in the plasma and brain. M. et al. reported that BCR-loaded lipid nanoparticles may be potential carriers as they can improve the drug's blood–brain barrier (BBB) penetration and contribute to the enhancement of BCR's bioavailability and therapeutic efficacy in the treatment of Parkinson's disease.172.4 Dendrimers
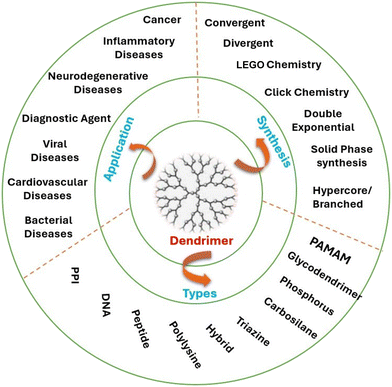
Dendrimers are highly branched tree-shaped macromolecules that have several benefits for drug delivery. By adhering to the ligands on their surface by straightforward ionic contact or chemical conjugation to the delivery system, dendrimers demonstrate the potential to transport pharmaceuticals to the target region via a passive mechanism by improving penetration retention and active targeting.18Their exact drug loading is made possible by their well-defined structure, and adding functional groups or targeting ligands to their surface is simple. Dendrimers are unique globular, hyper-branched, three-dimensional nano-polymeric structures. These are distinguished from other nanosystems by appealing characteristics such water solubility, nanoscaled size, narrow polydispersity indices, changeable molecular structure, internal cavity, and several peripheral functional groups. Drug conjugation and targeting are made possible by terminal functionality. Additionally, these peripheral functional groups provide them customized qualities that increase their adaptability.19 Polyamidoamine is the dendrimer that is most frequently studied for medication delivery.20 The amine group initiates its synthesis by interacting with methyl acrylate and helping to generate two new dendrimer branches that are terminated by esters.21 It is possible to create a “full-generation”, amine-terminated dendrimer by amidating the methyl ester with ethylene diamine later on. Small compounds, proteins, and nucleic acids are among the medicinal substances that have been delivered by dendrimers.22 They are perfect candidates for combination therapy because of their special design, which also enables controlled release and multiple-drug encapsulation. For transfection and bioimaging applications, CDs@PAMAM nanohybrids were created by self-assembling carbon dots (CDs) and G4–G6 PAMAM-NH2 dendrimers.23Fig. 2 shows various applications, synthesis methods and types of dendrimers.2.5 Micelles
Micelles are self-assembled nanoparticles that are created when amphiphilic molecules group together in aqueous fluids. Poorly water-soluble medications can be encapsulated within the hydrophobic core of micelles, increasing their stability and solubility.Micelles have been investigated for both passive and active targeting techniques and are especially helpful for the administration of hydrophobic medications, such as anticancer medicines. Ghezzi et al. reported that micelles are perfect for controlled release in certain conditions because of their capacity to react to variations in pH, temperature, or the presence of specific enzymes.24 By adding ligands on their surface, micelles may be made to target certain cells or tissues, increasing drug delivery accuracy and minimizing adverse effects. Micelles are most commonly administered via intravenous (i.v.) injection/infusion (mostly used for chemotherapy),25 although oral26 and topical (ocular, nasal, buccal)27 administration have also been shown to have highly intriguing outcomes in terms of enhanced drug bioavailability. Micelles reduce the systemic toxicity of medications by directing drug distribution to certain tissues or cells. This can lower the frequency of dosing and result in a longer-lasting therapeutic impact. Sun et al. reported that for the effective administration of topical ocular medications, PBA-CS-VE nanomicelles are a mucoadhesive option with improved transcorneal permeability and extended preocular retention.28 In order to support gene therapy, micelles can also be employed to transport nucleic acids, such as DNA or RNA. Micelles are useful for treating chronic illnesses with extended pharmacological activity because of their controlled release characteristics. It can be difficult to produce micelle-based medication delivery devices on a large scale. One important area of research is ensuring that micelles stay intact in the body until they arrive at their destination. Micelle-based formulations need to be thoroughly tested for safety and effectiveness, just like any other novel drug delivery method. Micelles are often used and recognized colloidal particles in drug administration, and bCN micelles constitute a flexible, biocompatible oral drug delivery platform. Use of micelles is a promising drug delivery method, and research is being done to improve their stability, functioning, and targeting abilities. Fig. 3 illustrates the efficiency of future multifunctional nanocarriers.
2.6 Polymeric nanoparticles
Usually, manmade or natural polymers make up polymeric nanoparticles. Poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), chitosan, and polyethylene glycol (PEG) are typical examples.29 Their usual diameters range from 10 nm to 1000 nm, which enable them to be both large enough to encapsulate a sizable quantity of medicine and tiny enough to pass through blood arteries and infiltrate tissues. While hydrophilic medications can be attached to the surface or integrated into the shell, hydrophobic pharmaceuticals can be enclosed within the core of the nanoparticle. It is possible to design polymers to regulate the encapsulated drug's release. This may occur as a result of drug diffusion over time or deterioration of the polymer matrix. These biodegradable polymer-based nanoparticles are becoming more and more popular due to their biocompatibility and adjustable drug release patterns. Selvam et al. reported that when paired with polyethylene glycol (PEG), hydroxyapatite (HAp) provides increased osteoblastic potential, fracture toughness, mechanical characteristics, biocompatibility, and Young's modulus.30 Because of their adaptability, polymeric nanoparticles may be customized for certain therapeutic uses, such gene therapy or cancer treatment. By shielding the antigens and guaranteeing their gradual release, polymeric nanoparticles can be used to deliver antigens in vaccinations, enhancing the immune response. Proteins or peptides that the body's enzymes could ordinarily break down can be delivered via polymers. Even while polymers like PLGA are biocompatible, some of their breakdown products might be harmful at larger doses or if they build up in particular organs. Polyethylene glycol (PLGA) NPs were used by Park et al.31 to encapsulate adriamycin. In order to generate multifunctional carriers with improved capabilities, researchers are investigating hybrid systems that mix polymeric nanoparticles with other drug delivery methods (such as liposomes or micelles). The extremely hydrophobic curcumin was encapsulated in PLGA NPs by Szymusiak et al.,32 which enhanced oral absorption and reduced the dosage of the medication required to produce similar amounts in mice's plasma and nervous system tissue following oral treatment by around twice as compared to unencapsulated curcumin. Bhattacharya assessed and reported docetaxel's and docetaxel-loaded polymeric nanoparticles’ cellular uptake efficiency percentages and IC50 values in a variety of human cancer cell lines, including U-87 MG, HeLa, C2BBe1, HCT-116, NCI-N87, and NCI-H929-Luc-mCh-Puro.33 However, difficulties in improving medication release and encapsulation continue to be major obstacles.3 Formulation strategies and drug release mechanisms
The qualities of the medication, the intended therapeutic target, and the appropriate release profile must all be carefully taken into account while formulating novel nano-based drug delivery systems (NDDS). To maximize the release of medications from NDDS and guarantee their timely and effective delivery, a number of strategies have been used.3.1 Controlled release systems
One of the most significant advantages of novel nano-based drug delivery systems (NDDS) is the ability to control the release of drugs over an extended period. Controlled release systems help maintain therapeutic drug levels within the body, minimizing fluctuations and reducing the risk of side effects. These systems can be designed to release drugs in a sustained or pulsatile manner, depending on the therapeutic needs. Polymeric nanoparticles can be engineered to distribute medications in a controlled and gradual manner. By lowering the frequency of dosing and minimizing peak plasma concentrations, this controlled release improves patient compliance. Table 2 shows a comparison between conventional and controlled drug delivery systems.| Parameters | Conventional systems | Controlled release systems | Ref. |
|---|---|---|---|
| Blood level | Fluctuating | Constant for a period of time | 54 |
| Bioavailability | Poor | Better | 55 |
| Dosing | Frequent | Less | 56 |
| Absorption | Poor absorption | Controlled drug release | 57 |
| Premature metabolism | Frequent | Protected | 58 |
| Rate limiting step | Absorption | Drug release from dosage form | 59 |
| Release | Immediate release | Slow release after a time duration | 60 |
| Patient compliance | Poor | High | 61 |
| Targeted drug delivery system | Targeting principle | Examples of NDDS developed | Ref. |
|---|---|---|---|
| Nanoparticles | The increased permeability and retention (EPR) effect is used; active targeting is possible by surface modification. | PLGA nanoparticles, chitosan nanoparticles, gold nanoparticles | 67 and 68 |
| Liposomes | Hydrophilic or hydrophobic medications are encapsulated in phospholipid vesicles, which can be PEGylated for extended circulation. | Doxil® (PEGylated liposomal doxorubicin), AmBisome® (liposomal amphotericin B) | 69 and 70 |
| Polymeric micelles | Hydrophobic medications can be dissolved using amphiphilic block copolymers, which self-assemble. | Genexol-PM® (paclitaxel loaded micelles) | 71 and 72 |
| Dendrimers | For drug conjugation, branching polymeric nanocarriers with many functional groups | PAMAM dendrimers loaded with methotrexate | 73 and 74 |
| Solid lipid nanoparticles (SLNs) | Poorly soluble medications are stabilized by a solid lipid core, allowing for regulated release. | SLN-based doxorubicin and curcumin carriers | 75 and 76 |
| Nanoemulsions | Nanosized emulsions of water in oil or oil in water improve solubility and bioavailability. | Cyclosporine nanoemulsion (Sandimmune®) | 77 and 78 |
| Nanocapsules | Drug reservoirs encapsulated in polymer shells are designed for continuous release | Poly(ε-caprolactone) nanocapsules | 48 and 79 |
| Gold nanoparticles (AuNPs) | Drug administration by photothermal and imaging guidance is made possible by surface plasmon resonance. | AuNPs loaded with doxorubicin, siRNA conjugates | 80 and 81 |
| Carbon nanotubes (CNTs) | Functionalized CNTs have a large surface area and can transport genes or medications into cells. | CNT–doxorubicin conjugates | 82 and 83 |
4 Challenges in nano-based drug delivery systems
Although medication delivery methods using nanoparticles show promise, a number of obstacles prevent their broad clinical use.4.1 Toxicity and biocompatibility
The possible toxicity of NDDS is one of the main issues. There are worries over the buildup of breakdown products over time, despite the fact that many of the polymers and components utilized in NDDS are biodegradable and biocompatible. These could be harmful or trigger allergic reactions. Certain nanoparticles have the ability to activate the immune system, which can result in allergic responses, inflammation, and other negative effects. When nanoparticles are employed often or in long-term therapy, this becomes particularly troublesome. There are worries about nanoparticles building up in organs like the liver, spleen, or kidneys, and long-term buildup might cause organ damage or poisoning. Therefore, in order to guarantee the safety of NDDS for human use, comprehensive biocompatibility studies and toxicity evaluations are necessary. Fig. 5 illustrates biomaterials’ toxicity and biocompatibility.4.2 Scalability and manufacturing
Ensuring consistency in the size, shape, and medication loading of nanoparticles is one of the primary challenges. Maintaining consistent quality on a wide scale can be challenging for nano-based systems, which need exact control during manufacturing. It can be costly to scale up the manufacturing of medicine delivery systems based on nanotechnology. In particular, when transferring lab-scale technologies to commercial production, high manufacturing costs might be a deterrent. The effectiveness, safety, and repeatability of the drug delivery system can all be significantly impacted by minor changes in the formulation or manufacturing process. To avoid negative immunological reactions, it is essential to ensure that nanoparticles are biocompatible. Toxicity requires thorough assessment and mitigation techniques, such as surface modification to lower clearance rates or promote excretion, especially when it results from the buildup of nanoparticles in organs.4.3 Overcoming biological barriers
The blood–brain barrier, cellular membranes, and the extracellular matrix are just a few of the biological barriers that nanoparticles must be able to get through. The blood–brain barrier (BBB) is a highly selective permeability barrier that separates the brain's tissue from its blood arteries. It keeps big molecules out of the brain, including the majority of medications. The lipid bilayer membrane that envelops each cell in the body serves as a selective barrier to keep undesirable things out. To overcome this obstacle, nanoparticles must be engineered with certain properties, such as proper size, charge, and surface changes. Some tactics include employing “smart” nanoparticles that can react to changes in the brain's microenvironment, such as pH shifts, or targeting ligands, such as transferrin or antibodies, that can attach to receptors on the BBB's endothelial cells. Mucus layers serve as a physical barrier to keep infections and external objects out of the body and are found on numerous epithelial surfaces, including the lungs, gastrointestinal system, and eyes. Cellular absorption can be improved by employing cationic particles or by covering the nanoparticles with lipids or proteins that resemble the cell membrane. Techniques such as receptor-mediated transcytosis, in which endothelial cells internalize nanoparticles and translocate them across the blood–brain barrier, and the application of nanoparticle coatings that can improve permeability are being investigated by researchers. One of the main challenges in clinical applications is creating nanoparticles that may successfully get through these barriers without eliciting immunological reactions.5 Recent developments and applications
Recent developments in medication delivery using nanoparticles have shown their therapeutic promise in a number of domains.5.1 Cancer treatment
In order to minimize harm to healthy organs, chemotherapeutic medicines have been delivered directly to tumor cells using nanoparticles. Furthermore, the creation of theranostic nanoparticles, which combine therapy and diagnosis, has been made easier by their capacity to transport imaging agents. Currently, a lot of clinical and preclinical research is being conducted on the topic of targeting macrophages in the development of cancer therapies.84Fig. 6 gives an idea about worldwide estimation of age-standardized incidence and mortality rates in cancer in 2024. The immune system can more successfully target cancers when medications like nivolumab (Opdivo) and pembrolizumab (Keytruda) disrupt checkpoint proteins that stop immune cells from attacking cancer cells.85 To attract monocytes to the tumor side, cancer cells release a range of cytokines and chemokines, such as CCL2, CCL3, CCL5, CCL20, CSF-1, CSF-2, IL-6, IL-8, IL-34, and C–X–C motif chemokine 12 (CXCL12).86 To attract monocytes, other tumor microenvironment (TME) cells may also release cytokines or chemokines. For instance, mesenchymal stem cells (MSCs) release CCL2, Th17 cells express IL-17, and cancer-associated fibroblasts (CAFs) secrete IL-8. The CSF-1–CSF-1R, CCL2–CCR2, CXCL12–CXCR4, and CCL5–CCR5 signaling axes are the main targets of current methods to stop monocyte recruitment and polarization towards the immunosuppressive M2 state. Liquid biopsies, which use blood sample analysis to find tumor DNA, have become popular as less intrusive methods for tracking the development of cancer and finding mutations.87 They are employed to inform treatment choices and identify cancer early. By altering a patient's own T-cells, chimeric antigen receptor T-cell (CAR-T) treatment enables them to identify and combat cancer cells.88 In particular, in blood malignancies like leukemia and lymphoma, it has demonstrated impressive outcomes. Cervarix and Gardasil are two vaccines that have previously demonstrated effectiveness in preventing cancer, particularly HPV-related cervical cancer.89 The development of novel medications like Selpercatinib (for RET-altered cancers) and Lenvatinib (for liver cancer) has brought renewed hope to patients with previously difficult to treat tumors.905.2 Vaccines for infectious diseases
In order to overcome issues such drug resistance and low absorption, nanoparticles have demonstrated potential in delivering antiviral and antibacterial medications with increased potency. Tariq et al. reported that the potential of protein nanoparticle-based vaccines and virus-like particle (VLP)-based vaccines as effective and viable immunization agents to produce immunity against virulent infectious agents, such as SARS-CoV-2, has been described.91Fig. 7 shows a comparison of the dangers of traditional vaccinations with those of VLP-based vaccines. Table 4 summarizes infectious diseases, the efficacy of NDDS, and the pace at which the diseases can be cured utilizing the nanodrug delivery technology.| Infectious disease | Curable rate using NDDS | Nano-based drug delivery technology | Effectiveness of the NDDS | Ref. |
|---|---|---|---|---|
| Tuberculosis (TB) | 70–90% (drug-resistant cases) | Liposomes, solid lipid NPs, polymeric NPs | Improves drug bioavailability and penetration, enhances efficacy in resistant strains | 92 |
| HIV/AIDS | Moderate (not curable) | NPs, dendrimers, liposomes | Increases drug stability, improves targeted delivery, reduces side effects | 93 |
| Malaria | 90%+ (if treated early) | Lipid-based NPs, nanosuspensions, micelles | Improves drug solubility, enhances targeting of infected cells | 94 |
| Hepatitis B and C | Moderate to high | Liposomes, polymeric NPs | Provides sustained release of antiviral agents, targets liver cells effectively | 95 |
| Bacterial infections | 90–95% (if treated with right antibiotics) | Silver NPs, gold NPs, antimicrobial peptides | Enhances antibiotic penetration, reduces bacterial resistance, targets infected tissues. | 96 |
| Fungal infections | 70–80% (depending on the type) | Polymeric NPs, liposomes, nanocomposites | Improves drug solubility, reduces toxicity, targets fungal cells. | |
| Leishmaniasis | 70–80% | Liposomes, polymeric NPs, nanoencapsulated antimonials | Enhances drug delivery, reduces side effects, improves treatment outcomes | 97 |
| Dengue fever | No cure yet | NPs, nanoemulsions | Aids in supportive treatments, drug delivery systems still under research | 98 |
| Pneumonia | 80–90% (with appropriate antibiotics) | NPs, nanoshells, antimicrobial peptides | Improves antibiotic delivery to the lungs, enhances the cure rate | 99 |
| Zika virus disease | No cure yet | Nanoemulsions, lipid-based NPs | Potential for developing antiviral therapies, still under research | 100 |
| COVID-19 (SARS-CoV-2) | Varies (mild to severe) | Nanoparticles, nanocarriers, vaccine delivery systems | Improves delivery of antivirals, enhances vaccine efficacy and immune response | 101 |
| Chagas disease | 60–80% (early treatment) | Nanoparticles for controlled release of antiparasitic drugs | Overcomes poor drug absorption, enhances efficacy | 102 |
5.3 Gene delivery
Because nanoparticles can transfer nucleic acids (DNA, RNA, and siRNA) directly to target cells, they are increasingly being used in gene therapy, offering promise for genetic diseases and customized treatment.6 Future directions in nano-based drug delivery
NDDS have a bright future thanks to continuing research aimed at solving present problems and investigating novel therapeutic uses. To increase efficacy and overcome resistance to treatment, it is being investigated to combine several treatment modalities, such as immunotherapy with chemotherapy or targeted therapy. Clinical trials of these combo treatments are yielding encouraging results. These advancements reflect a team effort to promote access to therapies, decrease side effects, improve survival rates, and customize cancer care. There is optimism that even more advancements will be made in cancer therapy in the years to come thanks to continuous research and clinical trials. Table 5 summarizes the comparison of major NDDS platforms (based on their size, composition, drug loading strategies, release triggers, and clinical status) (see also Table 6).| NDDS platform | Typical size range | Composition | Drug-loading strategies | Release triggers | Clinical status/examples | Ref. |
|---|---|---|---|---|---|---|
| Liposomes | 50–200 nm | Phospholipid bilayers with an aqueous core | Hydrophilic drugs in the core; hydrophobic drugs in the bilayers | pH, temperature, enzymatic degradation | Several FDA approved (e.g., Doxil®, AmBisome®) | 103–107 |
| Polymeric nanoparticles | 10–200 nm | Biodegradable polymers (e.g., PLGA, PLA, PEGylated polymers) | Encapsulation, adsorption, or covalent conjugation | pH, enzymatic, hydrolysis | Some in clinical trials (BIND-014, CRLX101) | 47 and 108–111 |
| Solid lipid nanoparticles (SLNs) | 50–500 nm | Solid lipids stabilized with surfactants | Drug dispersed or solubilized in a lipid matrix | Temperature, enzymatic | Some in clinical trials | 75 and 112–114 |
| Polymeric micelles | 10–100 nm | Amphiphilic block copolymers (e.g., PEG-PLA) | Hydrophobic drugs in the core, hydrophilic drugs at the corona | pH, redox potential, temperature | Clinical trials (e.g., Genexol-PM) | 29 and 115–118 |
| Dendrimers | 5–20 nm | Branched, highly ordered polymers (e.g., PAMAM dendrimers) | Surface functionalization, encapsulation in interior cavities | pH, enzymatic | Preclinical and some clinical candidates | 119–123 |
| Nanoemulsions | 20–200 nm | Oil-in-water or water-in-oil emulsions with surfactants | Solubilization of lipophilic drugs in the oil phase | Dilution, enzymatic digestion | Some marketed formulations (Neoral®) | 124–127 |
| Nanocapsules | 50–300 nm | Polymer shell encapsulating a liquid/solid core | Drug reservoir in the core, the polymer controls release | pH, hydrolysis, enzymatic | Preclinical and experimental trials | 128–132 |
| Gold nanoparticles (AuNPs) | 10–150 nm | Metallic gold core with surface modifications | Surface conjugation with drugs/ligands | Light (photothermal), pH | Preclinical and early-phase clinical trials | 133–136 |
| Carbon nanotubes (CNTs) | 1–100 nm (diameter) | Cylindrical carbon allotropes (single/multi-walled) | Adsorption, covalent functionalization, encapsulation | pH, NIR light, enzymatic | Mostly pre-clinical; under exploration for oncology | 137–140 |
| Drug delivery systems | Benefits | Drawbacks | Ref. |
|---|---|---|---|
| Pulmonary | Requires less dosage | Deposition issues in the throat | 141 |
| Transdermal | Prevents deterioration of drugs | May cause enzymatic deterioration | 142 |
| Intranasal | Rapid onset of action | Intolerance in the nasal mucosa | 143 |
| Oral drug | Cost effective | Difficulty in consumption | 144 |
| Rectal | Avoids enzymatic degradation | Rectal irritation | 145 |
| Intravenous | Short latent period | Undesirable immune reaction | 58 |
| Intramuscular | Rapid and uniform absorption | May cause hematoma | 54 |
| Subcutaneous | Self-administered | Low rate of absorption | 146 |
6.1 Personalized medicine
Personalized medicine is one of NDDS’ most interesting future opportunities. NDDS can assist maximize treatment success and reduce adverse effects by customizing drug delivery systems to each patient's unique requirements, including their genetic profiles and illness features. Patients with some malignancies are seeing better results thanks to proton therapy, which targets tumors while causing the least amount of harm to nearby healthy tissue. This is especially true for pediatric and head/neck cancers.147 High doses of radiation are delivered with pinpoint accuracy using stereotactic body radiotherapy (SBRT), which decreases the number of treatment sessions while increasing efficacy.1486.2 Gene therapy
NDDS have demonstrated considerable potential in the field of gene therapy, which entails delivering genetic material to target cells in order to treat or prevent illnesses. Potential cures for genetic diseases and tumors may be possible with the effective delivery of DNA, RNA, or gene-editing agents like CRISPR to cells via nanoparticles, liposomes, and dendrimers.6.3 Multi-drug delivery systems
To improve the treatment of complicated illnesses like HIV and cancer, multi-drug delivery systems which mix many therapeutic agents on a single platform are being intensively investigated. These systems can lower the likelihood of drug resistance, enhance patient compliance, and facilitate synergistic effects.7 Conclusion
Nano-based drug delivery systems represent a transformative advancement in modern therapeutics, offering precise, controlled, and efficient drug delivery. These methods greatly improve drug absorption, reduce side effects, and allow for custom treatment techniques using nanotechnology. With several formulations already licensed or undergoing late-stage clinical review, liposomes and polymeric nanoparticles stand out among the developed systems as the most clinically advanced, especially in the treatment of chronic diseases and cancer. Broader translation into clinical practice is currently limited by a number of significant issues that have not yet been addressed, such as worries about toxicity and long-term biocompatibility, challenges in achieving cost-effective large-scale production, and the lack of standardized international regulatory guidelines. Theranostic platforms that simultaneously combine therapeutic delivery and diagnostic imaging, the development of more predictive in vivo models to close the gap between laboratory success and patient outcomes, and the use of artificial intelligence and machine learning tools to speed up nanocarrier design and predict biological interactions are just a few of the exciting prospects for furthering NDDS research in the future. By getting beyond these obstacles and embracing new developments, NDDS can move closer to scalable, secure, and customized nanomedicine solutions that have the potential to revolutionize the way that cancer, infectious diseases, and chronic illnesses are treated.Author contributions
The sole author conceived the study and designed the structure of the article. She was responsible for the conceptualization, methodology, investigation, original draft writing, and review and editing.Conflicts of interest
The author declares that there are no conflicts of interest regarding the publication of this paper.Data availability
All the data supporting the findings of this study are included in this paper.Acknowledgements
The author would like to express her gratitude to the Kuwait College of Science and Technology for providing the necessary support and facilities to carry out this study.References
- M. T. Manzari, Y. Shamay, H. Kiguchi, N. Rosen, M. Scaltriti and D. A. Heller, Targeted drug delivery strategies for precision medicines, Nat. Rev. Mater., 2021, 6(4), 351–370 CrossRef PubMed.
- K. Yadav, K. K. Sahu, R. Yadav, W. Raza, S. Minz, M. R. Singh, D. Singh and M. Pradhan, et al., A complex molecular landscape to drug delivery concept for achieving precise therapy in psoriasis, Med. Drug Discovery, 2024, 100183 CrossRef.
- M. Ullah, M. I. Shah, M. W. Hasan, M. Jamshed, U. Mustafa and M. Inam, Engineered metal nanoparticles for precision drug delivery: Pioneering the future of medicine: Mini review, J. Chin. Chem. Soc., 2024, 71(11), 1358–1367 CrossRef.
- H. Moorthy and T. Govindaraju, Dendrimer architectonics to treat cancer and neurodegenerative diseases with implications in theranostics and personalized medicine, ACS Appl. Bio Mater., 2021, 4(2), 1115–1139 CrossRef PubMed.
- D. Wu, Q. Chen, X. Chen, F. Han, Z. Chen and Y. Wang, The blood–brain barrier: structure, regulation, and drug delivery, Signal Transduction Targeted Ther., 2023, 8(1), 217 CrossRef.
- I. Singh, S. Kumar, S. Singh and M. Y. Wani, Overcoming resistance: Chitosan-modified liposomes as targeted drug carriers in the fight against multidrug resistant bacteria-a review, Int. J. Biol. Macromol., 2024, 135022 CrossRef PubMed.
- N. Akram, M. Afzaal, F. Saeed, Y. A. Shah, Z. Faisal, A. Asghar, H. Ateeq, G. A. Nayik, S. H. Wani and M. Hussain, et al., Liposomes: a promising delivery system for active ingredients in food and nutrition, Int. J. Food Prop., 2023, 26(1), 2476–2492 CrossRef.
- C. Chai and J. Park, Food liposomes: Structures, components, preparations, and applications, Food Chem., 2024, 432, 137228 CrossRef.
- A. M. Alenzi, S. A. Albalawi, S. G. Alghamdi, R. F. Albalawi, H. S. Albalawi and M. Qushawy, Review on different vesicular drug delivery systems (vddss) and their applications, Recent Pat. Nanotechnol., 2023, 17(1), 18–32 CrossRef.
- J. Mall, N. Naseem, M. F. Haider, M. A. Rahman, S. Khan and S. N. Siddiqui, Nanostructured lipid carriers as a drug delivery system: A comprehensive review with therapeutic applications, Intell. Pharm., 2024, 3(4), 243–255 Search PubMed.
- S. Antimisiaris, A. Marazioti, M. Kannavou, E. Natsaridis, F. Gkartziou, G. Kogkos and S. Mourtas, Overcoming barriers by local drug delivery with liposomes, Adv. Drug Delivery Rev., 2021, 174, 53–86 CrossRef.
- X. Han, A. Alu, H. Liu, Y. Shi, X. Wei, L. Cai and Y. Wei, Biomaterial-assisted biotherapy: A brief review of biomaterials used in drug delivery, vaccine development, gene therapy, and stem cell therapy, Bioact. Mater., 2022, 17, 29–48 Search PubMed.
- W. Lu, W. Liu, A. Hu, J. Shen, H. Yi and Z. Cheng, Combinatorial polydopamine-liposome nanoformulation as an effective anti-breast cancer therapy, Int. J. Nanomed., 2023, 861–879 CrossRef.
- K. L. López, A. Ravasio, J. V. González-Aramundiz and F. C. Zacconi, Solid lipid nanoparticles (sln) and nanostructured lipid carriers (nlc) prepared by microwave and ultrasound-assisted synthesis: Promising green strategies for the nanoworld, Pharmaceutics, 2023, 15(5), 1333 CrossRef PubMed.
- N. Desai, D. Rana, S. Salave, R. Gupta, P. Patel, B. Karunakaran, A. Sharma, J. Giri, D. Benival and N. Kommineni, Chitosan: a potential biopolymer in drug delivery and biomedical applications, Pharmaceutics, 2023, 15(4), 1313 CrossRef CAS PubMed.
- N. U. Islam and E. Khan, Exploring salts, co-crystals, drug nanoparticles, and co-delivery in formulation development of ciprofloxacin: A review, ChemistrySelect, 2025, 10(2), 202405182 CrossRef.
- K. M. A. S. Sharif, M. Angolkar, M. Rahamathulla, K. Y. Thajudeen, M. M. Ahmed, S. A. Farhana, T. B. Shivanandappa, S. Paramshetti and R. A. M. Osmani, Box-behnken design-based optimization and evaluation of lipid-based nano drug delivery system for brain targeting of bromocriptine, Pharmaceuticals, 2024, 17(6), 720 CrossRef.
- R. J. Sarode and H. S. Mahajan, Dendrimers for drug delivery: An overview of its classes, synthesis, and applications, J. Drug Delivery Sci. Technol., 2024, 98, 105896 CrossRef CAS.
- V. Rastogi, P. Yadav, M. Porwal, S. Sur and A. Verma, Dendrimer as nanocarrier for drug delivery and drug targeting therapeutics: a fundamental to advanced systematic review, Int. J. Polym. Mater. Polym. Biomater., 2024, 73(4), 310–332 CrossRef CAS.
- P. Soltany, M. Miralinaghi and F. P. Shariati, Folic acid conjugated poly (amidoamine) dendrimer grafted magnetic chitosan as a smart drug delivery platform for doxorubicin: In vitro drug release and cytotoxicity studies, Int. J. Biol. Macromol., 2024, 257, 127564 CrossRef CAS.
- M. Delyanee, S. Akbari and A. Solouk, Amine-terminated dendritic polymers as promising nanoplatform for diagnostic and therapeutic agents’ modification: A review, Eur. J. Med. Chem., 2021, 221, 113572 CrossRef CAS PubMed.
- X. Li, A. Naeem, S. Xiao, L. Hu, J. Zhang and Q. Zheng, Safety challenges and application strategies for the use of dendrimers in medicine, Pharmaceutics, 2022, 14(6), 1292 CrossRef CAS PubMed.
- I. Martins, H. Tomás, F. Lahoz and J. Rodrigues, Engineered fluorescent carbon dots and g4-g6 pamam dendrimer nanohybrids for bioimaging and gene delivery, Biomacromolecules, 2021, 22(6), 2436–2450 CrossRef CAS PubMed.
- M. Ghezzi, S. Pescina, C. Padula, P. Santi, E. Del Favero, L. Cantù and S. Nicoli, Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions, J. Controlled Release, 2021, 332, 312–336 CrossRef CAS PubMed.
- G. S. Wahengbam, S. Nirmal, J. Nandwana, S. Kar, V. Kumari, R. Mishra and A. Singh, Polymeric nanoparticles revolutionizing brain cancer therapy: A comprehensive review of strategies and advances, Crit. Rev. Ther. Drug Carrier Syst., 2025, 42, 73–106 CrossRef.
- X. Hou, X. Ai, Z. Liu, J. Yang, Y. Wu, D. Zhang and N. Feng, Wheat germ agglutinin modified mixed micelles overcome the dual barrier of mucus/enterocytes for effective oral absorption of shikonin and gefitinib, Drug Delivery Transl. Res., 2024, 1–18 Search PubMed.
- S. Madane, B. Parande, S. More, S. Dhole and C. Dongaonkar, A critical review: Preparation and characterization of micellar gel, Indian J. Pharm. Sci., 2024, 86(3), 791–804 CAS.
- X. Sun, Y. Sheng, K. Li, S. Sai, J. Feng, Y. Li, J. Zhang, J. Han and B. Tian, Mucoadhesive phenylboronic acid conjugated chitosan oligosaccharide-vitamin e copolymer for topical ocular delivery of voriconazole: Synthesis, in vitro/vivo evaluation, and mechanism, Acta Biomater., 2022, 138, 193–207 CrossRef CAS.
- R. Mundel, T. Thakur and M. Chatterjee, Emerging uses of pla–peg copolymer in cancer drug delivery, 3 Biotech, 2022, 12(2), 41 CrossRef.
- S. P. Selvam, S. Ayyappan, S. I. Jamir, L. K. Sellappan and S. Manoharan, Recent advancements of hydroxyapatite and polyethylene glycol (peg) composites for tissue engineering applications–a comprehensive review, Eur. Polym. J., 2024, 215, 113226 CrossRef.
- J. Park, P. M. Fong, J. Lu, K. S. Russell, C. J. Booth, W. M. Saltzman and T. M. Fahmy, Pegylated plga nanoparticles for the improved delivery of doxorubicin, in Nanomedicine in Cancer, Jenny Stanford Publishing, 2017, pp. 575–596 Search PubMed.
- M. Szymusiak, X. Hu, P. A. L. Plata, P. Ciupinski, Z. J. Wang and Y. Liu, Bioavailability of curcumin and curcumin glucuronide in the central nervous system of mice after oral delivery of nano-curcumin, Int. J. Pharm., 2016, 511(1), 415–423 CrossRef PubMed.
- S. Bhattacharya, Fabrication of poly (sarcosine), poly (ethylene glycol), and poly (lactic-co-glycolic acid) polymeric nanoparticles for cancer drug delivery, J. Drug Delivery Sci. Technol., 2021, 61, 102194 CrossRef.
- S. Gad and N. Ghourab, et al., Nanoparticulate drug delivery systems in oncological treatment, Rec. Pharm. Biomed. Sci., 2024, 8(3), 193–200 Search PubMed.
- M. Souri, M. Soltani, F. M. Kashkooli and M. K. Shahvandi, Engineered strategies to enhance tumor penetration of drug-loaded nanoparticles, J. Controlled Release, 2022, 341, 227–246 CrossRef PubMed.
- A. Al-Jipouri, S. H. Almurisi, K. Al-Japairai, L. M. Bakar and A. A. Doolaanea, Liposomes or extracellular vesicles: a comprehensive comparison of both lipid bilayer vesicles for pulmonary drug delivery, Polymers, 2023, 15(2), 318 CrossRef PubMed.
- P. Trucillo, V. Nebbioso, R. Brancaccio and L. Gigante, Nanocarrier-embedded gels: Precision drug delivery via liposomal and niosomal platforms, Polym. Adv. Technol., 2024, 35(4), 6406 CrossRef.
- Y. V. Cardona, L. G. Muñoz, D. G. Cardozo and A. F. Chamorro, Recent applications of amphiphilic copolymers in drug release systems for skin treatment, Pharmaceutics, 2024, 16(9), 1203 CrossRef PubMed.
- S. Kotta, H. M. Aldawsari, S. M. Badr-Eldin, A. B. Nair and K. Yt, Progress in polymeric micelles for drug delivery applications, Pharmaceutics, 2022, 14(8), 1636 CrossRef PubMed.
- G. Naji, N. Fareed and W. Al-Zheery, Dendrimer as a recent drug delivery system: An overview, J. Biomed. Biochem., 2022, 1(3), 57–69 Search PubMed.
- T. Zhao, M. Zhou, R. Wu, H. Wang, C. C. Zouboulis, M. Zhu and M. Lee, Dendrimer-conjugated isotretinoin for controlled transdermal drug delivery, J. Nanobiotechnol., 2023, 21(1), 285 CrossRef PubMed.
- H. A. Mohammed, R. A. Khan, V. Singh, M. Yusuf, N. Akhtar, G. M. Sulaiman, S. Albukhaty, A. A. Abdellatif, M. Khan and S. A. Mohammed, et al., Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development, Nanotechnol. Rev., 2023, 12(1), 20220517 CrossRef.
- M. Munir, M. Zaman, M. A. Waqar, M. A. Khan and M. N. Alvi, Solid lipid nanoparticles: a versatile approach for controlled release and targeted drug delivery, J. Liposome Res., 2024, 34(2), 335–348 CrossRef.
- Y. Ozogul, G. T. Karsli, M. Durmuş, H. Yazgan, H. M. Oztop, D. J. McClements and F. Ozogul, Recent developments in industrial applications of nanoemulsions, Adv. Colloid Interface Sci., 2022, 304, 102685 CrossRef.
- H. A. Al-Hussaniy, Y. Q. lmajidi, A. I. Oraibi and A. Alkarawi, Nanoemulsions as medicinal components in insoluble medicines, Pharmacia, 2023, 70(3), 537–547 CrossRef.
- K. Elumalai, S. Srinivasan and A. Shanmugam, Review of the efficacy of nanoparticle-based drug delivery systems for cancer treatment, Biomed. Technol., 2024, 5, 109–122 CrossRef.
- M. A. Beach, U. Nayanathara, Y. Gao, C. Zhang, Y. Xiong, Y. Wang and G. K. Such, Polymeric nanoparticles for drug delivery, Chem. Rev., 2024, 124(9), 5505–5616 CrossRef.
- A. Mehandole, N. Walke, S. Mahajan, M. Aalhate, I. Maji, U. Gupta, N. K. Mehra and P. K. Singh, Core–shell type lipidic and polymeric nanocapsules: the transformative multifaceted delivery systems, AAPS PharmSciTech, 2023, 24(1), 50 CrossRef PubMed.
- P. Kumar, N. Yadav, B. Chaudhary, S. Umakanthan, V. K. Chattu, I. Kazmi, F. A. Al-Abbasi, S. I. Alzarea, O. Afzal and A. S. Altamimi, et al., Lipid nanocapsule: a novel approach to drug delivery system formulation development, Curr. Pharm. Biotechnol., 2024, 25(3), 268–284 Search PubMed.
- J.-J. Xu, W.-C. Zhang, Y.-W. Guo, X.-Y. Chen and Y.-N. Zhang, Metal nanoparticles as a promising technology in targeted cancer treatment, Drug Delivery, 2022, 29(1), 664–678 CrossRef.
- M. Wu, Y. Xiao, R. Wu, J. Lei, T. Li and Y. Zheng, Aggregable gold nanoparticles for cancer photothermal therapy, J. Mater. Chem. B, 2024, 8048–8061 RSC.
- L. Sonowal and S. Gautam, Advancements and challenges in carbon nanotube-based drug delivery systems, Nano-Struct. Nano-Objects, 2024, 38, 101117 CrossRef.
- K. Brindhadevi, H. A. Garalleh, A. Alalawi, E. Al-Sarayreh and A. Pugazhendhi, Carbon nanomaterials: Types, synthesis strategies and their application as drug delivery system for cancer therapy, Biochem. Eng. J., 2023, 192, 108828 CrossRef.
- H. Park, A. Otte and K. Park, Evolution of drug delivery systems: From 1950 to 2020 and beyond, J. Controlled Release, 2022, 342, 53–65 CrossRef PubMed.
- J. S. Kim, S. Cheon, M. R. Woo, S. Woo, J.-E. Chung, Y. S. Youn, K. T. Oh, S.-J. Lim, S. K. Ku and B. L. Nguyen, et al., Electrostatic spraying for fine-tuning particle dimensions to enhance oral bioavailability of poorly water-soluble drugs, Asian J. Pharm. Sci., 2024, 19(5), 100953 Search PubMed.
- T. C. Ezike, U. S. Okpala, U. L. Onoja, C. P. Nwike, E. C. Ezeako, O. J. Okpara, C. C. Okoroafor, S. C. Eze, O. L. Kalu and E. C. Odoh, et al., Advances in drug delivery systems, challenges and future directions, Heliyon, 2023, 9(6), e17488 CrossRef PubMed.
- A. L. Onugwu, C. S. Nwagwu, O. S. Onugwu, A. C. Echezona, C. P. Agbo, S. A. Ihim, P. Emeh, P. O. Nnamani, A. A. Attama and V. V. Khutoryanskiy, Nanotechnology based drug delivery systems for the treatment of anterior segment eye diseases, J. Controlled Release, 2023, 354, 465–488 CrossRef.
- A. Sultana, M. Zare, V. Thomas, T. S. Kumar and S. Ramakrishna, Nano-based drug delivery systems: Conventional drug delivery routes, recent developments and future prospects, Med. Drug Discovery, 2022, 15, 100134 CrossRef.
- S. Adepu and S. Ramakrishna, Controlled drug delivery systems: current status and future directions, Molecules, 2021, 26(19), 5905 CrossRef PubMed.
- P. Kesharwani, A. Bisht, A. Alexander, V. Dave and S. Sharma, Biomedical applications of hydrogels in drug delivery system: An update, J. Drug Delivery Sci. Technol., 2021, 66, 102914 CrossRef.
- I. Maji, S. Mahajan, A. Sriram, P. Medtiya, R. Vasave, D. K. Khatri, R. Kumar, S. B. Singh, J. Madan and P. K. Singh, Solid self emulsifying drug delivery system: Superior mode for oral delivery of hydrophobic cargos, J. Controlled Release, 2021, 337, 646–660 CrossRef.
- M. A. Rahim, N. Jan, S. Khan, H. Shah, A. Madni, A. Khan, A. Jabar, S. Khan, A. Elhissi and Z. Hussain, et al., Recent advancements in stimuli responsive drug delivery platforms for active and passive cancer targeting, Cancers, 2021, 13(4), 670 CrossRef PubMed.
- S. Shilpi, K. Saini, P. Chimaniya, E. Gurnany, K. Sharma, S. Dixit and A. K. Yadav, Stimuli-responsive drug delivery systems: Magnetically, thermally and ph assisted drug delivery system, in Novel Carrier Systems for Targeted and Controlled Drug Delivery, 2024, pp. 459–479 Search PubMed.
- C. J. Diehl and A. Ciulli, Discovery of small molecule ligands for the von hippel-lindau (vhl) e3 ligase and their use as inhibitors and protac degraders, Chem. Soc. Rev., 2022, 51(19), 8216–8257 RSC.
- T. Boch, J. Köhler, M. Janning and S. Loges, Targeting the egf receptor family in non-small cell lung cancer—increased complexity and future perspectives, Cancer Biol. Med., 2022, 19(11), 1543 CrossRef PubMed.
- C. Corvaja, A. Passaro, I. Attili, P. T. Aliaga, G. Spitaleri, E. Del Signore and F. Marinis, Advancements in fourth-generation egfr tkis in egfr-mutant nsclc: Bridging biological insights and therapeutic development, Cancer Treat. Rev., 2024, 102824 CrossRef.
- A. Abdelkawi, A. Slim, Z. Zinoune and Y. Pathak, Surface modification of metallic nanoparticles for targeting drugs, Coatings, 2023, 13(9), 1660 CrossRef.
- D. Essa, P. P. Kondiah, P. Kumar and Y. E. Choonara, Design of chitosan-coated, quercetin-loaded plga nanoparticles for enhanced psma-specific activity on lncap prostate cancer cells, Biomedicines, 2023, 11(4), 1201 CrossRef.
- E. Jaradat, A. Meziane and D. A. Lamprou, Conventional vs pegylated loaded liposomal formulations by microfluidics for delivering hydrophilic chemotherapy, Int. J. Pharm., 2024, 655, 124077 CrossRef PubMed.
- S. Basak and T. K. Das, Liposome-based drug delivery systems: from laboratory research to industrial production—instruments and challenges, ChemEngineering, 2025, 9(3), 56 CrossRef.
- O. Kontogiannis, D. Selianitis, N. Lagopati, N. Pippa, S. Pispas and M. Gazouli, Surfactant and block copolymer nanostructures: from design and development to nanomedicine preclinical studies, Pharmaceutics, 2023, 15(2), 501 CrossRef PubMed.
- Z. Binkhathlan, O. Yusuf, R. Ali, A. H. Alomrani, A. Alshamsan, A. K. Alshememry, A. Almomen, M. Alkholief, I. A. Aljuffali and F. Alqahtani, et al., Polycaprolactone–vitamin e tpgs micelles for delivery of paclitaxel: In vitro and in vivo evaluation, Int. J. Pharm., 2024, X7, 100253 Search PubMed.
- R. De, M. K. Mahata and K.-T. Kim, Structure-based varieties of polymeric nanocarriers and influences of their physicochemical properties on drug delivery profiles, Adv. Sci., 2022, 9(10), 2105373 CrossRef.
- A. Aghanejad, S. Kheiriabad, M. Ghaffari, S. Namvar Aghdash, N. Ghafouri, J. Ezzati Nazhad Dolatabadi, H. Andishmand and M. R. Hamblin, Targeted codelivery nanosystem based on methotrexate, curcumin, and pamam dendrimer for improvement of the therapeutic efficacy in cervical cancer, Sci. Rep., 2025, 15(1), 1813 CrossRef.
- M. d. C. V. Queiroz and L. A. Muehlmann, Characteristics and preparation of solid lipid nanoparticles and nanostructured lipid carriers, J. Nanotheranostics, 2024, 5(4), 188–211 CrossRef.
- D. Sivadasan, K. Ramakrishnan, J. Mahendran, H. Ranganathan, A. Karuppaiah and H. Rahman, Solid lipid nanoparticles: applications and prospects in cancer treatment, Int. J. Mol. Sci., 2023, 24(7), 6199 CrossRef CAS.
- S. Gad and R. Zidan, Nanoemulsion formulation for enhancing the aqueous solubility and systemic bioavailability of poorly soluble drugs, Rec. Pharm. Biomed. Sci., 2023, 7(3), 103–108 Search PubMed.
- S. Gharat, V. Basudkar and M. Momin, In vitro and in vivo evaluation of the developed curcumin-cyclosporine-loaded nanoemulgel for the management of rheumatoid arthritis, Immunol. Invest., 2024, 53(3), 490–522 CrossRef CAS PubMed.
- W. Deng, Y. Yan, P. Zhuang, X. Liu, K. Tian, W. Huang and C. Li, Synthesis of nanocapsules blended polymeric hydrogel loaded with bupivacaine drug delivery system for local anesthetics and pain management, Drug Delivery, 2022, 29(1), 399–412 CrossRef CAS.
- Q. Gao, J. Zhang, J. Gao, Z. Zhang, H. Zhu and D. Wang, Gold nanoparticles in cancer theranostics, Front. Bioeng. Biotechnol., 2021, 9, 647905 CrossRef PubMed.
- C.Ü Tunç and O. Aydin, Co-delivery of bcl-2 sirna and doxorubicin through gold nanoparticle-based delivery system for a combined cancer therapy approach, J. Drug Delivery Sci. Technol., 2022, 74, 103603 CrossRef.
- S. Das, S. Roy, S. C. Dinda, A. Bose, C. Mahapatra, B. Basu and B. Prajapati, Carbon nanotubes in brain targeted drug delivery: A comprehensive review, Results Chem., 2025, 102206 CrossRef CAS.
- S. Singhal, M. Gupta, M. S. Alam, M. N. Javed and J. R. Ansari, Carbon allotropes-based nanodevices: graphene in biomedical applications, in Nanotechnology, 2022, pp. 241–269 Search PubMed.
- X. Kang, Y. Huang, H. Wang, S. Jadhav, Z. Yue, A. K. Tiwari and R. J. Babu, Tumor-associated macrophage targeting of nanomedicines in cancer therapy, Pharmaceutics, 2023, 16(1), 61 CrossRef.
- G. Roozitalab, B. Abedi, S. Imani, R. Farghadani and P. Jabbarzadeh Kaboli, Comprehensive assessment of tecentriq® and opdivo®: analyzing immunotherapy indications withdrawn in triple-negative breast cancer and hepatocellular carcinoma, Cancer Metastasis Rev., 2024, 1–30 Search PubMed.
- Y. He, R. F. Araújo Júnior, L. J. Cruz and C. Eich, Functionalized nanoparticles targeting tumor-associated macrophages as cancer therapy, Pharmaceutics, 2021, 13(10), 1670 CrossRef CAS.
- R. Heestermans, R. Schots, A. De Becker and I. Van Riet, Liquid biopsies as non-invasive tools for mutation profiling in multiple myeloma: Application potential, challenges, and opportunities, Int. J. Mol. Sci., 2024, 25(10), 5208 CrossRef CAS.
- B. Spokeviciute, S. Kholia and M. F. Brizzi, Chimeric antigen receptor (car) t-cell therapy: harnessing extracellular vesicles for enhanced efficacy, Pharmacol. Res., 2024, 107352 CrossRef CAS.
- N. Khairkhah, A. Bolhassani and R. Najafipour, Current and future direction in treatment of hpv-related cervical disease, J. Mol. Med., 2022, 100(6), 829–845 CrossRef CAS PubMed.
- J. Li, Y. Zhang, F. Sun, L. Xing and X. Sun, Towards an era of precise diagnosis and treatment: Role of novel molecular modification-based imaging and therapy for dedifferentiated thyroid cancer, Front. Endocrinol., 2022, 13, 980582 CrossRef.
- H. Tariq, S. Batool, S. Asif, M. Ali and B. H. Abbasi, Virus-like particles: Revolutionary platforms for developing vaccines against emerging infectious diseases, Front. Microbiol., 2022, 12, 790121 CrossRef.
- Y.-N. Yang, M.-Q. Zhang, F.-L. Yu, B. Han, M.-Y. Bao, X. Li and Y. Zhang, et al., Peroxisome proliferator-activated receptor-γ coactivator-1α in neurodegenerative disorders: A promising therapeutic target, Biochem. Pharmacol., 2023, 115717 CrossRef PubMed.
- E. Owusu, Utilizing nanodiamond for antiretroviral drug delivery to hiv infected microglial cells, Master's thesis, The University of Texas Rio Grande Valley, 2024 Search PubMed.
- S. R. K. Pandian, T. Panneerselvam, P. Pavadai, S. Govindaraj, V. Ravishankar, P. Palanisamy, M. Sampath, M. Sankaranarayanan and S. Kunjiappan, Nano based approach for the treatment of neglected tropical diseases, Front. Nanotechnol., 2021, 3, 665274 CrossRef.
- T. G. Jain, Preclinical and translational applications of nanoparticulate drug delivery system in hepatocellular carcinoma, Afr. J. Biomed. Res., 2024, 27(4S), 4624–4632 Search PubMed.
- G. Joshi, S. S. Quadir and K. S. Yadav, Road map to the treatment of neglected tropical diseases: Nanocarriers interventions, J. Controlled Release, 2021, 339, 51–74 CrossRef PubMed.
- L. A. Ghenciu, A. C. Faur, S. L. Bolintineanu, M. C. Salavat and A. L. Maghiari, Recent advances in diagnosis and treatment approaches in fungal keratitis: A narrative review, Microorganisms, 2024, 12(1), 161 CrossRef PubMed.
- E. A. Ayeni, A. M. Aldossary, D. A. Ayejoto, L. A. Gbadegesin, A. A. Alshehri, H. A. Alfassam, H. K. Afewerky, F. A. Almughem and S. M. Bello, Tawfik, E.A.: Neurodegenerative diseases: implications of environmental and climatic influences on neurotransmitters and neuronal hormones activities, Int. J. Environ. Res. Public Health, 2022, 19(19), 12495 CrossRef PubMed.
- T.-X. Zong, A. P. Silveira, J. A. V. Morais, M. C. Sampaio, L. A. Muehlmann, J. Zhang, C.-S. Jiang and S.-K. Liu, Recent advances in antimicrobial nano-drug delivery systems, Nanomaterials, 2022, 12(11), 1855 CrossRef.
- A. L. Fymat, Pathogens in the brain and neurodegenerative diseases, J. Neurol. Psychol. Res., 2023, 5(1), 1–14 Search PubMed.
- A. De Giacomo, C. Pedaci, R. Palmieri, M. Simone, A. Costabile and F. Craig, Psychological impact of the sars-cov-2 pandemic in children with neurodevelopmental disorders and their families: Evaluation before and during covid-19 outbreak among an Italian sample, Riv. Psichiatr., 2021, 56(4), 205–210 Search PubMed.
- C. Vicidomini, F. Fontanella, T. D'Alessandro and G. N. Roviello, A survey on computational methods in drug discovery for neurodegenerative diseases, Biomolecules, 2024, 14(10), 1330 CrossRef PubMed.
- S. Pisani, D. Di Martino, S. Cerri, I. Genta, R. Dorati, G. Bertino, M. Benazzo and B. Conti, Investigation and comparison of active and passive encapsulation methods for loading proteins into liposomes, Int. J. Mol. Sci., 2023, 24(17), 13542 CrossRef.
- H. Nsairat, A. A. Ibrahim, A. M. Jaber, S. Abdelghany, R. Atwan, N. Shalan, H. Abdelnabi, F. Odeh, M. El-Tanani and W. Alshaer, Liposome bilayer stability: emphasis on cholesterol and its alternatives, J. Liposome Res., 2024, 34(1), 178–202 CrossRef.
- B. A. Nguyen, D. A. Purnamasari, N. Khan and J.-S. Park, A review of state-of-the-art strategies for encapsulating hydrophilic drugs into lipid nanocarriers, J. Pharm. Invest., 2025, 1–17 Search PubMed.
- S. Wang, X. Wang, Y. Luo and Y. Liang, A comprehensive review of conventional and stimuli-responsive delivery systems for bioactive peptides: from food to biomedical applications, Adv. Compos. Hybrid Mater., 2025, 8(1), 12 CrossRef.
- W. Mu, Q. Chu, Y. Liu and N. Zhang, A review on nano-based drug delivery system for cancer chemoimmunotherapy, Nano-Micro Lett., 2020, 12(1), 142 CrossRef PubMed.
- M. Elmowafy, K. Shalaby, M. H. Elkomy, O. A. Alsaidan, H. A. Gomaa, M. A. Abdel-gawad and E. M. Mostafa, Polymeric nanoparticles for delivery of natural bioactive agents: recent advances and challenges, Polymers, 2023, 15(5), 1123 CrossRef.
- L. Eltaib, Polymeric nanoparticles in targeted drug delivery: Unveiling the impact of polymer characterization and fabrication, Polymers, 2025, 17(7), 833 CrossRef PubMed.
- D. Ghosh, S. Yadav, S. Bag, A. I. Mallick and P. De, Antibacterial activity of hydrophobicity modulated cationic polymers with enzyme and ph-responsiveness, J. Mater. Chem. B, 2024, 12(11), 2894–2904 RSC.
- R. V. Kumarasamy, P. M. Natarajan, V. R. Umapathy, J. R. Roy, M. Mironescu and C. P. Palanisamy, Clinical applications and therapeutic potentials of advanced nanoparticles: a comprehensive review on completed human clinical trials, Front. Nanotechnol., 2024, 6, 1479993 CrossRef.
- C. Viegas, A. B. Patrício, J. M. Prata, A. Nadhman, P. K. Chintamaneni and P. Fonte, Solid lipid nanoparticles vs. nanostructured lipid carriers: a comparative review, Pharmaceutics, 2023, 15(6), 1593 CrossRef PubMed.
- T. Alloush and B. Demiralp, A review of formulation strategies for cyclodextrin-enhanced solid lipid nanoparticles (slns) and nanostructured lipid carriers (nlcs), Int. J. Mol. Sci., 2025, 26(13), 6509 CrossRef PubMed.
- E. Musielak, A. Feliczak-Guzik and I. Nowak, Optimization of the conditions of solid lipid nanoparticles (sln) synthesis, Molecules, 2022, 27(7), 2202 CrossRef.
- S. Perumal, R. Atchudan and W. Lee, A review of polymeric micelles and their applications, Polymers, 2022, 14(12), 2510 CrossRef.
- M. Sirazum, A. Abdelfattah, P. Pandey, A. Ashkarran, S. Tadjiki, S. Sharifi, H. Gharibi, A. A. Saei, M. Mahmoudi and A. Lavasanifar, Protein corona composition modulates uptake of polymeric micelles by colorectal cancer cells, Nanoscale Adv., 2025, 7(16), 4929–4946 RSC.
- Z. Zhang, L. Wang, Z. Guo, Y. Sun and J. Yan, A ph-sensitive imidazole grafted polymeric micelles nanoplatform based on ros amplification for ferroptosis-enhanced chemodynamic therapy, Colloids Surf., B, 2024, 237, 113871 CrossRef.
- A. Serras, C. Faustino and L. Pinheiro, Functionalized polymeric micelles for targeted cancer therapy: steps from conceptualization to clinical trials, Pharmaceutics, 2024, 16(8), 1047 CrossRef PubMed.
- G. Cavalieri, D. Marson, N. Giurgevich, R. Valeri, F. Felluga, E. Laurini and S. Pricl, Molecular ballet: Investigating the complex interaction between self-assembling dendrimers and human serum albumin via computational and experimental methods, Pharmaceutics, 2024, 16(4), 533 CrossRef PubMed.
- J. K. Patra, G. Das, L. F. Fraceto, E. V. R. Campos, M. d. P. Rodriguez-Torres, L. S. Acosta-Torres, L. A. Diaz-Torres, R. Grillo, M. K. Swamy and S. Sharma, et al., Nano based drug delivery systems: recent developments and future prospects, J. Nanobiotechnol., 2018, 16(1), 71 CrossRef PubMed.
- S. Habib, M. Talhami, A. Hassanein, E. Mahdi, M. Al-Ejji, M. K. Hassan, A. Altaee, P. Das and A. H. Hawari, Advances in functionalization and conjugation mechanisms of dendrimers with iron oxide magnetic nanoparticles, Nanoscale, 2024, 16(28), 13331–13372 RSC.
- S. Iraninasab, A. Homaei, E. Mosaddegh and M. Torkzadeh-Mahani, Polyamidoamine dendrimers functionalized with zno-chitosan nanoparticles as an efficient surface for l-asparaginase immobilization, Appl. Biochem. Biotechnol., 2024, 196(2), 971–991 CrossRef PubMed.
- K. Sztandera, J. L. Rodŕıguez-García and V. Ceña, In vivo applications of dendrimers: a step toward the future of nanoparticle-mediated therapeutics, Pharmaceutics, 2024, 16(4), 439 CrossRef.
- Preeti, S. Sambhakar, R. Malik, S. Bhatia, A. Al Harrasi, C. Rani, R. Saharan, S. Kumar, Geeta and R. Sehrawat, Nanoemulsion: an emerging novel technology for improving the bioavailability of drugs, Scientifica, 2023, 2023(1), 6640103 Search PubMed.
- A. Kishore, A. Jain, N. Asthana, R. Milan, S. M. Lakshmi, M. Gupta, A. K. Mahor and J. Kanoujia, Selection criteria for oils, surfactants, and co-surfactants in ocular nanoemulsion formulation: a mini review, Curr. Pharm. Des., 2025, 31(16), 1259–1269 CrossRef.
- A. A. Asmawi, N. Salim, E. Abdulmalek and M. B. Abdul Rahman, Size-controlled preparation of docetaxel-and curcumin-loaded nanoemulsions for potential pulmonary delivery, Pharmaceutics, 2023, 15(2), 652 CrossRef PubMed.
- G. Zuccari and S. Alfei, Development of phytochemical delivery systems by nanosuspension and nano-emulsion techniques, Int. J. Mol. Sci., 2023, 24(12), 9824 CrossRef PubMed.
- Q. Guo, Y. Liu, Y. Huang, G. Hu, G. Tang, X. Zhang, W. Yan, J. Xiao, G. Yan and J. Shi, et al., Nanocapsules bearing imide polymer as wall material for ph-responsive and synergistic fungicidal activity, Chem. Eng. J., 2025, 166144 CrossRef.
- R. A. Abdel-Emam, M. F. Ali, A. S. Hassan and R. B. Abd-Ellatief, Development and evaluation of dexamethasone-loaded bioadhesive polymeric nanocapsules for mitigating cardiac and gastric adverse effects of free dexamethasone, J. Pharm. Invest., 2024, 54(6), 825–844 CrossRef.
- Y. N. Kamel, E. M. El-Marakby and H. A. Gad, Intravenous delivery of furosemide using lipid-based versus polymer-based nanocapsules: in vitro and in vivo studies, Pharm. Dev. Technol., 2024, 29(7), 738–750 CrossRef.
- Y. Meng, C. Qiu, X. Li, D. J. McClements, S. Sang, A. Jiao and Z. Jin, Polysaccharide-based nano-delivery systems for encapsulation, delivery, and ph-responsive release of bioactive ingredients, Crit. Rev. Food Sci. Nutr., 2024, 64(1), 187–201 CrossRef PubMed.
- K. Zhu, L. Wang, Y. Xiao, X. Zhang, G. You, Y. Chen, Q. Wang, L. Zhao, H. Zhou and G. Chen, Nanomaterial-related hemoglobin-based oxygen carriers, with emphasis on liposome and nano-capsules, for biomedical applications: current status and future perspectives, J. Nanobiotechnol., 2024, 22(1), 336 CrossRef PubMed.
- P. Kesharwani, R. Ma, L. Sang, M. Fatima, A. Sheikh, M. A. Abourehab, N. Gupta, Z.-S. Chen and Y. Zhou, Gold nanoparticles and gold nanorods in the landscape of cancer therapy, Mol. Cancer, 2023, 22(1), 98 CrossRef PubMed.
- Y. Lyu, L. M. Becerril, M. Vanzan, S. Corni, M. Cattelan, G. Granozzi, M. Frasconi, P. Rajak, P. Banerjee and R. Ciancio, et al., The interaction of amines with gold nanoparticles, Adv. Mater., 2024, 36(10), 2211624 CrossRef.
- E. Alexander and K. W. Leong, Toxicity and biodistribution comparison of functionalized nanodiamonds, quantum dot nanocarbons and gold nanoparticles, Front. Nanotechnol., 2025, 7, 1512622 CrossRef.
- Y. Hang, A. Wang and N. Wu, Plasmonic silver and gold nanoparticles: shape- and structure-modulated plasmonic functionality for point-of-caring sensing, bio-imaging and medical therapy, Chem. Soc. Rev., 2024, 53(6), 2932–2971 RSC.
- L.-M. Peng, High-performance carbon nanotube thin-film transistor technology, ACS Nano, 2023, 17(22), 22156–22166 CrossRef PubMed.
- N. Vaidya, A. K. Bhatia and S. Dewangan, Carbon allotropes in air purification, in Carbon Allotropes and Composites: Materials for Environment Protection and Remediation, 2023, pp. 191–228 Search PubMed.
- M. Nabitabar, M. Shaterian, H. Danafar and M. Enhessari, Multi-wall carbon nanotube surface-based functional nanoparticles for stimuli-responsive dual pharmaceutical compound delivery, Sci. Rep., 2024, 14(1), 12073 CrossRef.
- S. Alfei, C. Reggio and G. Zuccari, Carbon nanotubes as excellent adjuvants for anticancer therapeutics and cancer diagnosis: A plethora of laboratory studies versus few clinical trials, Cells, 2025, 14(14), 1052 CrossRef.
- M. Kumar, A. R. Hilles, S. H. Almurisi, A. Bhatia and S. Mahmood, Micro and nano-carriers-based pulmonary drug delivery system: Their current updates, challenges, and limitations–a review, JCIS Open, 2023, 12, 100095 CrossRef.
- A. Kumar, K. S. Machhindra, K. Jain and A. K. Yadav, Transdermal drug delivery system, in Novel Carrier Systems for Targeted and Controlled Drug Delivery, 2024, pp. 115–133 Search PubMed.
- L.-A. Keller, O. Merkel and A. Popp, Intranasal drug delivery: opportunities and toxicologic challenges during drug development, Drug Delivery Transl. Res., 2022, 1–23 CAS.
- S. Z. Alshawwa, A. A. Kassem, R. M. Farid, S. K. Mostafa and G. S. Labib, Nanocarrier drug delivery systems: characterization, limitations, future perspectives and implementation of artificial intelligence, Pharmaceutics, 2022, 14(4), 883 CrossRef CAS PubMed.
- R. Rathi, Sanshita, A. Kumar, V. Vishvakarma, K. Huanbutta, I. Singh and T. Sangnim, Advancements in rectal drug delivery systems: clinical trials, and patents perspective, Pharmaceutics, 2022, 14(10), 2210 CrossRef CAS PubMed.
- A. Z. Alkilani, J. Nasereddin, R. Hamed, S. Nimrawi, G. Hussein, H. Abo-Zour and R. F. Donnelly, Beneath the skin: a review of current trends and future prospects of transdermal drug delivery systems, Pharmaceutics, 2022, 14(6), 1152 CrossRef CAS.
- S. Lillo, A. Mirandola, A. Vai, A. M. Camarda, S. Ronchi, M. Bonora, R. Ingar-giola, B. Vischioni and E. Orlandi, Current status and future directions of proton therapy for head and neck carcinoma, Cancers, 2024, 16(11), 2085 CrossRef CAS.
- L. Paoletti, C. Ceccarelli, C. Menichelli, C. Aristei, S. Borghesi, E. Tucci, P. Bastiani and S. Cozzi, Special stereotactic radiotherapy techniques: procedures and equipment for treatment simulation and dose delivery, Rep. Pract. Oncol. Radiother., 2022, 27(1), 1–9 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2026 |