Recent advances in applications of metal hydride hydrogen atom transfer for natural product synthesis
Yogesh G.
Shelke
 * and
Surasmita
Dhal
* and
Surasmita
Dhal
Department of Chemistry, Indian Institute of Technology Madras, Chennai-600036, India. E-mail: ygshelke@iitm.ac.in
First published on 27th November 2025
Abstract
Metal hydride hydrogen atom transfer (MHAT) has emerged as a powerful strategy for alkene hydrofunctionalization, offering high levels of chemo-, regio-, and stereocontrol. In recent years, this reaction has provided new retrosynthetic disconnections in the total synthesis of natural products. Its intrinsic Markovnikov selectivity, broad steric and functional group tolerance, and ability to enable polarity inversion make MHAT particularly well suited for tackling synthetic challenges in complex molecular architectures. This review highlights recent total syntheses that employ MHAT-induced reactions as the key steps, with particular emphasis on reaction optimization and the mechanistic basis of chemo- and stereoselectivity. The advantages and limitations of MHAT relative to traditional hydrofunctionalization approaches are also discussed. Together, these advances underscore the versatility and impact of MHAT as a central platform in natural product synthesis.
1. Introduction
The total synthesis of natural products remains a crucial testing ground for new methodologies and a source of inspiration for innovative chemical reactions.1 In this context, radical-based transformations have gained increasing importance, providing unique solutions to structural and selectivity challenges that are often beyond the reach of traditional two-electron processes.2 Among these, metal hydride hydrogen atom transfer (MHAT) reactions of alkenes have become valuable tools, allowing hydrofunctionalization with Markovnikov selectivity. Their exceptional chemoselectivity, combined with the ability to achieve polarity inversion (umpolung), broadens retrosynthetic options and often streamlines synthetic routes of complex natural products. Mechanistically, MHAT involves the in situ generation of a metal hydride, which transfers a hydrogen atom to the less substituted carbon of an alkene, generating a more stable carbon-centered radical. This radical, depending on its substitution pattern, can subsequently engage with a wide variety of electrophiles and nucleophiles, leading to the formation of C–C, C–H, and C–heteroatom bonds (Fig. 1). Notably, the initial step proceeds via an outer-sphere mechanism without the requirement for direct carbon–metal bond formation, rendering the process highly tolerant of sterically hindered substrates and electrophiles.14aThe foundations of the radical MHAT mechanism were established by pioneering studies from Iguchi,3 Halpern,4 Jackman,5 and Boger.6 Early milestones included Tabushi's 1979 report of Mn-catalyzed hydration of cyclohexene using NaBH4 and air,7 followed by Mukaiyama's 1989 demonstration of Co-catalysed olefin hydration employing alcohols or silanes as hydrogen sources.8 These contributions firmly laid the groundwork for the development of MHAT chemistry. In the mid-2000s, Carreira notably expanded this transformation by utilizing carbon-centered radicals, generated through metal hydride transfer, for various bond-forming reactions, including C–C, C–N, and C–halogen couplings.9
Boger was the first to apply MHAT hydrofunctionalization in the total synthesis of complex natural products, such as vindoline and vinblastine.6 Since then, the field has rapidly grown, with significant advances from Shenvi,10 Herzon,11 Baran,12 and others,13 demonstrating MHAT as a powerful tool in modern synthetic chemistry.
MHAT chemistry has rapidly become an indispensable addition to the synthetic toolbox. Its growing impact in natural product synthesis showcases how a mechanistic insight can mature into a powerful strategic platform—unlocking elegant solutions to the construction of complex and challenging architectures. In this review, we have discussed total syntheses of bioactive natural products reported in the past five years that harness MHAT-mediated olefin hydrofunctionalization as a key step. Particular attention is given to reaction optimization, mechanism, and chemo-, regio-, and stereoselectivity, as well as common side reactions and their implications.
There are excellent review articles in the literature by Shenvi and others, focusing on the hydrofunctionalization of alkenes and their mechanism.14 In 2021, Ma reviewed the applications of MHAT reactions in natural product synthesis.15 Since then, the field has expanded rapidly, with over 40 new total syntheses reported in the past five years. The remarkable growth in MHAT-mediated alkene hydrofunctionalization, along with advances in catalyst design and strategic applications in the total synthesis, warrants an updated review to capture these developments.
In this review, we do not discuss the application of second and third row transition metals hydrides16 or the generation of carbon-centered radicals from sources other than alkenes, such as alcohols, carbonyls, and epoxides.17 The discussion is organized based on the type of bond formed using the MHAT reaction and is divided into four sections: C–C, C–O, and C–H bond formation, and fragmentation. In some total syntheses, MHAT reactions are employed in multiple steps to construct different bonds, and these cases are discussed in one of the four sections based on the key step. The organization of this review is guided not strictly by chronology, but by a desire to provide clarity and coherence, particularly for non-specialist readers. We hope this review will inspire the synthetic community to expand the applications of this versatile transformation further.
2. C–C bond formation
Among all MHAT-mediated hydrofunctionalizations, C–C bond formation—most notably through conjugate addition—has been the most extensively explored in total synthesis, enabling the construction of 3–7-membered rings in diverse natural products. This section is organized according to the type of reaction of the carbon radical, generated after hydrogen atom transfer to an alkene, to form a C–C bond and is subdivided into four categories: conjugate addition, hydrobenzylation, hydroarylation, and SN2′ substitution.2.1 Conjugate addition
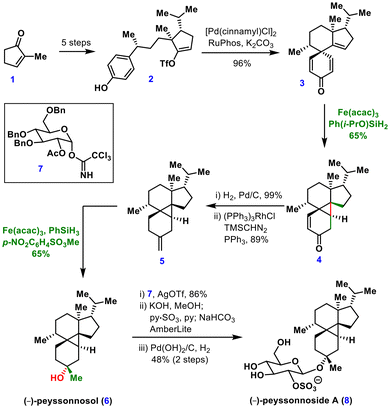
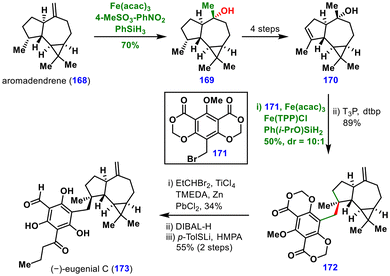
The synthesis began with 2-methylcyclopentenone 1, which was transformed into vinyl triflate 2 over five steps. Pd-catalysed dearomative cyclization of this intermediate furnished the spirocyclic compound 3 in excellent yield. The key cyclopropane was then formed using MHAT reaction. Initial experiments under Baran's protocol [Fe(acac)3, PhSiH3, EtOH, 60 °C] delivered the desired tetracyclic cyclopropane 4 in 65% yield.12b Notably, substituting PhSiH3 with Shenvi's silane Ph(i-PrO)SiH2 enabled the reaction to proceed with comparable yield at room temperature.10a
Further transformations included catalytic hydrogenation followed by ketone methylenation to give intermediate 5, which underwent Studer's anaerobic Mukaiyama hydration.20 Here, methyl 4-nitrobenzenesulfonate acted as the oxygen donor, affording the tertiary alcohol 6. Final installation of sulphated β-linked glucoside 7 and debenzylation furnished (−)-peyssonnoside A (8). This synthesis highlights the robustness of MHAT chemistry for the construction of sterically congested, radical-labile cyclopropanes that are prone to opening under traditional radical reactions.
Very recently the same group extrapolated MHAT initiated olefin–enone cyclization for the construction of a four membered ring in their total synthesis of (−)-psathyrin A, demonstrating the power of MHAT chemistry for the synthesis of strain rings.21
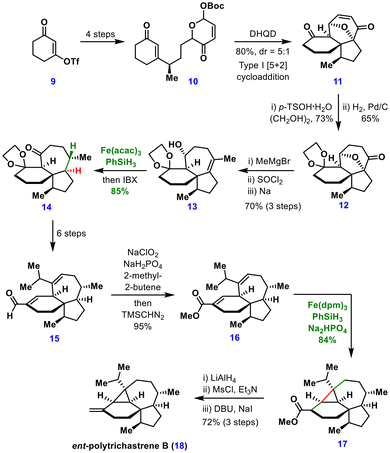
The synthesis commenced from alkenyl triflate 9, which was converted in four steps to the cyclohexanone derivative 10. An intramolecular type-I [5 + 2] cycloaddition mediated by DHQD furnished the spirotricyclic scaffold 11 in 80% yield (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Chemoselective carbonyl protection followed by hydrogenation afforded ketone 12, which, on methyl Grignard addition and reductive elimination, delivered alcohol 13.
1 dr). Chemoselective carbonyl protection followed by hydrogenation afforded ketone 12, which, on methyl Grignard addition and reductive elimination, delivered alcohol 13.
The diastereoselective hydrogenation of the tetrasubstituted alkene proved nontrivial. Both conventional conditions (PtO2, H2, AcOH, EtOAc) and MHAT conditions [(Mn(dpm)3, PhSiH3, TBHP, i-PrOH)] on the ketone substrate exclusively furnished the undesired cis-isomer. Remarkably, subjecting alcohol 13 to MHAT conditions delivered the desired trans-isomer 14 in 85% yield with a 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. The origin of this reversal in stereochemical outcome between the ketone and alcohol substrates remains unclear. Compound 14 was transformed into aldehyde 15 in six steps. Efforts to construct the cyclopropane ring directly via MHAT conjugate reactions on aldehyde 15 proved unsuccessful due to the decomposition of the starting material. To address this, the aldehyde was converted into a more stable ester 16 in two steps. Under optimized conditions [Fe(dpm)3, PhSiH3, Na2HPO4], ester 16 underwent a 3-exo-trig radical cyclization, efficiently generating tetracycle 17 in 84% yield. From this intermediate, the total synthesis of ent-polytrichastrene B (18) was completed in three further steps. This synthesis showcases the unique utility of MHAT reactions for both stereoselective hydrogenation and the construction of highly strained cyclopropane motifs.
1 dr. The origin of this reversal in stereochemical outcome between the ketone and alcohol substrates remains unclear. Compound 14 was transformed into aldehyde 15 in six steps. Efforts to construct the cyclopropane ring directly via MHAT conjugate reactions on aldehyde 15 proved unsuccessful due to the decomposition of the starting material. To address this, the aldehyde was converted into a more stable ester 16 in two steps. Under optimized conditions [Fe(dpm)3, PhSiH3, Na2HPO4], ester 16 underwent a 3-exo-trig radical cyclization, efficiently generating tetracycle 17 in 84% yield. From this intermediate, the total synthesis of ent-polytrichastrene B (18) was completed in three further steps. This synthesis showcases the unique utility of MHAT reactions for both stereoselective hydrogenation and the construction of highly strained cyclopropane motifs.
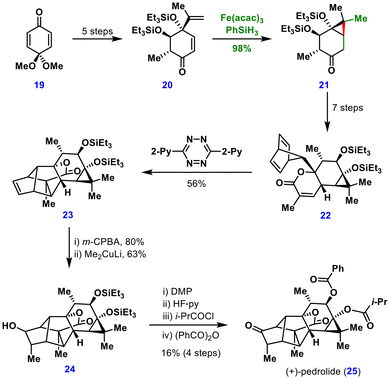
From this key intermediate, a seven-step sequence furnished the norbornadienyl species 22, which underwent a remarkable cascade comprising an inverse-electron-demand Diels–Alder reaction, nitrogen extrusion, a retro-Diels–Alder reaction, and a final intramolecular Diels–Alder cyclization to give compound 23 in 56% yield. Diastereoselective epoxidation of 23, followed by Me2CuLi-mediated epoxide opening, provided alcohol 24, which was converted to (+)-pedrolide (25) through oxidation, global desilylation, and selective acylation/benzoylation steps.
Together with the two preceding syntheses (Schemes 1 and 2), this work highlights novel MHAT-based retrosynthetic disconnections for the assembly of cyclopropane-containing natural products.
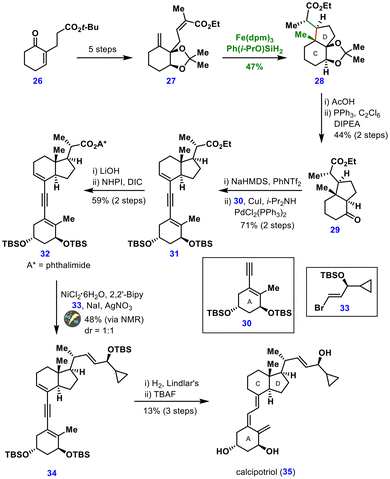
The key diene precursor 27 was prepared in five steps from enone 26. After extensive optimization, Fe(dpm)3 in combination with Ph(i-PrO)SiH2 in DCE/(CH2OH)2 was identified as the optimal system for the intramolecular MHAT annulation, affording the desired product 28 in 47% yield. Notably, phenylsilane gave no reaction, while manganese(III)-based catalysts provided only poor yields. Solvent effects were minimal, although EtOAc/i-PrOH failed to deliver the product. The highly diastereoselective installation of three contiguous stereocenters, including one quaternary center, highlights the synthetic power of the MHAT approach.
Subsequent deprotection of diol 28, followed by a semipinacol rearrangement, furnished ketone 29. Triflation of this ketone and Sonogashira coupling with the A-ring fragment 30 provided the en-yn-ene intermediate 31 in good yield. Transformation of the ethyl ester into the redox-active intermediate 32 enabled electrochemical coupling with vinyl bromide 33, affording the desired adduct 34 in 48% yield and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Final steps, including semihydrogenation, thermal isomerization and silyl deprotection, completed the synthesis of calcipotriol 35.
1 dr. Final steps, including semihydrogenation, thermal isomerization and silyl deprotection, completed the synthesis of calcipotriol 35.
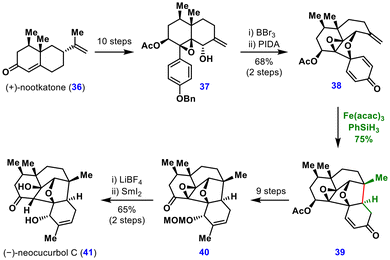
Subsequent benzyl deprotection followed by oxidative dearomatization–cyclization delivered intermediate 38. Treatment of this intermediate with Fe(acac)3 and PhSiH3 triggered the MHAT-mediated conjugate addition, constructing the complete neocucurbol skeleton 39 in 75% yield. From this framework, elaboration of the enone ring over nine steps furnished intermediate 40, which upon MOM deprotection and reductive epoxide opening completed the synthesis of (−)-neocucurbol C (41). This work demonstrates the power of MHAT chemistry in assembling challenging oxa-bridged architectures in natural product synthesis.
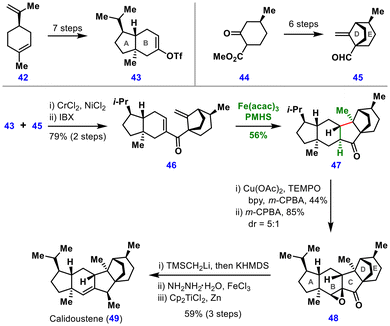
The central C-ring of (−)-calidoustene (49) was then synthesized via MHAT cyclization of substrate 46. Using Fe(acac)3 and PhSiH3 initially gave the desired ketone 47 in only 40% yield, accompanied by significant alkene reduction.27 This inefficiency was attributed to steric hindrance from the bicyclo[3.2.1]octane motif and excess Fe–H generated by the strongly reducing PhSiH3. Replacing PhSiH3 with poly(methylhydrosiloxane) (PMHS) provided a controlled hydride release, lowering Fe–H concentration and improving yield, scalability, and reproducibility. Under these conditions, the yield of ketone 47 was increased to 56%.
Subsequent regioselective dehydrogenation and epoxidation furnished intermediate 48, which underwent Peterson olefination and hydrogenation to introduce a β-methyl group. The epoxide served as an olefin-protecting group and was later reverted to the olefin, thereby completing the synthesis of (−)-calidoustene (49). Importantly, the MHAT-initiated conjugate addition reported here represents a rare example of a Baldwin-disfavored 5-endo-trig radical cyclization.28
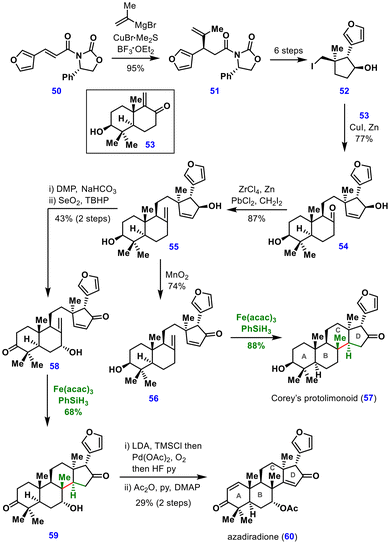
In 2025, Renata and co-workers reported a sclareolide-derived chemoenzymatic strategy that enabled the total synthesis of both ring-intact and seco-limonoids.30 MHAT-induced conjugate addition was used to construct the C-ring of azadiradione (60) and Corey's protolimonoid (57) (Scheme 7).
The synthesis began with a copper-mediated Michael addition to an Evan's auxiliary 50, affording a single diastereomer of intermediate 51, which was elaborated in six steps to chiral cyclopentanol 52. Cyclopentanol 52 was then coupled with the enone partner 53 under Luche's conditions to give ketone 54, which underwent C8-methylenation to furnish the common intermediate 55. This intermediate served as the branching point for the divergent syntheses of Corey's protolimonoid (57) and azadiradione (60).
For Corey's protolimonoid (57), allylic oxidation of intermediate 55 furnished enone 56, which, under Fe(acac)3/PhSiH3-promoted MHAT conjugate addition, delivered the target scaffold. In the case of azadiradione (60), global oxidation of alcohols followed by Riley allylic oxidation afforded diketone 58. Subjecting this diketone to MHAT conjugate addition afforded the intact limonoid framework 59 in 68% yield. A final one-pot sequence involving olefin installation and silyl deprotection then completed the synthesis of azadiradione (60). These total syntheses highlight the utility of MHAT reactions for constructing the sterol C-ring, thereby introducing new retrosynthetic disconnections within the sterol family of natural products.
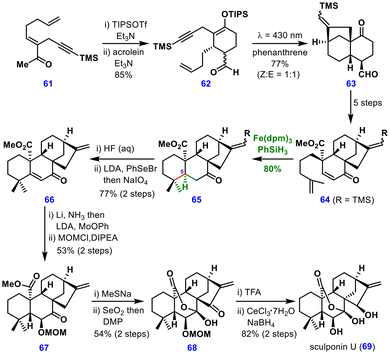
The synthesis commenced with the conversion of precursor 61 into its silyl enolate, which underwent a Diels–Alder reaction with acrolein to deliver adduct 62 in 85% yield with excellent diastereoselectivity. From this, the cyclohexanone-fused bicyclo[3.2.1]octane intermediate 63 was assembled through a photoinduced electron transfer (PET) process on silyl enolate 62, proceeding via a cascade 6-endo-trig followed by 5-exo-dig cyclization.
This bicyclo[3.2.1]octane intermediate 63 was transformed into enone 64 over five steps. The key MHAT step was then achieved using Fe(dpm)3 catalysis, which led to C–C bond formation, affording the cis-decalin intermediate 65 in 80% yield. Notably, the bulky TMS group preserved the exocyclic double bond within the bicyclic system during MHAT reaction, indicating the excellent functional group tolerance of the reaction.
Since the absolute configuration at C5 in this intermediate was opposite to that in the natural product, it was epimerized via enone 66, followed by Li/NH3-mediated alkene saturation. In the same pot, α-hydroxylation was carried out, and the product was subsequently protected as the MOM ether. Demethylation of ester 67 generated a cyclic hemiketal, which upon allylic oxidation furnished intermediate 68. Final deprotection of the MOM ether and carbonyl reduction completed the first total synthesis of sculponin U (69).
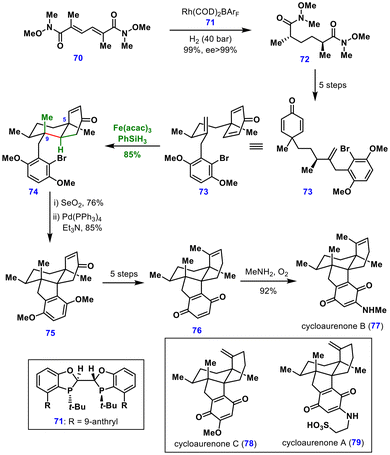
Cyclohexadienone 73 then underwent a Giese–Baran-type cyclization promoted by Fe(acac)3 and PhSiH3, delivering the cis-decalin scaffold 74 bearing three stereocenters via local desymmetrization in 85% yield and as a single diastereomer. The excellent diastereocontrol was attributed to the minimized pseudo-1,3-diaxial interactions between the C9 methyl and the C5 vinyl substituent, relative to the steric clash of C9 and C5 methyl groups.
Subsequent oxidative dehydrogenation of cyclohexenone 74, followed by an intramolecular reductive Heck-type cyclization, furnished tetracyclic intermediate 75, which was advanced to quinone 76 through five additional steps, including 1,3-ketone transposition, triflation, Stille coupling, and oxidation. Treatment of this quinone 76 with methylamine under aerobic conditions afforded cycloaurenone B (77) in 92% yield. The same strategy also enabled the total syntheses of cycloaurenones A (79) and C (78), along with dysiherbols A–E (not shown).
These studies showcase how subtle 1,3-diaxial interactions in six-membered transition states can be exploited to control stereochemistry in MHAT-induced conjugate additions.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exo
1 exo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
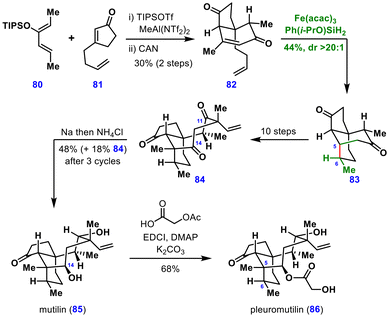
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) endo ratio. Oxidation of the desired exo-isomer with CAN yielded diene 82, which underwent MHAT-induced conjugate addition using Fe(acac)3 and Shenvi's silane [Ph(i-PrO)SiH2] to deliver the tricyclic core 83 in 44% yield as a single diastereomer. In this transformation, the metal hydride selectively engaged with a monosubstituted alkene, generating a secondary radical that underwent intramolecular conjugate addition to the tethered enone. The authors noted that the introduction of an additional electron-withdrawing group or other substituents α to the enone diminished the diastereoselectivity or yield of the products, respectively.
endo ratio. Oxidation of the desired exo-isomer with CAN yielded diene 82, which underwent MHAT-induced conjugate addition using Fe(acac)3 and Shenvi's silane [Ph(i-PrO)SiH2] to deliver the tricyclic core 83 in 44% yield as a single diastereomer. In this transformation, the metal hydride selectively engaged with a monosubstituted alkene, generating a secondary radical that underwent intramolecular conjugate addition to the tethered enone. The authors noted that the introduction of an additional electron-withdrawing group or other substituents α to the enone diminished the diastereoselectivity or yield of the products, respectively.
The resulting tricyclic diketone 83 was converted to triketone 84 in ten steps. Treatment of this intermediate with sodium in ethanol resulted in highly chemo- and stereoselective reduction of the C11 and C14 ketones, affording mutilin (85). Subsequent acylation and acetate deprotection furnished pleuromutilin (86), completing the total synthesis in 16 steps. Notably, while most MHAT-based reactions rely on 1,1-disubstituted olefins as hydrogen acceptors, this work demonstrated that even a monosubstituted olefin can serve as a viable radical precursor for conjugate addition in useful yields.
Next, MHAT-induced intramolecular conjugate addition on α,β-unsaturated aldehyde was explored. When iron(III)-based catalysts were tested for the MHAT reaction, the required product 90 was obtained in 50% yield, but significant decomposition was observed. Hence, the authors used Co(SaltBu,tBu)Cl, which afforded 90 in 45% yield. To further enhance efficiency, a photoredox cycle was explored. Remarkably, Co(TPP) catalysed the transformation in the presence of a photoredox catalyst and visible light, delivering the product smoothly. Control experiments revealed that, even in the absence of the photoredox catalyst, irradiation with a blue LED (465 nm) was crucial, providing 90 in 64% NMR yield. Since alkyl cobalt(III) porphyrins absorb in a similar wavelength range, it was proposed that photoexcitation specifically facilitated Co–C bond homolysis, thereby enabling turnover of the active catalytic species. Importantly, the reaction exhibited high efficiency at catalyst loadings as low as 0.1 mol%. Moreover, scale-up in a circulating flow system under irradiation with a slightly different blue LED (450 nm) significantly reduced the reaction time and furnished the desired product 90 in 68% yield as a single diastereomer. The light–dark-cycle experiment coupled with in situ IR spectroscopy revealed that the reaction stalled in the absence of light and restarted in the presence of light.
Deprotection of the silyl group in 90 followed by aldol cyclization and oxidative epoxide formation furnished 91 in 55% yield. Sequential epoxidations coupled with epimerization at C3 delivered 92 in 53% yield over two steps. Finally, a one-pot silylation–selenenylation–oxidation sequence established the C3–C4 alkene, completing the synthesis of (−)-triptonide (93). This synthesis demonstrated that visible light catalysis could be used to increase the efficiency of MHAT processes and opens new avenues for further exploration in this field.
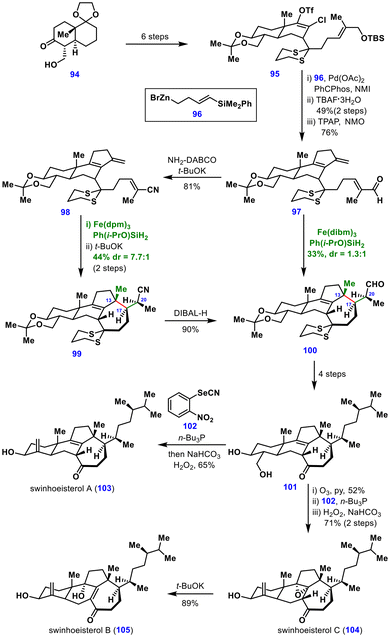
The synthesis began from 94, obtained in three steps from the (S)-Wieland–Miescher ketone, which was transformed in six additional steps into the chloroenol triflate 95. Coupling of this triflate with 96 under tandem Negishi–Heck conditions efficiently delivered the tetracyclic core. A silyl substituent was essential to suppress isomerization of the exocyclic olefin to its endocyclic counterpart. Subsequent desilylation and oxidation furnished the acrolein intermediate 97, the direct precursor for the MHAT transformation.
Given that the MHAT reaction was expected to forge the seven-membered ring and three contiguous stereocenters, achieving high diastereoselectivity at C13 and C17 was critical, though the C20 stereocenter could be epimerized under basic conditions. Treatment of acrolein 97 with Fe(dibm)3 and Ph(i-PrO)SiH2 afforded the cyclized product 100 in 33% yield with a 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr at C20, alongside additional diastereomers. Attempts to epimerize the C20 stereocenter with DBU were unsuccessful. Replacing the acrolein with an acrylate led to incorrect stereochemistry at both C17 and C20. Optimal results were obtained using a sterically less demanding acrylonitrile derivative 98, which under optimized conditions furnished the desired product in 48% yield with a 1
1 dr at C20, alongside additional diastereomers. Attempts to epimerize the C20 stereocenter with DBU were unsuccessful. Replacing the acrolein with an acrylate led to incorrect stereochemistry at both C17 and C20. Optimal results were obtained using a sterically less demanding acrylonitrile derivative 98, which under optimized conditions furnished the desired product in 48% yield with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 dr. Subsequent epimerization with t-BuOK improved the diastereomeric ratio to 7.7
1.5 dr. Subsequent epimerization with t-BuOK improved the diastereomeric ratio to 7.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the required isomer 99 was obtained in 44% yield over two steps. Remarkably, the tetrasubstituted olefin embedded in 97 and 98 remained untouched throughout the MHAT process, and the geometry of the C17–C20 olefin did not affect the stereochemical outcome in the conjugate addition.
1, and the required isomer 99 was obtained in 44% yield over two steps. Remarkably, the tetrasubstituted olefin embedded in 97 and 98 remained untouched throughout the MHAT process, and the geometry of the C17–C20 olefin did not affect the stereochemical outcome in the conjugate addition.
From this stage, the reduction of nitrile 99 provided aldehyde 100, which was advanced in four steps to intermediate 101. A Grieco–Sharpless elimination then completed the synthesis of swinhoeisterol A (103). Intermediate 101 was further functionalized to complete the total synthesis of Swinhoeisterols B (105) and C (104).
This synthesis highlights the exceptional chemoselectivity of MHAT chemistry: the hydrogen transfer occurred exclusively to the 1,1-disubstituted olefin, leaving the tetrasubstituted olefin intact.
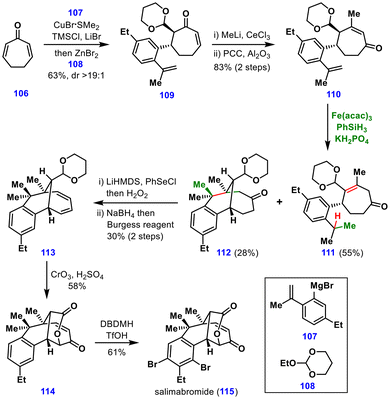
The synthesis began with cycloheptadienone 106, which underwent a tandem Michael/Mukaiyama aldol reaction to furnish intermediate 109. Oxidative rearrangement of the tertiary allylic alcohol affected ketone transposition, providing enone 110 in 83% yield. To construct the benzo-fused [4.3.1] framework, the authors screened multiple transition metals (Fe, Co, Mn, etc.) in combination with various silanes and solvents. The optimal conditions—Fe(acac)3, PhSiH3, and KH2PO4 in EtOH at 60 °C—afforded the desired product 112 in 28% yield, alongside an undesired alkene 111 (55% yield) arising from a competing [1,5]-HAT pathway. The low yield of the desired product 112 was attributed to steric effects due to vicinal quaternary stereocenters and the proximity of the tertiary radical to the γ-hydrogen of the enone.
Next, intermediate 112via a sequence of dehydrogenation, reduction, and dehydration was converted to diene 113 in moderate yield. Oxidation with the Jones reagent then produced the butyrolactone-fused enone 114, which upon dibromination furnished salimabromide (115) in good yield. In addition, the authors achieved a formal enantioselective total synthesis of (+)-salimabromide, employing an (S,R)-ZhaoPhos-catalysed asymmetric hydrogenation as the key enantioinduction step.
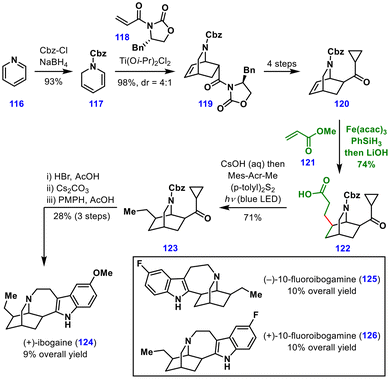 | ||
| Scheme 14 Olson's total synthesis of (+)-ibogaine, (−)-10-fluoroibogamine, and (+)-10-fluoroibogamine. | ||
The synthesis commenced with the protection and reduction of pyridine 116 to afford dihydropyridine 117 in 93% yield. A diastereoselective Diels–Alder reaction with the chiral oxazolidinone-derived enamide 118 delivered bicyclic intermediate 119, which was transformed into cyclopropyl ketone 120 over four steps. Chelation-controlled, exo-selective MHAT-induced conjugate addition, promoted by Fe(acac)3 and PhSiH3, proceeded smoothly and delivered the corresponding acid 122 after hydrolysis. A subsequent photoredox-catalysed hydrodecarboxylation furnished 123 in 71% yield. The final synthetic sequence of Cbz deprotection, cyclopropane opening, and Fischer indole cyclization delivered (+)-ibogaine (124) in overall 11 steps and 9% overall yield. The same strategy enabled the concise syntheses of (+)-10-fluoroibogamine (126) and (−)-10-fluoroibogamine (125). These syntheses underscore the potential of chelation control as a general strategy for stereochemical tuning in MHAT-mediated conjugate additions and thus expand the scope of the reaction.
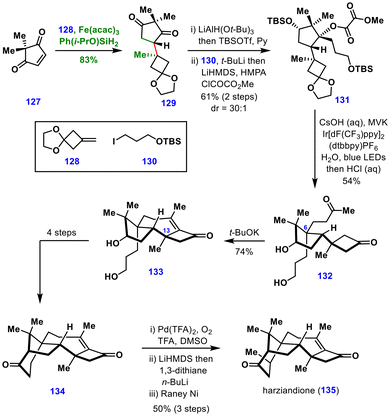
The crucial enone functionality and the C13 quaternary stereocenter in 133 were subsequently forged through a locally desymmetrizing intramolecular aldol reaction. From this intermediate, four additional steps provided ketone 134, which upon dehydrogenation and C5 methylation yielded harziandione (135). Harziandione (135) then served as a common intermediate, enabling divergent access to the broader family of harziane diterpenoids.
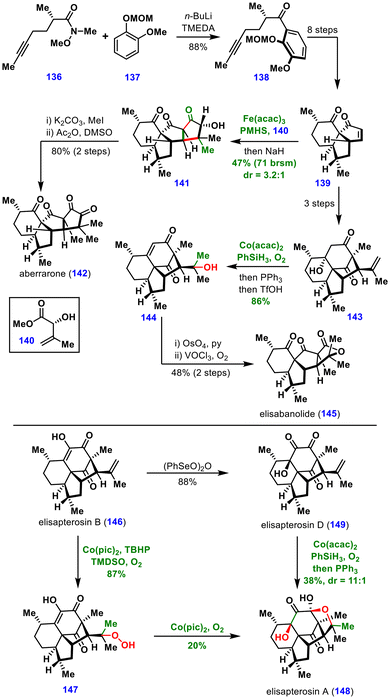
The diterpenoids whose syntheses involved MHAT-mediated transformations are discussed here. The common tricyclic precursor 139 was first prepared via ortho-lithiation of compound 137, followed by acylation with the Weinreb amide 136, affording ketone 138 in 88% yield. Subsequent key transformations—including ODI [5 + 2] cycloaddition/1,2-acyl migration, epoxidation, pinacol coupling, and Grobb fragmentation—produced the common tricyclic intermediate 139 in eight steps.
The pivotal MHAT-induced intermolecular conjugate addition between compounds 139 and 140, followed by NaH-mediated Dieckmann cyclization, delivered the tetracyclic compound 141 in 47% yield with 3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivity. Furthermore, methylation and oxidation of the resulting alcohol completed the total synthesis of aberrarone (142).
1 diastereoselectivity. Furthermore, methylation and oxidation of the resulting alcohol completed the total synthesis of aberrarone (142).
For the synthesis of elisabanolide (145), the common precursor 139 was converted to tetracyclic intermediate 143 in three steps. This intermediate underwent Mukaiyama hydration followed by elimination of the tertiary alcohol, generating enone 144 in 86% yield. Subsequent dihydroxylation and oxidative cleavage furnished elisabanolide (145).
In the same study, MHAT-based transformations were also applied to the synthesis of the revised structures of elisapterosins A (148) and D (149) from elisapterosin B (146). Chemoselective hydroperoxidation using Woerpel's protocol yielded the unstable hydroperoxide 147, which upon treatment with Co(pic)2 and O2 underwent intramolecular oxygen atom transfer to give elisapterosin A (148). Moreover, elisapterosin A (148) was also obtained by Mukaiyama hydration of elisapterosin D (149).
Collectively, these total syntheses highlight the versatility of MHAT-induced transformations in enabling divergent total syntheses, structural revisions, and late-stage diversification of complex natural products.
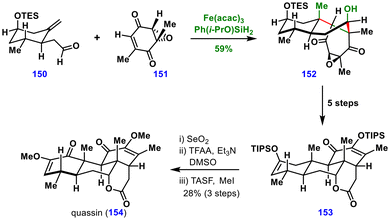
The key aldehyde fragment 150 was obtained from (+)-3-methylcyclohexanone in four steps, while the epoxy quinone fragment 151 was prepared in three steps from 2,6-dimethylbenzoquinone. In the presence of Fe(acac)3 and Shenvi's silane, the MHAT cascade between these two fragments proceeded efficiently to furnish the tricarbocyclic intermediate 152 in 59% yield. The resulting alcohol 152 was elaborated to advanced intermediate 153 in five steps. The synthesis was completed through an allylic hydroxylation, oxidation of the secondary alcohol, and final desilylation in the presence of MeI, delivering (+)-quassin (154) in just 14 steps from commercially available starting materials. Notably, here the MHAT reaction enabled the construction of two key carbon–carbon bonds as well as the central six-membered ring with excellent diastereoselectivity, offering a powerful strategy for the total synthesis of other terpenoids.
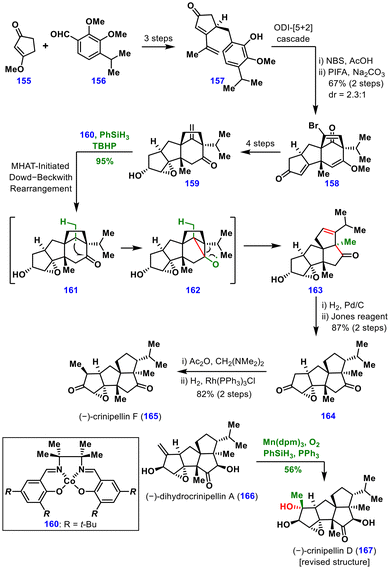
The synthesis began with cyclopentenone 155 and aldehyde 156, which were transformed in three steps into vinylphenol 157. An ODI [5 + 2] cycloaddition furnished intermediate 158 in 67% yield. Enone 158 was converted to epoxide 159 in four steps, including regioselective epoxidation, global reduction of the vinyl bromide and ketone, and methylenation of the ketone. For the pivotal Dowd–Beckwith rearrangement, several conditions were screened on the model substrate. Mn(dpm)3 led to undesired olefin reduction, while Fe(acac)3 and Fe(acac)2 gave complex mixtures. In contrast, the Co(Salen) catalyst 160 with PhSiH3 and TBHP in i-PrOH afforded the rearranged product in a quantitative yield. Under these optimized conditions, the actual substrate 159 was efficiently converted to tetraquinane 163 in 95% yield.
From this intermediate, substrate-controlled reduction followed by Jones oxidation produced diketone 164, which underwent regioselective methylenation and Wilkinson hydrogenation to furnish (−)-crinipellin F (165) in excellent yield. This versatile tetraquinane scaffold was further diversified to access seven additional crinipellins. Moreover, Mn(dpm)3-catalysed β-face selective hydration converted (−)-dihydrocrinipellin A (166) into (−)-crinipellin D (167), leading to a structural revision of the latter. Overall, this work underscores the power of MHAT-driven molecular rearrangements in generating common intermediates that can be leveraged for the collective total synthesis of complex natural products.
2.2 Hydrobenzylation
Optimization of the reaction conditions revealed that Fe(acac)3, Fe(TPP)Cl (TPP = tetraphenylporphyrin), and Ph(i-PrO)SiH2 provided the hydrobenzylated product in 50% yield with a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. This iron-catalysed MHAT hydrobenzylation proved to be general and exhibited a broad substrate scope. Subsequent phosphonic anhydride-mediated alcohol dehydration delivered intermediate 172. Finally, a reaction sequence involving modified Takai olefination, selective reduction and O-demethylation completed the total synthesis of (−)-eugenial C (173). This work significantly broadens the utility of MHAT chemistry for the construction of C(sp3)–C(sp3) bonds, offering valuable opportunities for pharmaceutical and drug synthesis.
1 dr. This iron-catalysed MHAT hydrobenzylation proved to be general and exhibited a broad substrate scope. Subsequent phosphonic anhydride-mediated alcohol dehydration delivered intermediate 172. Finally, a reaction sequence involving modified Takai olefination, selective reduction and O-demethylation completed the total synthesis of (−)-eugenial C (173). This work significantly broadens the utility of MHAT chemistry for the construction of C(sp3)–C(sp3) bonds, offering valuable opportunities for pharmaceutical and drug synthesis.
2.3 Hydroarylation
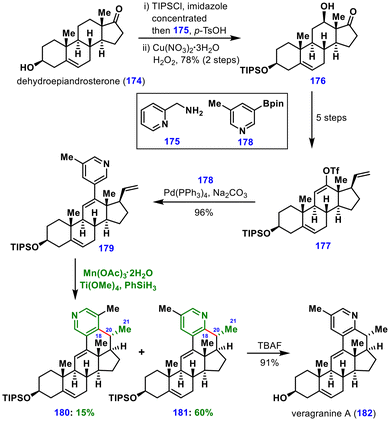
The synthesis commenced from dehydroepiandrosterone (174), which underwent alcohol protection, imine formation with 2-picolylamine 175, and Schönecker–Baran C–H oxidation to give ketone 176. Conversion of this ketone into triflate 177 over five steps, followed by a Pd-catalysed Suzuki–Miyaura coupling with boronoate 178, furnished intermediate 179 in 96% yield.
Optimization of the MHAT–Minisci cyclization established Mn(OAc)3, PhSiH3, and Ti(OMe)4 in THF/MeOH as optimal conditions, affording the desired product 181 in 60% yield along with the regioisomer 180 in 15% yield. Ti(OMe)4 acted as a Lewis acid, activating the pyridine ring to facilitate efficient cyclization. The stereochemical outcome at C20 was attributed to a 1,3-diaxial interaction between the C20 substituent and the axial C18 methyl group. Subsequent desilylation delivered (−)-veragranine A (182) in 91% yield.
This strategy was further extended to prepare a series of veragranine A analogs, whose biological evaluation identified new calcium and sodium channel blockers. Overall, this work showcased the power of MHAT chemistry in the medicinal chemistry of natural products and its potential in drug discovery.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 60 °C as the optimal system, while bulkier iron complexes such as Fe(dibm)3 and Fe(dpm)3 delivered the product in reduced yields.
1) at 60 °C as the optimal system, while bulkier iron complexes such as Fe(dibm)3 and Fe(dpm)3 delivered the product in reduced yields.
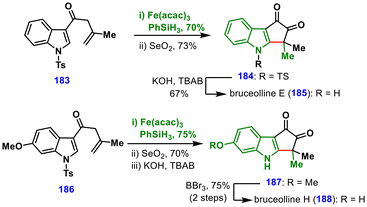
Under these optimized conditions, a broad range of N-fused indoles, N-fused pyrroles, and C-fused indoles were efficiently synthesized, underscoring the generality of this approach. The utility of the method was further highlighted in the total syntheses of bruceolline E (185) and bruceolline H (188). For bruceolline E (185), MHAT-induced cyclization of the C3-alkenylated indole 183 followed by α-oxidation furnished diketone 184 in 73% yield, and subsequent tosyl deprotection completed the total synthesis. Similarly, bruceolline H (188) was obtained from intermediate 186via MHAT cyclization, Riley oxidation, and sequential tosyl and methyl ether deprotections.
Together with Dai's semisynthesis of (−)-veragranine A (182) (Scheme 20), these studies demonstrate the versatility of MHAT-initiated hydroheteroarylation in constructing diverse heteroaryl-fused frameworks that are widely represented in natural products and bioactive molecules.
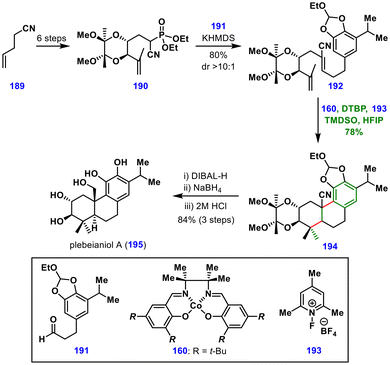
Subsequent DIBAL-H reduction of nitrile 194 afforded an aldehyde, which was further reduced to the corresponding alcohol. Final global deprotection delivered plebeianiol A (195). Comparison of the spectroscopic data of the synthetic material with that of the natural isolate led to a structural revision of plebeianiol A (195). This total synthesis demonstrated that MHAT-induced polyene cyclization is an effective alternative to cationic polyene cyclization, with broad applicability in natural product synthesis.
2.4 SN2′ substitution
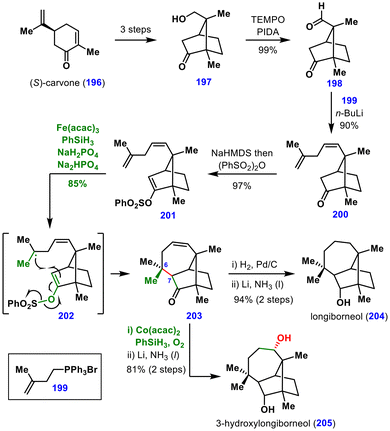
To achieve this, it was essential to identify a suitable radical acceptor derived from the ketone. Although enol ethers were a logical choice, their use was complicated by the fact that radical addition to enol ethers is rare and, moreover, enol ethers can function as H-atom acceptors in MHAT reactions. To overcome this issue, the authors employed electron-deficient enol derivatives. After extensive screening, vinyl sulfonates were identified as the most effective acceptors. Subjecting vinyl sulfonate 201 to MHAT conditions generated the nucleophilic tertiary radical 202 from the terminal alkene, which subsequently added to the electrophilic enol ether. Loss of the PhSO2 group then afforded the desired product 203 in 85% yield.
Final hydrogenation of the double bond, followed by ketone reduction, delivered longiborneol 204. Furthermore, this intermediate 203 was diversified to access other congeners, including 3-hydroxylongiborneol (205), obtained via MHAT-induced hydration reaction. This total synthesis demonstrated that electron-deficient enol ethers can serve as radical acceptors in MHAT reactions, offering a novel strategy for the α-functionalization of ketones.
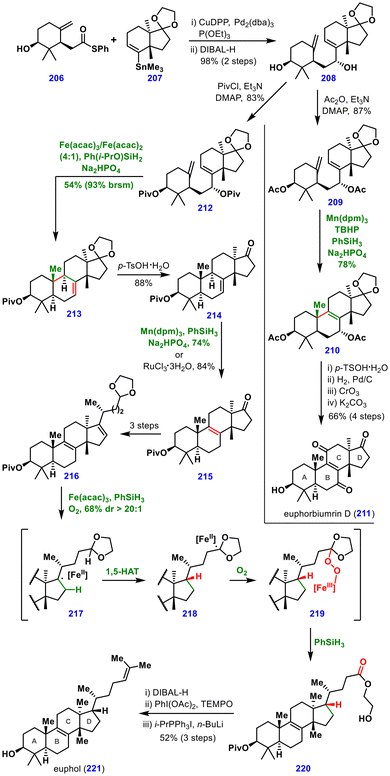
The synthesis commenced with the preparation of thioester 206 and stannane 207 from pent-4-ynal and the Wieland–Miescher ketone, respectively (not shown). A Liebeskind stannane–thioester coupling between these fragments, followed by DIBAL-H reduction, furnished diol 208 in 98% yield over two steps. This diol 208 served as a common precursor for the total syntheses of euphol (221), euphorbiumrin D (211), 25,26,27-trisnor-3β-hydroxy-euphan-24-al, and 3-oxo-tirucall-7-ene-3,20-dione. Importantly, MHAT reactions were exploited for the construction of the B-ring of the euphane framework. Screening of Fe, Mn, and Co catalysts with various silanes identified two sets of conditions: (A) Mn(dpm)3, TBHP, PhSiH3, and Na2HPO4 promoted cycloisomerization, while (B) Fe(acac)3, Fe(acac)2, Ph(i-PrO)SiH2, and Na2HPO4 gave the SN2′ substitution product.
For the synthesis of euphorbiumrin D (211), bis-acetylated diol 209 was subjected to MHAT conditions A, affording intermediate 210, which was advanced to the natural product in four steps, including ketal deprotection, hydrogenation, allylic oxidation, and acetate deprotection. In the case of euphol (221), the bis-pivalate-protected diol 212 was treated under conditions B to deliver the SN2′ substitution product 213 in 54% yield. Subsequent ketal deprotection gave ketone 214, which underwent either MHAT-induced or Ru-catalysed olefin isomerization to furnish intermediate 215 and was further elaborated in three steps to 216. A critical challenge was the selective hydrogenation of a trisubstituted alkene in the presence of a tetrasubstituted one in compound 216. The diimide reduction and Pd/C hydrogenation led to mixtures or incorrect diastereomers. In contrast, Fe-catalysed MHAT hydrogenation provided the desired product in 68% yield with >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivity. This sequence proceeded through intramolecular 1,5-HAT, oxygen capture, and decomposition of an orthoester intermediate (through 217 to 219), ultimately furnishing euphol (221) after three additional steps. Together, these syntheses underscore the tunability of MHAT conditions to access distinct products and demonstrate how multiple MHAT-based transformations can be orchestrated within a single synthesis to construct a wide variety of bonds.
1 diastereoselectivity. This sequence proceeded through intramolecular 1,5-HAT, oxygen capture, and decomposition of an orthoester intermediate (through 217 to 219), ultimately furnishing euphol (221) after three additional steps. Together, these syntheses underscore the tunability of MHAT conditions to access distinct products and demonstrate how multiple MHAT-based transformations can be orchestrated within a single synthesis to construct a wide variety of bonds.
3 C–O bond formation
The Mukaiyama hydration is a classic example of C–O bond formation via MHAT chemistry. In natural product synthesis, tertiary alcohols are often accessed by trapping carbon-centered radicals generated after MHAT with molecular oxygen or oxygen donors such as Studer's reagent. In contrast, examples involving intramolecular trapping of such radicals by tethered hydroxyl groups are relatively rare.3.1 Hydration
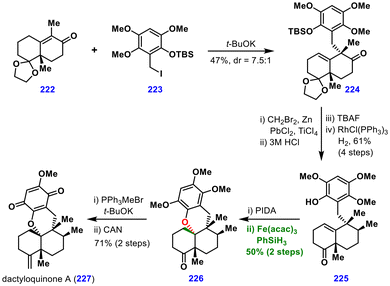
The authors proposed that the sterically hindered tertiary radical produced during MHAT was less likely to react at the carbon centre and was instead intercepted by oxygen. Mechanistically, two scenarios were proposed: (i) direct radical addition to the oxygen atom or (ii) a more probable pathway involving radical–polar crossover to form a tertiary carbocation, which was then trapped by tethered oxygen.
Finally, the Wittig olefination of ketone 226, followed by oxidation of the aromatic ring, completed the synthesis of dactyloquinone A (227). This total synthesis exemplifies the application of MHAT chemistry for the construction of oxa-cycles, which can be extended to aza-cycles as well.
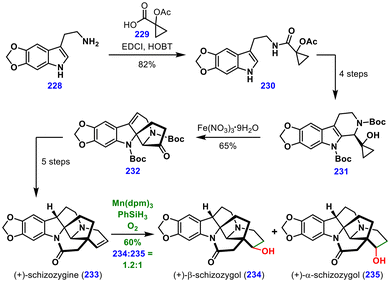
In four subsequent steps, intermediate 230 was transformed into 231, which then underwent a key dearomative cyclization mediated by Fe(NO3)3·9H2O, affording tetracyclic intermediate 232 in 65% yield. From this stage, a five-step sequence delivered the target (+)-schizozygine (233).
Crucially, late-stage MHAT functionalization of (+)-schizozygine (233) with Mn(dpm)3, PhSiH3, and O2 furnished a separable mixture of (+)-β-schizozygol (234) and (+)-α-schizozygol (235) in 60% combined yield with a 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivity. This transformation highlights the power of MHAT chemistry for the late-stage diversification of complex alkaloid frameworks.
1 diastereoselectivity. This transformation highlights the power of MHAT chemistry for the late-stage diversification of complex alkaloid frameworks.
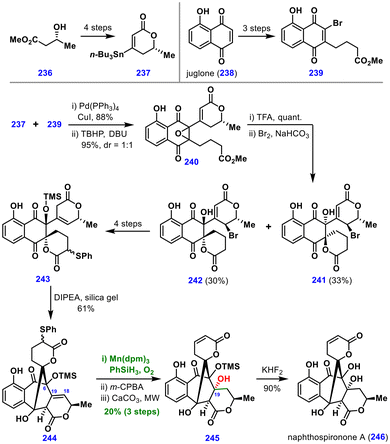
The synthesis commenced with the preparation of stannane 237 from chiral ester 236 and bromide 239 from juglone 238. A Stille coupling between these partners, followed by regioselective epoxidation, afforded intermediate 240 in 95% yield as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereomers. Intramolecular epoxide opening and γ-bromination then provided separable diastereomers 241 and 242. Lactone 242 was converted into sulphide 243 in four steps, which subsequently underwent intramolecular aldol cyclization to deliver a cage-like compound 244. Attempts to install the tertiary alcohol at C19 using traditional methods, such as acid-catalysed hydration or reductive epoxide opening, were unsuccessful. In contrast, treatment of 244 with Mn(dpm)3, PhSiH3, and O2 furnished the desired tertiary alcohol cleanly. Subsequent oxidation of the sulphide and elimination provided intermediate 245. Importantly, TMS protection of the C6 alcohol was critical for the success of this MHAT hydration: in its absence, undesired hemiketal formation via ring opening was observed. Finally, desilylation delivered naphthospironone A (246).
1 mixture of diastereomers. Intramolecular epoxide opening and γ-bromination then provided separable diastereomers 241 and 242. Lactone 242 was converted into sulphide 243 in four steps, which subsequently underwent intramolecular aldol cyclization to deliver a cage-like compound 244. Attempts to install the tertiary alcohol at C19 using traditional methods, such as acid-catalysed hydration or reductive epoxide opening, were unsuccessful. In contrast, treatment of 244 with Mn(dpm)3, PhSiH3, and O2 furnished the desired tertiary alcohol cleanly. Subsequent oxidation of the sulphide and elimination provided intermediate 245. Importantly, TMS protection of the C6 alcohol was critical for the success of this MHAT hydration: in its absence, undesired hemiketal formation via ring opening was observed. Finally, desilylation delivered naphthospironone A (246).
This work highlights the remarkable selectivity, functional-group tolerance, and utility of Mukaiyama hydration compared to conventional approaches for tertiary alcohol introduction.
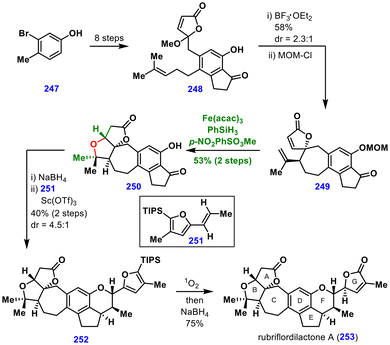
Ketone 249 was then subjected to a Mukaiyama hydration under Studer's conditions. In the same pot, the resulting tertiary alcohol underwent an oxa-Michael addition, efficiently constructing the AB ring system of rubriflordilactone A to produce 250.
The next major challenge was the stereoselective intermolecular ortho-quinone methide [4 + 2] cycloaddition to form the F ring. Indanone 250 was reduced to the corresponding alcohol, and the crude product was treated with 251 in the presence of Sc(OTf)3, delivering the cycloadduct 252 in 40% yield and dr = 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, via the top-face approach of 251. Finally, a photocatalytic oxidation of the butanolide, followed by reduction, completed the synthesis of (±)-rubriflordilactone A (253).
1, via the top-face approach of 251. Finally, a photocatalytic oxidation of the butanolide, followed by reduction, completed the synthesis of (±)-rubriflordilactone A (253).
This synthesis demonstrates the reduced susceptibility of the MHAT approach to steric hindrance, enabling the efficient formation of tertiary alcohols that can participate in further transformations such as Michael additions. In contrast, conventional hydration methods that proceed via carbocation intermediates are more prone to undesired Wagner–Meerwein rearrangements, underscoring the strategic advantage of the MHAT methodology in the total synthesis.
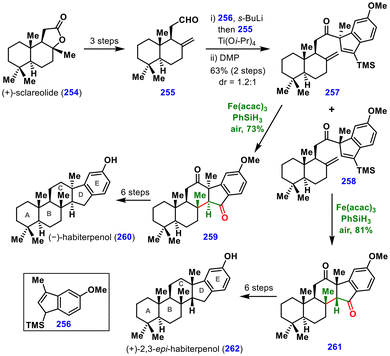 | ||
| Scheme 29 Total synthesis of (−)-habiterpenol and (+)-2,3-epi-habiterpenol by the Nagamitsu and Ohtawa group. | ||
(+)-Sclareolide (254) served as the starting material and was converted into aldehyde 255 in three steps. Coupling of aldehyde 255 with the indene fragment 256 in the presence of s-BuLi and Ti(i-OPr)4 furnished an alcohol, which upon oxidation delivered ketones 257 and 258 in 63% yield and 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. For this fragment coupling, the presence of the TMS group was critical for the regioselectivity of the reaction.
1 dr. For this fragment coupling, the presence of the TMS group was critical for the regioselectivity of the reaction.
The diastereomers 257 and 258 obtained from this coupling were separated, and the subsequent MHAT reaction was examined on each to form the C ring of (−)-habiterpenol (260) and (+)-2,3-epi-habiterpenol (262). Various conditions were screened, including Mn(dpm)3 or Fe(acac)3 as the catalyst, PhSiH3 as the silane, and tert-butyl hydroperoxide (TBHP) under N2. However, these conditions afforded only low yields of the desired product, presumably due to the steric bulk from the TMS group hindering efficient hydrogen trapping of the carbon radical. To address this, the reaction was instead carried out in the presence of air, allowing oxygen to trap the radical.
Further optimization identified Fe(acac)3 and PhSiH3 under air as the optimal conditions, delivering cyclized products 259 and 261 from 257 and 258 in 73% and 81% yields, respectively. Finally, diketones 259 and 261 were converted into (−)-habiterpenol (260) and (+)-2,3-epi-habiterpenol (262) in six steps, respectively. These total syntheses showcase the utility of MHAT chemistry for the formation of both C–C and C–O bonds in a single transformation.
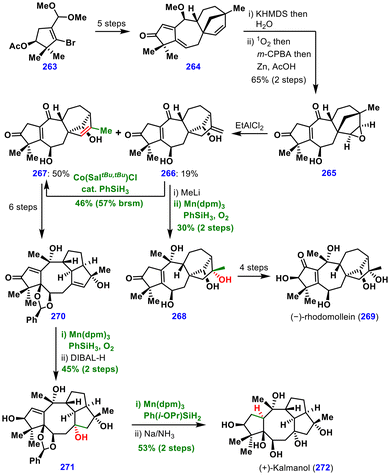
The synthesis began from chiral acetal 263, which was converted into tetracycle 264via a bridgehead carbocation cyclization as a key step. Regioselective deprotonation of 264, followed by protonation, furnished a diene that underwent cycloaddition with singlet oxygen. In the same pot, epoxidation and O–O bond cleavage provided intermediate 265. Subsequent ring rearrangement in the presence of EtAlCl2 yielded separable alkene regioisomers 266 and 267. Notably, the minor isomer 266 could be converted into the major isomer 267via olefin isomerization using Shenvi's reductive MHAT conditions.
For the synthesis of (−)-rhodomollein (269), the minor isomer 266 was treated with MeLi, followed by regioselective MHAT-induced hydration of the exocyclic olefin to afford tertiary alcohol 268, which was elaborated into (−)-rhodomollein (269) in four steps. From the major isomer 267, compound 270 was obtained in six steps and then subjected to regioselective MHAT-induced hydration, followed by carbonyl reduction to give 271. Finally, MHAT-induced hydrogenation and deprotection completed the total synthesis of (+)-kalmanol (272). These syntheses underscore the utility of MHAT chemistry for late-stage functional group manipulations.
4 C–H bond formation (hydrogenation)
Diastereoselective hydrogenation of alkenes is a pivotal transformation in natural product synthesis, particularly for constructing cis/trans-fused ring systems. While conventional Pd/Pt-catalysed hydrogenation typically delivers the cis-fused geometry, MHAT-induced hydrogenation often provides the complementary stereochemical outcome.644.1 Total synthesis of fusicoccane diterpenoids by the Xu group
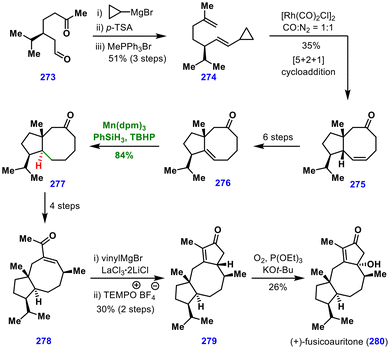
In 2024, Xu and co-workers reported the collective total synthesis of fusicoccane diterpenoids, where MHAT hydrogenation was used for making a trans-fused bicyclic system (Scheme 31).65 The synthesis commenced from chiral aldehyde 273, which was converted into intermediate 274 through cyclopropyl Grignard addition, dehydration, and a Wittig reaction sequence. Subjecting this substrate to Yu's [5 + 2 + 1] cycloaddition furnished the cis-fused [5,8]bicyclic scaffold 275. However, the natural products in this family possess a trans-fused [5,8]bicycle. To address this, olefin isomerization was carried out to deliver the trisubstituted alkene 276.Attempts at conventional Pd-or Pt-catalysed hydrogenation led only to the undesired cis-[5,8]bicycle. In contrast, MHAT-induced hydrogenation using Mn(dpm)3/PhSiH3/TBHP/i-PrOH delivered the desired trans-[5,8]bicycle 277 in 84% yield, thus overriding the inherent hydrogenation bias. From this intermediate, enone 278 was accessed in four steps. An oxidative Nazarov cyclization then constructed the critical cyclopentanone ring, affording intermediate 279 in moderate yield. Finally, γ-oxidation of the enone 279 completed the synthesis of (+)-fusicoauritone (280). Intermediate 279 also served as a branching point for the synthesis of other fusicoccane diterpenoid congeners.
4.2 Total synthesis of ent-trachylobane diterpenoids by the Dethe group
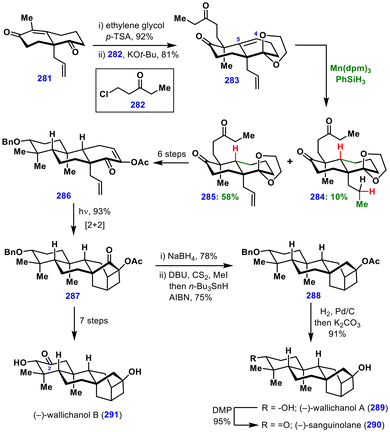
Dethe and co-workers reported a classic example of MHAT-induced hydrogenation for constructing a trans-decalin framework during their total syntheses of ent-trachylobane diterpenoids (Scheme 32).66 The synthesis commenced with a Wieland–Miescher ketone derivative, 281, which, upon ketone protection and subsequent alkylation with 282, yielded diketone 283.The next major challenge was the selective hydrogenation of the C4–C5 double bond in the presence of a monosubstituted alkene. Conventional transition metal–catalysed hydrogenation predominantly reduced the less hindered monosubstituted double bond. To overcome this, the authors exploited the thermodynamic preference for tertiary radical formation in MHAT chemistry. Treatment of 283 with Mn(dpm)3, phenylsilane, and tert-butyl hydroperoxide (TBHP) in degassed isopropanol delivered the trans-decalin 285 in 58% yield, along with 10% of the bis-olefin reduced byproduct 284.
From here, compound 285 was advanced in six steps to intermediate 286, which underwent a key photoinduced [2 + 2] cycloaddition, assembling the 6/6/6/4/6-fused pentacyclic skeleton 287 of the natural products. Subsequent ketone reduction and Barton–McCombie deoxygenation furnished acetate 288. Finally, global benzyl and acetate deprotection provided (–)-wallichanol A (289). Furthermore, oxidation of the secondary alcohol in (–)-wallichanol A (289) delivered (–)-sanguinolane (290). Similarly, (–)-wallichanol B (291), bearing an additional ketone group at C2, was synthesized from intermediate 287 in seven steps. This total synthesis revealed that, under MHAT conditions, a more substituted alkene can be selectively hydrogenated in the presence of a less substituted one—exhibiting the opposite selectivity compared to conventional Pd/Pt-catalysed hydrogenation.
4.3 Total synthesis of platensilin and its analogs by the Lou group
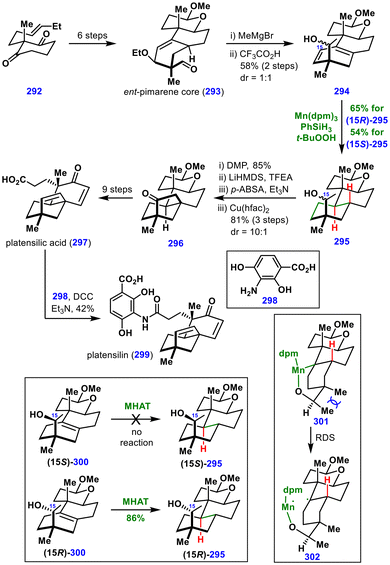
In 2024, Lou and co-workers reported the total syntheses of platensilin (299), platensimycin, platencin, and their analogs, where a key MHAT-induced hydrogenation of a diene was employed to access the trans-decalin framework (Scheme 33).67 The synthesis began with the symmetric diketone 292, which was converted to the ent-pimarene core 293 in six steps. Addition of a methyl Grignard reagent to 293, followed by ethoxy elimination, furnished the planar diene 294 as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereomers at C15.
1 mixture of diastereomers at C15.
To achieve diastereoselective hydrogenation of the diene, the authors first converted it to the tetrasubstituted olefins (15R)-300 and (15S)-300, which were then subjected to MHAT hydrogenation. Remarkably, the stereochemistry at C15 had a profound effect on the outcome. While (15R)-300 reacted under standard MHAT conditions—Mn(dpm)3, PhSiH3, and t-BuOOH—to provide the trans-decalin (15R)-295 in 86% yield, (15S)-300 failed to react. This discrepancy was rationalized by the transition state 301 in which alcohol coordination with the metal catalyst in the (15S)-300 isomer hinders productive hydrogen atom transfer.
In contrast, when diene 294 itself was subjected directly to MHAT hydrogenation conditions, both diastereomers reacted smoothly: (15S)-294 and (15R)-294 were transformed into (15S)-295 and (15R)-295 in 54% and 65% yields, respectively. The enhanced reactivity in this case was attributed to the greater stability of the radical generated from the diene compared to that from the corresponding alkene.
From this stage, oxidation of a mixture of diastereomers (15S)-295 and (15R)-295, followed by a carbenoid C–H insertion, furnished the bicyclic intermediate 296, which was further elaborated into platensilic acid 297. Final amide coupling with 298 provided platensilin 299. These total syntheses demonstrated that not only alkenes but also dienes can undergo highly stereoselective hydrogenation under MHAT conditions.
4.4 Total synthesis of (−)-caulamidine A by the Maimone group
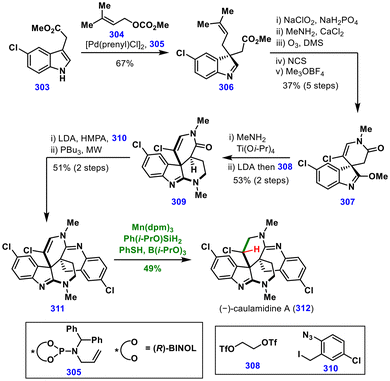
The functional group tolerance of MHAT chemistry was elegantly demonstrated in the late-stage hydrogenation of a vinyl chloride moiety, leaving the chlorine atom intact, during the total synthesis of (−)-caulamidine A (312) by Maimone and co-workers (Scheme 34).68 Their synthesis commenced from methyl-5-chloroindole-3-acetate 303, which underwent Pd-catalyzed asymmetric prenylation to furnish intermediate 306. Conversion of this species to imidate 307 over five steps, followed by treatment with methylamine in the presence of Ti(i-OPr)4, installed the C2 amine. Double alkylation with 308 delivered the tetracyclic scaffold 309, which upon alkylation with benzyl iodide 310 and subsequent Staudinger–aza-Wittig reaction furnished advanced intermediate 311.Next, the key challenge was hydrogenation of the vinyl chloride moiety. Traditional ionic hydrogenation was unsuccessful, while classical catalytic hydrogenation (Adam's catalyst, H2) primarily resulted in undesired hydrogenative dechlorination. After extensive optimization, MHAT-induced hydrogenation was found as a viable solution: employing Mn(dpm)3, Ph(i-PrO)SiH2, PhSH, and B(i-PrO)3 in i-PrOH furnished (−)-caulamidine A (312) in 49% yield, with 24% of the starting material being recovered. Mechanistic studies indicated that PhSH plays a crucial role as a reductant to suppress deleterious [1,5]-HAT of the α-chloro radical generated upon MHAT, while B(i-PrO)3 acts as a sacrificial Lewis acid, enabling productive hydrogenation. This total synthesis demonstrated that labile vinyl halides can be hydrogenated under MHAT conditions, highlighting the remarkable functional group tolerance of MHAT chemistry.
4.5 Total synthesis of asnovolins by the Porco Jr. group
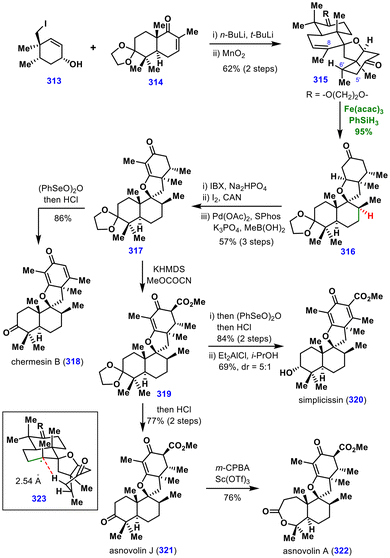
Generally, MHAT hydrogenation produces more stable equatorial methyl in terpenoid synthesis. However, if there is a possibility of intramolecular HAT to the radical generated after the first metal hydride transfer, the axial methyl group could be installed. The elegant example of the same was reported by John A. Porco Jr. and co-workers in their collective total synthesis of asnovolins (Scheme 35).69The synthesis began with the synthesis of iodide 313 and the decalin fragment 314 (obtained in 6 steps from (R)-carvone) on a gram scale. The anionic coupling of two fragments 313 and 314 followed by oxidation of alcohol furnishes the spirocyclic compound 315. Next, to install the axial C8-methyl group, the hydrogenation of trisubstituted alkene was attempted with H2, Pd/C, but it failed to give the required product.
Next, the authors attempted MHAT hydrogenation in the presence of Fe(acac)3, PhSiH3, and EtOH, which afforded the required product 316 in 95% yield and as a single diastereomer. Initially, based on a previous report by Yang,70 the authors proposed that under MHAT hydrogenation conditions, a tertiary radical generated could abstract the proximal C6′-methine hydrogen from the α-face, thereby placing the C8-methyl group in axial orientation. However, deuterium labelling experiments and DFT calculations indicated that the carbon radical abstracted the hydrogen alpha to carbonyl, which has a lower C–H bond dissociation energy as compared to C6′-methine hydrogen (shown in 323).
Furthermore, the spirocycle 316 was subjected to desaturation and methyl installation to deliver 317, which, on further desaturation, provided chermesin B (318). The intermediate 317 was converted to asnovolin J (321), asnovolin A (322), and simplicissin (320) in 2, 3, and 3 steps, respectively.
5 Fragmentation
5.1 Total synthesis of Δ3(4)-taxinin A, canataxapropellane, and dipropellane C by the Gaich group
In 2024, Gaich and co-workers reported a versatile synthetic strategy for the cyclotaxane diterpene family, centered on selective bond fragmentation within complex molecular frameworks assembled through two pericyclic steps (Scheme 36).71 This approach enabled the total synthesis of taxinine K (324) and several related congeners. Taxinine K (324) served as a central intermediate, providing access to six additional natural products.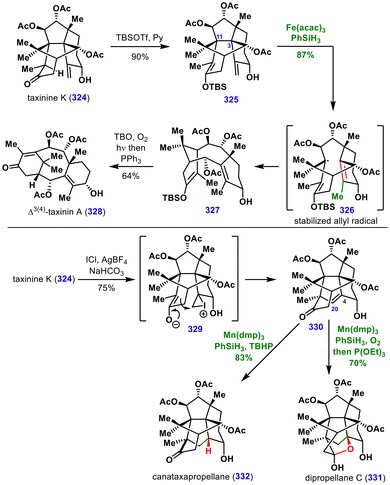 | ||
| Scheme 36 Total synthesis of Δ3(4)-taxinin A, canataxapropellane, and dipropellane C by the Gaich group. | ||
As this review focuses on MHAT-based transformations, we highlight the conversions of taxinine K (324) to Δ3(4)-taxinin A (328), canataxapropellane (332), and dipropellane C (331), all of which employ MHAT as a key step (Scheme 36). For the synthesis of Δ3(4)-taxinin A (328), taxinine K (324) was converted into silyl enol ether 325 and subjected to MHAT conditions, which induced C3–C11 bond fragmentation to give intermediate 327 in 87% yield. The enol ether played a dual role: ensuring the required stereoelectronic alignment of the two π-bonds for fragmentation and stabilizing the resulting allylic radical, thereby facilitating the process. Subsequent chemoselective oxidation of the enol ether furnished Δ3(4)-taxinine A (328).
To access canataxapropellane (332) and dipropellane C (331), taxinine K (324) was treated with ICl in the presence of AgBF4 to form the iodonium ion 329, which was then opened by an enolate followed by iodine elimination to generate olefin 330. Hydrogenation of the C4–C20 olefin under MHAT conditions provided canataxapropellane (332), while hydration under Mukaiyama conditions delivered dipropellane C (331). These total syntheses illustrate the power of late-stage MHAT-induced bond fragmentation for accessing complex natural products and their derivatives.
6 Overall conclusions and future outlook
This review has compiled and discussed recent total syntheses of natural products that employ MHAT-based transformations as the key steps. The discussion is organized into four sections based on the fate of the carbon-centered radical generated after the initial hydrogen atom transfer to the olefin: C–C bond formation, C–O bond formation, C–H bond formation, and fragmentation. Wherever possible, reaction optimization, observed stereoselectivity, and mechanistic hypotheses are discussed. The review highlights how MHAT reactions have enabled alternative retrosynthetic disconnections and simplified the synthesis of numerous natural products, particularly since 2020. Notably, Fe-based catalysts are commonly used for C–C bond formation, whereas Mn- and Co-based catalysts are frequently employed for C–O and C–H bond-forming transformations.Looking forward, continued development of MHAT chemistry will be essential to further expand its synthetic utility. The key challenges include exploring enantioselective variants, broadening the substrate scope to include functionalized alkenes, and integrating MHAT reactions into cascade sequences. Additionally, strategies to trap carbon-centered radicals with diverse heteroatoms such as N, S, and P, in both intra- and intermolecular fashion, remain largely unexplored. Furthermore, MHAT initiated radical cross couplings are powerful tools for C–C bond formation but remain unexplored. Advances in mechanistic understanding, computational modelling, and catalyst design are expected to accelerate progress in these areas.
Overall, the MHAT-based alkene hydrofunctionalization is a vibrant and evolving research area that is expected to receive considerable attention in the future, and we hope that this review will inspire the synthetic community to contribute to its continued development.
Author contributions
All authors wrote and approved the paper.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software, or code have been included, and no new data were generated or analysed as part of this review.Acknowledgements
We thank Indian Institute of Technology, Madras for the seed grant and the Department of Chemistry, IIT Madras, for the infrastructure and facilities. S. D. thanks CSIR-India for her doctoral fellowship.References
- (a) E. J. Corey and X.-M. Cheng, The Logic of Chemical Synthesis, Wiley, New York, 1989 Search PubMed; (b) S. Caille, S. Cui, M. M. Faul, S. M. Mennen, J. S. Tedrow and S. D. Walker, J. Org. Chem., 2019, 84, 4583–4603 CrossRef CAS PubMed, and references therein. (c) S. E. Reisman and T. J. Maimone, Acc. Chem. Res., 2021, 54, 1815–1816 CrossRef CAS PubMed.
- (a) A. W. Dombrowski, N. J. Gesmundo, A. L. Aguirre, K. A. Sarris, J. M. Young, A. R. Bogdan, M. C. Martin, S. Gedeon and Y. Wang, ACS Med. Chem. Lett., 2020, 11, 597–604 CrossRef CAS PubMed; (b) G. Laudadio, M. D. Palkowitz, T. E.-H. Ewing and P. S. Baran, ACS Med. Chem. Lett., 2022, 13, 1413–1420 CrossRef CAS PubMed; (c) L. E. Ehehalt, O. M. Beleh, I. C. Priest, J. M. Mouat, A. K. Olszewski, B. N. Ahern, A. R. Cruz, B. K. Chi, A. J. Castro, K. Kang, J. Wang and D. J. Weix, Chem. Rev., 2024, 124, 13397–13569 CrossRef CAS PubMed; (d) Y. Zhang, Y. Zhang and C. Li, Chem. Soc. Rev., 2025, 54, 10427–10486 RSC.
- M. Iguchi, Nippon Kagaku Kaishi, 1942, 63, 634–643 CrossRef CAS.
- (a) H. M. Feder and J. Halpern, J. Am. Chem. Soc., 1975, 97, 7186–7188 CrossRef CAS; (b) R. L. Sweany and J. Halpern, J. Am. Chem. Soc., 1977, 99, 8335–8337 CrossRef CAS; (c) J. Halpern, Adv. Chem. Ser., 1974, 70, 1–24 CrossRef; (d) J. Halpern and L.-Y. Wong, J. Am. Chem. Soc., 1968, 90, 6665–6669 CrossRef CAS; (e) L. Simandi and F. Nagy, Acta Chim. Hung., 1965, 46, 138–149 Search PubMed; (f) J. Halpern, Pure Appl. Chem., 1986, 58, 575–584 CrossRef CAS; (g) R. Sweany, S. C. Butler and J. Halpern, J. Organomet. Chem., 1981, 213, 487–492 CrossRef CAS; (h) J. Halpern, Science, 1985, 227, 869–875 CrossRef CAS PubMed.
- L. M. Jackman, J. A. Hamilton and J. M. Lawlor, J. Am. Chem. Soc., 1968, 90, 1914–1916 CrossRef CAS.
- (a) H. Ishikawa, D. A. Colby, S. Seto, P. Va, A. Tam, H. Kakei, T. J. Rayl, I. Hwang and D. L. Boger, J. Am. Chem. Soc., 2009, 131, 4904–4916 CrossRef CAS PubMed; (b) J. E. Sears and D. L. Boger, Acc. Chem. Res., 2015, 48, 653–662 CrossRef CAS PubMed; (c) X. Zeng, V. Shukla and D. L. Boger, J. Org. Chem., 2020, 85, 14817–14826 CrossRef CAS PubMed.
- I. Tabushi and N. Koga, J. Am. Chem. Soc., 1979, 101, 6456–6458 CrossRef CAS.
- (a) T. Mukaiyama, S. Isayama, S. Inoki, K. Kato, T. Yamada and T. Takai, Chem. Lett., 1989, 18, 449–452 CrossRef; (b) S. Inoki, K. Kato, T. Takai, S. Isayama, T. Yamada and T. Mukaiyama, Chem. Lett., 1989, 18, 515–518 CrossRef; (c) T. Mukaiyama and T. Yamada, Bull. Chem. Soc. Jpn., 1995, 68, 17–35 CrossRef CAS.
- (a) B. Gaspar and E. M. Carreira, Angew. Chem., Int. Ed., 2007, 46, 4519–4522 CrossRef CAS PubMed; (b) B. Gaspar and E. M. Carreira, J. Am. Chem. Soc., 2009, 131, 13214–13215 CrossRef CAS PubMed; (c) J. Waser, B. Gaspar, H. Nambu and E. M. Carreira, J. Am. Chem. Soc., 2006, 128, 11693–11712 CrossRef CAS PubMed; (d) J. Waser and E. M. Carreira, J. Am. Chem. Soc., 2004, 126, 5676–5677 CrossRef CAS PubMed; (e) J. Waser and E. M. Carreira, Angew. Chem., Int. Ed., 2004, 43, 4099–4102 CrossRef CAS PubMed; (f) J. Waser, J. C. González-Gómez, H. Nambu, P. Huber and E. M. Carreira, Org. Lett., 2005, 7, 4249–4252 CrossRef CAS PubMed.
- (a) S. W. M. Crossley, F. Barabé and R. A. Shenvi, J. Am. Chem. Soc., 2014, 136, 16788–16791 CrossRef CAS PubMed; (b) K. Iwasaki, K. K. Wan, A. Oppedisano, S. W. M. Crossley and R. A. Shenvi, J. Am. Chem. Soc., 2014, 136, 1300–1303 CrossRef CAS PubMed.
- S. M. King, X.-S. Ma and S. B. Herzon, J. Am. Chem. Soc., 2014, 136, 6884–6887 CrossRef CAS PubMed.
- (a) J. C. Lo, J.-H. Gui, Y. Yabe, C. M. Pan and P. S. Baran, Nature, 2014, 516, 343–348 CrossRef CAS PubMed; (b) M. Yan, J. C. Lo, J. T. Edwards and P. S. Baran, J. Am. Chem. Soc., 2016, 138, 12692–12714 CrossRef CAS PubMed; (c) J. C. Lo, Y. Yabe and P. S. Baran, J. Am. Chem. Soc., 2014, 136, 1304–1307 CrossRef CAS PubMed.
- (a) S. H. Gao and Y. Y. Qiu, Sci. China: Chem., 2016, 59, 1093–1108 CrossRef CAS; (b) J. E. Zweig, D. E. Kim and T. R. Newhouse, Chem. Rev., 2017, 117, 11680–11752 CrossRef CAS PubMed; (c) A. Simonneau and M. Oestreich, Angew. Chem., Int. Ed., 2015, 54, 3556–3558 CrossRef CAS PubMed.
- (a) S. W. M. Crossley, C. Obradors, R. M. Martinez and R. A. Shenvi, Chem. Rev., 2016, 116, 8912–9000 CrossRef CAS PubMed; (b) S. A. Green, S. W. M. Crossley, J. L. M. Matos, S. Vasquez-Cespedes, S. L. Shevick and R. A. Shenvi, Acc. Chem. Res., 2018, 51, 2628–2640 CrossRef CAS PubMed; (c) S. L. Shevick, C. V. Wilson, S. Kotesova, D. Kim, P. L. Holland and R. A. Shenvi, Chem. Sci., 2020, 11, 12401–12422 RSC.
- J. Wu and Z. Ma, Org. Chem. Front., 2021, 8, 7050–7076 RSC.
- (a) A. Maity and T. S. Teets, Chem. Rev., 2016, 116, 8873–8911 CrossRef CAS PubMed; (b) R. W. Hoffmann, Chem. Soc. Rev., 2016, 45, 577–583 RSC.
- (a) S. Crespi and M. Fagnoni, Chem. Rev., 2020, 120, 9790–9833 CrossRef CAS PubMed; (b) T. V. RajanBabu and W. A. Nugent, J. Am. Chem. Soc., 1994, 116, 986–997 CrossRef CAS; (c) T. E. O'Brien, A. O. Morris, L. F. Villela and L. Barriault, ChemCatChem, 2023, 15, e202300989 CrossRef; (d) A. Gansäuer, L. Shi, M. Otte, I. Huth, A. Rosales, I. Sancho-Sanz, N. M. Padial and J. E. Oltra, Top. Curr. Chem., 2011, 320, 93–120 CrossRef PubMed.
- B. Xu, C. Liu and M. Dai, J. Am. Chem. Soc., 2022, 144, 19700–19703 CrossRef CAS PubMed.
- A. L. J. Beckwith and V. W. Bowry, J. Am. Chem. Soc., 1994, 116, 2710–2716 CrossRef CAS.
- A. Bhunia, K. Bergander, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2021, 60, 8313–8320 CrossRef CAS PubMed.
- W. Zhao, R. Al-Ahmad and M. Dai, J. Am. Chem. Soc., 2025, 147, 32365–32369 CrossRef CAS PubMed.
- H. Wang, L. Wei, J. Huang, L. Wang, H. Peng, H. Jiang, J. Wu and Z. Ma, Angew. Chem., Int. Ed., 2025, 64, e202507961 CrossRef CAS PubMed.
- M. Fadel and E. M. Carreira, J. Am. Chem. Soc., 2023, 145, 8332–8337 CrossRef CAS PubMed.
- J. Gu, K. X. Rodriguez, Y. Kanda, S. Yang, M. Ociepa, H. Wilke, A. V. Abrishami, L. Jørgensen, T. Skak-Nielsen, J. S. Chen and P. S. Baran, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2200814119 CrossRef CAS PubMed.
- L.-P. Zhong, C. Gudeman, J. Zhen, O. A. Wanasinghe, J. Hellmig, M. J. E. Collins, J. Bacsa, A. Adibekian and M. Dai, J. Am. Chem. Soc., 2025, 147, 28589–28594 CrossRef CAS PubMed.
- W. Yao, Z. Liu, H. Ling, H. Wang, H. Zheng, S.-H. Wang, D.-Y. Zhu, S.-Y. Zhang and X. Chen, J. Am. Chem. Soc., 2025, 147, 15963–15969 CrossRef CAS PubMed.
- X. Chen, W. Yao, H. Zheng, H. Wang, P.-P. Zhou, D.-Y. Zhu and S.-H. Wang, J. Am. Chem. Soc., 2023, 145, 13549–13555 CrossRef CAS PubMed.
- Y. Zhou, S. Xu, X. Zhang, L. Zhou, H. Zheng and G. Zhu, Chem. Commun., 2024, 60, 10098–10111 RSC.
- Q.-G. Tan and X.-D. Luo, Chem. Rev., 2011, 111, 7437–7522 CrossRef CAS PubMed.
- F. Chen, J. Li and H. Renata, J. Am. Chem. Soc., 2025, 147, 21131–21142 CrossRef CAS PubMed.
- (a) P. Chen, C. Wang, R. Yang, H. Xu, J. Wu, H. Jiang, K. Chen and Z. Ma, Angew. Chem., Int. Ed., 2021, 60, 5512–5518 CrossRef CAS PubMed; (b) S. Deng, H. Xu, H. Jiang and Z. Ma, Org. Chem. Front., 2022, 9, 3961–3965 RSC.
- W. Cao, Z. Wang, Y. Hao, T. Wang, S. Fu and B. Liu, Angew. Chem. Int., Ed., 2023, 62, e202305516 CrossRef CAS PubMed.
- Y.-H. Huang, Q.-X. Gu, Q.-C. Chao, H.-Z. Xiao and H.-H. Lu, Angew. Chem., Int. Ed., 2025, 64, e202507638 CrossRef CAS PubMed.
- N. J. Foy and S. V. Pronin, J. Am. Chem. Soc., 2022, 144, 10174–10179 CrossRef CAS PubMed.
- X. Fang, N. Zhang, S.-C. Chen and T. Luo, J. Am. Chem. Soc., 2022, 144, 2292–2300 CrossRef CAS PubMed.
- F. von Kieseritzky, Y. Wang and M. Axelson, Org. Process Res. Dev., 2014, 18, 643–645 CrossRef CAS.
- J. Li, H. Tang, T. Kurtán, A. Mándi, C.-L. Zhuang, L. Su, G.-L. Zheng and W. Zhang, J. Nat. Prod., 2018, 81, 1645–1650 CrossRef CAS PubMed.
- G. Huang, X. Zhang, Y.-C. Gu and J. Gui, J. Am. Chem. Soc., 2025, 147, 20239–20245 CrossRef PubMed.
- (a) H.-H. Lu, K.-J. Gan, F.-Q. Ni, Z. Zhang and Y. Zhu, J. Am. Chem. Soc., 2022, 144, 18778–18783 CrossRef CAS PubMed; (b) K.-J. Gan, Y. Zhu, G. Shi, C. Wu, F.-Q. Ni, L.-H. Zhao, X. Dou, Z. Zhang and H.-H. Lu, Chin. J. Chem., 2025, 43, 79–89 CrossRef CAS.
- T. Tsuruta, K. Nishikawa, Y. Yoshino, D. Osada, T. Miwa, K. Nimura, T. Kamada and Y. Morimoto, Org. Lett., 2025, 27, 3489–3494 CrossRef CAS PubMed.
- R. N. Iyer, D. Favela, A. Domokos, G. Zhang, A. A. Avanes, S. J. Carter, A. G. Basargin, A. R. Davis, D. J. Tantillo and D. E. Olson, Nat. Chem., 2025, 17, 412–420 CrossRef CAS PubMed.
- J. Wu, J. Bao, J. Deng, H. Tian and J. Gui, J. Am. Chem. Soc., 2025, 147, 30599–30605 CrossRef CAS PubMed.
- W. P. Thomas and S. V. Pronin, J. Am. Chem. Soc., 2022, 144, 118–122 CrossRef CAS PubMed.
- (a) G. Liu, Z. Zhang, S. Fu and B. Liu, Org. Lett., 2021, 23, 290–295 CrossRef CAS PubMed; (b) B. Xu, W. Xun, S. Su and H. Zhai, Angew. Chem., Int. Ed., 2020, 59, 16475–16479 CrossRef CAS PubMed.
- Y. Zhao, J. Hu, R. Chen, F. Xiong, H. Xie and H. Ding, J. Am. Chem. Soc., 2022, 144, 2495–2500 CrossRef CAS PubMed.
- S. A. Green, T. R. Huffman, R. O. McCourt, V. van der Puyl and R. A. Shenvi, J. Am. Chem. Soc., 2019, 141, 7709–7714 CrossRef CAS PubMed.
- X.-C. Gan, S. Kotesova, A. Castanedo, S. A. Green, S. L. B. Møller and R. A. Shenvi, J. Am. Chem. Soc., 2023, 145, 15714–15720 CrossRef CAS PubMed.
- (a) C. Wang and Y. Guan, Synlett, 2021, 913–916 Search PubMed; (b) Y. Chen, Z. Xin, H. Wang, H. He and S. Gao, Org. Chem. Front., 2023, 10, 651–660 RSC; (c) G. Wen, S. Gu, J. Chen, H. He and S. Gao, Org. Lett., 2025, 27, 2310–2316 CrossRef CAS PubMed.
- D. Ma, P. Duran, R. Al-Ahmad, S. Hestehave, M. Joa, O. Alsbiei, E. J. Rodríguez-Palma, Y. Li, S. Wang, R. Khanna and M. Dai, J. Am. Chem. Soc., 2024, 146, 16698–16705 CrossRef CAS PubMed.
- S. J. Gharpure, R. S. Chavan and S. R. Narang, Org. Lett., 2024, 26, 4583–4588 CrossRef CAS PubMed.
- (a) D. Vrubliauskas and C. D. Vanderwal, Angew. Chem., Int. Ed., 2020, 59, 6115–6121 CrossRef CAS PubMed; (b) D. Vrubliauskas, B. M. Gross and C. D. Vanderwal, J. Am. Chem. Soc., 2021, 143, 2944–2952 CrossRef CAS PubMed.
- L. K. Johnson, S. W. Niman, D. Vrubliauskas and C. D. Vanderwal, Org. Lett., 2021, 23, 9569–9573 CrossRef CAS PubMed.
- (a) R. F. Lusi, G. Sennari and R. Sarpong, Nat. Chem., 2022, 14, 450–456 CrossRef CAS PubMed; (b) R. F. Lusi, G. Sennari and R. Sarpong, J. Am. Chem. Soc., 2022, 144, 17277–17294 CrossRef CAS PubMed.
- A. Masarwa, M. Weber and R. Sarpong, J. Am. Chem. Soc., 2015, 137, 6327–6334 CrossRef CAS PubMed.
- C. Zhou, W. Qin, C. Tu, Y. Chen, S. Fu and B. Liu, Angew. Chem., Int. Ed., 2025, 64, e202503943 CrossRef CAS PubMed.
- G. Schoenn, C. Kouklovsky, R. Guillot, T. Magauer and G. Vincent, Angew. Chem., Int. Ed., 2025, 64, e202505270 CrossRef CAS PubMed.
- N. J. Castellino, A. P. Montgomery, J. J. Danon and M. Kassiou, Chem. Rev., 2023, 123, 8127–8153 CrossRef CAS PubMed.
- W. Zhou, T. Zhou, M. Tian, Y. Jiang, J. Yang, S. Lei, Q. Wang, C. Zhang, H. Qiu, L. He, Z. Wang, J. Deng and M. Zhang, J. Am. Chem. Soc., 2021, 143, 19975–19982 CrossRef CAS PubMed.
- J.-X. Liu, S.-P. Zhang, F.-S. Sun, H. Li, Y.-L. Gong, S.-C. Lu and S. Xu, Angew. Chem., Int. Ed., 2023, 62, e202303229 CrossRef CAS PubMed.
- X. Zheng, X. Guo, H. Wang, P.-P. Zhou and X. Chen, J. Am. Chem. Soc., 2024, 146, 7198–7203 CrossRef CAS PubMed.
- K. Yu, F. Yao, Q. Zeng, H. Xie and H. Ding, J. Am. Chem. Soc., 2021, 143, 10576–10581 CrossRef CAS PubMed.
- H. Taguchi, M. Kawaguchi, T. Nagamitsu and M. Ohtawa, Org. Biomol. Chem., 2023, 21, 6129–6133 RSC.
- L. Kong, H. Yu, M. Deng, F. Wu, S.-C. Chen and T. Luo, J. Org. Chem., 2023, 88, 6017–6038 CrossRef CAS PubMed.
- (a) J. M. Aquilina and M. W. Smith, J. Am. Chem. Soc., 2022, 144, 11088–11093 CrossRef CAS PubMed; (b) S. Mazeh, M. D. Garcia-Fernandez, B. Pelletier, C. Moreau and P. Delair, Org. Biomol. Chem., 2023, 21, 817–822 RSC.
- S. Xie, Y. Chen, Y. Zhang, Z. Zhang, X. Hu, C. Yan and J. Xu, Cell Rep. Phys. Sci., 2024, 5, 101855 CrossRef CAS.
- D. H. Dethe, N. Sharma and S. Juyal, Angew. Chem., Int. Ed., 2025, 64, e202505766 CrossRef CAS PubMed.
- Z.-X. Gao, H. Wang, A.-H. Su, Q.-Y. Li, Z. Liang, Y.-Q. Zhang, X.-Y. Liu, M.-Z. Zhu, H.-X. Zhang, Y.-T. Hou, X. Li, L.-R. Sun, J. Li, Z.-J. Xu and H.-X. Lou, J. Am. Chem. Soc., 2024, 146, 18967–18978 CrossRef CAS PubMed.
- Z. Zhu and T. J. Maimone, J. Am. Chem. Soc., 2023, 145, 14215–14220 CrossRef CAS PubMed.
- F. Yang and J. A. Porco Jr, J. Am. Chem. Soc., 2022, 144, 12970–12978 CrossRef CAS PubMed.
- (a) Y. Qu, Z. Wang, Z. Zhang, W. Zhang, J. Huang and Z. Yang, J. Am. Chem. Soc., 2020, 142, 6511–6515 CrossRef CAS PubMed; (b) For a related transannular [1,5]-HAT, see: E. P. Farney, S. S. Feng, F. Schäfers and S. E. Reisman, J. Am. Chem. Soc., 2018, 140, 1267–1270 CrossRef CAS PubMed.
- L. Pan, F. Schneider, M. Ottenbruch, R. Wiechert, T. List, P. Schoch, B. Mertes and T. Gaich, Nature, 2024, 632, 543–549 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2026 |