Nitrones as directing groups in transition metal-catalysed C–H activation
Barbara
Stańska
,
Anjali
Dahiya
and
Rafał
Loska
 *
*
Institute of Organic Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland. E-mail: rafal.loska@icho.edu.pl
First published on 26th November 2025
Abstract
During about the last 15 years, nitrones have emerged as versatile directing groups for transition metal-catalysed C–H activation reactions. Apart from simple functionalization of the aryl ring attached to the nitrone moiety, the nitrone functional group can play the role of a transient directing group or an internal oxidant, allowing for the design of redox-neutral reactions initiated by a C–H activation step. Moreover, the dipolar character of nitrones has been exploited in cascade reactions involving C–H activation followed by 1,3-dipolar cycloaddition, resulting in the incorporation of the nitrone moiety into the structure of polycyclic, nitrogen-containing scaffolds found in biologically active compounds like aminoalcohols, indoles, carbazoles, quinolines, etc. Reactions of all of the above types, as well as the C(sp2)–H functionalization of the aldonitrone moiety itself, are the focus of this review. Apart from the already well-developed Rh, Ru and Pd-catalysed reactions, emerging applications of cobalt catalysis for nitrone functionalisation are discussed as well. Catalytic reactions of isoelectronic azomethine imines are also mentioned in connection with analogous reactions of nitrones.
1. Introduction
Nitrones are derivatives of carbonyl compounds and hydroxylamines, or N-oxides of imines (Scheme 1). They have some importance as pharmaceutical agents and bioactive compounds1 and are very rarely found in nature (Scheme 1(c)).2 The relatively high stability of nitrones (much higher than imines) and diverse reactivity make them versatile intermediates for the synthesis of nitrogen-containing target molecules, particularly of biologically active compounds such as unnatural amino acids, alkaloids and β-lactams.3 In accordance with the resonance structures of nitrones shown in Scheme 1(b), they are among the most frequently used dipoles in 1,3-dipolar cycloaddition reactions (1,3-DC),4 a process extensively used in the synthesis of natural products.3a,5 In analogy to carbonyl compounds, nitrones are also excellent substrates for nucleophilic addition,6 as well as carbon–carbon bond forming reductive coupling.7 A reaction unique to nitrones is the Kinugasa reaction for the synthesis of β-lactams.8a Other nitrone transformations leading to these important targets have been recently developed as well.8b,c Nitrones are also frequently employed in formal [3 + 3]9 and other10 cycloaddition reactions, electrocyclic reactions,11 rearrangements involving intramolecular oxygen atom transfer12 or Au-catalysed cyclisations.13 Other important, but less exploited, processes include asymmetric reduction,14 the addition of free radicals15 or processes initiated by nitrone deprotonation.16 This broad reactivity of nitrones makes them excellent synthetic intermediates for the development of cascade reactions in which complex nitrogen-containing molecules can be accessed efficiently.17 In recent years, reinvestigation of nitrone–allene cycloaddition4o led to the discovery of tandem 1,3-DC/electrocyclic rearrangement processes, which enable the preparation of a wide variety of heterocyclic compounds via transition metal-free functionalisation of the ortho position of the nitrone N-aryl ring.18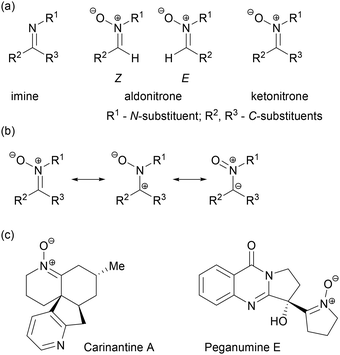 | ||
| Scheme 1 (a) The structures of imines, aldonitrones and ketonitrones. (b) Nitrone resonance structures. (c) Natural products containing the nitrone moiety. | ||
Transition metal-catalysed C–H activation reactions have become one of the key strategies of modern organic synthesis.19,20 They have been developed very actively over the last decades with the aim of providing unique opportunities for fast and efficient assembly of complex molecules without the need for pre-functionalized substrates, thereby improving atom economy and reducing the amount of resources used and the generation of waste associated with chemical synthesis. On the other hand, to ensure sufficient reactivity and selectivity, especially with electronically and sterically unbiased substrates, C–H activation reactions generally require the presence of a directing group (DG) in the substrate, which coordinates to the metal catalyst and enables C–H bond cleavage through the proximity effect.21 Such directing groups are usually redundant in the structure of the desired product, therefore considerable research efforts have been directed towards the development of directing groups that are easily removed,22 or of the so-called transient DGs, which bind to the substrate reversibly.23 Still, the feasibility of such binding also imposes some constraints on the substrate structure, for example the requirement for an aldehyde function to form an imine with 2-amino-3-picoline. Another approach, highly atom- and step-economical, is based on DGs that are incorporated into the desired structure of the target molecule, either directly following the C–H activation event or at a later stage of the synthesis. These reactions are usually annulations leading to polycyclic structures.24
The polar character and high reactivity of the nitrone functional group makes it an ideal directing group for transition metal-catalysed C–H activation, which is followed by further transformations of the DG and its incorporation into the structure of the final nitrogen-containing product. Moreover, the relatively labile N–O bond of nitrones may act as an internal oxidant, enabling the design of redox-neutral processes or the so-called migrating directing groups, which are (partially) relocated to the desired position in the target molecule after the C–H activation event.25 These features of nitrones resulted in the rapid development of transition metal-catalysed, nitrone-directed C–H activation reactions over about the last 15 years.
Our review provides an overview of modern applications of nitrones as directing groups for C–H activation reactions. First, palladium catalysed reactions are discussed, which are mainly C(sp2)–H functionalisations of the nitrone moiety itself. The next section presents Rh, Ru, Fe and Ir catalysed aryl C–H activation of the C- or N-bound aromatic rings of nitrones. A separate section is devoted to cascade C–H activation/1,3-dipolar cycloaddition reactions. Finally, the emerging applications of cobalt catalysis are presented. Additionally, similar reactions of azomethine imines, which are nitrogen analogues of nitrones with the same valence electronic structure (RCON− instead of O− in nitrone),26 are mentioned whenever they parallel the nitrone reactivity.
General reviews covering various aspects of nitrone chemistry in a comprehensive manner have appeared in recent years,3 but we are not aware of any review focused on nitrones as directing groups for C–H activation. A few years ago, Chupakhin, Maes and co-workers published an excellent review on the C(sp2)–H functionalization of compounds containing an imine bond, including nitrones, but it covered mainly the nucleophilic substitution of hydrogen, C-deprotonation, and the functionalization of nitrones by cycloaddition and isoxazolidine opening, with only a few transition metal-catalysed processes mentioned.27 In 2020, a book chapter on metal-mediated reactions leading to nonaromatic nitrogen heterocycles appeared in which some transition-metal catalysed annulation reactions were discussed.3e
2. Palladium-catalysed reactions
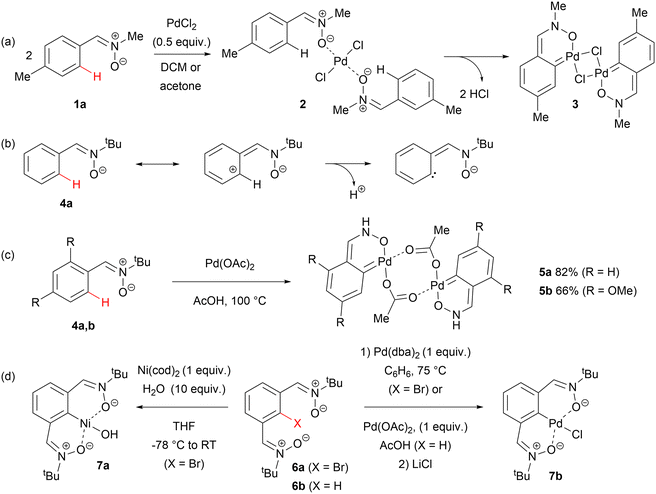
The first example of metalation of an aryl ring enabled by pre-coordination of the nitrone oxygen to a transition metal was observed in 2005 by Kukushkin, Pombeiro and co-workers.28 In the reaction between C-(4-methylphenyl)-N-methylnitrone 1a with [PdCl2(MeCN)2] in CH2Cl2 or acetone at room temperature, substitution of the acetonitrile ligands with two molecules of 1a occurred with the formation of an oxygen-coordinated, trans complex of palladium(II) (2). Its spontaneous dimerization was accompanied by metalation of the ortho positions in both nitrone ligands to form the arylpalladium complex 3 (Scheme 2(a)).Another milestone within the concept of a nitrone moiety acting as a directing group was associated with Yao's considerations of carbocyclic, unusual carbene ligands analogous to classical NHCs.29a One of the resonance structures of a C-arylnitrone deprotonated at the ortho position of its aromatic ring is a carbene substituted with a deprotonated hydroxylamine moiety, capable of acting as a chelating ligand (Scheme 2(b)). Indeed, heating of C-phenyl- and C-(2,4-dimethoxyphenyl)-N-tert-butylnitrone (4a,b) with a stoichiometric amount of Pd(OAc)2 in AcOH yielded dimeric, air-stable palladacycles 5a,b with carbocyclic carbene ligands (Scheme 2(c)). They both exhibited extraordinarily high activity as catalysts of Heck reactions of aryl bromides and chlorides with styrene, reaching turnover numbers greater than 106. A few years later, analogous complexes of nitrones 4 with iridium were synthesised and evaluated for their biological activity.29b
Soon afterwards, detailed studies on the OCO pincer complexes of Pd and Ni with 1,3-aryldinitrone ligands were published.30 The Ni complex 7a could be obtained by oxidative addition to the C–Br bond between the two nitrone groups of 6a and the Pd complex 7b either by oxidative addition to 6a or nitrone-directed C–H activation occurring in 6b (Scheme 2(d)). These new complexes showed high activity as catalysts in Kumada cross-coupling and in Heck reactions, but somewhat lower than C,O-chelates 5 described earlier by Yao,29a probably due to the stronger coordination properties of the pincer ligands. Later, similar pincer complexes but containing one nitrone and one N-(2-pyridylmethyl)amide arm were prepared by the Kantam group.31 Their catalytic activity was evaluated in decarboxylative Sonogashira-like cross-coupling reactions.
Despite the above promising preliminary studies, in the following years, palladium was seldom used in reactions relying upon the nitrone function as a directing group for C–H activation of the neighbouring aromatic ring. Instead, Rh, Ir, Ru and Co catalysts were used extensively for this purpose, as described in the following sections. In 2017, the group of Ukaji reported studies of the Pd-catalysed C–H alkenylation of C-aryl-N-tert-butylnitrones with acrylates.32 Initially, the dimeric carbene complex 5a was prepared and subjected to the reaction with an excess of ethyl acrylate in AcOH at 100 °C to give ortho-alkenylated N-tert-butyl amide 8 in 83% yield after two steps (Scheme 3(a)). The expected ortho-alkenylated nitrone 9a could be obtained in lower yield by changing the solvent to DCE, but upon heating in AcOH it further rearranged to amide 8. The intermediacy of nitrone 9a on the way to 8 was confirmed by the fact that benzamide gave only traces of a palladacyclic complex and no alkenylation product in the presence of Pd(OAc)2 and acrylate in AcOH. In the direct, catalytic reactions between C-arylnitrones and alkyl acrylates (used in large excess) moderate yields of alkenylation products 9 were obtained (lower with halogen substituents in the ring; Scheme 3(b)). A mixture of hexafluoroisopropanol (HFIP) and AcOH as the solvent suppressed the concurrent, unwanted nitrone–alkene cycloaddition.
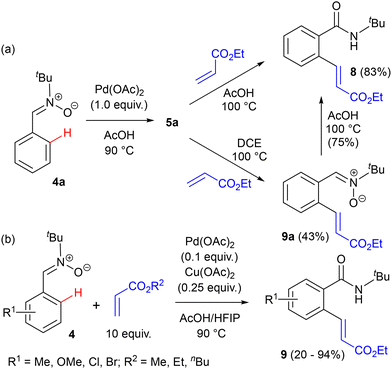 | ||
| Scheme 3 (a) Nitrone C-ring alkenylation in the reaction of nitrone–palladium complex 5a with acrylate. (b) Palladium-catalysed alkenylation of nitrones in the C-aryl ring. | ||
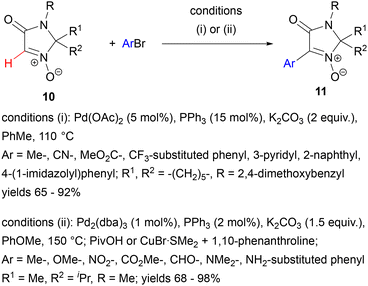
Palladium catalysis has gained importance in functionalizing the C–H bond of the C(sp2) carbon of aldonitrones. The first reports in this area were based upon the idea of the nitrone oxygen atom acting as a directing group for functionalization of the neighbouring C(sp2)–H in a cyclic nitrone. They were inspired by the postulated analogy between nitrones and aromatic N-oxides and by the series of seminal works of Fagnou33 and others,34 in which the palladium-catalysed C(2)-arylation of N-oxides of azines and azoles was described. In 2012, Zhao, Wang and co-workers investigated the Pd-catalysed arylation of imidazolone N-oxides 10.35 These substrates were non-aromatic, but their efficient C–H arylation with aryl bromides could be achieved in the presence of Pd(OAc)2, PPh3 and K2CO3 in toluene at 110 °C, with significantly lower yield with aryl iodides and very low yield with chlorides and triflates (Scheme 4, conditions (i)). No mechanistic investigations were performed within this study.
In the same year, a much more detailed study of cyclic nitrone arylation was published by Chavant, Blandin and co-workers, who also pointed to the parallel reactivity (nucleophilic addition, cycloaddition) of aromatic N-oxides and nitrones.36 Investigating the reaction of an imidazolone N-oxide derivative 10 with 4-bromotoluene in the presence of Pd2(dba)3 and phosphines, they found that the catalytic activity could be greatly enhanced by pivalic acid (PivOH) additive or a copper co-catalyst, CuBr·SMe2/1,10-phenanthroline (Scheme 4, conditions (ii)). The copper salt additive was less useful as it caused undesired dimerization of the starting nitrone. The reaction required rather harsh conditions (150 °C in anisole), but it gave high yields of cross-coupling products 11 with aryl and heteroaryl bromides, including aryls substituted with groups like OMe, CO2Me, CHO, NMe2 or even unprotected NH2. Aryl and heteroaryl dibromides gave bis-nitrone products in good yields.
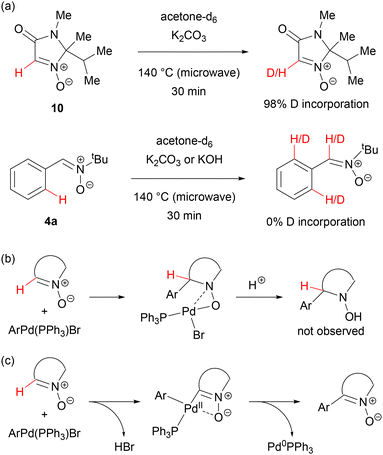
In contrast, no conversion was observed with acyclic nitrones: C-phenyl- and C-ethoxycarbonyl-N-tert-butylnitrone.
In experiments with deuterium-labelled substrates, a kinetic isotope effect (KIE) value of 3 was determined, indicating that hydrogen removal occurred during the rate-determining step of this arylation process.36 Two mechanistic hypotheses were considered: a Heck-type addition of an arylpalladium species to the carbon–nitrogen double bond of nitrone 10, followed by palladium hydride elimination (Scheme 5(b)), or a pathway initiated by deprotonation/metalation of the nitrone C(sp2) carbon, followed by reductive elimination to form the carbon–carbon bond (Scheme 5(c)). In the former pathway, the hydride elimination step would have to be the rate-limiting one. This pathway would also likely lead to the concurrent formation of N-hydroxylamine and imine products (in fact not observed), moreover, it should also be facile for acyclic nitrones, which exist in the Z configuration, particularly with a bulky tBu group on nitrogen. For these reasons, the Heck-type mechanism was discarded in favour of the direct metalation pathway. Its further confirmation came from the observation that the reactivity in the Pd-catalysed arylation parallels the relative acidity of nitrones and N-oxides: facile H/D exchange in nitrone 10 occurred even in the presence of K2CO3, whereas pyridine N-oxide required KOH and an acyclic nitrone was completely inert (Scheme 5(a)). Formation of the dimerization product of 10 in the presence of CuBr could be explained by the facile, deprotonative formation of an organocopper compound, which, in the absence of a Pd catalyst, undergoes nucleophilic addition to another molecule of 10 and elimination of water. DFT calculations further confirmed that cyclic nitrones metalated at the C(sp2) carbon were strongly stabilized by palladium–oxygen interactions, unlike Z isomers of acyclic nitrones.
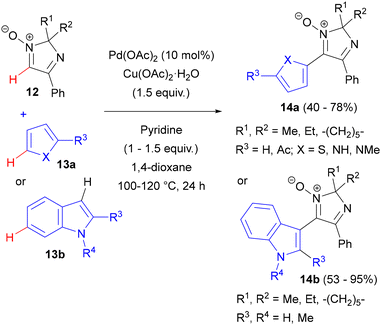
This methodology was later extended to the cross-dehydrogenative coupling of cyclic, imidazole-derived nitrones 12 with pyrroles and thiophenes (13a)37a and with indoles (13b).37b A series of 3-indolyl-, pyrrolyl- and thienyl-substituted 2H-imidazole 1-oxides 14 could be obtained in moderate to good yields (Scheme 6). Instead of oxidative addition to a carbon–halogen bond, this reaction was initiated by palladation of the C(2) position of the pyrrole or thiophene ring. The heteroaryl palladium complex formed participated in an oxygen- and acetate-assisted C–H activation of the nitrone ring, followed by reductive elimination. Deuterium labelling experiments indicated that the role of the copper additive was to re-oxidize Pd(0) to Pd(II).
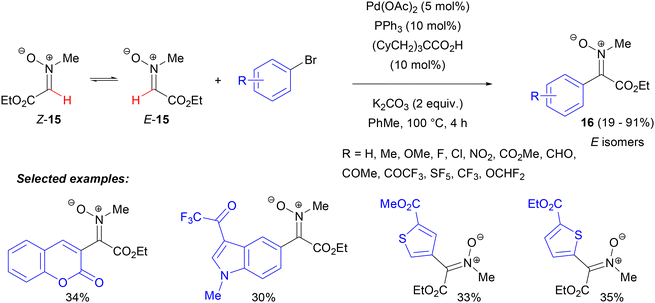
The success of the palladium-catalysed cross coupling reactions of nitrones described above clearly relied upon the directing effect of the nitrone oxygen atom, which in each case was in a Z configuration relative to the C–H bond undergoing metalation. This arrangement was forced by the cyclic structure of the starting nitrones, whereas acyclic aldonitrones generally exist in the opposite configuration. To address the challenge associated with arylation of such substrates, our group investigated the palladium-catalysed cross-coupling of aryl bromides with an aldonitrone derived from ethyl glyoxalate, which was known to exist in fast E–Z equilibrium (Scheme 7).38 The key to obtaining high yields of the ketonitrone products was the use of bulky pivalic or, better, tris(cyclohexylmethyl)acetic carboxylic acid as an additive, which was known to have a beneficial effect on certain Pd-catalysed cross-coupling reactions.39 A variety of aryl and heteroaryl bromides could be used, giving ketonitrones 16 in moderate to good yields and, initially, with high E selectivity (they isomerized slowly to E,Z mixtures upon storage).
From the mechanistic point of view, the reaction could proceed via the initial coordination of an arylpalladium complex to the nitrone oxygen, followed by the migratory insertion of the carbon–nitrogen double bond and β-hydride elimination, similarly to the Heck reaction. On the other hand, a moderate kinetic isotope effect and the fact that the products were initially formed as E isomers suggested that an oxygen-directed activation of the nitrone C(sp2)–H bond via a CMD mechanism should be considered. Importantly, only readily isomerizing C-methoxycarbonylnitrone participated in the reaction and not C-arylnitrones, which existed almost exclusively as Z isomers and isomerized slowly.
Strained, four-membered ketonitrones fused with a benzene ring (benzocyclobutenitrones) could be obtained in a similar reaction, but occurring in an intramolecular fashion, that is, starting with C-(2-bromoarylmethyl)-N-alkylnitrones 17 (Scheme 8).40 The reaction was found to proceed efficiently in the presence of Pd(OAc)2 and Cs2CO3 as a base and preferably a bidentate phosphine (dppe), under an Ar atmosphere in toluene. PivOH had no beneficial effect in this case. Considering the scope, an important requirement was the presence of at least one additional substituent on the sp3 carbon connecting the bromoaryl ring and the nitrone moiety, demonstrating the importance of the Thorpe–Ingold effect for this small ring closure. A variety of electron-donating and withdrawing substituents in the aryl ring and various alkyls on the nitrone nitrogen was tolerated. The four-membered nitrone ring of products 18 could further undergo nucleophilic addition and cycloaddition reactions, particularly with 2H-pentafluoropropene to give, after reductive cleavage of the N–O bond, trifluoromethyl-substituted polycyclic azaspiro[3.3]heptanes. Polycyclic products could also be obtained by combining intramolecular cross-coupling with 1,3-dipolar cycloaddition to a pendant olefin. A KIE value of only 1.06 was measured, which, together with the lack of PivOH influence, suggested a Heck-type mechanism rather than a deprotonation/metalation pathway, probably due to peculiar steric requirements of the four-membered ring closure.
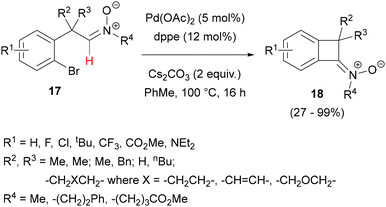 | ||
| Scheme 8 Synthesis of benzocyclobutanone-derived nitrones by Pd-catalysed intramolecular aldonitrone C–H arylation. | ||
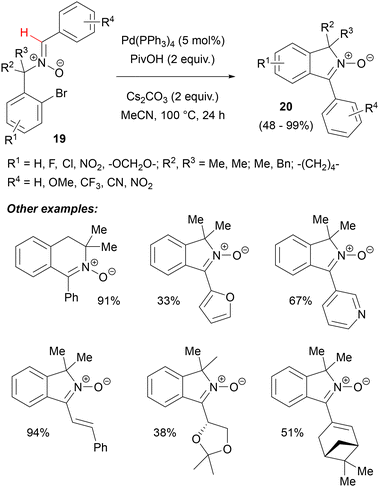
Changing the structure of the starting nitrones by moving the o-bromobenzyl group to the nitrone nitrogen atom allowed for this methodology to be extended to the synthesis of isoindole N-oxides 20 (Scheme 9). In this case, PivOH additive and a monodentate phosphine were crucial for achieving high yields.41 Together with a slightly higher KIE value (1.22), these observations might suggest that a C(sp2)–H metalation pathway could not be ruled out for this intramolecular nitrone arylation. Again, a variety of substituents from strongly electron-donating (1,3-dioxolane ring) to electron withdrawing (NO2) could be present in substrates 19 and a variety of aryl substituents (including very bulky ones) and even some alkyl groups could be introduced into the heterocyclic ring of 20 (Scheme 9). By elongating the linker between nitrone and bromoaryl, a dihydroisoquinoline N-oxide derivative was obtained in high yield. Compounds 20 could be further utilized in a Rh-catalysed tandem nitrone-directed C–H activation/1,3-DC with a Morita–Baylis–Hillman adduct, according to Hong and Kim.42
3. Rh, Ru, Fe and Ir-catalysed annulation and aryl ring functionalisation of nitrones
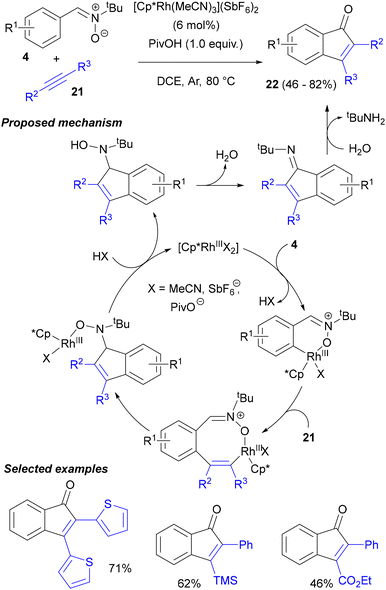
In 2013, Li and co-workers, for the first time, exploited an acyclic nitrone functionality as a traceless directing group in a catalytic process, namely Rh(III)-catalysed C–H activation of nitrones and their annulation with unsymmetrical alkynes 21, leading to indenones (Scheme 10).43 This concept was inspired by their earlier work utilizing azomethine imines to the same effect.44N-tert-Butyl aldonitrones 4, derived from aromatic aldehydes, reacted in the presence of [Cp*Rh(MeCN)3](SbF6)2 catalyst under an Ar atmosphere with internal alkynes, mostly symmetrical biaryl acetylenes, but also with TMS-protected phenylacetylene or a phenylpropiolic ester. The key to obtaining high yields of 22 was the use of PivOH as an additive (AcOH was less efficient). Notably, 40% yield of the annulation product was obtained with an N-methyl nitrone as the starting material. On the basis of experiments with D- and 18O-labelled substrates, it was postulated that the reaction commenced with coordination of rhodium to the nitrone oxygen, followed by the rate-limiting C–H activation step. Subsequent insertion of the alkyne forms a Rh–vinyl complex, which adds to the nitrone moiety, forming a Rh–hydroxylamine complex. Dehydration of this hydroxylamine and hydrolysis of the imine formed provides the final indenones 22.More recently, the Li group used a similar methodology for the enantioselective construction of atropoisomeric 3-aryl-1-hydroxyindolines.45
Soon after their pioneering report on the synthesis of indenones, the Li group demonstrated the usefulness of the nitrone directing group in the C–H alkynylation of the aryl ring with 1-(silylethynyl)-1,2-benziodoxol-3(1H)-ones (silyl-EBX), in the presence of Zn(OTf)2 and [Cp*RhCl2]2 precatalyst.46 An N-tert-butylnitrone directing group displayed comparable efficiency in this reaction to that of an oxime methyl ether, nitrosamine, azomethine imine or azo and azoxy compounds.
A few years later, with the aim of developing a new process in which a nitrone would serve as a directing group incorporated into the target product, the same authors reported the reaction of C-aryl-N-tert-butylnitrones 4 with cyclopropenones 23 (Scheme 11).47 Interestingly, the reaction outcome depended on the presence of a ring substituent ortho to the nitrone group: with a small substituent (H, F) the reaction led to 1-naphthols 24 and with a larger one (Cl, Br, Me) to bridged products of intramolecular 1,3-DC (25). Both classes of products were obtained in high yields using [Cp*RhCl2]2 and AgSbF6, with 4 Å molecular sieves as the key additive. Interestingly, unlike the annulation of nitrones with alkynes, this reaction was completely inhibited by PivOH.
 | ||
| Scheme 11 Synthesis of α-naphthols and bridged isoxazolines by annulation of nitrones with cyclopropenones. | ||
Deuterium labelling experiments, trapping of tBuNO with cyclohexadiene and fast 1,3-DC intramolecular cycloaddition of the intermediate ene–nitrone prepared by an alternative route led the authors to formulate a mechanistic cycle initiated by reversible, rate-determining, nitrone-directed formation of a rhodacycle (Scheme 11). After coordination of a cyclopropenone molecule, migratory insertion of its C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond and cleavage of the three-membered ring was then proposed to give a vinylrhodium complex. Depending on its steric requirements, it could undergo protodemetalation and intramolecular 1,3-DC to 25 or nucleophilic addition to the nitrone carbon–nitrogen double bond with the formation of the six-membered ring of 24. Interestingly, N,N-diarylnitrones were also viable substrates and reacted regioselectively in the N-aryl ring, showing preference for the formation of a five-membered rhodacycle in the C–H activation step rather than a six-membered one like in the reaction of N-tert-butyl substrates 4.
O bond and cleavage of the three-membered ring was then proposed to give a vinylrhodium complex. Depending on its steric requirements, it could undergo protodemetalation and intramolecular 1,3-DC to 25 or nucleophilic addition to the nitrone carbon–nitrogen double bond with the formation of the six-membered ring of 24. Interestingly, N,N-diarylnitrones were also viable substrates and reacted regioselectively in the N-aryl ring, showing preference for the formation of a five-membered rhodacycle in the C–H activation step rather than a six-membered one like in the reaction of N-tert-butyl substrates 4.
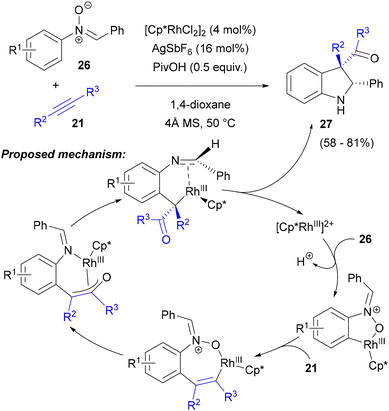
In accordance with Li's observation of the higher activity of the N-aryl ring in the Rh-catalysed C–H activation of C,N-diaryl aldonitrones,47 Chang reported a redox-neutral annulation of C,N-diarylnitrones 26 with internal alkynes 21, which involved an aryl C–H bond activation and nitrone oxygen atom transfer, in the presence of the [Cp*RhCl2]2 catalyst (Scheme 12).48a An optimization study of the reaction of diphenylacetylene with N-phenylnitrone in the presence of a catalytic amount of AgSbF6 showed that only the Rh(III) catalyst (and not Ru or Ir catalysts) led to the formation of the expected 2-aryl-3-aroylindoline 27 and that carboxylic acids, particularly PivOH, were crucial additives for obtaining high yields and diastereoselectivity. The structure of the major diastereoisomer of the product was confirmed by X-ray crystallographic analysis. The reaction scope included arylnitrones containing a 2-thienyl or a benzene ring with various substituents (Cl, Me, OMe, CF3) at nitrogen and symmetrical diarylacetylenes, as well as 1-phenylpropyne and 1-phenyl-1-hexyne, with the latter substrates giving exclusively 3-benzoylindolines rather than isomeric 3-acyloyl products. Dialkylacetylenes, enynes, propiolates and terminal alkynes were found to be unreactive.
The lack of 18O incorporation during the experiment in the presence of H218O and a considerable kinetic isotope effect observed with nitrone 26 deuterated at the ortho positions of the N-phenyl ring led to the mechanistic proposal in which coordination of the Rh(III) complex by the nitrone oxygen was followed by N-aryl C(sp2)–H activation (Scheme 12). Subsequent coordination of the alkyne and its regioselective insertion into the Rh–C bond (minimizing steric interactions in the case of unsymmetrical alkynes) leads to a seven-membered rhodacycle. Cleavage of the N–O bond transforms the nitrone function into an imine and leads to a rhodium enolate complex, which further undergoes nucleophilic cyclization to form 3-acylindoline 27. The observed diastereoselectivity could be explained by the formation of the less sterically hindered π-complex of Rh(III) with the imine. Additional details on this mechanism were provided by the DFT calculations performed by Houk, Lan and co-workers.48b Transformation of the seven-membered rhodacycle into the enolate imine complex was shown to proceed via reductive elimination, forming a carbon–oxygen bond of the Rh(I) complex, followed by oxidative addition of rhodium to the nitrogen–oxygen bond with the formation of an enolate imine complex. Insertion of the imine into the carbon–rhodium bond of this enolate accounts for the formation of the final indoline.
Concurrently, similar results were published by Li and co-workers.49 Also in the same year, Wang, Wan and co-workers reported that nitrone-directed C–H activation of the N-aryl ring allowed for the selective synthesis of N-unprotected 2,3-diarylindoles with two different aryl groups, starting from symmetrical diarylacetylenes 28 (Scheme 13).50 The key difference from Changs’ work outlined above was the use of a copper salt along with the Rh(III) catalyst. Under these conditions, symmetrical alkynes acted as one-carbon-atom units in a formal carbon–carbon triple bond cleavage. The reaction of C,N-diaryl nitrones 26 with 28 in the presence of catalytic amounts of [Cp*RhCl2]2 and AgSbF6 and 2 equiv. of Cu(OAc)2 led to a range of indoles 29 bearing aryl rings with a range of substituents (alkyl, OMe, halogen, CO2Me, NO2, CF3) at positions 2 and 3 of the indole in high yields. Isolation of 2-(4-methoxyphenyl)-3-benzoyl-3-phenyl-3H-indole 27 in high yield from the reaction between N-phenyl-C-(4-methoxyphenyl)nitrone and diphenylacetylene terminated after 1 h, as well as deuterium labelling experiments and control reactions with varying amounts of Cu(II) salt led the authors to propose a catalytic cycle analogous to the one presented earlier by Chang.48 The key difference was the further assistance of Cu(II) in regeneration of the rhodium catalyst and elimination of an aryl aldehyde molecule from 3-acylisoindole 27 to provide the final 2,3-diarylindole.
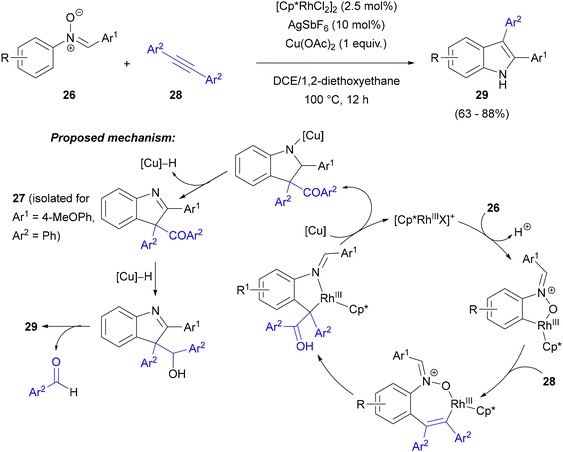 | ||
| Scheme 13 Annulation of nitrones with symmetrical acetylenes to form 2,3-diarylindoles with two different aryl substituents. | ||
An important step towards the development of nitrones as directing, oxidizing and transient groups for C–H activation was marked by a report from the Liu and Lu group, who employed C,N-diarylnitrones with bulky aryls at the carbon atom to synthesize indoles.51 In this Rh(III)-catalysed reaction, only the nitrogen atom of the nitrone moiety of 26 was incorporated into the target indole ring of indole 29, whereas carbon, oxygen and the C-aryl substituent (optimally mesityl) departed as the arylaldehyde (Scheme 14). PivOH was found to be the crucial additive and MeOH the preferred solvent over DCE. The addition of either water or 4 Å MS decreased the yield. The reaction scope encompassed symmetrical diarylacetylenes and alkyl aryl acetylenes, with the latter substrates giving 2-arylindoles selectively. Following detailed mechanistic studies, this reaction course (which was significantly different from the earlier observations of Chang described above) was ascribed to the different nature of the nitrone C-aryl substituent. After nitrone-directed C–H activation of the N-aryl ring and migratory insertion of the alkyne into the arylrhodium complex formed, reductive elimination of rhodium forms the C–O bond. Further cleavage of the N–O single bond is accompanied by reoxidation to Rh(III). The fate of the rhodium enolate formed depends on the stability of the imine function. With a bulky aryl substituent, imine hydrolysis is facile and it is followed by intramolecular amine–carbonyl condensation to form the indole's five-membered ring.
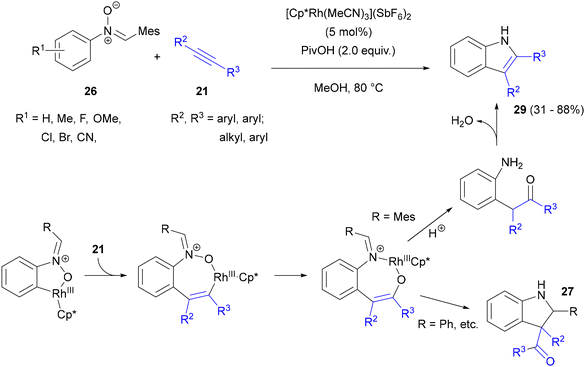 | ||
| Scheme 14 Annulation of nitrones with acetylenes to form 2,3-diarylindoles with two different aryl substituents. | ||
An example of nitrone annulation, which is peculiar from a mechanistic point of view, was disclosed by the Li group as a way of obtaining α-naphthols 31.52 They employed N-tert-butyl-C-benzoylnitrones 30, in which the benzoyl oxygen rather than the nitrone moiety served as the directing group assisting in the formation of the initial rhodacycle (Scheme 15). Further migratory insertion of an internal alkyne formed the cyclic vinylrhodium complex, which underwent nucleophilic addition to the nitrone carbon–nitrogen double bond, closing the carbocyclic ring. Aromatization by nitrosoalkane elimination (which could be trapped by cycloaddition to cyclohexadiene) led to the final 3,4-disubstituted 1-naphthols 31. Their formation could be observed using [Cp*RhCl2]2, AgSbF6 and AcOH as additives in DCE at 80 °C, but achieving high yields required [Cp*Rh(MeCN)3](SbF6)2, Ni(OTf)2 and PivOH and citric acid instead of AcOH. The reaction worked well for symmetrical diaryl- and di(heteroaryl)alkynes. Good yields and regioselectivity were also observed with 1-phenyl-1-butyne. Terminal or ester-substituted alkynes were unreactive. In the starting nitrones, various substituents were tolerated in the aryl ring, including those ortho to the carbonyl group. Good regioselectivity for the less hindered ring position was observed with meta-substituted nitrones.
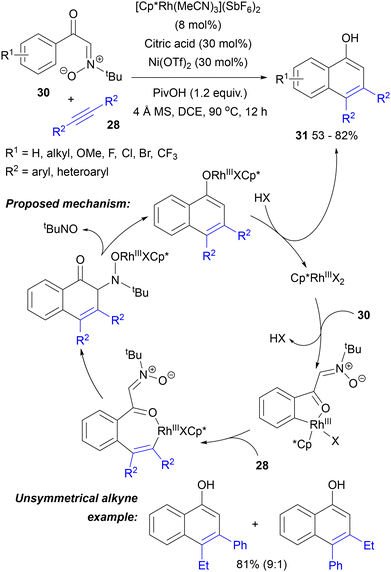 | ||
| Scheme 15 Carbonyl-directed aryl ring C–H activation followed by annulation involving the nitrone moiety. | ||
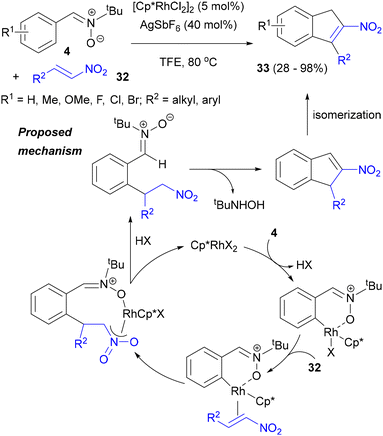
Inspired by the discoveries in the area of Rh-catalysed reactions of nitrones with alkynes, as well as a report on the successful annulation of acetophenone with nitroalkenes 32, the Li group developed Rh(III)-catalysed annulation of such alkenes with N-tert-butyl-C-arylnitrones 4 (Scheme 16).53 The use of the [Cp*RhCl2]2/AgSbF6 catalytic system in trifluoroethanol was crucial for achieving high yields in this reaction, whereas additives like PivOH, K2CO3, CsOAc, Cu(OAc)2 or AgOAc or different solvents had little or a detrimental effect and the reactions with Rh(I) precatalysts or without a rhodium complex or a silver salt failed altogether. A series of 3-alkyl- and 3-aryl-substituted 2-nitro-1H-indenes 33 was obtained in good yields, as well as 2-nitro-3-(2′-phenylethyl)-1H-indenes with alkyl, halogen or methoxy substituents on the indene's six-membered ring. Notably, 17% of product could be obtained from N-benzyl-C-phenylnitrone, but N,C-diphenylnitrone was recovered unchanged.
In the course of mechanistic experiments, a kinetic isotope effect of 5.7 was measured, and 40% of H/D exchange was observed when N-tBu-C-phenylnitrone 4 was subjected to the reaction conditions in the presence of D2O. On the other hand, when a nitroalkene was added together with D2O, some H/D exchange was observed at the indene CH2, but none at the ortho position of its benzene ring. The reaction was found to favour nitrones with electron-rich aromatic rings and electron-poor nitroalkenes. Therefore, the reaction probably proceeded via a nitrone directed formation of the rhodacycle, which underwent fast and irreversible coordination and migratory insertion of the nitroalkene (Scheme 16). Protonation of the alkylrhodium complex formed would then provide a C-(2-nitroethyl)arylnitrone and the final product via intramolecular condensation of the nitroalkane and nitrone, followed by isomerization of the double bond.
Nucleophilic addition to a nitrone group followed by hydroxylamine departure was also the final step of the catalytic cycle proposed for the formation of benzo[c]phenanthridines from C-arylnitrones and 7-azabenzonorbornadienes in the presence of the [Cp*RhCl2]2/AgSbF6 catalytic system.54 Importantly, the reaction proceeded efficiently with C-aryl-N-methylnitrones 1. The reaction was found to proceed in moderate yields with nitrones substituted with Me, MeO, NO2 and halogen groups in the aromatic ring. The use of dimethoxy- and methylenedioxy-substituted 7-azabenzonorbornadienes allowed for the direct synthesis of Decarine and other alkaloids with a benzo[c]phenanthridine core.
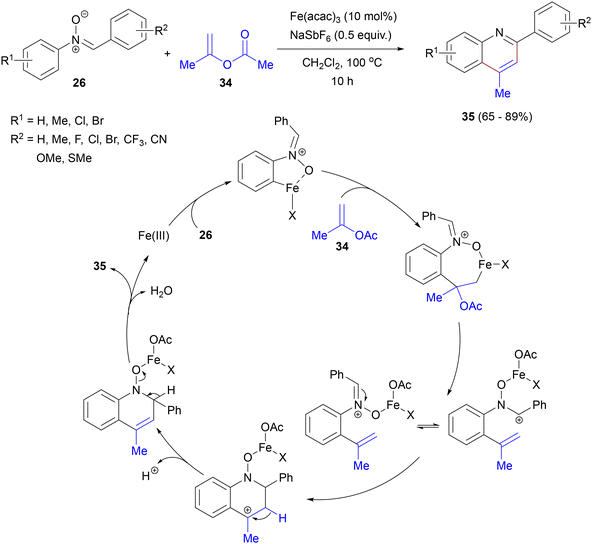
In 2016, Cheng, Shao and co-workers reported an iron-catalysed, external oxidant-free method for the synthesis of 2,4-disubstituted quinolines 35via [4 + 2] cyclization of N-arylnitrones 26 with geminal-substituted vinyl acetates 34 (Scheme 17).55 The salient features of this reaction include using an inexpensive iron catalyst, an internal oxidant, an air atmosphere and water and acetic acid as byproducts. It shows fairly good tolerance for various functional groups in both aryl rings of the nitrone, but fails with C-heteroaryl substrates and with nitrones containing ortho-substituted rings. The nitrone functionality of substrate 26 plays a dual role in this reaction: as a directing group and an internal oxidant. The Fe(III) catalyst initially participates in C–H activation of the N-aryl ring in arylnitrone 26, generating a 5-membered metallacycle in the proposed mechanism. Migratory insertion of vinylacetate 34, followed by β-O elimination and electrophilic cyclization of Fe(III)-activated nitrone onto the carbon–carbon double bond forms the heterocyclic ring. Next, the elimination of water generates the target quinoline 35.
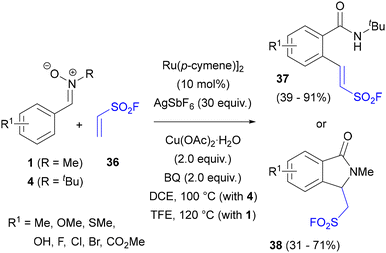
A particular type of Ru-catalysed alkenylation of the C-aryl ring in N-methyl and N-tert-butylnitrones was disclosed by the Zhao group who developed a protocol for introducing fluorosulfonylvinyl substituents.56 The reaction between nitrones 4 and ethenesulfonyl fluoride (36) gave a range of ortho-alkenylated amides 37, with a variety of other functional groups in the aromatic ring (Scheme 18). The formation of N-tert-butylamides could be explained by rearrangement of the initially formed alkenylated nitrones in the presence of water and protic acid at an elevated (100 °C) reaction temperature, similarly to Pd-catalysed alkenylation with acrylates in AcOH (see Scheme 3). Intriguingly, N-methylnitrones 1 under the same or very similar conditions (TFE instead of DCE as solvent) gave the products of further nucleophilic cyclisation onto the sulfonylvinyl group (38). Cyclisation onto 38 could be also effected with the N-tert-Bu-substituted amide 37 by the addition of DBU after the cross-coupling reaction. Concerning the catalytic cycle, its key steps were postulated to be migratory insertion of alkene 36 into the nitrone Ru(II) complex, followed by ruthenium hydride elimination and oxidation (with a Cu(II) salt and benzoquinone as the oxidants) of the Ru(0) complex to a Ru(II) acetate complex.
Isatogens are a peculiar type of cyclic nitrone. C–H activation of the aryl ring of 2-arylisatogens 39, followed by annulation with internal alkynes 21 to give spirocyclic indolinones 40, was described by Hai, Wu and co-workers (Scheme 19(a)).57 The reaction proceeded with complete regioselectivity with alkyl aryl acetylenes, giving the products with the aryl ring placed vicinally to the central carbon atom of the spirocyclic system.
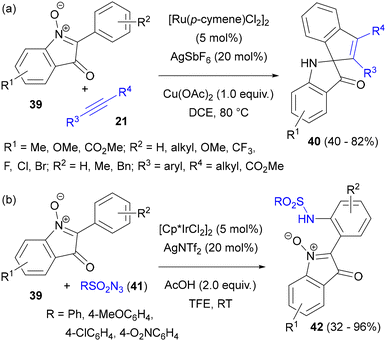 | ||
| Scheme 19 (a) Annulation of 2-arylisatogens with unsymmetrical alkynes; (b) sulfonamidation of 2-arylisatogens with sulfonyl azides. | ||
Another class of reagents that are useful in the metal-catalysed cross-coupling reactions of N-arylnitrones are diazo compounds.24b These resections usually result in the formation of a five membered indole or indoline ring annulated onto the N-aryl ring of the starting nitrone.
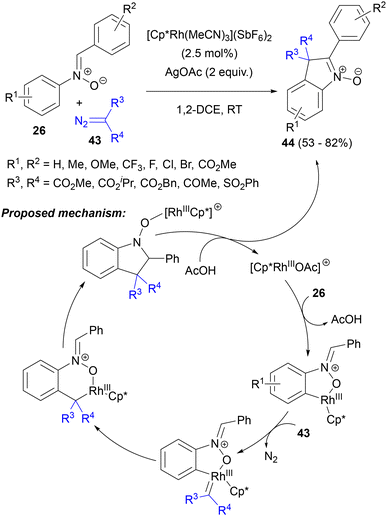
In 2015, Zhou's group demonstrated a Rh(III)-mediated synthesis of 3H-indole-N-oxides (44) via intermolecular coupling between C,N-diarylnitrones 26 and 1,3-dicarbonyl-2-diazo compounds 43 (Scheme 20).58a The reaction occurred in the presence of 2 equiv. of silver(I) acetate in DCE, notably at room temperature, to give a range of indole N-oxides bearing two ester or acyl substituents, or even an ester and a sulphonyl substituent at C(3). Mechanistic experiments, including H/D exchange at the ortho positions of the N-aryl ring of substrate 26 and KIE measurements, confirmed that initially, nitrone 26 underwent C–H metalation with the catalytically active rhodium species to give a rhodacyclic intermediate (Scheme 20). Next, the coordination of α-acyldiazoacetate 43 to rhodium via denitrogenation, followed by migratory insertion of the carbene ligand generates the six-membered rhodacyclic intermediate. Intramolecular nucleophilic attack on the imine moiety of nitrone leads to a Rh complex with N-hydroxyindoline. Protolytic cleavage of this complex and oxidation of the N-hydroxyindoline intermediate with Ag(I) gives the final N-oxide 44. The reaction stopped at the hydroxyindoline stage when PivOH was used as an additive instead of AgOAc. Recently, a very similar catalytic system – [Cp*RhCl2]2/AgSbF6/AgOAc – was used by Guo, Fan and co-workers in the reaction of nitrones 26 with 2-diazo-1,3-indandiones to obtain analogous, spirocyclic derivatives of indole N-oxides 37.58b
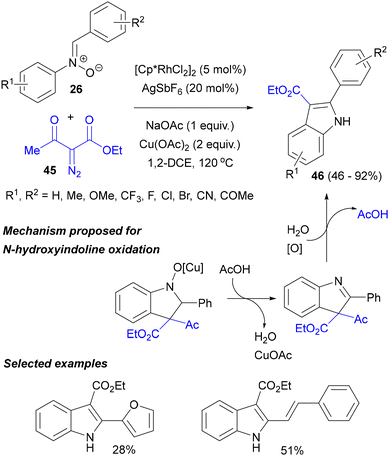
A similar reaction of nitrones 26 in the presence of the [Cp*RhCl2]2 precatalyst and only 12 mol% of AgSbF6 (as an activator of the rhodium precatalyst) and an excess of PivOH was later reported to be an efficient method of accessing 3,3-diacyl-N-hydroxyindolines.59 An interesting modification was then invented by Chen and co-workers, who performed the reaction with similar substrates in the presence of catalytic amounts of [Cp*RhCl2]2 and AgSbF6 and an excess of Cu(OAc)2, obtaining 2-arylindoles 46 in high yields (Scheme 21).60 The influence of the copper salt on the reaction course can be rationalized by transmetalation of the initially formed Rh complex of N-hydroxyindoline with copper, followed by water elimination to release a 3H-indole derivative. Then, oxidative cleavage of the 3-acetyl group, probably also assisted by copper salts, yields the anticipated indoles. The reaction worked also well with alkyl diazophosphonates.
Li and co-workers reported an innovative strategy towards enantioenriched spirocyclic nitrones 50 in which the central chirality of the target all-carbon stereocenter was governed by the axial chirality of atropomerically metastable biaryl intermediate 49, formed via C–H activation of C,N-diarylnitrone 47 and its reaction with ortho-quinone diazide 48 in the presence of a chiral Rh(III) complex (Scheme 22).61
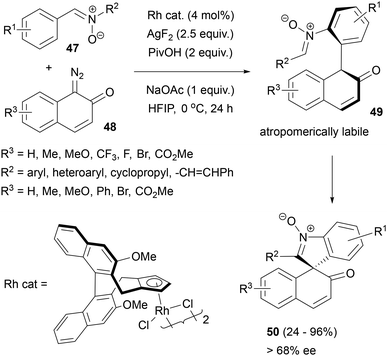 | ||
| Scheme 22 Enantioselective annulation of nitrones with diazo compounds to spirocyclic 3H-indole-N-oxides. | ||
Specifically, the high yield and enantioselectivity of this [4 + 1]-spiroannulation reaction could be obtained using Cramer's chiral CpXRh(III) catalyst,62 PivOH and AgF2 as an oxidant. A range of spirocyclic nitrones with various substituents on the aryl rings could be obtained by this method, with ee values usually exceeding 90%. Mechanistic studies established that the biaryl intermediate formed from C,N-diphenylnitrone and 7-bromoquinone diazide was characterized by a relatively low rotational barrier (t1/2 = 60.5 min), which was still sufficient to obtain the oxidative nucleophilic cyclisation product with high enantioselectivity. The oxidation step could be effected with AgF2 or electrochemically and did not require the presence of rhodium.
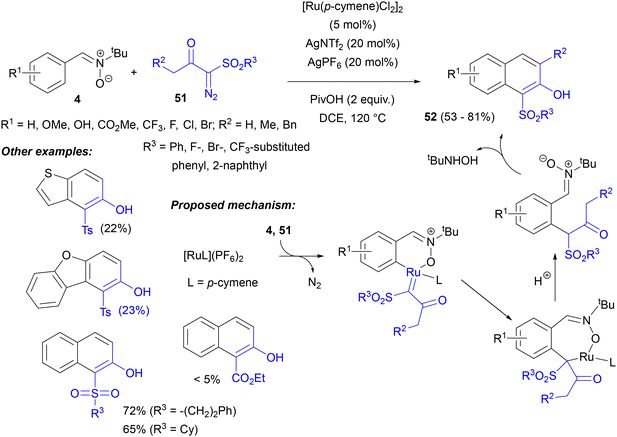
A Ru(II)-catalysed C–H activation of C-arylnitrones 4 and their subsequent reaction with α-diazo sulfonyl ketones 51 was elaborated by Li and co-workers.63 The intermolecular [3 + 3] annulation process gave 1-sulfonyl-2-naphthols 52 under redox-neutral conditions (Scheme 23). The reaction required the presence of carboxylic acids derivatives, particularly PivOH. The ruthenium catalyst gave the highest yields, while [Cp*RhCl2]2 and [Cp*RhCl2]2 were inferior and [Cp*Co(CO)I2] gave only traces of products 52. Concerning the mechanism, a typical formation of a ruthenacycle and its carbene complex, followed by carbene migratory insertion, was postulated to lead to ortho-acylalkyl nitrones, which could be isolated. Next, their intramolecular condensation with liberation of N-tBu-hydroxylamine to form the naphthol ring is feasible thanks to the sulfonyl substituent (an analogous ester substrate gave only traces of product). This cyclization step is likely the rate-determining step, which is consistent with the smaller KIE values measured for the initial C–H activation.
Similarly, the nitrone moiety of nitrones 4 acted as a traceless directing group in their Rh(III)-catalysed annulation with cyclic 2-diazo-1,3-diones.64 The reaction was found to proceed under air in the presence of relatively large amounts of silver salts and led to polycyclic chromendiones in moderate yields. This outcome could be rationalized by the Rh-catalysed introduction of the 1,3-dicarbonyl substituent at the C(2) position of the C-aryl ring in nitrones 4, followed by hydrolysis of the nitrone and condensation of the aldehyde formed to yield a cyclic hemiacetal. Oxidation of this intermediate, perhaps assisted by Ag salts, would give the final chromene products.
Ru- and Rh-catalysed annulations with diazo compounds have been also developed for azomethine imines.65
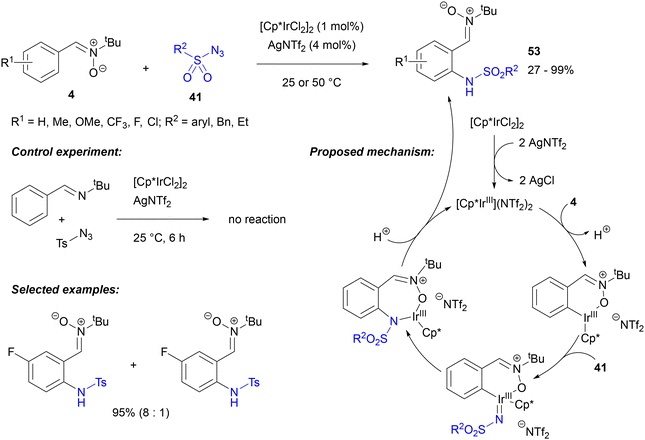
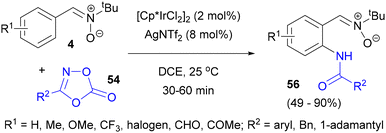
The formation of a carbon–nitrogen bond in the reactions of arylnitrones with diazo compounds and azides is also the basis of direct amidation of the nitrone aromatic ring. For example, nitrone-directed sulfamidation of N-tert-butyl-C-arylaldonitrones 4 was disclosed by Pi, Cui and Wu (Scheme 24).66 The reaction of such nitrones with sulfonyl azides 41 proceeded in the presence of just 1 mol% of an iridium precatalyst, notably at room temperature and with a silver salt as an activator of the precatalyst, without the need for any other additives or oxidants. For comparison, the [Ru(p-cymene)Cl2]2 catalyst could also be used, but only at elevated temperatures and in the presence of Cu(OAc)2, while Pd(OAc)2 and [Cp*RhCl2]2/AgSbF6 showed no activity. The authors observed excellent functional group tolerance, obtaining excellent yields and complete selectivity for mono-amidation with a range of ring substituted nitrones and azides 41 (with the exception of nitrobenzenesulfonyl azide), as well as with alkylsulfonyl substrates. One of the sulfonamides 53 could be further used in a 1,3-dipolar cycloaddition reaction with benzyne.
The reaction failed with an imine instead of a nitrone as the substrate, indicating that the nitrone oxygen was crucial for the directing group's activity and formation of an iridacycle intermediate. Further coordination of a sulfonyl azide and elimination of nitrogen produced a sulfonylnitrene iridium complex, which upon migratory insertion of the nitrene ligand and protolysis of the iridium imido complex formed gave the final functionalised aldonitrones 53 (Scheme 24).
A similar protocol has also been exploited for the sulfonamidation of isatogenes 39 to sulfonamides 42 (Scheme 19(b)).57
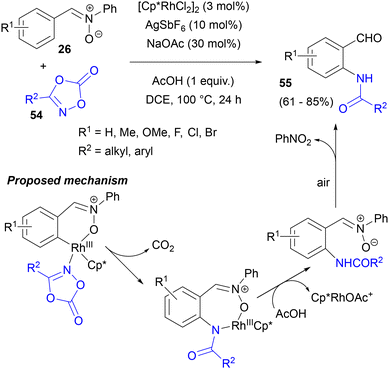
In 2017, the nitrone-directed amidation of the C-aryl rings of nitrones 26 in the presence of the [Cp*RhCl2]2/AgSbF6 catalyst system was disclosed (Scheme 25).67 The reaction with alkyl- and aryl-substituted 1,4,2-dioxazol-5-ones 54 was accompanied by nitrone hydrolysis to give N-acylated o-aminobenzaldehydes 55. The presence of NaOAc additive was found to be crucial to obtain any conversion, as well as a nitrone moiety in the substrate, since under the same conditions benzaldehyde gave the amidation product only in very low yield. Notably, the C-aryl ring of 26 participated in this reaction in the presence of N-phenyl.
Efficient Ir(III)-catalysed direct C–H amidation of C-arylnitrones 4 was described by Cui's group in 2019.68 The amidated nitrones 56 were achieved at room temperature in good to excellent yields and a broad range of functional groups were tolerated (Scheme 26). [Ru(p-cymene)Cl2]2 and [Cp*Co(CO)I2] were inactive in this reaction and [Cp*RhCl2]2 gave a slightly lower yield. Heterocycles 54 were used as a nitrogen source, generating CO2 as the sole byproduct. The reaction probably proceeded according to a mechanism analogous to the one in Scheme 25. The amidated nitrones obtained could be further converted into substituted benzisoxazolines, showing the practical utility of the method.
4. C–H activation of nitrones followed by 1,3-dipolar cycloaddition
Several creative domino sequences, allowing for the rapid construction of polycyclic isoxazolidine scaffolds, have been designed by exploiting the nitrones’ ability to act as both directing groups and dipoles in intramolecular 1,3-dipolar cycloaddition. The key step of these reactions is the transition metal-catalysed, nitrone-directed C–H activation and cross coupling with alkenes, alkynes or allenes, followed by an elimination step, which introduces an unsaturated moiety, positioned appropriately to further act as a dipolarophile to form an isoxazolidine ring. In all cases, the unsaturated partner of the reaction contained a leaving group and a multiple bond activated by the presence of groups withdrawing electrons via either resonance or inductive effects, or by ring strain. DFT calculations performed by the Kim group on amine-directed C–H activation and allylation revealed that the feasibility of the migratory insertion of olefinic substrates strongly depended on the electronic character of their double bonds.69Several C–H activation/1,3-DC sequences, analogous to those developed for nitrones, have also been described with azomethine imines.69a,70
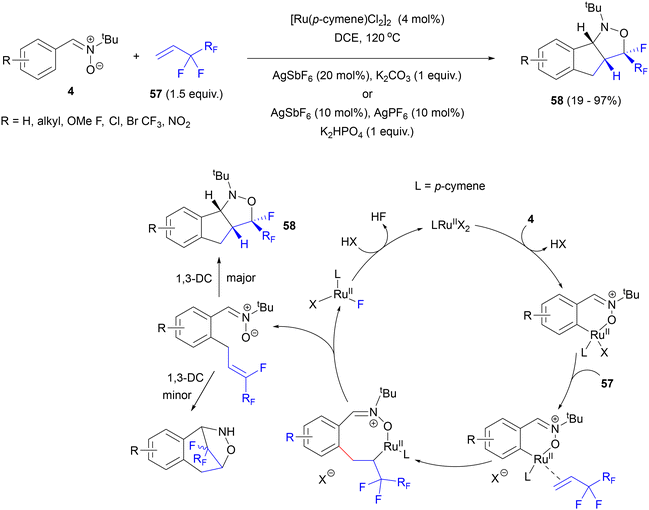
In 2018, the Li group presented the idea of Ru-catalysed, nitrone-directed C–H activation and cross-coupling with perfluoroalkylalkenes 57, which would be followed by Ru-mediated β-fluoride elimination to generate a double bond positioned appropriately to undergo dipolar cycloaddition with the nitrone moiety and provide polycyclic isoxazolidines with a quaternary fluorine-substituted stereocenter (Scheme 27).71 Specifically, N-tert-butylaldonitrones 4 were found to react with alkenes 57 in the presence of [Ru(p-cymene)Cl2]2, AgSbF6 and K2CO3 under a nitrogen atmosphere. Interestingly, higher endo/exo selectivity of the cycloaddition step was observed at elevated temperature (120 °C). In some cases, a mixture of silver salts (AgSbF6 and AgPF6) with K2HPO4 as the base was needed to achieve high yields and endo/exo stereoselectivity. Under the optimized conditions, a series of aldonitrones 4 derived from aromatic aldehydes with diverse substitution patterns (both electron-withdrawing and donating groups) reacted with alkenes 57 with various lengths of perfluoroalkyl chain to give polycyclic, fluorinated isoxazolidines 58 in high yields, with excellent diastereoselectivity and regioselectivity typical of the normal electron demand cycloadducts.
Apart from nitrones derived from m-CF3 and m-methylbenzaldehyde, low regioselectivities of C–H activation of non-equivalent ortho positions were observed in aldonitrones with meta substituents on the aryl ring. N-Cyclohexyl, N-benzyl and N-phenylnitrones failed to react. This observation was explained by the large steric hindrance of the tBu substituent, which stabilized the Z configuration of nitrone, facilitating the cyclometalation step. The reaction also failed with alkenes substituted with fluorine at the double bond or with alkyl or ester groups at the terminal carbon.
Based upon kinetic isotope effect measurements and competition experiments, a catalytic cycle was proposed in which reversible nitrone-directed C–H activation in the aromatic ring was followed by migratory insertion of the perfluoroalkene (Scheme 27). High E/Z selectivity of the subsequent fluoride elimination step ensures high diastereoselectivity of the final cycloadduct.
Most C–H activation/1,3-DC cascade reactions were realized by elimination of oxygen rather than fluorine leaving groups as the key elimination step, which formed the unsaturated dipolarophile. In many cases, Rh(III) catalysts could be used rather than ruthenium or iridium. For example, Hong, Kim and co-workers employed O-acetates of Morita–Baylis–Hillman (MBH) adducts 59 as cross-coupling partners to obtain bridged benzoxazepines, naphthalenes, and carbazoles from N-tert-butyl-C-arylnitrones (Scheme 28).42 The reactions of C-arylnitrones with various substitution patterns and electronic characteristics (OMe, NHAc, Me, halogen, CF3, CO2Me, NO2) with MBH acetates, performed in the presence of [Cp*RhCl2]2 and AgSbF6 and under an O2 atmosphere, gave benzoxazepines 60 in high yields and high regioselectivity, when nonequivalent positions of the ring were available. On the other hand, no products were observed with N-methyl- or N-phenyl-C-phenylaldonitrone.
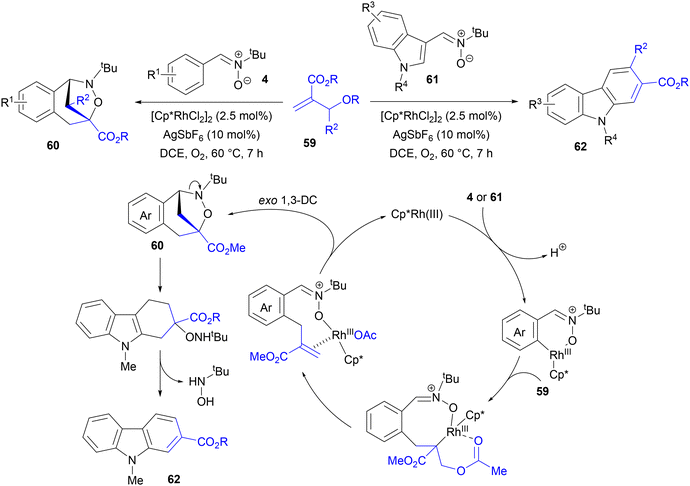 | ||
| Scheme 28 Alkenylation of nitrones with acetates of MBH adducts, followed by intramolecular 1,3-DC to carbazole derivatives. | ||
Interestingly, an entirely different product – a β-methoxycarbonylnaphthalene derivative – was obtained from nitrone with the strongly electron donating NMe2 group in the para position. Similarly, electron rich 3-indolinyl nitrones 61 gave a series of carbazoles 62 (Scheme 28). Based upon DFT studies, this outcome could be rationalized by C–N bond cleavage and elimination of N-tert-butylhydroxylamine from the initially formed benzoxazepines 60, facilitated by the strongly electron-rich characteristic of the aromatic ring.
The same group also developed a general synthesis of naphthalene derivatives (specifically, β-naphthols) via a one pot sequence involving a cascade Rh(III) cross-coupling of C-arylnitrones 4 with MBH adducts 59 and cycloaddition, followed by treatment with HCl at elevated temperature (Scheme 29).72 Protonation of the ring nitrogen atom of the bridged benzazepine cycloadducts facilitated further decarboxylation and concomitant N–O bond cleavage. Further elimination of tert-butylamine provided β-naphthols 63 in moderate to good yields.
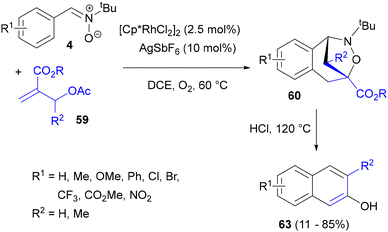 | ||
| Scheme 29 Alkenylation of nitrones with acetates of MBH adducts, followed by intramolecular 1,3-DC, decarboxylation and elimination to 2-naphthols. | ||
Very recently, similar Rh(III)-catalysed reactions of C-aryl-substituted azomethine imines with O-Boc-protected MBH adducts and with allyl acetate were reported.73
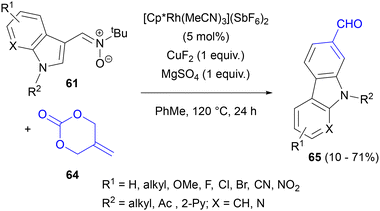
The concept of two oxygen substituents increasing the electrophilicity of a carbon–carbon double bond and acting as leaving groups was exploited in the reactions of 2-methylidenetrimethylene carbonate (64) with nitrones74a and azomethine imines.75 In the former case, N-tert-butyl-C-(3-indolyl)nitrones 61 reacted with 64 in air in the presence of the [Cp*Rh(MeCN)3](SbF6)2 catalyst and stoichiometric amounts of CuF2 and MgSO4 to give 2-formylcarbazoles 65 (Scheme 30). This result could be rationalized by the formation of bridged (exo) cycloadducts and elimination of N-tert-butylhydroxylamine to give hydroxymethylcarbazoles. These compounds could be further oxidized with air in moderate yields in the presence of either [Cp*Rh(MeCN)3](SbF6)2 or CuF2, or in high yields in the presence of both catalysts simultaneously. The product of nitrone allylation (before cycloaddition) could be isolated, further confirming the general mechanistic picture of C–H activation/1,3-DC cascades.
A year later, the group of Li and Liu described the synthesis of bridged benzoazepines with a hydroxymethyl group at the bridgehead carbon, starting from carbonate 64 and simple C-aryl-N-tert-butylnitrones 4 using the [Cp*RhCl2]2 precatalyst.74b The dichotomy of the product structure (benzoazepine vs. carbazole) is analogous to the observations of the Kim group shown in Scheme 28.
A cross-coupling partner similar to 64 is 4-vinyl-1,3-dioxolan-2-one (66). It provides hydroxymethyl-substituted oxazolidines 67 with a fused ring system in the Rh(III)-catalysed reactions with N-tert-butylaldonitrones 4, as reported by Liu, Chang and co-workers (Scheme 31).76 A wide range of substrates, including those functionalized with natural product fragments, could be used efficiently in this transformation. The observed stereochemistry could be explained by the Rh-assisted formation of the Z isomer of 4-arylbut-2-en-1-ol in the course of the β-elimination of oxygen. A similar Rh(III)-catalysed reaction of carbonate 66, but with azomethine imines, was reported a year later by the Zhang and Fan group, together with further studies on cross-coupling between these dipoles with carbonate 64.77
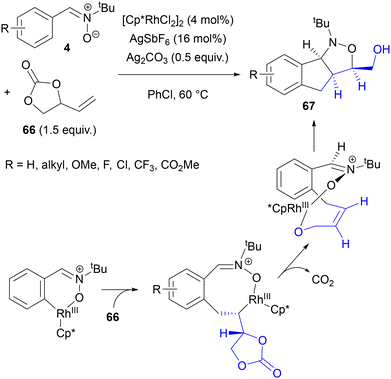 | ||
| Scheme 31 Alkenylation of nitrones with a vinyl-substituted cyclic carbonate, followed by intramolecular 1,3-DC. | ||
Unlike other C–H activation/1,3-DC cascades, which generally employed N-tert-butylnitrones, the Liu group succeeded recently in employing C,N-diarylnitrones 26 and simple allyl ethyl carbonate 68 in the Rh(III)-catalysed C–H activation/1,3-DC cascade for the synthesis of 1,4-epoxy-2-aryltetrahydro-1-benzazepines 69 (Scheme 32).78 The process requires stoichiometric amounts of AgOAc and is highly regio- and stereoselective, providing exclusively the cis isomers of 69, owing to the high preference of the intermediate ortho-allylated nitrones for the Z configuration. Compounds 69 were obtained in moderate yields, with a broad substrate scope and good functional group tolerance. The N–O bond could be further cleaved using zinc powder under mildly acidic conditions, showing the practical utility of the method. With a 2,6-dichlorophenyl substituent at nitrogen, the cycloaddition failed and the allylated, intermediate nitrone was isolated. Based upon deuterium labelling experiments and competition between substrates containing N-aryl rings with different electronic characteristics, the authors proposed that the catalytic cycle was initiated by the rate determining, electrophilic ortho C–H bond activation of nitrone 26 by the Rh(III) complex. The 5-membered rhodacycle intermediate generated coordinated to olefin 68. Subsequent migratory insertion of 68, β-oxygen elimination with carbonate acting as a good leaving group and 1,3-dipolar cycloaddition provides the final product 69. In some of the cases studied, doubly allylated products were observed.
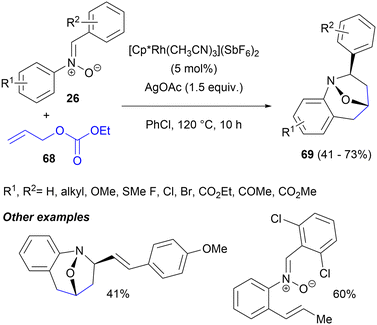 | ||
| Scheme 32 Alkenylation of the N-aryl ring of nitrones with allyl ethyl carbonate followed by intramolecular 1,3-DC. | ||
Very recently, our group disclosed a similar reaction (with allyl methyl instead of ethyl carbonate), but occurring at the C-aryl rings of nitrones 4.79 In the presence of [Cp*RhCl2]2, AgSbF6 and PivOH, tricyclic isoxazolidines with a condensed ring system were formed predominantly or exclusively, but in some cases bridged isomers dominated, depending on the substituents present at the ortho position of the aromatic ring.
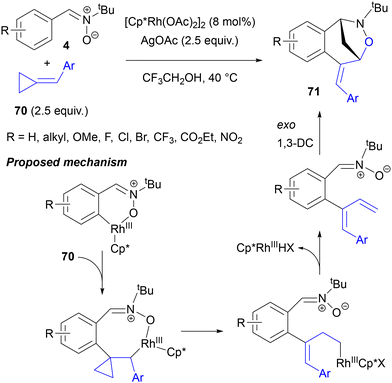
Bai, Li and co-workers reported the synthesis of bridged benzoxazepines 71 with an arylidene function by employing arylidene cyclopropanes 70 in a Rh-catalysed reaction with both C-arylnitrones 4 and analogous azomethine imines (Scheme 33).80a The reaction required an excess of AgOAc additive and a rhodium(III) precatalyst (preferably acetate rather than chloride) in CF3CH2OH at 40 °C and tolerated a range of substituents (halogens, CF3, CO2Me, CN, NO2) on the aromatic rings of both reaction partners. Its course could be rationalized by migratory insertion of the carbon–carbon double bond of arylidenecyclopropane 70 into the initial rhodacycle, strain-releasing ring opening to an alkylrhodium species, β-hydride elimination and 1,3-DC of the nitrone to the less substituted double bond of the resulting diene.
Analogous to the reactions of C-(3-indolyl)nitrones with MBH acetates and cyclic carbonates (see Schemes 28 and 30, respectively), the reaction of such nitrones with arylidenecyclopropanes 70 in the presence of [Cp*Rh(OAc)2] gave carbazoles as the products of elimination of the initially formed benzoxazepines.80b
Examples of tandem C–H activation/1,3-DC driven by the cyclopropane ring strain were reported recently for azomethine imines.81
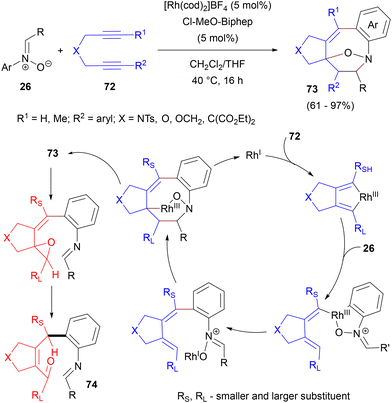
In 2014, Wan et al. reported an Rh-catalysed [2 + 2 + 5] approach for bridged eight-membered heterocycles 73 using nitrones 26 and diynes 72 with high regioselectivity (Scheme 34).82 The reaction probably proceeds via initial coordination of diyne 72 to rhodium and the formation of a rhodacyclopentadiene intermediate, which upon irreversible C–H activation of nitrone 26 (H/D KIE value 5.3) gives a dienyl-substituted rhodacycle. This step proceeds as an electrophilic attack of rhodium on the nitrone aryl and is faster for electron-rich nitrones. Reductive elimination affords the product of dienylation of the nitrone ring. Finally, intramolecular rhodium-assisted [3 + 2] cycloaddition and another reductive elimination step provides the final product 73 and regenerates the catalyst. In some of the cases, the release of ring tension resulted in retro-[3 + 2] cycloaddition followed by a 1,4-H shift to give the ring-opened product 74 (Scheme 34).
5. Co(III)-catalysed reactions
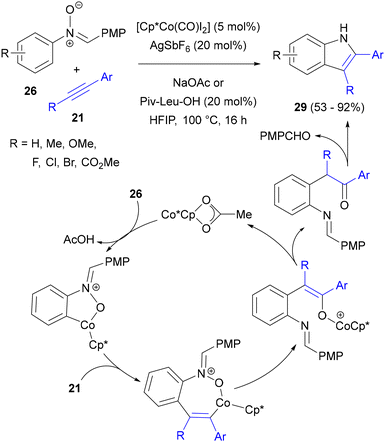
In recent years, reactions catalysed by cobalt(III) rather than rhodium(III) have been developed very actively, which is reasonable considering much better availability and abundance of the former metal.20j,83 In the context of nitrone C–H activation, in a pioneering study, Ackermann developed the synthesis of indoles from C-(p-methoxyphenyl)-N-arylnitrones 26 with symmetrical diaryl acetylenes and alkyl aryl acetylenes 21 (Scheme 35).84 High yields of annulation products 29 were obtained in the presence of the [Cp*CoI2(CO)] complex, AgSbF6, NaOAc and amides of amino acids, particularly Piv–Leu–OH, in air. Based on deuterium labelling experiments and competition reactions with substrates of various electronic characteristics, the reaction is believed to be initiated by acetate-assisted, electrophilic C–H activation of the N-aryl ring of 26 with the formation of a cobaltacycle intermediate. After migratory insertion of the triple bond of the alkyne, a vinylcobalt intermediate is formed. Next, it rearranges into a cobalt enolate complex, possibly via reductive elimination of cobalt to form a C–O bond, followed by oxidative insertion of Co(I) species into the N–O bond. In this step, the capability of the nitrone to act as an internal oxidant in an overall redox-neutral process is demonstrated. Further protolytic cleavage of the enolate complex and hydrolysis of the imine sets the stage for intramolecular condensation to furnish the final 2,3-diarylindoles 29.Owing to the higher electropositivity of cobalt compared to that of Rh and Ru and the resulting more nucleophilic character of the organocobalt species, cobalt catalysis has been increasingly applied in mechanistic scenarios involving nucleophilic attack on the nitrone group. For example, a Co(III)-catalysed reaction of nitrones with alkynes similar to the one described above (see Scheme 35) was studied by the Zhang group, who explored various aryl groups at the C terminus of C,N-diaryl aldonitrones 26 with the aim of directing the course of their reaction with alkynes 21 towards quinoline N-oxides 75, instead of indoles (Scheme 36).85 They found that the best selectivity for six-membered ring formation could be obtained with the 2,6-dichlorophenyl (DCP) group. In order to retain the N–O bond in the products, the reaction required an external stoichiometric oxidant; the optimal one was AgOAc (other Ag salts lacking an acetyl anion gave only traces of products). [Cp*RhCl2]2, [Ru(p-cymene)Cl2]2 and Pd(OAc)2 were found to be inactive under the same conditions. The reaction worked well with a broad range of substituents on the N-aryl ring of nitrone 26 and with symmetrical diaryl- and dialkylacetylenes.
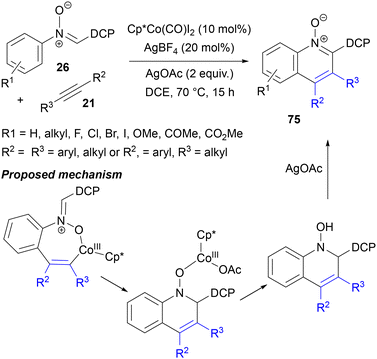 | ||
| Scheme 36 Co(III)-catalysed annulation of C-dichlorophenyl nitrones with alkynes to form quinoline N-oxides. DCP = 2,6-dichlorophenyl. | ||
Control experiments performed with an N-(o-vinylphenyl)nitrone derivative revealed that both [Cp*Co(CO)I2] and AgOAc were necessary for heterocyclic ring closure and a substoichiometric amount of AgOAc oxidant led to a quinoline N-oxide as the major product in air, whereas under a nitrogen atmosphere mainly free quinoline was formed. No oxidation of quinoline to its N-oxide was observed under standard conditions. Based upon these experiments, as well as DFT calculations, the authors proposed a catalytic cycle initiated by coordination of a [Cp*Co(III)(κ2-OAc)] complex to the nitrone oxygen, followed by acetate-assisted C–H activation (Scheme 36). Migratory insertion of the alkyne formed a vinylcobalt intermediate, which underwent intramolecular nucleophilic addition to the nitrone group. This step was found to be particularly strongly facilitated by the presence of a DCP group as compared to a simple phenyl. Liberation of N-hydroxydihydroquinoline and its oxidation with Ag+ gave the final quinoline N-oxides 75. Without oxidant, the same N-hydroxyquinoline intermediates liberated water to give free quinolines.
Recently, our group became interested in the possibility of employing cobalt(III) complexes as catalysts in nitrone-directed C–H activation/1,3-DC cascades.86 We expected that the addition of an arylcobalt intermediate to the central atom of an allene83c equipped with an appropriate leaving group would lead, after β-oxygen elimination, to dienylation of the nitrone C-aryl ring (Scheme 37). Intramolecular 1,3-DC of the nitrone–diene intermediates formed would lead to polycyclic nitrogen-containing systems, functionalized with an additional, exocyclic olefin. As model substrates, 1-arylisoindole N-oxides 20 were chosen (Scheme 37). Indeed, the reaction of cyclic nitrones 20 (isoindole N-oxides) with 2,3-butadiene-1-yl methyl carbonate 76 lead to regioisomeric mixtures of spirocyclic products 77. Interestingly, under identical conditions, [Cp*Co(CO)I2] proved superior to [Cp*RhCl2]2, while a ruthenium catalyst was completely inactive. The yields increased dramatically in the presence of Na or Cs acetates, AcOH or PivOH. Even more beneficial were more sterically hindered carboxylic acids, such as (1-methylcyclohexyl)carboxylic acid or adamantanecarboxylic acid. Regioselectivity of the fused-ring vs. bridged products could be significantly improved by increasing the reaction temperature from 80 to 100 °C. The reaction gave good yields with a variety of 1-arylisoindole-2-oxides 20 bearing a range of substituents on any of their benzene rings. We then found that other cyclic nitrones – 2-arylpyrroline-1-oxides – were viable substrates, giving products with condensed ring systems exclusively. Even a simple C-phenyl-N-tert-butyl reacted to give a reasonable yield and with completely opposite regioselectivity of the 1,3-DC step.
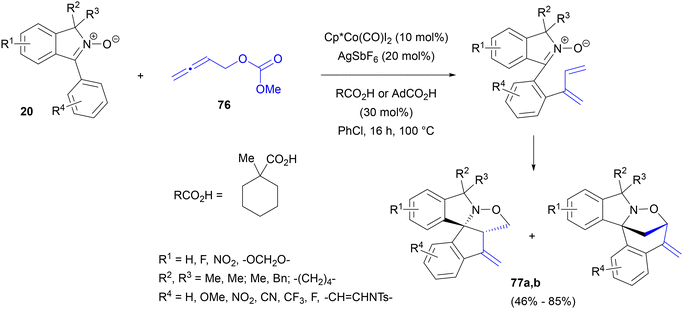 | ||
| Scheme 37 Synthesis of spirocyclic isoindolines by Co(III)-catalysed annulation of cyclic nitrones with allenylmethyl carbonates. | ||
Hydrogen–deuterium exchange under the reaction conditions in the presence of CD3CO2D, occurring at the ortho positions of the C-aryl ring, as well as a considerable KIE value, indicated that the reaction was indeed initiated by the nitrone-directed aryl C–H activation with a Co(III) complex. Additional, indirect confirmation was that a substrate with a 2,4-dimethoxyphenyl substituent connected to the nitrone ring failed to react, presumably due to its inability to adopt a planar conformation and form a cobaltacycle. Subsequent nucleophilic addition to the central carbon of the cumulene system, elimination of carbonate and intramolecular 1,3-DC account for the formation of the observed products 77. Their further transformations were demonstrated, particularly cleavage of the N–O bond to produce spirocyclic isoindolines.
6. Conclusions
Nitrone-directed C–H functionalization has become a versatile synthetic method for the preparation of nitrogen-containing targets, particularly polycyclic ones, by C–H activation-initiated annulation reactions or C–H activation/cycloaddition sequences. The large body of experimental data accumulated over the past few years provides a good background for designing new, advanced and atom-economical processes of this type. On the other hand, remarkably few of the works presented in this review supported their mechanistic proposals with computational methods. Future progress in nitrone-directed C–H functionalization will benefit from a more detailed mechanistic understanding supported by computational and kinetic studies. In particular, DFT modelling of key C–H activation and annulation steps could clarify the role of common additives, such as pivalic acid and acetates, which are known to influence both rate and selectivity strongly but remain mechanistically ambiguous. Quantitative insights into their function could enable predictive catalyst design and optimized reaction conditions for new substrate classes. Another interesting issue for computational studies would be the preference of the C- versus N-connected aryl ring of the nitrone to undergo C–H activation, depending on factors such as the particular metal used and the structure of the nitrone.The development of cobalt-catalysed reactions seems very promising as this metal is more Earth-abundant than other transition metals routinely employed for the catalysis of C–H activation processes. A systematic comparison of Co(III)–Cp complexes with different ancillary ligands (e.g., N,N-bidentate or phosphine-based) could reveal how electronic and steric parameters control reactivity and selectivity in nitrone-directed processes. Importantly, the development of cobalt catalysis with chiral cobalt complexes and chiral carboxylate additives is an intriguing area of research still in its infancy.87 Recent works on racemic reactions have proved that higher reactivity and unique chemo- and site selectivity can be achieved via electronic tuning of the Cp ligands.88 The hybridization of such electronically tuned Cp ligands along with chiral carboxylic acids would be an advantage and it is likely to open new exciting opportunities in nitrone-directed cascade reactions leading to chiral, non-planar products.
One possible way of developing such processes relies on the enantioselective generation of stereochemically labile nitrone intermediates in the C–H activation step, followed by their fast intramolecular transformations (annulation, cycloaddition) into the final enantioenriched products. This approach has been demonstrated in pioneering studies by the Li group56 (see section 3, Scheme 22). Another strategy could be the use of prochiral coupling partners that would introduce stereogenic centres to control the stereochemical course of the second, cycloaddition step, without direct participation of the chiral catalyst. Finally, in some cases of the synthesis of chiral, racemic indolines and their derivatives (see Schemes 12 and (Scheme 20)), the catalyst is proposed to participate in both C–H activation and annulation steps, so the use of a chiral Cp ligand in such cases appears to be a straightforward concept for achieving enantioselectivity.
Another important challenge in the area of nitrone-directed C–H activation is C(sp3)–H activation, which would considerably expand the structural diversity of the attainable products. Such processes have already been explored for similar directing groups, namely, aromatic N-oxides.89 Moreover, the discrimination of two identical substituents, for example alkyls attached at the N-terminus of nitrone, could be a useful strategy for the development of enantioselective C(sp3)–H activation reactions.
In summary, the extraordinary progress made during the last decade in the field of nitrone-directed C–H activation provides a strong basis for further developments in the synthetic application of nitrones in the area of designing cascade sequences for more efficient approaches for preparing complex nitrogen-containing molecules.
Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
Financial support from the National Science Centre, Poland (Grant Opus UMO-2022/47/B/ST4/01498) is gratefully acknowledged.References
- (a) I. Rozpara, J. Marco-Contelles, D. G. Piotrowska and I. E. Głowacka, Molecules, 2025, 30, 1333–1363 CrossRef CAS PubMed; (b) D. C. Santos, D. D. S. Dos Santos Costa, P. R. R. Costa, A. G. Dias, M. D. O. Silva, T. A. Aly, V. Del Colle, J. A. Xavier, M. O. F. Goulart and T. L. Silva, ChemElectroChem, 2025, 12, e202500068 CrossRef CAS; (c) M. Culcasi, C. Delehedde, M. Esgulian, M. Cassien, M. Chocry, A. Rockenbauer, S. Pietri and S. Thétiot-Laurent, ChemBioChem, 2023, 24, e202200749 CrossRef CAS PubMed; (d) J. Marco-Contelles, J. Med. Chem., 2020, 63, 13413–13427 CrossRef CAS PubMed; (e) C. Oliveira, D. Bagetta, F. Cagide, J. Teixeira, R. Amorim, T. Silva, J. Garrido, F. Remiao, E. Uriarte, P. J. Oliveira, S. Alcaro, F. Ortuso and F. Borges, Eur. J. Med. Chem., 2019, 174, 116–129 CrossRef CAS PubMed; (f) F. A. Villamena, A. Das and K. M. Nash, Future Med. Chem., 2012, 4, 1171–1207 CrossRef CAS PubMed.
- (a) Q. Wang, D.-K. Zheng, S.-P. Jiang, J. Li, Z. Xie, L.-H. He, L. Liu and Y. Liu, Chem. Biodiversity, 2023, 20, e202301449 CrossRef CAS PubMed; (b) M. Zhang, S. Liu, H. Li, Y. Guo, N. Li, M. Guan, H. Mehfooz, J. Zhao and Q. Zhang, Chem. – Eur. J., 2019, 25, 12620–12627 CrossRef CAS PubMed; (c) F. W. W. Hartrampf and D. Trauner, J. Org. Chem., 2017, 82, 8206–8212 CrossRef CAS PubMed; (d) P. S. Baran, C. A. Guerrero, B. D. Hafensteiner and N. B. Ambhaikar, Angew. Chem., Int. Ed., 2005, 44, 3892–3895 CrossRef CAS PubMed.
- For recent reviews on synthetic application of nitrones, see: (a) S. Yokoshima, Nat. Prod. Rep., 2025, 42, 1071–1090 RSC; (b) O. Tamura, Chem. Pharm. Bull., 2024, 72, 731–746 CrossRef CAS PubMed; (c) M. Abdulla, M. K. Hussain and S. Ahamad, Org. Biomol. Chem., 2024, 22, 5242–5256 RSC; (d) N. Zou, X. Qin, Z. Wang, W. Shi and D. Mo, Chin. J. Org. Chem., 2021, 41, 4535–4553 CrossRef CAS; (e) N. A. Bokach, D. S. Bolotin and V. Y. Kukushkin, in Synthetic Approaches to Nonaromatic Nitrogen Heterocycles, ed. A. M. M. M. Faisca Phillips, Wiley, 1st edn, 2020, pp. 533–548 Search PubMed; (f) S.-I. Murahashi and Y. Imada, Chem. Rev., 2019, 119, 4684–4716 CrossRef CAS PubMed; (g) L. L. Anderson, Asian J. Org. Chem., 2016, 5, 9–30 CrossRef CAS; (h) W.-M. Shi, X.-P. Ma, G.-F. Su and D.-L. Mo, Org. Chem. Front., 2016, 3, 116–130 RSC; (i) F. Cardona and A. Goti, Angew. Chem., Int. Ed., 2005, 44, 7832–7835 CrossRef CAS PubMed.
- (a) P.-W. Qiu, L.-B. Yan, L.-F. Ning, C.-H. Chen, H.-Y. Bi and D.-L. Mo, Org. Chem. Front., 2025, 12, 3403–3408 RSC; (b) Z.-N. Zhao, S. Li, S.-X. Zhu, G.-H. Liu, S.-Y. Li, Y.-C. Li, Y.-L. Wang and Y.-Y. Huang, Org. Biomol. Chem., 2025, 23, 3372–3379 RSC; (c) B.-Q. Huang, X.-X. Xu, B.-H. Wu, Y. Zou, X.-J. Zhang and M. Yan, J. Org. Chem., 2025, 90, 9189–9199 CrossRef CAS PubMed; (d) T. Phumjan, T. Yazawa, S. Harada and T. Nemoto, Org. Lett., 2025, 27, 1549–1554 CrossRef CAS PubMed; (e) C. Xu, Y. Wang, M. Xiao, R. Tian and Z. Duan, Chin. J. Chem., 2025, 43, 1161–1166 CrossRef CAS; (f) S. J. Malcolmson and F. Rahim, Chem. Rev., 2025, 125, 10765–10797 CrossRef CAS PubMed; (g) Y. Li, Y. Zhang, J. Wang, D. Xia, M. Zhuo, L. Zhu, D. Li, S.-F. Ni, Y. Zhu and W.-D. Zhang, Org. Lett., 2024, 26, 3130–3134 CrossRef CAS PubMed; (h) A. Mondal, L. R. Domingo and N. Acharjee, J. Phys. Org. Chem., 2024, 37, e4574 CrossRef CAS; (i) K. Stefkova, M. G. Guerzoni, Y. van Ingen, E. Richards and R. L. Melen, Org. Lett., 2023, 25, 500–505 CrossRef CAS PubMed; (j) S. Shivam, K. A. Chavan, A. N. S. Chauhan and R. D. Erande, Synlett, 2023, 709–728 CAS; (k) M. F. Jamali, M. Ahmad, S. P. Chandrasekharan and K. Mohanan, Org. Lett., 2023, 25, 7551–7556 CrossRef CAS PubMed; (l) D. A. Bilodeau, K. D. Margison, M. Serhan and J. P. Pezacki, Chem. Rev., 2021, 121, 6699–6717 CrossRef CAS PubMed; (m) S. Thakur, A. Das and T. Das, New J. Chem., 2021, 45, 11420–11456 RSC; (n) M. Bakthadoss and M. Mushaf, Org. Biomol. Chem., 2020, 18, 9653–9659 RSC; (o) A. I. Said and T. I. El-Emary, RSC Adv., 2020, 10, 845–850 RSC; (p) A. Ghosh, M. V. Mane, H. B. Rode, S. A. Patil, B. Sridhar and R. B. Dateer, Chem. – Asian J., 2020, 15, 899–903 CrossRef CAS PubMed; (q) A. Brar, D. K. Unruh, N. Ling and C. Krempner, Org. Lett., 2019, 21, 6305–6309 CrossRef CAS PubMed; (r) M. Berthet, T. Cheviet, G. Dujardin, I. Parrot and J. Martinez, Chem. Rev., 2016, 116, 15235–15283 CrossRef CAS PubMed; (s) F. Li, J. Chen, Y. Hou, Y. Li, X.-Y. Wu and X. Tong, Org. Lett., 2015, 17, 5376–5379 CrossRef CAS; (t) M. Cordaro, F. Risitano, A. Scala, A. Rescifina, U. Chiacchio and G. Grassi, J. Org. Chem., 2013, 78, 3972–3979 CrossRef CAS PubMed; (u) C. Qin and H. M. L. Davies, J. Am. Chem. Soc., 2013, 135, 14516–14519 CrossRef CAS PubMed; (v) K. V. Gothelf and K. A. Jørgensen, Chem. Commun., 2000, 1449–1458 RSC; (w) D. S. Tan, M. A. Foley, M. D. Shair and S. L. Schreiber, J. Am. Chem. Soc., 1998, 120, 8565–8566 CrossRef CAS.
- (a) Y. Wang, A. Hennig, T. Kuttler, C. Hahn, A. Jäger and P. Metz, Org. Lett., 2020, 22, 3145–3148 CrossRef CAS PubMed; (b) E. E. Stepanova, M. V. Dmitriev and A. N. Maslivets, Tetrahedron Lett., 2020, 61, 151595 CrossRef CAS; (c) F. Rouzier, R. Sillé, O. Montiège, A. Tessier, M. Pipelier, G. Dujardin, A. Martel, A. Nourry and S. Guillarme, Eur. J. Org. Chem., 2020, 6749–6757 CrossRef CAS; (d) A. Esteban, I. Izquierdo, N. García, M. J. Sexmero, N. M. Garrido, I. S. Marcos, F. Sanz, P. G. Jambrina, P. Ortega and D. Diez, Tetrahedron, 2020, 76, 130764 CrossRef CAS; (e) T. Pecchioli, F. Cardona, H.-U. Reissig, R. Zimmer and A. Goti, J. Org. Chem., 2017, 82, 5835–5844 CrossRef CAS PubMed; (f) J. M. E. Hughes and J. L. Gleason, Angew. Chem., Int. Ed., 2017, 56, 10830–10834 CrossRef CAS PubMed; (g) C. Parmeggiani, F. Cardona, L. Giusti, H.-U. Reissig and A. Goti, Chem. – Eur. J., 2013, 19, 10595–10604 CrossRef CAS PubMed; (h) A. Padwa and S. K. Bur, Tetrahedron, 2007, 63, 5341–5378 CrossRef CAS PubMed; (i) V. Nair and T. D. Suja, Tetrahedron, 2007, 63, 12247–12275 CrossRef CAS.
- (a) T. A. Pothi and C. V. Ramana, Org. Lett., 2024, 26, 2233–2237 CrossRef CAS PubMed; (b) P. Zhao, P. Li, J. Xiao and C. Liu, Chem. Commun., 2023, 59, 2624–2627 RSC; (c) T. D. Moseev, E. A. Nikiforov, M. V. Varaksin, V. N. Charushin and O. N. Chupakhin, J. Org. Chem., 2021, 86, 13702–13710 CrossRef CAS PubMed; (d) T. Lynch-Colameta, S. Greta and S. A. Snyder, Chem. Sci., 2021, 12, 6181–6187 RSC; (e) H. Xu, M. Ye, K. Yang and Q. Song, Org. Lett., 2021, 23, 7776–7780 CrossRef CAS PubMed; (f) A. Ghosh, R. V. Hegde, H. B. Rode, R. Ambre, M. V. Mane, S. A. Patil, B. Sridhar and R. B. Dateer, Org. Lett., 2021, 23, 8189–8193 CrossRef CAS PubMed; (g) M. Li, Y. Dong, C. Zhou, J. Bai, J. Cheng, J. Sun and S. Sun, Org. Lett., 2021, 23, 8229–8234 CrossRef CAS PubMed; (h) X. Meng, H. Pan, G. Zhu and X. Zhang, Tetrahedron, 2020, 76, 131167 CrossRef CAS; (i) T. D. Moseev, M. V. Varaksin, D. A. Gorlov, V. N. Charushin and O. N. Chupakhin, J. Org. Chem., 2020, 85, 11124–11133 CrossRef CAS PubMed; (j) Y. Arakawa, S. Ueta, T. Okamoto, K. Minagawa and Y. Imada, Synlett, 2020, 866–870 CAS; (k) X. Meng, H. Pan, G. Zhu and X. Zhang, Tetrahedron, 2020, 76, 131167 CrossRef CAS; (l) F. G. Adly, K. O. Marichev, J. A. Jensen, H. Arman and M. P. Doyle, Org. Lett., 2019, 21, 40–44 CrossRef CAS PubMed; (m) J. Flores-Ferrándiz, N. Carter, M. J. González-Soria, M. Wasinska, D. Gill, B. Maciá and V. Caprio, Org. Biomol. Chem., 2018, 16, 6961–6968 RSC; (n) X. Xu, M. O. Ratnikov, P. Y. Zavalij and M. P. Doyle, Org. Lett., 2011, 13, 6122–6125 CrossRef CAS PubMed.
- O. N. Burchak and S. Py, Tetrahedron, 2009, 65, 7333–7356 CrossRef CAS.
- (a) S. Stecko, B. Furman and M. Chmielewski, Tetrahedron, 2014, 70, 7817–7844 CrossRef CAS; (b) Y. Zhao, R. Xu, Z. Xu and X. Xu, Synthesis, 2022, 2433–2446 Search PubMed; (c) C. Wei, J. Zhu, J. Zhang, Q. Deng and D. Mo, Adv. Synth. Catal., 2019, 361, 3965–3973 CrossRef CAS.
- (a) Y.-Z. Wu, Y. Leng, Y.-X. Chen, S.-Q. Huang, N. Zou, C.-H. Chen and D.-L. Mo, Chin. J. Chem., 2025, 43, 417–422 CrossRef CAS; (b) N. Zou, Y.-Z. Wu, X.-Y. Zhong, C. Wei, L.-M. Liao, D.-L. Mo and W.-J. Zhou, Adv. Synth. Catal., 2024, 366, 4960–4965 CrossRef CAS; (c) Z. Liu, J. Pang, L. Che, L. Pang, C. Gan, J. Cui and Y. Huang, J. Heterocycl. Chem., 2024, 61, 474–482 CrossRef CAS; (d) N. Zou, Y.-Z. Wu, Z.-W. Shang, Y.-W. Cao, L.-M. Liao, C. Wei, D.-L. Mo and W.-J. Zhou, Org. Biomol. Chem., 2024, 22, 9047–9052 RSC; (e) W. Li, J. Lin, S. Huang, Q. Liu, W. Wei and X. Li, Org. Biomol. Chem., 2023, 21, 6778–6782 RSC; (f) Y. Xu, H. Gao, C. Pan, Y. Shi, C. Zhang, G. Huang and C. Feng, Angew. Chem., Int. Ed., 2023, 62, e202310671 CrossRef CAS PubMed; (g) T.-Z. Li, S.-J. Liu, Y.-W. Sun, S. Deng, W. Tan, Y. Jiao, Y.-C. Zhang and F. Shi, Angew. Chem., Int. Ed., 2021, 60, 2355–2363 CrossRef CAS PubMed; (h) H. Zhang, L.-Y. Cai, Z. Tang, X.-Z. Fan, H.-H. Wu, X.-F. Bi and H.-W. Zhao, Synlett, 2021, 1974–1980 CAS; (i) Y. Luo, C.-H. Chen, J.-Q. Zhang, C. Liang and D.-L. Mo, Synthesis, 2020, 424–432 CAS.
- (a) J. Zhang, J. Su, H. Zheng, H. Li and W. Deng, Angew. Chem., Int. Ed., 2024, 63, e202318476 CrossRef CAS PubMed; (b) C. Wei, X. Cheng, Y. Chen, C. Liang and D. Mo, Adv. Synth. Catal., 2023, 365, 2976–2981 CrossRef CAS; (c) C. Wei, J.-Q. Zhang, J.-J. Zhang, C. Liang and D.-L. Mo, Org. Chem. Front., 2020, 7, 1520–1526 RSC; (d) Z. Tian, J. Xu, B. Liu, Q. Tan and B. Xu, Org. Lett., 2018, 20, 2603–2606 CrossRef CAS PubMed; (e) X.-P. Ma, L.-G. Li, H.-P. Zhao, M. Du, C. Liang and D.-L. Mo, Org. Lett., 2018, 20, 4571–4574 CrossRef CAS PubMed.
- (a) L. Ding, Y. Lu, J. Pang, H. Lin, C. Chen, H. Bi and D. Mo, Chin. J. Chem., 2025, 43, 1287–1292 CrossRef CAS; (b) N. Zou, Z. Liu, G. Yan, Y. Wang, C. Liang and D. Mo, Adv. Synth. Catal., 2022, 364, 1671–1676 CrossRef CAS.
- (a) X. Li, L.-F. Liao, L.-Y. Ding, C.-H. Chen, C. Liang and D.-L. Mo, Org. Lett., 2024, 26, 3060–3064 CrossRef CAS PubMed; (b) Y.-J. Lu, F.-L. Lu, J.-Q. Zhang, C.-H. Chen, C. Liang, X.-P. Ma and D.-L. Mo, Org. Chem. Front., 2024, 11, 2277–2282 RSC; (c) K. Ishihara, T. Shioiri and M. Matsugi, Synlett, 2022, 781–784 CAS; (d) X. Yang, X. Guo, X. Yuan and B. Chen, Org. Chem. Front., 2022, 9, 4034–4040 RSC; (e) S. Bartlett, K. Keiter, B. Zavesky and J. Johnson, Synthesis, 2019, 203–212 CAS; (f) R. L. Sahani, M. D. Patil, S. B. Wagh and R. Liu, Angew. Chem., Int. Ed., 2018, 57, 14878–14882 CrossRef CAS PubMed; (g) D.-L. Mo and L. L. Anderson, Angew. Chem., Int. Ed., 2013, 52, 6722–6725 CrossRef CAS PubMed.
- (a) X. He, B. Pan, Y. Zhang, B. Chen, L. Jiang and L. Qiu, Adv. Synth. Catal., 2024, 366, 3105–3110 CrossRef CAS; (b) S. D. Tanpure, M. D. Dhole and R. Liu, Adv. Synth. Catal., 2023, 365, 2936–2942 CrossRef CAS; (c) B. Xu, Z. Zhang, J. Han, G. Gu and J. Zhang, Chin. J. Chem., 2022, 40, 1407–1412 CrossRef CAS; (d) R. L. Sahani and R.-S. Liu, ACS Catal., 2019, 9, 5890–5896 CrossRef CAS.
- L. Xu, T. Yang, H. Sun, J. Zeng, S. Mu, X. Zhang and G.-Q. Chen, Angew. Chem., Int. Ed., 2024, 63, e202319662 CrossRef CAS PubMed.
- A. A. Akulov, A. A. Pershin, A. A. Deleva, M. V. Chugaev, A. N. Tsmokaluk, D. G. Mazhukin, A. Y. Tikhonov, M. V. Varaksin and O. N. Chupakhin, J. Org. Chem., 2025 DOI:10.1021/acs.joc.5c02260.
- (a) G. Zhan, M.-L. Shi, W.-J. Lin, Q. Ouyang, W. Du and Y.-C. Chen, Chem. – Eur. J., 2017, 23, 6286–6289 CrossRef CAS PubMed; (b) Y.-R. Chen, G. Zhan, W. Du and Y.-C. Chen, Adv. Synth. Catal., 2016, 358, 3759–3764 CrossRef CAS.
- (a) D. Shang, T. Lin, Y. Shen, Y. Zhang, P. W. H. Chan and W. Rao, Org. Chem. Front., 2025, 12, 863–868 RSC; (b) W.-J. Han, F.-L. Yang, Z. Hu, W. Chen, S. Liu, K. Guo, X.-Y. Yang and B. Cheng, Adv. Synth. Catal., 2025, 367, e202401049 CrossRef CAS; (c) A. S. Alshreimi, E. J. Shim, D. J. Wink and L. L. Anderson, Org. Lett., 2025, 27, 5989–5994 CrossRef PubMed; (d) L. Wang, J. Qi, H. Li and Z. Xu, Chem. – Eur. J., 2025, 31, e202403722 CrossRef CAS PubMed; (e) P. Jovanovic, M. Jovanovic, M. Petkovic, M. Simic, G. Tasic, M. Rodic, S. Rakic, F. Vlahovic and V. Savica, Adv. Synth. Catal., 2024, 366, 3564–3571 CrossRef CAS; (f) F. Rao, M. Cheng, Z. Hu, X. Chen, S. Zhao and Z. Chen, Org. Chem. Front., 2024, 11, 3685–3691 RSC; (g) A. V. Sasane, C.-T. Chiou, M.-Y. Chang and W.-T. Li, Org. Lett., 2024, 26, 6675–6680 CrossRef CAS PubMed; (h) M.-F. Li, S.-Q. Shi, T. Xu, Q. Zhang, W.-J. Hao, S.-L. Wang, J. Wang, S.-J. Tu and B. Jiang, Chin. Chem. Lett., 2023, 34, 107751 CrossRef CAS; (i) H. Yuan, D. Lu, C. Liang and D. Mo, Adv. Synth. Catal., 2022, 364, 1409–1414 CrossRef CAS; (j) Z. Zhang, N. Sabat, G. Frison, A. Marinetti and X. Guinchard, ACS Catal., 2022, 12, 4046–4053 CrossRef CAS; (k) G. Zhang, A. S. Alshreimi, L. Alonso, A. Antar, H.-C. Yu, S. M. Islam and L. L. Anderson, Angew. Chem., Int. Ed., 2021, 60, 13089–13097 CrossRef CAS PubMed; (l) B. Hu, X. Zhang, Y. Mo, J. Li, L. Lin, X. Liu and X. Feng, Org. Lett., 2020, 22, 1034–1039 CrossRef CAS PubMed; (m) X.-P. Ma, C.-M. Nong, Y.-F. Liang, P.-P. Xu, X.-Y. Guo, C. Liang, C.-X. Pan, G.-F. Su and D.-L. Mo, Green Chem., 2020, 22, 3827–3834 RSC; (n) S. Qiu, R. Liang, Y. Wang and S. Zhu, Org. Lett., 2019, 21, 2126–2129 CrossRef CAS PubMed; (o) D. Qian and J. Zhang, Chem. – Eur. J., 2013, 19, 6984–6988 CrossRef CAS PubMed; (p) C. B. Huehls, T. S. Hood and J. Yang, Angew. Chem., Int. Ed., 2012, 51, 5110–5113 CrossRef CAS PubMed; (q) V. Capriati, S. Florio, R. Luisi, A. Salomone and C. Cuocci, Org. Lett., 2006, 8, 3923–3926 CrossRef CAS PubMed.
- (a) S.-G. Xin, S. Chen, J.-H. Qin, H.-Y. Bi, C. Liang, C.-H. Chen and D.-L. Mo, J. Org. Chem., 2025, 90, 1922–1933 CrossRef CAS PubMed; (b) R. Hammami, P. Maldivi, S. Carret, S. Touil, J. Poisson and B. Darses, Eur. J. Org. Chem., 2025, e202500359 CrossRef CAS; (c) P. Xu, S. Xin, X. Li, C. Liang and D. Mo, Adv. Synth. Catal., 2023, 365, 735–740 CrossRef CAS; (d) M. Liu, L. Xie, L. Hou, L. Lin and X. Feng, Chem. Commun., 2022, 58, 5482–5485 RSC; (e) M.-L. Luo, Q. Hou, S.-J. Liu, Q. Zhao, R. Qin, C. Peng, B. Han and G. Zhan, Org. Lett., 2022, 24, 8493–8497 CrossRef CAS PubMed; (f) P.-P. Xu, J.-Y. Liao, J.-J. Zhang, W.-M. Shi, C. Liang, G.-F. Su and D.-L. Mo, Org. Lett., 2021, 23, 7482–7486 CrossRef CAS PubMed; (g) T. Xu, N. Lin, W.-J. Hao, J. Zhang, M.-F. Li, S.-J. Tu and B. Jiang, Chem. Commun., 2020, 56, 11406–11409 RSC; (h) W. Lee, M. Yuan, C. Acha, A. Onwu and O. Gutierrez, Org. Biomol. Chem., 2019, 17, 1767–1772 RSC; (i) M. A. Kroc, A. Prajapati, D. J. Wink and L. L. Anderson, J. Org. Chem., 2018, 83, 1085–1094 CrossRef CAS PubMed; (j) W. H. Pace, D. Mo, T. W. Reidl, D. J. Wink and L. L. Anderson, Angew. Chem., Int. Ed., 2016, 55, 9183–9186 CrossRef CAS PubMed; (k) A. S. Kulandai Raj, B. S. Kale, B. D. Mokar and R.-S. Liu, Org. Lett., 2017, 19, 5340–5343 CrossRef CAS PubMed; (l) L. L. Anderson, M. A. Kroc, T. W. Reidl and J. Son, J. Org. Chem., 2016, 81, 9521–9529 CrossRef CAS PubMed.
- (a) Directed C–H Bond Functionalization: Concepts and Applications, ed. D. Maiti and S. Rej, Wiley, 1st edn, 2026 Search PubMed; (b) Functionalisation of Heterocycles through Transition Metal Catalyzed C-H Activation (Topics in Heterocyclic Chemistry vol. 60), ed. D. Maiti and U. Sharma, Springer, Cham, 2024 Search PubMed; (c) Handbook of CH-Functionalization, ed. D. Maiti, Wiley-VCH, 2023 Search PubMed.
- For recent reviews, see: (a) J. Le Bras and J. Muzart, Adv. Synth. Catal., 2023, 365, 3727–3773 CrossRef CAS; (b) J. Muzart, Adv. Synth. Catal., 2023, 365, 3784–3813 CrossRef CAS; (c) J. Muzart, Adv. Synth. Catal., 2023, 365, 3774–3783 CrossRef CAS; (d) S. Kaltenberger and M. van Gemmeren, Acc. Chem. Res., 2023, 56, 2459–2472 CrossRef CAS PubMed; (e) T. Rogge, N. Kaplaneris, N. Chatani, J. Kim, S. Chang, B. Punji, L. L. Schafer, D. G. Musaev, J. Wencel-Delord, C. A. Roberts, R. Sarpong, Z. E. Wilson, M. A. Brimble, M. J. Johansson and L. Ackermann, Nat. Rev. Methods Primers, 2021, 1, 43 CrossRef CAS; (f) S. W. Youn and C.-G. Cho, Org. Biomol. Chem., 2021, 19, 5028–5047 RSC; (g) K. M. Altus and J. A. Love, Commun. Chem., 2021, 4, 173 CrossRef PubMed; (h) G. Evano and C. Theunissen, Angew. Chem., Int. Ed., 2019, 58, 7202–7236 CrossRef CAS PubMed; (i) Y. Li and S. Xu, Chem. – Eur. J., 2018, 24, 16218–16245 CrossRef CAS PubMed; (j) P. Gandeepan, T. Muller, D. Zell, G. Cera, S. Warratz and L. Ackermann, Chem. Rev., 2019, 119, 2192–2452 CrossRef CAS PubMed.
- (a) J. Ma, X. Zhou, J. Chen, J. Shi, H. Cheng, P. Guo and H. Ji, Org. Biomol. Chem., 2022, 20, 7554–7576 RSC; (b) J. Das, D. K. Mal, S. Maji and D. Maiti, ACS Catal., 2021, 11, 4205–4229 CrossRef CAS; (c) S. Shabani, Y. Wu, H. G. Ryan and C. A. Hutton, Chem. Soc. Rev., 2021, 50, 9278–9343 RSC; (d) K. Murali, L. A. Machado, R. L. Carvalho, L. F. Pedrosa, R. Mukherjee, E. N. Da Silva Júnior and D. Maiti, Chem. – Eur. J., 2021, 27, 12453–12508 CrossRef CAS PubMed; (e) S. Rej, Y. Ano and N. Chatani, Chem. Rev., 2020, 120, 1788–1887 CrossRef CAS PubMed; (f) C. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes and M. Schnürch, Chem. Soc. Rev., 2018, 47, 6603–6743 RSC; (g) R.-Y. Zhu, M. E. Farmer, Y.-Q. Chen and J.-Q. Yu, Angew. Chem., Int. Ed., 2016, 55, 10578–10599 CrossRef CAS PubMed; (h) O. Daugulis, J. Roane and L. D. Tran, Acc. Chem. Res., 2015, 48, 1053–1064 CrossRef CAS PubMed.
- S. Rej and N. Chatani, Angew. Chem., Int. Ed., 2019, 58, 8304–8329 CrossRef CAS PubMed.
- (a) C. Jacob, B. U. W. Maes and G. Evano, Chem. – Eur. J., 2021, 27, 13899–13952 CrossRef CAS PubMed; (b) N. Goswami, T. Bhattacharya and D. Maiti, Nat. Rev. Chem., 2021, 5, 646–659 CrossRef CAS PubMed; (c) G. Rani, V. Luxami and K. Paul, Chem. Commun., 2020, 56, 12479–12521 RSC; (d) F.-L. Zhang, K. Hong, T.-J. Li, H. Park and J.-Q. Yu, Science, 2016, 351, 252–256 CrossRef CAS PubMed.
- (a) F. Xu, S.-Y. Zhang, Y.-P. Li, J.-Q. Huo and F.-W. Zeng, Chem. Commun., 2025, 61, 1729–1747 RSC; (b) J. Liu, R. Liang, Q. Yan, L. Zheng, Z.-Q. Liu and S. Pu, Org. Chem. Front., 2025, 12, 3065–3106 RSC; (c) X. Tang, E. V. Van der Eycken and L. Song, Chin. Chem. Lett., 2025, 111678 Search PubMed; (d) Q. Zhou, B. Li, X. Zhang and X. Fan, Org. Biomol. Chem., 2024, 22, 2324–2338 RSC; (e) O. Baudoin, Acc. Chem. Res., 2017, 50, 1114–1123 CrossRef CAS PubMed; (f) Q. Zhang, C. Chen, B. Liu, Y. Liu, Y. Gao, Q. Chen, Y. Huo and X. Li, ChemCatChem, 2023, 15, e202300891 CrossRef CAS; (g) D. R. Mishra, B. S. Panda, S. Nayak, J. Panda and S. Mohapatra, ChemistrySelect, 2022, 7, e202200531 CrossRef CAS; (h) M. Gulías and J. L. Mascareñas, Angew. Chem., Int. Ed., 2016, 55, 11000–11019 CrossRef PubMed.
- Y. Wu, C. Pi, Y. Wu and X. Cui, Chem. Soc. Rev., 2021, 50, 3677–3689 RSC.
- A. Deepthi, N. V. Thomas and S. L. Sruthi, New J. Chem., 2021, 45, 8847–8873 RSC.
- A. A. Akulov, M. V. Varaksin, P. Mampuys, V. N. Charushin, O. N. Chupakhin and B. U. W. Maes, Org. Biomol. Chem., 2021, 19, 297–312 RSC.
- N. A. Bokach, A. A. Krokhin, A. A. Nazarov, V. Y. Kukushkin, M. Haukka, J. J. R. Fraústo da Silva and A. J. L. Pombeiro, Eur. J. Inorg. Chem., 2005, 3042–3048 CrossRef CAS.
- (a) Q. Yao, M. Zabawa, J. Woo and C. Zheng, J. Am. Chem. Soc., 2007, 129, 3088–3089 CrossRef CAS PubMed; (b) X. Song, Y. Qian, R. Ben, X. Lu, H.-L. Zhu, H. Chao and J. Zhao, J. Med. Chem., 2013, 56, 6531–6535 CrossRef CAS PubMed.
- Y. Zhang, G. Song, G. Ma, J. Zhao, C.-L. Pan and X. Li, Organometallics, 2009, 28, 3233–3238 CrossRef CAS.
- P. V. Reddy, P. Srinivas, M. Annapurna, S. Bhargava, J. Wagler, N. Mirzadeh and M. L. Kantam, Adv. Synth. Catal., 2013, 355, 705–710 CrossRef CAS.
- M. Hasegawa, A. Nomoto, T. Suga, T. Soeta and Y. Ukaji, Chem. Lett., 2017, 46, 45–47 CrossRef CAS.
- (a) S. I. Gorelsky, D. Lapointe and K. Fagnou, J. Org. Chem., 2012, 77, 658–668 CrossRef CAS PubMed; (b) M. P. Huestis and K. Fagnou, Org. Lett., 2009, 11, 1357–1360 CrossRef CAS PubMed; (c) L.-C. Campeau, D. R. Stuart, J.-P. Leclerc, M. Bertrand-Laperle, E. Villemure, H.-Y. Sun, S. Lasserre, N. Guimond, M. Lecavallier and K. Fagnou, J. Am. Chem. Soc., 2009, 131, 3291–3306 CrossRef CAS PubMed; (d) L.-C. Campeau, M. Bertrand-Laperle, J.-P. Leclerc, E. Villemure, S. Gorelsky and K. Fagnou, J. Am. Chem. Soc., 2008, 130, 3276–3277 CrossRef CAS PubMed; (e) L.-C. Campeau, D. J. Schipper and K. Fagnou, J. Am. Chem. Soc., 2008, 130, 3266–3267 CrossRef CAS PubMed.
- (a) A. Bunnell, M. W. Milbauer, J. V. Bento, S. M. Taimoory, P. M. Zimmerman, D. Kalyani, T. Piou and M. S. Sanford, J. Am. Chem. Soc., 2025, 147, 20159–20167 CrossRef CAS PubMed; (b) V. V. Kouznetsov, L. Y. Vargas Méndez, C. E. Puerto Galvis and M. C. Ortiz Villamizar, New J. Chem., 2020, 44, 12–19 RSC; (c) M. Dabiri, S. I. Alavioon and S. K. Movahed, Eur. J. Org. Chem., 2019, 1479–1487 CrossRef CAS; (d) R. Rossi, F. Bellina, M. Lessi and C. Manzini, Adv. Synth. Catal., 2014, 356, 17–117 CrossRef CAS; (e) Y. Tan, F. Barrios-Landeros and J. F. Hartwig, J. Am. Chem. Soc., 2012, 134, 3683–3686 CrossRef CAS; (f) L. Ackermann and S. Fenner, Chem. Commun., 2011, 47, 430–432 RSC; (g) S. H. Cho, S. J. Hwang and S. Chang, J. Am. Chem. Soc., 2008, 130, 9254–9256 CrossRef CAS PubMed.
- H. Zhao, R. Wang, P. Chen, B. T. Gregg, M. M. Hsia and W. Zhang, Org. Lett., 2012, 14, 1872–1875 CrossRef CAS.
- E. Demory, D. Farran, B. Baptiste, P. Y. Chavant and V. Blandin, J. Org. Chem., 2012, 77, 7901–7912 CrossRef CAS PubMed.
- (a) A. A. Akulov, M. V. Varaksin, V. N. Charushin and O. N. Chupakhin, ACS Omega, 2019, 4, 825–834 CrossRef CAS PubMed; (b) M. V. Varaksin, I. A. Utepova, O. N. Chupakhin and V. N. Charushin, J. Org. Chem., 2012, 77, 9087–9093 CrossRef CAS PubMed.
- J. Brześkiewicz, B. Stańska, P. Dąbrowski and R. Loska, Eur. J. Org. Chem., 2021, 814–824 CrossRef.
- Y. Tanji, N. Mitsutake, T. Fujihara and Y. Tsuji, Angew. Chem., Int. Ed., 2018, 57, 10314–10317 CrossRef CAS PubMed.
- J. Brześkiewicz and R. Loska, Org. Lett., 2022, 24, 3960–3964 CrossRef PubMed.
- J. Brześkiewicz and R. Loska, J. Org. Chem., 2023, 88, 2385–2392 CrossRef.
- A. K. Pandey, D. Kang, S. H. Han, H. Lee, N. K. Mishra, H. S. Kim, Y. H. Jung, S. Hong and I. S. Kim, Org. Lett., 2018, 20, 4632–4636 CrossRef CAS PubMed.
- Z. Qi, M. Wang and X. Li, Org. Lett., 2013, 15, 5440–5443 CrossRef CAS PubMed.
- (a) Y. Chen, F. Wang, W. Zhen and X. Li, Adv. Synth. Catal., 2013, 355, 353–359 CrossRef CAS; (b) W. Zhen, F. Wang, M. Zhao, Z. Du and X. Li, Angew. Chem., Int. Ed., 2012, 51, 11819–11823 CrossRef CAS PubMed.
- F. Wang, J. Jing, Y. Zhao, X. Zhu, X.-P. Zhang, L. Zhao, P. Hu, W.-Q. Deng and X. Li, Angew. Chem., Int. Ed., 2021, 60, 16628–16633 CrossRef CAS PubMed.
- F. Xie, Z. Qi, S. Yu and X. Li, J. Am. Chem. Soc., 2014, 136, 4780–4787 CrossRef CAS.
- F. Xie, S. Yu, Z. Qi and X. Li, Angew. Chem., Int. Ed., 2016, 55, 15351–15355 CrossRef CAS PubMed.
- (a) R. B. Dateer and S. Chang, J. Am. Chem. Soc., 2015, 137, 4908–4911 CrossRef CAS; (b) Y. Li, C. Shan, Y.-F. Yang, F. Shi, X. Qi, K. N. Houk and Y. Lan, J. Phys. Chem. A, 2017, 121, 4496–4504 CrossRef CAS PubMed.
- L. Kong, F. Xie, S. Yu, Z. Qi and X. Li, Chin. J. Catal., 2015, 36, 925–932 CrossRef CAS.
- H. Yan, H. Wang, X. Li, X. Xi, Ch. Wang and B. Wan, Angew. Chem., Int. Ed., 2015, 54, 10613–10617 CrossRef CAS PubMed.
- Z. Zhou, G. Liu, Y. Chen and X. Lu, Adv. Synth. Catal., 2015, 357, 2944–2950 CrossRef CAS.
- Q. Wang, Y. Xu, X. Yang, Y. Li and X. Li, Chem. Commun., 2017, 53, 9640–9643 RSC.
- D. Bai, Q. Jia, T. Xu, Q. Zhang, F. Wu, C. Ma, B. Liu, J. Chang and X. Li, J. Org. Chem., 2017, 82, 9877–9884 CrossRef CAS PubMed.
- N. Aravindan and M. Jeganmohan, Org. Lett., 2023, 25, 3853–3858 CrossRef CAS PubMed.
- M. Zhong, S. Sun, J. Cheng and Y. Shao, J. Org. Chem., 2016, 81, 10825–10831 CrossRef CAS PubMed.
- T.-T. Wang and L.-M. Zhao, Chem. Commun., 2022, 58, 11099–11102 RSC.
- L. Guo, B. Tang, R. Nie, Y. Liu, S. Lv, H. Wang, L. Guo, L. Hai and Y. Wu, Chem. Commun., 2019, 55, 10623–10626 RSC.
- (a) Y. Yang, X. Wang, Y. Li and B. Zhou, Angew. Chem., Int. Ed., 2015, 54, 15400–15404 CrossRef CAS PubMed; (b) S. Guo, Z. Zhang, Z. Wei, Y. Zhu and X. Fan, J. Org. Chem., 2023, 88, 3845–3858 CrossRef CAS PubMed.
- R. B. Dateer and S. Chang, Org. Lett., 2016, 18, 68–71 CrossRef CAS PubMed.
- X. Guo, J. Han, Y. Liu, M. Qin, X. Zhang and B. Chen, J. Org. Chem., 2017, 82, 11505–11511 CrossRef CAS PubMed.
- L. Kong, X. Han, S. Liu, Y. Zou, Y. Lan and X. Li, Angew. Chem., Int. Ed., 2020, 59, 7188–7192 CrossRef CAS PubMed.
- C. G. Newton, D. Kossler and N. Cramer, J. Am. Chem. Soc., 2016, 138, 3935–3941 CrossRef CAS PubMed.
- L. Kong, X. Han and X. Li, Chem. Commun., 2019, 55, 7339–7342 RSC.
- Y. Wang and L. Zhao, Adv. Synth. Catal., 2023, 365, 3713–3717 CrossRef CAS.
- (a) D. G. Valapil, P. Mishra, K. Jungare and N. Shankaraiah, New J. Chem., 2023, 47, 17586–17591 RSC; (b) X. Cai, X. Song, Q. Zhu, X. Zhang and X. Fan, J. Org. Chem., 2022, 87, 11048–11062 CrossRef CAS PubMed; (c) Z. Shu, J. Zhou, J. Li, Y. Cheng, H. Liu, D. Wang and Y. Zhou, J. Org. Chem., 2020, 85, 12097–12107 CrossRef CAS PubMed.
- C. Pi, X. Cui and Y. Wu, J. Org. Chem., 2015, 80, 7333–7339 CrossRef CAS PubMed.
- H. Deng, H. Li, W. Zhang and L. Wang, Chem. Commun., 2017, 53, 10322–10325 RSC.
- X. Mi, W. Feng, C. Pi and X. Cui, J. Org. Chem., 2019, 84, 5305–5312 CrossRef CAS PubMed.
- (a) H. Lee, D. Kang, S. H. Han, R. Chun, A. K. Pandey, N. K. Mishra, S. Hong and I. S. Kim, Angew. Chem., Int. Ed., 2019, 58, 9470–9474 CrossRef CAS PubMed; (b) A. K. Pandey, S. H. Han, N. K. Mishra, D. Kang, S. H. Lee, R. Chun, S. Hong, J. S. Park and I. S. Kim, ACS Catal., 2018, 8, 742–746 CrossRef CAS.
- (a) N. K. Mishra, A. Rakshit, K. Moon, P. Singh and I. S. Kim, Bull. Korean Chem. Soc., 2024, 45, 131–144 CrossRef CAS; (b) M. Wu, H. Gao, H. Xu, W. Yi and Z. Zhou, Chin. Chem. Lett., 2022, 33, 842–846 CrossRef CAS; (c) L. Zhang, J. Zhao, Y. Jiang, X. Zhang and X. Fan, Org. Chem. Front., 2021, 8, 3734–3739 RSC; (d) M. Wu, R. Wang, F. Chen, W. Chen, Z. Zhou and W. Yi, Org. Lett., 2020, 22, 7152–7157 CrossRef CAS PubMed.
- Y. Li, F. Xie, Y. Liu, X. Yang and X. Li, Org. Lett., 2018, 20, 437–440 CrossRef CAS PubMed.
- S. H. Han, A. K. Pandey, H. Lee, S. Kim, D. Kang, Y. H. Jung, H. S. Kim, S. Hong and I. S. Kim, Org. Chem. Front., 2018, 5, 3210–3218 RSC.
- Y. Sun, Y. Wang, L. Zhang, R. Zhong and H. Wang, Asian J. Org. Chem., 2025, 14, e202400646 CrossRef CAS.
- (a) S. Min, T. Kim, T. Jeong, J. Yang, Y. Oh, K. Moon, A. Rakshit and I. S. Kim, Org. Lett., 2023, 25, 4298–4302 CrossRef CAS PubMed; (b) M. Zhu, M. Zhu, F. Wei, C. Shao, X. Li and B. Liu, J. Org. Chem., 2023, 88, 16330–16339 CrossRef CAS PubMed.
- J. Moon, N. Ko, S. Jang, P. Ghosh, H. S. Kim, N. K. Mishra and I. S. Kim, Org. Lett., 2022, 24, 8115–8119 CrossRef CAS PubMed.
- M. Zhu, Y. Zhang, M. Tian, X. Li, B. Liu and J. Chang, Org. Chem. Front., 2022, 9, 5879–5884 RSC.
- X. Cai, X. Song, X. Yang, X. Zhang and X. Fan, Org. Chem. Front., 2023, 10, 1015–1021 RSC.
- X. Wang, J. Li, H. Du, W. Liang, C. Luo, Y. Wu and B. Liu, Chem. Commun., 2024, 60, 2401–2404 RSC.
- B. Stańska, S. Ostrowska and R. Loska, Synlett, 2025 DOI:10.1055/a-2752-7699.
- (a) D. Bai, T. Xu, C. Ma, X. Zheng, B. Liu, F. Xie and X. Li, ACS Catal., 2018, 8, 4194–4200 CrossRef CAS; (b) T. Kim, C. Kim, P. Singh, T. Jeong, J. Park and I. S. Kim, J. Org. Chem., 2025, 90, 8376–8385 CrossRef PubMed.
- (a) F.-X. Liu, W. Chen, Y. Cai, Z. Zhou, W. Yi and K. Cheng, Org. Chem. Front., 2024, 11, 2512–2517 RSC; (b) X. Yang, X. Cai, X. Zhang and X. Fan, Adv. Synth. Catal., 2024, 366, 141–147 CrossRef CAS.
- C. Wang, D. Wang, H. Yan, H. Wang, B. Pan, X. Xin, X. Li, F. Wu and B. Wan, Angew. Chem., Int. Ed., 2014, 53, 11940–11943 CrossRef CAS PubMed.
- (a) Y. Li, X. Lu and Y. Fu, CCS Chem., 2024, 6, 1130–1156 CrossRef CAS; (b) S. Sunny and R. Karvembu, Adv. Synth. Catal., 2021, 363, 4309–4331 CrossRef CAS; (c) R. K. Shukla, A. M. Nair and C. M. R. Volla, Synlett, 2021, 1169–1178 CAS; (d) S. H. Kyne, G. Lefèvre, C. Ollivier, M. Petit, V.-A. Ramis Cladera and L. Fensterbank, Chem. Soc. Rev., 2020, 49, 8501–8542 RSC; (e) R. Mei, U. Dhawa, R. C. Samanta, W. Ma, J. Wencel-Delord and L. Ackermann, ChemSusChem, 2020, 13, 3306–3356 CrossRef CAS PubMed; (f) A. Guerinot and J. Cossy, Acc. Chem. Res., 2020, 53, 1351–1363 CrossRef CAS PubMed; (g) T. Michiyuki and K. Komeyama, Asian J. Org. Chem., 2020, 9, 343–358 CrossRef CAS; (h) A. Baccalini, S. Vergura, P. Dolui, G. Zanoni and D. Maiti, Org. Biomol. Chem., 2019, 17, 10119–10141 RSC; (i) K. Junge, V. Papa and M. Beller, Chem. – Eur. J., 2019, 25, 122–143 CrossRef CAS.
- H. Wang, M. Moselage, M. J. Gonzalez and L. Ackermann, ACS Catal., 2016, 6, 2705–2709 CrossRef CAS.
- Y. Liu, C. Wang, N. Lv, Z. Liu and Y. Zhang, Adv. Synth. Catal., 2017, 359, 1351–1358 CrossRef CAS.
- J. Brześkiewicz and R. Loska, Adv. Synth. Catal., 2023, 365, 4241–4247 CrossRef.
- (a) Q.-J. Yao and B.-F. Shi, Acc. Chem. Res., 2025, 58, 971–990 CrossRef CAS PubMed; (b) Z.-J. Zhang, S.-W. Li, J. C. A. Oliveira, Y. Li, X. Chen, S.-Q. Zhang, L.-C. Xu, T. Rogge, X. Hong and L. Ackermann, Nat. Commun., 2023, 14, 3149 CrossRef CAS PubMed; (c) P. Yang, Q. Wang, B.-H. Cui, X.-D. Zhang, H. Liu, Y.-Y. Zhang, J.-L. Liu, W.-Y. Huang, R.-X. Liang and Y.-X. Jia, J. Am. Chem. Soc., 2022, 144, 1087–1093 CrossRef CAS PubMed.
- T. Yoshino and S. Matsunaga, ACS Catal., 2021, 11, 6455–6466 CrossRef CAS.
- (a) C.-H. Yuan and L. Jiao, Org. Lett., 2024, 26, 29–34 CrossRef CAS PubMed; (b) S. Mandal, M. Barman, B. Debnath and T. Punniyamurthy, Org. Lett., 2024, 26, 7560–7564 CrossRef CAS PubMed; (c) C.-S. Wang, T. Roisnel, P. H. Dixneuf and J.-F. Soulé, Org. Lett., 2017, 19, 6720–6723 CrossRef CAS PubMed; (d) H. Sterckx, C. Sambiagio, V. Médran-Navarrete and B. U. W. Maes, Adv. Synth. Catal., 2017, 359, 3226–3236 CrossRef CAS; (e) L.-C. Campeau, D. J. Schipper and K. Fagnou, J. Am. Chem. Soc., 2008, 130, 3266–3267 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |