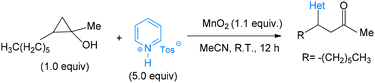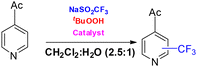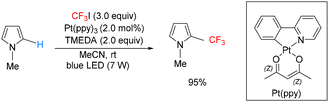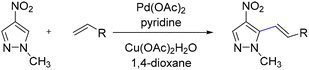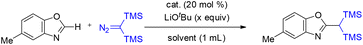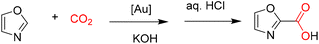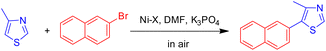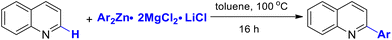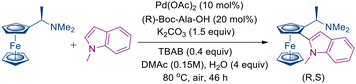Direct C–H functionalization on heteroaromatic rings
Nai-Xing
Wang
 *ab,
Shi
Tang
c,
Yu-Qiang
Zhou
d,
Dumitra
Lucan
*b,
Evan
Wu
e and
Yalan
Xing
*ab,
Shi
Tang
c,
Yu-Qiang
Zhou
d,
Dumitra
Lucan
*b,
Evan
Wu
e and
Yalan
Xing
 *e
*e
aTechnical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing, 100190, China. E-mail: nxwang@mail.ipc.ac.cn
bTechnical Sciences Academy of Romania ASTR, Dacia Avenue no.26, Bucharest, Romania. E-mail: dilucan@yahoo.com
cCollege of Chemistry and Chemical Engineering, Jishou University, Jishou 416000, China. E-mail: shitang@126.com
dCollege of Chemistry and Chemical Engineering, Xinyang Normal University, Xinyang, Henan 464000, China. E-mail: z1121@126.com
eDepartment of Chemistry, Hofstra University, Hempstead, NY 11549, USA. E-mail: Yalan.Xing@hofstra.edu
First published on 10th December 2025
Abstract
Direct C–H functionalization on heteroaromatic rings has emerged as a prominent topic in the field of catalytic organic synthesis. Among these, oxidative coupling methodologies involving C–H bond functionalization have gained significant attention due to their step economy and energy efficiency. This synopsis focuses on recent advances in coupling reactions on various heteroaromatic rings via C–H bond functionalization. We made significant contributions to C–H functionalization on heteroaromatic rings and aim to provide a conceptual summary that will be useful to researchers and inspire further developments in this rapidly growing field.
1. Introduction
Since the discovery of the Ullmann coupling reaction in the early twentieth century, there has been intense and sustained interest in the synthesis of biaryl compounds—an exciting area of organic chemistry with a broad range of applications.Nitrogen- and sulfur-containing heterocycles, along with their derivatives, exhibit remarkable versatility in materials science,1 agrochemicals,2 and pharmaceuticals,3 owing to their distinctive chemical and biological properties. The wide-ranging applications of these heterocycles have driven continuous efforts to develop efficient synthetic strategies for their construction.
Among these strategies, direct C–H functionalization of heteroaromatic rings has emerged as a powerful and atom-economical approach for accessing structurally diverse heteroaromatic derivatives. This review highlights recent advances (since 2015) in the direct coupling of pyridine, thiophene, pyrrole, azole, quinolone, and indole frameworks through C–H bond functionalization. It aims to provide a conceptual overview of key developments and inspire further progress in this rapidly evolving field.
The direct C–H functionalization of heteroaromatic rings holds both significant scientific interest and substantial practical value, as reflected by the increasing number of publications in recent years. Nonetheless, the oxidative coupling of inert C(sp3)–H bonds remains a formidable challenge due to their high bond dissociation energies. Despite these obstacles, the field continues to advance rapidly, and numerous innovative transformations are anticipated in the near future.
In particular, this review presents a detailed and critical analysis of the mechanistic pathways underlying many reported C–H functionalization reactions involving heteroaromatic systems.
2. Direct functionalization on pyridine heterocycles
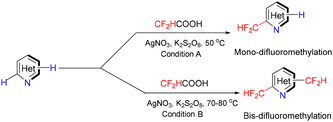
In 2017, Nielsen introduced difluoroacetic acid as a novel reagent for the direct C–H difluoromethylation of pyridine heterocycles.4 A technically simple procedure was developed in which commercially available difluoroacetic acid serves as the difluoromethylating agent. Control over mono- versus bis-difluoromethylation is achieved by adjusting the reaction temperature. Because this method grants access to a wide range of difluoromethylated heteroaromatic derivatives, it enables the synthesis of previously inaccessible tool compounds for biological evaluation and provides valuable new lead structures for drug discovery (see Scheme 1).4Lectka and colleagues reported a direct C–H to C–C bond functionalization of pyridine heterocycles via tandem C–C bond cleavage of cyclopropanols followed by oxidative aromatization with manganese(IV) oxide.5 This strategy affords a mild and efficient route to β-aryl carbonyl-containing products. Under the optimized conditions, a diverse array of heterocycles undergo regioselective C–C bond formation, while a broad scope of cyclopropanols bearing varied functional groups is well tolerated. Mechanistic investigations—including kinetic isotope effect (KIE) measurements, radical-scavenger experiments, and studies of manganese species in different oxidation states—were conducted to propose an initial mechanistic hypothesis. The products obtained serve as versatile intermediates for natural product synthesis and direct derivatization of complex, biologically relevant molecules. Evidence from radical-scavenger studies supports the involvement of a radical-based pathway (see Scheme 2).5
Baran and co-workers described an innate C–H trifluoromethylation of pyridine heterocycles.6 They developed a general protocol that employs a bench-stable trifluoromethyl radical source, applicable to both electron-rich and electron-deficient heteroaromatic systems with broad functional group tolerance. The method is operationally straightforward, proceeds under ambient conditions, and can be applied directly to unprotected substrates. The orthogonal reactivity of the trifluoromethyl radical, relative to aryl radicals, was further explored in the context of a complex natural product and a pharmaceutical compound. Extensive mechanistic studies were undertaken to probe the distinctive features of this reactivity, ultimately leading to the establishment of a robust and broadly useful protocol (see Scheme 3).6
In 2023, Tamura and co-workers disclosed an electrophilic C3–H alkenylation of 2,6-dialkoxypyridine derivatives employing a Pd(II)/Tl(III) catalytic system.7 The cooperative action of the Pd/thioether ligand catalyst and Tl(III) species enabled highly efficient C–H alkenylation across a range of nitrogen heteroaromatics with complete regioselectivity. Mechanistic investigations revealed that the transformation proceeds through electrophilic thallation of the heteroarene, followed by a Pd-catalyzed Heck-type coupling. Notably, the reaction tolerates the use of nitrogen heteroaromatics as the limiting reagent and was successfully applied to the late-stage functionalization of pyridine-containing anticancer agents, highlighting its synthetic utility (see Scheme 4).7
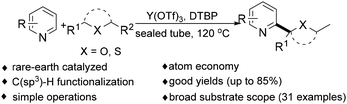
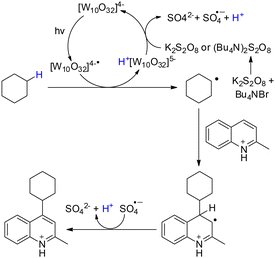
In 2019, our group reported a rare-earth-catalyzed coupling of ethers with azaarenes mediated by Y(OTf)3.8 We demonstrated that Y(OTf)3 effectively promotes the direct functionalization of pyridines with ethers, in particular chain ethers and thioethers. This method is operationally straightforward, accommodates a broad range of substrates (31 examples), delivers moderate to good yields (up to 85%), and exhibits favorable atom economy, underscoring its synthetic practicality (see Scheme 5).8
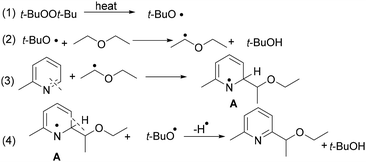
Based on mechanistic investigations, a plausible reaction pathway was proposed (Scheme 6).8 Upon heating, DTBP undergoes homolytic cleavage to produce two tert-butylperoxyl radicals. These peroxyl radicals abstract a hydrogen atom from the ether substrate, generating a carbon-centered ether radical. The resulting radical then couples with 2-methylpyridine to form intermediate A, which undergoes further oxidation by DTBP to afford the final product (see Scheme 6).8
The development of regioselective C–H functionalization methods for N-heterocycles has advanced steadily over the past two decades. Innovations in ligand design and Lewis-acidic metal precursors have enabled the creation of in situ cooperative catalytic systems capable of promoting C–H functionalization of pyridines at the C-2, C-3, and C-4 positions. In 2022, Shoshani reviewed representative examples highlighting the evolution of strategies aimed at enhancing cooperative interactions between Lewis-acidic metals and late transition metals to achieve regioselective C–H functionalization of pyridine (see Scheme 7).9
In 2017, Murakami and Itami published a comprehensive review on the C–H functionalization of azines.10 This survey focused on six-membered aromatic heterocycles containing one or more nitrogen atoms and categorized C–H functionalization strategies into four main classes: (1) nucleophilic aromatic substitution SNAr reactions, (2) radical-mediated processes, (3) deprotonation followed by functionalization, and (4) metal-catalyzed transformations.10
3. Direct functionalization on thiophene heterocycles
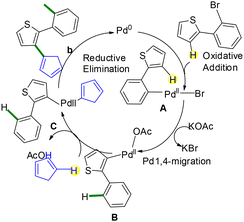
In 2023, Doucet and co-workers reported a double C–H bond functionalization strategy enabling C–C coupling at the β-position of thiophenes through a palladium-catalyzed 1,4-migration combined with direct arylation.11 Since C–H activation at the β-position of thiophenes is generally more challenging than at the α-position, this approach provides a valuable solution by harnessing Pd-catalyzed 1,4-migration under optimized conditions. The transformation involves the formation of a new C–C bond through the functionalization of two C–H bonds and proceeds with broad substrate scope, accommodating a variety of benzene substituents. The method employs readily available, air-stable palladium catalysts and inexpensive bases while avoiding the limitations of traditional β-bromothiophene-based protocols. In addition to enabling regio-divergent heteroarylations, this strategy is synthetically advantageous as it allows access to two distinct classes of products from the same molecular precursor (see Scheme 8).11Access to 1,2-bis(heteroaryl)benzenes is proposed to proceed through a classical Suzuki coupling pathway, while the formation of 2′-aryl-2,3′-biheteroarenes likely occurs via a Pd-mediated 1,4-migration followed by direct arylation, as outlined in Scheme 9.11 The catalytic cycle begins with the oxidative addition of 2-(2-bromoaryl)thiophene to generate intermediate A. In the case of Suzuki coupling with arylboronic acids, conventional transmetalation followed by reductive elimination affords the corresponding 1,2-bis(heteroaryl)benzenes (see Scheme 9).11
Berteina-Raboin and co-workers reported a direct C–H arylation at the C-2 and C-3 positions of the thiophene ring, along with a one-pot procedure for the synthesis of a variety of thieno[3,2-d]pyrimidines.12 This C–H activation strategy at the C-2 position of thiophene provided an improved approach for constructing annulated sulfur-containing heterocycles. The optimized reaction conditions were further demonstrated to be applicable to the synthesis of thienopyridines and thienopyrazines, underscoring the versatility of the methodology (see Scheme 10).12
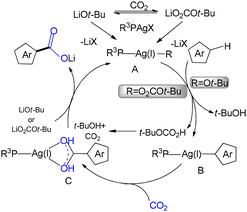
In 2021, Kwak reported an Ag(I)-catalyzed C–H carboxylation of thiophene derivatives.13 A key feature of this transformation is the direct utilization of CO2, offering a sustainable approach by converting it into value-added chemicals. The methodology, employing a phosphine ligand in combination with lithium tert-butoxide, enables the direct carboxylation of thiophenes under mild conditions. Experimental studies demonstrated that the presence of tert-butyl alkoxide is essential for the exergonic formation of an arylsilver intermediate. Further mechanistic investigations revealed that either a phosphine-ligated silver alkoxide species or a silver–TBC complex facilitates efficient C–H carboxylation of heteroaromatic substrates (Scheme 11).13
Drawing on experimental observations and DFT calculations, Kwak and co-workers proposed the reaction mechanism outlined in Scheme 12.13 In the presence of a silver precursor, a phosphine ligand, and lithium tert-butoxide, a phosphine-ligated silver alkoxide species (Ag–OtBu) is formed, which subsequently leads to the generation of an arylsilver intermediate (Scheme 12).13
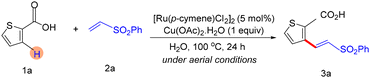
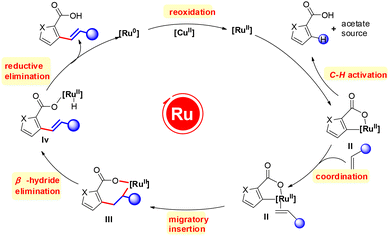
Very recently, Baidya and co-workers reported a regioselective C–H alkenylation and unsymmetrical bis-olefination of thiophene carboxylic acids using ruthenium catalysis in aqueous media.14 The transformation is operationally straightforward and exhibits broad substrate scope, accommodating diverse thiophene carboxylic acids as well as a wide range of olefins, delivering products in high yields (up to 87%). The ortho-C–H activation strategy was further applied to the tunable synthesis of densely functionalized heteroarenes via a challenging unsymmetrical bis-olefination process. Moreover, an efficient one-pot, sequential two-fold unsymmetrical bis-olefination of 3-substituted five-membered heteroarene carboxylic acids was achieved under a single catalytic system, providing rapid access to complex heteroaromatic scaffolds (Scheme 13).14
Mechanistic investigations indicated that the reaction proceeds through a reversible C–[Ru] bond-forming step, with evidence suggesting that C–H bond cleavage is unlikely to be the rate-determining step (Scheme 14).14
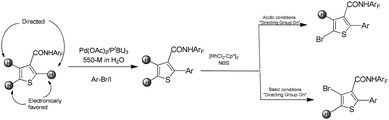
Tan and co-workers reported a sequential, regioselective C–H functionalization strategy for thiophenes.15 Utilizing a pH-sensitive directing group, the method enables access to both directed and non-directed C–H activation pathways, thereby allowing the synthesis of 2,3,4- and 2,4,5-substituted thiophenes. The C–H arylation was carried out in water, with an amphiphilic surfactant serving as a phase-transfer catalyst to facilitate the reaction under aqueous conditions. The resulting arylated heterocycles were subsequently amenable for further functionalization at the remaining unsubstituted positions via either directed or undirected rhodium-catalyzed bromination (Scheme 15).15
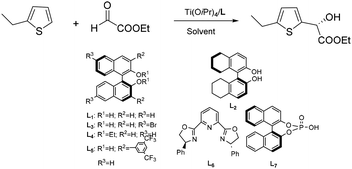
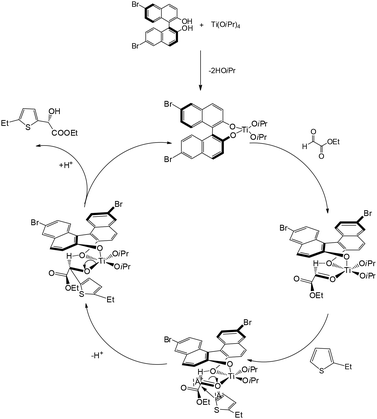
Our group reported an enantioselective Friedel–Crafts alkylation of thiophenes with ethyl glyoxylate, affording the corresponding chiral secondary alcohols.16 These reactions generally proceeded with good enantioselectivities and satisfactory isolated yields. Employing a titanium(IV)/BINOL catalytic system, the transformation delivered products with enantioselectivities of up to 91% ee. Notably, thiophenes bearing substituents at the 2-position exhibited significantly higher reactivity and selectivity compared to those substituted at the 3-position or unsubstituted thiophene (Scheme 16).16
Our group proposed the mechanism for the asymmetric Friedel–Crafts reaction of 2-ethylthiophene with ethyl glyoxylate, as shown in Scheme 17.16
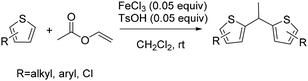
Our group also reported the direct alkylation of thiophenes through a bis-coupling reaction with vinyl acetates, representing the first example of an iron-catalyzed coupling between two thiophenes and vinyl groups.17 This methodology employs Earth-abundant, inexpensive, and non-toxic iron catalysts to convert simple thiophenes into symmetrical dithienylethane derivatives in a single step, proceeding under mild conditions with good yields (Scheme 18).17
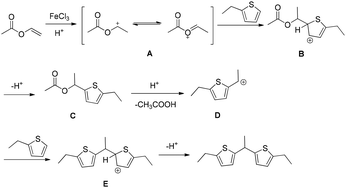
A plausible mechanism for this transformation was proposed (Scheme 19).17 In the presence of Fe(III) and TsOH, vinyl acetate undergoes protonation to generate an oxocarbenium cation, which subsequently attacks the thiophene. Activation of vinyl acetate by FeCl3 facilitates and accelerates this protonation step. The resulting intermediate eliminates acetic acid to produce a carbocation species that reacts with a second thiophene molecule. Final deprotonation furnishes the desired product (Scheme 19).17
4. Direct functionalization on pyrrole heterocycles
Pyrrole heterocycles are an important class of compounds found in a wide range of natural products, pharmaceutically active molecules, and organic materials. Recently, the direct functionalization of pyrrole heteroaromatic C–H bonds has become an efficient and attractive method to access substituted heteroarenes.Zhu and co-workers reported a direct arylation of pyrroles through an indirect electroreductive C–H functionalization strategy employing perylene bisimide as an electron-transfer mediator.18 In this approach, the electroreductive coupling of aryl halides with pyrroles was achieved using a catalytic amount of perylene bisimide in 1-ethyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide as the reaction medium. The process, carried out in a simple undivided cell at room temperature, employed a sacrificial zinc anode and proceeded without the need for a metal catalyst or external base. The high electron-transfer efficiency and stability of perylene bisimides enabled efficient electrocatalytic C–C bond formation under these mild conditions (Scheme 20).18
A plausible mechanism for the electrocatalytic coupling of aryl halides with pyrroles mediated by PDI-1 is illustrated in Scheme 21. Under constant-current electrolysis, PDI-1 undergoes reduction at the cathode to form PDI-1 radical anions, which serve as the key reactive intermediates in the process (Scheme 21).18
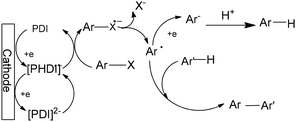 | ||
| Scheme 21 Plausible catalytic cycle for the electroreductive arylation of pyrroles using PDI as a mediator. | ||
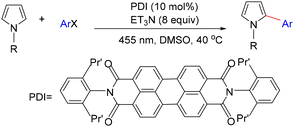
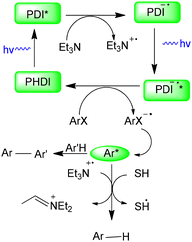
König and co-workers developed a visible-light-induced C–H arylation of pyrroles employing inexpensive aryl halides as coupling partners (Scheme 22).19 In this system, the fluorescent dye N,N′-bis(2,6-diisopropylphenyl)perylene-3,4,9,10-bis(dicarboximide) (PDI) served as the photocatalyst, enabling the reduction of stable aryl halides to reactive aryl radicals through a sequential photoinduced electron-transfer process. This methodology was effective not only for aryl iodides and aryl bromides but also for typically inert aryl chlorides, affording the corresponding C–C coupled pyrrole derivatives in good to excellent yields (Scheme 22).19
König and co-workers suggested a reaction mechanism for the visible-light-induced direct C–H arylation of pyrroles, as shown in Scheme 23.19
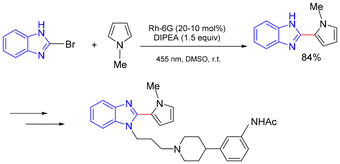
In 2016, König and co-workers reported a C–H heteroarylation of pyrroles employing heteroaryl bromides and chlorides as radical precursors (Scheme 24).20 In this protocol, the combination of rhodamine 6G (Rh-6G) with DIPEA proved essential for achieving high efficiency. A broad range of heteroaryl halides underwent smooth coupling with pyrroles to deliver the corresponding heteroarylated products in good yields. This strategy was further demonstrated through its successful application in the formal synthesis of a benzimidazole derivative (Scheme 24).20
Cho reported a radical trifluoromethylation strategy employing a ruthenium catalyst in combination with CF3I, which enabled the selective introduction of a trifluoromethyl group at the 2-position of pyrrole derivatives and related compounds (Scheme 25).21
Cho and You demonstrated that a cyclometalated platinum complex is capable of generating a CF3 radical from CF3I under visible-light irradiation, which was subsequently applied to the C–H trifluoromethylation of pyrrole derivatives (Scheme 26).22
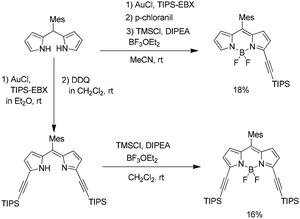
In 2020, Furuta and co-workers reported the regioselective α- and β-alkynylation of BODIPY dyes through a gold(I)-catalyzed direct C–H functionalization of pyrrole derivatives using ethynylbenziodoxolone (EBX) as the alkynylating agent.23 This atom-economical late-stage alkynylation strategy offers an alternative route to functionalized fluorescent dyes without requiring the preparation of unstable halogenated pyrrole precursors. The resulting α- and β-ethynyl-substituted BODIPYs exhibited distinct spectral properties that were strongly dependent on the site of substitution. This methodology therefore provides a valuable approach for tuning the photophysical characteristics of BODIPY dyes and for developing BODIPY-based cores in functional π-conjugated materials (Scheme 27).23
5. Direct functionalization on azole heterocycles
5.1. Functionalization of pyrazoles
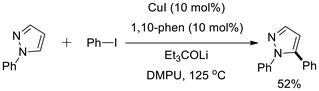
Joo and co-workers reported a direct C–H alkenylation of functionalized pyrazoles, which, through a sequence of C–H alkenylation followed by cyclization, afforded fused bicyclic pyrazoles.24 This approach provides a novel strategy for the annulation of readily accessible pyrazole derivatives without the need for specialized directing groups. Interestingly, the addition of pyridine—a stronger Lewis base than pyrazole—was found to enhance the oxidative alkenylation process. The methodology demonstrated broad functional group tolerance, highlighting its synthetic versatility. The ability to construct fused pyrazole frameworks through sequential incorporation of alkenyl substituents and functional groups into the parent pyrazole ring underscores the potential of this strategy for developing new routes to pyrazole annulation (Scheme 28).24Daugulis and co-workers reported the first example of C–H arylation of C-unsubstituted pyrazoles.25 In this Cu-catalyzed transformation, N-phenylpyrazole was employed as the substrate, and the combination of CuI with a lithium alkoxide base enabled the selective formation of the C5-arylated product, which was obtained in 52% yield (Scheme 29).
Nicewicz and co-workers developed a predictive model for site-selective aryl and heteroaryl functionalization mediated by organic photoredox catalysis.26 Their aim was to establish a framework for anticipating site selectivity and to expand this functionalization strategy to heteroaromatic systems of particular relevance to the pharmaceutical industry. By employing electron density calculations, they successfully predicted site selectivity across a range of heterocycles and identified generalizable trends within different heteroaromatic classes. This predictive approach proved applicable not only to diverse biologically active heterocyclic scaffolds but also to more complex molecules in late-stage functionalization, thereby underscoring its utility in drug discovery and development (Scheme 30).26
5.2. Functionalization of imidazole heterocycles
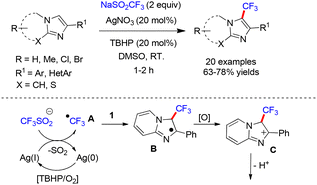
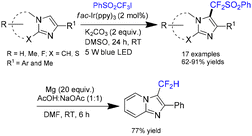
Hajra and co-workers reported an AgNO3-catalyzed oxidative trifluoromethylation of imidazo[1,2-a]pyridines, employing CF3SO2Na as the trifluoromethyl source and tert-butyl hydroperoxide (TBHP)/air as the oxidant in DMSO at room temperature (Scheme 31).27 Under the optimized conditions, a broad range of imidazoheterocycles underwent smooth trifluoromethylation with sodium triflinate, affording the corresponding products in 63–78% yields. Mechanistic studies suggest that the transformation proceeds through the generation of a CF3 radical (A) via the reaction of sodium triflinate with AgNO3. The radical then adds to the heteroarene substrate to form intermediate B, which subsequently undergoes single-electron transfer (SET) oxidation followed by deprotonation to furnish the final product (Scheme 31).27Fu and co-workers reported a visible-light-mediated C3-(phenylsulfonyl)difluoromethylation of imidazo[1,2-a]pyridines in DMSO at room temperature (Scheme 32).28 In this transformation, PhSO2CF2 served as the radical source, while fac-Ir(ppy) acted as the photocatalyst. Under the optimized conditions, a broad range of imidazo[1,2-a]pyridines as well as benzo[d]imidazo[2,1-b]thiazoles were efficiently converted to the corresponding products in yields ranging from 62% to 91% (Scheme 32).28
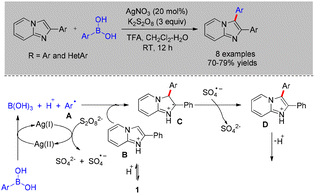
In 2017, Mahdavi reported a silver-catalyzed cross-coupling of imidazo[1,2-a]pyridines with arylboronic acids, employing K2S2O8 as the oxidant in a dichloromethane/water solvent system at room temperature (Scheme 33).29 This method afforded 3-arylimidazo[1,2-a]pyridines in moderate to good yields (70–79%). Mechanistic studies indicated that the reaction proceeds via a radical pathway: Ag(I) is first oxidized to Ag(II) by the persulfate anion, and the resulting Ag(II) species then reacts with the arylboronic acid to generate the corresponding aryl radical (Ar˙) (Scheme 33).29
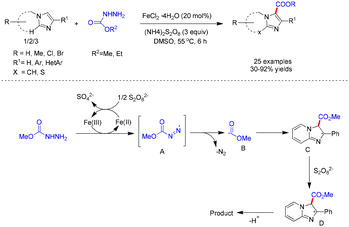
Sun and co-workers reported an alkoxycarbonylation of imidazo[1,2-a]pyridines with carbazates, employing FeCl2·4H2O as the catalyst and (NH4)2S2O8 as the oxidant in DMSO at 55 °C (Scheme 34).30 A variety of imidazo[1,2-a]pyridines, benzo[d]imidazo[2,1-b]thiazoles, and imidazo[2,1-b]thiazoles bearing diverse functional groups underwent smooth transformation with carbazates to afford the corresponding products in yields ranging from 32% to 92%. Mechanistic investigations indicated that the process proceeds through a radical pathway (Scheme 34).30
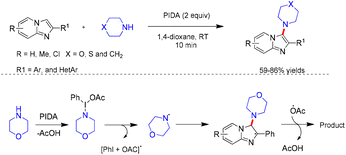
Hajra and co-workers reported a PIDA-mediated C3-amination of imidazo[1,2-a]pyridines with cyclic secondary amines, affording the aminated products in 59–86% yields (Scheme 35).31 This metal-free protocol employs morpholine, thiomorpholine, and piperidine as amine sources, with PIDA serving as the oxidant. Alternative oxidants, including (bis(trifluoroacetoxy)iodo)benzene (PIFA), K2S2O8, and TBHP, failed to provide the desired products. Likewise, several cyclic and acyclic amines such as pyrrolidine, dibenzylamine, aniline, and diisopropylamine were unreactive under the optimized conditions. The complete suppression of product formation in the presence of radical scavengers such as DDQ, TEMPO, BQ, and BHT strongly supported the involvement of a radical pathway. The proposed mechanism suggests that PIDA initially reacts with morpholine to generate a morpholine radical, along with iodobenzene and acetic acid (Scheme 35).31
5.3. Functionalization of oxazole heterocycles
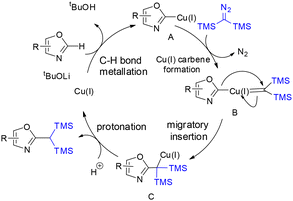
In 2019, Wang and co-workers reported a Cu(I)-catalyzed reaction of bis(trimethylsilyl)diazomethane with benzoxazoles and oxazoles, providing a new strategy for the direct introduction of a 1,1-bis(trimethylsilyl)methyl group into heteroaromatic C–H bonds (Scheme 37).32 This cross-coupling approach enabled the synthesis of a series of 1,1-bis(trimethylsilyl)methylated heteroaromatic compounds in moderate to good yields from readily accessible starting materials. The protocol employs inexpensive and commercially available CuI as the catalyst and exhibits broad functional group tolerance (Scheme 36).32On the basis of experimental studies, a mechanistic rationale was proposed.32 The transformation begins with deprotonation of the relatively acidic C–H bond of the oxazole substrate by LiOtBu, generating an oxazolyl anion, which undergoes transmetalation with Cu(I) to form intermediate A. This intermediate then reacts with the diazo compound to produce a Cu(I)–carbene species B. Migratory insertion of the carbene into the C–Cu bond affords intermediate C, which undergoes protonation to deliver the gem-disilylated product while regenerating the Cu(I) catalyst (Scheme 37).32
Nolan and co-workers reported the carboxylation of oxazoles via C–H bond functionalization, demonstrating that NHC–gold(I) hydroxide complexes efficiently catalyze the transformation with high regioselectivity at the most acidic C–H bond position.33 The observed selectivity can be rationalized on the basis of acid–base considerations. Several proposed intermediates in the Au(I)-catalyzed pathway were successfully isolated and characterized, providing strong support for the mechanistic rationale (Scheme 38).33
The postulated catalytic cycle for this gold(I)-mediated carboxylation was further validated under the reported conditions, and the proposed mechanism is illustrated in Scheme 39.33
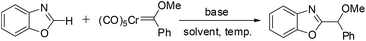
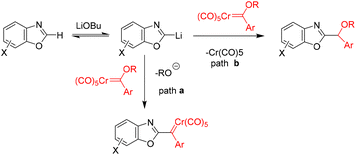
Wand and co-workers reported a C–H bond functionalization of benzoxazoles using chromium(0) Fischer carbene complexes.34 The reaction was found to be broadly compatible with a variety of benzoxazoles and chromium(0) carbene substrates, providing an efficient and straightforward method for the alkylation of benzoxazoles under mild conditions with good substrate tolerance. This transformation thus offers a direct route to substituted benzoxazole derivatives (Scheme 40).34
Mechanistic studies suggest that elimination of the Cr(CO)5 fragment is favored over alkoxy group elimination. The formation of a Cu(I)–carbene intermediate, followed by migratory insertion, is proposed to constitute the key steps in the catalytic cycle (Scheme 41).34
5.4. Functionalization of thiazole heterocycles
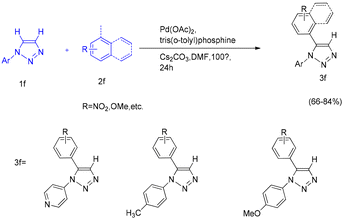
Benzobisthiazole and thiazolothiazole derivatives are valuable building blocks in organic electronic devices due to their favorable absorption, electroluminescence, and charge-transport properties. Blakey and co-workers reported a C–H functionalization of thiazole rings that enables the direct synthesis of benzobisthiazole derivatives through palladium/copper dual catalysis.35 Optimized conditions using a bromobenzene/benzobisthiazole system allowed for one-pot functionalization of both thioimidate positions of benzobisthiazole. This methodology provides an efficient approach for constructing these important heteroaromatic frameworks, with significant potential impact in both materials science and synthetic organic chemistry (Scheme 42).35Direct C–H arylation represents a highly efficient strategy for the synthesis of arylated heteroaromatics. Stefan and co-workers reported a regioselective direct C–H functionalization of thiazoles using an air- and moisture-stable iminopyridine-based α-diimine nickel(II) complex as the catalyst.36 This method enables selective C5–H arylation of thiazole derivatives, significantly reducing the number of synthetic steps and minimizing impurity formation. Under mild conditions—low catalyst loading (as little as 0.1 mol%), relatively low temperatures (80 °C), and an aerobic atmosphere—both mono- and diarylated thiazole products were efficiently obtained. Mechanistic investigations, supported by competition experiments and DFT calculations, indicated that C–H activation in 4-methylthiazole proceeds via an electrophilic aromatic substitution pathway. The reaction was accelerated by electron-donating substituents on the thiazole ring, whereas electron-withdrawing groups retarded the process. Notably, the direct coupling of 4-methylthiazole with 2-bromonaphthalene delivered the corresponding product in 91% yield under the optimized conditions (80 °C, air, DMF as solvent) (Scheme 43).36
Stefan and co-workers carried out an in-depth mechanistic investigation using DFT calculations to elucidate the direct C2–H/C5–H arylation of 4-methylthiazole catalyzed by an iminopyridine-based α-diimine nickel(II) complex.36 The study examined potential pathways for C2–H and C5–H activation with Ni(II)–ACc as the active catalytic species by comparing the corresponding rate-determining steps. The proposed catalytic cycle involves three key stages: oxidative addition, C–H bond activation at either C-2 or C-5, and reductive elimination. Computational analysis revealed that the transition state associated with C2–H activation is higher in energy than that for C5–H activation, thereby explaining the regioselectivity of the catalyst toward the C5 position and the higher yield of the corresponding product, 4-methyl-5-(naphthalen-2-yl)thiazole. Further insight from condensed Fukui function analysis of 4-methylthiazole supported these findings, indicating that electrophilic attack occurs more readily at the C5 position than at C2.36
5.5. Functionalization of triazole heterocycles
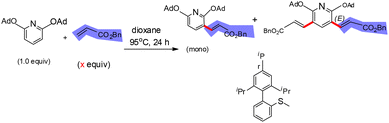
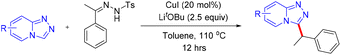
In 2019, Zou and co-workers reported a copper(I)-catalyzed benzylation of triazolopyridines via direct C–H functionalization.37 This transformation establishes a C(sp2)–C(sp3) bond through cross-coupling, affording 3-benzylated triazolopyridines in moderate to good yields. The methodology is synthetically valuable for the preparation of pharmaceutically relevant triazolopyridine derivatives, benefiting from the operational simplicity of generating N-tosylhydrazones from carbonyl compounds and the accessibility of an inexpensive copper(I) catalyst (Scheme 44).37A plausible mechanism was proposed (Scheme 45).37 The copper catalytic cycle begins with deprotonation of the acidic C–H bond in triazolopyridine by LiOtBu, followed by transmetalation with an excited Cu(I) species to produce a metallated triazolopyridinyl intermediate A. This species subsequently reacts with the reactive fragment derived from the tosylhydrazone, again activated by LiOtBu, to form a Cu(I)–carbene intermediate B, which then undergoes further steps to deliver the final product (Scheme 45).37
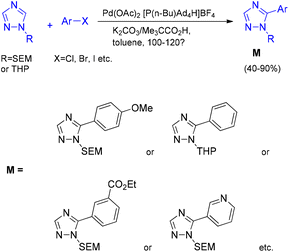
The synthesis of 1,2,3-triazole derivatives has emerged as a critical area of research owing to their wide-ranging applications in pharmaceutical and materials chemistry. To address the limitation that C–H arylation of 1,2,4-triazoles had previously been restricted largely to 1-methyltriazoles, Dalibor Sames and co-workers developed a strategy for the preparation of more complex arylated 1,2,4-triazoles.38 Their approach employed a simple benchtop protocol for the direct C–H arylation of 1-substituted 1,2,4-triazoles using air-stable phosphonium salts as arylating reagents (Scheme 46).
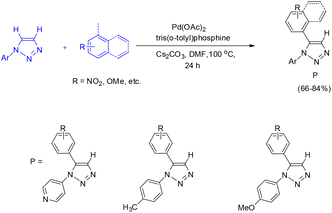
Patil and co-workers reported a direct palladium-catalyzed arylation of N-aryl-1,2,3-triazoles with aryl halides, enabling the synthesis of C5-substituted N-aryl-1,2,3-triazoles (Scheme 47).39 In this protocol, PPh3 proved to be less effective than the bulkier tris(o-tolyl)phosphine ligand. Substitution of Cs2CO3 with K2CO3 did not significantly affect the overall yield but led to a decrease in the reaction rate. Under an inert atmosphere, the optimal reaction conditions consisted of Pd(OAc)2 (10 mol%) as the catalyst, Cs2CO3 (2.0 equiv.) as the base, and tris(o-tolyl)phosphine (20 mol%) as the ligand in DMF at 100 °C for 24 h (Scheme 47).39
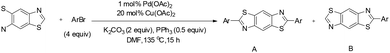
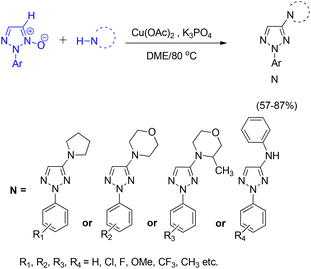
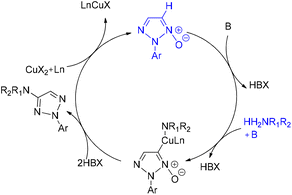
Chen and Liu developed an efficient copper-catalyzed strategy for the direct synthesis of 4-amino-2-aryl-1,2,3-triazole derivatives through C–H amination of 2-aryl-1,2,3-triazole N-oxides.40 This protocol accommodated a wide range of coupling partners, including both primary and secondary amines. Optimization studies revealed that solvent choice was critical to reaction efficiency: DME provided the highest yields of the desired product, whereas DMF completely suppressed product formation. Among the catalysts tested, including CuCl2, CuSO4, CuI, and various palladium and silver salts, Cu(OAc)2 exhibited superior performance. The optimized cross-deprotonative coupling (CDC) conditions involved 20 mol% Cu(OAc)2 as the catalyst and 2 equivalents of K3PO4 as the base in DME at 80 °C for 12 h, affording the aminated triazole products in good yields (Scheme 48).
A plausible mechanism for the copper-catalyzed direct coupling of 2-aryl-1,2,3-triazole N-oxides with amines, as proposed by Chen and Liu, is illustrated in Scheme 49.40 The reaction is initiated by coordination of the copper catalyst at the C5 position of the triazole N-oxide, accompanied by removal of the C5 hydrogen to generate an organocopper intermediate. This species subsequently reacts with the amine coupling partner to form intermediate 2n′. Reductive elimination from this intermediate furnishes the aminated product 3n′, while simultaneously producing a reduced copper species. Reoxidation of this lower-valent copper species to Cu(II) closes the catalytic cycle (Scheme 49).40
6. Direct functionalization of quinolone heterocycles
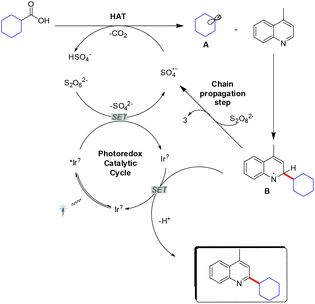
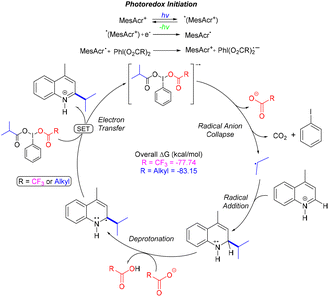
In 2021, Jiang and co-workers reported a direct C–H arylation and alkylation of electron-deficient quinolone rings.41 This transformation proceeds without the need for a transition-metal catalyst or external oxidants, enabling the efficient synthesis of a broad range of substituted heteroarenes with good functional group tolerance and in good yields. The method avoids prefunctionalization steps and minimizes waste generation, while also demonstrating scalability to the gram level (Scheme 50).41Glorius and co-workers reported a visible-light-mediated direct decarboxylative C–H functionalization of quinolone rings using aliphatic carboxylic acids as coupling partners.42 This mild protocol operates with very low catalyst loading (0.5 mol%), exhibits a broad substrate scope, and shows excellent functional group tolerance (Scheme 51). A variety of tertiary carboxylic acids, as well as amino and fatty acids, underwent smooth transformation under the standard conditions at room temperature (Scheme 51). Owing to its operational simplicity and efficiency, this strategy provides a valuable approach for the rapid and straightforward diversification of pharmacologically relevant quinolone scaffolds (Scheme 51).42
A mechanistic pathway for the visible-light-driven decarboxylative process was also proposed by Glorius and co-workers (Scheme 52).42
In 2018, Frenette and co-workers reported a metal-free, visible-light-mediated C–H alkylation of quinolone rings via hypervalent iodine-promoted decarboxylation.43 The methodology demonstrates broad functional group tolerance and is applicable to the late-stage functionalization of drugs and drug-like molecules. This light-induced chain process proceeds under mild conditions and makes use of a wide and readily accessible set of carboxylic acid substrates, highlighting its practicality for synthetic and medicinal chemistry applications (Scheme 53).43
The reaction mechanism was probed through control experiments, photophysical studies, and DFT calculations. A stepwise mechanistic pathway for the catalytic cycle was proposed, as illustrated in Scheme 54.43
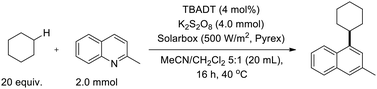
Ryu and co-workers reported a versatile cross-dehydrogenative coupling of quinolone rings, describing a straightforward sunlight-induced derivatization of heteroaromatics via photocatalyzed C–H functionalization with amides, ethers, alkanes, and aldehydes as coupling partners.44 The transformation was performed under mild conditions using tetrabutylammonium decatungstate (TBADT) as the photocatalyst (Scheme 55).44
A tentative mechanism was proposed to account for the reaction pathway (Scheme 56).44 Upon photoexcitation, TBADT is capable of homolytically cleaving the C–H bond of the coupling partner to generate a radical species, such as a cyclohexyl radical. This radical is then trapped by the protonated heteroaromatic substrate, forming an adduct radical that undergoes subsequent oxidation to deliver the final product. Control studies confirmed that the presence of TBADT is essential for the success of the transformation.44
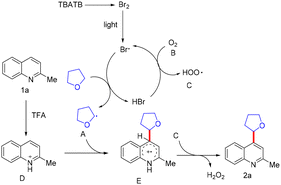
In 2024, Wei and co-workers reported a visible-light-induced bromine-radical-initiated direct C–H alkylation of quinolone rings.45 This protocol describes a photoinduced C(sp2)–H alkylation of N-heteroaromatics using commercially available tetrabutylammonium tribromide (TBATB) as a hydrogen atom transfer (HAT) reagent, with O2 serving as the oxidant. The methodology is metal-free, operates under mild conditions, exhibits broad functional group tolerance, and proceeds through a Minisci-type alkylation pathway (Scheme 57).45
A plausible mechanism was proposed (Scheme 58).45 Under light irradiation, TBATB releases catalytic amounts of elemental bromine, which are converted into bromine radicals (Br˙). These radicals abstract a hydrogen atom from THF to generate an alkyl radical A. The alkyl radical subsequently couples with the quinolone substrate to form intermediate E, which, upon further oxidation, furnishes the final product 2a while regenerating the catalytic cycle (Scheme 58).45
7. Direct functionalization of indole heterocycles
In 2023, Xu and co-workers reported a rhodium(I)-catalyzed direct enantioselective C–H functionalization of indoles.46 They developed a highly regioselective vinylogous carbene insertion strategy for asymmetric C–H activation of indole substrates. Using a simple Rh(I)/chiral diene catalytic system, the transformation proceeds exclusively at the vinylogous position of the vinylcarbenoid with remarkable E-selectivity and excellent enantiocontrol. This protocol provides efficient access to chiral indole scaffolds incorporating an α,β-unsaturated ester moiety and a gem-diaryl carbon stereocenter, delivering products in high yields (up to 99%) and outstanding enantioselectivities (up to 96%) under mild conditions at room temperature. Overall, this method offers a powerful approach for constructing chiral indole frameworks featuring diarylmethine-substituted α,β-unsaturated ester units (Scheme 59).46Wu and co-workers reported a palladium-catalyzed direct C–H functionalization of indoles that incorporates sulfur dioxide through a three-component reaction of 1-(pyridin-2-yl)indoles, DABCO·(SO2)2, and aryldiazonium tetrafluoroborates under mild conditions.47 Using palladium(II) bromide as the catalyst at room temperature, this method afforded a wide variety of 2-sulfonated indoles. The protocol also demonstrated that 2-pyrimidinyl can serve as an effective directing group for C–H sulfonylation, which can be readily removed after the transformation. This approach provides an efficient and straightforward strategy for the introduction of sulfonyl (–SO2–) groups, with the insertion of sulfur dioxide into C–H bonds offering a promising route to sulfonyl-containing compounds. Wu and co-workers further proposed a reaction mechanism to explain the C–H functionalization process of indoles (Scheme 60).47
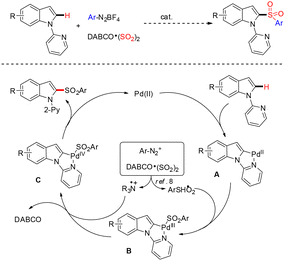 | ||
| Scheme 60 Palladium-catalyzed C–H functionalization of indoles with the insertion of sulfur dioxide. | ||
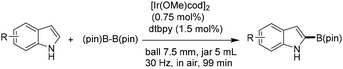
Kubota and Ito reported an iridium(I)-catalyzed C–H borylation of indoles carried out under mechanochemical conditions in air.48 The transformation proceeds with little to no solvent—requiring either none or only a catalytic amount of liquid—and enables the efficient C–H borylation of a range of heteroaromatic compounds, affording the corresponding arylboronates in good to excellent yields. Furthermore, a one-pot mechanochemical sequence combining C–H borylation with Suzuki–Miyaura cross-coupling was developed, providing direct access to 2-aryl indole derivatives. Optimization of the milling parameters, including the choice of jar, ball, and liquid-assisted grinding (LAG) additive, proved essential for achieving a solvent-free C–H borylation reaction that could be performed in air (Scheme 61).48
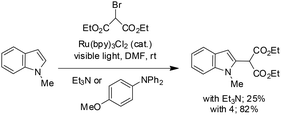
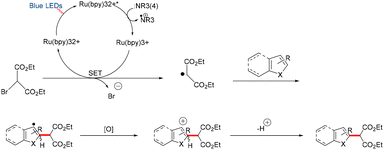
Stephenson and co-workers reported a visible-light-mediated intermolecular C–H functionalization of indoles with malonates, proceeding via the reductive quenching pathway of Ru(bpy)3Cl2.49 Through systematic evaluation of reductive quenchers and mechanistic studies, an optimized protocol was established that enabled heteroaromatic alkylations in good yields and regioselectivities, while effectively suppressing previously observed side reactions. The methodology demonstrated successful functionalization of dipeptide substrates and showed promising results when applied in aqueous media, highlighting the potential of this mild photoredox approach for future applications in protein modification and bioconjugation chemistry (Scheme 62).49
A plausible mechanism for the reaction is depicted in Scheme 63.49 Upon visible-light irradiation, Ru(bpy)32+ is excited to its photoactive state Ru(bpy)32+*, which undergoes reductive quenching by substrate 4 to yield Ru(bpy)31+ and an ammonium radical cation. Luminescence quenching experiments confirmed that substrate 4 is the sole quencher of the excited state. The Ru(I) species then reduces the activated C–Br bond via single-electron transfer, regenerating Ru(bpy)32+ and producing a carbon-centered radical. Subsequent coupling of this electron-deficient radical at the C2 position of the electron-rich arene generates a stabilized radical intermediate (benzylic or allylic). Final oxidation of this radical, followed by rearomatization, affords the desired product (Scheme 63).49
In 2021, Šebesta and co-workers reported a diastereoselective C–H functionalization of chiral ferrocenes with indoles.50 This strategy enabled a diastereoselective double C–H heteroarylation of chiral ferrocenes, affording multifunctionalized products under mild conditions with simple reagents. Palladium complexes bearing chiral mono-protected amino acid ligands promoted the transformation efficiently, delivering heteroarylated ferrocenyl amines in good yields and with high diastereomeric purity. Furthermore, by varying the configurations of the amino acid ligand and ferrocenyl amine, complementary diastereomeric products could be selectively obtained (Scheme 64).50
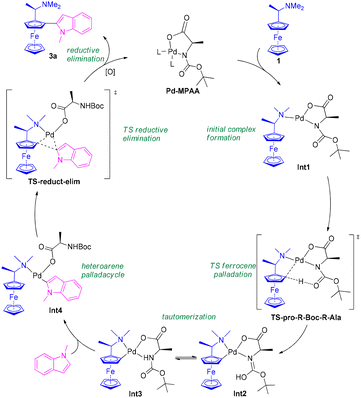
Based on DFT studies, a tentative mechanism for the oxidative double C–H activation has been proposed (Scheme 65).50 The process is initiated by the formation of the initial complex Int1 between the Pd–MPAA catalyst and the substrate amine 1. Palladation of the ferrocene unit then proceeds through the transition state TS-pro-R-Boc-R-Ala, with an associated energy barrier of 52.6 kJ mol−1. This step is suggested to occur via a concerted metalation–deprotonation pathway. Subsequent transfer of the hydrogen atom to the carbonyl group of the Boc moiety generates intermediate Int2, which can tautomerize to the more stable amide isomer Int3. At this stage, palladation of the heteroaryl partner—such as N-methylindole—can take place, giving rise to complex Int4 (Scheme 65).50
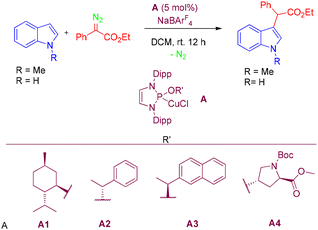
In 2024, Pérez and co-workers reported a selective C–H functionalization of unprotected indoles via donor–acceptor carbene insertion.51 Using copper catalysts bearing an alkoxy diaminophosphine (ADAP) ligand, the methodology enabled highly selective C3–H functionalization of indoles through carbene transfer from donor–acceptor diazo compounds, while leaving the N–H bond intact throughout the transformation (Scheme 66).51
Mechanistic investigations, supported by DFT calculations, suggest the presence of two competing pathways, neither of which proceeds via cyclopropane intermediates. The computational results further indicate that the reaction mechanism deviates from those proposed in earlier studies.51
8. Conclusions
In this review, we have summarized recent advances in the direct C–H functionalization of heteroaromatic rings, with a brief discussion of reaction patterns and mechanistic insights. The main content covers the direct functionalization of pyridine, thiophene, pyrrole, and azole heterocycles, as well as quinolone and indole derivatives. We believe that C–H bond activation for the direct functionalization of heteroaromatic rings represents a highly promising area of research. Our group has published numerous research papers in the field of C–H bond functionalization,52 making significant contributions to the functionalization of heteroaromatic rings and achieving notable results.8,16,17Although remarkable progress has been made in this area, several challenges and open questions remain at the forefront of current research:
1. Most reported C–H bond functionalization reactions require relatively high catalyst loadings (1–10 mol%). Additionally, high reaction temperatures, strong acidic conditions, and the use of powerful oxidizing agents often limit the scalability and practical application of these transformations.
2. In many cases, low product yields or poor functional group tolerance continue to hinder broader industrial applications.
3. Furthermore, greater attention is needed in the development of efficient and convenient asymmetric methods for the synthesis of chiral compounds with high enantioselectivity. To date, enantioselective C–H functionalization of heteroaromatic rings remains limited due to the challenges associated with controlling the stereochemistry of reactive and unstable radical intermediates.
Addressing these issues may require the development of new approaches that strike a balance between reactivity, stability, and stereocontrol. Clearly, further development of practical, highly controllable catalytic systems and methodologies for the direct C–H functionalization of heteroaromatic rings is highly desirable.
Conflicts of interest
There are no conflicts to declare.Notations and abbreviations
| Bn | Benzyl |
| B2pin2 | Bis(pinacolato)diboron |
| Bpy | Bipyridine |
| Bu | Butyl |
| nBuOAc | n-Butylacetate |
| dap | 2,9-Bis(p-anisyl)-1,10-phenanthroline |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| DCC | Dicyclohexylcarbodiimide |
| DCE | 1,2-Dichloroethane |
| DMF | N,N-Dimethylformamide |
| DEMPO | 2,2,6,6-Tetramethylpiperidine |
| DFT | Density functional theory |
| DLP | Dilauroyl peroxide |
| DIPEA | Diisopropylethylamine |
| DME | 1,2-Dimethoxyethane |
| DMSO | Dimethylsulfoxide |
| DTBP | Di-t-butyl peroxide |
| Equiv. | Equivalent |
| Et3N | Trimethylamine |
| Et | Ethyl |
| [Fe(Pc)] | Iron phthalocyanine |
| HAT | Hydrogen atom transfer |
| HOAc | Acetic acid |
| KOAc | Potassium acetate |
| Me | Methyl |
| NBS | N-Bromosaccharin |
| NFSI | N-Fluorobenzenesulfonimide |
| Nu | Nucleophile |
| OAc | Acetate |
| Ph | Phenyl |
| PDI | Perylene diimide |
| Phen | 1,10-Phenanthroline |
| PIDA | (Diacetoxy)iodobenzene |
| Ppy | 2-Phenylpyridine |
| i-PrOH | Isopropanol |
| SET | Single-electron transfer |
| TATB | 1,3,5-Triamino-2,4,6-trinitrobenzene |
| TBADT | Tetrabutylammonium decatungstate |
| TBATB | Tetrabutylammonium tribromide |
| TBHP | t-Butylhydroperoxide |
| TBPB | tert-Butyl peroxybenzoate |
| TBHP | t-Butylhydroperoxide |
| TEMPO | 2,2,6,6-Tetramethylpiperidine |
| TMEDA | N,N,N′,N′-Tetramethyl-1,2-ethanediamine |
| TMS | Trimethylsilyl |
| THF | Tetrahydrofuran |
| Tf | Trifluoromethyl |
| TFA | Trifluoroacetic |
| TIPS | Triisopropylsilyl |
| p-TsOH | p-Methylbenzene sulfonic acid |
| TTBP | 2,4,5-Tri-tert-butylpyrimidine |
Data availability
No primary research results, software or code have been included, and no new data were generated or analysed as part of this review.References
- (a) H. Guo and K. Ding, Tetrahedron Lett., 2000, 41, 10061 CrossRef CAS; (b) X. Shen, H. Guo and K. Ding, Tetrahedron: Asymmetry, 2000, 11, 4321 CrossRef CAS; (c) H.-J. Deussen, E. Hendrickx, C. Boutton, D. Krog, K. Clays, K. Bechgaard, A. Persoons and T. Bjørnholm, J. Am. Chem. Soc., 1996, 118, 6841 CrossRef CAS.
- (a) M. Rueping, B. J. Nachtsheim and W. Ieawsuwan, Adv. Synth. Catal., 2006, 348, 1033 CrossRef CAS; (b) I. Iovel, K. Mertins, J. Kischel, A. Zapf and M. Beller, Angew. Chem., Int. Ed., 2005, 44, 3913 CrossRef CAS.
- J. Majer, P. Kwiatkowski and J. Jurczak, Org. Lett., 2009, 11, 4636 CrossRef CAS PubMed.
- T. T. Tung, S. B. Christensen and J. Nielsen, Difluoroacetic Acid as a New Reagent for Direct C-H Difluoromethylation of Heteroaromatic Compounds, Chem. – Eur. J., 2017, 23, 18125–18128 CrossRef CAS.
- D. D. Bume, C. R. Pitts and T. Lectka, Tandem C–C Bond Cleavage of Cyclopropanols and Oxidative Aromatization by Manganese(IV) Oxide in a Direct C–H to C–C Functionalization of Heteroaromatics, Eur. J. Org. Chem., 2016, 26–30 CrossRef CAS.
- Y. Ji, T. Brueckl, R. D. Baxter, Y. Fujiwara, I. B. Seiple, S. Su, D. G. Blackmond and P. S. Baran, Innate C-H Trifluoromethylation of Heterocycles, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 14411–14415 CrossRef CAS PubMed.
- T. Yamada, K. Tanaka III, Y. Hashimoto, N. Morita and O. Tamura, Electrophilic C3 H Alkenylation of 2,6-Dialkoxypyridine Derivatives via Pd(II)/Tl(III) Reaction System, Adv. Synth. Catal., 2023, 365, 3138–3148 CrossRef CAS.
- Y. H. Wu, N. X. Wang, T. Zhang, L. Y. Zhang, X. W. Gao, B. C. Xu, Y. Xing and J. Y. Chi, Rare-Earth Y(OTf)3Ca talyzed Coupling Reaction of Ethers with Azaarenes, Org. Lett., 2019, 21, 7450–7454 CrossRef CAS.
- M. M. Shoshani, Cooperative Heterometallic Platforms Enabling Selective C–H Bond Activation and Functionalization of Pyridines, Cell Rep. Phys. Sci., 2023, 4, 101213–101224 CrossRef CAS.
- K. Murakami, S. Yamada, T. Kaneda and K. Itami, C−H Functionalization of Azines, Chem. Rev., 2017, 117, 9302–9332 CrossRef CAS.
- L. Liu, M. Cordier, T. Roisnel and H. Doucet, Double C–H Bond Functionalization for C–C Coupling at the β-position of Thiophenes Using Palladium-Catalyzed 1,4-Migration Associated with Direct Arylation, Org. Chem. Front., 2023, 10, 1441–1455 RSC.
- J. F. Campos, M.-J. R. P. Queiroz and S. Berteina-Raboin, The First Catalytic Direct C–H Arylation on C2 and C3 of Thiophene Ring Applied to Thieno-Pyridines, -Pyrimidines and –Pyrazines, Catalysts, 2018, 8, 137–151 CrossRef.
- M. Lee, Y. K. Hwang and J. Kwak, Ag(I)-Catalyzed C−H Carboxylation of Thiophene Derivatives, Organometallics, 2021, 40, 3136–3144 CrossRef CAS.
- A. Mandal, R. Bera and M. Baidya, Regioselective C−H Alkenylation and Unsymmetrical Bis-olefination of Heteroarene Carboxylic Acids with Ruthenium Catalysis in Water, J. Org. Chem., 2021, 86, 62–73 CrossRef CAS PubMed.
- M. H. Daniels, J. R. Armand and K. L. Tan, Sequential Regioselective C−H Functionalization of Thiophenes, Org. Lett., 2016, 18, 3310–3313 CrossRef CAS PubMed.
- Z. Huang, J. Zhang, Y. Zhou and N. X. Wang, Enantioselective Friedel–Crafts Alkylation of Thiophenes with Ethyl Glyoxylate: Easy Access to Chiral Secondary Alcohols, Eur. J. Org. Chem., 2011, 843–847 CrossRef CAS.
- T. Zhang, N. X. Wang, Y. H. Wu, Z. Yan, Y. Xing, J. L. Wen and X. W. Gao, Direct alkylation of thiophenesviabis-coupling with vinyl acetates, Tetrahedron Lett., 2018, 59, 4525–4527 CrossRef CAS.
- G. Sun, S. Ren, X. Zhu, M. Huang and Y. Wan, Direct Arylation of Pyrroles via Indirect Electroreductive C−H Functionalization Using Perylene Bisimide as an Electron-Transfer Mediator, Org. Lett., 2016, 18, 544–547 CrossRef CAS PubMed.
- I. Ghosh, T. Ghosh, J. I. Bardagi and B. König, Reduction of Aryl Halides by Consecutive Visible Light-Induced Electron Transfer Processes, Science, 2014, 346, 725–728 CrossRef CAS PubMed.
- L. Marzo, I. Ghosh, F. Esteban and B. König, Metal-Free Photocatalyzed Cross Coupling of Bromoheteroarenes with Pyrroles, ACS Catal., 2016, 6, 6780–6784 CrossRef CAS.
- N. Iqbal, S. Choi, E. Ko and E. J. Cho, Trifluoromethylation of Heterocycles via Visible Light Photoredox Catalysis, Tetrahedron Lett., 2012, 53, 2005–2008 CrossRef.
- W. J. Choi, S. Choi, K. Ohkubo, S. Fukuzumi, E. J. Cho and Y. You, Mechanisms and Applications of Cyclometalated Pt(Ii) Complexes in Photoredox Catalytic Trifluoromethylation, Chem. Sci., 2015, 6, 1454–1464 RSC.
- T. Shimada, S. Shigeki Mori, M. Masatoshi Ishida and H. Furuta, Regioselectively α- and β-alkynylated BODIPY dyes via gold(I)-catalyzed direct C–H functionalization and their photophysical properties, Beilstein J. Org. Chem., 2020, 16, 587–595 CrossRef CAS.
- S. J. Han, H. T. Kim and J. M. Joo, Direct C−H Alkenylation of Functionalized Pyrazoles, J. Org. Chem., 2016, 81, 689–698 CrossRef CAS PubMed.
- H.-Q. Do, R. M. K. Khan and O. Daugulis, A General Method for Copper-Catalyzed Arylation of Arene C−H Bonds, J. Am. Chem. Soc., 2008, 130, 15185–15192 CrossRef CAS PubMed.
- K. A. Margrey, J. B. McManus, S. Bonazzi, F. Zecri and D. A. Nicewicz, Predictive Modelfor Site-Selective Aryl and Heteroaryl C−H Functionalization via Organic Photoredox Catalysis, J. Am. Chem. Soc., 2017, 139, 11288–11299 CrossRef CAS PubMed.
- K. Monir, A. K. Bagdi, M. Ghosh and A. Hajra, Regioselective Oxidative Trifluoromethylation of Imidazoheterocycles via C(sp2)–H Bond Functionalization, J. Org. Chem., 2015, 80, 1332–1337 CrossRef CAS PubMed.
- G. Yin, M. Zhu and W. Fu, Visible-Light Mediated Regioselective (Phenylsulfonyl)-difluoromethylation of fused Imidazoles with Iododifluoromethyl Phenyl Sulfone, Heterocycl. Commun., 2017, 23, 275–279 CrossRef CAS.
- M. Khoshneviszadeh, M. Soheilizad, M. Fardpour and M. Mahdavi, An Efficient Access to 2,3-Diarylimidazo[1,2-a]pyridines via Silver(I)-catalyzed C-H Bond Functionalization, Monatsh. Chem., 2017, 148, 1817–1821 CrossRef CAS.
- Y. Gao, W. Lu, P. Liu and P. Sun, Iron-Catalyzed, Regioselective Alkoxycarbonylation of Imidazoheterocycles with Carbazates, J. Org. Chem., 2016, 81, 2482–2487 CrossRef CAS PubMed.
- S. Mondal, S. Samanta, S. Jana and A. Hajra, (Diacetoxy)iodobenzene-Mediated Oxidative C–H Amination of Imidazopyridines at Ambient Temperature, J. Org. Chem., 2017, 82, 4504–4510 CrossRef CAS.
- S. Wang, S. Xu, C. Cheng Yang, H. Sun and J. Wang, Formal Carbene C−H Bond Insertion in the Cu(I)-Catalyzed Reaction of Bis(trimethylsilyl)diazomethane with Benzoxazoles and Oxazoles, Org. Lett., 2019, 21, 1809–1812 CrossRef CAS PubMed.
- I. I. F. Boogaerts and S. P. Nolan, Carboxylation of C-H Bonds UsingN-Heterocyclic Carbene Gold(I) Complexes, J. Am. Chem. Soc., 2010, 132, 8858–8859 CrossRef CAS.
- F. Hu, J. Yang, Y. Xia, C. Ma, H. Xia, Y. Zhang and J. Wang, C−H Bond Functionalization of Benzoxazoles with Chromium(0) Fischer Carbene Complexes, Organometallics, 2016, 35, 1409–1414 CrossRef CAS.
- J. L. Bon, D. Feng, S. R. Marder and S. B. Blakey, A C−H Functionalization Protocol for the Direct Synthesis of Benzobisthiazole Derivatives, J. Org. Chem., 2014, 79, 7766–7771 CrossRef CAS.
- P. D. E. Arche, S. Chatterjee, M. M. Talukder, J. T. Miller, J. M. O. Cue, C. M. U. Gedara, R. L. Lord, M. C. Biewer, G. A. Cisneros and M. C. Stefan, Regioselective Direct C−H Bond Heteroarylation of Thiazoles Enabled by an Iminopyridine-Based αDiimine Nickel(II) Complex Evaluated by DFT Studies, J. Org. Chem., 2023, 88, 12319–12328 CrossRef CAS PubMed.
- M. R. Lonka, J. Zhang, T. Gogula and H. Zou, Copper(I)-Catalyzed Benzylation of Triazolopyridine Through Direct C–H Functionalization, Org. Biomol. Chem., 2019, 17, 7455–7460 RSC.
- (a) J. M. Joo, P. Guo and D. Sames, C–H Bonds as Ubiquitous Functionality: Preparation of Multiple Regioisomers of Arylated 1,2,4-Triazoles via C–H Arylation, J. Org. Chem., 2013, 78, 738–743 CrossRef CAS PubMed; (b) R. Goikhman, T. L. Jacques and D. Sames, C−H Bonds as Ubiquitous Functionality: A General Approach to Complex Arylated Pyrazoles via Sequential Regioselective C-Arylation and N-Alkylation Enabled by SEM-Group Transposition, J. Am. Chem. Soc., 2009, 131, 3042–3048 CrossRef CAS; (c) J. M. Joo, B. B. Toure and D. Sames, C−H Bonds as Ubiquitous Functionality: A General Approach to Complex Arylated Imidazoles via Regioselective Sequential Arylation of All Three C−H Bonds and Regioselective N-Alkylation Enabled by SEM-Group Transposition, J. Org. Chem., 2010, 75, 4911–4920 CrossRef CAS.
- K. D. B. Yamajala, M. Patil and S. Banerjee, Pd-Catalyzed Regioselective Arylation on the C-5 Position of N-Aryl 1,2,3-Triazoles, J. Org. Chem., 2015, 80, 3003–3011 CrossRef CAS PubMed.
- J. Zhu, Y. Kong, F. Lin, B. Wang, Z. Chen and L. Liu, Copper-Catalyzed Direct Amination of 1,2,3-Triazole N-Oxides by C–H Activation and C–N Coupling, Eur. J. Org. Chem., 2015, 1507–1515 CrossRef CAS.
- Z. Peng, C. Yu, Y. Wang, D. Wei and C. Jiang, Direct C−H Arylation and Alkylation of Electron-Deficient Heteroaromatic Compounds with Organozinc Reagents, Organometallics, 2021, 40, 3678–3688 CrossRef CAS.
- R. A. Garza-Sanchez, A. Tlahuext-Aca, G. Tavakoli and F. Glorius, Visible Light-Mediated Direct Decarboxylative C−H Functionalization f Heteroarenes, ACS Catal., 2017, 7, 4057–4061 CrossRef CAS.
- J. Genovino, Y. Lian, Y. Zhang, T. O. Hope, A. Juneau, Y. Gagné, G. Ingle and M. Frenette, Metal-Free-Visible Light C−H Alkylation of Heteroaromatics via Hypervalent Iodine-Promoted Decarboxylation, Org. Lett., 2018, 20, 3229–3232 CrossRef CAS PubMed.
- M. C. Quattrini, S. Fujii, K. Yamada, T. Fukuyama, D. Ravelli, M. Fagnoni and I. Ryu, Versatile Cross-Dehydrogenative Coupling of Heteroaromatics and Hydrogen Donorsvia Decatungstate Photocatalysis, Chem. Commun., 2017, 53, 2335–2338 RSC.
- X. Cao, L. Wei, J. Yang, H. Huanhuan Song and Y. Wei, A Visible-Light-Induced Bromine Radical Initiates Direct C–H Alkylation of Heteroaromatics, Org. Biomol. Chem., 2024, 22, 1157–1161 RSC.
- D. X. Zhu and M. H. Xu, Rhodium(I)-Catalyzed Direct Enantioselective C−H Functionalization of Indoles, J. Org. Chem., 2023, 88, 7844–7848 CrossRef CAS PubMed.
- T. Liu, W. Hou and J. Wu, Palladium-Catalyzed Direct C−H Functionalization of Indoles with the Insertion of Sulfur Dioxide: Synthesis of 2Sulfonated Indoles, Org. Lett., 2017, 19, 6638–6641 CrossRef CAS PubMed.
- Y. Pang, T. Ishiyama, K. Kubota and H. Ito, Iridium(I)-Catalyzed C-H Borylation in Air by Using Mechanochemistry, Chem. – Eur. J., 2019, 25, 4654–4659 CrossRef CAS PubMed.
- L. Furst, B. S. Matsuura, J. M. R. Narayanam, J. W. Tucker and C. R. J. Stephenson, Visible Light-Mediated Intermolecular C-H Functionalization of Electron-Rich Heterocycles with Malonates, Org. Lett., 2010, 12, 3104–3107 CrossRef CAS.
- K. Plevová, P. Kisszékelyi, D. DenisaVargová, S. Andrejčák, V. Tóth, L. Fertáľ, E. Rakovský, J. Filo and R. Šebesta, Diastereoselective Double C-H Functionalization of Chiral Ferrocenes with Heteroaromatics, Chem. – Eur. J., 2021, 27, 15501–15507 CrossRef.
- J. D. Pizarro, L. Morán-González, I. González-Fernández, F. Maseras, M. R. Fructos and O. J. Pérez, Selective C-H Bond Functionalization of Unprotected Indoles by Donor-Acceptor Carbene Insertion, Adv. Synth. Catal., 2024, 366, 844–851 CrossRef.
- (a) J. X. Zhang, Y. J. Wang, W. Wei Zhang, N. X. Wang, C. B. Bai, Y. Xing, Y. Li and J. L. Jia-Long Wen, Selective Nickel- and Manganese-Catalyzed Decarboxylative Cross Coupling of Some α,β-Unsaturated Carboxylic Acids with Cyclic Ethers, Sci. Rep., 2014, 4, 7446–7451 CrossRef PubMed; (b) W. Zhang, N. X. Wang, C. B. Bai, Y. J. Wang, X. W. Lan, Y. Xing, Y. H. Li and J. L. Wen, Manganese-Mediated Coupling Reaction of Vinylarenes and Aliphatic Alcohols, Sci. Rep., 2015, 5, 15250 CrossRef PubMed; (c) X. W. Lan, N. X. Wang, W. Zhang, J. L. Wen, C. B. Bai, Y. Xing and Y. H. Li, Copper/Manganese Cocatalyzed Oxidative Coupling of Vinylarenes with Ketones, Org. Lett., 2015, 17, 4460–4463 CrossRef CAS; (d) X. W. Lan, N. X. Wang, C. B. Bai, C. L. Lan, T. Zhang, S. L. Chen and Y. Xing, Unactivated C(sp3)−H Bond Functionalization of Alkyl Nitriles with Vinylarenes and Mechanistic Studies, Org. Lett., 2016, 18, 5986–5989 CrossRef CAS; (e) T. Zhang, X.-W. Lan, Y.-Q. Zhou, N. X. Wang, Y.-H. Wu, Y. Xing and J. L. Wen, C(sp3)–H Bond Functionalization of Non-Cyclic Ethers by Decarboxylative Oxidative Coupling with α,β-Unsaturated Carboxylic Acids, Sci. China: Chem., 2018, 61, 180–183 CrossRef CAS; (f) Z. Yan, N. X. Wang, X. W. Gao, J. L. Li, Y. H. Wu, T. Zhang, S. L. Chen and Y. Xing, A Copper(II) Acetate Mediated Oxidative-Coupling of Styrenes and Ethers Through an Unactivated C(sp3)-H Bond Functionalization, Adv. Synth. Catal., 2019, 361, 1007–1011 CrossRef CAS; (g) Z. Yan, N. X. Wang, L. Y. Zhang, Y. H. Wu, J. L. Li, M. Y. She, X. W. Gao, K. Feng and Y. Xing, The C(sp3)–H Bond Functionalization of Thioethers with Styrenes with Insight into the Mechanism, Org. Biomol. Chem., 2022, 20, 5845–5851 RSC.
| This journal is © The Royal Society of Chemistry 2026 |