Elucidating the mechanism, origin of regioselectivity and substrate scope of the synthesis of structurally diverse 1,2,4-triazoles via a rare type of diazo compound reactivity
Roman
Kuznetsov
,
Grigory
Kantin
 ,
Alexander
Sapegin
,
Alexander
Sapegin
 ,
Mikhail
Novikov
,
Mikhail
Novikov
 and
Dmitry
Dar'in
and
Dmitry
Dar'in
 *
*
Department of Medicinal Chemistry, Institute of Chemistry, Saint Petersburg State, University, 26 Universitetskiy pr., Saint Petersburg, 198504, Russian Federation. E-mail: d.dariin@spbu.ru
First published on 26th November 2025
Abstract
The 1,2,4-triazole scaffold plays a significant role in drug development, and efficient methods for synthesizing its derivatives are in high demand. In this work, we propose a convenient approach for the synthesis of polysubstituted 1H-1,2,4-triazoles under mild, metal-free conditions in a highly regioselective manner. The reaction proceeds with high yields and demonstrates tolerance towards a broad range of functional groups. Unlike previously reported methods, this approach does not require the prior preparation of azlactones and is effective with a wide variety of diazo reagents. A reaction mechanism supported by DFT calculations is proposed, and pathways for further transformation of functionalized 1,2,4-triazole derivatives are demonstrated.
Introduction
1,2,4-Triazoles, a class of polynitrogen aromatic five-membered heterocycles, are among the most highly sought-after scaffolds in the fields of medicinal chemistry and drug discovery.1 Molecules containing the 1,2,4-triazole motif exhibit a wide range of pharmacological activities. In recent years alone, numerous studies have demonstrated the active application of this heterocyclic scaffold in medicinal chemistry through the development of hit and lead compounds as galectin-3 inhibitors,2 dual carbonic anhydrase and VEGFR-2 inhibitors,3 neuroprotectants against cerebral ischemic injury,4 and molecules exhibiting anticancer,5 anti-inflammatory,6 anti-allergic,7 antifungal,8 antitubercular9 and anti-HIV10 activity. A more comprehensive overview of the biological properties of 1,2,4-triazole derivatives and their applications in medicinal chemistry can be found in several recent review articles.11To date, many important commercialized drug molecules containing the 1,2,4-triazole motif have been developed. Examples of these pharmaceuticals include letrozole (anticancer), anastrazole (anticancer), ribavirin (antiviral), voriconazole (antifungal), etizolam (anxiolytic),12 paclobutrazol (plant growth regulator),13 and fuzuloparib (anticancer) (Fig. 1).14
In view of such a high demand for 1,2,4-triazoles in drug design and other areas, methods for obtaining structurally diverse derivatives based on transformations of various types are actively being developed. Recently, a number of publications have reported approaches to synthesize 1,2,4-triazoles based on [3 + 2] cycloaddition reactions of nitrilimines15 and amidrazones,16 recyclizations,17 and electrochemical,18 ionic-liquid19 and visible-light20 promoted cyclizations. Among modern methods, approaches based on the use of diazo reagents in combination with arene diazonium salts, proceeding under copper21 or gold22 catalysis, or even in the absence of any catalyst,23 are notable. It should be noted, however, that most of the currently proposed methods are primarily focused on the synthesis of N-arylated derivatives and generally do not allow for extensive variation in the nature of the substituent at the nitrogen atom. A detailed examination of various synthetic approaches is presented in recently published reviews.24
In 2019, H.-W. Zhao and co-workers reported a reaction between diazooxindoles and oxazol-5-(4H)-ones (azlactones),25 representing a rare example of a diazo compound transformation involving solely nitrogen atoms (without the carbon atom of the diazo group) in the formation of a heterocycle (1,2,4-triazole) (Scheme 1B). Typically, diazo compounds are employed in organic synthesis – particularly for heterocycle construction26 – as sources of reactive carbenes (or metallocarbenoids) generated through thermal, photolytic, or catalytic decomposition of the diazo reagent. In addition, reactions involving retention of the dinitrogen fragment, in which the diazo compound acts as a 1,3-N,N,C-dipole, forming cycloaddition products (e.g. pyrazoles,27 1,2,3-triazoles,28 and tetrazoles29) with dipolarophiles of various nature are well known and widely documented in the literature (Scheme 1A). Conversely, reactions that proceed without loss of dinitrogen – where only the N–N fragment is incorporated into the assembled heterocyclic core – are limited to only a few examples (Scheme 1B).30
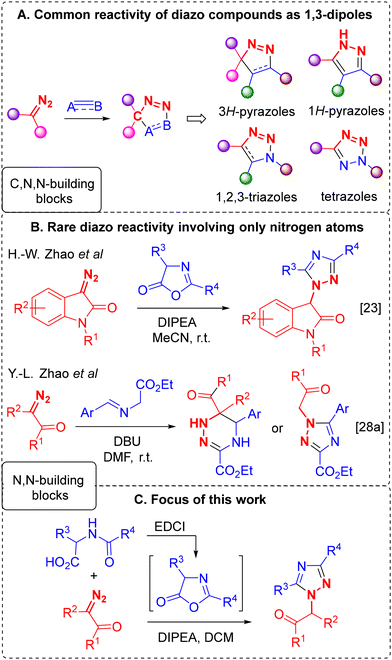 | ||
| Scheme 1 Reactions of diazo compounds without nitrogen loss leading to the formation of heterocycles. | ||
The reaction discovered by H.-W. Zhao et al. attracted our attention as a potentially powerful method, allowing independent variation of substituents at three positions of the 1,2,4-triazole ring. This transformation is characterized by absolute regioselectivity, allowing one to obtain a given regioisomer with a specific mutual arrangement of substituents. However, in order for this reaction to become a general method, it is necessary to address a number of important issues: (1) only one class of diazo compounds, namely diazooxindoles, are capable of participating in this reaction, while negative results were obtained for other tested diazo reagents, which greatly limits the application scope of the reaction; (2) the range of substituents in the azlactones used is limited, and the influence of the substitution nature on the reaction outcome has not been studied in detail; and (3) last but not least, the reaction mechanism is unknown and, as a consequence, there is no explanation for the regioselectivity of the process, as well as the unsuitability of α-unsubstituted azlactones for the reaction, which is one of the obvious limitations of the method.
It should be noted that during the course of our research, an article by W.-Y. Yu and colleagues was published, which can be regarded as an extension of this approach, utilizing activated esters of N-acetyl amino acids.31 However, in that study, only limited variation of substituents at positions 3 and 5 of the final 1,2,4-triazole was examined, the applicability boundaries of both types of substrates were not thoroughly investigated, and a credible and well-supported mechanistic pathway involving azlactones was not presented.
Considering the high demand for 1,2,4-triazoles in various fields of chemistry and medicine and recognizing the importance of developing methods that enable access to a wide range of novel 1,2,4-triazole derivatives, we undertook a comprehensive investigation of this new transformation. In the present work, we demonstrate the feasibility of synthesizing the target 1,2,4-triazoles directly from hippuric acids without the preliminary isolation of azlactones, thereby significantly expanding the scope of the reaction with respect to these substrates (Scheme 1C). Additionally, we showcase the versatility of the method towards the diazo reagents employed. A plausible mechanistic pathway is proposed, supported by DFT calculations, which elucidate the high regioselectivity observed in the process. Finally, we explore the potential applications of the functionalized products in various inter- and intramolecular transformations, thereby enabling access to an increased structural diversity of 1,2,4-triazole derivatives.
Results and discussion
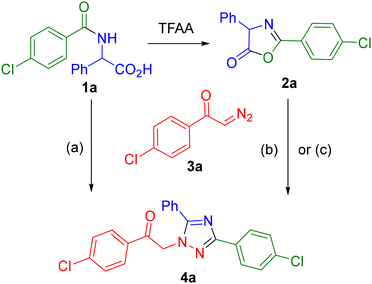
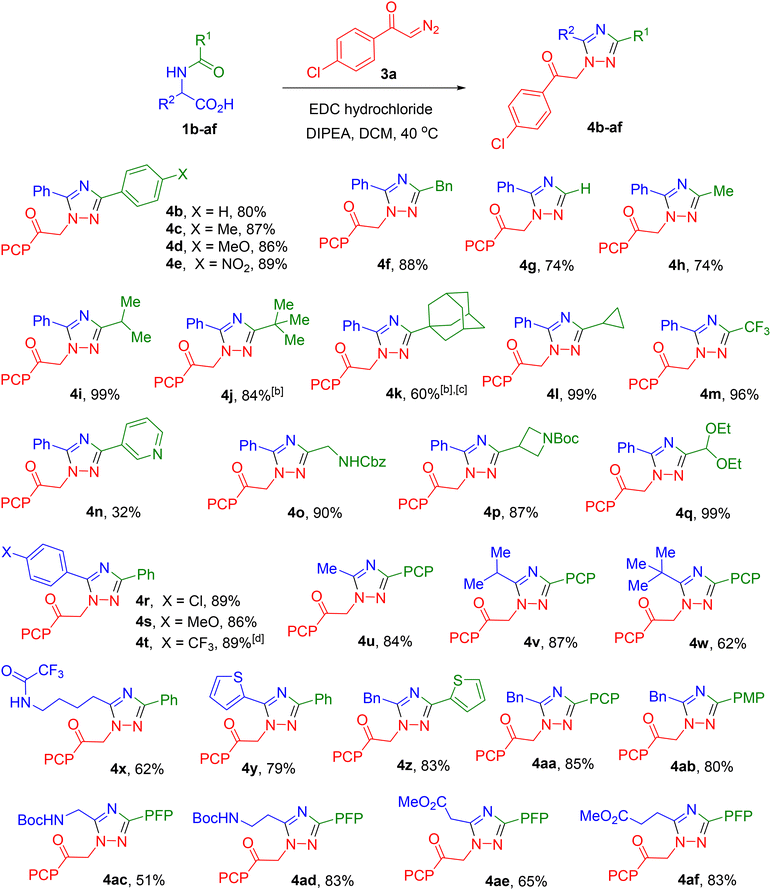
One of the significant advantages of the developed method is the accessibility of the starting compounds. Indeed, a wide variety of α-amino acids are readily available both from commercial sources and through synthetic routes. A second dimension of structural diversity is introduced via N-acylation of the amino acid. Diazo reagents, whose chemistry is currently undergoing active development, are also synthetically accessible in a broad range of variants. The ease of obtaining many of these compounds is associated with the use of previously developed protocols for diazo transfer in aqueous media.32Initially, we synthesized azlactones (e.g., 2a) for use in the target reaction by subjecting N-acylated α-amino acids (e.g., 1a) to dehydrative cyclization in the presence of trifluoroacetic anhydride (Scheme 2). As a model diazo reagent, p-chloro-α-diazoacetophenone33 (3a) was employed. The selection of the conditions for the target transformation, in particular the use of DIPEA as a base, was largely based on the results of previous studies.25 However, dichloromethane (DCM) was evaluated as the solvent in place of acetonitrile and gave excellent results in the model reaction (Scheme 2, conditions (b)). This procedural modification permits direct chromatographic purification of the reaction mixture without prior work-up. Since full conversion at room temperature required more than 24 h, the reaction was carried out under moderate heating (40 °C), which in most cases (vide infra) afforded complete conversion of the diazo reagent within 6–18 h. We were surprised to find that the reaction could proceed even in the absence of a base, albeit at an elevated temperature (130 °C). However, the yield of product 4a was significantly lower (Scheme 2, conditions (c)).
As previously demonstrated,25 the use of an excess of azlactone enhances the yield of the 1,2,4-triazole product. Through our investigation, we have established that this phenomenon is likely associated with the gradual degradation of the starting azlactone in the presence of a base. We also compared the results of several experiments involving variations in azlactone loading and concluded that to achieve the maximum yield of the target 1,2,4-triazole, a twofold excess of azlactone should be employed. Of course, in each specific case, this excess can be optimized if desired, as the stability of azlactone varies significantly depending on the nature of its substituents. For example, in the case of certain compounds bearing aryl or bulky alkyl substituents, we observed the presence of residual azlactone after the completion of the reaction. In contrast, for most of the starting materials, this excess reagent appeared to simply disappear “without a trace”, presumably undergoing oligomerization.
We hypothesized that the conversion of acid 1 to the corresponding azlactone 2 could be carried out directly under the conditions of the target reaction using a milder dehydrating agent. For this purpose, the water-soluble carbodiimide (EDC hydrochloride) was selected (Scheme 2, conditions (a)). Its use facilitates easy removal of the byproduct (urea) either by aqueous work-up or by flash chromatography. A test reaction involving acid 1a and diazo ketone 3a under the standard conditions afforded a high yield of the target triazole 4a, even slightly exceeding the yield previously obtained using the azlactone. This synthetic procedure eliminates the need for the prior preparation and storage of azlactones, which in some cases exhibit only moderate or even reduced stability.
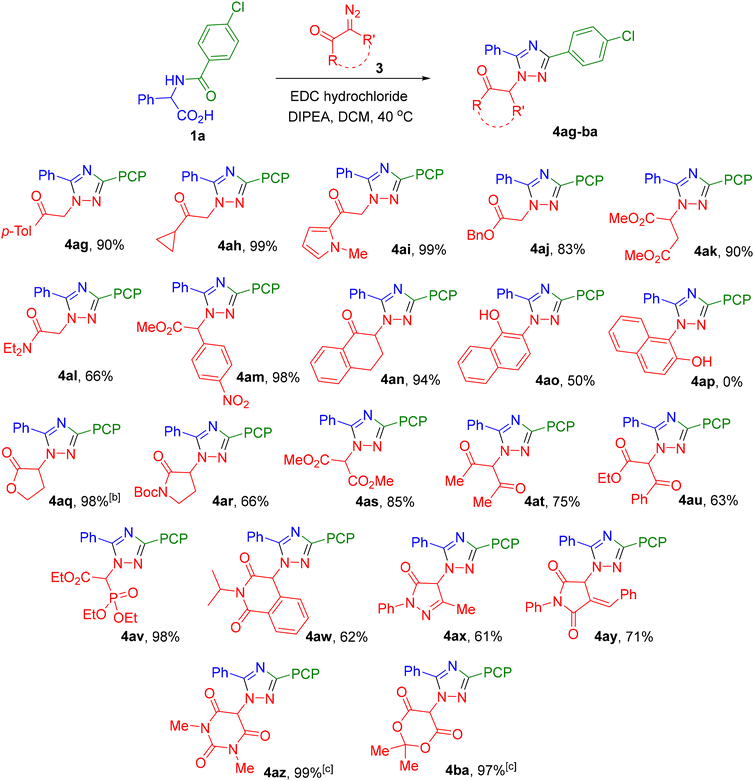
Subsequently, all syntheses were carried out without isolating azlactone 2, using N-acylated amino acids 1 as the starting materials. In the initial stage, under the standard reaction conditions, the influence of substituents in substrate 1 (i.e., at various positions of the intermediate azlactone 2) was investigated. The R1 substituent, corresponding to the fragment of the respective carboxylic acid or acid chloride, was varied over a rather wide range (Table 1). It was demonstrated that both aryl and alkyl substituents bearing groups of different nature – including protected functional groups such as protected amine (4o and 4p) and acetal (4q) – could be successfully employed in the synthesis. It should be noted that we observed the influence of steric factors, especially when using starting compounds with tert-butyl (4j) and adamantyl (4k) substituents. However, this primarily affected the time required to reach complete conversion (3 days instead of 18 hours), rather than the yield of the final product. Some decrease in yield was observed when using substrates with formyl (4g), acetyl (4h), and especially nicotinoyl (4n) groups.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| PCP – p-chlorophenyl, PMP – p-methoxyphenyl, PFP – p-fluorophenyl.a Reaction conditions: 0.4 mmol of diazo ketone 3a, 2 equiv. of acid 1, 2.25 equiv. of EDC hydrochloride, and 1 equiv. of DIPEA in dry DCM (3 mL) at 40 °C for 6–20 h.b The synthesis was carried out for 4 days.c The corresponding azlactone (2k) was used as the starting material.d Structure confirmed using single crystal X-ray analysis data. |
|---|
|
It was found that the nature of the α-substituent R2 exerts negligible influence on the yield of the target product. Specifically, triazoles bearing both alkyl and aryl substituents with either electron-donating or electron-withdrawing groups were obtained in high yields (Scheme 3). A slight decrease in yield was observed for derivatives of α-tert-butyl glycine (4w), lysine (4x), β-BocNH alanine (4ac), and aspartic acid (4ae). It should be noted that in the first case (4w), despite the presence of a bulky substituent, no reduction in the reaction rate was observed. The differing influence of the steric factors of substituents R1 and R2 (4j and 4kvs.4w) will be further elucidated upon discussion of the reaction mechanism (vide infra).
Interestingly, by using this method and selecting substituents R1 and R2 in the starting acid 1, it is possible to selectively obtain two regioisomeric 1,2,4-triazole derivatives, as demonstrated by the examples of products 4a and 4r.
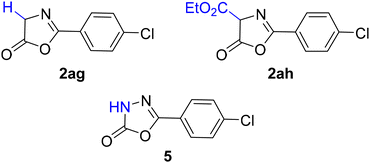
With certain substrates, namely glycine derivative 2ag, ester derivative 2ah, and an aza-analogue (oxadiazolone 5), the target products could not be obtained (Fig. 2). In the latter two cases, the reaction did not proceed under the standard conditions. In the case of substrate 2ag, after complete conversion of the azlactone, only the starting diazo ketone was detected in the reaction mixture; however, according to NMR data with an internal standard, the conversion of the diazo reagent was approximately 40%. A possible explanation is the rapid degradation of azlactone 2ag in the presence of a base. α-Substituted azlactones also decompose under basic conditions; however, they do so at a significantly slower rate, allowing them to participate in the reaction with the diazo compound. In certain cases – particularly with sterically hindered substituents (products 4v and 4w) – the residual azlactone was observed after complete conversion of the diazo reagent. In most cases, however, the excess azlactone degraded over the course of the reaction, converting into unidentified side products that could be readily separated from the desired product using flash chromatography.
Another reason for the negative outcome of the reaction involving the α-unsubstituted azlactone 2ag is related to the possible mechanism of the examined transformation (vide infra). It lies in the irreversibility of the addition of the diazo reagent at the α-position, which may lead to the formation of a hydrazone (a process impossible in the presence of an α-substituent). This hydrazone appears to be unstable under the reaction conditions and consequently decomposes. This explains the partial conversion of diazo ketone 3a in the reaction with azlactone 2ag, without the formation of any detectable products.
In a preceding work,25 it was reported that the reaction of azlactones with diazo reagents other than diazooxindoles (for example, diazobarbituric acid or α-diazo-β-keto-phosphonate) does not occur. However, we have demonstrated that this method exhibits considerable versatility and does not present substantial limitations in terms of the scope of diazo compounds. The transformation proceeds readily, yielding the target triazoles from a variety of diazo ketones as well as diazo esters and amides, featuring both terminal and internal diazo groups (Table 2). Furthermore, products with good to high yields were obtained using diazo compounds stabilized by two electron-withdrawing groups, such as dimethyl diazomalonate (4as), diazo diketone (4at), diazo ketoester (4au), and diazo ketophosphonate (4av). A wide range of various diazo heterocycles were also successfully engaged in the transformation, affording 1,2,4-triazole derivatives bearing a heterocyclic moiety at the N-1 atom (4aw-ba). Isomeric diazo naphthalenones exhibited different reactivities. Product 4ao, containing a hydroxy-naphthyl fragment, was isolated in 50% yield only in the case of 2-diazonaphthalen-1(2H)-one, whereas the use of 1-diazonaphthalen-2(1H)-one did not lead to the formation of the corresponding triazole 4ap. It is noteworthy that some products bearing a 1,3-dicarbonyl substituent (4az and 4ba), characterized by increased C–H acidity, were isolated by us in the form of salts with DIPEA.
The structures of the obtained products were confirmed using NOESY spectra, which show cross-peaks arising from the interaction between the protons of the substituent at the nitrogen atom (diazo component moiety) and the substituent at the 5-position of the triazole ring (R2) (Fig. 3). Additionally, single-crystal X-ray diffraction data were obtained for two compounds (4t and 4aq), reliably confirming the regioselectivity, which is consistent with earlier findings.25
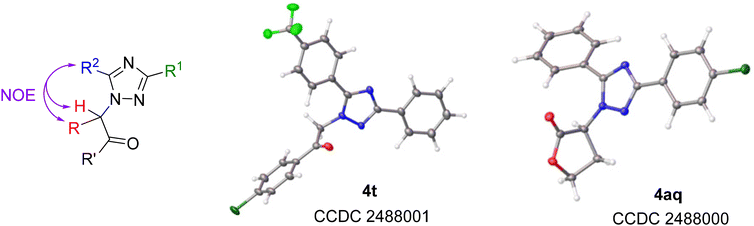 | ||
| Fig. 3 Interactions observed in the NOESY spectra of the obtained products 4 and solid-state structure of compounds 4t and 4aq (thermal ellipsoids are shown at 50% probability). | ||
An important objective was to elucidate how the formation of the 1,2,4-triazole ring occurs in this reaction, which would shed light on the reasons behind its exceptionally high regioselectivity. It should be noted that, upon interaction of azlactones with arenediazonium salts, predominantly an alternative regioisomer is formed, or mixtures of both regioisomeric products are obtained.34 Earlier work also described different chemoselectivity for this reaction, specifically the formation of products resulting from formal O–H insertion into azlactones upon reaction with diazo ketones.35
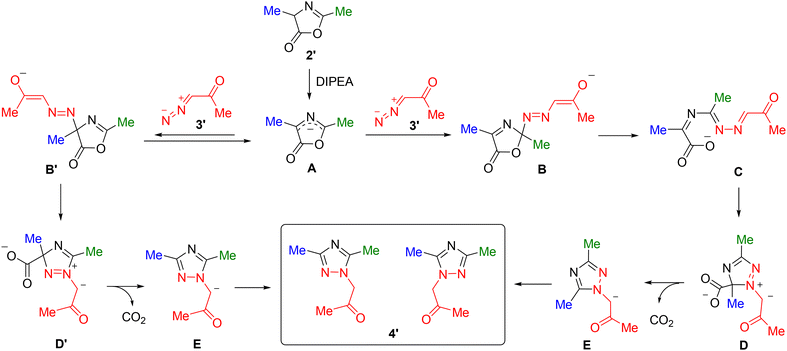
To establish the mechanism, we performed DFT calculations on the model substrates 2′ and 3′. The reaction begins with deprotonation of the azlactone, as in the absence of a base, the azlactone and diazo compound do not react at moderate temperatures (Scheme 3). In the subsequent step, the anion of azlactone (A) undergoes an attack by the electrophilic nitrogen atom of the diazo group (the diazo reagent acts as an N-terminal electrophile). Here, two potential sites for this attack should be considered: the α-position (C-4) and the C-2 atom. As is well known, the α-carbon atom in azlactones serves as a nucleophilic site, and azlactones predominantly react with electrophiles (such as alkylating agents or aldehydes) at this position. In our case, as demonstrated by computational studies, attachment of the diazo reagent at the α-position can indeed occur (the energy barriers leading to the formation of the alternative adducts B and B′ are comparable, 11.5 vs. 13.2 kcal mol−1, see Fig. 4). However, the resulting azo adduct readily dissociates back into the starting reagents, with a lower barrier for the reverse reaction of 7.7 kcal mol−1. At the same time, the barrier for the hypothetical cyclization of intermediate B′ into ylide D′, which leads to an alternative regioisomeric product, is considerably high (20.8 kcal mol−1).
 | ||
| Fig. 4 Energy profiles (Gibbs free energies, kcal mol−1, DFT B3LYP-D3/6-311+G(d,p), PCM for dichloromethane, T = 313 K) for the formation of enolate E from azlactone anion A and diazo acetone (3′). | ||
On the other hand, the addition of the diazo reagent at the C-2 position of the azlactone leads to the formation of intermediate B, which undergoes ring-opening to form the azine derivative C with almost no activation barrier (1.3 kcal mol−1). Subsequently, cyclization occurs with an activation barrier of 11.2 kcal mol−1, forming a 1,2,4-triazole ring in the ylide intermediate D, followed by rapid decarboxylation that yields the final product in the form of an enolate anion.
Thus, it can be concluded that the regioselectivity of the reaction is governed by the reversibility of the addition step at the azlactone's α-position and the high barrier for cyclization of this adduct. At the same time, the attack at the C-2 position, which proceeds at a comparable rate, is irreversible, as it is accompanied by the azlactone ring opening followed by the formation of the final 1,2,4-triazole product.
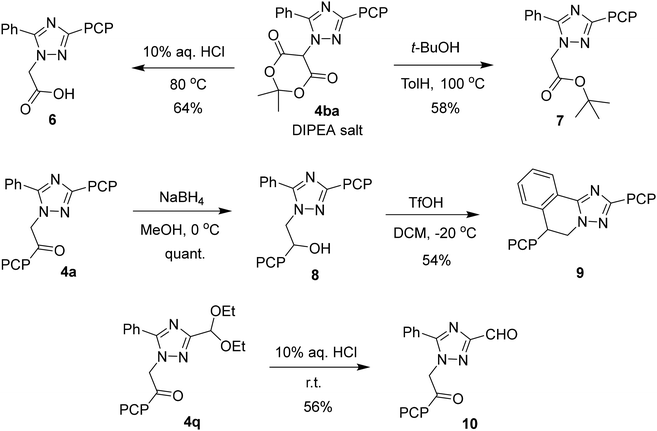
To confirm the synthetic applicability of the developed method, we performed the scale-up syntheses of compounds 4a and 4ba. Isolation of the products in approximately gram quantities was achieved without the use of chromatography (see the SI). Although this approach resulted in somewhat reduced yields, it significantly simplified the purification procedure. The obtained compounds were subjected to a series of subsequent transformations, demonstrating their synthetic versatility in expanding the structural diversity of 1,2,4-triazole derivatives through functional group modifications and an increase in molecular complexity (Scheme 4). Thus, heating Meldrum's acid derivative 4ba with diluted hydrochloric acid and tert-butyl alcohol afforded the acid 6 and tert-butyl ester 7, respectively. Ketone 4a was reduced to alcohol 8, which underwent cyclization in the presence of trifluoromethanesulfonic acid involving the phenyl ring, yielding the polycyclic structure 9. Additionally, acid hydrolysis of acetal 4q afforded aldehyde derivative 10.
Conclusion
In summary, based on the previously discovered rare type transformation of diazooxindoles, we have developed a convenient general approach to synthesize structurally diverse polysubstituted 1,2,4-triazoles. The method, which involves a decarboxylative cyclocondensation of diazocarbonyl compounds with in situ generated azlactones, provides access to a broad range of medicinally relevant heterocyclic derivatives. The transformation proceeds under mild conditions and is characterized by absolute regioselectivity, high yields, and tolerance towards a wide variety of functional groups, enabling the preparation of triazoles with various substituents at all positions. Product diversity is ensured both by the availability of the starting N-acyl amino acids and the versatility of the method with respect to the nature of the diazo reagent. The use of a broad scope of diazo carbonyls allows the introduction of various moieties at the 1-N position, including heterocyclic fragments. Unlike previously reported methods, this approach does not require prior modification of the starting acid to obtain the azlactone or any other activated form. Based on DFT calculations, a plausible reaction mechanism has been proposed, explaining the origin of the regioselectivity and certain substrate limitations. Post-modifications of selected products are also demonstrated, providing access to additional peripheral and scaffold diversity of 1,2,4-triazole derivatives.Experimental section
General procedure for the synthesis of 1,2,4-triazoles 4
A solution or suspension of the corresponding N-acylated amino acid 1 (0.8 mmol, 2 equiv.) and EDC hydrochloride (0.9 mmol, 2.25 equiv.) in dry DCM (3 mL) was placed in a screw-capped, thick-walled vial and stirred at 40 °C using a heating block. The corresponding diazo compound 3 (0.4 mmol, 1 equiv.) and DIPEA (0.4 mmol, 1 equiv.) were added sequentially. In most cases, gas evolution was observed upon the addition of DIPEA. The reaction mixture was then sealed and stirred at 40 °C for 6–20 hours. After the complete conversion of the diazo reagent (controlled by TLC), the reaction mixture was directly subjected to silica gel column chromatography (eluting with petroleum ether/acetone) to obtain the pure target compound.General procedure for the scale-up synthesis of 1,2,4-triazoles 4a and 4ba
A solution or suspension of the corresponding N-acylated amino acid 1a (4.8 mmol, 2 equiv.) and EDC hydrochloride (5.4 mmol, 2.2 equiv.) in dry DCM (18 mL) was placed in a screw-capped, thick-walled vial and stirred at 40 °C using a heating block. The corresponding diazo reagent (2.4 mmol, 1 equiv.) and DIPEA (2.4 mmol, 1 equiv.) were added sequentially. The reaction mixture was then sealed and stirred at 40 °C until the diazo reagent was completely consumed (monitored by TLC). The resulting mixture was washed with water (20 mL), 1 N aq. citric acid (20 mL), sat. aq. NaHCO3 (20 mL), and brine. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated to dryness under reduced pressure. The residual solid was triturated with diethyl ether (5 mL) and dried in vacuo.Conflicts of interest
The authors declare no competing financial interest.Data availability
The data underlying this study are available in the published article and its supplementary information (SI). Supplementary information: experimental procedures, complete characterization data, computation details, X-ray crystallography data for compounds 4t and 4aq and copies of 1H, 13C and NOESY NMR spectra. See DOI: https://doi.org/10.1039/d5ob01650a.CCDC 2488000 and 2488001 contain the supplementary crystallographic data for this paper.36a,b
Acknowledgements
This research was supported by the Russian Science Foundation (project grant 22-13-00005-P). We thank the Research Center for Magnetic Resonance, the Center for Chemical Analysis and Materials Research and the Center for X-ray Diffraction Methods of Saint Petersburg State University Research Park for obtaining the analytical data.References
- C. M. Marshall, J. G. Federice, C. N. Bell, P. B. Cox and J. T. Njardarson, An Update on the Nitrogen Heterocycle Compositions and Properties of U.S. FDA-Approved Pharmaceuticals (2013–2023), J. Med. Chem., 2024, 67, 11622–11655 CrossRef CAS PubMed.
- C. Liu, P. R. Jalagam, J. Feng, W. Wang, T. Raja, M. R. Sura, R. Manepalli, B. R. Aliphedi, S. Medavarapu, S. K. Nair, V. Muthalagu, R. Natesan, A. Gupta, B. Beno, M. Panda, K. Ghosh, J. K. Shukla, H. Sale, P. Haldar, N. Kalidindi, D. Shah, D. Patel, A. Mathur, B. A. Ellsworth, D. Cheng and A. Regueiro-Ren, Identification of Monosaccharide Derivatives as Potent, Selective, and Orally Bioavailable Inhibitors of Human and Mouse Galectin-3, J. Med. Chem., 2022, 65, 11084–11099 CrossRef CAS PubMed.
- A. E. Elsawi, M. M. Elbadawi, A. Nocentini, H. Almahli, S. Giovannuzzi, M. Shaldam, R. Salem, T. M. Ibrahim, H. A. Abdel-Aziz, C. T. Supuran and W. M. Eldehna, 1,5-Diaryl-1,2,4-triazole Ureas as New SLC-0111 Analogues Endowed with Dual Carbonic Anhydrase and VEGFR-2 Inhibitory Activities, J. Med. Chem., 2023, 66, 10558–10578 CrossRef CAS PubMed.
- (a) Y. Lao, P. Huang, J. Chen, Y. Wang, R. Su, W. Shao, W. Hu and J. Zhang, Discovery of 1,2,4-triazole derivatives as novel neuroprotectants against cerebral ischemic injury by activating antioxidant response element, Bioorg. Chem., 2022, 128, 106096 CrossRef CAS PubMed; (b) Y. Lao, Y. Wang, J. Chen, P. Huang, R. Su, J. Shi, C. Jiang and J. Zhang, Synthesis and biological evaluation of 1,2,4-triazole derivatives as potential Nrf2 activators for the treatment of cerebral ischemic injury, Eur. J. Med. Chem., 2022, 236, 114315 CrossRef CAS PubMed.
- (a) M. Luo, Q. Wu, Y. Yang, L. Sun, X. Huan, C. Tian, B. Xiong, Z. Miao, Y. Wang and D. Chen, Design and development of a novel series of oral bivalent BET inhibitors with potent anticancer activities, Eur. J. Med. Chem., 2022, 239, 114519 CrossRef CAS PubMed; (b) H. Gu, T. Zhang, T. Guan, M. Wu, S. Li, Y. Li, M. Guo, L. Zhang, Y. Peng, D. Mi, M. Liu, Z. Yi and Y. Chen, Discovery of a Highly Potent and Selective MYOF Inhibitor with Improved Water Solubility for the Treatment of Gastric Cancer, J. Med. Chem., 2023, 66, 16917–16938 CrossRef CAS PubMed; (c) N. Green, M. G. LaPorte, W. Paquette, S. Joyasawal, P. Mondal, Z. Vaughn, E. J. Carder, M. Liang, L. Shan, F. Wang, F. Tomaino, A. Srivastava, A. Flint, W. J. Moore, T. F. Chou, G. M. Stott, M. Wolf, P. Wipf and D. M. Huryn, Antitumor Efficacy of 1,2,4-Triazole-Based VCP/p97 Allosteric Inhibitors, J. Med. Chem., 2025, 68, 14465–14494 CrossRef CAS PubMed; (d) M. Mustafa, S. Anwar, F. Elgamal, E. R. Ahmed and O. M. Aly, Potent combretastatin A-4 analogs containing 1,2,4-triazole: Synthesis, antiproliferative, anti-tubulin activity, and docking study, Eur. J. Med. Chem., 2019, 183, 111697 CrossRef CAS PubMed.
- (a) E. A. Meyer, P. Aanismaa, E. A. Ertel, E. Huhn, D. S. Strasser, M. Rey, M. J. Murphy, M. M. Martinic, L. Pouzol, S. Froidevaux, M. P. Keller and E. Caroff, Discovery of Clinical Candidate ACT-777991, a Potent CXCR3 Antagonist for Antigen-Driven and Inflammatory Pathologies, J. Med. Chem., 2023, 66, 4179–4196 CrossRef CAS PubMed; (b) Y. Hao, H. Shao, Z. Qu, J. Li, Y. Shi, W. Zhang, J. Yu, P. Fu and C. Zhuang, Investigation on the chemical space of the substituted triazole thio-benzoxazepinone RIPK1 inhibitors, Eur. J. Med. Chem., 2022, 236, 114345 CrossRef CAS PubMed.
- L. Wang, Y. Geng, L. Liu, J. Wang, J. Chen, Y. Li, J. Wang, L. Song, K. Sun, Y. Yan, S. Zhou, D. Tian, R. Lin and H. Yao, Synthesis, anti-allergic rhinitis evaluation and mechanism investigation of novel 1,2,4-triazole-enamides as CB1 R antagonist, Eur. J. Med. Chem., 2025, 289, 117461 CrossRef CAS PubMed.
- T. Ni, X. Chi, H. Wu, F. Xie, J. Bao, J. Wang, Z. Ji, L. Li, X. Wang, L. Yan, Y. Hao, D. Zhang and Y. Jiang, Design, synthesis and evaluation of novel deferasirox derivatives with high antifungal potency in vitro and in vivo, Eur. J. Med. Chem., 2024, 264, 116026 CrossRef CAS PubMed.
- Y. Wen, S. Lun, Y. Jiao, W. Zhang, T. Hu, T. Liu, F. Yang, J. Tang, B. Zhang, W. R. Bishai and L.-F. Yu, Design, synthesis, and biological evaluation of 1,2,4-triazole derivatives as potent antitubercular agents, Chin. Chem. Lett., 2024, 35, 108464 CrossRef CAS.
- X. Jiang, P. P. Sharma, B. Rathi, X. Ji, L. Hu, Z. Gao, D. Kang, Z. Wang, M. Xie, S. Xu, X. Zhang, E. De Clercq, S. Cocklin, C. Pannecouque, A. Dick, X. Liu and P. Zhan, Discovery of novel 1,2,4-triazole phenylalanine derivatives targeting an unexplored region within the interprotomer pocket of the HIV capsid protein, J. Med. Virol., 2022, 94, 5975–5986 CrossRef CAS PubMed.
- (a) Q. Guan, S. Xing, L. Wang, J. Zhu, C. Guo, C. Xu, Q. Zhao, Y. Wu, Y. Chen and H. Sun, Triazoles in Medicinal Chemistry: Physicochemical Properties, Bioisosterism, and Application, J. Med. Chem., 2024, 67, 7788–7824 CrossRef CAS PubMed; (b) J. Khan, A. Rani, M. Aslam, R. S. Maharia, G. Pandey and B. Nand, Exploring triazole-based drugs: Synthesis, application, FDA approvals, and clinical trial updates–A comprehensive review, Tetrahedron, 2024, 162, 134122 CrossRef CAS; (c) O. Gupta, T. Pradhan and G. Chawla, An updated review on diverse range of biological activities of 1,2,4-triazole derivatives: Insight into structure activity relationship, J. Mol. Struct., 2023, 1274, 134487 CrossRef CAS; (d) B. Rathod and K. Kumar, Synthetic and Medicinal Perspective of 1,2,4-Triazole as Anticancer Agents, Chem. Biodivers., 2022, 19, e202200679 CrossRef CAS PubMed; (e) A. Sharma, A. K. Agrahari, S. Rajkhowa and V. K. Tiwari, Emerging impact of triazoles as anti-tubercular agent, Eur. J. Med. Chem., 2022, 238, 114454 CrossRef CAS PubMed; (f) Z. Kazeminejad, M. Marzi, A. Shiroudi, S. A. Kouhpayeh, M. Farjam and E. Zarenezhad, Novel 1, 2, 4-Triazoles as Antifungal Agents, BioMed. Res. Int., 2022, 4584846 CrossRef CAS PubMed; (g) Y. Wang and D. Liu, An Important Potential Anti-Epileptic/Anticonvulsant Active Group: A Review of 1,2,4-Triazole Groups and Their Action, Drug Res., 2022, 72, 131–138 CrossRef CAS PubMed.
- A. A. Al Bahri and H. J. Hamnett, Etizolam and Its Major Metabolites: A Short Review, J. Anal. Toxicol., 2023, 47, 216–226 CrossRef CAS PubMed.
- B. Desta and G. Amare, Paclobutrazol as a plant growth regulator, Chem. Biol. Technol. Agric., 2021, 8, 1 CrossRef CAS.
- A. Lee, Fuzuloparib: First Approval, Drugs, 2021, 81, 1221–1226 CrossRef CAS PubMed.
- (a) L. Song, Y. Lai, H. Li, J. Ding, H. Yao, Q. Su, B. Huang, M. A. Ouyang and R. Tong, Environmentally Benign and User-Friendly In Situ Generation of Nitrile Imines from Hydrazones for 1,3-Dipolar Cycloaddition, J. Org. Chem., 2022, 87, 10550–10554 CrossRef CAS PubMed; (b) D. Wang, X. Wan, Y. Zhou, J. Liu, J. Cai and G. J. Deng, Facile Synthesis of Fully Substituted 1,2,4−Triazoles via [3+2] Cycloaddition of Nitrileimines with Amidine under Transition Metal–Free Conditions, Asian J. Org. Chem., 2023, 12, e202200674 CrossRef CAS; (c) Y. Zhang, J. L. Zeng, Z. Chen and R. Wang, Base-Promoted (3 + 2) Cycloaddition of Trifluoroacetohydrazonoyl Chlorides with Imidates En Route to Trifluoromethyl-1,2,4-Triazoles, J. Org. Chem., 2022, 87, 14514–14522 CrossRef CAS PubMed; (d) S. K. Chilaka, K. P. Chinthapally, A. K. Soda, R. K. Chellu, S. Kurva, J. B. Nanubolu and S. Madabhushi, Synthesis of 1,2,4−Triazoles and 1,3,4−Thiadiazinones by [3+2] and [3+3] Domino Annulation Reactions of Nitrile Imines with Succinimide and Thiazolidine–2,4−dione, Asian J. Org. Chem., 2023, 12, e202300041 CrossRef; (e) Q. Zhang, S. Zhang, M. Tang, Q. Zhang and Y. Xia, Solvent–Free Synthesis of 1,2,4−Triazols from N–Tosylhydrazonyl Chlorides and Nitriles, Asian J. Org. Chem., 2023, 12, e202300081 CrossRef CAS.
- Y. Liu, K. Zhang, J. Zhang, S. Liu, Y. Song, X. Tan and L. Fang, Facile Synthesis of Diverse 1,3,5−Trisubstituted 1,2,4−Triazole using Hydrazones with Aldehydes, Alkenes, Amines, Asian J. Org. Chem., 2024, 13, e202400424 CrossRef CAS.
- A. Kumari, A. Jain, H. Sharma, S. Sermadurai and N. K. Rana, Catalyst-free efficient synthesis of functionalized 1,2,4-triazole via ring opening/cyclization of arylidene thiazolone with aryl/alkyl-hydrazine, Chem. Commun., 2025, 61, 11029–11032 RSC.
- P. Zhou, H. Li, R. Jiang, Y. Wan, J. Hou, Y. Hong and D. Tang, Electrochemical Cyclization of α,β-Alkynic Hydrazones and Primary Amines for the Synthesis of 3-Alkynyltriazoles, Asian J. Org. Chem., 2023, 12, e202300421 CrossRef CAS.
- S. Das, B. Choudhury, B. Maiti and K. Chanda, Ionic-liquid-supported copper-promoted synthesis of 3, 5-disubstituted-1,2,4-triazoles, Org. Biomol. Chem., 2025, 23, 2000–2009 RSC.
- L. Nicchio, L. Di Terlizzi, M. Fagnoni, L. Neuville, S. Protti and G. Masson, Visible–Light Enabled Synthesis of 1−Aryl–3−Sulfonylmethyl–1,2,4−Triazoles by Arylazo Sulfones, Adv. Synth. Catal., 2024, 367, e202401251 CrossRef.
- (a) H. Li, X. Wu, W. Hao, H. Li, Y. Zhao, Y. Wang, P. Lian, Y. Zheng, X. Bao and X. Wan, [3 + 2] Cycloaddition of Nitrile Ylides with Diazonium Salts: Copper-Catalyzed One-Pot Synthesis of Fully Substituted 1,2,4-Triazoles, Org. Lett., 2018, 20, 5224–5227 CrossRef CAS PubMed; (b) L. N. Zhou, F. F. Feng, C. W. Cheung and J. A. Ma, Cu-Enabled [3 + 2] Annulation of In Situ Formed Nitrile Ylides with Aryldiazonium Salts: Access to 5-Cyano-1,2,4-Triazoles, Org. Lett., 2021, 23, 739–744 CrossRef CAS PubMed.
- H. Díaz-Salazar, C. M. Ramírez-González, M. A. Rosas-Ortega and S. Porcel, Synthesis of 1,3,5-trisubstituted 1,2,4-triazoles enabled by a gold-catalyzed three-component reaction, Tetrahedron, 2024, 168, 134358 CrossRef.
- X. Li, X. Ye, C. Wei, C. Shan, L. Wojtas, Q. Wang and X. Shi, Diazo Activation with Diazonium Salts: Synthesis of Indazole and 1,2,4-Triazole, Org. Lett., 2020, 22, 4151–4155 CrossRef CAS PubMed.
- (a) S. C. Rodrigues, R. S. M. de Moraes, G. T. de Almeida Pinto, M. T. M. Martins, P. A. do Nascimento, D. L. A. Soares, A. B. M. Botelho, C. C. Cruz and A. C. Cunha, A Review on Chemistry and Methods of Synthesis of 1,2,4-Triazole Derivatives, Chem. Rec., 2025, 25, e202400190 CrossRef PubMed; (b) A. Abdelli, S. Azzouni, R. Plais, A. Gaucher, M. L. Efrit and D. Prim, Recent Advances in the Chemistry of 1,2,4-Triazoles: Synthesis, Reactivity and Biological Activities, Tetrahedron Lett., 2021, 86, 153518 CrossRef CAS.
- H. W. Zhao, Y. Y. Liu, Y. D. Zhao, N. N. Feng, J. Du, X. Q. Song, H. L. Pang and X. Q. Chen, Base-Catalyzed Formal [3+2] Cycloaddition of Diazooxindoles with Oxazol-5-(4H)-ones, Eur. J. Org. Chem., 2018, 341–346 CrossRef CAS.
- (a) A. Budeev, G. Kantin, D. Dar'in and M. Krasavin, Diazocarbonyl and Related Compounds in the Synthesis of Azoles, Molecules, 2021, 26, 2530 CrossRef CAS PubMed; (b) K. Malkova and D. Dar'in, Diazo–Based Construction of Heterocyclic Systems Via a X−H Insertion/Cyclization Cascade, Eur. J. Org. Chem., 2024, e202400909 CrossRef CAS; (c) Y. Xiang, C. Wang, Q. Ding and Y. Peng, Diazo Compounds: Versatile Synthons for the Synthesis of Nitrogen Heterocycles via Transition Metal-Catalyzed Cascade C–H Activation/Carbene Insertion/Annulation Reactions, Adv. Synth. Catal., 2018, 361, 919–944 CrossRef.
- (a) S. P. Chandrasekharan, A. Dhami, S. Kumar and K. Mohanan, Recent advances in pyrazole synthesis employing diazo compounds and synthetic analogues, Org. Biomol. Chem., 2022, 20, 8787–8817 RSC; (b) E. Y. Levashova, D. D. Zhukovsky, D. V. Dar'in and M. Y. Krasavin, Synthesis of Pyrazoles from α-Diazo-β-ketosulfones and α-Diazo-β-ketophosphonates, Chem. Heterocycl. Compd., 2020, 56, 806–808 CrossRef CAS.
- (a) A. Bubyrev, K. Malkova, G. Kantin, D. Dar'in and M. Krasavin, Metal-Free Synthesis of 1,5-Disubstituted 1,2,3-Triazoles, J. Org. Chem., 2021, 86, 17516–17522 CrossRef CAS PubMed; (b) S. Ahamad, R. Kant and K. Mohanan, Metal-Free Three-Component Domino Approach to Phosphonylated Triazolines and Triazoles, Org. Lett., 2016, 18, 280–283 CrossRef CAS PubMed.
- (a) M.-Y. Xiao, Z. Chen, F.-G. Zhang and J.-A. Ma, Regioselective synthesis of carboxylic and fluoromethyl tetrazoles enabled by silver-catalyzed cycloaddition of diazoacetates and aryl diazonium salts, Tetrahedron, 2020, 76, 131063 CrossRef CAS; (b) E. Levashova, O. Bakulina, D. Dar'in, A. Bubyrev, S. Chuprun and M. Krasavin, From Rare Reagents to Rare Products: Regiospecific Silver-Catalyzed [3+2] Cycloaddition of Aryl-, Alkyl- and Aminosulfonyl Diazomethanes with Arenediazonium Tosylates, Eur. J. Org. Chem., 2020, 4239–4242 CrossRef CAS; (c) S. Chuprun, D. Dar'in, G. Kantin and M. Krasavin, [3+2]-Cycloaddition of -Diazocarbonyl Compounds with Arenediazonium Salts Catalyzed by Silver Nitrate Delivers 2,5-Disubstituted Tetrazoles, Synthesis, 2019, 3998–4005 CAS; (d) X. Peng, M. Y. Xiao, J. L. Zeng, F. G. Zhang and J. A. Ma, Construction of Difluoromethylated Tetrazoles via Silver-Catalyzed Regioselective [3 + 2] Cycloadditions of Aryl Diazonium Salts, Org. Lett., 2019, 21, 4808–4811 CrossRef CAS PubMed.
- (a) L. Zhang, J. J. Chen, S. S. Liu, Y. X. Liang and Y. L. Zhao, DBU–Catalyzed [3+3] and [3+2] Annulation Reactions of Azomethine Ylides with α–Diazocarbonyls as N–Terminal Electrophiles: Modular, Atom–Economical Access to 1,2,4−Triazine and 1,2,4−Triazole Derivatives, Adv. Synth. Catal., 2018, 360, 2172–2177 CrossRef CAS; (b) L. Y. Mei, X. Y. Tang and M. Shi, One–Pot Tandem Diastereoselective and Enantioselective Synthesis of Functionalized Oxindole–Fused Spiropyrazolidine Frameworks, Chem. – Eur. J., 2014, 20, 13136–13142 CrossRef CAS PubMed.
- M. Luo, Q. Shen, H. Su, J. M. Li, C. M. Chan and W. Y. Yu, A Metal-Free Cycloaddition of α-Diazoacetates with Amino Acid-Derived NHPI Esters for the Facile Synthesis of 1,2,4-Triazoles, Org. Lett., 2024, 26, 5511–5516 CrossRef CAS PubMed.
- D. Dar'in, G. Kantin and M. Krasavin, A ‘sulfonyl-azide-free’ (SAFE) aqueous-phase diazo transfer reaction for parallel and diversity-oriented synthesis, Chem. Commun., 2019, 55, 5239–5242 RSC.
- D. Dar'in, G. Kantin and M. Krasavin, Practical Application of the Aqueous ‘Sulfonyl-Azide-Free’ (SAFE) Diazo Transfer Protocol to Less α-C–H Acidic Ketones and Esters, Synthesis, 2019, 4284–4290 Search PubMed.
- X. Y. Yu, W. J. Xiao and J. R. Chen, Synthesis of Trisubstituted 1,2,4-Triazoles from Azlactones and Aryldiazonium Salts by a Cycloaddition/Decarboxylation Cascade, Eur. J. Org. Chem., 2019, 6994–6998 CrossRef CAS.
- A. C. de Mello, P. B. Momo, A. C. B. Burtoloso and G. W. Amarante, Metal-Free Insertion Reactions of Diazo Carbonyls to Azlactones, J. Org. Chem., 2018, 83, 11399–11406 CrossRef CAS PubMed.
- (a) CCDC 2488000: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2phz28; (b) CCDC 2488001: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2phz39.
| This journal is © The Royal Society of Chemistry 2026 |