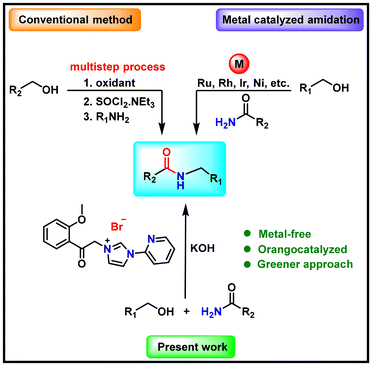
Transition metal-free organocatalyzed direct N-benzylation of amides via a hydrogen borrowing strategy
Deepak
Gautam
,
Puneet Singh
Gahlaut
,
Bhawana
Shekhawat
and
Barun
Jana
 *
*
Organometallic and Supramolecular Chemistry Laboratory (OMSCL), Department of Chemistry, Malaviya National Institute of Technology Jaipur, JLN Marg, Jhalana Gram, Malaviya Nagar, Jaipur, Rajasthan 302017, India. E-mail: barun.chy@mnit.ac.in
First published on 14th November 2025
Abstract
The development of a versatile methodology for the N-benzylation of amides is challenging due to the relatively poor nucleophilicity of the amide nitrogen. Herein, we report the first transition metal-free, broad catalytic methodology for the N-benzylation of amides using alcohols via a hydrogen borrowing methodology. The crucial step is the oxidation of the benzyl alcohol by the in situ generated N-heterocyclic carbene (NHC) to form 3-(2-(2-methoxyphenyl)-2-oxoethyl)-1-(pyridin-2-yl)-1H-imidazol-3-ium bromide (HL1Br). The carbene-based organocatalyst (HL1Br) efficiently sequesters hydrogen (as 2H+ + 2e−) produced during the dehydrogenation of the alcohol via a single-electron transfer (SET) process and returns the same to the in situ generated imine intermediate to yield the final N-benzylated product. The developed protocol is also applied for N-benzylation of aniline and selective mono-benzylation of benzene-1,2-diamine to form N1-benzylbenzene-1,2-diamine. Control experiments, available literature, and HRMS characterization support the plausible mechanism.
Introduction
A number of natural products, pharmaceuticals, and agrochemicals contain amine and amide skeletons,1 which demands their easy, atom-economical, and cost-effective synthesis. However, N-alkylation and N-benzylation of easily available amides are challenging due to the relatively poor nucleophilicity of the amide nitrogen in comparison with alkyl or aryl amines. Consequently, the most recently developed hydrogen borrowing strategy for the N-benzylation of amines using alcohols can be fruitful for the N-benzylation of amides as well. The hydrogen borrowing methodology for the N-benzylation of amines has several advantages in terms of the use of alcohols as green reagents, along with its atom-economical nature in marked contrast to the conventional N-benzylation methodologies that generally require harsh reagents and produce large amounts of waste chemicals2 (Scheme 1). Higher reaction temperatures and catalyst loadings are often applied to overcome these difficulties and generate N-benzylated amides in high yields.3 For example, the Watanabe4 and Jenner5 groups developed a method for N-alkylation of amides with alcohols using a Ru-catalyst [RuCl3·xH2O or Ru3(CO)12] at 180–210 °C. Later on, expensive phosphine or N-heterocyclic carbene ligands were combined with precious metals like Pd,6 Ru,7 and Ir8 to generate active catalysts for N-alkylation and N-benzylation of amides. Recently, Au/Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and Ag/Mo
9 and Ag/Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 metal composites have also been used for the N-benzylation of amides. Overall, the large-scale practical applicability of existing processes of N-benzylation of amides is rather constrained as they require expensive metal ions in relatively large quantities. Therefore, there is a strong need for the development of an inexpensive metal-based or metal-free green approach for the above-discussed reaction. Due to several advantages of organocatalysis, it would be advantageous to develop a high-yield metal-free methodology for the large-scale synthesis of N-benzylated amides from easily available amide molecules. Just to note, to the best of our knowledge, until now, there has been no metal-free approach reported for the N-benzylation of amides.11–13 Notably, several functionally designed imidazolium salts are found to be powerful organocatalysts in many homogeneous organic transformation reactions.14–16 Moreover, choices of the functional groups on the side wingtips of the N-heterocyclic carbene are the key to enabling certain reactivity towards an external molecule.17,18 The developed methodology is atom-economical and highly efficient for different substituted amides and various substituted benzyl alcohols.
10 metal composites have also been used for the N-benzylation of amides. Overall, the large-scale practical applicability of existing processes of N-benzylation of amides is rather constrained as they require expensive metal ions in relatively large quantities. Therefore, there is a strong need for the development of an inexpensive metal-based or metal-free green approach for the above-discussed reaction. Due to several advantages of organocatalysis, it would be advantageous to develop a high-yield metal-free methodology for the large-scale synthesis of N-benzylated amides from easily available amide molecules. Just to note, to the best of our knowledge, until now, there has been no metal-free approach reported for the N-benzylation of amides.11–13 Notably, several functionally designed imidazolium salts are found to be powerful organocatalysts in many homogeneous organic transformation reactions.14–16 Moreover, choices of the functional groups on the side wingtips of the N-heterocyclic carbene are the key to enabling certain reactivity towards an external molecule.17,18 The developed methodology is atom-economical and highly efficient for different substituted amides and various substituted benzyl alcohols.
Previously, we have introduced a functionalized imidazolium salt, HL1Br (HL1Br = 3-(2-(2-methoxyphenyl)-2-oxoethyl)-1-(pyridin-2-yl)-1H-imidazol-3-ium bromide), having methoxy substituted acetophenone and o-pyridine wingtip groups that showed tremendous catalytic activity for the N-arylation of amines in association with Cu(OAc)2 and in the absence of any base or solvent.19 Following the success of the N-arylation of amines, we attempted the N-benzylation of amides under comparable reaction conditions and successfully obtained the value-added N-benzylated product of benzamide. The results motivated us to optimize the reaction conditions, explore the substrate scope, and perform various control experiments to understand the possible mechanistic pathway.
Results and discussion
We initiated our investigation with a fusion of benzamide 1a (0.5 mmol), benzyl alcohol 2a (0.6 mmol), and KtOBu (0.5 mmol) along with 10 mol% of HL1Br at 130 °C for 16 hours in toluene. This reaction successfully produced N-benzyl benzamide (3a) in 68% yield (Table 1, entry 1). Delighted by the result, we screened different inorganic bases such as K2CO3, KOH, Cs2CO3, and NatOBu (Table 1, entries 2–5) to boost the yield of the desired product while retaining all other reaction parameters constant. We obtained the highest yield (82%) in the case of KOH, which allowed us to conclude that KOH is the best-suited base for the developed protocol. To check the importance of the organocatalyst and base, we carried out two distinct reactions: one without KOH (Table 1, entry 6) and another without HL1Br (Table 1, entry 7), keeping all other reaction parameters the same.| Entry | Base | Base equiv. | Organocatalyst | Organocatalyst (mol%) | Temp. (°C) | Isolated yield (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: benzamide 1a (0.5 mmol), benzyl alcohol 2a (0.6 mmol), organocatalyst (10 mol%), and KOH (0.5 mmol) at the indicated temperature for 16 h in toluene. b Ethanol used as a solvent. c Xylene used as a solvent. d Dioxane used as a solvent. e The reaction was allowed to run for 12 h. f The reaction was allowed to run for 8 h. | ||||||
| 1 | KtOBu | 1 | HL1Br | 10 | 130 | 68 |
| 2 | K2CO3 | 1 | HL1Br | 10 | 130 | 35 |
| 3 | KOH | 1 | HL1Br | 10 | 130 | 82 |
| 4 | Cs2CO3 | 1 | HL1Br | 10 | 130 | 45 |
| 5 | NatOBu | 1 | HL1Br | 10 | 130 | 65 |
| 6 | — | — | HL1Br | 10 | 130 | 0 |
| 7 | KOH | 1 | — | — | 130 | 0 |
| 8 | KOH | 1 | HL1PF6 | 10 | 130 | 55 |
| 9 | KOH | 1 | PhHL1Br | 10 | 130 | 68 |
| 10 | KOH | 1 | SIMes-Cl | 10 | 130 | 45 |
| 11 | KOH | 0.5 | HL1Br | 10 | 130 | 65 |
| 12 | KOH | 1.5 | HL1Br | 10 | 130 | 83 |
| 13 | KOH | 1 | HL1Br | 5 | 130 | 58 |
| 14 | KOH | 1 | HL1Br | 15 | 130 | 80 |
| 15b | KOH | 1 | HL1Br | 10 | 130 | 0 |
| 16c | KOH | 1 | HL1Br | 10 | 130 | 35 |
| 17d | KOH | 1 | HL1Br | 10 | 130 | 28 |
| 18 | KOH | 1 | HL1Br | 10 | 110 | 74 |
| 19 | KOH | 1 | HL1Br | 10 | 80 | 56 |
| 20e | KOH | 1 | HL1Br | 10 | 130 | 65 |
| 21f | KOH | 1 | HL1Br | 10 | 130 | 55 |
Unfortunately, no amount of 3a was formed in any of the above two reactions. These results showed that HL1Br and base are equally crucial for the generation of the desired product. In pursuit of finding the best-suited N-heterocyclic carbene, a number of imidazolium salts were screened (Fig. 1), where HL1-PF6, PhHL1-Br, and SIMes-Cl furnished the end product in 55%, 68%, and 45% yields, respectively (Table 1, entries 8–10). The obtained low yield while using the PhHL1-Br imidazolium salt could be due to the aromatic ring linked to the imidazole ring, which causes low nucleophilicity of the carbene carbon. Regarding HL1-PF6, the relatively higher stability of the formed KPF6 may have hindered its participation in the reaction mechanism to stabilize the formed cationic intermediate during the catalytic cycle. In the case of SIMes-Cl, the difference in performance is attributed to the distinct electronic and steric properties of the resulting NHCs. SIMes-Cl generates an NHC that is electron-rich and sterically hindered due to the electron-donating methyl groups on its phenyl rings. Conversely, the NHC from HL1Br is less sterically crowded because of the methylene-spacer on the imidazole nitrogen, and its carbene centre is more electron-deficient because the pyridine group has a strong electron-withdrawing inductive effect. The unique combination of a less crowded and more electron-deficient carbene centre is the key to the high yield in the case of HL1Br. Furthermore, to best optimise the amounts of KOH and HL1Br, a number of reactions were carried out (Table 1, entries 11–14), but no significant increase in yield was observed.
Along with toluene, several other solvents including ethanol, xylene and dioxane (Table 1, entries 15–17) were also tested, which gave the end products in 0, 35 and 28% yields, respectively. So, toluene is the ideal solvent for this reaction. In order to determine the impact of reaction temperature and time, more reactions were performed (Table 1, entries 18–21) and a decrement in yield was observed upon lowering the reaction temperature as well as the time duration. Overall, entry 3 is best suited for the developed protocol which includes benzamide 1a (0.5 mmol) and benzyl alcohol 2a (0.6 mmol) along with HL1Br (10 mol%) and KOH (1 equiv.) in toluene at 130 °C for 16 h.
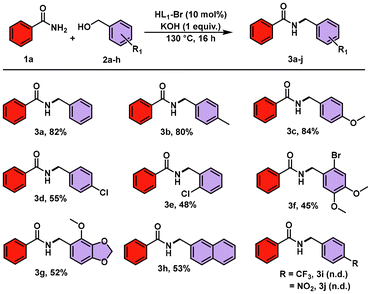
Having the optimized conditions in hand, the scope and efficiency of the HL1Br catalyzed N-benzylation of amides with benzyl alcohols were studied (Schemes 2 and 3). The library of N-benzylated products synthesized by the reaction of benzamide with different benzyl alcohols is presented in Scheme 2. As observed, when EDGs were attached to benzyl alcohols, the desired N-benzylated products were formed in good to excellent yields [3b (80%) and 3c (84%)]. However, upon attachment of EWGs to benzyl alcohols, the yields of the desired products were reduced relatively [3d (55%) and 3e (48%)] or there was no reaction (3i and 3j), which could be due to the low reactivity caused by the attached EWGs. Furthermore, N-(2-bromo-4,5-dimethoxy)benzyl alcohol and (4-methoxybenzo[d][1,3]dioxol-5-yl)methanol also reacted with benzamide and formed end products 3f and 3g in 45% and 52% yields, respectively. Moreover, naphthalen-2-ylmethanol reacted with benzamide and gave the desired product 3h in a good yield (53%). We also tried the reaction with 4-NO2 and 4-CF3 benzamides but found that the end products were furnished in trace amounts only.
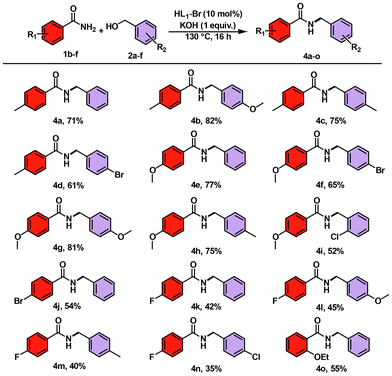
Furthermore, to explore the range of the developed methodology, various reactions were carried out in which various benzamides having electron-donating/withdrawing groups were reacted with different benzyl alcohols to synthesize mono-benzylated benzamide derivatives (Scheme 3).
Benzamides having electron-rich functionalities, such as p-methyl and p-methoxy, were well tolerated and furnished the desired products 4a to 4i in good to excellent isolated yields (52%–82%), but in the case of EWGs, the yields of the end products decreased as the electron-withdrawing groups on the ring lowered the reactivity of benzamide. We also carried out a gram-scale reaction of benzamide 1a (8.26 mmol) and 4-methyl benzyl alcohol 2b (9.91 mmol) under the optimized conditions, which furnished the desired product 3b in 61% (1.117 g) yield. The utility of the developed transformation was investigated with several other non-benzamide substrates, including aliphatic amides, heteroaryl-substituted amides and sulfonamides along with different substituted benzamides having functional groups like allyl ether, alkene or cyano. Unfortunately, under the optimized reaction conditions, none of these substrates yielded the desired product, indicating that the specific electronic and steric properties of the benzamide scaffold are crucial for the success of this protocol. In the case of 4-bromobenzamide, the yield of the final product 4j drops to 54%. p-Fluorobenzamides showed good tolerance in comparison with other EWGs and furnished the end products 4k–4n in good yields of 35%–45%. o-Ethyl benzamide also reacted with benzyl alcohol to form the final product 4o in 55% yield. Furthermore, similar to the N-benzylation of benzamides, the developed methodology was also applied for N-benzylation of aniline. Interestingly, the reaction of 4-methyl aniline 5a with benzyl alcohol 2a led to the formation of the N-benzylated amine product in an excellent yield [6a (88%)] (Scheme 4). Similarly, when N-benzylation of benzene-1,2-diamine 5b with benzyl alcohol 2a was tested, a selective mono-N-benzylation product, i.e. N1-benzylbenzene-1,2-diamine 6b, was formed in contrast to the cyclization product (Scheme 4). So, the developed methodology is applicable for N-benzylation of amines as well as selective N-benzylation of benzene-1,2-diamines, which further adds value to the reported work. Furthermore, to extend the scope of the developed methodology to N-benzylation of amides using secondary alcohols, benzamide was reacted with diphenylmethanol under the standard reaction conditions. However, no N-alkylated product was formed; instead, the formation of some unidentified compound (which appeared just below benzamide in TLC) occurred.
 | ||
| Scheme 4 Applications of the developed protocol. (For N-benzylation of aniline, the temperature is 110 °C). | ||
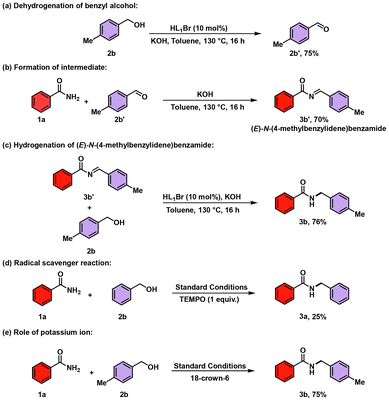
After successfully catalyzing N-benzylation of amides using different substituted benzyl alcohols, we performed control experiments to investigate the possible mechanism.
Initially, the oxidation reaction of p-methylbenzyl alcohol 2b was performed in the absence of benzamide 1a under the standard conditions, which successfully furnished the corresponding aldehyde 2b′, which indicates that the generation of p-methylbenzaldehyde 2b′ proceeds through the alcohol dehydrogenation process [Scheme 5, eqn (a)].
The synthesized p-methylbenzaldehyde 2b′ was later allowed to react with benzamide 1a under the standard conditions, which generated intermediate (E)-N-(4-methylbenzylidene)benzamide (3b′) in 70% yield [Scheme 5, eqn (b)]. Next, we performed one more reaction of an equimolar mixture of the intermediate (3b′) and p-methylbenzyl alcohol 2b; this reaction furnished the end product in 76% yield. These results demonstrated that intermediate 3b′ undergoes a hydrogen autotransfer (HAT) reaction, resulting in the desired N-alkylated product 3b [Scheme 5, eqn (c)]. Furthermore, to gain insights into the mechanism of whether the reaction proceeds through the radical or ionic pathway, we conducted a radical trapping reaction with one equivalent of a radical scavenger TEMPO (2,2,6,6-tetramethylpiperidin-1-yl)oxidanyl [Scheme 5, eqn (d)]. In this reaction, the yield of the end product 3a decreased drastically to 25%. This decrease in yield convinced us that the developed methodology proceeds through the radical pathway. After the above-mentioned reactions, a metal-ion-trapping experiment was performed with 18-crown-6 to check any possible potassium metal ion (K+) involvement. The reaction formed the desired N-benzylated amide product 3b in 75% yield, which concluded that there is no potassium ion involvement in the developed methodology [Scheme 5, eqn (e)].
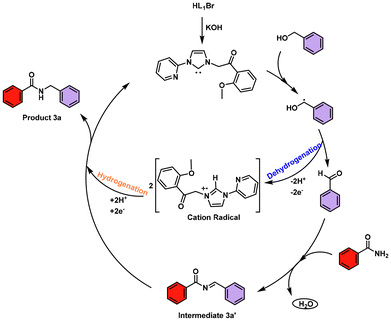
Based on the aforementioned experimental results and prior literature reports,20,21 a viable catalytic mechanism for the N-benzylation of amides has been proposed (Scheme 6). Firstly, in the presence of KOH, the imidazolium salt (HL1Br) in situ generates N-heterocyclic carbene (NHC). In the next step NHC causes the oxidation of benzyl alcohol via single electron transfer (SET).
The oxidation or dehydrogenation of benzyl alcohol involves a temporary hydrogen atom transfer (HAT) in the form of two protons (2H+) and two electrons (2e−) from benzyl alcohol to N-heterocyclic carbene. This temporary transfer subsequently generates the cation radical (unfortunately, no radical cation is detected as the formation of the proposed radical cation is extremely short-lived). Then, the generated corresponding aldehyde 2a′ reacts with benzamide 1a to furnish the intermediate by releasing a water molecule. For hydrogenation, the in situ generated cation radical delivers the two protons (2H+) and two electrons (2e−) to intermediate 3a′ along with the regeneration of the carbene in order to pave the way for the final N-benzylated amide product 3a.
Conclusion
We have developed an organocatalyzed, highly efficient, easily accessible, atom-economical, and comparatively greener approach for N-benzylation of amides by benzyl alcohols utilising an easy-to-synthesize, inexpensive, and bench-stable imidazolium salt as the NHC precursor. This methodology generates in situ NHC, which oxidizes the benzyl alcohol into the corresponding benzaldehyde via dehydrogenation by SET (as 2H+ + 2e−) and redelivers the hydrogen atom to the intermediate, which yields the end product. This protocol is greener than the already existing methods as it is organocatalyzed and produces water as the only side product. Along with the N-benzylation of amides, this methodology has excellent results for selective N-benzylation of benzene-1,2-diamine by alcohols, which makes this protocol more appealing and interesting. This strategy paves the way for more environmentally friendly and sustainable ways to use organocatalysts in furnishing C–C and C–N bonds.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ob01420d.Acknowledgements
B. J. gratefully acknowledges the Science and Engineering Research Board (SERB; CRG/2023/007224), New Delhi, for financial support to carry out this work. D. G. and B. S. thank UGC-SRF for providing a research fellowship. P. S. G. thanks MNIT Jaipur for providing a research fellowship.References
- C. S. Yeung and V. M. Dong, Catalytic dehydrogenative cross-coupling: forming carbon–carbon bonds by oxidizing two carbon–hydrogen bonds, Chem. Rev., 2011, 111(3), 1215–1292 CrossRef CAS PubMed.
- M. H. S. Hamid, C. L. Allen, G. W. Lamb, A. C. Maxwell, H. C. Maytum, A. J. Watson and J. M. Williams, Ruthenium-catalyzed N-alkylation of amines and sulfonamides using borrowing hydrogen methodology, J. Am. Chem. Soc., 2009, 131(5), 1766–1774 CrossRef CAS PubMed.
- P. Ruiz-Castillo and S. L. Buchwald, Applications of palladium-catalyzed C–N cross-coupling reactions, Chem. Rev., 2016, 116(19), 12564–12649 CrossRef CAS PubMed.
- Y. Watanabe, Y. Tsuji and Y. Ohsugi, The ruthenium catalyzed N-alkylation and N-heterocyclization of aniline using alcohols and aldehydes, Tetrahedron Lett., 1981, 22, 2667–2670 CrossRef CAS.
- A. Ben Taleb and G. Jenner, Scope of the N-alkylation of amides and the C-alkylation of malonates by methyl formate and dimethyl carbonate, J. Mol. Catal., 1993, 84(2), L131–L136 CrossRef CAS.
- P. Anandaraj and R. Ramesh, N-alkylation of benzamides/sulfonamides using alcohols via borrowing hydrogen approach by well-defined Pd (II) pincer complexes, Appl. Organomet. Chem., 2023, 37(10), e7228 CrossRef CAS.
- G. Chelucci, Ruthenium and osmium complexes in C–C bond-forming reactions by borrowing hydrogen catalysis, Coord. Chem. Rev., 2017, 331, 1–36 CrossRef CAS.
- M. Onoda and K.-i. Fujita, Iridium-Catalyzed C-Alkylation of Methyl Group on N-Heteroaromatic Compounds using Alcohols, Org. Lett., 2020, 22(18), 7295–7299 CrossRef CAS PubMed.
- G. C. Choo, H. Miyamura and S. Kobayashi, Synergistic cascade catalysis by metal nanoparticles and Lewis acids in hydrogen autotransfer, Chem. Sci., 2015, 6(3), 1719–1727 RSC.
- X. Cui, Y. Zhang, F. Shi and Y. Deng, Organic Ligand–Free Alkylation of Amines, Carboxamides, Sulfonamides, and Ketones by Using Alcohols Catalyzed by Heterogeneous Ag/Mo Oxides, Chem. – Eur. J., 2011, 17(3), 1021–1028 CrossRef CAS PubMed.
- S. Kumar, D. Singh and A. Rit, Cooperativity between metal centers in homobimetallic Pd II–NHC complexes: catalytic potential towards hydrodefluorination, Chem. Commun., 2024, 60(60), 7761–7764 RSC.
- D. Enders, O. Niemeier and A. Henseler, Organocatalysis by N-heterocyclic carbenes, Chem. Rev., 2007, 107(12), 5606–5655 CrossRef CAS PubMed.
- N. Marion, S. Díez-González and S. P. Nolan, N-heterocyclic carbenes as organocatalysts, Angew. Chem., Int. Ed., 2007, 46(17), 2988–3000 CrossRef CAS PubMed.
- D. M. Flanigan, F. Romanov-Michailidis, N. A. White and T. Rovis, Organocatalytic reactions enabled by N-heterocyclic carbenes, Chem. Rev., 2015, 115(17), 9307–9387 CrossRef CAS PubMed.
- S. Das, S. Santra, P. Mondal, A. Majee and A. Hajra, Zwitterionic imidazolium salt: recent advances in organocatalysis, Synthesis, 2016,(09), 1269–1285 CAS.
- S. Santra, A. Porey, B. Jana and J. Guin, N-Heterocyclic, carbenes as chiral Brønsted base catalysts: A highly diastereo- and enantioselective 1, 6-addition reaction, Chem. Sci., 2018, 9(30), 6446–6450 RSC.
- M. Paul, K. Peckelsen, T. Thomulka, J. Martens, G. Berden, J. Oomens, J. M. Neudörfl, M. Breugst, A. J. Meijer and M. Schäfer, Breslow Intermediates (Amino Enols) and Their Keto Tautomers: First Gas–Phase Characterization by IR Ion Spectroscopy, Chem. – Eur. J., 2021, 27(8), 2662–2669 CrossRef CAS PubMed.
- M. Paul, M. Breugst, J. r.-M. Neudörfl, R. B. Sunoj and A. Berkessel, Keto–enol thermodynamics of Breslow intermediates, J. Am. Chem. Soc., 2016, 138(15), 5044–5051 CrossRef CAS PubMed.
- D. Gautam, P. S. Gahlaut, K. Yadav and B. Jana, Functionalized imidazolium salt: an efficient catalyst for Buchwald–Hartwig type C–N cross-coupling of (hetero) aryl chlorides/bromides with amines under solvent-, inert gas-, and base-free ambience, New J. Chem., 2022, 46(47), 22841–22848 RSC.
- R. Sharma, A. Rahaman, J. Sen, I. V. Mashevskaya and S. Chaudhary, Discovering the role of N-heterocyclic carbene as hydrogen borrowing organocatalyst: metal-free, direct N-alkylation of amines with benzyl alcohols, Org. Chem. Front., 2023, 10(3), 730–744 RSC.
- J. Das and D. Banerjee, Nickel-catalyzed phosphine free direct N-alkylation of amides with alcohols, J. Org. Chem., 2018, 83(6), 3378–3384 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |