Fe(NO3)3·9H2O-mediated visible-light photocatalysis for the nitration of cyclobutanols: access to functionalized nitrocyclobutenes
Dongfang
Jiang
 *ac,
Can
Yang
a,
Xinying
Man
b,
Xin
Li
c,
Haifei
Wang
*a,
Yunlin
Song
*ac,
Can
Yang
a,
Xinying
Man
b,
Xin
Li
c,
Haifei
Wang
*a,
Yunlin
Song
 *d and
Zhenjie
Qi
*d and
Zhenjie
Qi
 *b
*b
aSchool of Biological Science and Medical Engineering, Hunan University of Technology, Zhuzhou 412007, P. R. China
bSchool of Resource & Environment and Safety Engineering, Jining University, Qufu, Shandong 273155, P. R. China
cHunan Provincial Key Laboratory of the Research and Development of Novel Pharmaceutical Preparations, Changsha Medical University, Provincial First-Class Applied Discipline (Pharmacy), Changsha, 410219, China
dMinistry of Agriculture, Nanjing Agricultural University, Nanjing 210095, China
First published on 26th November 2025
Abstract
A straightforward method for the synthesis of nitrocyclobutene derivatives from cyclobutanols with Fe(NO3)3·9H2O has been developed via a visible-light photocatalytic radical addition reaction. The reaction utilizes Fe(NO3)3·9H2O as the nitro source and RFTA as the photocatalyst, showing good functional group tolerance and furnishing the desired products in moderate to excellent yields. Preliminary mechanistic studies suggested that the nitro radical was involved in the reaction process.
Introduction
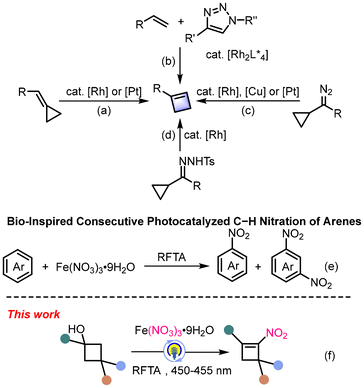
Cyclobutenes, common motifs in both natural products and pharmaceutical molecules, possess high chemical reactivity originating from the inherent ring strain (Fig. 1).1 Attributed to their high chemical reactivity originating from the inherent ring strain, cyclobutenes undergo various transformations, especially ring-opening and ring-expansion reactions, providing unique strategies to synthesize organic molecules with complex structures.2 The double bonds of cyclobutenes can also be selectively functionalized to afford cyclobutane derivatives.3 While methods for synthesizing the cyclobutene core are well-established (Scheme 1a–c),4–9 strategies for their direct functionalization, particularly via C–H or C–C bond activation, remain limited.The introduction of nitro groups into organic frameworks is a transformation of paramount importance, as nitro groups serve as versatile precursors to amines, amides, and other nitrogen-containing compounds.10 A protocol for the nitration of cyclobutenes has not been reported. Recently, Zhou et al. reported a visible-light-induced nitration of arenes using Fe(NO3)3·9H2O and RFTA (Scheme 1e).11 Inspired by this work and our ongoing interest in the functionalization of cycloalkanols, we herein report a visible-light-induced nitration of cyclobutanols. This transformation is distinct from arene C–H functionalization and involves a sequence of dehydration, radical addition, and ring-opening to directly access valuable nitrocyclobutene derivatives from readily available cyclobutanols (Scheme 1f).
Results and discussion
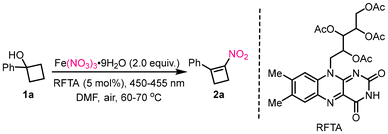
We initiated our study by employing 1-phenylcyclobutan-1-ol (1a) and Fe(NO3)3·9H2O as model substrates. The optimal conditions were obtained to furnish product 2a in 76% yield, after a comprehensive optimization of reaction conditions by screening solvents, nitro sources, and photocatalysts (Table 1, entry 1). The structure of 2a was unambiguously confirmed by X-ray crystallography (CCDC 2478060). To achieve consecutive nitration, several polar solvents including DMSO, MeCN and nonpolar solvents such as DCE, THF, and 1,4-dioxane were all inferior to DMF (Table 1, entry 2). Next, different nitro sources such as Cu(NO3)2·3H2O, AgNO3, Mn(NO3)2·4H2O, and NaNO2 were tested, but Fe(NO3)3·9H2O gave the best result with good yield (Table 1, entries 3 and 4). Replacing Fe(NO3)3·9H2O with TBN was not effective in this reaction (Table 1, entry 5). Various photocatalysts, including metal-containing Ru/Ir-tripyridines, organic dyes such as fluorescein, and 4-CzIPN salt, were screened but showed no reactivity (Table 1, entry 6). To investigate the role of molecular O2 or N2, the model reaction was conducted under an O2 or N2 atmosphere. The yield of 2a under these conditions (71% or 68%) was comparable to that obtained in air (76%), indicating that O2 is not essential for the reaction outcome (Table 1, entry 7). A control experiment was conducted in darkness, leading to the absence of the desired product 2a (Table 1, entry 8).| Entry | Variation from standard conditions | Yieldb (%) |
|---|---|---|
| a Reaction conditions: 1 (0.30 mmol, 1.0 equiv.), Fe(NO3)3·9H2O (0.60 mmol, 2.0 equiv.), RFTA (5 mol%), DMF (3 mL), 450–455 nm, 60–70 °C, 24 h, and air. b NMR yields using m-dinitrobenzene as the internal standard reference. | ||
| 1 | Standard conditions | 76 |
| 2 | DMSO, MeCN, DCE, THF, 1,4-dioxane instead of DMF | 52, 17, 9, 0, 0 |
| 3 | Cu(NO3)2·3H2O, AgNO3 and Mn(NO3)2·4H2O instead of Fe(NO3)3·9H2O | 48, 21, 35 |
| 4 | NaNO2 instead of Fe(NO3)3·9H2O | 60 |
| 5 | TBN instead of Fe(NO3)3·9H2O | Trace |
| 6 | [Ir(dFppy)2(dtbbpy)]PF6, Ru(phen)3(PF6)2, fluorescein, 4-CzIPN instead of RFTA | 0, 0, 0, 0 |
| 7 | O2, N2 instead of air | 71, 68 |
| 8 | Dark | Trace |
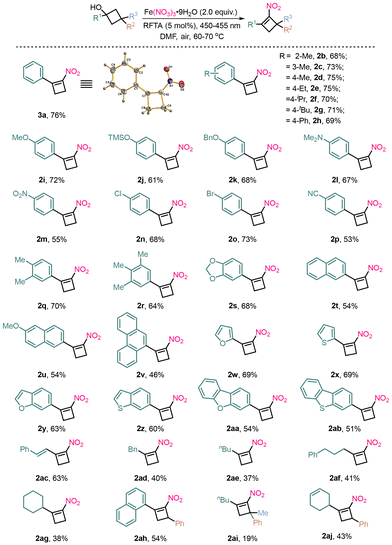
With the optimal conditions in hand, the scope of this cascade oxidative nitration of a range of cyclobutanols was investigated, as illustrated in Scheme 2. The scope of cyclobutanols with an aryl ring was evaluated. Cyclobutanols substituted with electron-donating groups of methyl at the ortho-, meta-, and para-positions of the aromatic ring (R1) showed excellent reactivity and provided the nitration products 2b–2d in 68–75% yields. Steric and electronic factors also influenced the efficiency, as evidenced by the lower yield of ortho-substituted substrate 2b compared to its para-counterpart. Meanwhile, the results demonstrated that the reaction was sensitive to the steric effect at the ortho-position, and product 2b was obtained in only 68% yield. Cyclobutanols containing an aromatic moiety bearing either electron-donating substituents (Et, iPr, tBu, Ph, OMe, OTMS, OBn, and NMe2) or electron-withdrawing groups (NO2, Cl, Br, and CN) at the para-position were all converted to the corresponding products 2e–2p in 53–75% yields. Generally, the cyclobutanols with electron-donating groups on the aryl substituents provided yields higher than those of electron-withdrawing cyclobutanols (2a–2p), consistent with the electrophilic nature of the nitro radical. Disubstituted and trisubstituted substrates bearing methyl groups provided products 2q–2s in 64–70% yields. 2-Naphthyl and 9-anthryl-substituted cyclobutanols were also viable in this transformation to give 2t–2v in 46–54% yields, demonstrating the method's utility for complex scaffolds. The reaction also demonstrates excellent compatibility with heterocycles such as furan, thiophene, benzofuran, benzothiophene, dibenzofuran and dibenzothiophene, all of which were obtained in satisfactory yields (2w–2ab).
Alkenylated cyclobutanol gave 2ac in 63% yield. It is worth mentioning that the alkene group remained stable during the formation of 2ac. The alkyl substituents were subjected to the reaction to afford nitrocyclobutenes 2ad–2ag, respectively, in a comparable efficiency. It is noteworthy that alkyl-substituted cyclobutanols generally afforded moderate yields, which may be attributed to the lower stability of the corresponding alkyl radicals and potential competitive β-scission pathways. Notably, yield variability was observed for sterically crowded or alkyl-substituted substrates. For example, 3,3-disubstituted cyclobutanol afforded 2ai in only 19% yield (vs. 54% for monosubstituted 2ah and 43% for 2ai), which is attributed to the significant steric congestion around the reactive center, likely impeding the formation or reactivity of the key radical intermediate. 2aj was synthesized and showed good reactivity without interference from the isolated alkene, confirming the chemoselectivity of the nitration process.
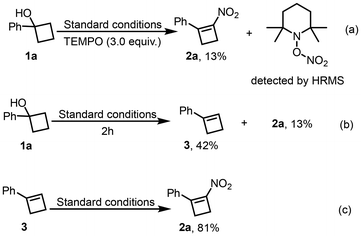
To shed light on the mechanism of this reaction, a series of several control experiments were conducted. The reaction was nearly completely inhibited by the addition of (2,2,6,6-tetramethylpiperidin-1-yl)oxyl (TEMPO). A TEMPO adduct derived from the nitro radical was detected by HRMS, which provides supporting evidence for the involvement of a nitro radical species in the mechanism (Scheme 3a). Further studies disclosed that compound 3 was obtained in 42% yield when the reaction was performed under the standard reaction conditions for 2 h (Scheme 3b). Then, compound 3 was also employed as the substrate, and the corresponding product 2a was afforded in 81% yield under the optimized conditions (Scheme 3c). Based on the above results, compound 3 should be the intermediate in this reaction.
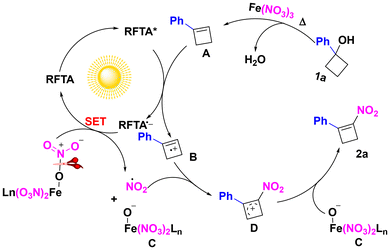
Based on these experimental results and previously reported studies,12 a possible mechanism is proposed in Scheme 4. Firstly, 1-phenylcyclobutanol 1a undergoes Fe-catalyzed thermal dehydration to form cyclobutene intermediate A. Subsequently, upon photoexcitation, RFTA* undergoes single-electron transfer (SET) oxidation of intermediate A, generating radical cation species B alongside the reduced photocatalyst radical anion RFTA˙−. The RFTA˙− species then mediates N–O bond homolysis in the ferric nitrate complex through a second SET event, regenerating the ground-state photocatalyst while releasing a nitro radical (˙NO2). Radical recombination between radical cation B and the ˙NO2 radical yields nitrated cyclobutyl intermediate D. Finally, deprotonation of D by the base present in the system affords the final nitrocyclobutene product 2a.
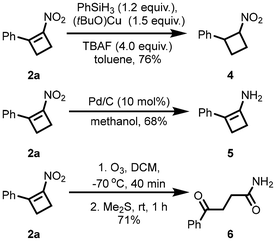
To demonstrate the synthetic utility of ring formation, we attempted a set of chemoselective transformations (Scheme 5). Nitrocyclobutene of 2a gave (2-nitrocyclobutyl)benzene 4 in 76% yield. Selective amination of 2a by using Pd/C afforded 2-phenylcyclobut-1-en-1-amine 5. Ozonization of 2a selectively gave 4-oxo-4-phenylbutanamide 6.
Conclusions
In summary, we have developed a novel and efficient protocol for the synthesis of nitrocyclobutenes via a photocatalytic nitration reaction of cyclobutanols with Fe(NO3)3·9H2O as the nitro source. The broad substrate scope and tolerance of various functional groups have been demonstrated. Mechanistic studies reveal that the reaction is more likely to undergo excitation of RFTA to generate a reactive triplet excited state, facilitating single electron transfer (SET) processes that lead to the formation of nitro radicals. Ongoing studies in our laboratory are dedicated to further synthetic applications of this transformation.Author contributions
Conceptualisation: Can Yang; methodology: Dongfang Jiang and Can Yang; investigation: Xinying Man; formal analysis: Xin Li; writing – original draft: Zhenjie Qi; writing – review & editing: Haifei Wang; funding acquisition: Dongfang Jiang; resources: Yunlin Song; supervision: Haifei Wang.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: synthetic details, experimental methods, and characterisation data (including copies of NMR spectra). See DOI: https://doi.org/10.1039/d5ob01627d.CCDC 2478060 contains the supplementary crystallographic data for this paper.13
Acknowledgements
This research was funded by the Natural Science Foundation of Hunan Province (2023JJ60492) and the National Natural Science Foundation of China (No. 82203235) with generous financial support.References
- (a) J. Li, K. Gao, M. Bian and H. Ding, Recent advances in the total synthesis of cyclobutane-containing natural products, Org. Chem. Front., 2020, 7, 136–154 RSC; (b) E. Lee-Ruff and G. Mladenova, Enantiomerically Pure Cyclobutane Derivatives and Their Use in Organic Synthesis, Chem. Rev., 2003, 103, 1449–1484 CrossRef CAS.
- (a) M. Murakami and N. Ishida, Cleavage of Carbon-Carbon σ-Bonds of Four-Membered Rings, Chem. Rev., 2021, 121, 264–299 CrossRef CAS; (b) B. Biletskyi, P. Colonna, K. Masson, J.-L. Parrain, L. Commeiras and G. Chouraqui, Small rings in the bigger picture: ring expansion of three- and four-membered rings to access larger all-carbon cyclic systems, Chem. Soc. Rev., 2021, 50, 7513–7538 RSC.
- (a) A. K. Simlandy, M.-Y. Lyu and M. K. Brown, Catalytic Arylboration of Spirocyclic Cyclobutenes: Rapid Access to Highly Substituted Spiro[3.n]alkanes, ACS Catal., 2021, 11, 12815–12820 CrossRef CAS; (b) L. Nvoa, L. Trulli, A. Parra and M. Tortosa, Stereoselective Diboration of Spirocyclobutenes: A Platform for the Synthesis of Spirocycles with Orthogonal Exit Vectors, Angew. Chem., Int. Ed., 2021, 60, 11763–11768 CrossRef; (c) F. W. Goetzke, A. M. L. Hell, L. van Dijk and S. P. Fletcher, A catalytic asymmetric cross-coupling approach to the synthesis of cyclobutanes, Nat. Chem., 2021, 13, 880–886 CrossRef CAS; (d) Y.-K. Liu, X.-W. Gu, Y.-H. Zhao and X.-F. Wu, Palladium-catalyzed selective alkoxycarbonylation of different alcohols toward the direct synthesis of cyclobutanecarboxylates, J. Catal., 2025, 443, 115956 CrossRef CAS; (e) X.-W. Gu, Y.-H. Zhao and X.-F. Wu, Ligand-controlled regiodivergent aminocarbonylation of cyclobutanols toward 1,1- and 1,2-substituted cyclobutanecarboxamides, Nat. Commun., 2024, 15, 9412 CrossRef CAS PubMed; (f) D. Zhuang, T. Gatera, Z. An and R. Yan, Iron-Catalyzed Ring Expansion of Cyclobutanols for the Synthesis of 1-Pyrrolines by Using MsONH3OTf, Org. Lett., 2022, 24, 771–775 CrossRef CAS.
- (a) Y. H. Liu, M. N. Liu and Z. Q. Song, Highly Regio- and Stereoselective Synthesis of Tetrasubstituted Cyclobutenes via Cyclodimerization of Alkynes Mediated by Zirconium, J. Am. Chem. Soc., 2005, 127, 3662–3663 CrossRef CAS; (b) V. López-Carrillo and A. M. Echavarren, Gold(I)-Catalyzed Intermolecular [2 + 2] Cycloaddition of Alkynes with Alkenes, J. Am. Chem. Soc., 2010, 132, 9292–9294 CrossRef; (c) C. Schotes and A. Mezzetti, Enantioselective Ficini Reaction: Ruthenium/PNNP-Catalyzed [2 + 2] Cycloaddition of Ynamides with Cyclic Enones, Angew. Chem., Int. Ed., 2011, 50, 3072–3074 CrossRef CAS; (d) A. Nishimura, M. Ohashi and S. Ogoshi, Nickel-Catalyzed Intermolecular [2 + 2] Cycloaddition of Conjugated Enynes with Alkenes, J. Am. Chem. Soc., 2012, 134, 15692–15695 CrossRef CAS; (e) M. M. Maturi and T. Bach, Enantioselective Catalysis of the Intermolecular [2 + 2] Photocycloaddition between 2-Pyridones and Acetylenedicarboxylates, Angew. Chem., Int. Ed., 2014, 53, 7661–7664 CrossRef CAS; (f) M. M. Parsutkar, V. V. Pagar and T. V. RajanBabu, Catalytic Enantioselective Synthesis of Cyclobutenes from Alkynes and Alkenyl Derivatives, J. Am. Chem. Soc., 2019, 141, 15367–15377 CrossRef CAS.
- (a) J. Barluenga, L. Riesgo, L. A. López, E. Rubio and M. Tomás, Discrimination of Diazo Compounds Toward Carbenoids: Copper(I)-Catalyzed Synthesis of Substituted Cyclobutenes, Angew. Chem., Int. Ed., 2009, 48, 7569–7572 CrossRef CAS; (b) J. Barluenga, L. Riesgo, G. Lonzi, M. Tomás and L. A. López, Copper(I)-Catalyzed [3 + 1] Cycloaddition of Alkenyldiazoacetates and Iminoiodinanes: Easy Access to Substituted 2-Azetines, Chem. – Eur. J., 2012, 18, 9221–9224 CrossRef CAS; (c) M. Shi, L. P. Liu and J. Tang, Palladium-Catalyzed Ring Enlargement of Aryl-Substituted Methylenecyclopropanes to Cyclobutenes, J. Am. Chem. Soc., 2006, 128, 7430–7431 CrossRef CAS PubMed; (d) A. Fürstner and C. Aissa, PtCl2-Catalyzed Rearrangement of Methylenecyclopropanes, J. Am. Chem. Soc., 2006, 128, 6306–6307 CrossRef; (e) H. Xu, W. Zhang, D. Shu, J. B. Werness and W. Tang, Synthesis of Cyclobutenes by Highly Selective Transition-Metal-Catalyzed Ring Expansion of Cyclopropanes, Angew. Chem., Int. Ed., 2008, 47, 8933–8936 CrossRef CAS; (f) R. Liu, M. Zhang, T. P. Wyche, G. N. WinstonMcPherson, T. S. Bugni and W. Tang, Stereoselective Preparation of Cyclobutanes with Four Different Substituents: Total Synthesis and Structural Revision of Pipercyclobutanamide A and Piperchabamide G, Angew. Chem., Int. Ed., 2012, 51, 7503–7506 CrossRef CAS PubMed; (g) S. Chuprakov, B. T. Worrell, N. Selander, R. K. Sit and V. V. Fokin, Stereoselective 1,3-Insertions of Rhodium(II) Azavinyl Carbenes, J. Am. Chem. Soc., 2014, 136, 195–202 CrossRef CAS PubMed; (h) S. C. Patel, M. W. Smith, J. A. M. Mercer, K. Suzuki and N. Z. Burns, Enantioselective Cyclobutenylation of Olefins Using N-Sulfonyl-1,2,3-Triazoles as Vicinal Dicarbene Equivalents, Org. Lett., 2021, 23, 6530–6535 CrossRef CAS PubMed.
- Y. Deng, L. A. Massey, P. Y. Zavalij and M. P. Doyle, Catalytic Asymmetric [3 + 1]-Cycloaddition Reaction of Ylides with Electrophilic Metallo-enolcarbene Intermediates, Angew. Chem., Int. Ed., 2017, 56, 7479–7483 CrossRef CAS PubMed.
- Y. Xu, Z. Wang and J. T. Sun, Asymmetric [3 + 1]-Cycloaddition Reaction via Diazo Discrimination, Org. Lett., 2021, 23, 7613–7617 CrossRef CAS.
- X. Q. Ning, Y. K. Chen, F. D. Hu and Y. Xia, Palladium-Catalyzed Carbene Coupling Reactions of Cyclobutanone N-Sulfonylhydrazones, Org. Lett., 2021, 23, 8348–8352 CrossRef CAS.
- W. Ouyang, J. Huo and J. Wang, Rh(II)-Catalyzed Ring Expansion of Cyclopropyl N-Tosylhydrazones to 1-Substituted Cyclobutenes, Synlett, 2023, 1507–1511 CAS.
- (a) C.-X. Zhao, T. Liu, M. Xu, H. Lin and C.-J. Zhang, A fundamental study on the fluorescence-quenching effect of nitro groups in tetraphenylethene AIE dyes with electron-withdrawing groups, Chin. Chem. Lett., 2021, 32, 1925–1928 CrossRef CAS; (b) D. Porwal and M. Oestreich, B(C6F5)3-Catalyzed Reduction of Aromatic and Aliphatic NitroGroups with Hydrosilanes, Eur. J. Org. Chem., 2016, 3307–3309 CrossRef CAS; (c) M. Orlandi, F. Tosi, M. Bonsignore and M. Benaglia, Metal-Free Reduction of Aromatic and Aliphatic Nitro Compounds to Amines: A HSiCl3-Mediated Reaction of Wide General Applicability, Org. Lett., 2015, 17, 3941–3943 CrossRef CAS PubMed.
- D.-L. An, C. Xu, M. Wang, Q. Shu and X. Zhou, Photocatalyzed C–H Nitration of Arenes, J. Org. Chem., 2025, 90, 4313–4324 CrossRef CAS.
- (a) Y. Zheng, Q.-Q. Hu, Q. Huang and Y. Xie, Late-Stage C-H Nitration of Unactivated Arenes by Fe(NO3)3·9H2O in Hexafluoroisopropanol, Org. Lett., 2024, 26, 3316–3320 CrossRef CAS; (b) Y. Zheng, Z. Liu, Q. Huang and Y. Xie, Ipso-Nitration of Boronic Esters Enabled by Ferric Nitrate Nonahydrate (Fe(NO3)3·9H2O) in HFIP, Org. Lett., 2025, 27, 2997–3002 CrossRef CAS; (c) S. J. S. Düsel and B. König, Visible-Light-Mediated Nitration of Protected Anilines, J. Org. Chem., 2018, 83(5), 2802–2807 CrossRef PubMed.
- CCDC 2478060: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p5mfy.
| This journal is © The Royal Society of Chemistry 2026 |