Mechanochemical synthesis of pyrrolo[1,2-a]indoles via consecutive C–C and C–N bond formation in the presence of an ionic liquid: antimicrobial and photophysical studies
Tanmay
Pramanik
 a,
Satyajit
Pal
a,
Satyajit
Pal
 a,
Nargis
Sultana
a,
Anupam
Kundu
b,
Anindita
Mukherjee
c,
Sougata
Santra
a,
Nargis
Sultana
a,
Anupam
Kundu
b,
Anindita
Mukherjee
c,
Sougata
Santra
 cd,
Naznin Ara
Begum
cd,
Naznin Ara
Begum
 a,
Jnanendra
Rath
b,
Grigory V.
Zyryanov
ce and
Adinath
Majee
a,
Jnanendra
Rath
b,
Grigory V.
Zyryanov
ce and
Adinath
Majee
 *a
*a
aDepartment of Chemistry, Visva-Bharati (A Central University), Santiniketan 731235, India. E-mail: adinath.majee@visva-bharati.ac.in
bDepartment of Botany, Visva-Bharati (A Central University), Santiniketan 731235, India
cDepartment of Organic & Biomolecular Chemistry, Chemical Engineering Institute, Ural Federal University, 19 Mira Str., Yekaterinburg, 620002, Russian Federation
dDepartment of Medicinal Biotechnology, Sirius University of Science and Technology, 1 Olympic Ave., Sirius Federal Territory, 354340, Russian Federation
eI. Ya. Postovskiy Institute of Organic Synthesis, Ural Division of the Russian Academy of Sciences, 22 S. Kovalevskoy Street, Yekaterinburg 620219, Russian Federation
First published on 18th November 2025
Abstract
Brønsted acidic ionic liquid (BAIL), 1-butane sulfonic acid-3-methylimidazolium tosylate ([BSMIM]OTs), is an efficient and useful organocatalyst for the two-component reaction of 3-substituted indoles with propargyl alcohols to synthesize biologically active pyrrolo[1,2-a]indole derivatives by the ball-milling technique. This mechanochemical technique has taken the reactions beyond the conventional grinding method to provide good to excellent yields of the corresponding products in a very short time. The catalyst enables the efficient formation of both C–C and C–N bonds under environmentally friendly conditions, conducted neat, without any additional base or ligand. Key advantages of this methodology include its simplicity, the use of easily accessible reagents, a broad substrate scope, and its metal-free, solvent-free, and environmentally friendly reaction conditions. Furthermore, the catalyst can be easily reused, contributing to the overall sustainability of the process. In addition, the present method is also applicable for gram-scale synthesis. This procedure is associated with high EcoScale metrics and a low E-factor. The synthesized compounds exhibited promising biological activities. Antimicrobial activity against various Gram-positive and Gram-negative bacteria was evaluated for some randomly selected synthesized compounds. In addition, we also studied the photophysical behavior of these unique and novel 3H-pyrrolo[1,2-a]indole derivatives.
Introduction
Nowadays, chemical industries prioritize sustainability as a key feature. In this context, in 1992, Roger Sheldon introduced the E-factor to point out the need for methods that are efficient, economical, and environmentally friendly, and EcoScale has also gained attention as a semi-quantitative tool for selecting organic methodology based on economic and ecological parameters.1 The concept of green chemistry encourages processes that minimize waste and environmental impact by using benign chemicals, catalysts, solvents, and avoiding hazardous conditions. By reducing the use of extreme reaction conditions, harsh solvents, and metals, chemical processes can become more environmentally friendly and economically adaptable. Solvent-free, room-temperature reactions can significantly reduce the release of harmful chemicals into the environment.Chemical reactions under ball-milling have received massive attention in academia and industry due to their specific advantages like shorter reaction time, usually solvent-free operation, better yield, ambient conditions, and often improved selectivity.2 A wide range of reactions have been efficiently developed by ball-milling, for example, carbon–carbon coupling,3 carbon–heteroatom bond formation,4 oxidation reactions,5 hydrogenation,6 polymerization reactions,7 crystal preparation,8 heterocycles synthesis,9 enantioselective synthesis,10 and multi-component organic reactions.11
Room-temperature ionic liquids (RTILs) have been analyzed as good, greener alternatives to volatile organic solvents due to their unique properties, including easy recyclability, negligible vapor pressure, high thermal stability, large liquid temperature range, and strong dissolving power for a wide range of organic and inorganic compounds.12 The properties of ionic liquids can be altered in many diverse ways by changing the cations and/or anions. So far, a large number of functionalized ILs have been prepared for different purposes by choosing appropriate cations and anions.13 Forbes and Davis introduced Brønsted acidic ionic liquids (BAILs), which have been receiving attention in catalysis due to their strong acidity.14 BAILs are preferred over mineral acids as BAILs show strong acidity with the usual properties of ionic liquids, such as non-flammability, stability and wide solvating ability. As a result, many transformations have been carried out involving acidic protons of these ILs. In this context, our group is also actively exploring catalysis using BAILs and imidazole-based zwitterions.15
Pyrrolo[1,2-a]indoles and their derivatives are commonly found in numerous natural products and biologically significant compounds. Substrates containing pyrrolo[1,2-a]indoles and their analogues constitute a crucial class of privileged structural units commonly encountered in natural products and pharmaceuticals.16 Especially, C3 carbon-derived pyrrolo-indoline shows a distinctive range of chemical and biological properties. For example, Calycanthaceae plant extracts have been used as traditional medicines for the treatment of fungal infection,17 melanogenesis, tumors, hypertension, and inflammatory conditions,18 while mytomycine-C is widely used as an antibacterial and anticancer drug.19 Thus, the synthesis of 3H-pyrrolo[1,2-a]indoles has been receiving much attention in the field of medicinal and pharmaceutical chemistry. Typically, very few methods are available for the synthesis of 3H-pyrrolo[1,2-a]indoles by cascade reaction between 3-substituted indoles and propargylic alcohols in the presence of different catalysts and reagents.20,21 However, these methodologies have been developed using expensive reagents, harsh reaction conditions and additives. Therefore, finding a new methodology for the synthesis of 3H-pyrrolo[1,2-a]indoles in terms of efficiency, green reaction conditions, operational simplicity and economic practicability is highly desirable.
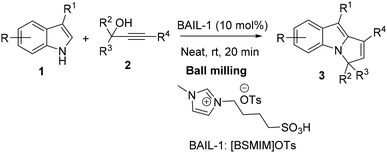
Herein, we are pleased to report a fast and convenient mechanochemical synthesis of 3H-pyrrolo[1,2-a]indoles in the presence of the Brønsted acidic ionic liquid (BAIL) 1-butane sulfonic acid-3-methylimidazolium tosylate ([BSMIM]OTs) under ball-milling conditions by the reaction between 3-substituted indoles and propargyl alcohols (Scheme 1). To the best of our knowledge, no other process method reported in the literature achieves this one-pot synthesis via mechanochemical activation within a short reaction time.
Results and discussion
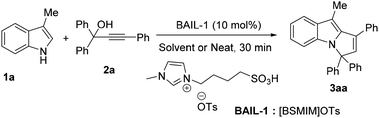
Initially, a mixture of 3-methyl-1H-indole (1a, 1 mmol) and propargylic alcohol (2a, 1 mmol) was reacted at room temperature for 30 min in the presence of 10 mol% of 1-butane sulfonic acid-3-methylimidazolium tosylate, [BSMIM]OTs (BAIL-1) in acetonitrile medium with the help of stirring (Table 1, entry 1). To our delight, a pure solid product of 3H-pyrrolo[1,2-a]indole 3aa was obtained in a moderate yield of 68%. Next, we observed that in an ethanolic medium, a very low yield was obtained (Table 1, entry 2). An aqueous medium for the same reaction did not afford any product (Table 1, entry 3). For further evaluation, some other common solvents such as dichloromethane, toluene, 1,2-DCE, THF, acetone, DMF, DMSO, acetonitrile, PET and EtOAc (Table 1, entries 4–12) were employed, which also did not improve the yield of the desired compound. After that, we examined the effect of temperature; we refluxed the reaction under the same catalytic conditions, but no considerable improvement of the desired product was observed (Table 1, entries 13 and 14). Therefore, we turned our attention to the concept of “no solvent is the best solvent” and carried out the reaction under solvent-free conditions, initially heating at 80 °C and then at room temperature (Table 1, entries 15 and 16). These observations showed that the yield improved, but the results were not very encouraging. Therefore, we carried out the reaction by switching from stirring to grinding with a pestle and mortar for 30 min, and the desired 3H-pyrrolo[1,2-a]indole derivative was generated in 68% yield (Table 1, entry 17). Next, we carried out the reaction under ball-milling conditions (Retsch PM 100). We observed that using four balls (10 mm) with 500 rpm for 20 min gave the best result (84%) (Table 1, entry 18) in the presence of 10 mol% of BAIL-1. By decreasing the time (10 min), a 55% yield was obtained (Table 1, entry 19). Furthermore, we have observed that 20 mol% of BAIL-1 did not alter the yield formation, while it was reduced when using 5 mol% of the catalyst (Table 1, entries 20 and 21). Finally, we also carried out the model reaction by increasing the milling time to 40 min in the presence of less BAIL-1 (5 mol%), but the yield was not satisfactory (52%) (Table 1, entry 22).| Entry | Catalyst | Solvent | Condition | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (1 mmol), 2a (1 mmol), and BAIL-1 (10 mol%); 2 mL of solvent was used where necessary. Conventional stirring was carried out for 30 min. b Isolated yields. c Manual grinding for 30 min. d Planetary ball-milling apparatus (Retsch PM 100) using four balls (size 10 mm), 500 rpm, 20 min. e Milling time 10 min. f 20 mol% of BAIL-1 was used. g 5 mol% of BAIL-1 was used. h Milling time 40 min in the presence of 5 mol% of BAIL-1. | |||||
| 1 | BAIL-1 | CH3CN | Stirring | rt | 68 |
| 2 | BAIL-1 | EtOH | Stirring | rt | 17 |
| 3 | BAIL-1 | H2O | Stirring | rt | nr |
| 4 | BAIL-1 | DCM | Stirring | rt | 58 |
| 5 | BAIL-1 | Toluene | Stirring | rt | 15 |
| 6 | BAIL-1 | 1,2-DCE | Stirring | rt | 60 |
| 7 | BAIL-1 | THF | Stirring | rt | 18 |
| 8 | BAIL-1 | Acetone | Stirring | rt | Trace |
| 9 | BAIL-1 | DMSO | Stirring | rt | 20 |
| 10 | BAIL-1 | DMF | Stirring | rt | 25 |
| 11 | BAIL-1 | PET | Stirring | rt | 30 |
| 12 | BAIL-1 | EtOAc | Stirring | rt | 23 |
| 13 | BAIL-1 | CH3CN | Stirring | Reflux | 66 |
| 14 | BAIL-1 | 1,2-DCE | Stirring | Reflux | 64 |
| 15 | BAIL-1 | Neat | Stirring | 80 °C | 75 |
| 16 | BAIL-1 | Neat | Stirring | rt | 74 |
| 17 | BAIL-1 | Neat | Grindingc | rt | 68 |
| 18 | BAIL-1 | Neat | Ball-millingd | rt | 84 |
| 19 | BAIL-1 | Neat | Ball-millinge | rt | 55 |
| 20 | BAIL-1 | Neat | Ball-milling | rt | 84f |
| 21 | BAIL-1 | Neat | Ball-milling | rt | 48g |
| 22 | BAIL-1 | Neat | Ball-milling | rt | 52h |
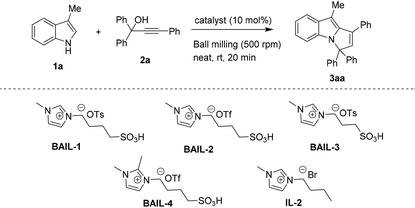
Next, we optimized the catalyst selection for this reaction, as shown in Table 2. All of these reactions were carried out under ball-milling. We prepared some other BAIL catalysts (BAIL-2, 3 and 4) in our laboratory and noticed that these were not as effective as BAIL-1 (Table 2, entries 1–4 ). IL-2 also did not give the desired product in satisfactory yield, apparently due to the lack of acidic properties (Table 2, entry 5). We also tested some other common Lewis and Brønsted acid catalysts, including AgOTf, FeCl3, Zn(OTf)2, Cu(OTf)2, BF3·Et2O, TfOH and p-TSA, but these were also not as effective as BAIL-1 (Table 2, entries 6–12). Here, we observed that imidazolium Brønsted acidic ionic liquids were more effective than other Brønsted acid catalysts (i.e. TfOH and p-TSA). This may be due to their tunable acidity and dual functionality as a catalyst and solvent, which allows for enhanced reaction rates and selectivity. Thus, the optimal reaction conditions were obtained using 3-methyl-1H-indole (1a, 1 mmol) and propargylic alcohol (2a, 1 mmol) in the presence of 10 mol% of 1-butane sulfonic acid-3-methylimidazolium tosylate ([BSMIM]OTs) BAIL-1 under ball-milling conditions (4 balls at 500 rpm) for 20 min (Table 1, entry 18).
| Entry | Catalyst | Yieldb |
|---|---|---|
| a Reaction conditions: carried out with 1 mmol of 1a and 1 mmol of 2a in the presence of various catalysts (10 mol%) under ball-milling in neat conditions at room temperature for 20 min. b Isolated yields. c ND = not detected in TLC. d p-TSA = para-toluenesulfonic acid. | ||
| 1 | BAIL-1 | 84% |
| 2 | BAIL-2 | 56% |
| 3 | BAIL-3 | 73% |
| 4 | BAIL-4 | 71% |
| 5 | IL-2 | 28% |
| 6 | AgOTf | 68% |
| 7 | FeCl3 | 55% |
| 8 | Zn(OTf)2 | NDc |
| 9 | Cu(OTf)2 | 72% |
| 10 | BF3·Et2O | 48% |
| 11 | TfOH | 39% |
| 12 | p-TSAd | 56% |
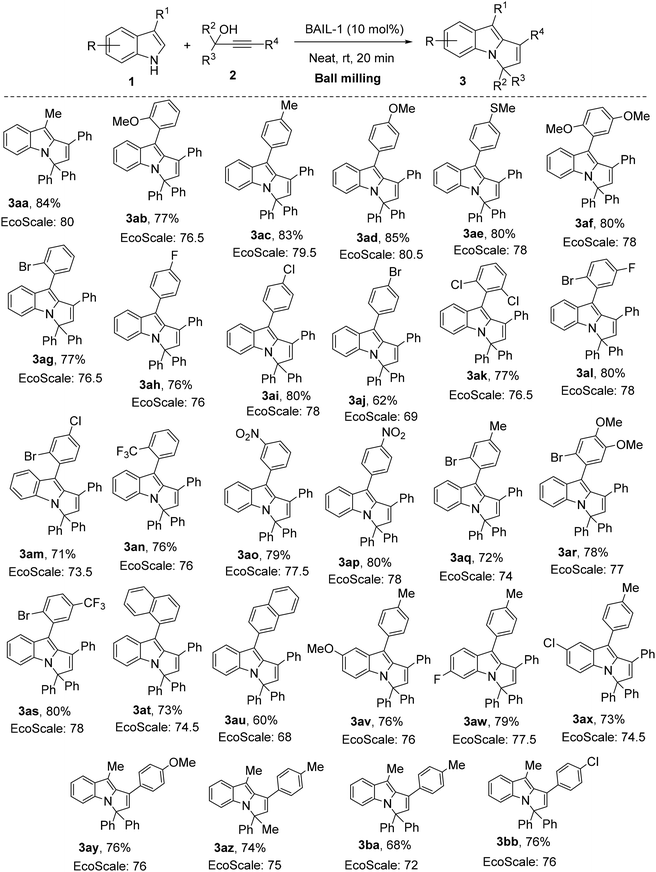
After optimizing the reaction conditions, we turned our attention to the scope of this reaction. As shown in Scheme 2, a wide range of 3-substituted indoles were reacted with diverse propargyl alcohols to demonstrate the broad utility of this methodology. During optimization, we already found that 3-methylindole (1a) produced the desired 3H-pyrrolo[1,2-a]indole derivative (3aa) in 84% yield when it reacted with 1,1,3-triphenylprop-2-yn-1-ol (2a). First, we varied substituents at the 3-position of the indole moiety. We found that the reaction yields were good in the case of both aliphatic and aromatic substituents (62–85%). Excellent yields were observed for halogens (3ag–3aj), electron-withdrawing groups (3an–3ap), electron-donating groups (3ab–3af) and naphthyl moieties (3at, 3au). Disubstituted aromatic moieties also gave very good yields (3ak–3am, 3aq, 3as). In addition, the sterically hindered tri-substituted aromatic moiety also produced the desired product in good yield (3ar). Next, we tried 3,5- and 3,6-disubstituted indoles, which also afforded satisfactory yields (3av–3ax). Finally, we tried a few different propargyl alcohols bearing both electron-withdrawing and electron-donating substituents to increase the generality of this method and we achieved moderate to good yields (3ay–3bb). All these reactions were conducted in an open atmosphere.
The known synthesized compounds were compared with existing spectral data, and the characterization of the new compounds was achieved using their spectral and analytical data. The X-ray crystallographic analysis of 9-methyl-1,3,3-triphenyl-3H-pyrrolo[1,2-a]indole (3aa) finally confirmed the structure as shown in Fig. 1 with CCDC 2457288.22
 | ||
| Fig. 1 X-ray crystallographic structure of 9-methyl-1,3,3-triphenyl-3H-pyrrolo[1,2-a]indole (3aa) with CCDC 2457288. | ||
We also investigated the efficiency of our method on a large scale, and for this, we employed 1.31 g of 3-methyl-1H-indole (1a), 2.84 g of propargylic alcohol (2a), and 10 mol% of BAIL-1, as shown in Scheme 3. Thereafter, performing the reaction using the ball-milling technique, we obtained 3.18 g of the desired product 9-methyl-1,3,3-triphenyl-3H-pyrrolo[1,2-a]indole (3aa).
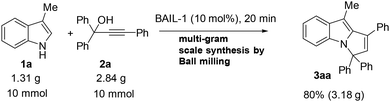 | ||
| Scheme 3 Multigram synthesis of 9-methyl-1,3,3-triphenyl-3H-pyrrolo[1,2-a]indole (3aa) by mechanochemical ball-milling. | ||
To check the reusability of our catalyst (BAIL-1), the reaction mixture was carefully washed with water (10 × 3 mL) and filtered after completion of the reaction. Then, the filtrate (aqueous part) was evaporated, and the ionic liquid catalyst was recovered, dried, and reused. It was observed that the catalyst is stable and conveniently recycled up to four times without any significant loss of catalytic activity (Table 3).
The compliance of this mechanochemical protocol with green reaction criteria is established using the green metrics EcoScale1b and E-factor.1a Conventionally, the EcoScale metric indicates the simplicity and general applicability of a method. This parameter considers cost, safety, technical setup, energy, and purification aspects, while the E-factor provides a quantitative measure of the waste generated, which in turn is used to assess the quality of an organic process. The calculated EcoScale values for the synthesis of compounds 3 are shown in Scheme 2. As shown in Scheme 2, the EcoScale metric is more than 75 for most of the compounds (see the SI). In addition, the calculated E-factor for synthesizing 3aa is 0.25 (see the SI). The detailed calculation of these green metrics is provided in the SI.
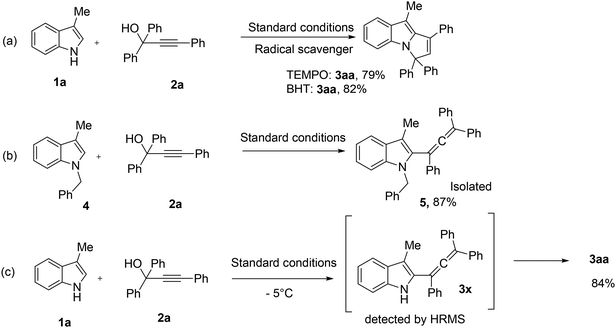
Two control experiments were conducted for the mechanistic investigation to predict the probable reaction pathway (Scheme 4). In the first experiment, the reaction yield did not drop significantly in the presence of radical scavengers such as 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO) and 2,6 di-tertbutyl-4-methyl phenol (BHT) (Scheme 4a). From this result, it can be concluded that the reaction goes through a polar or non-radical pathway. Secondly, we performed a reaction between 1-benzyl-3-methyl-1H-indole (4), i.e. N-protected indole, and 1,1,3-triphenylprop-2-yn-1-ol (2a) under our standard conditions. In this experiment, we observed the formation of the allene product 1-benzyl-3-methyl-2-(1,3,3-triphenylpropa-1,2-dien-1-yl)-1H-indole (5) as the final product in 87% yield, which confirms that the N-protected indole does not give the desired product. Finally, we also detected the formation of an allene intermediate 3-methyl-2-(1,3,3-triphenylpropa-1,2-dien-1-yl)-1H-indole (3x) (by HRMS) when we carried out the reaction between 1a and 2a under standard conditions at −5 °C (Scheme 4c).
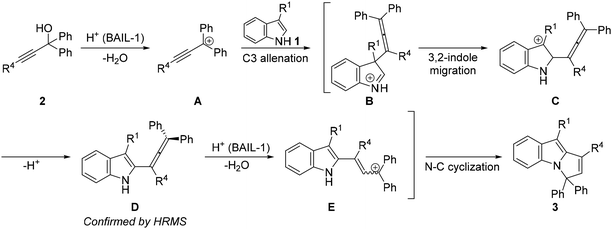
Based on the above control experiments and literature survey,20,21 we proposed a probable mechanistic pathway as shown in Scheme 5. The first step is the formation of a stabilized carbocation A, which is trapped by the indole, yielding 1,2-dihyrdoindolinium cation B.21 Migration of the allenyl group followed by protonation and N–C cyclization yields product 3 through intermediates C, D, and E, respectively. The formation of the cation E and subsequent endo-tet cyclization20a,c seems more likely than the endo-trig process,20b which is further complicated by the linear structure of the allenyl fragment. The formation of intermediate D was confirmed by HRMS.
In addition, we studied the antimicrobial activity of some selected synthesized compounds. Furthermore, we also carried out photophysical studies. The results are given in the SI.
Conclusions
In summary, we developed a mechanochemical method for the synthesis of 3H-pyrrolo[1,2-a]indole derivatives in the presence of Brønsted acidic ionic liquid 1-butane sulfonic acid-3-methylimidazolium tosylate ([BSMIM]OTs) in a short reaction time, and their antimicrobial properties have been studied. In addition, the photophysical behavior of the synthesized compounds was also studied. The reaction did not require any solvent or metal. Furthermore, the present methodology is also applicable for gram-scale synthesis without any significant loss of efficiency, demonstrating the potential application of the present method for the large-scale synthesis of 3H-pyrrolo[1,2-a]indole derivatives. The present reaction most likely occurs by the formation of an allene intermediate in the presence of the BAIL catalyst. The use of readily available starting materials, an organocatalyst, very fast reaction, a broad substrate scope, aerobic reaction conditions, and tolerance of a wide range of functional groups are the notable advantages of this present approach. The proposed approach is consistent with the principles of atom economy and the nature of the substituents in the final compounds can be varied over a fairly wide range. In addition, this protocol is associated with a low E-factor and high EcoScale metrics, which are consistent with the principles of green chemistry. These advantages render this protocol facile and suitable for creating a diverse library of pyrrolo[1,2-a]indole derivatives.Experimental section
General information
Melting points were determined on a glass disk with an electric hot plate and are uncorrected. 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were run in CDCl3 solutions. Commercially available substrates were freshly distilled before the reaction. Solvents, reagents, and chemicals were purchased from Aldrich, Fluka, Merck, SRL, Spectrochem, and Process Chemicals. Brønsted acidic ionic liquids were prepared according to the previously reported method (for details, see SI).23 A planetary Retsch™ PM100 ball-milling apparatus was employed using four balls (stainless steel, 10 mm). The reaction was performed in a 50 mL grinding jar. General procedures for the synthesis of the starting indoles and propargyl alcohols are given in SI.General procedure for the synthesis of compounds 3
A grinding beaker (50 mL) and milling balls (4 × 10 mm) were set as the reaction chamber. For each reaction, a mixture of propargyl alcohol (1 mmol), 3-substituted indole (1 mmol), and 10 mol% of Brønsted acidic ionic liquid was milled for 20 min at 500 rpm at room temperature. After completion of the reaction (monitored by TLC), the mixture was diluted with saturated saline water (3 × 15 mL), and extracted with ethyl acetate. The combined organic layer was collected and dried over anhydrous Na2SO4. The residue was purified by column chromatography on silica gel to obtain the desired products.Typical procedure for the synthesis of compounds 3aa on a large scale
A grinding beaker (50 mL) and milling balls (4 × 10 mm) were set as the reaction chamber. After that, a mixture of propargyl alcohol (2a, 2.84 g, 10 mmol), 3-methyl-1H-indole (1a, 1.31 g, 10 mmol), and 10 mol% of Brønsted acidic ionic liquid (3.90 g) was milled for 20 min at 500 rpm at room temperature. After completion of the reaction (monitored by TLC), the mixture was diluted with saturated saline water (3 × 100 mL), and extracted with ethyl acetate. The combined organic layer was collected and dried over anhydrous Na2SO4. The residue was purified by column chromatography on silica gel to obtain the desired product 9-methyl-1,3,3-triphenyl-3H-pyrrolo[1,2-a]indole (3aa) in 80% yield (3.18 g).Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: general information, experimental procedure, crystallographic data, antimicrobial studies, photophysical studies, green metrics calculations, 1H & 13C NMR spectra. See DOI: https://doi.org/10.1039/d5ob01489a.This study was carried out using publicly available data.
CCDC 2457288 (3aa) contains the supplementary crystallographic data for this paper.22
Acknowledgements
We are thankful to the DST-PURSE, DST-FIST and UGC-SAP programs. S. Santra acknowledges the Russian Science Foundation (Grant # 24-23-00516) for financial support.References
- (a) R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC; (b) K. Van Aken, L. Strekowski and L. Patiny, Beilstein J. Org. Chem., 2006, 2, 3 Search PubMed.
- R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437–1451 RSC.
- (a) R. Schmidt, R. Thorwirth, T. Szuppa, A. Stolle, B. Ondruschka and H. Hopf, Chem. – Eur. J., 2011, 17, 8129–8138 CrossRef CAS PubMed; (b) R. Thorwirth, A. Stolle and B. Ondruschka, Green Chem., 2010, 12, 985–991 RSC.
- (a) J. G. Hernández and E. Juaristi, J. Org. Chem., 2010, 75, 7107–7111 CrossRef; (b) J. Bonnamour, T.-X. Métro, J. Martinez and F. Lamaty, Green Chem., 2013, 15, 1116–1120 RSC; (c) Z. Zhang, H.-H. Wu and Y.-J. Tan, RSC Adv., 2013, 3, 16940–16944 RSC.
- (a) R. Thorwirth, F. Bernhardt, A. Stolle, B. Ondruschka and J. Asghari, Chem. – Eur. J., 2010, 16, 13236–13242 CrossRef CAS; (b) K. Kubota, Y. Pang, A. Miura and H. Ito, Science, 2019, 366, 1500–1504 CrossRef CAS.
- W. Su, J. Yu, Z. Li and Z. Jiang, J. Org. Chem., 2011, 76, 9144–9150 CrossRef CAS PubMed.
- (a) J. Li, C. Nagamani and J. S. Moore, Acc. Chem. Res., 2015, 48, 2181–2190 CrossRef CAS PubMed; (b) N. Willis-Fox, E. Rognin, T. A. Aljohani and R. Daly, Chem, 2018, 4, 2499–2537 CrossRef CAS.
- (a) D. Hasa, G. Schneider-Rauber, D. Voinovich and W. Jones, Angew. Chem., 2015, 127, 7479–7483 CrossRef; (b) T. Friščić, Chem. Soc. Rev., 2012, 41, 3493–3510 RSC.
- (a) H. Xu, H.-W. Liu, K. Chen and G.-W. Wang, J. Org. Chem., 2018, 83, 6035–6049 CrossRef CAS PubMed; (b) X. Jiang, J. Chen, W. Zhu, K. Cheng, Y. Liu, W.-K. Su and C. Yu, J. Org. Chem., 2017, 82, 10665–10672 CrossRef CAS PubMed; (c) J. Martínez, L. Sánchez, F. J. Pérez, V. Carranza, F. Delgado, L. Reyes and R. Miranda, J. Chem., 2016, 2016, 1–6 CrossRef.
- I. N. Egorov, S. Santra, D. S. Kopchuk, I. S. Kovalev, G. V. Zyryanov, A. Majee, B. C. Ranu, V. L. Rusinov and O. N. Chupakhin, Green Chem., 2020, 22, 302–315 RSC.
- (a) M. Leonardi, M. Villacampa and J. C. Menéndez, Chem. Sci., 2018, 9, 2042–2064 RSC; (b) T. K. Achar, A. Bose and P. Mal, Beilstein J. Org. Chem., 2017, 13, 1907–1931 CrossRef CAS PubMed; (c) A. Sarkar, S. Santra, S. K. Kundu, A. Hajra, G. V. Zyryanov, O. N. Chupakhin, V. N. Charushin and A. Majee, Green Chem., 2016, 18, 4475–4525 RSC.
- (a) Ionic liquids in synthesis: 2 Volume set, ed. P. Wasserscheid and T. Welton, Wiley-VCH Verlag, 2nd edn, 2008 Search PubMed; (b) J. Ranke, S. Stolte, R. Störmann, J. Arning and B. Jastorff, Chem. Rev., 2007, 107(6), 2183–2206 CrossRef CAS PubMed; (c) T. L. Greaves and C. J. Drummond, Chem. Rev., 2008, 108, 206–237 CrossRef CAS PubMed; (d) N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC; (e) J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed; (f) L. Myles, R. G. Gore, N. Gathergood and S. J. Connon, Green Chem., 2013, 15, 2740 RSC; (g) R. Sheldon, Chem. Commun., 2001, 2399–2407 RSC.
- (a) A. D. Sawant, D. G. Raut, N. B. Darvatkar and M. M. Salunkhe, Green Chem. Lett. Rev., 2011, 4, 41–54 CrossRef CAS; (b) B. C. Ranu and S. Banerjee, Org. Lett., 2005, 7, 3049–3052 CrossRef CAS PubMed; (c) A. K. Chakraborti and S. R. Roy, J. Am. Chem. Soc., 2009, 131, 6902–6903 CrossRef CAS PubMed; (d) L. Myles, N. Gathergood and S. J. Connon, Chem. Commun., 2013, 49, 5316–5318 RSC; (e) A. Kumar, S. Srivastava, G. Gupta, P. Kumar and J. Sarkar, RSC Adv., 2013, 11, 3548–3552 RSC.
- (a) A. C. Cole, J. L. Jensen, I. Ntai, K. L. T. Tran, K. J. Weaver, D. C. Forbes and J. H. Davis Jr, J. Am. Chem. Soc., 2002, 124, 5962–5963 CrossRef CAS PubMed; (b) D. Forbes, J. Mol. Catal. A: Chem., 2004, 214, 129–132 CrossRef CAS.
- (a) S. Mahato, S. Santra, R. Chatterjee, G. V. Zyryanov, A. Hajra and A. Majee, Green Chem., 2017, 19, 3282–3295 RSC; (b) S. Mondal, S. Sarkar, S. Pal, A. Kundu, J. Rath, S. Santra and A. Majee, J. Mol. Liq., 2024, 415, 126399 CrossRef CAS; (c) A. Majee, T. Pramanik, S. Pal and S. Santra, Synlett, 2024, 2447–2452 CrossRef.
- R. Kakadiya, H. Dong, P.-C. Lee, N. Kapuriya, X. Zhang, T.-C. Chou, T.-C. Lee, K. Kapuriya, A. Shah and T.-L. Su, Bioorg. Med. Chem., 2009, 17, 5614–5626 CrossRef CAS PubMed.
- M. S. C. Pedras and M. Suchy, Org. Biomol. Chem., 2005, 3, 2002–2007 RSC.
- (a) R.-Y. Gui, W.-W. Liang, S.-X. Yang, L. Llu and J.-C. Qin, Asian J. Chem., 2014, 26, 4445–4448 CrossRef CAS; (b) T. Araki, Y. Manabe, K. Fujioka, H. Yokoe, M. Kanematsu, M. Yoshida and K. Shishido, Tetrahedron Lett., 2013, 54, 1012–1014 CrossRef CAS; (c) J.-W. Zhang, J.-M. Gao, T. Xu, X.-C. Zhang, Y.-T. Ma, S. Jarussophon and Y. Konishi, Chem. Biodivers., 2009, 6, 838–845 CrossRef CAS PubMed.
- (a) U. Galm, M. H. Hager, S. G. Van Lanen, J. Ju, J. S. Thorson and B. Shen, Chem. Rev., 2005, 105, 739–758 CrossRef CAS PubMed; (b) W. T. Bradner, Cancer Treat. Rev., 2001, 27, 35–50 CrossRef CAS PubMed.
- (a) S. Pan, X. Wang, M. Liu, F. Fang, Y. Xia, P. Li and W. Li, ChemCatChem, 2024, 16, e202400900 CrossRef CAS; (b) X.-Z. Zhang, Z.-W. Qiu, G.-H. Wen, A.-J. Ma, P.-J. Bao, J.-Y. Du, X.-T. Xu, N. Feng and B. Q. Li, Synthesis, 2020, 3640–3649 CrossRef CAS; (c) J.-F. Bai, L. Zhao, F. Wang, F. Yan, T. Kano, K. Maruoka and Y. Li, Org. Lett., 2020, 22, 5439–5445 CrossRef CAS PubMed; (d) L. Hao, Y. Pan, T. Wang, M. Lin, L. Chen and Z.-P. Zhan, Adv. Synth. Catal., 2010, 352, 3215–3222 CrossRef CAS.
- A. Kale, S. J. Kwon, J. Lee, J. K. Lee and K. Lee, Org. Lett., 2024, 26, 1196–1200 CrossRef CAS PubMed.
- CCDC 2457288: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2nh0ck.
- Z. Du, Z. Li and Y. Deng, Synth. Commun., 2005, 35, 1343–1349 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |