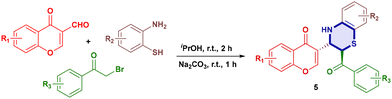Solvent-assisted one-pot green and diastereoselective synthesis of 1,4-oxazines and 1,4-thioxazines under metal-free conditions
Farukh
Ahmad
,
Rajeev
Singh
and
Naseem
Ahmed
 *
*
Department of Chemistry, Indian Institute of Technology Roorkee, Azad Bhawan, 7-Niti Nagar, Haridwar Highway, Roorkee, UK, India. E-mail: naseem.ahmed@cy.iitr.ac.in
First published on 3rd December 2025
Abstract
A novel and efficient three-component, one-pot green and diastereoselective synthesis of 1,4-oxazines and 1,4-thioxazines using formyl-chromone-based enamines under mild reaction conditions is presented. The desired products are afforded in anti-conformation and high yields (up to 95%). This method offers a significant improvement over traditional approaches, which often require harsh conditions, multiple steps, and expensive reagents. The broad substrate scope and high functional group tolerance make this protocol a powerful tool for highly diastereoselective synthesis of diverse classes of 1,4-oxazine and 1,4-thioxazine compounds, known for their wide range of biological activities. The synthetic route is environmentally friendly, aligning with the principles of green chemistry, as it avoids the use of toxic solvents and heavy metal catalysts.
Introduction
Heterocyclic compounds constitute a significant and diverse category of natural products, biologically and pharmaceutically active molecules and other synthetic compounds.1 Commonly, heteroatoms such as nitrogen (N), oxygen (O), and sulfur (S) are present in heterocyclic compounds, although other elements are also present in heterocyclic rings.2,3 The incorporation of nitrogen and oxygen atoms into cyclic structures imparts unique physico-chemical properties, which are crucial for their various functions, particularly of biological and pharmaceutical importance.4,5 For example, 1,4-oxazines and their saturated counterparts, morpholines, play key roles in medicinal chemistry (Fig. 1).6 N,O-Heterocycles are recognized for their stability and operational efficacy within the human body.7 In fact, approximately 60% of all FDA-approved small-molecule drugs contain at least one nitrogen-containing heterocyclic ring.8,9The development of reliable synthetic methods for the synthesis of 1,4-oxazine derivatives continues to be challenging owing to their broad applications.10 These heterocycles have been synthesised using traditional methods such as hydroamination or hydroalkoxylation of alkenes and alkynes, cyclisation of β-amino alcohols, and annulation of imine intermediates.11–13 Despite the above developments, these approaches often demand extreme conditions, operate over a restricted range of substrates, or provide poor regio- and stereoselectivity.
Significant advancements have been made in recent years due to the development of novel catalytic approaches. Under moderate circumstances, functionalised 1,4-oxazines might be obtained via tandem N–H insertion of α-arylamino ketones with diazo pyruvates, using ruthenium catalysts.14 Asymmetric transfer hydrogenation of alkynones using Ru- and Au-metal relay catalysts gave 3,4-dihydro-2H-1,4-oxazines in high yields, exhibiting high enantioselectivity.15 Through regio- and stereoselective tandem ring-opening and cyclisation of spiro-aziridines, spiro-fused frameworks, including spiro[3,4-dihydrobenzo[b][1,4]oxazine-2,3′-oxindole], have been obtained stereoselectively, yielding enantiopure scaffolds that have considerable synthetic and biological significance.16 Silver triflate-catalysed oxidation of N-propargyl N-sulfonyl amino alcohols has emerged as an effective method for obtaining 3,4-dihydro-2H-1,4-oxazines, thus broadening the synthetic scope. Crucially, solid-phase peptide synthesis (SPPS) has effectively used this technology, allowing for the late-stage integration of oxazine moieties into peptides at threonine and serine ends.17
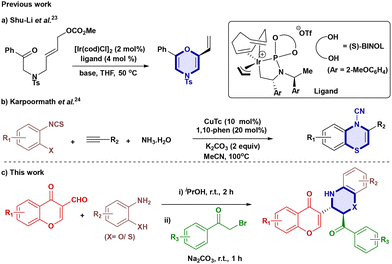
Furthermore, expanding the synthetic scope,18 metal-free [5 + 1] cycloaddition reactions,18d transition metal catalysts,19 metal carbenes,20 and catalyst-free reactions21 as well as microwave irradiation22 have been widely used for synthesising substituted 1,4-oxazines. Recently, enol was used as an O-nucleophile source for an intramolecular substitution reaction on the allylic site using an iridium catalyst to afford chiral 2H-1,4-oxazine derivatives (Scheme 1a)23 and a one-pot three-component synthesis of 1,4-benzothiazines was reported via cyclisation using copper(I) thiophene-2-carboxylate (Scheme 1b).24
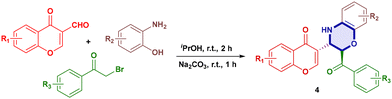
Our group has consistently focused on designing and synthesising biologically active heterocyclic compounds,25 such as 4-hydroxycoumarins, imidazo[1,2-a]pyridines, pyrroles, 1,4-dihydroquinolines and tetrazoles. Dual formyl-chromone and 1,4-oxazine scaffolds represent a new class of molecules with promising applications in medicinal chemistry, as both formyl-chromones and 1,4-oxazines are privileged structures in drug discovery. Chromone-based enamines were previously reported26via condensation of chromone derivatives with amines. We adopted a modified procedure to prepare these intermediates efficiently; herein, we report a novel and operationally simple method that enables the one-pot synthesis of 1,4-oxazine and 1,4-thioxazine derivatives under mild basic conditions, using a green and eco-friendly solvent system, with high atom economy and minimal waste generation (Scheme 1c).
Results and discussion
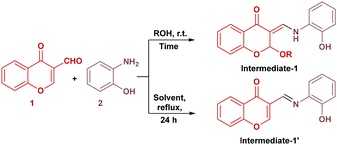
To optimize the reaction conditions, we initially screened various alcohols (ROH) at room temperature, of which primary alcohols such as methanol (MeOH), ethanol (EtOH), and isopropanol (iPrOH) provided high yields of the desired product (Table 1, entries 1–3). tert-Butanol (tBuOH), a sterically hindered alcohol, also gave an excellent yield of 95% (Table 1, entry 4), demonstrating the tolerance of the reaction to bulky solvents. In contrast, polyhydroxy alcohols such as propane-1,2-diol, ethylene glycol, and glycerol led to significantly reduced yields (Table 1, entries 5, 6 and 9). Phenol and benzyl alcohol (Table 1, entries 7 and 8) were found to be ineffective and failed to give the desired product. This might be due to their poor nucleophilicity and acidic nature. Similarly, propargyl alcohol, allylic alcohol and H2O gave lower yields of the desired product (Table 1, entries 10–12). The optimised reaction time for obtaining maximum product yield was 2 hours (Table 1, entries 13–15) when using EtOH, iPrOH, or tBuOH, confirming that the reaction proceeds efficiently and rapidly in these solvents. Isopropanol remained the optimal solvent, delivering 98% yield in just 2 hours (Table 1, entry 14). Other non-polar and aprotic solvents like toluene, xylene, dimethylsulfoxide, and dichloromethane gave intermediate-1′, which failed to undergo cyclization to give the desired product.| Entry | ROH | Time (h) | % Yieldb |
|---|---|---|---|
| a Reaction conditions: (i) 1 (0.5 mmol), 2 (0.5 mmol), ROH (3.0 ml) at room temperature. b Isolated yield. c No result. | |||
| 1 | MeOH | 12 | 89 |
| 2 | EtOH | 12 | 94 |
| 3 | iPrOH | 12 | 98 |
| 4 | t BuOH | 12 | 95 |
| 5 | Propane-1,2-diol | 24 | 71 |
| 6 | (CH2OH)2 | 24 | 64 |
| 7 | Phenol | 48 | NRc |
| 8 | Benzyl alcohol | 48 | 27 |
| 9 | Glycerol | 24 | 32 |
| 10 | Propargyl alcohol | 24 | 48 |
| 11 | Allylic alcohol | 24 | 41 |
| 12 | H2O | 24 | 78 |
| 13 | EtOH | 2 | 94 |
| 14 | i PrOH | 2 | 98 |
| 15 | t BuOH | 2 | 95 |
We also used different amines as solvents such as aniline, dimethylamine, trimethylamine, and dipropylamine. However, they failed to yield any product. Following solvent optimisation, various bases were also screened in EtOH, iPrOH, and tBuOH solvents to determine their influence on reaction efficiency (Table 2). Strong bases such as NaOH, KOtBu, KOH, and NaH (Table 2, entries 1–4) gave lower yields as compared to carbonates such as K2CO3, Cs2CO3, NaHCO3 and Na2CO3 (Table 2, entries 5–8), while organic bases such as DBU, NEt3, and NHEt2 gave poor yields (Table 2, entries 12–14) as compared to the carbonates. The use of Na2CO3 (1.5 equiv.) gave the highest yield in all three solvents, with 92% yield in iPrOH (Table 2, entry 9). Increasing the loading to 2.0 or 2.5 equivalents did not lead to any significant improvement, indicating 1.5 equivalents to be optimal. Furthermore, the reaction time study (Table 2, entries 16–18) confirmed that 1 hour is sufficient to achieve maximum yield, with no appreciable improvement beyond this time. Organic bases such as pyridine, DABCO, and piperidine were also tested but resulted in poor to low yields (10–20%), possibly due to weak basicity or steric hindrance. Overall, the combination of iPrOH as solvent and Na2CO3 (1.5 equiv.) as a base at room temperature provided the best outcome, delivering the product in excellent yield (92%) within 1 hour reaction time (Table 2, entry 17). These mild, metal-free, and efficient conditions underscore the practical and sustainable potential of the developed protocol.
| Entry | Base (equiv.) | Time (h) | % Yieldb | ||
|---|---|---|---|---|---|
| EtOH | iPrOH | t BuOH | |||
| a Reaction conditions: (i) 3 (0.5 mmol) and base at room temperature. b Isolated yield. Organic bases such as pyridine, pyrrolidine, DABCO, morpholine, and piperidine gave poor to low yields (10–20%). | |||||
| 1 | NaOH (2.0) | 6 | 48 | 57 | 51 |
| 2 | KOtBu (2.0) | 6 | 32 | 34 | 29 |
| 3 | KOH (2.0) | 6 | 45 | 58 | 50 |
| 4 | NaH (2.0) | 6 | 18 | 24 | 21 |
| 5 | K2CO3 (2.0) | 6 | 78 | 87 | 81 |
| 6 | CsCO3 (2.0) | 6 | 74 | 87 | 79 |
| 7 | NaHCO3 (2.0) | 12 | 68 | 72 | 70 |
| 8 | Na2CO3 (1.0) | 6 | 47 | 56 | 53 |
| 9 | Na2CO3 (1.5) | 6 | 85 | 92 | 88 |
| 10 | Na2CO3 (2.0) | 6 | 83 | 90 | 85 |
| 11 | Na2CO3 (2.5) | 6 | 81 | 85 | 82 |
| 12 | DBU (2.0) | 6 | 37 | 52 | 48 |
| 13 | NEt3 (2.0) | 6 | 29 | 41 | 37 |
| 14 | NHEt2 (2.0) | 6 | 21 | 28 | 26 |
| 15 | Na2CO3 (1.0) | 6 | 41 | 49 | 42 |
| 16 | Na2CO3 (1.5) | 0.5 | 57 | 62 | 59 |
| 17 | Na 2 CO 3 (1.5) | 1 | 85 | 92 | 88 |
| 18 | Na2CO3 (1.5) | 2 | 85 | 92 | 88 |
Using the optimised reaction conditions, a variety of electron-donating and electron-withdrawing groups were investigated on formyl-chromone, 2-aminophenol, 2-aminothiophenol and 2-bromoacetophenone, which showcased minor yield differences. The nitro group on 2-aminophenol gave lower yields of compounds 4d and 4h, 84% and 81%, respectively, and the chloro-substituent gave compounds 4g, 4i, and 4j in yields of 89%, 83% and 88%, respectively. Electron donating groups such as the methoxy-group gave compounds 4c, 4d, 4g, 4m, 4s, 4u and 4v in good to excellent yields (83–94%), and the methyl group compounds 4e, 4f, 4j, 4q and 4r were obtained in excellent yields (88–90%). Varying substituents on all three moieties gave compounds 4n, 4o, 4p, and 4t in 84%, 80%, 83% and 85% yields, respectively (Table 3).
| Entry | R1 | R2 | R3 | Product | Yield (%) |
|---|---|---|---|---|---|
| 1 | H | H | H | 4a | 92 |
| 2 | H | H | p-Br | 4b | 89 |
| 3 | H | H | p-OMe | 4c | 93 |
| 4 | H | p-NO2 | p-OMe | 4d | 84 |
| 5 | H | m-Me | p-Cl | 4e | 90 |
| 6 | H | H | p-Me | 4f | 90 |
| 7 | H | m-Cl | p-OMe | 4g | 89 |
| 8 | H | p-NO2 | p-Br | 4h | 81 |
| 9 | H | m-Cl | p-Cl | 4i | 83 |
| 10 | H | m-Cl | p-Me | 4j | 88 |
| 11 | p-Br | H | p-Br | 4k | 85 |
| 12 | p-Br | H | p-Cl | 4l | 84 |
| 13 | p-Br | H | p-OMe | 4m | 87 |
| 14 | p-Br | p-NO2 | p-OMe | 4n | 84 |
| 15 | p-Br | p-NO2 | p-Br | 4o | 80 |
| 16 | p-Br | m-Me | p-Cl | 4p | 83 |
| 17 | p-Me | H | p-Cl | 4q | 88 |
| 18 | p-Me | H | p-Br | 4r | 88 |
| 19 | p-Me | H | p-OMe | 4s | 94 |
| 20 | p-Me | p-NO2 | p-OMe | 4t | 85 |
| 21 | p-OMe | H | p-Br | 4u | 83 |
| 22 | p-OMe | H | p-OMe | 4v | 93 |
| 23 | p-OH | H | p-Br | 4w | 80 |
Similarly, the reaction of 2-aminothiophenols (Table 4) with chromones also afforded excellent product yields. The methyl and methoxy variants gave compounds 5d, 5e, 5f and 5j in higher yields of 95%, 88%, 90% and 95%, respectively. When 2-bromoacetophenone was replaced with ortho-phenyldiamine, propargyl bromide, allylic bromide and benzylic bromide, they all failed to give the desired products.
The synthesis of 4a (0.176 g, 92%) was evaluated using green chemistry criteria to assess the environmental friendliness and greenness of the method. Specifically, it was determined that the reaction atom economy was 79.49%, the atom efficiency was 73.13%, and the excellent E-factor was 0.69 (Fig. 2). i-PrOH, (isopropanol) which exhibits low-to-moderate toxicity, is readily biodegradable, and is flammable (flash point ≈12 °C), was used as the solvent, which improved the green profile of this solvent.
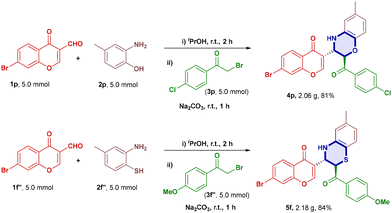
We also performed a scale up reaction and the desired products 4p (2.06 g) and 5f (2.18 g) were obtained in good yields of 81% and 84% respectively (Scheme 2).
Plausible mechanism
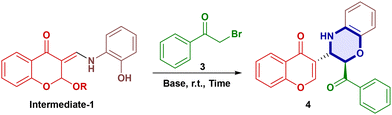
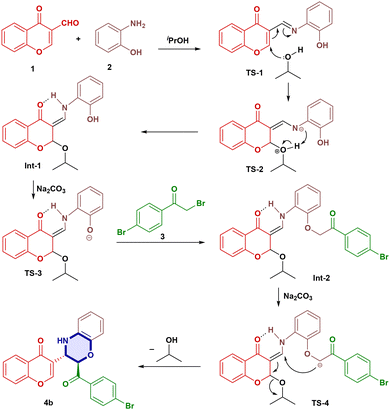
The plausible reaction mechanism began with the formation of an imine. Subsequent imine–enamine tautomerization by the attack of solvent afforded an α,β-unsaturated carbonyl moiety (TS-2). The proton shift gave a stable intermediate 1 (Int-1) due to H-bonding, which further reacted with base Na2CO3 to generate a phenoxide ion (TS-3). The reaction of TS-3 with 2-bromoacetopheneone (3) afforded intermediate 2 (Int-2). Then, intermediate 2 reacted with Na2CO3 to give the carbanion TS-4 and subsequent selective attack on the β-carbon from above the plane (Michael addition) resulted in a diastereoselective product. Subsequent elimination of propanol solvent afforded the desired product (4b) (Scheme 3). To confirm the reaction mechanism, intermediate-1 (Int-1) was isolated and analysed by 1H-NMR, 13C-NMR and HRMS, while intermediate-2 (Int-2) was observed in the HRMS spectrum as a peak at (M + H)+ = 522.0910. In the 1H-NMR spectrum for example, compound 4a gave two doublets at δ 5.24 (d, 1H) and δ 4.23 (d, 1H) with J-values of 5.0 Hz and 5.1 Hz, respectively, indicating its anti-geometry. Similarly, the NOESY experiment of 4a failed to show cross-peaks at δ 5.24 and δ 4.23 ppm, indicating that the diastereomeric protons are anti to each other (Fig. S4). The same was confirmed by single X-ray crystallography (Fig. 3). Crude 1H and 13C-NMR spectra of compound 4a were recorded, which showed only one set of signals, suggesting the formation of a single diastereomer (Fig. S3).Single-crystal X-ray structural analysis
A small amount of compound 4a was dissolved in acetonitrile and left to slowly evaporate at room temperature, affording single crystals of the molecule. A Bruker APEX-V CCD diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) at 300 K was used to collect crystallographic data. The monoclinic space group and bonding characteristics of 3-((2R,3R)-2-benzoyl-3,4-dihydro-2H-benzo[b][1,4]oxazin-3-yl)-4H-chromen-4-one (4a) were determined using single X-ray crystallography (CCDC 2484494) and the lattice parameters as follows: A = 20.836(4) Å, B = 7.1335(13) Å, and C = 21.837(5) Å and α = 90°, β = 146.592(5)°, and γ = 90° (Fig. 3).Conclusions
In conclusion, we have developed a highly efficient, green and diastereoselective method for the synthesis of formyl-chromone-based 1,4-oxazine and 1,4-thioxazine derivatives as anti-conformers, via a one-pot, three-component reaction. It is a practical route to access biologically active heterocyclic compounds. The ease of purification, high product yields, and a wide substrate scope of this method highlight its potential for large-scale synthesis.Experimental
General procedure
3.0 ml of isopropanol was added to a round-bottom flask containing 0.5 mmol of formylchromone (1) and 0.5 mmol of 2-aminophenol (2) or 2-aminothiophenol (2′), and the resulting mixture was stirred for 2 hours at room temperature, followed by the addition of 0.5 mmol of 2-bromoacetophenone (3) and 1.5 mmol of Na2CO3, and stirred at the same temperature for 1 hour. After completion of the reaction, 20.0 ml of ice-cold water was added to the reaction mixture to afford precipitates, which were filtered and recrystallised to get the desired product.Author contributions
Farukh Ahmad: experiments, original writing. Rajeev Singh: experiments, original writing. Naseem Ahmed: conceptualisation, editing and writing.Conflicts of interest
There are no conflicts to declare.Data availability
All data are available in the article and its supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ob01466b.CCDC 2484494 contains the supplementary crystallographic data for this paper.27
Acknowledgements
This research was supported by IIT Roorkee and DST-FIST [SR/FST/CS-II/2018/72(C)] (funding to the Chemistry Department, IIT Roorkee, for the provision of 500 MHz NMR and XRD facilities). Farukh Ahmad is grateful to the MHRD, IIT Roorkee, for providing a Junior Research Fellowship (JRF). We are also thankful to Md Azimuddin SK for the single-crystal X-ray structural analysis. The editor and reviewers are highly appreciated for their valuable suggestions.References
-
(a) R. Wijtmans, M. K. Vink, H. E. Schoemaker, F. L. van Delft, R. H. Blaauw and F. P. Rutjes, Synthesis, 2004, 641–662 CAS
; (b) I. Jabeen, P. Wetwitayaklung, F. Klepsch, Z. Parveen, P. Chiba and G. F. Ecker, Chem. Commun., 2010, 47, 2586–2588 RSC
; (c) K. F. Croom and K. L. Goa, Drugs, 2003, 63, 2769–2802 CrossRef CAS PubMed
; (d) Y. Asahina, M. Takei, T. Kimura and Y. Fukuda, J. Med. Chem., 2008, 51, 3238–3249 CrossRef CAS
; (e) D. J. Newman and G. M. Cragg, J. Nat. Prod., 2020, 83, 770–803 CrossRef CAS PubMed
; (f) J. O. Witt, A. L. McCollum, M. A. Hurtado, E. D. Huseman, D. E. Jeffries, K. J. Temple, H. C. Plumley, A. L. Blobaum, C. W. Lindsley and C. R. Hopkins, Bioorg. Med. Chem. Lett., 2016, 26, 2481–2488 CrossRef CAS PubMed
.
- P. Majumdar, A. Pati, M. Patra, R. K. Behera and A. K. Behera, Chem. Rev., 2014, 114, 2942–2977 CrossRef CAS PubMed
.
- P. Xu, B. Yu, F. L. Li, X. F. Cai and C. Q. Ma, Trends Microbiol., 2006, 14, 398–405 CrossRef CAS
.
- A. M. Waseem, R. M. Elmagzoub, M. M. M. Abdelgadir, A. A. Bahir, N. S. A. El-Gawaad, A. S. Abdel-Samea, D. P. Rao, K. Kossenas, S. Bräse and H. Hashem, Results Chem., 2024, 13, 101989 CrossRef
.
- Mallappa, M. Chahar, N. Choudhary, K. K. Yadav, M. T. Qasim, R. Zairov, A. Patel, V. K. Yadav and M. Jangir, J. Iran. Chem. Soc., 2024, 22, 1–33 Search PubMed
.
-
(a) M. Andrs, J. Korabecny, D. Jun, Z. Hodny, J. Bartek and K. Kuca, J. Med. Chem., 2014, 58, 41–71 CrossRef PubMed
; (b) G. Sonia, K. K. Thachil, M. K. Parameswaran and R. T. Kochupappy, Med. Chem. Res., 2013, 23, 1320–1326 CrossRef
; (c) A. S. Bourlot, I. Sánchez, G. Dureng, G. Guillaumet, R. Massingham, A. Monteil, E. Winslow, M. D. Pujol and J.-Y. Mérour, J. Med. Chem., 1998, 41, 3142–3158 CrossRef CAS PubMed
; (d) R. H. Chen, A. Burke, J.-G. Cho, J.-W. Alffenaar and L. D. Forsman, Pharmaceutics, 2024, 16, 818–818 CrossRef CAS PubMed
; (e) X. Li, N. Liu, H. Zhang, S. E. Knudson, R. A. Slayden and P. J. Tonge, Bioorg. Med. Chem. Lett., 2010, 20, 6306–6309 CrossRef CAS
; (f) P. J. Rybczynski, R. E. Zeck, J. Dudash, D. W. Combs, T. P. Burris, M. Yang, M. C. Osborne, X. Chen and K. T. Demarest, J. Med. Chem., 2003, 47, 196–209 CrossRef PubMed
; (g) M. Asif and M. Imran, Int. J. New Chem., 2020, 7, 60–73 CAS
.
- N. Cantini, A. I. Khlebnikov, L. Crocetti, I. A. Schepetkin, G. Floresta, G. Guerrini, C. Vergelli, G. Bartolucci, M. T. Quinn and M. P. Giovannoni, Bioorg. Med. Chem., 2021, 29, 115836–115836 CrossRef CAS PubMed
.
- M. M. Heravi and V. Zadsirjan, RSC Adv., 2020, 10, 44247–44311 RSC
.
- M. D. Delost, D. T. Smith, B. J. Anderson and J. T. Njardarson, J. Med. Chem., 2018, 61, 10996–11020 CrossRef CAS
.
-
(a) W. Liang, L.-J. Min, L. Han and X.-H. Liu, Curr. Org. Chem., 2021, 25, 2840–2855 CrossRef CAS
; (b) S. G. Antonsen, H. Gallantree-Smith, C. H. Görbitz, T. V. Hansen, Y. H. Stenstrøm and J. M. J. Nolsoe, Molecules, 2017, 22, 1720–1720 CrossRef PubMed
; (c) K. G. Ortiz, A. T. Brusoe, J. An, E. Chong and L. Wu, J. Am. Chem. Soc., 2024, 146, 29847–29856 CrossRef CAS PubMed
; (d) A. P. Kourounakis, C. Charitos, E. A. Rekka and P. N. Kourounakis, J. Med. Chem., 2008, 51, 5861–5865 CrossRef CAS PubMed
.
-
(a) M. Yamagishi, J. Okazaki, K. Nishigai, T. Hata and H. Urabe, Org. Lett., 2011, 14, 34–37 CrossRef PubMed
; (b) H. Zhai, A. Borzenko, Y. Y. Lau, S. H. Ahn and L. L. Schafer, Angew. Chem., 2012, 124, 12385–12389 CrossRef
; (c) Z. Lu and S. S. Stahl, Org. Lett., 2012, 14, 1234–1237 CrossRef CAS PubMed
; (d) B. Gabriele, G. Salerno, L. Veltri, R. Mancuso, Z. Li, A. Crispini and A. Bellusci, J. Org. Chem., 2006, 71, 7895–7898 CrossRef CAS
.
-
(a) H. Qin, Y. Miao, J. Xu, Q. Bi, W. Qu, W. Liu, F. Feng and H. Sun, Org. Chem. Front., 2019, 6, 205–208 RSC
; (b) K. Wen, Z. Wu, B. Chen, J. Chen and W. Zhang, Org. Biomol. Chem., 2018, 16, 5618–5625 RSC
; (c) J. V. Matlock, T. D. Svejstrup, P. Songara, S. Overington, E. M. McGarrigle and V. K. Aggarwal, Org. Lett., 2015, 17, 5044–5047 CrossRef CAS
.
-
(a) M. U. Luescher and J. W. Bode, Angew. Chem., 2015, 127, 11034–11038 CrossRef
; (b) W.-Y. Siau and J. W. Bode, J. Am. Chem. Soc., 2014, 136, 17726–17729 CrossRef CAS PubMed
.
- F. Sajjad, Y. Chen, X. Tian, S. Dong, A. G. K. Reddy, W. Hu and D. Xing, Org. Biomol. Chem., 2021, 19, 1769–1772 RSC
.
- D. Yang, C. Wang, Y. Wang, G. Li, T. Cheng and R. Li, Org. Chem. Front., 2022, 9, 102–108 RSC
.
- S. Hajra, A. Hazra and S. A. Saleh, J. Org. Chem., 2019, 84, 10412–10421 CrossRef CAS PubMed
.
- A. R. Patil and U. K. Marelli, Org. Lett., 2024, 26, 7584–7589 CrossRef CAS PubMed
.
-
(a) S. Bhukta, R. Chatterjee and R. Dandela, Green Chem., 2023, 25, 3034–3039 RSC
; (b) Z. Yang, J. Luo, W. Zang, C. Liu and Y. Li, Org. Biomol. Chem., 2025, 23, 323–327 RSC
; (c) Z. Yang, C. Liu, J. Lei, Y. Zhou, X. Gao and Y. Li, Chem. Commun., 2022, 58, 13483–13486 RSC
; (d) J. Zheng, J. Bi, L. Ma, L. Chen, L. Tang, D. Xiong and Y. Tian, Chem. Commun., 2023, 59, 8572–8575 RSC
.
-
(a) O. A. Davis and J. A. Bull, Angew. Chem., Int. Ed., 2014, 53, 14230–14234 CrossRef CAS PubMed
; (b) J. Q. Wu, Z. Yang, S.-S. Zhang, C.-Y. Jiang, Q. Li, Z. S. Huang and H. Wang, ACS Catal., 2015, 5, 6453–6457 CrossRef CAS
; (c) K. D. Jones, M. J. Nutt, E. Comninos, A. N. Sobolev, S. A. Moggach, T. Miura, M. Murakami and S. G. Stewart, Org. Lett., 2020, 22, 3490–3494 CrossRef CAS PubMed
; (d) F. Cambeiro, S. López, J. A. Varela and C. Saá, Angew. Chem., 2014, 126, 6069–6073 CrossRef
; (e) L.-F. Yao, Y. Wang and K.-W. Huang, Org. Chem. Front., 2015, 2, 721–725 RSC
; (f) B. Singh, M. Kumar, G. Goswami, I. Verma and M. K. Ghorai, J. Org. Chem., 2023, 88, 4504–4518 CrossRef CAS PubMed
.
-
(a) Q.-Q. Cheng, Y. Deng, M. Lankelma and M. P. Doyle, Chem. Soc. Rev., 2017, 46, 5425–5443 RSC
; (b) S. Bera, H. Khan, D. Barman, R. Bhattacharya, K. Verma, P. Rai, S. Banerjee and S. Sen, J. Org. Chem., 2025, 90, 8457–8465 CrossRef CAS PubMed
; (c) A. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire and M. A. McKervey, Chem. Rev., 2015, 115, 9981–10080 CrossRef CAS PubMed
; (d) J. Min, G. Xu and J. Sun, J. Org. Chem., 2017, 82, 5492–5498 CrossRef CAS PubMed
; (e) J. Yang, C. Ke, D. Zhang, X. Liu and X. Feng, Org. Lett., 2018, 20, 4536–4539 CrossRef CAS PubMed
.
-
(a) B. W. Wan, W. Liu, Y. Xiao, L. Lv, X. Li, F. Lin, Y. Zhao, Y. He, S. Nie, X. Yang and H. X. Yuan, Bioorg. Chem., 2025, 156, 108201 CrossRef CAS PubMed
; (b) N. D. Kushwaha, B. Kushwaha, R. Karpoormath, M. C. Mahlalela and S. R. Shinde, J. Org. Chem., 2020, 85, 8221–8229 CrossRef CAS PubMed
; (c) B. Teli, M. A. Waseem, S. Rashid, B. A. Ganaie and B. A. Bhat, J. Heterocycl. Chem., 2021, 58, 1541–1545 CrossRef CAS
.
- B. K. Zhimomi, M. Phom, P. Achumi, P. Imchen, K. Yanthan, S. Tumtin and T. Phucho, ARKIVOC, 2024, 8, 202412276 Search PubMed
.
- Y. Wang, W. Y. Zhang and S. L. You, J. Am. Chem. Soc., 2019, 141, 2228–2232 CrossRef CAS PubMed
.
- J. J. Chu, B. L. Hu, Z. Y. Liao and X. G. Zhang, J. Org. Chem., 2016, 81, 8647–8652 CrossRef CAS PubMed
.
-
(a) M. Waheed, N. Ahmed, M. A. Alsharif, M. I. Alahmdi and S. Mukhtar, ChemistrySelect, 2017, 2, 7946–7950 CrossRef CAS
; (b) M. Waheed, N. Ahmed, M. A. Alsharif, M. I. Alahmdi and S. Mukhtar, Org. Biomol. Chem., 2018, 16, 3428–3437 RSC
; (c) M. I. Alahmdi, A. Bhakta, M. A. Alsharif, S. Mukhtar, H. Parveen, A. Singh, N. E. Abo-Dya, Y. H. Y. Almalky, M. Y. Wani and N. Ahmed, J. Mol. Struct., 2025, 1328, 141427–141427 CrossRef CAS
; (d) N. Ahmed and R. Singh, Synlett, 2024, A–F Search PubMed
; (e) A. Bhakta, S. Mukhtar, S. Anwar, S. Haider, M. I. Alahmdi, H. Parveen, M. A. Alsharif, M. Y. Wani, A. Chakrabarty, M. I. Hassan and N. Ahmed, RSC Med. Chem., 2024, 15, 1942–1958 RSC
.
- I. Sigg, G. Haas and T. Winkler, Helv. Chim. Acta, 1982, 65, 275–279 CrossRef CAS
.
-
CCDC 2484494: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2pd9zd
.
| This journal is © The Royal Society of Chemistry 2026 |