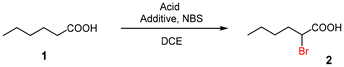
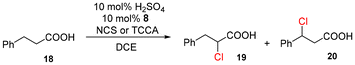Direct α-halogenation of carboxylic acids with N-bromosuccinimide (NBS) or trichloroisocyanuric acid (TCCA) in the presence of catalytic amounts of 4-trifluoromethylbenzoic anhydride and H2SO4
Kiyoshi
Tanemura

Chemical Laboratory, School of Life Dentistry at Niigata, Nippon Dental University, Hamaura-cho, Niigata 951-8580, Japan. E-mail: tanemura@ngt.ndu.ac.jp
First published on 1st December 2025
Abstract
α-Halogenation of carboxylic acids with N-bromosuccinimide (NBS) or trichloroisocyanuric acid (TCCA) in the presence of catalytic amounts of 4-trifluoromethylbenzoic anhydride and H2SO4 in 1,2-dichloroethane was devised. The method does not need prior conversion to acyl chlorides, and the reactions could be applied to a gram-scale synthesis.
Halogenation of organic substrates such as ketones,1 aromatics,2 double bonds3 and allyl compounds4 is a widely employed and significant transformation in synthetic organic chemistry, because the resulting halogenated compounds serve as valuable synthetic intermediates for diverse subsequent conversions.5 Among these reactions, α-halogenation of carboxylic acids also represents a particularly important process. However, this transformation is generally more difficult than halogenation of ketones due to the lower reactivity of the carbonyl group in carboxylic acids. Nevertheless, several methods for achieving α-halogenation of carboxylic acids have been developed.5,6 A traditional example is the Hell–Volhard–Zelinsky (HVZ) reaction, which involves the use of Br2 in the presence of a catalytic amount of P (red phosphorus) or PCl3.7 While this method is effective, it typically requires long reaction times, high temperature and strict anhydrous conditions. Furthermore, Br2 is difficult to handle and a large amount of HBr is generated. Alternative approaches which utilize SOCl2 as a solvent have been devised.8 These reactions proceed more rapidly and work-up procedure is quite simple, but they require the in situ conversion of carboxylic acids to acyl chlorides and the hydrolysis of the resulting α-brominated acyl chlorides. This method causes the release of large amounts of toxic gases such as SO2 and HCl by the decomposition of SOCl2, raising concerns regarding a safety and environmental point.
α-Chlorination of carboxylic acids is more difficult than α-bromination.9 Although several methods have been reported until now, these reactions require longer reaction times than bromination and often cause undesired reactions. Moreover, Cl2 gas is hazardous and more difficult to handle. An efficient procedure of the halogenation in SOCl2 using N-chlorosuccinimide (NCS), N-bromosuccinimide (NBS) or I2 has been developed.10 NXS is advantageous due to their ease of handling and relatively mild reactivities. Notably, benzylic positions are not affected in this method. However, this method also needs the conversion of carboxylic acids to the corresponding acyl chlorides and the hydrolysis of the resulting α-halogenated acyl chlorides to regenerate carboxylic acids. Large amounts of toxic SO2 and HCl gases are released by the decomposition of SOCl2 during the reactions and work-up. Thus, direct and environmentally benign procedures using easy-to-handle reagents have been desired to develop.
In this paper, we report a direct method for the α-halogenation of carboxylic acids using NBS or trichloroisocyanuric acid (TCCA) in the presence of catalytic amounts of 4-trifluoromethylbenzoic anhydride (8) and H2SO4 in 1,2-dichloroethane (DCE).
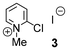
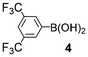
First, we examined the α-bromination of hexanoic acid (1) using 1.1 mmol of NBS in the presence of catalytic amounts of various additives and TsOH·H2O in DCE. The results are summarized in Table 1. In the presence of I2 which is known to catalyse α-halogenation of carboxylic acids,8a no reaction occurred (entry 2). Similarly, I2-copper salts11 which are effective additives in the α-iodination of carboxylic acids, failed to promote the reaction (entries 3 and 4). 2-Chloro-1-methylpyridinium iodide (3)12 and 3,5-bis(trifluoromethyl)phenylboronic acid (4)13 which activate carboxylic acids in esterification and amidation, respectively, also showed no activity (entries 5 and 6).
| Entry | Additive | NBS (mmol) | Acid | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1 (1.0 mmol), acid, additive, NBS, DCE (0.5 mL), 80 °C. b Determined by 1H NMR spectroscopy based on 1. c MeCN was used instead of DCE. d CH3(CH2)4COOMe (1.0 mmol) was used instead of 1. | |||||
| 1 | None | 1.1 | TsOH·H2O (10 mol%) | 2 | 0 |
| 2 | I2 | 1.1 | TsOH·H2O (10 mol%) | 2 | 0 |
| 3 | CuCl | 1.1 | TsOH·H2O (10 mol%) | 2 | 0 |
| 4 | CuCl2 | 1.1 | TsOH·H2O (10 mol%) | 2 | 0 |
| 5 |
|
1.1 | TsOH·H2O (10 mol%) | 2 | 0 |
| 6 |
|
1.1 | TsOH·H2O (10 mol%) | 2 | 0 |
| 7 |
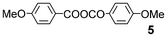
|
1.1 | TsOH·H2O (10 mol%) | 2 | 0 |
| 8 |
|
1.1 | TsOH·H2O (10 mol%) | 2 | 0 |
| 9 |
|
1.1 | TsOH·H2O (10 mol%) | 2 | 0 |
| 10 |
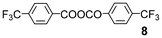
|
1.1 | TsOH·H2O (10 mol%) | 2 | 52 |
| 11 | 1.5 | TsOH·H2O (10 mol%) | 5 | 68 | |
| 12 | 2.0 | TsOH·H2O (10 mol%) | 5 | 92 | |
| 13 | 2.2 | TsOH·H2O (10 mol%) | 4 | 100 | |
| 14 | 1.1 | H2SO4 (10 mol%) | 1.5 | 79 | |
| 15 | 1.3 | H2SO4 (10 mol%) | 2.5 | 91 | |
| 16 | 1.4 | H2SO4 (10 mol%) | 3 | 100 | |
| 17 | 2.8 | H2SO4 (10 mol%) | 4 | 100 | |
| 18 | 1.4 | — | 3 | 0 | |
| 19 | 1.4 | H2SO4 (5 mol%) | 3 | 73 | |
| 20c | 1.4 | H2SO4 (10 mol%) | 3 | 35 | |
| 21d | 1.4 | H2SO4 (10 mol%) | 10 | 0 | |
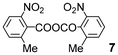
We further explored the reactions using catalytic amounts of benzoic anhydrides 714 and 815 bearing electron-withdrawing substituents on the benzene ring, anticipating the activation of carbonyl groups in carboxylic acids (entries 9 and 10). Indeed, the use of anhydride 8 afforded the desired product 2 (2 h, 52%) (entry 10). In contrast, anhydride 7 did not produce 2, probably due to steric hindrance caused by the ortho-substituted nitro and methyl groups during the bromination process although bearing a nitro group as an electron-withdrawing substituent (entry 9).13 2.2 equivalents of NBS were required for complete conversion when TsOH·H2O was used (entry 13).
It would be attributed to the hydrolysis of 8 to the corresponding carboxylic acid. We employed H2SO4 which does not possess water of crystallization, so that the reaction proceeded faster to give the product 2 in better yield (79%) within the shorter reaction time (1.5 h) (entry 14). Complete bromination realised with 1.4 equivalents of NBS (entry 16). The use of a large excess amount of NBS (2.8 equivalents) exclusively led to monobromide 2 with no detectable formation of the α,α-dibromo derivative (entry 17). No reaction was observed in the absence of H2SO4 (entry 18). When MeCN was used as a solvent, the reaction proceeded more slowly (entry 20). It is noteworthy that the methyl ester of 1 was unreactive under the employed conditions (entry 21).
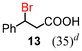
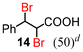
Next, the α-bromination of various carboxylic acids was carried out using 1.4 equivalents of NBS. As shown in Table 2, most of the corresponding α-bromides except 12 were produced in excellent yields. During the reactions, the formation of a small amount of molecular bromine was observed. Carboxylic acids bearing electron-withdrawing groups such as the bromo substituent as well as α,α-disubstituted ones, required longer reaction times to achieve satisfactory conversions (entries 7–9). Because the bromination of 3-phenylpropanoic acid (18) gave a large amount of dibromide 14 (50%) (entry 5), we conducted the reaction using 1.05 equivalents of NBS. Under the conditions, bromination occurred preferentially at the benzylic position via the radical mechanism (entry 6). In the HVZ procedure of compound 18, it has been reported that a mixture of compounds 12 and 13 yielded.16
| Entry | Time (h) | Product | Yieldb (%) | |
|---|---|---|---|---|
| a Reaction conditions: carboxylic acid (1.0 mmol), H2SO4 (10 mol%), anhydride 8 (10 mol%), NBS (1.4 mmol), DCE (0.5 mL), 80 °C. b Isolated yield. c NBS (1.05 mmol). d Determined by 1H NMR spectroscopy based on the starting material. | ||||
| 1 | 5 |
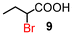
|
88 (100)d | |
| 2 | 3 |
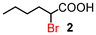
|
87 (100)d | |
| 3 | 3 |

|
91 (100)d | |
| 4 | 5 |
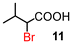
|
89 (100)d | |
| 5 | 4 |
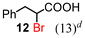
|
|
|
| 6c | 1.5 | (13)d | (69)d | (17)d |
| 7 | 10 |
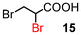
|
80 (90)d | |
| 8 | 9 |
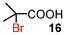
|
87 (100)d | |
| 9 | 10 |
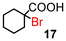
|
87 (100)d | |
The chlorination of compound 18 under various conditions was investigated (Table 3). We compared the reactions under normal, solvent-free17 and highly concentrated18 conditions (entries 1–6). TCCA exhibited higher reactivity than NCS in every case although it also afforded the benzylic substitution product 20. Under highly concentrated conditions, chlorination proceeded most rapidly (entry 6). The prolonged reaction using TCCA led to almost quantitative conversion (entry 9), whilst the prolonged reaction using NCS did not increase the yield (entry 8). An attempt to accelerate the reaction by the addition of 10 mol% of I2 under normal conditions resulted in the low yields of 19 and 20 in spite of the accelerating effect of I2 (entry 10).
| Entry | Reagent | DCE (mL) | 19 (%) | 20 (%) |
|---|---|---|---|---|
| a Conditions: 18 (1.0 mmol), H2SO4 (10 mol%), anhydride 8 (10 mol%), reagent (2.4 equiv.), 60 °C, 20 min, 80 °C, 4 h. b Determined by 1H NMR spectroscopy based on 18. c 60 °C, 20 min, 80 °C, 8 h. d 60 °C, 20 min, 80 °C, 14 h. e I2 (10 mol%) was added. | ||||
| 1 | NCS | 0.5 | 12 | 0 |
| 2 | TCCA | 0.5 | 10 | 7 |
| 3 | NCS | — | 8 | 0 |
| 4 | TCCA | — | 31 | 30 |
| 5 | NCS | 0.1 | 19 | 0 |
| 6 | TCCA | 0.1 | 32 | 40 |
| 7c | TCCA | 0.1 | 41 | 47 |
| 8d | NCS | 0.1 | 17 | 0 |
| 9d | TCCA | 0.1 | 43 | 50 |
| 10d,e | TCCA | 0.5 | 9 | 12 |
The results for the α-chlorination of various carboxylic acids are summarized in Table 4. Since the chlorination proceeded more slowly than bromination, 2.4 equivalents of chlorinating reagents were employed.10a Most of the α-chlorinated compounds except 19 and 27 were produced in excellent yields using TCCA under highly concentrated conditions. During the reactions, the formation of a small amount of chlorine was observed, which was detected by DPD (diethyl-p-phenylenediamine) and syringaldazine (3,5-dimethoxy-4-hydroxybenzaldazine) methods. Carboxylic acids bearing electron-withdrawing substituents such as the chloro group as well as α,α-disubstituted ones, required longer reaction times (entries 6–8). The addition of 10 mol% of I2 increased the yield of 27 probably due to the formation of more active ICl (entry 9).2f,19 The prolonged reaction did not improve the yield (entry 10). α-Iodination of carboxylic acid 18 using N-iodosuccinimide (NIS) or I2 was examined at 80 or 130 °C under normal, solvent-free and highly concentrated conditions, but the reactions did not occur (SI, Table S1).
| Entry | Time (h) | Product | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: carboxylic acid (1.0 mmol), H2SO4 (10 mol%), anhydride 8 (10 mol%), TCCA (0.8 mmol), DCE (0.1 mL), 80 °C. b Isolated yield. c 60 °C, 20 min, 80 °C, 14 h. d I2 (10 mol%) was added. e 60 °C, 20 min, 80 °C, 24 h. f 60 °C, 20 min, 80 °C, 96 h. g Determined by 1H NMR spectroscopy based on the starting material. | |||
| 1 | 12 |
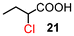
|
87 (96)g |
| 2 | 10 |
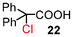
|
92 (100)g |
| 3c | 14 |
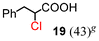
|
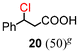
|
| 4 | 12 |
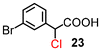
|
83 (93)g |
| 5 | 12 |
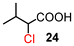
|
93 (l00)g |
| 6 | 15 |
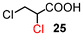
|
85 (96)g |
| 7 | 27 |
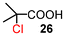
|
88 (93)g |
| 8 | 24 |
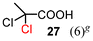
|
|
| 9d,e | 24 | (44)g | |
| 10d,f | 96 | (48)g | |
α-Bromination could also be performed under highly concentrated conditions.18 The results are summarised in Table 5. On the other hand, under solvent-free conditions, the reaction mixture did not form a melt at 80 °C because of the insolubility and high melting point of NBS (175–178 °C). All α-brominated carboxylic acids except 12 were produced in good yields under highly concentrated conditions. Under these conditions, the amount of the solvent could be reduced. When compound 18 was brominated, α-brominated carboxylic acid 12 was formed in a higher yield (53%) compared with the reaction under normal conditions probably due to the increased polarity resulting from the smaller amount of less polar solvent DCE (entry 5). In these cases, carboxylic acids bearing electron-withdrawing groups such as bromo as well as α,α-disubstituted ones, also required longer reaction times (entries 6–8).
| Entry | Time (h) | Product | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: carboxylic acid (1.0 mmol), H2SO4 (10 mol%), anhydride 8 (10 mol%), NBS (1.4 mmol), DCE (0.1 mL), 80 °C. b Isolated yield. c NBS (1.05 mmol). d Determined by 1H NMR spectroscopy based on the starting material. | |||
| 1 | 5 |
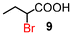
|
86 (100)d |
| 2 | 4 |

|
88 (100)d |
| 3 | 3 |

|
86 (100)d |
| 4 | 5 |
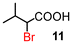
|
85 (100)d |
| 5c | 1 |
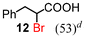
|
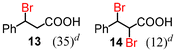
|
| 6 | 7 |
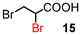
|
77 (88)d |
| 7 | 7 |
|
86 (100)d |
| 8 | 7 |
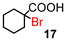
|
89 (100)d |
In order to expand the scope of this method, the current procedure was successfully scaled up to 20-fold. Under these conditions, bromides 2 and 17 as well as chlorides 24 and 26 could be isolated in good yields without any difficulty (2: 4.5 h, 90%, 17: 15 h, 90%, 24: 18 h, 94%, 26: 40 h, 86%).
The reaction of 1 under the employed conditions was monitored by 1H NMR spectroscopy. As shown in Fig. 1, the bromination using NBS was much faster than that using Br2, indicating that most of the α-bromination was mediated by NBS rather than liberated Br2.
A plausible mechanism for bromination is depicted in Scheme 1.9c Carboxylic acid A undergoes an equilibrium with the catalyst 8, affording the corresponding mixed anhydride C and compound B.20 In the presence of a catalytic amount of H2SO4, mixed anhydride C is transformed to its enol form D, which subsequently undergoes the bromination with NBS to generate E. The intermediate E reacts with A to yield the α-brominated product F along with the regeneration of mixed anhydride C.
In order to verify the involvement of an enol intermediate in the catalytic cycle, H/D scrambling experiments were conducted in the presence of CD3OD. Under the reaction conditions without halogenating agents, 12% deuterium incorporation at the α-position of the produced CD3 ester of 1 was observed. This is considered to be formed before esterification of 1.
In conclusion, an efficient procedure for the α-halogenation of carboxylic acids was realised using NBS or TCCA in the presence of catalytic amounts of 4-trifluoromethylbenzoic anhydride and H2SO4. This method does not require prior conversion of carboxylic acids to the corresponding acyl chlorides, nor the hydrolysis of the resulting α-halogenated acyl chlorides. The process does not release a large amount of toxic gas such as SO2 or HCl. In addition, NBS and TCCA are convenient and easy to handle, and furthermore, the use of highly toxic and corrosive reagents such as PCl3 and SOCl2etc. is avoided.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ob01483b.Acknowledgements
We thank CCRF, Institute for Research Promotion, Niigata University for NMR measurements.References
-
(a) W. Wang, S. Song and N. Jiao, Acc. Chem. Res., 2024, 57, 3161 CrossRef CAS PubMed
; (b) I. Pravst, M. Zupan and S. Stavber, Tetrahedron, 2008, 64, 5191 CrossRef CAS
; (c) J. C. Lee, Y. H. Bae and S.-K. Chang, Bull. Korean Chem. Soc., 2003, 24, 407 CrossRef CAS
; (d) K. Tanemura, T. Suzuki, Y. Nishida, K. Satsumabayashi and T. Horaguchi, Chem. Commun., 2004, 470 RSC
.
-
(a) W. Wang, X. Yang, R. Dai, Z. Yan, J. Wei, X. Dou, X. Qiu, H. Zhang, C. Wang, Y. Liu, S. Song and N. Jiao, J. Am. Chem. Soc., 2022, 144, 13415 CrossRef CAS PubMed
; (b) C. N. Kona, R. Oku, S. Nakamura, M. Miura, K. Hirano and Y. Nishii, Chem, 2024, 10, 402 CrossRef CAS
; (c) Y. Nishii, M. Ikeda, Y. Hayashi, S. Kawauchi and M. Miura, J. Am. Chem. Soc., 2019, 142, 1621 CrossRef PubMed
; (d) Y. Hirose, M. Yamazaki, M. Nogata, A. Nakamura and T. Maegawa, J. Org. Chem., 2019, 84, 7405 CrossRef CAS PubMed
; (e) J. Matsuoka, Y. Yano, Y. Hirose, K. Mashiba, N. Sawada, A. Nakamura and T. Maegawa, J. Org. Chem., 2023, 89, 770 CrossRef PubMed
; (f) K. Tanemura, Tetrahedron, 2025, 176, 134544 CrossRef CAS
; (g) K. Tanemura, Org. Biomol. Chem., 2024, 22, 5105 RSC
; (h) K. Tanemura, Tetrahedron Lett., 2025, 155, 155419 CrossRef CAS
; (i) K. Tanemura, T. Suzuki, Y. Nishida, K. Satsumabayashi and T. Horaguchi, Chem. Lett., 2003, 32, 932 CrossRef CAS
.
-
(a) J. Bock, S. Guria, V. Wedek and U. Hennecke, Chem. – Eur. J., 2021, 27, 4517 CrossRef CAS PubMed
; (b) U. Hennecke, Chem. – Asian J., 2012, 7, 456 CrossRef CAS PubMed
; (c) K. Tanemura, Tetrahedron Lett., 2018, 59, 4293 CrossRef CAS
.
-
(a) H. J. Dauben Jr and L. L. McCoy, J. Am. Chem. Soc., 1959, 81, 4863 CrossRef
; (b) S. F. Reed Jr., J. Org. Chem., 1965, 30, 3258 CrossRef
.
- M. Okimoto and Y. Takahashi, Synthesis, 2002, 2215 CrossRef CAS
.
-
(a) Y. Ogata and S. Watanabe, J. Org. Chem., 1980, 45, 2831 CrossRef CAS
; (b) H. Harwood, Chem. Rev., 1962, 62, 99 CrossRef CAS
; (c) Y. Ogata and S. Watanabe, J. Org. Chem., 1979, 44, 2768 CrossRef CAS
; (d) L. H. Zhang, J. Duan, Y. Xu and W. R. Dolbier Jr, Tetrahedron Lett., 1998, 39, 9621 CrossRef CAS
; (e) G. Farinelli, M. Coha, D. Vione, M. Minella and A. Tiraferri, Environ. Sci. Technol., 2022, 56, 5123 CrossRef CAS PubMed
; (f) J. G. Gleason and D. N. Harpp, Tetrahedron Lett., 1970, 11, 3431 CrossRef
; (g) J. Wang, X. Wu, B. Wang, X. Yuan, S. Yang and H. Hu, Eur. J. Org. Chem., 2024, e202400962 CrossRef CAS
; (h) X. Xue, Y. Zhang, C. Wang, M. Zhang, Q. Xiang, J. Wang, A. Wang, C. Li, C. Zhang, L. Zou, R. Wang, S. Wu, Y. Lu, H. Chen, K. Ding, G. Li and Y. Xu, Eur. J. Med. Chem., 2018, 152, 542 CrossRef CAS PubMed
; (i) H. Wack, A. E. Taggi, A. M. Hafez, W. J. Drury and T. Lectka, J. Am. Chem. Soc., 2001, 123, 1531 CrossRef CAS PubMed
; (j) H. A. Hassan, M. Abdel-Aziz, G. E.-D. A. Abuo-Rahma and H. H. Farag, Bioorg. Med. Chem., 2009, 17, 1681 CrossRef CAS PubMed
; (k) F. M. Steudel, E. Ubasart, L. Leanza, M. Pujals, T. Parella, G. M. Pavan, X. Ribas and M. von Delius, Angew. Chem., Int. Ed., 2023, 62, e202309393 CrossRef CAS PubMed
; (l) Y. Yamamoto, A. Tabuchi, K. Hosono, T. Ochi, K. Yamazaki, S. Kodama, A. Nomoto and A. Ogawa, Beilstein J. Org. Chem., 2021, 17, 2906 CrossRef CAS PubMed
.
-
(a) C. Hell, Ber. Dtsch. Chem. Ges., 1881, 14, 891 CrossRef
; (b) J. Volhard, Justus Liebigs Ann. Chem., 1887, 242, 141 CrossRef
; (c) N. Zelinsky, Ber. Dtsch. Chem. Ges., 1887, 20, 2026 CrossRef
; (d) H. Reinheckel, Chem. Ber., 1960, 93, 2222 CrossRef CAS
; (e) X. Chen and S. Z. Zard, Org. Lett., 2020, 22, 3628 CrossRef CAS PubMed
.
-
(a) A. Galat, J. Am. Chem. Soc., 1947, 69, 86 CrossRef CAS PubMed
; (b) X. Li, F. Xing, Q. Xu, X. Sun, Y. Wang, L. Wang and P. Wang, J. Surfactants Deterg., 2015, 19, 129 CrossRef
; (c) A. A. Homon, O. V. Hryshchuk, S. Trofymchuk, O. Michurin, Y. Kuchkovska, D. S. Radchenko and O. O. Grygorenko, Eur. J. Org. Chem., 2018, 5596 CrossRef CAS
.
-
(a) S. France, H. Wack, A. E. Taggi, A. M. Hafez, T. R. Wagerle, M. H. Shah, C. L. Dusich and T. Lectka, J. Am. Chem. Soc., 2004, 126, 4245 CrossRef CAS PubMed
; (b) S. T. Purrington and D. L. Woodard, J. Org. Chem., 1990, 55, 3423 CrossRef CAS
; (c) C. Morano, A. Giraudo, G. Roda, E. Armano, G. Nasta, M. Sipala, M. Pallavicini and C. Bolchi, RSC Adv., 2025, 15, 12298 RSC
; (d) Y. Ogata, T. Sugimoto and M. Inaishi, Bull. Chem. Soc. Jpn., 2006, 52, 255 CrossRef
; (e) V. Bertolini, M. Pallavicini, G. Tibhe, G. Roda, S. Arnoldi, L. Monguzzi, M. Zoccola, G. Di Nardo, G. Gilardi and C. Bolchi, ACS Omega, 2021, 6, 31901 CrossRef CAS PubMed
.
-
(a) D. N. Harpp, L. Bao, C. J. Black, J. G. Gleason and R. A. Smith, J. Org. Chem., 1975, 40, 3420 CrossRef CAS
; (b) D. N. Harpp, L. Bao, C. J. Black, R. A. Smith and J. G. Gleason, Tetrahedron Lett., 1974, 15, 3235 CrossRef
.
- C. A. Horiuchi and J. Y. Satoh, Chem. Lett., 1984, 13, 1509 CrossRef
.
- T. Mukaiyama, Angew. Chem.,
Int. Ed. Engl., 1979, 18, 707 CrossRef
.
- K. Ishihara, S. Ohara and H. Yamamoto, J. Org. Chem., 1996, 61, 4196 CrossRef CAS PubMed
.
- I. Shiina, M. Kubota and R. Ibuka, Tetrahedron Lett., 2002, 43, 7535 CrossRef CAS
.
- I. Shiina, Tetrahedron, 2004, 60, 1587 CrossRef CAS
.
-
(a) E. Brenna, F. G. Gatti, A. Manfredi, D. Monti and F. Parmeggiani, Eur. J. Org. Chem., 2011, 4015 CrossRef CAS
; (b) H. Reinheckel, A. Jovtscheff and S. Spassov, Monatsh. Chem., 1965, 96, 1185 CrossRef CAS
.
-
(a) F. Toda, Synlett, 1993, 303 CrossRef CAS
; (b) K. Tanemura, Tetrahedron, 2023, 140, 133470 CrossRef CAS
; (c) K. Kubota, N. Shizukuishi, S. Kubo and H. Ito, Chem. Lett., 2024, 53, upae056 CrossRef CAS
; (d) K. Tanemura, Results Chem., 2022, 4, 100316 CrossRef CAS
; (e) S. Sbi, V. Mkpenie, K. Tanemura and T. Rohand, Synlett, 2020, 31, 903 CrossRef CAS
; (f) K. Tanemura, Tetrahedron Lett., 2019, 60, 1924 CrossRef CAS
; (g) T. Rohand and K. Tanemura, Acta Chim. Slov., 2021, 68 Search PubMed
; (h) K. Tanemura and T. Rohand, Tetrahedron Lett., 2020, 61, 152142 CrossRef CAS
.
-
(a) P. J. Walsh, H. Li and C. A. de Parrodi, Chem. Rev., 2007, 107, 2503 CrossRef CAS PubMed
; (b) K. Tanemura, Tetrahedron Lett., 2021, 82, 153391 CrossRef CAS
; (c) F. Garnes-Portoles and A. Leyva-Perez, ACS Catal., 2023, 13, 9415 CrossRef CAS
; (d) K. Tanemura, Results Chem., 2022, 4, 100486 CrossRef CAS
.
- P. K. Pramanick, Z.-L. Hou and B. Yao, Tetrahedron, 2017, 73, 7105 CrossRef CAS
.
-
(a) J. M. Wallace Jr and J. E. Copenhaver, J. Am. Chem. Soc., 1941, 63, 699 CrossRef
; (b) C. D. Hurd, R. Christ and C. L. Thomas, J. Am. Chem. Soc., 1933, 55, 2589 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2026 |