Metal- and photocatalyst-free C3–H functionalization of quinoxalin-2(1H)-ones enabled by electron donor–acceptor complex photoactivation
An
Chen
,
Yating
Du
,
Xinchang
Wang
,
Yongqi
Xu
,
Xiaoyang
Yao
,
Yu
Hong
and
Wanmei
Li
 *
*
College of Material, Chemistry and Chemical Engineering, Key Laboratory of Organosilicon Chemistry and Material Technology, Ministry of Education, Zhejiang Key Laboratory of Organosilicon Material Technology, Hangzhou Normal University, Hangzhou, 311121, P. R. China. E-mail: liwanmei@hznu.edu.cn
First published on 2nd December 2025
Abstract
A straightforward, metal- and photocatalyst-free method for the C3-arylation and alkylation of quinoxalin-2(1H)-ones has been developed. This protocol exploits the photoactivation of electron donor–acceptor (EDA) complexes between quinoxalinones and boronic acids, facilitated by p-toluenesulfonic acid. The reaction proceeds under mild conditions (violet LED irradiation, air atmosphere) and exhibits broad substrate scope, accommodating a wide range of quinoxalinones and (hetero)aryl/alkyl boronic acids to deliver the products in moderate to excellent yields. Mechanistic studies confirm the involvement of a radical pathway and the critical role of the EDA complex. The practicality of this method is demonstrated by its scalability and successful late-stage functionalization of drug-like molecules.
Introduction
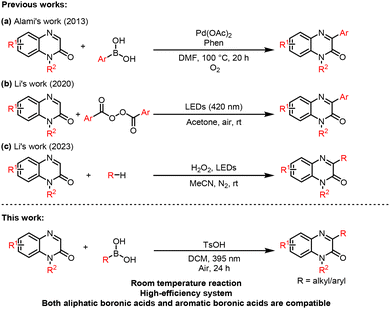
Quinolin-2(1H)-ones represent a privileged class of nitrogen-containing heterocycle that are widely found in pharmaceuticals, agrochemicals, and functional materials.1 The biological and physical properties of this scaffold can be significantly modulated by introducing substituents at the C3 position, which alters both electronic distribution and molecular conformation.2 As a result, the development of efficient methods for C3-functionalization has attracted considerable attention in synthetic chemistry.Traditional approaches to C3 alkylation and arylation have relied on transition-metal catalysis, photoredox catalysis, or radical pathways.3 For instance, Alami and co-workers reported a pioneering Pd-catalyzed C3-arylation using arylboronic acids, albeit requiring elevated temperatures.4 Subsequently, Zhang et al. employed diaryliodonium salts as arylating reagents,5 while Qu et al. demonstrated arylation with arylamines under tert-butyl nitrite catalysis.6 In parallel, visible-light-promoted alkylation via cross-dehydrogenative coupling with ethers was achieved by the Wang group.7 Although these methods provide valuable access to C3-substituted quinoxalin-2(1H)-ones, they often suffer from limitations such as the use of precious metals, stoichiometric oxidants, expensive photocatalysts, or stringent reaction conditions.
In response to these challenges, our group has been committed to developing greener and more sustainable alternatives. In 2020, we reported a metal- and photocatalyst-free arylation of quinoxalin-2(1H)-ones using aryl peroxides under mild conditions (Scheme 1b).8 More recently, we described a visible-light-driven alkylation employing H2O2 as a green oxidant (Scheme 1c).9 Despite these advances, a general and unified strategy that enables both arylation and alkylation under identical mild and catalyst-free conditions remains elusive.
Boronic acids are among the most versatile and widely used coupling partners in organic synthesis, notably in Suzuki–Miyaura cross-couplings.10 Their application in C–H functionalization of heteroarenes, however, often still depends on transition-metal catalysis.11 Therefore, developing a metal-free approach for the direct use of boronic acids in C–H functionalization would be highly desirable from both economic and environmental perspectives.
Recently, photoreactions via electron donor–acceptor (EDA) complexes have emerged as a powerful strategy for generating radicals under mild conditions, without the need for external photocatalysts or metal additives.12 In such processes, complexation between an electron-rich donor and an electron-accepting partner leads to a visible-light-absorbing aggregate, which upon irradiation undergoes single-electron transfer to generate radical species.13 Notable examples include Sanford's work on C–H pyridination using arene-pyridine EDA complexes,14 as well as our own studies on EDA complex-enabled functionalization of heteroaromatics.15 Building on this foundation, we hypothesized that quinoxalin-2(1H)-ones could act as electron acceptors, while boronic acids, upon activation, might serve as donors to form a photoactive EDA complex.
Herein, we report a unified and practical method for the direct C3-arylation and alkylation of quinoxalin-2(1H)-ones using commercially available aryl and alkyl boronic acids. This reaction is driven by visible-light irradiation of an in situ formed EDA complex, requires no transition metal or external photocatalyst, and proceeds under ambient atmosphere. The protocol exhibits broad substrate scope, excellent functional group tolerance, and is applicable to late-stage functionalization of complex molecules, offering a sustainable and efficient strategy for the diversification of quinoxalin-2(1H)-one derivatives.
Results and discussion
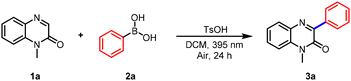
We initiated our investigation by selecting 1-methylquinoxalin-2(1H)-one (1a) and phenylboronic acid (2a) as model substrates to optimize the reaction conditions. A systematic evaluation of key parameters, including atmosphere, light wavelength, solvent, additive, and reaction time, was conducted. Optimal results were achieved by irradiating a mixture of the substrates with 395 nm violet LEDs in dichloromethane (DCM) under air for 24 hours, using p-toluenesulfonic acid (TsOH) as an additive, affording the desired product 3a in 85% yield (Table 1, entry 1). Further screening revealed that replacing air with an oxygen or nitrogen atmosphere markedly diminished the yield (Table 1, entry 2). It is noteworthy that when pure oxygen is used, a decrease in yield is observed alongside the formation of byproducts. This indicates that while oxygen is essential for the reaction, excessively high concentrations promote inefficient over-oxidation pathways. The use of other acids, such as trifluoroacetic acid (TFA) or acetic acid, led to slightly lower efficiencies, while stronger acids (e.g., HCl) or basic additives strongly suppressed the reaction (Table 1, entries 3 and 4). Control experiments confirmed that TsOH was essential, as omitting the additive significantly reduced the yield (Table 1, entry 5). Meanwhile, neither increasing nor decreasing the amount of additives resulted in an improvement in the reaction yield (Table 1, entries 6 and 7). Although productive within the 365–470 nm range, the reaction performed best at 395 nm (Table 1, entries 8–12). It is noteworthy that a control experiment conducted in the absence of light resulted in no product formation, underscoring the essential role of photoexcitation in this transformation (Table 1, entries 13). Screening of solvents indicated that DCM was superior to acetonitrile, methanol, and ethyl acetate (EA) (Table 1, entry 14). Varying the reaction time did not lead to further improvement (Table 1, entry 15).| Entry | Variation from given conditions | Yieldb [%] |
|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (2.0 equiv.), TsOH (1.0 equiv.), DCM (2.0 mL), 395 nm LEDs, 24 h, air. b Isolated yields. | ||
| 1 | None | 85 |
| 2 | O2 or N2 instead of Air | 16, trace |
| 3 | TFA, HCl, or CH3COOH instead of TsOH | 72, 25, 55 |
| 4 | Et3N or Na2CO3 instead of TsOH | 11, 34 |
| 5 | No TsOH | 46 |
| 6 | 2 equiv. of TsOH was used | 83 |
| 7 | 0.5 equiv. of TsOH was used | 71 |
| 8 | 365 nm LED instead of 395 nm | 56 |
| 9 | 410 nm LED instead of 395 nm | 47 |
| 10 | 420 nm LED instead of 395 nm | 60 |
| 11 | 455 nm LED instead of 395 nm | 55 |
| 12 | 470 nm LED instead of 395 nm | 43 |
| 13 | In dark | 0 |
| 14 | MeCN, MeOH or EA instead of DCM | 15, 10, 37 |
| 15 | Reaction proceeds for 18 or 30 h | 40, 56 |
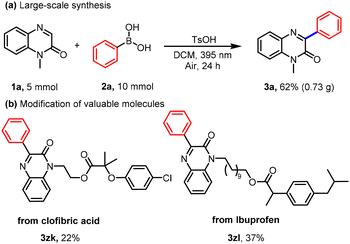
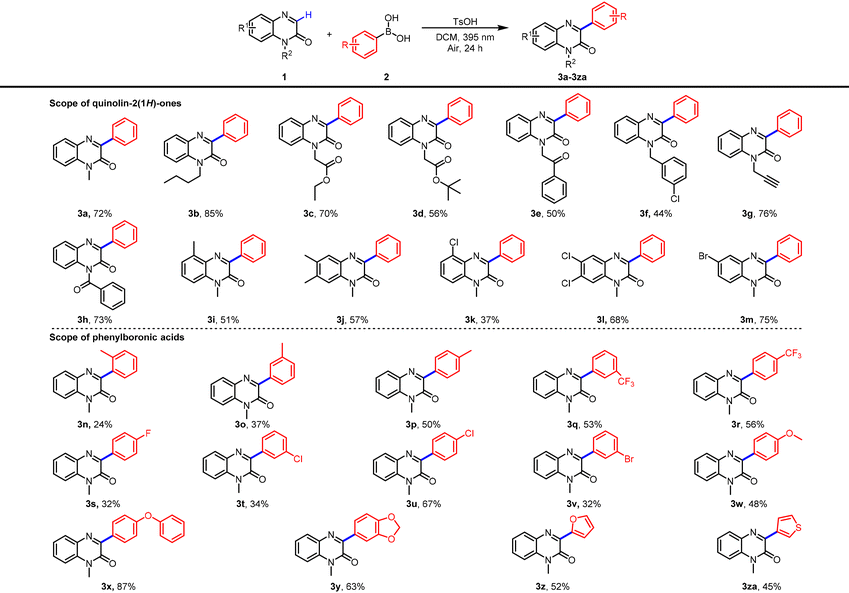
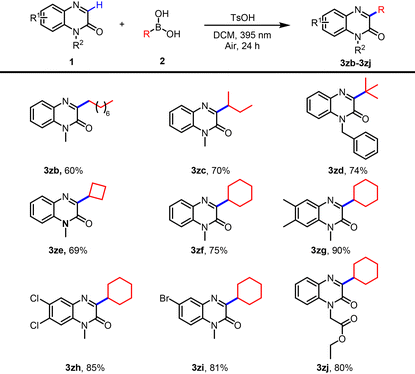
With the optimized conditions established, we first evaluated the substrate scope with respect to quinoxalin-2(1H)-ones by coupling with phenylboronic acid. As summarized in Table 2, a range of N-protecting groups, including alkyl, ester, ketone, and phenyl, were all well tolerated, providing the corresponding products 3a–3f in 44–85% yields. Notably, several sensitive functional groups such as alkyne and acyl also remained intact under the standard conditions, affording 3g–3i smoothly. Furthermore, 1-methylquinoxalin-2(1H)-ones bearing alkyl or halogen substituents at the C5, C6, or C7 positions underwent efficient arylation to give products 3j–3m in 37–75% yields. We next turned our attention to the scope of boronic acids. Arylboronic acids bearing substituents such as alkyl, trifluoromethyl, halogen, methoxy, and phenoxy at the para, meta, or ortho positions proved to be suitable coupling partners, delivering products 3n–3x in 24–87% yields. As expected, ortho-substituted arenes gave lower yields, likely due to steric encumbrance. Beyond (hetero)arenes, heteroaryl boronic acids were also compatible, furnishing product 3y–3za in 45–63% yield. We further evaluated the applicability of aliphatic boronic acids under the standard conditions. A variety of straight-chain, branched, and cycloalkyl boronic acids reacted smoothly, delivering the corresponding C3-alkylated products 3zb–3zf in 60–75% yields. The scope was also extended with respect to the quinoxalinone component: diversely substituted 1-methylquinoxalin-2(1H)-ones exhibited high reactivity toward cyclohexylboronic acid. Substrates bearing alkyl or halogen groups at C5, C6, or C7 were efficiently converted into products 3zg–3zi in excellent yields (81–90%). An N-ester-protected quinoxalin-2(1H)-one derivative was also compatible, furnishing 3zj in 80% yield (Table 3). To further demonstrate the synthetic utility, late-stage functionalization of several drug-like molecules was carried out under the optimized conditions, affording the target compounds 3zk–3zl in 22–37% yields (Scheme 2b).
The practicality of this method was further demonstrated through a gram-scale reaction. Using 1a (5 mmol) and 2a (10 mmol) under the standard conditions, the desired product was obtained in 62% isolated yield (0.73 g), confirming the robustness and scalability of the protocol (Scheme 2a).
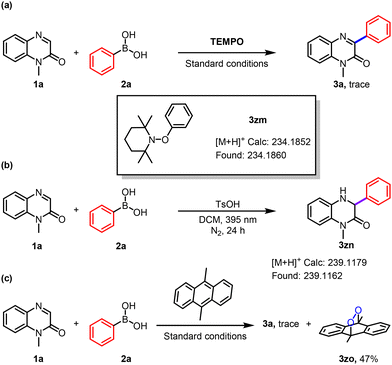
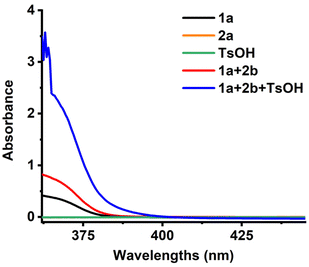
To gain insight into the reaction mechanism, a series of control experiments were performed. The addition of 2.0 equivalents of TEMPO as a radical scavenger markedly suppressed the model reaction. High-resolution mass spectrometry (HRMS) analysis detected the formation of a TEMPO-adducted radical species 3zm, providing direct evidence for the involvement of radical intermediates (Scheme 3a). When the reaction was conducted under a nitrogen atmosphere, intermediate 3zn was observed by HRMS. Further experiments revealed that 3zn could be converted to the final product only under both light and aerobic conditions, suggesting that singlet oxygen is essential for the transformation (Scheme 3b). At the same time, 9,10-dimethylanthracene, which acts as a singlet oxygen scavenger, was incorporated into our system. As expected, the adduct (3zo) was obtained with a yield of 47% (Scheme 3c). Additionally, UV-vis absorption spectroscopy of a mixture containing 1-methylquinoxalin-2(1H)-one, phenylboronic acid, and TsOH in DCM showed a distinct bathochromic shift relative to the individual components, supporting the formation of an EDA complex in solution (Scheme 4).
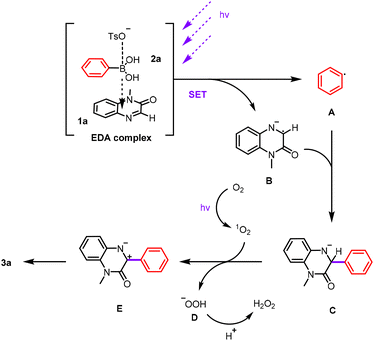
Based on the experimental evidence, a plausible reaction mechanism is proposed (Scheme 5). Upon visible-light irradiation, an EDA complex is formed from phenylboronic acid (2a), p-toluenesulfonic acid, and quinoxalin-2(1H)-one (1a). The complex undergoes photoinduced single-electron transfer, generating a phenyl radical A, while the quinolinone receives an electron to form a quinolinone radical anion B.16 Radical cross-coupling between A and B affords the intermediate C. Subsequent photoexcited singlet oxygen abstracts a hydride from C, yielding intermediates D and E. Finally, E undergoes bond reorganization to furnish the final product 3a (Scheme 5). Additionally, we considered the potential role of TsOH in activating quinoline through protonation. Therefore, we proposed an alternative acid-assisted mechanism (see Scheme S1 in the SI).
Conclusions
In summary, we have developed a novel and sustainable strategy for the direct C3-functionalization of quinoxalin-2(1H)-ones by leveraging the photoactivity of EDA complexes. This metal- and photocatalyst-free method enables both arylation and alkylation under mild conditions, employing readily available boronic acids as radical precursors. The reaction exhibits broad substrate scope and excellent functional group tolerance, affording a wide range of C3-substituted quinoxalinones in useful yields. Mechanistic studies support a radical pathway initiated by visible-light-induced EDA complex formation. The practical value of this protocol is underscored by its scalability and successful application in the late-stage diversification of complex molecules, offering a green and efficient alternative for the synthesis of biologically relevant heterocycles.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: detailed experimental procedures and analytical data. See DOI: https://doi.org/10.1039/d5ob01618e.Acknowledgements
We thank the Natural Science Foundation of Zhejiang Province (No. LMS25B060007) and the Key Research & Development Project of Science Technology Department of Zhejiang Province (No. 2024C01203) for financial support.References
- (a) J. A. Pereira, A. M. Pessoa, M. N. D. S. Cordeiro, R. Fernandes, C. Prudêncio, J. P. Noronha and M. Vieira, Eur. J. Med. Chem., 2015, 97, 664–672 CrossRef CAS PubMed; (b) R. Liu, Z. Huang, M. G. Murray, X. Guo and G. Liu, J. Med. Chem., 2011, 54, 5747–5768 CrossRef CAS PubMed; (c) J.-W. Yuan, J.-H. Fu, S.-N. Liu, Y.-M. Xiao, P. Mao and L.-B. Qu, Org. Biomol. Chem., 2018, 16, 3203–3212 RSC; (d) X. Jiang, K. Wu, R. Bai, P. Zhang and Y. Zhang, Eur. J. Med. Chem., 2022, 229, 114085 CrossRef CAS PubMed; (e) S. A. Galal, S. H. M. Khairat, F. A. F. Ragab, A. S. Abdelsamie, M. M. Ali, S. M. Soliman, J. Mortier, G. Wolber and H. I. El Diwani, Eur. J. Med. Chem., 2014, 86, 122–132 CrossRef CAS PubMed; (f) B. Chen, J. Xu, Z. Wang, L. Yang, Q. Liu and P. Zhang, Green Chem., 2025, 27, 10656–10663 RSC; (g) Q.-H. Peng, J. Jiang, Z.-H. Liao, Z.-L. Wu, L.-J. Ou, H. Dai, J. Peng and W.-M. He, ACS Sustainable Chem. Eng., 2025, 13, 10971–10977 CrossRef CAS; (h) X.-M. Chen, L. Song, J. Pan, F. Zeng, Y. Xie, W. Wei and D. Yi, Chin. Chem. Lett., 2024, 35, 110112 CrossRef CAS; (i) W.-T. Ouyang, J. Jiang, Y.-F. Jiang, T. Li, Y.-Y. Liu, H.-T. Ji, L.-J. Ou and W.-M. He, Chin. Chem. Lett., 2024, 35, 110038 CrossRef CAS; (j) S. Gao, H. Zhu, Q. Jin, L. Jin, X. Wang and L. Dai, Chin. J. Org. Chem., 2024, 44, 1264–1275 CrossRef CAS.
- (a) R. S. K. Lalji, Prince, M. Gupta, S. Kumar, A. Kumar and B. K. Singh, RSC Adv., 2023, 13, 6191–6198 RSC; (b) Y. Ramli, A. Moussaif, K. Karrouchi and E. M. Essassi, J. Chem., 2014, 2014, 1–21 CrossRef; (c) T. Guo, C.-C. Wang, X.-H. Fu, Y. Liu and P.-K. Zhang, Org. Biomol. Chem., 2019, 17, 3333–3337 RSC; (d) D. Xie, R.-G. Tian, X.-T. Zhang and S.-K. Tian, Org. Biomol. Chem., 2022, 20, 4518–4521 RSC; (e) A. Carrër, J. Brion, M. Alami and S. Messaoudi, Adv. Synth. Catal., 2014, 356, 3821–3830 CrossRef; (f) Y. Gao, Z. Wu, L. Yu, Y. Wang and Y. Pan, Angew. Chem., Int. Ed., 2020, 59, 10859–10863 CrossRef CAS PubMed; (g) Y. Wang, J. Wang, C. Chen, L. Song, Z.-L. Wang, W. Wei and D. Yi, Chem. Commun., 2025, 61, 10812–10815 RSC; (h) Y.-C. Wen, J.-C. Hou, Q. Zhou, S.-H. Wang, J. Jiang, Z. Yang, H.-T. Zhu, Z.-L. Wang and W.-M. He, Chin. Chem. Lett., 2025, 36, 111795 CrossRef CAS; (i) Z. Zhang, F. Cheng, X. Ma, K. Sun, X. Huang, J. An, M. Peng, X. Chen and B. Yu, Green Chem., 2024, 26, 7331–7336 RSC; (j) Q.-H. Peng, N.-B. Li, J.-C. Hou, C.-J. He, Y.-X. Yang, C.-L. Zhuang, L.-J. Ou, M. Yuan and W.-M. He, Chin. Chem. Lett., 2025, 36, 111402 CrossRef CAS; (k) Y. Zhu, H. Wang, P. Shu, K. Zhang and Q. Wang, Chin. J. Org. Chem., 2024, 44, 1–17 CrossRef CAS.
- (a) L. Wang, P. Yang, J. Yuan, W. Lian, X. Jin, S. Zhang, L. Yang and D. Xing, J. Org. Chem., 2024, 89, 6334–6344 CrossRef CAS PubMed; (b) H. Bao, Z. Lin, M. Jin, H. Zhang, J. Xu, B. Chen and W. Li, Tetrahedron Lett., 2021, 66, 152841 CrossRef CAS; (c) S. Liu, Y. Huang, F.-L. Qing and X.-H. Xu, Org. Lett., 2018, 20, 5497–5501 CrossRef CAS PubMed; (d) S. Paul, J. H. Ha, G. E. Park and Y. R. Lee, Adv. Synth. Catal., 2017, 359, 1515–1521 CrossRef CAS; (e) Z. Deng, T. Yang, K.-H. Wang, W. Xue, D. Huang, P. Li, J. Wang, Y. Su and Y. Hu, Tetrahedron, 2020, 76, 131185 CrossRef CAS; (f) X. Zeng, C. Liu, X. Wang, J. Zhang, X. Wang and Y. Hu, Org. Biomol. Chem., 2017, 15, 8929–8935 RSC; (g) J. Xu, C. Liang, J. Shen, Q. Chen, W. Li and P. Zhang, Green Chem., 2023, 25, 1975–1981 RSC; (h) L. Wu, Z. Wang, Y. Qiao, L. Xie and Q. Wang, Chem. Commun., 2024, 60, 11311–11314 RSC; (i) H. Zhang, J. Xu, Y. Ouyang, X. Yue, C. Zhou, Z. Ni and W. Li, Chin. Chem. Lett., 2022, 33, 2036–2040 CrossRef CAS; (j) Y. Shen, Y. Li, X. Wang, J. Wei, Y. Shen, L. Wu and K. Luo, Org. Biomol. Chem., 2025, 23, 1683–1688 RSC; (k) N. B. Dutta, M. Bhuyan and G. Baishya, RSC Adv., 2020, 10, 3615–3624 RSC; (l) L. Wang, Y. Zhang, F. Li, X. Hao, H. Zhang and J. Zhao, Adv. Synth. Catal., 2018, 360, 3969–3977 CrossRef CAS; (m) K. Zheng, C. Chen, H. Xu, J. Mao and C. Shen, J. Org. Chem., 2025, 90, 15567–15577 CrossRef CAS PubMed; (n) L. Huang, J. Xu, L. He, C. Liang, Y. Ouyang, Y. Yu, W. Li and P. Zhang, Chin. Chem. Lett., 2021, 32, 3627–3631 CrossRef CAS.
- A. Carrër, J.-D. Brion, S. Messaoudi and M. Alami, Org. Lett., 2013, 15, 5606–5609 CrossRef.
- K. Yin and R. Zhang, Org. Lett., 2017, 19, 1530–1533 CrossRef CAS PubMed.
- J. Yuan, S. Liu and L. Qu, Adv. Synth. Catal., 2017, 359, 4197–4207 CrossRef CAS.
- W. Wei, L. Wang, H. Yue, P. Bao, W. Liu, C. Hu, D. Yang and H. Wang, ACS Sustainable Chem. Eng., 2018, 6, 17252–17257 CrossRef CAS.
- J. Xu, H. Zhang, J. Zhao, Z. Ni, P. Zhang, B.-F. Shi and W. Li, Org. Chem. Front., 2020, 7, 4031–4042 RSC.
- C. Liang, Y. Guo, Y. Zhang, Z. Wang, L. Li and W. Li, Org. Chem. Front., 2023, 10, 611–623 RSC.
- (a) C. Zhu and J. R. Falck, Adv. Synth. Catal., 2014, 356, 2395–2410 CrossRef CAS PubMed; (b) Z. Deng, J. Hu and S. Liu, Macromol. Rapid Commun., 2017, 38, 1600685 CrossRef PubMed; (c) T. Sun, J. Wang, C. Qiu, X. Ling, B. Tian, W. Chen and C. Su, Adv. Sci., 2018, 5, 1800036 CrossRef PubMed; (d) L. Yao, D. Zhu, L. Wang, J. Liu, Y. Zhang and P. Li, Chin. Chem. Lett., 2021, 32, 4033–4037 CrossRef CAS; (e) A. Bisoyi, A. Panuganti, R. Poolamanna, A. R. Tripathy and V. R. Yatham, Adv. Synth. Catal., 2025, 367, e202500191 CrossRef CAS; (f) C.-Y. Xu, X.-L. Luan, Y.-Y. Cui and C.-X. Yang, Chin. Chem. Lett., 2025, 36, 110937 CrossRef CAS; (g) B. Geng, X. Chu, L. Liu, L. Zhang, S. Zhang, X. Wang, S. Song and H. Zhang, Chin. Chem. Lett., 2024, 35, 108924 CrossRef CAS.
- (a) L. Cao, H. Zhao, R. Guan, H. Jiang, P. H. Dixneuf and M. Zhang, Nat. Commun., 2021, 12, 4206 CrossRef CAS PubMed; (b) Y. Li, Z. Peng, D. Liu, M. Pan, Y. Shen, H. You, M. Zhao and W. Li, J. Org. Chem., 2024, 89, 13296–13307 CrossRef CAS PubMed; (c) D. G. Thakur, M. A. Sonawane, R. N. Patel, N. B. Rathod, S. D. Patel, D. M. Patel and S. C. Ghosh, Adv. Synth. Catal., 2024, 366, 4994–5000 CrossRef.
- (a) J. L. Tu, Y. Zhu, P. Li and B. Huang, Org. Chem. Front., 2024, 11, 5278–5305 RSC; (b) C. G. S. Lima, T. De, M. Lima, M. Duarte, I. D. Jurberg and M. W. Paixão, ACS Catal., 2016, 6, 1389–1407 CrossRef CAS; (c) A. K. Wortman and C. R. J. Stephenson, Chem, 2023, 9, 2390–2415 CrossRef CAS PubMed; (d) Y. Sumida and H. Ohmiya, Chem. Soc. Rev., 2021, 50, 6320–6332 RSC; (e) X. Cui and W. He, Chin. J. Org. Chem., 2024, 44, 2955–2957 CrossRef; (f) J. Yang, Y. Zhao and L. Shi, Chin. J. Org. Chem., 2025, 45, 559–573 CrossRef CAS; (g) R. Xie, F. Xie, R. Sun, X. Wang, Y. Wang, L. Li and H. Wang, Chin. J. Org. Chem., 2025, 45, 2913–2922 CrossRef CAS.
- (a) L. Liu, J. Liu, S. Li, M. Yang, X. Zhao and K. Lu, Org. Biomol. Chem., 2025, 23, 629–637 RSC; (b) D. J. Castillo-Pazos, J. D. Lasso, E. Hamzehpoor, J. Ramos-Sánchez, J. M. Salgado, G. Cosa, D. F. Perepichka and C.-J. Li, Chem. Sci., 2023, 14, 3470–3481 RSC; (c) J. Xu, Y. Hong, R. Xu, Y. Wang, Y. Guo, J. Shen, Y. Zhao and W. Li, Org. Chem. Front., 2024, 11, 6166–6176 RSC; (d) G. E. M. Crisenza, D. Mazzarella and P. Melchiorre, J. Am. Chem. Soc., 2020, 142, 5461–5476 CrossRef CAS PubMed; (e) W. Huang, T. Wang, S.-Y. Chen, Q. Zhou and L. Wang, Org. Biomol. Chem., 2025, 23, 5564–5568 RSC; (f) C. Zheng, G. Wang and R. Shang, Adv. Synth. Catal., 2019, 361, 4500–4505 CrossRef CAS; (g) P. Meher, S. P. Panda, S. K. Mahapatra, K. R. Thombare, L. Roy and S. Murarka, Org. Lett., 2023, 25, 8290–8295 CrossRef CAS PubMed.
- M. R. Lasky, T. K. Salvador, S. Mukhopadhyay, M. S. Remy, T. P. Vaid and M. S. Sanford, Angew. Chem., Int. Ed., 2022, 61, e202208741 CrossRef CAS PubMed.
- (a) Y. Li, J. Xu, Y. Wang, R. Xu, Y. Zhao and W. Li, J. Org. Chem., 2025, 90, 1683–1696 CrossRef CAS PubMed; (b) R. Xu, B. Cao, Y. Wang, J. Zhang, Y. Huang and W. Li, Green Chem., 2025, 27, 9223–9229 RSC; (c) J. Zhu, Y. Guo, Y. Zhang, W. Li, P. Zhang and J. Xu, Green Chem., 2023, 25, 986–992 RSC; (d) Y. Hong, J. Xu, A. Chen, Y. Du, G. Wang, J. Shen and P. Zhang, Org. Lett., 2025, 27, 2526–2531 CrossRef CAS.
- R. Maity, A. Bankura and I. Das, Green Chem., 2023, 25, 7774–7781 RSC.
| This journal is © The Royal Society of Chemistry 2026 |