Cationic halogenated BODIPYs as water-soluble photosensitizers for photodynamic therapy
Arrhon Mae
Bongo†
a,
Yeji
Kim†
a,
Duy Khuong
Mai
a,
Jung Suk
Kim
*c,
Sung
Cho
*b and
Ho-Joong
Kim
 *a
*a
aDepartment of Chemistry, Chosun University, Gwangju 61452, Korea. E-mail: hjkim@chosun.ac.kr
bDepartment of Chemistry, Chonnam National University, Gwangju, 61186, Korea. E-mail: scho@chonnam.ac.kr
cDepartment of Orthopedic Surgery, National Police Hospital, Seoul 05715, Korea. E-mail: maeno70@daum.net
First published on 5th December 2025
Abstract
This paper reports the synthesis of three water-soluble halogenated boron-dipyrromethene (BODIPY) photosensitizers (BHTM, BBrTM, and BITM) functionalized with cationic groups to improve biological performance. Introduction of bromine and iodine at the 2,6-positions promoted singlet-oxygen generation through the heavy-atom effect, with quantum yields (ϕΔ) of 0.59 for BBrTM and 0.91 for BITM, higher than that (0.11) for the non-halogenated analog BHTM. Incorporation of cationic substituents enhanced hydrophilicity, enabling effective dispersion in aqueous environments and facilitating cellular uptake. Biological evaluations in MCF-7 and HeLa cancer cells revealed minimal dark cytotoxicity (>95% viability at 400 nM) but pronounced phototoxicity under 530 nm light irradiation, confirming efficient photodynamic therapy activity. Confocal laser scanning microscopy further demonstrated favorable fluorescence imaging capability and cytoplasmic localization. Collectively, these findings highlight the potential of water-soluble halogenated BODIPY dyes as dual-function agents for fluorescence-imaging-guided photodynamic therapy.
1. Introduction
Cancer is a significant health concern and one of the leading causes of mortality worldwide, as reported by the International Agency for Research on Cancer (IARC).1–3 Conventional treatment strategies, such as chemotherapy, surgery, radiotherapy, and immunotherapy, are often accompanied by severe adverse effects, including vomiting, weakened immune systems, nausea, and hair loss.1,4–6 Photodynamic therapy (PDT), introduced in the 20th century,7,8 is a clinically approved, minimally invasive, and widely recognized therapeutic approach for diagnosing and treating various diseases, especially cancer.8–10 For cancer treatment, the benefits of PDT include higher selectivity toward tumor cells, reduced aftereffects, and the potential of repeated treatments.11 PDT involves three major components: a photosensitizer (PS), light, and oxygen.12 Upon light irradiation at a wavelength corresponding to its highest absorption peak, the PS is excited to the singlet excited state (S1) and undergoes intersystem crossing to the triplet state (T1). From this point, the PS can react with nearby molecules through two mechanisms: Type I and Type II. In Type I reactions, the excited PS transfers an electron or proton to nearby oxygen molecules, generating reactive oxygen species (ROS) such as hydroxyl radicals, hydrogen peroxide, or superoxide anions. In Type II reactions, the light-activated PS directly transfers energy to molecular oxygen, producing highly reactive singlet oxygen (1O2), which is the main cytotoxic agent in PDT. Ultimately, the generated cytotoxic ROS damages tumor cells and prevents disease progression.10,12 Notably, in cancer treatment, PDT can disrupt the vascular structure surrounding the tumor cells and trigger an immunological response against them.13 Cyclic tetrapyrroles (porphyrins, chlorins, and bacteriochlorins) are representative examples of clinically approved PDT agents. Since their discovery in 1968, there has been growing interest in non-porphyrin PSs based on small photoactive molecules such as boron-dipyrromethene (BODIPY).14,15BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) dyes are an emerging class of PSs and well-known fluorophores with excellent properties, such as a high absorption coefficient, fluorescence quantum yield, and photostability. However, they exhibit several drawbacks, including high lipophilicity, low singlet oxygen yield, and absorption limited to the short-wavelength range of the visible spectrum. The low singlet oxygen yield is attributable to the preferential release of absorbed energy through fluorescence rather than through ISC to the T1 state, a critical step for effective singlet oxygen production. To promote the use of BODIPY dyes, the singlet oxygen yield, which is a key factor influencing the effectiveness of PDT, must be enhanced. The versatile and modular structure of BODIPY enables modifications to improve performance. A conventional strategy to enhance singlet-oxygen generation is the incorporation of heavy atoms, such as bromine or iodine, into the BODIPY core. Electrophilic substitution at the 2,6-positions of the core was first demonstrated by Treibs and Kreuzer via sulfonation using chlorosulfonic acid.16 The electron-rich nature of the BODIPY core facilitates the introduction of halogens like bromine and iodine. These heavy atoms enhance ISC through spin–orbit coupling (SOC), a phenomenon known as the heavy-atom effect. This effect, involving the amplification of spin-forbidden processes owing to the presence of high-atomic-number elements in the excited molecule, transforms BODIPY from a highly fluorescent dye with low triplet transition capability into an efficient PS. The modification reduces fluorescence intensity and lifetime while enhancing and stabilizing ISC to T1. This results in a long-lived, high-yield triplet state that promotes singlet-oxygen generation.14,17–19 In addition to halogenation, heavy chalcogen substitutions have also been employed to promote intersystem crossing. For example, electrochemical 2,6-diselenation of BODIPY fluorophores affords selenium-containing derivatives with red-shifted emission, efficient triplet formation, and singlet-oxygen sensitization suitable for bioimaging applications.20
Conventional BODIPY dyes exhibit low solubility in aqueous environments, limiting their biological applications. In 1985, Wories et al.21 synthesized a BODIPY dye bearing a chlorosulfonic acid group, initiating efforts to enhance water solubility. Since then, various hydrophilic groups such as sulfonates, phosphonates, and quaternary ammonium salts have been introduced to improve aqueous compatibility.22–24 More recently, boron-centered hydrophilization strategies have been reported, in which the BF2 unit is replaced by hydrophilic acyloxy groups to generate highly water-soluble COO–BODIPY dyes without significantly perturbing photophysical properties.25 In parallel, sugar-based GlycoBODIPYs have been developed, in which mono-, di-, or trisaccharides are directly integrated into the BODIPY scaffold to generate neutral, highly water-soluble, and bright probes with tunable cellular localization.26 Remote sulfonation of BODIPY photocages has also been shown to markedly improve water solubility while preserving photorelease efficiency and enabling control over cellular permeability.27 Among the hydrophilic approaches, quaternary ammonium compounds (QACs) are notable for their cationic nature, amphiphilicity, and broad biological compatibility.28–30 Considering these aspects, we developed a series of cationic BODIPY PSs featuring a quaternized diaminophenyl moiety at the meso position of the BODIPY core.31 These compounds exhibited excellent mitochondrial-targeting ability, positioning them as effective PSs for PDT for cancer treatment.
In this study, we synthesized a series of water-soluble cationic BODIPY PSs (BHTM, BBrTM, and BITM), functionalized with a quaternary ammonium moiety and an alkyl linker via the copper iodide (CuI)-catalyzed azide–alkyne cycloaddition (CuAAC) click reaction. Photophysical properties of these PSs were investigated using UV–visible (UV–vis) absorbance, fluorescence spectroscopy, fluorescence quantum yield, and singlet oxygen quantum yield measurements. In addition, their PDT activity and fluorescence imaging ability were assessed in two cancer cell lines (human breast adenocarcinoma (MCF-7) and human cervical adenocarcinoma (HeLa)). The results provide valuable insights into the potential of quaternary-ammonium-functionalized BODIPY dyes for biological and therapeutic applications.
2. Results and discussion
2.1 Synthesis and photophysical characterization of BODIPY PSs
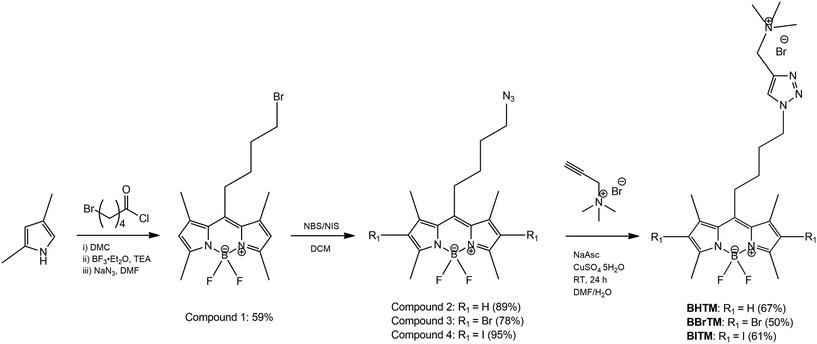
A three-step synthetic route for the tumor-targeting cationic BODIPY PSs is outlined in Scheme 1. The synthesis of the BODIPY core began with the reaction of 2,4-dimethyl pyrrole with 5-bromovaleryl chloride, yielding a dipyrromethene hydrochloride salt intermediate. This unstable intermediate was then complexed with boron trifluoride diethyl etherate in the presence of triethylamine, producing the BODIPY core. Next, the alkyl halide constituent of the BODIPY was converted into its corresponding alkyl azide (compound 2) by treating the BODIPY core with sodium azide (NaN3). In the second step, heavy atoms (bromine and iodine) were introduced at the 2,6-positions of the BODIPY core using N-bromosuccinimide (NBS) and N-iodosuccinimide (NIS), respectively. In the final step, compounds 2, 3, and 4 were reacted with N,N,N-trimethyl-N-propargylammonium bromide via the simple and straightforward CuAAC reaction. All synthesized BODIPY compounds were characterized using proton nuclear magnetic resonance (1H-NMR) spectroscopy. The final cationic BODIPY PSs, BHTM, BBrTM, and BITM were further characterized by 13C-NMR and mass spectrometry (see SI).2.2 Photophysical and theoretical characterizations of BODIPY PSs
Fig. 1 shows the absorbance and fluorescence spectra of the cationic BODIPY PSs (BHTM, BBrTM, and BITM) in methanol and water. The sharp and narrow S1-state absorption bands observed at 450–550 nm are characteristic of BODIPY chromophores.32,33 Based on optimized molecular structures, the simulated electronic excited states confirmed that the S1 state of the cationic BODIPY PSs arises predominantly from the highest occupied molecular orbital (HOMO)–lowest unoccupied molecular orbital (LUMO) transition (Chart S1 in the SI). Relative to BHTM, the halogenated analogs BBrTM and BITM exhibited red-shifted S1-state absorption bands. This bathochromic shift is attributable to the electron-withdrawing effects of β-substituted bromine and iodine atoms, which stabilize the frontier molecular orbitals of BBrTM and BITM. However, the LUMOs are less stabilized owing to enhanced electronic coupling between the BODIPY core and halogen substituents (Fig. 2). Consequently, the HOMO–LUMO gaps of BBrTM and BITM are reduced, resulting in the observed bathochromic shifts. The fluorescence spectra of the cationic BODIPY PSs are mirror images of their absorption spectra, reflecting their rigid structure. Notably, the fluorescence quantum yields (ΦF) of BBrTM and BITM in methanol decreased significantly to 0.26 and 0.05, respectively (Table 1), as discussed in the following section. | ||
| Fig. 1 Steady-state absorption and fluorescence spectra of cationic BODIPY PSs in methanol (solid lines) and in water (dashed lines). | ||
| Sample | Solvent | λ maxabs nm−1 (FWHM (cm−1)) | λ maxem nm−1 (FWHM (cm−1)) | Stokes shift cm−−1 | ϕ F | ϕ Δ | ε/M−1 cm−1 |
|---|---|---|---|---|---|---|---|
| BHTM | MeOH | 496 (668) | 511 (846) | 573 | 0.65 | 0.11 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
| Water | 494 (730) | 507 (864) | 540 | ||||
| BBrTM | MeOH | 520 (823) | 539 (934) | 662 | 0.26 | 0.59 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
| Water | 520 (1000) | 539 (1130) | 680 | ||||
| BITM | MeOH | 526 (898) | 550 (1000) | 808 | 0.05 | 0.91 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
| Water | 526 (1060) | 541 (1770) | 541 |
2.3 Singlet-oxygen generation of cationic BODIPY PSs
To evaluate the singlet-oxygen generation efficiency of the cationic BODIPY PSs, we mixed 1,3-diphenylisobenzofuran (DPBF) in the sample solution and monitored its photobleaching under 517 nm light-emission diode (LED) irradiation. DPBF undergoes a [4 + 2] cycloaddition with singlet oxygen, producing a non-absorbing monocyclic product; thus, the decrease in its characteristic absorption band at approximately 400 nm serves as a direct indicator of singlet-oxygen generation. Upon 517 nm LED irradiation, halogenated BBrTM and BITM induced significant photobleaching of DPBF, with complete loss of absorbance within 5 min. In contrast, the non-halogenated BHTM required up to 30 min to achieve this effect under identical conditions. The singlet-oxygen generation quantum yields (ΦΔ) of BBrTM (0.59) and BITM (0.91) significantly exceeded that of BHTM (0.11), highlighting the effect of β-substituted bromine and iodine atoms (Fig. 3).To elucidate the origin of the enhanced singlet-oxygen generation efficiency for halogenated BBrTM and BITM, we calculated the energy differences between the S1 and Tn (n = 1–3) states and their SOC matrix elements (Fig. 4 and Table 2). Although halogenation reduced the energy levels of the S1 and Tn states, the relative energy differences remained nearly unchanged, indicating minimal energetic perturbation of the excited states. In contrast, the calculated SOC matrix elements dramatically increased by 81- and 310-times for the S1–T2 transition and by 41- and 130-times for the S1–T1 transition in BBrTM and BITM, respectively. Because the T2 and T3 states are energetically close to the S1 state, ISC from S1 to these triplet states is strongly accelerated relative to radiative decay and internal conversion from S1. Collectively, these theoretical results confirm that the β-halogen substitution of bromine and iodine atoms induces a strong heavy-atom effect, facilitating ISC and markedly enhancing singlet-oxygen generation.
| Sample | Manifold | E S1 (eV) | E Tn (eV) | ΔES1–Tn (eV) | SOC (cm−1) |
|---|---|---|---|---|---|
| a Calculated at the B3LYP/ZORA-def2-TZVP level of theory implemented in the ORCA 6.0.1 software package, based on the optimized molecular structures at the B3LYP/Def2TZVP level in the Gaussian 16 software package. | |||||
| BHOM | S1–T3 | 3.020 | 2.966 | 0.054 | 1.30 |
| S1–T2 | 2.822 | 0.198 | 0.057 | ||
| S1–T1 | 1.610 | 1.410 | 0.014 | ||
| BBrOM | S1–T3 | 2.793 | 2.753 | 0.040 | 1.42 |
| S1–T2 | 2.581 | 0.212 | 4.70 | ||
| S1–T1 | 1.570 | 1.223 | 0.59 | ||
| BIOM | S1–T3 | 2.729 | 2.688 | 0.041 | 2.49 |
| S1–T2 | 2.541 | 0.188 | 17.5 | ||
| S1–T1 | 1.572 | 1.157 | 1.85 | ||
2.4 Cytotoxicity of cationic BODIPY PSs
For effective PDT, a PS should exhibit minimal cytotoxicity in the dark while inducing significant toxicity under light irradiation. The cytotoxicity of the synthesized cationic BODIPY PSs was assessed against two cancer cell lines, MCF-7 and HeLa. Cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. The two cancer cell lines were treated with various concentrations of the BODIPY PSs and evaluated both in the absence and presence of 530 nm LED illumination. Background absorbance values from wells containing MTS solutions alone were subtracted from those of the treated and control cell wells to ensure accurate viability measurements.As shown in Fig. 5a–c, the synthesized cationic BODIPY PSs exhibited negligible dark toxicity against MCF-7 cells, with cell viability ≥95% even at the maximum tested concentration of 400 nM. Similarly, low cytotoxicity in the dark was observed against HeLa cells (Fig. 5d–f), where all cationic BODIPY PSs maintained cell viabilities of at least 95%, even at the highest concentration of 400 nM. These results indicate that the synthesized cationic BODIPY PSs exhibit excellent biocompatibility in the absence of light irradiation.
 | ||
| Fig. 5 Cell survival rates of MCF-7 cells (a–c) and HeLa (d–f) after treatment with BHTM, BBrTM, and BITM under dark conditions. | ||
2.5 Photodynamic anticancer activity of cationic BODIPY PSs
The photodynamic toxicity of BHTM, BBrTM, and BITM was evaluated after LED irradiation at 530 nm (9 mW cm−2) for approximately 20 min. As shown in Fig. 6b and c, in MCF-7 cells, the heavy-atom-containing PSs (BBrTM and BITM) exhibited cytotoxic effects even at the lowest concentration of 50 nM, with toxicity increasing as the concentration increased. Cell viability dropped significantly to below 25% at 200 nM and approached 0% at 400 nM for both PSs. Similarly, in HeLa cells (Fig. 6e and f), BBrTM and BITM exhibited notable toxic effects at a concentration of 50 nM, with cytotoxicity intensifying at higher concentrations. Additionally, the incorporation of an amine group in the alkylated triazole chain at the meso position of the diaza-s-indacene ring enhanced the amphiphilicity of these compounds. This modification resulted in cationic substituents, improving their solubility in biological environments and facilitating adhesion and permeation into cell membranes, thereby enhancing their photodynamic efficacy.30In contrast, the control BODIPY PS BHTM did not exhibit significant cytotoxic effects on the tested tumor cells, even under LED irradiation (Fig. 6a and d), with cell viabilities remaining at 95% or higher. Overall, these findings demonstrate that the synthesized cationic BODIPY PSs, BBrTM and BITM, are biocompatible and non-toxic in the absence of light, while effectively inducing cytotoxicity upon LED irradiation through reactive singlet-oxygen generation.
2.6 Fluorescence cell imaging of BODIPY dyes
The cellular uptake and subcellular localization of the synthesized BODIPY derivatives (BHTM, BBrTM, and BITM) were examined in HeLa and MCF-7 cells using confocal laser scanning microscopy (CLSM). In this assessment, 4′,6-diamidino-2-phenylindole (DAPI) was used as a nuclear counterstain (blue), while BODIPY fluorescence was observed in the green channel. As shown in Fig. 7, all three dyes efficiently penetrated both cell lines and were predominantly localized in the cytoplasmic region, with negligible nuclear accumulation. In HeLa cells, BHTM and BBrTM displayed intense punctate fluorescence distributed across the cytoplasm, whereas BITM exhibited more diffuse fluorescence with visible cytoplasmic uptake. Similar patterns were observed in MCF-7 cells, where all three dyes accumulated primarily in the cytoplasm, with punctate signals suggesting association with intracellular compartments. These results confirm that the cationic BODIPY derivatives are readily taken up by cancer cells and exhibit favorable intracellular localization. The punctate fluorescence of BHTM, BBrTM and BITM is consistent with the well-documented behavior of cationic, lipophilic dyes, which are often associated with subcellular organelles.34,35The observed fluorescence distribution is significant for PDT, as cytoplasmic and organelle-associated localization enhances the potential for ROS-mediated cell death upon photoactivation.36 Importantly, the consistent behavior of the dyes in both HeLa and MCF-7 cells highlights their broad applicability across cancer models. Our earlier reports have highlighted the robust fluorescence imaging performance of halogenated BODIPY derivatives.31–33
Collectively, these findings support that BHTM, BBrTM, and BITM efficiently accumulate in cancer cells and display favorable intracellular localization, providing a strong basis for their use as PSs in PDT.
3. Materials and methods
3.1 Materials
All chemicals were procured from commercial sources. Boron trifluoride diethyl etherate (BF3·Et2O) and 2,4-dimethyl pyrrole were purchased from Sigma–Aldrich (St Louis, MO, USA). Triethylamine (TEA), 5-bromovaleryl chloride, NBS, NIS, propargyl bromide, trimethylamine, and sodium ascorbate (NaAsc) were obtained from TCI Chemicals (Tokyo, Japan). NaN3, magnesium sulfate (MgSO4), copper sulfate (CuSO4·5H2O), toluene, ethyl ether, and N,N-dimethylformamide were acquired from Daejung Chemical (Gyeonggi-do, Republic of Korea). Methylene chloride (CH2Cl2), ethyl acetate (EtOAc), tetrahydrofuran (THF), methanol, and other solvents were of analytical grade. All solvents, except THF were dried with calcium hydride prior to use.All compounds were characterized by 1H and 13C-NMR spectroscopy using a Bruker AM 250 spectrometer (Billerica, MA, USA). Product purity was assessed via thin-layer chromatography (TLC, silica gel 60 mesh). UV spectra were measured using a Shimadzu UV-1650PC spectrometer, and fluorescence spectra were recorded on a Hitachi F-7000 spectrometer. High-resolution electrospray ionization mass spectrometry (HR-ESI-MS) data of the final cationic BODIPY compounds was measured using a SYNAPT G2-Si high-definition mass spectrometer (Wilmslow, UK).
3.2 Synthesis of cationic BODIPY PSs
1H-NMR (400 MHz, CDCl3, δ, ppm): 6.06 (s, 2H), 3.37–3.34 (t, 2H), 2.99–2.95 (t, 2H), 2.51 (s, 6H), 2.41 (s, 6H), 1.81–1.70 (m, 4H).
13C NMR (101 MHz, CDCl3) δ 154.44, 145.82, 140.68, 131.76, 122.16, 51.27, 31.37, 30.13, 29.64, 29.17, 28.09, 16.72, 14.90, 14.87, 14.85.
HRMS (ESI): m/z calcd for C17H22BN5F2 [M + Na]+ 368.1834, found 368.1831.
1H NMR (400 MHz, CDCl3) δ 3.36 (t, 2H), 2.93 (d, 2H), 2.56 (s, 6H), 2.39 (s, 6H), 1.72 (d, 4H).
13C NMR (101 MHz, CDCl3) δ 152.38, 145.73, 137.43, 130.19, 111.99, 50.55, 29.85, 29.48, 28.92, 28.34, 27.97, 26.90, 22.52, 19.53, 15.19, 13.96, 13.50.
HRMS (ESI): m/z calcd for C17H20BN5F2Br2 [M + Na]+ 524.0044, found 524.0046.
1H NMR (400 MHz, CDCl3) δ 3.38 (t, 2H), 3.05–2.98 (m, 2H), 2.61 (s, 6H), 2.47 (s, 6H), 1.82–1.68 (m, 4H).
13C NMR (101 MHz, CDCl3) δ 155.52, 144.96, 142.07, 131.25, 86.56, 50.76, 37.03, 31.87, 29.98, 29.65, 29.10, 28.54, 27.03, 22.64, 18.89, 16.09, 14.08.
HRMS (ESI): m/z calcd for C17H20BN5F2I2 [M + Na]+ 619.9766, found 619.9767.
BHTM . Compound 2 (70 mg, 0.203 mmol), N,N,N-trimethyl-N-propargylammonium bromide (Scheme S1; 46.94 mg, 0.264 mmol), NaAsc (200 mg, 1 mmol), and CuSO4·5H2O (64.73 mg, 0.406 mmol) were dissolved in a mixture of DMF/H2O (10/2 mL, v/v). The resulting mixture was stirred for 48 h at room temperature, extracted with EtOAc and brine three times, and dried over MgSO4. Following filtration and solvent removal on a rotary evaporator, the crude product was purified by recrystallization from MeOH/diethyl ether to afford an orange solid (67 mg, 63.14% yield).
1H-NMR (400 MHz, CD3OD, δ, ppm): 8.33 (s, 1H), 6.07 (s, 2H) 4.63 (s, 2H), 4.51–4.48 (t, 2H), 3.09 (s, 9H), 2.39 (s, 6H), 2.30 (s, 6H), 2.13–2.09 (m, 4H), 1.58–1.51 (m, 2H).
13C-NMR (100 MHz, CD3OD, δ, ppm): 155.10, 147.19, 142.27, 132.51, 129.7, 122.75, 61.13, 53.38, 51.23, 49.85, 31.26, 29.71, 28.47, 16.50, 14.48.
HRMS (ESI): m/z 443.2905, calculated mass for C23H34N6BF2 443.29.
BBrTM . Following the same procedure described above using compound 3 afforded a black-red solid (34 mg, 50.2% yield).
1H-NMR (400 MHz, CD3OD, δ, ppm): 8.35 (s, 1H), 4.65 (s, 2H), 4.54–4.51 (t, 2H), 3.10 (s, 9H), 2.46 (s, 6H), 2.35 (s, 6H), 2.17–2.12 (m, 4H), 1.61–1.53 (m, 2H).
13C-NMR (100 MHz, CD3OD, δ, ppm): 153.43, 148.28, 139.51, 136.80, 131.64, 129.62, 112.72, 61.06, 53.34, 51.12, 31.11, 29.46, 29.19, 15.72, 13.80.
HRMS (ESI): m/z 559.1117, calcd for C23H32BN6F2Br2: 599.11.
BITM . Following the same procedure described above using compound 4 afforded a red-orange solid (55 mg, 60.52% yield).
1H-NMR (400 MHz, CD3OD, δ, ppm): 8.31 (s, 1H), 4.62 (s, 2H), 4.54–4.51 (t, 2H), 3.09 (s, 9H), 2.50 (s, 6H), 2.40 (s, 6H), 2.21–2.16 (m, 4H), 1.61–1.54 (m, 2H).
13C-NMR (100 MHz, CD3OD, δ, ppm): 156.37, 147.21, 144.04, 132.48, 129.69, 87.03, 61.07, 53.36, 51.13, 31.10, 29.41, 24.21, 19.29, 16.87, 16.34.
HRMS (ESI): m/z 695.0838, calcd for C23H32BN6F2Br2: 695.08.
3.3 Measurement of photophysical properties
UV–vis absorption spectra were analyzed in a 1 cm path length quartz cuvette using a double-beam UV-2800 UV–vis spectrophotometer (Shimadzu, Kyoto, Japan) at room temperature. An F-4500 steady-state fluorometer (Hitachi, Tokyo, Japan), equipped with a xenon arc lamp and photomultiplier detection system, was used to obtain the steady-state fluorescence spectra. All spectra were evaluated at 300–700 nm, in triplicate, and corrected for background intensities by subtracting the spectra of the pure solvent measured under similar conditions.3.4 Fluorescence quantum yield measurements
Fluorescence emission intensities were measured using a Hitachi F-7000 fluorescence spectrophotometer. Absolute fluorescence quantum yields were measured using a Quantarius–QY Absolute PL quantum yield spectrometer (Hamamatsu Photonics K.K., Hamamatsu, Japan).3.5 Structural optimization and excited-state simulations
The molecular structures of the cationic BODIPY PSs were optimized using the density functional theory (DFT) method with Becke's three-parameter hybrid exchange functional and the Lee–Yang–Parr correlation functional (B3LYP), employing the Def2-TZVP basis set in the Gaussian 16 software package. The calculations were performed on a supercomputer (KISTI, Nurion, Daejeon, Korea). Excited-state simulations and SOC calculations were conducted at the B3LYP/ZORA-def2-TZVP level of theory implemented in the ORCA 6.0.1 software package, based on the optimized molecular structures at the B3LYP/Def2TZVP level in Gaussian 16.3.6 Singlet oxygen quantum yield assessments
Singlet oxygen quantum yields (ΦΔ) of the cationic BODIPY PSs BHTM, BBrTM, and BITM were assessed using DPBF as a chemical quencher.37 Briefly, a mixture of the BODIPY dye (absorbance 0.10–0.15 at 517 nm in MeOH) and DPBF (absorbance ∼0.5 at 400 nm in MeOH) was subjected to laser irradiation (λirr = 517 nm). DPBF photooxidation was examined over 0–30 min, depending on the efficiency of the cationic BODIPY PSs. The singlet oxygen quantum yield was calculated using eosin Y in ethanol at room temperature (ΦΔ = 0.3) as the reference standard, following eqn (1):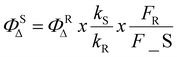 | (1) |
 is the singlet oxygen quantum yield of the reference; k is the slope of the photodegradation rate of DPBF; and S and R represent the sample and reference, respectively. F indicates the absorption correction factor, defined as F = 1–10−OD (where OD represents the absorption value at the irradiation wavelength).
is the singlet oxygen quantum yield of the reference; k is the slope of the photodegradation rate of DPBF; and S and R represent the sample and reference, respectively. F indicates the absorption correction factor, defined as F = 1–10−OD (where OD represents the absorption value at the irradiation wavelength).
3.7 Cells and cell culture
The MCF-7 and HeLa cell lines were procured from the Korean Cell Line Bank and preserved according to the provider's instructions. The cells were cultured under standard culture conditions (5% CO2 and 95% air at 37 °C) in the RPMI-1640 medium (Gibco, Carlsbad, CA, USA) enriched with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics (100 U mL−1 penicillin and 100 mg mL−1 streptomycin) (Welgene Inc., Gyeongsangbuk-do, Korea).3.8 Cell proliferation assay
Cell proliferation was evaluated using the CellTiter 96® Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA), following the manufacturer's instructions. Briefly, MCF-7 and HeLa cells (3 × 103 cells per well) were seeded in 96-well plates. After 24 h of cell incubation, the cells were treated with cationic BODIPY PSs BHTM, BBrTM, and BITM at various concentrations (0, 50, 100, 200, 400 nM) for 2 h. Absorbance was measured at 490 nm using an ELISA plate reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA).3.9 Photodynamic anticancer activity
MCF-7 and HeLa cells were seeded at 3 × 103 cells per well in 96-well plates and incubated at 37 °C in 5% CO2. After 24 h, the cells were treated with various concentrations of BHTM, BBrTM, and BITM (0, 50, 100, 200, 400 nM) and incubated again at 37 °C in 5% CO2 for an additional 2 h under dark conditions. After a 2 h incubation to allow uptake of the BODIPY compounds, the media in all plates were replaced with RPMI-1640 without phenol red. The cells were irradiated using a green LED at 9 mW cm−2 (530 nm) for 20 min. Following irradiation, the cells were incubated for an additional 24 h, and cell proliferation was measured using the CellTiter 96® Aqueous One Solution Cell Proliferation Assay, as described in section 3.8.3.10 Statistical analysis
All results are expressed as mean ± standard deviation. Statistical comparisons were performed using one-way analysis of variance, followed by Tukey's post hoc analysis, in GraphPad Prism 6 software (San Diego, CA, USA). Statistical significance was set at p < 0.05.4. Conclusions
We synthesized a series of water-soluble, cationic BODIPY PSs (BHTM, BBrTM, and BITM) and evaluated their photophysical and photodynamic performance. Quaternary ammonium functionalization conferred excellent aqueous solubility, while 2,6-halogenation enhanced photodynamic activity via the heavy-atom effect. Consistent with this design, the halogenated derivatives exhibited markedly higher singlet oxygen quantum yields (ΦΔ = 0.59 for BBrTM and 0.91 for BITM) relative to the non-halogenated BHTM (ΦΔ = 0.11). In cell studies (MCF-7 and HeLa), the compounds showed minimal dark cytotoxicity and pronounced photocytotoxicity under green-light irradiation, aligning with their elevated singlet-oxygen generation. CLSM analysis confirmed robust cellular uptake and cytoplasmic fluorescence, underscoring their dual role in imaging and therapy. Collectively, these results highlight the potential of halogenated cationic BODIPYs as agents for fluorescence-imaging-guided PDT, overcoming the limitations of conventional BODIPY dyes (low water solubility and ΦΔ).Author contributions
Arrhon Mae Bongo: writing – original draft, conceptualization. Yeji Kim: methodology, investigation, formal analysis. Duy Khuong Mai methodology, investigation. Jung Suk Kim: writing – review & editing, supervision, conceptualization Sung Cho: writing – review & editing, supervision, conceptualization. Ho-Joong Kim: writing – review & editing, funding acquisition, conceptualization.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. The supplementary information contains the synthetic scheme for the preparation of N,N,N-trimethylprop-2-yn-1-aminium bromide; full NMR spectra (1H, 13C), HR-ESI mass spectra, and characterization data for all intermediates and final BODIPY compounds (BHTM, BBrTM, BITM); time-dependent DPBF absorption spectra under 517 nm irradiation; frontier molecular orbital diagrams; and TD-DFT excited-state tables computed at the B3LYP/Def2-TZVP level. See DOI: https://doi.org/10.1039/d5ob01499a.Acknowledgements
This study was supported by a research fund from Chosun University (2023).References
- T. Xu, L. Mi, T. Namulinda, Y.-J. Yan, G. A. Meerovich, I. V. Reshetov, E. A. Kogan and Z.-L. Chen, Quaternary Ammonium Cations Conjugated 5,15-Diaryltetranaphtho[2,3]Porphyrins as Photosensitizers for Photodynamic Therapy, Eur. J. Med. Chem., 2024, 267, 116228, DOI:10.1016/j.ejmech.2024.116228.
- R. L. Siegel, K. D. Miller, N. S. Wagle and A. Jemal, Cancer Statistics, Ca-Cancer J. Clin., 2023, 73(1), 17–48, DOI:10.3322/caac.21763.
- H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal and F. Bray, Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, Ca-Cancer J. Clin., 2021, 71(3), 209–249, DOI:10.3322/caac.21660.
- K. Engle and G. Kumar, Cancer Multidrug-Resistance Reversal by ABCB1 Inhibition: A Recent Update, Eur. J. Med. Chem., 2022, 239, 114542, DOI:10.1016/j.ejmech.2022.114542.
- C. Holohan, S. Van Schaeybroeck, D. B. Longley and P. G. Johnston, Cancer Drug Resistance: An Evolving Paradigm, Nat. Rev. Cancer, 2013, 13(10), 714–726, DOI:10.1038/nrc3599.
- R. K. Rej, J. E. I. Thomas, R. K. Acharyya, J. M. Rae and S. Wang, Targeting the Estrogen Receptor for the Treatment of Breast Cancer: Recent Advances and Challenges, J. Med. Chem., 2023, 66(13), 8339–8381, DOI:10.1021/acs.jmedchem.3c00136.
- M. R. Hamblin, Fullerenes as Photosensitizers in Photodynamic Therapy: Pros and Cons, Photochem. Photobiol. Sci., 2018, 17(11), 1515–1533, 10.1039/c8pp00195b.
- A.-G. Niculescu and A. M. Grumezescu, Photodynamic Therapy—An Up-to-Date Review, Appl. Sci., 2021, 11(8), 3626, DOI:10.3390/app11083626.
- M. Lan, S. Zhao, W. Liu, C.-S. Lee, W. Zhang and P. Wang, Photosensitizers for Photodynamic Therapy, Adv. Healthcare Mater., 2019, 8(13), 1900132, DOI:10.1002/adhm.201900132.
- J. Piskorz, W. Porolnik, M. Kucinska, J. Dlugaszewska, M. Murias and J. Mielcarek, BODIPY-Based Photosensitizers as Potential Anticancer and Antibacterial Agents: Role of the Positive Charge and the Heavy Atom Effect, ChemMedChem, 2021, 16(2), 399–411, DOI:10.1002/cmdc.202000529.
- M. L. Agazzi, M. B. Ballatore, A. M. Durantini, E. N. Durantini and A. C. Tomé, BODIPYs in Antitumoral and Antimicrobial Photodynamic Therapy: An Integrating Review, J. Photochem. Photobiol., C, 2019, 40, 21–48, DOI:10.1016/j.jphotochemrev.2019.04.001.
- Z. Yu, J. Zhou, X. Ji, G. Lin, S. Xu, X. Dong and W. Zhao, Discovery of a Monoiodo Aza-BODIPY Near-Infrared Photosensitizer: In Vitro and in Vivo Evaluation for Photodynamic Therapy, J. Med. Chem., 2020, 63(17), 9950–9964, DOI:10.1021/acs.jmedchem.0c00882.
- A. Kamkaew, S. H. Lim, H. B. Lee, L. K. Kiew, L. Y. Chung and K. Burgess, BODIPY Dyes in Photodynamic Therapy, Chem. Soc. Rev., 2013, 42(1), 77–88, 10.1039/C2CS35216H.
- A. Filatov and M. Heavy-Atom-Free, BODIPY Photosensitizers with Intersystem Crossing Mediated by Intramolecular Photoinduced Electron Transfer, Org. Biomol. Chem., 2020, 18(1), 10–27, 10.1039/C9OB02170A.
- S. Callaghan and M. O. Senge, The Good, the Bad, and the Ugly – Controlling Singlet Oxygen through Design of Photosensitizers and Delivery Systems for Photodynamic Therapy, Photochem. Photobiol. Sci., 2018, 17(11), 1490–1514, 10.1039/C8PP00008E.
- A. Treibs and F.-H. Kreuzer, Difluorboryl-Komplexe von Di- und Tripyrrylmethenen, Justus Liebigs Ann. Chem., 1968, 718(1), 208–223, DOI:10.1002/jlac.19687180119.
- J. Piskorz, W. Porolnik, M. Kucinska, J. Dlugaszewska, M. Murias and J. Mielcarek, BODIPY-Based Photosensitizers as Potential Anticancer and Antibacterial Agents: Role of the Positive Charge and the Heavy Atom Effect, ChemMedChem, 2021, 16(2), 399–411, DOI:10.1002/cmdc.202000529.
- G. S. Awuah and Y. You, Boron Dipyrromethene (BODIPY)-Based Photosensitizers for Photodynamic Therapy, RSC Adv., 2012, 2(30), 11169–11183, 10.1039/C2RA21404K.
- C. S. Kue, S. Y. Ng, S. H. Voon, A. Kamkaew, L. Y. Chung, L. V. Kiew and H. B. Lee, Recent Strategies to Improve Boron Dipyrromethene (BODIPY) for Photodynamic Cancer Therapy: An Updated Review, Photochem. Photobiol. Sci., 2018, 17(11), 1691–1708, 10.1039/c8pp00113h.
- Í.AO. Bozzi, L. A. Machado, E. B. T. Diogo, F. G. Delolo, L. O. F. Barros, G. A. P. Graça, M. H. Araujo, F. T. Martins, L. F. Pedrosa, L. C. da Luz, E. S. Moraes, F. S. Rodembusch, J. S. F. Guimarães, A. G. Oliveira, S. H. Röttger, D. B. Werz, C. P. Souza, F. Fantuzzi, J. Han, T. B. Marder, H. Braunschweig and E. N. da Silva Júnior, Electrochemical Diselenation of BODIPY Fluorophores for Bioimaging Applications and Sensitization of 1O2, Chem. – Eur. J., 2024, 30(11), e202303883, DOI:10.1002/chem.202303883.
- H. J. Wories, J. H. Koek, G. Lodder, J. Lugtenburg, R. Fokkens, O. Driessen and G. R. Mohn, A Novel Water-Soluble Fluorescent Probe: Synthesis, Luminescence and Biological Properties of the Sodium Salt of the 4-Sulfonato-3,3′,5,5′-Tetramethyl-2,2′-Pyrromethen-1,1′-BF2 Complex, Recl. Trav. Chim. Pays-Bas, 1985, 104(11), 288–291, DOI:10.1002/recl.19851041104.
- P. Deng, F. Xiao, Z. Wang and G. Jin, A Novel BODIPY Quaternary Ammonium Salt-Based Fluorescent Probe: Synthesis, Physical Properties, and Live-Cell Imaging, Front. Chem., 2021, 9, 650006, DOI:10.3389/fchem.2021.650006.
- E. K. Choi, S. H. Park, J. A. Lim, S. W. Hong, K. H. Kwak, S.-S. Park, D. G. Lim and H. Jung, Beneficial Role of Hydrogen Sulfide in Renal Ischemia Reperfusion Injury in Rats, Yonsei Med. J., 2018, 59(8), 960–967, DOI:10.3349/ymj.2018.59.8.960.
- X. Y. Koh, X. H. Koh, L. Hwang, F. J. Ferrer, S. A. B. Rahmat, D. Lama and D. P. Lane, Therapeutic Anti-Cancer Activity of Antibodies Targeting Sulfhydryl Bond Constrained Epitopes on Unglycosylated RON Receptor Tyrosine Kinase, Oncogene, 2019, 38(48), 7342–7356, DOI:10.1038/s41388-019-0946-8.
- C. Schad, C. Ray, C. Díaz-Norambuena, S. Serrano-Buitrago, F. Moreno, B. L. Maroto, I. García-Moreno, M. Muñoz-Úbeda, I. López-Montero, J. Bañuelos and S. de la Moya, Water-Soluble BODIPY Dyes: A Novel Approach for Their Sustainable Chemistry and Applied Photonics, Chem. Sci., 2025, 16(18), 8030–8039, 10.1039/D5SC01295C.
- L. J. Patalag, S. Ahadi, O. Lashchuk, P. G. Jones, S. Ebbinghaus and D. B. Werz, GlycoBODIPYs: Sugars Serving as a Natural Stock for Water-Soluble Fluorescent Probes of Complex Chiral Morphology, Angew. Chem., Int. Ed., 2021, 60(16), 8766–8771, DOI:10.1002/anie.202016764.
- D. Kand, P. Liu, M. X. Navarro, L. J. Fischer, L. Rousso-Noori, D. Friedmann-Morvinski, A. H. Winter, E. W. Miller and R. Weinstain, Water-Soluble BODIPY Photocages with Tunable Cellular Localization, J. Am. Chem. Soc., 2020, 142(11), 4970–4974, DOI:10.1021/jacs.9b13219.
- S. Mohapatra, J. L. L. Xian, A. Galvez-Rodriguez, O. S. Ekande, J. E. Drewes and K. Y.-H. Gin, Photochemical Fate of Quaternary Ammonium Compounds (QACs) and Degradation Pathways Prediction through Computational, J. Hazard. Mater., 2024, 465, 133483, DOI:10.1016/j.jhazmat.2024.133483.
- S. Mohapatra, L. Yutao, S. G. Goh, C. Ng, Y. Luhua, N. H. Tran and K. Y.-H. Gin, Quaternary Ammonium Compounds of Emerging Concern: Classification, Occurrence, Fate, Toxicity and Antimicrobial Resistance, J. Hazard. Mater., 2023, 445, 130393, DOI:10.1016/j.jhazmat.2022.130393.
- M. L. Agazzi, M. B. Ballatore, E. Reynoso, E. D. Quiroga and E. N. Durantini, Synthesis, Spectroscopic Properties and Photodynamic Activity of Two Cationic BODIPY Derivatives with Application in the Photoinactivation of Microorganisms, Eur. J. Med. Chem., 2017, 126, 110–121, DOI:10.1016/j.ejmech.2016.10.001.
- I. W. Badon, J.-P. Jee, T. P. Vales, C. Kim, S. Lee, J. Yang, S. K. Yang and H.-J. Kim, Cationic BODIPY Photosensitizers for Mitochondrion-Targeted Fluorescence Cell-Imaging and Photodynamic Therapy, Pharmaceutics, 2023, 15(5), 1512, DOI:10.3390/pharmaceutics15051512.
- D. K. Mai, B. Kang, T. P. Vales, I. W. Badon, S. Cho, J. Lee, E. Kim and H.-J. Kim, Synthesis and Photophysical Properties of Tumor-Targeted Water-Soluble BODIPY Photosensitizers for Photodynamic Therapy, Molecules, 2020, 25(15), 3340, DOI:10.3390/molecules25153340.
- T. P. Vales, S. Cho, J. Lee, H. T. Bui, D. K. Mai, I. W. Badon, H. Lim, W. Jeong, J.-L. Kim, H.-K. Kim and H.-J. Kim, Functionalization of 4,4-Difluoro-4-Bora-3a,4a-Diaza-s-Indacene (BODIPY)-Based Photosensitizers with Triphenylphosphonium (TPP) for Mitochondria-Targeted Fluorescence Bioimaging and Photodynamic Therapy, J. Mol. Struct., 2021, 1246, 131284, DOI:10.1016/j.molstruc.2021.131284.
- T. Gao, H. He, R. Huang, M. Zheng, F.-F. Wang, Y.-J. Hu, F.-L. Jiang and Y. Liu, BODIPY-Based Fluorescent Probes for Mitochondria-Targeted Cell Imaging with Superior Brightness, Low Cytotoxicity and High Photostability, Dyes Pigm., 2017, 141, 530–535, DOI:10.1016/j.dyepig.2017.03.009.
- S. H. Alamudi and Y.-A. Lee, Design Strategies for Organelle-Selective Fluorescent Probes: Where to Start?, RSC Adv., 2025, 15(3), 2115–2131, 10.1039/D4RA08032G.
- X. Du, S. Huang, Z. Lin, G. Chen, Y. Jiang and H. Zhang, Organelle-Targeted Small Molecular Photosensitizers for Enhanced Photodynamic Therapy: A Minireview for Recent Advances and Potential Applications, Chem. Commun., 2025, 61(40), 7236–7252, 10.1039/D5CC01642H.
- S. Zhu, J. Zhang, G. Vegesna, F.-T. Luo, S. A. Green and H. Liu, Highly Water-Soluble Neutral BODIPY Dyes with Controllable Fluorescence Quantum Yields, Org. Lett., 2011, 13(3), 438–441, DOI:10.1021/ol102758z.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2026 |