Selective synthesis of 4,5-dihydropyrenes by a Brønsted acid-catalyzed cyclization cascade of biphenyl-embedded enynes
Jaime
Tostado
,
Lucía
Sánchez-Jiménez
 and
Manuel A.
Fernández-Rodríguez
and
Manuel A.
Fernández-Rodríguez
 *
*
Universidad de Alcalá (IRYCIS). Departamento de Química Orgánica y Química Inorgánica, Instituto de Investigación Química “Andrés M. del Río” (IQAR), 28805 Alcalá de Henares, Madrid, Spain. E-mail: mangel.fernandezr@uah.es
First published on 28th November 2025
Abstract
A metal-free Brønsted acid-catalyzed cascade cyclization enables the selective synthesis of 4,5-dihydropyrenes from biphenyl-embedded trienynes. Proceeding under mild conditions with a low E-factor and broad substrate scope, it provides a sustainable approach to access these scarcely explored scaffolds.
Introduction
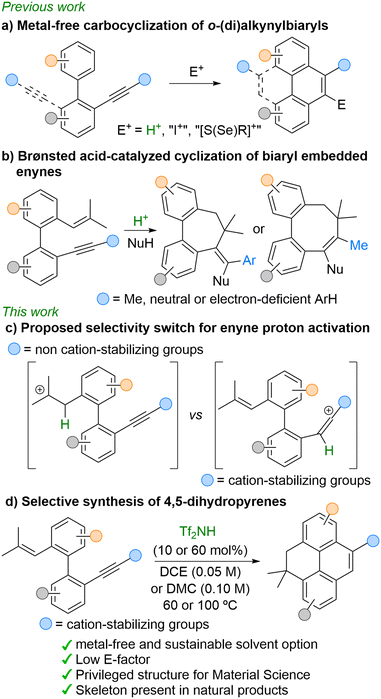
Dihydropyrenes, a class of polycyclic aromatic hydrocarbons (PAHs), have shown promising applications in optoelectronics1 and, due to their structural similarities to natural products (Fig. 1a), are also potential bioactive compounds.2 Additionally, 4,5-dihydropyrenes have been identified as pollution indicators, as they are excreted by certain bacteria and fungi during the metabolism of PAHs like pyrene (Fig. 1a),3 a well-known toxic and environmentally harmful compound. | ||
| Fig. 1 Natural products and metabolites related to 4,5-dihydropyrenes and traditional synthetic approaches. | ||
This class of PAHs has seen limited development due to a lack of selective and efficient synthetic methods. Existing strategies rely on pyrene reduction using alkali metals in ammonia (Birch reduction),4 strontium with oxidants,5 or metal-catalyzed hydrogenation.6 However, these approaches have several drawbacks, including the formation of dihydropyrene isomer mixtures and, from a sustainability perspective, dependence on expensive metal complexes, hazardous and strictly dry conditions, high temperatures, and/or elevated hydrogen pressure (Fig. 1b). Therefore, designing environmentally friendly synthetic methods to access these molecules is highly desirable.
Over the past decade, Brønsted acids have emerged as valuable catalysts in organic synthesis, providing an efficient and sustainable tool for the construction of novel carbo- and heterocycles via the electrophilic activation of unsaturated carbon–carbon bonds.7 Compared to related and widely developed metal-catalyzed transformations, Brønsted acid catalysis offers notable advantages, including commercial availability, cost-effectiveness, and the avoidance of toxic and expensive metal complexes.
In this context, the metal-free carbocyclization of ortho-alkynylbiaryls, triggered by Brønsted acids or electrophilic iodine, sulfur, or selenium reagents, has emerged as a robust and widely utilized approach for the construction of polycyclic frameworks (Scheme 1a). These transformations typically proceed through initial activation of the alkyne functionality, followed by an intramolecular nucleophilic attack from one of the aryl rings within the biaryl skeleton. This sequence has been extensively exploited for the synthesis of various PAHs, including phenanthrenes, pyrenes, pyrenoids, as well as more elaborate architectures such as helicenes and graphene nanoribbons.8 Despite these advances, analogous strategies for the synthesis of dihydropyrenes remain notably underexplored.
We recently described a Brønsted acid-catalyzed cationic cyclization of biaryl-based enynes in the presence of suitable O- and C-nucleophiles, enabling the selective formation of dibenzofused seven- and eight-membered carbocycles (Scheme 1b).9 These transformations proceed via selective initial protonation of the trisubstituted olefin,10 generating a tertiary carbocation that cyclizes intramolecularly with the alkyne, followed by incorporation of an external nucleophile. The size of the newly formed ring is governed by the stability of the vinyl cation intermediate. Guided by these insights and aiming to expand the reactivity of fully conjugated biaryl-embedded enynes,9,11 we envisioned that shifting the site of initial protonation—from the olefin to the alkyne—could open access to new polycyclic frameworks. To promote selective alkyne activation under Brønsted acid catalysis, we introduced cation-stabilizing groups—such as electron-rich arenes—at the alkyne terminus (Scheme 1c).
Herein, we report a Brønsted acid-catalyzed cyclization of ortho-alkenyl-ortho′-alkynylbiaryls for the selective synthesis of 4,5-dihydropyrenes (Scheme 1d). This strategy proceeds under mild and environmentally friendly conditions, using a catalytic amount of Brønsted acid.12 In addition, the transformation shows excellent sustainability metrics, including a notably low E-factor, making it a valuable contribution to the development of greener synthetic approaches to polycyclic aromatic frameworks.
Results and discussion
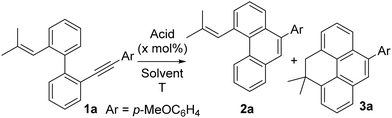
We began our investigation into the acid-catalyzed synthesis of 4,5-dihydropyrenes using 1a as a model substrate, treating it with 10 mol% of TfOH in DCE (0.05 M) at 60 °C. Under these conditions, the desired product, 4,4-dimethyl-9-(4-methoxyphenyl)-4,5-dihydropyrene (3a), was obtained in 66% yield (Table 1, entry 1). Notably, the use of Tf2NH (10 mol%) as the catalyst significantly improved the efficiency, affording 3a in quantitative yield (Table 1, entry 2). When the reaction was conducted under open-air conditions, the yield dropped slightly to 63%; nevertheless, this still represents a favourable outcome for the selective synthesis of 3a (Table 1, entry 3).| Entry | Acid (x mol%) | Solvent | T (°C) | Time (h) |
2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3ab (yield)c 3ab (yield)c |
|---|---|---|---|---|---|
| a All reactions were conducted with 0.15 mmol of 1a. Concentration was 0.05 M, except for entry 13 (0.06 M) and entries 14 and 15 (0.10 M). b Conversion estimated by 1H NMR (300 MHz). c In brackets, isolated yield. d Open-to-air atmosphere. e Heating by MW. DMC = dimethyl carbonate. | |||||
| 1 | TfOH (10) | DCE | 60 | 16 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (66) 100 (66) |
| 2 | Tf2NH (10) | DCE | 60 | 16 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (99) 100 (99) |
| 3d | Tf2NH (10) | DCE | 60 | 16 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (63) 100 (63) |
| 4 | Tf2NH (10) | DMC | 60 | 16 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 5 | Tf2NH (10) | EtOAc | 60 | 16 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 6 | Tf2NH (10) | DMC | 140e | 1 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
| 7 | Tf2NH (10) | Ethyl lactate | 140e | 1 | — |
| 8 | Tf2NH (10) | Acetone | 140e | 1 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 9 | Tf2NH (10) | DMC | 100 | 16 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 64 64 |
| 10 | Tf2NH (20) | DMC | 100 | 16 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
| 11 | Tf2NH (30) | DMC | 100 | 16 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 75 |
| 12 | Tf2NH (30) | DMC | 100 | 72 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 78 |
| 13 | Tf2NH (40) | DMC | 100 | 16 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 82 82 |
| 14 | Tf2NH (50) | DMC | 100 | 16 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
| 15 | Tf2NH (60) | DMC | 100 | 24 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (90) 100 (90) |
At this stage, we assessed the feasibility of conducting the reaction under more environmentally benign conditions. To this end, we employed dimethyl carbonate (DMC) and ethyl acetate (EtOAc)—two solvents widely recognized for their green profiles in sustainable chemistry.13 In both cases, the reaction furnished an inseparable mixture of the desired 4,5-dihydropyrene and phenanthrene 2a, with the target compound as the minor component (Table 1, entries 4 and 5).
No significant improvement was observed when the reaction was conducted at 140 °C under microwave heating in DMC, while no reaction occurred under the same conditions when using ethyl lactate as solvent (Table 1, entries 6 and 7). Nevertheless, selective formation of the vinyl-functionalized phenanthrene intermediate 2a was achieved in acetone, a notable result given its structural similarity to certain bioactive molecules (Fig. 1a).14 Upon exposure to the reaction conditions in DCE (entry 2), 2a was completely converted into 3a, supporting its role as a plausible intermediate in the transformation. When raising the temperature to 100 °C under conventional heating in DMC to selectively obtain 3a, we observed that the yield increased progressively with higher loadings of Tf2NH, reaching an optimum at 60 mol% (Table 1, entries 9–15). When combined with an extended reaction time, this catalyst loading effectively minimized the accumulation of intermediate 2a by promoting its cyclization to the desired product in 90% yield (entry 15). Although comparable results were obtained using DMC and DCE solvents (entry 15 vs. entry 2), and despite DMC being considered greener, we selected DCE with 10 mol% Tf2NH at 60 °C as the optimal conditions for further studies due to its superior efficiency—achieving the transformation at a lower temperature, shorter reaction time, and reduced acid loading.
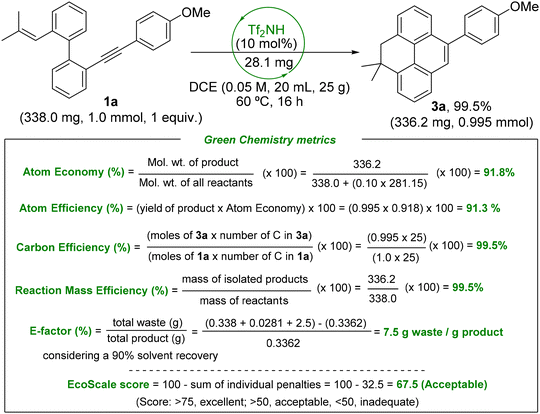
To demonstrate the practicality of the developed synthetic approach, a reaction on the millimole scale was carried out using 1a (338 mg) under the optimized conditions. The product 3a was obtained in almost quantitative yield (336.2 mg), proving the straightforward scalability of the process (Scheme 2). Moreover, the structure of the model dihydropyrene 3a was unambiguously confirmed by X-ray crystallography.
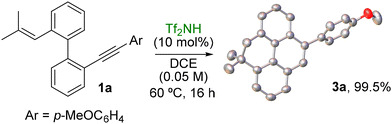 | ||
| Scheme 2 1 mmol-scale synthesis of 3a and its X-ray structure (the hydrogens are removed for clarity and the thermal ellipsoids are displayed at 50% probability). | ||
Based on the experimental observations and previous literature,8 a plausible mechanism for the cascade cyclization is proposed in Scheme 3. In the presence of a catalytic amount of Tf2NH, 1a would undergo selective protonation at the electron-rich alkyne to generate the vinyl cation intermediate Ia, stabilized by the electron-donating aryl group. This species would then evolve via a Friedel–Crafts-type benzannulation with the appropriately positioned aromatic ring of the biaryl core, producing phenanthrene intermediate 2a. The formation of this species is strongly supported by its isolation under certain reaction conditions and its further transformation into 3a under the optimized conditions. Subsequently, activation of the olefin by the Brønsted acid would generate intermediate IIa,15 bearing a tertiary carbocation, which would undergo a second intramolecular Friedel–Crafts-type reaction with the phenanthrene unit, ultimately furnishing 4,5-dihydropyrene 3a and regenerating the catalyst for a new catalytic cycle.
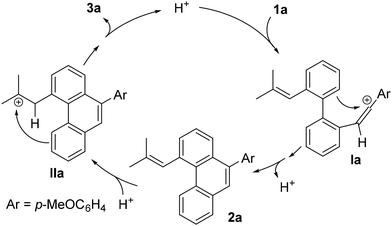 | ||
| Scheme 3 Proposed mechanism for the catalytic cascade cyclization of 1a for the selective synthesis of 3a. | ||
Next, the scope of the Brønsted acid-catalyzed cascade cyclization was explored under the optimized conditions, as summarized in Scheme 4. A series of trienyne substrates 1 bearing a p-methoxyphenyl group at the alkyne terminus and various electron-donating or electron-withdrawing substituents on the biaryl core were synthesized and subjected to the reaction. The corresponding functionalized 4,5-dihydropyrenes 3b–g were obtained in good to excellent yields. Notably, compounds 3d–f, featuring chlorine atoms at distinct positions on the tetracyclic framework, offer synthetic handles for further derivatization. Biaryl-embedded enynes 1h–p bearing electron-rich or cation-stabilizing substituents at the alkyne underwent smooth cyclization. Even less electron-rich substrates, such as the p-tolyl derivative, delivered the desired product, albeit in moderate yield, due to the competing formation of a dibenzocycloheptene byproduct—resulting from the known selective alkene activation pathway previously reported for such enyne systems (see Scheme 1b).9 Substrate 1i, containing an ortho-methoxyphenyl group at the alkyne, afforded 3i in moderate yield, suggesting that steric effects near the alkyne terminus can significantly influence the reaction outcome. Furthermore, the developed methodology allows access to dihydropyrenes bearing heteroarenes (3j–l). In these cases, the electronic properties of the heteroarene attached to the alkyne proved crucial. The 2-thienyl-substituted substrate 1k delivered product 3k in high yield, while substrates bearing less electron-rich heteroarenes—connected through the C3 position—led to moderate yields of 3j,l, along with partial decomposition. In addition, alkenyl and cyclopropyl groups at the alkyne were well tolerated. In particular, cyclopropyl-substituted compound 3n was obtained in high yield, likely due to the known ability of cyclopropanes to stabilize adjacent carbocationic intermediates. The protocol also accommodated heteroatom-containing substituents, such as sulfides and amines, enabling efficient access to functionalized dihydropyrenes 3o–p.
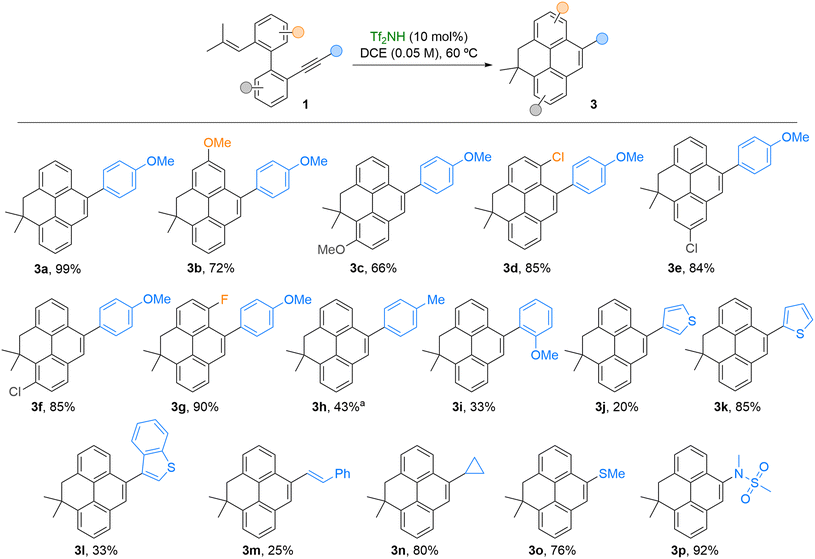 | ||
Scheme 4 Substrate scope for the synthesis of functionalized 4,5-dihydropyrenes 3. Reactions conducted using 0.15–0.3 mmol of 1. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Accompanied by 25% of the corresponding benzocycloheptadiene (Scheme 1b, ref. 9) formed by initial activation of the alkene. Accompanied by 25% of the corresponding benzocycloheptadiene (Scheme 1b, ref. 9) formed by initial activation of the alkene. | ||
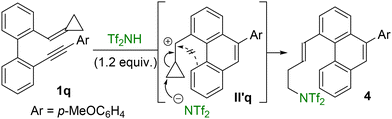
Finally, substrate 1q, bearing a methylenecyclopropane as the olefin unit, showed negligible conversion to phenanthrene 2q or dihydropyrene 3q under the optimized catalytic conditions. This outcome may result from competing activation of the Brønsted acid to the alkyne and alkene moieties, which could slow down or even suppress the reactivity observed for the other substrates 1. In contrast, when an excess of the acid was employed, enyne 1q was fully converted into alkenylphenanthrene 4, incorporating an NTf2 group in its structure (Scheme 5).
The formation of this product can be rationalized by an initial acid-mediated cyclization followed by alkene activation—analogous to the pathway observed with other substrates—leading to the most stable intermediate II′q. Rather than undergoing a Friedel–Crafts-type cyclization with the phenanthrene core to produce a cyclopentane-fused phenanthrene tetracycle,14 this intermediate instead undergoes an intermolecular nucleophilic attack by the NTf2− anion on the cyclopropane ring. This triggers ring opening and ultimately leads to the observed product 4.16
To further evaluate the sustainability of the newly developed methodology, we assessed several green chemistry metrics associated with the synthesis of compound 3a. The relevant data are summarized in Scheme 6. The process exhibits excellent performance across key indicators: a high atom economy (91.8%) and atom efficiency (91.3%), along with almost maximal values for both carbon efficiency and reaction mass efficiency (99.5% each), indicating minimal waste and optimal utilization of starting materials. Furthermore, the E-factor—a measure of waste generation in chemical processes—is remarkably low at 7.5,18 underscoring the minimal amount of non-recoverable byproducts produced in this transformation.
Another important sustainability indicator, the EcoScale score,17c which considers factors such as yield, cost, safety, technical setup, and environmental impact, was also determined. The synthesis of 3a achieved a favorable EcoScale score of 67.5, as detailed in Table S1 of the SI, placing the process in the range considered “acceptable” according to green chemistry standards. Collectively, these metrics highlight the efficiency and environmental compatibility of the methodology, reinforcing its potential for broader application in sustainable synthetic chemistry.
Conclusions
In summary, we have developed a metal-free, cost-effective, scalable, and sustainable synthetic approach for the selective construction of 4,5-dihydropyrenes via a Brønsted acid-catalyzed cascade cyclization of biphenyl-embedded trienynes. The method relies on the initial activation of the alkyne moiety, facilitated by cation-stabilizing substituents at the alkyne terminus, which triggers a sequence of intramolecular Friedel–Crafts-type cyclizations. Importantly, the reaction can be efficiently carried out under mild and environmentally friendly conditions, including catalytic amounts of triflimide, an open-air atmosphere, and green solvents such as dimethyl carbonate. A wide range of functionalized polycyclic aromatic hydrocarbons were obtained in moderate to excellent yields, demonstrating broad substrate scope and functional group tolerance. The sustainability of the process is underscored by key green chemistry metrics, including a low E-factor and a favourable EcoScale score. The synthesized 4,5-dihydropyrenes are of particular relevance due to their structural similarity to emerging bioactive compounds and their potential applications in materials science and medicinal chemistry. Additionally, given their role as biomarkers of PAH metabolism in biological systems, the development of efficient and selective synthetic strategies to access these frameworks is especially valuable. Overall, this methodology provides a practical and environmentally benign alternative to conventional metal-catalyzed routes for the synthesis of functionalized polycyclic aromatic hydrocarbons.Author contributions
M. A. F.-R. conceived and supervised the investigation. J. T. optimized the reaction conditions. J. T. and L. S.-J. conducted all experiments and characterized the novel compounds. J. T. and M. A. F.-R. prepared the original draft of the manuscript. All the authors reviewed and edited the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental details, NMR spectra of new compounds and X-ray crystallographic data for 3a. See DOI: https://doi.org/10.1039/d5ob01626f.CCDC 2475375 contains the supplementary crystallographic data for this paper.19
Acknowledgements
We are grateful to MCIN/AEI/10.13039/501100011033 and “E. Union NextGeneration EU/PRTR” (grants PID2023-146343NB-I00 and TED2021-129843BI00) and Instituto de Salud Carlos III (FEDER funds, ISCIIIRICORS2040/RD21/0005/0005) for financial support. J. T. and L. S.-J. thank the University of Alcalá for predoctoral contracts.References
- (a) S. Molla and S. Bandyopadhyay, J. Mater. Chem. C, 2024, 12, 17511 RSC; (b) Z. Ziani, S. Cobo, F. Loiseau, D. Jouvenot, E. Lognon, M. Boggio-Pasqua and G. Royal, JACS Au, 2023, 3, 131 CrossRef CAS; (c) A. Gillespie, M. Roemer, D. Jago, A. Sobolev, G. Nealon, P. Spackman, S. Moggach and G. Koutsantonis, Dalton Trans., 2023, 52, 14549 RSC; (d) K. Klaue, W. Han, P. Liesfeld, F. Berger, Y. Garmshausen and S. Hecht, J. Am. Chem. Soc., 2020, 142, 11857 CrossRef CAS.
- (a) S. Ullah, Y. Amen and K. Shimizu, Nat. Prod. Res., 2023, 13, 3253 Search PubMed; (b) J.-W. Zhang, Y. Xiong, F. Wang, F.-M. Zhang, X. Yang, G.-Q. Lin, P. Tian, G. Ge and D. Gao, Eur. J. Med. Chem., 2022, 228, 114030 CrossRef CAS PubMed; (c) W. Zhao, L.-L. Xu, X. Zhang, X.-W. Gong, D.-L. Zhu, X.-H. Xu, F. Wang and X.-L. Yang, Fitoterapia, 2018, 130, 247 CrossRef CAS PubMed.
- (a) E. Bonatti, A. dos Santos, W. Garcia Birolli and E. Rodrigues-Filho, World J. Microbiol. Biotechnol., 2023, 39, 152 CrossRef CAS; (b) M. Qutob, M. Rafatullah, S. A. Muhammad, A. M. Alosaimi, H. S. Alorfi and M. A. Hussein, Fermentation, 2022, 8, 260 CrossRef CAS; (c) X. Li, H. Liu, W. Yang, H. Sheng, F. Wang, J. D. Harindintwali, H. M. S. K. Herath and Y. Zhang, Chemosphere, 2022, 288, 132613 CrossRef CAS; (d) J. Lu, C. Guo, M. Zhang, G. Lu and Z. Dang, Int. Biodeterior. Biodegrad., 2014, 87, 75 CrossRef CAS.
- P. W. Rabideau and Z. Marcinow, in Organic Reactions, The Birch Reduction of Aromatic Compounds, Wiley Online Library, 2004, vol. 42, pp. 1–334 Search PubMed.
- S. D. Ohmura, M. Ueno and N. Miyoshi, Tetrahedron Lett., 2018, 23, 2268 CrossRef.
- Selected articles: (a) C. Bai, Q. Luyao, Y. L. Jia and X. X. Ma, Fuel, 2024, 365, 131133 CrossRef; (b) P. Stegner, C. Färber, U. Zenneck, C. Knüpfer, J. Eyselein, M. Wiesinger and S. Harder, Angew. Chem., Int. Ed., 2021, 60, 4252 CrossRef CAS; (c) E. Bresó-Femenia, B. Chaudret and S. Castillón, Catal. Sci. Technol., 2015, 5, 2741 RSC; (d) Q. Lin, K.-I. Shimizu and A. Satsuma, Appl. Catal., 2010, 387, 166 CrossRef CAS.
- (a) O. García-Pedrero and F. Rodríguez, Chem. Commun., 2022, 58, 1089 RSC; (b) P. Hermange, J. Gicquiaud, M. Barbier, A. Karnat and P. Y. Toullec, Synthesis, 2022, 5360 CAS; (c) Y. Yamamoto, A. I. Almansour, N. Arumugam and R. S. Kumar, Arkivoc, 2016, 2016, 9 Search PubMed.
- For revisions, see: (a) A. V. Gulevskaya and D. I. Tonkoglazova, Adv. Synth. Catal., 2022, 364, 2502 CrossRef CAS; (b) A. S. Pankova, A. N. Shestakov and M. A. Kuznetsov, Russ. Chem. Rev., 2019, 88, 594 CrossRef CAS; (c) E. Aguilar, R. Sanz, M. A. Fernández-Rodríguez and P. García-García, Chem. Rev., 2016, 116, 8256 CrossRef CAS. For selected Brønsted acid-mediated cyclizations, see: (d) X.-Y. Fan, X. Liu, Y.-Z. Kong, B.-H. Zhu, J. Lin, P.-C. Qian, B. Zhou and L.-W. Ye, Org. Chem. Front., 2023, 10, 2766 RSC; (e) K. Magiera, V. Aryal and W. A. Chalifoux, Org. Biomol. Chem., 2020, 18, 2372 RSC; (f) I. Takahashi, T. Fujita, N. Shoji and J. Ichiwaka, Chem. Commun., 2019, 55, 9267 RSC; (g) J. Gicquiaud, A. Hacihasanoglu, P. Hermange, J. Sotiropoulos and P. Toullec, Adv. Synth. Catal., 2019, 361, 2025 CrossRef CAS; (h) R. Bam, W. Yang, G. Longhi, S. Abbate, A. Lucotti, M. Tommasini, R. Franzini, C. Villani, V. J. Catalano, M. M. Olmstead and W. A. Chalifoux, Org. Lett., 2019, 21, 8652 CrossRef CAS; (i) J. Zhang, S. Li, C. Peng, X. Wang and J. Chang, Chem. Commun., 2018, 54, 12455 RSC; (j) W. Yang and W. A. Chalifoux, Synlett, 2017, 625 CAS; (k) W. Yang, J. Monteiro, A. de Bettencourt-Dias, V. J. Catalano and W. A. Chalifoux, Angew. Chem., Int. Ed., 2016, 55, 10427 CrossRef CAS; (l) W. Yang, A. Lucotti, M. Tommasini and W. A. Chalifoux, J. Am. Chem. Soc., 2016, 138, 9137 CrossRef CAS PubMed; (m) M. B. Goldfinger and T. M. Swager, J. Am. Chem. Soc., 1994, 116, 7895 CrossRef CAS. For iodine-, sulfur- or selenium-promoted reactions, see for example: (n) Q. X. Luo, H. T. Ji, Y. H. Lu, K. L. Wang, L. J. Ou and W. M. He, J. Org. Chem., 2023, 88, 16790 CrossRef CAS; (o) N. Mukherjee, A. N. V. Satyanarayana, P. Singh, M. Dixit and T. Chatterjee, Green Chem., 2022, 24, 7029 RSC; (p) N. Mukherjee and T. Chatterjee, Green Chem., 2021, 23, 10006 RSC; (q) T.-L. Yao, M. A. Campo and R. C. Larock, J. Org. Chem., 2005, 70, 3511 CrossRef CAS; (r) T. Yao, M. A. Campo and R. C. Larock, Org. Lett., 2004, 6, 2677 CrossRef CAS PubMed.
- J. Tostado, A. Milián, J. J. Vaquero and M. A. Fernández-Rodríguez, Org. Lett., 2024, 26, 3343–3348 CrossRef CAS PubMed.
- Selected studies on cationic cyclization of enynes promoted by initial activation of the alkene by Brønsted acids: (a) X. Liu, Y. Wang, J. Zhou, Y. Yu and H. Cao, J. Org. Chem., 2020, 85, 2406 CrossRef CAS PubMed; (b) P. Alonso, R. Fontaneda, P. Pardo, F. J. Fañanás and F. Rodríguez, Org. Lett., 2018, 20, 1659 CrossRef CAS PubMed; (c) P. Alonso, P. Pardo, R. Fontaneda, F. J. Fañanás and F. Rodríguez, Chem. – Eur. J., 2017, 23, 12158 CrossRef; (d) P. Alonso, P. Pardo, A. Galván, F. J. Fañanás and F. Rodríguez, Angew. Chem., Int. Ed., 2015, 54, 15506 CrossRef CAS; (e) P. Alonso, P. Pardo, F. J. Fañanás and F. Rodríguez, Chem. Commun., 2014, 50, 14364 RSC; (f) T. Jin, J. Uchiyama, M. Himuro and Y. Yamamoto, Tetrahedron Lett., 2011, 52, 2069 CrossRef CAS; (g) T. Jin, M. Himuro and Y. Yamamoto, J. Am. Chem. Soc., 2010, 132, 5590 CrossRef CAS PubMed; (h) T. Jin, M. Himuro and Y. Yamamoto, Angew. Chem., Int. Ed., 2009, 48, 5893 CrossRef CAS.
- (a) A. Milián, L. Sánchez-Jiménez, J. Tostado, J. J. Vaquero, M. A. Fernández-Rodríguez and P. García-García, Adv. Synth. Catal., 2024, 366, 232 CrossRef; (b) A. Milián, M. A. Fernández-Rodríguez, E. Merino, J. J. Vaquero and P. García-García, Angew. Chem., Int. Ed., 2022, 61, e202205651 CrossRef; (c) A. Milián, P. García-García, J. J. Vaquero, R. Sanz and M. A. Fernández-Rodríguez, Adv. Synth. Catal., 2022, 364, 3960 CrossRef; (d) A. Milián, P. García-García, A. Pérez-Redondo, R. Sanz, J. J. Vaquero and M. A. Fernández-Rodríguez, Org. Lett., 2020, 22, 8464 CrossRef.
- For selected recent examples of metal-free Brønsted acid-catalyzed reactions, see: (a) M. Lamba, P. R. Singh, S. Bhatt and A. Goswami, Green Chem., 2024, 26, 448 RSC; (b) S. Chen, Z. Chen, T. Zhang, B. Zhao, B. You, M. Li and Y. Gu, Green Chem., 2022, 24, 7376 RSC.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288 RSC.
- Very recently, TfOH-mediated cyclization of related biaryl-embedded enynes bearing an acrylate moiety as the olefin component has been reported, selectively affording cyclopenta[def]phenanthrenes. see: K. Goel, D. Shree and G. Satyanarayana, Chem. Commun., 2025, 61, 11457 RSC.
- For a single example of a related Friedel–Crafts cyclization involving a biheteroaryl system, see: D. A. Klumpp, P. J. Kindelin and A. Li, Tetrahedron Lett., 2005, 46, 2931 CrossRef CAS.
- Alternatively, it cannot be ruled out that the methylenecyclopropane unit is first selectively activated, followed by ring opening through nucleophilic attack of the NTf2− anion. The resulting alkynylbiphenyl intermediate could then potentially undergo intramolecular cyclization via the alkyne moiety to give the observed product 4.
- (a) R. A. Sheldon, ACS Sustainable Chem. Eng., 2018, 6, 32 CrossRef CAS; (b) M. Tobiszewski, M. Marć, A. Gałuszka and J. Namieśnik, Molecules, 2015, 20, 10928 CrossRef CAS PubMed; (c) K. V. Aken, L. Strekowski and L. Patiny, Beilstein J. Org. Chem, 2006, 2, 3 Search PubMed; (d) D. J. C. Constable, A. D. Curzons and V. L. Cunningham, Green Chem., 2002, 4, 521 RSC.
- The E-factor was calculated without accounting for catalyst recovery, while a 90% recovery of the reaction solvent was considered.
- CCDC 2475375: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p2ttf.
| This journal is © The Royal Society of Chemistry 2026 |