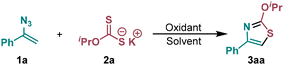Synthesis of thiazoles from vinyl azides and xanthates under the action of an Mn(III)-oxidant
Lada A.
Zaikina
ab,
Mikhail M.
Doronin
 a,
Oleg O.
Segida
a,
Oleg O.
Segida
 a,
Olga M.
Mulina
a,
Olga M.
Mulina
 a,
Igor B.
Krylov
a,
Igor B.
Krylov
 ab,
Liang-Nian
He
ab,
Liang-Nian
He
 c and
Alexander O.
Terent'ev
c and
Alexander O.
Terent'ev
 *ab
*ab
aZelinsky Institute of Organic Chemistry, Russian Academy of Sciences, 47 Leninsky prospect, Moscow, 119991, Russian Federation. E-mail: terentev@ioc.ac.ru
bMendeleev University of Chemical Technology of Russia, 9 Miusskaya Square, Moscow 125047, Russian Federation
cState Key Laboratory and Institute of Elemento-Organic Chemistry, Frontiers Science Center for New Organic Matter, College of Chemistry, Nankai University, Tianjin 300071, P. R. China
First published on 18th November 2025
Abstract
Reaction of xanthates and vinyl azides under the action of Mn(OAc)3 results in the formation of alkoxy thiazoles. In this transformation, potassium xanthate undergoes Mn-mediated oxidation, generating the corresponding xanthyl radical. The latter interacts with the double bond of the vinyl azide, and after N2 elimination, a β-xanthylated iminyl radical is formed. The quenching of the iminyl radical by an Mn(II)-ion with subsequent cyclization into a 5-membered ring, an unexpected elimination of a sulfur-containing fragment and aromatization lead to thiazoles. It is important to mention that cyclization with the formation of a 6-membered ring is not observed in the disclosed process. The obtained thiazoles demonstrate antifungal activity surpassing that of commercially available fungicides.
Introduction
The thiazole ring is a known and important heterocyclic core and thiazoles are widely used in fine chemical synthesis1,2 and materials science.3 In addition, numerous compounds with thiazole fragments exhibit valuable biological activities.4 Thiazole-based molecules are already used in medicine. Some of them are febuxostat (treatment of chronic gout and hyperuricemia), ritonavir (treatment of HIV/AIDS), and abafungin (a broad-spectrum antifungal agent). Thiazole is also a key fragment of vitamin B1. Despite their great importance, the number of versatile synthetic approaches to thiazoles remains quite limited. There are three classical methods for the construction of the thiazole core (Scheme 1, right). The Hantzsch synthesis uses α-halocarbonyl compounds or their analogues and thiocarbamic acid derivatives as the reactants (Scheme 1a).5–7 Modification of the Robinson–Gabriel synthesis involving the cyclization of α-acylthioketone with ammonia also leads to thiazoles (Scheme 1b).5 They can also be obtained by the reaction between TosMIC and carbon disulfide, followed by S-alkylation (Scheme 1c).8 In the last few years, research on the oxidative methods for thiazole synthesis has intensified.9–11Vinyl azides have been known for many years due to their utility in the construction of various N-heterocyclic compounds.12,13 However, over the last decade, the number of published papers on vinyl azide chemistry have increased dramatically.14–16 This increase is due to the discovery of their new reactivity in free radical reactions.17 Generally, attack of an external radical on the double bond of a vinyl azide results in rapid loss of an N2 molecule and the generation of the corresponding iminyl radical, which participates in subsequent transformations depending on the reaction conditions and the nature of the external radical.18–20 Applying this strategy, various substituted ketones,21–23 enamines,24–26 imines,27 amines,28 azines,29 and heterocycles30–32 have already been prepared.
Herein, we report a versatile method for the construction of the thiazole core through the reaction of vinyl azides with xanthates under the action of Mn(III) (Scheme 1, left). Xanthates have been used in organic synthesis for a long time for the construction of various sulfur-containing heterocyclic compounds via ionic pathways33 and as sources of C-centered34,35 and S-centered radicals.36–39 The reactivity of xanthates as precursors of radicals for oxidative coupling with C–S bond formation has hardly been explored prior to this report. Despite the possibility of numerous transformations of intermediate iminyl radicals generated after radical addition to the vinyl azide double bond, thiazoles can be selectively constructed under the developed conditions. The use of Mn(III) plays a key role in achieving this chemoselectivity by directing the process toward the formation of aromatic thiazoles instead of non-aromatic thiazole-2(3H)-thiones, which were previously obtained from similar starting materials (vinylazides and xanthates) in the absence of an oxidant.40 One of the key features of the disclosed method is the ability to incorporate alkoxy substituents at the second position of the ring, which greatly expands the scope of this transformation. Examples of such processes are very rare,41,42 yet they enable the incorporation of various oxygen-substituted groups into the thiazole structure, effectively increasing molecular complexity and allowing fine-tuning of the pharmacophoric properties of the molecule.
Results and discussion
Our study of the newly developed method for thiazole synthesis began with the optimization of the reaction conditions using (1-azidovinyl)benzene 1a and potassium O-isopropyl xanthate 2a as the model substrates. During optimization, the influence of the molar ratio of the starting reagents, the nature of the oxidant and solvent, reaction time, and temperature on the yield of thiazole 3aa was tested (Table 1).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| No. | Molar ratio 1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a 2a |
Oxidant (equiv.) | Solvent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
3aa yield,b % |
|---|---|---|---|---|
a General procedure: to a solution of vinyl azide 1a (1.0 or 1.5 mmol) and xanthate 2a (1.0 or 1.5 mmol) in 10 mL of THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (1 DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), DMSO, THF 1), DMSO, THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O(1 H2O(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), MeCH 1), MeCH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), MeCN 1), MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (1 DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), EtOH 1), EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), EtOH, MeCN 1), EtOH, MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF (1 DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), or DMF 1), or DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), an oxidant (1.0 or 1.5 mmol) was added under magnetic stirring at room temperature (23–25 °C). The reaction mixture was stirred for 30 min at room temperature (23–25 °C).
b The yield was determined by 1H NMR using 1,4-dinitrobenzene as an internal standard; isolated yields are given in parentheses.
c The reaction mixture was stirred for 90 min at room temperature (23–25 °C).
d The reaction mixture was stirred at 0 °C.
e The reaction mixture was stirred at 40 °C.
f Reaction was conducted with a catalytic (20 mol%) amount of Mn(OAc)3·2H2O in the presence of PhI(OAc)2 as the terminal oxidant. 1), an oxidant (1.0 or 1.5 mmol) was added under magnetic stirring at room temperature (23–25 °C). The reaction mixture was stirred for 30 min at room temperature (23–25 °C).
b The yield was determined by 1H NMR using 1,4-dinitrobenzene as an internal standard; isolated yields are given in parentheses.
c The reaction mixture was stirred for 90 min at room temperature (23–25 °C).
d The reaction mixture was stirred at 0 °C.
e The reaction mixture was stirred at 40 °C.
f Reaction was conducted with a catalytic (20 mol%) amount of Mn(OAc)3·2H2O in the presence of PhI(OAc)2 as the terminal oxidant.
|
||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Mn(OAc)3·2H2O (1) | THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO DMSO |
53 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Mn(OAc)3·2H2O (1) | DMSO | 46 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Mn(OAc)3·2H2O (1) | THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
24 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Mn(OAc)3·2H2O (1) | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
50 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Mn(OAc)3·2H2O (1) | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO DMSO |
61(53) |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Mn(OAc)3·2H2O (1) | EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
15 |
| 7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Mn(OAc)3·2H2O (1) | EtOH | 37 |
| 8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Mn(OAc)3·2H2O (1) | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF DMF |
51 |
| 9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Mn(OAc)3·2H2O (1) | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
32 |
| 10c | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Mn(OAc)3·2H2O (1) | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO DMSO |
59 |
| 11d | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Mn(OAc)3·2H2O (1) | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO DMSO |
44 |
| 12e | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Mn(OAc)3·2H2O (1) | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO DMSO |
40 |
| 13 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Mn(OAc)3·2H2O (1.5) | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO DMSO |
38 |
| 14 | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Mn(OAc)3·2H2O (1) | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO DMSO |
65(57) |
| 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
Mn(OAc)3·2H2O (1) | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO DMSO |
58 |
| 16 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
Mn(OAc)3·2H2O (1.5) | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO DMSO |
73(68) |
| 17 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
Mn(acac)3 (1.5) | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO DMSO |
69(61) |
| 18 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
Fe(ClO4)3·8H2O (1.5) | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO DMSO |
19 |
| 19 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
PhI(OAc)2 (1.5) | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO DMSO |
0 |
| 21 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
H2O2 (35 wt% in Et2O) | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO DMSO |
0 |
| 22f | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
PhI(OAc)2 (1.5) | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO DMSO |
Trace |
| 23 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
— | MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO DMSO |
0 |
The initial experiment was carried out at room temperature with equimolar amount of the starting reagents, Mn(OAc)3·2H2O as the oxidant, and a THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO mixture as the solvent. After 30 min of stirring, thiazole 3aa was formed in 53% yield (entry 1). Based on the literature data24,43,44 for similar processes, an extensive screening of solvents (entries 2–9) was performed, which demonstrated that the MeCN
DMSO mixture as the solvent. After 30 min of stirring, thiazole 3aa was formed in 53% yield (entry 1). Based on the literature data24,43,44 for similar processes, an extensive screening of solvents (entries 2–9) was performed, which demonstrated that the MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO mixture was the optimal one (entry 5, 61%). Increasing the reaction time to 90 min (entry 10) did not improve reaction efficiency (yield of 3aa was 59%). Cooling (entry 11) and heating (entry 12) the reaction mixture decreased the yield of the desired product 3aa to 44% and 40%, respectively. The influence of reagents and molar ratio of oxidants was further explored (entries 13–16). The highest yield of 3aa was achieved when 1.0 equiv. of vinyl azide 1a, 1.5 equiv. of xanthate 2a, and 1.5 equiv. of Mn(OAc)3·2H2O were applied (entry 16, 73%). Using Mn(acac)3 as an alternative source of Mn(III) gave comparable results (entry 17, 69%). Employment of Fe(ClO4)3·8H2O as the single-electron oxidant dramatically reduced the yield of 3aa (entry 18, 19%). When the reaction was carried out in the presence of PhI(OAc)2, thiazole 3aa was not formed. Usage of hydrogen peroxide also did not lead to the formation of the target product (entry 21, 0%). A similar outcome was observed in the absence of an oxidant (entry 23). We also attempted to perform this transformation with catalytic amounts of Mn(OAc)3·2H2O, using PIDA as the terminal oxidizer. In this case, only trace amounts of the target product were observed (entry 22). Thus, the optimal conditions for the construction of thiazole 3aa from vinyl azide 1a and xanthate 2a were identified as follows: 1.5-fold excess of 2a over 1a, 1.5 equiv. of Mn(OAc)3·2H2O as the oxidant, MeCN
DMSO mixture was the optimal one (entry 5, 61%). Increasing the reaction time to 90 min (entry 10) did not improve reaction efficiency (yield of 3aa was 59%). Cooling (entry 11) and heating (entry 12) the reaction mixture decreased the yield of the desired product 3aa to 44% and 40%, respectively. The influence of reagents and molar ratio of oxidants was further explored (entries 13–16). The highest yield of 3aa was achieved when 1.0 equiv. of vinyl azide 1a, 1.5 equiv. of xanthate 2a, and 1.5 equiv. of Mn(OAc)3·2H2O were applied (entry 16, 73%). Using Mn(acac)3 as an alternative source of Mn(III) gave comparable results (entry 17, 69%). Employment of Fe(ClO4)3·8H2O as the single-electron oxidant dramatically reduced the yield of 3aa (entry 18, 19%). When the reaction was carried out in the presence of PhI(OAc)2, thiazole 3aa was not formed. Usage of hydrogen peroxide also did not lead to the formation of the target product (entry 21, 0%). A similar outcome was observed in the absence of an oxidant (entry 23). We also attempted to perform this transformation with catalytic amounts of Mn(OAc)3·2H2O, using PIDA as the terminal oxidizer. In this case, only trace amounts of the target product were observed (entry 22). Thus, the optimal conditions for the construction of thiazole 3aa from vinyl azide 1a and xanthate 2a were identified as follows: 1.5-fold excess of 2a over 1a, 1.5 equiv. of Mn(OAc)3·2H2O as the oxidant, MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (1
DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture as the solvent, room temperature, and reaction time of 30 min (Table 1, entry 16). We propose that the key role of the manganese-based oxidant can be explained by its reactivity toward the iminyl radical derived from the vinyl azide.45
1) mixture as the solvent, room temperature, and reaction time of 30 min (Table 1, entry 16). We propose that the key role of the manganese-based oxidant can be explained by its reactivity toward the iminyl radical derived from the vinyl azide.45
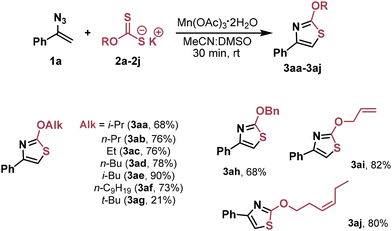
With the optimized reaction conditions in hand, we studied the scope of the starting reagents that were compatible with the developed transformation. Firstly, a variety of xanthates 2 were tested (Table 2). Alkyl-substituted xanthates 2a–2f with different chain lengths provided the desired thiazoles 3aa–3af in yields ranging from 68% to 90%. When the sterically hindered tert-butyl xanthate 2g was used, the corresponding thiazole 3ag was obtained in only 21% yield. The BnO-substituted thiazole 3ah was also efficiently synthesized in 68% yield. It should be especially noted that alkyl xanthates with double C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds (2i and 2j) gave the desired thiazoles 3ai and 3aj selectively and in high yields. Formation of side products resulting from C
C bonds (2i and 2j) gave the desired thiazoles 3ai and 3aj selectively and in high yields. Formation of side products resulting from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond oxidation can be expected in this case. In the case of PhO-xanthate, no final thiazole was observed.
C bond oxidation can be expected in this case. In the case of PhO-xanthate, no final thiazole was observed.
The thiazoles 3 synthesized from different vinyl azides 1 are shown in Table 3. Firstly, a number of aryl-substituted vinyl azides 1b–1k were tested. The presence of alkyl groups in the aromatic ring of vinyl azides 1b and 1c enabled the formation of the corresponding thiazoles 3bc and 3cc in high yields. When a strong electron-donating methoxy group was present at either the para- or ortho-position, the yield of the desired products 3dc and 3ec also exceeded 70%. The halogen-substituted aromatic vinyl azides 1f–1h afforded the target thiazoles 3fc–3hc in 77–82% yields. para-Azidomethylphenyl and naphthyl vinyl azides 1i and 1j were also compatible with the developed synthetic approach, affording thiazoles 3ic and 3jc in 69% and 85% yields, respectively. A significant decrease in the yield of the desired product was observed when β-methyl phenyl vinyl azide 1k was used as the starting reagent, which can be explained by its steric hindrance. Various alkyl-substituted vinyl azides (1l–1n) also participated in this reaction, delivering thiazoles 3lc–3nc derived from both linear and cyclic alkyl vinyl azides in 70–82% yields. The carboethoxy-substituted vinyl azide 1o gave the corresponding thiazole 3oc in 55% yield.
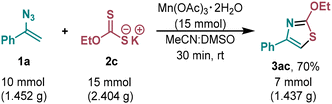
The scalability of the developed transformation was also demonstrated. A 10-fold increase in the loading of the starting materials had no effect on reaction efficiency. Thiazole 3ac was obtained from vinyl azide 1a and potassium O-ethyl xanthate 2c in 70% yield (Scheme 2).
To gain insight into the mechanism of the developed process, a number of control experiments were conducted (Scheme 3). Firstly, the synthesis of thiazole 3ac was carried out in the presence of TEMPO as the radical trap (Scheme 3a). As a result, molecular ions with the expected mass were detected by HRMS. However, the yield of the desired thiazole 3ac was approximately the same as that obtained in the absence of TEMPO. These observations suggest that the reaction proceeds via a free-radical pathway, but radical intermediates are quite reactive and engage in subsequent processes immediately after generation. An aerobic oxidation pathway was excluded by performing the experiment under an inert atmosphere (Scheme 3b). The synthesis of thiazole 3ac was also conducted in the presence of Mn(II) instead of Mn(III) (Scheme 3c). The yield of the target product 3ac was only 15% in this case. When the reaction was performed using Mn(II) under an inert atmosphere, no thiazole 3ac formation was observed (Scheme 3d). To test the possibility of Mn(III) catalysis, we conducted the experiment in which the starting compounds were added in two portions and oxidant load was halved. In this case, we were able to obtain the final thiazole in 33% yield (vs. 68% under optimal conditions). Based on these experiments, we concluded that Mn(III) is required for the efficient formation of thiazoles and that after the formation of thiazole 3, Mn(III) ions likely convert into the deactivated Mn–S complex. Presumably, the Mn(II) xanthate complex can be partially oxidized under air to the Mn(III) complex,46,47 which then triggers the desired heterocycle construction. Under an inert atmosphere, the oxidation of Mn(II) into Mn(III) is impossible. Nevertheless, the possibility of Mn(III)-oxidant turnover was excluded by the experiment with the addition of potassium xanthate and vinyl azide after the completion of the reaction. For this reason, Mn(II)-ions can supposedly initiate reduction of iminyl radicals, but the formed Mn(III)-intermediate lacks the ability to initiate the next step of xanthate oxidation (see the SI for details).
Based on the literature and experimental data, we proposed the mechanism of thiazole 3 formation (Scheme 4). Initially, Mn(III) oxidizes the xanthate anion 1 into the xanthyl radical A; its reaction with a double bond of vinyl azide leads to the carbon-centered radical B. The latter rapidly undergoes N2 elimination resulting in the iminyl radical C. Previously, we showed that Mn(II) ions can reduce iminyl radicals with the formation of Mn(III) complexes.45 Thus, we suppose that the quenching of the iminyl radical C by Mn(II) ions leads to intermediate D. Cyclization of D to E, followed by deprotonation and desulfuration leads to thiazole 3.
In vitro fungicidal activity of the synthesized thiazoles
The thiazole ring is a known key fragment in several fungicides.48–50 Thus, the synthesized thiazoles 3 were tested for their in vitro fungicidal activity against 6 pathogenic fungi that cause significant damage to agriculture crop production: Venturia inaequalis (V.i.) (causes apple scab disease), Rhizoctonia solani (R.s.) (causes black scurf of potatoes), Fusarium oxysporum (F.o.) (causes rotting of the roots and wilting of lucerne, pea, soybean, wheat, and cucumber, and affects the vascular system of tomatoes), Fusarium moniliforme (F.m.) (causes fusarium of corn cob), Bipolaris sorokiniana (B.s.) (causes root rot of wheat, barley, rye, and oats), and Sclerotinia sclerotiorum (S.s.) (causes white rot of sunflower) (Table 4). The commercially available fungicide triadimefon was used as the reference compound.| Compound | Mycelium growth inhibition, % (C = 30 mg L−1) | |||||
|---|---|---|---|---|---|---|
| V. i. | R. s. | F. o. | F. m. | B. s. | S. s. | |
| a Values given in bold indicate activity superior to that of triadimefon. | ||||||
| 3aa | 69 | 93 | 48 | 78 | 79 | 48 |
| 3ac | 79 | 100 | 55 | 90 | 78 | 53 |
| 3ad | 61 | 68 | 32 | 66 | 67 | 28 |
| 3ae | 75 | 100 | 36 | 59 | 74 | 19 |
| 3ag | 38 | 65 | 18 | 57 | 45 | 12 |
| 3bc | 75 | 100 | 35 | 71 | 66 | 40 |
| 3dc | 80 | 100 | 42 | 86 | 68 | 52 |
| 3ec | 74 | 95 | 44 | 75 | 75 | 48 |
| 3fc | 100 | 100 | 61 | 86 | 83 | 64 |
| 3hc | 44 | 67 | 33 | 70 | 62 | 37 |
| 3ic | 56 | 99 | 32 | 58 | 53 | 38 |
| 3kc | 79 | 100 | 46 | 73 | 88 | 42 |
| 3nc | 56 | 87 | 45 | 72 | 52 | 29 |
| Triadimefon | 70 | 59 | 64 | 86 | 71 | 71 |
As can be seen from Table 4, compounds 3ac, 3ae, 3dc, 3fc and 3kc exhibit the highest activity against the tested phytopathogenic fungi. In general, the greatest activity was observed for thiazoles that are either unsubstituted on the aryl ring (3ac and 3kc) or bearing alkyl (3bc), methoxy (3dc and 3ec), or fluorine (3fc) substituents at the p- or o-positions. Compounds with isopropoxy (3aa), n-butoxy (3ad), or tert-butoxy (3ag) substituents at the 2-position of the thiazole ring did not show significant activity compared to thiazoles with ethoxy (3ac) or isobutoxy (3ae) substituents. The most pronounced fungicidal effects are observed for compounds 3ac and 3fc, whose activity is, in most cases, comparable to or higher than that of the commercial compound triadimefon.
Conclusions
A novel method for the synthesis of alkoxy thiazoles has been disclosed. In the reaction of potassium xanthates with Mn(III), xanthyl radicals are produced. Their subsequent reaction with vinyl azides leads to the formation of iminyl radicals upon elimination of dinitrogen. Intramolecular cyclization of these iminyl radicals to form a 5-membered ring, followed by an unexpected elimination of a sulfur fragment and aromatization affords the final thiazole products. Remarkably, cyclization with the formation of a 6-membered ring was not observed. The proposed mechanism was confirmed with various control experiments and literature data. The developed approach is compatible with various vinyl azides and potassium xanthates containing substituents sensitive to radical reactions. Gram scale application of the developed method was shown. The resulting thiazoles were evaluated in vitro for fungicidal activity and they showed activity similar to or higher than that of a commercial compound.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available (experimental procedures, characterization data and copies of NMR and HRMS spectra). See DOI: https://doi.org/10.1039/d5ob01543j.Acknowledgements
This work was supported by the Russian Science Foundation (Grant No. 24-43-00111).References
- V. N. Charushin, E. V. Verbitskiy, O. N. Chupakhin, D. y. V. Vorobyeva, P. S. Gribanov, S. N. Osipov, A. V. Ivanov, S. V. Martynovskaya, E. F. Sagitova and V. D. Dyachenko, Usp. Khim., 2024, 93, 1–366 Search PubMed
.
- A. Dondoni and A. Marra, Chem. Rev., 2004, 104, 2557–2600 CrossRef CAS PubMed
.
- Y. Lin, H. Fan, Y. Li and X. Zhan, Adv. Mater., 2012, 24, 3087–3106 CrossRef CAS
.
- A. Rouf and C. Tanyeli, Eur. J. Med. Chem., 2015, 97, 911–927 CrossRef CAS
.
- H. S. Kim, I. C. Kwon and O. H. Kim, J. Heterocycl. Chem., 1995, 32, 937–939 CrossRef CAS
.
- A. Babadjamian, R. Gallo, J. Metzger and M. Chanon, J. Heterocycl. Chem., 1976, 13, 1205–1208 CrossRef CAS
.
- R. M. Moriarty, B. Vaid, M. Duncan, S. G. Levy, O. Prakash and S. Goyal, Synthesis, 1992, 845–846 CrossRef CAS
.
- A. M. van Leusen and J. Wildeman, Synthesis, 1977, 501–502 CrossRef CAS
.
- P. Zhao, Y. Zhou, C. Wang and A. X. Wu, J. Org. Chem., 2024, 89, 2505–2515 CrossRef CAS PubMed
.
- Q. Huang, J. Liu and J. P. Wan, Org. Lett., 2024, 26, 5263–5268 CrossRef
.
- R. Zheng, X. Hu, H. Jiang, H. Guo and L. Wang, Synthesis, 2023, 2091–2098 CAS
.
- G. L'abbé, Angew. Chem., Int. Ed. Engl., 1975, 14, 775–782 CrossRef
.
- G. Smolinsky and C. A. Pryde, J. Org. Chem., 1968, 33, 2411–2416 CrossRef CAS
.
- B. Saxena, R. I. Patel, J. Tripathi and A. Sharma, Org. Biomol. Chem., 2023, 21, 4723–4743 RSC
.
- F. Gholami, F. Yousefnejad, B. Larijani and M. Mahdavi, RSC Adv., 2023, 13, 990–1018 RSC
.
- J. Fu, G. Zanoni, E. A. Anderson and X. Bi, Chem. Soc. Rev., 2017, 46, 7208–7228 RSC
.
- H. Hayashi, A. Kaga and S. Chiba, J. Org. Chem., 2017, 82, 11981–11989 CrossRef CAS PubMed
.
- Z. Fang, Y. Zhang, Z. Zhang, Q. Song, Y. Wu, Z. Liu and Y. Ning, Org. Lett., 2022, 24, 6374–6379 CrossRef CAS PubMed
.
- L. Lu, B. Zhou, H. Jin and Y. Liu, Chin. J. Org. Chem., 2019, 39, 515–520 CrossRef CAS
.
- D. Mou, Q. Li and Z. Du, ChemistrySelect, 2023, 8, e202203486 CrossRef CAS
.
- C.-M. Luo, Y.-B. Sun, L.-T. Wang, N.-N. Dai, J.-Z. Li, J. Zhang, J.-Y. Chen, W.-T. Wei and G.-P. Ge, Org. Chem. Front., 2023, 10, 4023–4029 RSC
.
- W.-H. Yang, Y. Zhou, Y. Tong, Z.-Y. Wang, H.-L. Wu, L. Mei, Q. Li, L.-Y. Xie, J.-Q. Zhang and W.-T. Wei, ACS Sustainable Chem. Eng., 2024, 12, 5046–5051 CrossRef CAS
.
- S. Sarkar, S. Pal, S. Santra, G. V. Zyryanov and A. Majee, J. Org. Chem., 2024, 89, 8137–8145 CrossRef CAS PubMed
.
- O. M. Mulina, M. M. Doronin and A. O. Terent'ev, Tetrahedron Lett., 2021, 84, 153436 CrossRef CAS
.
- X. Wang, H. Li, G. Qiu and J. Wu, Chem. Commun., 2019, 55, 2062–2065 RSC
.
- T. Wang, Y. Y. Zong, B. Yang, T. Huang, X. L. Jin and Q. Liu, Org. Lett., 2024, 26, 1683–1687 CrossRef CAS
.
- S. A. Paveliev, A. I. Churakov, L. S. Alimkhanova, O. O. Segida, G. I. Nikishin and A. O. Terent'ev, Adv. Synth. Catal., 2020, 362, 3864–3871 CrossRef CAS
.
- S. Li, H.-W. Du, P. W. Davies and W. Shu, CCS Chem., 2024, 6, 1060–1070 CrossRef CAS
.
- Y. F. Wang, G. H. Lonca and S. Chiba, Angew. Chem., Int. Ed., 2014, 53, 1067–1071 CrossRef CAS PubMed
.
- R. Chatterjee, S. Bhukta, K. K. Angajala and R. Dandela, Org. Biomol. Chem., 2023, 21, 5419–5423 RSC
.
- Y. Zhang, B. Jiang, P. Liu and X. Liu, J. Org. Chem., 2023, 88, 14634–14639 CrossRef CAS
.
- C. M. Luo, M. Q. Yang, D. Q. Yang, Z. Q. Wu, Y. Zhou, W. C. Tian, J. Zhang, Q. Li, C. Deng and W. T. Wei, Org. Lett., 2024, 26, 6859–6865 CrossRef CAS PubMed
.
- F. Gholami, S. Ansari, B. Larijani and M. Mahdavi, J. Organomet. Chem., 2023, 992, 122663 CrossRef CAS
.
- S. Z. Zard, Chem. – Eur. J., 2020, 26, 12689–12705 CrossRef CAS
.
- S. Z. Zard, Acc. Chem. Res., 2018, 51, 1722–1733 CrossRef CAS PubMed
.
- D. I. Fomenkov, R. A. Budekhin, O. M. Mulina, O. A. Komarova, M. M. Doronin, Y. Y. Belyakova, L.-N. He, I. A. Yaremenko and A. O. Terent'ev, Eur. J. Org. Chem., 2025, 28, e202401198 CrossRef CAS
.
- E. V. Ignatov, L. E. Zelenkov, S. V. Baykov, M. K. Shurikov, A. V. Semenov, N. A. Bokach, P. S. Postnikov and V. Y. Kukushkin, Inorg. Chem., 2025, 64, 11867–11879 CrossRef CAS
.
- D. I. Bugaenko, A. A. Volkov, V. V. Andreychev and A. V. Karchava, Org. Lett., 2023, 25, 272–276 CrossRef CAS
.
- D. I. Bugaenko, A. A. Volkov and A. V. Karchava, J. Org. Chem., 2023, 88, 9968–9972 CrossRef CAS PubMed
.
- K. Rajaguru, A. Mariappan, S. Muthusubramanian and N. Bhuvanesh, ChemistrySelect, 2016, 1, 1636–1640 CrossRef CAS
.
- Z. Zhu, X. Tang, J. Cen, J. Li, W. Wu and H. Jiang, Chem. Commun., 2018, 54, 3767–3770 RSC
.
-
R. Schaumann, in Category 2, Hetarenes and Related Ring Systems, ed. R. Schaumann, 2002, DOI:10.1055/sos-sd-011-00783
.
- O. M. Mulina, M. M. Doronin, V. A. Kostyagina and G. P. Timofeev, Russ. J. Org. Chem., 2021, 57, 1302–1308 CrossRef CAS
.
- O. M. Mulina, M. M. Doronin and A. O. Terent'ev, ChemistrySelect, 2021, 6, 10250–10252 CrossRef CAS
.
- M. M. Doronin, A. S. Klikushin, O. M. Mulina, M. Medvedev, V. A. Vil, L.-N. He and A. O. Terent’ev, Org. Chem. Front., 2025, 12, 5271–5278 RSC
.
- V. Kirichenko, L. Glinskaya, R. Klevtsova, T. Leonova and S. Larionov, J. Struct. Chem., 1994, 35, 242–247 CrossRef
.
- D. Holah and C. Murphy, Can. J. Chem., 1971, 49, 2726–2732 CrossRef CAS
.
- K. B. Gangurde, R. A. More, V. A. Adole and D. S. Ghotekar, Results Chem., 2024, 7, 101380 CrossRef CAS
.
- Q.-F. Wu, B. Zhao, Z.-J. Fan, J.-B. Zhao, X.-F. Guo, D.-Y. Yang, N.-L. Zhang, B. Yu, T. Kalinina and T. Glukhareva, RSC Adv., 2018, 8, 39593–39601 RSC
.
- A. Biernasiuk, A. Berecka-Rycerz, A. Gumieniczek, M. Malm, K. Z. Łączkowski, J. Szymańska and A. Malm, Appl. Microbiol. Biotechnol., 2021, 105, 6355–6367 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2026 |