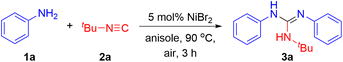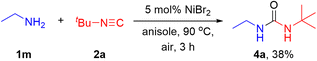A nickel-catalyzed isocyanide insertion reaction with aromatic amines: direct access to open-chain guanidines
Zhe
Zheng
a,
Hongqiang
Ma
a,
Hongwei
Jin
ab,
Bingwei
Zhou
 a and
Yuanyuan
Hu
a and
Yuanyuan
Hu
 *a
*a
aCollege of Chemical Engineering, Zhejiang University of Technology, Chaowang Road 18#, Hangzhou, 310014, China. E-mail: jhwei828@zjut.edu.cn; zhoubw@zjut.edu.cn
bEco-Industrial Innovation Institute, Zhejiang University of Technology, Quzhou, 324400, China
First published on 28th November 2025
Abstract
Herein, a nickel-catalyzed isocyanide insertion reaction with aromatic amines under open-air conditions is disclosed. A number of open-chain guanidines are successfully synthesized in moderate to good yields and their structures are confirmed by X-ray diffraction analysis. This reaction features mild reaction conditions, readily available starting materials and easy operation. Halogen atoms including F, Cl, Br and I are all compatible with the reaction. Mechanistic studies reveal that oxygen in the air might act as an oxidant.
Guanidines are an important class of organic compounds characterized by a unique “CN3” structural motif. This parent structure contained in some natural products and pharmaceuticals exhibits multiple biological activities.1 Owing to the strong electron-donating nature of the N atom, guanidines are extensively used as organosuperbases2 and chelating ligands in organometallic chemistry.3 Moreover, chiral guanidines and their derivatives can also be used as organocatalysts to trigger various asymmetric transformations with moderate to high ee values.4 Thus, great effort has been devoted to the efficient synthesis of guanidines. Typically, guanidines are constructed from the reaction of amines with various guanylating reagents such as carbodiimides, thioureas, isothioureas, cyanamides and amidines.5 However, most of these guanylating reagents need to be pre-prepared and activated with Lewis acids. The choice of guanylating reagent always depends on the nucleophilicity of the used amine. Recently, transition-metal-catalyzed approaches toward guanidine synthesis have attracted much attention.6–14 Among them, the cascade cyclization reaction of isocyanides15 with amines has emerged as a convenient method to synthesise guanidine-containing heterocycles. In 2012, Maes, Orru and Ruijter et al. developed a palladium-catalyzed aerobic oxidative isocyanide insertion reaction with diamines, in which various guanidine-containing heterocycles such as 2-aminobenzimidazoles, 2-amino-quinazolines and 2-aminoimidazolines were achieved (Scheme 1a).7 Afterwards, Ji,8 Cai9 and others10 successively reported similar tandem coupling reactions of isocyanides with bisnucleophiles to afford guanidine-containing heterocycles. In contrast, the catalytic synthesis of open-chain guanidines from isocyanides and amines has been far less developed. In 2015, Ji's group elegantly described a cobalt-catalyzed oxidative isocyanide insertion reaction with two amines under ultrasound irradiation conditions (Scheme 1b, i). One aromatic amine and one aliphatic amine were used as nucleophiles to afford N-aryl guanidines.11tert-Butyl benzoperoxoate was proved to be an important oxidant in the reaction. Later, the same group described a cobalt-catalyzed nitrene radical coupling reaction of azides with isocyanides and amines to achieve sulfonyl guanidines successfully.12 Subsequently, a palladium-catalyzed cascade reaction of isocyanides with azides and amines was also disclosed by Zhang and co-workers (Scheme 1b, ii).13 Specifically, guanidines bearing N-sulfonyl, N-phosphoryl or N-acyl groups could be prepared smoothly. More recently, Phukan et al. documented a rhodium-catalyzed tandem coupling of isocyanides with sulfonamides and amines in the presence of iodobenzene diacetate (IBD) to afford a sulfonyl guanidine (Scheme 1b, iii).14 This protocol replaced the azide with a sulfonamide, which was preferable in terms of security and practical manipulation. Experimental studies showed that IBD was indispensable and played a crucial role in the reaction. Despite the fruitful progress, the continuous development of highly efficient methods for the synthesis of open-chain guanidines is still in demand. Note that the nickel catalyst, known for its low cost, great sustainability and diverse catalytic reactivity, has been widely used in various organic transformations.16 Based on our continued interest in isocyanide chemistry17 and nickel catalysis,18 we herein describe a novel nickel-catalyzed isocyanide insertion reaction with two aromatic amines to access various N-aryl guanidines (Scheme 1c). The reaction is characterized by mild reaction conditions and easy operation. External addition of any additives or oxidants is not required in this reaction.
Initially, we set out to optimize the reaction conditions using aniline 1a and tert-butyl isocyanide 2a as standard substrates (Table 1). We envisioned that the reaction might proceed through an oxidative cross-coupling process. In this regard, we first performed the model reaction under air conditions in the presence of 5 mol% NiBr2 in anisole at 90 °C. After 3 hours, the expected guanidine 3a was obtained in 75% yield (entry 1). A further study revealed that the nickel catalyst was crucial to the reaction since the product could not be formed at all in the absence of NiBr2 (entry 2). Screening of other nickel catalysts such as NiBr2(DME), Ni(OH)2 and Ni(OAc)2 failed to give higher yields (entries 3–5). Evaluation of solvents showed that anisole was still the optimal one (entries 6–8). Raising or lowering the reaction temperature decreased the yield of 3a to 73% and 65%, respectively (entries 9 and 10). Unfortunately, further adjustment of reaction time and concentration could not increase the yield, either (entries 11–14). When the amount of NiBr2 was decreased to 1 mol%, the isocyanide insertion reaction was almost inhibited (entry 15). However, a comparable yield of 3a (76%) was obtained if the catalyst loading was increased to 10 mol% (entry 16). Finally, the reaction was conducted under a nitrogen atmosphere, while only a trace amount of product could be detected (entry 17). It was suggested that an oxidative process as a key step might be involved in this reaction.
| Entry | Variation | Yield (%) |
|---|---|---|
| a Reaction conditions: 1a (0.6 mmol), 2a (0.2 mmol), NiBr2 (5 mol%), anisole (0.5 mL), at 90 °C in air for 3 h. | ||
| 1 | None | 75 |
| 2 | w/o NiBr2 | 0 |
| 3 | NiBr2(DME) instead of NiBr2 | 57 |
| 4 | Ni(OH)2 instead of NiBr2 | 0 |
| 5 | Ni(OAc)2 instead of NiBr2 | 25 |
| 6 | Toluene instead of anisole | 58 |
| 7 | THF instead of anisole | 30 |
| 8 | DMF instead of anisole | Trace |
| 9 | 120 °C instead of 90 °C | 73 |
| 10 | 60 °C instead of 90 °C | 65 |
| 11 | 1 h instead of 3 h | 49 |
| 12 | 5 h instead of 3 h | 72 |
| 13 | 0.2 mL of anisole used as a solvent | 57 |
| 14 | 1.0 mL of anisole used as a solvent | 45 |
| 15 | 1 mol% of NiBr2 used as a catalyst | Trace |
| 16 | 10 mol% of NiBr2 used as a catalyst | 76 |
| 17 | Under a N2 atmosphere | Trace |
With the optimal reaction conditions in hand, we started to explore the substrate scope of the reaction. As shown in Scheme 2, a variety of aromatic amines with electron-donating or electron-withdrawing substituents were well tolerated in the reaction, affording diverse guanidines in moderate to good yields (3a–k). The structure of guanidine 3a was confirmed by X-ray diffraction analysis (CCDC 2472788). It was concluded that steric hindrance exerted a negative impact on the reaction efficiency since product 3c derived from 2-methylaniline was generated in a lower yield than that from 4-methylaniline. To our delight, 2,4,6-trimethylaniline was a viable substrate to afford product 3d in 55% yield. Halogen atoms such as F, Cl, Br and even I were all compatible and remained intact in the reaction. It should be noteworthy that these halogen atoms especially the Br and I atoms were generally recognized to be highly susceptible in previously reported nickel-catalyzed cross-coupling reactions (3f–k). Bisnucleophiles such as 2-aminophenol could also participate in the reaction successfully, giving the corresponding heterocycle 3l in 45% yield, while benzene-1,2-diamine and 2-aminobenzenethiol could not be transformed into the corresponding aromatic heterocycles. In addition, the reactivity of aliphatic amines such as ethanamine was also investigated under the standard reaction conditions (Scheme 3). Of note, 1-(tert-butyl)-3-ethylurea 4a was isolated in 38% yield other than an alkyl guanidine. Furthermore, the reactivity of a range of heteroaromatic amines was evaluated. Unfortunately, five-membered heteroaromatic amines bearing pyrazole, thiadiazole or indole skeletons resulted in a messy mixture (see the SI for details).
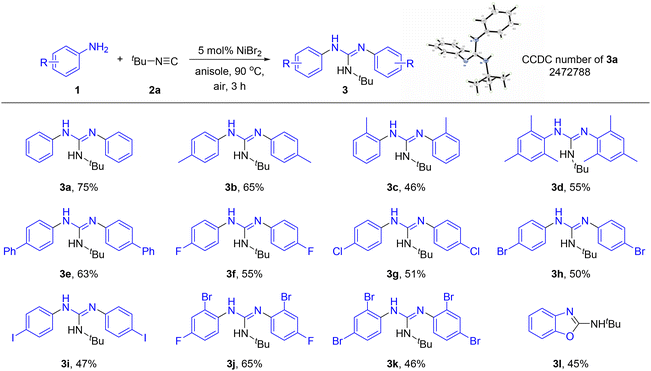 | ||
| Scheme 2 Substrate scope of amines. Reaction conditions: 1a (0.6 mmol), 2a (0.2 mmol), NiBr2 (5 mol%), anisole (0.5 mL), at 90 °C under air for 3 h. | ||
Then, we examined the scope of isocyanides (Scheme 4). Some representative isocyanides were subjected to the reaction with aniline. The 2-isocyano-2,4,4-trimethylpentane 2b participated successfully in the reaction, albeit in a lower reactivity to give the product in 36% yield. Primary alkyl isocyanides, secondary alkyl isocyanides and isocyanobenzenes were unfortunately proved to be invalid and they failed to give any corresponding guanidines (2c–e). Note that the cross-over reaction of two different amines with isocyanides was also conducted (see the SI for details).
To prove the synthetic utility of this nickel-catalyzed isocyanide insertion reaction, a large-scale experiment on a 5.0 mmol scale was conducted (Scheme 5). To our delight, the reaction proceeded smoothly and delivered the expected product 3a in 68% yield. This indicated that the protocol exhibited potential for practical applications in guanidine synthesis.
To clarify the possible reaction mechanism, we first conducted radical inhibition reactions using TEMPO or BHT as a radical scavenger (Scheme 6a). As shown, both the reactions were completely suppressed, which suggested that a radical pathway might be involved in the catalytic cycle. Next, we carried out the model reaction under an oxygen atmosphere with an O2 balloon, and product 3a was obtained in 77% isolated yield (Scheme 6b). We thus assumed that the oxygen in the air might have served as an oxidant and be critical to the reaction efficiency.
Based on the experimental study and previous literature,10a,c a tentative reaction mechanism was postulated; see Scheme 7. Initially, nickel bromide coordinates with isocyanide 2a to afford intermediate I, which undergoes ligand exchange with anilines 1a to give intermediate II. Then, intramolecular isocyanide insertion occurs to deliver intermediate III, followed by reductive elimination to form nickel species IV and guanidine V. Nickel species IV is further oxidised by air to regenerate nickel catalyst I, which enters the next catalytic cycle. Guanidine V ultimately isomerizes to the target product 3a.
Conclusions
In conclusion, we have developed an efficient nickel-catalyzed isocyanide insertion reaction with aromatic amines. A series of open-chain N-aryl guanidines are successfully synthesized under an air atmosphere, thus avoiding the requirement of an external oxidant. The reaction features mild reaction conditions, readily available starting materials and easy operation. Notably, halogen atoms especially the Br and I atoms, which are usually vulnerable in nickel catalysis, are all amenable in this isocyanide insertion reaction.Author contributions
Z. Z. and H. M. conducted the experiments. H. J., B. Z. and Y. H. provided instructions for the experiments. B. Z. and Y. H. did the analysis and wrote the manuscript. B. Z. supervised the whole project.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: experimental procedures, characterization data and NMR spectra of compounds and X-ray crystallographic data for 3a. See DOI: https://doi.org/10.1039/d5ob01509j.CCDC 2472788 contains the supplementary crystallographic data for this paper.19
Acknowledgements
We gratefully acknowledge the funding from the National Natural Science Foundation of China (22001231 and 22001232) and the Project KYY-HX-20240675.References
-
(a)
J. W. Shaw, D. H. Grayson and I. Rozas, in Guanidines as Reagents and Catalysts I, ed. P. Selig, Springer, 2017, vol. 3 Search PubMed
; (b) R. G. S. Berlinck and S. Romminger, Nat. Prod. Rep., 2016, 33, 456 RSC
.
-
T. Ishikawa, Superbases for Organic Synthesis: Guanidines, Amidines, Phosphazenes and Organocatalysts, John Wiley & Sons, 2009 Search PubMed
.
- X.-Y. Cui, C.-H. Tan and D. Leow, Org. Biomol. Chem., 2019, 17, 4689 RSC
.
-
(a) S. Dong, X. Feng and X. Liu, Chem. Soc. Rev., 2018, 47, 8525 RSC
; (b) H.-C. Chou, D. Leow and C.-H. Tan, Chem. – Asian J., 2019, 14, 3803 CrossRef CAS PubMed
.
-
(a) C. Alonso-Moreno, A. Antinolo, F. Carrillo-Hermosilla and A. Otero, Chem. Soc. Rev., 2014, 43, 3406 RSC
; (b) W.-X. Zhang, L. Xua and Z. Xi, Chem. Commun., 2015, 51, 254 RSC
.
- J. Li and L. Neuville, Org. Lett., 2013, 15, 6124 CrossRef CAS PubMed
.
- T. Vlaar, R. C. Cioc, P. Mampuys, B. U. W. Maes, R. V. A. Orru and E. Ruijter, Angew. Chem., Int. Ed., 2012, 51, 13058 CrossRef CAS PubMed
.
- T.-H. Zhu, S.-Y. Wang, G.-N. Wang and S.-J. Ji, Chem. – Eur. J., 2013, 19, 5850 CrossRef CAS PubMed
.
- F. Ji, M.-F. Lv, W.-B. Yi and C. Cai, Org. Biomol. Chem., 2014, 12, 5766 RSC
.
-
(a) G.-N. Wang, T.-H. Zhu, S.-Y. Wang, T.-Q. Wei and S.-J. Ji, Tetrahedron, 2014, 70, 8079 CrossRef CAS
; (b) F. Ahmadi and A. Bazgir, RSC Adv., 2016, 6, 61955 RSC
; (c) A. H. Shinde, S. Arepally, M. D. Baravkar and D. S. Sharada, J. Org. Chem., 2017, 82, 331 CrossRef CAS PubMed
; (d) A. J. Ansari, R. S. Pathare, A. K. Maurya, V. K. Agnihotri, S. Khan, T. K. Roy, D. M. Sawant and R. T. Pardasani, Adv. Synth. Catal., 2018, 360, 290 CrossRef CAS
; (e) J. Escudero, P. Mampuys, C. Mensch, C. B. Bheeter, R. Vroemans, R. V. A. Orru, J. Harvey and B. U. W. Maes, ACS Catal., 2022, 12, 6857 CrossRef CAS
; (f) J. Xiong, H.-T. He, H.-Y. Yang, Z.-G. Zeng, C.-R. Zhong, H. Shi, M.-L. Ouyang, Y.-Y. Tao, Y.-L. Pang, Y.-H. Zhang, B. Hu, Z.-X. Fu, X.-L. Miao, H.-L. Zhu and G. Yao, J. Org. Chem., 2022, 87, 9488 CrossRef CAS PubMed
; (g) X. Ma, Z. Gao, J. Niu, S. Zhang, L. Luo, S. Yan, Q. Zhang and W. Zhang, J. Org. Chem., 2025, 90, 2707 CrossRef CAS PubMed
.
- T.-H. Zhu, S.-Y. Wang, T.-Q. Wei and S.-J. Ji, Adv. Synth. Catal., 2015, 357, 823 CrossRef CAS
.
- Z.-Y. Gu, Y. Liu, F. Wang, X. Bao, S.-Y. Wang and S.-J. Ji, ACS Catal., 2017, 7, 3893 CrossRef CAS
.
- G. Qiao, Z. Zhang, B. Huang, L. Zhu, F. Xiao and Z. Zhang, Synthesis, 2018, 330 CAS
.
- P. Phukan, M. Bhattacharjee and P. Phukan, Asian J. Org. Chem., 2025, 14, e202500176 CrossRef CAS
.
-
(a) Z. Gu and S. Ji, Acta Chim. Sin., 2008, 76, 347 CrossRef
; (b) G. Qiu, Q. Ding and J. Wu, Chem. Soc. Rev., 2013, 42, 5257 RSC
; (c) W. Hao, J. Tian, W. Li, R. Shi, Z. Huang and A. Lei, Chem. - Asian J., 2016, 11, 1664 CrossRef CAS PubMed
; (d) J. W. Collet, T. R. Roose, E. Ruijter, B. U. W. Maes and R. V. A. Orru, Angew. Chem., Int. Ed., 2020, 59, 540 CrossRef CAS PubMed
; (e) W. Wang, T. Liu, C.-H. Ding and B. Xu, Org. Chem. Front., 2021, 8, 3525 RSC
.
-
(a) S. Z. Tasker, E. A. Standley and T. F. Jamison, Nature, 2014, 509, 299 CrossRef CAS PubMed
; (b) V. P. Ananikov, ACS Catal., 2015, 5, 1964 CrossRef CAS
; (c) D. J. Weix, Acc. Chem. Res., 2015, 48, 1767 CrossRef CAS PubMed
; (d) X. Wang, Y. Dai and H. Gong, Top. Curr. Chem., 2016, 374, 43 CrossRef PubMed
; (e) T. Iwasaki and N. Kambe, Top. Curr. Chem., 2016, 374, 66 CrossRef PubMed
; (f) A. Kommoju, K. Snehita, K. Sowjanya, S. B. Mukkamala and K. Padala, Chem. Commun., 2024, 60, 8946 RSC
.
-
(a) Q. Li, Y. Cai, H. Jin, Y. Liu and B. Zhou, Tetrahedron Lett., 2020, 61, 152605 CrossRef CAS
; (b) Q. Li, H. Jin, Y. Liu and B. Zhou, Synthesis, 2020, 3466 CrossRef CAS
; (c) Y. Zhang, Z. Zhang, Y. Hu, Y. Liu, H. Jin and B. Zhou, Org. Biomol. Chem., 2022, 20, 8049 RSC
; (d) H. Mao, H. Qian, Z. He, Z. Zhang, H. Jin, Y. Liu and B. Zhou, Org. Biomol. Chem., 2023, 21, 3360 RSC
; (e) B. Zhou, Z. Huang, Z. Gao and Y. Hu, Org. Lett., 2024, 26, 10511 CrossRef CAS PubMed
; (f) Y. Yang, Z. Gao and B. Zhou, Tetrahedron Lett., 2024, 146, 155194 CrossRef CAS
.
-
(a) Y. Hu, H. Luo, X. Tu, H. Xue, H. Jin, Y. Liu and B. Zhou, Chem. Commun., 2021, 57, 4686 RSC
; (b) Q. Li, Y. Cai, Y. Hu, H. Jin, F. Chen, Y. Liu and B. Zhou, Chem. Commun., 2021, 57, 11657 RSC
; (c) Z. Wang, Y. Hu, H. Jin, Y. Liu and B. Zhou, J. Org. Chem., 2021, 86, 466 CrossRef CAS PubMed
; (d) X. Chen, Z. Wang, H. Jin, Y. Liu and B. Zhou, Org. Biomol. Chem., 2021, 19, 8021 RSC
; (e) Z. Jin, Y. Cai, Z. Wang, H. Jin, Y. Liu and B. Zhou, Org. Biomol. Chem., 2022, 20, 8838 RSC
; (f) Z. He, Z. Wang, Z. Gao, H. Qian, W. Ding, H. Jin, Y. Liu and B. Zhou, Org. Biomol. Chem., 2023, 21, 6493 RSC
; (g) Z. Jin, Y. Yang, Z. He, Z. Huang, Y. Hu, H. Jin and B. Zhou, Org. Lett., 2024, 26, 4520 CrossRef CAS PubMed
; (h) Z. Wang, Z. Jin and B. Zhou, Org. Chem. Front., 2024, 11, 1382 RSC
; (i) B. Zhou, L. Cai, Z. Jin and Y. Hu, Org. Lett., 2025, 27, 1118 CrossRef CAS PubMed
; (j) Y. Hu, L. Cai, Z. Wang, H. Jin and B. Zhou, Chem. Commun., 2025, 61, 14185 RSC
.
-
CCDC 2472788: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2p04c7
.
| This journal is © The Royal Society of Chemistry 2026 |