Photoredox/NHC-catalyzed remote alkylation of γ-functionalized enals with N-substituted pyridinium salts
Yuan-Yuan
Xu
 *ab,
Chang-Chun
Liu
a,
Zhi-Hao
Shen
a,
Ben-Cai
Dai
a,
Jun-An
Ma
a and
Yang
Zhou
*ab
*ab,
Chang-Chun
Liu
a,
Zhi-Hao
Shen
a,
Ben-Cai
Dai
a,
Jun-An
Ma
a and
Yang
Zhou
*ab
aHenan Province Key Laboratory of Environmentally Friendly Functional Materials, Institute of Chemistry Co. Ltd, Henan Academy of Science, Zhengzhou 450002, China
bHenan Academy of Sciences, Zhengzhou 450002, China. E-mail: xuyyuan@hnas.ac.cn; zhouyang636.happy@163.com
First published on 26th November 2025
Abstract
We report a dual photoredox/NHC-catalyzed method for the regioselective remote alkylation of γ-functionalized enals. Using abundant Katritzky pyridinium salts as alkyl radical precursors and γ-functionalized enals to form trienolate intermediates, this method delivers the corresponding ε-alkyl-α,β-γ,δ-unsaturated esters in moderate to good yields. This strategy features mild conditions and a broad substrate scope, providing an efficient approach for remote alkylation reactions.
Introduction
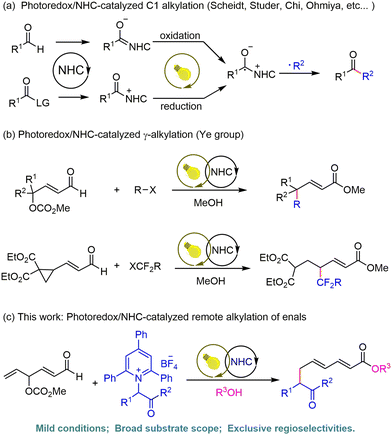
Alkylation reactions stand as one of the most fundamental and indispensable transformations in organic synthesis, serving as a cornerstone for constructing carbon–carbon and carbon–heteroatom bonds—key steps in the synthesis of pharmaceuticals, natural products, and functional materials.1 Among various alkylation strategies, remote alkylation has garnered particular attention due to its ability to selectively modify specific distant sites in complex molecules, addressing the challenge of “poor site selectivity” in traditional alkylation reactions. However, classical remote alkylation methods, which rely on nucleophilic/electrophilic substitution2 or transition metal catalysis,3 often suffer from critical limitations: the need for toxic or expensive reagents and harsh reaction conditions (e.g., high temperature and strong oxidants), and poor functional group tolerance. Therefore, the development of mild, highly selective, and green remote alkylation protocols remains an urgent demand in synthetic chemistry.Over the past few decades, N-heterocyclic carbenes (NHCs) have emerged as powerful catalysts to enable diverse transformations via versatile intermediates,4 such as the classic benzoin condensation,5 the Stetter reaction6 and so on. Despite these advances, a single NHC catalytic system has inherent limitations. Meanwhile, photocatalytic reactions, which leverage light energy to generate free radicals under mild conditions, have attracted growing interest from the chemistry community.7 The combination of NHC catalysis and photoredox catalysis has proven to be a transformative strategy, as it integrates the substrate activation ability of NHCs with the radical generation capability of photocatalysts. In 2012, Rovis and coworkers reported the first NHC/photoredox dual-catalyzed asymmetric α-acylation,8 highlighting the potential of light mediated NHC catalysis in organic synthesis. Subsequently, numerous research groups, including Scheidt,9 Chi,10 Studer,11 Ohmiya,12 and other groups,13 have expanded this field by achieving alkylation reactions using diverse radical precursors. Notably, most of these dual-catalyzed alkylations proceed exclusively at the C1-position, while alkylations at remote sites have rarely been reported (Scheme 1a). The Ye group reported the γ-alkylation and γ-difluoroalkylation of γ-preoxidized enals or cyclopropane enals via NHC/photoredox catalysis14 (Scheme 1b). Following these studies, ε-benzylation via cooperative photoredox and NHC catalysis was also developed, although the method is largely limited to electron-deficient benzyl bromides.15 Despite these reports, the development of general and efficient strategies for remote alkylation via dual photoredox/NHC catalysis remains a significant challenge.
In recent years, Katritzky pyridinium salts have re-emerged as ideal radical precursors due to their high stability.16 Furthermore, unsaturated esters serve as versatile building blocks17 that can form extended trienolate intermediates upon NHC activation. Leveraging these features, we herein report a dual photoredox/NHC-catalyzed remote alkylation of γ-functionalized enals using Katritzky pyridinium salts as radical precursors (Scheme 1c). The method features mild conditions, a broad substrate scope and exclusive regioselectivity and delivers moderate to good yields, offering an efficient and green approach for the precise construction of unsaturated ester derivatives.
Results and discussion
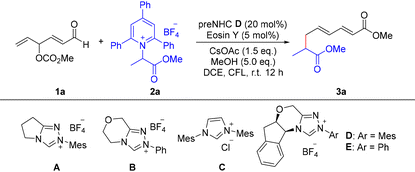
In this study, we first investigated the model reaction between γ-oxidized enal 1a and Katritzky salt 2a, with methanol serving as a nucleophile, under NHC/photoredox dual catalysis (Table 1). After screening a variety of condition, we successfully isolated the ε-alkyl-α,β-γ,δ-unsaturated ester 3a in 65% yield when the reaction was conducted in the presence of triazolium preNHC D (20 mol%) and Eosin Y (5 mol%), using CsOAc (1.5 equiv.) as a base and MeOH (5.0 equiv.) as a nucleophile in DCE, under CFL irradiation at room temperature (Table 1, entry 1). Next, we found that when triazolium preNHC D was replaced with thiazolium preNHC B or imidazolium preNHC C, no product was detected or only a trace amount of the desired product 3a was obtained, respectively. When triazolium preNHC A and E were used, product 3a was obtained in 13 and 40% yields (entries 2–5). Screening of photocatalysts showed that Eosin Y proved to be superior to 4CzIPN, Ir[dF(CF3)2(PPy)(dtbbpy)]PF6− and Ru(bpy)3Cl2·6H2O (entries 6–8). Upon examining different bases, we found that inorganic bases, such as Cs2CO3, K2CO3, NaOAc, showed no significant improvement in product yields (entries 9–11), while organic bases, such as DIPEA and DABCO, -were less or ineffective (entries 12 and 13). Additionally, evaluation of other solvents revealed that reactions conducted in CH3CN or THF did not perform as well as those conducted in DCE (entries 14 and 15).| Entry | Deviation from standard conditions | Yieldb (%) |
|---|---|---|
| ND = not determined. DIPEA = N,N-diisopropylethylamine; DABCO = 1,4-diazabicyclo[2.2.2]octane; CFL = compact fluorescence lamp.a Standard conditions: 1a (0.3 mmol, 2.0 eq.), 2a (0.15 mmol), preNHC D (20 mol%), Eosin Y (5 mol%), CsOAc (1.5 eq.), MeOH (5.0 eq.), and DCE (1.5 mL); irradiation with a 30 W CFL at room temperature for 12 h under a nitrogen atmosphere.b Isolated yields. | ||
| 1 | None | 65 |
| 2 | A, instead of D | 13 |
| 3 | B, instead of D | Trace |
| 4 | C, instead of D | ND |
| 5 | E, instead of D | 40 |
| 6 | 4CzIPN, instead of Eosin Y | Trace |
| 7 | Ir[dF(CF3)2(PPy)(dtbbpy)]PF6−, instead of Eosin Y | 16 |
| 8 | Ru(bpy)3Cl2·6H2O, instead of Eosin Y | 54 |
| 9 | Cs2CO3, instead of CsOAc | 39 |
| 10 | K2CO3, instead of CsOAc | 48 |
| 11 | NaOAc, instead of CsOAc | 38 |
| 12 | DIPEA, instead of CsOAc | Trace |
| 13 | DABCO, instead of CsOAc | ND |
| 14 | CH3CN, instead of DCE | Trace |
| 15 | THF, instead of DCE | 57 |
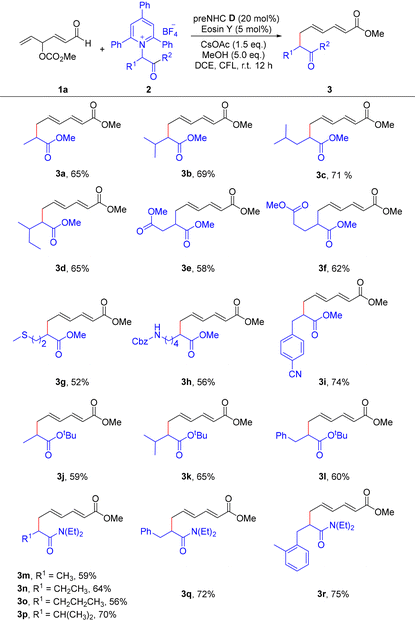
Under the optimized reaction conditions (Table 1, entry 1), we next investigated a range of Katritzky salts to explore the substrate scope of this ε-alkylation process (Table 2). It was found that Katritzky salts derived from natural and unnatural amino acid esters all worked well under standard conditions, affording the corresponding ε-alkyl-α,β-γ,δ-unsaturated esters (3a–3f) in medium to good yields. Notably, the use of methionine and cbz-lysine-derived Katritzky salts allows for the incorporation of thioether and amino moieties into the final products 3g and 3h, respectively—which may serve as useful handles for further derivatization. It is noteworthy that the Katritzky salt derived from methyl 4-cyanophenylalaninate was also tolerated to give the desired product 3i in 74% yield. Moreover, Katritzky salts derived from amino acid tert-butyl esters also showed good reactivity, affording the target products 3j–3l in good yields. Besides, pyridinium salts bearing amido functional groups, including methyl, ethyl, isopropyl, or benzyl amide moieties, were also suitable substrates, yielding the corresponding complex ε-alkylated unsaturated esters 3m–3r in good yields. However, the reaction yields with these aliphatic amine-derived Katritzky pyridinium salts were relatively low (for details see section 6 in the SI), possibly due to the lower stability of alkyl radicals generated from simple aliphatic amine-derived Katritzky salts.
| a Standard conditions: 1a (0.3 mmol, 2.0 eq.), 2 (0.15 mmol), preNHC D (20 mol%), Eosin Y (5 mol%), CsOAc (1.5 eq.), MeOH (5.0 eq.), and DCE (1.5 mL); irradiation with a 30 W CFL at room temperature for 12 h under a nitrogen atmosphere. Isolated yields. |
|---|
|
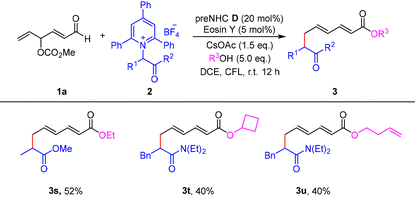
In addition, different nucleophiles were then briefly investigated (Table 3). It was found that alkyl alcohols, cyclic alcohols, and allylic alcohols all worked well in the reaction, albeit affording the corresponding products (3s–3u) in moderate yields. These results further underscore the broad applicability of this methodology.
| a Standard conditions: 1a (0.3 mmol, 2.0 eq.), 2 (0.15 mmol), preNHC D (20 mol%), Eosin Y (5 mol%), CsOAc (1.5 eq.), R3OH (5.0 eq.), and DCE (1.5 mL); irradiation with a 30 W CFL at room temperature for 12 h under a nitrogen atmosphere. Isolated yields. |
|---|
|
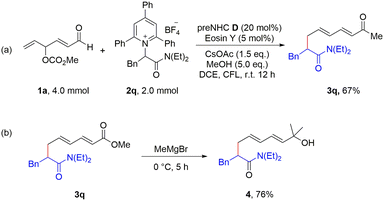
To highlight the practicality of this strategy, a scale-up study and a meaningful transformation were carried out. When the reaction was scaled up tenfold, the desired product was isolated in 67% yield (Scheme 2a). Furthermore, the reaction of 3q with methylmagnesium bromide afforded tertiary alcohol 4 in 76% yield (Scheme 2b).
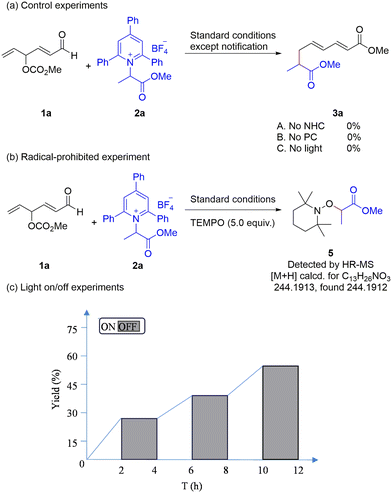
To gain insights into the reaction mechanism, a series of exploratory experiments were conducted. First, the desired product was not observed in the absence of NHC, photocatalyst, or light, indicating that all three components are essential (Scheme 3a). Second, the addition of a radical scavenger 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) to the reaction under standard conditions resulted in complete suppression of product formation, accompanied by the detection of radical adduct 5 by HRMS (Scheme 3b). Finally, the light on/off experiment showed that the light plays an essential role in the reaction (Scheme 3c).
Based on the previous knowledge and the results of our mechanistic studies, we propose a plausible mechanism for the ε-alkylation reaction (Scheme 4). The addition of NHC I, generated in situ from its triazolium precursor in the presence of a base, to the γ-oxidized enal 1a affords the trienolate intermediate II. The extended conjugated system of II provides access to the remote ε-reaction site. Meanwhile, the alkyl radical III, which is generated from Katritzky salt 2via a photoredox process, reacts with the trienolate intermediate II, delivering the radical anion intermediate V. The radical anion intermediate V subsequently undergoes single-electron transfer (SET) oxidation via the PC radical cation IV to afford the acyl azolium intermediate VI, completing the photoredox catalytic cycle. Finally, the acyl azolium intermediate VI is trapped by alcohol, furnishing the final ε-alkyl-α,β-γ,δ-unsaturated ester 3 and regenerating the NHC catalyst I to complete the NHC catalytic cycle.
Conclusions
In summary, we have developed an efficient dual photoredox/NHC-catalyzed method for the ε-selective remote alkylation of γ-functionalized enals. This transformation employs versatile Katritzky pyridinium salts as radical precursors and γ-functionalized enals as precursors for trienolate intermediates, ultimately affording a series of ε-alkylated products in moderate to good yields with excellent regioselectivity. Notably, this protocol exhibits tolerance toward alcohol nucleophiles, not only toward common alkyl and cyclic alcohols but also functionalized alcohols, further demonstrating its practical utility. Moreover, this study employs mild conditions to provide a new, green, and efficient strategy for remote alkylation reactions. Further investigations on remote functionalization reactions are currently ongoing in our laboratory.Author contributions
L. C. C., S. Z. H., D. B. C., and M. J. A., performed the experiments. X. Y.Y. and Z. Y. conceived the study, directed the project, and wrote the manuscript with the assistance of all the authors.Conflicts of interest
There are no conflicts to declare.Data availability
The authors confirm that the data supporting the findings of this study are available within the paper and its supplementary information (SI). Supplementary information: experimental procedures and NMR and HRMS analyses. See DOI: https://doi.org/10.1039/d5ob01714a.Acknowledgements
We are grateful for the financial support from the Research and Development Program projects of the Henan Academy of Sciences (No. 230503007, 241803075, and 20250603006) and the Natural Science Foundation of Henan Province (No. 252300423702).References
- H. Peng, D. Wang, J. Ye, Z.-S. Liu, Z. Zhu, X. Fu, C. Liu, H. Cong, H.-G. Cheng and Q. Zhou, J. Am. Chem. Soc., 2025, 147, 3670 CrossRef CAS PubMed.
- S. Singh and C. K. Hazra, Synthesis, 2023, 56, 368 Search PubMed.
- (a) C. Pradhan and B. Punji, Org. Chem. Front., 2024, 11, 2397 RSC; (b) Z.-S. Liu, D. Wang, H.-G. Cheng and Q. Zhou, ChemCatChem, 2024, 16, e202301764 CrossRef CAS; (c) T. Irrgang and R. Kempe, Chem. Rev., 2019, 119, 2524 CrossRef CAS PubMed; (d) H. Xie, J. Guo, Y.-Q. Wang, K. Wang, P. Guo, P.-F. Su, X. Wang and X.-Z. Shu, J. Am. Chem. Soc., 2020, 142, 16787 CrossRef CAS; (e) R. Babu, M. Subaramanian, S. P. Midya and E. Balaraman, Org. Lett., 2021, 23, 3320 CrossRef CAS PubMed.
- (a) S. Chakraborty, S. Barik and A. T. Biju, Chem. Soc. Rev., 2025, 54, 1102 RSC; (b) Z.-Z. Zhang, R. Zeng, Y.-Q. Liu and J.-L. Li, ChemCatChem, 2024, 16, e202400063 CrossRef CAS; (c) M. Zhang, X. Yang, X. Peng, X. Li and Z. Jin, Sci. China: Chem., 2024, 68, 815 CrossRef; (d) J. Sun, S. Jiang, Y. Liu, L. Pan, Y.-G. Liu and B. Zeng, Chem. Rec., 2024, 24, e202400125 CrossRef CAS PubMed; (e) Y. He, J. Chen, Y. Jiang, X. Fang, J. Liu and J.-L. Yan, Chem. Rec., 2024, 24, e202400165 CrossRef CAS; (f) P. M. D. A. Ewing, P. K. Majhi, C. Prentice, C. M. Young, K. van Rees, P. L. Arnold, E. Zysman-Colman and A. D. Smith, Chem. Sci., 2024, 15, 9369 RSC; (g) M. N. Hopkinson, C. Richter, M. Schedler and F. Glorius, Nature, 2014, 510, 485 CrossRef CAS; (h) D. M. Flanigan, F. Romanov-Michailidis, N. A. White and T. Rovis, Chem. Rev., 2015, 115, 9307 CrossRef CAS PubMed.
- (a) R. Breslow, J. Am. Chem. Soc., 1958, 80, 3719 CrossRef CAS; (b) D. Enders, K. Breuer and J. H. Teles, Helv. Chim. Acta., 1996, 79, 1217 CrossRef CAS; (c) P. Noppawan, B. Phungpis and K. Worawut, Chim. Techno Acta, 2023, 10, 2411 Search PubMed.
- (a) H. Stetter, Angew. Chem., Int. Ed. Engl., 1976, 15, 639 CrossRef; (b) M. M. Heravi, V. Zadsirjan, K. Kafshdarzadeh and Z. Amiri, Asian J. Org. Chem., 2020, 9, 1999 CrossRef CAS.
- (a) G. E. M. Crisenza and P. Melchiorre, Nat. Commun., 2020, 11, 803 CrossRef PubMed; (b) Y.-F. Wang, M.-Y. Qi, M. Conte, Z.-R. Tang and Y.-J. Xu, Angew. Chem., Int. Ed., 2023, 62, e202304306 CrossRef CAS.
- D. A. DiRocco and T. Rovis, J. Am. Chem. Soc., 2012, 134, 8094 CrossRef CAS PubMed.
- (a) A. V. Bay, K. P. Fitzpatrick, R. C. Betori and K. A. Scheidt, Angew. Chem., Int. Ed., 2020, 59, 9143 CrossRef CAS; (b) A. V. Bay, K. P. Fitzpatrick, G. A. González-Montiel, A. O. Farah, P. H.-Y. Cheong and K. A. Scheidt, Angew. Chem., Int. Ed., 2021, 60, 17925 CrossRef CAS PubMed; (c) C. R. Schull, J. Cao, S. R. Mitton-Fry, M. Mrksich and K. A. Scheidt, ACS Catal., 2025, 15, 1287 CrossRef CAS.
- S.-C. Ren, W.-X. Lv, X. Yang, J.-L. Yan, J. Xu, F.-X. Wang, L. Hao, H. Chai, Z. Jin and Y. R. Chi, ACS Catal., 2021, 11, 2925 CrossRef CAS.
- (a) Q.-Y. Meng, N. Döben and A. Studer, Angew. Chem., Int. Ed., 2020, 59, 19956 CrossRef CAS PubMed; (b) Q.-Y. Meng, L. Lezius and A. Studer, Nat. Commun., 2021, 12, 2068 CrossRef CAS.
- (a) Y. Sato, Y. Goto, K. Nakamura, Y. Miyamoto, Y. Sumida and H. Ohmiya, ACS Catal., 2021, 11, 12886 CrossRef CAS; (b) T. Ishii, Y. Kakeno, K. Nagao and H. Ohmiya, J. Am. Chem. Soc., 2019, 141, 3854 CrossRef CAS PubMed.
- (a) K. Scheidt, J. Cao and C. Schull, Angew. Chem., Int. Ed., 2025, 64, e202507542 CrossRef PubMed; (b) X. Liu, S. Xu, H. Chen and Y. Yang, ACS Catal., 2024, 14, 9144 CrossRef CAS; (c) S. Li, C. Zhang, S. Wang, W. Yang, X. Fang, S. Fan, Q. Zhang, X.-X. Li and Y.-S. Feng, Org. Lett., 2024, 26, 1728 CrossRef CAS PubMed; (d) X. Wang, B. Zhu, Y. Liu and Q. Wang, ACS Catal., 2022, 12, 2522 CrossRef CAS; (e) H. Huang, Q.-S. Dai, H.-J. Leng, S.-L. Yang, Y.-M. Tao, X. Zhang, T. Qi and J.-L. Li, Chem. Sci., 2022, 13, 2584 RSC; (f) D. Du, K. Zhang, R. Ma, L. Chen, J. Gao, T. Lu, Z. Shi and J. Feng, Org. Lett., 2020, 22, 6370 CrossRef CAS PubMed.
- (a) L. Dai, Z.-H. Xia, Y.-Y. Gao, Z.-H. Gao and S. Ye, Angew. Chem., Int. Ed., 2019, 58, 18124 CrossRef CAS PubMed; (b) L. Dai, Y.-Y. Xu, Z.-H. Xia and S. Ye, Org. Lett., 2020, 22, 8173 CrossRef CAS PubMed; (c) L. Dai and S. Ye, Org. Lett., 2020, 22, 986 CrossRef CAS.
- Y.-Y. Xu, L. Dai, Z.-H. Gao and S. J. Ye, Org. Chem., 2022, 87, 14970 CrossRef CAS PubMed.
- (a) J. Yi, S. O. Badir, L. M. Kammer, M. Ribagorda and G. A. Molander, Org. Lett., 2019, 21, 3346 CrossRef CAS; (b) D. Moser, Y. Duan, F. Wang, Y. Ma, M. J. O'Neill and J. Cornella, Angew. Chem., Int. Ed., 2018, 57, 11035 CrossRef CAS PubMed.
- (a) Y. Muramatsu, A. Takasu, M. Higuchi and M. Hayashi, J. Polym. Sci., 2020, 58, 2936 CrossRef CAS; (b) K. Maekawa, N. Komine, S. Kiyota and M. Hirano, Org. Biomol. Chem., 2024, 22, 2098 RSC; (c) F. Sakamoto, E. Arata, R. Saito, S. Kiyota, N. Komine and M. J. Hirano, Org. Chem., 2025, 90, 5924 CrossRef CAS PubMed; (d) Y. Hosoi, A. Takasu, S.-i. Matsuoka and M. Hayashi, J. Am. Chem. Soc., 2017, 139, 15005 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |