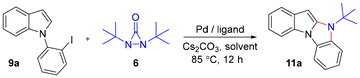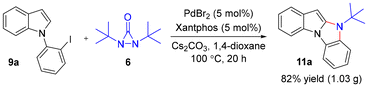Pd-catalyzed efficient synthesis of benzo[4,5]imidazo[1,2-a]indoles with diaziridinone
Jianjun
Wang
 ,
Xiao
Zhang
,
Shiwei
Zhang
,
Wei
Liu
and
Yian
Shi
,
Xiao
Zhang
,
Shiwei
Zhang
,
Wei
Liu
and
Yian
Shi
 *
*
Jiangsu Key Laboratory of Advanced Catalytic Materials & Technology, School of Petrochemical Engineering, Institute of Natural and Synthetic Organic Chemistry, Changzhou University, Changzhou 213164, P. R. China. E-mail: shiyian@cczu.edu.cn
First published on 27th November 2025
Abstract
This work describes an efficient Pd-catalyzed synthesis of benzo[4,5]imidazo[1,2-a]indoles from readily available 1-(2-iodophenyl)-1H-indoles and di-t-butyldiaziridinone. A variety of benzo[4,5]imidazo[1,2-a]indoles can be obtained in up to 96% yield. The reaction likely proceeds via an intramolecular aryl C–H activation to form a pallada(II)cycle, which is subsequently bisaminated with di-t-butyldiaziridinone.
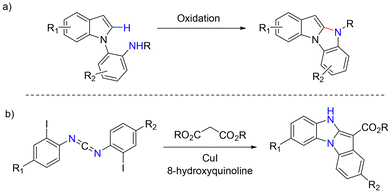
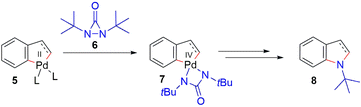
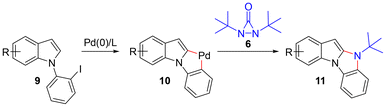
Indoles are some of the most common nitrogen heterocycles. Indole fused N-heterocycles such as benzo[4,5]imidazo[1,2-a]indole (1) and its related derivatives widely exist in various natural products, biologically active molecules, and materials (Fig. 1).1–3 Benzo[4,5]imidazo[1,2-a]indoles containing an indole and an imidazole have displayed unique properties and have great potential in organic light-emitting devices (OLEDs) as well as in drug development. The construction of structurally diverse benzo[4,5]imidazo[1,2-a]indoles and their related derivatives is of interest. A number of synthetic methods have been reported.4 In most cases, the reaction processes involve cyclization via C–C or C–N bond formation from precursors with all nitrogen atoms already being incorporated. In a few cases, only one example of benzo[4,5]imidazo[1,2-a]indole was reported.4a,b,d There are several reports showing that benzo[4,5]imidazo[1,2-a]indoles can be oxidatively formed from 2-(1H-indol-1-yl)anilines with oxidants or via electrochemical oxidation (Scheme 1a).4e–h It has also been reported that benzo[4,5]imidazo[1,2-a]indoles can be synthesized from bis(o-iodoaryl)carbodiimides and malonates via copper-catalyzed domino addition/double cyclization reaction processes (Scheme 1b).4c Each method appears to have its own substrate scope and limitation. New and efficient reaction processes are still highly desirable. As part of our ongoing efforts in constructing nitrogen-heterocycles via bisamination of pallada(II)cycles with diaziridinone (Scheme 2),5–7 we wish to report that benzo[4,5]imidazo[1,2-a]indoles 11 can be efficiently generated from 1-(2-iodophenyl)-1H-indoles 9 and di-t-butyldiaziridinone 6 with simultaneous formation of two C–N bonds8 (Scheme 3).
Indole 9a was used as a test substrate. Various ligands were initially examined for the reaction with 5 mol% Pd(OAc)2, di-t-butyldiaziridinone (6) (1.5 equiv.), and Cs2CO3 (2.0 equiv.) in dioxane at 85 °C for 12 h (Table 1, entries 1–16). To our delight, the desired benzo[4,5]imidazo[1,2-a]indoles 11a were obtained in all cases, with xantphos being the best, affording 11a in 75% yield as determined by 1H NMR (Table 1, entry 16). A slightly higher yield (86%) was obtained when PdBr2 was used as a catalyst (Table 1, entry 18). The yield increased to 96% when the reaction temperature was raised to 100 °C (Table 1, entry 23). 1,4-Dioxane appeared to be the best for the reaction among the solvents examined (Table 1, entry 23 vs. entries 26–30).
| Entry | [Pd] | Ligand | Temperature | Solvent | Yieldb (%) |
|---|---|---|---|---|---|
| a All reactions were carried out with indole 9a (0.10 mmol), di-t-butyldiaziridinone 6 (0.15 mmol), Pd (0.0050 mmol), ligand (0.0050–0.010 mmol, Pd/P = 1/2), and Cs2CO3 (0.20 mmol) in 1,4-dioxane (0.5 mL) at 85 °C under N2 for 12 h unless otherwise stated. b The yield was determined by 1H NMR analysis of the crude reaction mixture with BnOMe as the internal standard. | |||||
| 1 | Pd(OAc)2 | PPh3 | 85 °C | 1,4-Dioxane | 47 |
| 2 | Pd(OAc)2 | (o-Tolyl)3P | 85 °C | 1,4-Dioxane | 21 |
| 3 | Pd(OAc)2 | (p-Tolyl)3P | 85 °C | 1,4-Dioxane | 48 |
| 4 | Pd(OAc)2 | (p-MeOPh)3P | 85 °C | 1,4-Dioxane | 26 |
| 5 | Pd(OAc)2 | (p-FPh)3P | 85 °C | 1,4-Dioxane | 58 |
| 6 | Pd(OAc)2 | (p-CF3Ph)3P | 85 °C | 1,4-Dioxane | 45 |
| 7 | Pd(OAc)2 | CyPPh2 | 85 °C | 1,4-Dioxane | 46 |
| 8 | Pd(OAc)2 | Cy2PPh | 85 °C | 1,4-Dioxane | 46 |
| 9 | Pd(OAc)2 | Cy3P | 85 °C | 1,4-Dioxane | 20 |
| 10 | Pd(OAc)2 | (2-Furyl)3P | 85 °C | 1,4-Dioxane | 22 |
| 11 | Pd(OAc)2 | Dppm | 85 °C | 1,4-Dioxane | 13 |
| 12 | Pd(OAc)2 | Dppe | 85 °C | 1,4-Dioxane | 18 |
| 13 | Pd(OAc)2 | Dpph | 85 °C | 1,4-Dioxane | 13 |
| 14 | Pd(OAc)2 | Dppf | 85 °C | 1,4-Dioxane | 40 |
| 15 | Pd(OAc)2 | BINAP | 85 °C | 1,4-Dioxane | 13 |
| 16 | Pd(OAc)2 | Xantphos | 85 °C | 1,4-Dioxane | 75 |
| 17 | PdCl2 | Xantphos | 85 °C | 1,4-Dioxane | 31 |
| 18 | PdBr2 | Xantphos | 85 °C | 1,4-Dioxane | 86 |
| 19 | PdI2 | Xantphos | 85 °C | 1,4-Dioxane | 64 |
| 20 | Pd(TFA)2 | Xantphos | 85 °C | 1,4-Dioxane | 83 |
| 21 | Pd(CH3CN)4(BF4)2 | Xantphos | 85 °C | 1,4-Dioxane | 28 |
| 22 | Pd(dba)2 | Xantphos | 85 °C | 1,4-Dioxane | 34 |
| 23 | PdBr2 | Xantphos | 100 °C | 1,4-Dioxane | 96 |
| 24 | PdBr2 | Xantphos | 115 °C | 1,4-Dioxane | 95 |
| 25 | PdBr2 | Xantphos | 130 °C | 1,4-Dioxane | 87 |
| 26 | PdBr2 | Xantphos | 100 °C | DMF | 87 |
| 27 | PdBr2 | Xantphos | 100 °C | DMSO | 83 |
| 28 | PdBr2 | Xantphos | 100 °C | THF | 94 |
| 29 | PdBr2 | Xantphos | 100 °C | PhMe | 78 |
| 30 | PdBr2 | Xantphos | 100 °C | CH3CN | 64 |
Having the optimized reaction conditions in hand, the reaction generality was subsequently investigated with a range of 1-(2-iodophenyl)-1H-indoles 9. As shown in Table 2, the reaction can be extended to various 5-substituted indoles, affording the corresponding benzo[4,5]imidazo[1,2-a]indoles 11a–k in 39–96% yields (Table 2).9 The reaction was compatible with various substituents including Me (11b), OMe (11e), F (11f), Cl (11g), CHO (11h), CO2Me (11i), CN (11j), and NO2 (11k). Electron-withdrawing groups such as CN and CO2Me appeared to be beneficial for the reaction, while a lower yield was obtained with an electron-donating OMe group. The reaction can also be applied to various 6-substituted (Table 2, 9l–q), 4-substituted (Table 2, 9r–t), and 7-substituted (Table 2, 9u) indoles, affording the corresponding benzo[4,5]imidazo[1,2-a]indoles 11l–u in 49–90% yields. When 3-methyl indole 9v was subjected to the reaction conditions, the corresponding product was obtained in only 29% yield, possibly due to the steric bulkiness of the 3-methyl group. The reaction was also effective for pyridine fused indole 9w and benzo[d]imidazole 9x, affording the corresponding products 11w and 11x in 88% and 81% yields, respectively. The reaction was also applicable to indoles bearing substituted N-phenyl rings 9y and 9z as well as N-pyridine ring 9aa, affording the corresponding benzo[4,5]imidazo[1,2-a]indoles 11y, 11z, and 11aa in 61%, 38% and 92% yields, respectively.10,11
| a All reactions were carried out with indole 9 (0.30 mmol), di-t-butyldiaziridinone 6 (0.45 mmol), PdBr2 (0.015 mmol), xantphos (0.015 mmol), and Cs2CO3 (0.60 mmol) in 1,4-dioxane (1.0 mL) at 100 °C under N2 for 12 h unless otherwise stated. b Isolated yield. c With PdBr2 (0.030 mmol) and xantphos (0.030 mmol) at 120 °C. d For 24 h. |
|---|
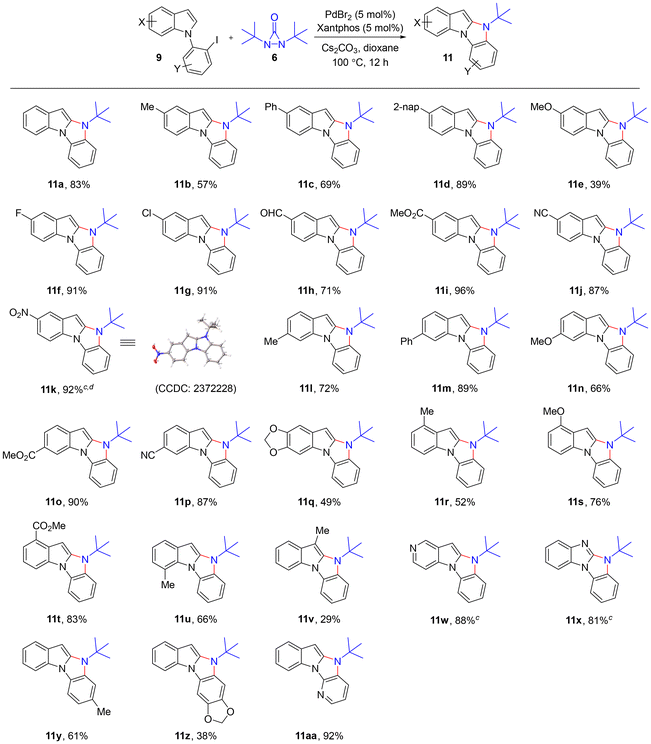
|
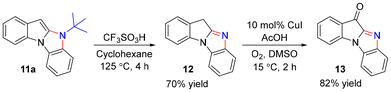
As exemplified with 9a, the reaction can be carried out on a gram scale, affording benzo[4,5]imidazo[1,2-a]indole 11a in 82% yield (Scheme 4). Treating 11a with CF3SO3H/cyclohexane led to the formation of imidazole 12 in 70% yield (Scheme 5).4h,5b
The oxidation of 12 with O2 gave 11H-benzo[4,5]imidazo[1,2-a]indol-11-one 13 in 82% yield (Scheme 5).4h,12
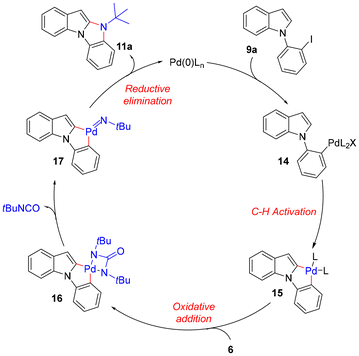
A precise understanding of the reaction mechanism awaits further studies. A plausible catalytic cycle is outlined in Scheme 6.5 The oxidative addition of Pd(0) to iodophenyl indole 9a gave Pd(II) intermediate 14, which subsequently underwent intramolecular C–H activation to form pallada(II)cycle 15.13 The oxidative addition of di-t-butyldiaziridione (6) to 15 led to the formation of pallada(IV)cycle 16, which gave Pd(IV)-nitrene 17 upon release of t-butyl isocyanate. Benzo[4,5]imidazo[1,2-a]indole 11a was eventually formed from 17via two consecutive reductive elimination steps with regeneration of the Pd(0) catalyst.
Conclusions
In summary, we have developed an efficient synthesis of benzo[4,5]imidazo[1,2-a]indoles from readily available 1-(2-iodophenyl)-1H-indoles and di-t-butyldiaziridinone in the presence of a Pd(0) catalyst. A wide variety of benzo[4,5]imidazo[1,2-a]indoles bearing various functional groups can be obtained in up to 96% yields. The reaction can be performed on a gram scale, and the resulting benzo[4,5]imidazo[1,2-a]indoles can be further transformed into benzoimidazoles. The reaction was postulated to proceed via an intramolecular C–H activation to form the corresponding pallada(II)cycle, which was subsequently bisaminated with di-t-butyldiaziridinone to afford the benzo[4,5]imidazo[1,2-a]indole. The current amination process further illustrates the versatility of di-t-butyldiaziridinone for the synthesis of polycyclic heterocycles.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI): experimental details, NMR spectra of new compounds and X-ray crystallographic data for 11k. See DOI: https://doi.org/10.1039/d5ob01335f.CCDC 2372228 contains the supplementary crystallographic data for this paper.14
Acknowledgements
The authors thank the National Natural Science Foundation of China (22271024 and 21632005) and Changzhou University for financial support.References
- T. V. Sravanthi and S. L. Manju, Indoles – A Promising Scaffold for Drug Development, Eur. J. Pharm. Sci., 2016, 91, 1–10 CrossRef CAS PubMed.
- P. R. Nitha, S. Soman and J. John, Indole Fused Heterocycles as Sensitizers in Dye-Sensitized Solar Cells: An Overview, Mater. Adv., 2021, 2, 6136–6168 RSC.
- (a) S. Layek, J. Feldman, P. Wolohan and T. Lu, Organic Electroluminescent Materials and Devices, US patent, US2019/0252623A1, 2019 Search PubMed; (b) W.-C. Chen, Y. Yuan, Z.-L. Zhu, S.-F. Ni, Z.-Q. Jiang, L.-S. Liao, F.-L. Wong and C.-S. Lee, A Novel Spiro-Annulated Benzimidazole Host for Highly Efficient Blue Phosphorescent Organic Light-Emitting Devices, Chem. Commun., 2018, 54, 4541–4544 RSC; (c) A. S. Filatov, Y. A. Pronina, S. I. Selivanov, S. V. Shmakov, A. A. Uspenski, V. M. Boitsov and A. V. Stepakov, 11H-Benzo[4,5]imidazo[1,2-a]indol-11-one as a New Precursor of Azomethine Ylides: 1,3-Dipolar Cycloaddition Reactions with Cyclopropenes and Maleimides, Int. J. Mol. Sci., 2022, 23, 13202 CrossRef CAS PubMed.
- (a) R. R. Naredla, C. Zheng, S. O. N. Lill and D. A. Klumpp, Charge Delocalization and Enhanced Acidity in Tricationic Superelectrophiles, J. Am. Chem. Soc., 2011, 133, 13169–13175 CrossRef CAS PubMed; (b) X. Shang, C. Chen, H. Qiu and W. Chen, Silver(I)-Promoted Intramolecular Addition of N-Heterocyclic Carbenes towards Unsaturated Esters in Water, Tetrahedron, 2014, 70, 3073–3077 CrossRef CAS; (c) G. Yuan, H. Liu, J. Gao, K. Yang, Q. Niu, H. Mao, X. Wang and X. Lv, Copper-Catalyzed Domino Addition/Double Cyclization: An Approach to Polycyclic Benzimidazole Derivatives, J. Org. Chem., 2014, 79, 1749–1757 CrossRef CAS PubMed; (d) K. Tong, X. Liu, Y. Zhang and S. Yu, Visible-Light–Induced Direct Oxidative C-H Amidation of Heteroarenes with Sulfonamides, Chem. – Eur. J., 2016, 22, 15669–15673 CrossRef CAS PubMed; (e) S. Badigenchala, V. Rajeshkumar and G. Sekar, Iodine Mediated Intramolecular C2-Amidative Cyclization of Indoles: a Facile Access to Indole Fused Tetracycles, Org. Biomol. Chem., 2016, 14, 2297–2305 RSC; (f) X. Wang, N. Li, Z. Li and H. Rao, Copper-Catalyzed Dehydrogenative C(sp2)-N Bond Formation via Direct Oxidative Activation of an Anilidic N-H Bond: Synthesis of Benzoimidazo[1,2-a]indoles, J. Org. Chem., 2017, 82, 10158–10166 CrossRef CAS PubMed; (g) Y. Yu, Y. Yuan, H. Liu, M. He, M. Yang, P. Liu, B. Yu, X. Dong and A. Lei, Electrochemical Oxidative C-H/N-H Cross-Coupling for C-N Bond Formation with Hydrogen Evolution, Chem. Commun., 2019, 55, 1809–1812 RSC; (h) Y. Sharma, S. Kaur, H. Kandhasamy, S. C. Sahoo and V. D. Chaudhari, Palladium-Catalyzed Intramolecular C−H Amination via Oxidative Coupling on Indole Derivatives: Access to 11H-Benzo[4,5]imidazo[1,2-a]indoles, Org. Lett., 2025, 27, 517–521 CrossRef CAS PubMed.
- (a) T. A. Ramirez, Q. Wang, Y. Zhu, H. Zheng, X. Peng, R. G. Cornwall and Y. Shi, Pd(0)-Catalyzed Sequential C-N Bond Formation via Allylic and Aromatic C-H Amination of α-Methylstyrenes with Diaziridinone, Org. Lett., 2013, 15, 4210–4213 CrossRef CAS PubMed; (b) H. Zheng, Y. Zhu and Y. Shi, Palladium(0)-Catalyzed Heck Reaction/C-H Activation/Amination Sequence with Diaziridinone: A Facile Approach to Indolines, Angew. Chem., Int. Ed., 2014, 53, 11280–11284 CrossRef CAS PubMed; (c) S. Debnath, L. Liang, M. Lu and Y. Shi, Domino C-N Bond Formation via a Palladacycle with Diaziridinone. An Approach to Indolo[3,2-b]indoles, Org. Lett., 2021, 23, 3237–3242 CrossRef CAS PubMed; (d) J. Li, J. Chen, L. Wang and Y. Shi, Palladium-Catalyzed Sequential C-H Activation/Amination with Diaziridinone: An Approach to Indoles, Org. Lett., 2021, 23, 3646–3651 CrossRef CAS PubMed; (e) J. Wang, X. Sun, D. Hu and Y. Shi, Pd-Catalyzed Indole Synthesis via C−H Activation and Bisamination Sequence with Diaziridinone, Org. Lett., 2021, 23, 7561–7565 CrossRef CAS PubMed; (f) J. Li, Y. Liu and Y. Shi, Palladium-catalyzed amination of alkynes with diaziridinone via a palladacycle. An approach to polysubstituted pyrroles, Tetrahedron Lett., 2024, 147, 155158 CrossRef CAS; (g) J. Wang, E. Yang, W. Liu, S. Zhang and Y. Shi, Pd-Catalyzed Efficient Synthesis of 3-Formylindole Derivatives with Diaziridinone, Org. Biomol. Chem., 2025, 23, 5006–5015 RSC; (h) J. Wang, W. Liu, J. Liu, H. Hong, Y. Shi, E. Yang and Y. Shi, Pd-Catalyzed Regioselective Tandem Heck/C–H Activation/Bisamination Reactions with Ynamides and Diaziridinone. Rapid Access to 3-Aminoindoles, Org. Lett., 2025, 27, 5399–5405 CrossRef CAS PubMed; (i) J. Li, Y. Fu and Y. Shi, A Rapid Access to Fused Polycyclic Indolo[2,1-a]isoquinolins via Pd-Catalyzed Sequential Heck /C-H activation/Amination Reaction with Diaziridinone, Chin. Chem. Lett., 2025 DOI:10.1016/j.cclet.2025.111376.
- For related works, see: (a) C. Shao, B. Zhou, Z. Wu, X. Ji and Y. Zhang, Synthesis of Carbazoles from 2-Iodobiphenyls by Palladium-Catalyzed C-H Activation and Amination with Diaziridinone, Adv. Synth. Catal., 2018, 360, 887–892 CrossRef CAS; (b) B. Zhou, Z. Wu, D. Ma, X. Ji and Y. Zhang, Synthesis of Indoles through Palladium-Catalyzed Three-Component Reaction of Aryl Iodides, Alkynes, and Diaziridinone, Org. Lett., 2018, 20, 6440–6443 CrossRef CAS PubMed; (c) X. Sun, Z. Wu, W. Qi, X. Ji, C. Cheng and Y. Zhang, Synthesis of Indolines by Palladium-Catalyzed Intermolecular Amination of Unactivated C(sp3)-H Bonds, Org. Lett., 2019, 21, 6508–6512 CrossRef CAS PubMed; (d) D. Ma, X. Ji, Z. Wu, C. Cheng, B. Zhou and Y. Zhang, Synthesis of Benzimidazoles through Palladium-Catalyzed Amination of 2-Iodobenzimines with Diaziridinone, Adv. Synth. Catal., 2019, 361, 739–746 CrossRef CAS; (e) C. Cheng, X. Zuo, D. Tu, B. Wan and Y. Zhang, Synthesis of 3,4-Fused Tricyclic Indoles through Cascade Carbopalladation and C-H Amination: Development and Total Synthesis of Rucaparib, Org. Lett., 2020, 22, 4985–4989 CrossRef CAS PubMed; (f) L. Zhang, J. Chen, T. Zhong, X. Zheng, J. Zhou, X. Jiang and C. Yu, Palladium-Catalyzed [2+2+1] Annulation of Alkyne-Tethered Aryl Iodides with Diaziridinone: Synthesis of 3,4-Fused Tricyclic Indoles, J. Org. Chem., 2020, 85, 10823–10834 CrossRef CAS PubMed; (g) J. Bajohr, A. Dupeux, D. Schenk, C. Jans and M. Lautens, Pd-Catalyzed Domino Narasaka–Heck/C–H Activation/Amination Reactions: Synthesis of Bis-heterocyclic Spirocycles, Org. Lett., 2023, 25, 5361–5365 CrossRef CAS PubMed.
- For related indole synthesis via palladacycles with different nitrogen sources, see: (a) F. Jafarpour, M. Ghasemi, H. Navid, N. Safaie, S. Rajai-Daryasarei, A. Habibi and R. C. Ferrier Jr., Assembly of Indole Cores through a Palladium-Catalyzed Metathesis of Ar-X σ-Bonds, Org. Lett., 2020, 22, 9556–9561 CrossRef CAS PubMed; (b) L. Fan, J. Hao, J. Yu, X. Ma, J. Liu and X. Luan, Hydroxylamines as Bifunctional Single-Nitrogen Sources for the Rapid Assembly of Diverse Tricyclic Indole Scaffolds, J. Am. Chem. Soc., 2020, 142, 6698–6707 CrossRef CAS PubMed.
- For leading reviews on C–N bond formation, see: (a) J. F. Hartwig, Transition Metal Catalyzed Synthesis of Arylamines and Aryl Ethers from Aryl Halides and Triflates: Scope and Mechanism, Angew. Chem., Int. Ed., 1998, 37, 2046–2067 CrossRef CAS; (b) J. P. Wolfe, S. Wagaw, J.-F. Marcoux and S. L. Buchwald, Rational Development of Practical Catalysts for Aromatic Carbon-Nitrogen Bond Formation, Acc. Chem. Res., 1998, 31, 805–818 CrossRef CAS; (c) J. X. Qiao and P. Y. S. Lam, Copper-Promoted Carbon-Heteroatom Bond Cross-Coupling with Boronic Acids and Derivatives, Synthesis, 2011, 29–856 Search PubMed; (d) S. Bhunia, G. G. Pawar, S. V. Kumar, Y. Jiang and D. Ma, Selected Copper-Based Reactions for C-N, C-O, C-S, and C-C Bond Formation, Angew. Chem., Int. Ed., 2017, 56, 16136–16179 CrossRef CAS PubMed; (e) M. M. Heravi, Z. Kheilkordi, V. Zadsirjan, M. Heydari and M. Malmir, Buchwald-Hartwig Reaction: An overview, J. Organomet. Chem., 2018, 861, 17–104 CrossRef CAS; (f) M. J. West, J. W. B. Fyfe, J. C. Vantourout and A. J. B. Watson, Mechanistic Development and Recent Applications of the Chan-Lam Amination, Chem. Rev., 2019, 119, 12491–12523 CrossRef CAS PubMed; (g) Y.-A. Xu, S.-H. Xiang, J.-T. Che, Y.-B. Wang and B. Tan, Skeletal Editing of Cyclic Molecules Using Nitrenes, Chin. J. Chem., 2024, 42, 2656–2667 CrossRef CAS.
- Subjecting 1-(2-bromophenyl)-1H-indole to the same reaction conditions gave only trace amounts of benzo[4,5]imidazo[1,2-a]indole 11a as judged by 1H NMR. However, 11a was isolated in 31% yield when the reaction was carried out with 10 mol% PdBr2, xantphos (10 mol%), di-t-butyldiaziridinone (6) (1.5 equiv.), and Cs2CO3 (2.0 equiv.) in dioxane at 130 °C for 36 h.
- As previously reported, attempts to make other substituted diaziridinones such as di-n-butyldiaziridinone, di-benzyldiaziridinone, and di-phenyldiaziridinone were unsuccessful (see note 14 in ref. 11).
- J. Wang, H. Hong, D. Hu, J. Liu, W. Liu and Y. Shi, Pd-Catalyzed Vinyl C-H Amination with Diaziridinone, Org. Front. Chem., 2024, 11, 484–488 RSC.
- H. Sterckx, J. De Houwer, C. Mensch, I. Caretti, K. A. Tehrani, W. A. Herrebout, S. V. Doorslaer and B. U. W. Maes, Mechanism of the CuII-Catalyzed Benzylic Oxygenation of (Aryl)(Heteroaryl)methanes with Oxygen, Chem. Sci., 2016, 7, 346–357 RSC.
- For leading references, see: (a) C. Xie, Y. Zhang, Z. Huang and P. Xu, Synthesis of Indolo[1,2-f]phenanthridines from Palladium-Catalyzed Reactions of Arynes, J. Org. Chem., 2007, 72, 5431–5434 CrossRef CAS PubMed; (b) L. Liu, J. Qiang, S. Bai, Y. Li, C. Miao and J. Li, Palladium-Catalyzed Cyclocarbonylation of Cyclic Diaryliodoniums: Synthesis of Fluorenones, Appl. Organomet. Chem., 2017, 31, e3817 CrossRef; (c) C. Hu, D. Mou, X. Zhang, Y. Fu, C. Huo and Z. Du, Synthesis of 10H-indolo[1,2-a]indol-10-ones via palladium-Catalyzed C-H Bond Activation and Difluorocarbene transfer, Org. Biomol. Chem., 2022, 20, 8120–8124 RSC; (d) X. Liu, Y.-L. Ban, Y. Liu, M. Zhuang and Y. Zhou, Palladium-Catalyzed C-H Bond Activation and Decarboxylation for the Assembly of Indolo[1,2-f]phenanthridine, Org. Biomol. Chem., 2024, 22, 9188–9191 RSC; (e) W. Li, W. Li, X. Hu, Y. Yu, M. Cao, H. Sheng, X. Zhang, J. Tao and T. Li, Palladium-Catalyzed Dual C-H Activation for the Synthesis of Indolo[1,2-f]phenanthridines, Org. Biomol. Chem., 2025, 23, 5882–5886 RSC.
- CCDC 2372228: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2kmhh6.
| This journal is © The Royal Society of Chemistry 2026 |