Fusion of β-enaminones and 2-aminopyridines to 3-oyl-imidazo[1,2-a]pyridines induced by iodine: a mechanochemical approach
Medishetti
Nagaraju†
ab,
Chintha
Saikrishna†
 ab and
Atmakur
Krishnaiah
ab and
Atmakur
Krishnaiah
 *ab
*ab
aFluoro & Agrochemicals Department, CSIR-Indian Institute of Chemical Technology, Hyderabad, 500007, India. E-mail: krishnu@iict.res.in
bAcademy of Scientific and Innovative Research, Ghaziabad, 201002, India
First published on 21st November 2025
Abstract
Metal and additive-free, one-step synthesis of 3-oyl-imidazo[1,2-a]pyridines was accomplished by the solid-state manual grinding of β enaminones, 2-aminopyridines and molecular iodine. The reaction proceeds by the addition of 2-aminopyridine to β-enaminone in a Michael fashion, and the in situ formed adduct undergoes intramolecular endo-cyclization induced by molecular iodine leading to the title product. Simple reaction conditions, short reaction time, no column purification and excellent yields are the advantages of the protocol. Furthermore, this methodology also offers superior yields for previously reported compounds, and this is the first report on the synthesis of the title compounds from β-enaminones, through grindstone chemistry.
Introduction
Enaminones are vinylogous amides consisting of conjugated ambiphilic sites and are considered as unique synthons with three nucleophilic and two electrophilic centres (Fig. 1a),1 which promoted the renewed blossoming in enaminone-based chemistry that led to the synthesis of versatile biological and pharmaceutically active intermediates in organic synthesis.2 The majority of the reactions of β-enaminones proceed via a vinylic C–H bond,3 the π-bond of an alkene,4 or an alkenyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond,5 and with/without cleavage of the C–N bond.6 This enables them to be utilized as precursors for bicyclic and aza-heterocyclic compounds, such as indoles,7 quinolines,8 pyrazolopyridines,9 imidazopyridines,10 and pyrazolopyrimidines,11 which are prominent scaffolds in pharmaceutical and medicinal fields.
C double bond,5 and with/without cleavage of the C–N bond.6 This enables them to be utilized as precursors for bicyclic and aza-heterocyclic compounds, such as indoles,7 quinolines,8 pyrazolopyridines,9 imidazopyridines,10 and pyrazolopyrimidines,11 which are prominent scaffolds in pharmaceutical and medicinal fields.
On the other hand, imidazopyridines are fused aza-bicyclic heterocyclic compounds with exceptional pharmacological properties;10,12 for example, saripidem,13a alpidem,13b zolimidine,13c olprinone,13d zolpidem,13b necopidem,13e minodronic acid and TP-003 are clinically proved imidazo[1,2-a]pyridine drugs (Fig. 1b).13 Owing to the importance of these scaffolds, a good number of methods have been well-documented for these compounds using some classical reactions,14 such as oxidative coupling, amino oxygenation, condensation and hydroamination.15 However, a few reports demonstrated the synthesis of 3-aroylimidazo[1,2-a]pyridine substructures, which have shown great impact as drug candidates, such as calcium channel blockers16a and anticancer agents.16b
Jiang et al. reported the synthesis of imidazo[1,2-a]pyridines by the intermolecular oxidative conversion of pyridine17a or 2-aminopyridine17b in the presence of copper. Furthermore, 3-benzoyl-imidazo[1,2-a]pyridines were directly synthesized by the aminooxygenation of 2-aminopyridines with 3-phenylpropiolaldehyde promoted by a transition metal [Cu, Au/Ag, Fe]18a–c or NIS18d (Scheme 1A).18
He et al.19a and J.-P. Wan and co-workers19b delivered 3-aroylimidazo[1,2-a]pyridines by the intramolecular oxidative α-amination of carbonyl compounds employing I2/H2O2 or CuI (Scheme 1B).19 In 2012, Schmitt et al. reported the highly functionalized imidazo[1,2-a]pyridines by activating secondary amides obtained from N-acylamidine and phenacyl bromide.20a In 2013, Adimurthy's group reported the intramolecular silver-catalyzed aminooxygenation of N-(3-phenylprop-2-yn-1-yl)pyridin-2-amine in the presence of TMEDA.20b In 2019, Chen21a and Li21b demonstrated the two-step synthesis of 3-benzoyl-imidazo[1,2-a]pyridines from 2-aminopyridine and propiophenone (Scheme 1C).21 Furthermore, Y. Ma reported the I2-catalyzed multi-component sequential synthesis of 3-aroylimidazo[1,2-a]pyridines using an excess of oxidants/additives [K2S2O8/NaOAc] at 110 °C for longer reaction times (Scheme 1D).22 All these reports have a few limitations, such as the usage of expensive metal catalysts, excess oxidants/additives, longer reaction time and tedious workup procedures. Therefore, developing a simple methodology for 3-aroyl-imidazo[1,2-a]pyridines with readily available starting materials without expensive catalysts in high yields is enticing.
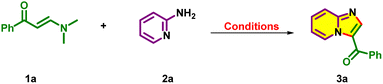
Herein, we wish to report a simple methodology for the synthesis of 3-oyl-imidazo[1,2-a]pyridines, which emerged from our renewed research activity on exploring the enaminones23 and synthesis of aza-heterocyclics.24 This time, β-enaminones and 2-aminopyridines were subjected to mechanochemistry, as it is an attractive protocol, allowing innovation for solution-state organic synthesis,25 unlocking the reactivity of insoluble substrates, being insensitive to oxygen and significantly mitigating the harsh conditions, waste generation and reaction time. Furthermore, grinding/ball milling/mechanochemistry offers a new opportunity for various organic transformations, such as oxidative reactions,26a coupling reactions,26b condensation reactions,26c SNAr reactions,26d and multicomponent reactions,26e and to the best of our knowledge, the grindstone chemistry of β-enaminones has been sparsely explored. Thus, we have achieved the synthesis of 3-oyl-imidazo[1,2-a]pyridines by grinding β-enaminones, 2-aminopyridines and molecular iodine (Scheme 2).
Results and discussion
Being ambiphilic synthons, β-enaminones can undergo a Michael type addition/elimination followed by cyclization with another suitable partner in the presence of acid catalysts. In this instance, we chose the readily synthesizable β-enaminone23 and the commercially available 2-aminopyridine as the model substrates to investigate the grinding reaction (Table 1). Initially, solvent-free grinding of β-enaminone 1a (1.0 equiv.), 2-aminopyridine 2a (1.05 equiv.) and N-chloro/bromosuccinimide (1.0 equiv.) in the presence of p-toluenesulfonic acid (p-TSA, 0.5 equiv.) at room temperature for 20 minutes (Table 1, entries 1 and 2) yielded the cyclized product 3a as the minor product and aza-Michael addition product 4a as the major product (Scheme 3). The structures of 3a22 and 4a23c were elucidated using the spectral data and were comparable with similar reported data.| Entry | Reagent (equiv.) | Additive (equiv.) | Time (min.) | Yield (%)b of 3a |
|---|---|---|---|---|
| a Reaction conditions: 1a (1.0 equiv.), 2a (1.05 equiv.), grinding at room temperature (25 °C). b Yields of isolated product 3a; equiv.: equivalent; time in minutes. | ||||
| 1 | NCS (1.0) | p-TSA (0.5) | 20 | 28 |
| 2 | NBS (1.0) | p-TSA (0.5) | 20 | 25 |
| 3 | NCS (1.0) | p-TSA (1.0) | 30 | 37 |
| 4 | NCS (1.0) | p-TSA (2.0) | 30 | 40 |
| 5 | NCS (1.0) | AlCl3 (1.0) | 25 | 23 |
| 6 | NCS (1.0) | AcOH (2.0) | 30 | 40 |
| 7 | NCS (1.0) | HCl (2.0) | 30 | 20 |
| 8 | NCS (1.0) | NH4OAc (2.0) | 20 | 60 |
| 9 | IBDA (1.0) | NH4OAc (2.0) | 20 | 52 |
| 10 | PhIO (1.0) | NH4OAc (2.0) | 20 | 34 |
| 11 | NIS (1.0) | NH4OAc (2.0) | 20 | 65 |
| 12 | I2 (1.0) | NH4OAc (2.0) | 20 | 78 |
| 13 | I2 (1.5) | NH4OAc (2.0) | 20 | 83 |
| 14 | I2 (1.0) | NH4OAc (1.0) | 20 | 83 |
| 15 | I2 (1.0) | NH4OAc (0.5) | 20 | 87 |
| 16 | I2 (1.0) | — | 20 | 92 |
| 17 | I2 (0.5) | — | 30 | 58 |
| 18 | — | — | 30 | - |
To enrich the formation of 3a, grinding was performed by employing various reagents (1.0 equiv.) with varying mole ratios of additives, and the results are tabulated in Table 1 (entries 3–12). In particular, encouraging results were obtained with the combination of I2 and NH4OAc (Table 1, entry 12). Additionally, the dosage of I2 and NH4OAc was further investigated (Table 1, entries 13–15) and the yield of 3a went up to 87%. Then, the same reaction was also conducted by employing only iodine. Interestingly, this reaction also gave the desired product 3a in an excellent yield of 92% (Table 1, entry 16). Furthermore, this reaction was much cleaner than other reaction conditions and did not require any column purification except anti-solvent precipitation with a suitable solvent (DCM and pentane/hexane) after quenching iodine. Having obtained the desired product 3a in 92% yield, the dosage of iodine was optimized by conducting a reaction with 0.5 equivalents and without iodine also. However, it was observed that the yield of 3a significantly dropped with half equivalents (Table 1, entry 17), and the reaction did not occur in the absence of iodine (Table 1, entry 18).
Next, having established the optimum parameters to obtain 3a in excellent yields, the scope and generality of this reaction were explored by employing a variety of β-enaminones (1a–1r) and 2-aminopyridines (2a–2i)/heteroaryl amines (2j–2k) as shown in Scheme 4. To start with, a series of substituted aryl enaminones (1a–1i) reacted with 2-aminopyridine (2a) affording the desired products 3a–3i in 55–92% yields. Next, fused aryl (1l–1m)/heteroaryl enaminones (1n–1p) were also well-tolerated in the reaction with 2-aminopyridine (2a) and gave the corresponding products 3j–3n in good yields. However, aliphatic substrates of β-enaminones (1q and 1r) resulted in moderate yields of 3o and 3p. Likewise, β-enaminones (1a–1c, 1f, 1j–1n) were reacted with different substituents of 2-aminopyridines (2b–2i). In this case, -alkyl and -alkoxy 2-aminopyridines (2b–2f) delivered the corresponding products 3q–3z in very good yields. However, –Br- and –CF3-substituted 2-aminopyridines (2g–2i) furnished the products 3za, 3zb and 3zc in 67%, 78% and 75% yields, respectively.
Finally, other heteroaryl amines were also tested: 2-aminopyrazine (2j) and 2-aminopyrimidine (2k) smoothly converted into products 3zd and 3ze in good yields. All the products 3a–3ze were obtained without any column purification, except 3o and 3p. It is notable to say that we successfully carried out the previously22 failed reactions under the standard conditions with 2-pyrrolyl (3m), 2-pyridyl (3n), aliphatic (3o and 3p), trifluoro methyl (3zc), and pyrazinyl (3zd) substituents. However, meta/para-OH and meta-CN/para-NO2 substituents of 1 did not give any products.
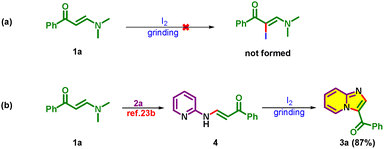
To gain insight into the formation of 3a, control experiments were conducted, as shown in Scheme 5. First, β-enaminone (1a) and I2 were ground, which failed to give any product (Scheme 5a).23c Next, I2 on grinding with readily prepared (E)-1-phenyl-3-(pyridin-2-ylamino)prop-2-en-1-one (4)23b from 1a and 2a resulted in the exclusive formation of 3a (Scheme 5b). With these, it is understood that the reaction proceeds through Michael addition/elimination (C–N formation) between 1a and 2a followed by intramolecular cyclisation in the presence of I2, leading to the desired product 3a.
A plausible mechanism is proposed for 3 based on the control experiments (Scheme 5) and previous reports27 as shown in Scheme 6. Initially, the reaction between β-enaminone 1 and 2-aminopyridine 2 leads to C–N bond formation through a Michael addition followed by elimination of dimethylamine (DMA). Next, iodine is added to the in situ formed Michael adduct 4 resulting in iodonium ion intermediate A, which may rearrange to the desired product 3 on intramolecular endo-cyclization.
This strategy was adopted for a gram-scale synthesis of the anticancer compound (7-methoxyimidazo[1,2-a]pyridin-3-yl)(3,4,5-trimethoxyphenyl)methanone (3v) where compounds 1f and 2f were subjected to grinding in the presence of iodine and yielded 3v in superior yield of 87% (route-A) over reported routes16b,22 (route-B22 and C16b), as shown in Scheme 7a. Furthermore, derivatization of 3a to trifluoro methylated alcohols 5a (76%) and tert-propargylic alcohols 6a (83%) was accomplished with CF3TMS and phenyl acetylene (Scheme 7b).
Conclusions
In conclusion, we developed a simple, metal/additive-free manual grinding synthesis of 3-oyl-imidazo[1,2-a]pyridines from easily accessible β-enaminones, 2-aminopyridines and molecular iodine. This protocol allows the synthesis of a variety of imidazo [1,2-a]pyridines/pyrazines/pyrimidines without requiring any column purification with high compatibility. Additionally, gram-scale experiments and late-stage functionalization of imidazopyridines have also been well demonstrated. We believe that this grinding/mechanochemical approach strategy of using enaminones will be of interest to construct a variety of heterocycles in future.Conflicts of interest
There are no conflicts to declare.Data availability
The supporting data are enclosed in the supplementary information (SI). Supplementary information: 1H-NMR, 13C and mass spectral data acquired for this work. See DOI: https://doi.org/10.1039/d5nj03149d.Acknowledgements
We are grateful to Anusandhan National Research Foundation (ANRF), New Delhi, for the financial support (grant no. EEQ/2021/000039) and also thank Director CSIR-IICT for the support (IICT/Pubs./2025/209). We gratefully acknowledge the help received from the Centre for NMR and Structural Chemistry, Analytical Chemistry and Mass Spectrometry, CSIR-IICT.References
- (a) J. V. Greenhill, Chem. Soc. Rev., 1977, 6, 277 RSC; (b) P. Lue and J. V. Greenhill, Adv. Heterocycl. Chem., 1997, 67, 207 CrossRef.
- (a) Z. Wang, B. Zhao, Y. Liu and J.-P. Wan, Adv. Synth. Catal., 2022, 364, 1508 CrossRef CAS; (b) X.-F. Xia and Y.-N. Niu, Org. Biomol. Chem., 2022, 20, 282 RSC; (c) I. J. Maye, R. D. Haywood, E. M. Mandzo, J. J. Wirick and P. L. Jackson-Ayotunde, Tetrahedron, 2021, 83, 131984 CrossRef.
- (a) J. Zeng, J.-P. Wan and Y. J. Liu, Org. Chem., 2022, 87, 13195 CrossRef CAS PubMed; (b) Q. Yu, Y. Liu and J.-P. Wan, Chin. Chem. Lett., 2021, 32, 3514 CrossRef CAS; (c) X. Duan, H. Li, W. Li, J. Wang and N. Liu, ChemistrySelect, 2021, 6, 6478 CrossRef CAS; (d) Y. Yuan, W. Hou, D. Zhang-Negrerie, K. Zhao and Y. Du, Org. Lett., 2014, 16, 5410 CrossRef CAS PubMed; (e) D. Li, S. Li, C. Peng, L. Lu, S. Wang, P. Wang, Y.-H. Chen, H. Cong and A. Lei, Chem. Sci., 2019, 10, 2791 RSC.
- (a) Z. Xu, L. Fu, X. Fang, B. Huang, L. Zhou and J.-P. Wan, Org. Lett., 2021, 23, 5049 CrossRef CAS PubMed; (b) X. Li, Z. Chen, Y. Liu, N. Luo, W. Chen, C. Liu, F. Yu and J. Huang, J. Org. Chem., 2022, 87, 10349 CrossRef PubMed; (c) E.-Z. Yao, G.-L. Chai, P. Zhang, B. Zhu and J. Chang, Org. Chem. Front., 2022, 9, 2375 RSC.
- (a) J. Chen, P. Guo, J. Zhang, J. Rong, W. Sun, Y. Jiang and T.-P. Loh, Angew. Chem., Int. Ed., 2019, 58, 12674 CrossRef CAS PubMed; (b) P. Zhou, B. Hu, L. Li, K. Rao, J. Yang and F. Yu, J. Org. Chem., 2017, 82, 13268 CrossRef CAS PubMed.
- (a) T. Liu, L. Wei, B. Zhao, Y. Liu and J.-P. Wan, J. Org. Chem., 2021, 86, 9861 CrossRef CAS PubMed; (b) H. Wu, T. Luo, J.-P. Wan, J. Jiang and Y. Liu, Eur. J. Org. Chem., 2022, e202200552 CrossRef CAS.
- (a) L. Zhao, C. Qiu, L. Zhao, G. Yin, F. Li, C. Wang and Z. Li, Org. Biomol. Chem., 2021, 19, 5377 RSC; (b) S. Würtz, S. Rakshit, J. J. Neumann, T. Dröge and F. Glorius, Angew. Chem., Int. Ed., 2008, 47, 7230 CrossRef PubMed; (c) X. Li, Y. Du, Z. Liang, X. Li, Y. Pan and K. Zhao, Org. Lett., 2009, 11, 2643 CrossRef CAS PubMed; (d) B. V. S. Reddy, M. R. Reddy, Y. G. Rao, J. S. Yadav and B. Sridhar, Org. Lett., 2013, 15, 464 CrossRef CAS PubMed.
- (a) Z.-Y. Gu, T.-H. Zhu, J.-J. Cao, X.-P. Xu, S.-Y. Wang and S.-J. Ji, ACS Catal., 2014, 4, 49 CrossRef CAS; (b) X. F. Xia, W. He and D. Wang, Adv. Synth. Catal., 2019, 361, 2959 CrossRef CAS.
- (a) T. Maqbool, A. Nazeer, M. N. Khan, M. C. Elliott, M. A. Khan, M. Ashraf, M. Nasrullah, S. Arshad and M. A. Munawar, Asian J. Chem., 2014, 26, 2870 CrossRef CAS; (b) J. Sindhu, H. Singh, J. M. Khurana, J. K. Bhardwaj, P. Saraf and C. Sharma, Med. Chem. Res., 2016, 25, 1813 CrossRef CAS; (c) M. A. El-Borai, H. F. Rizk, D. M. Beltagy and I. Y. El-Deeb, Eur. J. Med. Chem., 2013, 66, 415 CrossRef CAS PubMed; (d) H. S. P. Rao, L. N. Adigopula and K. Ramadas, ACS Comb. Sci., 2017, 19, 279 CrossRef CAS PubMed.
- (a) N. M. Shukla, D. B. Salunke, E. Yoo, C. A. Mutz, R. Balakrishna and S. A. David, Bioorg. Med. Chem., 2012, 20, 5850 CrossRef CAS PubMed; (b) T. H. Al-Tel, R. A. Al- Qawasmeh and R. Zaarour, Eur. J. Med. Chem., 2011, 46, 1874 CrossRef CAS PubMed.
- (a) A. Damont, V. Médran-Navarrete, F. Cacheux, B. Kuhnast, G. Pottier, N. Bernards, F. Marguet, F. Puech, R. Boisgard and F. Dollé, J. Med. Chem., 2015, 58, 7449 CrossRef CAS PubMed; (b) S. Cherukupalli, R. Karpoormath, B. Chandrasekaran, G. A. Hampannavar, N. Thapliyal and V. N. Palakollu, Eur. J. Med. Chem., 2017, 126, 298 CrossRef CAS PubMed; (c) D. W. Engers, A. Y. Frist, C. W. Lindsley, C. C. Hong and C. R. Hopkins, Bioorg. Med. Chem. Lett., 2013, 23, 3248 CrossRef CAS PubMed.
- (a) J.-B. Xi, Y.-F. Fang, B. Frett, M.-L. Zhu, T. Zhu, Y.-N. Kong, F.-J. Guan, Y. Zhao, X.-W. Zhang, H.-Y. Li, M.-L. Ma and W. Hu, Eur. J. Med. Chem., 2017, 126, 1083 CrossRef CAS PubMed; (b) O. Kim, Y. Jeong, H. Lee, S.-S. Hong and S. Hong, J. Med. Chem., 2011, 54, 2455 CrossRef CAS PubMed; (c) S. Lei, Y. Mai, C. Yan, J. Mao and H. Cao, Org. Lett., 2016, 18, 3582 CrossRef CAS PubMed; (d) I. D. Luke, M. A. Carroll, T. J. Gregson, G. J. Ellames, R. W. Harrington and W. Clegg, Org. Biomol. Chem., 2013, 11, 5877 RSC; (e) M. A. Ismail, R. K. Arafa, T. Wenzler, R. Brun, F. A. Tanious, W. D. Wilson and D. W. Boykin, Bioorg. Med. Chem. Lett., 2008, 16, 683 CrossRef CAS; (f) M. A. Ismail, R. Brun, T. Wenzler, F. A. Tanious, W. D. Wilson and D. W. Boykin, J. Med. Chem., 2004, 47, 3658 CrossRef CAS PubMed.
- (a) A. Maleki, S. Javanshirand and M. Naimabadi, RSC Adv., 2014, 4, 30229 RSC; (b) S. Z. Langer, S. Arbilla, J. Benavides and B. Scatton, Adv. Biochem. Psychopharmacol., 1990, 46, 61–72 CAS; (c) L. Almirante, L. Polo, A. Mugnaini, E. Provinciali, P. Rugarli, A. Biancotti, A. Gamba and W. Murmann, J. Med. Chem., 1965, 8, 305 CrossRef CAS PubMed; (d) K. Mizushige, T. Ueda, K. Yukiiri and H. Suzuki, Cardiovasc. Drug Rev., 2002, 20, 163 CrossRef CAS PubMed; (e) N. C. Anilkumar, M. S. Sundaram, C. D. Mohan, S. Rangappa, K. C. Bulusu, J. E. Fuchs, K. S. Girish, A. Bender, B. Basappa and K. S. Rangappa, PLoS One, 2015, 10, e0131896 CrossRef PubMed.
- (a) K. Zhang, A. El Bouakher, H. Levaique, J. Bignon, P. Retailleau, M. Alami and A. Hamze, Adv. Synth. Catal., 2020, 362, 3243 CrossRef CAS; (b) B. Li, N. Shen, Y. Yang, X. Zhang and X. Fan, Org. Chem. Front., 2020, 7, 919 RSC; (c) S. Wang, S. Zhang, M. Liu, J. Zang, G. Jiang and F. Ji, Org. Chem. Front., 2020, 7, 697 RSC; (d) J.-J. Ji, Z.-Q. Zhu, L.-J. Xiao, D. Guo, X. Zhu, J. Tang, J. Wu, Z.-B. Xie and Z.-G. Le, Org. Chem. Front., 2019, 6, 3693 RSC; (e) Z. Chen, P. Liang, F. Xu, R. Qiu, Q. Tan, L. Long and M. Ye, J. Org. Chem., 2019, 84, 9369 CrossRef CAS PubMed; (f) Y. Ren, B. Xu, Z. Zhong, Jr., C. U. Pittman and A. Zhou, Org. Chem. Front., 2019, 6, 2023 RSC; (g) M.-L. Feng, S.-Q. Li, H.-Z. He, L.-Y. Xi, S.-Y. Chen and X.-Q. Yu, Green Chem., 2019, 21, 1619 RSC.
- A. K. Bagdi, S. Santra, K. Monir and A. Hajra, Chem. Commun., 2015, 51, 1555 RSC.
- (a) P. J. Sanfilippo, M. Urbanski, J. B. Press, B. Dubinsky and J. B. Moore Jr., J. Med. Chem., 1988, 31, 2221 CrossRef CAS; (b) Y.-S. Tung, M. S. Coumar, Y.-S. Wu, H.-Y. Shiao, J.-Y. Chang, J.-P. Liou, P. Shukla, C.-W. Chang, C.-Y. Chang, C.-C. Kuo, T.-K. Yeh, C.-Y. Lin, J.-S. Wu, S.-Y. Wu, C.-C. Liao and H.-P. Hsieh, J. Med. Chem., 2011, 54, 3076 CrossRef CAS.
- (a) H. W. Huang, X. C. Ji, X. D. Tang, M. Zhang, X. W. Li and H. F. Jiang, Org. Lett., 2013, 15, 6254 CrossRef CAS; (b) Y. Gao, M. Z. Yin, W. Q. Wu, H. W. Huang and H. F. Jiang, Adv. Synth. Catal., 2013, 355, 2263 CrossRef CAS.
- (a) H. Cao, X. Liu, J. Liao, J. Huang, H. Qiu, Q. Chen and Y. Chen, J. Org. Chem., 2014, 79, 11209 CrossRef CAS; (b) H. Zhan, L. Zhao, J. Liao, N. Li, Q. Chen, S. Qiu and H. Cao, Adv. Synth. Catal., 2015, 357, 46 CrossRef CAS; (c) Z. Chen, B. Liu, P. Liang, Z. Yang and M. Ye, Tetrahedron Lett., 2018, 59, 667 CrossRef CAS; (d) K. R. Reddy, A. P. Gupta and P. Das, Asian J. Org. Chem., 2016, 5, 900 CrossRef CAS.
- (a) L. Huang, W. Yin, J. Wang, C. Gan, Y. Huang, C. Huang and Y. He, RSC Adv., 2019, 9, 2381 RSC; (b) J.-P. Wan, D. Hu, Y. Liu, L. Li and C. Wen, Tetrahedron Lett., 2016, 57, 2880 CrossRef CAS.
- (a) A. Basilio-Lopes, T. M. de Aquino, A. Mongeot, J.-J. Bourguignon and M. Schmitt, Tetrahedron Lett., 2012, 53, 2583 CrossRef CAS; (b) D. Chandra Mohan, S. Nageswara Rao and S. Adimurthy, J. Org. Chem., 2013, 78, 1266 CrossRef CAS.
- (a) W. Wu, Z. Wang, Q. Shen, Q. Liu and H. Chen, Org. Biomol. Chem., 2019, 17, 6753 RSC; (b) T. Wang, G. Chen, Y.-J. Lu, Q. Chen, Y. Huo and X. Li, Adv. Synth. Catal., 2019, 361, 3886 CrossRef CAS.
- Y. Zhang, R. Chen, Z. Wang, L. Wang and Y. Ma, J. Org. Chem., 2021, 86, 6239 CrossRef CAS PubMed.
- (a) B. Bhanu Prasad, M. Nagaraju, N. Jagadeesh Babu and A. Krishnaiah, Org. Biomol. Chem., 2023, 21, 4434 RSC; (b) C. Saikrishna, M. Nagaraju, K. Maneesha and A. Krishnaiah, ChemistrySelect, 2024, 9, e202401554 CrossRef; (c) K. Ashok, M. Nagaraju, N. Jagadeesh Babu and A. Krishnaiah, Tetrahedron Lett., 2018, 59, 4168 CrossRef; (d) N. Medishetti, A. Kale, J. B. Nanubolu and K. Atmakur, Synth. Commun., 2020, 50, 3642–3651 CrossRef CAS; (e) B. Chiranjeevi, K. Ashok, N. Jagadeesh Babu and A. Krishnaiah, RSC Adv., 2015, 5, 106860 RSC; (f) B. Chiranjeevi, K. Ashok, N. Jagadeesh Babu and A. Krishnaiah, Synth. Commun., 2017, 47, 1332 CrossRef.
- (a) M. Nagaraju, B. Bhanu Prasad and A. Krishnaiah, Org. Biomol. Chem., 2024, 22, 7854 RSC; (b) M. Nagaraju, B. Bhanu Prasad, N. Jagadeesh Babu and A. Krishnaiah, ChemistrySelect, 2023, 8, e202204964 CrossRef; (c) I. Chaitanya, Ch Saikrishna, M. Nagaraju, N. Jagadeesh Babu and A. Krishnaiah, Eur. J. Org. Chem., 2023, e202300357 Search PubMed; (d) M. Nagaraju, I. Chaitanya, N. Jagadeesh Babu and A. Krishnaiah, ChemistrySelect, 2022, 7, e202200063 CrossRef; (e) K. Ashok, M. Nagaraju, K. Sirisha, C. Ganesh Kumar and A. Krishnaiah, Org. Biomol. Chem., 2019, 17, 3186 RSC; (f) K. Ashok, B. Chiranjeevi, R. Nagarjuna Chary, S. Prabhakar, T. Prabhakar Rao and A. Krishnaiah, Synthesis, 2016, 1603 Search PubMed; (g) C. Madhu, E. Narender Reddy, B. Chiranjeevi and A. Krishnaiah, RSC Adv., 2015, 5, 19418 RSC.
- (a) S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413 RSC; (b) G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668 RSC; (c) A. L. Garay, A. Pichon and S. L. James, Chem. Soc. Rev., 2007, 36, 846 RSC; (d) R. Kumar, A. Sharma and A. Sharma, ACS Sustainable Chem. Eng., 2024, 12, 12808 CrossRef CAS.
- (a) G. Cravotto, D. Garella, D. Carnaroglio, E. C. Gaudino and O. Rosati, Chem. Commun., 2012, 48, 11632 RSC; (b) Y. Hou, H. Wang, J. Xi, R. Jiang, L. Zhang, X. Li, F. Sun, Q. Liu, Z. Zhao and H. Liu, Green Chem., 2023, 25, 2279 RSC; (c) J. M. dos Santos Filho and S. M. Pinheiro, Green Chem., 2017, 19, 2212 RSC; (d) D. Langerreiter, N. L. Attallah, I. Schlapp-Hackl, M. A. Kostiainen and S. Kaabel, Green Chem., 2024, 26, 9823 RSC; (e) M. Leonardi, M. Villacampa and J. C. Menéndez, Chem. Sci., 2018, 9, 2042 RSC.
- (a) D. Kour, A. Gupta, K. K. Kapoor, V. K. Gupta, Rajnikant, D. Singh and P. Das, Org. Biomol. Chem., 2018, 16, 1330 RSC; (b) O. T. K. Nguyen, P. T. Ha, H. V. Dang, Y. H. Vo, T. T. Nguyen, N. T. H. Le and N. T. S. Phan, RSC Adv., 2019, 9, 5501 RSC; (c) M.-M. Xing, M. Xin, C. Shen, J.-R. Gao, J.-H. Jia and Y.-J. Li, Tetrahedron, 2016, 72, 4201 CrossRef CAS; (d) Y. Li, H. Xu, M. Xing, F. Huang, J. Jia and J. Gao, Org. Lett., 2015, 17, 3690 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2026 |






![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reaction conditions: 1a–1r (1.0 equiv.), 2a–2k (1.05 equiv.), and I2 (1.0 equiv.) grinding at room temperature for 15–20 min;
Reaction conditions: 1a–1r (1.0 equiv.), 2a–2k (1.05 equiv.), and I2 (1.0 equiv.) grinding at room temperature for 15–20 min;