Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Engineering high-capacity hydrogen storage in pristine Ca12O12 nanocages via cooperative adsorption
Saeedeh
Kamalinahad†‡
 a,
Aritra
Roy‡
a,
Aritra
Roy‡
 b,
Pablo
Gamallo
b,
Pablo
Gamallo
 *a and
Felipe
Fantuzzi
*a and
Felipe
Fantuzzi
 *c
*c
aDepartament de Ciència de Materials i Química Física & Institut de Química Teòrica i Computacional (IQTCUB), Universitat de Barcelona, C/ Martí i Franquès 1, Barcelona 08028, Spain. E-mail: gamallo@ub.edu
bDepartment of Chemical and Energy Engineering, London South Bank University, 103 Borough Rd, London SE1 0AA, UK
cChemistry and Forensic Science, School of Natural Sciences, University of Kent, Park Wood Rd, Canterbury CT2 7NH, UK. E-mail: f.fantuzzi@kent.ac.uk
First published on 28th November 2025
Abstract
A comprehensive in silico investigation of pristine Ca12O12 nanocages as potential hydrogen storage materials is presented. Our study focuses on assessing the structural, electronic, and adsorption properties of Ca12O12 relative to its Be12O12 and Mg12O12 analogs. The larger internal cavity, resulting from increased interatomic distances in Ca12O12, not only supports stable endohedral adsorption—a capability not observed in Be12O12 or Mg12O12—but also facilitates a greater number of exohedral H2 adsorption events compared to the more compact nanocages. Notably, our results reveal a clear preference for end-on coordination in Ca12O12, in contrast to the side-on adsorption observed for the smaller cages. Although the overall adsorption process remains exothermic across a broad range of hydrogen loadings, the most favorable cooperative effects occur at moderate coverages, with the lowest normalized adsorption enthalpy observed at 13H2 molecules and favorable interactions maintained up to 32. Additionally, mixed adsorption configurations incorporating one endohedral H2 alongside multiple exohedral H2 molecules yield a maximum hydrogen uptake of 9.24 wt%, exceeding the U.S. DOE target of 5.5 wt%. These findings highlight the potential of pristine Ca12O12 nanocages as effective hydrogen storage media and offer strategic guidance for the development of high-performance storage systems.
1 Introduction
The rapid and continuous growth of the global population, coupled with ongoing deforestation and heavy reliance on fossil fuels, has intensified environmental concerns and raised fears of future energy shortages. To mitigate these challenges, considerable efforts from diverse research groups worldwide have been directed toward developing alternative energy sources that are both sustainable and eco-friendly. Among these, hydrogen gas has emerged as a promising, safe, and versatile energy carrier that can be efficiently converted into usable power without generating harmful emissions. Its combustion yields only water as a byproduct, positioning it as an environmentally sustainable alternative for addressing global energy demands.1–5Despite its promise, the primary obstacle in hydrogen technology remains the development of compact, safe, and cost-effective storage systems. An ideal hydrogen storage material should achieve high gravimetric and volumetric densities under ambient conditions.6 Solid-state hydrogen storage has emerged as a compelling strategy in this regard, as it enables storage in molecular or atomic forms while ensuring fast kinetics and enhanced safety.7–9 However, weak physisorption interactions typically lead to desorption at low temperatures, and chemisorption can sometimes be too strong or irreversible, highlighting the need for adsorption energies in the intermediate range of 0.01–0.5 eV per hydrogen atom.10 Progress has been made through research on various nanomaterials,11–17 but from a practical standpoint, no single medium has yet met the U.S. Department of Energy (DOE)'s 2025 target of 5.5 wt%.18
The discovery of buckminsterfullerene C60 in 198519 catalyzed an intensive exploration of fullerene-like architectures, encompassing both homoatomic (An) and heteroatomic, binary (AB)n nanocages. These hollow clusters offer unique potential as molecular containers, a feature extensively studied for fullerenes.20–27 Beyond carbon-based systems, homoatomic nanocages such as those formed by gold (Aun) and boron (Bn) have sparked significant interest, particularly for their encapsulation capabilities.28–31 For instance, Aun nanocages have demonstrated applications in catalysis and photochemistry,30 as well as in biological fields including sensing, imaging, diagnosis, and treatment.32–34 Similarly, Bn nanocages are attractive for biomedical imaging,35 anti-cancer drug delivery,36 and sensing applications.37 Notable milestones include the first all-boron fullerene (B40−) reported by Wang and co-workers38 and the recent discovery of the fullerene-like 80-atom boron cluster (B80−), whose electronic structure and bonding closely resemble C60.39
While homoatomic nanocages have been extensively studied, heteroatomic nanocages, particularly those composed of main group elements, have emerged as a focal point for research due to their chemical diversity and potential applications. Examples include systems such as CSi,40,41 BN,40,42–47 BP,46–48 AlN,40,46,47,49,50 AlP,40,46,47 BeO,10,40,51–54 MgO,40,50,51,55 CaO,50,54,56–58 ZnO,56,59 and LiF.60,61 Particular attention has been devoted to (AB)12 nanocages, as sodalite-type structures featuring this stoichiometry are recognized for their exceptional stability,42 even though they are not always the global minimum.62,63 This inherent stability has spurred extensive analysis of (AB)12 nanocages as potential materials for diverse applications, including hydrogen storage.
Among the sodalite-type nanocages, a notable example is Ca12O12, which has a radius (3.55 Å) comparable to that of C60.56 This structural similarity enables Ca12O12 to encapsulate small molecules and toxic gases, such as phosgene (Cl2CO), H2S, CO2, SO2, and NO2,50,57,64 highlighting its potential as a nanosensor or molecular trap. Furthermore, its applicability as a nanocarrier for drug delivery has been demonstrated with compounds like 5-fluorouracil and thymine derivatives,54,65 and adsorption studies have further emphasized its capability for storing molecules such as sulforaphane.66 In the context of hydrogen storage, Louis and co-workers computationally investigated pristine and transition-metal-decorated Ca12O12 (M−Ca12O12), reporting the favorable adsorption of up to ten H2 molecules.58 Their analysis, however, was limited to low-coverage adsorption and did not explore cooperative effects, multi-molecule occupation of individual sites, or the interplay between exohedral and endohedral adsorption modes—factors that can critically influence hydrogen uptake.
Herein, we extend that investigation by examining pristine Ca12O12 for both endohedral and exohedral hydrogen adsorption, considering a broader range of adsorption scenarios. In addition, we present a comparative analysis with Be12O12 and Mg12O12 to assess how cage size and metal character affect hydrogen accommodation. This study employs dispersion-corrected and range-separated density functional theory (DFT), together with complementary wave-function-based benchmarks, to evaluate the structural, energetic, and electronic features that govern hydrogen adsorption on these nanocages. Our findings refine the understanding of Ca12O12 as a hydrogen host and contribute to the broader search for high-performance materials for reversible hydrogen storage.
2 Computational details
Full geometry optimizations were carried out using dispersion-corrected DFT with the Gaussian 16, Revision C.01 software package.67 The B3LYP hybrid functional,68–71 combined with Grimme's zero-damping D3 dispersion correction72 (with the default Axilrod–Teller–Muto three-body dispersion term) and the 6-31G(d,p) basis set, was employed. This combination was chosen based on preliminary benchmark results, which demonstrated comparable structural accuracy to the range-separated ωB97X-D functional73 but with significantly lower computational costs. To obtain more reliable electronic energies, we performed single-point calculations on the optimized geometries using a range of DFT functionals, including B3LYP-D3, BP86-D3,74,75 M06-D3,76 PBE0-D3,77,78 and ωB97X-D, all with the 6-311++G(d,p) basis set. These values were benchmarked against high-level DLPNO-CCSD(T)/6-311++G(d,p) calculations79 performed with Orca 5.0.3,80 employing the NormalPNO threshold settings. Among the functionals tested, ωB97X-D provided the closest agreement with the DLPNO-CCSD(T) reference. Indeed, previous studies on hydrogen storage materials have also highlighted the superior performance of ωB97X-D for accurately capturing long-range noncovalent interactions.81,82 Based on these findings, we have adopted the ωB97X-D/6-311++G(d,p)//B3LYP-D3/6-31G(d,p) level of theory for all subsequent calculations in this work. More details about the benchmark calculations done can be found in Section S1 of the SI.Harmonic vibrational frequency calculations were conducted on all optimized geometries to confirm their characterization as local minima on the potential energy surface and to compute thermochemical corrections. Key features of the electronic structure were elucidated through the analysis of frontier molecular orbitals (FMOs). Additionally, adsorption energy (ΔEads), adsorption enthalpy (ΔHads), and HOMO–LUMO energy gap (Eg) calculations were performed to evaluate the thermodynamic and electronic properties associated with hydrogen adsorption.
The adsorption energy (ΔEads) for the adsorption of n hydrogen molecules on the Ca12O12 nanocage was calculated as:
| ΔEads[n] = E[Ca12O12·nH2] − {E[Ca12O12] + n × E[H2]} | (1) |
The adsorption enthalpy (ΔHads) was calculated using:
| ΔHads[n] = H[Ca12O12·nH2] − {H[Ca12O12] + n × H[H2]} | (2) |
To evaluate the enthalpic cooperativity in the adsorption of multiple hydrogen molecules, we define a normalized adsorption enthalpy:
| ΔHnormads[n] = ΔHads[n] − nΔHads[1] | (3) |
The energy gap (Eg) was calculated as the energy difference between the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO), determined at the ωB97X-D/6-311++G(d,p)//B3LYP-D3/6-31G(d,p) level of theory:
 | (4) |
The weight percentage (wt%) of hydrogen adsorption was calculated using the following expression:
 | (5) |
Building upon the adsorption energy, enthalpy, and electronic gap evaluations detailed above, it is essential to acknowledge that the focus on enthalpic and electronic properties represents only one facet of the overall thermodynamic behavior of hydrogen adsorption. Recent findings by Wang and Chen83 highlight that low-frequency vibrational modes can yield significant entropy contributions during hydrogen adsorption, potentially compensating for relatively modest enthalpy values. While these observations emphasize the importance of anharmonic vibrational effects for accurate free-energy estimates, many computational studies (including the present one) primarily concentrate on adsorption enthalpies. This focus is largely due to the increased complexity and computational cost associated with capturing entropic contributions beyond the harmonic approximation. Moreover, when screening a wide array of potential materials, harmonic-based enthalpy calculations are often sufficient to identify promising candidates. Therefore, although a complete thermodynamic analysis (i.e., obtaining ΔGads) would involve evaluating both ΔHads and ΔSads at an anharmonic level, our study is limited to the enthalpy term in line with standard practices for initial screening.
3 Results and discussion
3.1 Structural, electronic, and adsorptive properties of pristine M12O12 (M = Be, Mg, Ca) nanocages
Fig. 1a displays the optimized structures of the M12O12 nanocages (Be12O12, Mg12O12, and Ca12O12). Although these clusters have been examined previously (vide supra), a systematic comparison at a uniform computational level enables a rigorous evaluation of their relative stabilities, electronic properties, and potential as hydrogen-storage hosts. This subsection outlines the key structural and electronic characteristics of the nanocages, with a focus on the attributes that render Ca12O12 especially suitable for H2 adsorption. Comprehensive electronic structure and thermochemical data are summarized in Table 1.| Structure | n | ΔEads (ΔHads) | ΔHnormads | wt% |
|---|---|---|---|---|
| Be12O12 | 0 | — | — | 0.00 |
| Mg12O12 | 0 | — | — | 0.00 |
| Ca12O12 | 0 | — | — | 0.00 |
| Be12O12·H2 | 1 | −4.4 (−5.3) | — | 0.67 |
| Mg12O12·H2 (M) | 1 | −6.8 (−7.3) | — | 0.42 |
| Mg12O12·H2 (O) | 1 | −1.5 (−3.6) | — | 0.42 |
| Ca12O12·H2 (M) | 1 | −0.9 (−0.5) | — | 0.30 |
| Ca12O12·H2 (O) | 1 | −1.8 (−4.3) | 0.0 | 0.30 |
| (H2@Ca12O12) | 1 | −9.0 (−9.2) | −4.8 | 0.30 |
| Ca12O12·2H2 | 2 | −4.1 (−9.9) | −1.3 | 0.60 |
| (2H2@Ca12O12) | 2 | 57.3 (49.2) | 57.8 | 0.60 |
| Ca12O12·3H2 | 3 | −6.5 (−14.9) | −1.9 | 0.89 |
| Ca12O12·4H2 | 4 | −9.0 (−20.5) | −3.2 | 1.18 |
| Ca12O12·5H2 | 5 | −10.9 (−25.3) | −3.7 | 1.48 |
| Ca12O12·6H2 | 6 | −13.9 (−31.2) | −5.3 | 1.77 |
| Ca12O12·7H2 | 7 | −16.2 (−36.5) | −6.2 | 2.05 |
| Ca12O12·8H2 | 8 | −19.5 (−42.7) | −8.2 | 2.34 |
| Ca12O12·9H2 | 9 | −21.8 (−47.8) | −8.9 | 2.63 |
| Ca12O12·10H2 | 10 | −25.5 (−54.8) | −11.6 | 2.91 |
| Ca12O12·11H2 | 11 | −26.9 (−59.3) | −11.7 | 3.19 |
| Ca12O12·12H2 | 12 | −29.2 (−64.9) | −13.0 | 3.47 |
| (H2@Ca12O12)·12H2 | 13 | −40.0 (−75.8) | −19.6 | 3.75 |
| Ca12O12·13H2 | 13 | −30.9 (−69.3) | −13.1 | 3.75 |
| (H2@Ca12O12)·13H2 | 14 | −40.8 (−83.4) | −22.9 | 4.03 |
| Ca12O12·14H2 | 14 | −32.4 (−73.3) | −12.7 | 4.03 |
| Ca12O12·15H2 | 15 | −33.9 (−77.7) | −12.8 | 4.30 |
| Ca12O12·16H2 | 16 | −33.9 (−80.5) | −11.4 | 4.57 |
| Ca12O12·17H2 | 17 | −34.7 (−84.3) | −10.8 | 4.85 |
| Ca12O12·18H2 | 18 | −35.9 (−87.7) | −9.9 | 5.12 |
| Ca12O12·19H2 | 19 | −36.6 (−91.3) | −9.2 | 5.39 |
| Ca12O12·20H2 | 20 | −35.8 (−94.5) | −8.0 | 5.65 |
| Ca12O12·21H2 | 21 | −36.7 (−98.2) | −7.4 | 5.92 |
| Ca12O12·22H2 | 22 | −37.9 (−102.1) | −7.0 | 6.18 |
| Ca12O12·23H2 | 23 | −37.4 (−104.8) | −5.4 | 6.45 |
| Ca12O12·24H2 | 24 | −41.8 (−110.6) | −6.9 | 6.71 |
| (H2@Ca12O12)·24H2 | 25 | −50.6 (−120.3) | −12.2 | 6.97 |
| Ca12O12·25H2 | 25 | −42.7 (−113.5) | −5.4 | 6.97 |
| Ca12O12·26H2 | 26 | −43.7 (−115.6) | −3.2 | 7.23 |
| Ca12O12·27H2 | 27 | −44.6 (−123.4) | −6.7 | 7.48 |
| Ca12O12·28H2 | 28 | −45.8 (−126.4) | −5.4 | 7.74 |
| Ca12O12·29H2 | 29 | −42.3 (−126.7) | −1.3 | 7.99 |
| Ca12O12·30H2 | 30 | −47.7 (−133.6) | −3.9 | 8.25 |
| Ca12O12·31H2 | 31 | −47.0 (−131.6) | 2.4 | 8.50 |
| Ca12O12·32H2 | 32 | −52.9 (−140.7) | −2.3 | 8.75 |
| (H2@Ca12O12)·32H2 | 33 | −60.7 (−144.0) | −1.4 | 9.00 |
| Ca12O12·33H2 | 33 | −44.7 (−138.8) | 3.8 | 9.00 |
| (H2@Ca12O12)·33H2 | 34 | −53.5 (−148.7) | −1.7 | 9.24 |
| Ca12O12·34H2 | 34 | −51.2 (−140.1) | 6.9 | 9.24 |
| (H2@Ca12O12)·34H2 | 35 | −51.1 (−150.9) | 0.4 | 9.49 |
| Ca12O12·35H2 | 35 | −51.6 (−146.7) | 4.6 | 9.49 |
Each M12O12 nanocage adopts a framework reminiscent of a sodalite unit, featuring eight 6-membered rings and six 4-membered rings arranged in a near-spherical geometry with Th symmetry. Inspection of Fig. 1a reveals two distinct types of M–O bonds: dhh, connecting two hexagonal faces, and dhs, bridging a hexagonal and a square face. The dhh bond length in Be12O12 is approximately 1.521 Å, increasing to 1.891 Å in Mg12O12 and reaching 2.220 Å in Ca12O12. A similar upward trend is observed for the dhs bond, which measures 1.579 Å in Be12O12, 1.946 Å in Mg12O12, and 2.264 Å in Ca12O12.
These increases in bond lengths reflect the larger ionic radii of the heavier Group 2 metals relative to beryllium, consistent with periodic trends. The corresponding expansion of the cage volume indicates that the internal cavity of Ca12O12 is substantially more spacious, a feature that not only benefits endohedral adsorption but also enhances the adsorption characteristics of exohedral H2 molecules. In the larger Ca12O12 framework, the cluster atoms are positioned further apart, which leads to greater separation between adjacent exohedral adsorption sites and, consequently, reduced repulsive interactions among adsorbed H2 molecules. In addition, the difference between dhh and dhs highlights variations in the local bonding environments, which may influence site-specific adsorption geometries. Overall, the structural analysis confirms that Be12O12 possesses the most compact framework, while Ca12O12 exhibits the most open structure.
Fig. 1b presents the frontier molecular orbitals (FMOs) of the pristine nanocages, and Table 1 provides the numerical values of the HOMO–LUMO gaps (Eg). In particular, Be12O12 exhibits the largest gap (11.32 eV), indicative of a highly insulating character. In contrast, Mg12O12 has a gap of 8.71 eV, and Ca12O12 the smallest at 7.48 eV. A narrower gap is generally associated with increased polarizability and, in some instances, enhanced chemical reactivity—factors that are significant for molecular adsorption and charge-transfer processes.
Further examination of the LUMOs in the M12O12 nanocages shows that they are predominantly localized within the interior of the cages. This observation suggests that these clusters can serve as internal electron reservoirs, analogous to perfluorinated fullerenes known for endohedral electron trapping.84 Such an orbital configuration may also facilitate the stabilization of small molecules inside the cage. Among the systems examined, Ca12O12 is particularly notable due to its narrower Eg value and larger interior volume, attributes that could promote effective H2 adsorption. Although calcium is heavier than beryllium or magnesium, its enhanced capacity to accommodate multiple adsorbates may compensate for the increased mass, making Ca12O12 a promising scaffold for hydrogen storage applications.
A natural follow-up question is whether moving further down Group 2, to hypothetical Sr12O12 or Ba12O12 sodalite-type cages, would provide an additional benefit. While larger metals would be expected to further expand the cage cavity and ease steric crowding between neighboring exohedral adsorption sites, two considerations suggest that calcium already represents an effective balance. First, continued bond elongation in heavier alkaline earth oxides could compromise the mechanical rigidity of the M12O12 framework, whose stability is well established for calcium but remains uncertain for strontium- and barium-based analogs. Second, the much higher atomic mass of Sr and Ba would directly penalize the gravimetric hydrogen capacity, even if a comparable absolute number of H2 molecules could in principle be accommodated. Taken together, these factors indicate that Ca12O12 offers a favorable compromise between cavity size, structural stability, and mass efficiency, whereas heavier congeners may deliver diminishing returns—an aspect that warrants future computational assessment.
Fig. 1c illustrates the adsorption preferences of a single H2 molecule on the Be12O12, Mg12O12, and Ca12O12 nanocages. For Be12O12, only a single adsorption mode was found in which the H2 molecule binds in a side-on configuration with a Be atom; the absence of any endohedral adsorption minima indicates that the small cavity is unsuitable for internal adsorption. In contrast, Mg12O12 exhibits two exohedral modes. In the more favorable configuration, denoted as Mg12O12·H2 (M), the H2 molecule adopts a side-on orientation similar to that of Be12O12, whereas a less stable mode, Mg12O12·H2 (O), features an end-on coordination where one hydrogen atom interacts with an oxygen atom. This end-on configuration shows less negative adsorption energy and enthalpy values (−1.5 and −3.6 kJ mol−1, respectively) compared to −6.8 and −7.3 kJ mol−1 for the side-on mode. Notably, no stable endohedral adsorption was observed for Mg12O12.
For Ca12O12, three adsorption modes were identified: two exohedral modes analogous to those in Mg12O12 and an additional endohedral mode. Among these, the configuration corresponding to endohedral adsorption exhibits the most negative adsorption energy and enthalpy (−9.0 and −9.2 kJ mol−1, respectively), followed by the exohedral end-on mode, Ca12O12·H2 (O), with values of −1.8 and −4.3 kJ mol−1. The exohedral side-on mode, Ca12O12·H2 (M), shows the least favorable binding (−0.9 and −0.5 kJ mol−1). These results indicate that the energetic preference for an end-on adsorption configuration increases from Be12O12 (where it is absent) to Mg12O12 (where it is less stable) and is most pronounced in Ca12O12. Consequently, the exohedral end-on mode in Ca12O12 is adopted as the reference state for the ΔHnormads calculations discussed in subsequent sections.
An additional observation pertains to the impact of H2 adsorption on the Eg values. As summarized in Table 1, the adsorption of a single H2 molecule induces subtle but distinct changes in Eg across the different nanocages. For Be12O12, Eg decreases by 0.014 eV (from 11.319 to 11.305 eV) upon adsorption. In contrast, for Mg12O12 the gap increases by 0.013 eV in the side-on mode and by 0.026 eV in the end-on mode, while for Ca12O12 the increases are 0.001 eV for the side-on, 0.025 eV for the end-on, and 0.022 eV for the endohedral adsorption modes. These variations underscore the sensitivity of the electronic structure to the adsorption geometry and the nature of the metal center.
Finally, it is also important to highlight the limits of endohedral adsorption in Ca12O12. Although a single H2 molecule can be favorably adsorbed within the cavity, the introduction of a second H2 molecule results in a positive adsorption enthalpy of 49.2 kJ mol−1. This unfavorable energy change clearly indicates that the accommodation of more than one H2 molecule within the Ca12O12 cage is not energetically viable.
As discussed in subsequent sections, these structural and electronic distinctions result in Ca12O12 offering both endohedral and exohedral sites for H2 adsorption. This balanced geometry suggests that multiple H2 molecules can be accommodated simultaneously, potentially achieving the density targets necessary for practical fuel-cell applications.
3.2 Multiple H2 adsorption on Ca12O12: exohedral adsorption
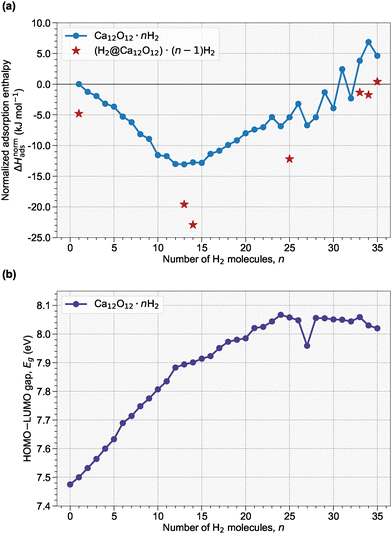
Next, we turn our attention to the multiple adsorption of H2 molecules on the Ca12O12 nanocage, focusing exclusively on exohedral adsorption. The exohedral adsorption process was systematically investigated for configurations containing up to 48H2 molecules. Key adsorption properties for the first 35 adsorption events are summarized in Table 1, with complete data for all 48 adsorptions provided in Table S2 of the SI.As highlighted in Section 3.1, our preliminary single-molecule studies identified the oxygen sites as the most active centers for H2 uptake. Each oxygen site can formally accommodate up to three H2 molecules via favorable interactions—a capacity enabled by the larger cavity of the Ca12O12 nanocage, which is not observed in the analogous Be12O12 and Mg12O12 systems. In combination with one H2 molecule adsorbed on each calcium site, the theoretical maximum capacity of the nanocage is 48H2 molecules. To properly assess the cooperative effects during sequential adsorption, we evaluated both the overall adsorption enthalpy, ΔHads, and the normalized adsorption enthalpy, ΔHnormads, defined as the deviation from the sum of n independent single-molecule adsorption events (see Section 2 for further details).
Our analysis reveals that ΔHads remains negative throughout the entire adsorption interval, confirming that the overall exohedral adsorption process is exothermic. However, the cooperative behavior, as captured by ΔHnormads, exhibits more nuanced trends (see Fig. 2a). For exohedral adsorption, negative ΔHnormads values are observed up to n = 32, indicating that each additional H2 molecule is adsorbed more favorably than predicted by an independent adsorption model. The most pronounced cooperative effect is observed at n = 13, where ΔHnormads = −13.1 kJ mol−1 and the cumulative adsorption energy reaches −69.3 kJ mol−1. For n = 31 and for all systems beyond n = 32, ΔHnormads becomes positive, signaling that further H2 adsorption is less exothermic than anticipated, likely due to increased steric hindrance and repulsive interactions among the adsorbed molecules.
In parallel, the Eg values (Fig. 2b) exhibit an increasing trend with successive H2 adsorption, reflecting a gradual shift toward more insulating behavior. Notably, this increase in Eg plateaus after the adsorption of 24H2 molecules, suggesting that the electronic structure of the nanocage reaches a saturation point with respect to further H2 loading.
Furthermore, hydrogen uptake was quantified in terms of weight percentage (wt%). As shown in Table 1, all structures with n > 19H2 molecules exceed the U.S. DOE's target of 5.5 wt%, with many of these configurations still featuring negative ΔHnormads values. Collectively, these findings illustrate that while the Ca12O12 nanocage is theoretically predicted to be an effective host for exohedral H2 adsorption—exhibiting favorable cooperative interactions at moderate loadings—the breakdown of cooperativity (as indicated by positive ΔHnormads values) at higher coverages defines a distinct limit for additional adsorption. It is noteworthy that the per-molecule adsorption enthalpies at moderate coverages lie in the few-kJ mol−1 range, which is generally regarded as favorable for reversible physisorption-based hydrogen storage: sufficiently attractive to retain H2 on the scaffold, but not so strong as to require harsh conditions for desorption.
From an electronic perspective, the adsorbed H2 molecules considered above remain intact, with H−H bond lengths close to that of free H2 and no evidence of dissociative chemisorption, even at high coverage. The preferred end-on orientation at oxygen sites in Ca12O12 can be rationalized by attractive electrostatic and induction interactions between the partially negative O centers and the quadrupole of H2, whereas side-on approaches to the metal sites are generally less stabilizing. In Ca12O12, the relatively narrow HOMO–LUMO gap and higher metal polarizability enhance these induced-dipole interactions. This behavior is consistent with the notion that heavier alkaline earth elements, including Ca, can access low-lying d-like acceptor character,85 enabling stronger yet still non-dissociative interactions with H2. These features help explain why Ca12O12 supports both high-density exohedral loading and distinct endohedral stabilization, outperforming Be12O12 and Mg12O12.
From an application standpoint, the magnitudes of the adsorption enthalpies reported here (on the order of a few kJ mol−1 per H2, and up to ∼10 kJ mol−1 for the most favorable individual events) are consistent with weak, largely physisorptive binding rather than dissociative chemisorption. Such interaction strengths are generally considered attractive for reversible hydrogen storage, insofar as they are strong enough to stabilize molecular H2 on the scaffold but not so strong as to make desorption prohibitively energy-intensive. We emphasize, however, that the present analysis is based on enthalpic trends at 298 K and does not explicitly include entropic contributions or kinetic barriers. As a result, assigning a precise working temperature or pressure window for hydrogen uptake and release lies beyond the scope of this study and will require future free-energy and finite-temperature simulations.
3.3 Multiple H2 adsorption on Ca12O12: mixed exohedral-endohedral adsorption
In the previous section, we determined that systems with purely exohedral adsorption maintained favorable cooperative interactions, evidenced by negative ΔHnormads values, up to 32H2 molecules. However, as noted in Section 3.1, the Ca12O12 nanocage can also accommodate an additional H2 molecule in an endohedral position, a feature that is absent in the Be12O12 and Mg12O12 cases. This observation motivated an investigation into how the adsorption energetics are affected when both exohedral and endohedral H2 adsorption modes are combined.The mixed adsorption configurations are denoted as (H2@Ca12O12)·nH2, with n representing the number of exohedrally adsorbed H2 molecules. Specifically, we examined structures with 12, 13, 24, 32, 33, and 34 exohedral H2 molecules, each accompanied by one endohedral H2 (see Fig. 3 for their optimized structures). The results reveal that the highly negative adsorption energy associated with the first endohedral H2 substantially enhances the overall adsorption process. In fact, this favorable endohedral contribution promotes the adsorption of additional exohedral H2 molecules, leading to a maximum configuration with 33 exohedral and one endohedral H2 (i.e., a total of 34H2 molecules). This configuration exhibits an adsorption energy of −148.7 kJ mol−1 and a normalized adsorption enthalpy of −1.7 kJ mol−1, thereby favorably achieving a hydrogen uptake of 9.24 wt%.
In turn, when a 34th exohedral H2 molecule is added, yielding the system (H2@Ca12O12)·34H2 (total of 35H2 molecules), the normalized adsorption enthalpy sharply increases to a positive value of 6.9 kJ mol−1. This positive shift indicates that the cooperative benefits are lost and that further H2 adsorption becomes energetically unfavorable, likely due to enhanced steric hindrance and repulsive interactions. Additionally, the most negative normalized adsorption enthalpy was observed for a configuration with 14 adsorbed molecules (corresponding to a hydrogen uptake of 4.03 wt%), with a value of −22.9 kJ mol−1, highlighting the strong cooperative interactions at intermediate loadings. The mixed exohedral–endohedral adsorption strategy on Ca12O12, therefore, enhances the overall adsorption capacity by leveraging the favorable energetics of endohedral H2 adsorption. However, these cooperative interactions break down beyond a critical coverage level, delineating an energetic limit for further H2 uptake.
This enhancement arises from two contributions. First, the initial endohedral H2 is strongly stabilized by the electrostatic potential within the cage cavity, contributing an enthalpically favorable term (ΔHads ≈ −9 kJ mol−1) that lowers the overall adsorption enthalpy of the mixed systems. Second, filling the interior slightly redistributes electron density toward the cage center, which mitigates intermolecular repulsion between neighboring exohedral H2 molecules at high surface coverage. As a result, introducing one endohedral H2 effectively promotes one additional shell of exohedral adsorption before steric and repulsive penalties dominate.
4 Conclusions
In summary, we performed a comprehensive in silico investigation of pristine Ca12O12 nanocages as potential hydrogen storage materials, utilizing dispersion-corrected and range-separated DFT methods, benchmarked against coupled-cluster calculations. The structural, electronic, and adsorptive properties of Ca12O12 were systematically compared with those of its Be12O12 and Mg12O12 analogs, revealing that the larger framework of Ca12O12 is particularly advantageous for hydrogen uptake. Single H2 adsorption studies demonstrated that Ca12O12 exhibits distinct exohedral adsorption modes, with oxygen sites providing the most favorable interactions and even enabling stable endohedral adsorption—a feature not observed in the smaller Be12O12 and Mg12O12 nanocages. In multiple H2 adsorption analyses, the overall adsorption enthalpy, ΔHads, remains negative across the entire loading range, confirming an exothermic process. However, examination of the normalized adsorption enthalpy, ΔHnormads, reveals that while cooperative adsorption is favorable (i.e., ΔHnormads is negative) up to 32H2 molecules, this benefit diminishes at higher coverages—with positive ΔHnormads values observed for configurations beyond n = 32. Notably, the most pronounced cooperative effect is observed at n = 13, where ΔHnormads reaches −13.1 kJ mol−1 and the cumulative adsorption energy attains −69.3 kJ mol−1. Furthermore, mixed adsorption configurations combining exohedral and endohedral H2 molecules were explored. The incorporation of a single endohedral H2 molecule significantly enhances the overall adsorption energetics, yielding an optimal configuration with 33 exohedral plus one endohedral H2 (a total of 34H2 molecules) that exhibits ΔHads = −148.7 kJ mol−1 and ΔHnormads = −1.7 kJ mol−1. This configuration corresponds to a hydrogen uptake of 9.24 wt%, which comfortably exceeds the U.S. DOE target of 5.5 wt%. Importantly, the calculated adsorption enthalpies fall in the regime typically considered optimal for reversible storage media, supporting the practical relevance of Ca12O12 as a physisorptive hydrogen host. The evolution of the Eg gap with increasing H2 coverage further suggests that the electronic structure of the nanocage gradually shifts toward a more insulating character, although this effect plateaus beyond moderate adsorption levels. Collectively, these findings establish Ca12O12 as a promising candidate for hydrogen storage due to its capacity to support both exohedral and endohedral H2 adsorption, its favorable cooperative adsorption behavior at moderate loadings, and its potential to achieve high gravimetric hydrogen densities. While this study provides key information on the thermodynamic and electronic characteristics of hydrogen adsorption on Ca12O12, it is based primarily on adsorption enthalpies and does not yet incorporate full finite-temperature free-energy profiles or desorption kinetics. Future work should therefore address anharmonic vibrational effects, entropic contributions under realistic pressures, finite-temperature molecular dynamics, and explicit many-body dispersion contributions, in order to evaluate stability and reversibility under working conditions and to establish practical operating windows (temperature and pressure ranges) for reversible hydrogen uptake and release. Overall, the present work contributes to the growing body of research on nanostructured hydrogen storage materials and offers a robust computational framework for the design and evaluation of advanced storage systems.Author contributions
Saeedeh Kamalinahad: conceptualization (equal); methodology (equal); formal analysis (equal); investigation (equal); data curation (equal); writing − original draft (equal); writing − review & editing (equal). Aritra Roy: methodology (equal); formal analysis (equal); investigation (equal); data curation (equal); writing − original draft (equal); writing − review & editing (equal). Pablo Gamallo: conceptualization (equal); methodology (equal); resources (equal); writing − review & editing (equal); supervision (equal); project administration (equal); funding acquisition (equal). Felipe Fantuzzi: conceptualization (equal); methodology (equal); validation (lead); resources (equal); writing − original draft (equal); writing − review & editing (equal); visualization (lead); supervision (equal); project administration (equal); funding acquisition (equal).Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available within the article and its supplementary information (SI). Additional data are available from the corresponding authors upon reasonable request. Supplementary information: Benchmarking; summary of key structural, energetic, and electronic properties of all the investigated systems; cartesian coordinates. See DOI: https://doi.org/10.1039/d5nj04215a.Acknowledgements
S. K. thanks Dr Motahareh Noormohammadbeigi and Dr Masoud Haratian for insightful scientific discussions. P. G. acknowledges support from the Spanish Ministry of Science and Innovation (MICIN) through the project PID2022-138180OB-I00 funded by MCIN/AEI/10.13039/501100011033 and by the European Union through ERDF A way of making Europe. Additional support has been provided by the CEX2021-001202-M María de Maeztu Unit of Excellence and by the Generalitat de Catalunya (project 2021SGR00079 and P. G. Serra Hunter Associate Professorship). F. F. gratefully acknowledges the University of Kent and Julius-Maximilians-Universität Würzburg for providing complementary computational resources whereas P. G. and S. K. also acknowledges the computational resources provided by Red Española de Supercomputación (RES) and by Consorci de Serveis Universitaris de Catalunya (CSUC).Notes and references
- S. Studer, S. Stucki and J. D. Speight, Hydrogen as a Future Energy Carrier, John Wiley & Sons, Ltd, 2008, pp. 23–69 Search PubMed.
- Energy White Paper: Our Energy Future: Creating a Low Carbon Economy, 2024, https://assets.publishing.service.gov.uk/media/5a7c1f5940f0b645ba3c6d4f/5761.pdf.
- R. B. Gupta, Hydrogen Fuel: Production, Transport, and Storage, CRC Press, 2008 Search PubMed.
- L. Schlapbach and A. Züttel, Nature, 2001, 414, 353–358 CrossRef CAS PubMed.
- N. Ma, W. Zhao, W. Wang, X. Li and H. Zhou, Int. J. Hydrogen Energy, 2024, 50, 379–396 CrossRef CAS.
- R. A. Varin, T. Czujko and Z. S. Wronski, Nanomaterials for Solid State Hydrogen Storage, Springer Science & Business Media, 2009 Search PubMed.
- F. Birol, International Energy Agency, The Future of Hydrogen, IEA technical report, 2019.
- I. Hassan, H. S. Ramadan, M. A. Saleh and D. Hissel, Renewable Sustainable Energy Rev., 2021, 149, 111311 CrossRef CAS.
- E. Harrak Abdechafik, H. Ait Ousaleh, S. Mehmood, Y. Filali Baba, I. Bürger, M. Linder and A. Faik, Int. J. Hydrogen Energy, 2024, 52, 1182–1193 CrossRef.
- J. Beheshtian and I. Ravaei, Appl. Surf. Sci., 2016, 368, 76–81 CrossRef CAS.
- Q. Wang and J. K. Johnson, J. Phys. Chem. B, 1999, 103, 277–281 CrossRef CAS.
- C. Liu, Y. Fan, M. Liu, H. Cong, H. Cheng and M. S. Dresselhaus, Science, 1999, 286, 1127–1129 CrossRef CAS PubMed.
- E. Beheshti, A. Nojeh and P. Servati, Carbon, 2011, 49, 1561–1567 CrossRef CAS.
- M. F. Fellah, Appl. Surf. Sci., 2017, 394, 9–15 CrossRef CAS.
- A. Blomqvist, C. M. Araújo, P. Srepusharawoot and R. Ahuja, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 20173–20176 CrossRef CAS PubMed.
- P. Weis, P. R. Kemper and M. T. Bowers, J. Phys. Chem. A, 1997, 101, 2809–2816 CrossRef CAS.
- M. D. Ganji, S. Hosseini-Khah and Z. Amini-Tabar, Phys. Chem. Chem. Phys., 2015, 17, 2504–2511 RSC.
- U.S. Department of Energy, Target Explanation Document: Onboard Hydrogen Storage for Light-Duty Fuel Cell Vehicles, U.S. Department of Energy technical report, 2017.
- H. W. Kroto, J. R. Heath, S. C. O’Brien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162–163 CrossRef CAS.
- Y. Rubin, T. Jarrosson, G.-W. Wang, M. D. Bartberger, K. Houk, G. Schick, M. Saunders and R. J. Cross, Angew. Chem., Int. Ed., 2001, 40, 1543–1546 CrossRef CAS PubMed.
- K. Komatsu, M. Murata and Y. Murata, Science, 2005, 307, 238–240 CrossRef CAS PubMed.
- T. B. Lee and M. L. McKee, J. Am. Chem. Soc., 2008, 130, 17610–17619 CrossRef CAS PubMed.
- O. V. Pupysheva, A. A. Farajian and B. I. Yakobson, Nano Lett., 2008, 8, 767–774 CrossRef CAS PubMed.
- K. Kurotobi and Y. Murata, Science, 2011, 333, 613–616 CrossRef CAS PubMed.
- A. Kaiser, C. Leidlmair, P. Bartl, S. Zöttl, S. Denifl, A. Mauracher, M. Probst, P. Scheier and O. Echt, J. Chem. Phys., 2013, 138, 074311 CrossRef CAS PubMed.
- A. A. Popov, S. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989–6113 CrossRef CAS PubMed.
- X. Chang, Y. Xu and M. von Delius, Chem. Soc. Rev., 2024, 53, 47–83 RSC.
- M. P. Johansson, D. Sundholm and J. Vaara, Angew. Chem., Int. Ed., 2004, 43, 2678–2681 CrossRef CAS PubMed.
- Y. Gao and X. C. Zeng, J. Am. Chem. Soc., 2005, 127, 3698–3699 CrossRef CAS PubMed.
- B. Yin and Z. Luo, Coord. Chem. Rev., 2021, 429, 213643 CrossRef CAS.
- T. N. Gribanova, R. M. Minyaev, V. I. Minkin and A. I. Boldyrev, Struct. Chem., 2020, 31, 2105–2128 CrossRef CAS.
- S. E. Skrabalak, J. Chen, Y. Sun, X. Lu, L. Au, C. M. Cobley and Y. Xia, Acc. Chem. Res., 2008, 41, 1587–1595 CrossRef CAS PubMed.
- Y. Xia, W. Li, C. M. Cobley, J. Chen, X. Xia, Q. Zhang, M. Yang, E. C. Cho and P. K. Brown, Acc. Chem. Res., 2011, 44, 914–924 CrossRef CAS PubMed.
- V. Alimardani, G. Farahavar, S. Salehi, S. Taghizadeh, M. R. Ghiasi and S. S. Abolmaali, Front. Mater. Sci., 2021, 15, 494–511 CrossRef.
- E. Shakerzadeh, E. Tahmasebi, L. Van Duong and M. Tho Nguyen, Charact. Appl. Boron, IntechOpen, 2022, ch. 6, pp. 1–31 Search PubMed.
- L. Zhang, Y.-L. Ye, X.-H. Li, J.-H. Chen and W.-M. Sun, J. Mol. Liq., 2021, 342, 117533 CrossRef CAS.
- H. Kaur, J. Kaur and R. Kumar, Mater. Today Proc., 2022, 48, 1095–1102 CrossRef CAS.
- H.-J. Zhai, Y.-F. Zhao, W.-L. Li, Q. Chen, H. Bai, H.-S. Hu, Z. A. Piazza, W.-J. Tian, H.-G. Lu, Y.-B. Wu, Y.-W. Mu, G.-F. Wei, Z.-P. Liu, J. Li, S.-D. Li and L.-S. Wang, Nat. Chem., 2014, 6, 727–731 CrossRef CAS PubMed.
- H. W. Choi, Y.-Y. Zhang, D. Kahraman, C.-Q. Xu, J. Li and L.-S. Wang, Boron Buckminsterfullerene, 2024, https://chemrxiv.org/engage/chemrxiv/article-details/676da75efa469535b9e4a03b Search PubMed.
- H. Jouypazadeh and H. Farrokhpour, J. Mol. Struct., 2018, 1164, 227–238 CrossRef CAS.
- H. O. Edet, H. Louis, I. Benjamin, M. Gideon, T. O. Unimuke, S. A. Adalikwu, A. D. Nwagu and A. S. Adeyinka, Chem. Phys. Impact, 2022, 5, 100107 CrossRef.
- D. L. Strout, J. Phys. Chem. A, 2000, 104, 3364–3366 CrossRef CAS.
- Q. Sun, Q. Wang and P. Jena, Nano Lett., 2005, 5, 1273–1277 CrossRef CAS PubMed.
- N. S. Venkataramanan, R. V. Belosludov, R. Note, R. Sahara, H. Mizuseki and Y. Kawazoe, Chem. Phys., 2010, 377, 54–59 CrossRef CAS.
- S. Giri, A. Chakraborty and P. Chattaraj, Nano Rev., 2011, 2, 5767 CrossRef PubMed.
- K. Ayub, J. Mater. Chem. C, 2016, 4, 10919–10934 RSC.
- A. S. Rad and K. Ayub, J. Mol. Liq., 2017, 238, 303–309 CrossRef CAS.
- R. Baloach, K. Ayub, T. Mahmood, A. Asif, S. Tabassum and M. A. Gilani, J. Inorg. Organomet. Polym. Mater., 2021, 31, 3062–3076 CrossRef CAS.
- Y. Zhang, X. Zheng, S. Zhang, S. Huang, P. Wang and H. Tian, Int. J. Hydrogen Energy, 2012, 37, 12411–12419 CrossRef CAS.
- H. Louis, I. O. Amodu, T. O. Unimuke, T. E. Gber, B. B. Isang and A. S. Adeyinka, Mater. Today Commun., 2022, 32, 103946 CrossRef CAS.
- N. Kosar, S. Gul, K. Ayub, A. Bahader, M. A. Gilani, M. Arshad and T. Mahmood, Mater. Chem. Phys., 2020, 242, 122507 CrossRef CAS.
- M. Rezaei-Sameti and S. Abdoli, J. Mol. Struct., 2020, 1205, 127593 CrossRef CAS.
- S. Gul, Kainat, Q. Ali, M. Khan, M. Ur Rehman, A. F. AlAsmari, F. Alasmari and M. Alharbi, Sci. Rep., 2023, 13, 18481 CrossRef CAS PubMed.
- J. S. Al-Otaibi, Y. S. Mary, Y. S. Mary, A. Mondal, N. Acharjee and D. S. Rajendran Nair, Spectrochim. Acta, Part A, 2024, 308, 123728 CrossRef CAS PubMed.
- Y. Zhang and H. Chen, Int. J. Hydrogen Energy, 2018, 43, 16609–16616 CrossRef CAS.
- O. V. de Oliveira, J. M. Pires, A. C. Neto and J. D. dos Santos, Chem. Phys. Lett., 2015, 634, 25–28 CrossRef CAS.
- A. S. Rad, S. M. Aghaei, H. Pazoki, E. Binaeian and M. Mirzaei, Surf. Interface Anal., 2018, 50, 411–419 CrossRef CAS.
- M. D. Mohammadi, H. Louis, O. J. Ikenyirimba, G. E. Mathias, T. O. Unimuke and M. E. Rasaki, Int. J. Hydrogen Energy, 2023, 48, 29446–29460 CrossRef CAS.
- M. Kazemi and A. S. Rad, Superlattices Microstruct., 2017, 106, 122–128 CrossRef CAS.
- Y. Zhang and X. Cheng, Chem. Phys. Lett., 2017, 672, 105–111 CrossRef CAS.
- F. Fantuzzi, R. R. Oliveira, A. V. Henkes, J. Rubayo-Soneira and M. A. C. Nascimento, Chem. – Eur. J., 2019, 25, 5269–5279 CrossRef CAS PubMed.
- M. R. Farrow, Y. Chow and S. M. Woodley, Phys. Chem. Chem. Phys., 2014, 16, 21119–21134 RSC.
- R. R. Oliveira, F. Fantuzzi and M. A. C. Nascimento, J. Appl. Phys., 2021, 129, 205102 CrossRef CAS.
- H. Louis, T. C. Egemonye, T. O. Unimuke, B. E. Inah, H. O. Edet, E. A. Eno, S. A. Adalikwu and A. S. Adeyinka, ACS Omega, 2022, 7, 34929–34943 CrossRef CAS PubMed.
- G. J. Ogunwale, H. Louis, T. O. Unimuke, G. E. Mathias, A. E. Owen, H. O. Edet, O. C. Enudi, E. O. Oluwasanmi, A. S. Adeyinka and M. Doust Mohammadi, ACS Omega, 2023, 8, 13551–13568 CrossRef CAS PubMed.
- S. Wu, L. Li, Q. Liang, H. Gao, T. Tang and Y. Tang, Mater. Today Commun., 2024, 38, 107687 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16 Revision C.01, 2016 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS PubMed.
- S. H. Vosko, L. Wilk and M. Nusair, Can. J. Phys., 1980, 58, 1200–1211 CrossRef CAS.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 RSC.
- J. P. Perdew, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 33, 8822–8824 CrossRef PubMed.
- A. D. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098–3100 CrossRef CAS PubMed.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- M. Ernzerhof and G. E. Scuseria, J. Chem. Phys., 1999, 110, 5029–5036 CrossRef CAS.
- C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS.
- Y. Guo, C. Riplinger, U. Becker, D. G. Liakos, Y. Minenkov, L. Cavallo and F. Neese, J. Chem. Phys., 2018, 148, 011101 Search PubMed.
- F. Neese, Wiley Interdiscip. Rev.:Comput. Mol. Sci., 2022, 12, e1606 Search PubMed.
- Y.-J. Wang, L. Xu, L.-H. Qiao, J. Ren, X.-R. Hou and C.-Q. Miao, Int. J. Hydrogen Energy, 2020, 45, 12932–12939 Search PubMed.
- H.-R. Li, C. Zhang, W.-B. Ren, Y.-J. Wang and T. Han, Int. J. Hydrogen Energy, 2023, 48, 25821–25829 CrossRef CAS.
- X. Wang and H. Chen, Appl. Phys. Lett., 2018, 112, 093903 CrossRef.
- Y. Wang, Z. Li, D. Wu, C. Sun and F. Gu, J. Comput. Chem., 2010, 31, 195–203 CrossRef CAS PubMed.
- M. Zhou and G. Frenking, Acc. Chem. Res., 2021, 54, 3071–3082 CrossRef CAS PubMed.
Footnotes |
| † Present address: Department of Chemistry Education, Farhangian University, P.O. Box 14665-889, Tehran, Iran. |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2026 |