Peripheral tailoring of pentacene: developing next-generation organic sonosensitizers for cancer sonodynamic therapy
Nan
Han†
,
Yu
Zhang†
*,
Chunyuan
Hou
,
Jun
Gu
and
Jun
Luo
 *
*
School of Chemistry and Chemical Engineering, Nanjing University of Science and Technology, Nanjing, 210094, China. E-mail: y_zhang@njust.edu.cn; luojun@njust.edu.cn
First published on 13th November 2025
Abstract
Sonodynamic therapy (SDT) is an innovative, non-invasive, and effective method for cancer treatment. However, exploring sonosensitizers with high sonosensitivity and biosafety remains a significant challenge. Recent investigations have demonstrated that the excellent delocalized π-electron conjugation system and narrow HOMO–LUMO gap characteristic of acenes endow them with intrinsic sonoactivity, providing an opportunity for advancing novel sonosensitizers. Herein, two pentacene derivatives, 4Br-PEN and 4Br-CN-PEN, were successfully synthesized through site-specific peripheral tailoring of the pentacene backbone with bromine atoms and cyano groups. Both in vitro and in vivo therapeutic outcomes demonstrated that the synthesized compounds could generate singlet oxygen (1O2) under ultrasound irradiation, effectively eradicating cancer cells while exhibiting significant anti-proliferative effects and excellent biocompatibility. Notably, because of the synergistic inductive and conjugative effects of the cyano group, 4Br-CN-PEN exhibited superior sonodynamic activity to 4Br-PEN. These findings collectively suggest that pentacene derivatives hold promising potential as highly effective and safe sonosensitizers for SDT applications.
Introduction
Cancer, as a major global public health challenge in the 21st century, poses a formidable medical hurdle in terms of its treatment and ultimate cure.1,2 Given the adverse effects associated with conventional cancer therapies, non-invasive treatments, as a novel therapeutic approach, can largely avoid damaging normal cells and tissues, offering a significantly enhanced safety profile.3,4 Sonodynamic therapy (SDT) is a treatment modality that utilizes ultrasound (US) to activate sonosensitizers, thereby inducing the production of reactive oxygen species (ROS) to kill tumor cells.5 Compared to photodynamic therapy (PDT), SDT offers superior tissue penetration depth, making it particularly suitable for treating deep-seated tumors.6–13 Due to its superior biosafety and patient tolerability, SDT has rapidly advanced in biomedical applications.14–16The primary determinant of SDT efficacy is the sonosensitizer, which is mainly categorized into two classes: inorganic sonosensitizers and organic sonosensitizers.17–22 The commonly used inorganic sonosensitizers include titanium dioxide (TiO2),23 ferroferric oxide (Fe3O4),24 manganese dioxide (MnO2),25,26 cuprous oxide (Cu2O),27 and black phosphorus (BP).28 Although inorganic sonosensitizers exhibit lower toxicity toward normal cells and tissues and remain stable under physiological conditions, their widespread application is significantly hindered by challenges in poor biodegradation and low ROS generation efficiency.29 In contrast, organic sonosensitizers not only possess well-defined structures and facile biodegradability but also demonstrate higher ROS generation efficiency and superior biosafety, making them a focal point of current research efforts.30 Common organic sonosensitizers include hematoporphyrin,31 rose bengal,32 and chlorin e6.33 Nevertheless, the clinical translation of organic sonosensitizers has been significantly hindered by their inherent instability and tendency to aggregate under physiological conditions, as documented in prior studies.34 This critical limitation underscores the urgent demand for innovative molecular design strategies to engineer next-generation organic sonosensitizers with enhanced structural integrity, superior sonodynamic activity, and favorable biosafety profiles.35,36
pentacene, an organic small molecule, features an extensive conjugated structure with highly delocalized π-electrons and an exceptionally small energy gap between its HOMO and LUMO orbitals.37,38 First created in the early 1900s, this material has remained a popular subject in scientific research due to its unique electronic properties.39,40 Recent studies have demonstrated that pentacene possesses promising sonodynamic activity. However, poor stability and low solubility make it difficult to use in real-world applications.41 To tackle these issues, the pentacene backbone was strategically tailored with various substituents at peripheral positions. For instance, installation of electron-withdrawing groups or sterically bulky substituents at the 6,13-positions, which are susceptible to oxygen addition, enables precise modulation of electron density distribution along the conjugated backbone, thereby significantly enhancing both stability and solubility.42,43 Notably, the strongly electron-withdrawing cyano moieties, characterized by their linear configuration and compact spatial dimensions, induce minimal disruption to the molecular planarity, rendering them ideal functional groups for modifying the pentacene backbone.44–47 Factually, it has been demonstrated that the site-specific cyano-functionalization at the 6,13-positions of the pentacene backbone significantly reduces the electron density at these reactive sites, thereby markedly enhancing the stability of the pentacene derivatives.48–51 Importantly, the cyano groups can conjugate with the π-electrons of the pentacene backbone, extending electron delocalization,52–54 which significantly narrows the HOMO–LUMO energy gap, further facilitating molecular transitions from the ground state (S0) to the excited state (S1). This means that the cyano-substituted derivatives can generate higher levels of ROS under constant US excitation. In addition, the bromination at both ends of the pentacene backbone can also lower the electron density of the conjugated system and improve the antioxidant capacity of the molecule.55–57 Particularly, the incorporation of bromine atoms can facilitate intersystem crossing (ISC) via the spin–orbit coupling effect, which may enhance US-responsiveness. These findings offer valuable insights for designing sonosensitizers that possess both high stability and superior sonodynamic activity.
Herein, we strategically synthesized two pentacene derivatives, 4Br-PEN and 4Br-CN-PEN, via site-specific peripheral tailoring of the pentacene backbone with bromine atoms and cyano groups. Their potential as sonosensitizers for SDT was systematically evaluated both in vitro and in vivo. Owing to the synergistic inductive and conjugative effects of the cyano groups, 4Br-CN-PEN demonstrated superior sonosensitivity, elevated ROS production, and a stronger capability for tumor growth suppression (Fig. 1).
Results and discussion
Synthesis and optical performance of 4Br-PEN and 4Br-CN-PEN
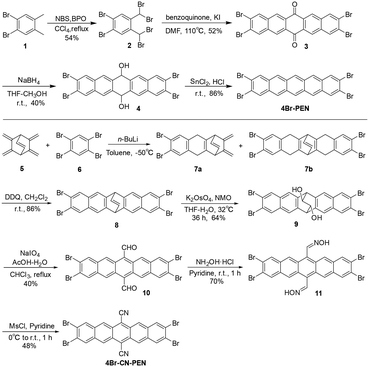
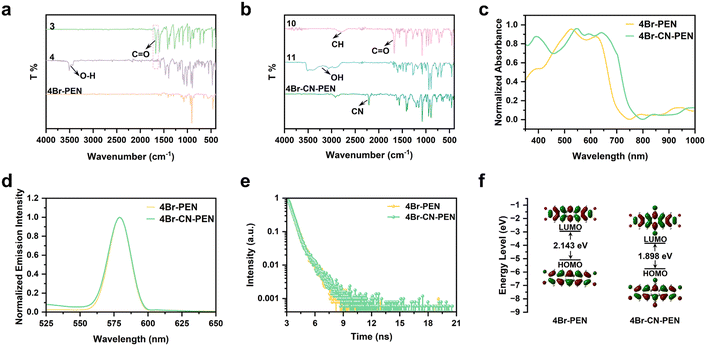
The synthetic routes for 4Br-PEN and 4Br-CN-PEN were designed based on ref. 58–60. In the synthesis of 4Br-PEN, starting from 1,2-dibromo-4,5-dimethylbenzene, sequential bromination and a Diels–Alder reaction were employed to afford 2,3,9,10-tetrabromopentacene-6,13-dione (3). Then, 4Br-PEN was synthesized from compound 3 through a series of reduction steps. To obtain 4Br-CN-PEN, the freshly prepared compound 5,6,7,8-tetramethylenebicyclo[2.2.2]oct-2-ene (ref. 61–64) underwent a Diels–Alder reaction with 1,2,4,5-tetrabromobenzene to construct the pentacyclic skeleton, affording 2,3,9,10-tetrabromo-5,6,7,12,13,14-hexahydro-6,13-ethenopentacene (7b). Compound 7b was converted to 2,3,9,10-tetrabromopentacene-6,13-dicarbaldehyde (10) through sequential oxidative aromatization, oxidative dihydroxylation, and oxidative cleavage. At the end, compound 10 underwent oximation followed by dehydration, resulting in the final product 4Br-CN-PEN (Scheme 1).Given the modification of functional groups during the synthesis of 4Br-PEN and 4Br-CN-PEN, to more comprehensively characterize these two compounds, Fourier-transform infrared spectroscopy (FTIR) was employed to analyze the changes in functional groups during the reaction process (Fig. 2a and b). The FTIR spectrum of compound 3 exhibits a characteristic carbonyl absorption peak at 1680 cm−1. In contrast, compound 4 shows no absorption at 1680 cm−1 but displays a hydroxyl peak at 3500 cm−1, consistent with the reduction of the carbonyl group to a hydroxyl group. Notably, the FTIR spectrum of 4Br-PEN exhibits exclusively the characteristic absorption peaks of the benzene ring. In the synthesis of 4Br-CN-PEN, the FTIR spectrum of compound 10 displays characteristic aldehyde group absorption bands at 1690 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch) and 2820 cm−1 (C–H stretch). In contrast, compound 11 demonstrates a complete disappearance of these aldehyde signals, along with the emergence of a new broad hydroxyl peak at 3500 cm−1, corresponding to the oxime group. The FTIR spectrum of 4Br-CN-PEN shows a sharp nitrile (C
O stretch) and 2820 cm−1 (C–H stretch). In contrast, compound 11 demonstrates a complete disappearance of these aldehyde signals, along with the emergence of a new broad hydroxyl peak at 3500 cm−1, corresponding to the oxime group. The FTIR spectrum of 4Br-CN-PEN shows a sharp nitrile (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) stretching vibration at 2210 cm−1, providing direct spectroscopic evidence for the dehydration of compound 11 to form the target cyano-containing product.
N) stretching vibration at 2210 cm−1, providing direct spectroscopic evidence for the dehydration of compound 11 to form the target cyano-containing product.
The UV-vis absorption peaks of 4Br-PEN and 4Br-CN-PEN were observed in the range of 500–700 nm. A distinct red shift was observed in the absorption maximum of 4Br-CN-PEN compared to that of 4Br-PEN (Fig. 2c). This red shift is attributed to the enhanced π-conjugation resulting from the incorporation of the cyano group into the pentacene, which not only expands π-electron delocalization but also lowers the optical bandgap (Fig. S18). In contrast to their distinct UV-vis absorption profiles, both compounds exhibited nearly identical fluorescence emission spectra (Fig. 2d), accompanied by characteristically short fluorescence lifetimes (Fig. 2e). This fluorescence quenching phenomenon likely arises from the heavy atom effect caused by bromine substituents, which facilitates faster ISC and consequently weakens the fluorescence emission. To investigate the photostability of 4Br-PEN and 4Br-CN-PEN, solid samples of these two compounds were exposed to natural light for 5 days. The UV-vis spectra (Fig. S19 and S20) exhibited no discernible differences compared to their initial spectra, indicating that the peripheral tailoring with bromine atoms and cyano groups significantly enhances the pentacene stability.
The Frontier molecular orbitals of the two compounds were calculated using density functional theory (DFT) with the B3LYP functional and the 6-31G(d,p) basis set. The electron density of both 4Br-PEN and 4Br-CN-PEN is predominantly localized on the pentacene backbone, with significant delocalization extending through the entire π-conjugated system (Fig. 2f). 4Br-CN-PEN exhibits a smaller HOMO–LUMO gap than 4Br-PEN, which aligns with the trend observed in the UV-vis absorption spectra. Generally, the sonosensitizer molecule is sono/photo-excited from the ground state (S0) to the first singlet excited state (S1), followed by efficient ISC that converts neighboring triplet oxygen molecules (3O2) into the first excited singlet oxygen species (1O2) (Fig. 1). The energy gap (ΔEST) between the S1 and triplet excited state (T1) of 4Br-PEN and 4Br-CN-PEN was calculated using time-dependent density functional theory (TD-DFT) at the M06-2X/def2 SVP level with the Gaussian 16 program. Table S3 (see SI) indicates that 4Br-CN-PEN demonstrates a reduced ΔEST compared to 4Br-PEN. Therefore, the modification of pentacene with bromine atoms and cyano groups facilitates the generation of sono/photo-induced excitons and enhances the ISC efficiency, which may endow them with promising sonoactivity.
Sonosensitive activities of 4Br-PEN and 4Br-CN-PEN
Based on the superior adaptability and safety performance demonstrated at an intensity of 1.0 W cm−2, a frequency of 1.0 MHz, and a 50% duty cycle, these US parameters were selected as the optimal settings for subsequent investigations.65,66 As a fluorescent probe for ROS detection, 1,3-diphenylisobenzofuran (DPBF) enables quantitative monitoring of US-induced ROS generation in 4Br-PEN and 4Br-CN-PEN through time-dependent absorbance measurements under varying sonication durations. The absorbance decrease observed in DPBF aqueous solutions containing 4Br-CN-PEN was significantly greater than that in solutions containing 4Br-PEN, demonstrating that 4Br-CN-PEN generated more ROS under US exposure (Fig. 3a–c). Both 4Br-PEN and 4Br-CN-PEN exhibited no detectable thermal effects during US irradiation, with their US-responsiveness demonstrating temperature-independent characteristics (Fig. S21). 1O2, superoxide anion (O2˙−), and hydroxyl radical (·OH) constitute the three predominant ROS generated during sonodynamic therapy. To elucidate the specific ROS generated by 4Br-PEN and 4Br-CN-PEN, distinct fluorescent probes combined with colorimetric assay were employed to detect the production profiles of 1O2, O2˙−, and ·OH. Singlet oxygen sensor green (SOSG) is a well-established probe for detecting 1O2. Relative to control samples, both the 4Br-PEN and 4Br-CN-PEN groups exhibited progressive increases in SOSG fluorescence intensity (Fig. 3d–f), manifesting 1O2 production. Notably, 4Br-CN-PEN displayed significantly stronger SOSG fluorescence than 4Br-PEN, confirming that enhanced π-electron conjugation effectively promotes 1O2 generation efficiency.O2˙− can be quantified using a sulfonamide-based colorimetric assay by monitoring the absorbance at 530 nm. Fig. 3g shows no statistically significant difference between the control group and either the 4Br-PEN or 4Br-CN-PEN group. Importantly, no blue or green coloration developed during sonication, indicating the absence of O2˙− production in these compounds under US irradiation. ·OH is commonly detected using the 3,3′,5,5′-tetramethylbenzidine (TMB) colorimetric probe. Fig. 3h shows no significant absorbance changes at 652 nm for either 4Br-PEN or 4Br-CN-PEN groups during sonication, which confirms that neither compound produces ·OH under US stimulation. In addition, electron paramagnetic resonance (EPR) spectroscopy exclusively captured characteristic 1O2 signals from both compounds, further confirming that 1O2 constitutes the predominant ROS generated by 4Br-PEN and 4Br-CN-PEN under US irradiation. Moreover, the 1O2 signal intensity of 4Br-CN-PEN significantly surpassed that of 4Br-PEN (Fig. 3i), demonstrating its superior sonosensitizing capability to yield greater 1O2 production under identical sonication parameters. Based on our calculations, 4Br-PEN and 4Br-CN-PEN demonstrated singlet oxygen yields 1.35 and 1.65 times that of Ce6 (Fig. S22), respectively.
SDT in vitro and in vivo
The SDT efficacy of 4Br-PEN and 4Br-CN-PEN against 4T1 mouse breast cancer cells was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Fig. 4a). In the absence of US irradiation, the viability of 4T1 cells remained stable even with increasing concentrations of 4Br-PEN and 4Br-CN-PEN up to 140 μmol L−1, indicating that 1O2 generation is US-dependent. Conversely, under US conditions, both compounds exhibited concentration-dependent cytotoxicity, with 4Br-CN-PEN showing significantly enhanced cell-killing efficacy compared to 4Br-PEN at equivalent concentrations. Notably, both 4Br-PEN and 4Br-CN-PEN demonstrated minimal cytotoxic effects against normal cell lines, highlighting their excellent biocompatibility (Fig. S23 and S24).Quantitative analysis showed that the IC50 values of 4Br-PEN and 4Br-CN-PEN were 134.9 μmol L−1 and 112.8 μmol L−1, respectively, which were lower than that of Ce6 (168.8 μmol L−1) (Fig. S25). The lower IC50 value of 4Br-CN-PEN suggests superior anti-proliferative efficacy against tumor cells compared to its counterpart.67 Based on the determined IC50 concentration of 4Br-CN-PEN, the cytotoxic effects on 4T1 cells were further assessed using live/dead cell staining, and corresponding fluorescence images acquired with an inverted fluorescence microscope. Under non-US conditions, both the 4Br-PEN and 4Br-CN-PEN treatment groups showed no significant cytotoxicity toward 4T1 cells, which was comparable to that of the untreated control group. Importantly, US exposure alone (in the absence of drugs) also resulted in negligible cell death, validating the safety of the selected acoustic parameters. Notably, when combined with US irradiation, the 4Br-CN-PEN group demonstrated approximately 50% cell mortality, whereas the 4Br-PEN group displayed comparatively lower cytotoxic efficacy under identical experimental conditions (Fig. 4b).
To systematically evaluate the SDT efficacy of 4Br-PEN and 4Br-CN-PEN under US irradiation, intracellular ROS generation was quantitatively assessed using the dichlorodihydrofluorescein diacetate (DCFH-DA) fluorescent probe. Fluorescence imaging analysis showed that the 4Br-CN-PEN group exhibited a markedly stronger green fluorescence intensity than the 4Br-PEN group (Fig. 4c). Quantitative analysis of fluorescence signals further confirmed the superior ROS-producing capability of 4Br-CN-PEN under US activation, as evidenced by its significantly higher signal intensity compared to that of the control (Fig. 4d). It has been demonstrated that ROS generation can trigger mitochondrial dysfunction, ultimately inducing cellular apoptosis through a series of cascade reactions. Given the critical role of the mitochondrial membrane potential as a key physiological parameter, we employed the JC-1 fluorescent assay to quantitatively monitor its dynamic changes throughout the therapeutic intervention. The membrane-permeable JC-1 dye preferentially aggregates in healthy mitochondria, emitting red fluorescence, whereas it exists as monomers and produces green fluorescence upon mitochondrial membrane disruption. Carbonyl cyanide 3-chlorophenylhydrazone (CCCP), a widely used uncoupler of oxidative phosphorylation, was employed as a positive control to induce mitophagy in mammalian cells. The groups of 4Br-PEN and 4Br-CN-PEN without US treatment exhibited weak green fluorescence, whereas strong green signals were observed in cells treated with sonicated 4Br-PEN and 4Br-CN-PEN, indicating that the combined treatment effectively induced mitochondrial membrane potential loss (Fig. 4e). All in vitro results demonstrated that 4Br-CN-PEN possessed satisfactory biocompatibility in the absence of US, while exhibiting enhanced SDT efficacy under US irradiation. Quantitative analysis of fluorescence signals demonstrated that 4Br-PEN and 4Br-CN-PEN can significantly disrupt mitochondrial function under US exposure (Fig. 4f).
Encouraged by the satisfactory in vitro cytotoxic effect of 4Br-CN-PEN, we further investigated its antitumor efficacy in 4T1 tumor-bearing BALB/c mice (primary tumor volume ≈ 100 mm3).68 Tumor-bearing mice were randomly allocated into three treatment groups (n = 6 per group): 1) control (saline + US), 2) 4Br-PEN + US, and 3) 4Br-CN-PEN + US. Treatments were administered every other day via intratumoral injection, followed by 2 min of US irradiation (Fig. 5a). The therapeutic regimen lasted for a total of 14 days. Following saline + US treatment, tumors exhibited rapid and uninhibited growth, ultimately reaching a mean volume of approximately 745 mm3 (Fig. 5b and c). In contrast, significant tumor growth suppression was observed in both 4Br-PEN + US and 4Br-CN-PEN + US groups (Fig. 5d and e). The 4Br-CN-PEN + US group demonstrated the most effective tumor growth inhibition, with a minimal increase in mean volume of to 262 mm3. Following the completion of the 14 day treatment regimen, the mice in each group were humanely euthanized. Tumors were subsequently excised, weighed, and photographed for comparative analysis (Fig. 5f). ROS generation was assessed by staining cryosectioned tumor tissues (Fig. 5h).
Notably, the 4Br-CN-PEN + US group exhibited significantly enhanced fluorescence intensity, indicative of elevated ROS levels. Hematoxylin and eosin (H&E) staining combined with TUNEL assays revealed inhibition of cellular proliferation in both the 4Br-PEN + US and 4Br-CN-PEN + US treatment groups compared to the control group. Throughout the treatment period, no significant reduction in body weight was observed in the mice, indicating good tolerance of the treatment (Fig. 5g). To evaluate systemic biocompatibility, histopathological analysis of major organs (heart, liver, spleen, lungs, and kidneys) was performed using H&E and TUNEL staining (Fig. S26). No observable histomorphological abnormalities or pathological lesions were detected across all treatment groups, further corroborating the excellent in vivo biosafety profiles of both 4Br-PEN and 4Br-CN-PEN.
Conclusions
In summary, we have successfully synthesized and comprehensively characterized two US-sensitive pentacene derivatives, 4Br-PEN and 4Br-CN-PEN. The introduction of two cyano groups in the 6,13-positions of 4Br-PEN extends the electron conjugation area, resulting in a narrowed HOMO–LUMO energy gap and a reduced ΔEST. These electronic modifications collectively enhance the propensity for 1O2 generation compared to that of 4Br-PEN. Comparative evaluation of the sonosensitizing capabilities between the two compounds revealed that the pentacene derivatives demonstrate enhanced sonodynamic performance under US irradiation. The in vitro SDT results demonstrated that, under identical US conditions, 4Br-CN-PEN exhibited enhanced cytotoxicity against 4T1 cells compared to 4Br-PEN. The in vivo SDT results revealed that both compounds exhibited potent anti-proliferative effects and excellent biocompatibility. Particularly, 4Br-CN-PEN demonstrated the most pronounced therapeutic efficacy, with a significant suppression of tumor growth being observed. This study demonstrates that pentacene derivatives hold considerable promise as highly efficient organic sonosensitizers, establishing a new paradigm for the design of sonosensitizers with improved therapeutic efficacy and biosafety.Experimental
Materials
1,2-Dibromo-4,5-dimethylbenzene, potassium iodide, stannous chloride, 1,2,4,5-tetrabromobenzene, potassium osmate(VI) dihydrate, tetramethylbenzidine (TMB) and 2,2,6,6-tetramethyl-4-piperidone hydrochloride (TEMP) were purchased from Bide Pharmatech Co., Ltd. N-Bromosuccinimide, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, benzoyl peroxide, 1,4-benzoquinone, hydroxylamine hydrochloride and sodium periodate were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. 4-Methylmorpholine N-oxide, phosphate buffered saline (PBS), singlet oxygen sensor green (SOSG) and diphenylisobenzofuran (DPBF) were purchased from Shanghai Macklin Biochemical Technology Co., Ltd. n-Butyllithium was purchased from Shanghai Titan Scientific Co., Ltd. Superoxide anion radical detection kit was purchased from Shanghai Yuanye Bio-Technology Co., Ltd. Methyl thiazolyl tetrazolium (MTT), Hoechst33342 and 2′-7′dichlorofluorescin diacetate (DCFH-DA) were purchased from Beyotime Institute of Biotechnology Co. LLC. All starting chemicals and solvents were purchased from commercial suppliers without further purification. 4T1, NCTC 1469 and L02 cell lines were obtained from Cell Bank of Chinese Academy of Sciences and cultured in RPMI 1640 medium supplemented with 10% FBS (Gibco) and penicillin/streptomycin (1%, w/v). Female BALB/c mice were purchased from Nanjing Qinglongshan Experimental Animal Center. Mice used in experiments were 6–8 weeks old. All animal experiments were performed in accordance with the National Institute of Health Guidelines under the protocols approved by the Animal Ethical and Welfare Committee of Nanjing University of Science and Technology (approval no.: AUCU-NUST2023012).Evaluation of sonodynamic effects
DPBF was used as a probe to detect the production of ROS. DPBF was dispersed in a DMF solution (1 mg mL−1), and then 100 μL of DPBF solution was mixed with 8 mL of 4Br-PEN or 4Br-CN-PEN aqueous solution. A 200 μL mixed solution was placed into each well of 96-well plates. Each well was exposed to US treatment (1 W cm−2, 1 MHz, 50% duty cycle) for different times. The absorbance of DPBF at 410 nm was then measured to detect ROS generation after US treatment.Detection of 1O2
The fluorescent probe SOSG was used to detect 1O2. 18 μL of the SOSG storage solution (5 mM) was diluted to 1800 μL with water to obtain the SOSG working solution (50 μM). Subsequently, 20 μL SOSG working solution was mixed with 180 μL of H2O, 4Br-PEN and 4Br-CN-PEN aqueous solutions in each well of a 96-well plate. Each well was exposed to US (1 W cm−2, 1 MHz, 50% duty cycle) for different times. The fluorescence intensity of the mixed solution was measured (λex = 504 nm, λem = 525 nm) after US treatment.ESR measurements of 1O2
TEMP was employed as a spin-trapping agent to detect 1O2. 4Br-PEN and 4Br-CN-PEN were dissolved in water at a dilution of 10 μM, respectively, and then 25 mM TEMP was added. The solution was irradiated with US (1 W cm−2, 1 MHz, 50% duty cycle), and the EPR signal was recorded at room temperature. As a comparison, a control group was also tested.Detection of ·OH
TMB was used as a probe to detect ·OH. TMB was dispersed in DMF solution (5 mg mL−1), and then 40 μL of the TMB solution was mixed with 3 mL of H2O, 4Br-PEN and 4Br-CN-PEN aqueous solutions. 200 μL mixed solution was placed in each well of a 96-well plate. Each well was exposed to US (1 W cm−2, 1 MHz, 50% duty cycle) for different durations. The absorbance of TMB at 652 nm was then measured for ·OH detection after US treatment.Detection of O2˙−
20 μL of H2O, 4Br-PEN and 4Br-CN-PEN aqueous solutions were added to each well in a 96-well plate. Each well was treated for different times with US (1 W cm−2, 1 MHz, 50% duty cycle). O2˙− lysis buffer (1 eq.) and hydroxylamine solution (2 eq.) were then added and mixed. After 20 min, sulfanilic acid solution (2 eq.) and naphthylamine solution (2 eq.) were added. The absorbance at 530 nm was measured after 30 min for O2˙− detection.In vitro cytotoxicity
4T1 cells were seeded into 96-well plates with 100 μL of culture medium at a density of 5000 cells per well and cultured for 12 h. Each well was washed 3 times with PBS, and the medium was replaced with serum-free medium containing different concentrations of 4Br-PEN and 4Br-CN-PEN for 8 h, followed by US treatment (1 min, 1 W cm−2, 1 MHz, 50% duty cycle). After 16 h of incubation, MTT solution (20 μL) was added to the culture medium, and the cells were further incubated for 4 h. The medium was then removed, and dimethyl sulfoxide (200 μL) was added to completely dissolve the product (formazan), after which the absorbance was measured at 570 nm using the microplate reader.Evaluation of biocompatibility
NCTC 1469 cells and L02 cells were seeded into 96-well plates with 100 μL of culture medium at a density of 5000 cells per well and cultured for 12 h. Each well was washed 3 times with PBS, and the medium was replaced with serum-free medium containing different concentrations of 4Br-PEN and 4Br-CN-PEN for 24 h of incubation. Then, MTT solution (20 μL) was added to the culture medium, and the cells were further incubated for 4 h. The medium was removed, and dimethyl sulfoxide (200 μL) was added to completely dissolve the product (formazan), after which the absorbance was measured at 570 nm using the microplate reader.Live/dead staining assay
4T1 cells were seeded into 6-well plates with 2 mL of culture medium at a density of 6 × 105 cells per well and cultured for 12 h. Each well was washed 3 times with PBS, followed by incubating with 4Br-PEN (112.8 μM) and 4Br-CN-PEN (112.8 μM) for 8 h. Cells were then treated with US (1 min, 1 W cm−2, 1 MHz, 50% duty cycle) and continued to incubate for 16 h. Afterwards, cells were washed with PBS and stained with calcein-AM (2 μM) and propidium iodide (PI, 8 μM) for 30 min. Finally, the 4T1 cells were observed by an inverted fluorescence microscope.Measurement of intracellular ROS levels
4T1 cells were seeded into 6-well plates with 2 mL of culture medium at a density of 6 × 105 cells per well and cultured for 12 h. Each well was washed 3 times with PBS, followed by incubating with 4Br-PEN (112.8 μM) and 4Br-CN-PEN (112.8 μM) for 10 h. After washing with PBS, the DCFH-DA probe was added and incubated for 15 min. Then, the US, 4Br-PEN + US, and 4Br-CN-PEN + US groups were treated with US (1 min, 1 W cm−2, 1 MHz, 50% duty cycle). Finally, the 4T1 cells were observed by an inverted fluorescence microscope.In vivo antitumor effects
For in vivo experiments, 1 × 107 4T1 cells were subcutaneously injected into female BALB/c mice. When the tumor volume reached ≈100 mm3, the mice were randomly assigned into three groups (n = 6 for each group) as follows: control (saline) + US; 4Br-PEN (112.8 μM) + US; 4Br-CN-PEN (112.8 μM) + US. The treatment process was as follows: after intratumoral injection of the corresponding drugs, US treatment (2 min, 1 W cm−2, 1 MHz, 50% duty cycle) was performed in each group every 2 days for 14 days. Tumor volumes and body weights were measured every 2 days. At the end of treatment, mice were sacrificed, and tumors were collected, weighted, imaged, and analyzed by H&E and TUNEL staining. The major organs, including heart, liver, spleen, lung, and kidneys, were also harvested and analyzed by H&E staining.Statistical analysis
All experimental results shown in this article were based on data from at least three separate experiments, and all data are presented as mean ± SD. Graphpad Prism 9.5 software was used for all statistical comparisons and data were analyzed via Student's t-test: *P < 0.05, **P < 0.005, ***P < 0.0005.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI).Supplementary information: instruments; experimental details; DFT calculations; NMR spectrum of compounds; additional figures including UV-vis spectral of 4Br-PEN and 4Br-CN-PEN, fluorescence intensity of 4Br-PEN, 4Br-CN-PEN and Ce6, temperature changes of 4Br-PEN and 4Br-CN-PEN under US irradiation, cytotoxicity of 4Br-PEN, 4Br-CN-PEN and Ce6, H&E and TUNEL staining major organ tissues. See DOI: https://doi.org/10.1039/d5md00851d.
Acknowledgements
This work was sponsored by the National Natural Science Foundation of China [grant number 22301136 and 22075144], the Natural Science Foundation of Jiangsu Province (BK20230942) and the Fundamental Research Funds for the Central Universities (30924010910).References
- A. N. Giaquinto, H. Sung, L. A. Newman, R. A. Freedman, R. A. Smith, J. Star, A. Jemal and R. L. Siegel, Breast cancer statistics, Ca-Cancer J. Clin., 2024, 74, 477–495 CrossRef PubMed
.
- Y. L. Liang, M. Z. Zhang, Y. J. Zhang and M. X. Zhang, Ultrasound Sonosensitizers for Tumor Sonodynamic Therapy and Imaging: A New Direction with Clinical Translation, Molecules, 2023, 28, 6484 CrossRef CAS PubMed
.
- W. P. Fan, B. Yung, P. Huang and X. Y. Chen, Nanotechnology for Multimodal Synergistic Cancer Therapy, Chem. Rev., 2017, 117, 13566–13638 CrossRef CAS PubMed
.
- Q. Y. Guo, Y. N. Tang, S. M. Wang and X. H. Xia, Applications and enhancement strategies of ROS-based non-invasive therapies in cancer treatment, Redox Biol., 2025, 80, 103515 CrossRef CAS PubMed
.
- H. B. Wang, D. Li, H. Q. Wang, Q. Y. Ren, Y. Pan, A. Y. Dao, D. L. Wang, Z. G. Wang, P. Y. Zhang and H. Y. Huang, Enhanced Sonodynamic Therapy for Deep Tumors Using a Self-Assembled Organoplatinum(II) Sonosensitizer, J. Med. Chem., 2024, 67(20), 18356–18367 CrossRef CAS PubMed
.
- J. Y. Zhu, A. Ouyang, J. Q. He, J. Xie, S. Banerjee, Q. L. Zhang and P. Y. Zhang, An ultrasound activated cyanine-rhenium(i) complex for sonodynamic and gas synergistic therapy, Chem. Commun., 2022, 58, 3314–3317 RSC
.
- S. Son, J. H. Kim, X. W. Wang, C. L. Zhang, S. A. Yoon, J. Shin, A. Sharma, M. H. Lee, L. Cheng, J. S. Wu and J. S. Kim, Multifunctional sonosensitizers in sonodynamic cancer therapy, Chem. Soc. Rev., 2020, 49, 3244–3261 RSC
.
- Q. Liu, Z. Lei, F. J. Shang, X. Y. Dong, E. W. Yang, Y.-M. Zhang, Q. L. Yu, X. F. Xu and Y. Liu, Harnessing Photoredox Cascade to Enhance Photodynamic Oncotherapy by Nanoformulated Macrocyclic Photosensitizer, CCS Chem., 2025, 1–18 Search PubMed
.
- X. T. Pan, H. Y. Wang, S. H. Wang, X. Sun, L. J. Wang, W. W. Wang, H. Y. Shen and H. Y. Liu, Sonodynamic therapy (SDT): a novel strategy for cancer nanotheranostics, Sci. China: Life Sci., 2018, 61, 415–426 CrossRef PubMed
.
- J. Guo, X. T. Pan, C. H. Wang and H. Y. Liu, Molecular Imaging-Guided Sonodynamic Therapy, Bioconjugate Chem., 2022, 33, 993–1010 CrossRef CAS PubMed
.
- D. G. You, V. G. Deepagan, W. Um, S. Jeon, S. Son, H. Chang, H. I. Yoon, Y. W. Cho, M. Swierczewska, S. Lee, M. G. Pomper, I. C. Kwon, K. Kim and J. H. Park, ROS-generating TiO2 nanoparticles for non-invasive sonodynamic therapy of cancer, Sci. Rep., 2016, 6, 23200 CrossRef CAS PubMed
.
- R. G. Liu, Q. Y. Zhang, Y. H. Lang, Z. Z. Peng and L. B. Li, Sonodynamic therapy, a treatment developing from photodynamic therapy, Photodiagn. Photodyn. Ther., 2017, 19, 159–166 CrossRef PubMed
.
- R. Kushwaha, V. Singh, S. Peters, A. K. Yadav, T. Sadhukhan, B. Koch and S. Banerjee, Comparative Study of Sonodynamic and Photoactivated Cancer Therapies with Re(I)-Tricarbonyl Complexes Comprising Phenanthroline Ligands, J. Med. Chem., 2024, 67, 6537–6548 CrossRef CAS PubMed
.
- N. Yang, J. M. Li, S. J. Yu, G. Y. Xia, D. Y. Li, L. L. Yuan, Q. L. Wang, L. J. Ding, Z. X. Fan and J. Y. Li, Application of Nanomaterial-Based Sonodynamic Therapy in Tumor Therapy, Pharmaceutics, 2024, 16, 603 CrossRef CAS PubMed
.
- J. J. Chen, Q. Zhou and W. W. Cao, Multifunctional Porphyrin-Based Sonosensitizers for Sonodynamic Therapy, Adv. Funct. Mater., 2024, 34, 2405844 CrossRef CAS
.
- B. B. Zhang, L. F. Mo, R. Huang, G. Chen, S. Y. Yang, X. Zhang, M. M. Sang, Y. Y. Zhao, Z. S. Li, R. F. Li, L. Z. Zhang and Y. C. Wang, Ultrasound-Enabled Sonogenetics: Pioneering Advances in Cancer Theranostics, Adv. Funct. Mater., 2024, 2418748 Search PubMed
.
- Y. M. Zhou, M. X. Wang and Z. F. Dai, The molecular design of and challenges relating to sensitizers for cancer sonodynamic therapy, Mater. Chem. Front., 2020, 4, 2223–2234 RSC
.
- X. H. Lin, J. B. Song, X. Y. Chen and H. H. Yang, Ultrasound-Activated Sensitizers and Applications, Angew. Chem., Int. Ed., 2020, 59, 14212–14233 CrossRef CAS PubMed
.
- S. Liang, J. J. Yao, D. Liu, L. Rao, X. Y. Chen and Z. H. Wang, Harnessing Nanomaterials for Cancer Sonodynamic Immunotherapy, Adv. Mater., 2023, 35, 2211130 CrossRef CAS PubMed
.
- F. H. Yang, J. Q. Lv, W. Ma, Y. L. Yang, X. M. Hu and Z. Yang, Engineering Sonosensitizer-Derived Nanotheranostics for Augmented Sonodynamic Therapy, Small, 2024, 20, 2402669 CrossRef CAS PubMed
.
- D. Li, Y. J. Zhu, W. W. Yin, X. T. Lin, G. Kim, Z. Y. Liu, S. Jung, J. Seo, S. Kim, J. S. Kim, H. Y. Huang and P. Y. Zhang, Small molecule sonosensitizers in cancer therapy: recent advances and clinical prospects, Chem. Soc. Rev., 2025, 54, 7610–7653 RSC
.
- A. A. Mandal, R. Kushwaha, A. K. Yadav and S. Banerjee, Metal Complexes for Cancer Sonodynamic Therapy, ChemBioChem, 2023, 24, e202200597 CrossRef CAS PubMed
.
- Q. Yu, J. Zhou, Q. Q. Tao, Y. Liu, H. Zhou, B. Kang and J.-J. Xu, Ultrasound-Activated Copper Matrix Nanosonosensitizer for Cuproptosis-Based Synergy Therapy, ACS Appl. Bio Mater., 2025, 8, 1503–1510 CrossRef CAS PubMed
.
- P. Chen, P. Zhang, N. H. Shah, Y. Y. Cui and Y. L. Wang, A Comprehensive Review of Inorganic Sonosensitizers for Sonodynamic Therapy, Int. J. Mol. Sci., 2023, 24, 12001 CrossRef CAS PubMed
.
- M. X. Li, Q. Y. Liu, S. Z. Xie, D. S. Weng, J. J. He, X. Y. Yang, Y. Y. Liu, J. Q. You, J. H. Liao, P. Wang, X. H. Lu and J. J. Zhao, Transformable Tumor Microenvironment-Responsive Oxygen Vacancy-Rich MnO2@Hydroxyapatite Nanospheres for Highly Efficient Cancer Sonodynamic Immunotherapy, Adv. Sci., 2025, 12, 2414162 CrossRef CAS PubMed
.
- A. K. Yadav, V. Singh, S. Acharjee, S. Saha, R. Kushwaha, A. Dutta, B. Koch and S. Banerjee, Sonodynamic Cancer Therapy by Mn(I)-tricarbonyl Complexes via Ultrasound-triggered CO Release and ROS Generation, Chem. – Eur. J., 2025, 31, e202403454 CrossRef PubMed
.
- L. Yan, L. Chang, Y. J. Tian, J. Y. Hu, Z. Cao, X. Guo and B. J. Geng, Graphene Quantum Dot Sensitized Heterojunctions Induce Tumor-Specific Cuproptosis to Boost Sonodynamic and Chemodynamic Enhanced Cancer Immunotherapy, Adv. Sci., 2025, 12, 2410606 CrossRef CAS PubMed
.
- Y. J. Liu, Z. Y. Li, F. Fan, X. J. Zhu, L. B. Jia, M. Q. Chen, P. W. Du, L. H. Yang and S. F. Yang, Boosting Antitumor Sonodynamic Therapy Efficacy of Black Phosphorus via Covalent Functionalization, Adv. Sci., 2021, 8, 2102422 CrossRef CAS PubMed
.
- D. H. Liao, J. F. Huang, C. Y. Jiang, L. Y. Zhou, M. B. Zheng, A. Nezamzadeh-Ejhieh, N. Qi, C. Y. Lu and J. Q. Liu, A Novel Platform of MOF for Sonodynamic Therapy Advanced Therapies, Pharmaceutics, 2023, 15, 2071 CrossRef CAS PubMed
.
- L. C. Nene and H. Abrahamse, Design consideration of phthalocyanines as sensitizers for enhanced sono-photodynamic combinatorial therapy of cancer, Acta Pharm. Sin. B, 2024, 14, 1077–1097 CrossRef CAS PubMed
.
- F. Y. Zheng, P. X. Zhang, Y. L. Zhang, H. X. Long, F. Y. Zhu, H. J. Chen and Y. Gao, Aptamer-Modified Mesoporous Silica Nanoparticle for Nitric Oxide-Enhanced Targeted Sonodynamic Therapy against Lung Cancer, ACS Appl. Nano Mater., 2025, 8, 5179–5192 CrossRef CAS
.
- Y. H. Li, X. J. Yang, Z. Wei, H. Niu, L. Y. Wu, C. J. Chen, H. N. Liu, T. Cai and H. D. Fan, Sulforaphane Wrapped in Self-Assembled Nanomicelle Enhances the Effect of Sonodynamic Therapy on Glioma, Pharmaceutics, 2025, 17, 34 CrossRef CAS PubMed
.
- Y. H. Zhu, Y. J. Li, X. W. Li, Y. Yu, L. P. Zhang, H. C. Zhang, C. Chen, D. Chen, M. S. Wang, N. Z. Xing, F. Y. Yang, W. Wasilijiang and X. J. Ye, Targeting Hypoxia and Autophagy Inhibition via Delivering Sonodynamic Nanoparticles With HIF-2α Inhibitor for Enhancing Immunotherapy in Renal Cell Carcinoma, Adv. Healthcare Mater., 2024, 13, 2402973 CrossRef CAS PubMed
.
- F. H. Yang, J. Q. Lv, W. Ma, Y. L. Yang, X. M. Hu and Z. Yang, Engineering Sonosensitizer-Derived Nanotheranostics for Augmented Sonodynamic Therapy, Small, 2024, 20, 2402669 CrossRef CAS PubMed
.
- X. D. Li, X. B. Sun, H. Chen, X. Y. Chen, Y. M. Li, D. M. Li, Z. Z. Zhang, H. J. Chen and Y. Gao, Exploring BODIPY derivatives as sonosensitizers for anticancer sonodynamic therapy, Eur. J. Med. Chem., 2024, 264, 116035 CrossRef CAS PubMed
.
- W. W. Zeng, Y. Xu, W. T. Yang, K. Liu, K. X. Bian and B. B. Zhang, An Ultrasound-Excitable Aggregation-Induced Emission Dye for Enhanced Sonodynamic Therapy of Tumors, Adv. Healthcare Mater., 2020, 9, 2000560 CrossRef CAS PubMed
.
- A. G. Santos, G. O. da Rocha and J. B. de Andrade, Occurrence of the potent mutagens 2-nitrobenzanthrone and 3-nitrobenzanthrone in fine airborne particles, Sci. Rep., 2019, 9, 1 CrossRef CAS PubMed
.
- H. F. Bettinger and C. Tönshoff, The Longest Acenes, Chem. Rec., 2015, 15, 364–369 CrossRef CAS PubMed
.
- W. H. Mills and M. Mills, CCXXX.—The synthetical production of derivatives of dinaphthanthracene, J. Chem. Soc., Trans., 1912, 101, 2194–2208 RSC
.
- H. Chung and Y. Diao, Polymorphism as an emerging design strategy for high performance organic electronics, J. Mater. Chem. C, 2016, 4, 3915–3933 RSC
.
- Q. Ye and C. Y. Chi, Recent Highlights and Perspectives on Acene Based Molecules and Materials, Chem. Mat., 2014, 26, 4046–4056 CrossRef CAS
.
- W. Q. Chen, F. Yu, Q. Xu, G. F. Zhou and Q. C. Zhang, Recent Progress in High Linearly Fused Polycyclic Conjugated Hydrocarbons (PCHs, n > 6) with Well-Defined Structures, Adv. Sci., 2020, 7, 1903766 CrossRef CAS PubMed
.
- B. S. Basel, C. Hetzer, J. Zirzlmeier, D. Thiel, R. Guldi, F. Hampel, A. Kahnt, T. Clark, D. M. Guldi and R. R. Tykwinski, Davydov splitting and singlet fission in excitonically coupled pentacene dimers, Chem. Sci., 2019, 10, 3854–3863 RSC
.
- L. Ueberricke, I. Ciubotaru, F. Ghalami, F. Mildner, F. Rominger, M. Elstner and M. Mastalerz, Di- and Tetracyano-Substituted Pyrene-Fused Pyrazaacenes: Aggregation in the Solid State, Chem. – Eur. J., 2020, 26, 11634–11642 CrossRef CAS PubMed
.
- T. Wang, R. Q. An, M. Q. Cao, H. Y. Shu, X. F. Wu, H. Tong and L. X. Wang, Nonfullerene acceptors with cyano-modified terminal groups for organic solar cells: Effect of substitution position on photovoltaic properties, Dyes Pigm., 2022, 206, 110661 CrossRef CAS
.
- A. Sharma, K. R. J. Thomas, K. K. Kesavan, I. Siddiqui, M. Ram Nagar and J.-H. Jou, Effect of Cyano on the Functional Properties of Phenanthroimidazole-Substituted Carbazole Derivatives, ACS Appl. Electron. Mater., 2021, 3, 3876–3888 CrossRef CAS
.
- F. Glöcklhofer, M. Lunzer and J. Fröhlich, Facile Synthesis of Cyanoarenes from Quinones by ReductiveAromatization of Cyanohydrin Intermediates, Synlett, 2015, 26, 950–952 CrossRef
.
- H. Miyamoto, K. Okada, K. Tada, R. Kishi and Y. Kitagawa, Theoretical Study on Singlet Fission Dynamics and Triplet Migration Process in Symmetric Heterotrimer Models, Molecules, 2024, 29, 5449 CrossRef CAS PubMed
.
- I. Kaur, W. L. Jia, R. P. Kopreski, S. Selvarasah, M. R. Dokmeci, C. Pramanik, N. E. McGruer and G. P. Miller, Substituent Effects in pentacenes: Gaining Control over HOMO–LUMO Gaps and Photooxidative Resistances, J. Am. Chem. Soc., 2008, 130, 16274–16286 CrossRef CAS PubMed
.
- F. Glöcklhofer, A. Petritz, E. Karner, M. J. Bojdys, B. Stadlober, J. Fröhlich and M. M. Unterlass, Dicyano- and tetracyanopentacene: foundation of an intriguing new class of easy-to-synthesize organic semiconductors, J. Mater. Chem. C, 2017, 5, 2603–2610 RSC
.
- M.-Y. Kuo, H.-Y. Chen and I. Chao, Cyanation: Providing a Three-in-One Advantage for the Design of n-Type Organic Field-Effect Transistors, Chem. – Eur. J., 2007, 13, 4750–4758 CrossRef CAS PubMed
.
- S. Oda, T. Sugitani, H. Tanaka, K. Tabata, R. Kawasumi and T. Hatakeyama, Development of Pure Green Thermally Activated Delayed Fluorescence Material by Cyano Substitution, Adv. Mater., 2022, 34, 2201778 CrossRef CAS PubMed
.
- M. H. Yang, H. C. Jin, J. H. Kim and D. W. Chang, Synthesis of Cyano-Substituted Conjugated Polymers for Photovoltaic Applications, Polymers, 2019, 11, 746 CrossRef CAS PubMed
.
- E. A. Margulies, N. Kerisit, P. Gawel, C. M. Mauck, L. Ma, C. E. Miller, R. M. Young, N. Trapp, Y.-L. Wu, F. Diederich and M. R. Wasielewski, Substituent Effects on Singlet Exciton Fission in Polycrystalline Thin Films of Cyano-Substituted Diaryltetracenes, J. Phys. Chem. C, 2017, 121, 21262–21271 CrossRef CAS
.
- T. Okamoto, C. Reese, M. L. Senatore, M. L. Tang, Y. Jiang, S. R. Parkin and Z. N. Bao, 2,9-Dibromopentacene: Synthesis and the role of substituent and symmetry on solid-state order, Synth. Met., 2010, 160, 2447–2451 CrossRef CAS
.
- C.-T. Chien, M. Watanabe and T. J. Chow, The synthesis of 2-halopentacenes and their charge transport properties, Tetrahedron, 2015, 71, 1668–1673 CrossRef CAS
.
- C. Tönshoff and H. F. Bettinger, The Influence of Terminal Push–Pull Substitution on the Electronic Structure and Optical Properties of pentacenes, Chem. – Eur. J., 2012, 18, 1789–1799 CrossRef PubMed
.
- T. Okamoto, M. L. Senatore, M.-M. Ling, A. B. Mallik, M. L. Tang and Z. Bao, Synthesis, Characterization, and Field-Effect Transistor Performance of pentacene Derivatives, Adv. Mater., 2007, 19, 3381–3384 CrossRef CAS
.
- H. Yamada, Y. Yamashita, M. Kikuchi, H. Watanabe, T. Okujima, H. Uno, T. Ogawa, K. Ohara and N. Ono, Photochemical Synthesis of pentacene and its Derivatives, Chem. – Eur. J., 2005, 11, 6212–6220 CrossRef CAS PubMed
.
- S. Katsuta, D. Miyagi, H. Yamada, T. Okujima, S. Mori, K.-i. Nakayama and H. Uno, Synthesis, Properties, and Ambipolar Organic Field-Effect Transistor Performances of Symmetrically Cyanated pentacene and Naphthacene as Air-Stable Acene Derivatives, Org. Lett., 2011, 13, 1454–1457 CrossRef CAS PubMed
.
- H. Uno, H. Watanabe, Y. Yamashita and N. Ono, Extremely large cavity assembled by self-interlocking of distorted biconcave porphyrins, Org. Biomol. Chem., 2005, 3, 448–453 RSC
.
- K. Hermann, S. Sardini, Y. Ruan, R. J. Yoder, M. Chakraborty, S. Vyas, C. M. Hadad and J. D. Badjić, Method for the Preparation of Derivatives of Heptiptycene: Toward Dual-Cavity Baskets, J. Org. Chem., 2013, 78, 2984–2991 CrossRef CAS PubMed
.
- C. T. Lin and T. C. Chou, 3,4,8,9-Tetramethylene-endo-2,5: endo-7,10-dietheno-cis-decalin. A new exocyclic diene bridged polycyclic hydrocarbon, J. Org. Chem., 1990, 55, 2252–2254 CrossRef CAS
.
- R. Gabioud and P. Vogel, Synthesis and Diels-Alder Reactivity of 5,6,7,8-Tetramethylidene-2-bicyclo[2.2.2]octanol and -octanone. Selective Oxidations of the Corresponding Bis(irontricarbonyl) Complexes, Helv. Chim. Acta, 1983, 66, 1134–1147 CrossRef CAS
.
- G. Y. Hu, J. R. Wang, L. L. Chen, H. Q. Zheng, Y. P. Lan, K. Y. Wang, X. J. Liu, H. P. You, Y. Luo and L. L. Dong, Small-Bandgap Transducers Induced Carrier Separation for In Situ Dual-Amplified Tumor Oxidative Stress, Adv. Funct. Mater., 2024, 34, 2401897 CrossRef CAS
.
- Y. Z. He, T. X. Wang, Y. R. Song, C. Fang, Y. Wang, X. L. Dong, Y. R. Wang, T. Y. Yu, Y. Shi, F. Zhang, K. Zhang and F. Wang, Targeting Vascular Destruction by Sonosensitizer-Free Sonocatalytic Nanomissiles Instigates Thrombus Aggregation and Nutrition Deprivation to Starve Pancreatic Cancer, Adv. Funct. Mater., 2024, 34, 2315394 CrossRef CAS
.
- Y. Zhang, S. J. Feng, X. Meng, J. Luo, Y. R. Xu and X. H. Ning, Identifying urotropine derivatives as co-donors of formaldehyde and nitric oxide for improving antitumor therapy, Chem. Commun., 2021, 57, 7581–7584 RSC
.
- Y. Q. Duan, Y. Yu, P. L. Liu, Y. Gao, X. Y. Dai, L. Zhang, L. Chen and Y. Chen, Reticular Chemistry-Enabled Sonodynamic Activity of Covalent Organic Frameworks for Nanodynamic Cancer Therapy, Angew. Chem., Int. Ed., 2023, e202302146 CAS
.
Footnote |
| † These authors contributed equally |
| This journal is © The Royal Society of Chemistry 2026 |