Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structure–activity relationships of hydrophobic small molecule irreversible inhibitors of tissue transglutaminase
Daniel A.
Wallace
,
Sarah
Tribe
 ,
Pauline
Navals
,
Christina
Bi
,
Tarasha
Sharma
and
Jeffrey W.
Keillor
,
Pauline
Navals
,
Christina
Bi
,
Tarasha
Sharma
and
Jeffrey W.
Keillor
 *
*
Department of Chemistry and Biomolecular Sciences, University of Ottawa, Ottawa, ON N6H 1N5, Canada. E-mail: jkeillor@uottawa.ca
First published on 11th November 2025
Abstract
Tissue transglutaminase (TG2) is both an enzyme and a G-protein that is implicated in many diseases, such that small molecule inhibitors of TG2 have broad potential as drugs or research tools. Previous work has demonstrated how the structure of EB-2-16, a highly potent irreversible inhibitor of TG2, has been optimised with respect to its warhead, tether and bridge moieties. In this work, we studied the structure–activity relationships of the pendant hydrophobic group of the scaffold. This confirmed the superior affinity conferred by the parent adamantyl moiety, over other cycloalkyl, aryl, biaryl and bridged biaryl groups. Additionally, some substituted adamantyl derivatives were shown to exhibit superior inhibitory efficiency over the parent inhibitor, with kinact/KI values over 106 M−1 min−1. The best inhibitors were shown to exhibit excellent lipid membrane permeability, but evaluation of their human hepatocyte stability revealed a sharp distinction between them. Despite the bromo- and iodoadamantyl derivatives being more efficient inhibitors, chloroadamantyl inhibitor 25b exhibits the best overall properties (kinact = 1.69 min−1, KI = 1.79 μM, kinact/KI = 941 × 103 M−1 min−1, Pe = 1.41 × 10−6 cm s−1, CLint = 6.91 μL min−1/106 cells) and suitability for potential applications in vivo.
Introduction
Tissue transglutaminase 2 (TG2) is a dynamic enzyme present in all tissue types of the human body. It is one of eight enzymes belonging to the transglutaminase (TGase) family (TG1–TG7 and Factor XIII), all of which catalyze transamidation reactions between peptide-bound glutamine and lysine residues, leading to the formation of Nε(γ-glutaminyl)lysine isopeptide bonds. This reaction, leading to the cross-linking of protein substrates via the side chains of their Gln and Lys residues, is mediated by an active site comprising a cysteine catalytic triad. TG2 is also uniquely multifunctional among the TGases; in addition to its catalytic activity, it has the ability to act as a G-protein, participating in cell signalling pathways and hydrolyzing GTP to GDP in the process.1,2 These two functions are mutually exclusive, as each requires a unique (and substantially dissimilar) enzyme conformation.3 TG2 can act as a transamidase only in its “open” conformation (Fig. 1B), which is favoured in the extracellular space due to relatively high Ca2+ concentrations. Conversely, the lower Ca2+ environment within cells favours the “closed” conformation (Fig. 1A),4 allowing TG2 to function as a G-protein and disfavouring the catalysis of transamidation.5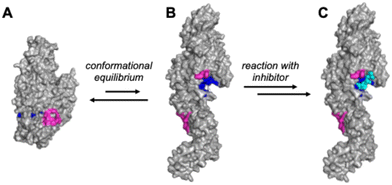 | ||
| Fig. 1 Conformational equilibrium and inhibition of TG2. A) Closed conformation (based on PDB code 4PYG)4 with assembled GTP-binding site shown in magenta. B) Open conformation (based on PDB code 2Q3Z) with substrate binding site shown in blue. C) Reaction with an irreversible inhibitor (cyan) locks TG2 in the open conformation. | ||
Dysregulation of either the transamidase or G-protein functions of TG2 has been found to contribute to multiple diseases, including celiac disease,6 fibrosis,7,8 and several types of cancer.9,10 Individuals with these conditions generally have few—if any—available treatment options. Small molecule TG2 inhibitors have been shown to hold therapeutic potential for the treatment of these diseases, with ZED1227 advancing to clinical trials for the treatment of celiac disease.11 Thus, the development of novel therapeutic agents that target TG2 function is considered a crucial avenue of research.
Inhibition of TG2 has been studied extensively, prompting the discovery of inhibitors with a wide range of structures and modes of inhibition.12–14 Some of the most effective inhibitors found to date are small molecules that irreversibly inactivate TG2's catalytic activity. These small molecule inhibitors often have Michael-acceptor warheads (such as acrylamides and α,β-unsaturated esters) that form an enzyme-inhibitor adduct via 1,4-addition of the electrophilic warhead with the thiolate15 form of the enzyme's active site cysteine residue (Cys277).16 Evidence suggests this covalent bond formation not only prevents Cys277 from catalyzing transamidation reactions, but also locks the enzyme in its open conformation (Fig. 1C), thereby abolishing its G-protein activity, regardless of the overall size of the inhibitor.17 The ability to inhibit both of the main activities of TG2 makes this form of covalent inhibition very promising for future research on—and treatment of—celiac disease, fibrosis, and cancer.
The structure of TG2 in complex with a peptidic inhibitor (PDB code 2Q3Z),5 determined using X-ray crystallography, has been useful in the design of peptidomimetic inhibitors. A hydrophobic pocket near the enzyme's catalytic site, sometimes referred to as the “D-site,” was identified and recognized as having significant potential for binding hydrophobic moieties. Many peptidomimetic TG2 inhibitors have subsequently been designed to bear a hydrophobic moiety intended to promote affinity with this D-site.18–25 However, we have shown26 that this crystallographic structure cannot be used to account for the relative affinity of small molecule inhibitors through docking studies. The structure of TG2 in the absence of a ligand may differ from the crystallographic structure obtained after reaction with a peptidic inhibitor, such that the structure derived from the latter cannot be used to predict how the free enzyme may bind small molecule inhibitors that do not resemble the crystallographic ligand.
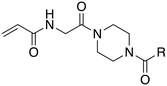
In the absence of structure-guided inhibitor design, we have undertaken more empirical studies in the exploration of small molecule inhibitor structures. One of the most effective TG2 inhibitors to date, known as EB-2-16 (Fig. 2), was discovered by the Griffin group in 2015.16 This small molecule possesses an acrylamide warhead attached to a glycine linker, piperazine bridge, and adamantane hydrophobic group. EB-2-16 is a highly efficient inhibitor that shows low toxicity and whose structure is amenable to facile modification. In previous studies, we have investigated the optimization of some of EB-2-16's four moieties. In a paper published by Mader et al.,27 a library of inhibitors with different warheads, including some reversible covalent, were evaluated to determine if any were superior to the original acrylamide. Only three warheads were found to be more efficient than acrylamide (chloroacrylamide, bromoacrylamide, and chloroacetamide), but each had higher off-target reactivity, suggesting the standard acrylamide remains the best warhead option. Similarly, in a study by Rangaswamy et al.,26 the glycine tether portion of the inhibitor was compared to tethers with longer carbon chains. Here, the original glycine tether was also the most effective. Recently, Ryan et al.28 explored variants of the bridge moiety and confirmed that the piperazine linker is superior to a large number of alkyl, cycloalkyl, and spirocyclic diamines. However, although some sections of this inhibitor scaffold have been investigated, a robust exploration into the hydrophobic moiety is still required. This missing scope inspired us to undertake the present SAR study, where we synthesized TG2 inhibitors bearing various hydrophobic groups on the scaffold of EB-2-16 and evaluated their inhibition efficiency. The most efficient inhibitors of this study were subjected to permeability and stability assays to further evaluate their feasibility as potential in vivo tools.
Results and discussion
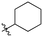
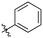
Our investigation into the structure–activity relationships of the hydrophobic moiety of this inhibitor scaffold was divided into three series wherein the hydrophobic group was varied, and the rest of the inhibitor remained unchanged. The first series replaced the parent adamantyl group with cycloalkyl and phenyl rings. The second series replaced adamantyl with modified biphenyl groups. The final series maintained the adamantyl group but bearing new substituents. Each novel inhibitor was evaluated under established continuous assay conditions,28 yielding the inhibition parameters kinact and KI, which were combined to produce the inhibition efficiency parameter, kinact/KI. The parameters of each inhibitor were compared to each other and to the parent inhibitor EB-2-16, to obtain insight into which characteristics of the inhibitor's hydrophobic group are favourable for inhibition of TG2.Though the adamantyl group of the parent compound was proposed16 to be one of the best hydrophobic moieties found for this scaffold, we noted that many other cyclic aliphatic moieties at this position had not been tested. To determine whether the bulky, three-dimensional adamantyl group is optimal, a series of inhibitors with different cycloalkyl hydrophobic groups was prepared, incorporating 3- to 7-membered rings (6a–e). Additionally, one inhibitor with a phenyl ring (6f) was included, to observe how aromaticity and planarity of the hydrophobic group may affect binding.
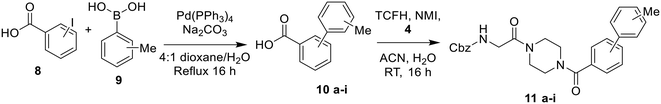
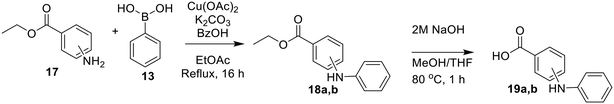
The synthesis of most inhibitors in this study passed through common intermediate 4, which was made via amide coupling between Cbz-glycine and Boc-piperazine, followed by removal of the Boc group to yield the hydrochloride salt of the free piperazine (Scheme 1). Next, this amine was coupled with each carboxylic acid bearing a hydrophobic group, under various conditions (Scheme 2). The final inhibitors were then obtained in two steps: removal of the Cbz group by hydrogenolysis, followed by coupling between the primary amine and acryloyl chloride to install the acrylamide warhead.
The inhibitors of this first series were synthesized via amide coupling between the free piperazine nitrogen of the common intermediate 4 and the commercially available cycloalkyl carboxylic acids to give 5a–f, followed by a deprotection reaction and a final coupling to the acrylamide warhead.
The cycloalkyl inhibitors of the first series did not show any improved inhibition over the parent compound, but a clear trend was observed showing a preference towards six-membered rings (cyclohexyl and phenyl), with inhibitory efficiencies decreasing as the rings become larger or smaller. Additionally, the increase in efficiency as the six-membered hydrophobic rings gained three-dimensional bulk (from phenyl to cycloalkyl to adamantyl) suggests the volume of the hydrophobic group also plays an important role in binding (Table 1).
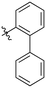
The next series of inhibitors included hydrophobic groups containing a biphenyl motif, starting with each biphenyl regioisomer—ortho, meta, and para—without further modifications. These designs were partially inspired by a discovery by the Griffin group16 of a very potent inhibitor known as EB-1-155 (kinact/KI = 1508 × 103 M−1 min−1)26 bearing dansyl as the hydrophobic group. The high efficiency of the dansyl inhibitor gives precedence to the idea of having large aromatic structures in this position (Table 2).
For these three regioisomers, the inhibition efficiency increased as the angle of projection of the pendant phenyl ring decreased, with the ortho biphenyl inhibitor (7a) having similar efficiency to the cyclohexyl (6d), and much improved efficiency over the mono-phenyl inhibitor (6f). Interestingly, though the first hydrophobic ring appears to be limited to the size of a six-membered ring, extension from that ring can improve inhibition, as is seen by the relatively high kinact/KI ratios of the ortho and meta inhibitors compared to the phenyl inhibitor (6f).
Additional biphenyl inhibitors were therefore prepared, introducing methyl substituents at different positions of the distal phenyl ring as well as bridging atoms between the two rings. Both the methyl additions and bridging atoms were chosen to probe for additional hydrophobic interactions. Incorporation of bridging atoms also sought to determine if additional freedom of rotation between the rings would be beneficial and if the presence of heteroatoms within the D-site could provide additional opportunities for binding.
The synthesis of the methyl-substituted inhibitors followed Scheme 3. Methyl-substituted biphenyl carboxylic acids were obtained from Suzuki couplings between the respective iodobenzoic acids and tolylboronic acids (Scheme 3). The resulting methyl-substituted hydrophobic groups were coupled to intermediate 4 as was done before.
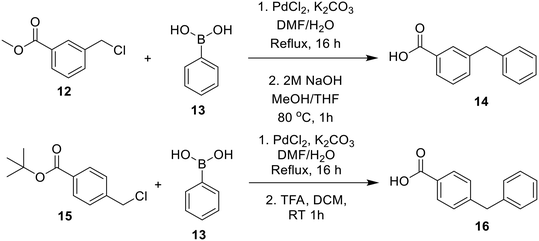
Bridged biphenyl carboxylic acids were obtained from a modified Suzuki coupling between the respective chloromethyl benzoate esters and boronic acid, followed by ester deprotection (Scheme 4). The remaining phenylaminobenzoic acids were synthesized from ethyl aminobenzoates and phenylboronic acid via Chan–Lam coupling. The resulting ethyl-protected phenylaminobenzoates were deprotected with aqueous NaOH to give the phenylaminobenzoic acids (Scheme 5). All the remaining bridged biphenyl precursors were purchased from chemical suppliers.
After synthesis of the bridged biphenyl carboxylic acids, they were coupled with intermediate 4 (Scheme 6) and then reacted following Scheme 2 to obtain the final inhibitors.
As shown in Table 3, the addition of methyl substituents to the distal phenyl rings was beneficial at some positions and detrimental at others. Compound 21d was the most efficient inhibitor of this series (kinact/KI = 636 × 103 M−1 min−1) and was the only inhibitor without an adamantyl group shown herein to approach the efficiency of EB-2-16. This result was unexpected, as 21d has the same meta-biphenyl backbone as 7b, which was less favoured than the unmodified ortho biphenyl inhibitor, 7a. Methyl additions to the ortho-biphenyl inhibitor only led to a slight improvement in efficiency when introduced at the 3′ position, and offered little overall improvement of the poor efficiency shown by the para-biphenyl scaffold.
Generally, incorporation of a bridging atom lowered inhibition efficiency, relative to the corresponding parent biphenyl compounds (22a–i, Table 4). This finding suggests either the extra freedom of rotation or increased length of the molecule (or a combination of the two) is not beneficial for binding. In most cases, the identity of the bridging group had a modest effect, with –O– and –NH– slightly increasing efficiency at the 2 position, –NH– decreasing efficiency at the 3 position, and both having little effect at the 4 position. These results provide some evidence that there are limited significant dipole interactions that can be exploited with heteroatoms at these positions, but does not necessarily rule out their potential with other hydrophobic motifs.
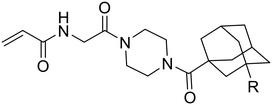
The final series of inhibitors maintained the adamantyl group of EB-2-16, but introduced substitutions on the ring, including the halogens and a phenyl group, to investigate any steric and electronic effects of substituents on the adamantane ring. An adamantyl sulfonamide was also synthesized to determine how alteration of the bond angle, from carboxamide to sulfonamide, would affect binding and inhibitory efficiency.
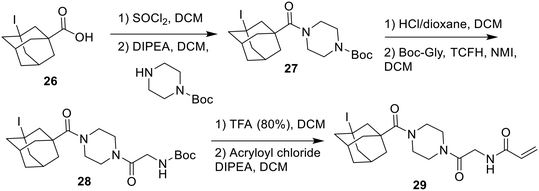
After unsuccessful attempts to couple adamantane carboxylic acids according to Scheme 2, it was found that these inhibitors could only be synthesized from the adamantane acid chloride (Scheme 7). After the in situ formation of the acid chloride from the adamantane carboxylic acid, the same procedure was followed as shown in Scheme 2.
The only inhibitor in this series that could not be accessed via this synthetic pathway was the iodo-adamantyl inhibitor (29). Iodine is known to act as a poison for palladium catalysts, suggesting that Cbz deprotection would not be facile synthetically.29 The iodo compound was therefore made following a stepwise procedure similar to that of Scheme 7, but using acid-labile protecting groups (Scheme 8).
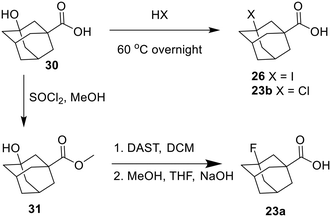
The halogenated adamantyl carboxylic acids had to be synthesized before incorporating them into the inhibitor. The I, Cl, and F inhibitors were all synthesized from a 3-hydroxyadamantane-1-carboxylic acid starting material, substituting the hydroxy for each halogen as shown in Scheme 9.30,31
3-Bromoadamantanecarboxylic acid was synthesized via a two-step in situ reaction between 1-adamantanecarboxylic acid and a mixture of concentrated H2SO4/HNO3 which selectively oxidized the tertiary 3-position of the adamantyl group to an alcohol. Next, HBr/AcOH was added to the mixture, replacing the tertiary alcohol with bromide, presumably via SN1 substitution.32 Then, 3-phenyladamantane-1-carboxylic acid was made via Friedel-Crafts arylation with benzene, catalyzed by AlBr3 (Scheme 10).33
This series gave the best inhibitory activities of all compounds tested; as shown in Table 5, all these substituents resulted in better inhibition than the parent compound (R = H). We also observed a clear trend whereby the efficiency of inhibition (as reflected in the kinact/KI ratio) increases with the size of the halide substituent. It should be noted that this trend is in contrast to the apparent efficiency reflected in the IC50 values reported in a recent patent, where the values of 54 nM, 58 nM and 119 nM were measured for the fluoro, chloro and bromo derivatives, respectively.34 This apparent discrepancy may be due to the IC50 values being measured by a different assay, with a different source of enzyme, and without taking time-dependence into account.35 It is also worth noting that the inhibitor bearing the largest substituent of the series, the phenyl group (25d), showed worse inhibitory efficiency than the bromo (25c) and iodo (29) derivatives. Taken together, we hypothesize that a substituent presenting a certain volume of diffuse electron density appears to be best suited for binding, over a larger volume such as phenyl or a smaller dense one such as fluoro. This size dependency was examined further by plotting the log(kinact/KI) values of the halide inhibitors against the relative size of the halide substituent. As shown in Fig. 3, this correlation illustrated that larger halogens result in more efficient inhibition, according to a linear free energy relationship with respect to their size parameter (v).36
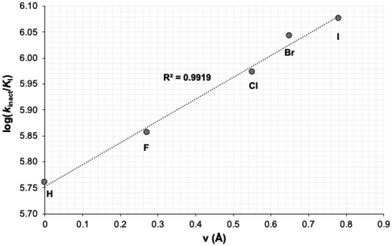 | ||
| Fig. 3 The linear relationship between log(kinact/KI) (in M−1 min−1) and the size index (v)36 of the halide substituent. | ||
Finally, an adamantane sulfonamide derivative was synthesized to test the importance of the bond angle of the parent amide inhibitor. Originally, we envisioned proceeding via a coupling reaction between the sulfonic acid and the scaffold amine (4), but all attempts to perform the coupling between the hindered sulfonyl chloride and the secondary amine failed. Therefore, the sulfonamide derivative (38) was synthesised by way of the sulfinamide intermediate (35), which was prepared by coupling the less hindered sulfinyl chloride (34) with the secondary amine, followed by oxidation to the sulfonamide using m-CBPA (Scheme 11).37
Evaluation of the sulfonamide inhibitor (38) provided a kinact of (0.97 ± 0.14) min−1 and KI of (7.56 ± 1.87) μM for a kinact/KI ratio of (129 ± 36) × 103 M−1 min−1. Compared to other derivatives, 38 proved to be a much less efficient inhibitor, due to its substantially higher KI value. This suggests the smaller C–S–N bond angle of the sulfonamide linkage cannot be accommodated in the binding site as easily as the C–C–N bond angle of amide linkage.
Once the efficiency of the compounds had been determined, the pharmacokinetic properties of the best inhibitors were tested. We have shown previously that EB-2-16 shows excellent bidirectional diffusion across the membrane of MDCK-MDR1 cells, and it is not susceptible to P-glycoprotein-mediated efflux.28 Considering the similarity of the adamantyl derivatives studied herein, and the structural requirements for P-glycoprotein affinity,38 all of them would be expected to show similarly excellent permeability properties. All the halide derivatives, the phenyl derivative (25d), and the parent EB-2-16 were evaluated (at Pharmaron, Inc.) in a parallel artificial membrane permeability assay (PAMPA). This assay is a measure of the compound's ability to pass through a lipid membrane, such as a cell membrane. In the case of the PAMPA testing, a lower −log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pe value indicates a more membrane permeable compound. All the tested compounds, except for the fluoro derivative, have −log
Pe value indicates a more membrane permeable compound. All the tested compounds, except for the fluoro derivative, have −log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pe values less than 6 (i.e. Pe >10−6 cm s−1), which may be considered excellent permeability. The phenyl substituted adamantane (25d) was the most cell permeable with a −log
Pe values less than 6 (i.e. Pe >10−6 cm s−1), which may be considered excellent permeability. The phenyl substituted adamantane (25d) was the most cell permeable with a −log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pe value of 4.88. The permeabilities of all the other derivatives were similar to that of the parent inhibitor EB-2-16, as expected (Table 6).
Pe value of 4.88. The permeabilities of all the other derivatives were similar to that of the parent inhibitor EB-2-16, as expected (Table 6).
| Compound | −log[Pe (cm s−1)] |
|---|---|
| EB-2-16 | 5.81 |
| 25a | 6.45 |
| 25b | 5.85 |
| 25c | 5.66 |
| 29 | 5.28 |
| 25d | 4.88 |
Next, the stability of the compounds in human hepatocytes was studied (at Pharmaron, Inc.). Mader et al. reported that the parent compound EB-2-16 has a half-life of over 120 minutes in human hepatocytes.27 In order to be able to make a direct comparison with this reference, we also evaluated the stability of our inhibitors in human hepatocytes (Table 7). We reasoned that any compound that may be pharmacologically useful should be at least as stable as the parent compound EB-2-16. Two of the most potent compounds, the iodo (29) and the bromo (25c) were first incubated in phosphate buffer (0.1 M, pH = 8.0), and were found to degrade after 24 hours, even with no hepatocytes present. This instability was also reflected in a 20 minute half-life in the presence of hepatocytes for the iodo compound (29). The phenyl derivative (25d) was slightly more stable, with a half-life in hepatocytes of around 47 minutes, but was still not as stable as the parent compound. The chloro derivative (25b), on the other hand, has a half-life of 200 minutes in human hepatocytes, and is at least as stable as the parent compound. Therefore, despite the bromo (25c) and iodo (29) derivatives being more efficient and having slightly better membrane permeability, the chloro compound (25b) appears to be the most suitable for in vivo application. It is important to note that this chloroadamantyl inhibitor, as well as inhibitors 25a, c and d, were also divulged in a more recent patent by Griffin et al.34 This patent revealed that inhibitor 25b has a low IC50 (58 nM), as measured in an assay monitoring the incorporation of biotinylated cadaverine (0.25 mM) into immobilized DMC using rhTG2; however, that inhibitor was not subjected to further evaluation. In the current study, our kinetic evaluations confirm its potency, and our study of its membrane permeability and hepatocyte stability confirm its suitability for future applications.
| Compound | In vitro t 1/2 (min) | In vitro CLint (μL min−1/106 cells) |
|---|---|---|
| EB-2-16 | >120 (ref. 27) | |
| 25b | 200 | 6.91 |
| 25d | 47.2 | 29.3 |
| 29 | 20.0 | 69.2 |
Conclusions
We present an extensive SAR study of the hydrophobic portion of the EB-2-16 scaffold. This study replaced the adamantane group of the parent compound with a range of cycloalkyl, biphenyl, bridged biphenyl, substituted biphenyl, and substituted adamantyl groups. Of the 33 compounds studied herein, 4 were previously divulged in a recent patent34 and 29 are novel compounds. Using a continuous assay, each of these compounds was rigorously evaluated to determine their kinetic parameters kinact and KI, for the first time. While most of the cycloalkyl and biphenyl modifications resulted in worse inhibition, it was found that substituting the adamantyl group of the parent compound gave better inhibition. The iodo (29), bromo (25c), and phenyl (25d) adamantane derivatives gave significantly increased inhibition efficiencies and had good membrane permeability; however, these compounds had much worse stability than the parent compound. On the other hand, the chloro derivative 25b also showed improved inhibition efficiency, increased stability and similar permeability relative to the parent compound. Based on these results, the chloroadamantyl inhibitor 25b appears to be the most suitable for future studies, and potentially for in vivo applications.Materials and methods
Synthesis
General procedure A: synthesis of methyl-substituted biphenyl carboxylic acids. To a round bottom flask equipped with a magnetic stir bar was added the iodobenzoic acid (1.0 eq.), tolylboronic acid (1.5 eq.), and Na2CO3 (3.0 eq.). A 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of dioxane/H2O was then added to the flask followed by Pd(PPh3)4 (0.05 eq.). The reaction mixture was stirred at reflux overnight (approx. 16 h). Upon completion, the mixture was diluted with equal volumes of EtOAc and H2O until the organic and aqueous phases separate. This mixture was then filtered through celite and transferred to a separatory funnel where it was acidified to pH = 1–2 using conc. HCl. and extracted with EtOAc (×3). The combined organic phases were dried over MgSO4, filtered and evaporated under vacuum. The resulting crude mixture was purified via column chromatography (5% MeOH/DCM on silica).
1 mixture of dioxane/H2O was then added to the flask followed by Pd(PPh3)4 (0.05 eq.). The reaction mixture was stirred at reflux overnight (approx. 16 h). Upon completion, the mixture was diluted with equal volumes of EtOAc and H2O until the organic and aqueous phases separate. This mixture was then filtered through celite and transferred to a separatory funnel where it was acidified to pH = 1–2 using conc. HCl. and extracted with EtOAc (×3). The combined organic phases were dried over MgSO4, filtered and evaporated under vacuum. The resulting crude mixture was purified via column chromatography (5% MeOH/DCM on silica).
General procedure B: synthesis of Cbz–Gly–PZ-hydrophobic intermediates. To a round bottom flask equipped with a magnetic stir bar was added the carboxylic acid (1.0 eq.) and TCFH (1.1 eq.), then solubilized in 10 mL ACN. NMI (3.5 eq.) was then added, and the mixture was left to stir for 15 minutes at R.T. Benzyl N-[2-oxo-2-(piperazin-1-yl)ethyl]carbamate HCl (4) (1.2 eq.) was then added to the flask with enough drops of H2O to dissolve the salt. The reaction was allowed to stir overnight (approx. 16 h) at R.T. under open atmosphere. Upon completion, the reaction mixture was diluted with 50 mL EtOAc and transferred to a separatory funnel where it was washed with 10% AcOH (×3), brine (×3), and 2 M NaOH (×3). The organic phase was dried over MgSO4, filtered, and the solvent was evaporated under vacuum. The resulting crude product was purified via column chromatography.
General procedure C: synthesis of Cbz–Gly–PZ-substituted adamantane intermediates. The product was obtained by adding the acid (1.0 eq.) to a round bottom flask charged with a stir bar and dissolving in DCM. Thionyl chloride (2.0 eq.) was added dropwise slowly and left to stir for 4 hours at room temperature in open atmosphere. The solvent was evaporated under vacuum, then co-evaporated with DCM (×3). This powder was dissolved directly in DCM. To this mixture was added DIPEA (2.0 eq.) and benzyl N-[2-oxo-2-(piperazin-1-yl)ethyl]carbamate HCl (4) (1.2 eq.), then left to stir overnight (approx. 16 h). The product was worked up using either work up A or B. The crude product was purified via flash silica column chromatography (if required).
Work up A: the solvent was removed and the residue was dissolved in EtOAc, and washed with 2 M NaOH (×3) and brine (×3). The organic phase was then dried over MgSO4, filtered, and evaporated under vacuum.
Work up B: the solvent was removed and the residue was dissolved in EtOAc, and washed with 10% AcOH (×3), brine (×2), saturated NaHCO3 (×3), and brine (×3). The organic phase was then dried over MgSO4, filtered, and evaporated under vacuum.
General procedure D: synthesis of final acrylamide inhibitors. A round bottom flask equipped with a magnetic stir bar was purged with N2 and sealed with a rubber stopper. To this flask was added the Cbz-protected Gly–PZ-Hyd intermediate (1.0 eq.), palladium on carbon (10% w/w), and dry MeOH. The atmosphere of the flask replaced with H2 with a balloon and the reaction was stirred until deemed complete by TLC (5% MeOH/DCM) and ninhydrin staining (between 2 and 16 h). Upon completion, the reaction mixture was filtered through celite, and the solvent was evaporated under vacuum. To this flask containing the free-amine intermediate was added a magnetic stir bar and dry DCM. DIPEA (3.0 eq.) was then added to the mixture, followed by acryloyl chloride (1.1 eq.) which was added dropwise slowly, either at room temperature or at 0 °C (the equivalencies were determined based on the amount of Cbz-protected starting material used). The reaction was allowed to stir for 4 h at R.T. or allowed to warm to room temperature, then washed using work up B or C. The product from the work up was washed with hexanes (×3) and purified via column chromatography.
Work up B: the solvent was removed and the product was dissolved in EtOAc, and washed with 10% AcOH (×3), brine (×2), saturated NaHCO3 (×3), and brine (×3). The organic phase was then dried over MgSO4, filtered, and evaporated under vacuum.
Work up C: the reaction was diluted with diluted with EtOAc and transferred to a separatory funnel where it was washed with 10% AcOH (×3) and brine (×3). The organic phase was dried over MgSO4, filtered, and the solvent was evaporated under vacuum.
1H NMR (600 MHz, CDCl3) δ 7.38–7.28 (m, 5H), 5.77 (br s, 1H), 5.12 (s, 2H), 4.06–3.94 (m, 2H), 3.64–3.56 (m, 2H), 3.51–3.40 (m, 4H), 3.40–3.28 (m, 2H), 1.47 (s, 9H). 13C NMR (150 MHz, CDCl3) δ 166.6, 156.2, 154.4, 136.3, 128.5, 136.3, 128.5, 128.1, 128.0, 80.5, 67.0, 44.2, 42.7, 41.8, 28.3.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C19H27N3O5Na: 400.1848, found: 400.1832.
1H NMR (600 MHz, D2O) δ 7.50–7.39 (m, 5H), 5.19–5.12 (m, 2H), 4.10 (s, 2H), 3.88–3.69 (m, 4H), 3.39–3.10 (m, 4H). 13C NMR (150 MHz, D2O) δ 169.7, 158.6, 136.3, 128.8, 128.5, 127.7, 67.3, 42.9, 42.8, 41.9, 41.4, 39.8.
HRMS (ESI-QTOF) m/z (M + H)+ calcd for C14H19N3O3H: 278.1505, Found: 278.1555.
1H NMR (600 MHz, CDCl3) δ 7.40–7.28 (m, 5H), 5.75 (br s, 1H), 5.13 (s, 2H), 4.08–3.97 (m, 2H), 3.79–3.56 (m, 6H), 3.53–3.30 (m, 2H), 1.75–1.67 (m, 1H), 1.04–1.00 (m, 2H), 0.86–0.76 (m, 2H). 13C NMR (150 MHz, CDCl3) δ 172.3, 156.2, 136.3, 128.6, 128.2, 128.1, 67.0, 45.1, 42.7, 42.0, 11.0, 7.8.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C18H23N3O4Na: 368.1586, found: 368.1592.
1H NMR (600 MHz, CDCl3) δ 7.39–7.28 (m, 5H), 5.80–5.69 (m, 1H), 5.12 (s, 2H), 4.08–3.95 (m, 2H), 3.68–3.54 (m, 4H), 3.42–3.30 (m, 4H), 3.30–3.19 (m, 1H), 2.40–2.29 (m, 2H), 2.21–2.12 (m, 2H), 2.03–1.93 (m, 1H), 1.92–1.84 (m, 1H). 13C NMR (150 MHz, CDCl3) δ 173.4, 173.3, 166.8, 166.6, 156.2, 136.3, 128.5, 128.2, 128.0, 67.0, 44.7, 44.5, 44.2, 42.6, 42.1, 41.8, 41.4, 41.2, 37.1, 37.1, 25.0, 17.9.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C19H25N3O4Na: 382.1743, found: 382.1778.
1H NMR (600 MHz, CDCl3) δ 7.39–7.28 (m, 5H), 5.83–5.70 (m, 1H), 5.13 (s, 2H), 4.08–3.96 (m, 2H), 3.70–3.58 (m, 4H), 3.58–3.47 (m, 2H), 3.44–3.35 (m, 2H), 2.92–2.83 (m, 1H), 1.86–1.78 (m, 4H), 1.78–1.70 (m, 2H), 1.62–1.54 (m, 2H). 13C NMR (150 MHz, CDCl3) δ 174.9, 174.7, 166.8, 166.6, 156.2, 136.3, 128.5, 128.1, 128.0, 67.0, 45.1, 45.0, 44.6, 44.2, 42.6, 42.1, 41.9, 41.5, 41.0, 30.1, 26.0.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C20H27N3O4Na: 396.1899, found: 396.1877.
1H NMR (600 MHz, CDCl3) δ 7.40–7.29 (m, 5H), 5.82–5.66 (m, 1H), 5.13 (s, 2H), 4.08–3.95 (m, 2H), 3.71–3.29 (m, 8H), 2.49–2.39 (s, 1H), 1.87–1.75 (m, 2H), 1.75–1.64 (m, 3H), 1.56–1.48 (m, 2H), 1.33–1.19 (m, 3H). 13C NMR (150 MHz, CDCl3) δ 174.9, 166.8, 156.2, 136.3, 128.5, 128.2, 128.0, 67.0, 44.9, 44.3, 42.6, 42.2, 41.9, 41.2, 40.5, 20.3, 25.7, 25.7.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C21H29N3O4Na: 410.2056, found: 410.2072.
1H NMR (600 MHz, CDCl3) δ 7.39–7.30 (m, 5H), 5.81–5.67 (m, 1H), 5.13 (s, 2H), 4.08–3.96 (m, 2H), 3.70–3.56 (m, 4H), 3.56–3.34 (m, 4H), 2.66–2.57 (m, 1H), 1.84–1.74 (m, 4H), 1.74–1.65 (m, 2H), 1.62–1.53 (m, 4H), 1.49–1.39 (m, 2H). 13C NMR (150 MHz, CDCl3) δ 176.1, 166.8, 156.2, 136.3, 128.5, 128.2, 128.1, 67.0, 45.1, 44.3, 42.6, 42.1, 41.5, 41.2, 31.2, 28.2, 26.6.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C22H31N3O4Na: 424.2212, found: 424.2216.
1H NMR (400 MHz, CDCl3) δ 7.48–7.38 (m, 5H), 7.38–7.29 (m, 5H), 5.75 (br s, 1H), 5.13 (s, 2H), 4.05 (s, 2H), 3.91–3.22 (m, 8H). 13C NMR (100 MHz, CDCl3) δ 170.7, 166.8, 156.2, 136.3, 134.9, 130.2, 128.7, 128.5, 128.2, 128.0, 127.1, 67.0, 47.3, 44.3, 42.6, 42.1.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C21H23N3O4Na: 404.1586, found: 404.1591.
1H NMR (600 MHz, CDCl3) δ 6.68 (br s, 1H), 6.32 (dd, J = 17.0, 1.4 Hz, 1H), 6.20 (dd, J = 17.0, 10.3 Hz, 1H), 5.69 (dd, J = 10.2, 1.6 Hz, 1H), 4.19–4.15 (m, 2H), 3.79–3.61 (m, 6H), 3.56–3.40 (m, 2H), 1.75–1.70 (m, 1H), 1.04–1.01 (m, 2H), 0.84–0.80 (m, 2H). 13C NMR (150 MHz, CDCl3) δ 172.4, 166.7, 165.4, 130.3, 127.0, 45.1, 44.3, 42.0, 41.7, 41.3, 11.0, 7.8.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C13H19N3O3Na: 288.1324, found: 288.1339.
1H NMR (600 MHz, CDCl3) δ 6.72–6.59 (m, 1H), 6.31 (d, J = 17.0 Hz, 1H), 6.19 (dd, J = 17.0, 10.3 Hz, 1H), 5.69 (d, J = 10.3 Hz, 1H), 4.18–4.09 (m, 2H), 3.70–3.59 (m, 4H), 3.45–3.34 (m, 4H), 3.26 (quint, J = 8.5 Hz, 1H), 2.35 (quint, J = 9.6 Hz, 2H), 2.21–2.13 (m, 2H), 2.04–1.94 (m, 1H), 1.93–1.85 (m, 1H). 13C NMR (150 MHz, CDCl3) δ 173.4, 173.3, 166.8, 166.5, 165.4, 130.3, 127.0, 44.7, 44.6, 44.5, 44.3, 42.2, 41.9, 41.4, 41.3, 41.2, 37.1, 25.3, 18.0.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C14H21N3O3Na: 302.1481, found: 302.1489.
1H NMR (600 MHz, CDCl3) δ 6.75–6.64 (m, 1H), 6.34 (dd, J = 17.0, 1.2 Hz, 1H), 6.22 (dd, J = 17.0, 10.3 Hz, 1H), 5.71 (d, J = 10.3 Hz, 1H), 4.22–4.16 (m, 2H), 3.74–3.55 (m, 6H), 3.50–3.42 (m, 2H), 2.91 (quint, J = 7.9 Hz, 1H), 1.90–1.81 (m, 4H), 1.80–1.72 (m, 2H), 1.66–1.59 (m, 2H). 13C NMR (150 MHz, CDCl3) δ 174.9, 169.4, 165.4, 130.3, 127.0, 44.3, 42.0, 41.3, 41.3, 41.1, 39.8, 39.2, 30.1, 26.0.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C15H23N3O3Na: 316.1637, found: 316.1618.
1H NMR (600 MHz, CDCl3) δ 6.73–6.60 (m, 1H), 6.32 (d, J = 17.1 Hz, 1H), 6.19 (dd, J = 17.0, 10.3 Hz, 1H), 5.69 (d, J = 10.3 Hz, 1H), 4.16 (m, 1H), 4.18–4.14 (m, 2H), 3.72–3.38 (m, 8H), 2.50–2.41 (m, 1H), 1.86–1.77 (m, 2H), 1.74–1.66 (m, 3H), 1.60–1.49 (m, 2H), 1.34–1.21 (m, 3H). 13C NMR (150 MHz, CDCl3) δ 174.9, 166.8, 165.4, 130.3, 127.0, 45.0, 44.9, 44.7, 44.3, 42.3, 42.0, 41.3, 40.5, 29.4, 25.8, 25.7.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C16H25N3O3Na: 330.1794, found: 330.1784.
1H NMR (600 MHz, CDCl3) δ 6.73–6.61 (m, 1H), 6.32 (dd, J = 17.1, 1.3 Hz, 1H), 6.19 (dd, J = 17.0, 10.3 Hz, 1H), 5.69 (d, J = 10.4 Hz, 1H), 4.19–4.14 (m, 2H), 3.72–3.60 (m, 4H), 3.57–3.50 (m, 2H), 3.49–3.39 (m, 2H), 2.66–2.59 (m, 1H), 1.84.1.75 (m, 4H), 1.74–1.66 (m, 2H), 1.64–1.56 (m, 5H), 1.50–1.41 (m, 2H). 13C NMR (150 MHz, CDCl3) δ 176.0, 166.6, 165.4, 130.3, 127.0, 45.1, 44.4, 44.3, 42.1, 41.6, 41.3, 31.2, 28.2, 26.6.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C17H27N3O3Na: 344.1950, found: 344.1933.
1H NMR (600 MHz, CDCl3) δ 7.49–7.39 (m, 5H), 6.66, (br s, 1H), 6.32 (dd, J = 17.0, 1.2 Hz, 1H), 6.19 (dd, J = 17.0, 10.3 Hz, 1H), 5.69 (dd, J = 10.3, 1.3 Hz, 1H), 4.17 (s, 2H), 3.93–3.31 (m, 8H). 13C NMR (150 MHz, CDCl3) δ 170.7, 166.7, 134.9, 130.3, 130.3, 128.7, 127.1, 127.0, 44.4, 42.0, 41.3.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C16H19N3O3Na: 324.1324, found: 324.1327.
1H NMR (600 MHz, CDCl3) δ 7.55–7.40 (m, 8H), 7.39–7.35 (m, 1H), 6.77–6.64 (m, 1H), 6.29 (dd, J = 17.1, 7.1 Hz, 1H), 6.16 (dd, J = 17.0, 10.3 Hz, 1H), 5.69 (d, J = 10.4 Hz, 1H), 4.14–3.83 (m, 4H), 3.50–3.40 (m, 1H), 3.39–3.27 (m, 1H), 3.00–2.88 (m, 2H), 2.87–2.79 (m, 1H), 2.16–1.95 (m, 1H). 13C NMR (150 MHz, CDCl3) δ 170.5, 170.4, 166.2, 166.2, 165.7, 139.5, 138.5, 138.3, 134.0, 130.2, 130.1, 129.9, 129.9, 129.4, 129.3, 128.8, 128.8, 128.8, 128.8, 128.2, 128.2, 128.1, 128.1, 128.0, 127.8, 127.5, 127.5, 46.0, 45.9, 43.6, 41.3, 41.2, 41.1.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C22H23N3O3Na: 400.1637, found: 400.1616.
1H NMR (600 MHz, CDCl3) δ 7.64–7.60 (m, 1H), 7.57–7.55 (m, 1H), 7.53–7.50 (m, 2H), 7.45 (t, J = 7.7 Hz, 1H), 7.39 (t, J = 7.9 Hz, 2H), 7.34–7.30 (m, 2H), 6.70 (br s, 1H), 6.26 (dd, J = 17.1 Hz, 1H), 6.13 (dd, J = 17.0, 10.3 Hz, 1H), 5.65 (d, J = 10.3 Hz, 1H), 4.12 (s, 2H), 3.86–3.26 (m, 8H). 13C NMR (150 MHz, CDCl3) δ 170.8, 166.7, 165.7, 141.9, 140.0, 135.2, 130.0, 129.2, 129.1, 129.0, 127.9, 127.5, 127.1, 125.8, 125.7, 47.2, 44.4, 42.1, 41.3.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C22H23N3O3Na: 400.1637, found: 400.1611.
1H NMR (600 MHz, CDCl3) δ 7.66 (d, J = 8.1 Hz, 2H), 7.60 (d, J = 7.8 Hz, 2H), 7.50 (d, J = 8.2 Hz, 2H), 7.47 (t, J = 7.5 Hz, 2H), 7.39 (t, J = 7.3 Hz, 1H), 6.68 (br s, 1H), 6.32 (d, J = 16.9 Hz, 1H), 6.20 (dd, J = 17.0, 10.2 Hz, 1H), 5.69 (d, J = 10.2 Hz, 1H), 4.24–4.11 (m, 2H), 3.95–3.37 (m, 8H). 13C NMR (150 MHz, CDCl3) δ 170.5, 166.7, 165.4, 143.3, 140.0, 133.5, 130.2, 128.9, 128.0, 127.7, 127.4, 127.2, 127.0, 44.5, 42.1, 41.3.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C22H23N3O3Na: 400.1637, found: 400.1634.
1H NMR (400 MHz, CDCl3) δ 8.04 (dd, J = 7.9, 1.4 Hz, 1H), 7.57 (td, J = 7.5, 1.4 Hz, 1H), 7.43 (td, J = 7.7, 1.2 Hz, 1H), 7.29–7.17 (m, 4H), 7.08 (d, J = 7.5 Hz, 1H), 2.07 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 171.1, 143.4, 141.1, 135.4, 132.4, 131.2, 130.8, 129.6, 128.9, 128.5, 127.4, 127.2, 125.3, 20.0.
Characterization is consistent with literature data.39
1H NMR (600 MHz, CDCl3) δ 7.93 (dd, J = 7.8, 1.2 Hz, 1H), 7.55 (td, J = 7.5, 1.3 Hz, 1H), 7.42 (td, J = 7.4, 1.1 Hz, 1H), 7.37 (d, J = 7.8 Hz, 1H), 7.28 (t, J = 8.0 Hz, 1H), 7.17 (d, J = 10.8 Hz, 2H), 7.14 (d, J = 7.7 Hz, 1H), 2.39 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 171.7, 143.3, 140.9, 137.7, 132.0, 131.4, 130.6, 129.2, 129.1, 128.2, 128.0, 127.1, 125.6, 21.5.
Characterization is consistent with literature data.39
1H NMR (600 MHz, CDCl3) δ 7.93 (d, J = 7.7 Hz, 1H), 7.55 (t, J = 7.6 Hz, 1H), 7.41 (t, J = 7.5 Hz, 1H), 7.37 (d, J = 7.8 Hz, 1H), 7.25 (d, J = 8.0 Hz, 2H), 7.21 (d, J = 7.9 Hz, 2H), 2.40 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 171.2, 143.2, 138.0, 137.2, 132.0, 131.2, 130.6, 129.1, 128.9, 128.4, 127.0, 21.2.
Characterization is consistent with literature data.40
1H NMR (600 MHz, CDCl3) δ 8.11–8.08 (m, 2H), 7.60–7.57 (m, J = 7.6 Hz, 1H), 7.53 (t, J = 7.8 Hz, 1H), 7.31–7.23 (m, 4H), 2.28 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 170.8, 142.4, 140.6, 135.3, 134.5, 130.9, 130.5, 129.7, 129.1, 128.6, 128.3, 127.8, 125.9, 20.4.
Characterization is consistent with literature data.41
1H NMR (600 MHz, CDCl3) δ 8.34 (t, J = 1.7 Hz, 1H), 8.08 (dt, J = 7.7, 1.3 Hz, 1H), 7.84 (d, J = 7.7, 1H), 7.55 (t, J = 7.7 Hz, 1H), 7.45 (m, 2H), 7.37 (t, J = 8.6 Hz, 1H), 7.21 (d, J = 7.5 Hz, 1H), 2.44 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 170.3, 141.8, 139.9, 138.6, 132.4, 129.5, 128.9, 128.9, 128.8, 128.6, 128.0, 124.3, 21.5.
Characterization is consistent with literature data.42
1H NMR (600 MHz, CDCl3) δ 8.33 (s, 1H), 8.06 (d, J = 7.7 Hz, 1H), 7.83 (d, J = 7.7 Hz, 1H), 7.56–7.52 (m, 3H), 7.28 (d, J = 8.0 Hz, 2H), 2.41 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 169.9, 141.6, 137.7, 137.0, 132.1, 129.7, 129.5, 129.0, 128.7, 128.6, 127.0, 21.1.
Characterization is consistent with literature data.43
1H NMR (600 MHz, CDCl3) δ 8.15 (d, J = 8.3 Hz, 2H), 7.44 (d, J = 8.4 Hz, 2H), 7.31–7.26 (m, 3H), 7.24 (d, J = 7.4 Hz, 1H), 2.28 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 170.0, 147.6, 140.7, 135.2, 130.6, 130.5, 130.1, 129.5, 129.4, 127.9, 127.4, 125.9, 125.2, 20.4.
Characterization is consistent with literature data.44
1H NMR (600 MHz, CDCl3) δ 8.17 (d, J = 8.4 Hz, 2H), 7.69 (d, J = 8.4 Hz, 2H), 7.45 (d, J = 10.2 Hz, 1H), 7.37 (t, J = 7.5 Hz, 1H), 7.23 (d, J = 7.4 Hz), 2.33 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 169.8, 146.6, 139.9, 138.6, 130.7, 129.1, 128.9, 128.1, 127.6, 127.2, 124.4, 21.5.
Characterization is consistent with literature data.45
1H NMR (600 MHz, CDCl3) δ 8.16 (d, J = 8.6 Hz, 2H), 7.69 (d, J = 8.5 Hz, 2H), 7.55 (d, J = 8.1 Hz, 2H), 7.29 (d, J = 7.9 Hz, 2H), 2.42 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 169.8, 146.4, 138.3, 137.0, 130.7, 129.7, 127.4, 127.2, 126.9, 21.2.
Characterization is consistent with literature data.46
1H NMR (600 MHz, CDCl3) δ 7.50–7.26 (m, 11H), 7.25–7.16 (m, 2H), 5.76–5.61 (m, 1H), 5.10 (s, 2H), 4.10–2.66 (m, 10), 2.34–2.12 (m, 3H). 13C NMR (150 MHz, CDCl3) δ 170.0, 166.3, 156.2, 137.3, 136.3, 135.5, 130.6, 129.6, 128.9, 128.5, 128.3, 128.3, 128.2, 128.1, 128.0, 127.8, 127.8, 125.4, 67.0, 45.9, 43.9, 42.5, 41.5, 41.2, 41.1, 39.0, 20.3, 20.2.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C28H29N3O4Na: 494.2056, found: 494.2072.
1H NMR (600 MHz, CDCl3) δ 7.52–7.46 (m, 1H), 7.46–7.38 (m, 3H), 7.37–7.27 (m, 8H), 7.20–7.15 (m, 1H), 5.66 (m, 1H), 5.10 (m, 2H), 4.02–3.75 (m, 4H), 3.49–3.24 (m, 2H), 3.00–2.77 (m, 3H), 2.39 (s, 3H), 2.16–1.90 (m, 1H). 13C NMR (150 MHz, CDCl3) δ 170.2, 170.1, 156.1, 139.5, 138.4, 136.3, 134.3, 129.9, 129.3, 128.9, 128.8, 128.5, 128.2, 128.1, 128.0, 127.9, 127.9, 125.9, 46.0, 45.9, 43.6, 42.5, 41.2, 41.1, 21.5.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C28H29N3O4Na: 494.2056, found: 494.2079.
1H NMR (600 MHz, CDCl3) δ 7.53–7.45 (m, 1H), 7.44–7.39 (m, 3H), 7.39–7.28 (m, 7H), 7.22 (t, J = 7.8 Hz, 2H), 5.70–5.61 (m, 2H), 5.12–5.07 (m, 2H), 3.96–3.93 (m, 1H), 3.86–3.73 (m, 3H), 3.48–3.30 (m, 2H), 3.10–2.85 (m, 2H), 2.85–2.73 (m, 1H), 2.38 (s, 3H), 2.31–2.02 (m, 1H). 13C NMR (150 MHz, CDCl3) δ 170.3, 170.2, 166.3, 166.3, 156.2, 138.4, 138.3, 138.1, 138.0, 136.6, 136.3, 134.3, 134.2, 129.9, 129.9, 129.5, 129.4, 129.3, 129.3, 128.6, 128.5, 128.2, 128.1, 128.9, 127.9, 127.8, 67.0, 46.0, 45.9, 43.6, 42.5, 42.5, 41.4, 41.2, 41.1, 41.1, 21.2.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C28H29N3O4Na: 494.2056, found: 494.2065.
1H NMR (600 MHz, CDCl3) δ 7.49 (t, J = 7.5 Hz, 1H), 7.44–7.38 (m, 2H), 7.38–7.30 (m, 6H), 7.29–7.23 (m, 3H), 7.21 (d, J = 7.3 Hz, 1H), 5.76 (br s, 1H), 5.12 (s, 2H), 4.05 (s, 2H), 3.89–3.34 (m, 8H), 2.27 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 170.6, 166.8, 156.2, 142.4, 140.6, 136.3, 135.1, 134.8, 131.1, 130.5, 129.7, 128.6, 128.2, 128.0, 127.8, 127.7, 126.0, 125.6, 47.1 44.4, 42.6, 42.1, 20.4.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C28H29N3O4Na: 494.2056, found: 494.2064.
1H NMR (600 MHz, CDCl3) δ 7.71–7.68 (m, 1H), 7.64 (s, 1H), 7.52 (t, J = 7.6 Hz, 1H), 7.43–7.31 (m, 9H), 7.22 (d, J = 7.4 Hz, 1H), 5.76 (br s, 1H), 5.15 (s, 2H), 4.08 (s, 2H), 3.94–3.29 (m, 8H), 2.45 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 170.7, 166.8, 156.2, 142.0, 140.0, 138.6, 136.3, 135.4, 129.1, 129.0, 128.9, 128.6, 128.6, 128.2, 128.1, 127.9, 125.8, 125.7, 124.2, 47.4, 44.4, 42.7, 21.5.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C28H29N3O4Na: 494.2056, found: 494.2072.
1H NMR (600 MHz, CDCl3) δ 7.66 (d, J = 7.8 Hz, 1H), 7.61 (s, 1H), 7.50–7.46 (m, 3H), 7.40–7.29 (m, 6H), 7.29–7.26 (m, 2H), 5.73 (br s, 1H), 5.13 (s, 2H), 4.06 (s, 2H), 3.92–3.24 (m, 8H), 2.40 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 170.7, 166.8, 156.2, 141.8, 137.8, 137.1, 136.3, 135.4, 129.7, 129.1, 128.7, 128.5, 128.2, 128.1, 126.9, 125.6, 125.5, 67.0, 44.4, 42.7, 42.2, 21.1.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C28H29N3O4Na: 494.2056, found: 494.2068.
1H NMR (400 MHz, CDCl3) δ 7.50–7.44 (m, 2H), 7.43–7.26 (m, 10H), 7.25–7.19 (m, 1H), 5.76 (br s, 1H), 5.13 (s, 2H), 4.07 (s, 2H), 3.89–3.41 (m, 8H), 2.28 (s, 3H). 13C NMR (100 MHz, CDCl3) δ 170.7, 167.3, 166.8, 156.2, 144.1, 140.8, 136.3, 135.2, 133.2, 130.5, 129.6, 129.5, 128.5, 128.2, 128.1, 127.8, 127.0, 125.9, 42.7, 20.4.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C28H29N3O4Na: 494.2056, found: 494.2060.
1H NMR (600 MHz, CDCl3) δ 7.64 (d, J = 8.2 Hz, 2H), 7.48 (d, J = 8.2 Hz, 2H), 7.43–7.27 (m, 8H), 7.19 (d, J = 7.3 Hz, 1H), 5.74 (br s, 1H), 5.13 (s, 3H), 4.07 (s, 2H), 3.88–3.33 (m, 8H), 2.43 (s,3 H). 13C NMR (100 MHz, CDCl3) δ 170.6, 167.3, 166.8, 156.2, 143.4, 140.0, 138.6, 136.3, 133.4, 128.8, 128.7, 128.5, 128.2, 128.1, 127.9, 127.7, 127.4, 124.3, 42.7, 67.0, 42.7, 21.5.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C28H29N3O4Na: 494.2056, found: 494.2076.
1H NMR (600 MHz, CDCl3) δ 7.63 (d, J = 8.2 Hz, 2H), 7.51–7.46 (m, 4H), 7.39–7.30 (m, 5H), 7.27 (d, J = 8.0 Hz, 2H), 5.75 (br s, 1H), 5.13 (s, 2H), 4.09 (s, 2H), 3.88–3.36 (m, 8H), 2.41 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 170.6, 166.8, 156.2, 143.2, 137.9, 137.1, 136.3, 133.2, 129.7, 128.7, 128.3, 128.2, 128.1, 127.7, 127.1, 127.0, 67.0, 42.7, 21.1.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C28H29N3O4Na: 494.2056, found: 494.2073.
1H NMR (600 MHz, CDCl3) δ 7.98–7.94 (m, 2H), 7.44 (d, J = 7.8 Hz, 1H), 7.39 (t, J = 7.6 Hz, 1H), 7.30 (t, J = 7.5 Hz, 2H), 7,24–7.18 (m, 3H), 4.05 (s, 2H). 13C NMR (150 MHz, CDCl3) δ 171.4, 141.7, 140.3, 134.4, 130.6, 129.3, 128.9, 128.7, 128.6, 128.1, 126.4, 41.7.
Characterization is consistent with literature data.47
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution of MeOH and H2O to give a white powder (155 mg, 49%).
1 solution of MeOH and H2O to give a white powder (155 mg, 49%).
1H NMR (600 MHz, CDCl3) δ 8.00 (d, J = 8.2 Hz, 2H), 7.32–7.27 (m, 4H), 7.23 (t, J = 7.4 Hz, 1H), 7.18 (d, J = 7.4 Hz, 2H), 4.05 (s, 2H). 13C NMR (150 MHz, CDCl3) δ 170.2, 147.5, 139.9, 130.5, 129.1, 129.0, 128.6, 127.0, 126.4, 42.0.
Characterization is consistent with literature data.48
1H NMR (400 MHz, CDCl3) δ 7.74–7.70 (m, 1H), 7.60–7.59 (m, 1H), 7.34–7.23 (m, 4H), 7.09 (d, J = 7.6 Hz, 2H), 6.98 (t, J = 7.4 Hz, 1H), 5.86 (br s, 1H), 4.37 (q, J = 7.1 Hz, 2H), 1.38 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 166.6, 143.5, 142.5, 131.7, 129.5, 129.3, 121.8, 121.7, 121.4, 118.3, 118.3, 61.0, 14.3.
Characterization is consistent with literature data.49
1H NMR (600 MHz, CDCl3) δ 7.93 (d, J = 8.7 Hz, 2H), 7.30–7.27 (m, 2H), 7.17 (d, J = 7.7 Hz, 2H), 7.06 (t, J = 7.5 Hz, 1H), 6.99 (d, J = 8.7 Hz, 2H), 6.01 (br s, 1H), 4.34 (q, J = 7.1 Hz, 2H), 1.38 (t, J = 7.1 Hz, 3H). 13C NMR (150 MHz, CDCl3) δ 166.5, 147.9, 140.9, 131.4, 129.5, 123.0, 121.5, 120.3, 114.6, 60.4, 14.4.
Characterization is consistent with literature data.50
1H NMR (400 MHz, CDCl3) δ 7.78 (s, 1H), 7.64 (d, J = 7.5 Hz, 1H), 7.38–7.28 (m, 4H), 7.11 (d, J = 7.5 Hz, 2H), 7.00 (t, J = 7.4 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 171.3, 143.7, 142.1, 130.3, 129.5, 129.5, 122.3, 122.1, 122.0, 118.6, 118.4.
Characterization is consistent with literature data.51
1H NMR (600 MHz, CDCl3) δ 7.98 (d, J = 8.9 Hz, 2H), 7.37–7.34 (m, 2H), 7.20 (d, J = 7.8 Hz, 2H), 7.10 (t, J = 7.4 Hz, 1H), 7.00 (d, J = 8.8 Hz, 2H). 13C NMR (150 MHz, CDCl3) δ 171.5, 148.9, 140.5, 132.3, 129.5, 123.5, 120.8, 119.9, 114.4.
Characterization is consistent with literature data.50
1H NMR (600 MHz, CDCl3) δ 7.41–7.29 (m, 7H), 7.29–7.26 (m, 1H), 7.25–7.20 (m, 2H), 7.18–7.13 (m, 1H), 7.13–7.08 (m, 3H), 5.74–5.66 (m, 1H), 5.16–5.08 (m, 2H), 4.23 (t, J = 14.0 Hz, 1H), 4.01 (s, 1H), 3.93–3.78 (m, 2H), 3.72–3.65 (m, 0.5H), 3.65–3.56 (m, 2H), 3.50–3.42 (m, 0.5 H), 3.40–3.32 (m, 0.5H), 3.30–3.20 (m, 1H), 3.02–2.89 (m, 1.5H), 2.80–2.72 (m, 0.5H), 2.55–2.43 (m, 1H), 2.38–2.28 (m, 0.5H). 13C NMR (150 MHz, CDCl3) δ 169.9, 169.8, 166.6, 166.5, 156.2, 140.3, 140.2, 138.6, 136.3, 135.3, 135.2, 131.4, 131.3, 129.5, 129.2, 129.2, 128.5, 128.5, 128.4, 128.2, 128.0, 126.5, 126.3, 67.0, 46.2, 46.2, 43.8, 43.6, 42.6, 42.5, 41.5, 41.3, 40.9, 40.8, 39.1.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C28H29N3O4Na: 494.2056, found: 494.2045.
1H NMR (600 MHz, CDCl3) δ 7.42–7.28 (m, 9H), 7.20–7.11 (m, 2H), 6.98 (d, J = 7.9 Hz, 2H), 6.89 (t, J = 7.9 Hz, 1H), 5.80–5.69 (m, 1H), 5.12 (s, 2H), 4.13–3.21 (m, 10H). 13C NMR (150 MHz, CDCl3) δ 167.3, 167.2, 166.7, 166.5, 156.2, 156.1, 153.1, 152.9, 136.3, 131.0, 130.0, 130.0, 129.0, 128.9, 128.5, 128.2, 128.1, 128.0, 124.1, 124.0, 123.8, 119.0, 118.9, 118.3, 118.1, 46.7, 46.6, 44.6, 44.2, 42.6, 42.3, 41.8, 41.5, 41.1.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C27H27N3O5Na: 496.1848, found: 496.1844.
1H NMR (600 MHz, CDCl3) δ 7.41 (d, J = 8.3 Hz, 1H), 7.39–7.32 (m, 5H), 7.32–7.26 (m, 3H), 7.18 (d, J = 7.4 Hz, 1H), 7.10 (d, J = 8.0 Hz, 2H), 6.99 (t, J = 7.4 Hz, 1H), 6.92 (t, J = 7.5 Hz, 1H), 5.75 (br s, 1H), 5.14 (s, 2H), 4.08–3.97 (m, 2H), 3.80–3.56 (m, 6H), 3.48–3.34 (m, 2H). 13C NMR (150 MHz, CDCl3) δ 170.2, 166.7, 156.2, 142.6, 141.9, 136.3, 131.0, 129.4, 128.5, 128.2, 128.1, 128.0, 122.3, 121.9, 119.8, 119.0, 117.4, 67.0, 44.4, 42.6, 42.1.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C27H28N4O4Na: 495.2032, found: 495.2038.
1H NMR (600 MHz, CDCl3) δ 7.38–7.26 (m, 9H), 7.25–7.20 (m, 2H), 7.20–7.16 (m, 3H), 5.73 (br s, 1H), 5.13 (s, 2H), 4.04 (s, 2H), 4.01 (s, 2H), 3.88–3.19 (m, 8H). 13C NMR (150 MHz, CDCl3) δ 170.7, 166.8, 156.2, 142.0, 140.2, 136.3, 135.0, 130.8, 129.0, 128.8, 128.6, 128.5, 128.2, 128.1, 127.6, 126.4, 124.9, 67.0, 47.2, 44.3, 42.6, 41.7.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C28H29N3O4Na: 494.2056, found: 494.2082.
1H NMR (600 MHz, CDCl3) δ 7.42–7.28 (m, 8H), 7.16 (t, J = 11.2 Hz, 1H), 7.13–7.06 (m, 2H), 7.06–6.99 (m, 3H), 5.75 (br s, 1H), 5.12 (s, 2H), 4.04 (s, 2H), 3.89–3.24 (m, 8H). 13C NMR (150 MHz, CDCl3) δ 169.8, 166.8, 157.9, 156.2, 136.5, 136.3, 130.2, 130.0, 128.5, 128.2, 128.0, 124.1, 121.4, 120.0, 119.5, 116.8, 67.0, 42.6.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C27H27N3O5Na: 496.1848, found: 496.1848.
1H NMR (600 MHz, CDCl3) δ 7.38–7.27 (m, 9H), 7.25–7.21 (m, 2H), 7.20–7.16 (m, 3H), 5.74 (br s, 1H), 5.13 (s, 2H), 4.08–4.02 (m, 2H), 3.87–3.15 (m, 8H). 13C NMR (150 MHz, CDCl3) δ 170.7, 166.8, 156.2, 142.0, 140.2, 136.3, 135.0, 130.8, 129.0, 128.8, 128.6, 128.5, 128.2, 128.1, 127.6, 126.4, 124.9, 67.0, 44.3, 42.6, 41.7.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C27H28N4O4Na: 495.2008, found: 495.2013.
1H NMR (600 MHz, CDCl3) δ 7.38–7.28 (m, 9H), 7.25 (d, J = 8.1 Hz, 2H), 7.22 (t, J = 7.5 Hz, 1H), 7.18 (d, J = 7.6 Hz, 2H), 5.74 (br s, 1H), 5.12 (s, 2H), 4.04 (s, 2H), 4.01 (s, 2H), 3.88–3.20 (m, 8H). 13C NMR (150 MHz, CDCl3) δ 170.7, 166.8, 156.2, 143.7, 140.2, 136.3, 132.6, 129.2, 129.0, 128.6, 128.5, 128.0, 127.4, 126.4, 67.0, 44.4, 42.6, 42.0, 41.8.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C28H29N3O4Na: 494.2056, found: 494.2036.
1H NMR (600 MHz, CDCl3) δ 7.44–7.37 (m, 4H), 7.37–7.29 (m, 5H), 7.18 (t, J = 7.4 Hz, 1H), 7.05 (d, J = 7.7 Hz, 1H), 7.02 (d, J = 8.6 Hz, 1H), 5.74 (br s, 1H), 5.13 (s, 2H), 4.06 (s, 2H), 3.79–3.38 (m, 8H). 13C NMR (150 MHz, CDCl3) δ 170.4, 166.8, 159.5, 156.2, 155.9, 136.3, 130.0, 129.3, 128.8, 128.5, 128.2, 128.1, 124.3, 119.8, 118.0, 67.0, 44.4, 42.6, 42.1.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C27H27N3O5Na: 496.1872, found: 496.1865.
1H NMR (600 MHz, CDCl3) δ 7.38–7.29 (m, 9H), 7.14 (d, J = 7.6 Hz, 2H), 7.06–7.00 (m, 3H), 5.93 (br s, 1H), 5.76 (br s, 1H), 5.13 (s, 2H), 4.08–3.99 (m, 2H), 3.75–3.58 (m, 6H), 3.49–3.38 (m, 2H). 13C NMR (150 MHz, CDCl3) δ 170.8, 166.8, 156.2, 145.8, 141.3, 136.3, 129.5, 129.4, 128.5, 128.2, 128.1, 125.7, 122.7, 119.7, 115.5, 67.0, 44.4, 42.7, 42.1.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C27H28N4O4Na: 495.2008, found: 495.1980.
1H NMR (600 MHz, CDCl3) δ 7.51–6.99 (m, 8H), 6.68–6.50 (m, 1H), 6.29 (d, J = 17.0 Hz, 1H), 6.16 (dd, J = 17.0, 10.3 Hz, 1H), 5.67 (d, J = 10.2 Hz, 1H), 4.14–3.93 (m, 2H), 3.91–2.65 (m, 8H), 2.40–2.10 (m, 3H). 13C NMR (150 MHz, CDCl3) δ 170.6, 166.7, 165.4, 142.0, 140.0, 138.6, 135.4, 130.2, 129.1, 129.0, 128.9, 128.6, 127.9, 127.0, 125.8, 125.7, 124.2, 44.4, 42.0, 41.2, 21.5.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C23H25N3O3Na: 414.1794, found: 414.1776.
1H NMR (600 MHz, CDCl3) δ 7.52–7.47 (m, 1H), 7.46–7.40 (m, 3H), 7.33–7.26 (m, 3H), 7.18 (d, J = 7.0 Hz, 1H), 6.59 (m, 1H), 6.28 (dd, J = 17.3, 7.8 Hz, 1H), 6.15 (dd, J = 17.0, 10.3 Hz, 1H), 5.66 (d, J = 10.4 Hz, 1H), 4.12–4.01 (m, 1H), 3.98–3.82 (m, 2.5H), 3.50–3.39 (m, 1H), 3.39–3.25 (m, 1H), 3.06–3.00 (m, 0.5H), 2.97–2.87 (m, 2H), 2.87–2.77 (m, 1H), 2.39 (s, 3H), 2.18–1.94 (m, 1H). 13C NMR (150 MHz, CDCl3) δ 170.2, 170.1, 166.2, 166.1, 165.3, 139.5, 138.5, 138.5, 138.4, 138.4, 134.3, 130.2, 130.2, 129.9, 129.9, 129.4, 129.4, 129.3, 129.3, 128.9, 128.8, 128.7, 128.6, 128.9, 127.9, 127.9, 127.0127.0, 125.9, 46.0, 45.9, 43.6, 43.6, 41.3, 41.2, 41.1, 41.1, 41.1, 21.5.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C23H25N3O3Na: 414.1794, found: 414.1783.
1H NMR (600 MHz, CDCl3) δ 7.51–7.46 (m, 1H), 7.45–7.39 (m, 3H), 7.39–7.35 (m, 2H), 7.25–7.19 (m, 2H), 6.64–6.53 (m, 1H), 6.28 (dd, J = 17.1, 7.2 Hz, 1H), 6.15 (dd, J = 17.0, 10.1 Hz, 1H), 5.66 (dd, J = 10.3 Hz, 1H), 4.07 (d, J = 4.1 Hz, 1H), 3.94 (d, J = 3.9 Hz, 1H), 3.88–3.75 (m, 1H), 3.48–3.31 (m, 2H), 3.11–2.86 (m, 3H), 2.86–2.75 (m, 1H), 2.38 (s, 3H), 2.33–2.06 (m, 1H). 13C NMR (150 MHz, CDCl3) δ 170.3, 170.2, 166.3, 166.2, 165.3, 138.4, 138.3, 138.1, 138.0, 136.6, 134.2, 130.2, 130.2, 129.9, 129.9, 128.6, 128.6, 127.9, 127.8, 127.8, 127.8, 127.0, 127.0, 46.0, 45.9, 43.7, 41.4, 41.2, 41.2, 41.1, 41.1, 41.0.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C23H25N3O3Na: 414.1794, found: 414.1794.
1H NMR (600 MHz, CDCl3) δ 7.49 (t, J = 7.6 Hz, 1H), 7.45–7.39 (m, 2H), 7.36 (s, 1H), 7.31–7.27 (m, 2H), 7.27–7.24 (m, 2H), 7.23–7.19 (m, 1H), 6.67 (br s, 1H), 6.32 (d, J = 17.1 Hz, 1H), 6.19 (dd, J = 17.0, 10.3 Hz, 1H), 5.69 (d, J = 10.3 Hz, 1H), 4.17 (s, 2H), 3.95–3.35 (m, 8H), 2.27 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 170.6, 166.7, 165.4, 142.4, 140.6, 135.2, 134.7, 131.1, 130.5, 130.2, 129.7, 128.6, 127.8, 127.7, 127.0, 126.0, 125.6, 44.4, 42.1, 41.3, 20.5.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C23H25N3O3Na: 414.1794, found: 414.1798.
1H NMR (600 MHz, CDCl3) δ 7.68 (m, 1H), 7.62 (m, 1H), 7.50 (t, J = 7.7 Hz, 1H), 7.41–7.32 (m, 4H), 7.20 (d, J = 7.2 Hz, 1H), 6.66 (br s, 1H), 6.32 (dd, J = 1.2, 17.0 Hz, 1H), 6.19 (dd, J = 10.3, 17.0 Hz, 1H), 5.69 (dd, J = 1.4, 10.2 Hz, 1H), 4.17 (s, 2H), 3.95–3.31 (m, 8H), 2.43 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 170.7, 166.7, 165.4, 142.0, 140.0, 138.6, 135.4, 130.2, 129.1, 129.0, 128.9, 128.6, 127.9, 127.1, 125.8, 125.7, 124.2, 41.3, 21.5.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C23H25N3O3Na: 414.1794, found: 414.1819.
1H NMR (600 MHz, CDCl3) δ 7.68–7.65 (m, 1H), 7.62–7.60 (m, 1H), 7.51–7.47 (m, 3H), 7.36–7.34 (m, 1H), 7.28–2.26 (m, 2H), 6.66 (br s, 1H), 6.32 (dd, J = 1.3, 17.1 Hz, 1H), 6.19 (dd, J = 10.3, 17.0 Hz, 1H), 5.69 (dd, J = 1.3, 10.3 Hz, 1H), 4.17 (s, 2H), 3.95–3.35 (m, 8H), 2.40 (s, 3H)13C NMR (150 MHz, CDCl3) δ 170.7, 166.7, 165.4, 141.8, 137.8, 137.1, 135.4, 130.2, 129.7, 129.1, 128.8, 127.0, 126.9, 125.6, 125.5, 47.3, 42.1, 41.3, 21.1.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C23H25N3O3Na: 414.1794, found: 414.1796.
1H NMR (600 MHz, CDCl3) δ 7.47 (d, J = 8.3 Hz, 2H), 7.40 (d, J = 8.3 Hz, 2H), 7.30–7.25 (m, 3H), 7.23–7.20 (m, 1H), 6.66 (br s, 1H), 6.33 (dd, J = 17.0, 1.3 Hz, 1H), 6.20 (dd, J = 17.0, 10.3 Hz, 1H), 5.70 (dd, J = 10.3, 1.4 Hz, 1H), 4.19 (s, 1H), 3.91–3.42 (m, 8H), 2.28 (s, 3H). 13C NMR (150 MHz, CDCl3) 170.7, 166.7, 165.4, 144.2, 135.2, 133.2, 130.5, 130.2, 129.6, 129.5, 127.8, 127.1, 127.0, 125.9, 41.3, 20.4.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C23H25N3O3Na: 414.1794, found: 414.1791.
1H NMR (600 MHz, CDCl3) δ 7.67 (d, J = 8.2 Hz, 2H), 7.51 (d, J = 8.3 Hz, 2H), 7.44–7.40 (m, 2H), 7.38 (t, J = 7.5 Hz, 1H), 7.23 (d, J = 7.3 Hz, 1H), 6.68 (br s, 1H), 6.35 (dd, J = 17.0, 1.2 Hz, 1H), 6.20 (dd, J = 17.0, 10.3 Hz, 1H), 5.72 (dd, J = 10.3, 1.3 Hz, 1H), 4.20 (s, 2H), 3.97–3.38 (m, 8H), 2.46 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 170.6, 166.7, 165.4, 143.4, 140.0, 138.6, 133.4, 130.3, 128.9, 128.7, 127.9, 127.7, 127.4, 127.0, 124.3, 41.3, 21.5.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C23H25N3O3Na: 414.1794, found: 414.1782.
1H NMR (400 MHz, CDCl3) δ 7.64 (d, J = 8.2 Hz, 2H), 7.52–7.46 (m, 4H), 7.28 (d, J = 8.1 Hz, 2H), 6.67 (br s, 1H), 6.33 (dd, J = 17.2, 1.5 Hz, 1H), 6.20 (dd, J = 16.9, 10.2 Hz, 1H), 5.70 (dd, J = 10.1, 1.5 Hz, 1H), 4.18 (s, 2H), 3.91–3.40 (m, 8H), 2.41 (s, 3H). 13C NMR (150 MHz, CDCl3) δ 170.6, 166.7, 165.4, 143.2, 137.9, 137.1, 133.2, 130.3, 129.7, 127.7, 127.2, 127.1, 127.0, 41.3, 21.1.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C23H25N3O3Na: 414.1794, found: 414.1797.
1H NMR (600 MHz, CDCl3) δ 7.42–7.34 (m, 2H), 7.30–7.26 (m, 1H), 7.25–7.22 (m, 2H), 7.18–7.09 (m, 4H), 6.64–6.58 (m, 1H), 6.31 (dd, J = 16.9, 8.2 Hz, 1H), 6.17 (dd, J = 17.0, 10.2 Hz, 1H), 5.68 (d, J = 10.4 Hz, 1H), 4.24 (t, J = 12.5 Hz, 1H), 4.17–4.08 (m, 1H), 4.02–3.87 (m, 2H), 3.74–3.57 (m, 2.5H), 3.52–3.36 (m, 1H), 3.52–3.36 (m, 1H), 3.34–3.24 (m, 1H), 2.82–2.72 (m, 0.5H), 2.57–2.45 (m, 1H), 2.40–2.32 (m, 0.5H). 13C NMR (150 MHz, CDCl3) δ 169.9, 169.9, 166.5, 166.4, 165.3, 140.3, 140.2, 138.7, 135.3, 135.2, 131.4, 131.3, 130.3, 130.2, 129.6, 129.5, 129.2, 129.2, 128.5, 128.4, 127.0, 127.0, 126.5, 126.5, 126.4, 126.4, 126.3, 46.2, 46.1, 43.9, 43.7, 41.5, 41.4, 41.2, 41.2, 40.9, 40.8, 39.1, 39.1.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C23H25N3O3Na: 414.1794, found: 414.1813.
1H NMR (600 MHz, CDCl3) δ 7.42–7.38 (m, 1H), 7.38–7.32 (m, 3H), 7.20–7.11 (m, 2H), 6.99 (d, J = 8.1 Hz, 2H), 6.91–6.87 (m, 1H), 6.66 (m, 1H), 6.31 (d, J = 17.2 Hz, 1H), 6.19 (dd, J = 17.1, 10.3 Hz, 1H), 5.68 (dd, J = 10.3, 1.3 Hz, 1H), 4.25–4.09 (m, 2H), 4.00–3.26 (m, 8H). 13C NMR (150 MHz, CDCl3) δ 167.3, 167.2, 166.6, 166.4, 165.3, 156.2, 153.1, 153.0, 131.1, 131.1, 130.3, 130.3, 130.1, 130.0, 129.0, 128.9, 127.1, 127.1, 127.0, 127.0, 124.1, 124.1, 123.9, 123.9, 119.0, 118.9, 118.2, 118.2, 46.7, 46.6, 44.7, 44.3, 42.3, 41.8, 41.5, 41.4, 41.3.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C22H23N3O4Na: 416.1586, found: 416.1596.
1H NMR (600 MHz, CDCl3) δ 7.39 (d, J = 8.3 Hz, 1H), 7.31–7.26 (m, 3H), 7.17 (d, J = 7.5 Hz, 1H), 7.09 (d, J = 8.1 Hz, 1H), 6.98 (t, J = 7.4 Hz, 1H), 6.91 (t, J = 7.4 Hz, 1H), 6.66 (br s, 1H), 6.31 (d, J = 17.1 Hz, 1H), 6.18 (dd, J = 17.0, 10.1 Hz, 1H), 5.69 (d, J = 10.0 Hz, 1H), 4.15 (s, 2H), 3.79–3.59 (m, 6H), 3.49–3.40 (m, 2H). 13C NMR (150 MHz, CDCl3) δ 170.2, 166.7, 165.4, 142.6, 141.9, 131.0, 130.2, 129.4, 128.1, 127.1, 122.3, 121.9, 119.9, 118.9, 117.5, 44.6, 42.1, 41.3.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C22H24N4O3Na: 415.1770, found: 415.1766.
1H NMR (600 MHz, CDCl3) δ 7.35 (t, J = 7.6 Hz, 1H), 7.32–7.28 (m, 3H), 7.25–7.22 (m, 2H), 7.22–7.16 (m, 3H), 6.65 (br s, 1H), 6.32 (dd, J = 17.0, 1.4 Hz, 1H), 6.19 (dd, J = 17.0, 10.3 Hz, 1H), 5.69 (dd, J = 10.3, 1.4 Hz, 1H), 4.15 (s, 2H), 4.01 (s, 2H), 3.90–3.17 (m, 8H). 13C NMR (150 MHz, CDCl3) δ 170.7, 166.7, 165.4, 142.0, 140.2, 134.9, 130.8, 130.2, 129.0, 128.8, 128.6, 127.6, 127.0, 126.4, 124.9, 47.1, 44.4, 41.7, 41.3.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C23H25N3O3Na: 414.1794, found: 414.1803.
1H NMR (600 MHz, CDCl3) δ 7.41–7.34 (m, 3H), 7.16 (t, J = 7.4 Hz, 1H), 7.13–7.06 (m, 2H), 7.06–6.99 (m, 3H), 6.66 (br s, 1H), 6.32 (dd, J = 17.0, 1.6 Hz, 1H), 6.19 (dd, J = 17.1, 10.2 Hz, 1H), 5.69 (dd, J = 10.2, 1.3 Hz, 1H), 4.16 (s, 2H), 3.89–3.29 (m, 8H). 13C NMR (150 MHz, CDCl3) δ 169.9, 166.7, 165.4, 157.9, 156.2, 136.5, 130.3, 130.2, 130.0, 127.1, 124.1, 121.4, 120.1, 119.5, 116.8, 41.3.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C22H23N3O4Na: 416.1586, found: 416.1571.
1H NMR (600 MHz, CDCl3) δ 7.33–7.27 (m, 2H), 7.12–7.08 (m, 3H), 7.07–7.05 (m, 1H), 7.00 (t, J = 7.4 Hz, 1H), 6.88 (d, J = 7.5 Hz, 1H), 6.66 (br s, 1H), 6.32 (dd, J = 17.0, 1.3 Hz, 1H), 6.19 (dd, J = 17.0, 10.3 Hz, 1H), 5.69 (dd, J = 10.3, 1.2 Hz, 1H), 4.17 (s, 2H), 3.90–3.33 (m, 8H). 13C NMR (150 MHz, CDCl3) δ 170.6, 166.7, 165.4, 144.1, 141.9, 136.1, 130.2, 129.8, 129.5, 127.0, 122.2, 119.1, 118.6, 118.5, 115.0, 41.3.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C22H24N4O3Na: 415.1746, found: 415.1760.
1H NMR (600 MHz, CDCl3) δ 7.34 (d, J = 8.0 Hz, 2H), 7.32–7.29 (m, 2H), 7.26–7.24 (m, 2H), 7.22 (t, J = 7.4 Hz, 1H), 7.19–7.17 (m, 2H), 6.68 (br s, 1H), 6.31 (dd, J = 17.0, 1.4 Hz, 1H), 6.19 (dd, J = 17.0, 10.2 Hz, 1H), 5.69 (dd, J = 10.2, 1.4 Hz, 1H), 4.16 (s, 2H), 4.01 (s, 2H), 3.85–3.36 (m, 8H). 13C NMR (150 MHz, CDCl3) δ 170.7, 166.7, 165.4, 143.7, 140.2, 132.6, 130.2, 129.2, 129.0, 128.6, 127.4, 127.0, 126.4, 44.4, 42.1, 41.8, 41.3.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C23H25N3O3Na: 414.1794, found: 414.1780.
1H NMR (400 MHz, CDCl3) δ 7.44–7.34 (m, 4H), 7.18 (t, J = 7.4 Hz, 1H), 7.09–6.99 (m, 4H), 6.66 (br s, 1H), 6.32 (dd, J = 17.0, 1.5 Hz, 1H), 6.19 (dd, J = 17.0, 10.1 Hz, 1H), 5.70 (dd, J = 10.2, 1.4 Hz, 1H), 4.22–4.14 (m, 2H), 3.83–3.40 (m, 8H). 13C NMR (150 MHz, CDCl3) δ 170.3, 166.7, 165.4, 159.5, 155.9, 130.3, 130.0, 129.3, 129.0, 127.0, 124.3, 119.8, 118.0, 44.5, 42.1, 41.3.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C22H23N3O4Na: 416.1586, found: 416.1592.
1H NMR (400 MHz, CDCl3) δ 7.37–7.29 (m, 4H), 7.15 (d, J = 7.9 Hz, 1H), 7.06–7.1 (m, 3H), 6.70 (br s, 1H), 6.32 (dd, J = 17.1, 1.5 Hz, 1H), 6.19 (dd, J = 17.0, 10.1, Hz, 1H), 5.69 (dd, J = 10.1, 1.5 Hz, 1H), 4.21–4.13 (m, 2H), 3.77–3.61 (m, 6H), 3.52–3.44 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 170.9, 166.7, 165.4, 145.9, 141.3, 130.3, 129.5, 129.4, 127.0, 125.6, 122.7, 119.7, 155.5, 44.5, 42.2, 41.3.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C22H24N4O3Na: 415.1746, found: 415.1754.
1H NMR (400 MHz, CDCl3) δ 2.36 (d, J = 4.3 Hz, 2H), 2.05 (d, J = 5.6 Hz, 2H), 1.93–1.81 (m, 8H), 1.61 (d, J = 3.0 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 181.8 (d, JCF = 2.2 Hz), 91.2 (d, JCF = 183.4 Hz), 44.8 (d, JCF = 10.1 Hz), 43.5 (d, JCF = 20.2 Hz), 41.9 (d, JCF = 17.3 Hz), 37.5 (d, JCF = 2.2 Hz), 34.9 (d, JCF = 2.2 Hz), 30.9 (d, JCF = 10.1 Hz). 19F NMR (282 MHz, CDCl3) δ −132.7.
HRMS (EI) m/z (M)+ calcd for C11H15FO2: 198.1056, found: 198.1056.
1H NMR (400 MHz, CDCl3) δ 2.33–2.20 (m, 4H), 2.11 (s, 4H), 1.87 (d, J = 3.1 Hz, 4H), 1.72–1.61 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 181.5, 66.9, 48.1, 46.7, 44.3, 37.1, 34.6, 31.0.
HRMS (EI) m/z (M)+ calcd for C11H15ClO2: 214.0761, found: 214.0768.
1H NMR (400 MHz, CDCl3) δ 2.49 (s, 2H), 2.36–2.26 (m, 4H), 2.25–2.19 (m, 2H), 1.95–1.88 (m, 4H), 1.76–1.65 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 181.1, 63.1, 49.3, 48.0, 44.6, 36.9, 34.4, 31.6.
Characterization is consistent with literature data.31
1H NMR (600 MHz, CDCl3) δ 7.38–7.35 (m, 2H), 7.35–7.31 (m, 2H), 7.20 (t, J = 7.1 Hz, 1H), 2.28–2.23 (m, 2H), 2.07 (s, 2H), 2.00–1.87 (m, 8H), 1.75 (s, 2H). 13C NMR (150 MHz, CDCl3) δ 182.8, 149.8, 128.2, 125.9, 124.8, 43.8, 42.1, 41.6, 37.9, 36.4, 35.5, 28.6.
Characterization is consistent with literature data.52
1H NMR (600 MHz, CDCl3) δ 7.38–7.29 (m, 5H), 5.74 (t, J = 4.5 Hz, 1H), 5.12 (s, 2H), 4.04 (d, J = 4.3 Hz, 2H), 3.73–3.66 (m, 4H), 3.65–3.60 (m, 2H), 3.43–3.37 (m, 2H), 2.38 (m, 2H), 2.10 (d, J = 6.1 Hz, 2H), 1.93–1.85 (m, 9H), 1.62 (d, J = 3.2 Hz, 2H). 13C NMR (151 MHz, CDCl3) δ 174.16 (d, JCF = 1.9 Hz), 166.98, 156.37, 128.68, 128.31, 128.18, 92.4 (d, JCF = 184.4 Hz), 67.15, 46.15 (d, JCF = 9.7 Hz), 45.14 (d, JCF = 87.4 Hz), 44.57, 44.41 (d, JCF = 19.6 Hz), 42.52 (d, JCF = 76.0 Hz), 41.95 (d, JCF = 17.4 Hz), 37.78 (d, JCF = 1.9 Hz), 34.96 (d, JCF = 2.2 Hz), 31.14 (d, JCF = 10.0 Hz). 19F NMR (376 MHz, CDCl3) δ −131.01.
HRMS (ESI-QTOF) m/z (M + H)+ calcd for C25H33FN3O4: 458.2455, found: 458.2477.
1H NMR (400 MHz, CDCl3) δ 7.39–7.29 (m, 5H), 5.73 (s, 1H), 5.13 (s, 2H), 4.04 (d, J = 4.4 Hz, 2H), 3.72–3.60 (m, 6H), 3.41 (t, J = 5.3 Hz, 2H), 2.34 (s, 2H), 2.28 (s, 2H), 2.17–2.07 (m, 4H), 1.94 (d, J = 3.4 Hz, 4H), 1.67 (d, J = 8.1 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 174.1, 167.0, 156.4, 136.5, 128.7, 128.3, 128.2, 67.4, 67.2, 48.9, 46.8, 45.7, 45.5, 42.8, 42.3, 37.50, 34.7, 31.4.
HRMS (ESI-QTOF) m/z (M + H)+ calcd for C25H33ClN3O4: 474.2160, found: 474.2156.
1H NMR (600 MHz, CDCl3) δ 7.38–7.29 (m, 5H), 5.73 (br s, 1H), 5.13 (s, 2H), 4.06–3.97 (m, 6H), 3.44–3.32 (m, 2H), 2.55 (s, 2H), 2.33 (m, 4H), 2.25 (m, 2H), 2.02–1.95 (m, 4H), 1.76–1.67 (m, 2H). 13C NMR (150 MHz, CDCl3) δ 173.8, 166.8, 156.2, 136.3, 128.5, 136.3, 128.5, 128.2, 128.0, 67.0, 63.7, 50.1, 48.1, 46.0, 45.3, 44.7, 44.4, 42.6, 42.1, 37.3, 34.5, 31.9.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C25H32BrN3O4Na: 542.1456, found: 542.1453.
1H NMR (400 MHz, CDCl3) δ 7.39–7.29 (m, 9H), 7.24–7.18 (m, 1H), 5.75 (br s, 1H), 5.12 (s, 2H), 4.07–3.96 (m, 2H), 3.75–3.66 (m, 4H), 3.65–3.59 (m, 2H), 3.44–3.32 (m, 2H), 2.31–2.25 (m, 2H), 2.11 (s, 2H), 2.07–1.97 (m, 4H), 1.97–1.89 (m, 4H), 1.79–1.72 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 175.7, 166.8, 156.2, 149.7, 136.3, 128.5, 128.3, 128.2, 128.0, 126.0, 124.7, 67.0, 45.3, 44.7, 44.5, 42.9, 42.6, 42.2, 42.1, 38.3, 36.8, 35.7, 29.0.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C31H37N3O4Na: 538.2682, found: 538.2673.
1H NMR (400 MHz, CDCl3) δ 6.79 (s, 1H), 6.29 (dd, J = 17.1, 1.8 Hz, 1H), 6.18 (dd, J = 17.0, 10.0 Hz, 1H), 5.66 (dd, J = 10.1, 1.8 Hz, 1H), 4.14 (d, J = 4.0 Hz, 2H), 3.71–3.60 (m, 6H), 3.48–3.39 (m, 2H), 2.36 (t, J = 5.4 Hz, 2H), 2.08 (d, J = 6.1 Hz, 2H), 1.87 (s, 8H), 1.60 (d, J = 3.1 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 174.1 (d, JCF = 1.8 Hz), 166.9, 165.5, 130.4, 127.1, 92.4 (d, JCF = 183.0 Hz), 46.1 (d, JCF = 9.4 Hz), 45.1 (d, JCF = 37.5 Hz), 44.6, 44.4 (d, JCF = 19.5 Hz), 42.2, 41.9 (d, JCF = 17.3 Hz), 41.3, 37.7 (d, JCF = 2.2 Hz), 34.9 (d, JCF = 2.2 Hz), 31.1 (d, JCF = 10.1 Hz). 19F NMR (376 MHz, CDCl3) δ −130.75.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C20H28FN3O3Na: 400.2012, found: 400.1997.
1H NMR (400 MHz, CDCl3) δ 6.82 (s, 1H), 6.28 (dd, J = 17.1, 1.8 Hz, 1H), 6.18 (dd, J = 17.0, 10.0 Hz, 1H), 5.65 (dd, J = 10.0, 1.9 Hz, 1H), 4.14 (d, J = 4.1 Hz, 2H), 3.71–3.58 (m, 6H), 3.44 (dd, J = 6.8, 3.6 Hz, 2H), 2.30 (s, 2H), 2.28–2.22 (m, 2H), 2.14–2.04 (m, 4H), 1.90 (s, 4H), 1.70–1.57 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 174.0, 166.9, 165.5, 130.4, 127.0, 67.4, 48.8, 46.7, 45.6, 45.2, 44.9, 44.6, 42.2, 41.3, 37.4, 34.6, 31.3.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C20H28ClN3O3Na: 416.1717, found: 416.1739.
1H NMR (400 MHz, CDCl3) δ 6.67 (br s, 1H), 6.32 (dd, J = 17.0, 1.5 Hz, 1H), 6.19 (dd, J = 17.0, 10.2 Hz, 1H), 5.69 (dd, J = 10.1, 1.5 Hz, 1H), 4.18–4.13 (m, 2H), 3.75–3.41 (m, 6H), 3.49–3.41 (m, 2H), 2.55 (s, 2H), 2.39–2.27 (m, 4H), 2.28–2.21 (m, 2H), 2.04–1.93 (m, 4H), 1.79–1.66 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 173.8, 166.7, 165.4, 130.2, 127.0, 63.7, 50.1, 48.1, 46.0, 45.2, 44.8, 44.5, 42.1, 41.3, 37.3, 34.5, 31.9.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C20H28BrN3O3Na: 462.1191, found: 462.1194.
1H NMR (400 MHz, CDCl3) δ 7.38–7.31 (m, 4H), 7.24–7.18 (m, 1H), 6.66 (br s, 1H), 6.32 (dd, J = 17.0, 1.5 Hz, 1H), 6.18 (dd, J = 17.0, 10.2 Hz, 1H), 5.69 (dd, J = 10.2, 1.5 Hz, 1H), 4.18–4.12 (m, 2H), 3.78–3.69 (m, 4H), 3.68–3.61 (m, 2H), 3.47–3.40 (m, 2H), 2.32–2.25 (m, 2H), 2.12 (s, 2H), 2.08–1.98 (m, 4H), 1.97–1.89 (m, 4H), 1.79–1.72 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 175.7, 166.7, 165.3, 149.7, 130.3, 128.3, 127.0, 126.0, 124.7, 45.2, 44.8, 44.5, 42.9, 42.2, 42.1, 41.3, 38.4, 36.9, 35.7, 29.0.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C26H33N3O3Na: 458.2420, found: 458.2397.
1H NMR (300 MHz, CDCl3) δ 2.75 (s, 2H), 2.57 (m, 4H), 2.06 (m, 2H), 1.99 (s, 4H), 1.79 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 181.8, 52.4, 51.2, 45.7, 44.8, 37.0, 34.6, 32.1.
Characterization is consistent with literature data.30
1H NMR (400 MHz, CDCl3) δ 3.55 (s, 4H), 3.35 (s, 4H), 2.73 (s, 2H), 2.50 (s 4H), 1.99 (m, 6H), 1.71 (s, 2 H), 1.22 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 173.3, 154.5, 80.1, 53.0, 51.1, 47.1, 46.0, 45.1, 37.6, 32.3, 28.3.
HRMS (ESI-QTOF) m/z (M + H)+ calcd for C20H32IN2O3: 475.1458, found: 475.1481.
1H NMR (400 MHz, CDCl3) δ 5.45 (bs, 1H), 3.97 (s, 2H), 3.66 (m, 6H), 3.41 (s, 2H), 2.82 (s, 2H), 2.58 (m, 4H), 2.05 (m, 6H), 1.79 (s, 2H), 1.45 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 173.7, 167.5, 155.9, 80.0, 53.1, 51.3, 46.6, 46.2, 45.5, 44.9, 44.5, 42.4, 42.2, 37.4, 34.7, 32.5, 28.5.
HRMS (ESI-QTOF) m/z (M + H)+ calcd for C22H35IN3O4: 532.1672, found: 532.1665.
1H NMR (400 MHz, CDCl3) δ 6.83 (s, 1H), 6.28 (dd, J = 17.0, 1.9 Hz, 1H), 6.18 (dd, J = 17.0, 10.0 Hz, 1H), 5.65 (dd, J = 9.9, 1.8 Hz, 1H), 4.14 (d, J = 4.1 Hz, 2H), 3.71–3.58 (m, 6H), 3.47–3.37 (m, 2H), 2.77 (s, 2H), 2.55 (q, J = 9.4 Hz, 4H), 2.10–1.94 (m, 6H), 1.76 (t, J = 2.9 Hz, 2H). 13C NMR (100 MHz, CDCl3) δ 173.6, 166.9, 165.5, 130.3, 127.0, 53.0, 51.2, 46.65, 46.1, 45.2, 44.9, 44.6, 42.2, 41.3, 37.4, 34.6, 32.4.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C20H28IN3O3Na: 508.1073, found: 508.1101.
1H NMR (400 MHz, CDCl3) δ 3.64 (s, 3H), 2.23 (s, 2H), 1.82–1.57 (m, 11H), 1.57 (s, 2H). 13C NMR (100 MHz, CDCl3) δ 177.0, 68.4, 51.9, 46.4, 44.4, 44.2, 37.6, 35.1, 30.3.
HRMS (EI) m/z (M)+ calcd for C12H18O3: 210.1256, found: 210.1253.
1H NMR (400 MHz, CDCl3) δ 2.26–2.19 (m, 2H), 1.94 (d, J = 1.6 Hz, 4H), 1.86–1.81 (m, 1H), 1.80–1.62 (m, 8H). 13C NMR (101 MHz, CDCl3) δ 66.2, 37.7, 36.0, 34.5, 28.8, 28.3.
HRMS (EI) m/z (M)+ calcd for C10H15OS: 183.0838, found: 183.0821 (Fragmentation of Cl).
1H NMR (400 MHz, CDCl3) δ 3.43 (t, J = 5.4 Hz, 4H), 3.04 (m, 4H), 2.08 (m, 3H), 1.80 (m, 3H), 1.66 (m, 9H), 1.41 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 154.7, 80.1, 60.4, 47.3, 36.4, 35.3, 28.6, 28.4.
HRMS (ESI-QTOF) m/z (M + H)+ calcd for C19H33N2O3S: 369.2212, found: 369.2217.
1H NMR (400 MHz, CDCl3) δ 3.58–3.20 (m, 8H), 2.13 (s, 2H), 1.98 (s, 6H), 1.70 (q, J = 12.8 Hz, 6H), 1.46 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 154.7, 80.4, 62.9, 47.4, 36.2, 36.0, 28.5, 28.3.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C19H32N2O4SNa: 407.1980, found: 407.1983.
1H NMR (600 MHz, CDCl3) δ 3.96 (s, 2H), 3.43 (m, 6H), 2.14 (s, 3H), 1.97 (d, J = 3.3 Hz, 6H), 1.73 (dt, J = 12.7, 3.0 Hz, 3H), 1.67 (d, J = 10.5 Hz, 3H), 1.45 (s, 9H). 13C NMR (151 MHz, CDCl3) δ 167.2, 155.9, 80.0, 63.0, 47.4, 47.3, 45.5, 43.0, 42.4, 36.2, 35.9, 28.5, 28.2.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C21H35N3O5SNa: 464.2195, found: 464.2189.
1H NMR (400 MHz, CDCl3) δ 6.75 (s, 1H), 6.30 (dd, J = 17.0, 1.8 Hz, 1H), 6.19 (dd, J = 17.0, 10.0 Hz, 1H), 5.67 (dd, J = 10.1, 1.7 Hz, 1H), 4.13 (d, J = 4.0 Hz, 2H), 3.92–3.11 (m, 8H), 2.13 (s, 3H), 1.96 (d, J = 3.4 Hz, 6H), 1.76–1.60 (m, 6H). 13C NMR (101 MHz, CDCl3) δ 166.7, 165.5, 130.4, 127.1, 63.0, 47.3, 45.6, 43.0, 41.4, 36.1, 35.9, 28.2.
HRMS (ESI-QTOF) m/z (M + Na)+ calcd for C19H29N3O4SNa: 418.1776, found: 418.1761.
TG2 enzyme expression and purification protocol
The enzyme was expressed and purified according to a modified procedure developed by Han et al.53 A swab of Novagen Rosetta 2 (DE3) E. coli cells, which had been transformed with a plasmid containing the C-terminal His-tag TG2 gene (purchased from Addgene, plasmid #100719), was added to two test tubes with 5 mL of sterile Terrific Broth (TB) medium and 5 μL each of kanamycin (50 mg mL−1) and chloramphenicol (34 mg mL−1) stock solutions. The test tubes were incubated at 37 °C for 16 h while shaking at 250 RPM. The contents were added to 500 mL of TB medium and 500 μL each of kanamycin (50 mg mL−1) and chloramphenicol (34 mg mL−1) stock solutions in a single 1 L Erlenmeyer flask. The mixture was incubated at 37 °C while shaking at 250 RPM until the optical density at 600 nm measure between 0.5 and 0.7 (approximately 2.75 h). Protein expression was induced by addition of isopropyl β-D-thiogalactopyranoside, which was added for a final concentration of 1 mM in the flask, then incubated at 18 °C for 20 h while shaking at 250 RPM. The induced mixture was centrifuged at 4000 RPM for 20 min at 4 °C, and the supernatant liquid was removed to leave a pellet which was resuspended with 12 mL of lysis buffer (50 mM Na3PO4, 400 mM NaCl, 5 mM imidazole, 0.5% v/v Triton-X100, pH 7.5). The suspension was homogenized using an Avestin EmulsiFlex-B15 homogenizer at 60 PSI. The lysate was then centrifuged at 19000 RPM for 1 h at 4 °C and the resulting supernatant liquid was filtered through 0.45 μm PFTE sterile filters and incubated with 0.5 mL nickel-NTA agarose resin for 1 h at 4 °C. The expressed protein was purified by washing the resin in a gravity-flow column twice with 10 mL lysis buffer then twice with 10 mL wash buffer (50 mM Na3PO4, 500 mM NaCl, 60 mM imidazole, pH 7.5). Purified enzyme was eluted twice with 10 mL of elution buffer (50 mM HEPES, 100 mM NaCl, 300 mM imidazole, 10% v/v glycerol, pH 7.0), aliquoted, and flash frozen in an acetone/dry ice bath.Inhibition assay protocol
For each inhibitor molecule, the following assay was performed in triplicate. A 125 μL volume of buffer (111.11 mM MOPS, 15.56 mM CaCl2, pH 6.9) was added to seven 1.5 mL microcentrifuge tubes designated to contain five different inhibitor concentrations, a positive control (no inhibitor), and a negative control (no inhibitor or enzyme). Separately, inhibitor stock solutions were prepared by dissolving the inhibitor in DMSO and water such that the DMSO concentration in the final assay mixture (250 μL total) does not exceed 6%. All inhibitors tested were soluble in their concentrated stock solutions at concentrations up to 500 μM. Inhibitor stock solutions were added to five of the microcentrifuge tubes to reach the target inhibitor concentrations. Water was then added to each microcentrifuge tube to reach a volume of 245 μL. 5 μL of 5.56 mM AL5 in DMSO was added last to each microcentrifuge tube, which was vortexed to fully mix the solution. An aliquot of 180 μL of each solution was transferred from their respective microcentrifuge tubes into separate wells of a 96-well transparent polystyrene microplate. The assay was initiated by the addition of 20 μL of 50 mU mL−1 C-terminal His-tag TG2 using a multichannel pipette for a final enzyme concentration of 5 mU mL−1 in each well (except the negative control) simultaneously. The hydrolysis of AL5 by TG2 to produce p-nitrophenolate was monitored immediately after enzyme addition using a BioTek Synergy H4 Hybrid Multi-Mode microplate reader (20 min at 25 °C) to measure absorbance at 405 nm against time. GraphPad Prism's one-phase association analysis was used to obtain the kobs inactivation rate constants for each inhibitor concentration and positive control. The KI and kinact values for the inhibitor were obtained by plotting kobs against [I]/α (where α = (1 + ([AL5]/KM)), [AL5] = 100 μM, KM = 10 μM), and fitting this plot, using GraphPad Prism, by non-linear regression to the following hyperbolic equation:Author contributions
Conceptualization: J. W. K. and P. N.; funding acquisition: J. W. K.; supervision: J. W. K.; investigation and methodology: D. A. W., S. T., P. N., C. B. and T. S.; writing – original draft: D. A. W., S. T. and J. W. K.; writing – reviewing and editing: all authors.Conflicts of interest
There are no conflicts of interest to declare.Data availability
The data supporting this article have been included as part of the SI. Supplementary information (SI): including kinetic data, HPLC traces and NMR spectra. See DOI: https://doi.org/10.1039/d5md00815h.Acknowledgements
J. W. K. is grateful to the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canadian Institutes of Health Research (CIHR) for funding. S. T. thanks NSERC for a CGS-M Award.References
- S. Gundemir, G. Colak, J. Tucholski and G. V. Johnson, Biochim. Biophys. Acta, 2012, 1823, 406 CrossRef CAS.
- J. W. Keillor, C. M. Clouthier, K. Apperley, A. Akhbar and A. Mulani, Acyl transfer mechanisms of tissue transglutaminase, Bioorg. Chem., 2014, 57, 186–197 CrossRef CAS PubMed.
- N. S. Caron, L. N. Munsie, J. W. Keillor and R. Truant, PLoS One, 2012, 7, e44159 CrossRef CAS PubMed.
- T.-H. Jang, D.-S. Lee, K. Choi, E. M. Jeong, I.-G. Kim, Y. W. Kim, J. N. Chun, J.-H. Jeon and H. H. Park, Crystal Structure of Transglutaminase 2 with GTP Complex and Amino Acid Sequence Evidence of Evolution of GTP Binding Site, PLoS One, 2014, 9(9), e107005 CrossRef PubMed.
- D. M. Pinkas, P. Strop, A. T. Brunger and C. Khosla, Transglutaminase 2 Undergoes a Large Conformational Change upon Activation, PLoS Biol., 2007, 5, e327 CrossRef PubMed.
- W. Dieterich, T. Ehnis, M. Bauer, P. Donner, U. Volta, E. O. Riecken and D. Schuppan, Nat. Med., 1997, 3, 797 CrossRef CAS PubMed.
- T. S. Johnson, M. Fisher, J. L. Haylor, Z. Hau, N. J. Skill, R. Jones, R. Saint, I. Coutts, M. E. Vickers, A. M. El Nahas and M. Griffin, J. Am. Soc. Nephrol., 2007, 18, 3078 CrossRef CAS PubMed.
- N. Shweke, N. Boulos, C. Jouanneau, S. Vandermeersch, G. Melino, J. C. Dussaule, C. Chatziantoniou, P. Ronco and J. J. Boffa, Am. J. Pathol., 2008, 173, 631 CrossRef CAS PubMed.
- Z. Szondy, I. Korponay-Szabó, R. Király, Z. Sarang and G. J. T. Tsay, Biomedicine, 2017, 7, 15 CrossRef.
- R. L. Eckert, M. L. Fisher, D. Grun, G. Adhikary, W. Xu and C. Kerr, Transglutaminase is a tumor cell and cancer stem cell survival factor, Mol. Carcinog., 2015, 54, 947–958 CrossRef CAS PubMed.
- D. Schuppan, M. Mäki, K. E. A. Lundin, J. Isola, T. Friesing-Sosnik, J. Taavela, A. Popp, J. Koskenpato, J. Langhorst, Ø. Hovde, M.-L. Lähdeaho, S. Fusco, M. Schumann, H. P. Török, J. Kupcinskas, Y. Zopf, A. W. Lohse, M. Scheinin, K. Kull, L. Biedermann, V. Byrnes, A. Stallmach, J. Jahnsen, J. Zeitz, R. Mohrbacher, R. Greinwald and Group, C.-3 T., A Randomized Trial of a Transglutaminase 2 Inhibitor for Celiac Disease, N. Engl. J. Med., 2021, 385, 35–45 CrossRef CAS PubMed.
- J. W. Keillor, K. Y. P. Apperley and A. Akbar, Inhibitors of Tissue Transglutaminase, Trends Pharmacol. Sci., 2015, 36, 417 CrossRef CAS.
- J. W. Keillor and K. Y. P. Apperley, Transglutaminase Inhibitors: A Patent Review, Expert Opin. Ther. Pat., 2016, 26, 49–63 CrossRef CAS PubMed.
- M. Song, H. Hwang, C. Y. Im and S.-Y. Kim, Recent Progress in the Development of Transglutaminase 2 (TGase2) Inhibitors, J. Med. Chem., 2017, 60, 554–567 CrossRef CAS.
- S. K. I. Watt, J. G. Charlebois, C. N. Rowley and J. W. Keillor, A Mechanistic Study of Thiol Addition to N-Acryloylpiperidine, Org. Biomol. Chem., 2023, 21, 2204–2212 RSC.
- E. Badarau and M. Griffin, Development of Potent and Selective Tissue Transglutaminase Inhibitors: Their Effect on TG2 Function and Application in Pathological Conditions, Chem. Biol., 2015, 22, 1347–1361 CrossRef CAS PubMed.
- P. Navals, A. M. M. Rangaswamy, P. Kasyanchyk, M. V. Berezovski and J. W. Keillor, Conformational Modulation of Tissue Transglutaminase via Active Site Thiol Alkylating Agents: Size Does Not Matter, Biomolecules, 2024, 14, 496 CrossRef CAS.
- J. Wityak, M. E. Prime, F. A. Brookfield, S. M. Courtney, S. Erfan, S. Johnsen, P. D. Johnson, M. Li, R. W. Marston, L. Reed, D. Vaidya, S. Schaertl, A. Pedret-Dunn, M. Beconi, D. Macdonald, I. Muñoz-Sanjuan and C. Dominguez, SAR Development of Lysine-Based Irreversible Inhibitors of Transglutaminase 2 for Huntington's Disease, ACS Med. Chem. Lett., 2012, 3(12), 1024–1028 CrossRef CAS.
- B. van der Wildt, M. M. M. Wilhelmus, J. Bijkerk, L. Y. F. Haveman, E. J. M. Kooijman, R. C. Schuit, J. G. J. M. Bol, C. A. M. Jongenelen, A. A. Lammertsma, B. Drukarch and A. D. Windhorst, Development of Carbon-11 Labeled Acryl Amides for Selective PET Imaging of Active Tissue Transglutaminase, Nucl. Med. Biol., 2016, 43(4), 232–242 CrossRef CAS.
- A. Akbar, N. M. R. McNeil, M. R. Albert, V. Ta, G. Adhikary, K. Bourgeois, R. L. Eckert and J. W. Keillor, Structure–Activity Relationships of Potent, Targeted Covalent Inhibitors That Abolish Both the Transamidation and GTP Binding Activities of Human Tissue Transglutaminase, J. Med. Chem., 2017, 60(18), 7910–7927 CrossRef CAS.
- R. Wodtke, C. Hauser, G. Ruiz-Gómez, E. Jäckel, D. Bauer, M. Lohse, A. Wong, J. Pufe, F.-A. Ludwig, S. Fischer, S. Hauser, D. Greif, M. T. Pisabarro, J. Pietzsch, M. Pietsch and R. Löser, Nε-Acryloyllysine Piperazides as Irreversible Inhibitors of Transglutaminase 2: Synthesis, Structure–Activity Relationships, and Pharmacokinetic Profiling, J. Med. Chem., 2018, 61(10), 4528–4560 CrossRef CAS PubMed.
- R. Wodtke, J. Wodtke, S. Hauser, M. Laube, D. Bauer, R. Rothe, C. Neuber, M. Pietsch, K. Kopka, J. Pietzsch and R. Löser, Development of an 18F-Labeled Irreversible Inhibitor of Transglutaminase 2 as Radiometric Tool for Quantitative Expression Profiling in Cells and Tissues, J. Med. Chem., 2021, 64(6), 3462–3478 CrossRef CAS.
- N. M. R. McNeil, E. W. J. Gates, N. Firoozi, N. J. Cundy, J. Leccese, S. Eisinga, J. D. A. Tyndall, G. Adhikary, R. L. Eckert and J. W. Keillor, Structure-Activity Relationships of N-Terminal Variants of Peptidomimetic Tissue Transglutaminase Inhibitors, Eur. J. Med. Chem., 2022, 232, 114172 CrossRef CAS.
- E. W. J. Gates, N. D. Calvert, N. J. Cundy, F. Brugnoli, P. Navals, A. Kirby, N. Bianchi, G. Adhikary, A. J. Shuhendler, R. L. Eckert and J. W. Keillor, Cell-Impermeable Inhibitors Confirm That Intracellular Human Transglutaminase 2 Is Responsible for the Transglutaminase-Associated Cancer Phenotype, Int. J. Mol. Sci., 2023, 24(16), 12546 CrossRef CAS.
- N. J. Cundy, J. Arciszewski, E. W. J. Gates, S. L. Acton, K. D. Passley, E. Awoonor-Williams, E. K. Boyd, N. Xu, É. Pierson, C. Fernandez-Ansieta, M. R. Albert, N. M. R. McNeil, G. Adhikary, R. L. Eckert and J. W. Keillor, Novel Irreversible Peptidic Inhibitors of Transglutaminase 2, RSC Med. Chem., 2023, 14(2), 378–385 RSC.
- A. M. M. Rangaswamy, P. Navals, E. W. J. Gates, S. Shad, S. K. I. Watt and J. W. Keillor, Structure–activity relationships of hydrophobic alkyl acrylamides as tissue transglutaminase inhibitors, RSC Med. Chem., 2022, 13, 413–428 RSC.
- L. Mader, S. K. I. Watt, H. R. Iyer, L. Nguyen, H. Kaur and J. W. Keillor, The war on hTG2: warhead optimization in small molecule human tissue transglutaminase inhibitors, RSC Med. Chem., 2023, 14, 277–298 RSC.
- B. Ryan, A. M. M. Rangaswamy, S. Shad and J. W. Keillor, Diamino Variants of Piperazine-Based Tissue Transglutaminase Inhibitors, Bioorg. Med. Chem. Lett., 2025, 119, 130078 CrossRef CAS.
- S. Yang, T. Guo, H. Fu, S. Zheng, J. Sun and X. Qu, Catalytic Hydrodehalogenation Activity and Selectivity of Polyiodinated Phenolic Disinfection Byproducts at Ambient Conditions, Sci. Total Environ., 2024, 944, 173905 CrossRef CAS PubMed.
- V. J. Jasys, F. Lombardo, T. A. Appleton, J. Bordner, M. Ziliox and R. A. Volkmann, Preparation of Fluoroadamantane Acids and Amines: Impact of Bridgehead Fluorine Substitution on the Solution- and Solid-State Properties of Functionalized Adamantanes, J. Am. Chem. Soc., 2000, 122, 466–473 CrossRef CAS.
- D. G. Harman and S. J. Blanksby, Investigation of the Gas Phase Reactivity of the 1-adamantyl Radical Using a Distonic Radical Anion Approach, Org. Biomol. Chem., 2007, 5, 3495–3503 RSC.
- M. C. Leech, D. Nagornîi, J. M. Walsh, C. Kiaku, D. L. Poole, J. Mason, I. C. A. Goodal, P. Devo and K. Lam, eFluorination Using Cheap and Readily Available Tetrafluoroborate Salts, Org. Lett., 2023, 25, 1353–1358 CrossRef CAS.
- H. Stetter and J. Mayer, Über Verbindungen mit Urotropin-Struktur, XXI. Herstellung und Eigenschaften von in 3-Stellung substituierten Adamantan-carbonsäuren-(1), Chem. Ber., 1962, 95, 667–672 CrossRef CAS.
- M. Griffin, Z. Wang, D. L. Rathbone, R. Aqil and A. J. Ratcliffe, Inhibitors of Transglutaminase, WO Pat., WO2023/135425A1, 2023 Search PubMed.
- L. K. Mader, J. E. Borean and J. W. Keillor, A Practical Guide for the Assay-Dependent Characterisation of Irreversible Inhibitors, RSC Med. Chem., 2025, 16(1), 63–76 RSC.
- M. Charton, The Nature of the ortho Effect. II. Composition of the Taft Steric Parameters, J. Am. Chem. Soc., 1969, 93(3), 615–618 CrossRef.
- M. Plewe, E. Brown, V. Gantla, G. Henkel, K. McCormack and N. Sokolova, Adamantane Derivatives for the Treatment of Filovirus Infection, US Pat., US2020/0017514A1, 2020 Search PubMed.
- Z. Bikadi, I. Hazai, D. Malik, K. Jemnitz, Z. Veres, P. Hari, Z. Ni, T. W. Loo, D. M. Clarke, E. Hazai and Q. Mao, Predicting P-Glycoprotein-Mediated Drug Transport Based On Support Vector Machine and Three-Dimensional Crystal Structure of P-Glycoprotein, PLoS One, 2011, 6(10), e25815 CrossRef CAS PubMed.
- A.-L. Jiang, G. Zhou, B.-Y. Jiang, T. Zhou and B.-F. Shi, Pd-Catalyzed Atroposelective C–H Olefination: Diverse Synthesis of Axially Chiral Biaryl-2-carboxylic Acids, Org. Lett., 2024, 26, 5670–5675 CrossRef CAS.
- J.-J. Dai, W.-T. Xu, Y.-A. Wu, W.-M. Zhang, Y. Gong, X.-P. He, X.-Q. Zhang and H.-J. Xu, Silver-Catalyzed C(sp2)–H Functionalization/C–O Cyclization Reaction at Room Temperature, J. Org. Chem., 2015, 80, 911–919 CrossRef CAS.
- K. Anderson and S. Buchwald, General Catalysts for the Suzuki–Miyaura and Sonogashira Coupling Reactions of Aryl Chlorides and for the Coupling of Challenging Substrate Combinations in Water, Angew. Chem., Int. Ed., 2005, 44, 6173–6177 CrossRef CAS.
- S. Akhter, B. Lund, A. Ismael, M. Langer, J. Isaksson, T. Christopeit, H.-K. Leiros and A. Bayer, A focused fragment library targeting the antibiotic resistance enzyme – Oxacillinase-48: Synthesis, structural evaluation and inhibitor design, Eur. J. Med. Chem., 2018, 145, 634–648 CrossRef CAS PubMed.
- A. Asachenko, K. Sorochkina, P. Dzhevakov, M. Topchiy and M. Nechaev, Suzuki–Miyaura Cross-Coupling under Solvent-Free Conditions, Adv. Synth. Catal., 2013, 355, 3553–3557 CrossRef CAS.
- G. Edwards, M. Trafford, A. Hamilton, A. Buxton, M. Bardeaux and J. Chalker, Melamine and Melamine-Formaldehyde Polymers as Ligands for Palladium and Application to Suzuki–Miyaura Cross-Coupling Reactions in Sustainable Solvents, J. Org. Chem., 2014, 79, 2094–2104 CrossRef CAS.
- J. Pollinger, S. Schierle, L. Gellrich, J. Ohmdorf, A. Kaiser, P. Heitel, A. Chaikuad, S. Knapp and D. Merk, A Novel Biphenyl-based Chemotype of Retinoid X Receptor Ligands Enables Subtype and Heterodimer Preferences, ACS Med. Chem. Lett., 2019, 10, 1346–1352 CrossRef CAS PubMed.
- Y. Lui, Z. She, X. Zheng, T. Wang and G. Gao, Rigid Chelating Dicarbene Ligands Based on Naphthyridine-Fused Bisimidazolium Salts, Chin. Chem. Lett., 2022, 33, 2993–2996 CrossRef.
- R. Gui and C.-J. Li, Ruthenium(ii)-catalyzed deoxygenation of ketones, Chem. Commun., 2022, 75, 10572–10575 RSC.
- I. Fuji, K. Sembra, Q.-Z. Li, S. Sakaki and Y. Nakao, Magnesiation of Aryl Fluorides Catalyzed by a Rhodium–Aluminum Complex, J. Am. Chem. Soc., 2020, 142, 11647–11652 CrossRef.
- C. L. Higgins, S. V. Filip, A. Afsar and W. Hayes, Increasing the antioxidant capability via the synergistic effect of coupling diphenylamine with sterically hindered phenol, Tetrahedron, 2019, 75, 130759 CrossRef CAS.
- R. S. Villatoro, J. R. Belfield, H. D. Arman, L. W. Hernandez, E. M. Simmons, Z. J. Garlets, S. R. Wisniewski, J. R. Coombs and D. E. Frantz, General Method for Ni-Catalyzed C–N Cross-Couplings of (Hetero)Aryl Chlorides with Anilines and Aliphatic Amines under Homogeneous Conditions Using a Dual-Base Strategy, Organometallics, 2023, 42, 3164–3172 CrossRef CAS.
- Y. Yoshida, S. Otsuka, K. Nogi and H. Yorimitus, Palladium-Catalyzed Amination of Aryl Sulfoxides, Org. Lett., 2018, 20, 1134–1137 CrossRef CAS PubMed.
- W. Feng, K. Ye and R. Hou, Synthesis and Characterization of 3-Phenyl-adamantane-1-carboxylic Acid, Chem. Res. Chin. Univ., 2019, 35, 556–559 CrossRef CAS.
- B.-G. Han, J.-W. Cho, Y. D. Cho, K.-J. Jeong, S.-Y. Kim and B. I. Lee, Crystal structure of human transglutaminase 2 in complex with adenosine triphosphate, Int. J. Biol. Macromol., 2010, 47, 190–195 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |