Design, synthesis and structure–activity relationship analysis of dibenzodiazepinone derivatives against Osimertinib resistant NSCLC
Chengliu
Jin
,
Zhen
Zhang
,
Peng
Liao
,
Chen
Zhang
,
Hua
Cao
 * and
Yan-Long
Ma
* and
Yan-Long
Ma
 *
*
School of Chemistry and Chemical Engineering, Guangdong Cosmetics Engineering & Technology Research Center, Guangdong Pharmaceutical University, Zhongshan, 528458, P. R. China
First published on 16th October 2025
Abstract
Lung cancer, particularly NSCLC, is the leading cause of cancer-related deaths worldwide, accounting for over 80% of cases. Mutations in the epidermal growth factor receptor (EGFR) are key drivers of NSCLC. Although three generations of EGFR tyrosine kinase inhibitors (TKIs) have been developed, resistance limits their efficacy. The dibenzodiazepinone scaffold exhibits diverse biological activities, however, reports on its derivatives for treating NSCLC with EGFR mutations, particularly triple mutations, are rare. Guided by the binding model of DDC4002 with EGFRT790M/V948R, this study synthesized and characterized 36 dibenzodiazepinone analogues, evaluating their antiproliferative activity against NSCLC cell lines. Structure–activity relationship analysis highlighted the importance of substituents at the C2 and N10 positions. Compound 33 exhibited the strongest inhibitory effects, especially for H1975™ cells (EGFRL858R/T790M/C797S) with a 2.4-fold lower IC50 (2.7 μM) than osimertinib (6.5 μM). It effectively inhibited colony formation, migration of H1975™ cells, induced G0/G1 arrest, and promoted apoptosis through suppressing EGFR and AKT phosphorylation. These findings demonstrate the potential of optimizing the dibenzodiazepinone framework for developing novel potent molecules against osimertinib resistant NSCLC cells, providing valuable insights for future research.
1. Introduction
Recent global statistics indicate that lung cancer is a leading cause of cancer-related deaths worldwide, with an estimated 2 million new cases and 1.8 million deaths in 2020. It is the primary cause of cancer mortality in men and the second leading cause in women after breast cancer.1 Non-small cell lung cancer (NSCLC) accounts for over 80% of all lung cancer cases,2 with epidermal growth factor receptor (EGFR) overexpression and activating mutations being major oncogenic drivers.3 Over the past two decades, targeting mutant EGFR has become the most effective therapeutic strategy for NSCLC in clinical practice.4,5 Three generations of EGFR tyrosine kinase inhibitors (TKIs) have been developed to treat NSCLC patients with EGFR mutations.6,7Despite the widespread use of these drugs, drug resistance remains a significant challenge. Osimertinib, a third-generation irreversible EGFR-TKI, has become a first-line treatment for NSCLC patients with EGFR19del/T790M or EGFRL858R/T790M mutations. However, 20–25% of patients with T790M mutations develop C797S mutations after 9–13 months of treatment, leading to resistance. Several ATP-competitive EGFR TKIs, including BLU-945, BLU-701, TBQ3804, BBT-176, BPI-361175, BDTX-1535 and Jin-A02, are currently in clinical trials.8–10 Nevertheless, there are no approved therapies for NSCLC patients with EGFR19del/T790M/C797S or EGFRL858R/T790M/C797S mutations.3 Therefore, there is an urgent need for developing novel and potent small molecules to address this challenge.11
The dibenzodiazepinone scaffold (Fig. 1A) exhibits diverse biological activities, including anti-inflammatory, anti-microbial, anti-tumor and other efficacies.12,13 However, reports on its derivatives for treating NSCLC with EGFR mutations, particularly triple mutations, are rare. In 2019, Eck et al.14 identified compound EAI002 (Fig. 1A) as an allosteric EGFR TKI that selectively inhibits EGFRL858R/T790M with an IC50 of 52 nM. Subsequent optimization led to DDC4002, which shows mutant-selective activity against EGFRL858R/T790M and EGFRL858R/T790M/C797S at nanomolar concentrations. However, DDC4002 exhibits poor activity against mutant EGFR cells as a single agent. Introducing a 4-(piperazinyl)phenyl group at the C2 position yielded three derivatives that inhibit mutant EGFR-expressing Ba/F3 cells with IC50 values of 2–7.2 μM in vitro. Notably, compound D1 (Fig. 1A) shows enhanced antiproliferative effects when combined with cetuximab.
In 2024, Heppner and colleagues15 incorporated ATP inhibitors at the C9 or N10 position of dibenzodiazepinone to develop four bivalent EGFR TKIs. Among them, compound D2 (Fig. 1A) demonstrated potent inhibition of mutant EGFR TKs, with an IC50 of 0.06 nM for EGFRL858R/T790M/C797S TK. These findings demonstrate that dibenzodiazepinone can function as an allosteric EGFR TKI by binding to the allosteric site near the ATP-binding pocket of EGFR (Fig. 1B), inducing a conformational change that inhibits EGFR activity and downstream signalling pathways.16,17 However, the potential of dibenzodiazepinone derivatives in treating NSCLC, particularly in cases with EGFR19del/T790M/C797S or EGFRL858R/T790M/C797S mutations, is limited.
To explore its potential, the crystal structure of the DDC4002@EGFRT790M/V948R complex was chosen as a binding model for the rational design of its derivatives, based on two key considerations: limited crystallographic data exists for allosteric EGFR TKIs in EGFR19del/T790M/C797S or EGFRL858R/T790M/C797S contexts, and the allosteric site lies outside the conserved ATP-binding pocket, unaffected by mutations within it.18,19DDC4002 (ref. 20) binds to the allosteric site via hydrophobic interactions and a hydrogen bond between the diazepinone N–H and the backbone carbonyl of F856 in the DFG motif (Fig. 1C). The fluorobenzene ring occupies a hydrophobic pocket near the loop connecting the αC-helix and β4-strand in the N-lobe, suggesting limited space for additional large substituents. The benzyl group (N10 position, Fig. 1C and E) is situated within a pocket formed by β2, β3, and β5-strands, indicating potential for introducing hydrophilic or hydrophobic substitutions.
The unsubstituted benzene ring is situated within a hydrophobic channel formed by the αC-helix and activation segment, oriented towards the solvent. This orientation indicates sufficient space to accommodate hydrophobic substituents with varying rigidity, flexibility, lengths, and polar groups at the C2 position (R1 positions, Fig. 1C and E). This observation is further supported by the structural accommodation of the 4-(4-methylpiperazin-1-yl)phenoxy group from compound D1, as well as the 4-(1-methylpiperidin-4-yl)phenyl group of another allosteric EGFR TKI, JBJ-09-063,21 which fits within this hydrophobic channel (Fig. 1D). Based on these observations, we introduced various substituents at the R1 (C2) and R2 (N10) positions within the DDC4002 framework to investigate its structure–activity relationships, action mechanism and explore its potential for treating osimertinib resistant NSCLC.
2. Results and discussion
2.1. Chemical synthesis
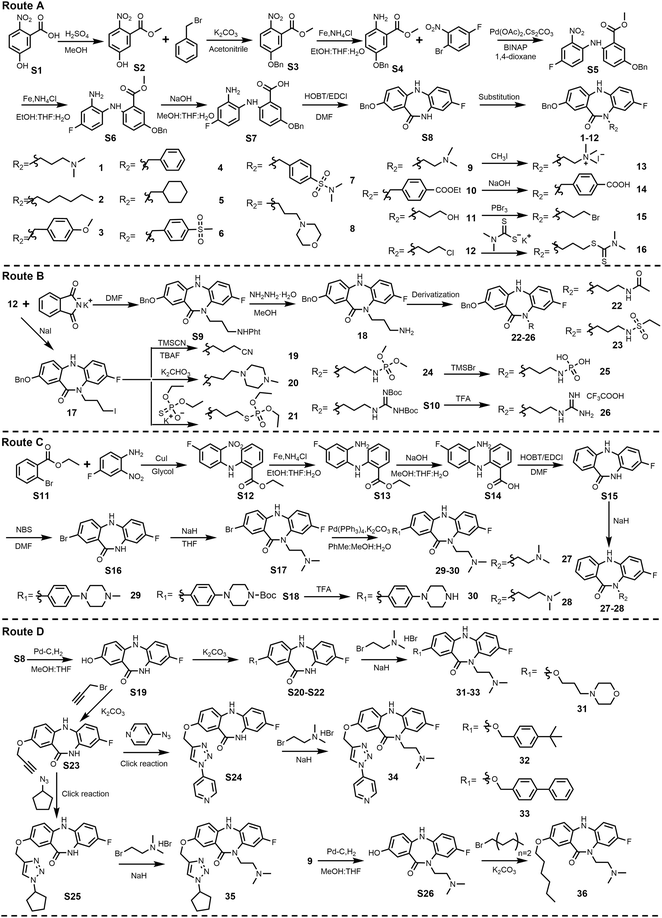
Based on the design strategy, a series of DDC4002 derivatives (36 dibenzodiazepinone analogues) were constructed and synthesized as outlined in Scheme 1. Target compounds 1–16 were synthesized according to route A. 5-Hydroxy-2-nitrobenzoic acid (S1) as the starting material underwent esterification and then reacted with benzyl bromide to give S3, which was reduced by iron powder and then reacted with 1-bromo-4-fluoro-2-nitrobenzene through Buchwald–Hartwig cross coupling to afford S5. S5 further underwent iron powder reduction and hydrolysis to produce S7, which underwent intramolecular amide condensation reaction using EDCI as a condensation agent to afford the seven-membered ring intermediate S8. S8 was then reacted with different brominated substrates to give target compounds 1–12. Compounds 9–12 further underwent quaternarization, hydrolysis, hydroxylation–bromination reaction and substitution reaction with potassium dimethyldithiocarbamate to give target compounds 13–16, respectively. What's more, the crystal structure of compound 13 (CDCC: 2243568, Fig. 2) confirmed these target compounds' structure, and brominated substrates were grafted on the N10 position of dibenzodiazepinone instead of the N5 position under these reaction conditions.Compounds 17–26 were derived from 12. As depicted in route B, 12 was reacted with NaI through the Finkelstein reaction to give target compound 17, which further underwent substitution reactions with TMSCN, 1-methylpiperazine and potassium O,O-dimethyl phosphorothioate to give target compounds 18–21, respectively. Moreover, 12 could also undergo the Gabriel reaction to afford amino target compound 18, which could further be derivatized into target compounds 22–26.
Compounds 27–30 were prepared following synthetic route C. Initially, ethyl 2-bromobenzoate (S11) was reacted with 4-fluoro-2-nitroaniline through the Ullmann reaction to give compound S12, which then underwent iron powder reduction, hydrolysis and intramolecular amide condensation reaction to afford the seven-membered ring intermediate S15. On the one hand, S15 was then reacted with brominated substrates to give target compounds 27 and 28. On the other hand, S15 underwent bromination and substitution reaction with 2-bromo-N,N-dimethylethan-1-amine hydrobromide to give S17, which further underwent the Suzuki–Miyaura reaction to give target compounds 29 and 30.
Compounds 31–36 were derived from S8. As shown in route D, the benzyl group of S8 was removed by a carbon-supported palladium catalyst under H2 to give intermediate S19. On the one hand, S19 was reacted with different brominated substrates and then reacted with 2-bromo-N,N-dimethylethan-1-amine hydrobromide to give target compounds 31–33. On the other hand, S19 was reacted with propargyl bromide to afford S23, which was reacted with 4-azidopyridine or azidocyclopentane via click reaction and then reacted with 2-bromo-N,N-dimethylethan-1-amine hydrobromide to give target compounds 34–35, respectively. Compound 36 was obtained by reacting 1-bromobutane and S26, which was produced from debenzylation of compound 9. DDC4002 was synthesized according to the reported literature.14 All the intermediates and the final target compounds were characterized by 1H and 13C NMR and mass spectroscopy, and their 1H and 13C NMR spectra are shown in SI Fig. S1–S123.
2.2. Anti-proliferative activity and structure–activity relationship of the target compounds
To investigate the antiproliferative activity of these target compounds against NSCLC cells with EGFR mutations in vitro and their structure–activity relationship (SAR), four human NSCLC cell lines were selected: A549 (EGFRWT), HCC827 (EGFR19del), H1975 (EGFRL858R/T790M), and H1975™ (EGFRL858R/T790M/C797S). Antiproliferative activities were quickly screened through MTT or CCK-8 assays at specified concentrations. Osimertinib (Osi) served as the control group.The influence of substituents at the N10 position (R2 position) of the dibenzodiazepinone framework (with a benzyloxy group at the C2/R1 position) was systematically explored. The inhibitory activities of compounds 1 to 26 were evaluated, and the results are summarized in Fig. 3 (Table S1). Among these, compound 18 exhibited the strongest antiproliferative activity against NSCLC cells, followed by compounds 4, 5, 9, 12, 14, 20 and 25. However, all these compounds showed lower activity compared to Osi. Other compounds either had no inhibitory effect or only inhibited the proliferation of one of the two cell types (HCC827 or H1975). Compound 18, despite its strong inhibitory effect, lacked selectivity between wild-type and mutant EGFR, with inhibitory rates of approximately 73%, 98%, and 51% for HCC827, H1975, and H1975™ cells, respectively, at 10 μM, and 98% for A549 cells at 30 μM. Compounds 4, 5, 9, 12, 14, 20 and 25 showed similar antiproliferative activity against HCC827 and H1975 cells (around 50% inhibition at 10 μM) but had minimal effect on H1975™ cells. Compounds 20 and 25 also lacked selectivity toward wild-type and mutant EGFR.
The SAR of R2 substituents could be summarized as follows: 1) larger hydrophobic groups, such as methylcyclohexane (compound 5) or a planar rigid benzyl group (compound 4), enhanced activity, while benzyl groups with hydrophilic functional groups at the para position were detrimental to activity (entries 1–7); 2) alkyl chains with terminal functional groups significantly influenced activity; only specific groups, such as chlorine (compound 12), dimethylamine (compound 9), amino (compound 18), O,O-dimethyl phosphorothioate (compound 21), and 1-methylpiperazine (compound 20), improved activity (entries 8–22); 3) electronegative hydrophilic groups were more favorable for activity than electropositive hydrophilic groups (entries 23–26).
To investigate the influence of C2 substituents (R1 position) on antiproliferative activity, a 2-(dimethylamino)ethyl group was selected at the R2 position of the dibenzodiazepinone framework, based on the inhibitory activity and selectivity of the aforementioned compounds. The results are summarized in Fig. 4 (Table S2). Removing the benzyloxy group at the R1 position led to reduced activity (entries 1–4). Replacing the benzyloxy group with more hydrophobic substituents, such as a (4-(tert-butyl)benzyl)oxy group (compound 32) or a [1,1′-biphenyl]-4-ylmethoxy group (compound 33), significantly enhanced antiproliferative activity against HCC827, H1975, and H1975™ cells. Conversely, introducing hydrophilic functional groups or flexible hydrophobic alkyl chains at the R1 position did not improve activity and had no effect on H1975™ cell proliferation (entries 5–10). Additionally, substituting the flexible benzyloxy group with rigid groups, such as a 4-(4-methylpiperazin-1-yl)phenyl group (compound 29) or a 4-(piperazin-1-yl)phenyl group (compound 30), also improved activity, though to a lesser extent than compound 33. Compound 33 exhibited the highest inhibitory rates of 92%, 91%, and 96% against HCC827, H1975, and H1975™ cells, respectively, at 10 μM. While its activity was lower than osimertinib against HCC827 and H1975 cells, it showed superior activity against H1975™ cells. These findings suggest that larger, more hydrophobic substituents at the R1 position enhance antiproliferative activity against H1975™ cells, as exemplified by compound 33.
Target compounds exhibiting superior antiproliferative activity against NSCLC cells with EGFR mutations, including compounds 18, 29, 30, 32 and 33, were selected for IC50 determination. As summarized in Table 1 (Fig. S125–S132), these compounds exhibited micromolar-level inhibitory activity across all tested NSCLC cell lines, with IC50 values ranging from 2.7 to 33.2 μM. Notably, compound 33 demonstrated the most potent inhibitory activity, with IC50 values of 3.1 μM and 5.2 μM against HCC827 and H1975 cells, respectively. However, its activity was significantly lower than that of Osi, which showed IC50 values of 0.4 μM and 0.5 μM against HCC827 and H1975 cells, respectively. Conversely, compound 33 exhibited superior activity against H1975™ cells, with an IC50 of 2.7 μM, compared to Osi's IC50 of 6.5 μM. Furthermore, the antiproliferative activity of compound 33 against all the tested NSCLC cell lines is comparable to that of JBJ-09-063,21 but significantly greater than that of DDC4002. Compound 33 also exhibited selectivity towards cells with the L858R/T790M/C797S mutation, as evidenced by its higher IC50 of 10.2 μM against A549 cells. These findings indicate that modifying the framework structure of DDC4002 (dibenzodiazepinone) can lead to the discovery of novel potent molecules against NSCLC cells harboring mutant EGFR, particularly for osimertinib-resistant EGFRL858R/T790M/C797S mutations. Based on these results, compound 33 was selected for subsequent in vitro cell experiments to study its action mechanism.
| Compound | HCC827 | H1975 | H1975™ | A549 |
|---|---|---|---|---|
| 18 | 9.5 ± 0.2 | 5.2 ± 0.1 | 14.1 ± 0.2 | 9.8 ± 0.5 |
| 29 | 6.9 ± 0.5 | 4.4 ± 0.3 | 9.6 ± 0.1 | 12.3 ± 0.3 |
| 30 | 4.6 ± 0.3 | 5.4 ± 0.1 | 10.4 ± 0.3 | 6.8 ± 0.1 |
| 32 | 3.2 ± 0.4 | 5.4 ± 0.2 | 10.7 ± 0.3 | 33.2 ± 0.8 |
| 33 | 3.1 ± 0.1 | 5.2 ± 0.1 | 2.7 ± 0.1 | 10.2 ± 0.8 |
| Osi | 0.4 ± 0.1 | 0.5 ± 0.1 | 6.5 ± 0.1 | 5.4 ± 0.1 |
| JBJ-09-063 | 3.6 ± 0.5 | 2.1 ± 0.1 | 3.1 ± 0.4 | 3.6 ± 0.2 |
| DDC4002 | <30 | <30 | <30 | <30 |
2.3. EGFR inhibitory activity and EGFR-mediated signalling pathways assay of compound 33
To verify whether compound 33 acts as an allosteric EGFR TKI by inhibiting NSCLC cell proliferation, its enzymatic activity against EGFR tyrosine kinases (EGFRWT, EGFR19del, EGFRL858R/T790M and EGFRL858R/T790M/C797S) was evaluated using an HTRF KinEASE TK assay kit. Notably, the 6xHis-glutathione S-transferase (His6-GST) tag must be removed from all EGFR kinases prior to testing allosteric EGFR TKI activity, as the kinases will not respond to inhibitors otherwise. EGFRL858R/T790M/C797S kinase (His6-GST tag removed) was co-incubated with JBJ-09-063 or DDC4002 for 30 min, followed by reaction with the substrate under 100 μM ATP for 30 min. IC50 values were determined according to the kit protocol. IC50 values for JBJ-09-063 and DDC4002 against EGFRL858R/T790M/C797S were approximately 0.65 nM and 65 nM, respectively (Fig. 5A and B, Table 2), closely matching literature values (0.08 nM and 59 nM).14,21 Subsequently, this method was used to test compound 33's inhibitory activity against EGFR kinases. Surprisingly, compound 33 showed negligible inhibitory activity against EGFRWT, EGFR19del, EGFRL858R/T790M and EGFRL858R/T790M/C797S, with IC50 values of approximately 100, 20, 4, and 44 μM, respectively (Fig. 5C and Table 2). This suggests that compound 33 does not function as an allosteric EGFR TKI.To further understand the antiproliferative mechanism of compound 33 at the molecular level, the expression levels of related proteins in EGFR signaling pathways, such as EGFR, p-EGFR, Akt and p-Akt,22 were determined in H1975™ cells using western blot analysis after treatment with different concentrations of compound 33 (2, 6 and 10 μM) for 20 h. The expression levels of GAPDH served as the internal reference protein expression level of each treatment group. As shown in Fig. 5D and E, compound 33 suppressed the phosphorylation of EGFR and its downstream proteins Akt in H1975™ cells in a concentration dependent manner. At the concentration of 10 μM, compound 33 significantly decreased the expression of p-EGFR by 59% and p-Akt by 57% compared with the control group. Its inhibitory effect on p-EGFR was comparable to that of Osi, but was better than that of Osi for p-Akt (WB's Raw Data is displayed in the SI). These results suggest that compound 33 exhibits strong antiproliferative effects on H1975™ cells, not by acting as an allosteric inhibitor, but by suppressing the EGFR signaling pathway. Furthermore, kinase inhibition assays revealed that compound 33 does not target the downstream PI3Kα and AKT1 kinases of the EGFR pathway, as evidenced by its IC50 values of approximately 11.81 μM and 100 μM, respectively (Fig. 5F and Table 2).
To investigate why compound 33 does not function as an allosteric EGFR TKI, we conducted molecular docking studies to analyse its interaction with the EGFRT790M/V948R mutant and compared it to the interaction of JBJ-09-063 (PDB: 7JXQ). As illustrated in Fig. 5G, compound 33 effectively bound to the allosteric site of the EGFR TK domain. Specifically, the N–H group at the 5-position of the dibenzodiazepinone core formed a critical hydrogen bond with the backbone carbonyl of F856 (Phe856) in the DFG motif, an interaction pattern similar to that observed with DDC4002 (Fig. 1B). The [1,1′-biphenyl]-4-ylmethoxy group at the C2 (R1) position extended toward the solvent along a hydrophobic channel formed by the αC-helix and the activation segment, while the 2-(dimethylamino)ethyl group at the N10 (R2) position occupied a hydrophobic pocket formed by the β2, β3, and β5 strands and AMP-PNP. Importantly, this binding mode closely overlapped with that of JBJ-09-063 when bound to EGFRT790M/V948R (Fig. 5H). These results indicate that compounds designed based on the original skeletal structure may exhibit distinct mechanisms of action.
2.4. Colony formation inhibition assay of compound 33
To further verify that compound 33 can significantly inhibit the proliferation of NSCLC cells in vitro, its colony formation assay on H1975™ cells was well conducted. This assay reflects the ability of a single cell to differentiate and proliferate into a colony, as well as its tumorigenicity in vivo.23 As shown in Fig. 6A, compound 33 inhibited colony formation of H1975™ cells in a concentration dependent manner after incubation for 10 days. When the concentration of compound 33 was higher than its IC50 value (2.66 μM), it exhibited a good inhibitory effect on the colony formation of H1975™ cells. At the concentration of 9 μM, there were almost no colony formation.2.5. Cell migration inhibition assay of compound 33
Tumor cell migration represents a hallmark feature of metastatic cancers, which is significantly driven by the activation of the EGFR signalling pathway.24 Therefore, a wound healing assay was used to evaluate the migration inhibitory activity of compound 33 against H1975™ cells. H1975™ cells were treated with varying concentrations of compound 33 (2, 4, 6 and 10 μM) for 0, 12 and 24 h. As depicted in Fig. 6B, the scratch of the control group gradually closed through cell migration within 24 h, and its wound closure was up to 78% (Fig. 6C). After exposure to compound 33, the migration of H1975™ cells in the horizontal direction was significantly inhibited compared with that of the control group. At the concentration of 10 μM, the wound closure of compound 33 was only 12% and 22% after treatment 12 and 24 h, respectively. These results demonstrated that compound 33 effectively inhibited H1975™ cells' migration in a concentration-dependent manner.2.6. Cell cycle analysis of compound 33
It is well known that the activation of the EGFR signalling pathway by EGR can activate the G1/S cell cycle progression.25 So, the effect of compound 33 on the tumor cell cycle was evaluated using flow cytometry. H1975™ cells were treated with different concentrations of compound 33 (1, 3, 6 and 9 μM) for 48 h, and then the cells were fixed in ethanol and stained with propidium iodide (PI). Fig. 6D and Table 3 clearly showed that the percentage of H1975™ cells at the G0/G1 phase obviously increased with the increase of concentration of compound 33, while the S and G2/M phases decreased with its concentration increasing. For example, after treatment with 9 μM of compound 33 for 48 h, the G0/G1 phase increased from 30.8% (control) to 59.7%, while S and G2/M phases declined from 50.3% and 18.9% to 31.1% and 9.3%, respectively. These results demonstrated that compound 33 arrested the cell cycle progression of H1975™ cells at the G0/G1 phase in a concentration-dependent manner.| Compound | Con. | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|---|
| Control | 30.8 ± 0.2 | 50.3 ± 0.1 | 18.9 ± 0.3 | |
| 33 | 1 μM | 35.2 ± 2.1 | 48.1 ± 0.5 | 16.8 ± 1.7 |
| 3 μM | 39.5 ± 2.7 | 46.1 ± 1.3 | 14.3 ± 1.5 | |
| 6 μM | 46.2 ± 2.1 | 43.1 ± 1.9 | 10.7 ± 0.4 | |
| 9 μM | 59.7 ± 2.7 | 31.1 ± 2.7 | 9.3 ± 0.6 |
2.7. Cell apoptosis assay of compound 33
Dysregulation of apoptosis is one of the hallmark features of cancer cells, triggering the tumor occurrence and progression. As a consequence, inducing cancer cell apoptosis is an attractive strategy for treating cancer. To understand the effect of compound 33 on tumor cell apoptosis, H1975™ cells were treated with different concentrations of compound 33 (2, 6 and 8 μM) for 32 h, and then evaluated using annexin V-FITC and PI double staining by flow cytometry. As depicted in Fig. 6E and F, the early and late apoptosis states of H1975™ cells both increased with the increase of concentration of compound 33. For instance, after treatment with 8 μM of compound 33 for 32 h, the early and late apoptosis states increased from 1.11% and 2.51% (control) to 18.26% and 55.05%, respectively. These results demonstrated that compound 33 inhibited cell growth through inducing cell apoptosis in a concentration-dependent manner.3. Conclusion
In summary, we explored the potential of DDC4002 derivatives against NSCLC. According to the design strategy, a new series of DDC4002 derivatives (36 dibenzodiazepinone analogues) were synthesized and characterized, and their antiproliferative activities were evaluated against four human NSCLC cell lines: A549 cells (EGFRwt), HCC827 cells (EGFR19del), H1975 cells (EGFRL858R/T790M) and H1975™ cells (EGFRL858R/T790M/C797S). The SAR analysis revealed that larger hydrophobic groups (compounds 4 and 5), alkyl chains with terminal functional groups (compounds 9, 12, 18, 20 and 21) and electronegative hydrophilic groups (compounds 14 and 25) at the N10 position (R2 position), as well as larger volume and more hydrophobic substituents (compounds 29, 30, 32 and 33) at the C2 position (R1 position), were more favorable for enhancing its antiproliferative activity (Fig. 7).Among them, compound 33 exhibited the best inhibitory activity against NSCLC cells harboring EGFR mutations. Especially for H1975™ cells bearing L858R/T790M/C797S EGFR mutation, its IC50 value (2.7 μM) was 2.4-fold lower than that of Osi (6.5 μM), while for HCC827 cells with deletions like E746-A750 mutation in exon 19 and H1975 cells with L858R/T790M mutation, its IC50 values (3.1 and 5.2 μM) were much worse than that of Osi (0.4 and 0.5 μM). Furthermore, the antiproliferative activity of compound 33 is comparable to that of JBJ-09-063, but significantly greater than that of DDC4002. Enzymatic and WB assays revealed that compound 33 exhibits strong antiproliferative effects on H1975™ cells, not by acting as an allosteric inhibitor, but by suppressing the EGFR signaling pathway. It effectively inhibited the colony formation and migration of H1975™ cells, induced G0/G1 arrest, and promoted apoptosis through suppressing EGFR and AKT phosphorylation in the EGFR signaling pathways.
Overall, these findings suggest that modifying the framework structure of DDC4002 (dibenzodiazepinone) can lead to the discovery of novel potent molecules against NSCLC cells harboring mutant EGFR, particularly for osimertinib-resistant EGFR19del/T790M/C797S or EGFRL858R/T790M/C797S mutations. In addition, this study provides valuable insights and inspiration for the further development and optimization of the dibenzodiazepinone scaffold in the treatment of Osimertinib resistant NSCLC.
4. Experimental section
4.1. Chemistry
Methyl 5-hydroxy-2-nitrobenzoate (S2). A mixture of S1 (20 g, 109 mmol) and sulfuric acid (12 mL) in MeOH (200 mL) was stirred at 70 °C for 24 h. After completion, the solvent was evaporated under reduced pressure using a rotary evaporator. The resulting residue was suspended in deionized water (150 mL) and extracted with ethyl acetate (3 × 100 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to afford the crude product. Final purification was performed by silica gel column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 5
EA = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound S2 (17.64 g, 82%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ [ppm] = 8.01 (d, J = 9.06 Hz, 1H), 6.92 (dd, J = 9.06 Hz, 2.59 Hz, 1H), 6.87 (d, J = 2.49 Hz, 1H), 3.81 (s, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 167.8, 161.3, 139.5, 131.3, 127.3, 117.6, 115.8, 53.9. HRMS GC/QTOF (m/z): calcd for C8H7NO5+ [M]+: 197.0324; found 197.0318.
1) to give compound S2 (17.64 g, 82%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ [ppm] = 8.01 (d, J = 9.06 Hz, 1H), 6.92 (dd, J = 9.06 Hz, 2.59 Hz, 1H), 6.87 (d, J = 2.49 Hz, 1H), 3.81 (s, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 167.8, 161.3, 139.5, 131.3, 127.3, 117.6, 115.8, 53.9. HRMS GC/QTOF (m/z): calcd for C8H7NO5+ [M]+: 197.0324; found 197.0318.
Methyl 5-(benzyloxy)-2-nitrobenzoate (S3). A mixture of S2 (8.6 g, 43.77 mmol), K2CO3 (9.04 g, 65.48 mmol) and benzyl bromide (8.98 g, 52.52 mmol) in MeCN (150 mL) was stirred at room temperature for 12 h. After completion, the mixture was filtered, and the filtrate was concentrated. The residue was dissolved in EtOAc (200 mL), washed sequentially with water (2 × 100 mL) and saturated brine (1 × 100 mL), dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to afford the crude product. The crude product was purified by recrystallization from n-hexane/EtOAc to give compound S3 (11.8 g, 94%) as a white solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 8.03 (d, J = 9.04 Hz, 1H), 7.48–7.35 (m, 5H), 7.15 (d, J = 2.70 Hz, 1H), 7.08 (dd, J = 9.07 Hz, 2.74, 1H), 5.16 (s, 2H), 3.93 (s, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 166.6, 162.5, 140.2, 135.1, 131.2, 128.9 (2C), 128.7, 127.6 (2C), 126.8, 116.5, 115.1, 71.0, 53.4. HRMS GC/QTOF (m/z): calcd for C15H13NO5+ [M]+: 287.0794; found 287.0791.
Methyl 2-amino-5-(benzyloxy)benzoate (S4). A mixture of S3 (11.8 g, 41.1 mmol) and NH4Cl (21.7 g, 411.1 mmol) in 300 mL THF/MeOH/H2O (V
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V
V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V = 1
V = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5) was added iron dust (11.5 g, 205.5 mmol). The reaction mixture was heated to 70 °C under a nitrogen atmosphere for 12 h. After completion, the mixture was filtered and the filtrate was concentrated in vacuo. The residue was dissolved with EtOAc (200 mL), washed sequentially with water (1 × 100 mL) and saturated brine (1 × 100 mL), dried over anhydrous Na2SO4, filtered, and concentrated to afford the crude product. The crude product was purified by column chromatography (PE
2.5) was added iron dust (11.5 g, 205.5 mmol). The reaction mixture was heated to 70 °C under a nitrogen atmosphere for 12 h. After completion, the mixture was filtered and the filtrate was concentrated in vacuo. The residue was dissolved with EtOAc (200 mL), washed sequentially with water (1 × 100 mL) and saturated brine (1 × 100 mL), dried over anhydrous Na2SO4, filtered, and concentrated to afford the crude product. The crude product was purified by column chromatography (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 4
EA = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound S4 (8.2 g, 77.6%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.49–7.30 (m, 6H), 7.02 (dd, J = 8.89 Hz, 3.01, 1H), 6.65 (s, 1H), 5.00 (s, 2H), 3.88 (s, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.4, 149.8, 145.3, 137.2, 128.7 (2C), 128.1, 127.8 (2C), 124.2, 118.4, 114.9, 110.9, 70.9, 51.8. HRMS GC/QTOF (m/z): calcd for C15H15NO3+ [M]+: 257.1052; found 257.1049.
1) to give compound S4 (8.2 g, 77.6%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.49–7.30 (m, 6H), 7.02 (dd, J = 8.89 Hz, 3.01, 1H), 6.65 (s, 1H), 5.00 (s, 2H), 3.88 (s, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.4, 149.8, 145.3, 137.2, 128.7 (2C), 128.1, 127.8 (2C), 124.2, 118.4, 114.9, 110.9, 70.9, 51.8. HRMS GC/QTOF (m/z): calcd for C15H15NO3+ [M]+: 257.1052; found 257.1049.
Methyl 5-(benzyloxy)-2-((4-fluoro-2-nitrophenyl)amino)benzoate (S5). A mixture of S4 (7.48 g, 29.10 mmol), 1-bromo-4-fluoro-2-nitrobenzene (6.37 g, 29.10 mmol), Cs2CO3 (14.2 g, 43.7 mmol), BINAP (1.36 g, 2.18 mmol) and Pd(OAc)2 (325 mg, 1.45 mmol) in 1,4-dioxane (200 mL) was stirred at 110 °C under a nitrogen atmosphere for 24 h. After completion, the mixture was filtered and the filtrate was concentrated in vacuo. The residue was dissolved in EtOAc (200 mL), washed sequentially with deionized water (2 × 100 mL) and saturated brine (1 × 100 mL), dried over anhydrous Na2SO4, filtered, and concentrated to afford the crude product. The crude product was purified by recrystallization from n-hexane/EtOAc to give compound S5 (7.47 g, 64.9%) as a red solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 10.73 (s, 1H), 7.88 (d, J = 8.65 Hz, 1H), 7.64 (s, 1H), 7.47–7.35 (m, 7H), 7.20–7.11 (m, 2H), 5.09 (s, 2H), 3.94 (s, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 167.0, 154.5 (d, J = 241.7 Hz), 154.2, 137.5, 136.5, 135.4 (d, J = 8.2 Hz), 135.3, 128.8 (2C), 128.3, 127.7 (2C), 123.5 (d, J = 23.2 Hz), 122.2, 121.4, 121.1, 119.1 (d, J = 7.4 Hz), 116.7, 112.5 (d, J = 26.5 Hz), 70.7, 52.6. HRMS GC/QTOF (m/z): calcd for C21H17FN2O5+ [M]+: 396.1121; found 396.1132.
Methyl 2-((2-amino-4-fluorophenyl)amino)-5-(benzyloxy)benzoate (S6). S6 was synthesized following the procedure described for S4. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 3
EA = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound S6 (6.3 g, 65%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 8.50 (s, 1H), 7.45–7.29 (m, 5H), 7.00 (t, J = 7.40 Hz, 2H), 6.51–6.38 (m, 3H), 4.98 (s, 2H), 3.88 (s, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.8, 161.8 (d, J = 242.5 Hz), 149.8, 145.3 (d, J = 11.5 Hz), 144.9, 137.1, 129.3 (d, J = 10.2 Hz), 128.6 (2C), 128.0, 127.7 (2C), 123.9, 122.4 (d, J = 2.5 Hz), 115.5, 115.3, 111.0, 105.1 (d, J = 22.6 Hz), 102.2 (d, J = 25.7 Hz), 70.9, 51.9. HRMS GC/QTOF (m/z): calcd for C21H19FN2O3+ [M]+: 366.138; found 366.1381.
1) to afford compound S6 (6.3 g, 65%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 8.50 (s, 1H), 7.45–7.29 (m, 5H), 7.00 (t, J = 7.40 Hz, 2H), 6.51–6.38 (m, 3H), 4.98 (s, 2H), 3.88 (s, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.8, 161.8 (d, J = 242.5 Hz), 149.8, 145.3 (d, J = 11.5 Hz), 144.9, 137.1, 129.3 (d, J = 10.2 Hz), 128.6 (2C), 128.0, 127.7 (2C), 123.9, 122.4 (d, J = 2.5 Hz), 115.5, 115.3, 111.0, 105.1 (d, J = 22.6 Hz), 102.2 (d, J = 25.7 Hz), 70.9, 51.9. HRMS GC/QTOF (m/z): calcd for C21H19FN2O3+ [M]+: 366.138; found 366.1381.
2-((2-Amino-4-fluorophenyl)amino)-5-(benzyloxy)benzoic acid (S7). A mixture of S6 (5 g, 13.66 mmol) and NaOH (1.09 g, 27.32 mmol) in 100 mL THF/MeOH/H2O (V
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V
V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V = 1
V = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5) was stirred at 70 °C for 12 h. After completion, the organic solvents were removed under reduced pressure. The aqueous solution was acidified to pH 2–3 with 2 N HCl, and the product was extracted with EtOAc (3 × 50 mL). The combined organic layers were washed with saturated brine (1 × 50 mL), dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography (DCM
2.5) was stirred at 70 °C for 12 h. After completion, the organic solvents were removed under reduced pressure. The aqueous solution was acidified to pH 2–3 with 2 N HCl, and the product was extracted with EtOAc (3 × 50 mL). The combined organic layers were washed with saturated brine (1 × 50 mL), dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography (DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 4
MeOH = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound S7 (4.2 g, 87.3%) as a yellow oil. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 7.46 (d, J = 3.05 Hz, 1H), 7.43 (d, J = 7.21 Hz, 2H), 7.38 (t, J = 7.42 Hz, 2H), 7.34–7.30 (m, 1H), 7.16–7.06 (m, 2H), 6.91 (dd, J = 10.38 Hz, 2.93 Hz, 1H), 6.71–6.63 (m, 1H), 6.55–6.50 (m, 1H), 5.03 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 170.2, 161.3 (d, J = 238.6 Hz), 149.3, 146.9 (d, J = 12.0 Hz), 144.9, 137.8, 128.8 (2C), 128.4 (d, J = 10.5), 128.2, 128.0 (2C), 123.6, 122.2 (d, J = 1.9 Hz), 116.1, 115.3, 112.0, 102.8 (d, J = 22.6 Hz), 101.5 (d, J = 25.4 Hz), 70.3. HRMS ESI-TOF (m/z): calcd for C20H18FN2O3+ [M + H]+: 353.1301; found 353.1297.
1) to afford compound S7 (4.2 g, 87.3%) as a yellow oil. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 7.46 (d, J = 3.05 Hz, 1H), 7.43 (d, J = 7.21 Hz, 2H), 7.38 (t, J = 7.42 Hz, 2H), 7.34–7.30 (m, 1H), 7.16–7.06 (m, 2H), 6.91 (dd, J = 10.38 Hz, 2.93 Hz, 1H), 6.71–6.63 (m, 1H), 6.55–6.50 (m, 1H), 5.03 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 170.2, 161.3 (d, J = 238.6 Hz), 149.3, 146.9 (d, J = 12.0 Hz), 144.9, 137.8, 128.8 (2C), 128.4 (d, J = 10.5), 128.2, 128.0 (2C), 123.6, 122.2 (d, J = 1.9 Hz), 116.1, 115.3, 112.0, 102.8 (d, J = 22.6 Hz), 101.5 (d, J = 25.4 Hz), 70.3. HRMS ESI-TOF (m/z): calcd for C20H18FN2O3+ [M + H]+: 353.1301; found 353.1297.
2-(Benzyloxy)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (S8). A mixture of S7 (10 g, 28.4 mmol), HOBt (4.22 g, 31.3 mmol) and EDCI (5.98 g, 31.3 mmol) was dissolved in 100 mL DMF. The reaction mixture was stirred at room temperature for 24 h. Upon completion, the mixture was diluted with deionized water (200 mL) and extracted with DCM (3 × 200 mL). The combined organic layers were washed sequentially with deionized water (2 × 50 mL) and saturated brine (1 × 30 mL), dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by silica gel column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 2
EA = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound S8 (8.25 g, 87%) as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 9.98 (s, 1H), 7.59 (s, 1H), 7.48–7.29 (m, 5H), 7.26 (t, J = 2.39 Hz, 1H), 7.07 (dd, J = 8.83 Hz, 2.85, 1H), 7.03–6.91 (m, 2H), 6.87–6.71 (m, 2H), 5.04 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 167.7, 157.9 (d, J = 237.6 Hz), 152.7, 144.3, 137.3 (d, J = 1.9), 137.1, 131.3 (d, J = 10.6 Hz), 128.4 (2C), 127.8, 127.6 (2C), 123.8, 121.3, 120.5 (2C, t, J = 4.7 Hz), 116.3, 110.7 (d, J = 22.0 Hz), 107.8 (d, J = 25.6 Hz), 69.6. HRMS GC/QTOF (m/z): calcd for C20H15FN2O2+ [M]+: 334.1118; found 334.1116.
1) to give compound S8 (8.25 g, 87%) as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 9.98 (s, 1H), 7.59 (s, 1H), 7.48–7.29 (m, 5H), 7.26 (t, J = 2.39 Hz, 1H), 7.07 (dd, J = 8.83 Hz, 2.85, 1H), 7.03–6.91 (m, 2H), 6.87–6.71 (m, 2H), 5.04 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 167.7, 157.9 (d, J = 237.6 Hz), 152.7, 144.3, 137.3 (d, J = 1.9), 137.1, 131.3 (d, J = 10.6 Hz), 128.4 (2C), 127.8, 127.6 (2C), 123.8, 121.3, 120.5 (2C, t, J = 4.7 Hz), 116.3, 110.7 (d, J = 22.0 Hz), 107.8 (d, J = 25.6 Hz), 69.6. HRMS GC/QTOF (m/z): calcd for C20H15FN2O2+ [M]+: 334.1118; found 334.1116.
2-(Benzyloxy)-10-(3-(dimethylamino)propyl)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e] [1,4]diazepin-11-one (1). A mixture of S8 (213 mg, 0.64 mmol) and NaH (60% dispersion in mineral oil, 54 mg, 1.34 mmol) in 8 mL dry THF was stirred at 0 °C under a nitrogen atmosphere for 30 min. A solution of 3-bromo-N,N-dimethylpropan-1-amine hydrobromide (173 mg, 0.70 mmol) in dry THF (2 mL) was added dropwise to the reaction mixture. After addition, the reaction warmed to room temperature and was stirred for 24 h under a nitrogen atmosphere. Upon completion, the organic solvent was removed under reduced pressure. The mixture was diluted with deionized water (50 mL) and extracted with dichloromethane (DCM, 3 × 20 mL). The combined organic layers were washed with saturated brine (1 × 20 mL), dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 1
EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to give compound 1 (150 mg, 56%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.47–7.27 (m, 7H), 6.98 (dd, J = 9.89 Hz, 2.72, 1H), 6.92 (dd, J = 8.65 Hz, 2.98, 1H), 6.87 (dd, J = 8.70 Hz, 5.48 Hz, 1H), 6.80–6.70 (m, 2H), 5.00 (s, 2H), 4.14 (d, J = 33.72 Hz, 2H), 2.44–2.29 (m, 2H), 2.15 (d, J = 10.65 Hz, 6H), 1.87–1.76 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.3, 159.4 (d, J = 242.3 Hz), 154.6, 144.8, 141.9 (d, J = 2.5 Hz), 136.8, 135.3, 135.2, 128.7 (2C), 128.1, 127.7 (2C), 126.2, 121.2 (d, J = 9.1 Hz), 120.8, 119.8, 116.5, 112.8 (d, J = 22.5 Hz), 111.4 (d, J = 24.9 Hz), 70.5, 56.7, 48.2, 45.4, 26.1. HRMS ESI (m/z): calcd for C25H27FN3O2+ [M + H]+: 420.2082; found 420.2078.
2) to give compound 1 (150 mg, 56%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.47–7.27 (m, 7H), 6.98 (dd, J = 9.89 Hz, 2.72, 1H), 6.92 (dd, J = 8.65 Hz, 2.98, 1H), 6.87 (dd, J = 8.70 Hz, 5.48 Hz, 1H), 6.80–6.70 (m, 2H), 5.00 (s, 2H), 4.14 (d, J = 33.72 Hz, 2H), 2.44–2.29 (m, 2H), 2.15 (d, J = 10.65 Hz, 6H), 1.87–1.76 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.3, 159.4 (d, J = 242.3 Hz), 154.6, 144.8, 141.9 (d, J = 2.5 Hz), 136.8, 135.3, 135.2, 128.7 (2C), 128.1, 127.7 (2C), 126.2, 121.2 (d, J = 9.1 Hz), 120.8, 119.8, 116.5, 112.8 (d, J = 22.5 Hz), 111.4 (d, J = 24.9 Hz), 70.5, 56.7, 48.2, 45.4, 26.1. HRMS ESI (m/z): calcd for C25H27FN3O2+ [M + H]+: 420.2082; found 420.2078.
2-(Benzyloxy)-8-fluoro-10-hexyl-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (2). Compound 2 was synthesized following the procedure described for compound 1. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 5
EA = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound 2 (94 mg, 64%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.43 (s, 1H), 7.39–7.32 (m, 4H), 7.30 (d, J = 6.2 Hz, 1H), 6.99–6.81 (m, 3H), 6.79–6.69 (m, 2H), 5.34 (s, 1H), 4.98 (s, 2H), 4.04 (s, 2H), 1.69–1.60 (m, 2H), 1.39–1.28 (m, 2H), 1.24 (s, 4H), 0.83 (t, J = 5.8 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.2, 159.3 (d, J = 241.9 Hz), 154.5, 144.8, 141.8 (d, J = 2.6 Hz), 136.8, 135.3 (d, J = 9.7 Hz), 128.6 (2C), 128.0, 127.6 (2C), 126.2, 121.1 (d, J = 9.2 Hz), 120.8, 119.7, 116.5, 112.6 (d, J = 22.5 Hz), 111.2 (d, J = 24.9 Hz), 70.5, 50.0, 31.4, 28.0, 26.3, 22.6, 14.0. HRMS ESI-TOF (m/z): calcd for C26H27FN2O2Na+ [M + Na]+: 441.1954; found 441.1956.
1) to afford compound 2 (94 mg, 64%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.43 (s, 1H), 7.39–7.32 (m, 4H), 7.30 (d, J = 6.2 Hz, 1H), 6.99–6.81 (m, 3H), 6.79–6.69 (m, 2H), 5.34 (s, 1H), 4.98 (s, 2H), 4.04 (s, 2H), 1.69–1.60 (m, 2H), 1.39–1.28 (m, 2H), 1.24 (s, 4H), 0.83 (t, J = 5.8 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.2, 159.3 (d, J = 241.9 Hz), 154.5, 144.8, 141.8 (d, J = 2.6 Hz), 136.8, 135.3 (d, J = 9.7 Hz), 128.6 (2C), 128.0, 127.6 (2C), 126.2, 121.1 (d, J = 9.2 Hz), 120.8, 119.7, 116.5, 112.6 (d, J = 22.5 Hz), 111.2 (d, J = 24.9 Hz), 70.5, 50.0, 31.4, 28.0, 26.3, 22.6, 14.0. HRMS ESI-TOF (m/z): calcd for C26H27FN2O2Na+ [M + Na]+: 441.1954; found 441.1956.
2-(Benzyloxy)-8-fluoro-10-(4-methoxybenzyl)-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (3). Compound 3 was synthesized following the procedure described for compound 1. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 5
EA = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound 3 (120 mg, 44.1%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.48 (d, J = 2.96 Hz, 1H), 7.42–7.22 (m, 7H), 6.94 (dd, J = 8.63 Hz, 2.97, 1H), 6.90 (dd, J = 9.97 Hz, 2.72, 1H), 6.86–6.76 (m, 3H), 6.75–6.65 (m, 2H), 5.19 (s, 2H), 5.01 (s, 2H), 3.76 (s, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.5, 159.3 (d, J = 242.0 Hz), 158.7, 154.7, 144.7, 141.2 (d, J = 2.7 Hz), 136.8, 135.5 (d, J = 9.8 Hz), 129.3, 128.7 (2C), 128.3 (2C), 128.1, 127.7 (2C), 125.9, 121.1 (d, J = 2.3 Hz), 121.0, 119.9, 116.8, 114.1 (2C), 112.8 (d, J = 22.6 Hz), 111.1 (d, J = 25.4 Hz), 70.6, 55.3, 53.3. HRMS ESI (m/z): calcd for C28H24FN2O3+ [M + H]+: 455.1765; found 455.1761.
1) to afford compound 3 (120 mg, 44.1%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.48 (d, J = 2.96 Hz, 1H), 7.42–7.22 (m, 7H), 6.94 (dd, J = 8.63 Hz, 2.97, 1H), 6.90 (dd, J = 9.97 Hz, 2.72, 1H), 6.86–6.76 (m, 3H), 6.75–6.65 (m, 2H), 5.19 (s, 2H), 5.01 (s, 2H), 3.76 (s, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.5, 159.3 (d, J = 242.0 Hz), 158.7, 154.7, 144.7, 141.2 (d, J = 2.7 Hz), 136.8, 135.5 (d, J = 9.8 Hz), 129.3, 128.7 (2C), 128.3 (2C), 128.1, 127.7 (2C), 125.9, 121.1 (d, J = 2.3 Hz), 121.0, 119.9, 116.8, 114.1 (2C), 112.8 (d, J = 22.6 Hz), 111.1 (d, J = 25.4 Hz), 70.6, 55.3, 53.3. HRMS ESI (m/z): calcd for C28H24FN2O3+ [M + H]+: 455.1765; found 455.1761.
10-Benzyl-2-(benzyloxy)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (4). Benzyl bromide (37 mg, 0.2 mmol) was added to a mixture of S8 (67 mg, 0.2 mmol) and LiOH (7.2 mg, 0.3 mmol) in THF (5 mL). The reaction mixture was then stirred at 50 °C for 24 h. Upon completion, the organic solvent was removed under reduced pressure. The reaction mixture was diluted with water (10 mL) and extracted with DCM (3 × 10 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 5
EA = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 4 (60 mg, 70.7%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.50 (d, J = 2.94 Hz, 1H), 7.42–7.28 (m, 9H), 7.27–7.19 (m, 1H), 6.96 (dd, J = 8.62 Hz, 2.94, 1H), 6.90 (dd, J = 9.95 Hz, 2.70, 1H), 6.82 (dd, J = 8.68 Hz, 5.48 Hz, 1H), 6.78–6.66 (m, 1H), 5.38 (s, 1H), 5.27 (s, 2H), 5.01 (s, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.6, 159.2 (d, J = 242.1 Hz), 154.6, 144.8, 141.1 (d, J = 2.5 Hz), 137.1, 136.8, 135.5 (d, J = 9.7 Hz), 128.7 (4C, d, J = 3.5 Hz), 128.1 (2C), 127.7 (2C), 127.2, 126.8 (2C), 125.8, 121.1 (t, J = 4.4 Hz), 120.0, 116.7, 112.8 (d, J = 22.6 Hz), 110.9 (d, J = 25.4 Hz), 70.5, 54.0. HRMS ESI (m/z): calcd for C27H22FN2O2+ [M + H]+: 425.1660; found 425.1655.
1) to give compound 4 (60 mg, 70.7%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.50 (d, J = 2.94 Hz, 1H), 7.42–7.28 (m, 9H), 7.27–7.19 (m, 1H), 6.96 (dd, J = 8.62 Hz, 2.94, 1H), 6.90 (dd, J = 9.95 Hz, 2.70, 1H), 6.82 (dd, J = 8.68 Hz, 5.48 Hz, 1H), 6.78–6.66 (m, 1H), 5.38 (s, 1H), 5.27 (s, 2H), 5.01 (s, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.6, 159.2 (d, J = 242.1 Hz), 154.6, 144.8, 141.1 (d, J = 2.5 Hz), 137.1, 136.8, 135.5 (d, J = 9.7 Hz), 128.7 (4C, d, J = 3.5 Hz), 128.1 (2C), 127.7 (2C), 127.2, 126.8 (2C), 125.8, 121.1 (t, J = 4.4 Hz), 120.0, 116.7, 112.8 (d, J = 22.6 Hz), 110.9 (d, J = 25.4 Hz), 70.5, 54.0. HRMS ESI (m/z): calcd for C27H22FN2O2+ [M + H]+: 425.1660; found 425.1655.
2-(Benzyloxy)-10-(cyclohexylmethyl)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (5). Compound 5 was synthesized following the procedure described for compound 1. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 5
EA = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound 5 (58 mg, 67.4%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 9.01 (s, 1H), 7.47–7.27 (m, 6H), 7.08 (dd, J = 8.87 Hz, 3.04 Hz, 1H), 7.01 (t, J = 7.85 Hz, 2H), 6.82 (dd, J = 13.25 Hz, 5.56 Hz, 2H), 5.04 (q, J = 11.54 Hz, 2H), 3.56–3.31 (m, 2H), 1.84 (t, J = 13.32 Hz, 2H), 1.70–1.47 (m, 4H), 1.10 (s, 3H), 0.89 (q, J = 11.21 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 170.2, 159.1 (d, J = 243.0 Hz), 154.8, 146.3, 140.7 (d, J = 2.5 Hz), 136.8, 134.4 (d, J = 10.1 Hz), 128.7 (2C), 128.3, 128.2, 127.7 (2C), 121.4 (d, J = 9.2 Hz), 121.1, 120.3, 115.7, 111.9 (d, J = 22.3 Hz), 108.5 (d, J = 25.0 Hz), 70.5, 56.8, 35.2, 31.3 (2C), 26.8, 26.0 (2C). HRMS ESI (m/z): calcd for C27H28FN2O2+ [M + H]+: 431.2129; found 431.2127.
1) to afford compound 5 (58 mg, 67.4%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 9.01 (s, 1H), 7.47–7.27 (m, 6H), 7.08 (dd, J = 8.87 Hz, 3.04 Hz, 1H), 7.01 (t, J = 7.85 Hz, 2H), 6.82 (dd, J = 13.25 Hz, 5.56 Hz, 2H), 5.04 (q, J = 11.54 Hz, 2H), 3.56–3.31 (m, 2H), 1.84 (t, J = 13.32 Hz, 2H), 1.70–1.47 (m, 4H), 1.10 (s, 3H), 0.89 (q, J = 11.21 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 170.2, 159.1 (d, J = 243.0 Hz), 154.8, 146.3, 140.7 (d, J = 2.5 Hz), 136.8, 134.4 (d, J = 10.1 Hz), 128.7 (2C), 128.3, 128.2, 127.7 (2C), 121.4 (d, J = 9.2 Hz), 121.1, 120.3, 115.7, 111.9 (d, J = 22.3 Hz), 108.5 (d, J = 25.0 Hz), 70.5, 56.8, 35.2, 31.3 (2C), 26.8, 26.0 (2C). HRMS ESI (m/z): calcd for C27H28FN2O2+ [M + H]+: 431.2129; found 431.2127.
2-(Benzyloxy)-8-fluoro-10-(4-(methylsulfonyl)benzyl)-5,10-dihydro-11H-dibenzo[b,e][1,4] diazepin-11-one (6). Compound 6 was synthesized following the procedure described for compound 1. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 5
EA = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound 6 (55 mg, yield 54.7%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.88 (d, J = 8.26 Hz, 2H), 7.57 (d, J = 8.20 Hz, 2H), 7.44 (d, J = 2.92 Hz, 1H), 7.41–7.30 (m, 5H), 6.99 (dd, J = 8.63 Hz, 2.91 Hz, 1H), 6.94–6.82 (m, 2H), 6.81–6.72 (m, 2H), 5.32 (s, 2H), 5.02 (s, 2H), 3.02 (s, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.5, 159.4 (d, J = 243.3 Hz), 154.8, 144.7, 143.8, 141.2 (d, J = 2.6 Hz), 139.4, 136.7, 135.2 (d, J = 9.5 Hz), 128.7 (2C), 128.2, 127.9 (2C), 127.8 (2C), 127.7 (2C), 125.3, 121.5 (d, J = 9.6 Hz), 121.5, 120.2, 116.7, 113.3 (d, J = 22.5 Hz), 110.8 (d, J = 25.1 Hz), 70.6, 53.7, 44.7. HRMS ESI (m/z): calcd for C28H24FN2O4S+ [M + H]+: 503.1435; found 503.1432.
1) to afford compound 6 (55 mg, yield 54.7%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.88 (d, J = 8.26 Hz, 2H), 7.57 (d, J = 8.20 Hz, 2H), 7.44 (d, J = 2.92 Hz, 1H), 7.41–7.30 (m, 5H), 6.99 (dd, J = 8.63 Hz, 2.91 Hz, 1H), 6.94–6.82 (m, 2H), 6.81–6.72 (m, 2H), 5.32 (s, 2H), 5.02 (s, 2H), 3.02 (s, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.5, 159.4 (d, J = 243.3 Hz), 154.8, 144.7, 143.8, 141.2 (d, J = 2.6 Hz), 139.4, 136.7, 135.2 (d, J = 9.5 Hz), 128.7 (2C), 128.2, 127.9 (2C), 127.8 (2C), 127.7 (2C), 125.3, 121.5 (d, J = 9.6 Hz), 121.5, 120.2, 116.7, 113.3 (d, J = 22.5 Hz), 110.8 (d, J = 25.1 Hz), 70.6, 53.7, 44.7. HRMS ESI (m/z): calcd for C28H24FN2O4S+ [M + H]+: 503.1435; found 503.1432.
4-((2-(Benzyloxy)-8-fluoro-11-oxo-5,11-dihydro-10H-dibenzo[b,e][1,4]diazepin-10-yl) methyl)-N,N-dimethylbenzenesulfonamide (7). Compound 7 was synthesized following the procedure described for compound 4. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 5
EA = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound 7 (55 mg, 51.7%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.71 (d, J = 7.96 Hz, 2H), 7.53 (d, J = 7.94 Hz, 2H), 7.45 (d, J = 2.50 Hz, 1H), 7.43–7.28 (m, 5H), 7.08–6.95 (m, 1H), 6.93–6.63 (m, 4H), 5.32 (s, 2H), 5.02 (s, 2H), 2.68 (s, 6H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.6, 159.3 (d, J = 242.9 Hz), 154.8, 144.7, 142.5, 141.3 (d, J = 2.8 Hz), 136.7, 135.1 (d, J = 9.7 Hz), 134.3, 128.7 (2C), 128.2 (2C), 128.2, 127.7 (2C), 127.4 (2C), 125.4, 121.5 (d, J = 9.2 Hz), 121.3, 120.2, 116.7, 113.2 (d, J = 22.6 Hz), 110.8 (d, J = 25.1 Hz), 70.6, 53.6, 38.0 (2C). HRMS ESI (m/z): calcd for C29H27FN3O4S+ [M + H]+: 532.1701; found 532.1699.
1) to afford compound 7 (55 mg, 51.7%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.71 (d, J = 7.96 Hz, 2H), 7.53 (d, J = 7.94 Hz, 2H), 7.45 (d, J = 2.50 Hz, 1H), 7.43–7.28 (m, 5H), 7.08–6.95 (m, 1H), 6.93–6.63 (m, 4H), 5.32 (s, 2H), 5.02 (s, 2H), 2.68 (s, 6H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.6, 159.3 (d, J = 242.9 Hz), 154.8, 144.7, 142.5, 141.3 (d, J = 2.8 Hz), 136.7, 135.1 (d, J = 9.7 Hz), 134.3, 128.7 (2C), 128.2 (2C), 128.2, 127.7 (2C), 127.4 (2C), 125.4, 121.5 (d, J = 9.2 Hz), 121.3, 120.2, 116.7, 113.2 (d, J = 22.6 Hz), 110.8 (d, J = 25.1 Hz), 70.6, 53.6, 38.0 (2C). HRMS ESI (m/z): calcd for C29H27FN3O4S+ [M + H]+: 532.1701; found 532.1699.
2-(Benzyloxy)-8-fluoro-10-(3-morpholinopropyl)-5,10-dihydro-11H-dibenzo[b,e][1,4] diazepin-11-one (8). Compound 8 was synthesized following the procedure described for compound 4. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 2
EA = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound 8 (140 mg, 53.2%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.41 (s, 1H), 7.39–7.30 (m, 4H), 7.30–7.24 (m, 1H), 6.98 (d, J = 9.7 Hz, 1H), 6.93–6.82 (m, 2H), 6.73 (d, J = 8.5 Hz, 2H), 5.62 (s, 1H), 4.96 (s, 2H), 4.11 (s, 2H), 3.62 (s, 4H), 2.37 (t, J = 6.9 Hz, 2H), 2.32 (s, 4H), 1.91–1.72 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.3, 159.3 (d, J = 241.9 Hz), 154.5, 145.1, 141.9, 136.8, 135.4 (d, J = 9.7 Hz), 128.6 (2C), 128.1, 127.6 (2C), 126.1, 121.2 (d, J = 9.0 Hz), 120.8, 119.9, 116.4, 112.6 (d, J = 22.4 Hz), 111.3 (d, J = 24.9 Hz), 70.4, 67.0 (2C), 55.5, 53.6 (2C), 48.2, 25.0. HRMS ESI-TOF (m/z): calcd for C27H29FN3O3+ [M + H]+: 462.2193. Found 462.2201.
1) to afford compound 8 (140 mg, 53.2%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.41 (s, 1H), 7.39–7.30 (m, 4H), 7.30–7.24 (m, 1H), 6.98 (d, J = 9.7 Hz, 1H), 6.93–6.82 (m, 2H), 6.73 (d, J = 8.5 Hz, 2H), 5.62 (s, 1H), 4.96 (s, 2H), 4.11 (s, 2H), 3.62 (s, 4H), 2.37 (t, J = 6.9 Hz, 2H), 2.32 (s, 4H), 1.91–1.72 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.3, 159.3 (d, J = 241.9 Hz), 154.5, 145.1, 141.9, 136.8, 135.4 (d, J = 9.7 Hz), 128.6 (2C), 128.1, 127.6 (2C), 126.1, 121.2 (d, J = 9.0 Hz), 120.8, 119.9, 116.4, 112.6 (d, J = 22.4 Hz), 111.3 (d, J = 24.9 Hz), 70.4, 67.0 (2C), 55.5, 53.6 (2C), 48.2, 25.0. HRMS ESI-TOF (m/z): calcd for C27H29FN3O3+ [M + H]+: 462.2193. Found 462.2201.
2-(Benzyloxy)-10-(2-(dimethylamino)ethyl)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4] diazepin-11-one (9). Compound 9 was synthesized following the procedure described for compound 1. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 1
EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to afford compound 9 (1.3 g, 60.6%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.43–7.29 (m, 6H), 7.15 (d, J = 9.9 Hz, 1H), 6.95–6.90 (m, 1H), 6.89–6.82 (m, 1H), 6.81–6.69 (m, 2H), 4.99 (s, 2H), 4.12 (t, J = 6.6 Hz, 2H), 2.66 (t, J = 6.9 Hz, 2H), 2.29 (s, 6H); 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.3, 159.4 (d, J = 242.0 Hz), 154.6, 144.7, 141.4 (d, J = 2.6 Hz), 136.8, 135.5 (d, J = 9.8 Hz), 128.6 (2C), 128.0, 127.6 (2C), 126.0, 121.1 (d, J = 9.3 Hz), 120.9, 119.8, 116.5, 112.7 (d, J = 22.5 Hz), 111.4 (d, J = 25.4 Hz), 70.5, 57.3, 49.1, 45.6 (2C). HRMS ESI-TOF (m/z): calcd for C24H25FN3O2+ [M + H]+: 406.1931; found 406.1930.
2) to afford compound 9 (1.3 g, 60.6%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.43–7.29 (m, 6H), 7.15 (d, J = 9.9 Hz, 1H), 6.95–6.90 (m, 1H), 6.89–6.82 (m, 1H), 6.81–6.69 (m, 2H), 4.99 (s, 2H), 4.12 (t, J = 6.6 Hz, 2H), 2.66 (t, J = 6.9 Hz, 2H), 2.29 (s, 6H); 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.3, 159.4 (d, J = 242.0 Hz), 154.6, 144.7, 141.4 (d, J = 2.6 Hz), 136.8, 135.5 (d, J = 9.8 Hz), 128.6 (2C), 128.0, 127.6 (2C), 126.0, 121.1 (d, J = 9.3 Hz), 120.9, 119.8, 116.5, 112.7 (d, J = 22.5 Hz), 111.4 (d, J = 25.4 Hz), 70.5, 57.3, 49.1, 45.6 (2C). HRMS ESI-TOF (m/z): calcd for C24H25FN3O2+ [M + H]+: 406.1931; found 406.1930.
Ethyl 4-((2-(benzyloxy)-8-fluoro-11-oxo-5,11-dihydro-10H-dibenzo[b,e][1,4]diazepin-10-yl) methyl)benzoate (10). Compound 10 was synthesized following the procedure described for compound 4. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 3
EA = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound 10 (64.2 mg, 64.7%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.98 (d, J = 8.34 Hz, 2H), 7.47 (d, J = 2.95 Hz, 1H), 7.45–7.28 (m, 7H), 6.98 (dd, J = 8.64 Hz, 2.97, 1H), 6.90–6.81 (m, 2H), 6.80–6.67 (m, 2H), 5.31 (s, 2H), 5.02 (s, 2H), 4.35 (q, J = 7.13 Hz, 2H), 1.37 (t, J = 7.13 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.5, 166.5, 159.4 (d, J = 242.7 Hz), 154.8, 144.6, 142.3, 141.0 (d, J = 1.6 Hz), 136.8, 135.3 (d, J = 9.7 Hz), 130.1 (2C), 129.5, 128.7 (2C), 128.2, 127.7 (2C), 126.9 (2C), 125.6, 121.3, 121.2, 120.0, 116.8, 113.0 (d, J = 22.4 Hz), 110.9 (d, J = 25.2 Hz), 70.6, 61.1, 53.7, 14.5. HRMS ESI (m/z): calcd for C30H26FN2O4+ [M + H]+: 497.1871; found 497.1869.
1) to afford compound 10 (64.2 mg, 64.7%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.98 (d, J = 8.34 Hz, 2H), 7.47 (d, J = 2.95 Hz, 1H), 7.45–7.28 (m, 7H), 6.98 (dd, J = 8.64 Hz, 2.97, 1H), 6.90–6.81 (m, 2H), 6.80–6.67 (m, 2H), 5.31 (s, 2H), 5.02 (s, 2H), 4.35 (q, J = 7.13 Hz, 2H), 1.37 (t, J = 7.13 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.5, 166.5, 159.4 (d, J = 242.7 Hz), 154.8, 144.6, 142.3, 141.0 (d, J = 1.6 Hz), 136.8, 135.3 (d, J = 9.7 Hz), 130.1 (2C), 129.5, 128.7 (2C), 128.2, 127.7 (2C), 126.9 (2C), 125.6, 121.3, 121.2, 120.0, 116.8, 113.0 (d, J = 22.4 Hz), 110.9 (d, J = 25.2 Hz), 70.6, 61.1, 53.7, 14.5. HRMS ESI (m/z): calcd for C30H26FN2O4+ [M + H]+: 497.1871; found 497.1869.
2-(Benzyloxy)-8-fluoro-10-(3-hydroxypropyl)-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (11). A mixture of S8 (334 mg, 1.0 mmol) and NaH (60% dispersion in mineral oil, 60 mg, 1.5 mmol) in 50 mL dry THF was stirred at 0 °C under a nitrogen atmosphere for 30 min. (3-Iodopropoxy)triisopropylsilane (410 mg, 1.2 mmol) was added to the mixture. After addition, the reaction mixture warmed to room temperature and was stirred for 24 h under a nitrogen atmosphere. Upon completion, the organic solvent was removed under reduced pressure. The reaction mixture was diluted with water (20 mL) and extracted with DCM (3 × 20 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude intermediate was purified by silica gel column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 10
EA = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 260 mg of a yellow solid. The intermediate (260 mg) was dissolved in anhydrous MeOH (8 mL), and a 10% aqueous HCl solution (1 mL) was added dropwise at 0 °C. After completion, the mixture was stirred at room temperature for 4 h and concentrated under reduced pressure. Then, H2O (20 mL) was added and the aqueous layer was extracted with DCM (3 × 20 mL). The organic phase was dried over anhydrous Na2SO4, and the solvent was removed under reduced pressure. The crude product was purified by column chromatography (PE
1) to afford 260 mg of a yellow solid. The intermediate (260 mg) was dissolved in anhydrous MeOH (8 mL), and a 10% aqueous HCl solution (1 mL) was added dropwise at 0 °C. After completion, the mixture was stirred at room temperature for 4 h and concentrated under reduced pressure. Then, H2O (20 mL) was added and the aqueous layer was extracted with DCM (3 × 20 mL). The organic phase was dried over anhydrous Na2SO4, and the solvent was removed under reduced pressure. The crude product was purified by column chromatography (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 1
EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to give compound 11 (167 mg, 42.7%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.40–7.29 (m, 6H), 6.98 (dd, J = 9.78 Hz, 2.50, 2H), 6.93–6.86 (m, 2H), 6.79–6.72 (m, 2H), 4.97 (s, 2H), 4.19 (s, 2H), 3.68 (t, J = 5.61 Hz, 2H), 2.98 (s, 1H), 1.86–1.77 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 169.5, 159.6 (d, J = 242.5 Hz), 154.9, 145.3, 142.5 (d, J = 2.1 Hz), 137.0, 135.1 (d, J = 9.6 Hz), 128.9 (2C), 128.4, 127.9 (2C), 126.4, 121.8 (d, J = 9.0 Hz), 121.1, 120.3, 116.7, 113.4 (d, J = 22.4 Hz), 111.7 (d, J = 25.0 Hz), 70.8, 59.7, 47.1, 31.3. HRMS ESI (m/z): calcd for C23H21FN2O3+ [M + H]+: 393.1609; found 393.1603.
2) to give compound 11 (167 mg, 42.7%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.40–7.29 (m, 6H), 6.98 (dd, J = 9.78 Hz, 2.50, 2H), 6.93–6.86 (m, 2H), 6.79–6.72 (m, 2H), 4.97 (s, 2H), 4.19 (s, 2H), 3.68 (t, J = 5.61 Hz, 2H), 2.98 (s, 1H), 1.86–1.77 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 169.5, 159.6 (d, J = 242.5 Hz), 154.9, 145.3, 142.5 (d, J = 2.1 Hz), 137.0, 135.1 (d, J = 9.6 Hz), 128.9 (2C), 128.4, 127.9 (2C), 126.4, 121.8 (d, J = 9.0 Hz), 121.1, 120.3, 116.7, 113.4 (d, J = 22.4 Hz), 111.7 (d, J = 25.0 Hz), 70.8, 59.7, 47.1, 31.3. HRMS ESI (m/z): calcd for C23H21FN2O3+ [M + H]+: 393.1609; found 393.1603.
2-(Benzyloxy)-10-(3-chloropropyl)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (12). Compound 12 was synthesized following the procedure described for compound 1. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 3
EA = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound 12 (5.2 g, 70.6%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.44 (d, J = 2.96 Hz, 1H), 7.41–7.29 (m, 5H), 7.04–6.82 (m, 3H), 6.83–6.70 (m, 2H), 4.99 (s, 2H), 4.23 (s, 2H), 3.62 (t, J = 6.36 Hz, 2H), 2.15 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.3, 159.6 (d, J = 242.8 Hz), 154.7, 144.7 (d, J = 3.4 Hz), 141.7 (d, J = 2.6 Hz), 136.8, 135.4 (d, J = 9.5 Hz), 128.7 (2C), 128.2, 127.7 (2C), 125.9, 121.3 (d, J = 9.2 Hz), 121.0, 119.9, 116.6, 113.1 (d, J = 22.5 Hz), 111.4 (d, J = 24.8 Hz), 70.6, 47.6, 42.3, 30.8. HRMS ESI (m/z): calcd for C23H21ClFN2O2+ [M + H]+: 411.1270; found 411.1266.
1) to afford compound 12 (5.2 g, 70.6%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.44 (d, J = 2.96 Hz, 1H), 7.41–7.29 (m, 5H), 7.04–6.82 (m, 3H), 6.83–6.70 (m, 2H), 4.99 (s, 2H), 4.23 (s, 2H), 3.62 (t, J = 6.36 Hz, 2H), 2.15 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.3, 159.6 (d, J = 242.8 Hz), 154.7, 144.7 (d, J = 3.4 Hz), 141.7 (d, J = 2.6 Hz), 136.8, 135.4 (d, J = 9.5 Hz), 128.7 (2C), 128.2, 127.7 (2C), 125.9, 121.3 (d, J = 9.2 Hz), 121.0, 119.9, 116.6, 113.1 (d, J = 22.5 Hz), 111.4 (d, J = 24.8 Hz), 70.6, 47.6, 42.3, 30.8. HRMS ESI (m/z): calcd for C23H21ClFN2O2+ [M + H]+: 411.1270; found 411.1266.
2-(2-(Benzyloxy)-8-fluoro-11-oxo-5,11-dihydro-10H-dibenzo[b,e][1,4]diazepin-10-yl)-N,N,N-trimethylethan-1-aminium iodide (13). Compound 9 (183 mg, 0.45 mmol) and CH3I (70 mg, 0.5 mmol) were added into MeCN (5 mL). The reaction mixture was then stirred at room temperature for 24 h. Upon completion, the mixture was filtered and the filtrate was concentrated. The crude product was purified by recrystallization to give compound 13 (120 mg, 63.5%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 7.74 (s, 1H), 7.44–7.35 (m, 5H), 7.35–7.30 (m, 1H), 7.27–7.21 (m, 2H), 7.14–7.03 (m, 3H), 5.06 (s, 2H), 4.40 (s, 2H), 3.65 (t, J = 6.8 Hz, 2H), 3.17 (s, 9H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 168.2, 158.9 (d, J = 239.2 Hz), 153.7, 146.4, 142.9 (d, J = 2.3 Hz), 137.4, 134.5 (d, J = 9.9 Hz), 128.9 (2C), 128.3, 128.0 (2C), 125.0, 122.2 (d, J = 9.4 Hz), 121.3, 120.7, 116.7, 113.7 (d, J = 22.2 Hz), 111.3 (d, J = 25.6 Hz), 70.0, 62.0, 53.0 (3C), 44.1. HRMS ESI-TOF (m/z): calcd for C25H27FN3O2+ [M]+: 420.2082. Found 420.2081.
4-((2-(Benzyloxy)-8-fluoro-11-oxo-5,11-dihydro-10H-dibenzo[b,e][1,4]diazepin-10-yl) methyl)benzoic acid (14). A mixture of compound 10 (440 mg, 0.88 mmol) and NaOH (71 mg, 1.77 mmol) in 10 mL THF/MeOH/H2O (V
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V
V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V = 1
V = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5) was stirred at 70 °C for 12 hours. Upon completion, the organic solvent was removed under reduced pressure. The aqueous solution was adjusted to pH 2–3 with 2 N HCl. After completion of pH adjustment, extraction was done with EtOAc (3 × 15 mL). Then the organic solvent was removed under reduced pressure. The crude product was purified by column chromatography (DCM
2.5) was stirred at 70 °C for 12 hours. Upon completion, the organic solvent was removed under reduced pressure. The aqueous solution was adjusted to pH 2–3 with 2 N HCl. After completion of pH adjustment, extraction was done with EtOAc (3 × 15 mL). Then the organic solvent was removed under reduced pressure. The crude product was purified by column chromatography (DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 3
MeOH = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 14 (400 mg, 94.9%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 12.89 (s, 1H), 7.86 (d, J = 8.24 Hz, 2H), 7.70 (s, 1H), 7.53–7.28 (m, 7H), 7.23 (dd, J = 12.78 Hz, 2.74 Hz, 2H), 7.17–6.97 (m, 3H), 6.89 (td, J = 8.35 Hz, 2.80, 1H), 5.34 (s, 2H), 5.06 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 167.7, 167.2, 158.2 (d, J = 238.6 Hz), 153.4, 146.0, 142.6 (d, J = 2.0 Hz), 137.1, 134.4 (d, J = 10.0 Hz), 129.6 (2C), 128.5 (4C), 127.9, 127.7 (2C), 126.9 (2C), 125.1, 121.5 (d, J = 9.2 Hz), 120.7, 120.2, 116.6, 112.8 (d, J = 21.9 Hz), 110.9 (d, J = 25.0 Hz), 69.7, 55.0, 51.7. HRMS ESI (m/z): calcd for C28H22FN2O4+ [M + H]+: 469.1554; found 469.1558.
1) to give compound 14 (400 mg, 94.9%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 12.89 (s, 1H), 7.86 (d, J = 8.24 Hz, 2H), 7.70 (s, 1H), 7.53–7.28 (m, 7H), 7.23 (dd, J = 12.78 Hz, 2.74 Hz, 2H), 7.17–6.97 (m, 3H), 6.89 (td, J = 8.35 Hz, 2.80, 1H), 5.34 (s, 2H), 5.06 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 167.7, 167.2, 158.2 (d, J = 238.6 Hz), 153.4, 146.0, 142.6 (d, J = 2.0 Hz), 137.1, 134.4 (d, J = 10.0 Hz), 129.6 (2C), 128.5 (4C), 127.9, 127.7 (2C), 126.9 (2C), 125.1, 121.5 (d, J = 9.2 Hz), 120.7, 120.2, 116.6, 112.8 (d, J = 21.9 Hz), 110.9 (d, J = 25.0 Hz), 69.7, 55.0, 51.7. HRMS ESI (m/z): calcd for C28H22FN2O4+ [M + H]+: 469.1554; found 469.1558.
2-(Benzyloxy)-10-(3-bromopropyl)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (15). PBr3 (224 mg, 0.83 mmol) was added to a solution of compound 11 (130 mg, 0.33 mmol) in dry DCM (10 mL). The mixture was stirred at 0 °C under a nitrogen atmosphere for 2 h, then warmed to room temperature and stirred for 24 h. Upon completion, the reaction mixture was concentrated in vacuo. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 2
EA = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 15 (120 mg, 80.1%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.42 (d, J = 2.96 Hz, 1H), 7.41–7.27 (m, 5H), 7.00–6.87 (m, 3H), 6.83–6.71 (m, 2H), 5.00 (s, 2H), 4.22 (s, 2H), 3.48 (t, J = 6.49 Hz, 2H), 2.23 (p, J = 6.40 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.3, 159.5 (d, J = 242.8 Hz), 154.7, 144.7, 141.7 (d, J = 2.5 Hz), 136.8, 135.3 (d, J = 9.6 Hz), 128.7 (2C), 128.1, 127.6 (2C), 125.9, 121.3 (d, J = 9.3 Hz), 121.0, 119.9, 116.5, 113.1 (d, J = 22.6 Hz), 111.4 (d, J = 25.0 Hz), 70.5, 48.7, 30.9, 30.7. HRMS ESI (m/z): calcd for C23H21BrFN2O2+ [M + H]+: 455.0765; found 455.0760.
1) to give compound 15 (120 mg, 80.1%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.42 (d, J = 2.96 Hz, 1H), 7.41–7.27 (m, 5H), 7.00–6.87 (m, 3H), 6.83–6.71 (m, 2H), 5.00 (s, 2H), 4.22 (s, 2H), 3.48 (t, J = 6.49 Hz, 2H), 2.23 (p, J = 6.40 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.3, 159.5 (d, J = 242.8 Hz), 154.7, 144.7, 141.7 (d, J = 2.5 Hz), 136.8, 135.3 (d, J = 9.6 Hz), 128.7 (2C), 128.1, 127.6 (2C), 125.9, 121.3 (d, J = 9.3 Hz), 121.0, 119.9, 116.5, 113.1 (d, J = 22.6 Hz), 111.4 (d, J = 25.0 Hz), 70.5, 48.7, 30.9, 30.7. HRMS ESI (m/z): calcd for C23H21BrFN2O2+ [M + H]+: 455.0765; found 455.0760.
3-(2-(Benzyloxy)-8-fluoro-11-oxo-5,11-dihydro-10H-dibenzo[b,e][1,4]diazepin-10-yl)propyl dimethylcarbamodithioate (16). A mixture of 12 (200 mg, 0.44 mmol) and sodium dimethyldithiocarbamate (95 mg, 0.66 mmol) in 15 mL THF was stirred at 65 °C for 24 h. Upon completion, the reaction mixture was concentrated in vacuo The reaction mixture was diluted with water (100 mL) and extracted with DCM (3 × 100 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 2
EA = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 16 (120 mg, 55%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.54–7.28 (m, 6H), 7.11–6.84 (m, 3H), 6.83–6.70 (m, 2H), 4.98 (s, 2H), 4.18 (s, 2H), 3.50 (s, 3H), 3.34 (t, J = 7.10 Hz, 3H), 3.29 (s, 2H), 2.09 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 197.0, 168.3, 159.4 (d, J = 242.2 Hz), 154.5, 144.9, 141.9 (d, J = 2.4 Hz), 136.8, 135.0 (d, J = 9.6 Hz), 128.6 (2C), 128.0, 127.6 (2C), 125.9, 121.4 (d, J = 9.1 Hz), 120.8, 119.9, 116.5, 112.8 (d, J = 22.4 Hz), 111.3 (d, J = 25.0 Hz), 70.5, 48.6, 45.3 (2C), 41.5, 34.3, 27.1. HRMS ESI (m/z): calcd for C26H27FN3O2S2+ [M + H]+: 496.1523; found 496.1517.
1) to give compound 16 (120 mg, 55%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.54–7.28 (m, 6H), 7.11–6.84 (m, 3H), 6.83–6.70 (m, 2H), 4.98 (s, 2H), 4.18 (s, 2H), 3.50 (s, 3H), 3.34 (t, J = 7.10 Hz, 3H), 3.29 (s, 2H), 2.09 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 197.0, 168.3, 159.4 (d, J = 242.2 Hz), 154.5, 144.9, 141.9 (d, J = 2.4 Hz), 136.8, 135.0 (d, J = 9.6 Hz), 128.6 (2C), 128.0, 127.6 (2C), 125.9, 121.4 (d, J = 9.1 Hz), 120.8, 119.9, 116.5, 112.8 (d, J = 22.4 Hz), 111.3 (d, J = 25.0 Hz), 70.5, 48.6, 45.3 (2C), 41.5, 34.3, 27.1. HRMS ESI (m/z): calcd for C26H27FN3O2S2+ [M + H]+: 496.1523; found 496.1517.
2-(Benzyloxy)-8-fluoro-10-(3-iodopropyl)-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (17). A mixture of 12 (2.2 g, 5.36 mmol) and NaI (3.2 g, 21.46 mmol) in 50 mL MeCN was stirred at 70 °C for 24 h. Upon completion, the reaction mixture was concentrated in vacuo. The reaction mixture was diluted with water (100 mL) and extracted with DCM (3 × 100 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 2
EA = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 17 (2 g, 74.3%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.44–7.28 (m, 6H), 7.01–6.92 (m, 2H), 6.89 (dd, J = 8.65 Hz, 5.44 Hz, 1H), 6.84–6.78 (m, 1H), 6.74 (d, J = 8.66 Hz, 1H), 5.01 (s, 2H), 4.17 (s, 2H), 3.25 (t, J = 6.80 Hz, 2H), 2.26–2.13 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.2, 159.7 (d, J = 243.0 Hz), 154.8, 144.6, 141.7 (d, J = 2.8 Hz), 136.8, 135.4 (d, J = 9.7 Hz), 128.7 (2C), 128.2, 127.7 (2C), 125.9, 121.3 (d, J = 9.2 Hz), 121.1, 119.9, 116.6, 113.2 (d, J = 22.4 Hz), 111.6 (d, J = 25.0 Hz), 70.6, 50.6, 31.8, 2.9. HRMS ESI (m/z): calcd for C23H21FIN2O2+ [M + H]+: 503.0626; found 503.0619.
1) to give compound 17 (2 g, 74.3%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.44–7.28 (m, 6H), 7.01–6.92 (m, 2H), 6.89 (dd, J = 8.65 Hz, 5.44 Hz, 1H), 6.84–6.78 (m, 1H), 6.74 (d, J = 8.66 Hz, 1H), 5.01 (s, 2H), 4.17 (s, 2H), 3.25 (t, J = 6.80 Hz, 2H), 2.26–2.13 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.2, 159.7 (d, J = 243.0 Hz), 154.8, 144.6, 141.7 (d, J = 2.8 Hz), 136.8, 135.4 (d, J = 9.7 Hz), 128.7 (2C), 128.2, 127.7 (2C), 125.9, 121.3 (d, J = 9.2 Hz), 121.1, 119.9, 116.6, 113.2 (d, J = 22.4 Hz), 111.6 (d, J = 25.0 Hz), 70.6, 50.6, 31.8, 2.9. HRMS ESI (m/z): calcd for C23H21FIN2O2+ [M + H]+: 503.0626; found 503.0619.
2-(3-(2-(Benzyloxy)-8-fluoro-11-oxo-5,11-dihydro-10H-dibenzo[b,e][1,4]diazepin-10-yl)propyl)isoindoline-1,3-dione (S9). A mixture of 12 (3.08 g, 7.51 mmol) and potassium phthalimide (1.88 g, 10.16 mmol) in 50 mL DMF was stirred at 80 °C for 24 h. Upon completion, the mixture was diluted with deionized water (100 mL) and extracted with EtOAc (3 × 100 mL). The combined organic layers were washed with saturated brine (1 × 100 mL), dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 5
MeOH = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound S9 (2.6 g, 66.4%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 7.92–7.73 (m, 4H), 7.60 (s, 1H), 7.43–7.32 (m, 4H), 7.32–7.27 (m, 1H), 7.27–7.20 (m, 1H), 7.17 (d, J = 2.85 Hz, 1H), 7.12 (dd, J = 8.83 Hz, 5.87 Hz, 1H), 7.06–6.89 (m, 3H), 5.02 (s, 2H), 4.05 (s, 2H), 3.59 (t, J = 7.17 Hz, 2H), 1.84 (p, J = 7.18 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 167.9, 167.4, 158.4 (d, J = 238.5 Hz), 157.2, 153.3, 146.0, 143.1 (d, J = 2.3 Hz), 137.2, 134.4 (2C), 134.2 (d, J = 10.0 Hz), 131.8 (2C), 128.5 (2C), 127.9, 127.7 (2C), 125.4, 123.1 (2C), 121.5 (d, J = 9.2 Hz), 120.4, 120.2 (d, J = 25.6 Hz), 116.4, 112.7 (d, J = 22.2 Hz), 111.1 (d, J = 25.0 Hz), 69.7, 46.3, 35.4, 26.6. HRMS ESI (m/z): calcd for C31H25FN3O4+ [M + H]+: 522.1824; found 522.1820.
1) to give compound S9 (2.6 g, 66.4%) as a white solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 7.92–7.73 (m, 4H), 7.60 (s, 1H), 7.43–7.32 (m, 4H), 7.32–7.27 (m, 1H), 7.27–7.20 (m, 1H), 7.17 (d, J = 2.85 Hz, 1H), 7.12 (dd, J = 8.83 Hz, 5.87 Hz, 1H), 7.06–6.89 (m, 3H), 5.02 (s, 2H), 4.05 (s, 2H), 3.59 (t, J = 7.17 Hz, 2H), 1.84 (p, J = 7.18 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 167.9, 167.4, 158.4 (d, J = 238.5 Hz), 157.2, 153.3, 146.0, 143.1 (d, J = 2.3 Hz), 137.2, 134.4 (2C), 134.2 (d, J = 10.0 Hz), 131.8 (2C), 128.5 (2C), 127.9, 127.7 (2C), 125.4, 123.1 (2C), 121.5 (d, J = 9.2 Hz), 120.4, 120.2 (d, J = 25.6 Hz), 116.4, 112.7 (d, J = 22.2 Hz), 111.1 (d, J = 25.0 Hz), 69.7, 46.3, 35.4, 26.6. HRMS ESI (m/z): calcd for C31H25FN3O4+ [M + H]+: 522.1824; found 522.1820.
10-(3-Aminopropyl)-2-(benzyloxy)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (18). Compound S9 (3 g, 5.75 mmol) was dissolved in 50 mL MeOH, and N2H4·H2O (9 mL) was added dropwise. The reaction mixture was stirred at room temperature under a nitrogen atmosphere for 12 hours. Upon completion, the solvent was removed in vacuo, and the residue was adjusted to pH 9–10 with 2 M NaOH. The aqueous layer was extracted with EtOAc (3 × 50 mL). The combined organic layers were washed with saturated brine (1 × 30 mL), dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 2
MeOH = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 18 (1.98 g, 87.9%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.47–7.28 (m, 6H), 7.00–6.66 (m, 5H), 5.67 (s, 1H), 4.99 (s, 2H), 4.13 (s, 2H), 2.79 (d, J = 42.20 Hz, 4H), 1.81–1.62 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.8, 159.4 (d, J = 242.8 Hz), 154.6, 145.1, 142.4 (d, J = 2.7 Hz), 136.8, 134.7 (d, J = 9.4 Hz), 128.7 (2C), 128.1, 127.7 (2C), 126.1, 121.7 (d, J = 9.1 Hz), 120.8, 120.1, 116.5, 113.1 (d, J = 22.6 Hz), 111.4 (d, J = 25.0 Hz), 70.5, 46.9, 38.6, 30.1. HRMS ESI (m/z): calcd for C23H23FN3O2+ [M + H]+: 392.1769; found 392.1765.
1) to give compound 18 (1.98 g, 87.9%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.47–7.28 (m, 6H), 7.00–6.66 (m, 5H), 5.67 (s, 1H), 4.99 (s, 2H), 4.13 (s, 2H), 2.79 (d, J = 42.20 Hz, 4H), 1.81–1.62 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.8, 159.4 (d, J = 242.8 Hz), 154.6, 145.1, 142.4 (d, J = 2.7 Hz), 136.8, 134.7 (d, J = 9.4 Hz), 128.7 (2C), 128.1, 127.7 (2C), 126.1, 121.7 (d, J = 9.1 Hz), 120.8, 120.1, 116.5, 113.1 (d, J = 22.6 Hz), 111.4 (d, J = 25.0 Hz), 70.5, 46.9, 38.6, 30.1. HRMS ESI (m/z): calcd for C23H23FN3O2+ [M + H]+: 392.1769; found 392.1765.
4-(2-(Benzyloxy)-8-fluoro-11-oxo-5,11-dihydro-10H-dibenzo[b,e][1,4]diazepin-10-yl) butanenitrile (19). A mixture of compound 17 (251 mg, 0.5 mmol), TBAF (1 M in THF, 1 mL, 1 mmol), and TMSCN (99 mg, 1 mmol) in MeCN (10 mL) was stirred at room temperature under a nitrogen atmosphere for 12 h. Upon completion, the solvent was removed in vacuo. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 3
EA = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 19 (110 mg, 54.9%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.48–7.28 (m, 6H), 7.04–6.88 (m, 3H), 6.86–6.69 (m, 2H), 5.35 (s, 1H), 5.00 (s, 2H), 4.20 (s, 2H), 2.46 (t, J = 7.40 Hz, 2H), 2.11–1.90 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.4, 159.5 (d, J = 243.4 Hz), 154.7, 144.8, 141.9, 136.7, 134.7 (d, J = 9.7 Hz), 128.7 (2C), 128.1, 127.6 (2C), 125.7, 121.6 (d, J = 9.1 Hz), 121.1, 120.0, 119.3, 116.5, 113.4 (d, J = 22.5 Hz), 111.4 (d, J = 24.8 Hz), 70.5, 48.3, 24.0, 14.4. HRMS ESI (m/z): calcd for C24H21FN3O2+ [M + H]+: 402.1612; found 402.1609.
1) to give compound 19 (110 mg, 54.9%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.48–7.28 (m, 6H), 7.04–6.88 (m, 3H), 6.86–6.69 (m, 2H), 5.35 (s, 1H), 5.00 (s, 2H), 4.20 (s, 2H), 2.46 (t, J = 7.40 Hz, 2H), 2.11–1.90 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.4, 159.5 (d, J = 243.4 Hz), 154.7, 144.8, 141.9, 136.7, 134.7 (d, J = 9.7 Hz), 128.7 (2C), 128.1, 127.6 (2C), 125.7, 121.6 (d, J = 9.1 Hz), 121.1, 120.0, 119.3, 116.5, 113.4 (d, J = 22.5 Hz), 111.4 (d, J = 24.8 Hz), 70.5, 48.3, 24.0, 14.4. HRMS ESI (m/z): calcd for C24H21FN3O2+ [M + H]+: 402.1612; found 402.1609.
2-(Benzyloxy)-8-fluoro-10-(3-(4-methylpiperazin-1-yl)propyl)-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (20). A mixture of 17 (201 mg, 0.4 mmol), K2CO3 (98 mg, 0.71 mmol) and 1-methylpiperazine (52 mg, 0.52 mmol) in 150 mL MeCN was stirred at 60 °C for 12 h. Upon completion, the solvent was removed in vacuo. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 1
EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to give compound 20 (160 mg, 84.4%) as a white solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.32 (d, J = 2.92 Hz, 1H), 7.26 (q, J = 7.72 Hz, 4H), 7.23–7.16 (m, 1H), 6.89 (dd, J = 9.91 Hz, 2.64 Hz, 1H), 6.86–6.74 (m, 2H), 6.69–6.60 (m, 2H), 5.52 (s, 1H), 4.88 (s, 2H), 4.01 (s, 2H), 2.51–2.19 (m, 8H), 2.17 (s, 3H), 1.76 (p, J = 6.90 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.2, 159.2 (d, J = 241.9 Hz), 154.4, 144.9, 141.8 (d, J = 2.4 Hz), 136.7, 135.3 (d, J = 9.6 Hz), 128.6 (2C), 128.0, 127.5 (2C), 126.1, 121.1 (d, J = 9.1 Hz), 120.7, 119.8, 116.4, 112.6 (d, J = 22.3 Hz), 111.3 (d, J = 24.9 Hz), 70.4, 54.9, 54.8 (2C), 52.7 (2C), 48.1, 45.7, 25.2. HRMS ESI (m/z): calcd for C28H31FN4O2+ [M + H]+: 475.2504; found 475.2500.
2) to give compound 20 (160 mg, 84.4%) as a white solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.32 (d, J = 2.92 Hz, 1H), 7.26 (q, J = 7.72 Hz, 4H), 7.23–7.16 (m, 1H), 6.89 (dd, J = 9.91 Hz, 2.64 Hz, 1H), 6.86–6.74 (m, 2H), 6.69–6.60 (m, 2H), 5.52 (s, 1H), 4.88 (s, 2H), 4.01 (s, 2H), 2.51–2.19 (m, 8H), 2.17 (s, 3H), 1.76 (p, J = 6.90 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.2, 159.2 (d, J = 241.9 Hz), 154.4, 144.9, 141.8 (d, J = 2.4 Hz), 136.7, 135.3 (d, J = 9.6 Hz), 128.6 (2C), 128.0, 127.5 (2C), 126.1, 121.1 (d, J = 9.1 Hz), 120.7, 119.8, 116.4, 112.6 (d, J = 22.3 Hz), 111.3 (d, J = 24.9 Hz), 70.4, 54.9, 54.8 (2C), 52.7 (2C), 48.1, 45.7, 25.2. HRMS ESI (m/z): calcd for C28H31FN4O2+ [M + H]+: 475.2504; found 475.2500.
S-(3-(2-(Benzyloxy)-8-fluoro-11-oxo-5,11-dihydro-10H-dibenzo[b,e][1,4]diazepin-10-yl)propyl)O,O-diethyl phosphorothioate (21). A mixture of 17 (100 mg, 0.20 mmol) and potassium O,O-diethyl phosphorothioate (62 mg, 0.30 mmol) in 15 mL THF was stirred at 50 °C for 24 h. Upon completion, the solvent was removed in vacuo. The mixture was diluted with deionized water (10 mL) and extracted with EtOAc (3 × 10 mL). The combined organic layers were washed with saturated brine (1 × 10 mL), dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 2
EA = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 21 (70 mg, 64.3%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.42–7.28 (m, 6H), 6.99–6.87 (m, 3H), 6.83–6.72 (m, 2H), 5.00 (s, 2H), 4.35–3.97 (m, 6H), 3.03–2.80 (m, 2H), 2.13–1.98 (m, 2H), 1.34–1.27 (m, 6H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.3, 159.5 (d, J = 242.6 Hz), 154.7, 144.7, 141.9 (d, J = 1.6 Hz), 136.8, 135.1 (d, J = 9.6 Hz), 128.7 (2C), 128.2, 127.7 (2C), 125.9, 121.5 (d, J = 9.0 Hz), 121.0, 119.9, 116.6, 113.1 (d, J = 22.5 Hz), 111.4 (d, J = 24.8 Hz), 70.6, 63.8 (2C), 63.7, 48.4, 29.1 (d, J = 4.5 Hz), 28.0 (d, J = 3.9 Hz), 16.2 (2C), 16.1. HRMS ESI (m/z): calcd for C27H31FN3O5PS+ [M + H]+: 545.1670; found 545.1667.
1) to give compound 21 (70 mg, 64.3%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.42–7.28 (m, 6H), 6.99–6.87 (m, 3H), 6.83–6.72 (m, 2H), 5.00 (s, 2H), 4.35–3.97 (m, 6H), 3.03–2.80 (m, 2H), 2.13–1.98 (m, 2H), 1.34–1.27 (m, 6H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.3, 159.5 (d, J = 242.6 Hz), 154.7, 144.7, 141.9 (d, J = 1.6 Hz), 136.8, 135.1 (d, J = 9.6 Hz), 128.7 (2C), 128.2, 127.7 (2C), 125.9, 121.5 (d, J = 9.0 Hz), 121.0, 119.9, 116.6, 113.1 (d, J = 22.5 Hz), 111.4 (d, J = 24.8 Hz), 70.6, 63.8 (2C), 63.7, 48.4, 29.1 (d, J = 4.5 Hz), 28.0 (d, J = 3.9 Hz), 16.2 (2C), 16.1. HRMS ESI (m/z): calcd for C27H31FN3O5PS+ [M + H]+: 545.1670; found 545.1667.
N-(3-(2-(Benzyloxy)-8-fluoro-11-oxo-5,11-dihydro-10H-dibenzo[b,e][1,4]diazepin-10-yl)propyl)acetamide (22). A mixture of 18 (200 mg, 0.51 mmol), TEA (56 mg, 0.55 mmol) and acetic anhydride (52 mg, 0.51 mmol) in 10 mL DCM was stirred at room temperature under a nitrogen atmosphere for 12 h. Upon completion, the solvent was removed in vacuo. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 2
EA = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 22 (160 mg, 72.4%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.44–7.28 (m, 6H), 6.99–6.88 (m, 3H), 6.82–6.74 (m, 2H), 6.49 (t, J = 5.71 Hz, 1H), 5.62 (s, 1H), 5.00 (s, 2H), 4.16 (s, 2H), 3.32 (d, J = 4.32 Hz, 2H), 1.95 (s, 3H), 1.77–1.62 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 170.5, 169.2, 159.4 (d, J = 242.8 Hz), 154.8, 145.0, 142.5 (d, J = 2.4 Hz), 136.7, 134.4 (d, J = 9.5 Hz), 128.7 (2C), 128.2, 127.6 (2C), 126.2, 121.6 (d, J = 9.2 Hz), 120.8, 120.1, 116.3, 113.3 (d, J = 22.4 Hz), 111.4 (d, J = 25.0 Hz), 70.5, 46.7, 36.3, 27.5, 23.5. HRMS ESI (m/z): calcd for C25H25FN3O3+ [M + H]+: 434.1874; found 434.1870.
1) to give compound 22 (160 mg, 72.4%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.44–7.28 (m, 6H), 6.99–6.88 (m, 3H), 6.82–6.74 (m, 2H), 6.49 (t, J = 5.71 Hz, 1H), 5.62 (s, 1H), 5.00 (s, 2H), 4.16 (s, 2H), 3.32 (d, J = 4.32 Hz, 2H), 1.95 (s, 3H), 1.77–1.62 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 170.5, 169.2, 159.4 (d, J = 242.8 Hz), 154.8, 145.0, 142.5 (d, J = 2.4 Hz), 136.7, 134.4 (d, J = 9.5 Hz), 128.7 (2C), 128.2, 127.6 (2C), 126.2, 121.6 (d, J = 9.2 Hz), 120.8, 120.1, 116.3, 113.3 (d, J = 22.4 Hz), 111.4 (d, J = 25.0 Hz), 70.5, 46.7, 36.3, 27.5, 23.5. HRMS ESI (m/z): calcd for C25H25FN3O3+ [M + H]+: 434.1874; found 434.1870.
N-(3-(2-(Benzyloxy)-8-fluoro-11-oxo-5,11-dihydro-10H-dibenzo[b,e][1,4]diazepin-10-yl)propyl)ethanesulfonamide (23). A mixture of 18 (102 mg, 0.26 mmol), TEA (34 mg, 0.34 mmol) and ethanesulfonyl chloride (33 mg, 0.26 mmol) in 5 mL DCM was stirred at room temperature under a nitrogen atmosphere for 12 h. Upon completion, the reaction mixture was concentrated in vacuo. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 2
EA = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 23 (65 mg, 51.7%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.41–7.27 (m, 6H), 6.99–6.91 (m, 3H), 6.84–6.74 (m, 2H), 5.79 (t, J = 6.18 Hz, 1H), 5.70 (s, 1H), 4.98 (s, 2H), 4.20 (s, 2H), 3.19 (dd, J = 12.14, 6.10, 2H), 2.91 (q, J = 7.38 Hz, 2H), 1.88–1.74 (m, 2H), 1.22 (t, J = 7.38 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.9, 160.7, 159.5 (d, J = 243.0 Hz), 154.7, 144.8, 142.3 (d, J = 2.7 Hz), 136.7, 134.5 (d, J = 9.6 Hz), 128.6 (2C), 128.1, 127.6 (2C), 125.9, 121.9 (d, J = 9.2 Hz), 120.8, 120.1, 116.4, 113.4 (d, J = 22.5 Hz), 111.3 (d, J = 24.9 Hz), 70.5, 46.9, 46.7, 40.8, 28.3, 8.2. HRMS ESI (m/z): calcd for C25H27FN3O4S+ [M + H]+: 484.1701; found 484.1697.
1) to give compound 23 (65 mg, 51.7%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.41–7.27 (m, 6H), 6.99–6.91 (m, 3H), 6.84–6.74 (m, 2H), 5.79 (t, J = 6.18 Hz, 1H), 5.70 (s, 1H), 4.98 (s, 2H), 4.20 (s, 2H), 3.19 (dd, J = 12.14, 6.10, 2H), 2.91 (q, J = 7.38 Hz, 2H), 1.88–1.74 (m, 2H), 1.22 (t, J = 7.38 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.9, 160.7, 159.5 (d, J = 243.0 Hz), 154.7, 144.8, 142.3 (d, J = 2.7 Hz), 136.7, 134.5 (d, J = 9.6 Hz), 128.6 (2C), 128.1, 127.6 (2C), 125.9, 121.9 (d, J = 9.2 Hz), 120.8, 120.1, 116.4, 113.4 (d, J = 22.5 Hz), 111.3 (d, J = 24.9 Hz), 70.5, 46.9, 46.7, 40.8, 28.3, 8.2. HRMS ESI (m/z): calcd for C25H27FN3O4S+ [M + H]+: 484.1701; found 484.1697.
Dimethyl(3-(2-(benzyloxy)-8-fluoro-11-oxo-5,11-dihydro-10H-dibenzo[b,e][1,4]diazepin-10-yl)propyl)phosphoramidate (24). A mixture of 18 (160 mg, 0.41 mmol), TEA (45 mg, 0.45 mmol) and dimethyl phosphorochloridate (59 mg, 0.41 mmol) in 5 mL DCM was stirred at room temperature under a nitrogen atmosphere for 12 h. Upon completion, the reaction mixture was concentrated in vacuo. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 5
EA = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 24 (130 mg, 63.5%) as a white solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.43–7.27 (m, 6H), 7.01–6.86 (m, 3H), 6.87–6.68 (m, 2H), 5.91 (d, J = 5.31 Hz, 1H), 4.97 (s, 2H), 4.17 (s, 2H), 3.60 (d, J = 11.14 Hz, 6H), 3.10–2.91 (m, 2H), 2.59 (s, 1H), 1.81–1.66 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.8, 159.3 (d, J = 242.37 Hz), 154.5, 145.1, 142.44 (d, J = 2.35 Hz), 136.7, 134.6 (d, J = 9.63 Hz), 128.6 (2C), 128.1, 127.6 (2C), 126.0, 121.7 (d, J = 9.03 Hz), 120.8, 120.1, 116.3, 113.0 (d, J = 22.33 Hz), 111.2 (d, J = 24.95 Hz), 70.5, 53.00 (d, J = 5.50 Hz), 46.8, 38.8, 29.5, 29.5. HRMS ESI (m/z): calcd for C25H28FN3O5P+ [M + H]+: 500.1745; found 500.1740.
1) to give compound 24 (130 mg, 63.5%) as a white solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.43–7.27 (m, 6H), 7.01–6.86 (m, 3H), 6.87–6.68 (m, 2H), 5.91 (d, J = 5.31 Hz, 1H), 4.97 (s, 2H), 4.17 (s, 2H), 3.60 (d, J = 11.14 Hz, 6H), 3.10–2.91 (m, 2H), 2.59 (s, 1H), 1.81–1.66 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.8, 159.3 (d, J = 242.37 Hz), 154.5, 145.1, 142.44 (d, J = 2.35 Hz), 136.7, 134.6 (d, J = 9.63 Hz), 128.6 (2C), 128.1, 127.6 (2C), 126.0, 121.7 (d, J = 9.03 Hz), 120.8, 120.1, 116.3, 113.0 (d, J = 22.33 Hz), 111.2 (d, J = 24.95 Hz), 70.5, 53.00 (d, J = 5.50 Hz), 46.8, 38.8, 29.5, 29.5. HRMS ESI (m/z): calcd for C25H28FN3O5P+ [M + H]+: 500.1745; found 500.1740.
(3-(2-(Benzyloxy)-8-fluoro-11-oxo-5,11-dihydro-10H-dibenzo[b,e][1,4]diazepin-10-yl)propyl)phosphoramidic acid (25). A mixture of 24 (80 mg, 0.16 mmol) and TMSBr (32 mg, 0.21 mmol) in 5 mL DCM was stirred at room temperature under a nitrogen atmosphere for 12 h. Upon completion, the reaction mixture was concentrated in vacuo. The crude product was purified by column chromatography (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 5
MeOH = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 25 (50 mg, 66.3%) as a red solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.4–7.2 (m, 7H), 7.2–7.1 (m, 2H), 7.0 (d, J = 2.3 Hz, 2H), 6.9–6.8 (m, 1H), 5.0 (s, 2H), 4.2 (s, 2H), 3.1–2.9 (m, 2H), 2.1–1.9 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 169.8, 159.3 (d, J = 242.1 Hz), 154.5, 145.9, 143.1 (d, J = 2.0 Hz), 136.7, 134.0 (d, J = 9.7 Hz), 128.7 (2C), 128.2, 127.7 (2C), 125.8, 122.6 (d, J = 9.2 Hz), 120.9, 120.5, 116.3, 113.8 (d, J = 22.2 Hz), 111.3 (d, J = 23.6 Hz), 70.5, 37.7, 29.8, 25.6. HRMS ESI (m/z): calcd for C23H22FN3O5P− [M–HPO2]−: 390.1623; found 390.1632.
1) to give compound 25 (50 mg, 66.3%) as a red solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.4–7.2 (m, 7H), 7.2–7.1 (m, 2H), 7.0 (d, J = 2.3 Hz, 2H), 6.9–6.8 (m, 1H), 5.0 (s, 2H), 4.2 (s, 2H), 3.1–2.9 (m, 2H), 2.1–1.9 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 169.8, 159.3 (d, J = 242.1 Hz), 154.5, 145.9, 143.1 (d, J = 2.0 Hz), 136.7, 134.0 (d, J = 9.7 Hz), 128.7 (2C), 128.2, 127.7 (2C), 125.8, 122.6 (d, J = 9.2 Hz), 120.9, 120.5, 116.3, 113.8 (d, J = 22.2 Hz), 111.3 (d, J = 23.6 Hz), 70.5, 37.7, 29.8, 25.6. HRMS ESI (m/z): calcd for C23H22FN3O5P− [M–HPO2]−: 390.1623; found 390.1632.
Tert-Butyl (E)-(N-(3-(2-(benzyloxy)-8-fluoro-11-oxo-5,11-dihydro-10H-dibenzo[b,e][1,4]diazepin-10-yl)propyl)-N'-(tert-butoxycarbonyl)carbamimidoyl)-l2-azanecarboxylate (S10). A mixture of 18 (440 mg, 1.12 mmol), 1,3-di-Boc-2-methylisothiourea (442 mg, 1.52 mmol) and DIPEA (400 mg, 3.08 mmol) was stirred in 10 mL DCM at room temperature under nitrogen atmosphere for 24 h. Upon completion, the reaction mixture was concentrated in vacuo. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 2
EA = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound S10 (400 mg, 56.4%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 11.41 (s, 1H), 8.37 (s, 1H), 7.48–7.27 (m, 6H), 6.98–6.83 (m, 3H), 6.81–6.68 (m, 2H), 5.38 (s, 1H), 4.99 (s, 2H), 4.11 (s, 2H), 3.51 (dd, J = 11.68 Hz, 5.70 Hz, 2H), 1.96–1.83 (m, 2H), 1.48 (d, J = 14.02 Hz, 18H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.4, 163.5, 159.5 (d, J = 242.5 Hz), 156.2, 154.6, 153.2, 144.9, 142.1 (d, J = 2.6 Hz), 136.9, 135.2 (d, J = 10.0 Hz), 128.7 (2C), 128.1, 127.7 (2C), 126.1, 121.5 (d, J = 9.3 Hz), 120.9, 119.9, 116.5, 113.0 (d, J = 22.6 Hz), 111.4 (d, J = 24.8 Hz), 83.2, 79.4, 70.5, 47.5, 37.9, 28.5 (3C), 28.1 (3C), 27.7. HRMS ESI (m/z): calcd for C34H41FN5O6+ [M + H]+: 634.3035; found 634.3032.
1) to give compound S10 (400 mg, 56.4%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 11.41 (s, 1H), 8.37 (s, 1H), 7.48–7.27 (m, 6H), 6.98–6.83 (m, 3H), 6.81–6.68 (m, 2H), 5.38 (s, 1H), 4.99 (s, 2H), 4.11 (s, 2H), 3.51 (dd, J = 11.68 Hz, 5.70 Hz, 2H), 1.96–1.83 (m, 2H), 1.48 (d, J = 14.02 Hz, 18H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.4, 163.5, 159.5 (d, J = 242.5 Hz), 156.2, 154.6, 153.2, 144.9, 142.1 (d, J = 2.6 Hz), 136.9, 135.2 (d, J = 10.0 Hz), 128.7 (2C), 128.1, 127.7 (2C), 126.1, 121.5 (d, J = 9.3 Hz), 120.9, 119.9, 116.5, 113.0 (d, J = 22.6 Hz), 111.4 (d, J = 24.8 Hz), 83.2, 79.4, 70.5, 47.5, 37.9, 28.5 (3C), 28.1 (3C), 27.7. HRMS ESI (m/z): calcd for C34H41FN5O6+ [M + H]+: 634.3035; found 634.3032.
1-(3-(2-(Benzyloxy)-8-fluoro-11-oxo-5,11-dihydro-10H-dibenzo[b,e][1,4]diazepin-10-yl)propyl)guanidine trifluoroacetate (26). A mixture of S11 (200 mg, 0.31 mmol) and TFA (2 mL) in 10 mL DCM was stirred at room temperature for 6 h. Upon completion, the reaction mixture was concentrated in vacuo to afford compound 26 (167 mg, 98%). 1H NMR (400 MHz, CD3OD) δ [ppm] = 7.38–7.18 (m, 6H), 7.10–7.00 (m, 2H), 6.94 (d, J = 1.20 Hz, 2H), 6.87–6.79 (m, 1H), 4.90 (s, 2H), 4.12 (s, 2H), 3.20 (t, J = 6.78 Hz, 2H), 1.93–1.73 (m, 2H). 13C NMR (101 MHz, CD3OD) δ [ppm] = 170.7, 161.7, 159.3, 158.5 (d, J = 5.2 Hz), 155.5, 147.4, 144.4 (d, J = 2.4 Hz), 138.3, 135.6 (d, J = 9.7 Hz), 129.4, 128.8 (2C), 128.5, 126.8 (2C), 122.7 (d, J = 9.0 Hz), 121.7, 121.1, 117.5, 114.3 (d, J = 22.6 Hz), 112.2 (d, J = 25.4 Hz), 71.3, 47.5, 39.5, 28.0. HRMS ESI (m/z): calcd for C26H25F4N5O4+ [M + H]+: 434.1915; found 434.1986.
Ethyl 2-((4-fluoro-2-nitrophenyl)amino)benzoate (S12). S12 was synthesized following the procedure described for S5. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 5
EA = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound S12 (5.6 g, 83%) as a red solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 11.01 (s, 1H), 8.05 (d, J = 7.96 Hz, 1H), 7.87 (dd, J = 8.54 Hz, 2.45, 1H), 7.60 (dd, J = 9.26 Hz, 4.72, 1H), 7.42 (s, 2H), 7.23 (dd, J = 11.67 Hz, 4.86, 1H), 7.07–6.98 (m, 1H), 4.42 (q, J = 7.10 Hz, 2H), 1.42 (t, J = 7.11 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 167.3, 155.2 (d, J = 243.6 Hz), 142.6, 137.3 (d, J = 8.1 Hz), 135.8, 133.6, 132.2, 123.0 (d, J = 23.4 Hz), 121.8, 120.9 (d, J = 7.3 Hz), 118.7, 118.4, 112.7 (d, J = 26.5 Hz), 61.5, 14.4. HRMS GC/QTOF (m/z): calcd for C15H13FN2O4+ [M]+: 304.0859; found 304.0852.
1) to afford compound S12 (5.6 g, 83%) as a red solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 11.01 (s, 1H), 8.05 (d, J = 7.96 Hz, 1H), 7.87 (dd, J = 8.54 Hz, 2.45, 1H), 7.60 (dd, J = 9.26 Hz, 4.72, 1H), 7.42 (s, 2H), 7.23 (dd, J = 11.67 Hz, 4.86, 1H), 7.07–6.98 (m, 1H), 4.42 (q, J = 7.10 Hz, 2H), 1.42 (t, J = 7.11 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 167.3, 155.2 (d, J = 243.6 Hz), 142.6, 137.3 (d, J = 8.1 Hz), 135.8, 133.6, 132.2, 123.0 (d, J = 23.4 Hz), 121.8, 120.9 (d, J = 7.3 Hz), 118.7, 118.4, 112.7 (d, J = 26.5 Hz), 61.5, 14.4. HRMS GC/QTOF (m/z): calcd for C15H13FN2O4+ [M]+: 304.0859; found 304.0852.
Ethyl 2-((2-amino-4-fluorophenyl)amino)benzoate (S13). S13 was synthesized following the procedure described for S6. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 5
EA = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound S13 (950 mg, 63%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 8.86 (s, 1H), 8.06–7.91 (m, 1H), 7.23 (d, J = 6.85 Hz, 1H), 7.03 (dd, J = 8.41 Hz, 6.10 Hz, 1H), 6.68 (t, J = 7.37, 1H), 6.52 (d, J = 8.43 Hz, 1H), 6.49–6.39 (m, 2H), 4.35 (q, J = 7.11 Hz, 2H), 3.96 (s, 2H), 1.40 (t, J = 7.12 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.8, 162.1 (d, J = 242.8 Hz), 150.0, 145.3 (d, J = 11.6 Hz), 134.5, 131.5, 129.8 (d, J = 10.3 Hz), 121.8, 116.5, 113.6, 111.4, 105.3 (d, J = 22.6 Hz), 102.4 (d, J = 25.8 Hz). 60.7, 14.5. HRMS GC/QTOF (m/z): calcd for C15H15FN2O2+ [M]+: 274.1118; found 274.1111.
1) to afford compound S13 (950 mg, 63%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 8.86 (s, 1H), 8.06–7.91 (m, 1H), 7.23 (d, J = 6.85 Hz, 1H), 7.03 (dd, J = 8.41 Hz, 6.10 Hz, 1H), 6.68 (t, J = 7.37, 1H), 6.52 (d, J = 8.43 Hz, 1H), 6.49–6.39 (m, 2H), 4.35 (q, J = 7.11 Hz, 2H), 3.96 (s, 2H), 1.40 (t, J = 7.12 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.8, 162.1 (d, J = 242.8 Hz), 150.0, 145.3 (d, J = 11.6 Hz), 134.5, 131.5, 129.8 (d, J = 10.3 Hz), 121.8, 116.5, 113.6, 111.4, 105.3 (d, J = 22.6 Hz), 102.4 (d, J = 25.8 Hz). 60.7, 14.5. HRMS GC/QTOF (m/z): calcd for C15H15FN2O2+ [M]+: 274.1118; found 274.1111.
2-((2-Amino-4-fluorophenyl)amino)benzoic acid (S14). S14 was synthesized following the procedure described for S7. The crude product was purified by column chromatography (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 3
MeOH = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound S14 (750 mg, 88%) as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 12.88 (s, 1H), 8.91 (s, 1H), 7.85 (d, J = 7.86 Hz, 1H), 7.27 (t, J = 7.65 Hz, 1H), 7.00 (t, J = 7.31 Hz, 1H), 6.65 (t, J = 7.41 Hz, 1H), 6.57 (d, J = 11.08 Hz, 1H), 6.46 (d, J = 8.41 Hz, 1H), 6.34 (t, J = 8.39 Hz, 1H), 5.23 (s, 2H). 13C NMR (101 MHz, DMSO) δ [ppm] = 170.0, 161.0 (d, J = 238.9 Hz), 149.6, 146.6 (d, J = 12.2 Hz), 134.0, 131.4, 128.5 (d, J = 10.5 Hz), 122.3, 115.7, 112.8, 111.1, 102.2 (d, J = 22.7 Hz), 100.9 (d, J = 25.5 Hz). HRMS GC/QTOF (m/z): calcd for C13H11FN2O2+ [M]+: 246.0805; found 246.0800.
1) to afford compound S14 (750 mg, 88%) as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 12.88 (s, 1H), 8.91 (s, 1H), 7.85 (d, J = 7.86 Hz, 1H), 7.27 (t, J = 7.65 Hz, 1H), 7.00 (t, J = 7.31 Hz, 1H), 6.65 (t, J = 7.41 Hz, 1H), 6.57 (d, J = 11.08 Hz, 1H), 6.46 (d, J = 8.41 Hz, 1H), 6.34 (t, J = 8.39 Hz, 1H), 5.23 (s, 2H). 13C NMR (101 MHz, DMSO) δ [ppm] = 170.0, 161.0 (d, J = 238.9 Hz), 149.6, 146.6 (d, J = 12.2 Hz), 134.0, 131.4, 128.5 (d, J = 10.5 Hz), 122.3, 115.7, 112.8, 111.1, 102.2 (d, J = 22.7 Hz), 100.9 (d, J = 25.5 Hz). HRMS GC/QTOF (m/z): calcd for C13H11FN2O2+ [M]+: 246.0805; found 246.0800.
8-Fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (S15). S15 was synthesized following the procedure described for S8. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 2
EA = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound S15 (410 mg, 53%) as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 9.94 (s, 1H), 7.85 (s, 1H), 7.66 (dd, J = 7.84 Hz, 1.70 Hz, 1H), 7.37–7.32 (m, 1H), 7.07–6.94 (m, 2H), 6.93–6.87 (m, 1H), 6.85–6.74 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 167.8, 158.0 (d, J = 237.6 Hz), 150.4, 136.3, 133.5 (2C), 132.1 (2C), 131.2 (d, J = 10.4 Hz), 122.6, 120.9, 120.7 (d, J = 8.9 Hz), 119.1, 110.7 (d, J = 22.0 Hz), 107.7 (d, J = 25.7 Hz). HRMS GC/QTOF (m/z): calcd for C13H9FN2O+ [M]+: 228.0699; found 228.0692.
1) to afford compound S15 (410 mg, 53%) as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 9.94 (s, 1H), 7.85 (s, 1H), 7.66 (dd, J = 7.84 Hz, 1.70 Hz, 1H), 7.37–7.32 (m, 1H), 7.07–6.94 (m, 2H), 6.93–6.87 (m, 1H), 6.85–6.74 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 167.8, 158.0 (d, J = 237.6 Hz), 150.4, 136.3, 133.5 (2C), 132.1 (2C), 131.2 (d, J = 10.4 Hz), 122.6, 120.9, 120.7 (d, J = 8.9 Hz), 119.1, 110.7 (d, J = 22.0 Hz), 107.7 (d, J = 25.7 Hz). HRMS GC/QTOF (m/z): calcd for C13H9FN2O+ [M]+: 228.0699; found 228.0692.
10-(2-(Dimethylamino)ethyl)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (27). 27 was synthesized following the procedure described for 9. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 1
EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to afford compound 27 (342 mg, 45%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.80 (d, J = 7.8 Hz, 1H), 7.25 (t, J = 7.6 Hz, 1H), 7.16 (d, J = 9.7 Hz, 1H), 6.99 (t, J = 7.5 Hz, 1H), 6.95–6.88 (m, 1H), 6.83 (d, J = 7.9 Hz, 1H), 6.75 (t, J = 7.7 Hz, 1H), 5.85 (s, 1H), 4.13 (t, J = 6.8 Hz, 2H), 2.67 (t, J = 6.9 Hz, 2H), 2.28 (s, 6H); 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.7, 159.4 (d, J = 242.0 Hz), 151.2, 140.9 (d, J = 2.6 Hz), 135.4 (d, J = 9.8 Hz), 132.5, 132.5, 125.3, 122.7, 121.4 (d, J = 9.2 Hz), 118.6, 112.6 (d, J = 22.5 Hz), 111.3 (d, J = 25.4 Hz), 57.3, 49.0, 45.7 (2C). HRMS ESI-TOF (m/z): calcd for C17H19FN3O+ [M + H]+: 300.1512; found 300.150.
2) to afford compound 27 (342 mg, 45%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.80 (d, J = 7.8 Hz, 1H), 7.25 (t, J = 7.6 Hz, 1H), 7.16 (d, J = 9.7 Hz, 1H), 6.99 (t, J = 7.5 Hz, 1H), 6.95–6.88 (m, 1H), 6.83 (d, J = 7.9 Hz, 1H), 6.75 (t, J = 7.7 Hz, 1H), 5.85 (s, 1H), 4.13 (t, J = 6.8 Hz, 2H), 2.67 (t, J = 6.9 Hz, 2H), 2.28 (s, 6H); 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.7, 159.4 (d, J = 242.0 Hz), 151.2, 140.9 (d, J = 2.6 Hz), 135.4 (d, J = 9.8 Hz), 132.5, 132.5, 125.3, 122.7, 121.4 (d, J = 9.2 Hz), 118.6, 112.6 (d, J = 22.5 Hz), 111.3 (d, J = 25.4 Hz), 57.3, 49.0, 45.7 (2C). HRMS ESI-TOF (m/z): calcd for C17H19FN3O+ [M + H]+: 300.1512; found 300.150.
10-(3-(Dimethylamino)propyl)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (28). 28 was synthesized following the procedure described for 1. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 1
EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to afford compound 28 (150 mg, 56%) as a yellow solid. 1H NMR (400 MHz, CD3OD) δ [ppm] = 7.83 (dd, J = 7.83 Hz, 1.48, 1H), 7.36–7.20 (m, 2H), 7.12–6.94 (m, 4H), 6.82 (d, J = 7.97 Hz, 1H), 5.54 (s, 1H), 4.15 (t, J = 6.87 Hz, 2H), 2.98 (s, 2H), 2.49–2.35 (m, 2H), 2.20 (s, 6H), 1.92–1.76 (m, 2H). 13C NMR (101 MHz, CD3OD) δ [ppm] = 180.5, 171.0, 160.6 (d, J = 240.7 Hz), 153.8, 143.9 (d, J = 2.6 Hz), 135.8 (d, J = 9.7 Hz), 133.9, 133.0, 126.1, 123.2, 122.8 (d, J = 9.2 Hz), 119.9, 114.1 (d, J = 22.6 Hz), 112.2 (d, J = 25.5 Hz), 57.2, 44.9 (2C), 25.9, 24.3. HRMS ESI (m/z): calcd for C18H21FN3O+ [M + H]+: 314.1663; found 314.1659.
2) to afford compound 28 (150 mg, 56%) as a yellow solid. 1H NMR (400 MHz, CD3OD) δ [ppm] = 7.83 (dd, J = 7.83 Hz, 1.48, 1H), 7.36–7.20 (m, 2H), 7.12–6.94 (m, 4H), 6.82 (d, J = 7.97 Hz, 1H), 5.54 (s, 1H), 4.15 (t, J = 6.87 Hz, 2H), 2.98 (s, 2H), 2.49–2.35 (m, 2H), 2.20 (s, 6H), 1.92–1.76 (m, 2H). 13C NMR (101 MHz, CD3OD) δ [ppm] = 180.5, 171.0, 160.6 (d, J = 240.7 Hz), 153.8, 143.9 (d, J = 2.6 Hz), 135.8 (d, J = 9.7 Hz), 133.9, 133.0, 126.1, 123.2, 122.8 (d, J = 9.2 Hz), 119.9, 114.1 (d, J = 22.6 Hz), 112.2 (d, J = 25.5 Hz), 57.2, 44.9 (2C), 25.9, 24.3. HRMS ESI (m/z): calcd for C18H21FN3O+ [M + H]+: 314.1663; found 314.1659.
2-Bromo-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (S16). A solution of S15 (2.4 g, 10.5 mmol) in 20 mL DMF was cooled to 0 °C under a nitrogen atmosphere. To this solution, NBS (2.06 g, 11.57 mmol) dissolved in DMF (10 mL) was added dropwise. The reaction mixture was stirred at 0 °C for 4 h. Upon completion, the reaction mixture was diluted with water (100 mL) and extracted with DCM (3 × 50 mL). The combined organic layers were dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 5
EA = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound S16 (2 g, 62%) as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 10.08 (s, 1H), 8.06 (s, 1H), 7.74 (d, J = 2.49 Hz, 1H), 7.52 (dd, J = 8.61 Hz, 2.51 Hz, 1H), 7.04–6.90 (m, 2H), 6.88–6.73 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 166.4, 158.2 (d, J = 238.1 Hz), 149.6, 135.9, 135.5, 134.1, 130.9 (d, J = 10.4 Hz), 124.1, 121.3, 120.9 (d, J = 9.4 Hz), 112.0, 111.0 (d, J = 22.3 Hz), 107.9 (d, J = 25.7 Hz). HRMS ESI (m/z): calcd for C13H9BrFN2O+ [M + H]+: 306.9877; found 306.9877.
1) to give compound S16 (2 g, 62%) as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 10.08 (s, 1H), 8.06 (s, 1H), 7.74 (d, J = 2.49 Hz, 1H), 7.52 (dd, J = 8.61 Hz, 2.51 Hz, 1H), 7.04–6.90 (m, 2H), 6.88–6.73 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 166.4, 158.2 (d, J = 238.1 Hz), 149.6, 135.9, 135.5, 134.1, 130.9 (d, J = 10.4 Hz), 124.1, 121.3, 120.9 (d, J = 9.4 Hz), 112.0, 111.0 (d, J = 22.3 Hz), 107.9 (d, J = 25.7 Hz). HRMS ESI (m/z): calcd for C13H9BrFN2O+ [M + H]+: 306.9877; found 306.9877.
2-Bromo-10-(2-(dimethylamino)ethyl)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4] diazepin-11-one (S17). S17 was synthesized following the procedure described for 9. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 1
EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to afford compound S17 (900 mg, 61%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.89 (d, J = 2.33 Hz, 1H), 7.35 (dd, J = 8.46 Hz, 2.36 Hz, 1H), 7.18 (dd, J = 9.97 Hz, 2.68 Hz, 1H), 6.87 (dd, J = 8.71 Hz, 5.46 Hz, 1H), 6.81–6.74 (m, 1H), 6.68 (d, J = 8.47 Hz, 1H), 5.47 (s, 1H), 4.09 (t, J = 6.89 Hz, 2H), 2.63 (t, J = 6.89 Hz, 2H), 2.27 (s, 6H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 167.2, 159.7 (d, J = 242.9 Hz), 149.9, 140.2 (d, J = 2.8 Hz), 135.3 (2C, dd, J = 14.1 Hz, 6.7 Hz), 127.1, 121.5 (2C, d, J = 9.3 Hz), 120.3, 115.4, 113.0 (d, J = 22.7 Hz), 111.7 (d, J = 25.5 Hz), 57.4, 49.3, 45.8 (2C). HRMS ESI (m/z): calcd for C17H18BrFN3O+ [M + H]+: 378.0612; found 378.0612.
2) to afford compound S17 (900 mg, 61%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.89 (d, J = 2.33 Hz, 1H), 7.35 (dd, J = 8.46 Hz, 2.36 Hz, 1H), 7.18 (dd, J = 9.97 Hz, 2.68 Hz, 1H), 6.87 (dd, J = 8.71 Hz, 5.46 Hz, 1H), 6.81–6.74 (m, 1H), 6.68 (d, J = 8.47 Hz, 1H), 5.47 (s, 1H), 4.09 (t, J = 6.89 Hz, 2H), 2.63 (t, J = 6.89 Hz, 2H), 2.27 (s, 6H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 167.2, 159.7 (d, J = 242.9 Hz), 149.9, 140.2 (d, J = 2.8 Hz), 135.3 (2C, dd, J = 14.1 Hz, 6.7 Hz), 127.1, 121.5 (2C, d, J = 9.3 Hz), 120.3, 115.4, 113.0 (d, J = 22.7 Hz), 111.7 (d, J = 25.5 Hz), 57.4, 49.3, 45.8 (2C). HRMS ESI (m/z): calcd for C17H18BrFN3O+ [M + H]+: 378.0612; found 378.0612.
10-(2-(Dimethylamino)ethyl)-8-fluoro-2-(4-(4-methylpiperazin-1-yl)phenyl)-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (29). A mixture of S17 (110 mg, 0.29 mmol), Pd(PPh3)4 (24 mg, 0.02 mmol), 1-methyl-4-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)piperazine (264 mg, 0.875 mmol) and K2CO3 (48 mg, 0.35 mmol) in 10 mL toluene/MeOH/H2O (V
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V
V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V = 8
V = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was heated at 110 °C under a nitrogen atmosphere for 24 hours. Upon completion, the mixture was filtered and the filtrate was concentrated in vacuo. The residue was diluted with deionized water (10 mL) and extracted with ethyl acetate (3 × 10 mL). The combined organic layers were washed with saturated brine (1 × 10 mL), dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography (DCM
1) was heated at 110 °C under a nitrogen atmosphere for 24 hours. Upon completion, the mixture was filtered and the filtrate was concentrated in vacuo. The residue was diluted with deionized water (10 mL) and extracted with ethyl acetate (3 × 10 mL). The combined organic layers were washed with saturated brine (1 × 10 mL), dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography (DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 3
MeOH = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 29 (100 mg, 72.9%) as a white solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 8.00 (d, J = 2.13 Hz, 1H), 7.51–7.39 (m, 3H), 7.19 (dd, J = 10.03 Hz, 2.65 Hz, 1H), 6.95 (d, J = 8.77 Hz, 2H), 6.90–6.74 (m, 3H), 5.34 (s, 1H), 4.13 (t, J = 6.89 Hz, 2H), 3.28–3.20 (m, 4H), 2.69 (t, J = 6.93 Hz, 2H), 2.61–2.54 (m, 4H), 2.35 (s, 3H), 2.30 (s, 6H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.5, 159.6 (d, J = 242.4 Hz), 150.5, 149.3, 140.6, 135.9, 135.7 (d, J = 9.9 Hz), 130.9, 130.5 (d, J = 14.6 Hz), 127.4 (2C), 125.6, 121.3 (d, J = 9.1 Hz), 119.0, 116.1 (2C), 112.7 (d, J = 22.6 Hz), 111.6 (d, J = 25.5 Hz), 57.5, 55.2 (2C), 49.3, 48.9 (2C), 46.3, 45.8 (2C). HRMS ESI (m/z): calcd for C28H33FN5O+ [M + H]+: 474.2664; found 474.2662.
1) to give compound 29 (100 mg, 72.9%) as a white solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 8.00 (d, J = 2.13 Hz, 1H), 7.51–7.39 (m, 3H), 7.19 (dd, J = 10.03 Hz, 2.65 Hz, 1H), 6.95 (d, J = 8.77 Hz, 2H), 6.90–6.74 (m, 3H), 5.34 (s, 1H), 4.13 (t, J = 6.89 Hz, 2H), 3.28–3.20 (m, 4H), 2.69 (t, J = 6.93 Hz, 2H), 2.61–2.54 (m, 4H), 2.35 (s, 3H), 2.30 (s, 6H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.5, 159.6 (d, J = 242.4 Hz), 150.5, 149.3, 140.6, 135.9, 135.7 (d, J = 9.9 Hz), 130.9, 130.5 (d, J = 14.6 Hz), 127.4 (2C), 125.6, 121.3 (d, J = 9.1 Hz), 119.0, 116.1 (2C), 112.7 (d, J = 22.6 Hz), 111.6 (d, J = 25.5 Hz), 57.5, 55.2 (2C), 49.3, 48.9 (2C), 46.3, 45.8 (2C). HRMS ESI (m/z): calcd for C28H33FN5O+ [M + H]+: 474.2664; found 474.2662.
Tert-Butyl 4-(4-(10-(2-(dimethylamino)ethyl)-8-fluoro-11-oxo-10,11-dihydro-5H-dibenzo[b,e][1,4]diazepin-2-yl)phenyl)piperazine-1-carboxylate (S18). S18 was synthesized following the procedure described for 29. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 1
EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound S18 (128 mg, 78.9%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.99 (d, J = 2.20 Hz, 1H), 7.44 (dd, J = 10.84 Hz, 5.35, 3H), 7.17 (dd, J = 10.00 Hz, 2.68, 1H), 6.96–6.83 (m, 4H), 6.81–6.70 (m, 1H), 5.70 (s, 1H), 4.13 (t, J = 6.87 Hz, 2H), 3.67–3.50 (m, 4H), 3.21–3.07 (m, 4H), 2.68 (t, J = 6.92 Hz, 2H), 2.29 (s, 6H), 1.48 (s, 9H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.6, 159.5 (d, J = 242.2 Hz), 158.3, 154.8, 150.4, 149.6, 140.8, 135.5 (d, J = 5.9 Hz), 131.4 (2C), 130.5 (d, J = 16.4 Hz), 127.4 (2C), 125.5, 121.3 (d, J = 9.3 Hz), 119.1, 116.6, 112.7 (d, J = 22.6 Hz), 111.4 (d, J = 25.3 Hz), 80.0, 57.4, 49.2 (2C), 45.7 (2C), 28.5 (3C). HRMS ESI (m/z): calcd for C32H39FN5O3+ [M + H]+: 560.3031; found 560.3027.
1) to afford compound S18 (128 mg, 78.9%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.99 (d, J = 2.20 Hz, 1H), 7.44 (dd, J = 10.84 Hz, 5.35, 3H), 7.17 (dd, J = 10.00 Hz, 2.68, 1H), 6.96–6.83 (m, 4H), 6.81–6.70 (m, 1H), 5.70 (s, 1H), 4.13 (t, J = 6.87 Hz, 2H), 3.67–3.50 (m, 4H), 3.21–3.07 (m, 4H), 2.68 (t, J = 6.92 Hz, 2H), 2.29 (s, 6H), 1.48 (s, 9H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.6, 159.5 (d, J = 242.2 Hz), 158.3, 154.8, 150.4, 149.6, 140.8, 135.5 (d, J = 5.9 Hz), 131.4 (2C), 130.5 (d, J = 16.4 Hz), 127.4 (2C), 125.5, 121.3 (d, J = 9.3 Hz), 119.1, 116.6, 112.7 (d, J = 22.6 Hz), 111.4 (d, J = 25.3 Hz), 80.0, 57.4, 49.2 (2C), 45.7 (2C), 28.5 (3C). HRMS ESI (m/z): calcd for C32H39FN5O3+ [M + H]+: 560.3031; found 560.3027.
10-(2-(Dimethylamino)ethyl)-8-fluoro-2-(4-(piperazin-1-yl)phenyl)-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (30). To a solution of S18 (100 mg, 0.178 mmol) in 5 mL DCM cooled to 0 °C, TFA (1 mL) was added dropwise under a nitrogen atmosphere. The mixture was stirred at room temperature for 6 h, then concentrated in vacuo. The residue was added with 1 M NaOH (10 mL) and then extracted with EtOAc three times (3 × 15 mL). The organic layer was separated, washed with deionized water and saturated brine, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 1
EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to afford compound 30 (67 mg, 81.6% yield). 1H NMR (400 MHz, CD3OD) δ [ppm] = 7.57 (d, J = 2.05 Hz, 1H), 7.17 (dd, J = 8.36 Hz, 2.04 Hz, 1H), 7.08 (d, J = 8.68 Hz, 2H), 6.86–6.66 (m, 3H), 6.59–6.46 (m, 3H), 3.87–3.72 (m, 2H), 2.80–2.61 (m, 4H), 2.63–2.44 (m, 4H), 2.25 (t, J = 7.18, 2H), 1.88 (s, 6H). 13C NMR (101 MHz, CD3OD) δ [ppm] = 170.8, 160.4 (d, J = 240.7 Hz), 152.1 (d, J = 8.8 Hz), 143.4, 136.0 (t, J = 4.8 Hz), 132.0, 131.6, 130.5, 127.9 (2C), 126.2, 122.6 (d, J = 9.0 Hz), 120.3, 117.4 (2C), 114.0 (d, J = 22.5 Hz), 112.0 (d, J = 25.5 Hz), 57.7, 50.7, 49.1 46.4, 45.6 (3C). HRMS ESI (m/z): calcd for C27H31FN5O+ [M + H]+: 460.2507; found 460.2499.
3) to afford compound 30 (67 mg, 81.6% yield). 1H NMR (400 MHz, CD3OD) δ [ppm] = 7.57 (d, J = 2.05 Hz, 1H), 7.17 (dd, J = 8.36 Hz, 2.04 Hz, 1H), 7.08 (d, J = 8.68 Hz, 2H), 6.86–6.66 (m, 3H), 6.59–6.46 (m, 3H), 3.87–3.72 (m, 2H), 2.80–2.61 (m, 4H), 2.63–2.44 (m, 4H), 2.25 (t, J = 7.18, 2H), 1.88 (s, 6H). 13C NMR (101 MHz, CD3OD) δ [ppm] = 170.8, 160.4 (d, J = 240.7 Hz), 152.1 (d, J = 8.8 Hz), 143.4, 136.0 (t, J = 4.8 Hz), 132.0, 131.6, 130.5, 127.9 (2C), 126.2, 122.6 (d, J = 9.0 Hz), 120.3, 117.4 (2C), 114.0 (d, J = 22.5 Hz), 112.0 (d, J = 25.5 Hz), 57.7, 50.7, 49.1 46.4, 45.6 (3C). HRMS ESI (m/z): calcd for C27H31FN5O+ [M + H]+: 460.2507; found 460.2499.
8-Fluoro-2-hydroxy-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (S19). A mixture of S8 (1.7 g, 5.1 mmol) and 10% Pd/C (1.1 g) in 20 mL MeOH/THF (V
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V = 1
V = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was evacuated and backfilled with hydrogen three times and then charged with hydrogen. The resulting mixture was stirred at 50 °C for 16 h. Then the mixture was filtered and the filtrate was concentrated in vacuo to give S19 (1.2 g, 96.4%) as a red oil. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 9.90 (s, 1H), 9.19 (s, 1H), 7.41 (s, 1H), 7.05 (d, J = 2.72, 1H), 6.96 (dd, J = 8.38 Hz, 5.76 Hz, 1H), 6.86–6.70 (m, 4H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 167.7, 157.6 (d, J = 237.1 Hz), 151.5, 142.4, 137.6, 131.2 (d, J = 10.5 Hz), 123.9, 120.8, 120.2, 120.1, 116.8, 110.4 (d, J = 22.0 Hz), 107.6 (d, J = 25.4 Hz). HRMS ESI (m/z): calcd for C13H10FN2O2+ [M + H]+: 245.0721; found 245.0718.
1) was evacuated and backfilled with hydrogen three times and then charged with hydrogen. The resulting mixture was stirred at 50 °C for 16 h. Then the mixture was filtered and the filtrate was concentrated in vacuo to give S19 (1.2 g, 96.4%) as a red oil. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 9.90 (s, 1H), 9.19 (s, 1H), 7.41 (s, 1H), 7.05 (d, J = 2.72, 1H), 6.96 (dd, J = 8.38 Hz, 5.76 Hz, 1H), 6.86–6.70 (m, 4H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 167.7, 157.6 (d, J = 237.1 Hz), 151.5, 142.4, 137.6, 131.2 (d, J = 10.5 Hz), 123.9, 120.8, 120.2, 120.1, 116.8, 110.4 (d, J = 22.0 Hz), 107.6 (d, J = 25.4 Hz). HRMS ESI (m/z): calcd for C13H10FN2O2+ [M + H]+: 245.0721; found 245.0718.
8-Fluoro-2-(3-morpholinopropoxy)-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (S20). A mixture of S19 (500 mg, 2.06 mmol), K2CO3 (331 mg, 2.4 mmol) and 4-(3-iodopropyl)morpholine (1.275 g, 5.0 mmol) in anhydrous MeCN (30 mL) was stirred at 70 °C for 12 h. Upon completion, the solvent was removed in vacuo, and the residue was suspended in water (30 mL). The aqueous phase was extracted with DCM (3 × 20 mL), and the combined organic layers were washed with saturated brine (1 × 15 mL), dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 3
EA = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound S20 (400 mg, 52.3%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 9.45–9.23 (m, 1H), 7.39 (d, J = 2.5 Hz, 1H), 6.94–6.87 (m, 1H), 6.80–6.73 (m, 2H), 6.72–6.58 (m, 2H), 5.41 (s, 1H), 3.97 (t, J = 6.2 Hz, 2H), 3.73–3.67 (m, 4H), 2.53–2.35 (m, 6H), 1.97–1.86 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 169.6, 159.2 (d, J = 241.7 Hz), 154.3, 143.4, 136.6 (d, J = 2.5 Hz), 131.0 (d, J = 10.1 Hz), 123.7, 121.8 (2C), 120.5 (d, J = 6.3 Hz), 120.5, 116.1 (2C), 111.7 (d, J = 22.3 Hz), 108.6 (d, J = 25.4 Hz), 66.9, 66.6 (2C), 55.5, 53.6 (2C), 26.2. HRMS ESI-TOF (m/z): calcd for C20H23FN3O3+ [M + H]+: 372.1723. Found 372.1719.
1) to afford compound S20 (400 mg, 52.3%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 9.45–9.23 (m, 1H), 7.39 (d, J = 2.5 Hz, 1H), 6.94–6.87 (m, 1H), 6.80–6.73 (m, 2H), 6.72–6.58 (m, 2H), 5.41 (s, 1H), 3.97 (t, J = 6.2 Hz, 2H), 3.73–3.67 (m, 4H), 2.53–2.35 (m, 6H), 1.97–1.86 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 169.6, 159.2 (d, J = 241.7 Hz), 154.3, 143.4, 136.6 (d, J = 2.5 Hz), 131.0 (d, J = 10.1 Hz), 123.7, 121.8 (2C), 120.5 (d, J = 6.3 Hz), 120.5, 116.1 (2C), 111.7 (d, J = 22.3 Hz), 108.6 (d, J = 25.4 Hz), 66.9, 66.6 (2C), 55.5, 53.6 (2C), 26.2. HRMS ESI-TOF (m/z): calcd for C20H23FN3O3+ [M + H]+: 372.1723. Found 372.1719.
2-((4-(Tert-Butyl)benzyl)oxy)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (S21). A mixture of S19 (1.22 g, 5.02 mmol), potassium carbonate (1.04 g, 7.5 mmol), and 4-tert-butylbenzyl bromide (1.36 g, 6.0 mmol) in anhydrous acetonitrile (50 mL) was stirred at 60 °C for 12 h. Upon completion, the solvent was removed in vacuo, and the residue was suspended in water (60 mL). The aqueous phase was extracted with DCM (3 × 60 mL), and the combined organic layers were washed with saturated brine (1 × 40 mL), dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 3
EA = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound S21 (960 mg, 49.2%). 1H NMR (400 MHz, CDCl3) δ [ppm] = 9.98 (s, 1H), 7.58 (s, 1H), 7.45–7.36 (m, 2H), 7.32 (d, J = 8.38 Hz, 2H), 7.24 (d, J = 3.05 Hz, 1H), 7.05 (dd, J = 8.75 Hz, 3.06 Hz, 1H), 7.01–6.88 (m, 2H), 6.85–6.72 (m, 2H), 4.99 (s, 2H), 1.26 (s, 4H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 167.7, 157.9 (d, J = 237.7 Hz), 152.8, 150.3, 144.3, 137.4 (d, J = 1.7 Hz), 134.1, 131.3 (d, J = 10.5 Hz), 127.5 (2C), 125.2, 123.8 (2C), 121.3, 120.5 (t, J = 4.3 Hz), 116.2, 110.8 (d, J = 21.7 Hz), 107.8 (d, J = 24.9 Hz), 69.4, 34.3, 31.2 (3C). HRMS ESI (m/z): calcd for C24H24FN2O2+ [M + H]+: 391.1816; found 391.1813.
1) to afford compound S21 (960 mg, 49.2%). 1H NMR (400 MHz, CDCl3) δ [ppm] = 9.98 (s, 1H), 7.58 (s, 1H), 7.45–7.36 (m, 2H), 7.32 (d, J = 8.38 Hz, 2H), 7.24 (d, J = 3.05 Hz, 1H), 7.05 (dd, J = 8.75 Hz, 3.06 Hz, 1H), 7.01–6.88 (m, 2H), 6.85–6.72 (m, 2H), 4.99 (s, 2H), 1.26 (s, 4H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 167.7, 157.9 (d, J = 237.7 Hz), 152.8, 150.3, 144.3, 137.4 (d, J = 1.7 Hz), 134.1, 131.3 (d, J = 10.5 Hz), 127.5 (2C), 125.2, 123.8 (2C), 121.3, 120.5 (t, J = 4.3 Hz), 116.2, 110.8 (d, J = 21.7 Hz), 107.8 (d, J = 24.9 Hz), 69.4, 34.3, 31.2 (3C). HRMS ESI (m/z): calcd for C24H24FN2O2+ [M + H]+: 391.1816; found 391.1813.
2-([1,1′-Biphenyl]-4-ylmethoxy)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (S22). A mixture of S19 (1.22 g, 5.02 mmol), potassium carbonate (1.04 g, 7.5 mmol), and 4-(bromomethyl)biphenyl (1.48 g, 6.0 mmol) in anhydrous acetonitrile (50 mL) was stirred at 60 °C for 12 h. Upon completion, the solvent was removed in vacuo, and the residue was suspended in water (60 mL). The aqueous phase was extracted with DCM (3 × 60 mL), and the combined organic layers were washed with saturated brine (1 × 40 mL), dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 3
EA = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound S22 (1.32 g, 64.1%). 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 10.01 (s, 1H), 7.72–7.58 (m, 5H), 7.56–7.42 (m, 4H), 7.35 (t, J = 7.35 Hz, 1H), 7.28 (d, J = 3.00 Hz, 1H), 7.09 (dd, J = 8.73 Hz, 3.03 Hz, 1H), 7.02–6.92 (m, 2H), 6.86–6.73 (m, 2H), 5.09 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 167.6, 157.9 (d, J = 237.1 Hz), 152.7, 144.3, 139.8, 139.6, 137.3 (d, J = 2.1 Hz), 136.3, 131.3 (d, J = 10.4 Hz), 129.0 (2C), 128.2 (2C), 127.5, 126.7 (4C, d, J = 6.0 Hz), 123.8, 121.3, 120.5 (t, J = 4.3 Hz), 116.3, 110.7 (d, J = 21.9 Hz), 107.8 (d, J = 25.5 Hz), 69.2. HRMS ESI (m/z): calcd for C26H20FN2O2+ [M + H]+: 411.1503; found 411.1500.
1) to afford compound S22 (1.32 g, 64.1%). 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 10.01 (s, 1H), 7.72–7.58 (m, 5H), 7.56–7.42 (m, 4H), 7.35 (t, J = 7.35 Hz, 1H), 7.28 (d, J = 3.00 Hz, 1H), 7.09 (dd, J = 8.73 Hz, 3.03 Hz, 1H), 7.02–6.92 (m, 2H), 6.86–6.73 (m, 2H), 5.09 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 167.6, 157.9 (d, J = 237.1 Hz), 152.7, 144.3, 139.8, 139.6, 137.3 (d, J = 2.1 Hz), 136.3, 131.3 (d, J = 10.4 Hz), 129.0 (2C), 128.2 (2C), 127.5, 126.7 (4C, d, J = 6.0 Hz), 123.8, 121.3, 120.5 (t, J = 4.3 Hz), 116.3, 110.7 (d, J = 21.9 Hz), 107.8 (d, J = 25.5 Hz), 69.2. HRMS ESI (m/z): calcd for C26H20FN2O2+ [M + H]+: 411.1503; found 411.1500.
10-(2-(Dimethylamino)ethyl)-8-fluoro-2-(3-morpholinopropoxy)-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (31). 31 was synthesized following the procedure described for 9. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 1
EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to afford compound 31 (200 mg, 55%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.30 (t, J = 2.6 Hz, 1H), 7.19–7.13 (m, 1H), 6.90–6.84 (m, 2H), 6.80–6.70 (m, 2H), 4.12 (t, J = 6.6 Hz, 2H), 3.96 (t, J = 6.2 Hz, 2H), 3.73–3.67 (m, 4H), 2.68 (t, J = 6.9 Hz, 2H), 2.49–2.41 (m, 6H), 2.30 (s, 6H), 1.95–1.87 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.3, 159.3 (d, J = 242.0 Hz), 154.7, 144.4, 141.5 (d, J = 2.1 Hz), 135.5 (d, J = 9.8 Hz), 125.9 (2C), 121.1 (d, J = 9.1 Hz), 120.6, 119.7, 115.9 (2C), 112.6 (d, J = 22.5 Hz), 111.4 (d, J = 25.4 Hz), 67.0, 66.6 (2C), 57.3 (2C), 55.4, 53.7, 49.1, 45.6 (2C), 26.4. HRMS (ESI-TOF) m/z: calcd for C24H32FN4O3+ [M + H]+: 443.2458. Found 443.2461.
2) to afford compound 31 (200 mg, 55%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.30 (t, J = 2.6 Hz, 1H), 7.19–7.13 (m, 1H), 6.90–6.84 (m, 2H), 6.80–6.70 (m, 2H), 4.12 (t, J = 6.6 Hz, 2H), 3.96 (t, J = 6.2 Hz, 2H), 3.73–3.67 (m, 4H), 2.68 (t, J = 6.9 Hz, 2H), 2.49–2.41 (m, 6H), 2.30 (s, 6H), 1.95–1.87 (m, 2H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.3, 159.3 (d, J = 242.0 Hz), 154.7, 144.4, 141.5 (d, J = 2.1 Hz), 135.5 (d, J = 9.8 Hz), 125.9 (2C), 121.1 (d, J = 9.1 Hz), 120.6, 119.7, 115.9 (2C), 112.6 (d, J = 22.5 Hz), 111.4 (d, J = 25.4 Hz), 67.0, 66.6 (2C), 57.3 (2C), 55.4, 53.7, 49.1, 45.6 (2C), 26.4. HRMS (ESI-TOF) m/z: calcd for C24H32FN4O3+ [M + H]+: 443.2458. Found 443.2461.
2-((4-(Tert-Butyl)benzyl)oxy)-10-(2-(dimethylamino)ethyl)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (32). 32 was synthesized following the procedure described for 9. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 1
EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to afford compound 32 (316 mg, 68.5%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.15 (d, J = 9.93 Hz, 1H), 6.94 (dd, J = 8.32 Hz, 2.34 Hz, 1H), 6.90–6.83 (m, 1H), 6.82–6.76 (m, 2H), 5.21 (s, 1H), 4.96 (s, 2H), 4.14 (s, 2H), 2.72 (s, 2H), 2.34 (d, J = 11.61 Hz, 6H), 1.31 (s, 9H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.4, 159.5 (d, J = 242.7 Hz), 154.9, 151.2, 144.6, 141.5, 135.6, 133.8, 127.7 (2C), 125.7 (2C), 121.3, 121.0, 119.9, 116.4, 112.9 (d, J = 21.2 Hz), 111.5 (d, J = 25.5 Hz), 70.5, 57.3 (d, J = 9.6 Hz), 48.9, 45.6, 45.5, 34.7 (2C), 31.5 (3C). HRMS ESI (m/z): calcd for C28H33FN3O2+ [M + H]+: 462.2551; found 462.2549.
2) to afford compound 32 (316 mg, 68.5%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.15 (d, J = 9.93 Hz, 1H), 6.94 (dd, J = 8.32 Hz, 2.34 Hz, 1H), 6.90–6.83 (m, 1H), 6.82–6.76 (m, 2H), 5.21 (s, 1H), 4.96 (s, 2H), 4.14 (s, 2H), 2.72 (s, 2H), 2.34 (d, J = 11.61 Hz, 6H), 1.31 (s, 9H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.4, 159.5 (d, J = 242.7 Hz), 154.9, 151.2, 144.6, 141.5, 135.6, 133.8, 127.7 (2C), 125.7 (2C), 121.3, 121.0, 119.9, 116.4, 112.9 (d, J = 21.2 Hz), 111.5 (d, J = 25.5 Hz), 70.5, 57.3 (d, J = 9.6 Hz), 48.9, 45.6, 45.5, 34.7 (2C), 31.5 (3C). HRMS ESI (m/z): calcd for C28H33FN3O2+ [M + H]+: 462.2551; found 462.2549.
2-([1,1′-Biphenyl]-4-ylmethoxy)-10-(2-(dimethylamino)ethyl)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (33). 33 was synthesized following the procedure described for 9. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 1
EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to afford compound 33 (180 mg, 55.3%) as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 7.67 (dd, J = 8.0 Hz, 2.1 Hz, 4H), 7.59 (s, 1H), 7.51–7.41 (m, 5H), 7.39–7.34 (m, 1H), 7.21 (d, J = 3.0 Hz, 1H), 7.12 (dd, J = 8.9 Hz, 5.8 Hz, 1H), 7.06 (dd, J = 8.7, 3.0, 1H), 7.01–6.92 (m, 2H), 5.09 (s, 2H), 4.02 (t, J = 6.7, 2H), 2.42 (t, J = 6.7, 2H), 2.14 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] 167.8, 158.7 (d, J = 238.0 Hz), 153.6, 146.3, 143.2, 140.2 (d, J = 16.1 Hz), 136.8, 135.2 (d, J = 10.3), 129.4 (2C), 128.6 (2C), 128.0, 127.2 (4C, d, J = 7.3 Hz), 126.0, 121.7 (d, J = 9.6 Hz), 120.5 (d, J = 23.9 Hz), 116.8, 112.9 (d, J = 22.5), 111.6 (d, J = 25.3), 69.7, 57.5, 48.3, 45.8 (2C). HRMS ESI (m/z): calcd for C30H29FN3O2+ [M + H]+: 482.2238; found 482.2236.
2) to afford compound 33 (180 mg, 55.3%) as a yellow solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 7.67 (dd, J = 8.0 Hz, 2.1 Hz, 4H), 7.59 (s, 1H), 7.51–7.41 (m, 5H), 7.39–7.34 (m, 1H), 7.21 (d, J = 3.0 Hz, 1H), 7.12 (dd, J = 8.9 Hz, 5.8 Hz, 1H), 7.06 (dd, J = 8.7, 3.0, 1H), 7.01–6.92 (m, 2H), 5.09 (s, 2H), 4.02 (t, J = 6.7, 2H), 2.42 (t, J = 6.7, 2H), 2.14 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] 167.8, 158.7 (d, J = 238.0 Hz), 153.6, 146.3, 143.2, 140.2 (d, J = 16.1 Hz), 136.8, 135.2 (d, J = 10.3), 129.4 (2C), 128.6 (2C), 128.0, 127.2 (4C, d, J = 7.3 Hz), 126.0, 121.7 (d, J = 9.6 Hz), 120.5 (d, J = 23.9 Hz), 116.8, 112.9 (d, J = 22.5), 111.6 (d, J = 25.3), 69.7, 57.5, 48.3, 45.8 (2C). HRMS ESI (m/z): calcd for C30H29FN3O2+ [M + H]+: 482.2238; found 482.2236.
8-Fluoro-2-(prop-2-yn-1-yloxy)-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (S23). S23 was synthesized following the procedure described for S20. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 5
EA = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound S23 (600 mg, 71%) as a red solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 9.17 (s, 1H), 7.53 (s, 1H), 7.01 (d, J = 6.9 Hz, 1H), 6.81–6.66 (m, 4H), 5.30 (s, 1H), 4.67 (s, 2H), 2.53 (s, 1H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 169.2, 159.3 (d, J = 242.1 Hz), 152.9, 144.0, 136.1 (d, J = 4.8 Hz), 131.0 (d, J = 9.8 Hz), 123.7, 122.4, 120.6 (d, J = 3.7 Hz), 120.5 (2C), 117.1, 111.8 (d, J = 22.5 Hz), 108.6 (d, J = 25.8 Hz), 78.3, 75.9, 56.4. HRMS (GC/QTOF) m/z: calcd for C16H11FN2O2+ [M]+: 282.0805. Found 282.0802.
1) to afford compound S23 (600 mg, 71%) as a red solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 9.17 (s, 1H), 7.53 (s, 1H), 7.01 (d, J = 6.9 Hz, 1H), 6.81–6.66 (m, 4H), 5.30 (s, 1H), 4.67 (s, 2H), 2.53 (s, 1H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 169.2, 159.3 (d, J = 242.1 Hz), 152.9, 144.0, 136.1 (d, J = 4.8 Hz), 131.0 (d, J = 9.8 Hz), 123.7, 122.4, 120.6 (d, J = 3.7 Hz), 120.5 (2C), 117.1, 111.8 (d, J = 22.5 Hz), 108.6 (d, J = 25.8 Hz), 78.3, 75.9, 56.4. HRMS (GC/QTOF) m/z: calcd for C16H11FN2O2+ [M]+: 282.0805. Found 282.0802.
8-Fluoro-2-((1-(pyridin-4-yl)-1H-1,2,3-triazol-4-yl)methoxy)-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (S24). A mixture of 4-iodopyridine (1 g, 4.9 mmol) and NaN3 (476 mg, 7.3 mmol) in 30 mL 1,4-dioxane/H2O (V
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V = 1
V = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was stirred at 100 °C for 48 h. Upon completion, the mixture was filtered and the filtrate was concentrated in vacuo. The crude product was purified by column chromatography to give 4-azidopyridine (0.5 g, yield 85%). A mixture of S23 (1 g, 3.5 mmol), 4-azidopyridine (0.5 g, 4.2 mmol), Cu(MeCN)4PF6 (2 g, 5.3 mmol) and 2,6-lutidine (86 mg, 0.8 mmol) was stirred in 30 mL DCM at 40 °C for 24 hours. Upon completion, CH2Cl2 (20 mL) was added and the resulting mixture was washed with EDTA solution (0.1 M, 3 × 50 mL) and then H2O (3 × 50 mL). The organic layer was dried over anhydrous Na2SO4 and the solvent was removed under reduced pressure. The crude product was purified by column chromatography (PE
1) was stirred at 100 °C for 48 h. Upon completion, the mixture was filtered and the filtrate was concentrated in vacuo. The crude product was purified by column chromatography to give 4-azidopyridine (0.5 g, yield 85%). A mixture of S23 (1 g, 3.5 mmol), 4-azidopyridine (0.5 g, 4.2 mmol), Cu(MeCN)4PF6 (2 g, 5.3 mmol) and 2,6-lutidine (86 mg, 0.8 mmol) was stirred in 30 mL DCM at 40 °C for 24 hours. Upon completion, CH2Cl2 (20 mL) was added and the resulting mixture was washed with EDTA solution (0.1 M, 3 × 50 mL) and then H2O (3 × 50 mL). The organic layer was dried over anhydrous Na2SO4 and the solvent was removed under reduced pressure. The crude product was purified by column chromatography (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 3
EA = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give S24 (0.7 g, 50%) as a gray solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 10.06 (s, 1H), 9.17 (s, 1H), 8.82 (s, 2H), 8.03 (s, 2H), 7.67 (s, 1H), 7.37 (s, 1H), 7.15 (s, 1H), 7.00 (s, 2H), 6.82 (s, 2H), 5.24 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 168.0, 158.4 (d, J = 237.3 Hz), 152.7, 152.1 (2C), 145.0, 143.0 (2C), 137.7 (d, J = 2.1 Hz), 131.7 (d, J = 10.4 Hz), 124.3, 123.2, 121.7, 121.0 (d, J = 7.1 Hz), 121.0, 117.1, 117.0, 114.16, 111.2 (d, J = 22.1 Hz), 108.3 (d, J = 25.4 Hz), 61.9. HRMS ESI-TOF (m/z): calcd for C21H15FN6O2Na+ [M + Na]+: 425.1138. Found 425.1129.
1) to give S24 (0.7 g, 50%) as a gray solid. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 10.06 (s, 1H), 9.17 (s, 1H), 8.82 (s, 2H), 8.03 (s, 2H), 7.67 (s, 1H), 7.37 (s, 1H), 7.15 (s, 1H), 7.00 (s, 2H), 6.82 (s, 2H), 5.24 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 168.0, 158.4 (d, J = 237.3 Hz), 152.7, 152.1 (2C), 145.0, 143.0 (2C), 137.7 (d, J = 2.1 Hz), 131.7 (d, J = 10.4 Hz), 124.3, 123.2, 121.7, 121.0 (d, J = 7.1 Hz), 121.0, 117.1, 117.0, 114.16, 111.2 (d, J = 22.1 Hz), 108.3 (d, J = 25.4 Hz), 61.9. HRMS ESI-TOF (m/z): calcd for C21H15FN6O2Na+ [M + Na]+: 425.1138. Found 425.1129.
10-(2-(Dimethylamino)ethyl)-8-fluoro-2-((1-(pyridin-4-yl)-1H-1,2,3-triazol-4-yl)methoxy)-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (34). 34 was synthesized following the procedure described for 9. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 1
EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to afford compound 34 (100 mg, 42%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 8.76 (d, J = 4.7 Hz, 2H), 8.21 (s, 1H), 7.72 (d, J = 5.0 Hz, 2H), 7.41 (s, 1H), 7.14 (d, J = 9.7 Hz, 1H), 6.94–6.86 (m, 2H), 6.79–6.72 (m, 2H), 5.21 (s, 2H), 4.12 (t, J = 6.1 Hz, 2H), 2.67 (t, J = 6.7 Hz, 2H), 2.29 (s, 6H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.1, 159.4 (d, J = 242.5 Hz), 153.6, 151.7 (2C), 145.4, 145.2, 142.9 (2C), 141.3 (d, J = 2.6 Hz), 135.4 (d, J = 9.8 Hz), 126.1, 121.2 (d, J = 9.2 Hz), 120.5, 119.9, 116.9 (2C), 113.8, 112.8 (d, J = 22.4 Hz), 111.4 (d, J = 25.5 Hz), 62.1, 57.2, 49.0, 45.5 (2C). HRMS ESI-TOF (m/z): calcd for C25H24FN7O2 Na+ [M + Na]+: 496.1873. Found 496.1880.
2) to afford compound 34 (100 mg, 42%) as a yellow solid. 1H NMR (400 MHz, CDCl3) δ [ppm] = 8.76 (d, J = 4.7 Hz, 2H), 8.21 (s, 1H), 7.72 (d, J = 5.0 Hz, 2H), 7.41 (s, 1H), 7.14 (d, J = 9.7 Hz, 1H), 6.94–6.86 (m, 2H), 6.79–6.72 (m, 2H), 5.21 (s, 2H), 4.12 (t, J = 6.1 Hz, 2H), 2.67 (t, J = 6.7 Hz, 2H), 2.29 (s, 6H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.1, 159.4 (d, J = 242.5 Hz), 153.6, 151.7 (2C), 145.4, 145.2, 142.9 (2C), 141.3 (d, J = 2.6 Hz), 135.4 (d, J = 9.8 Hz), 126.1, 121.2 (d, J = 9.2 Hz), 120.5, 119.9, 116.9 (2C), 113.8, 112.8 (d, J = 22.4 Hz), 111.4 (d, J = 25.5 Hz), 62.1, 57.2, 49.0, 45.5 (2C). HRMS ESI-TOF (m/z): calcd for C25H24FN7O2 Na+ [M + Na]+: 496.1873. Found 496.1880.
2-((1-Cyclopentyl-1H-1,2,3-triazol-4-yl)methoxy)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e] [1,4]diazepin-11-one (S25). S25 was synthesized following the procedure described for 9. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 3
EA = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound S25 (600 mg, 60%) as a yellow oil. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 10.01 (s, 1H), 8.25 (s, 1H), 7.62 (s, 1H), 7.29 (d, J = 2.8 Hz, 1H), 7.11–7.06 (m, 1H), 7.02–6.92 (m, 2H), 6.84–6.76 (m, 2H), 5.06 (s, 2H), 5.01–4.92 (m, 1H), 2.22–2.11 (m, 2H), 1.99–1.89 (m, 2H), 1.85–1.74 (m, 2H), 1.73–1.62 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 168.2, 158.4 (d, J = 237.5 Hz), 152.9, 144.9, 143.0, 137.8 (d, J = 2.1 Hz), 131.7 (d, J = 10.5 Hz), 124.3, 123.7, 121.7, 121.0 (d, J = 9.2 Hz), 120.9, 116.9, 111.3 (d, J = 22.1 Hz), 108.3 (d, J = 25.3 Hz), 62.0, 61.5, 33.3 (2C), 24.0 (2C). HRMS ESI-TOF (m/z): calcd for C21H20FN5O2Na+ [M + Na]+: 416.1499. Found 416.1505.
1) to afford compound S25 (600 mg, 60%) as a yellow oil. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 10.01 (s, 1H), 8.25 (s, 1H), 7.62 (s, 1H), 7.29 (d, J = 2.8 Hz, 1H), 7.11–7.06 (m, 1H), 7.02–6.92 (m, 2H), 6.84–6.76 (m, 2H), 5.06 (s, 2H), 5.01–4.92 (m, 1H), 2.22–2.11 (m, 2H), 1.99–1.89 (m, 2H), 1.85–1.74 (m, 2H), 1.73–1.62 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 168.2, 158.4 (d, J = 237.5 Hz), 152.9, 144.9, 143.0, 137.8 (d, J = 2.1 Hz), 131.7 (d, J = 10.5 Hz), 124.3, 123.7, 121.7, 121.0 (d, J = 9.2 Hz), 120.9, 116.9, 111.3 (d, J = 22.1 Hz), 108.3 (d, J = 25.3 Hz), 62.0, 61.5, 33.3 (2C), 24.0 (2C). HRMS ESI-TOF (m/z): calcd for C21H20FN5O2Na+ [M + Na]+: 416.1499. Found 416.1505.
2-((1-Cyclopentyl-1H-1,2,3-triazol-4-yl)methoxy)-10-(2-(dimethylamino)ethyl)-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (35). 35 was synthesized following the procedure described for 9. The crude product was purified by column chromatography (PE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 1
EA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) to afford compound 35 (120 mg, 45%) as a yellow oil. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 8.22 (s, 1H), 7.62 (s, 1H), 7.45–7.38 (m, 1H), 7.22 (d, J = 2.7 Hz, 1H), 7.17–7.10 (m, 1H), 7.06–6.97 (m, 2H), 6.97–6.89 (m, 1H), 5.04 (s, 2H), 4.98–4.90 (m, 1H), 4.02 (t, J = 5.9 Hz, 2H), 2.42 (t, J = 6.6 Hz, 2H), 2.13 (s, 8H), 1.97–1.84 (m, 2H), 1.83–1.70 (m, 2H), 1.70–1.56 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 167.8158.78 (d, J = 238.2 Hz), 153.38, 146.4, 143.2 (d, J = 1.5 Hz), 143.0, 135.2 (d, J = 10.1 Hz), 126.0, 123.7, 121.8 (d, J = 9.4 Hz), 120.5, 120.4, 116.9, 112.9 (d, J = 22.3 Hz), 111.6 (d, J = 25.5 Hz), 62.1, 61.5, 57.5, 48.3, 45.8 (2C), 33.3 (2C), 24.0 (2C). HRMS ESI-TOF (m/z): calcd for C25H29FN6O2Na+ [M + Na]+: 487.2234. Found 487.2230.
2) to afford compound 35 (120 mg, 45%) as a yellow oil. 1H NMR (400 MHz, DMSO-d6) δ [ppm] = 8.22 (s, 1H), 7.62 (s, 1H), 7.45–7.38 (m, 1H), 7.22 (d, J = 2.7 Hz, 1H), 7.17–7.10 (m, 1H), 7.06–6.97 (m, 2H), 6.97–6.89 (m, 1H), 5.04 (s, 2H), 4.98–4.90 (m, 1H), 4.02 (t, J = 5.9 Hz, 2H), 2.42 (t, J = 6.6 Hz, 2H), 2.13 (s, 8H), 1.97–1.84 (m, 2H), 1.83–1.70 (m, 2H), 1.70–1.56 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ [ppm] = 167.8158.78 (d, J = 238.2 Hz), 153.38, 146.4, 143.2 (d, J = 1.5 Hz), 143.0, 135.2 (d, J = 10.1 Hz), 126.0, 123.7, 121.8 (d, J = 9.4 Hz), 120.5, 120.4, 116.9, 112.9 (d, J = 22.3 Hz), 111.6 (d, J = 25.5 Hz), 62.1, 61.5, 57.5, 48.3, 45.8 (2C), 33.3 (2C), 24.0 (2C). HRMS ESI-TOF (m/z): calcd for C25H29FN6O2Na+ [M + Na]+: 487.2234. Found 487.2230.
10-(2-(Dimethylamino)ethyl)-8-fluoro-2-hydroxy-5,10-dihydro-11H-dibenzo[b,e][1,4] diazepin-11-one (S26). S26 was synthesized following the procedure described for 9. The crude product was purified by column chromatography (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 3
MeOH = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford compound S26 (200 mg, 70%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.24 (d, J = 2.1 Hz, 1H), 6.99–6.92 (m, 1H), 6.88–6.81 (m, 1H), 6.78–6.59 (m, 3H), 5.70 (s, 2H), 4.12 (t, J = 6.2 Hz, 2H), 2.67 (t, J = 6.6 Hz, 2H), 2.27 (s, 6H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.9, 159.3 (d, J = 242.0 Hz), 152.7, 143.7, 141.8 (d, J = 2.5 Hz), 135.1 (d, J = 9.8 Hz), 125.6, 121.3 (d, J = 9.1 Hz), 120.9, 119.9, 118.0, 113.0 (d, J = 22.2 Hz), 111.1 (d, J = 25.4 Hz), 56.7, 48.3, 45.2 (2C). HRMS (ESI-TOF) m/z: calcd for C17H19FN3O2+ [M + H]+: 316.1461. Found 316.146.
1) to afford compound S26 (200 mg, 70%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.24 (d, J = 2.1 Hz, 1H), 6.99–6.92 (m, 1H), 6.88–6.81 (m, 1H), 6.78–6.59 (m, 3H), 5.70 (s, 2H), 4.12 (t, J = 6.2 Hz, 2H), 2.67 (t, J = 6.6 Hz, 2H), 2.27 (s, 6H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.9, 159.3 (d, J = 242.0 Hz), 152.7, 143.7, 141.8 (d, J = 2.5 Hz), 135.1 (d, J = 9.8 Hz), 125.6, 121.3 (d, J = 9.1 Hz), 120.9, 119.9, 118.0, 113.0 (d, J = 22.2 Hz), 111.1 (d, J = 25.4 Hz), 56.7, 48.3, 45.2 (2C). HRMS (ESI-TOF) m/z: calcd for C17H19FN3O2+ [M + H]+: 316.1461. Found 316.146.
10-(2-(Dimethylamino)ethyl)-8-fluoro-2-(hexyloxy)-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (36). A solution of compound S26 (126 mg, 0.44 mmol) and 1-bromohexane (73 mg, 0.44 mmol) in 3 mL ACN was stirred at 70 °C for 12 h. Upon completion, the reaction mixture was concentrated in vacuo. The crude product was purified by column chromatography (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 3
MeOH = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 36 (116 mg, 66%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.30 (d, J = 2.6 Hz, 1H), 7.18–7.13 (m, 1H), 6.90–6.82 (m, 2H), 6.80–6.70 (m, 2H), 4.12 (t, J = 6.7 Hz, 2H), 3.89 (t, J = 6.5 Hz, 2H), 2.67 (t, J = 6.9 Hz, 2H), 2.30 (s, 6H), 1.76–1.67 (m, 2H), 1.44–1.36 (m, 2H), 1.33–1.27 (m, 4H), 0.88 (t, J = 6.5 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.4, 159.3 (d, J = 242.0 Hz), 155.0, 144.3, 141.5 (d, J = 2.7 Hz), 135.5 (d, J = 9.8 Hz), 125.9, 121.1 (d, J = 9.2 Hz), 120.7, 119.7, 115.9, 112.6 (d, J = 22.5 Hz), 111.4 (d, J = 25.4 Hz), 68.5, 57.3, 49.1, 45.6 (2C), 31.5, 29.2, 25.7, 22.6, 14.05. HRMS ESI-TOF (m/z): calcd for C23H31FN3O2+ [M + H]+: 400.2400. Found 400.2401.
1) to give compound 36 (116 mg, 66%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ [ppm] = 7.30 (d, J = 2.6 Hz, 1H), 7.18–7.13 (m, 1H), 6.90–6.82 (m, 2H), 6.80–6.70 (m, 2H), 4.12 (t, J = 6.7 Hz, 2H), 3.89 (t, J = 6.5 Hz, 2H), 2.67 (t, J = 6.9 Hz, 2H), 2.30 (s, 6H), 1.76–1.67 (m, 2H), 1.44–1.36 (m, 2H), 1.33–1.27 (m, 4H), 0.88 (t, J = 6.5 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ [ppm] = 168.4, 159.3 (d, J = 242.0 Hz), 155.0, 144.3, 141.5 (d, J = 2.7 Hz), 135.5 (d, J = 9.8 Hz), 125.9, 121.1 (d, J = 9.2 Hz), 120.7, 119.7, 115.9, 112.6 (d, J = 22.5 Hz), 111.4 (d, J = 25.4 Hz), 68.5, 57.3, 49.1, 45.6 (2C), 31.5, 29.2, 25.7, 22.6, 14.05. HRMS ESI-TOF (m/z): calcd for C23H31FN3O2+ [M + H]+: 400.2400. Found 400.2401.
10-Benzyl-8-fluoro-5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one (DDC4002). DDC4002 was synthesized according to the reported literature.141H NMR (400 MHz, DMSO) δ [ppm] = 7.91 (s, 1H), 7.66 (dd, J = 7.8 Hz, 1.6, 1H), 7.39–7.34 (m, 1H), 7.32–7.22 (m, 5H), 7.22–7.16 (m, 1H), 7.14–7.05 (m, 2H), 7.02–6.96 (m, 1H), 6.93–6.85 (m, 1H), 5.28 (s, 2H). 13C NMR (101 MHz, DMSO) δ [ppm] = 167.9, 158.3 (d, J = 238.9 Hz), 152.1, 141.8 (d, J = 2.4 Hz), 137.4, 134.5 (d, J = 10.1 Hz), 132.7, 132.2, 128.4 (2C), 126.9, 126.7 (2C), 124.3, 121.8, 121.6 (d, J = 9.3 Hz), 118.8, 112.5 (d, J = 22.4 Hz), 110.8 (d, J = 25.2 Hz), 51.5.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1,2-dichloroethane and petroleum ether, and slowly volatilized to obtain single crystals. Crystal data was collected on a Bruker D8 VENTURE with Mo Kα radiation (λ = 0.71073 Å) at 150 K. The structure was solved by direct methods and different Fourier syntheses. All calculations were performed by full-matrix least-squares methods on F2 by using the SHELXS-97 and SHELXL-97 programs.26 All non-hydrogen atoms were refined with anisotropic thermal parameters and the hydrogen atoms were fixed at calculated positions and refined by a riding mode. The SQUEEZE routine implemented on PLATON was used to remove electron densities corresponding to disordered solvent molecules in crystal data.
1 mixture of 1,2-dichloroethane and petroleum ether, and slowly volatilized to obtain single crystals. Crystal data was collected on a Bruker D8 VENTURE with Mo Kα radiation (λ = 0.71073 Å) at 150 K. The structure was solved by direct methods and different Fourier syntheses. All calculations were performed by full-matrix least-squares methods on F2 by using the SHELXS-97 and SHELXL-97 programs.26 All non-hydrogen atoms were refined with anisotropic thermal parameters and the hydrogen atoms were fixed at calculated positions and refined by a riding mode. The SQUEEZE routine implemented on PLATON was used to remove electron densities corresponding to disordered solvent molecules in crystal data.
Crystal data for compound 13: CCDC: 2243568. C26H29ClFIN3O2, M = 596.87 g mol−1, monoclinic, space group C2/c (no. 15), a = 37.035(3) Å, b = 9.0602(6) Å, c = 15.6080(11) Å, V = 5222.1(7) Å3, Z = 8, T = 150 K, μ(MoKα) = 1.363 mm−1, Dcalc = 1.518 g cm−3, 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 359 reflections measured (4.412° ≤ 2Θ ≤ 52.822°), 5302 unique (Rint = 0.0694, Rsigma = 0.0645) which were used in all calculations. The final R1 was 0.0526 (I > 2σ(I)) and wR2 was 0.1133 (all data).
359 reflections measured (4.412° ≤ 2Θ ≤ 52.822°), 5302 unique (Rint = 0.0694, Rsigma = 0.0645) which were used in all calculations. The final R1 was 0.0526 (I > 2σ(I)) and wR2 was 0.1133 (all data).
4.2. Biological experiment
The human non-small cell lung cancer (NSCLC) cell lines HCC827, A549, and H1975 were purchased from iCell Bioscience Inc. (Shanghai, China). The EGFR triple-mutant (L858R/T790M/C797S) NCI-H1975 subline (referred to as H1975™ in this study) was purchased from Cobioer Biotechnology Co., Ltd. (Nanjing, China). All cell lines were maintained in a humidified incubator at 37 °C with 5% CO2. HCC827, A549, H1975 and H1975™ were sub-cultured regularly using 0.25% trypsin/EDTA, and cultured in the following media formulations: HCC827: RPMI-1640 supplemented with 10% FBS, 1% sodium pyruvate, and 1% penicillin–streptomycin. H1975: RPMI-1640 supplemented with 10% FBS and 1% penicillin–streptomycin. A549: F12K supplemented with 10% FBS and 1% penicillin–streptomycin. H1975™: RPMI-1640 supplemented with 10% FBS, 1% penicillin–streptomycin, and 1% puromycin.
CCK-8: H1975™ cells were seeded in 96-well plates at a density of 3 × 103 cells per well (100 μL medium per well) and incubated overnight under standard culture conditions (37 °C, 5% CO2). Cells were then exposed to graded concentrations of test compounds for 72 h. Cell viability was assessed using the Cell Counting Kit-8 (CCK-8) according to the manufacturer's protocol. Absorbance at 490 nm was measured using a Varioskan LUX microplate reader (Thermo Scientific), and IC50 values were calculated by GraphPad Prism 8.0 software. Data represent mean values from three independent experiments.
The human PI3Kα and AKT1 kinases without the His6-GST tag were provided by PreceDo Pharmaceuticals Co., Ltd. (Hefei, China). Inhibition assays were performed using the ADP-Glo™ assay kit (Promega) according to the manufacturer's protocol. Compound 33 from 20 mM DMSO stocks was dispensed into black 384-well plates and normalized to 0.5% final DMSO concentration. Assay buffer containing purified PI3Kα or AKT1 kinase at specified concentrations (3 ng per well for PI3Kα and AKT1) was incubated with different concentrations of these compounds at room temperature for 10 min, followed by reaction with the substrate under 25 (PI3Kα) or 50 μM (AKT1) of ATP for 60 min, and adding the ADP-Glo™ Reagent over 40 min, and then adding the kinase detection reagent from the ADP-Glo™ assay kit over 30 min. The chemiluminescence signal was measured using a PerkinElmer microplate reader. Data were processed using GraphPad Prism 8 and fit to a dose–response model.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g, 4 °C, 20 min), and supernatants were collected. The protein concentrations of all the samples were quantified with a BCA kit (Beyotime, Shanghai, China). Afterwards, the obtained proteins (20 μg) for each group were separated by SDS-PAGE, transferred to PVDF membranes (Millipore), and blocked with 5% BSA. Membranes were incubated overnight at 4 °C with primary antibodies: anti-EGFR (1
000 × g, 4 °C, 20 min), and supernatants were collected. The protein concentrations of all the samples were quantified with a BCA kit (Beyotime, Shanghai, China). Afterwards, the obtained proteins (20 μg) for each group were separated by SDS-PAGE, transferred to PVDF membranes (Millipore), and blocked with 5% BSA. Membranes were incubated overnight at 4 °C with primary antibodies: anti-EGFR (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), anti-p-EGFR (1
000), anti-p-EGFR (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000), anti-AKT (1
5000), anti-AKT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), anti-p-AKT (1
1000), anti-p-AKT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), and anti-GAPDH (1
1000), and anti-GAPDH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000). After HRP-conjugated secondary antibody (1
2000). After HRP-conjugated secondary antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) incubation, signals were detected by enhanced chemiluminescence reagents (FDbio-Dura ECL, FUDE Biology Technology, Hangzhou, China) and photographed by a chemiluminescence imaging system (LF-C900, Longfangxinyu, Beijing, China) and quantified with SWE Image Gray Analysis Software. Each experiment was repeated three times, and the reported results were the mean values.
1000) incubation, signals were detected by enhanced chemiluminescence reagents (FDbio-Dura ECL, FUDE Biology Technology, Hangzhou, China) and photographed by a chemiluminescence imaging system (LF-C900, Longfangxinyu, Beijing, China) and quantified with SWE Image Gray Analysis Software. Each experiment was repeated three times, and the reported results were the mean values.
4.3. Statistical analysis
All data were expressed as mean ± standard deviation (SD) from three independent replicate experiments. Statistical analysis was conducted by one-way analysis of variance (ANOVA) for comparison of multiple groups using GraphPad Prism 8 software. *p < 0.05 was considered to indicate a statistically significant result.Author contributions
Chengliu Jin: investigation. Zhen Zhang: investigation. Peng Liao: investigation. Chen Zhang: investigation. Hua Cao: review & editing, supervision. Yan-Long Ma: conceptualization, writing – original draft.Conflicts of interest
The authors declare no conflicts of interest.Data availability
Data will be made available on request.Supplementary information (SI): NMR spectra of the intermediates and the final target compounds, HPLC chromatogram of the compound 33, inhibitory activities of target compounds against NSCLC cells and WB's raw data can be found in the SI. See DOI: https://doi.org/10.1039/d5md00759c.
CCDC 2243568 contains the supplementary crystallographic data for this paper.27
Acknowledgements
This work is financially supported by Scientific Research Platforms and Projects of Guangdong Provincial Education Department (Youth Innovation Talent Project: 51309447024).References
- A. Leiter, R. R. Veluswamy and J. P. Wisnivesky, Nat. Rev. Clin. Oncol., 2023, 20, 624–639 CrossRef.
- U. Testa, G. Castelli and E. Pelosi, Cancers, 2018, 10, 248–248 CrossRef PubMed.
- P. Maity, J. Chatterjee, K. T. Patil, S. Arora, M. K. Katiyar, M. Kumar, A. Samarbakhsh, G. Joshi, P. Bhutani, M. Chugh, N. S. Gavande and R. Kumar, J. Med. Chem., 2023, 66, 3135–3172 CrossRef CAS PubMed.
- L. Xu, B. Xu, J. Wang, Y. Gao, X. He, T. Xie and X. Y. Ye, Eur. J. Med. Chem., 2023, 245, 114900 CrossRef CAS PubMed.
- T. Hirano, H. Yasuda, J. Hamamoto, S. Nukaga, K. Masuzawa, I. Kawada, K. Naoki, T. Niimi, S. Mimasu, H. Sakagami, K. Soejima and T. Betsuyaku, Mol. Cancer Ther., 2018, 17, 740–750 CrossRef CAS.
- J. Rotow and T. G. Bivona, Nat. Rev. Cancer, 2017, 17, 637–658 CrossRef CAS PubMed.
- D. Hong, B. Zhou, B. Zhang, H. Ren, L. Zhu, G. Zheng, M. Ge and J. Ge, Eur. J. Med. Chem., 2022, 239, 114533 CrossRef CAS PubMed.
- H. Y. Zhao, X. X. Xi, M. Xin and S. Q. Zhang, Bioorg. Chem., 2022, 128, 106057 CrossRef CAS PubMed.
- D. Shaban, A. Kamashev, A. Emelianova and A. Buzdin, Cell, 2024, 13, 47 CrossRef PubMed.
- D. Das, L. Xie and J. Hong, RSC Med. Chem., 2024, 15, 3371–3394 RSC.
- X. Hu, Q. Xun, T. Zhang, S.-J. Zhu, Q. Li, L. Tong, M. Lai, T. Huang, C.-H. Yun, H. Xie, K. Ding and X. Lu, Chin. Chem. Lett., 2020, 31, 1281–1287 CrossRef CAS.
- K. Cao, J. Yan, F. Yan and T. Yin, Mol. Diversity, 2021, 25, 1111–1122 CrossRef CAS PubMed.
- C. P. Kumar, T. S. Reddy, P. S. Mainkar, V. Bansal, R. Shukla, S. Chandrasekhar and H. M. Hügel, Eur. J. Med. Chem., 2016, 108, 674–686 CrossRef PubMed.
- D. J. H. De Clercq, D. E. Heppner, C. To, J. Jang, E. Park, C. H. Yun, B. H. Mushajiang, T. W. Shin, T. W. Gero, D. A. Scott, P. A. Jänne, M. J. Eck and N. S. Gray, ACS Med. Chem. Lett., 2019, 10, 1549–1553 CrossRef CAS PubMed.
- F. Wittlinger, B. C. Ogboo, T. Shevchenko, T. Damghani, C. D. Pham, I. K. Schaeffner, B. T. Oligny, S. P. Chitnis, T. S. Beyett, A. Rasch, D. B. Buckley, D. A. Urul, T. Shaurova, E. W. May, E. M. Schaefer, M. J. Eck, P. A. Hershberger, A. Poso, S. A. Laufer and D. E. Heppner, Commun. Chem., 2024, 7, 38 CrossRef CAS PubMed.
- S. K. Tripathi and B. K. Biswal, Drug Discovery Today, 2021, 26, 1466–1472 CrossRef CAS PubMed.
- T. Amelia, R. E. Kartasasmita, T. Ohwada and D. H. Tjahjono, Molecules, 2022, 27, 819 CrossRef CAS PubMed.
- X. Y. Lu, J. B. Smaill and K. Ding, Angew. Chem., 2020, 59, 13764–13776 CrossRef CAS.
- Y. Pan and M. M. Mader, J. Med. Chem., 2022, 65, 5288–5299 CrossRef CAS.
- I. Ahmad and H. Patel, In Silico Research in Biomedicine, 2025, 1, 100011 CrossRef.
- C. To, T. S. Beyett, J. Jang, W. W. Feng, M. Bahcall, H. M. Haikala, B. H. Shin, D. E. Heppner, J. K. Rana, B. A. Leeper, K. M. Soroko, M. J. Poitras, P. C. Gokhale, Y. Kobayashi, K. Wahid, K. J. Kurppa, T. W. Gero, M. D. Cameron, A. Ogino, M. Mushajiang, C. Xu, Y. Zhang, D. A. Scott, M. J. Eck, N. S. Gray and P. A. Janne, Nat. Cancer, 2022, 3, 402–417 CrossRef CAS PubMed.
- V. G. Deshmukh, S. B. Sapkal, S. S. Gadekar and V. Deshmukh, J. Mol. Struct., 2025, 1339, 142326 CrossRef CAS.
- N. A. Franken, H. M. Rodermond, J. Stap, J. Haveman and C. van Bree, Clonogenic assay of cells in vitro, Nat. Protoc., 2006, 1, 2315–2319 CrossRef CAS.
- L. Wang, X. Ding, K. Wang, R. Sun, M. Li, F. Wang and Y. Xu, J. Mol. Struct., 2023, 1274, 134499 CrossRef CAS.
- P. Wee and Z. Wang, Cancers, 2017, 9, 52 CrossRef PubMed.
- R. C. Clark and J. S. Reid, Acta Crystallogr., Sect. A, 1995, 51, 887–897 CrossRef.
- CCDC 2243568: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2f9m5k.
| This journal is © The Royal Society of Chemistry 2026 |