Discovery of indole- and quinolone-based inhibitors of the mTOR/Akt/Pi3K pathway for the potential treatment of autism and certain types of cancer
Ahmad
Elshahary
a,
Hesham
Safwan
a,
Ahmad
Abdelwaly
ab,
Reem K.
Arafa
 a and
Mohamed A.
Helal
a and
Mohamed A.
Helal
 *ac
*ac
aBiomedical Sciences Program, University of Science and Technology, Zewail City of Science and Technology, Giza 12587, Egypt. E-mail: mhelal@zewailciy.edu.eg
bInstitute for Computational Molecular Science, and, Department of Chemistry, Temple University, Philadelphia, Pennsylvania 19122, USA
cMedicinal Chemistry Department, Faculty of Pharmacy, Suez Canal University, Ismailia 41522, Egypt
First published on 8th October 2025
Abstract
Mammalian target of rapamycin (mTOR) is a serine/threonine kinase that belongs to the PI3K-related protein kinase family. It is an integral part of two functionally distinct protein complexes: mTOR complex 1 and mTOR complex 2. Its signaling pathway is linked to cell survival, growth, proliferation, and motility. Deregulation of the mTOR pathway has been reported in many types of cancer. Hence, mTOR is an attractive target for the treatment of certain cancers such as renal cell carcinoma and pancreatic tumors. In addition, hyperactivity in mTOR-mediated signaling is associated with the pathogenesis of autism spectrum disorder (ASD) and Alzheimer's disease. Recently, mTOR inhibitors have been considered as emerging pharmacotherapy for these disorders. In this research, we have used molecular modeling techniques to design three series of compounds, indoles, β-carbolines, and 4-aminoquinolines, targeting the ATP site of the mTOR kinase. Based on insights from molecular docking, we developed twenty eight derivatives of these scaffolds to explore the SAR and optimize their affinities. The prepared compounds were evaluated for their inhibitory activity against mTOR as well as other closely related kinases such as PI3K and AKt. To our delight, twenty compounds have shown sub-micromolar activities towards the mTOR kinase. Compounds HA-2l and HA-2c showed a superior IC50 of 66 and 75 nM, respectively, for mTOR, while being selective against AKt and Pi3K. Upon optimization, these selective inhibitors could be useful for the management of ASD due to their relatively higher safety and, hence, suitability for long-term use. On the other hand, derivatives HA-1e, HA-2g, and HA-3d exhibited high affinities for the three enzymes, suggesting their potential utility as anticancer agents. Also, the cytotoxicity of the most active compounds was assessed using different cell-lines. Compounds HA-2g, HA-2l, and HA-3d showed sub-micromolar inhibition, in the range of 0.610–0.780 μM, against the tested cancer cell lines MDA-MB231 and HCT-116. The discovery of a clinically useful mTOR inhibitor would represent a new hope for patients of two important non-communicable diseases, cancer and ASD.
1. Introduction
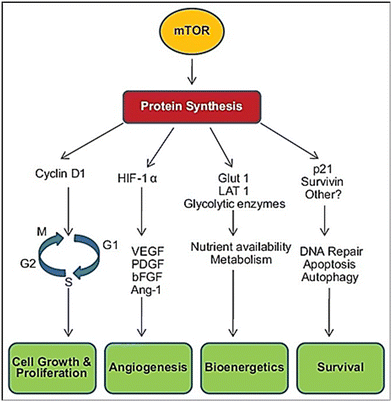
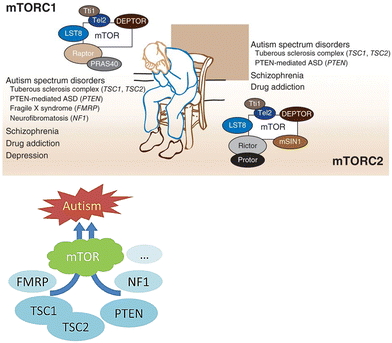
Mammalian target of rapamycin (mTOR) is a serine/threonine kinase highly conserved across several species. It belongs to the phosphatidylinositol 3-kinase-related kinase family of protein kinases. Through the formation of two unique protein complexes, mTORC1 and mTORC2, it is believed to control metabolism and cell growth, modulated by nutrients, growth factors, and cellular energy.1 mTOR is involved in the regulation of several cellular processes, including translation, transcription, ribosome biogenesis, nutrient transport, and autophagy. Both mTOR complexes are found in different subcellular compartments. mTORC1 controls protein synthesis and serves as a nutrient/energy sensor. Its activity is thought to be regulated by rapamycin, insulin, growth factors, phosphatidic acid, and specific amino acids. mTORC2 has been implicated in the regulation of the actin cytoskeleton through its stimulation of F-actin stress fibers, paxillin, RhoA, Rac1, Cdc42, and protein kinase C α (PKCα). Rapamycin was found to inhibit mTORC1 leading to most of the beneficial effects of the drug (Fig. 1).2Over-activation of mTOR signaling is implicated in many types of cancer such as breast, prostate, lung, melanoma, bladder, brain, and renal carcinomas. Dysregulation of mTOR can occur due to several reasons, including mutations in the tumor suppressor PTEN gene, increased activity of phosphoinositide-dependent kinase (PI3K) or Akt, and overexpression of certain effectors downstream to mTOR such as 4E-BP1, S6K and eIF4E. Increased mTOR activity could lead to a surge in cell proliferation, enhancement of angiogenesis, and inhibition of autophagy. Based on previous observations, mTOR has attracted the attention of pharmaceutical companies as a promising target for cancer treatment. Inhibitors of mTOR were proved to be useful in the management of several types of cancer.3 In addition, and more interestingly, mTOR has recently been tagged as a potential target to control autism spectrum disorder (ASD). This disease is frequently accompanied by monogenic disorders, such as tuberous sclerosis complex, phosphatase and tensin homolog tumor hamartoma syndrome, neurofibromatosis 1, and fragile X syndrome, in which mTOR is hyperactive. Mutations in the genes involved in the mTOR-mediated signaling pathway have been identified in some cases of syndromic ASD. Evidence indicates a pathologic role in hyperactive mTOR-mediated signaling in ASD associated with these monogenic disorders.4,5 An in depth review published by Costa-Mattioli and Monteggia in Nature Neuroscience in 2013 provided strong supporting evidence correlating ASD and mTOR overexpression.6 Thus, it can be well argued that mTOR inhibitors should be envisaged as putative promising therapeutic agents for ASD. The use of mTOR inhibitors as a potential therapeutic approach for ASD is of particular interest in syndromic forms such as tuberous sclerosis complex (TSC) and PTEN-related disorders, where hyperactivation of the mTOR signaling pathway contributes to abnormal synaptic development, neuronal overgrowth, and dysregulated protein synthesis. Modulating this pathway, mTOR inhibitors such as everolimus and sirolimus have the potential to regain synaptic homeostasis, enhance neurodevelopmental progress, and lessen the associated comorbidities including dementia and/or epilepsy.7 Nevertheless, their clinical utility in idiopathic autism still needs conclusive clinical evidence, as ASD is highly heterogeneous and mTOR pathway dysregulation is not observed in all patients. Furthermore, mTOR inhibitors carry significant limitations, including immunosuppression, metabolic adverse effects, potential cytotoxicity of multi-kinase inhibitors, and questionable long-term safety when administered during critical periods of brain development. Current evidence is largely restricted to small clinical studies and preclinical models, highlighting the need for large scale, well-designed trials to clearly judge the efficacy, appropriate dosing for age groups, and treatment protocols (Fig. 2).8
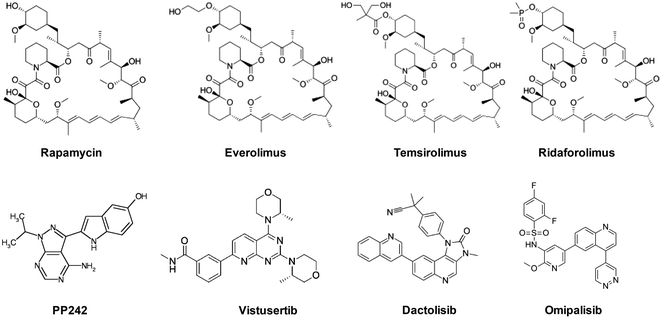
Over the last decade, numerous mTOR inhibitors were reported by industry and academia for treatment of several malignancies such as breast, renal, pancreatic, and non-small-cell lung cancer. Several chemical scaffolds have been exploited for the design of mTOR inhibitors, including pyrimidine, quinoxaline, imidazoquinoline, and pyrazolopyrimidine. As the name implies, the most well-known inhibitor of mTOR is rapamycin. Interestingly, this drug does not block the kinase activity of mTOR in a direct classic fashion. Instead, it binds, together with a small protein, an immunophilin termed FKBP12, to a domain adjacent to the kinase active site, blocking the effect of mTORC1 on some substrates. Several rapamycin analogs (rapalogs) have been developed to enhance the pharmacokinetic properties of rapamycin. These efforts resulted in the discovery of everolimus (Novartis), temsirolimus (Pfizer), and ridaforolimus (Merck) as immunosuppressive and anti-cancer drugs (Fig. 3).9–11
Due to the limited efficacy of rapalogs in treating certain types of cancer and their illusive mechanism of binding to mTORC1, several research laboratories have invested in developing small-molecule ATP-competitive inhibitors that target the kinase domain of mTOR. The first compound in this series, PP242, was reported by Apsel et al. in 2008,9 and has shown activity against some rapamycin-resistant tumors. In 2009, scientists from AstraZeneca have reported two important mTOR inhibitors with nanomolar activity, Torin-1 and Torin-2. The latter showed 100-fold selectivity over several other kinases. Later, the same company has developed AZD2014 (vistusertib) which was tested clinically for breast and lung cancer but was, unfortunately, discontinued. Parallel to these efforts, Novartis has developed NVP-BEZ235, renamed later as dactolisib as a dual inhibitor of mTOR and PI3K. This was followed by the discovery of GSK2126458 (omipalisib) by GalxoSmithKline and XL765 by Aventis. These dual acting inhibitors are believed to be more beneficial in some aggressive tumors as they target two essential signaling pathways for cancer cell growth.9,12,13
Dual PI3K/mTOR or pan PI3K/mTOR/Akt inhibitors are also ATP-competitive molecules designed to block both upstream class I PI3K isoforms and the downstream mTOR complexes (mTORC1/2), with the purpose of limiting adaptive re-activation of the mTOR enzyme.14–16 Several prototype compounds have entered clinical development: voxtalisib (SAR245409) showed early single-agent activity and combination potential in phase I/II studies but was limited by pharmacokinetic issues and on-target toxicities; BEZ235 has been tested in combination studies in advanced solid tumors. Apitolisib (GDC-0980) progressed to randomized trials but failed to demonstrate superiority over mTOR inhibition in some settings. More recently, gedatolisib (PF-05212384/PKI-587) has been the most clinically active dual inhibitor, receiving FDA fast-track designation for certain breast cancer settings and advancing into later-phase studies. Overall, despite the numerous trials, no dual PI3K/mTOR small molecule has yet achieved broad regulatory approval.17–19
Herein, we report the design and synthesis of novel ATP-competitive mTOR inhibitors for treatment of cancer as well as ASD using a ligand simplification strategy. Despite the great interest of both industry and academia in the development of 2nd generation mTOR inhibitors, none of the developed ligands have reached the market yet. Also, rapalogs have shown limited clinical efficacy in certain cancers and poor pharmacokinetics. Moreover, most of the reported small-molecule mTOR inhibitors exhibit poor selectivity towards other kinases, especially PI3K, leading to significant toxicity in clinical trials. Notably, some recent clinical trials have justified the toxicity of dual mTOR/PI3K for the management of certain tumors. Nevertheless, for a long-term treatment of a neurodevelopmental disorder such as autism, a safer selective mTOR inhibitor is essential and would act, for the first time, as a treatment “tailored” for this condition. These findings highlight the need for novel mTOR inhibitors with improved safety and pharmacokinetic profiles. We have also studied the selectivity and cytotoxicity of our prepared analogs. This would guide the translational process of these leads to the appropriate pharmacotherapeutic group, directing the selective mTOR inhibitors for autism and the non-selective agents for chemotherapy.20,21
2. Results and discussion
2.1. Compound design
We have noticed that despite the large number and the diversity of the chemical scaffolds used for the design of mTOR inhibitors, none of the reported ligands was based on a β-carboline nucleus, even though a large number of β-carboline or tetrahydro-β-carboline derivatives were developed as inhibitors of many other kinases.22–24 Moreover, this nucleus has a straightforward synthesis and could be easily accessed from the readily available tryptophan amino acids via a one-step Pictet–Spengler reaction. In addition, the tetrahydro-β-carboline nucleus has been safely used in some drugs such as tadalafil for a long time which makes this system an attractive target for redesign because of its extensively studied pharmacokinetics. Interestingly, in silico alignment of the tetrahydro-β-carboline nucleus with some of the mTOR inhibitors in clinical trials such as dactolisib and PI-103 revealed very similar steric and electronic arrangement (Fig. 4). These observations have motivated us to design a series of tetrahydro-β-carboline derivatives targeting mTOR inhibition (series HA-1). Moreover, we investigated an even more simplified scaffold by opening the piperidine ring of the tetrahydro-β-carboline to obtain a 5-arylindole amide/imine series (HA-2). Selection of the exact derivatives for synthesis was guided by docking and MD simulations.9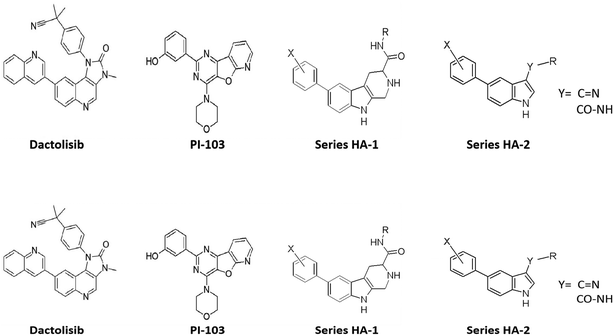 | ||
| Fig. 4 Steric and electronic similarity between our proposed series HA-1 and some clinically studied mTOR inhibitors. | ||
Additionally, Torin-2 (Fig. 5) is known as a potent and selective mTOR inhibitor with 800-fold greater selectivity for mTOR than PI3K and improved pharmacokinetic properties.25 We proposed a third series of compounds (HA-3) with structural similarity to Torin-2 but based on structural simplification of the benzonaphthyridine backbone via its replacement with a 4-aminoquinoline nucleus, thus retaining the key pharmacophoric elements. As such, we hope to achieve simpler synthesis while maintaining the mTOR affinity of this newly proposed series of derivatives. Also expected is an enhancement of the pharmacokinetic profile of the proposed series with respect to delivery to both cancer cells and the brain for management of ASD.
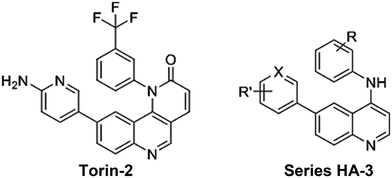 | ||
| Fig. 5 The potent mTOR inhibitor Torin-2 and our proposed series HA-2 designed using a scaffold simplification strategy. | ||
2.2. Chemistry
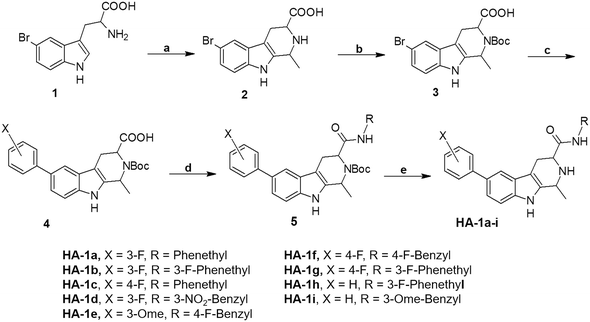
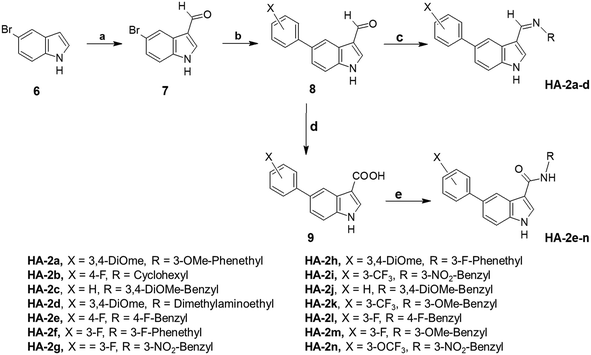
Synthesis of the phenyl tetrahydro-β-carboline derivatives started with Pictet–Spengler cyclization of the commercially available 5-bromotryptophan to obtain the substituted tetrahydro-β-carboline nucleus 2, followed by Boc-protection of the amine. Then, diversity was introduced on both sides of this nucleus. First, Suzuki coupling was performed with diverse aryl boronic acids. Second, a standard amide coupling procedure was applied to furnish intermediate 5. Finally, Boc deprotection of the ring amine provided the target compounds HA-1a–i (Scheme 1).The synthetic route for the indole-based Schiff bases HA-2a–d began with the Vilsmeier formylation of the commercially available 5-bromoindole, using phosphorus oxychloride and dimethylformamide, to afford aldehyde derivative 7, which showed a deshielded aldehydic proton at δ 9.95 ppm (1H-NMR) (Scheme 2). Diversity at the 5 position was introduced via Suzuki–Miyaura coupling with the appropriate phenylboronic acid derivatives. Care has been taken to select boronic acid derivatives with one or more H-bonding groups to target the hinge region backbone amides as required by most kinase inhibitors. Finally, the introduced formyl functionality handled structural diversity at the indole's C3 position through Schiff base condensation with different alkyl or aralkyl amines to yield the final products HA-2a–d.
In addition, another linker at position 3 of the indole ring was explored by oxidizing the aldehydes with permanganate solution to yield acid 9 (Scheme 2). This was followed by coupling with the appropriate aralkyl amines using EDCI as a coupling agent to yield the target amides HA-2e–n. These amide derivatives possess a similar length compared to the Schiff bases. However, the amide linker shows different electronic and H-bonding characteristics.
Finally, we turned our attention towards the synthesis of the quinoline series which is depicted in Scheme 3. First, 6-bromo-2-chloroquinoline was subjected to Suzuki coupling with the appropriate phenylboronic acid derivatives to give 6-(substituted)phenylquinoline derivative 11. Then, nucleophilic substitution with a series of anilines under basic conditions gave 4-(substituted-aniline)-6-aryl-quinolines HA-3a–e as the target compounds. Despite the successful preparation of certain target compounds, the yield of this reaction was found to be very poor and the purification of the product was challenging and required multiple flash columns. Therefore, we opted to perform the amination reaction using the Buchwald–Hartwig coupling in basic medium and tris(dibenzylideneacetone)dipalladium as the catalyst. To our delight, the reaction proceeded well under the Buchwald coupling conditions with an improved purity of the products.
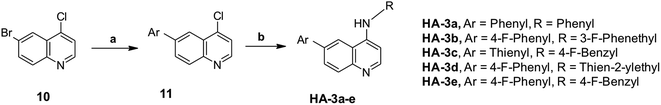 | ||
| Scheme 3 (a) Aryl boronic acid, Pd(PPh3)4, K2CO3, reflux, toluene, methanol, 18 h; (b) aryl halide, Xphos, (Pd(OAc)2), CsCO3, toluene, reflux, 24 h. | ||
2.3. Biological evaluation and SAR study
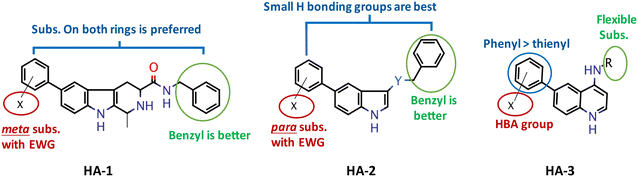
As mentioned earlier, selective mTOR inhibitors could be useful as a novel and safe treatment approach for autism, while dual mTOR/PI3K inhibitors would act as more potent anticancer agents. Therefore, several biological examinations are required to guide the translational efforts for these compounds. Thus, first the in vitro enzyme assay to estimate the abilities of our compounds to inhibit the mTOR kinase was conducted on all derivatives. The compounds' codes, structures, and IC50 values are illustrated in Table 1. Interestingly, all the prepared compounds showed an overall promising activity profile where the 28 newly synthesized compounds showed IC50 values in the low micromolar range, 15 of which achieved an mTOR inhibitory activity at the submicromolar level. With regard to the β-carboline series, the most active compound (HA-1e) was able to inhibit the mTOR activity with an IC50 of 56 nM. On the other hand, HA-1b was the least potent amongst the members of this chemical class (IC50 = 5.33 μM). Structure–activity relationship analysis of the mTOR inhibitory activity of this series demonstrated that, in general, those derivatives with an N-benzyl substitution on the amide group were more potent than the N-phenethyl counterparts as demonstrated by the inhibitory efficacies of derivatives HA-1f and HA-1b (IC50 = 0.193 and 5.33 μM, respectively) (Fig. 6). Additionally, findings related to the conducted chemical variation suggest that the substituent type and position are critical. Within the N-phenethyl analogs, meta-substitution with an electron donating group viz. OCH3 in HA-1c enhanced the mTOR inhibition by this compound (IC50 = 0.107 μM), while the para-fluoro counterpart HA-1g elicited an IC50 of 0.641 μM. Additionally, it can be inferred that substitution on both terminal phenyl rings is in favor of activity as demonstrated by the mTOR IC50s of the disubstituted derivative HA-1g in contrast to its monosubstituted analog HA-1h (IC50 = 0.641 and 1.664 μM, respectively).| Ser. | Codea | IC50b (μM) | Ser. | Code | IC50 (μM) |
|---|---|---|---|---|---|
| a HA-1: β-carboline series; HA-2: indole series; HA-3: quinoline series. b Compounds were tested for inhibitory effects on mTOR, PI3K, and AKt using the Transcreener fluorescence polarization method with a concentration of 10 μM ATP. | |||||
| 1 | HA-1a | 1.268 | 16 | HA-2g | 0.088 |
| 2 | HA-1b | 5.33 | 17 | HA-2h | 1.586 |
| 3 | HA-1c | 0.107 | 18 | HA-2i | 6.38 |
| 4 | HA-1d | 0.443 | 19 | HA-2j | 2.4 |
| 5 | HA-1e | 0.056 | 20 | HA-2k | 0.119 |
| 6 | HA-1f | 0.193 | 21 | HA-2l | 0.066 |
| 7 | HA-1g | 0.641 | 22 | HA-2m | 4.027 |
| 8 | HA-1h | 1.664 | 23 | HA-2n | 0.273 |
| 9 | HA-1i | 0.196 | 24 | HA-3a | 2.145 |
| 10 | HA-2a | 1.268 | 25 | HA-3b | 1.354 |
| 11 | HA-2b | 0.718 | 26 | HA-3c | 0.592 |
| 12 | HA-2c | 0.075 | 27 | HA-3d | 0.189 |
| 13 | HA-2d | 1.243 | 28 | HA-3e | 1.184 |
| 14 | HA-2e | 0.955 | Rapamycin | 133 | |
| 15 | HA-2f | 3.247 | |||
Concerning the Schiff bases HA-2a–d, the most potent derivative was HA-2c (IC50 = 0.075 μM), reflecting that while substitution at the Schiff base phenyl ring is well tolerated, it is less likely so when on the 5-phenyl ring on the indole core (cf.HA-2a, b and d). Reflecting on the results of the amides HA-2e–n, the highest inhibitory activity was demonstrated by HA-2l followed by HA-2g (IC50 = 0.066 and 0.088 μM, respectively). Notably, both compounds have an N-substituted amide, benzyl for the former and phenethyl for the latter, suggesting that both groups are well tolerated. However, the whole pattern of functionalization impacts the biological activity of this series as witnessed by the drop of activity of HA-2i (IC50 = 6.38 μM) bearing an N-meta-nitrobenzylamide moiety compared to its N-meta-methoxybenzylamide analog HA-2k (IC50 = 0.119 μM). In brief, it can be inferred that generally N-benzylamides bear better activity than the N-phenethyl counterparts. In addition, the para substitution imparts higher potency to these derivatives than the meta substitution. Also, relatively small hydrogen bonding groups potentiate the activity, possibly by contributing additional interactions to the target enzyme's active site amino acids, as will be further investigated in the docking study.
Finally, looking at the Torin-2 quinoline analogs HA-3a–e, members of this series were generally less potent than the β-carboline derivatives, with the most effective mTOR inhibitor being HA-3d (IC50 = 0.189 μM). SAR findings suggest that more flexible substituents on the 4-amino group are better for activity, as confirmed by the low activity of HA-3a (IC50 = 2.145 μM) in comparison with other members of the series. Yet, an excessive increase of flexibility of the linker can negatively affect the biological activity as seen by the drop of activity of HA-3b (IC50 = 1.354). Finally, for the positional isomers HA-3c and HA-3d, it is clear that accommodation of the phenyl ring at the 6-position of the quinoline, with a hydrogen bond accepting group, imparts better activity than a thiophene bioisosteric ring (IC50 = 0.592 and 0.189 μM, respectively).
Thereafter, we decided to further test the most active compounds representing the different series, viz. the 5 compounds: HA-1e, HA-2c, HA-2g, HA-2l, and HA-3d in the following assays; (i) IC50 on PI3K and AKt and (ii) cytotoxicity on two cancer cell lines, the MDA-MB231 breast cancer cell line and the HCT-116 colon cancer cell line. The results of these tests are described in Table 2. It was noticed that the selected compounds showed variable degrees of inhibition for the three enzymes, mTOR, PI3K, and AKt, with HA-2c and HA-2l being the most selective for mTOR. This could be attributed to the bulky size of HA-2l allowing this derivative to make favorable hydrophobic contacts and fill the relatively large ATP pocket of the mTOR nicely. While in the case of HA-2c which is not bulky enough, we may rationalize its moderate selectivity with the presence of the dimethoxy groups in the optimum position required for bonding with Gln194 in the back pocket. It is noteworthy that HA-2g demonstrated pan inhibition with relatively similar IC50 on all three tested kinases. For evaluating the cytotoxic effect of these five selected compounds on cancer, the breast cancer cell line MDA-MB231 and the colon cancer cell line HCT-116 were treated with the test compounds. The rationale behind the selection of these two cell lines depends on the role of the mTOR pathway as a key regulator of their growth and proliferation, where its dysregulation was also observed to be associated with the constitutively activated PI3K/AkT pathway.26,27All tested compounds showed good cytotoxicity in the low to sub-micromolar range against the colon cancer cell line HCT-116, as well as the breast cancer cells MDA-MB231.
| Compound | mTOR IC50 (nM) | PI3K IC50 (nM) | AKt IC50 (nM) | Cytotoxicity (μM) | |
|---|---|---|---|---|---|
| MDA-MB231 | HCT-116 | ||||
| HA-1e | 56 | 190 | 437 | 1.200 | 0.710 |
| HA-2c | 75 | 155 | 1326 | 1.190 | 0.740 |
| HA-2g | 88 | 72 | 81 | 0.780 | 0.710 |
| HA-2l | 66 | 890 | 1089 | 0.660 | 0.630 |
| HA-3d | 189 | 382 | 794 | 0.680 | 0.610 |
The scientific literature extensively documents that strong inhibition of the mTOR kinase results in considerable cytotoxic effects, primarily mediated by the activation of apoptotic pathways. The PI3K/Akt/mTOR pathway serves as a master regulator of cell survival and proliferation; thus, when this signaling cascade is inhibited, the cell forfeits its vital pro-survival signals.28 mTOR inhibition primarily accomplishes this by downregulating its essential substrates, particularly S6K and 4E-BP1. The consequent loss of 4E-BP1 phosphorylation obstructs the translation of crucial pro-survival proteins, such as Myc and Cyclin D, which leads to cell-cycle arrest and cessation of proliferation. Concurrently, mTOR inhibition frequently activates the Bim/Bax/Bcl-2 axis, a pivotal switch for intrinsic apoptosis. By altering the balance from anti-apoptotic proteins (Bcl-2) to pro-apoptotic proteins (Bim and Bax), the mitochondrial membrane becomes destabilized, resulting in the release of cytochrome c, subsequent activation of the caspase cascade (Caspase-9, -7, and -3), and ultimately, programmed cell death. This mechanism is essential for the effectiveness of mTOR inhibitors such as rapamycin and its analogs (rapalogs), as well as the more potent second-generation compounds like Torin-2, establishing a clear connection between the biochemical inhibition observed in enzyme assays and the resultant cell death in our cytotoxicity studies.29,30
2.4. Molecular modeling
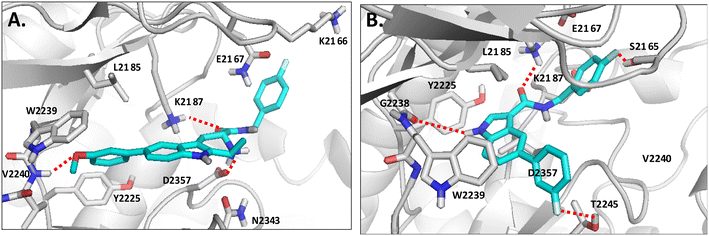
To gain insights into the binding determinants of these series of compounds, we have docked the prepared compounds into the ATP-binding site of the mTOR enzyme (PDB ID: 4JSX). Docking simulation has been performed using the FRED module within the OpenEye software with the default settings.31Fig. 7 shows the proposed binding mode of the two most active compounds HA-1e and HA-2l belonging to the β-carboline and the indoleamide series, respectively. It was noticed that, in both molecules, the aromatic scaffold fits into the ATP binding site, forming a π-stacking interaction, characteristic for many mTOR inhibitors, with Trp2239. In addition, Tyr2225 forms a “hydrophobic clamp” with Trp2239 helping in the ligand stabilization within the active site. Also, the hydrophobic nuclei of both ligands are anchored in their places by interactions with the non-polar residues Leu2185, Ile2237, and Ile2356. The critical interaction with the hinge region residues, characteristic for most of the kinase inhibitors, is noticed with the methoxy group of HA-1e (Fig. 7A), while the relatively smaller indole core of HA-2l was able to approach the hinge region with its NH (Fig. 7B). As we envisioned in our design, installing H-bonding groups on both sides of the ligand could help the binding to the polar residues at the perimeter of the active site. Interestingly, the basic nitrogen of the tetrahydro-β-carboline nucleus showed a strong charge-assisted H bond with Asp2357 of the DFG motif, and another H bond with Lys2187 using its amide oxygen (Fig. 7A). Similarly, compound HA-2l was noticed to form an extensive network of H bonds with residues on the edge of the active site. It interacts with Lys2187, using its amide oxygen as well, while the fluorine atoms on both ends of the molecule show H bonds with Ser2165 and Thr2245. This observation highlights the importance of installing H-bonding groups on one or both sides of the molecules. It can also rationalize the superior activity of benzyl derivatives over their phenethyl counterparts as the former provide the ligand with the optimum distance to reach the aforementioned polar residues Ser2165 and Thr2245.2.5. Pharmacokinetic study
Finally, the pharmacokinetic and drug likeness properties of the most active compound from each chemotype were evaluated in silico using the Swiss ADME server (Table 3). To our delight, all the investigated compounds showed acceptable predicted pharmacokinetics with no violation of Lipinski's rule of five. The compounds also showed high gastrointestinal absorption and variable BBB penetration. Overall, the favorable physicochemical properties and the relatively low molecular weight of most of them would allow room for further lead optimization studies in the future.| Compound | HA-1e | HA-2c | HA-2l | HA-3d |
|---|---|---|---|---|
| M.W | 444.52 | 370.44 | 362.37 | 334.41 |
| HBA | 3 | 3 | 3 | 2 |
| HBD | 3 | 1 | 2 | 1 |
Mlog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
2.96 | −0.44 | 4.27 | 4 |
| Lipinski's violations | 0 | 0 | 0 | 0 |
| Rotatable bonds | 6 | 7 | 5 | 4 |
| TPSA | 70.73 | 46.61 | 44.89 | 53.16 |
| Solubility | −5.48 | −5.35 | −5.26 | −5.52 |
| GI absorption | High | High | High | High |
| BBB penetration | Yes | Yes | Yes | No |
3. Conclusion
In summary, we have discovered novel lead compounds HA-1e, HA-2c, HA-2g, HA-2l, and HA-3d with promising inhibitory affinities for the mTOR kinase and other related kinases in the pathway: PI3K and AKt. We have used a combined ligand-based and receptor-based approach which is more efficient in designing ligands for targets with large and challenging binding sites.32 These compounds possess common pharmacophoric features with dactolisib and Torin-2 in agreement with the previously reported SAR of these molecules. We started with isosteric modifications of these ligands and then optimized the designed scaffold based on the molecular modeling results. Our top derivatives showed good mTOR inhibition in the nanomolar range with variable selectivities. Our future work will focus on optimizing these lead compounds to enhance their safety, cytotoxic selectivity, and clinical efficacy for cancer treatment.On the other hand, while the idea of using mTOR inhibitors as a treatment for ASD has a compelling biological rationale, translating that into safe and effective treatments for the broader autism population remains challenging. Moreover, research over the last two decades has suggested that dysregulation of mTOR signaling contributes to the development and progression of Alzheimer's disease. Many of the animal studies show promising biomarkers and behavioral rescue, but human trials have so far had mixed or negative results when evaluated for core symptoms like social communication and IQ scores. The obstacles include heterogeneity of this syndrome, accurate developmental window to intervene, long-term adverse effects, and ethical issues when children with ASD are involved. The challenge is to identify who is likely to respond, when to intervene, and how to do so safely over the long term.
Overall, our study revealed a new generation of mTOR ligands that, upon future optimization, could lead to the discovery of clinically useful agents for treatment of cancer and/or autism. Although our in vitro findings offer a persuasive argument for the therapeutic promise of these inhibitors in specific cancers and ASD, the study currently faces mechanistic constraints. While we have validated pathway inhibition, future investigations must concentrate on thorough mechanistic characterization to uncover the resulting apoptotic implications of mTOR inhibition, along with the complete range of cellular targets and possible off-target effects. Looking ahead, the translational advancement of these compounds necessitates three vital subsequent steps: (1) comprehensive ADME/Tox profiling: it is crucial to ascertain the pharmacokinetic properties, including solubility, metabolic stability, and overall toxicity, to validate drug-like attributes; (2) evaluation of CNS penetration: from the ASD therapeutic perspective, it is essential to demonstrate that the inhibitors can effectively traverse the blood–brain barrier; (3) in vivo efficacy testing: confirming their efficacy and safety in pertinent preclinical animal models, especially those that simulate mTOR-driven cancers and ASD, will be the conclusive step toward clinical implementation. These powerful and innovative inhibitors serve as significant chemical instruments for investigating the mTOR pathway and represent promising lead compounds for the creation of targeted therapies.
4. Experimental
4.1. Chemistry
Reagents and solvents were obtained from commercial sources and used without any further purification. Reaction progress was monitored by thin layer chromatography (TLC) analysis on Merck silica gel 60 F254 plates. Visualization was performed with UV light (254 nm) or iodine. Yields are of purified compounds and were not optimized. Flash column chromatography was performed on Sigma Aldrich silica gel (200–400 mesh) as a stationary phase. Melting points (mp) were determined in open capillaries on an Electrothermal IA9100 melting point apparatus and were uncorrected. Low-resolution mass spectra (MS) were recorded with a Shimadzu QP2010-Plus gas chromatograph/mass spectrometer (GC/MS) in electron impact (EI+) mode at 70 eV maintained at 250 °C. Infrared (IR) spectra were recorded on a Bruker Tensor 27 FT-IR spectrophotometer, driven by OPUSTM version 1.1 managing software (Vmax in cm−1, using KBr pellets). The purity of the final compounds, subjected to biological assays, was evaluated using HPLC and all the tested compounds showed acceptable purities with area under the curve (AUC) values of less than 5%.Proton and carbon nuclear magnetic resonance (1H and 13C NMR) spectra were recorded in CDCl3 or DMSO-d6 as a solvent on a Joel Delta 500 (500 MHz) spectrometer or a Bruker Ascend Aeon 400 (400 MHz) spectrometer at room temperature within the range of 20–25 °C. Chemical shifts (δ) are expressed as parts per million (ppm), using tetramethylsilane (TMS) as the internal standard. For signal multiplicities, the following abbreviations are used: s (singlet), d (doublet), dd (doublets of doublet), t (triplet), q (quartet), br s (broad singlet) and m (multiplet). Coupling constants (J values) are given in hertz (Hz). The acidic protons of the amidic –NH in the thiazolidinedione ring, alcohols or aromatic amines were not frequently observed in the 1H-NMR spectra.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DMF
1 DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, respectively. 0.3 ml of triethylamine (TEA) (4 equivalents) was added dropwise and the mixture was left to stir for 10 minutes. Then, 0.185 g of BOC (1.5 equivalents) was transferred to the flask and the reaction was left overnight at room temperature. When the reaction ended, the workup was performed. During the workup, some acid was added to neutralize the base (TEA), and a suitable amount of water was used to dissolve DMF. Sodium bisulfate (NaHSO4 20%) and ethyl acetate were used in the first step of the workup, then water and ethyl acetate were used, and this step was done twice. The separated ethyl acetate layer was collected as it contained the compound. After that, brine was added to extract water from the solution. These were performed using a separating funnel. Also, sodium sulfate was used to absorb any excess water in the extracted solution containing the compound. The mixture was filtered to remove residues of the salt and finally, the compound was dried using a rotavapor device. To obtain the pure product, column chromatography was performed using silica gel as a stationary phase and dichloromethane (DCM)/methanol as a mobile phase. The sample was loaded on the silica using wet packing, and the development was done using stepwise gradient elution.
water, respectively. 0.3 ml of triethylamine (TEA) (4 equivalents) was added dropwise and the mixture was left to stir for 10 minutes. Then, 0.185 g of BOC (1.5 equivalents) was transferred to the flask and the reaction was left overnight at room temperature. When the reaction ended, the workup was performed. During the workup, some acid was added to neutralize the base (TEA), and a suitable amount of water was used to dissolve DMF. Sodium bisulfate (NaHSO4 20%) and ethyl acetate were used in the first step of the workup, then water and ethyl acetate were used, and this step was done twice. The separated ethyl acetate layer was collected as it contained the compound. After that, brine was added to extract water from the solution. These were performed using a separating funnel. Also, sodium sulfate was used to absorb any excess water in the extracted solution containing the compound. The mixture was filtered to remove residues of the salt and finally, the compound was dried using a rotavapor device. To obtain the pure product, column chromatography was performed using silica gel as a stationary phase and dichloromethane (DCM)/methanol as a mobile phase. The sample was loaded on the silica using wet packing, and the development was done using stepwise gradient elution.
6-Bromo-1-methyl-2,3,4,9-tetrahydro-1H-b-carboline-3-carboxylic acid (2). The compound was prepared from 1.059 mmol of 2-amino-3-(5-bromo-1H-indol-3-yl)propanoic acid, 3 mL of H2O, 2 mL of H2SO4, and 5.44 mmol of CH3CHO as mentioned in general procedure A. The final product was obtained as crystals. Yield of 91.46%; mp: 247 °C; 1H NMR (400 MHz, DMSO-d6) d ppm 1.24 (s, 1 H) 1.48–1.65 (m, 3 H) 2.68–2.82 (m, 1 H) 3.16 (dd, J = 16.08, 3.97 Hz, 1 H) 3.63 (dd, J = 11.80, 4.58 Hz, 1 H) 4.53 (d, J = 6.11 Hz, 1 H) 7.14–7.23 (m,1 H) 7.29–7.37 (m, 1 H) 7.60–7.69 (m, 1 H) 11.48 (br. s., 1 H).
6-Bromo-2-(tert-butoxycarbonyl)-1-methyl-2,3,4,9-tetrahydro-1H-b-carboline-3-carboxylic acid (3). The compound was prepared from 0.485 mmol of 6-bromo-1-methyl-2,3,4,9-tetrahydro-1H-b-carboline-3-carboxylic acid (1.1), 5 mL of DMF and H2O in a ratio of 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3 mL of TEA, and 1.829 mmol of BOC as mentioned in general procedure B. The final product was obtained as a yellow sticky solid. Yield of 96.8%; 1H NMR (400 MHz, DMSO-d6) δ ppm 1.40–1.47 (m, 1 H) 1.50 (br. s., 3 H) 1.57–1.78 (m, 1 H) 2.56 (br. s., 1 H) 3.53 (br. s., 1 H).
1, 3 mL of TEA, and 1.829 mmol of BOC as mentioned in general procedure B. The final product was obtained as a yellow sticky solid. Yield of 96.8%; 1H NMR (400 MHz, DMSO-d6) δ ppm 1.40–1.47 (m, 1 H) 1.50 (br. s., 3 H) 1.57–1.78 (m, 1 H) 2.56 (br. s., 1 H) 3.53 (br. s., 1 H).
6-Phenyl-2-(tert-butoxycarbonyl)-1-methyl-2,3,4,9-tetrahydro-1H-b-carboline-3-carboxylic acid (4a). The compound was prepared from 0.3664 mmol of 6-bromo-2-(tert-butoxycarbonyl)-1-methyl-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylic acid (1.2), 10 mL methanol, 20 mL toluene, 2 ml K2CO3, 0.0129 mmol of Pd(PPh3)4, and 0.403 mmol of phenyl boronic acid (1.1) as mentioned in general procedure I. The final product was obtained as a light brown solid, mp: 214 °C. Yield of 92.34%; 1H NMR (400 MHz, DMSO-d6) δ ppm 1.24 (br. s., 1 H) 1.34–1.42 (m, 2 H) 1.48 (br. s., 5 H) 3.34–3.42 (m, 1 H) 3.54 (br. s., 1 H) 4.97–5.08 (m, 1 H) 7.26–7.31 (m, 1 H) 7.31–7.37 (m, 1 H) 7.39–7.45 (m, 1 H) 7.62–7.69 (m, 2 H) 10.90–11.08 (m, 1 H).
6-(3-Fluorophenyl)-2-(tert-butoxycarbonyl)-1-methyl-2,3,4,9-tetrahydro-1H-b-carboline-3-carboxylic acid (4b). The compound was prepared from (0.12 g, 0.3664 mmol) 6-bromo-2-(tert-butoxycarbonyl)-1-methyl-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylic acid (1), 10 mL methanol, 20 mL toluene, 2 ml K2CO3, 0.0129 mmol of Pd(PPh3)4, and (0.0564 g, 0.403 mmol) 3-fluorophenylboronic acid (1.1) as mentioned in general procedure C. The final product was obtained as a light beige solid, mp: 182 °C. Yield of 93.2%.
6-(3-Methoxyphenyl)-2-(tert-butoxycarbonyl)-1-methyl-2,3,4,9-tetrahydro-1H-b-carboline-3-carboxylic acid (4c). The compound was prepared from (0.12 g, 0.3664 mmol) 6-bromo-2-(tert-butoxycarbonyl)-1-methyl-2,3,4,9-tetrahydro-1H-b-carboline-3-carboxylic acid (1), 10 mL methanol, 20 mL toluene, 2 ml K2CO3, 0.0129 mmol of Pd(PPh3)4, and (0.0612 g, 0.403 mmol) 3-methoxyphenylboronic acid (1.1) as mentioned in general procedure C. The final product was obtained as a light beige solid, mp: 182 °C. Yield of 85.2%.
N-Phenethyl-6-(3-fluorophenyl)-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxamide (HA-1a). The title compound HA-1a was prepared from 5a (0.15 g, 0.29 mmol) with phenethylamine using procedure J to give a sticky oil. Yield: 54.2%. 1H NMR (400 MHz, DMSO-d6) δ 1.39–1.51 (m, 3 H), 2.60–2.69 (m, 1 H), 2.77–3.09 (m, 4 H), 3.46–3.58 (m, 2 H), 4.16 (d, J = 7.34 Hz, 1 H), 7.15–7.45 (m, 8 H), 7.46–7.70 (m, 3 H), 8.05 (d, J = 5.87 Hz, 1 H), 10.98 (s, 1 H),13C NMR (101 MHz, DMSO-d6) δ 20.36, 20.37, 48.85, 48.88, 57.75, 109.17–109.30, 111.84, 111.86, 113.38, 113.41, 113.58, 113.60, 120.23, 120.26, 122.97, 123.15–123.56, 126.61, 128.02, 128.74–129.27, 128.85, 129.05, 129.17, 131.10, 131.13, 136.26, 139.95; MS (EI+) m/z: 427.21 [M+˙]. Analysis C27H26FN3O (427.52) calculated % C 75.85, % H 6.13, % F 4.44, % N 9.83, % O 3.74; found % C (76.23%), % H (5.816%), % N (10.05%).
N-(3-Fluorophenethyl)-6-(3-fluorophenyl)-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxamide (HA-1b). The title compound HA-1b was prepared from 5b (0.12 g, 0.277 mmol) with 3-fluorophenethyl amine using procedure J to give a sticky oil. Yield: 44.2%.1H NMR (400 MHz, DMSO-d6) δ 1.57 (d, J = 6.36 Hz, 3 H), 2.55 (br. s., 2 H), 2.73 (t, J = 6.85 Hz, 2 H), 3.29–3.34 (m, 2 H), 5.07–5.23 (m, 2 H), 6.95–7.05 (m, 3 H), 7.16 (t, J = 8.44 Hz, 1 H), 7.20–7.26 (m, 1 H), 7.41–7.47 (m, 2 H), 7.48–7.60 (m, 3 H), 7.76 (s, 1 H), 11.01 (br. s., 1 H); MS (EI+) m/z: 445.2 [M+˙]. Analysis C27H25F2N3O (427.52) calculated % C 72.79, % H 5.66, % F 8.53, % N 9.43, % O 3.59; found % C (73.156%), % H (6.34%), % N (10.556%).
N-Phenethyl-6-(4-fluorophenyl)-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxamide (HA-1c). The title compound HA-1c was prepared from 5c (0.11 g, 0.265 mmol) with phenethylamine using procedure J to give a sticky oil. Yield: 47.34%.1H NMR (400 MHz, DMSO-d6) δ 1.61–1.72 (m, 3 H), 2.56 (s, 2 H), 2.77–2.95 (m, 2 H), 3.37–3.45 (m, 2 H), 5.01–5.28 (m, 4 H), 6.97–7.13 (m, 3 H), 7.26–7.51 (m, 5 H), 7.56–7.83 (m, 4 H), 10.93 (br. s., 1 H), 13C NMR (101 MHz, DMSO-d6) δ 10.21, 28.61, 45.61, 79.80, 111.43, 118.23, 118.87, 121.31, 128.89, 129.19, 129.31, 131.93, 132.52, 174.16; MS (EI+) m/z: 427.21 [M+˙]. HPLC (20 min), tR = 1.38 min, HPLC purity >88%.
N-(3-Nitrobenzyl)-6-(3-fluorophenyl)-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxamide (HA-1d). The title compound HA-1d was prepared from 5d (0.1 g, 0.224 mmol) with 3-nitrobenzyl amine using procedure J to give a sticky oil. Yield: 53.24%.1H NMR (400 MHz, DMSO-d6) δ 2.53–2.57 (m, 3 H), 2.58–2.79 (m, 2 H), 3.11 (dd, J = 16.14, 3.67 Hz, 1 H), 4.06–4.23 (m, 1 H), 4.44–4.57 (m, 2 H), 7.11–7.18 (m, 1 H), 7.40–7.47 (m, 2 H), 7.47–7.60 (m, 3 H), 7.70 (t, J = 7.83 Hz, 1 H), 7.85 (d, J = 8.31 Hz, 1 H), 8.15–8.28 (m, 2 H), 8.62–8.72 (m, 1 H), 10.97 (s, 1 H); MS (EI+) m/z: 458.18 [M+˙]. Analysis C26H23FN4O3 (458.49) calculated % C 68.11, % H 5.06, % F 4.14, % N 12.22, % O 10.47; found % C (68.151%), % H (5.116%), % N (12.453%).
N-(4-Fluorobenzyl)-6-(3-methoxyphenyl)-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxamide (HA-1e). The title compound HA-1e was prepared from 5e (0.125 g, 0.3 mmol) with 4-fluorobenzyl amine using procedure J to give a sticky oil. Yield: 43.24%.1H NMR (400 MHz, DMSO-d6) δ 1.30 (s, 3 H), 1.51–1.60 (m, 2 H), 3.49–3.57 (m, 3 H), 3.74–3.82 (m, 1 H), 3.89 (s, 1 H), 4.36–4.50 (m, 2 H), 6.89–6.98 (m, 1 H), 6.98–7.09 (m, 1 H), 7.09–7.18 (m, 1 H), 7.18–7.34 (m, 3 H), 7.35–7.53 (m, 4 H), 7.77 (d, J = 9.54 Hz, 1 H), 11.01–11.10 (m, 1 H); MS (EI+) m/z: 443.2 [M+˙]. Analysis C27H26FN3O2 (443.52) calculated % C 73.12, % H 5.91, % F 4.28, % N 9.47, % O 7.2; found % C (72.98%), % H (6.153%), % N (10.61%).
N-(4-Fluorobenzyl)-6-(4-fluorophenyl)-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxamide (HA-1f). The title compound HA-1f was prepared from 5f (0.15 g, 0.357 mmol) with 4-fluorobenzyl amine using procedure J to give a sticky oil. Yield: 53.24%.1H NMR (400 MHz, DMSO-d6) δ 1.58 (dd, J = 6.48, 3.30 Hz, 3 H), 2.52–2.57 (m, 1 H), 2.92 (s, 1 H), 4.23 (t, J = 5.87 Hz, 2 H), 5.05–5.34 (m, 2 H), 6.97–7.05 (m, 2 H), 7.11–7.17 (m, 2 H), 7.23–7.52 (m, 4 H), 7.70–7.75 (m, 2 H), 8.25 (br. s., 1 H), 10.91 (br. s., 1 H), 13C NMR (101 MHz, DMSO-d6) δ 28.58, 41.95, 80.23, 80.26, 115.10, 115. 31, 115.83, 116.04, 116.46, 120.52, 128.81, 129.38, 129.46, 136.00, 171.63; MS (EI+) m/z: 431.18 [M+˙]. HPLC (20 min), tR = 4.01 min, HPLC purity >95%.
N-(3-Fluorophenethyl)-6-(4-fluorophenyl)-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxamide (HA-1g). The title compound HA-1g was prepared from 5g (0.14 g, 0.323 mmol) with 3-fluorophenethyl amine using procedure J to give a sticky oil. Yield: 48.64%.1H NMR (400 MHz, DMSO-d6) d ppm 2.51 (s, 1.83 Hz, 3 H) 2.74–2.83 (m, 3 H) 2.89–2.97 (m, 1 H) 3.82 (dd, J = 7.21, 3.55 Hz, 1 H) 3.99–4.15 (m, 2 H) 4.40 (br. s., 1 H) 5.16 (dd, J = 8.44, 2.08 Hz, 1 H) 7.03–7.12 (m, 4 H) 7.25–7.35 (m, 4 H) 7.67–7.71 (m, 2 H) 7.96 (t, J = 5.38 Hz, 1 H) 10.85 (s, 1 H) 11.24 (d, J = 4.65 Hz, 1 H); MS (EI+) m/z: 445.2 [M+˙]. Analysis C27H25F2N3O (445.51) calculated % C 72.79, % H 5.66, % F 8.53, % N 9.48, % O 3.59; found % C (73.541%), % H (5.965%), % N (9.5661%).
N-(3-Fluorophenethyl)-6-phenyl-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxamide (HA-1h). The title compound HA-1h was prepared from 5h (0.12 g, 0.23 mmol) with 3-fluorophenethyl amine using procedure J to give a sticky oil. Yield: 55.48%.1H NMR (400 MHz, DMSO-d6) δ 1.30 (s, 3 H), 2.66–2.78 (m, 2 H), 2.82–2.92 (m, 2 H), 3.25–3.38 (m, 2 H), 4.30–4.42 (m, 1 H), 5.11–5.29 (m, 1 H), 7.09–7.20 (m, 3 H), 7.33–7.59 (m, 6 H), 7.73 (d, J = 7.09 Hz, 2 H), 7.82–7.96 (m, 1 H), 10.95 (br. s., 1 H); MS (EI+) m/z: 427.21 [M+˙]. Analysis C27H26FN3O (427.52) calculated % C 75.85, % H 6.13, % F 4.44, % N 9.83, % O 3.74; found % C (76.351%), % H (6.451%), % N (9.903%).
N-(3-Methoxybenzyl)-6-phenyl-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxamide (HA-1i). The title compound HA-1i was prepared from 5i (0.14 g, 0.34 mmol) with 3-methoxybenzyl amine using procedure J to give a sticky oil. Yield: 56.44%.1H NMR (400 MHz, DMSO-d6) δ 1.59 (d, J = 6.60 Hz, 3 H), 2.88–3.02 (m, 1 H), 3.43–3.53 (m, 2 H), 3.60 (s, 3 H), 4.18–4.25 (m, 2 H), 5.09–5.15 (m, 1 H), 6.67–6.75 (m, 3 H), 7.06–7.11 (m, 1 H), 7.29–7.34 (m, 1 H), 7.39 (s, 2 H), 7.45 (t, J = 7.64 Hz, 2 H), 7.67–7.70 (m, 2 H), 8.22 (br. s., 1 H), 10.96 (br. s., 1 H); MS (EI+) m/z: 425.21 [M+˙]. Analysis C27H27N3O2 (425.53) calculated % C 76.21, % H 6.4, % N 9.87, % O 7.52; found % C (76.349%), % H (6.46%), % N (10.609%).
5-Bromo-1H-indole-3-carbaldehyde (7). The compound was prepared from 0.5 g 5-bromo-1H-indole (1.1) (2.55 mmol), 1.5 ml dimethylformamide (DMF), and 0.5 ml phosphorus oxychloride (POCl3) (5.65 mmol) as described in general procedure A. The final product was obtained as a white solid. Yield of 100% (0.577 g); mp: 78 °C; 1H NMR (400 MHz, DMSO-d6) d ppm 7.41 (dd, J = 8.56, 1.71 Hz, 1 H) 7.51 (d, J = 8.56 Hz, 1 H) 8.19–8.30 (m, 1 H) 8.36 (s, 1 H) 9.94 (s, 1 H) 12.28 (br. s., 1 H).
5-(3-Fluorophenyl)-1H-indole-3-carbaldehyde (8a). The compound was prepared from 0.5 g 5-bromo-1H-indole-3-carbaldehyde (1.2), tetrakis(triphenylphosphine)palladium(0) (0.03 equivalent), and 3-fluorophenyl boronic acid (1.2 equivalents) in 10 ml methanol, 20 mL toluene, and 2 mL potassium carbonate aqueous solution (2 equivalents) as described in general procedure I. The final product was obtained as a white solid. Yield of 85%; mp: 99–101 °C; 1H NMR (400 MHz, DMSO-d6) d ppm 7.19 (d, J = 2.20 Hz, 1 H) 7.43–7.58 (m, 3 H) 7.58–7.67 (m, 2 H) 8.27–8.49 (m, 2 H) 10.00 (s, 1 H) 12.27 (br. s., 1 H).
5-(4-Fluorophenyl)-1H-indole-3-carbaldehyde (8b). The compound was prepared from 0.5 g 5-bromo-1H-indole-3-carbaldehyde, tetrakis(triphenylphosphine)palladium(0) (0.03 equivalent), and 4-fluorophenyl boronic acid (1.2 equivalents) in 10 ml methanol, 20 ml toluene, and 2 mL potassium carbonate aqueous solution (2 equivalents) as described in general procedure I. The final product was obtained as a white solid. Yield: 89.65%; mp: 103–105 °C; 1H NMR (400 MHz, DMSO-d6) d ppm 7.16 (t, J = 9.05 Hz, 1 H) 7.26–7.32 (m, 1 H) 7.49–7.63 (m, 2 H) 7.68–7.72 (m, 1 H) 7.85 (dd, J = 8.31, 6.60 Hz, 1 H) 8.12 (s, 1 H) 8.34 (d, J = 12.72 Hz, 1 H) 9.85–10.12 (m, 1 H) 12.24 (br. s., 1 H).
5-(Phenyl)-1H-indole-3-carbaldehyde (8c). The compound was prepared from 0.5 g 5-bromo-1H-indole-3-carbaldehyde, tetrakis(triphenylphosphine)palladium(0) (0.03 equivalent), and phenyl boronic acid (1.2 equivalents) in 10 ml methanol, 20 ml toluene, and 2 mL potassium carbonate aqueous solution (2 equivalents) as described in general procedure I. The final product was obtained as a white solid. Yield: 91.2%; mp: 112–115 °C.
5-(3-Trifluoromethylphenyl)-1H-indole-3-carbaldehyde (8d). The compound was prepared from 0.5 g 5-bromo-1H-indole-3-carbaldehyde, tetrakis(triphenylphosphine)palladium (0) (0.03 equivalent), and 3-trifluoromethylphenyl boronic acid (1.2 equivalents) in 10 mL methanol, 20 mL toluene, and 2 mL potassium carbonate aqueous solution (2 equivalents) as described in general procedure I. The final product was obtained as a white solid. Yield: 92.4%; mp: 98–101 °C.
5-(3,4-Dimethoxyphenyl)-1H-indole-3-carbaldehyde (8e). The compound was prepared from 0.5 g 5-bromo-1H-indole-3-carbaldehyde, tetrakis(triphenylphosphine)palladium(0) (0.03 equivalent), and 3,4-dimethoxyphenyl boronic acid (1.2 equivalents) in 10 ml methanol, 20 mL toluene, and 2 mL potassium carbonate aqueous solution (2 equivalents) as described in general procedure I. The final product was obtained as a white solid. Yield: 88.3%; mp: 106–109 °C.
5-(3-Trifluoromethoxyphenyl)-1H-indole-3-carbaldehyde (8f). The compound was prepared from 0.5 g 5-bromo-1H-indole-3-carbaldehyde, tetrakis(triphenylphosphine)palladium(0) (0.03 equivalent), and 3-trifluoromethoxyphenyl boronic acid (1.2 equivalents) in 10 ml methanol, 20 mL toluene, and 2 mL potassium carbonate aqueous solution (2 equivalents) as described in general procedure I. The final product was obtained as a white solid. Yield: 94.7%; mp: 91–95 °C.
(E)-{[5-(3,4-dimethoxyphenyl)-1H-indol-3-yl]methylidene}[2-(3-methoxyphenyl)ethyl]amine (HA-2a). The title compound HA-2a was prepared from 8e (0.2 g, 0.71 mmol) with 3-methoxyphenethyl amine using procedure C to give a white solid. Yield: 70.78%; mp: 118–122 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.07 (t, J = 5.43 Hz, 2 H), 3.69–3.72 (m, 5 H), 3.73 (s, 3 H), 3.79 (s, 3 H), 6.75 (t, J = 2.44 Hz, 1 H), 6.87 (dt, J = 8.18, 2.84 Hz, 1 H), 6.91–6.95 (m, 1 H), 6.97 (d, J = 8.79 Hz, 1 H), 7.18–7.22 (m, 1 H), 7.32 (d, J = 1.83 Hz, 1 H), 7.51–7.55 (m, 3 H), 7.63 (s, 1 H), 7.89–7.90 (m, 1 H), 8.17 (s, 1 H), 13C NMR (101 MHz, DMSO-d6) δ 23.09, 35.68, 55.36, 56.04, 111.14, 112.02, 112.75, 113.20, 114.65, 118.80, 119.48, 121.32, 123.30, 125.27, 129.19, 129.31, 129.79, 132.02, 132.54 (d, J = 2.20 Hz, 1 C), 134.59, 135.33, 136.79, 139.46, 148.56, 149.51, 185.50; MS (EI+) m/z: 414.19 [M+˙]. HPLC (20 min), tR = 1.12 min, HPLC purity >97%.
(E)-N-cyclohexyl-1-(5-(4-fluorophenyl)-1H-indol-3-yl)methanimine (HA-2b). The title compound HA-2b was prepared from 8b (0.18 g, 0.753 mmol) with cyclohexyl amine using procedure C to give a red solid. Yield: 68.52%; mp: 112–116 °C. 1H NMR (400 MHz, DMSO-d6) δ 1.32–1.51 (m, 3 H), 1.52–1.59 (m, 3 H), 1.66–1.71 (m, 1 H), 1.72–1.76 (m, 1 H), 1.93 (q, J = 2.81 Hz, 1 H), 1.97 (q, J = 2.83 Hz, 1 H), 3.30 (t, J = 10.19 Hz, 1 H), 7.25 (ddd, J = 8.77, 6.82, 1.50 Hz, 2 H), 7.52–7.59 (m, 2 H), 7.59–7.63 (m, 2 H), 7.73 (s, 1 H), 7.94 (d, J = 2.62 Hz, 1 H), 8.27 (s, 1 H), 13C NMR (101 MHz, DMSO-d6) δ 11.28, 24.73, 25.08, 25.73, 25.89, 30.89, 33.00, 69.56, 112.63, 114.84, 115.98, 116.19, 120.19, 122.14, 128.93, 129.01, 129.19, 132.28, 136.94, 137.01, 153.53; MS (EI+) m/z: 320.17 [M+˙]. Analysis C21H21FN2 (320.41) calculated % C 78.72, % H 6.61, % F 5.93, % N 8.74; found % C (78.121%), % H (6.43%), % N (7.931%).
(E)-N-(3,4-dimethoxybenzyl)-1-(5-phenyl-1H-indol-3-yl)methanimine (HA-2c). The title compound HA-2c was prepared from 8c (0.22 g, 0.995 mmol) with 3,4-dimethoxybenzyl amine using procedure C to give a green solid. Yield: 77.23%; mp: 117–119 °C. 1H NMR (400 MHz, DMSO-d6) δ ppm 3.73–3.76 (m, 6 H) 4.67 (d, J = 13.69 Hz, 2 H) 6.87–6.93 (m, 3 H) 6.99–7.07 (m, 2 H) 7.25–7.50 (m, 5 H) 7.63 (dd, J = 8.31, 1.22 Hz, 1 H) 7.77 (dd, J = 7.95, 1.59 Hz, 1 H) 7.83–7.87 (m, 1 H) 8.51–8.59 (m, 1 H); MS (EI+) m/z: 370.17 [M+˙]. Analysis C24H22N2O2 (370.45) calculated % C 77.81, % H 5.99, % N 7.56, % O 8.64; found % C (78.06%), % H (6.131%), % N (7.343%).
(E)‐{[5‐(3,4‐dimethoxyphenyl)‐1H‐indol‐3‐yl]methylidene}[2‐(2‐dimethylaminoethyl)ethyl]amine (HA-2d). The title compound HA-2d was prepared from 8e (0.2 g, 0.71 mmol) with N,N-dimethylethane-1,2-diamine using procedure C to give an off-white solid. Yield: 75.43%; mp: 107–109 °C. 1H NMR (400 MHz, DMSO-d6) δ ppm 2.15 (s, 3 H) 2.12 (s, 3 H) 2.26 (t, J = 6.72 Hz, 1 H) 2.32–2.39 (m, 1 H) 2.73 (br. s., 1 H) 3.54 (t, J = 6.72 Hz, 1 H) 3.79–3.82 (m, 3 H) 3.85–3.88 (m, 3 H) 7.00–7.09 (m, 1 H) 7.13–7.22 (m, 2 H) 7.44–7.48 (m, 1 H) 7.51–7.62 (m, 2 H) 8.28–8.32 (m, 1 H) 8.46 (d, J = 17.36 Hz, 1 H) 9.97 (s, 1 H), 13C NMR (101 MHz, DMSO-d6) δ ppm 22.73, 22.93, 24.83, 39.19, 39.39, 39.60, 39.81, 40.02, 40.23, 44.61, 44.95, 56.02, 111.03, 112.75, 113.30, 118.70, 118.72, 119.52, 123.31, 125.16, 134.49, 135.34, 136.77, 139.65, 148.51, 149.44, 185.92; MS (EI+) m/z: 351.19 [M+˙]. HPLC (20 min), tR = 1.08 min, HPLC purity >98%.
N-(4-Fluorobenzyl)-5-(4-fluorophenyl)-1H-indole-3-carboxamide (HA-2e). The title compound HA-2e was prepared from 9b (0.2 g, 0.784 mmol) with 4-fluorobenzyl amine using procedure E to give a beige solid. Yield: 70.43%; mp: 115–119 °C; 1H NMR (400 MHz, DMSO-d6) δ ppm 4.23 (d, J = 5.87 Hz, 2 H) 7.11–7.19 (m, 2 H) 7.25–7.36 (m, 3 H) 7.52–7.64 (m, 2 H) 7.71 (dd, J = 8.44, 5.50 Hz, 2 H) 8.30–8.37 (m, 2 H) 9.93–10.11 (s, 1 H) 12.24 (br. s., 1 H), 13C NMR (101 MHz, DMSO-d6) δ 42.58, 114.41, 115.26–115.91, 115.33, 115.49, 115.54, 115.70, 122.39, 123.67, 124.92, 129.60, 129.68, 130.55, 130.63, 130.88–131.43, 130.88–131.43, 131.19, 135.29, 136.96, 166.70–167.18, 166.70–167.18, 167.21–167.33; MS (EI+) m/z: 362.12 [M+˙]. Analysis C22H16F2N2O (362.38) calculated % C 72.92, % H 4.45, % F 10.49, % N 7.73, % O 4.41; found % C (73.06%), % H (5.56%), % N (7.843%).
N-(3-Fluorophenethyl)-5-(3-fluorophenyl)-1H-indole-3-carboxamide (HA-2f). The title compound HA-2f was prepared from 9a (0.2 g, 0.784 mmol) with 3-fluorophenethyl amine using procedure E to give a white solid. Yield: 92.23%; mp: 105–108 °C. 1H NMR (400 MHz, DMSO-d6) δ 4.22–4.37 (m, 2 H), 4.51 (d, J = 5.87 Hz, 2 H), 6.84–6.91 (m, 2 H), 6.94–7.02 (m, 3 H), 7.26–7.36 (m, 3 H), 7.48 (d, J = 8.56 Hz, 1 H), 8.19 (d, J = 2.69 Hz, 1 H), 8.38 (s, 1 H), 8.60 (t, J = 5.99 Hz, 1 H), 11.85 (br. s., 1 H); MS (EI+) m/z: 376.14 [M+˙]. Analysis C23H18F2N2O (376.41) calculated % C 73.39, % H 4.82, % F 10.09, % N 7.44, % O 4.25; found % C (73.66%), % H (5.06%), % N (7.643%).
N-(3-Nitrobenzyl)-5-(3-fluorophenyl)-1H-indole-3-carboxamide (HA-2g). The title compound HA-2g was prepared from 9a (0.2 g, 0.784 mmol) with 3-nitrobenzyl amine using procedure E to give a yellow solid. Yield: 95.11%; mp: 115–121 °C. 1H NMR (400 MHz, DMSO-d6) δ 4.68 (d, J = 5.87 Hz, 2 H), 7.19 (t, J = 7.34 Hz, 1 H), 7.49–7.62 (m, 4 H), 7.70 (t, J = 7.95 Hz, 1 H), 7.88 (d, J = 7.58 Hz, 1 H), 8.16 (s, 1 H), 8.19 (d, J = 2.93 Hz, 1 H), 8.27 (s, 1 H), 8.48 (s, 1 H), 8.77 (d, J = 6.60 Hz, 1 H), 11.71–11.90 (br. s., 1 H), 13C NMR (101 MHz, DMSO-d6) δ 41.90, 111.06, 112.99, 113.67, 119.69, 121.87, 122.15–122.19, 122.15–122.19, 122.17, 123.29, 129.64, 130.29–130.36, 130.32, 131.13–131.27, 131.20, 134.54, 134.51, 136.54, 143.45, 144.76, 148.29, 165.13; MS (EI+) m/z: 389.12 [M+˙]. HPLC (20 min), tR = 1.11 min, HPLC purity >96%.
N-(3-Fluorophenethyl)-5-(3,4-dimethoxyphenyl)-1H-indole-3-carboxamide (HA-2h). The title compound HA-2h was prepared from 9e (0.15 g, 0.51 mmol) with 3-fluorophenethyl amine using procedure E to give a yellow solid. Yield: 77.36%; mp: 98–101 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.08 (t, J = 7.09 Hz, 2 H), 3.24–3.33 (m, 2 H), 3.83–3.86 (s, 3 H), 3.90 (s, 3 H), 7.06–7.14 (m, 2 H), 7.22–7.28 (m, 2 H), 7.32–7.44 (m, 1 H), 7.58–7.64 (m, 2 H), 7.65–7.70 (m, 3 H), 7.83 (d, J = 8.56 Hz, 1 H), 8.18 (s, 1 H), 10.71–10.80 (br. s., 1 H), 13C NMR (101 MHz, DMSO-d6) δ 44.72, 55.99, 56.02, 110.31–110.41, 112.66–112.74, 118.78, 118.88, 125.27–125.35, 125.51–125.60, 129.20, 129.16–129.22, 129.16–129.22, 129.32, 129.28–129.33, 129.28–129.33, 131.90–132.04, 131.90–132.04, 131.90–132.04, 131.90–132.04, 131.90–132.04, 131.96–132.06, 132.02, 133.70, 148.65–148.71, 149.52–149.62, 161.43–161.56; MS (EI+) m/z: 418.17 [M+˙]. HPLC (20 min), tR = 3.32 min, HPLC purity >96%.
N-(3-Nitrobenzyl)-5-(3-(trifluoromethyl) phenyl)-1H-indole-3-carboxamide (HA-2i). The title compound HA-2i was prepared from 9d (0.19 g, 0.62 mmol) with 3-nitrobenzyl amine using procedure E to give a beige solid. Yield: 81.44%; mp: 105–108 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.95 (s, 2 H), 7.29–7.37 (m, 3 H), 7.44–7.51 (m, 3 H), 7.64 (t, J = 7.83 Hz, 1 H), 8.06 (s, 2 H), 8.13–8.16 (m, 3 H), 8.31 (s, 1 H), 11.92–12.13 (br. s., 1 H), 13C NMR (101 MHz, DMSO-d6) δ 44.35–44.44, 49.04–49.13, 49.04–49.13, 49.04–49.13, 49.08, 55.36–55.46, 107.66–107.75, 114.22–114.30, 114.27, 114.74–114.86, 114.74–114.86, 114.80, 123.13–123.24, 123.13–123.24, 123.18, 125.12–125.23, 125.18, 128.25–128.33, 130.04–130.13, 133.91–134.05, 133.97, 135.54–135.70, 166.08–166.22 (m, 1 C); MS (EI+) m/z: 439.11 [M+˙]. HPLC (20 min), tR = 1.08 min, HPLC purity >95%.
N-(3,4-Dimethoxybenzyl)-5-phenyl-1H-indole-3-carboxamide (HA-2j). The title compound HA-2j was prepared from 9c (0.22 g, 0.93 mmol) with 3,4-dimethoxybenzyl amine using procedure E to give an orange solid. Yield: 79.89%; mp: 113–118 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.73 (s, 3 H), 3.80 (s, 3 H), 4.45 (s, 2 H), 6.63 (d, J = 8.42 Hz, 1 H), 6.76 (d, J = 2.69 Hz, 1 H), 6.83 (dd, J = 8.45, 2.72 Hz, 1 H), 7.40–7.52 (m, 3 H), 7.52–7.55 (m, 1 H), 7.56–7.62 (m, 2 H), 7.68 (dt, J = 8.06, 2.17 Hz, 1 H), 7.94 (s, 1 H), 8.16 (d, J = 2.32 Hz, 1 H); MS (EI+) m/z: 386.16 [M+˙]. Analysis C24H22N2O3 (386.45) calculated % C 74.59, % H 5.74, % N 7.25, % O 12.42; found % C (74.69%), % H (5.8%), % N (7.468%).
N-(3-Methoxybenzyl)-5-(3-(trifluoromethyl)phenyl)-1H-indole-3-carboxamide (HA-2k). The title compound HA-2k was prepared from 9d (0.23 g, 0.75 mmol) with 3-methoxybenzyl amine using procedure E to give a beige solid. Yield: 77.44%; mp: 99–101 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.75–3.83 (s, 3 H), 4.67 (d, J = 5.62 Hz, 2 H), 6.48 (d, J = 5.38 Hz, 1 H), 6.89 (d, J = 7.83 Hz, 1 H), 7.01–7.13 (m, 2 H), 7.29–7.35 (m, 1 H), 7.85 (d, J = 4.89 Hz, 1 H), 7.96 (d, J = 8.56 Hz, 1 H), 8.15 (d, J = 8.80 Hz, 1 H), 8.26 (s, 2 H), 8.41 (d, J = 5.38 Hz, 1 H), 8.79 (s, 1 H); MS (EI+) m/z: 424.14 [M+˙]. Analysis C25H23FN2O3 (424.42) calculated % C 67.92, % H 4.51, % F 13.43, % N 6.6, % O 7.54; found % C (68.561%), % H (4.598%), % N (6.659%).
N-(4-Fluorobenzyl)-5-(3-fluorophenyl)-1H-indole-3-carboxamide (HA-2l). The title compound HA-2l was prepared from 9a (0.2 g, 0.784 mmol) with 4-fluorobenzyl amine using procedure E to give a yellow solid. Yield: 90.21%; mp: 120–122 °C; 1H NMR (400 MHz, DMSO-d6) δ 4.50 (d, J = 5.75 Hz, 2 H), 7.12–7.22 (m, 3 H), 7.41 (dd, J = 8.31, 5.75 Hz, 2 H), 7.45–7.57 (m, 5 H), 8.14 (d, J = 2.69 Hz, 1 H), 8.46 (s, 1 H), 8.57 (t, J = 5.93 Hz, 1 H), 11.72 (br. s., 1 H); MS (EI+) m/z: 362.12 [M+˙]. HPLC (20 min), tR = 1.72 min, HPLC purity >95%.
N-(3-Methoxybenzyl)-5-(3-fluorophenyl)-1H-indole-3-carboxamide (HA-2m). The title compound HA-2m was prepared from 9a (0.2 g, 0.784 mmol) with 3-methoxybenzyl amine using procedure E to give a reddish brown solid. Yield: 78.71%; mp: 105–107 °C; 1H NMR (400 MHz, DMSO-d6) δ 3.74 (s, 3 H), 4.49 (d, J = 5.87 Hz, 2 H), 6.80–6.84 (m, 1 H), 6.92–6.97 (m, 2 H), 7.12–7.20 (m, 1 H), 7.26 (t, J = 8.01 Hz, 1 H), 7.45–7.57 (m, 5 H), 8.15 (d, J = 2.69 Hz, 1 H), 8.46 (s, 1 H), 8.54 (t, J = 5.99 Hz, 1 H), 11.69–11.77 (br. s., 1 H); MS (EI+) m/z: 374.14 [M+˙]. HPLC (20 min), tR = 1.53 min, HPLC purity >97%.
N-(3-Nitrobenzyl)-5-(3-(trifluoromethoxy)phenyl)-1H-indole-3-carboxamide (HA-2n). The title compound HA-2l was prepared from 9f (0.25 g, 0.78 mmol) with 3-nitrobenzyl amine using procedure E to give a yellowish white solid. Yield: 75.21%; mp: 88–91 °C. 1H NMR (400 MHz, DMSO-d6) δ 3.13 (br. s., 2 H), 7.44–7.48 (m, 3 H), 7.49–7.55 (m, 2 H), 7.55–7.63 (m, 2 H), 7.76–7.80 (m, 3 H), 8.09 (d, J = 2.93 Hz, 1 H), 8.28 (s, 1 H), 11.94–11.99 (br. s., 1 H), 13C NMR (101 MHz, DMSO-d6) δ 41.85–41.97, 108.20, 113.32, 119.30, 119.51–119.78, 121.82–122.01, 121.82–122.01, 121.82–122.01, 121.95, 122.06, 122.19, 127.08, 128.97, 129.00–129.12, 129.05, 129.32, 130.30, 132.61, 133.73, 136.69, 141.41, 147.74, 166.39; MS (EI+) m/z: 455.11 [M+˙]. Analysis C23H16F3N3O4 (455.39) calculated % C 60.66, % H 3.54, % F 12.52, % N 9.23, % O 14.05; found C (59.937%), % H (3.391%), % N (13.887%).
4-Chloro-6-phenylquinoline (11a). The compound was prepared from 0.5 g 6-bromo-4-chloroquinoline (10), tetrakis(triphenylphosphine)palladium(0) (0.03 equivalent), and phenyl boronic acid (1 equivalent) in 10 ml methanol, 20 ml toluene, and 2 ml potassium carbonate aqueous solution (2 equivalents) as described in general procedure B. Column chromatography was performed using silica gel with stepwise gradient elution (n-hexane/ethyl acetate (100
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 95
0, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 90
5, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 77.5
10, 77.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.5)). At the 12.5% ethyl acetate mark, the purest final product could be collected as a white solid. The yield is 85–92%; mp: 79–81 °C; 1H NMR (400 MHz, methanol-d4) δ ppm 7.14–7.23 (m, 2 H) 7.24–7.35 (m, 3 H) 7.37–7.43 (m, 1 H) 7.53–7.57 (m, 1 H) 7.84–7.91 (m, 2 H) 8.10 (d, J = 1.47 Hz, 1 H).
12.5)). At the 12.5% ethyl acetate mark, the purest final product could be collected as a white solid. The yield is 85–92%; mp: 79–81 °C; 1H NMR (400 MHz, methanol-d4) δ ppm 7.14–7.23 (m, 2 H) 7.24–7.35 (m, 3 H) 7.37–7.43 (m, 1 H) 7.53–7.57 (m, 1 H) 7.84–7.91 (m, 2 H) 8.10 (d, J = 1.47 Hz, 1 H).
4-Chloro-6-(4-fluorophenyl)quinoline (11b). The compound was prepared from 0.5 g 6-bromo-4-chloroquinoline (10), tetrakis(triphenylphosphine)palladium(0) (0.03 equivalent), and 4-fluorophenylboronic acid (1 equivalent) in 10 ml methanol, 20 ml toluene, and 2 ml potassium carbonate aqueous solution (2 equivalents) as described in general procedure B. Column chromatography was performed using silica gel with stepwise gradient elution (n-hexane/ethyl acetate (100
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 95
0, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 90
5, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 77.5
10, 77.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.5)). At the 12.5% ethyl acetate mark, the purest final product could be collected. The final product is a white solid. The yield is 83%; mp: 138–140 °C; 1H NMR (500 MHz, DMSO-d6) δ ppm 406.37 (t, J = 8.82 Hz, 2 H) 406.80 (d, J = 4.81 Hz, 1 H) 406.89 (dd, J = 8.81, 5.61 Hz, 2 H) 407.17 (s, 2 H) 407.32 (s, 1 H) 407.83 (d, J = 4.81 Hz, 1 H).
12.5)). At the 12.5% ethyl acetate mark, the purest final product could be collected. The final product is a white solid. The yield is 83%; mp: 138–140 °C; 1H NMR (500 MHz, DMSO-d6) δ ppm 406.37 (t, J = 8.82 Hz, 2 H) 406.80 (d, J = 4.81 Hz, 1 H) 406.89 (dd, J = 8.81, 5.61 Hz, 2 H) 407.17 (s, 2 H) 407.32 (s, 1 H) 407.83 (d, J = 4.81 Hz, 1 H).
4-Chloro-6-(thiophen-3-yl)quinoline (11c). The compound was prepared from 0.5 g 6-bromo-4-chloroquinoline (10), tetrakis(triphenylphosphine)palladium(0) (0.03 equivalent), and thiophen-3-ylboronic acid (1 equivalent) in 10 ml methanol, 20 ml toluene, and 2 ml potassium carbonate aqueous solution (2 equivalents) as described in general procedure B. Column chromatography was performed using silica gel with stepwise gradient elution (n-hexane/ethyl acetate (100
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 95
0, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 90
5, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 77.5
10, 77.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.5)). At the 12.5% ethyl acetate mark, the purest final product could be collected. The final product is a white solid. The yield is 84%; mp: 145–147 °C; 1H NMR (500 MHz, DMSO-d6) δ ppm 6.88–6.94 (m, 3 H) 7.28 (d, J = 8.82 Hz, 1 H) 7.33–7.35 (m, 1 H) 7.42 (dd, J = 8.82, 1.60 Hz, 1 H) 7.55 (d, J = 1.60 Hz, 1 H) 7.96 (d, J = 4.01 Hz, 1 H).
12.5)). At the 12.5% ethyl acetate mark, the purest final product could be collected. The final product is a white solid. The yield is 84%; mp: 145–147 °C; 1H NMR (500 MHz, DMSO-d6) δ ppm 6.88–6.94 (m, 3 H) 7.28 (d, J = 8.82 Hz, 1 H) 7.33–7.35 (m, 1 H) 7.42 (dd, J = 8.82, 1.60 Hz, 1 H) 7.55 (d, J = 1.60 Hz, 1 H) 7.96 (d, J = 4.01 Hz, 1 H).
4-Chloro-6-(4-(trifluoromethyl)phenyl)quinoline. The compound was prepared from 0.5 g 6-bromo-4-chloroquinoline (10), tetrakis(triphenylphosphine)palladium(0) (0.03 equivalent), and 4-(trifluoromethyl)phenyl boronic acid (1 equivalent) in 10 ml methanol, 20 ml toluene, and 2 ml potassium carbonate aqueous solution (2 equivalents) as described in general procedure B. Column chromatography was performed using silica gel with stepwise gradient elution (n-hexane/ethyl acetate (100
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 95
0, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 90
5, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 77.5
10, 77.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12.5)). At the 12.5% ethyl acetate mark, the purest final product could be collected. The final product is a white solid. The yield is 85–92%; 1H NMR (500 MHz, DMSO-d6) δ ppm 7.78–7.86 (m, 1 H) 7.89 (d, J = 7.21 Hz, 2 H) 8.08 (d, J = 8.01 Hz, 2 H) 8.18–8.29 (m, 2 H) 8.43 (d, J = 4.01 Hz, 1 H) 8.88 (dd, J = 4.41, 2.00 Hz, 1 H).
12.5)). At the 12.5% ethyl acetate mark, the purest final product could be collected. The final product is a white solid. The yield is 85–92%; 1H NMR (500 MHz, DMSO-d6) δ ppm 7.78–7.86 (m, 1 H) 7.89 (d, J = 7.21 Hz, 2 H) 8.08 (d, J = 8.01 Hz, 2 H) 8.18–8.29 (m, 2 H) 8.43 (d, J = 4.01 Hz, 1 H) 8.88 (dd, J = 4.41, 2.00 Hz, 1 H).
N,6-Diphenylquinolin-4-amine (HA-3a). The compound was prepared from 1 equivalent of 4-chloro-6-phenylquinoline, 1.1 equivalents of aniline, 0.04 equivalent of Xphos, 0.04 equivalent of palladium(II) acetate (Pd(OAc)2), and 2.5 equivalents of cesium carbonate as described in general procedure F. The final product is a beige solid. Yield: 63%; mp: 227–229 °C; 1H NMR (500 MHz, DMSO-d6) δ 6.58 (d, J = 4.81 Hz, 1 H), 7.06–7.12 (m, 1 H), 7.19–7.49 (m, 5 H), 7.51–7.54 (t, J = 8.02 Hz, 2 H), 7.65–7.69 (m, 2 H), 7.72 (d, J = 8.01 Hz, 1 H), 7.9 (dd, J = 8.81, 2.40 Hz, 1 H), 8.17 (d, J = 4.81 Hz, 1 H), 8.42 (d, J = 2.41 Hz, 1 H), 8.75 (s, 1 H); MS (EI+) m/z: 360.14 [M+˙]. Analysis C22H18N2 (310.15) calculated % C 85.13, % H 5.85, % N 9.03; found % C (85.221%), % H (5.301%), % N (10.566%).
N-(3-Fluorophenethyl)-6-(4-fluorophenyl)quinolin-4-amine (HA-3b). The compound was prepared from 1 equivalent of 4-chloro-6-(4-fluorophenyl)quinoline, 1.1 equivalents of 2-(3-fluorophenyl)ethanamine, 0.04 equivalent of Xphos, 0.04 equivalent of palladium(II) acetate (Pd(OAc)2), and 2.5 equivalents of cesium carbonate with 10 ml toluene under argon as described in general procedure F. The final product is a grey solid. Yield: 64%; mp: 213–216 °C; 1H NMR (400 MHz, DMSO-d6) δ 3.07 (t, J = 7.46 Hz, 2 H), 3.58–3.65 (m, 2 H), 6.64 (d, J = 5.38 Hz, 1 H), 7.10 (td, J = 8.62, 2.20 Hz, 1 H), 7.19–7.29 (m, 2 H), 7.36–7.45 (m, 3 H), 7.59 (t, J = 5.32 Hz, 1 H), 7.87–8.01 (m, 4 H), 8.46 (d, J = 5.38 Hz, 1 H), 8.51–8.57 (s, 1 H), 13C NMR (101 MHz, DMSO-d6) δ 99.22, 113.33–113.67, 115.86–116.41, 119.31, 119.75, 125.48, 128.32, 129.34–129.67, 130.60–130.81, 135.19, 136.69, 142.80, 147.43, 150.66–150.82; MS (EI+) m/z: 360.14 [M+˙]. HPLC (20 min), tR = 3.32 min, HPLC purity >96%.
N-(4-Fluorobenzyl)-6-(thiophen-3-yl)quinolin-4-amine (HA-3c). The compound was prepared from 1 equivalent of 4-chloro-6-(thiophen-3-yl)quinoline, 1.1 equiv. of 4-(fluorophenyl)methanamine, 0.04 equivalent of Xphos, 0.04 equivalent of palladium(II) acetate (Pd(OAc)2), and 2.5 equivalents of cesium carbonate with 10 ml toluene under argon as described in general procedure F. The final product is a brown solid. Yield: 74%; mp: 224–226 °C; 1H NMR (400 MHz, DMSO-d6) δ 4.59 (d, J = 5.87 Hz, 2 H), 6.37 (d, J = 5.38 Hz, 1 H), 7.18 (t, J = 8.93 Hz, 2 H), 7.47 (dd, J = 8.56, 5.62 Hz, 2 H), 7.74 (dd, J = 4.89, 2.93 Hz, 1 H), 7.80–7.84 (m, 2 H), 8.01–8.07 (m, 3 H), 8.30 (d, J = 5.38 Hz, 1 H), 8.64–8.67 (s, 1 H), 13C NMR (101 MHz, DMSO-d6) δ 99.78, 115.47–115.93, 118.80, 119.52, 121.59, 126.98, 127.64, 127.80, 129.21–129.64, 130.11, 131.20–131.44, 135.47, 141.82, 148.03, 150.22, 150.87; MS (EI+) m/z: 348 [M+˙]. Analysis C21H17FN2S (348.44) calculated % C 72.39, % H 4.92, % F 5.45, % N 8.04, % S 9.2; found % C (72.56%), % H (5.12%), % N (7.943%).
N-(2-(Thiophen-2-yl)ethyl)-6-(4-fluorophenyl)quinolin-4-amine (HA-3d). The compound was prepared from 1 equivalent of 4-chloro-6-(4-fluorophenyl)quinoline, 1.1 equivalents of 2-thiophenethyl amine, 0.04 equivalent of Xphos, 0.04 equiv. of palladium(II) acetate (Pd(OAc)2), and 2.5 equivalents of cesium carbonate with 10 ml toluene under argon as described in general procedure F. The final product is a brown solid. Yield: 74%; mp: 224–226 °C; 1H NMR (400 MHz, DMSO-d6) δ 3.28 (t, J = 7.09 Hz, 2 H), 3.65 (q, J = 6.72 Hz, 2 H), 6.62 (d, J = 5.38 Hz, 1 H), 7.01–7.06 (m, 2 H), 7.39–7.45 (m, 3 H), 7.72 (t, J = 4.89 Hz, 1 H), 7.89–7.96 (m, 3 H), 7.98–8.03 (m, 1 H), 8.46 (d, J = 5.38 Hz, 1 H), 8.57 (s, 1 H), 13C NMR (101 MHz, DMSO-d6) δ 28.63, 29.52, 44.61, 99.08, 116.08–116.54, 119.15, 119.88, 124.80, 126.01, 127.50, 128.78, 129.51, 135.47, 136.54, 141.71, 149.95, 151.19; MS (EI+) m/z: 348.11 [M+˙]. Analysis C21H17FN2S (348.44) calculated % C 72.39, % H 4.92, % F 5.45, % N 8.04, % S 9.2; found % C (72.356%), % H (4.53%), % N (8.567%).
N-(4-Fluorobenzyl)-6-(4-fluorophenyl)quinolin-4-amine (HA-3e). The compound was prepared from 1 equivalent of 4-chloro-6-(4-fluorophenyl)quinoline, 1.1 equivalents of 4-fluorobenzyl amine, 0.04 equivalent of Xphos, 0.04 equiv. of palladium(II) acetate (Pd(OAc)2), and 2.5 equivalents of cesium carbonate with 10 ml toluene under argon as described in general procedure F. The final product is a yellow solid. Yield: 73%; mp: 254–256 °C; 1H NMR (400 MHz, DMSO-d6) δ 4.62 (d, J = 5.62 Hz, 2 H), 6.42 (d, J = 5.38 Hz, 1 H), 7.20 (t, J = 8.74 Hz, 2 H), 7.41 (t, J = 8.68 Hz, 2 H), 7.50 (t, J = 8.40 Hz, 2 H), 7.89 (d, J = 8.80 Hz, 1 H), 7.92–8.02 (m, 3 H), 8.19 (t, J = 5.38 Hz, 1 H), 8.36 (d, J = 5.26 Hz, 1 H), 8.65 (s, 1 H), 13C NMR (101 MHz, DMSO-d6) δ 45.34, 99.76, 115.50–115.85, 116.11, 116.33, 119.42–119.80, 128.17, 129.33–129.53, 129.97, 135.16–135.52, 136.74, 147.82, 150.51, 150.95; MS (EI+) m/z: 346.13 [M+˙]. HPLC (20 min), tR = 1.05 min, HPLC purity >98%.
4.2. Biological evaluation
4.3. Molecular docking
The two most active compounds HA-1e and HA-2l belonging to the betacarboline and the indole amide series, respectively, were docked into the ATP site of the mTOR kinase. Molecular docking was performed using the FRED module of OpenEye software 2023.2.3.34 Receptor grids were generated using the make-receptor command as implemented in FRED. Then, a conformer library of each ligand was prepared using the OE Omega module with a maximum number of 100 conformers per ligand. The crystal structure of the mTOR kinase (PDB ID: 4JSX) was employed for the molecular docking procedure. The docking box was centered on the sidechain nitrogen of Lys2187, with a volume of 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 Å2. The Chemgauss4 scoring function was applied in the FRED module to rank the docked poses based on shape complementarity. The score comprises the following terms: steric, acceptor, donors, coordinating groups, metals, lone pairs, polar hydrogens and chelator coordinating groups. Poses were visualized using the Pymol 2.5.5 software module.
560 Å2. The Chemgauss4 scoring function was applied in the FRED module to rank the docked poses based on shape complementarity. The score comprises the following terms: steric, acceptor, donors, coordinating groups, metals, lone pairs, polar hydrogens and chelator coordinating groups. Poses were visualized using the Pymol 2.5.5 software module.
Author contributions
Conceptualization, M. A. H. and R. K. A.; data curation, A. E. and H. S.; formal analysis, all authors; funding acquisition, M. A. H. and R. K. A.; methodology, A. E. and A. A.; writing – original draft, all authors; writing – review & editing, all authors.Conflicts of interest
The authors declare no competing interests.Data availability
All data associated with the manuscript are available upon reasonable request from the corresponding authors.Supplementary information: NMR spectra and the HPLC chromatograms are included in the SI. Supplementary data to this article can be found online. See DOI: https://doi.org/10.1039/d5md00641d.
Acknowledgements
This study has been funded by grant number 28936, Science, Technology, and Innovation Funding Authority (STDF), Egypt.References
- N. Sobhani, D. Generali, F. Zanconati, M. Bortul and B. Scaggiante, Current status of PI3K-mTOR inhibition in hormone-receptor positive, HER2-negative breast cancer, World J. Clin. Oncol., 2018, 9(8), 172–179, DOI:10.5306/wjco.v9.i8.172.
- C. H. Aylett, E. Sauer, S. Imseng, D. Boehringer, M. N. Hall, N. Ban and T. Maier, Architecture of human mTOR complex 1, Science, 2016, 351(6268), 48–52, DOI:10.1126/science.aaa3870.
- K. A. Papavassiliou, I. Zoi, A. N. Gargalionis and M. Koutsilieris, Polycystin-1 affects cancer cell behaviour and interacts with mTOR and Jak signalling pathways in cancer cell lines, J. Cell. Mol. Med., 2019, 23(9), 1–13, DOI:10.1111/jcmm.14506.
- A. Sato, mTOR, a Potential Target to Treat Autism Spectrum Disorder, CNS Neurol. Disord.: Drug Targets, 2016, 15(5), 533–543 CrossRef CAS PubMed.
- A. Kilincaslan, B. E. Kok, P. Tekturk, C. Yalcinkaya, C. Ozkara and Z. Yapici, Beneficial Effects of Everolimus on Autism and Attention-Deficit/Hyperactivity Disorder Symptoms in a Group of Patients with Tuberous Sclerosis Complex, J. Child Adolesc. Psychopharmacol., 2017, 27(4), 383–388, DOI:10.1089/cap.2016.0100.
- M. Costa-Mattioli and L. M. Monteggia, mTOR complexes in neurodevelopmental and neuropsychiatric disorders, Nat. Neurosci., 2013, 16(11), 1537–1543, DOI:10.1038/nn.3546.
- M. Wang, X. Zhang, L. Zhong, L. Zeng, L. Li and P. Yao, Understanding autism: Causes, diagnosis, and advancing therapies, Brain Res. Bull., 2025, 227, 111411, DOI:10.1016/j.brainresbull.2025.111411.
- I. Drehmer, J. Santos-Terra, C. Gottfried and I. Deckmann, mTOR signaling pathway as a pathophysiologic mechanism in preclinical models of autism spectrum disorder, Neuroscience, 2024, 563, 33–42, DOI:10.1016/j.neuroscience.2024.10.050.
- J. Xie, X. Wang and C. G. Proud, mTOR inhibitors in cancer therapy, F1000Research, 2016, 5 DOI:10.12688/f1000research.9207.1.
- S. Faes, T. Santoro, N. Demartines and O. Dormond, Evolving Significance and Future Relevance of Anti-Angiogenic Activity of mTOR Inhibitors in Cancer Therapy, Cancers, 2017, 9(11), 152, DOI:10.3390/cancers9110152.
- , vii A. Gomez-Pinillos and A. C. Ferrari, mTOR signaling pathway and mTOR inhibitors in cancer therapy, Hematol./Oncol. Clin. North Am., 2012, 26(3), 483–505, DOI:10.1016/j.hoc.2012.02.014.
- A. M. Eiden, S. Zhang, J. M. Gary, J. K. Simmons and B. A. Mock, Molecular Pathways: Increased Susceptibility to Infection Is a Complication of mTOR Inhibitor Use in Cancer Therapy, Clin. Cancer Res., 2016, 22(2), 277–283, DOI:10.1158/1078-0432.CCR-14-3239.
- Y. Shiose, Current development status and prospect of dual PI3K/mTOR inhibitors for cancer therapy, Nihon Yakurigaku Zasshi, 2013, 142(4), 156–161 CrossRef CAS.
- M. Suhail, W. M. AlZahrani, S. Shakil, M. Tarique, S. Tabrez, T. A. Zughaibi and M. Rehan, Analysis of some flavonoids for inhibitory mechanism against cancer target phosphatidylinositol 3-kinase (PI3K) using computational tool, Front. Pharmacol., 2023, 14, 1236173, DOI:10.3389/fphar.2023.1236173.
- M. Rehan, I. A. Sheikh, M. Suhail, S. Tabrez and S. Shakil, Computational Exploration of a Diverse Flavonoid Library for Targeted Allosteric Inhibition of AKT1 in Cancer Therapy, Anticancer Res., 2025, 45(2), 593–604, DOI:10.21873/anticanres.17446.
- I. Ahmad, M. Hoque, S. S. M. Alam, T. A. Zughaibi and S. Tabrez, Curcumin and Plumbagin Synergistically Target the PI3K/Akt/mTOR Pathway: A Prospective Role in Cancer Treatment, Int. J. Mol. Sci., 2023, 24(7), 6651, DOI:10.3390/ijms24076651.
- B. Ul Islam, M. S. Khan, F. M. Husain, M. T. Rehman, T. A. Zughaibi, A. M. Abuzenadah, M. Urooj, M. A. Kamal and S. Tabrez, mTOR Targeted Cancer Chemoprevention by Flavonoids, Curr. Med. Chem., 2021, 28(39), 8068–8082, DOI:10.2174/0929867327666201109122025.
- B. Ul Islam, M. Suhail, M. S. Khan, A. Ahmad, T. A. Zughaibi, F. M. Husain, M. T. Rehman and S. Tabrez, Flavonoids and PI3K/Akt/mTOR Signaling Cascade: A Potential Crosstalk in Anticancer Treatment, Curr. Med. Chem., 2021, 28(39), 8083–8097, DOI:10.2174/0929867328666210804091548.
- M. Rehan, M. M. Mahmoud, S. Tabrez, H. M. A. Hassan and G. M. Ashraf, Exploring Flavonoids for Potential Inhibitors of a Cancer Signaling Protein PI3Kγ Kinase Using Computational Methods, Anticancer Res., 2020, 40(8), 4547–4556, DOI:10.21873/anticanres.14460.
- G. I. Manfredi, A. Dicitore, G. Gaudenzi, M. Caraglia, L. Persani and G. Vitale, PI3K/Akt/mTOR signaling in medullary thyroid cancer: a promising molecular target for cancer therapy, Endocrine, 2015, 48(2), 363–370, DOI:10.1007/s12020-014-0380-1.
- E. Pons-Tostivint, B. Thibault and J. Guillermet-Guibert, Targeting PI3K Signaling in Combination Cancer Therapy, Trends Cancer, 2017, 3(6), 454–469, DOI:10.1016/j.trecan.2017.04.002.
- K. Rüben, A. Wurzlbauer, A. Walte, W. Sippl, F. Bracher and W. Becker, Selectivity Profiling and Biological Activity of Novel β-Carbolines as Potent and Selective DYRK1 Kinase Inhibitors, PLoS One, 2015, 10(7), e0132453, DOI:10.1371/journal.pone.0132453.
- K. Ohishi, K. Toume, M. A. Arai, T. Koyano, T. Kowithayakorn, T. Mizoguchi, M. Itoh and M. Ishibashi, 9-Hydroxycanthin-6-one, a β-Carboline Alkaloid from Eurycoma longifolia, Is the First Wnt Signal Inhibitor through Activation of Glycogen Synthase Kinase 3β without Depending on Casein Kinase 1α, J. Nat. Prod., 2015, 78(5), 1139–1146, DOI:10.1021/acs.jnatprod.5b00153.
- C. Wang, R. Hu, T. Wang, L. Duan, Q. Hou, J. Wang and Z. Yang, A bivalent β-carboline derivative inhibits macropinocytosis-dependent entry of pseudorabies virus by targeting the kinase DYRK1A, J. Biol. Chem., 2023, 299(4), 104605, DOI:10.1016/j.jbc.2023.104605.
- C. Wang, X. Wang, Z. Su, H. Fei, X. Liu and Q. Pan, The novel mTOR inhibitor Torin-2 induces autophagy and downregulates the expression of UHRF1 to suppress hepatocarcinoma cell growth, Oncol. Rep., 2015, 34(4), 1708–1716, DOI:10.3892/or.2015.4146.
- H. Zhang, J. K. Yi, H. Huang, S. Park, W. Kwon, E. Kim, S. Jang, S. Y. Kim, S. K. Choi and S. H. Kim, et al., Rhein Suppresses Colorectal Cancer Cell Growth by Inhibiting the mTOR Pathway In Vitro and In Vivo, Cancers, 2021, 13(9), 2176, DOI:10.3390/cancers13092176.
- P. Yellen, M. Saqcena, D. Salloum, J. Feng, A. Preda, L. Xu, V. Rodrik-Outmezguine and D. A. Foster, High-dose rapamycin induces apoptosis in human cancer cells by dissociating mTOR complex 1 and suppressing phosphorylation of 4E-BP1, Cell Cycle, 2011, 10(22), 3948–3956, DOI:10.4161/cc.10.22.18124.
- J. Cham, A. R. Venkateswaran and M. Bhangoo, Targeting the PI3K-AKT-mTOR Pathway in Castration Resistant Prostate Cancer: A Review Article, Clin. Genitourin. Cancer, 2021, 19(6), 563.e561–563.e567, DOI:10.1016/j.clgc.2021.07.014.
- P. Garg, S. Ramisetty, M. Nair, P. Kulkarni, D. Horne, R. Salgia and S. S. Singhal, Strategic advancements in targeting the PI3K/AKT/mTOR pathway for Breast cancer therapy, Biochem. Pharmacol., 2025, 236, 116850, DOI:10.1016/j.bcp.2025.116850.
- P. K. Senapati, K. K. Mahapatra, A. Singh and S. K. Bhutia, mTOR inhibitors in targeting autophagy and autophagy-associated signaling for cancer cell death and therapy, Biochim. Biophys. Acta, Rev. Cancer, 2025, 1880(3), 189342, DOI:10.1016/j.bbcan.2025.189342.
- S. Vilar, G. Cozza and S. Moro, Medicinal chemistry and the molecular operating environment (MOE): application of QSAR and molecular docking to drug discovery, Curr. Top. Med. Chem., 2008, 8(18), 1555–1572, DOI:10.2174/156802608786786624.
- M. A. Helal and M. A. Avery, Combined receptor-based and ligand-based approach to delineate the mode of binding of guaianolide-endoperoxides to PfATP6, Bioorg. Med. Chem. Lett., 2012, 22(17), 5410–5414, DOI:10.1016/j.bmcl.2012.07.053.
- H. Jia, G. Dai, W. Su, K. Xiao, J. Weng, Z. Zhang, Q. Wang, T. Yuan, F. Shi and W. Chen, et al., Discovery, Optimization, and Evaluation of Potent and Highly Selective PI3Kγ-PI3Kδ Dual Inhibitors, J. Med. Chem., 2019, 62(10), 4936–4948, DOI:10.1021/acs.jmedchem.8b02014.
- M. McGann, FRED pose prediction and virtual screening accuracy, J. Chem. Inf. Model., 2011, 51(3), 578–596, DOI:10.1021/ci100436p.
| This journal is © The Royal Society of Chemistry 2026 |