Synthesis and evaluation of lupeol-derived triterpenic azines as potential neuroprotective agents
Florencia A.
Musso
 ab,
Natalia P.
Alza
ab,
Natalia P.
Alza
 *cd,
Gabriela A.
Salvador
*cd,
Gabriela A.
Salvador
 cd and
María Belén
Faraoni
cd and
María Belén
Faraoni
 *ab
*ab
aInstituto de Química del Sur (INQUISUR, CONICET – UNS), Av. Alem 1253, Bahía Blanca, 8000, Argentina. E-mail: bfaraoni@criba.edu.ar
bDepartamento de Química, Universidad Nacional del Sur (UNS), Av. Alem 1253, Bahía Blanca, 8000, Argentina
cInstituto de Investigaciones Bioquímicas de Bahía Blanca (INIBIBB), Camino La Carrindanga Km. 7, Bahía Blanca, 8000, Argentina
dDepartamento de Biología, Bioquímica y Farmacia, Universidad Nacional del Sur (UNS), San Juan 670, Bahía Blanca, 8000, Argentina. E-mail: natalia.alza@uns.edu.ar
First published on 6th November 2025
Abstract
Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized by the selective loss of dopaminergic neurons and the accumulation of α-synuclein aggregates. Current treatments are primarily symptomatic, highlighting the need for new neuroprotective strategies. Natural triterpenes have shown promise in neurodegenerative diseases, and structural modifications can enhance their bioactivity. In this study, we obtained a series of new triterpenic azines (4a–4p) from lupeol, optimizing reaction conditions through microwave-assisted synthesis. The neuroprotective potential of these derivatives was evaluated in human neuroblastoma IMR-32 cells exposed to 6-hydroxydopamine (6-OHDA), a widely used in vitro model of PD. Compounds 4c, 4m, and 4n significantly prevented 6-OHDA-induced cytotoxicity, restoring cell viability at 10 and 50 μM to control levels. Since ferroptosis is a cell death mechanism implicated in PD, we further examined the effects of these compounds in N27 dopaminergic neurons exposed to the ferroptosis inducers RSL3 and erastin. Among the tested derivatives, 4c exhibited a remarkable protective effect against RSL3-induced ferroptosis, which was comparable to ferrostatin-1, displaying an IC50 value of 9.1 μM. These findings support the development of triterpenic azines as neuroprotective agents and warrant further investigation in preclinical PD models.
1. Introduction
Parkinson's disease (PD) is the second most prevalent neurodegenerative disorder after Alzheimer's disease, affecting 2–3% of individuals over 65 years old. While the precise cause of PD remains unclear, alterations in mitochondrial function, iron regulation in the brain, neuroinflammation, and energy metabolism have been identified as main contributors to its underlying mechanisms.1–6 Neuropathologically, PD is primarily characterized by the progressive loss of dopaminergic neurons in the substantia nigra pars compacta and the accumulation of α-synuclein protein aggregates, forming intracellular Lewy bodies.1,7 PD patients exhibit motor symptoms as a consequence of the dopamine depletion, including resting tremor, bradykinesia, and postural instability, among others.Despite decades of intense efforts to develop specific therapeutic strategies for PD, current treatments primarily focus on symptomatic relief, aiming to restore dopamine levels. These treatments include the gold-standard drug levodopa, dopamine agonists, and surgical procedures.8 In 2024, a review stated that there are sixty disease-modifying therapies for PD under clinical trials,9 targeting a spectrum of triggering mechanisms, such as neuroinflammation, α-synuclein aggregation, oxidative stress, ferroptosis, mitochondrial dysfunction, and dysregulation of the gut–brain axis.2,4,5,8,10 A widely used cellular model for studying the mechanisms of neuronal death in PD and for screening of potential therapeutic agents is the induction with the neurotoxicant 6-hydroxydopamine (6-OHDA), which is an oxidative metabolite of dopamine.11 When dopaminergic neurons are exposed to 6-OHDA, cell death is promoted by a rise in the formation of reactive oxygen species and alterations in the mitochondrial respiratory chain.12,13
As natural products (NPs) play a crucial role in the treatment of various diseases, including neurodegenerative disorders,14–18 they are promising candidates for the development of new PD treatments. Triterpenes are among the most abundant NPs in the plant kingdom and exhibit a broad spectrum of biological activities, including neuroprotective properties.19–24 Despite their structural diversity, they are predominantly found as tetra- or pentacyclic structures in nature, with the latter gaining significant pharmacological interest in recent years.25–28 Lupeol (1) is a natural pentacyclic triterpene found in various plant species, including those of the Asteraceae family.29–34 In previous studies, we successfully isolated lupeol in substantial quantities as one of the major pentacyclic triterpenes from the ethanolic extract of the aerial parts of Chuquiraga erinacea (Asteraceae), an endemic species of Argentina.35 This triterpene exhibits significant biological properties, including anti-diabetic, anti-inflammatory, anti-arthritic, anti-oxidant, anti-infective, and anti-angiogenic activities.36–39 However, its low bioavailability limits its therapeutic application.38 Chemical modification of its structure is a widely used strategy to enhance this property and improve its pharmacological parameters.37,40,41 Although numerous semisynthetic analogues of lupeol have been reported,26,41–49 the development of an α,β-unsaturated carbonyl system is particularly attractive not only due to its biological relevance but also for its significant reactivity, making it a valuable building block for synthesizing novel chemical structures.42,43 Recently, Pokorny et al. synthesized a series of novel triterpenic azines through condensation reactions between an α,β-unsaturated carbonyl group of a pentacyclic triterpene and aromatic hydrazones with high yields using conventional synthesis methods.50 Azines are a class of organic compounds that have garnered significant attention for their chemical versatility and bioactive potential. These molecules, derived from the condensation of aldehydes or ketones with hydrazines, are 2,3-diaza analogues of 1,3-butadiene, also known as N–N linked diimines (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N
N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). Recent research on azines has demonstrated a remarkable range of biological activities, including antimicrobial, antitumor, antioxidant, and, particularly, neuroprotective properties.51–54 Based on this background, this work aims to identify the most promising compounds to advance toward preclinical trials for PD treatment. For this purpose, we synthesized the α,β-unsaturated aldehyde, 3β-hydroxy-lup-20(29)-en-30-al (2), from lupeol isolated from the natural source C. erinacea.46 This building block was employed in the synthesis of a series of triterpenic azines (4a–p), optimizing the reaction proposed by Pokorny et al. through microwave (MW)-assisted irradiation. Subsequently, the neuroprotective activity of the synthesized azines was evaluated using an in vitro PD model induced by the neurotoxin 6-OHDA. Furthermore, the effect of the most active compounds was explored in a ferroptosis model in dopaminergic neurons, showing the most promising results for compound 4c.
C). Recent research on azines has demonstrated a remarkable range of biological activities, including antimicrobial, antitumor, antioxidant, and, particularly, neuroprotective properties.51–54 Based on this background, this work aims to identify the most promising compounds to advance toward preclinical trials for PD treatment. For this purpose, we synthesized the α,β-unsaturated aldehyde, 3β-hydroxy-lup-20(29)-en-30-al (2), from lupeol isolated from the natural source C. erinacea.46 This building block was employed in the synthesis of a series of triterpenic azines (4a–p), optimizing the reaction proposed by Pokorny et al. through microwave (MW)-assisted irradiation. Subsequently, the neuroprotective activity of the synthesized azines was evaluated using an in vitro PD model induced by the neurotoxin 6-OHDA. Furthermore, the effect of the most active compounds was explored in a ferroptosis model in dopaminergic neurons, showing the most promising results for compound 4c.
2. Results and discussion
Synthesis of triterpene azines 4a–p
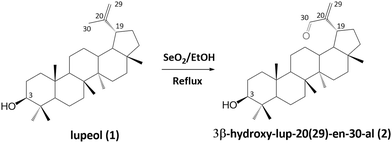
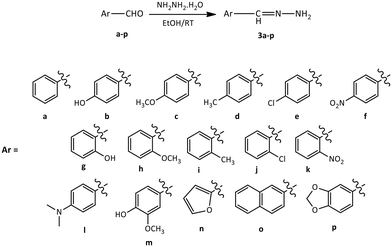
The synthesis of new azine derivatives of the natural triterpene lupeol (1), isolated from C. erinacea, followed the reactions depicted in Schemes 1–3. The reaction between 3β-hydroxy-lup-20(29)-en-30-al (2), our building block, and diverse aromatic hydrazones (3a–p) yielded sixteen triterpene azines (4a–p). As shown in Scheme 1, compound 2 was obtained through the allylic oxidation of 1 with SeO2 (2.5 eq.) in ethanol under reflux, with 70% yield. Previous studies have reported allylic oxidation as a method to obtain α,β-unsaturated aldehydes from 1 (ref. 42, 43, 45, 55 and 56) and other triterpenes.50,57–59The aromatic hydrazones 3a–p were synthesized through the condensation of aromatic aldehydes (a–p) and hydrazine hydrate at room temperature for 30–60 minutes (Scheme 2). The reaction was monitored by thin layer chromatography (TLC), observing the disappearance of the aldehyde and the appearance of a new spot with a lower Rf. The final product was confirmed by 1H-NMR, and the hydrazones were used without further purification.
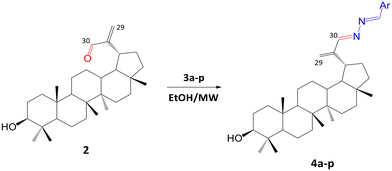
A first approach to obtain azine derivatives of 1 was explored using the protocol reported by Pokorny et al.50 To a solution of compound 2 in ethanol, the corresponding aromatic hydrazones (3a and b) were added, and the mixture was heated under reflux for 5–6 h, yielding compounds 4a and b (32% and 39%, respectively) (see method A in the Experimental section). Notably, the reaction exhibited a higher yield compared to the synthesis of azine derivatives of betulinic acid reported by Pokorny et al.50
To optimize the synthesis of lupeol azines, a MW-assisted reaction was adapted (Scheme 3). After the addition of 3a and b to a solution of 2 in ethanol, the mixture was irradiated at 70 °C with a power of 280 W for 30 min in a MW oven. Under these conditions, the yield of 4a was comparable to the reaction described above; however, 4b was obtained with a higher yield. A major advantage of the MW-assisted method is the shorter reaction times (Table 1). Therefore, derivatives 4c–p were also synthesized using MW irradiation, with reaction times ranging from 5 to 30 min (see method B in the Experimental section). The reaction yield after purification was variable (6.6–59.5%), considering that in most experiments, 20% of compound 2 remained unreacted. Additionally, a dimeric azine was obtained as a side product, which is consistent with previous reports on this type of reaction.50
The purity of the synthesized compounds was evaluated by TLC, which showed a single spot under UV light after staining with p-anisaldehyde and heating. In addition, 1H and 13C NMR spectroscopy, melting point measurements, and HPLC analysis were performed. The identity of all compounds was confirmed by high-resolution mass spectrometry (HRMS). Detailed spectral data and images are provided in the SI.
The isolated azines 4a–p were identified as E isomers at both newly formed double bonds, as confirmed by a two-dimensional NOESY NMR experiment on the selected derivative 4a (see SI file). Symmetrical azines can exist in three configurational isomers: (E,E), (E,Z), and (Z,Z), with the (E,E) configuration being the most thermodynamically stable, as reported by Safari et al.51 This stereochemical preference has also been observed in structurally related compounds by Pokorny et al.50 The absence of key spatial correlations in the NOESY spectrum of 4a further supports that the synthesized azines predominantly adopt the (E,E) configuration.
Evaluation of the protective effect of triterpene derivatives 4a–p against 6-OHDA toxicity and ferroptosis
Our first biological evaluation was focused on the screening of azines 4a–p based on the well-established in vitro 6-OHDA model,12 which was selected because it closely reproduces the oxidative stress and mitochondrial dysfunction characteristic of PD, providing a relevant system for evaluating neuroprotective activity. For this purpose, we used human neuroblastoma IMR-32 cells, which express neuronal markers such as neurofilament subunits and dopamine transporters and provide a human-derived system for the 6-OHDA model.12Neuroprotection was assessed by cell viability measurement when IMR-32 cells were exposed to the neurotoxin 6-OHDA in the presence of the compounds at two concentrations (10 and 50 μM) for 24 h. First, we confirmed that the working concentrations of the compounds were not cytotoxic. Based on preliminary dose–response experiments, a concentration of 25 μM 6-OHDA was established as a submaximal, marked, and moderate toxicity (45%).12 As the natural triterpene 1, the derivatives 4d, 4e, 4g, 4i–l, 4o and 4p at 10 μM did not revert the cytotoxicity of 6-OHDA. Interestingly, when IMR-32 cells were pretreated with compounds 4c (Ar = p-methoxyphenyl), 4m (Ar = 4-hydroxy-3-methoxyphenyl), and 4n (Ar = furanyl), the cytotoxic effect of 6-OHDA was prevented (Fig. 1), restoring cell viability to the control conditions even at a concentration of 10 μM. A lower efficacy was observed for compounds 4a, 4b, 4f and 4h (Fig. 1).
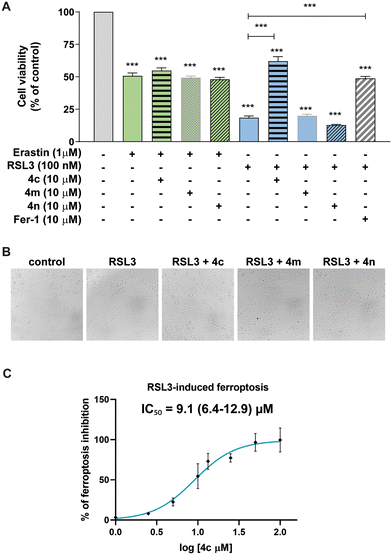
Taking into account the protective activity against 6-OHDA exerted by compounds 4c, 4m and 4n, and considering that ferroptosis is a well-described mechanisms of cell death induced by this neurotoxin,2 we decided to study how the derivatives affect neurons challenged with ferroptosis inducers. Subsequent experiments addressing ferroptosis inhibition were conducted at 10 μM of derivatives 4c, 4m and 4n in N27 dopaminergic neurons exposed to RSL3 (GPX4 inhibitor) or erastin (which inhibits cystine–glutamate antiporter system XC−). The concentrations of ferroptosis inducers were fixed in preliminary dose–response assays based on their ability to elicit a reproducible decrease in neuronal viability, thereby establishing conditions suitable for assessing the neuroprotective potential of the tested compounds. At 1 μM, erastin reduced cell viability by 50%, and none of the compounds neither enhanced neuronal damage nor exerted neuroprotection. Differentially, when RSL3 was used (reducing cell viability by 80%), compound 4c efficiently rescued cells from ferroptosis, resulting in a 344% increase in viability (Fig. 2A). The effect was comparable to that of the reference ferroptosis inhibitor ferrostatin-1 (Fer-1). This finding was corroborated by microscopic analysis, as shown in Fig. 2B. While cells exposed to 4m and 4n exhibited morphological changes consistent with ferroptotic death after RSL3 treatment, neurons pretreated with 4c preserved normal morphology, resembling control conditions. To further characterize its anti-ferroptotic potential, compound 4c was tested at increasing concentrations in the RSL3-induced ferroptosis model (Fig. 2C). The compound displayed a dose-dependent inhibition, with an IC50 value of 9.1 μM, indicating moderate potency. Although less potent than Cu(II)(atsm), a clinically explored ferroptosis modulator with IC50 values below 1 μM,60,614c effectively prevented RSL3-induced neuronal death, suggesting that 4c could serve as a promising scaffold for the development of novel ferroptosis inhibitors targeting dopaminergic neurons.
3. Conclusions
In this study, we successfully synthesized and characterized a series of novel triterpenic azines derived from lupeol (4a–4p), optimizing the reaction conditions through MW-assisted synthesis. The neuroprotective potential of these compounds was evaluated in a previously described in vitro model of PD using 6-OHDA-induced cytotoxicity in IMR-32 cells. Among the synthesized derivatives, compounds 4c, 4m, and 4n exhibited significant protective effects against 6-OHDA-induced cell death, suggesting their potential as neuroprotective agents.Further evaluation using N27 dopaminergic neurons exposed to ferroptosis inducers revealed that compound 4c effectively prevented ferroptotic cell death induced by the GPX4 inhibitor RSL3, while 4m and 4n failed to exert such an effect. Compound 4c efficiently preserved cell viability and morphology at micromolar concentrations, displaying an IC50 of 9.1 μM, which indicates a moderate but biologically relevant anti-ferroptotic potency. These findings suggest that compound 4c may display its neuroprotective activity, at least in part, by counteracting ferroptosis, a regulated cell death mechanism increasingly recognized as a key contributor to neurodegeneration in PD.62 Although less potent than Cu(II)(atsm), a well-characterized ferroptosis inhibitor with submicromolar activity,60,61 compound 4c achieved comparable protection to Fer-1. These results highlight 4c as a promising scaffold for the development of novel neuroprotective agents targeting ferroptosis-related pathways, potentially relevant for the treatment of neurodegenerative disorders such as PD. Overall, our results highlight the promising pharmacological potential of triterpenic azines as neuroprotective agents and provide a basis for further studies aimed at elucidating their mechanisms of action and evaluating their therapeutic potential in preclinical models of PD. Future investigations will focus on in vivo validation and structure–activity relationship studies to optimize their efficacy and bioavailability.
4. Experimental section
Materials and methods
All reagents used for chemical synthesis were of analytical grade. Masses were measured using an analytical balance (model: AUW220D, manufacturer: Shimadzu) with a readability of 0.00001 g. The balance was calibrated internally and verified with certified external weights (class E2) prior to use. Yields refer to purified products. Microwave reactions were performed with a CEM Discover Synthesis Unit (CEM Corp., Matthews, NC) with a continuous focused microwave power delivery system in glass vessels (10 mL) sealed with a septum, under magnetic stirring. The temperature of the reaction mixture inside the vessel was monitored using a calibrated infrared temperature control under the reaction vessel. Silica gel 60 (200–425 mesh, Aldrich) was used for column chromatography. The separation was monitored by TLC on silica gel 60 F254 sheets (Merck), visualized under UV light and/or using p-anisaldehyde–acetic acid in ethanol spray reagent. Nuclear magnetic resonance (NMR) spectra were recorded at 300 MHz for 1H-NMR and 75 MHz for 13C-NMR using deuterated chloroform (CDCl3, 99.8% D, Aldrich) as the solvent and tetramethyl silane (TMS) as an internal reference. Chemical shifts (δ) are reported in parts per million (ppm) from TMS using the residual solvent resonance (CDCl3: 7.26 ppm for 1H-NMR, 77.16 ppm for 13C-NMR). Multiplicities are abbreviated as follows: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br s = broad signal. Coupling constants (J) were reported in hertz (Hz). The identity of all compounds was confirmed by high-resolution mass spectrometry (HRMS) using a Bruker micrOTOF-QII mass spectrometer. Purity was assessed by HPLC analysis using a Thermo Scientific Dionex Ultimate 3000 equipped with a diode-array detector. Samples were detected at a wavelength of 254 nm following separation on a Phenomenex Luna C18 column (250 × 4.6 mm, 5 μm). The mobile phase consisted of acetonitrile and methanol in a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ratio, with a flow rate of 1 mL min−1. Melting points were determined using a Büchi 510 apparatus and are not corrected.
30 ratio, with a flow rate of 1 mL min−1. Melting points were determined using a Büchi 510 apparatus and are not corrected.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, #M2128), 6-OHDA hydrochloride (#162957) and Fer-1 (#SML0583) were obtained from Sigma-Aldrich (St. Louis, MO, USA). RSL3 (#19288) and erastin (#17754) were purchased from Cayman (Vienna, Austria). Dulbecco's modified Eagle medium (DMEM) high glucose medium (#52100047), RPMI 1640 medium (#31800022), and antibiotic–antimycotic (#15240062) were purchased from Gibco, CABA, Argentina. Fetal bovine serum (FBS) was obtained from Internegocios, Mercedes, Argentina.
Synthesis of compound 2
Compound 1 (0.5 g; 1.17 mmol) was dissolved in absolute EtOH (20 mL), and SeO2 (2.5 eq.) was added. The reaction mixture was stirred under reflux (78 °C) for 24 h. Then, the reaction mixture was cooled to room temperature and filtered, and water (80 mL) was added to the reaction mixture. The hydroalcoholic phase was separated and extracted thrice with dichloromethane (120 mL). The combined organic phases were dried over MgSO4. The organic solvent was removed under reduced pressure and the residue was purified by flash chromatography using hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (90
ethyl acetate (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as an eluent system.
10) as an eluent system.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 80
EtOAc 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20). Mp: 215–218 °C. 1H (CDCl3, 300 MHz): 9.50 (s, 1H, H-30), 6.28 (br s, 1H, H-29a), 5.90 (br s, 1H, H-29b), 3.17 (dd, 1H, J = 10.6, 5.3 Hz, H-3), 2.73 (ddd, 1H, J = 10.9, 10.9, 5.6 Hz, H-19), 2.24–2.06 (m, 1H, J = 11.1 Hz, H-21a), 1.76–1.57 (m, 5H); 1.50 (br s, 3H), 1.47–1.39 (m, 5H), 1.37 (br s, 4H), 1.27–1.12 (m, 5H), 1.00 (s, 3H, H-23), 0.95 (s, 3H, H-26), 0.91 (s, 3H, H-27), 0.81 (s, 3H, H-25), 0.80 (s, 3H, H-28), 0.74 (s, 3H, H-24), 0.65 (d, 1H, J = 9.0 Hz, H-5). 13C (CDCl3, 75 MHz): 195.2 (C-30), 157.3 (C-20), 133.3 (C-29), 79.1 (C-3), 55.4 (C-5), 51.3 (C-18), 50.3 (C-9), 43.4 (C-17), 42.8 (C-14), 40.9 (C-8), 40.0 (C-22), 39.0 (C-4), 38.8 (C-1), 37.8 (C-13), 37.2 (C-10, C-19), 35.5 (C-16), 34.4 (C-7), 32.7 (C-21), 28.1 (C-23), 27.7 (C-12), 27.5 (C-15), 27.4 (C-2), 21.0 (C-11), 18.4 (C-6), 17.9 (C-28), 16.2 (C-25), 16.0 (C-26), 15.5 (C-24), 14.5 (C-27).
20). Mp: 215–218 °C. 1H (CDCl3, 300 MHz): 9.50 (s, 1H, H-30), 6.28 (br s, 1H, H-29a), 5.90 (br s, 1H, H-29b), 3.17 (dd, 1H, J = 10.6, 5.3 Hz, H-3), 2.73 (ddd, 1H, J = 10.9, 10.9, 5.6 Hz, H-19), 2.24–2.06 (m, 1H, J = 11.1 Hz, H-21a), 1.76–1.57 (m, 5H); 1.50 (br s, 3H), 1.47–1.39 (m, 5H), 1.37 (br s, 4H), 1.27–1.12 (m, 5H), 1.00 (s, 3H, H-23), 0.95 (s, 3H, H-26), 0.91 (s, 3H, H-27), 0.81 (s, 3H, H-25), 0.80 (s, 3H, H-28), 0.74 (s, 3H, H-24), 0.65 (d, 1H, J = 9.0 Hz, H-5). 13C (CDCl3, 75 MHz): 195.2 (C-30), 157.3 (C-20), 133.3 (C-29), 79.1 (C-3), 55.4 (C-5), 51.3 (C-18), 50.3 (C-9), 43.4 (C-17), 42.8 (C-14), 40.9 (C-8), 40.0 (C-22), 39.0 (C-4), 38.8 (C-1), 37.8 (C-13), 37.2 (C-10, C-19), 35.5 (C-16), 34.4 (C-7), 32.7 (C-21), 28.1 (C-23), 27.7 (C-12), 27.5 (C-15), 27.4 (C-2), 21.0 (C-11), 18.4 (C-6), 17.9 (C-28), 16.2 (C-25), 16.0 (C-26), 15.5 (C-24), 14.5 (C-27).
General procedure for the synthesis of compounds 3a–p
Hydrazine hydrate (2 eq.) was added to a solution of each aromatic aldehyde a–p in absolute EtOH (1 mL). The reaction mixture was stirred at room temperature for 30–60 min until complete disappearance of the starting material. After that time, the solvent was removed under reduced pressure, affording the substituted aromatic hydrazones 3a–p quantitatively. TLC confirmed complete consumption of the aldehydes and formation of the corresponding hydrazones, which was further corroborated by NMR analysis. The products were used without further purification, in line with the methodology previously reported by Pokorny et al.50General procedure for the synthesis of compounds 4a and b (method A)
Compound 2 (1 eq.) was dissolved in absolute EtOH (2 mL), and the corresponding aromatic hydrazones 3a and b (1 eq.) were added. The reaction mixture was stirred under reflux (78 °C) for 5–6 h. After completion the solvent was removed under reduced pressure and the crude product was purified by flash chromatography on a silica gel column with hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate as the eluent to obtain pure compounds 4a and b.
ethyl acetate as the eluent to obtain pure compounds 4a and b.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (90
ethyl acetate (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as an eluent system. Rf 0.40 (hexane
10) as an eluent system. Rf 0.40 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 70
EtOAc 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Mp: 126–128 °C. 1H (CDCl3, 300 MHz): 8.55 (s, 1H, H-31), 8.21 (s, 1H, H-30), 7.80 (dd, 2H, J = 6.6, 3.0 Hz, H-2′, H-6′), 7.44 (dd, 3H, J = 5.1, 1.9 Hz, H-3′, H-4′, H-5′), 5.66 (s, 1H, H-29a), 5.47 (s, 1H, H-29b), 3.17 (dd, 1H, J = 10.8, 5.2 Hz, H-3), 3.06–2.89 (m, 1H, H-19), 2.36–2.15 (m, 1H, H-21), 1.87–1.64 (m, 5H), 1.61 (br s, 3H), 1.54–1.43 (m, 5H), 1.39 (br s, 4H), 1.33–1.15 (m, 5H), 1.03 (s, 3H, H-23), 0.96 (s, 6H, H-26, H-27), 0.86 (s, 3H, H-25), 0.80 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.7 Hz, H-5). 13C (CDCl3, 75 MHz): 164.9 (C-30, C-31), 161.5 (C-1′), 152.6 (C-20), 134.3 (C-3′), 131.2 (C-5′), 128.9 (C-6′), 128.6 (C-4′), 123.5 (C-29), 79.1 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.4 (C-7), 32.9 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.6 (C-27). HRMS (ESI) m/z: 543.4302 calcd. for (C37H55N2O), found 573.4309 [M + H]+. HPLC purity: >99%.
30). Mp: 126–128 °C. 1H (CDCl3, 300 MHz): 8.55 (s, 1H, H-31), 8.21 (s, 1H, H-30), 7.80 (dd, 2H, J = 6.6, 3.0 Hz, H-2′, H-6′), 7.44 (dd, 3H, J = 5.1, 1.9 Hz, H-3′, H-4′, H-5′), 5.66 (s, 1H, H-29a), 5.47 (s, 1H, H-29b), 3.17 (dd, 1H, J = 10.8, 5.2 Hz, H-3), 3.06–2.89 (m, 1H, H-19), 2.36–2.15 (m, 1H, H-21), 1.87–1.64 (m, 5H), 1.61 (br s, 3H), 1.54–1.43 (m, 5H), 1.39 (br s, 4H), 1.33–1.15 (m, 5H), 1.03 (s, 3H, H-23), 0.96 (s, 6H, H-26, H-27), 0.86 (s, 3H, H-25), 0.80 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.7 Hz, H-5). 13C (CDCl3, 75 MHz): 164.9 (C-30, C-31), 161.5 (C-1′), 152.6 (C-20), 134.3 (C-3′), 131.2 (C-5′), 128.9 (C-6′), 128.6 (C-4′), 123.5 (C-29), 79.1 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.4 (C-7), 32.9 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.6 (C-27). HRMS (ESI) m/z: 543.4302 calcd. for (C37H55N2O), found 573.4309 [M + H]+. HPLC purity: >99%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (80
ethyl acetate (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) as an eluent system. Rf 0.25 (hexane
20) as an eluent system. Rf 0.25 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 70
EtOAc 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Mp: 174–176 °C. 1H (CDCl3, 300 MHz): 8.51 (s, 1H, H-31), 8.21 (s, 1H, H-30), 7.68 (d, 2H, J = 8.7 Hz, H-2′, H-6′), 6.86 (d, 2H, J = 8.7 Hz, H-3′, H-5′), 6.71 (br s, 1H, BzOH), 5.63 (s, 1H, H-29a), 5.45 (s, 1H, H-29b), 3.19 (dd, 1H, J = 10.8, 5.0 Hz, H-3), 3.03–2.86 (m, 1H, H-19), 2.34–2.13 (m, 1H, H-21), 1.85–1.62 (m, 5H), 1.60 (br s, 3H), 1.53–1.42 (m, 5H), 1.38 (br s, 4H), 1.00 (s, 5H), 0.96 (s, 3H, H-23), 0.94 (s, 3H, H-26, H-27), 0.84 (s, 3H, H-25), 0.79 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.5 Hz, H-5). 13C (CDCl3, 75 MHz): 164.5 (C-30, C-31), 161.6 (C-4′), 159.0 (C-20), 152.5 (C-1′), 130.6 (C-2′), 126.6 (C-6′), 123.3 (C-29), 116.0 (C-3′, C-5′), 79.3 (C-3), 55.4 (C-5), 50.4 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 40.9 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.0 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.4 (C-7), 32.9 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.1 (C-11), 18.4 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.6 (C-27). HRMS (ESI) m/z: 559.4240 calcd. for (C37H55N2O2), found 559.4258 [M + H]+. HPLC purity: 95%.
30). Mp: 174–176 °C. 1H (CDCl3, 300 MHz): 8.51 (s, 1H, H-31), 8.21 (s, 1H, H-30), 7.68 (d, 2H, J = 8.7 Hz, H-2′, H-6′), 6.86 (d, 2H, J = 8.7 Hz, H-3′, H-5′), 6.71 (br s, 1H, BzOH), 5.63 (s, 1H, H-29a), 5.45 (s, 1H, H-29b), 3.19 (dd, 1H, J = 10.8, 5.0 Hz, H-3), 3.03–2.86 (m, 1H, H-19), 2.34–2.13 (m, 1H, H-21), 1.85–1.62 (m, 5H), 1.60 (br s, 3H), 1.53–1.42 (m, 5H), 1.38 (br s, 4H), 1.00 (s, 5H), 0.96 (s, 3H, H-23), 0.94 (s, 3H, H-26, H-27), 0.84 (s, 3H, H-25), 0.79 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.5 Hz, H-5). 13C (CDCl3, 75 MHz): 164.5 (C-30, C-31), 161.6 (C-4′), 159.0 (C-20), 152.5 (C-1′), 130.6 (C-2′), 126.6 (C-6′), 123.3 (C-29), 116.0 (C-3′, C-5′), 79.3 (C-3), 55.4 (C-5), 50.4 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 40.9 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.0 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.4 (C-7), 32.9 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.1 (C-11), 18.4 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.6 (C-27). HRMS (ESI) m/z: 559.4240 calcd. for (C37H55N2O2), found 559.4258 [M + H]+. HPLC purity: 95%.
General procedure for the synthesis of compounds 4a–p (method B)
Compound 2 (1 eq.) was dissolved in absolute EtOH (1 mL), and aromatic hydrazones 3a–p (1 eq.) were added. The reaction was irradiated in a microwave oven at 280 W for 5–30 min at 70 °C. The reaction mixture was treated as mentioned in method A to obtain compounds 4a–p.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (90
ethyl acetate (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as an eluent system. Rf 0.56 (hexane
10) as an eluent system. Rf 0.56 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 70
EtOAc 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Mp: 122–125 °C. 1H (CDCl3, 300 MHz): 8.52 (s, 1H, H-31), 8.21 (s, 1H, H-30), 7.75 (d, 2H, J = 8.7 Hz, H-2′, H-6′), 6.95 (d, 2H, J = 8.6 Hz, H-3′, H-5′), 5.63 (s, 1H, H-29a), 5.45 (s, 1H, H-29b), 3.86 (s, 3H, Bz-OCH3), 3.17 (dd, 1H, J = 10.9, 5.1 Hz, H-3), 3.06–2.86 (m, 1H, H-19), 2.37–2.16 (m, 1H, H-21), 1.90–1.63 (m, 5H), 1.61 (br s, 3H), 1.54–1.42 (m, 5H), 1.39 (br s, 4H), 1.13–1.32 (m, 5H), 1.00 (s, 3H, H-23), 0.95 (s, 6H, H-26, H-27), 0.85 (s, 3H, H-25), 0.80 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.66 (d, 1H, J = 9.7 Hz, H-5). 13C (CDCl3, 75 MHz): 164.4 (C-30, C-31), 162.1 (C-4′), 161.3 (C-20), 130.2 (C-2′, C-6′), 127.0 (C-1′), 123.0 (C-29), 114.4 (C-3′, C-5′), 79.1 (C-3), 55.5 (C-5), 55.4 (C-18), 50.5 (C-9), 43.3 (C-17), 42.9 (C-14), 40.9 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 29.8 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.1 (C-11), 18.4 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 573.4435 calcd. for (C38H57N2O2), found 573.4415 [M + H]+. HPLC purity: 95%.
30). Mp: 122–125 °C. 1H (CDCl3, 300 MHz): 8.52 (s, 1H, H-31), 8.21 (s, 1H, H-30), 7.75 (d, 2H, J = 8.7 Hz, H-2′, H-6′), 6.95 (d, 2H, J = 8.6 Hz, H-3′, H-5′), 5.63 (s, 1H, H-29a), 5.45 (s, 1H, H-29b), 3.86 (s, 3H, Bz-OCH3), 3.17 (dd, 1H, J = 10.9, 5.1 Hz, H-3), 3.06–2.86 (m, 1H, H-19), 2.37–2.16 (m, 1H, H-21), 1.90–1.63 (m, 5H), 1.61 (br s, 3H), 1.54–1.42 (m, 5H), 1.39 (br s, 4H), 1.13–1.32 (m, 5H), 1.00 (s, 3H, H-23), 0.95 (s, 6H, H-26, H-27), 0.85 (s, 3H, H-25), 0.80 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.66 (d, 1H, J = 9.7 Hz, H-5). 13C (CDCl3, 75 MHz): 164.4 (C-30, C-31), 162.1 (C-4′), 161.3 (C-20), 130.2 (C-2′, C-6′), 127.0 (C-1′), 123.0 (C-29), 114.4 (C-3′, C-5′), 79.1 (C-3), 55.5 (C-5), 55.4 (C-18), 50.5 (C-9), 43.3 (C-17), 42.9 (C-14), 40.9 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 29.8 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.1 (C-11), 18.4 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 573.4435 calcd. for (C38H57N2O2), found 573.4415 [M + H]+. HPLC purity: 95%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (94
ethyl acetate (94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) as an eluent system. Rf 0.75 (hexane
6) as an eluent system. Rf 0.75 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 70
EtOAc 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Mp: 110–113 °C. 1H (CDCl3, 300 MHz): 8.53 (s, 1H, H-31), 8.21 (s, 1H, H-30), 7.69 (d, 2H, J = 7.8 Hz, H-2′, H-6′), 7.24 (d, 2H, J = 7.9 Hz, H-3′, H-5′), 5.64 (s, 1H, H-29a), 5.46 (s, 1H, H-29b), 3.24–3.09 (m, 1H, H-3), 3.06–2.88 (m, 1H, H-19), 2.41 (s, 3H, Bz-CH3), 2.32–2.15 (m, 1H, H-21), 1.87–1.60 (m, 5H), 1.57 (br s, 3H), 1.54–1.42 (m, 5H), 1.39 (br s, 4H), 1.14–1.30 (m, 5H), 1.03 (s, 3H, H-23), 0.96 (s, 6H, H-26, H-27), 0.86 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.6 Hz, H-5). 13C (CDCl3, 75 MHz): 164.6 (C-30, C-31), 161.6 (C-20), 141.6 (C-1′), 131.6 (C-4′), 129.6 (C-3′, C-5′), 128.6 (C-2′, C-6′), 123.2 (C-29), 79.2 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.2 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10), 35.7 (C-16), 34.5 (C-7), 34.2 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.8 (C-19), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 557.4462 calcd. for (C38H57N2O), found 557.4465 [M + H]+. HPLC purity: 98%.
30). Mp: 110–113 °C. 1H (CDCl3, 300 MHz): 8.53 (s, 1H, H-31), 8.21 (s, 1H, H-30), 7.69 (d, 2H, J = 7.8 Hz, H-2′, H-6′), 7.24 (d, 2H, J = 7.9 Hz, H-3′, H-5′), 5.64 (s, 1H, H-29a), 5.46 (s, 1H, H-29b), 3.24–3.09 (m, 1H, H-3), 3.06–2.88 (m, 1H, H-19), 2.41 (s, 3H, Bz-CH3), 2.32–2.15 (m, 1H, H-21), 1.87–1.60 (m, 5H), 1.57 (br s, 3H), 1.54–1.42 (m, 5H), 1.39 (br s, 4H), 1.14–1.30 (m, 5H), 1.03 (s, 3H, H-23), 0.96 (s, 6H, H-26, H-27), 0.86 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.6 Hz, H-5). 13C (CDCl3, 75 MHz): 164.6 (C-30, C-31), 161.6 (C-20), 141.6 (C-1′), 131.6 (C-4′), 129.6 (C-3′, C-5′), 128.6 (C-2′, C-6′), 123.2 (C-29), 79.2 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.2 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10), 35.7 (C-16), 34.5 (C-7), 34.2 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.8 (C-19), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 557.4462 calcd. for (C38H57N2O), found 557.4465 [M + H]+. HPLC purity: 98%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (95
ethyl acetate (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as an eluent system. Rf 0.53 (hexane
5) as an eluent system. Rf 0.53 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 70
EtOAc 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Mp: 113–115 °C. 1H (CDCl3, 300 MHz): 8.51 (s, 1H, H-31), 8.20 (s, 1H, H-30), 7.74 (d, 2H, J = 8.3, H-2′, H-6′), 7.41 (d, 2H, J = 8.5 Hz, H-3′, H-5′), 5.67 (s, 1H, H-29a), 5.48 (s, 1H, H-29b), 3.26–3.10 (m, 1H, H-3), 3.06–2.88 (m, 1H, H-19), 2.36–2.15 (m, 1H, H-21), 1.86–1.63 (m, 5H), 1.58 (br s, 3H), 1.54–1.41 (m, 5H), 1.39 (br s, 4H), 1.11–1.32 (m, 5H), 1.02 (s, 3H, H-23), 0.96 (s, 6H, H-26, H-27), 0.85 (s, 3H, H-25), 0.80 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.7 Hz, H-5). 13C (CDCl3, 75 MHz): 165.3 (C-30, C-31), 160.2 (C-20), 137.1 (C-4′), 132.8 (C-1′), 129.7 (C-3′, C-5′), 129.2 (C-2′, C-6′), 123.9 (C-29), 79.1 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10), 36.7 (C-19), 35.7 (C-16), 34.5 (C-7), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 24.8 (C-21), 21.2 (C-11), 18.4 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 577.3919 calcd. for (C37H54ClN2O), found 577.3919 [M + H]+. HPLC purity: 95%.
30). Mp: 113–115 °C. 1H (CDCl3, 300 MHz): 8.51 (s, 1H, H-31), 8.20 (s, 1H, H-30), 7.74 (d, 2H, J = 8.3, H-2′, H-6′), 7.41 (d, 2H, J = 8.5 Hz, H-3′, H-5′), 5.67 (s, 1H, H-29a), 5.48 (s, 1H, H-29b), 3.26–3.10 (m, 1H, H-3), 3.06–2.88 (m, 1H, H-19), 2.36–2.15 (m, 1H, H-21), 1.86–1.63 (m, 5H), 1.58 (br s, 3H), 1.54–1.41 (m, 5H), 1.39 (br s, 4H), 1.11–1.32 (m, 5H), 1.02 (s, 3H, H-23), 0.96 (s, 6H, H-26, H-27), 0.85 (s, 3H, H-25), 0.80 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.7 Hz, H-5). 13C (CDCl3, 75 MHz): 165.3 (C-30, C-31), 160.2 (C-20), 137.1 (C-4′), 132.8 (C-1′), 129.7 (C-3′, C-5′), 129.2 (C-2′, C-6′), 123.9 (C-29), 79.1 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10), 36.7 (C-19), 35.7 (C-16), 34.5 (C-7), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 24.8 (C-21), 21.2 (C-11), 18.4 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 577.3919 calcd. for (C37H54ClN2O), found 577.3919 [M + H]+. HPLC purity: 95%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (92
ethyl acetate (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) as an eluent system. Rf 0.47 (hexane
8) as an eluent system. Rf 0.47 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 70
EtOAc 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Mp: 123–125 °C. 1H (CDCl3, 300 MHz): 8.59 (s, 1H, H-31), 8.22 (s, 1H, H-30), 8.29 (d, 2H, J = 8.6 Hz, H-3′, H-5′), 7.97 (d, 2H, J = 8.5 Hz, H-2′, H-6′), 5.74 (s, 1H, H-29a), 5.54 (s, 1H, H-29b), 3.17 (dd, 1H, J = 10.9, 5.2 Hz, H-3), 3.05–2.87 (m, 1H, H-19), 2.39–2.16 (m, 1H, H-21); 1.90–1.60 (m, 5H), 1.56 (br s, 3H), 1.50–1.42 (m, 5H), 1.40 (br s, 4H), 1.12–1.32 (m, 5H), 1.03 (s, 3H, H-23); 0.96 (s, 6H, H-26, H-27), 0.86 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.3 Hz, H-5). 13C (CDCl3, 75 MHz): 166.3 (C-30, C-31), 158.7 (C-20), 149.2 (C-1′), 140.2 (C-4′), 129.1 (C-2′, C6′), 125.0 (C-29), 124.2 (C-3′, C-5′), 79.1 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 29.8 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.4 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 588.4160 calcd. for (C37H54N3O3), found 588.4160 [M + H]+. HPLC purity: >99%.
30). Mp: 123–125 °C. 1H (CDCl3, 300 MHz): 8.59 (s, 1H, H-31), 8.22 (s, 1H, H-30), 8.29 (d, 2H, J = 8.6 Hz, H-3′, H-5′), 7.97 (d, 2H, J = 8.5 Hz, H-2′, H-6′), 5.74 (s, 1H, H-29a), 5.54 (s, 1H, H-29b), 3.17 (dd, 1H, J = 10.9, 5.2 Hz, H-3), 3.05–2.87 (m, 1H, H-19), 2.39–2.16 (m, 1H, H-21); 1.90–1.60 (m, 5H), 1.56 (br s, 3H), 1.50–1.42 (m, 5H), 1.40 (br s, 4H), 1.12–1.32 (m, 5H), 1.03 (s, 3H, H-23); 0.96 (s, 6H, H-26, H-27), 0.86 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.3 Hz, H-5). 13C (CDCl3, 75 MHz): 166.3 (C-30, C-31), 158.7 (C-20), 149.2 (C-1′), 140.2 (C-4′), 129.1 (C-2′, C6′), 125.0 (C-29), 124.2 (C-3′, C-5′), 79.1 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 29.8 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.4 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 588.4160 calcd. for (C37H54N3O3), found 588.4160 [M + H]+. HPLC purity: >99%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (95
ethyl acetate (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as an eluent system. Rf 0.67 (hexane
5) as an eluent system. Rf 0.67 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 70
EtOAc 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Mp: 125–127 °C. 1H (CDCl3, 300 MHz): 11.76 (s, 1H, Bz-OH), 8.69 (s, 1H, H-31), 8.20 (s, 1H, H-30), 7.39–7.29 (m, 2H, H-4′, H-6′), 7.00 (d, 1H, J = 8.2 Hz, H-3′), 6.93 (t, 1H, J = 8.2 Hz, H-5′), 5.71 (s, 1H, H-29a), 5.51 (s, 1H, H-29b), 3.24–3.08 (m, 1H, H-3), 3.05–2.87 (m, 1H, H-19), 2.35–2.15 (m, 1H, H-21), 2.03–1.64 (m, 5H), 1.59 (br s, 3H), 1.54–1.42 (m, 5H), 1.39 (br s, 4H), 1.31–1.19 (m, 5H), 1.03 (s, 3H, H-23), 0.96 (s, 6H, H-26, H-27), 0.86 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.8 Hz, H-5). 13C (CDCl3, 75 MHz): 165.6 (C-30, C-31), 164.9 (C-20), 159.9 (C-2′), 152.3 (C-1′), 132.9 (C-20), 132.3 (C-6′), 124.7 (C-29), 119.5 (C-5′), 117.9 (C-4′), 117.1 (C-3′), 79.1 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 32.8 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 559.4258 calcd. for (C37H55N2O2), found 559.4258 [M + H]+. HPLC purity: >99%.
30). Mp: 125–127 °C. 1H (CDCl3, 300 MHz): 11.76 (s, 1H, Bz-OH), 8.69 (s, 1H, H-31), 8.20 (s, 1H, H-30), 7.39–7.29 (m, 2H, H-4′, H-6′), 7.00 (d, 1H, J = 8.2 Hz, H-3′), 6.93 (t, 1H, J = 8.2 Hz, H-5′), 5.71 (s, 1H, H-29a), 5.51 (s, 1H, H-29b), 3.24–3.08 (m, 1H, H-3), 3.05–2.87 (m, 1H, H-19), 2.35–2.15 (m, 1H, H-21), 2.03–1.64 (m, 5H), 1.59 (br s, 3H), 1.54–1.42 (m, 5H), 1.39 (br s, 4H), 1.31–1.19 (m, 5H), 1.03 (s, 3H, H-23), 0.96 (s, 6H, H-26, H-27), 0.86 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.8 Hz, H-5). 13C (CDCl3, 75 MHz): 165.6 (C-30, C-31), 164.9 (C-20), 159.9 (C-2′), 152.3 (C-1′), 132.9 (C-20), 132.3 (C-6′), 124.7 (C-29), 119.5 (C-5′), 117.9 (C-4′), 117.1 (C-3′), 79.1 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 32.8 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 559.4258 calcd. for (C37H55N2O2), found 559.4258 [M + H]+. HPLC purity: >99%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (92
ethyl acetate (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) as an eluent system. Rf 0.67 (hexane
8) as an eluent system. Rf 0.67 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 70
EtOAc 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Mp: 113–116 °C. 1H (CDCl3, 300 MHz): 8.96 (s, 1H, H-31), 8.20 (s, 1H, H-30), 8.04 (dd, 1H, J = 7.4, 1.8 Hz, H-6′), 7.41 (ddd, 1H, J = 8.7, 7.4, 1.8 Hz, H-5′), 7.00 (t, 1H, J = 7.5 Hz, H-4′), 6.93 (d, 1H, J = 8.3 Hz, H-3′), 5.63 (s, 1H, H-29a), 5.45 (s, 1H, H-29b), 3.89 (s, 3H, Bz-OCH3), 3.17 (dd, 1H, J = 10.9, 5.2 Hz, H-3), 3.07–2.90 (m, 1H, H-19), 2.36–2.18 (m, 1H, H-21), 1.98–1.63 (m, 5H), 1.59 (br s, 3H), 1.53–1.42 (m, 5H), 1.39 (br s, 4H), 1.29–1.12 (m, 5H), 1.02 (s, 3H, H-23), 0.95 (s, 6H, H-26, H-27), 0.86 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.9 Hz, H-5). 13C (CDCl3, 75 MHz): 164.3 (C-30, C-31), 159.1 (C-20), 157.4 (C-2′), 132.5 (C-1′), 127.4 (C-6′), 122.9 (C-4′), 120.9 (C-5′), 111.3 (C-29), 79.1 (C-3), 55.7 (C-18), 55.4 (C-5), 50.5 (C-9), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 32.8 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.6 (C-27). HRMS (ESI) m/z: 573.4414 calcd. for (C38H57N2O2), found 573.4415 [M + H]+. HPLC purity: 97%.
30). Mp: 113–116 °C. 1H (CDCl3, 300 MHz): 8.96 (s, 1H, H-31), 8.20 (s, 1H, H-30), 8.04 (dd, 1H, J = 7.4, 1.8 Hz, H-6′), 7.41 (ddd, 1H, J = 8.7, 7.4, 1.8 Hz, H-5′), 7.00 (t, 1H, J = 7.5 Hz, H-4′), 6.93 (d, 1H, J = 8.3 Hz, H-3′), 5.63 (s, 1H, H-29a), 5.45 (s, 1H, H-29b), 3.89 (s, 3H, Bz-OCH3), 3.17 (dd, 1H, J = 10.9, 5.2 Hz, H-3), 3.07–2.90 (m, 1H, H-19), 2.36–2.18 (m, 1H, H-21), 1.98–1.63 (m, 5H), 1.59 (br s, 3H), 1.53–1.42 (m, 5H), 1.39 (br s, 4H), 1.29–1.12 (m, 5H), 1.02 (s, 3H, H-23), 0.95 (s, 6H, H-26, H-27), 0.86 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.9 Hz, H-5). 13C (CDCl3, 75 MHz): 164.3 (C-30, C-31), 159.1 (C-20), 157.4 (C-2′), 132.5 (C-1′), 127.4 (C-6′), 122.9 (C-4′), 120.9 (C-5′), 111.3 (C-29), 79.1 (C-3), 55.7 (C-18), 55.4 (C-5), 50.5 (C-9), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 32.8 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.6 (C-27). HRMS (ESI) m/z: 573.4414 calcd. for (C38H57N2O2), found 573.4415 [M + H]+. HPLC purity: 97%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (95
ethyl acetate (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as an eluent system. Rf 0.62 (hexane
5) as an eluent system. Rf 0.62 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 70
EtOAc 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Mp: 119–122 °C. 1H (CDCl3, 300 MHz): 8.84 (s, 1H, H-31), 8.23 (s, 1H, H-30), 8.00 (dd, 1H, J = 7.6, 1 Hz, H-3′), 7.38–7.16 (m, 3H, H-4′, H-5′, H-6′), 5.65 (s, 1H, H-29a), 5.48 (s, 1H, H-29b), 3.25–3.09 (m, 1H, H-3), 3.06–2.88 (m, 1H, H-19), 2.54 (s, 3H, CH3), 2.38–2.17 (m, 1H, H-21), 1.90–1.60 (m, 5H), 1.58 (br s, 3H), 1.54–1.42 (m, 5H), 1.39 (br s, 4H), 1.14–1.32 (m, 5H), 1.03 (s, 3H, H-23), 0.96 (s, 6H, H-26, H-27), 0.87 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.4 Hz, H-5). 13C (CDCl3, 75 MHz): 164.8 (C-30, C-31), 160.1 (C-20), 138.5 (C-1′), 132.4 (C-2′), 131.1 (C-3′), 130.8 (C-6′), 127.6 (C-4′), 126.4 (C-5′), 123.2 (C-29), 79.1 (C-3), 55.4 (C-5), 53.6 (C-18), 50.5 (C-9), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 33.0 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 19.8 (Bz-CH3), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.6 (C-27). HRMS (ESI) m/z: 557.4442 calcd. for (C38H57N2O), found 557.4465 [M + H]+. HPLC purity: >99%.
30). Mp: 119–122 °C. 1H (CDCl3, 300 MHz): 8.84 (s, 1H, H-31), 8.23 (s, 1H, H-30), 8.00 (dd, 1H, J = 7.6, 1 Hz, H-3′), 7.38–7.16 (m, 3H, H-4′, H-5′, H-6′), 5.65 (s, 1H, H-29a), 5.48 (s, 1H, H-29b), 3.25–3.09 (m, 1H, H-3), 3.06–2.88 (m, 1H, H-19), 2.54 (s, 3H, CH3), 2.38–2.17 (m, 1H, H-21), 1.90–1.60 (m, 5H), 1.58 (br s, 3H), 1.54–1.42 (m, 5H), 1.39 (br s, 4H), 1.14–1.32 (m, 5H), 1.03 (s, 3H, H-23), 0.96 (s, 6H, H-26, H-27), 0.87 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.4 Hz, H-5). 13C (CDCl3, 75 MHz): 164.8 (C-30, C-31), 160.1 (C-20), 138.5 (C-1′), 132.4 (C-2′), 131.1 (C-3′), 130.8 (C-6′), 127.6 (C-4′), 126.4 (C-5′), 123.2 (C-29), 79.1 (C-3), 55.4 (C-5), 53.6 (C-18), 50.5 (C-9), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 33.0 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 19.8 (Bz-CH3), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.6 (C-27). HRMS (ESI) m/z: 557.4442 calcd. for (C38H57N2O), found 557.4465 [M + H]+. HPLC purity: >99%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (95
ethyl acetate (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as an eluent system. Rf 0.65 (hexane
5) as an eluent system. Rf 0.65 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 70
EtOAc 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Mp: 83–86 °C. 1H (CDCl3, 300 MHz): 8.95 (s, 1H, H-31), 8.20 (s, 1H, H-30), 8.14 (dd, 1H, J = 7.4, 2.0 Hz, H-3′), 7.46–7.27 (m, 3H, H-4′, H-5′, H-6′), 5.68 (s, 1H, H-29a), 5.49 (s, 1H, H-29b), 3.24–3.10 (m, 1H, H-3), 3.07–2.91 (m, 1H, H-19), 2.38–2.17 (m, 1H, H-21), 1.97–1.64 (m, 5H), 1.57 (br s, 3H), 1.54–1.42 (m, 5H), 1.40 (br s, 4H), 1.10–1.32 (m, 5H), 1.03 (s, 3H, H-23), 0.96 (s, 6H, H-26, H-27), 0.87 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.5 Hz, H-5). 13C (CDCl3, 75 MHz): 165.2 (C-30, C-31), 157.9 (C-20), 135.7 (C-1′), 132.0 (C-2′), 131.8 (C-3′), 130.2 (C-6′), 128.2 (C-4′), 127.1 (C-5′), 123.9 (C-29), 79.1 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10), 36.7 (C-19), 35.7 (C-16), 34.5 (C-7), 32.1 (C-21), 29.8 (C-23), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.6 (C-27). HRMS (ESI) m/z: 577.3940 calcd. for (C37H54ClN2O), found 577.3919 [M + H]+. HPLC purity: 90%.
30). Mp: 83–86 °C. 1H (CDCl3, 300 MHz): 8.95 (s, 1H, H-31), 8.20 (s, 1H, H-30), 8.14 (dd, 1H, J = 7.4, 2.0 Hz, H-3′), 7.46–7.27 (m, 3H, H-4′, H-5′, H-6′), 5.68 (s, 1H, H-29a), 5.49 (s, 1H, H-29b), 3.24–3.10 (m, 1H, H-3), 3.07–2.91 (m, 1H, H-19), 2.38–2.17 (m, 1H, H-21), 1.97–1.64 (m, 5H), 1.57 (br s, 3H), 1.54–1.42 (m, 5H), 1.40 (br s, 4H), 1.10–1.32 (m, 5H), 1.03 (s, 3H, H-23), 0.96 (s, 6H, H-26, H-27), 0.87 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.5 Hz, H-5). 13C (CDCl3, 75 MHz): 165.2 (C-30, C-31), 157.9 (C-20), 135.7 (C-1′), 132.0 (C-2′), 131.8 (C-3′), 130.2 (C-6′), 128.2 (C-4′), 127.1 (C-5′), 123.9 (C-29), 79.1 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10), 36.7 (C-19), 35.7 (C-16), 34.5 (C-7), 32.1 (C-21), 29.8 (C-23), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.6 (C-27). HRMS (ESI) m/z: 577.3940 calcd. for (C37H54ClN2O), found 577.3919 [M + H]+. HPLC purity: 90%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (87
ethyl acetate (87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13) as an eluent system. Rf 0.45 (hexane
13) as an eluent system. Rf 0.45 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc, 70
EtOAc, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Mp: 117–120 °C. 1H (CDCl3, 300 MHz): 8.96 (s, 1H, H-31), 8.21 (dd, 1H, J = 7.8, 1.6 Hz, H-3′), 8.16 (s, 1H, H-30), 8.04 (dd, 1H, J = 8.1, 1.4 Hz, H-6′), 7.68 (t, 1H, J = 7.3 Hz, H-5′), 7.58 (t, 1H, J = 8.2 Hz, H-4′), 5.71 (s, 1H, H-29a), 5.50 (s, 1H, H-29b), 3.17 (dd, 1H, J = 10.8, 5.3 Hz, H-3), 3.05–2.88 (m, 1H, H-19), 2.37–2.14 (m, 1H, H-21), 1.87–1.60 (m, 5H), 1.64–1.54 (m, 3H), 1.53–1.42 (m, 5H), 1.39 (br s, 4H), 1.18–1.31 (m, 5H), 1.04 (s, 3H, H-23), 0.96 (s, 6H, H-26, H-27), 0.86 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.7 Hz, H-5). 13C (CDCl3, 75 MHz): 165.5 (C-30, C-31), 156.5 (C-20), 149.1 (C-2′), 133.5 (C-1′), 131.1 (C-5′), 129.5 (C-6′), 129.3 (C-4′), 124.8 (C-3′), 124.6 (C-29), 79.1 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 33.0 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.6 (C-27). HRMS (ESI) m/z: 588.4132 calcd. for (C37H54N3O3), found 588.4160 [M + H]+. HPLC purity: 96%.
30). Mp: 117–120 °C. 1H (CDCl3, 300 MHz): 8.96 (s, 1H, H-31), 8.21 (dd, 1H, J = 7.8, 1.6 Hz, H-3′), 8.16 (s, 1H, H-30), 8.04 (dd, 1H, J = 8.1, 1.4 Hz, H-6′), 7.68 (t, 1H, J = 7.3 Hz, H-5′), 7.58 (t, 1H, J = 8.2 Hz, H-4′), 5.71 (s, 1H, H-29a), 5.50 (s, 1H, H-29b), 3.17 (dd, 1H, J = 10.8, 5.3 Hz, H-3), 3.05–2.88 (m, 1H, H-19), 2.37–2.14 (m, 1H, H-21), 1.87–1.60 (m, 5H), 1.64–1.54 (m, 3H), 1.53–1.42 (m, 5H), 1.39 (br s, 4H), 1.18–1.31 (m, 5H), 1.04 (s, 3H, H-23), 0.96 (s, 6H, H-26, H-27), 0.86 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.7 Hz, H-5). 13C (CDCl3, 75 MHz): 165.5 (C-30, C-31), 156.5 (C-20), 149.1 (C-2′), 133.5 (C-1′), 131.1 (C-5′), 129.5 (C-6′), 129.3 (C-4′), 124.8 (C-3′), 124.6 (C-29), 79.1 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 33.0 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.6 (C-27). HRMS (ESI) m/z: 588.4132 calcd. for (C37H54N3O3), found 588.4160 [M + H]+. HPLC purity: 96%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (92
ethyl acetate (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) as an eluent system. Rf 0.55 (hexane
8) as an eluent system. Rf 0.55 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 70
EtOAc 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Mp: 123–125 °C. 1H (CDCl3, 300 MHz): 8.50 (s, 1H, H-31), 8.22 (s, 1H, H-30), 7.67 (d, 2H, J = 8.9 Hz, H-2′, H-6′), 6.70 (d, 2H, J = 8.9 Hz, H-3′, H-5′), 5.58 (s, 1H, H-29a), 5.41 (s, 1H, H-29b), 3.23–3.11 (m, 1H, H-3), 3.04 (s, 6H, Bz-N(CH3)2), 3.03–2.98 (m, 1H, H-19), 2.37–2.14 (m, 1H, H-21), 1.99–1.64 (m, 5H), 1.61 (br s, 3H), 1.58–1.46 (m, 5H), 1.39 (br s, 4H), 1.12–1.31 (m, 5H), 1.02 (s, 3H, H-23), 0.95 (s, 6H, H-26, H-27), 0.85 (s, 3H, H-25), 0.80 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.66 (d, 1H, J = 9.5 Hz, H-5). 13C (CDCl3, 75 MHz): 163.4 (C-30, C-31), 162.3 (C-20), 152.4 (C-4′), 130.2 (C-2′, C-6′), 122.1 (C-29), 122.0 (C-1′), 111.8 (C-3′, C-5′), 79.2 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.3 (Bz-N(CH3)2), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.8 (C-16), 34.5 (C-7), 33.0 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 586.4736 calcd. for (C39H60N3O), found 586.4731 [M + H]+. HPLC purity: 98%.
30). Mp: 123–125 °C. 1H (CDCl3, 300 MHz): 8.50 (s, 1H, H-31), 8.22 (s, 1H, H-30), 7.67 (d, 2H, J = 8.9 Hz, H-2′, H-6′), 6.70 (d, 2H, J = 8.9 Hz, H-3′, H-5′), 5.58 (s, 1H, H-29a), 5.41 (s, 1H, H-29b), 3.23–3.11 (m, 1H, H-3), 3.04 (s, 6H, Bz-N(CH3)2), 3.03–2.98 (m, 1H, H-19), 2.37–2.14 (m, 1H, H-21), 1.99–1.64 (m, 5H), 1.61 (br s, 3H), 1.58–1.46 (m, 5H), 1.39 (br s, 4H), 1.12–1.31 (m, 5H), 1.02 (s, 3H, H-23), 0.95 (s, 6H, H-26, H-27), 0.85 (s, 3H, H-25), 0.80 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.66 (d, 1H, J = 9.5 Hz, H-5). 13C (CDCl3, 75 MHz): 163.4 (C-30, C-31), 162.3 (C-20), 152.4 (C-4′), 130.2 (C-2′, C-6′), 122.1 (C-29), 122.0 (C-1′), 111.8 (C-3′, C-5′), 79.2 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.3 (Bz-N(CH3)2), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.8 (C-16), 34.5 (C-7), 33.0 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 586.4736 calcd. for (C39H60N3O), found 586.4731 [M + H]+. HPLC purity: 98%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (82
ethyl acetate (82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18) as an eluent system. Rf 0.25 (hexane
18) as an eluent system. Rf 0.25 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 70
EtOAc 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Mp: 123–125 °C. 1H (CDCl3, 300 MHz): 8.48 (s, 1H, H-31), 8.21 (s, 1H, H-30), 7.50 (d, 1H, J = 1.8 Hz, H-6′), 7.18 (dd, 1H, J = 8.1, 1.8 Hz, H-2′), 6.95 (d, 1H, J = 8.1 Hz, H-5′), 5.95 (s, 1H, Bz-OH), 5.63 (s, 1H, H-29a), 5.45 (s, 1H, H-29b), 3.96 (s, 3H, Bz-OCH3), 3.23–3.11 (m, 1H, H-3), 3.03–2.89 (m, 1H, H-19), 2.35–2.15 (m, 1H, H-21), 1.84–1.63 (m, 5H), 1.58 (br s, 3H), 1.54–1.41 (m, 5H), 1.40 (br s, 4H), 1.11–1.31 (m, 5H), 1.02 (s, 3H, H-23), 0.95 (s, 6H, H-26, H-27), 0.85 (s, 3H, H-25), 0.80 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.5 Hz, H-5). 13C (CDCl3, 75 MHz): 164.3 (C-30, C-31), 161.6 (C-20), 148.8 (C-3′), 147.1 (C-4′), 126.9 (C-1′), 124.8 (C-6′), 123.0 (C-29), 114.5 (C-5′), 108.5 (C-2′), 79.1 (C-3), 56.2 (C-5), 55.4 (C-18), 50.5 (C-9), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 29.7 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 589.4376 calcd. for (C38H57N2O3), found 589.4364 [M + H]+. HPLC purity: >99%.
30). Mp: 123–125 °C. 1H (CDCl3, 300 MHz): 8.48 (s, 1H, H-31), 8.21 (s, 1H, H-30), 7.50 (d, 1H, J = 1.8 Hz, H-6′), 7.18 (dd, 1H, J = 8.1, 1.8 Hz, H-2′), 6.95 (d, 1H, J = 8.1 Hz, H-5′), 5.95 (s, 1H, Bz-OH), 5.63 (s, 1H, H-29a), 5.45 (s, 1H, H-29b), 3.96 (s, 3H, Bz-OCH3), 3.23–3.11 (m, 1H, H-3), 3.03–2.89 (m, 1H, H-19), 2.35–2.15 (m, 1H, H-21), 1.84–1.63 (m, 5H), 1.58 (br s, 3H), 1.54–1.41 (m, 5H), 1.40 (br s, 4H), 1.11–1.31 (m, 5H), 1.02 (s, 3H, H-23), 0.95 (s, 6H, H-26, H-27), 0.85 (s, 3H, H-25), 0.80 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.5 Hz, H-5). 13C (CDCl3, 75 MHz): 164.3 (C-30, C-31), 161.6 (C-20), 148.8 (C-3′), 147.1 (C-4′), 126.9 (C-1′), 124.8 (C-6′), 123.0 (C-29), 114.5 (C-5′), 108.5 (C-2′), 79.1 (C-3), 56.2 (C-5), 55.4 (C-18), 50.5 (C-9), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 29.7 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 589.4376 calcd. for (C38H57N2O3), found 589.4364 [M + H]+. HPLC purity: >99%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (90
ethyl acetate (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) as an eluent system. Rf 0.45 (hexane
10) as an eluent system. Rf 0.45 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 70
EtOAc 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Mp: 107–104 °C. 1H (CDCl3, 300 MHz): 8.43 (s, 1H, H-31), 8.26 (s, 1H, H-30), 7.59 (d, 1H, J = 1.7 Hz, H-5′), 6.86 (d, 1H, J = 3.5 Hz, H-3′), 6.53 (dd, 1H, J = 3.5, 1.8 Hz, H-4′), 5.64 (s, 1H, H-29a), 5.46 (s, 1H, H-29b), 3.17 (dd, 1H, J = 10.8, 5.2 Hz, H-3), 3.01–2.83 (m, 1H, H-19), 2.35–2.10 (m, 1H, H-21), 1.90–1.63 (m, 5H), 1.60 (br s, 3H), 1.57–1.42 (m, 5H), 1.39 (br s, 4H), 1.11–1.30 (m, 5H), 1.02 (s, 3H, H-23), 0.96 (s, 6H, H-26, H-27), 0.85 (s, 3H, H-25), 0.80 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.66 (d, 1H, J = 9.5 Hz, H-5). 13C (CDCl3, 75 MHz): 165.7 (C-30, C-31), 150.5 (C-20), 149.8 (C-2′), 145.7 (C-5′), 123.7 (C-29), 116.3 (C-3′), 112.3 (C-4′), 79.1 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 27.4 (C-21), 21.1 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.6 (C-27). HRMS (ESI) m/z: 533.4109 calcd. for (C35H53N2O2), found 533.4102 [M + H]+. HPLC purity: >99%.
30). Mp: 107–104 °C. 1H (CDCl3, 300 MHz): 8.43 (s, 1H, H-31), 8.26 (s, 1H, H-30), 7.59 (d, 1H, J = 1.7 Hz, H-5′), 6.86 (d, 1H, J = 3.5 Hz, H-3′), 6.53 (dd, 1H, J = 3.5, 1.8 Hz, H-4′), 5.64 (s, 1H, H-29a), 5.46 (s, 1H, H-29b), 3.17 (dd, 1H, J = 10.8, 5.2 Hz, H-3), 3.01–2.83 (m, 1H, H-19), 2.35–2.10 (m, 1H, H-21), 1.90–1.63 (m, 5H), 1.60 (br s, 3H), 1.57–1.42 (m, 5H), 1.39 (br s, 4H), 1.11–1.30 (m, 5H), 1.02 (s, 3H, H-23), 0.96 (s, 6H, H-26, H-27), 0.85 (s, 3H, H-25), 0.80 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.66 (d, 1H, J = 9.5 Hz, H-5). 13C (CDCl3, 75 MHz): 165.7 (C-30, C-31), 150.5 (C-20), 149.8 (C-2′), 145.7 (C-5′), 123.7 (C-29), 116.3 (C-3′), 112.3 (C-4′), 79.1 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 27.4 (C-21), 21.1 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.6 (C-27). HRMS (ESI) m/z: 533.4109 calcd. for (C35H53N2O2), found 533.4102 [M + H]+. HPLC purity: >99%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (95
ethyl acetate (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as an eluent system. Rf 0.45 (hexane
5) as an eluent system. Rf 0.45 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 70
EtOAc 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Mp: 128–131 °C. 1H (CDCl3, 300 MHz): 8.71 (s, 1H, H-31), 8.27 (s, 1H, H-30), 8.13–8.02 (m, 2H, H-1′, H-4′), 7.94–7.81 (m, 3H, H-3′, H-6′, H-9′), 7.59–7.47 (m, 2H, H-7′, H-8′), 5.68 (s, 1H, H-29a), 5.50 (s, 1H, H-29b), 3.23–3.10 (m, 1H, H-3), 3.08–2.91 (m, 1H, H-19), 2.36–2.19 (m, 1H, H-21), 1.92–1.64 (m, 5H), 1.58 (br s, 3H), 1.55–1.45 (m, 5H), 1.40 (br s, 4H), 1.10–1.32 (m, 5H), 1.04 (s, 3H, H-23), 0.97 (s, 6H, H-26, H-27), 0.88 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.7 Hz, H-5). 13C (CDCl3, 75 MHz): 165.0 (C-30, C-31), 161.6 (C-20), 135.0 (C-10′), 133.3 (C-5′), 132.1 (C-2′), 130.7 (C-1′), 128.8 (C-8′), 128.7 (C-4′), 128.1 (C-5′), 127.6 (C-3′), 126.7 (C-6′), 123.9 (C-7′), 123.6 (C-29), 79.2 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.2 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.8 (C-16), 34.5 (C-7), 32.1 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 593.4439 calcd. for (C41H57N2O), found 593.4465 [M + H]+. HPLC purity: 98%.
30). Mp: 128–131 °C. 1H (CDCl3, 300 MHz): 8.71 (s, 1H, H-31), 8.27 (s, 1H, H-30), 8.13–8.02 (m, 2H, H-1′, H-4′), 7.94–7.81 (m, 3H, H-3′, H-6′, H-9′), 7.59–7.47 (m, 2H, H-7′, H-8′), 5.68 (s, 1H, H-29a), 5.50 (s, 1H, H-29b), 3.23–3.10 (m, 1H, H-3), 3.08–2.91 (m, 1H, H-19), 2.36–2.19 (m, 1H, H-21), 1.92–1.64 (m, 5H), 1.58 (br s, 3H), 1.55–1.45 (m, 5H), 1.40 (br s, 4H), 1.10–1.32 (m, 5H), 1.04 (s, 3H, H-23), 0.97 (s, 6H, H-26, H-27), 0.88 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.67 (d, 1H, J = 9.7 Hz, H-5). 13C (CDCl3, 75 MHz): 165.0 (C-30, C-31), 161.6 (C-20), 135.0 (C-10′), 133.3 (C-5′), 132.1 (C-2′), 130.7 (C-1′), 128.8 (C-8′), 128.7 (C-4′), 128.1 (C-5′), 127.6 (C-3′), 126.7 (C-6′), 123.9 (C-7′), 123.6 (C-29), 79.2 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.2 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.8 (C-16), 34.5 (C-7), 32.1 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 593.4439 calcd. for (C41H57N2O), found 593.4465 [M + H]+. HPLC purity: 98%.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate (93
ethyl acetate (93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) as an eluent system. Rf 0.42 (hexane
7) as an eluent system. Rf 0.42 (hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc 70
EtOAc 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Mp: 106–109 °C. 1H (CDCl3, 300 MHz): 8.46 (s, 1H, H-31), 8.19 (s, 1H, H-30), 7.42 (d, 1H, J = 1.6 Hz, H-4′), 7.18 (dd, 1H, J = 8.0, 1.6 Hz, H-1′), 6.85 (d, 1H, J = 7.9 Hz, H-6′), 6.02 (s, 2H, H-7′), 5.64 (s, 1H, H-29a), 5.45 (s, 1H, H-29b), 3.17 (dd, 1H, J = 10.8, 5.1 Hz, H-3), 3.04–2.86 (m, 1H, H-19), 2.35–2.14 (m, 1H, H-21), 1.91–1.61 (m, 5H), 1.58 (br s, 3H), 1.53–1.41 (m, 5H), 1.39 (br s, 4H), 1.12–1.30 (m, 5H), 1.02 (s, 3H, H-23), 0.95 (s, 6H, H-26, H-27), 0.85 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.66 (d, 1H, J = 9.6 Hz, H-5). 13C (CDCl3, 75 MHz): 164.6 (C-30, C-31), 161.1 (C-20), 150.4 (C-3′), 148.5 (C-2′), 128.9 (C-5′), 125.2 (C-6′), 123.2 (C-29), 108.5 (C-1′), 106.8 (C-4′), 101.7 (C-7′), 79.2 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 32.9 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 587.4208 calcd. for (C38H55N2O3), found 587.4207 [M + H]+. HPLC purity: >99%.
30). Mp: 106–109 °C. 1H (CDCl3, 300 MHz): 8.46 (s, 1H, H-31), 8.19 (s, 1H, H-30), 7.42 (d, 1H, J = 1.6 Hz, H-4′), 7.18 (dd, 1H, J = 8.0, 1.6 Hz, H-1′), 6.85 (d, 1H, J = 7.9 Hz, H-6′), 6.02 (s, 2H, H-7′), 5.64 (s, 1H, H-29a), 5.45 (s, 1H, H-29b), 3.17 (dd, 1H, J = 10.8, 5.1 Hz, H-3), 3.04–2.86 (m, 1H, H-19), 2.35–2.14 (m, 1H, H-21), 1.91–1.61 (m, 5H), 1.58 (br s, 3H), 1.53–1.41 (m, 5H), 1.39 (br s, 4H), 1.12–1.30 (m, 5H), 1.02 (s, 3H, H-23), 0.95 (s, 6H, H-26, H-27), 0.85 (s, 3H, H-25), 0.81 (s, 3H, H-28), 0.75 (s, 3H, H-24), 0.66 (d, 1H, J = 9.6 Hz, H-5). 13C (CDCl3, 75 MHz): 164.6 (C-30, C-31), 161.1 (C-20), 150.4 (C-3′), 148.5 (C-2′), 128.9 (C-5′), 125.2 (C-6′), 123.2 (C-29), 108.5 (C-1′), 106.8 (C-4′), 101.7 (C-7′), 79.2 (C-3), 55.4 (C-5), 50.5 (C-9, C-18), 43.3 (C-17), 42.9 (C-14), 41.0 (C-8), 40.1 (C-22), 39.0 (C-4), 38.8 (C-1), 38.1 (C-13), 37.3 (C-10, C-19), 35.7 (C-16), 34.5 (C-7), 32.9 (C-21), 28.1 (C-23), 27.6 (C-2, C-12), 27.5 (C-15), 21.2 (C-11), 18.5 (C-6), 18.1 (C-28), 16.2 (C-25), 16.1 (C-26), 15.5 (C-24), 14.7 (C-27). HRMS (ESI) m/z: 587.4208 calcd. for (C38H55N2O3), found 587.4207 [M + H]+. HPLC purity: >99%.
Biological evaluation of compounds 4a–p
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well for 6-OHDA-induced cytotoxicity and 5000 cells per well for RSL3/erastin-induced ferroptosis, and after treatments, cellular viability was measured by the colorimetric MTT assay.63 The tetrazolium salt MTT is reduced by metabolically viable cells to a colored water-insoluble formazan salt. Following treatments, the MTT was incubated for 2 h at 37 °C in a 5% CO2 atmosphere (final concentration 0.5 mg ml−1). Then, the formazan crystals were dissolved with 200 μl of 20% sodium dodecyl sulfate (pH 4.7), and measured spectrophotometrically at 570 and 650 nm using a microplate reader (Multiskan GO, Thermo Scientific). Results are expressed as the mean ± SD (percentage of the control). Control wells containing each compound in the absence of cells and reagents were included in all experiments to exclude interferences with the colorimetric measurements.
000 cells per well for 6-OHDA-induced cytotoxicity and 5000 cells per well for RSL3/erastin-induced ferroptosis, and after treatments, cellular viability was measured by the colorimetric MTT assay.63 The tetrazolium salt MTT is reduced by metabolically viable cells to a colored water-insoluble formazan salt. Following treatments, the MTT was incubated for 2 h at 37 °C in a 5% CO2 atmosphere (final concentration 0.5 mg ml−1). Then, the formazan crystals were dissolved with 200 μl of 20% sodium dodecyl sulfate (pH 4.7), and measured spectrophotometrically at 570 and 650 nm using a microplate reader (Multiskan GO, Thermo Scientific). Results are expressed as the mean ± SD (percentage of the control). Control wells containing each compound in the absence of cells and reagents were included in all experiments to exclude interferences with the colorimetric measurements.
Author contributions
F. A. M. conducted the synthesis, purification, and identification of the compounds, performed data analysis and evaluation, and contributed to investigation, validation, visualization, drafting of the original manuscript, and editing. N. P. A. carried out the biological experiments, interpreted and evaluated the results, and contributed to conceptualization, formal analysis, investigation, validation, visualization, drafting of the original manuscript, and editing. M. B. F. contributed to writing – review and editing, original draft preparation, visualization, validation, supervision, funding acquisition, conceptualization, investigation, project administration, and resource management. G. A. S. contributed similarly to M. B. F., providing support in writing – review and editing, original draft preparation, visualization, validation, supervision, funding acquisition, conceptualization, investigation, project administration, and resource management. All authors critically reviewed and approved the final version of the manuscript and accept responsibility for all aspects of the work.Conflicts of interest
The authors declare no competing interests.Data availability
The data supporting this article have been included in the supplementary information (SI).Supplementary information is available. See DOI: https://doi.org/10.1039/d5md00753d.
Acknowledgements
G. A. S. acknowledges financial support from the National Scientific and Technical Research Council (CONICET, grant PIP11220210100175CO), the National Agency for the Promotion of Science and Technology (ANPCyT, grant PICT 2021-0143), and Universidad Nacional del Sur (grants PGI 24B292 and PGI 24B358), Argentina. M. B. F. acknowledges financial support of ANPCyT (grant PICT 2017-1443) and Universidad Nacional del Sur (grant PGI-UNS 24/Q105), Argentina. G. A. S. and N. P. A. are Researcher Members of CONICET, and M. B. F. is a Research Member of CIC. F. A. M. gratefully acknowledges CONICET for her doctoral fellowship. The authors acknowledge the use of the image of Chuquiraga erinacea (photo by John Doe), available at Wikimedia Commons under the Creative Commons Attribution 2.0 Generic License (CC BY 2.0).Notes and references
- A. J. Lees, J. Hardy and T. Revesz, Lancet, 2009, 373, 2055–2066 CrossRef CAS PubMed.
- L. Mahoney-Sánchez, H. Bouchaoui, S. Ayton, D. Devos, J. A. Duce and J. C. Devedjian, Prog. Neurobiol., 2021, 196(101890), 101890 CrossRef PubMed.
- K. J. Lin, S. D. Chen, K. L. Lin, C. W. Liou, M. Y. Lan and Y. C. Chuang, et al. , Cells, 2022, 11(23), 3829 CrossRef CAS PubMed.
- L. Liu, Y. Cui, Y.-Z. Chang and P. Yu, Sci. Rep., 2023, 13(1), 15365 CrossRef CAS.
- X. Dong-Chen, C. Yong, X. Yang, S. Chen-Yu and P. Li-Hua, Signal Transduction Targeted Ther., 2023, 8(1), 73 CrossRef PubMed.
- N. Teferi, M. Challa, T. Woodiwiss, B. Allen and M. Petronek, Redox Experimental Medicine, 2023, 1 Search PubMed.
- M. B. H. Youdim, L. Kupershmidt, T. Amit and O. Weinreb, Parkinsonism Relat. Disord., 2014, 20, S132–S136 CrossRef PubMed.
- Z. L. Wang, L. Yuan, W. Li and J. Y. Li, Trends Mol. Med., 2022, 28(4), 258–269 CrossRef CAS PubMed.
- K. McFarthing, S. Buff, G. Rafaloff, K. Pitzer, B. Fiske and A. Navangul, et al. , J. Parkinson's Dis., 2024, 14, 899–912 CrossRef PubMed.
- N. P. Alza, P. A. Iglesias González, M. A. Conde, R. M. Uranga and G. A. Salvador, Front. Cell. Neurosci., 2019, 13, 175 CrossRef CAS PubMed.
- D. Hernandez-Baltazar, L. M. Zavala-Flores and A. Villanueva-Olivo, Neurologia, 2017, 32, 533–539 CrossRef CAS PubMed.
- P. A. Iglesias González, M. A. Conde, V. González-Pardo, R. M. Uranga and G. A. Salvador, Toxicol. In Vitro, 2019, 60, 400–411 CrossRef PubMed.
- N. Simola, M. Morelli and A. R. Carta, Neurotoxic. Res., 2007, 11, 151–167 CrossRef CAS PubMed.
- H. Z. Long, Y. Cheng, Z. W. Zhou, H. Y. Luo, D. D. Wen and L. C. Gao, Front. Pharmacol., 2021, 12, 648636 CrossRef CAS PubMed.
- H. H. Rahman, J. Bajgai, A. Fadriquela, S. Sharma, T. T. Trinh, R. Akter, Y. J. Jeong, S. H. Goh, C. S. Kim and K. J. Lee, Molecules, 2021, 26(17), 5327 CrossRef PubMed.
- S. Andrade, D. Nunes, M. Dabur, M. J. Ramalho, M. C. Pereira and J. A. Loureiro, Pharmaceutics, 2023, 15(1), 212 CrossRef PubMed.
- D. Cui, Y. Chen, B. Ye, W. Guo, D. Wang and J. He, Phytomedicine, 2023, 121, 155101 CrossRef PubMed.
- R. Goyal, P. Mittal, R. K. Gautam, M. A. Kamal, A. Perveen, V. Garg, A. Alexiou, M. Saboor, S. Haque, A. Farhana, M. Papadakis and G. M. Ashraf, Nutr. Metab., 2024, 21, 26 CrossRef PubMed.
- R. A. Hill and J. D. Connolly, Nat. Prod. Rep., 2013, 30, 1028–1065 RSC.
- B. Bednarczyk-Cwynar and L. Zaprutko, Phytochem. Rev., 2014, 14, 203–231 CrossRef PubMed.
- A. Hordyjewska, A. Ostapiuk and A. Horecka, J. Pre-Clin. Clin. Res., 2018, 12, 72–75 CrossRef.
- W. Wang, Y. Wang, M. Liu, Y. Zhang, T. Yang, D. Li, Y. Huang, Q. Li, G. Bai and L. Shi, J. Cell. Mol. Med., 2018, 23, 586–595 CrossRef PubMed.
- L. González-Cofrade, B. de las Heras, L. Apaza Ticona and O. M. Palomino, Planta Med., 2019, 85, 1304–1315 CrossRef PubMed.
- N. V. Heise, J. A. Schüler, T. E. Orlamünde, B. Brandes, H. P. Deigner, A. Al-Harrasi and R. Csuk, Mediterr. J. Chem., 2022, 12(2), 188–200 CrossRef CAS.
- N. H. Harun, A. W. Septama, W. A. N. W. Ahmad and R. Suppian, Chin. Herb. Med., 2020, 12, 118–124 Search PubMed.
- G. Nistor, C. Trandafirescu, A. Prodea, A. Milan, A. Cristea, R. Ghiulai, R. Racoviceanu, A. Mioc, M. Mioc, V. Ivan and C. Şoica, Molecules, 2022, 27(19), 6552 CrossRef CAS PubMed.
- H. Hussain, J. Xiao, A. Ali, I. R. Green and B. Westermann, Nat. Prod. Rep., 2023, 40, 412–451 RSC.
- J. Liu, X. Yin, C. Kou, R. Thimmappa, X. Hua and Z. Xue, Plant Commun., 2024, 5(4), 100845 CrossRef CAS PubMed.
- D. D. Duan, C. Y. Bu, J. Cheng, Y. N. Wang and G. L. Shi, J. Econ. Entomol., 2011, 104, 375–378 CrossRef CAS PubMed.
- L. B. Silva, R. L. B. Strapasson, D. Riva, M. J. Salvador and M. É. A. Stefanello, Rev. Bras. Farmacogn., 2011, 21, 556–559 CrossRef CAS.
- M. A. Haque, M. M. Hassan, A. Das, B. Begum, M. Y. Ali and H. Morshed, J. Appl. Pharm. Sci., 2012, 2(6), 79–83 Search PubMed.
- J. Asgarpanah, N. Dakhili, F. Mirzaee, M. Salehi, M. Janipour and E. Rangriz, Pharmacogn. J., 2015, 8, 42–43 CrossRef.
- E. Bouattour, J. Fakhfakh, D. Frikha Dammak, K. Jabou, M. Damak and R. Mezghani Jarraya, Chem. Biodiversity, 2016, 13, 1674–1684 CrossRef CAS PubMed.
- D. Romo-Asunción, M. A. Ávila-Calderón, M. A. Ramos-López, J. E. Barranco-Florido, S. Rodríguez-Navarro, S. Romero-Gomez, E. J. Aldeco-Pérez, J. R. Pacheco-Aguilar and M. A. Rico-Rodríguez, Fla. Entomol., 2016, 99, 345–351 CrossRef.
- M. Gurovic, M. Castro, V. Richmond, M. Faraoni, M. Maier and A. Murray, Planta Med., 2009, 76, 607–610 CrossRef PubMed.
- A. H. Laghari, S. Memon, A. Nelofar and K. M. Khan, Ind. Crops Prod., 2011, 34, 1141–1145 CrossRef CAS.
- H. R. Siddique and M. Saleem, Life Sci., 2011, 88, 285–293 CrossRef CAS PubMed.
- N. J. C. Furtado, L. Pirson, H. Edelberg, L. M. Miranda, C. Loira-Pastoriza, V. Preat, Y. Larondelle and C. André, Molecules, 2017, 22, 400 CrossRef PubMed.
- F. P. Beserra, A. J. Vieira, L. F. S. Gushiken, E. O. de Souza, M. F. Hussni, C. A. Hussni, R. H. Nóbrega, E. R. M. Martinez, C. J. Jackson, G. L. de Azevedo Maia, A. L. Rozza and C. H. Pellizzon, Oxid. Med. Cell. Longevity, 2019, 1–20 Search PubMed.
- S. Kumar, N. Misra, K. Raj, K. Srivastava and S. K. Puri, Nat. Prod. Res., 2008, 22, 305–319 CrossRef CAS PubMed.
- M. Saini, M. F. Khan, R. Sangwan, M. A. Khan, A. Kumar, R. Verma, T. Ahamad and S. Jain, ChemistrySelect, 2019, 4, 1800–1805 CrossRef CAS.
- M. F. Khan, C. K. Maurya, K. Dev, D. Arha, A. K. Rai, A. K. Tamrakar and R. Maurya, Bioorg. Med. Chem. Lett., 2014, 24, 2674–2679 CrossRef CAS PubMed.
- M. F. Khan, D. P. Mishra, E. Ramakrishna, A. K. Rawat, A. Mishra, A. K. Srivastava and R. Maurya, Med. Chem. Res., 2014, 23, 4156–4166 CrossRef CAS.
- B. Bednarczyk-Cwynar, T. Wiecaszek and P. Ruszkowski, Nat. Prod. Commun., 2016, 11(9), 1237–1238 CrossRef PubMed.
- V. R. Machado, L. P. Sandjo, G. L. Pinheiro, M. H. Moraes, M. Steindel, M. G. Pizzolatti and M. W. Biavatti, Nat. Prod. Res., 2017, 32, 275–281 CrossRef PubMed.
- M. J. Castro, F. Musso, V. Vetere and M. B. Faraoni, J. Organomet. Chem., 2019, 880, 333–340 CrossRef CAS.
- K. Liu, X. Zhang, L. Xie, M. Deng, H. Chen, J. Song, J. Long, X. Li and J. Luo, Pharmacol. Res., 2021, 164, 105373 CrossRef CAS PubMed.
- H. V. T. Phan, T. H. Duong, D. D. Pham, H. A. Pham, V. K. Nguyen, T. P. Nguyen and J. Sichaem, Nat. Prod. Res., 2020, 36(1), 1–7 CrossRef PubMed.
- H. T. T. Le, Q. C. Chau, T. H. Duong, Q. T. P. Tran, N. K. T. Pham, T. H. T. Nguyen, N. H. Nguyen and J. Sichaem, Molbank, 2021, M1306 CrossRef CAS.
- J. Pokorny, S. Krajcovicova, M. Hajduch, M. Holoubek, S. Gurska, P. Dzubak, T. Volna, I. Popa and M. Urban, Future Med. Chem., 2018, 10(5), 483–491 CrossRef CAS PubMed.
- J. Safari and S. Gandomi-Ravandi, RSC Adv., 2014, 4, 46224–46249 RSC.
- L. Subedi, O. W. Kwon, C. Pak, G. Lee, K. Lee, H. Kim and S. Y. Kim, BMC Neurosci., 2017, 18, 82 CrossRef PubMed.
- S. S. Chourasiya, D. Kathuria, A. A. Wani and P. V. Bharatam, Org. Biomol. Chem., 2019, 17, 8486–8521 RSC.
- L. Muzio, R. Sirtori, D. Gornati, S. Eleuteri, A. Fossaghi, D. Brancaccio, L. Manzoni, L. Ottoboni, L. De Feo, A. Quattrini, E. Mastrangelo, L. Sorrentino, E. Scalone, G. Comi, L. Marinelli, N. Riva, M. Milani, P. Seneci and G. Martino, Nat. Commun., 2020, 11(1), 3848 CrossRef PubMed.
- A. Wal, P. Wal, A. K. Rai and K. Raj, J. Pharm. Sci. Res., 2010, 2(1), 13–25 CAS.
- M. J. Castro, V. Richmond, M. B. Faraoni and A. P. Murray, Bioorg. Chem., 2018, 79, 301–309 CrossRef CAS PubMed.
- H. Neukirch, M. D'Ambrosio, S. Sosa, G. Altinier, R. Della Loggia and A. Guerriero, Chem. Biodiversity, 2005, 2, 657–671 CrossRef CAS PubMed.
- M. J. Castro, V. Richmond, C. Romero, M. S. Maier, A. Estévez-Braun, Á. G. Ravelo, M. B. Faraoni and A. P. Murray, Bioorg. Med. Chem., 2014, 22, 3341–3350 CrossRef CAS PubMed.
- M. C. Sousa, R. Varandas, R. C. Santos, M. Santos-Rosa, V. Alves and J. A. R. Salvador, PLoS One, 2014, 9, e89939 CrossRef PubMed.
- C. Scarpellini, G. Klejborowska, C. Lanthier, B. Hassannia, T. Vanden Berghe and K. Augustyns, Trends Pharmacol. Sci., 2023, 44(12), 902–916 CrossRef CAS PubMed.
- A. Southon, K. Szostak, K. M. Acevedo, K. A. Dent, I. Volitakis, A. A. Belaidi, K. J. Barnham, P. J. Crouch, S. Ayton, P. S. Donnelly and A. I. Bush, Br. J. Pharmacol., 2020, 177(3), 656–667 CrossRef CAS PubMed.
- A. Maniscalchi, O. N. Benzi Juncos, M. A. Conde, M. I. Funk, M. E. Fermento, M. M. Facchinetti, A. C. Curino, R. M. Uranga, N. P. Alza and G. A. Salvador, Redox Biol., 2024, 71, 103074 CrossRef CAS PubMed.
- N. P. Alza, A. P. Murray and G. A. Salvador, Naunyn-Schmiedeberg's Arch. Pharmacol., 2017, 390(12), 1229–1238 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |