 Open Access Article
Open Access ArticleReline-assisted synthesis of fcc-hcp Ni/Ni(OH)2 nanocatalyst for effective reductive hydrogenation of 4-nitrophenol
Md. Minhajul Alam
Khan
 a,
Shawon
Saha
a,
Shawon
Saha
 a,
Sumaya Nur
Mithila
a,
Yeasin Arafat
Tarek
a,
Sumaya Nur
Mithila
a,
Yeasin Arafat
Tarek
 b,
Akter Hossain
Reaz
b,
Akter Hossain
Reaz
 c and
Shakhawat H.
Firoz
c and
Shakhawat H.
Firoz
 *a
*a
aDepartment of Chemistry, Bangladesh University of Engineering and Technology (BUET), Dhaka-1000, Bangladesh. E-mail: shfiroz@chem.buet.ac.bd
bSchool of Mechanical and Mining Engineering, The University of Queensland, St Lucia, Queensland 4072, Australia
cDepartment of Chemistry, Michigan State University, East Lansing, MI 48824, USA
First published on 3rd January 2026
Abstract
This study presents a facile, eco-friendly, and controlled reline-assisted chemical reduction method to synthesize fcc-hcp Ni/Ni(OH)2 nanocomposites. The conventional chemical reduction process was modified by replacing water with reline to produce the Ni/Ni(OH)2 nanocomposite (Ni-reline). The incorporation of precursor salt in reline significantly influenced its physicochemical properties. Specifically, the optimized concentration of nickel salt in reline (0.4 mol dm−3) reduced its viscosity from 573 cP to 57 cP and increased its conductivity, which played a crucial role in the nucleation and growth of nanoparticles. As a consequence, coral reef-like nanostructures (∼39 nm) developed in reline, whereas in aqueous media, spherical Ni nanoparticles (Ni-Aq.) with a significantly larger particle size (∼92 nm) were formed. Elemental analysis further confirmed the presence of carbon in Ni-reline, derived from the DES matrix. Ni-Aq. and Ni-reline were evaluated as catalysts for the reductive hydrogenation of 4-nitrophenol to 4-aminophenol. Ni-reline exhibited superior catalytic activity, achieving 95% conversion in 20 minutes with a rate constant of 0.1336 min−1, 11 times higher than Ni-Aq. This enhancement is attributed to the mixed-phase structure, increased surface area, and surface-bound carbon species promoting adsorption and electron transfer. Moreover, Ni-reline demonstrated excellent reusability over five consecutive cycles due to easy magnetic separation with minimal catalyst loss. This study highlights the potential of reline to modify nanostructure growth and phase behavior for efficient, environmentally friendly catalytic applications.
1. Introduction
Rapid industrialization has resulted in increased environmental pollution, especially water contamination, emerging as a critical threat to human health and the ecosystem.1 Despite global awareness, industrial activities continue to discharge hazardous pollutants, especially in developing countries.2,3 Among the diverse pollutants affecting water bodies, nitrophenol and its derivatives are one of the most detrimental contaminants. These compounds are released during the production of synthetic dyes, medicines, pesticides, herbicides, and insecticides.4 The high toxicity of nitrophenols, particularly their potential to cause severe damage to human organs such as the kidney, liver, and central nervous system, has led to their classification as one of the “worst 114 organic contaminants” by the United States Environmental Protection Agency (USEPA).5 Given the risks associated with nitrophenol, it is crucial to find effective methods for its degradation or removal, as well as to explore opportunities for converting it into value-added chemical products. Hence, various remediation techniques have already been explored, including oxidation,6 microbial degradation,7 electrochemical removal,8 adsorption,9 and catalytic hydrogenation.10,11Among these, catalytic hydrogenation is remarkable due to its cost-effectiveness, high efficiency, and conversion ability of nitrophenols into less toxic aromatic amines.12–14 Metal nanoparticles (NPs), especially noble metals such as gold, silver, platinum, and palladium, have exhibited exceptional catalytic performance in the reduction of 4-NP.4,15 Although noble metals exhibit excellent catalytic activity, their limited availability and high cost severely limit their wider use, leading to a search for suitable catalysts that are abundant and cost-effective.16 Thus, transition metal nanoparticles (TMNPs) appear to be a viable alternative to noble metals in the role as catalysts. Among them, nickel nanoparticles show promise due to their cost, easy reusability, and good catalytic activity.17,18 However, developing Ni NPs with enhanced catalytic properties is hindered by their intrinsic instability. The magnetic dipole interactions in Ni NPs and strong van der Waals forces tend to promote the agglomeration of NPs, which is detrimental in reducing the active surface area and inducing some unwanted phase transitions.19,20 This may compromise the structural integrity of the NP, leading to a decrease in its catalytic efficiency. As a result, in order to alleviate Ni NP limitations, attempts to improve their catalytic characteristics have emerged as a primary focus.
Catalytic properties of a catalyst are intrinsically related to its shape and particle size, as these factors have a direct impact on surface area, active site distribution, and electronic structure.21 For example, Wang et al.22 synthesized Ni NPs with chemical modifiers like citric acid and CTAB and tested their catalytic activity in the hydrogenation of 4-NP to 4-AP. The citric acid-modified Ni NPs showed increased catalytic activity due to their smaller particle size and improved dispersion properties. While Darband et al.23 used Ni nanocones as a very efficient and stable catalyst for the electrochemical hydrogen evolution reaction. Aside from size and shape, phase structure can provide additional flexibility for modulating the catalytic performance of desired materials because different crystal phases have different spatial atomic arrangements and coordination status, which can affect substrate adsorption or activation and product desorption.24,25 For instance, Zhang et al.26 examined the hydrogenation of nitrophenols using unsupported Ni nanocrystals with fcc and hcp phases. The hcp-phase Ni nanocrystals showed superior catalytic activity and selectivity, because of their enhanced surface energy and higher active site concentration.
In addition, Yang et al.27 synthesized a Ni catalyst with both hcp and fcc crystal phases, demonstrating remarkable catalytic hydrogenation efficacy across a wide range of substrates, including nitro compounds, aldehydes, ketones, alkenes, and nitrogen-containing heterocycles. It is expected that the mixed crystal phase exerts a synergistic effect, considerably increasing the catalyst's intrinsic activity. However, phase stability remains a concern, as metastable domains may relax over time, diminishing catalytic efficiency.28 Ni exhibits two allotropes: stable fcc and metastable hcp. The hcp phase is less energetically favorable and typically requires external stabilization.29 In contrast, Ni(OH)2 in its hcp crystal is well stable, and the hcp-phase Ni(OH)2 provides strong adsorption sites for reaction intermediates and facilitates proton-coupled electron transfer, ultimately enhancing catalytic properties.30,31 So, Ni/Ni(OH)2 in fcc-hcp mixed phase with controlled morphology has high potential to perform as a prominent catalyst for 4-NP reduction.
However, controlled synthesis of NPs often requires adding stabilizers or capping agents to control particle size and prevent aggregation.32 For instance, Sidhaye et al.33 utilized sodium dodecyl sulfate and oleic acid as surfactants and capping agents to regulate the growth of Ni NPs. Xu et al.34 achieved a uniform, monomorphic, self-assembled flower-like Ni microstructure using polyvinylpyrrolidone. Kim et al.35 synthesized ultrafine Ni powders in the presence of sodium carboxymethyl cellulose, while Nandi et al.36 prepared Ni using sulfonated polybutadiene. But these additives often contain long-chain hydrocarbons or synthetic polymers that are harmful and toxic, and may cause environmental contamination.37 Hence, there is a growing interest in developing alternatives to conventional stabilizers or capping agents that will be environmentally friendly and enable precise control over NPs growth.
In search for alternatives to conventional stabilizers or capping agents, deep eutectic solvents (DESs), a class of green solvents, have emerged. DESs are formed by the combination of hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs), resulting in stable liquids with unique and tunable physicochemical properties. It has gained significant attention, particularly in the field of NPs synthesis, due to its ability to regulate NPs nucleation and growth by stabilizing surface charges, modulating reduction potentials, and altering chemical activities.38 For example, Gomes-Junior et al.39 reported ultra-small CeO2 NPs (∼0.73 nm) with controlled spherical morphology in reline DES, where reline governed reaction kinetics and stabilized nascent surfaces. In related work, Datta et al.40 showed that reline facilitates Au NP formation by (i) acting as a mild reducing environment via ammonia released from urea hydrolysis and (ii) tuning gold-precursor speciation to stabilize Au+ and suppress rapid disproportionation, thereby enabling controlled reduction. So, DES is now an established system for controlling the growth dynamics of NPs, particularly reline, a eutectic mixture of choline chloride and urea in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratios with a melting point between 12 °C and 24 °C, which is significantly lower than their individual melting point,41 has been widely studied. Because of its supramolecular structure and strong hydrogen-bonding network, reline can act as both a solvent medium and a stabilizing agent, effectively controlling NP shape and size.42,43 This dual role is particularly advantageous for developing NPs with unique surface characteristics and specific properties essential for catalytic applications.44,45 Given reline's ability to control the size of particles, morphology, and even phase structure, using reline as a growth medium in chemical reduction methods presents a promising, environmentally friendly strategy for synthesizing Ni/Ni(OH)2 nanocomposite hydrogenation catalysts with increased activity and selectivity. However, the significance of reline in NP morphology and phase structure optimization, notably in Ni NPs, remains unclear.
2 molar ratios with a melting point between 12 °C and 24 °C, which is significantly lower than their individual melting point,41 has been widely studied. Because of its supramolecular structure and strong hydrogen-bonding network, reline can act as both a solvent medium and a stabilizing agent, effectively controlling NP shape and size.42,43 This dual role is particularly advantageous for developing NPs with unique surface characteristics and specific properties essential for catalytic applications.44,45 Given reline's ability to control the size of particles, morphology, and even phase structure, using reline as a growth medium in chemical reduction methods presents a promising, environmentally friendly strategy for synthesizing Ni/Ni(OH)2 nanocomposite hydrogenation catalysts with increased activity and selectivity. However, the significance of reline in NP morphology and phase structure optimization, notably in Ni NPs, remains unclear.
Herein, this study focuses on developing of a simple chemical reduction approach for synthesizing fcc-hcp Ni/Ni(OH)2 mixed-phase nanocomposite with reline as the growth medium. Unique properties of reline allow for the formation of a mixed crystal phase and precise control over composite shape. In addition, the physicochemical features of reline are investigated in the controlled production of the Ni/Ni(OH)2 nanocomposite. This fcc-hcp Ni/Ni(OH)2 nanocomposite is then used as a hydrogenation catalyst to reduce 4-NP, with performance comparable to that of fcc Ni.
2 Experimental
2.1 Materials
Nickel chloride hexahydrate (NiCl2·6H2O; Sigma-Aldrich, Germany), sodium hydroxide pellets (NaOH; Sigma-Aldrich, Germany), hydrazine hydrate 99% (N2H4·H2O; Sigma-Aldrich, Germany), choline chloride (C5H14ClNO; TCI, Japan), urea crystal (CO(NH2)2; Merck, Germany), sodium borohydride (NaBH4; Sigma-Aldrich, Germany) and 4-nitrophenol (C6H5NO3; Merck, Germany) were procured from commercial sources and utilized directly without any further purification. Deionized water (approximately 0.66 µS cm−1) was utilized for preparing the solutions in the experiment. The DI water was obtained from a from a DI-water purification system (Barnstead NanoPure, Thermo Fisher Scientific, USA).2.2 Synthesis of materials
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, heating and stirring the mixture at 80 °C for 2 hours, resulting in a transparent liquid. Subsequently, the formed DES was cooled to room temperature, tightly sealed, and stored under ambient conditions.
2, heating and stirring the mixture at 80 °C for 2 hours, resulting in a transparent liquid. Subsequently, the formed DES was cooled to room temperature, tightly sealed, and stored under ambient conditions.
2.3 Characterization
The surface morphology with elemental composition of the synthesized samples was characterized using a field emission scanning electron microscopy (FESEM, model JSM-7600F, JEOL, Japan) integrated with energy dispersive X-ray spectroscopy (EDS). Phase identification and crystallinity were examined through X-ray diffraction (XRD) analysis using a Smartlab diffractometer (Rigaku, Japan). Functional groups present in the nanoparticles were determined via Fourier transform infrared (FTIR) spectroscopy, with spectra collected in transmission mode over the range of 4000–400 cm−1 using an FTIR-8400 spectrophotometer (Shimadzu, Japan). Surface chemical composition was further analyzed by X-ray photoelectron spectroscopy (XPS) employing a Kratos AXIS Nova system (Kyoto, Japan) equipped with a monochromatic Al Kα source (photon energy = 1486.6 eV).2.4 Surface charge analysis
The surface charge characteristics of the material were assessed using the point of zero charge (PZC) method, which identifies the pH at which the net surface charge becomes neutral under controlled conditions of temperature, pressure, and ionic strength.48,49 For this analysis, 0.2 g of the sample was suspended in 40 mL of 0.1 M NaNO3 solution within 50 mL centrifuge tubes. The pH of each suspension was adjusted to target values of 2, 4, 6, 7, 8, 10, and 12 (±0.1) using 0.1 M NaOH. Before initiating the agitation process, the initial pH (pHi) of the supernatants was measured. Next, the tubes were placed on an orbital shaker (Stuart-SSL1) and shaken for 24 hours at 200 rpm. After settling, each supernatant's final pH (pHf) was determined, and to calculate the PZC, the pH change (ΔpH = pHi − pHf) was plotted against the initial pH. To ensure consistency, the operation was performed again with a 0.05 M NaNO3 solution. All measurements were carried out in triplicate, and the mean result was used for further analysis.2.5 Investigation of the catalytic activity in the reductive hydrogenation of 4-NP
The catalytic properties of Ni-Aq. and Ni-reline was investigated in the reductive hydrogenation of 4-NP to 4-AP through a UV-vis spectroscopic method. To confirm the presence of 4-NP, a 1 mM solution (300 µL) was added to 6 mL of DI water, and the absorbance at 317 nm was measured. While, in the absence of a catalyst, mixing NaBH4 (10 mM, 1 mL) with the 4-NP solution resulted in an immediate color shift to a bright yellow, indicating the formation of phenolate ions, as detected at 400 nm by UV-vis spectroscopy. Then, catalytic tests involved adding 1 mL of a 5 mg mL−1 nanocatalyst suspension to the mixture of 4-NP, NaBH4, and water, with reaction progress tracked via absorbance decrease at 400 nm until complete reduction. Effects of pH (pH 3 to pH 11) and catalyst dosage (3 to 5 mg mL−1) on reduction efficiency were investigated. Catalyst stability and reusability were assessed over multiple cycles by magnetic separation, washing, and reused without drying.3 Results and discussion
3.1 Characterization
The surface characteristics and morphology of the synthesized materials were analyzed in greater detail using field emission scanning electron microscopy (FESEM), as shown in Fig. 2. Fig. 2(a) and (b) display the FESEM images of Ni-Aq. at low and high magnifications, respectively, while Fig. 2(c) and (d) present the FESEM images of Ni-reline at low and high magnifications, respectively. In Fig. 2(a) and (b), it is observed that monodispersed Ni nanospheres were formed with an average particle size of ∼92 nm, while Fig. 2(c) and (d) depicts that Ni-reline possessed a coral reef like morphology, with an average particle size of ∼39 nm, which is smaller than Ni-Aq., and other than particle size, the nanostructure developed high porosity and surface defects. A comparative histogram of particle size distribution for both samples is provided in Fig. S1. These results demonstrated a notable difference in both morphology and particle size between the Ni-Aq. and Ni-reline. This variation is attributed to the distinct physicochemical properties of the reline system, which affect the nucleation and growth dynamics of the nanoparticles.50Additionally, the elemental composition of Ni-reline was analyzed using energy dispersive X-ray spectroscopy (EDS), as shown in Fig. 2(e). The EDS spectrum of Ni-reline exhibited prominent peaks at 0.525 keV and 0.851 keV, corresponding to the K-shell of oxygen (O) and the L-shell of nickel (Ni), respectively. The O content was found to be 15.68%, while the Ni content was 68.13%. These findings suggest the possibility of Ni oxide or hydroxide being present, in addition to metallic Ni. Moreover, two distinct peaks at 0.277 keV and 0.392 keV were observed, which are attributed to the K-shell of carbon (C) and nitrogen (N), likely originating from the reline counterparts. Based on these observations, it is indicative of the possible formation of a Ni/Ni(OH)2 nanocomposite, enriched with carbon content.
Furthermore, the crystalline structure and phase purity of the synthesized materials were analyzed using X-ray diffraction (XRD) as depicted in Fig. 3(a). Fig. 3(a(i)) represents Ni-Aq. (blue line), displayed three distinct peaks at 2θ = 44.5°, 51.8°, and 76.4°, which correspond to the (111), (200), and (220) planes of pure face-centered cubic (fcc) Ni (JCPDS card: 96-900-9863).51 As no additional peaks were present, this indicated the purity of the synthesized Ni NPs in the aqueous system. In contrast, Fig. 3(a(ii)) represents Ni-reline (red line) showcasing new peaks at 33.4°, 38.5°, and 59.3° denoted by (*), corresponding to the (100), (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) and (110) planes referred to the formation of β-Ni(OH)2 with brucite type hexagonal close-packed (hcp) crystal system (JCPDS card: 96-154-8812), along with the characteristic fcc Ni peaks.52 The presence of both fcc and hcp phases suggested that the Ni-reline sample comprises a mixed crystal system and confirmed the formation of a Ni/Ni(OH)2 nanocomposite.
) and (110) planes referred to the formation of β-Ni(OH)2 with brucite type hexagonal close-packed (hcp) crystal system (JCPDS card: 96-154-8812), along with the characteristic fcc Ni peaks.52 The presence of both fcc and hcp phases suggested that the Ni-reline sample comprises a mixed crystal system and confirmed the formation of a Ni/Ni(OH)2 nanocomposite.
 | ||
| Fig. 3 (a) XRD spectra of (i) Ni-Aq. and (ii) Ni-reline, (b) FTIR spectra of (i) Ni-Aq. and (ii) Ni-reline, and High-resolution XPS spectra of (c) Ni 2p and (d) O 1s for Ni-reline. | ||
Moreover, the XRD spectrum indicated that Ni-reline exhibited broad peaks with decreasing intensity compared to Ni-Aq., suggesting that Ni-reline possessed lower crystallinity and smaller crystallite size. Ni-Aq. remained in a highly crystalline state with 98% crystallinity, while the crystallinity of Ni-reline decreased to 80%. The decrease in crystallinity was likely due to the presence of the highly viscous reline system, which inhibited crystal growth and disrupted the reduction process, ultimately leading to the formation of a new crystal system of hcp Ni(OH)2. The average crystallite size of Ni-Aq. and Ni-reline was found to be 16 nm and 7 nm, respectively, as calculated from the significant diffraction peaks using the Debye–Scherrer equation, as shown in eqn (1).53
 | (1) |
To further investigate the surface functional group present on the synthesized materials, Fourier transform infrared (FTIR) spectroscopy was performed. The corresponding FTIR spectra for Ni-Aq. and Ni-reline are shown in Fig. 3(b). In Fig. 3(b), distinctive peaks in the 400–650 cm−1 range, corresponding to Ni–O stretching vibrations, confirmed the formation of Ni in both samples.54 A band around 2200–2300 cm−1 was attributed to the stretching modes of CO2 absorbed from the air,55 while the broad peak at 3400–3600 cm−1 indicated –OH vibrations, suggesting hydrogen bonding and water absorption.56 In Ni-reline, additional peaks were observed, including those at 1000–1100 cm−1 (νC–O and νC–N vibrations), 1347 cm−1 (C–N stretching),57,58 and a band around 1630 cm−1, likely originating from the vibrations of the C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O bond (carbonyl group from urea), as well as an N–H vibration band, possibly due to ammonia formation during urea hydrolysis.59 The peak around 2800–3000 cm−1 corresponded to C–H stretching vibrations, linked to the alkyl chains in the DES structure, suggesting that carbon contents were present on the surface of the materials, originating from the adsorption of DES components.60 Therefore, the FTIR analysis suggested that carbon and nitrogen contents were likely present on the materials' surface, which correlated with the observations from the EDS analysis.
O bond (carbonyl group from urea), as well as an N–H vibration band, possibly due to ammonia formation during urea hydrolysis.59 The peak around 2800–3000 cm−1 corresponded to C–H stretching vibrations, linked to the alkyl chains in the DES structure, suggesting that carbon contents were present on the surface of the materials, originating from the adsorption of DES components.60 Therefore, the FTIR analysis suggested that carbon and nitrogen contents were likely present on the materials' surface, which correlated with the observations from the EDS analysis.
To further confirm the presence of Ni(OH)2 and metallic Ni in the Ni-reline, X-ray Photoelectron Spectroscopy (XPS) was performed. In Fig. 3(c), two distinct peaks at 873.5 eV and 855.5 eV corresponded to the Ni 2p1/2 and Ni 2p3/2 spin–orbit components of Ni(OH)2, respectively. These peaks were further accompanied by two satellite (sat.) peaks, which were associated with the Ni 2p1/2 and Ni 2p3/2 peaks. Upon deconvolution of the spectrum, additional signals at 867.2 eV and 852.6 eV were identified, which corresponded to the Ni 2p1/2 and Ni 2p3/2 binding energies of metallic Ni, indicating the presence of metallic Ni alongside Ni(OH)2. Further investigation of the O 1s spectra, displayed in Fig. 3(d), located a strong peak at 531.5 eV, indicating the existence of Ni(OH)2. In addition, a weaker signal at 529.5 eV revealed the presence of NiO with lower content. Furthermore, the XPS survey spectrum (Fig. S2(a)) revealed the presence of Ni, O, C, and N, with elemental composition information provided in Table S1. On top of that, the C 1s and N 1s XPS spectra, displayed in Fig. S2(b) and (c), confirmed the existence of C and N in the sample, which were obtained during synthesis. The C 1s spectra revealed peaks corresponding to C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O and C–C bonds, obtained from DES counterparts and the N 1s spectra also verified the existence of primary amine groups that are produced from the urea molecules. Thus, the XPS analysis established the formation of a Ni/Ni(OH)2 nanocomposite in the reline system, as evidenced by the presence of characteristic peaks for Ni(OH)2 and metallic Ni, along with the presence of C and N components from the reline system during the synthesis process.
O and C–C bonds, obtained from DES counterparts and the N 1s spectra also verified the existence of primary amine groups that are produced from the urea molecules. Thus, the XPS analysis established the formation of a Ni/Ni(OH)2 nanocomposite in the reline system, as evidenced by the presence of characteristic peaks for Ni(OH)2 and metallic Ni, along with the presence of C and N components from the reline system during the synthesis process.
3.2 Possible mechanism for DES-mediated Ni/Ni(OH)2 formation
In the present study, metallic Ni NP was obtained from the aqueous system, while in the reline system, Ni/Ni(OH)2 nanocomposite was formed. This obvious distinction emphasizes the unique function of reline in controlling nanoparticle growth dynamics. DESs play a significant role in nucleation and growth processes by neutralizing charges, modulating reduction potentials, changing chemical actions, altering crystal facets, and directing growth along distinct crystallographic directions.61,62 The distinctive physicochemical features and complex framework of DESs lead to nanomaterial growth dynamics that are markedly different from those of conventional aqueous systems.In aqueous media, as hydrazine hydrate was introduced to the NiCl2 solution, a stable Ni complex formed instantly. Upon addition of NaOH, this complex dissociated, resulting in the formation of Ni(OH)2via the sol–gel process.63 Afterwards, Ni(OH)2 was reduced by excess N2H4, resulting in the recrystallization of monodispersed spherical Ni NPs. The readily produced Ni complex acts as a protective matrix during particle formation, which prevents new particles from aggregating. Consequently, uncontrolled agglomeration was avoided during nucleation and growth, resulting in monodisperse spherical NPs. The reaction scheme of the formation of Ni NPs in an aqueous system is shown here as described by Choi et al.47
Step 1: Formation of pale violate gel
NiCl2(aq.) + 3N2H4(aq.) → [Ni(N2H4)3Cl2](thick![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) gel) gel) |
Step 2: Stabilization of complex
| [Ni(N2H4)3]Cl2 → [Ni(N2H4)2]Cl2 + N2H4 |
| [Ni(N2H4)3]Cl2 + 5/2N2H4 → [Ni(NH3)6]Cl2 + 3/2N2 |
Step 3: Ligand exchange via introducing OH− to form Ni NPs
| Ni2+ + N2H4 + OH− → 2Ni + N2 + 4H2O |
Reline possesses a considerably greater viscosity than most commonly used DES systems.64 The increased viscosity in reline was caused by the effect of urea as a hydrogen bond donor (HBD). Urea's strong hydrogen bonding characteristic assisted in the formation of a substantial and dense hydrogen bonding network with ChCl, effectively reducing the average free volume or void size within the solvent matrix.65 The ensuing structural compactness and reduced molecular mobility directly led to the reline system's enhanced viscosity.66 Interestingly, the incorporation of NiCl2 salt (Ni2+ ion) into the reline system resulted in a sudden decrease in viscosity, deviating from the conventional behavior found in most molecular solvents, where solute addition typically increases viscosity by promoting liquid structuring. As shown in Fig. 4(a), the addition of Ni2+ ions to an aqueous system resulted in a modest increase in viscosity, which is consistent with usual solvent–solute interactions. Conversely, the addition of Ni2+ ions significantly decreased the viscosity of the reline system, with a minimum of 0.4 mol dm−3 of Ni2+ ions. This drop in viscosity can be ascribed to the disruption of the reline system's native hydrogen bonding network. Such disruption effectively increases the average void size in the solvent matrix and facilitates enhancing molecular mobility and mass transport.67 In addition to the viscosity trends, Fig. 4(b) shows that the electrical conductivity of the reline system increased with Ni2+ ion concentration. The decreased viscosity with elevated metal ion concentrations enabled increased ion mobility, which improved overall mass transport throughout the system. All together, these results indicate that the reline's physicochemical properties were very sensitive to precursor concentration, which affected the system's viscosity and conductivity.
 | ||
| Fig. 4 Effect of Ni2+ concentration on (a) viscosity, (b) conductivity of aqueous and reline systems and (c) schematic illustration of the Ni/Ni(OH)2 formation in reline system. | ||
The native robust hydrogen bonding of the reline system was rapidly disrupted with the incorporation of Ni2+, resulting in the release of urea molecules. Additionally, the hygroscopic nature of pure reline, which contained trace amounts of water, led to the hydrolysis of the free urea molecules, resulting in the formation of ammonia.40
| NH2CONH2 + H2O → 2NH3 + CO2 |
(i) Step 1: The hydrogen bonding network of reline was disrupted, causing the generation of ammonia, which served as an agile reducing agent, aiding the reduction of Ni2+ ions to metallic Ni NPs. Initially, Ni2+ remains in high concentrations that facilitate fast nucleation of Ni NPs. Though there was a drop in viscosity as precursor salt was incorporated, the solvent's viscosity remained high enough to impede ionic mobility, resulting in partial reduction of some Ni2+ ions.
(ii) Step 2: The remaining unreacted Ni2+ ions formed a stable coordination complex with the hydrazine hydrate. Following the addition of NaOH, this compound underwent ligand exchange to decompose the Ni complex into Ni(OH)2. The excess hydrazine present in aqueous media could further decrease Ni(OH)2, however, in reline, the high viscosity persisted that limited ionic mobility and prevented excess hydrazine from reducing Ni(OH)2. As a consequence, Ni/Ni(OH)2 mixed-phase nanocomposite formed in place of pure metallic Ni. In Ni-reline, XRD analysis revealed that the Ni(OH)2 phase has a brucite-type hcp crystal structure. Each Ni2+ ion in this structure was octahedrally coordinated by six OH− ions, resulting in two-dimensional layers. These layers were tightly connected within the plane but very weakly kept together between planes due to van der Waals forces or hydrogen bonding. This feature facilitated anisotropic growth, culminating in the development of Ni(OH)2 nanosheet.69 A schematic illustration of the Ni/Ni(OH)2 formation in the reline system is represented in Fig. 4(c).
3.3 Catalytic hydrogenation of 4-NP to 4-AP
The catalytic hydrogenation of 4-NP to 4-AP in the presence of NaBH4 was carried out utilizing Ni-Aq. and Ni reline as hydrogenation catalyst. As shown in Fig. S3(a), UV-vis spectra demonstrated that the 4-NP solution initially exhibited an absorption peak at 317 nm, which shifted to 400 nm upon the addition of NaBH4, indicating the formation of the 4-nitrophenolate ion. Upon introduction of the catalyst, a new peak at 300 nm appeared, which confirms the formation of 4-AP, while the 400 nm peak gradually decreased, demonstrating the reduction process. In the 4-NP reduction process, NaBH4 can act as the reducing agent by dissociating in aqueous media to form borohydride ions (BH4−). These BH4− ions are capable of donating hydride ions (H−), which are essential for the reduction of the nitro group in 4-NP. This reaction is thermodynamically feasible, as the reduction potential of BH4− is sufficiently negative to reduce the nitro group into an amine group. However, in the absence of a catalyst, this reaction is kinetically restricted due to the high kinetic barrier caused by the electrostatic repulsion between the negatively charged BH4− and the phenolate ion (the deprotonated form of 4-nitrophenol under alkaline conditions).70 As a result, without a catalyst, the 400 nm peak remained unchanged after 120 minutes, indicating no significant reduction of 4-NP, as shown in Fig. S3(b). This highlights the necessity of a suitable catalyst for efficient reduction. To address this, Ni-Aq. and Ni-reline nanocatalysts were employed in the reduction process. As demonstrated in Fig. S3(c) and (d), Ni-Aq. showed a slow conversion rate, with the 400 nm peak decreasing over 90 minutes without reaching full conversion. In contrast, Ni-reline caused the 400 nm peak to disappear within 20 minutes, demonstrating its superior catalytic efficiency.A comparative catalytic performance of Ni-Aq. and Ni-reline after 20 minutes, depicted in Fig. 5(a), and UV-vis spectral analysis confirmed that Ni-reline exhibited remarkable catalytic performance in the reductive hydrogenation of 4-NP.
As Ni-reline possessed coral reef like morphology with smaller particle size compared to Ni-Aq., resulting in an increased active surface area for catalysis, additionally, the presence of carbon in Ni-reline enhanced its adsorption capability, which in turn promoted the reduction process. Moreover, the presence of a mixed-phase of Ni-reline (fcc Ni and hcp Ni(OH)2) enhanced electrical properties by developing a synergistic interface that facilitated charge transfer and increased the density of active sites. The coexistence of fcc and hcp phases resulted in different atomic configurations and electronic states, which boosted conductivity by allowing electrons to move more efficiently across phase boundaries. This heterogeneity also modified the local work function and surface potential, promoting faster electron exchange during catalytic reactions.30,71 So, the enhanced catalytic activity of Ni-reline can be ascribed to the following key features, its reduced particle size, distinct surface properties, and the presence of a mixed-phase crystal structure.
The kinetics of the reactions were considered to follow pseudo-first-order kinetics as there was a significant difference in the concentration of NaBH4 and 4-NP. Furthermore, to assess the efficacy of the synthesized nanocatalysts, pseudo-first-order kinetics was applied, as represented in eqn (2).72
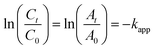 | (2) |
Another important parameter that affects the catalyst's performance is the pH of the reaction media. Hence, the most suitable pH condition for Ni-reline in the catalytic hydrogenation reaction was analyzed. At various pH conditions, the catalytic hydrogenation reaction of 4-NP to 4-Ap by Ni-reline was observed. Fig. 6(a) shows the effect of the solution's pH on the catalytic performance of Ni-reline in the hydrogenation reaction of 4-NP.
It was observed that Ni-reline exhibited superior catalytic activity at pH 3. At pH 3, the catalytic hydrogenation reaction was completed after 12 minutes, reaching equilibrium with an efficiency of 98%. In contrast, at pH 5, the catalytic hydrogenation reaction completed after 16 minutes, reaching equilibrium with an efficiency of 97%. At pH 7, after 20 minutes, the efficiency of Ni-reline was around 95%, but it did not reach equilibrium. On the other hand, as the solution pH increased and solution basicity rose, the efficiency of Ni-reline decreased, as shown in Table S4. Therefore, it could be said that as the pH of the solution decreased and the acidity increased, the catalytic performance of Ni-reline was significantly enhanced. The enhancement in performance was due to the modification in the catalyst surface charge. The catalyst's surface charge varied drastically as the pH of the solution changed, affecting its catalytic efficiency. In catalytic hydrogenation, the effective adsorption of reactant molecules onto the surface of the catalyst is crucial for accelerating the reduction reaction. Modifying the surface charge of the catalyst can significantly influence its adsorption behavior, thereby impacting overall catalytic performance.
Therefore, the surface charge of a material can be investigated using the PZC test, which determines the pH at which the surface charge of components become neutral under controlled conditions of temperature, pressure, and solution composition.73 The PZC test was conducted on Ni-reline and demonstrated an isoelectric point of 9.01, as shown in Fig. 6(b). Once the pH of the solution is less than PZC, the net surface charge on the solid surface for the adsorbent becomes positive because of the adsorption of excess protons. On the other hand, the net surface charge becomes negative when the pH of the solution is higher than PZC. Hence, Ni-reline remained positively charged in neutral aqueous media due to proton adsorption. It was observed that the reduction rate of 4-NP increased with decreasing solution pH (Fig. 6(a)). When the solution pH was below the PZC, the catalyst surface acquired a net positive charge, which enhanced the electrostatic adsorption of the negatively charged nitrophenolate ions. This increased adsorption affinity facilitated a more rapid reduction of nitrophenolate ions by molecular hydrogen.
3.4 A possible mechanism of the catalytic hydrogenation of 4-NP by Ni-reline
Based on the observations described above, a possible mechanism is proposed for the catalytic reduction of 4-NP by Ni-reline and illustrated in Fig. 7. It is anticipated that at the start of the catalytic hydrogenation reaction, the adsorption of the reductant and reactant on the surface of Ni-reline bears the most significance. As a reducing agent, NaBH4 in aqueous medium can react with water at ambient temperature, yielding H2 and the sodium metaborate (NaBO2) by-product, causing 4-NP to convert into its corresponding 4-nitrophenolate ion that is the reactant.74 It was observed from the surface charge analysis that in neutral condition, surface of the catalyst remained positive due to the adsorption of the hydronium ion. Consequently, the negatively charged 4-nitrophenolate ions were electrostatically adsorbed onto the positively charged surface of the catalyst. Furthermore, adsorption of nitrophenolate ion significantly improved due to the deposition of carbon on the surface of the Ni-reline from DES media, resulting in a synergistic effect. Previous reports suggested that the presence of carbon content synergistically improves materials' adsorptive properties.75Conversely, in the presence of a catalyst, the newly formed hydrogen molecules dissociate and adsorb onto the metal surface, resulting in the formation of Metal–H species. These Ni-reline-H intermediates initiate the reduction by transferring electrons from the hydride to the catalyst, which are then directed toward the positively charged nitrogen atom in the nitro group of 4-NP. This electron transfer reduces the nitro group to a nitroso intermediate. Subsequent hydrogenation transforms the nitroso molecule into hydroxylamine, which further reduced into the corresponding aniline derivative.76
Ni-reline has superior catalytic efficiency than Ni-Aq., owing to its unique mixed-phase structure, which comprises both fcc and hcp crystal systems. These structural characteristics have a substantial impact on the catalyst's electronic properties, contributing to increase its performance. The fcc phase of Ni imparts strong electrical conductivity, allowing for efficient electron transport over the catalyst surface. Meanwhile, the hcp phase of Ni(OH)2 offers structural heterogeneity and interfacial barriers, which improve charge separation and localized electron accumulation.77 The coexistence of these two crystal systems results in synergistic interfacial interactions that reduce the overall electron flow resistance while shortening charge migration paths. This structural design shifts the reduction potential of Ni, allowing faster electron transport during the hydrogenation process.78 Furthermore, phase barriers and lattice mismatches between the fcc and hcp domains can cause crystal defects and increase the density of electronic states (DOS) around the Fermi level.79 These electronic optimizations enhance the material's ability to adsorb and activate hydrogen species, allowing for a faster conversion of 4-NP into 4-AP.
3.6 Reusability of the Ni-reline catalyst
To assess the reusability and stability of the Ni-reline catalyst, it was employed in the catalytic reduction of 4-NP over five successive reaction cycles. After each reaction cycle, the catalyst was recovered from the reaction mixture, thoroughly rinsed, and then reintroduced under the same experimental conditions for reuse. The catalytic efficiency after each cycle, as shown in Fig. 8(a), demonstrates the catalyst's excellent stability. There is only a marginal decrease in performance, from 95% to 91%, after five repeated catalytic processes. This remarkable retention of performance can be attributed to the well-structured crystal nature and magnetic properties of the Ni-reline catalyst, which allows for easy separation without significant loss of the sample. For clarity, the magnetic separation process of nanocatalyst is schematically represented in Fig. S5. | ||
| Fig. 8 (a) Analysis of the reusability of Ni-reline in the hydrogenation of 4-NP (b) FTIR spectra of Ni-reline catalyst before and after five consecutive catalytic cycles. | ||
Furthermore, to evaluate the structural integrity and stability of the Ni-reline catalyst, FTIR spectroscopy was performed for the Ni-reline catalyst before and after being employed in five consecutive catalytic cycles, as shown in Fig. 8(b). The FTIR spectra revealed no significant variations between the two samples. Key peaks observed in the 400–650 cm−1 range in both the fresh and recycled catalysts correspond to Ni–O stretching vibrations, confirming the presence of Ni.53 Additionally, a narrow and strong band at 3634 cm−1, which is characteristic of the O–H stretching vibration of Ni(OH)2,79 was observed in both samples, suggesting that the Ni/Ni(OH)2 structure remained intact. However, in the recycled Ni-reline catalyst, a slight haziness was observed in the high wavenumber region of the FTIR spectrum. This suggests the presence of carbonaceous residues, despite this, no new peaks were detected, indicating that the catalyst remained structurally stable and retained its integrity for further use.
4. Conclusion
This study demonstrated a strategic and environmentally benign approach for synthesizing Ni/Ni(OH)2 heterostructures with a mixed fcc-hcp crystal system by modulating a conventional chemical reduction method with reline DES. In the conventional aqueous system, metallic Ni with a pure fcc crystal structure was obtained, while replacing the reaction medium with reline resulted in the formation of a Ni/Ni(OH)2 nanocomposite with a mixed fcc-hcp crystal structure. The structural variation observed in the reline system was attributed to the modulation of the physicochemical properties of reline upon the addition of Ni salt. Specifically, the addition of the precursor salt reduced the viscosity of reline from 573 cP to 57 cP while increasing its ionic conductivity. These alterations resulted in a coral reef-like morphology with smaller particles (∼39 nm) in Ni-reline, compared to the spherical morphology with increase particles size (∼92 nm) in the aqueous system (Ni-Aq.). In the reductive hydrogenation of 4-NP, Ni-reline showed a substantially higher rate constant (0.1336 min−1), nearly 11 times greater than Ni-Aq. This improved catalytic activity is caused by the synergistic effects of the mixed-phase structure, abundant surface defects, and increased surface area. Furthermore, elemental analysis confirmed the presence of carbonaceous species in Ni-reline, which improves adsorption, while surface charge analysis revealed a positively charged surface that promotes stronger electrostatic interaction with negatively charged nitrophenolate ions, speeding up the reaction. These findings highlight the versatility of reline as a sustainable solvent capable of regulating nanoparticle phase, shape, and surface chemistry.Author contributions
Md. Minhajul Alam Khan: conceptualization, methodology, data curation, formal analysis, investigation, visualization, writing – original draft. Shawon Saha: formal analysis, investigation, writing – review and editing. Sumaya Nur Mithila: formal analysis, methodology. Yeasin Arafat Tarek: visualization, writing – review and editing. Akter Hossain Reaz: visualization, writing – review and editing. Shakhawat H. Firoz: conceptualization, project administration, resources, supervision, writing – review and editing.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data that is used to support the conclusions of this research may be found inside the article as well as the files that are associated with its supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ma01275a. Additional data that support the findings of this study are available from the corresponding author upon reasonable request.Acknowledgements
The authors would like to acknowledge the laboratory and financial support from Bangladesh University of Engineering & Technology (BUET).References
- L. Xiong, Y. Fu, Y. Luo, Y. Wei, Z. Zhang, C. Wu, S. Luo, G. Wang, D. Sawtell and K. Xie, J. Mater. Res., 2022, 37, 2109–2123 CrossRef.
- J.-R. Chiou, B.-H. Lai, K.-C. Hsu and D.-H. Chen, J. Hazard. Mater., 2013, 248, 394–400 CrossRef PubMed.
- N. P. P. Pabbathi, N. M. Nathani, I. R. Gadhvi and M. Chandrashekar, Environmental Biotechnology, Springer, 2020, vol. 1, pp. 197–225 Search PubMed.
- X.-X. Yan, Y.-F. Zhang, Y. Chen and B. Wang, Colloids Surf., A, 2020, 598, 124826 CrossRef.
- S. Balgude, K. Patil, S. Moharil, M. Puranik, S. Kadam, P. Lokhande, S. Patange and P. More, ChemistrySelect, 2022, 7, e202200221 CrossRef.
- J. D. Donaldson, S. M. Grimes, N. G. Yasri, B. Wheals, J. Parrick and W. E. Errington, J. Chem. Technol., 2002, 77, 756–760 Search PubMed.
- R. Dai, J. Chen, J. Lin, S. Xiao, S. Chen and Y. Deng, J. Hazard. Mater., 2009, 170, 141–143 CrossRef PubMed.
- N. Kaur and V. Singh, New J. Chem., 2017, 41, 2844–2868 RSC.
- E. Marais and T. Nyokong, J. Hazard. Mater., 2008, 152, 293–301 CrossRef PubMed.
- Z. Wu, J. Chen, Q. Di and M. Zhang, Catal. Commun., 2012, 18, 55–59 Search PubMed.
- R. K. Narayanan and S. J. Devaki, Ind. Eng. Chem. Res., 2015, 54, 1197–1203 Search PubMed.
- Y. Shaoqing, H. Jun and W. Jianlong, Radiat. Phys. Chem., 2010, 79, 1039–1046 Search PubMed.
- R. Begum, R. Rehan, Z. H. Farooqi, Z. Butt and S. Ashraf, J. Nanopart. Res., 2016, 18, 231 Search PubMed.
- K. Naseem, R. Begum and Z. H. Farooqi, Environ. Sci. Pollut. Res., 2017, 24, 6446–6460 Search PubMed.
- C. Kästner and A. F. Thünemann, Langmuir, 2016, 32, 7383–7391 CrossRef PubMed.
- K. Zhang, J. M. Suh, J.-W. Choi, H. W. Jang, M. Shokouhimehr and R. S. Varma, ACS Omega, 2019, 4, 483–495 CrossRef PubMed.
- A. Wang, H. Yin, H. Lu, J. Xue, M. Ren and T. Jiang, Langmuir, 2009, 25, 12736–12741 Search PubMed.
- S. N. Mithila, A. H. Reaz, F. Z. Farhana, M. J. Shiddiky and S. H. Firoz, RSC Adv., 2024, 14, 38605–38614 RSC.
- S. Senapati, S. Srivastava, S. B. Singh and K. Biswas, Cryst. Growth Des., 2010, 10, 4068–4075 CrossRef.
- A. Loiacono, F. Fioravanti, G. Bertossi, E. A. Franceschini and G. I. Lacconi, Discovery Chem., 2025, 2, 88 Search PubMed.
- H. Yang, Y. Wu, Z. Zhuang, Y. Li and C. Chen, Chin. J. Chem., 2022, 40, 515–523 Search PubMed.
- A. Bhattacharjee and M. Ahmaruzzaman, RSC Adv., 2016, 6, 41348–41363 RSC.
- G. B. Darband, M. Aliofkhazraei and A. S. Rouhaghdam, Int. J. Hydrogen Energy, 2017, 42, 14560–14565 CrossRef.
- A. Serrà, S. Grau, C. Gimbert-Suriñach, J. Sort, J. Nogués and E. Vallés, Appl. Catal., B, 2017, 217, 81–91 CrossRef.
- F. Feng, C. Ma, S. Han, X. Ma, C. He, H. Zhang, W. Cao, X. Meng, J. Xia and L. Zhu, Angew. Chem., Int. Ed., 2024, 63, e202405173 Search PubMed.
- H. Ye, Q. Wang, M. Catalano, N. Lu, J. Vermeylen, M. J. Kim, Y. Liu, Y. Sun and X. Xia, Nano Lett., 2016, 16, 2812–2817 CrossRef PubMed.
- Y. Lv, X. Mao, W. Gong, D. Wang, C. Chen, P. Liu, Y. Lin, G. Wang, H. Zhang and A. Du, Sci. China Mater., 2022, 65, 1252–1261 Search PubMed.
- Y. Du, C. Zhao, S. Li, T. Dai, X. Yang, Y. Zhu and Q. Shao, Chem. Soc. Rev., 2025, 54, 7706–7739 RSC.
- X. Gong, Z. Li, A. S. Pattamatta, T. Wen and D. J. Srolovitz, Commun. Mater., 2024, 5, 157 CrossRef.
- X. Yi, V. Celorrio, H. Zhang, N. Robertson and C. Kirk, J. Mater. Chem. A, 2023, 11, 22275–22287 RSC.
- J. Hu, S. Li, Y. Li, J. Wang, Y. Du, Z. Li, X. Han, J. Sun and P. Xu, J. Mater. Chem. A, 2020, 8, 23323–23329 Search PubMed.
- X. Zhang, H. Yin, X. Cheng, Z. Jiang, X. Zhao and A. Wang, Appl. Surf. Sci., 2006, 252, 8067–8072 CrossRef CAS.
- D. S. Sidhaye, T. Bala, S. Srinath, H. Srikanth, P. Poddar, M. Sastry and B. Prasad, J. Phys. Chem. C, 2009, 113, 3426–3429 CrossRef CAS.
- W. Xu, K. Y. Liew, H. Liu, T. Huang, C. Sun and Y. Zhao, Mater. Lett., 2008, 62, 2571–2573 Search PubMed.
- K. H. Kim, Y. B. Lee, S. G. Lee, H. Park and S. S. Park, Mater. Sci. Eng., A, 2004, 381, 337–342 Search PubMed.
- A. Nandi, M. DuttaGupta and A. Banthia, Mater. Lett., 2002, 52, 203–205 Search PubMed.
- H. Duan, D. Wang and Y. Li, Chem. Soc. Rev., 2015, 44, 5778–5792 Search PubMed.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 Search PubMed.
- P. C. Gomes-Junior, G. P. Longatto, K. K. de Lima Augusto, J. da Silveira Rocha, E. Piccin and O. Fatibello-Filho, Microchim. Acta, 2024, 191, 425 Search PubMed.
- S. Datta, J. Mahin, E. Liberti, I. Manasi, K. J. Edler and L. Torrente-Murciano, ACS Sustainable Chem. Eng., 2023, 11, 10242–10251 CrossRef CAS PubMed.
- O. S. Hammond, K. J. Edler, D. T. Bowron and L. Torrente-Murciano, Nat. Commun., 2017, 8, 14150 CrossRef CAS.
- X. Meng, K. Ballerat-Busserolles, P. Husson and J.-M. Andanson, New J. Chem., 2016, 40, 4492–4499 RSC.
- S. Datta, C. Jo, M. De Volder and L. Torrente-Murciano, ACS Appl. Mater. Interfaces, 2020, 12, 18803–18812 CrossRef CAS.
- M. Espino, M. de los Ángeles Fernández, F. J. Gomez and M. F. Silva, TrAC, Trends Anal. Chem., 2016, 76, 126–136 Search PubMed.
- Y. Nahar and S. C. Thickett, Polymers, 2021, 13, 447 Search PubMed.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef CAS.
- J. Y. Choi, Y. K. Lee, S. M. Yoon, H. C. Lee, B. K. Kim, J. M. Kim, K. M. Kim and J. H. Lee, J. Am. Ceram. Soc., 2005, 88, 3020–3023 Search PubMed.
- M. S. Che Zain, J. X. Yeoh, S. Y. Lee and K. Shaari, Sustainability, 2021, 13, 12981 CrossRef CAS.
- A. Abbott, G. Frisch, S. Gurman, A. Hillman, J. Hartley, F. Holyoak and K. Ryder, Chem. Commun., 2011, 47, 10031–10033 Search PubMed.
- S. Sáringer, P. Rouster and I. Szilagyi, J. Colloid Interface Sci., 2021, 590, 28–37 CrossRef.
- D. Lundqvist, Arkiv for Kemi, Mineralogi Och Geologi, 1947, 24, 12 Search PubMed.
- Y. Li, Y. Cao and D. Jia, J. Nanopart. Res., 2018, 20, 1–8 CrossRef.
- M. S. Bakshi, Cryst. Growth Des., 2016, 16, 1104–1133 CrossRef CAS.
- Y. Khan, S. Durrani, M. Mehmood, A. Jan and M. A. Abbasi, Mater. Chem. Phys., 2011, 130, 1169–1174 CrossRef CAS.
- L. Wang, M. Zhang and S. A. Redfern, Clays Clay Miner., 2003, 51, 439–444 CrossRef CAS.
- S. Bagheri, K. Shameli and S. B. Abd Hamid, J. Chem., 2013, 2013, 848205 Search PubMed.
- H. Shekaari, M. T. Zafarani-Moattar, A. Shayanfar and M. Mokhtarpour, J. Mol. Liq., 2018, 249, 1222–1235 Search PubMed.
- T. B. Pushpa, J. Vijayaraghavan, S. S. Basha, V. Sekaran, K. Vijayaraghavan and J. Jegan, Ecotoxicol. Environ. Saf., 2015, 118, 177–182 CrossRef PubMed.
- K.-I. Sotowa, T. Amamoto, A. Sobana, K. Kusakabe and T. Imato, Diamond Relat. Mater., 2004, 13, 145–150 CrossRef CAS.
- M. Menelaou, K. Georgoula, K. Simeonidis and C. Dendrinou-Samara, Dalton Trans., 2014, 43, 3626–3636 Search PubMed.
- O. Ejeromedoghene, J. I. Orege, O. Oderinde, C. O. Okoye, M. Alowakennu, M. O. Nnyia and G. Fu, Eur. Polym. J., 2022, 181, 111711 Search PubMed.
- M. Francisco, A. van den Bruinhorst and M. C. Kroon, Angew. Chem., Int. Ed., 2013, 52, 3074–3085 CrossRef CAS PubMed.
- J. Gao, F. Guan, Y. Zhao, W. Yang, Y. Ma, X. Lu, J. Hou and J. Kang, Mater. Chem. Phys., 2001, 71, 215–219 CrossRef CAS.
- G. Gygli, X. Xu and J. Pleiss, Sci. Rep., 2020, 10, 21395 Search PubMed.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- A. P. Abbott, ChemPhysChem, 2005, 6, 2502–2505 Search PubMed.
- A. P. Abbott, ChemPhysChem, 2004, 5, 1242–1246 CrossRef CAS PubMed.
- K. M. Udert, T. A. Larsen, M. Biebow and W. Gujer, Water Res., 2003, 37, 2571–2582 Search PubMed.
- S. Kettaf, O. Guellati, A. Harat, H. Kennaz, D. Momodu, J. Dangbegnon, N. Manyala and M. Guerioune, SN Appl. Sci., 2019, 1, 34 CrossRef.
- M. Li and G. Chen, Nanoscale, 2013, 5, 11919–11927 RSC.
- F. Bao, F. Tan, W. Wang, X. Qiao and J. Chen, RSC Adv., 2017, 7, 14283–14289 Search PubMed.
- Y. Kumar, S. Rani, J. Shabir and L. S. Kumar, ACS Omega, 2020, 5, 13250–13258 CrossRef CAS PubMed.
- M. Kosmulski, Adv. Colloid Interface Sci., 2020, 275, 102064 CrossRef CAS PubMed.
- A. Chinnappan, S. K. Eshkalak, C. Baskar, M. Khatibzadeh, E. Kowsari and S. Ramakrishna, Nanoscale Adv., 2019, 1, 305–313 Search PubMed.
- D. Mohan and C. U. Pittman Jr, J. Hazard. Mater., 2007, 142, 1–53 Search PubMed.
- J. Sun, Y. Fu, G. He, X. Sun and X. Wang, Catal. Sci. Technol., 2014, 4, 1742–1748 Search PubMed.
- D. Shao, Q. Wang, X. Yao, Y. Zhou and X.-Y. Yu, J. Mater. Chem. A, 2022, 10, 21848–21855 RSC.
- F. Zhang, S. Zhao, K. Jin, H. Bei, D. Popov, C. Park, J. C. Neuefeind, W. J. Weber and Y. Zhang, Appl. Phys. Lett., 2017, 110, 011902 CrossRef.
- C. Zhang, J. Yang, Y. Liu, Y. Li, Z. Dai, M. Han and J. Bao, ChemistrySelect, 2019, 4, 42–48 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |





