Open Access Article
Open Access ArticleInnovative chromene-based disperse dyes for concurrent dyeing: molecular docking, biochemical assessment, and repellent efficacy against Culex pipiens mosquitoes
Mohamed S. A.
El-Gaby
a,
M. A.
Habib
b,
Nadeem
Raza
b,
Ahmed B. M.
Ibrahim
b,
Ali A.
Ali
 *a,
Walid E.
Elgammal
*a,
Walid E.
Elgammal
 a,
Ahmed H.
Halawa
a,
Mostafa A.
Ismail
c,
Tharwat A.
Selim
d,
Ahmed I.
Hasaballah
d,
Mohamed A. M.
El-Tabakh
d and
Gameel A. M.
Elhagali
a
a,
Ahmed H.
Halawa
a,
Mostafa A.
Ismail
c,
Tharwat A.
Selim
d,
Ahmed I.
Hasaballah
d,
Mohamed A. M.
El-Tabakh
d and
Gameel A. M.
Elhagali
a
aDepartment of Chemistry, Faculty of Science (Boys), Al-Azhar University, Cairo 11884, Egypt. E-mail: dr_ali055@azhar.edu.eg
bDepartment of Chemistry, College of Science, Imam Mohammad Ibn Saud Islamic University (IMSIU), 11623 Riyadh, Saudi Arabia
cDepartment of Chemistry, College of Science, King Khalid University, Abha 61413, Saudi Arabia
dDepartment of Zoology and Entomology, Faculty of Science (boys), Al-Azhar University, Cairo 11884, Egypt
First published on 9th December 2025
Abstract
Integrating a heterocyclic component into the azo dye framework substantially improves the bioactivity of the synthesized compounds. This structural modification facilitates the optimization of drug-like molecules for diverse biological and pharmacological applications. A synthetic route was adopted in this study to develop the target compounds, specifically the 4H-chromene containing azo benzophenones 6a–d, and their behavior on polyester fabrics was examined by optimizing the dyeing conditions. An increase in the color strength of the dyed polyester fabrics was observed with rising dyeing temperatures (100–130 °C) and prolonged dyeing durations (15–60 min). These results imply that synthetic dyes are viable options for adding a wide range of colors to polyester fabrics. In addition to their dyeing performance, bioassay evaluations demonstrated that dyes 6a–d possess repellent efficacy against the mosquito vector Culex pipiens. Compound 6a exhibited the highest repellent activity, reaching approximately 60% after 30 minutes of treatment and maintaining effectiveness for two hours, with a slight decline to 56%. In contrast, compounds 6b, 6c, and 6d showed repellent effects of 53–44%, 56–45%, and 35–44%, respectively. The toxicological potential of these dyes was further investigated by assessing the performance of acetylcholinesterase (AChE) along with glutathione S-transferase (GST) enzymes in Culex pipiens insects following exposure. The synthesized 4H-chromene-based azo dyes show promise for polyester coloration and as bioactive agents with mosquito-repellent and enzyme-inhibitory effects.
1. Introduction
Undoubtedly, mosquitoes pose a significant global health threat due to their role in transmitting numerous infectious diseases. Culex species are considered the most prevalent in Africa and Asia and serve as the primary vectors of filariasis, with millions of cases reported worldwide in recent years.1Culex pipiens is widely distributed2 and is a well-established vector of West Nile virus,3Wuchereria bancrofti,4 and the virus responsible for Rift Valley fever.5 Furthermore, several recent studies have investigated the potential role of mosquitoes in transmitting the hepatitis C virus.6,7 Therefore, effective mosquito control is crucial for reducing the transmission of mosquito-transported diseases and protecting the health of society. A variety of strategies have been explored for mosquito control.8–11 These include the direct application of insect repellents to the skin, clothing, or household materials, as well as the use of protective barriers such as mosquito nets and screens. However, prolonged use of commercial mosquito coils, mats, and fumigators containing chemicals such as N,N-diethyl-m-toluamide (DEET) and synthetic pyrethroids (e.g., pyrethrin, allethrin, and permethrin) has been associated with adverse health effects.12 As a safer and more sustainable alternative, researchers have investigated the use of natural materials such as fresh moringa leaves, castor oil, and mint leaves for the development of mosquito-repellent textiles. This innovation represents a promising and creative approach to enhancing the textile industry while addressing the urgent need for effective mosquito-repellent fabrics.13Chromenes represent a significant class of naturally occurring molecules, encompassing diverse forms such as tocopherols, alkaloids, anthocyanins, and flavones. The chromene nucleus is recognized as an essential structural motif in the pursuit of new drug candidates.14 Interestingly, 4H-chromenes have demonstrated an extensive variety of medicinal properties, among which are antimicrobial,15 antifungal,16 antiviral,17 anticancer,18 antitumor,19 analgesic,20 antioxidant,21 anti-influenza,22 anti-inflammatory,23 antidiabetic,24 anticonvulsant,25 anticholinesterase,26 herbicidal,27 antituberculosis,28 and anticoagulant features.
Pigments and azo dyes constitute two major classes of chemical colouring agents, distinguished by their broad applications throughout numerous industrial fields.29 Among these, azo dyes hold particular importance in the textile industry, primarily owing to their characteristic nitrogen–nitrogen double bond, which is central to their chromophoric properties and functional versatility.30 Azo dyes have historically been extensively utilized to color a diverse array of things, such as food, paper, textiles, cosmetics, pharmaceuticals, and various consumer goods.31 The synthesis of azo compounds can be achieved through various chemical reactions.32 These salts, typically generated through conventional diazotization reactions, readily react with diazo-coupling nucleophiles such as phenols, naphthols, or amines under low-temperature conditions in the presence of acids and salts.33
Benzophenone is an essential organic scaffold that functions as a flexible precursor in the generation of insecticides, pharmaceuticals, perfumes, and organic pigments.34 Structurally, benzophenone consists of two rings of benzene connected through an inner carbonyl functional group and is commonly referred to as benzoyl benzene.35 Numerous naturally occurring compounds incorporate the benzophenone framework, which contributes to its prevalence in medicinal chemistry owing to its diverse biological activities.36 Naturally occurring benzophenones display diverse medicinal benefits, including antioxidant, antifungal, anti-HIV, and antibacterial, along with antiviral effects.37 In agriculture, the benzophenone nucleus is of particular importance, as it constitutes the core structure of certain fungicides, such as flumorph.38 In addition, several benzophenone-based compounds are commercially available as pharmaceuticals and sunscreens (Fig. 1). For example, ketoprofen is a powerful nonsteroidal anti-inflammatory substance with both antipyretic and analgesic actions.39 Tolcapone, a dihydroxy benzophenone derivative, is employed in the management of Parkinson's disease,40 while fenofibrate (Tricor) is commonly prescribed to reduce cholesterol levels in patients at risk of cardiovascular disorders.41 Beyond therapeutic applications, benzophenones are extensively utilized as photosensitizers and constitute one of the more significant categories of materials in photochemistry. In sun protection products, they serve as UV-A/B filters, with 2-hydroxybenzophenones such as oxybenzone and sulisobenzone widely employed to prevent the penetration of harmful ultraviolet radiation into the skin.42
As part of our continuing investigation on the incorporation of heterocyclic constituents into azo colorant architectures,19,43–49 we have planned and generated a new class of disperse dyes by integrating a benzophenone fragment conjugated with a 4H-chromene core. The target disperse dyes 6a–d were obtained through the cyclocondensation of compound 4 with arylidene-malononitriles 5 in ethanol under reflux using piperidine as a catalyst. Comprehensive characterization of the synthesized compounds was performed using a combination of analytical and spectroscopic techniques. Owing to their remarkable biological characteristics, benzophenone-based azo dyes have gained significant attention for applications in diverse fields, particularly in printing and biomedicine. When applied to polyester fabrics, the synthesized dyes produced shades ranging from 1 to 5 using dyeing settings at temperatures between 100 and 130 °C and pH values of 2, 4, 6, and 8. Bioassay evaluations revealed that dyes 6a–d exhibit repellent impact towards the mosquito vector Culex pipiens. Among them, compound 6a exhibited the strongest repellent effect, while compound 6d showed the weakest. To gain deeper insight into their biological potential, the actions of glutathione S-transferase (GST) and acetylcholinesterase (AChE) were assessed in Culex pipiens larvae after exposure to these dyes. Molecular docking studies validated the adopted protocol and revealed that compounds 6a–d displayed stronger binding affinities than the reference ligands, with compound 6a being the most effective against AChE and compound 6d against GST. Taken together, these findings highlight the potential of the synthesized disperse dyes not only as efficient colorants for polyester fabrics but also as promising bioactive agents with mosquito-repellent properties.
2. Experimental
2.1. Materials and instruments
The SI provides detailed information on the instruments and chemical reagents employed for the characterization of the generated molecules. The starting materials, including para-aminobenzophenone and m-dihydroxybenzene (resorcinol), were provided by Sigma-Aldrich. Sodium nitrite (NaNO2), sodium acetate trihydrate (CH3COONa·3H2O), sodium carbonate (purchased from Alpha Chemika, India), and concentrated hydrochloric acid (HCl) were obtained from a variety of sources, including CDH (Central Drug House) and SD Fine. Lobachem provided organic solvents, including glacial acetic acid and ethanol, without any additional purification. Using pre-coated silica gel aluminum plates (Macherey-Nagel) and DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (95%
MeOH (95%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5%) as the eluting solution, we performed thin layer chromatography (TLC) analysis. We used UV light (254 nm) to observe and visualize the reactions. The 100% polyester fabric (140 gm m−2, plain weave, Yarn 48 × 150 denier, the threads are 110 × 80 warp in weft, width: 66 inches, and thickness: 0.39 mm cm−1) was supplied by El-Nahawy Textile Company, Egypt.
5%) as the eluting solution, we performed thin layer chromatography (TLC) analysis. We used UV light (254 nm) to observe and visualize the reactions. The 100% polyester fabric (140 gm m−2, plain weave, Yarn 48 × 150 denier, the threads are 110 × 80 warp in weft, width: 66 inches, and thickness: 0.39 mm cm−1) was supplied by El-Nahawy Textile Company, Egypt.
Method 2: a solution of compound 4 (0.01 mmol) in ethanol (30 ml) containing piperidine (0.1 ml) was treated with a mixture of aromatic aldehyde (0.01 mmol) and malononitrile (0.01 mmol). The reaction mixture was refluxed for 30 minutes, and the resulting solid was collected by filtration, washed with water, and recrystallized from the appropriate solvent.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and 1593 (N
O), and 1593 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, DMSO-d6, δ ppm): 12.11 (s, 1H, OH, exchangeable by D2O), 8.13 (d, J = 8.5 Hz, 2H, AB-system), 7.89 (d, J = 8.6 Hz, 2H, AB-system), 7.82 (s, 1H, resorcinol-H), 7.81–7.75 (m, 2H, phenyl group), 7.75–7.67 (m, 1H, phenyl group), 7.59 (t, J = 7.7 Hz, 2H, phenyl group), 7.39 (d, J = 8.4 Hz, 2H, AB-system),7.24 (d, J = 8.5 Hz, 2H, AB-system), 7.14 (s, 2H, NH2, exchangeable by D2O), 6.83 (d, J = 9.0 Hz, 1H, resorcinol-H), 4.81 (s, 1H, pyrane-H). 13C NMR (101 MHz, DMSO-d6, δ ppm): 195.57, 159.94, 154.46, 153.44, 153.31, 144.45, 138.81, 137.30, 135.72, 133.39, 131.72, 131.33, 130.11, 129.61, 129.14, 128.95, 124.56, 123.01, 120.45, 112.57, 109.23, 57.20, 36.50. MS (m/z): 506 (M+, 21.98%). Anal. calcd for C29H19ClN4O3 (506.11): C, 68.71; H, 3.78; N, 11.05; found: C, 68.59; H, 3.69; N, 10.92%.
N). 1H NMR (300 MHz, DMSO-d6, δ ppm): 12.11 (s, 1H, OH, exchangeable by D2O), 8.13 (d, J = 8.5 Hz, 2H, AB-system), 7.89 (d, J = 8.6 Hz, 2H, AB-system), 7.82 (s, 1H, resorcinol-H), 7.81–7.75 (m, 2H, phenyl group), 7.75–7.67 (m, 1H, phenyl group), 7.59 (t, J = 7.7 Hz, 2H, phenyl group), 7.39 (d, J = 8.4 Hz, 2H, AB-system),7.24 (d, J = 8.5 Hz, 2H, AB-system), 7.14 (s, 2H, NH2, exchangeable by D2O), 6.83 (d, J = 9.0 Hz, 1H, resorcinol-H), 4.81 (s, 1H, pyrane-H). 13C NMR (101 MHz, DMSO-d6, δ ppm): 195.57, 159.94, 154.46, 153.44, 153.31, 144.45, 138.81, 137.30, 135.72, 133.39, 131.72, 131.33, 130.11, 129.61, 129.14, 128.95, 124.56, 123.01, 120.45, 112.57, 109.23, 57.20, 36.50. MS (m/z): 506 (M+, 21.98%). Anal. calcd for C29H19ClN4O3 (506.11): C, 68.71; H, 3.78; N, 11.05; found: C, 68.59; H, 3.69; N, 10.92%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and 1593 (N
O), and 1593 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, DMSO-d6, δ ppm): 12.12 (s, 1H, OH, exchangeable by D2O), 8.12 (d, J = 8.6 Hz, 2H, AB-system), 7.88 (d, J = 8.6 Hz, 2H, AB-system), 7.82 (s, 1H, resorcinol-H), 7.80–7.75 (m, 3H, phenyl group), 7.75–7.66 (m, 1H, phenyl group), 7.63–7.56 (m, 2H, phenyl group), 7.52 (d, J = 8.5 Hz, 2H, AB-system), 7.18 (d, J = 8.5 Hz, 2H, AB-system), 7.14 (s, 2H, NH2, exchangeable by D2O), 6.83 (d, J = 9.0 Hz, 1H, resorcinol-H), 4.80 (s, 1H, pyrane-H). 13C NMR (101 MHz, DMSO-d6, δ ppm): 195.61, 159.94, 154.42, 153.39, 153.28, 144.85, 138.80, 137.28, 135.69, 133.40, 131.87, 131.34, 130.10, 129.99, 129.15, 124.82, 122.97, 120.45, 120.21, 112.47, 109.27, 57.13, 36.55. MS (m/z): 550 (M+, 30.14%). Anal. calcd for C29H19BrN4O3 (550.06): C, 63.17; H, 3.47; N, 10.16; found: C, 63.06; H, 3.38; N, 10.02%.
N). 1H NMR (300 MHz, DMSO-d6, δ ppm): 12.12 (s, 1H, OH, exchangeable by D2O), 8.12 (d, J = 8.6 Hz, 2H, AB-system), 7.88 (d, J = 8.6 Hz, 2H, AB-system), 7.82 (s, 1H, resorcinol-H), 7.80–7.75 (m, 3H, phenyl group), 7.75–7.66 (m, 1H, phenyl group), 7.63–7.56 (m, 2H, phenyl group), 7.52 (d, J = 8.5 Hz, 2H, AB-system), 7.18 (d, J = 8.5 Hz, 2H, AB-system), 7.14 (s, 2H, NH2, exchangeable by D2O), 6.83 (d, J = 9.0 Hz, 1H, resorcinol-H), 4.80 (s, 1H, pyrane-H). 13C NMR (101 MHz, DMSO-d6, δ ppm): 195.61, 159.94, 154.42, 153.39, 153.28, 144.85, 138.80, 137.28, 135.69, 133.40, 131.87, 131.34, 130.10, 129.99, 129.15, 124.82, 122.97, 120.45, 120.21, 112.47, 109.27, 57.13, 36.55. MS (m/z): 550 (M+, 30.14%). Anal. calcd for C29H19BrN4O3 (550.06): C, 63.17; H, 3.47; N, 10.16; found: C, 63.06; H, 3.38; N, 10.02%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and 1586 (N
O), and 1586 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (400 MHz, DMSO-d6, δ ppm): 12.15 (s, 1H, OH, exchangeable by D2O), 8.12 (d, J = 8.6 Hz, 2H, AB-system), 7.89 (d, J = 8.6 Hz, 2H, AB-system), 7.81 (s, 1H, resorcinol-H), 7.79–7.76 (m, 2H, phenyl group), 7.75–7.67 (m, 1H, phenyl group), 7.59 (t, J = 7.6 Hz, 2H, phenyl group), 7.13 (d, J = 8.7 Hz, 2H, AB-system), 7.05 (s, 2H, NH2, exchangeable by D2O), 6.87 (d, J = 8.7 Hz, 2H, AB-system), 6.82 (d, J = 9.0 Hz, 1H, resorcinol-H), 4.73 (s, 1H, pyrane-H), 3.71 (s, 3H, OCH3). 13C NMR (101 MHz, DMSO-d6, δ ppm): 195.59, 159.92, 158.49, 154.28, 153.39, 153.31, 138.77, 137.56, 137.29, 135.64, 133.39, 131.35, 130.10, 129.14, 128.73, 124.87, 122.94, 120.69, 114.33, 113.46, 109.23, 57.96, 55.53, 36.19. MS (m/z): 502 (M+, 21.98%). Anal. calcd for C30H22N4O4 (502.16): C, 71.70; H, 4.41; N, 11.15; found: C, 71.59; H, 4.31; N, 11.03%.
N). 1H NMR (400 MHz, DMSO-d6, δ ppm): 12.15 (s, 1H, OH, exchangeable by D2O), 8.12 (d, J = 8.6 Hz, 2H, AB-system), 7.89 (d, J = 8.6 Hz, 2H, AB-system), 7.81 (s, 1H, resorcinol-H), 7.79–7.76 (m, 2H, phenyl group), 7.75–7.67 (m, 1H, phenyl group), 7.59 (t, J = 7.6 Hz, 2H, phenyl group), 7.13 (d, J = 8.7 Hz, 2H, AB-system), 7.05 (s, 2H, NH2, exchangeable by D2O), 6.87 (d, J = 8.7 Hz, 2H, AB-system), 6.82 (d, J = 9.0 Hz, 1H, resorcinol-H), 4.73 (s, 1H, pyrane-H), 3.71 (s, 3H, OCH3). 13C NMR (101 MHz, DMSO-d6, δ ppm): 195.59, 159.92, 158.49, 154.28, 153.39, 153.31, 138.77, 137.56, 137.29, 135.64, 133.39, 131.35, 130.10, 129.14, 128.73, 124.87, 122.94, 120.69, 114.33, 113.46, 109.23, 57.96, 55.53, 36.19. MS (m/z): 502 (M+, 21.98%). Anal. calcd for C30H22N4O4 (502.16): C, 71.70; H, 4.41; N, 11.15; found: C, 71.59; H, 4.31; N, 11.03%.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and 1589 (N
O), and 1589 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR (300 MHz, DMSO-d6, δ ppm): 12.18 (s, 1H, OH, exchangeable by D2O), 8.09 (d, J = 7.9 Hz, 2H, AB-system), 7.95 (d, J = 8.3 Hz, 1H, resorcinol-H), 7.87 (d, J = 7.9 Hz, 2H, AB-system), 7.82–7.64 (m, 3H, phenyl group), 7.57 (t, J = 7.2 Hz, 2H, phenyl group), 7.10 (d, J = 8.0 Hz, 2H, AB-system), 7.01 (s, 2H, NH2, exchangeable by D2O), 6.84 (d, J = 9.0 Hz, 2H, AB-system), 6.79 (s, 1H, resorcinol-H), 4.71 (s, 1H, pyrane-H), 3.96 (q, J = 6.7 Hz, 2H, OCH2), 1.28 (t, J = 6.8 Hz, 3H, CH3). 13C NMR (101 MHz, DMSO-d6, δ ppm): 195.04, 160.39, 159.46, 157.31, 153.79, 152.84, 138.26, 136.84, 135.11, 133.42, 132.87, 130.87, 129.62, 128.64, 128.26, 124.69, 122.42, 120.23, 115.51, 114.30, 108.75, 62.97, 57.55, 35.74, 14.67. MS (m/z): 516 (M+, 7.79%). Anal. calcd for C31H24N4O4 (516.18): C, 72.08; H, 4.68; N, 10.85; found: C, 71.97; H, 4.59; N, 10.73%.
N). 1H NMR (300 MHz, DMSO-d6, δ ppm): 12.18 (s, 1H, OH, exchangeable by D2O), 8.09 (d, J = 7.9 Hz, 2H, AB-system), 7.95 (d, J = 8.3 Hz, 1H, resorcinol-H), 7.87 (d, J = 7.9 Hz, 2H, AB-system), 7.82–7.64 (m, 3H, phenyl group), 7.57 (t, J = 7.2 Hz, 2H, phenyl group), 7.10 (d, J = 8.0 Hz, 2H, AB-system), 7.01 (s, 2H, NH2, exchangeable by D2O), 6.84 (d, J = 9.0 Hz, 2H, AB-system), 6.79 (s, 1H, resorcinol-H), 4.71 (s, 1H, pyrane-H), 3.96 (q, J = 6.7 Hz, 2H, OCH2), 1.28 (t, J = 6.8 Hz, 3H, CH3). 13C NMR (101 MHz, DMSO-d6, δ ppm): 195.04, 160.39, 159.46, 157.31, 153.79, 152.84, 138.26, 136.84, 135.11, 133.42, 132.87, 130.87, 129.62, 128.64, 128.26, 124.69, 122.42, 120.23, 115.51, 114.30, 108.75, 62.97, 57.55, 35.74, 14.67. MS (m/z): 516 (M+, 7.79%). Anal. calcd for C31H24N4O4 (516.18): C, 72.08; H, 4.68; N, 10.85; found: C, 71.97; H, 4.59; N, 10.73%.
2.2. Dyeing and preparing dye dispersions
Polyester was dyed using AHIBA's infrared dyeing equipment in laboratory. The dye bath (20 mL) also contained 2.0 mL L−1 of Kimi-levelling ES, a fatty acid ester-based leveling and dispersing agent supplied by an Egyptian chemical company.50 The previously mentioned disperse dye was prepared. Glacial acetic acid was employed to adjust the dye bath pH to 2, 4, and 6, whereas sodium hydroxide was used to increase it to 10.50 The polyester textiles were immersed in the dye bath, which was gradually heated at a rate of 2 °C per minute. The temperature was raised gradually to the range of 100–130 °C. This high temperature is kept in the dye bath for 15, 30, 45, or 60 minutes depending on the desired temperature.51,52 An aqueous solution of 2 g L−1 sodium hydroxide and 2 g L−1 sodium hydrosulfite with a liquor ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 was utilized for the reduction clearing operation, which was carried out at 85 °C for 20 minutes.53,54
20 was utilized for the reduction clearing operation, which was carried out at 85 °C for 20 minutes.53,54
2.3. Biological activity of dyed-fabric samples
To test the dyed-fabric samples for their possible repellency properties against the blood sucking animal model, Culex pipiens mosquitoes of well-known public health significance were selected to investigate this possibility.3. Results and discussion
3.1. Organic synthesis
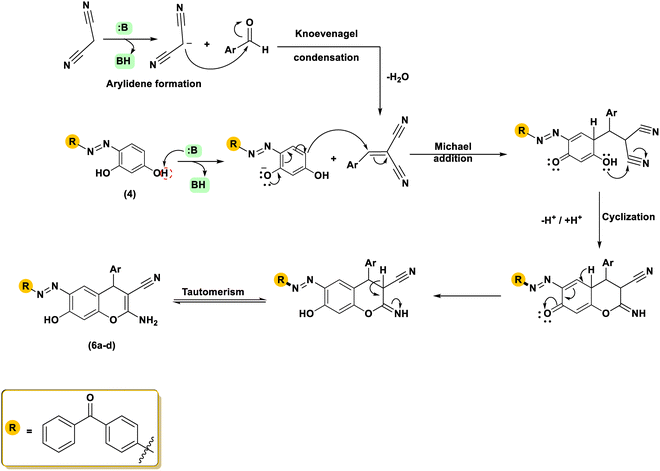
The therapeutic potential of the target molecules was enhanced once the heterocyclic component was integrated into the azo dye framework. Drugs can be easily adjusted for an assortment of biological and pharmacological purposes by including a heterocyclic moiety. The procedure adopted in this study for the synthesis of the desired 4H-chromene substituted azo benzophenones 6a–d is depicted in Scheme 2. As outlined in Scheme 1, the requisite compound 4 was first synthesized by diazotising 4-aminobenzophenone 1 and then coupling with resorcinol 3.59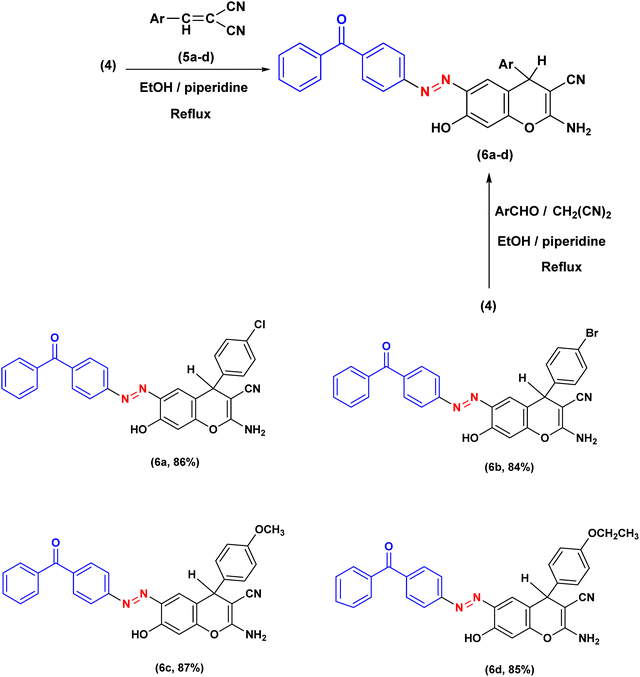 | ||
| Scheme 2 The synthetic approach employed for the preparation of 2-amino-4H-chromene-3-carbonitriles. | ||
The 2-amino-4H-chromene-3-carbonitriles 6a–d were efficiently synthesized via a one-pot reaction of compound 4 with arylidene-malononitriles 5 in ethanol using piperidine as a catalyst under reflux conditions. High yields were achieved for all the created molecules, falling within the range of 84–87%. The chemical structures of the isolated chromenes were demonstrated through extensive spectroscopic investigations. Elemental evaluation provided results consistent with the theoretical percentages, indicating the high purity of the obtained products. The infrared spectra of the isolated products indicated distinctive absorption bands corresponding to amino, cyano, carbonyl, and azo functional groups. The infrared spectrum of compound 6a, utilized as an illustrative model, displayed prominent absorption bands corresponding to the OH, NH2, CN, and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N functional groups at 3300, 3251, 3191, 2198, 1647, and 1589 cm−1, respectively. The proton nuclear magnetic resonance spectrum of molecule 6d presented a singlet at δH 4.71 ppm, which is attributed to the methine proton of the chromene ring. Additionally, two downfield signals were observed at δH 12.18 and 7.01 ppm, which are exchangeable with D2O and correspond to the hydroxyl and NH2 protons, respectively. Two doublets at δH 7.87 (J = 7.9 Hz) and 8.09 (J = 6.8 Hz) ppm were designated to an AB system, while a triplet at δH 1.28 ppm (J = 6.8 Hz) and a quartet at δH 3.96 ppm (J = 6.7 Hz) were observed and attributed to the ethyl group. Furthermore, the remaining aromatic protons were observed as a doublet at δH 7.95 (J = 8.3 Hz) and a singlet at δH 6.79 ppm, corresponding to the resorcinol protons; two doublets at δH 6.84 (J = 9.0 Hz) and 7.10 (J = 8.0 Hz) ppm, a triplet at δH 7.57 ppm (J = 7.2 Hz), as well as a multiplet at δH 7.82–7.64 ppm were also identified. In the carbon nuclear magnetic resonance spectrum of 6d, the downfield resonance at δC 195.04 ppm was assigned to the carbonyl moiety, whereas the signal at δC 115.51 ppm was attributed to the nitrile carbon at position C-3. Furthermore, the singlet at δC 62.97 ppm was attributed to the methylene carbon, while the terminal carbon of the ethoxy group appeared at δC 14.67 ppm. The peak at 35.74 ppm in the spectrum is attributed to the benzylic C-4 carbon located within the pyran ring. The 13C NMR spectrum also displayed signals at δC = 160.39, 159.46, 157.31, 153.79, 152.84, 138.26, 136.84, 135.11, 133.42, 132.87, 130.87, 129.62, 128.64, 128.64, 128.64, 128.64, 128.26, 124.69, 122.42, 120.23, 114.30, 108.75, and 57.55 (C3), representing the several kinds of carbon atoms that make up the molecule. The mass spectroscopy of compound 6c revealed a noticeable molecular ion at m/z 502 (21.98%), which is in agreement with the proposed molecular formula C30H22N4O4. The achievable multi-step mechanism for the synthesis of 4H-chromene-3-carbonitriles 6 under organo-base conditions is proposed and illustrated in Scheme 3. The mechanism of this reaction involves sequential steps, including a Knoevenagel condensation, a Michael addition, and an intramolecular cyclization, which collectively lead to the formation of the final product.21 The development of 4H-chromene 6 is assumed through the deprotonation of resorcinol 4 to generate the reactive intermediate A, subsequently followed by Michael addition to the double bond in 5. The Michael adduct B subsequently underwent efficient cyclisation through intramolecular attack of the oxygen atom on the cyano carbon, affording the heterocyclisation products 6a–d. Further confirmation of the structure of 2-amino-4H-chromenes 6 was obtained via independent synthesis using a one-pot, ternary reaction of resorcinol 4, an aromatic aldehyde, and malononitrile (1
N functional groups at 3300, 3251, 3191, 2198, 1647, and 1589 cm−1, respectively. The proton nuclear magnetic resonance spectrum of molecule 6d presented a singlet at δH 4.71 ppm, which is attributed to the methine proton of the chromene ring. Additionally, two downfield signals were observed at δH 12.18 and 7.01 ppm, which are exchangeable with D2O and correspond to the hydroxyl and NH2 protons, respectively. Two doublets at δH 7.87 (J = 7.9 Hz) and 8.09 (J = 6.8 Hz) ppm were designated to an AB system, while a triplet at δH 1.28 ppm (J = 6.8 Hz) and a quartet at δH 3.96 ppm (J = 6.7 Hz) were observed and attributed to the ethyl group. Furthermore, the remaining aromatic protons were observed as a doublet at δH 7.95 (J = 8.3 Hz) and a singlet at δH 6.79 ppm, corresponding to the resorcinol protons; two doublets at δH 6.84 (J = 9.0 Hz) and 7.10 (J = 8.0 Hz) ppm, a triplet at δH 7.57 ppm (J = 7.2 Hz), as well as a multiplet at δH 7.82–7.64 ppm were also identified. In the carbon nuclear magnetic resonance spectrum of 6d, the downfield resonance at δC 195.04 ppm was assigned to the carbonyl moiety, whereas the signal at δC 115.51 ppm was attributed to the nitrile carbon at position C-3. Furthermore, the singlet at δC 62.97 ppm was attributed to the methylene carbon, while the terminal carbon of the ethoxy group appeared at δC 14.67 ppm. The peak at 35.74 ppm in the spectrum is attributed to the benzylic C-4 carbon located within the pyran ring. The 13C NMR spectrum also displayed signals at δC = 160.39, 159.46, 157.31, 153.79, 152.84, 138.26, 136.84, 135.11, 133.42, 132.87, 130.87, 129.62, 128.64, 128.64, 128.64, 128.64, 128.26, 124.69, 122.42, 120.23, 114.30, 108.75, and 57.55 (C3), representing the several kinds of carbon atoms that make up the molecule. The mass spectroscopy of compound 6c revealed a noticeable molecular ion at m/z 502 (21.98%), which is in agreement with the proposed molecular formula C30H22N4O4. The achievable multi-step mechanism for the synthesis of 4H-chromene-3-carbonitriles 6 under organo-base conditions is proposed and illustrated in Scheme 3. The mechanism of this reaction involves sequential steps, including a Knoevenagel condensation, a Michael addition, and an intramolecular cyclization, which collectively lead to the formation of the final product.21 The development of 4H-chromene 6 is assumed through the deprotonation of resorcinol 4 to generate the reactive intermediate A, subsequently followed by Michael addition to the double bond in 5. The Michael adduct B subsequently underwent efficient cyclisation through intramolecular attack of the oxygen atom on the cyano carbon, affording the heterocyclisation products 6a–d. Further confirmation of the structure of 2-amino-4H-chromenes 6 was obtained via independent synthesis using a one-pot, ternary reaction of resorcinol 4, an aromatic aldehyde, and malononitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) with a catalytic amount of piperidine at reflux temperature in ethanol melting point (m.p), Thin Layer Chromatography (TLC) and IR (Infrared) spectrum.
1 molar ratio) with a catalytic amount of piperidine at reflux temperature in ethanol melting point (m.p), Thin Layer Chromatography (TLC) and IR (Infrared) spectrum.
3.2. ClogP computation
The ClogP values, representing the N-octanol/water partition coefficients of the synthesized dyes, were calculated using Chem-Draw. The effectiveness of polyester dyeing depends on how disperse dyes are distributed between the polyester fibers and the surrounding water. Consequently, dyes exhibiting lower solubility in the aqueous dye bath (i.e., higher hydrophobicity) tend to penetrate more efficiently into the hydrophobic polyester matrix. Thus, the relevance of ClogP values is discussed in the following section. Generally, dyes with lower ClogP values are more soluble in the aqueous dye bath, which tends to result in lower K/S values.60 Consistent with this relationship, dyes 6d and 6c, which exhibit the lowest ClogP values (7.62446 and 7.09546, respectively), demonstrated the highest solubility. Conversely, dyes 6b and 6a, with higher ClogP values (8.03946 and 7.88946, respectively), are expected to yield higher K/S ratios due to their reduced aqueous solubility. These findings confirm that the ClogP values are in good agreement with the observed K/S values.3.3. Characteristics of dyeing polyester fabrics
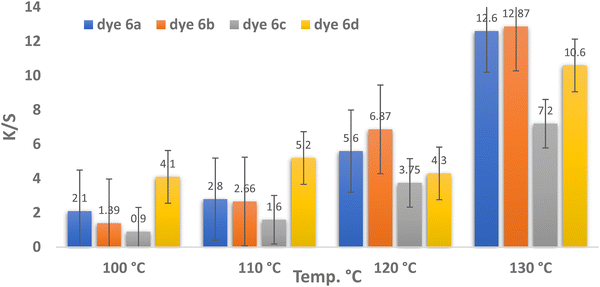 | ||
| Fig. 2 Temperature and color strength (K/S) relationship for dyes 6a–d (pH = 4, time = 30 min, and 3% shade). | ||
Fig. 2 shows that the optimum dyeing temperature for dyes 6a–d was 130 °C, corresponding to K/S values of 12.6, 12.87, 7.2, and 10.6, respectively. This implies that the restriction temperature of 130 °C is ideal for dispersing all dyes. These phenomena can be attributed to the high kinetic energy of the dye molecules and their rapid dispersion rates. The highest K/S values for dyes 6a and 6b were observed at 130 °C, recording 12.6 and 12.87, respectively. This behavior may be attributed to their chemical structures, which incorporate chlorine and bromine substituents that enhance the electron-accepting ability, thereby promoting stronger interactions with polyester fibers.63 As the temperature increases for all dyes, the reflectance values decrease, as shown in Table 1 and Fig. 2.
 | ||
| Fig. 3 pH and color strength (K/S) relationship for dyes 6a–d (temperature = 130 °C, time = 30 min, and 3% shade). | ||
However, polyester dyeing is commonly carried out under acidic pH conditions. As shown in Fig. 3 and Table 2, the findings indicate that the optimum pH for dyeing polyester with dyes 6a–c is 2, whereas for dye 6d it is 4.
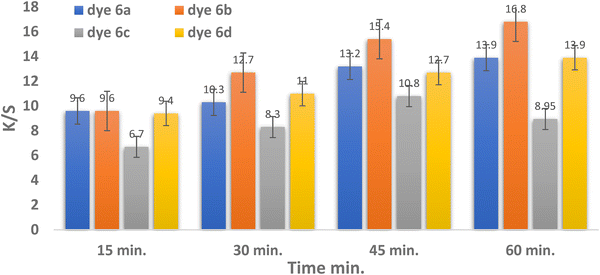 | ||
| Fig. 4 Time and color strength (K/S) relationship for dyes 6a–d (temperature = 130 °C, pH = 2 for dyes 6a–c and pH = 4 in the case of dye 6d, and 3% shade). | ||
3.4. Repellency assessment of the dyed fabrics
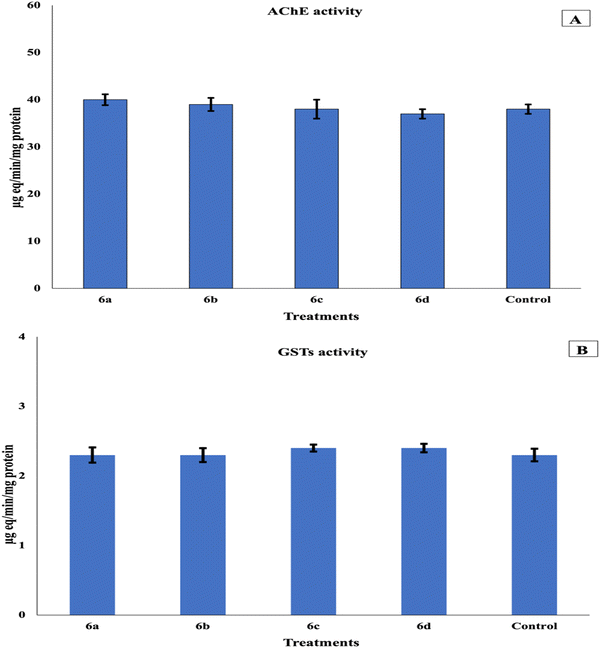
Mosquitoes can travel up to 200 miles from their birthplace.70 Consequently, numerous researchers, industries, and medical organizations are investigating mosquito repellents, which are finishing agents or treatments applied to skin, clothing, and other surfaces to deter mosquitoes. One of the sectors of the global textile industry that is expanding the fastest right now is smart or functional textiles. These smart technology applications also include protective textiles.71 The purpose of protective textiles is to shield the wearer from insects, bacteria, fungi, heat, cold, and mosquitoes. Because medical textiles have a direct connection to people, they are also an area that needs a lot of attention.72 Both the domestic and international markets for medical textiles have enormous potential. In addition to being classified as medical textiles, fabrics treated with natural materials to offer defense against bacteria and insect bites are becoming more and more significant to consumers.73 Within this general framework, our research seeks to find natural alternatives. The results revealed that the repellent effect of 6a reached about 60% after 30 minutes of treatment and continued for two hours until it became 56%. This time-dependent decline is likely attributable to volatilization because the active compound evaporates from the tissue substrate over time. Additionally, exposure to ambient light, particularly the UV spectrum, can induce chemical transformations, such as photolysis or photo-oxidation, of the active molecules.74 The active compound may also slowly diffuse from the surface layer into the bulk structure of the fabric material; this reduces the surface concentration required for immediate contact and vapor release.69 The repellent effect percentages of compounds 6b, 6c, and 6d became 53–44%, 56–45% and 35–44%, respectively. In general, 6a had the highest repellent effect, while 6d had the least effect (Fig. 6). Our findings concur with those of ref. 74, who found that textile materials, such as door curtains, military uniforms, bed linens, tablecloths, and sofa coverings, can be treated with insect repellent chemicals to provide external protection against mosquito bites. Consistent with our findings, three medical insect pests (A. aegypti, A. albopictus, and Musca domestica) were effectively knocked down and adulticidally controlled using essential oils (EOs) of Cymbopogon citratus and Eucalyptus globulus, as well as their mixtures.75 Using the WHO cone test, several essential oils have been evaluated for their efficacy in repelling and killing adult mosquitoes to help prevent disease transmission.76 In agreement with our findings, another study reported that the essential oils of Citrus aurantium, Cymbopogon citratus, and Cinnamomum verum were highly effective in killing both adults and eggs of A. aegypti and A. albopictus, with C. verum oil showing an inhibition rate of 91.0–93.0%.77 Additionally, a study78 revealed that clove oil exhibited the highest effectiveness against A. aegypti when formulated in coconut and olive oils, providing protection durations of 96.00 and 76.50 minutes, respectively. Likewise, citronella grass oil in coconut oil, citronella grass oil in olive oil, and lemongrass oil in coconut oil afforded protection against Culex quinquefasciatus for 165.00, 105.00, and 112.50 minutes, respectively. Their research made it very evident that lemongrass, citronella, and clove oils held the greatest promise for mosquito repellents. However, it has been demonstrated that being essential for stress physiology, GST and acetylcholinesterase (AChE), which protect insects against insecticidal chemicals, are engaged in intracellular transport and several biosynthetic activities.79 The levels of AChE and GST enzymes were also measured after testing these dyes on C. pipiens mosquito larvae to determine their toxicity, where AChE and GST were 40, 39, 38, and 37 and 2.3, 2.3, 2.4, and 2.4 µg eg min−1 mg−1 protein in the case of 6a–d compared to 38 and 2.3 µg eg min−1 mg−1 protein in the control group. The results demonstrated that these dyes exerted no significant effect on the two enzymes with respect to the reference, as illustrated in Fig. 7. In the same context,80 it was reported that the extract of Lantana camara considerably decreased AChE function in larvae compared with those fed on other plant extracts and/or positive controls. These findings support the hypothesis that the L. camara extract significantly reduces antioxidant synthesis compared to other plant extracts, which may account for the higher mosquito mortality rate.81 Furthermore, a previous study80 demonstrated that the L. camara extract exerted the strongest larvicidal effect, likely due to its capacity to suppress GST activity, which enabled its toxic components to disrupt the larval immune system more rapidly than those of other plant extracts.82 Unlike GST, which was markedly elevated in all examined samples, AChE exhibited variable activity patterns, characterized by a significant increase in response to columbamine and β-sitosterol treatments and a decrease following exposure to AC, palmatine, and jatrorrhizine.9 | ||
| Fig. 6 Repellent effect of the tested materials for two consecutive hours against adult female Culex pipiens mosquitoes. | ||
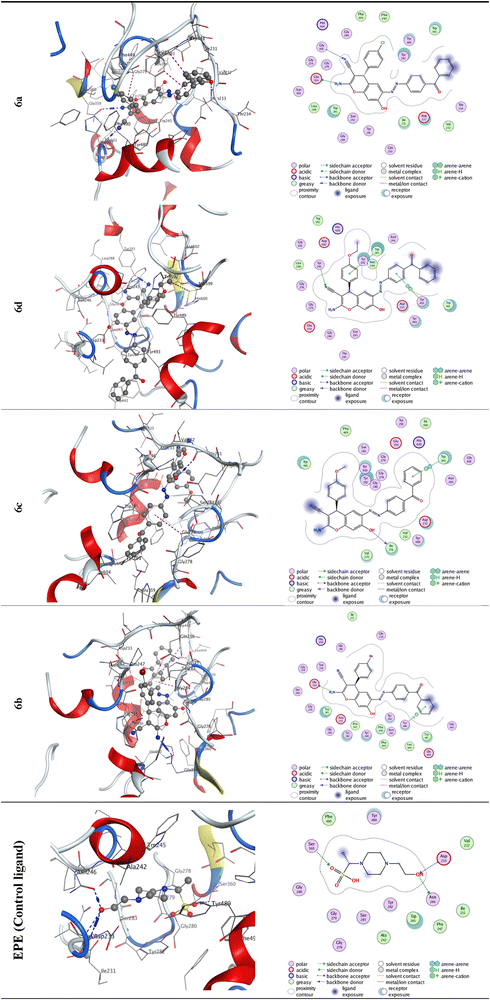
3.5. Docking investigation
| Molecule | Ligand | Receptor | Interaction | Distance (in Å from the main residue) | E (kcal mol−1) | S (kcal mol−1) |
|---|---|---|---|---|---|---|
| 6a | N54 | GLU 359 | H-donor | 3.03 | −2 | −8.4233 |
| N15 | GLY 601 | H-acceptor | 3.41 | −1.2 | ||
| 6d | N15 | TYR 291 | H-acceptor | 3.02 | −1.9 | −7.8642 |
| 6-Ring | TRP 245 | pi–pi | 3.75 | 0 | ||
| 6-Ring | TRP 245 | pi–pi | 3.99 | 0 | ||
| 6-Ring | TYR 493 | pi–pi | 3.81 | 0 | ||
| 6c | O31 | ILE 231 | H-donor | 3.39 | −0.7 | −7.7104 |
| 6-Ring | TRP 245 | pi–pi | 3.54 | 0 | ||
| 6b | N54 | GLU 359 | H-donor | 2.96 | −4.6 | −7.427 |
| 6-Ring | TYR 494 | pi–H | 4.17 | −0.6 | ||
| EPE (control ligand) | O32 | ASN 246 | H-donor | 2.62 | −0.6 | −5.3571 |
| O23 | SER 360 | H-acceptor | 2.77 | −1.8 | ||
| O32 | ASP 233 | H-acceptor | 2.92 | −2.8 |
| Molecule | Ligand | Receptor | Interaction | Distance (in Å from the main residue) | E (kcal mol−1) | S (kcal mol−1) |
|---|---|---|---|---|---|---|
| 6d | N61 | GLU 64 | H-donor | 3.01 | −4.3 | −6.6624 |
| N61 | GLN 49 | H-acceptor | 3.23 | 5.9 | ||
| 6a | N54 | GLU 64 | H-donor | 3.36 | −1.4 | −6.2752 |
| 6c | O31 | MET 34 | H-donor | 3.14 | −3.2 | −6.2494 |
| N15 | SER 9 | H-acceptor | 3.38 | −1.3 | ||
| N15 | ALA 10 | H-acceptor | 3.5 | −1.5 | ||
| 6b | O53 | GLY 8 | H-acceptor | 3.34 | −0.7 | −6.0302 |
| GTX (control ligand) | O11 | SER 65 | H-acceptor | 3.27 | −2.5 | −5.7243 |
4. Conclusions
Our approach to the design and synthesis of four novel disperse dyes involved the confirmation of their chemical compositions. The synthesized dyes were subsequently applied to polyester fabrics in shades 1–5 at temperatures ranging from 100 to 130 °C under various pH conditions (2, 4, 6, 8, and 10). With respect to color levelness and shade depth, the resulting color palette was suitable, exhibiting tones of orange, light brown, reddish brown, and dark brown. High colour strength (13.9, 16.8,12.2, and 13.9) was achieved by dispersing dyes (6a, b, and d) under the optimum dyeing conditions of 60 minutes, pH = 2, and 130 °C at a shade level of 3%. These values achieved the required dyeing levelness and shade depth for dyes 6a–d. Importantly, this study emphasizes optimizing dyeing conditions to achieve the best color outcomes for polyester fabrics using the previously described disperse dyes. This study demonstrated that textile fabrics can be finished with insect-repellent agents to provide external protection against mosquito bites, making them suitable for use in door curtains, military uniforms, bed linens, tablecloths, and sofa covers.Author contributions
Mohamed S. A. El-Gaby: supervision, conceptualization, and writing. M. A. Habib: supervision, writing and review. Nadeem Raza: preparation of the final research report. Ahmed B. M. Ibrahim: writing. Ali A. Ali: conceptualization, methodology, data curation, editing, writing, and review. Walid E. Elgammal: conceptualization, methodology, and data curation. Ahmed H. Halawa: resources, data curation, validation, and formal analysis. Mostafa A. Ismail: investigation, resources, and formal analysis. Tharwat A. Selim: biological activity of dyed-fabric samples, methodology, writing, and review. Ahmed I. Hasaballah: biochemical assay, methodology, writing, and review. Mohamed A. M. El-Tabakh: molecular docking software, writing, and review. Gameel A. M. Elhagali: conceptualization and review.Conflicts of interest
The authors declare that they have no known financial or personal conflicts of interest that could have influenced the work reported in this paper.Data availability
Data will be made available upon request.All authors confirm that the data which supporting the findings of this study are available within the article and its supplementary information (SI). Supplementary information: Fig. S1–S20. See DOI: https://doi.org/10.1039/d5ma01162k.
Supplementary data has been deposited with Protein Data Bank: 5x61 and 1PN9.83a,b
Acknowledgements
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant no. IMSIU-DDRSP250-04).References
- B. Sartorius, J. Cano, H. Simpson, L. S. Tusting, L. B. Marczak, M. K. Miller-Petrie, B. Kinvi, H. Zoure, P. Mwinzi and S. I. Hay, Lancet Global Health, 2021, 9, e52–e60 CrossRef CAS PubMed.
- T. A. Selim, S. H. Ragab, S. A. Riad, R. I. Eltaly, S. H. Mohammed, S. E. Sharawi, N. A. Alkenani, R. S. Almahallawi, H. S. Al-Rashidi and M. A. El-Tabakh, Insects, 2025, 16, 433 CrossRef PubMed.
- M. M. El-Bahnasawy, L. A. Megahed, H. A. A. Saleh and T. A. Morsy, J. Egypt. Soc. Parasitol., 2013, 43, 41–56 Search PubMed.
- S. Zotzmann, A. Steinbrink, K. Schleich, F. Frantzmann, C. Xoumpholphakdy, M. Spaeth, C. V. Moro, P. Mavingui and S. Klimpel, Parasitol. Res., 2017, 116, 1899–1906 CrossRef PubMed.
- M. M. El-Bahnasawy, M. K. M. Khater and T. A. Morsy, J. Egypt. Soc. Parasitol., 2013, 43, 87–102 Search PubMed.
- A. Gabarty, T. A. Selim and A. I. Hasaballah, J. Radiat. Res. Appl. Sci., 2022, 15, 1–6 CAS.
- F. I. Abdallah, B. A. Merdan, F. A. Shaarawi, A. F. Mohamed, T. A. Selim, S. M. Dahesh and M. H. Rady, Beni-Suef Univ. J. Basic Appl. Sci., 2024, 13, 119 CrossRef.
- A. Hasaballah, T. Selim, M. Tanani and E. Nasr, Afr. Entomol., 2021, 29, 479–490 Search PubMed.
- T. A. Selim, I. E. Abd-El Rahman, H. A. Mahran, H. A. Adam, V. Imieje, A. A. Zaki, M. A. Bashar, H. Hwihy, A. Hamed and A. A. Elhenawy, Insects, 2022, 13, 676 CrossRef PubMed.
- A. H. Hashem, T. A. Selim, M. H. Alruhaili, S. Selim, D. H. M. Alkhalifah, S. K. Al Jaouni and S. S. Salem, J. Funct. Biomater., 2022, 13, 112 CrossRef CAS PubMed.
- A. E. Mekky, E. Saied, M. M. Al-Habibi, Z. A. Shouaib, A. I. Hasaballah, M. E. Rashed, A. F. Khaled, A. N. Alshammari, F. S. Youssef and A. M. Al-Shahat, Sci. Rep., 2025, 15, 27845 CrossRef CAS PubMed.
- S. K. Maguranyi, C. E. Webb, S. Mansfield and R. C. Russell, J. Am. Mosq. Control Assoc., 2009, 25, 292–300 CrossRef PubMed.
- R. Prabha and R. Vasugi, Indian J. Sci., 2012, 1, 74–76 Search PubMed.
- U. Agarwal, S. Verma and R. K. Tonk, Bioorg. Med. Chem. Lett., 2024, 129912 CrossRef CAS PubMed.
- M. K. Vanga, R. Bhukya, V. Thumma, V. Tamalapakula, L. S. Boddu and V. Manga, Chem. Biodiversity, 2024, 21, e202401583 CrossRef CAS PubMed.
- Y. Li, T. Ma, Y. Yang, X. Zhong, G. Zhu, J. Wang, W. Chen, J. Fan, L. Tang and W. Liu, J. Agric. Food Chem., 2024, 72, 26983–26995 CrossRef CAS PubMed.
- X. Yang, D. Liu, C. Wei, J. Li, C. Zhao, Y. Tian, X. Li, B. Song and R. Song, iScience, 2024, 27, 111210 CrossRef CAS PubMed.
- F. F. Alblewi, M. H. Alsehli, Z. M. Hritani, A. Eskandrani, W. H. Alsaedi, M. O. Alawad, A. A. Elhenawy, H. Y. Ahmed, M. S. El-Gaby and T. H. Afifi, Int. J. Mol. Sci., 2023, 24, 16716 CrossRef CAS PubMed.
- R. M. Okasha, M. Alsehli, S. Ihmaid, S. S. Althagfan, M. S. El-Gaby, H. E. Ahmed and T. H. Afifi, Bioorg. Chem., 2019, 92, 103262 CrossRef PubMed.
- N. Cai, X. Gao, L. Jia, Y. Liu, J. Zhao, J. Qu and Y. Zhou, Bioorg. Chem., 2025, 154, 108050 CrossRef CAS PubMed.
- S. Abdolmohammadi and B. Mirza, Results Chem., 2025, 102060 CrossRef CAS.
- M. T. Chen, H. Y. Chen, Y. F. Luo, J. Zhou, J. J. Yang, Z. P. Jiang and L. Huang, Chem. Biodiversity, 2024, e202402465 Search PubMed.
- D. Sangeetha, J. Mol. Struct., 2025, 1322, 140562 CrossRef.
- M. Z. Saif, N. J. I. Esha, S. T. Quayum, S. Rahman, M. A. Al-Gawati, G. Alsowygh, H. Albrithen, A. N. Alodhayb, R. A. Poirier and K. M. Uddin, Sci. Rep., 2024, 14, 13221 CrossRef CAS PubMed.
- B. N. Sudha, V. G. Sastry, M. S. Harika and N. Yellasubbaiah, Indian J. Chem., 2018, 57, 737–745 Search PubMed.
- M. Saeedi, M. Safavi, E. Karimpour-Razkenari, M. Mahdavi, N. Edraki, F. H. Moghadam, M. Khanavi and T. Akbarzadeh, Bioorg. Chem., 2017, 70, 86–93 CrossRef CAS PubMed.
- W. Yuanhui, L. Na, H. Jincai, L. Hao, L. Zuren, L. Dingfeng and B. Lianyang, Chin. J. Pestic. Sci., 2024, 26, 857–869 Search PubMed.
- W. Zhao, B. Wang, Y. Liu, L. Fu, L. Sheng, H. Zhao, Y. Lu and D. Zhang, Eur. J. Med. Chem., 2020, 189, 112075 CrossRef PubMed.
- K. Mezgebe and E. Mulugeta, RSC Adv., 2022, 12, 25932–25946 RSC.
- E. El-Sayed, A. El-Aziz, H. Othman and A. G. Hassabo, Egypt. J. Chem., 2024, 67, 87–97 CrossRef.
- S. Benkhaya, S. M’rabet and A. El Harfi, Heliyon, 2020, 6, e03271 CrossRef CAS PubMed.
- F. Hamon, F. Djedaini-Pilard, F. Barbot and C. Len, Tetrahedron, 2009, 65, 10105–10123 CrossRef CAS.
- C. Patil, D. S. Talele, S. P. Talele, P. R. Pohekar and D. Kolhe, J. Pharm. Sci. Res., 2019, 11, 2213–2219 CAS.
- B. Zhang, L. Song, C. Feng and W. Tian, ChemistrySelect, 2022, 7, e202203948 CrossRef CAS.
- C. Lei, W. Yang, Z. Lin, Y. Tao, R. Ye, Y. Jiang, Y. Chen and B. Zhou, RSC Adv., 2024, 14, 20339–20350 RSC.
- T. Marinov, Z. Kokanova-Nedialkova and P. T. Nedialkov, Diversity, 2023, 15, 1030 CrossRef CAS.
- K. Surana, B. Chaudhary, M. Diwaker and S. Sharma, MedChemComm, 2018, 9, 1803–1817 RSC.
- S. Sheng Zhu, X. Li Liu, P. Fei Liu, Y. Li, J. Qiang Li, H. Min Wang, S. Kui Yuan and N. Guo Si, Phytopathology, 2007, 97, 643–649 CrossRef PubMed.
- M. C. Cuquerella, V. Lhiaubet-Vallet, J. Cadet and M. A. Miranda, Acc. Chem. Res., 2012, 45, 1558–1570 CrossRef CAS PubMed.
- J. Leegwater-Kim and C. Waters, Expert Rev. Neurother., 2007, 7, 1649–1657 CrossRef CAS PubMed.
- R. S. Rosenson, Expert Rev. Cardiovasc. Ther., 2008, 6, 1319–1330 CrossRef CAS PubMed.
- K. Sivakumar and A. Nalini, J. Mol. Liq., 2024, 395, 123905 CrossRef CAS.
- M. El-Gaby, A. Atalla, A. Gaber and K. Abd Al-Wahab, Il Farmaco, 2000, 55, 596–602 CrossRef CAS PubMed.
- M. S. El-Gaby, N. M. Taha, J. A. Micky and M. A. El-Sharief, Acta Chim. Slov., 2002, 49, 159–171 CAS.
- N. M. Saleh, M. S. El-Gaby, K. El-Adl and N. E. Abd El-Sattar, Bioorg. Chem., 2020, 104, 104350 CrossRef CAS PubMed.
- A. M. Sayed, F. A. Taher, M. R. Abdel-Samad, M. S. El-Gaby, K. El-Adl and N. M. Saleh, Bioorg. Chem., 2021, 108, 104669 CrossRef CAS PubMed.
- M. El-Gaby, M. Hussein, M. A. Reheim, A. Abdou, A. Fahmy, A. Drar and M. Gad, Russ. J. Bioorg. Chem., 2024, 50, 917–933 CrossRef CAS.
- M. El-Gaby, G. E.-H. Ali, M. A. Reheim, A. Abdou, M. Bakry, A. Drar and M. Gad, Russ. J. Bioorg. Chem., 2024, 50, 1037–1048 CrossRef CAS.
- M. El-Gaby, M. Hussein, F. Faraghally, A. Drar and M. Gad, Curr. Chem. Lett., 2023, 12, 599–606 CrossRef.
- A. A. Ali, M. Abass, S. El-Molla, S. A. Halim and E.-S. Ibrahim, Pigm. Resin Technol., 2024, 53, 1017–1027 CrossRef CAS.
- S. Yoon, B. Choi, M. M. Rahman, S. Kumar, S. M. Mamun Kabir and J. Koh, Materials, 2019, 12, 4209 CrossRef CAS PubMed.
- A. A. Ali, H. Abd El-Wahab, M. S. Abusaif, A. Ragab, O. A. Abdel-Jaid, E. Eldeeb and Y. A. Ammar, Pigm. Resin Technol., 2024, 53, 557–568 CrossRef CAS.
- A. A. Ali, M. M. Elsawy, S. S. Salem, A. A. El-Henawy and H. Abd El-Wahab, Pigm. Resin Technol., 2023, 52, 19–32 CrossRef CAS.
- A. A. Ali, M. Alshukur, A. M. Ashmawy, A. M. Mahmoud, A. Saleh, H. S. Nassar and B. Yao, Res. J. Text. Apparel, 2024, 28, 478–492 CrossRef CAS.
- M. Larvicides, Google Scholar, 2005 Search PubMed.
- D. Simpson, D. Bull and D. Lindquist, Ann. Entomol. Soc. Am., 1964, 57, 367–371 CrossRef CAS.
- C.-H. Kao, C.-F. Hung and C.-N. Sun, J. Econ. Entomol., 1989, 82, 1299–1304 CrossRef CAS.
- W. S. Abbott, J. Econ. Entomol., 1925, 18, 265–267 CrossRef CAS.
- A. A. Ali, M. A. Ismail, W. E. Elgammal, A. Belal, A. J. Obaidullah, A. K. Khalil, G. A. Elhagali and M. S. El-Gaby, Sci. Rep., 2025, 15, 4360 CrossRef CAS PubMed.
- J. Chen, L. Pei, W. Shi, J. Yi and J. Wang, Polymers, 2023, 15, 1046 CrossRef CAS PubMed.
- A. A. Ali, M. Elsawy, N. M. Saleh, A. A. El-Henawy, F. A. M. Al-Zahrani and H. A. El-Wahab, Pigm. Resin Technol., 2025 Search PubMed.
- N. H. Bahtiti, W. E. Elgammal, A. A. Ali, A. Belal, O. Abdullah, M. M. Ghoneim, M. S. Qenawy and M. M. Abdou, ACS Omega, 2023, 9, 447–455 CrossRef PubMed.
- M. M. Abdou, A. A. Ali, H. A. El-Wahab, H. F. Al Shareef and F. A. Al-Zahrani, Fibers Polym., 2024, 1–16 Search PubMed.
- S. S. Pawar, S. Maiti, S. Biranje, K. Kulkarni and R. V. Adivarekar, Heliyon, 2019, 5, e01606 CrossRef PubMed.
- W. E. Elgammal, A. A. Ali, A. E. Hassan, F. A. M. Al-Zahrani, E. K. Alenezy and H. A. El-Wahab, ACS Omega, 2025, 10, 6876–6890 CrossRef CAS PubMed.
- A. A. Ali, F. A. M. Al-Zahrani, W. E. Elgammal, M. Ali, A. M. Mahmoud and H. Abd El-Wahab, Pigm. Resin Technol., 2025, 54, 982–993 CrossRef.
- O. A. A. Ali, H. F. Al Shareef, R. Felaly, A. A. Ali, M. Elsawy, A. Belal, R. I. Alsantali, A. B. Mehany, S. Al-Shomar and N. Abdelshafi, J. Mol. Struct., 2025, 1337, 142075 CrossRef CAS.
- W. E. Elgammal, A. A. Ali, G. A. Elhagali, M. A. Ismail, A. Belal, N. K. Albezrah, M. A. Ali, M. A. El-Tabakh, M. A. Alrayyani and M. S. El-Gaby, ACS Omega, 2025, 10, 39567–39579 CrossRef CAS PubMed.
- O. A. Abu Ali, A. A. Ali, N. M. Saleh, M. Elsawy, A. A. El-Henawy and H. Abd El-Wahab, Chem. Afr., 2025, 8, 995–1014 CrossRef CAS.
- A. M. Lutambi, M. A. Penny, T. Smith and N. Chitnis, Math. Biosci., 2013, 241, 198–216 CrossRef PubMed.
- R. Mia, M. Selim, A. Shamim, M. Chowdhury, S. Sultana, M. Armin, M. Hossain, R. Akter, S. Dey and H. Naznin, J. Text. Eng. Fashion Technol., 2019, 5, 220–226 Search PubMed.
- M. D. Teli and P. P. Chavan, J. Text. Inst., 2018, 109, 427–434 CrossRef CAS.
- D. Saber and K. Abd El-Aziz, J. Ind. Text., 2022, 51, 246S–271S CrossRef CAS PubMed.
- M. M. Baz, A. S. Montaser and R. M. Ali, J. Text. Inst., 2025, 116, 293–308 CrossRef CAS.
- M. Soonwera and S. Sittichok, Environ. Sci. Pollut. Res., 2020, 27, 20201–20214 CrossRef CAS PubMed.
- M.-X. Li, Y.-P. Ma, H.-X. Zhang, H.-Z. Sun, H.-H. Su, S.-J. Pei and Z.-Z. Du, Plant Diversity, 2021, 43, 317–323 CrossRef PubMed.
- T. Moungthipmalai, C. Puwanard, J. Aungtikun, S. Sittichok and M. Soonwera, Sci. Rep., 2023, 13, 2119 CrossRef CAS PubMed.
- M. Sharififard, I. Alizadeh, E. Jahanifard, C. Wang and M. E. Azemi, J. Arthropod.-Borne Dis., 2018, 12, 387 Search PubMed.
- M. T. Rehman, M. F. AlAjmi, A. Hussain, G. M. Rather and M. A. Khan, Int. J. Mol. Sci., 2019, 20, 819 CrossRef CAS PubMed.
- H. M. Al-Solami, J. King Saud Univ., Sci., 2021, 33, 101371 CrossRef.
- N. Abutaha, F. A. Al-Mekhlafi, L. A. Al-Keridis, M. Farooq, F. A. Nasr and M. Al-Wadaan, Entomol. Res., 2018, 48, 362–371 CrossRef CAS.
- İ. Gulçin, P. Taslimi, A. Aygün, N. Sadeghian, E. Bastem, O. I. Kufrevioglu, F. Turkan and F. Şen, Int. J. Biol. Macromol., 2018, 119, 741–746 CrossRef PubMed.
- (a) Q. Han, D. M. Wong, H. Robinson, H. Ding, P. C. H. Lam, M. M. Totrov, P. R. Carlier and J. Li, Crystal structure of acetylcholinesterase catalytic subunits of the malaria vector Anopheles gambiae, Insect Sci, 2017 DOI:10.1111/1744-7917.12450; (b) L. Chen, P. R. Hall, X. E. Zhou, H. Ranson, J. Hemingway and E. J. Meehan, Crystal structure of an insect delta-class glutathione S-transferase from a DDT-resistant strain of the malaria vector Anopheles gambiae, Acta Crystallogr D Biol Crystallogr, 2003, 59, 2211–2217 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2026 |