Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Exploring facet-engineered anatase nanoparticles for amplification of sensitivity in heavy metal ion detection and other applications
Md. Anayet
Ullah
ab,
Fataha Nur
Robel
b,
Newaz Mohammed
Bahadur
c,
Dipa
Islam
d,
Subarna
Sandhani dey
e,
Samina
Ahmed
 *a and
Md.
Sahadat Hossain
*a and
Md.
Sahadat Hossain
 *a
*a
aInstitute of Glass & Ceramic Research and Testing, Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka 1205, Bangladesh. E-mail: shanta_samina@yahoo.com; saz8455@gmail.com
bDepartment of Applied Chemistry and Chemical Engineering, Noakhali Science and Technology University, Noakhali, Bangladesh
cDepartment of Chemistry, Noakhali Science and Technology University, Noakhali 3814, Bangladesh
dBiomedical and Toxicological Research Institute, Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka 1205, Bangladesh
eInstitute of Food Science and Technology, Bangladesh Council of Scientific and Industrial Research (BCSIR), Dhaka 1205, Bangladesh
First published on 3rd December 2025
Abstract
TiO2 is one of the most extensively studied nanomaterials due to its remarkable surface, catalytic, and electronic properties, which are further enhanced when synthesized with exposed facets. In this study, anatase-phase TiO2 nanoparticles with different crystal facets were synthesized using inorganic modifiers through a hydrothermal method. The synthesized samples were characterized using various analytical techniques. X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and Raman spectroscopy confirmed the formation of anatase TiO2. The crystallite size, estimated from XRD data, ranged between 1–50 nm, except for the linear straight-line method (LSLM), which exhibited a higher value. Growth preference, texture coefficient and Raman data and structural analysis verified the facet formation. Additionally, thermogravimetric analysis (TGA) revealed enhanced thermal stability of the samples. Photocatalytic studies demonstrated that {001}-faceted and {101}/{001} co-faceted TiO2 achieved complete degradation of Congo Red (CR) dye within 30 min, significantly outperforming {101}-faceted TiO2 (69.36% degradation). Scavenging experiments confirmed that ˙OH radicals played the dominant role in CR degradation over the {001}-faceted TiO2. Kinetic studies based on the Langmuir–Hinshelwood model revealed apparent rate constants of 0.0202 min−1 for {101}-faceted TiO2, 0.0081 min−1 for {001}-faceted TiO2, and 0.0127 min−1 for {101}/{001}-co-faceted TiO2. Furthermore, {001}-faceted TiO2 exhibited significant antimicrobial efficacy against Gram-positive bacteria, attributed to enhanced surface activity. Electrochemical studies revealed the superior sensing capabilities of {001}-faceted TiO2 for Pb2+ ion detection, with its enhanced electroactive surface area contributing to a limit of detection (LOD) of 9.378 ppm and a limit of quantification (LOQ) of 31.162 ppm.
Introduction
Nowadays, nanomaterials are widely valued for their exceptional properties over bulk materials. For example, nanostructured titanium dioxide (TiO2), with its remarkable optical and electronic features, has revolutionized applications in photocatalysis, sensors, photovoltaics, and photonic devices.1 This affordable, non-toxic, and stable semiconductor is extensively used in dye sensitized solar cells,2 pollutant remediation,3 charge spreading devices,4 gas sensors,5 photocatalytic degradation, photoluminescence,6 bactericides,7 optical coatings,8 opto-electronic devices,9 electrochemistry,10 textiles11etc. TiO2 is found predominantly in three crystalline forms: anatase, brookite, and rutile. Anatase is notable for its high catalytic efficiency, attributed to its vast surface area, minimal charge recombination, strong oxygen affinity, and broad bandgap, making it highly effective under UV light.12 The performance of TiO2 is influenced by its physical and chemical properties, including crystal structure, grain size, morphology, and surface characteristics, prompting interest in synthesizing TiO2 with specific crystal structures to enhance highly photoactive facets. Different facets of a single crystal exhibit distinct properties, affecting reactivity.13 Anatase TiO2, with its wide band gap, demonstrates photocatalytic activity through photogenerated electrons (e−) and holes (h+) at the surface, initiating redox reactions. However, electron–hole recombination during transport reduces carrier efficiency, with charge separation relying on trapping holes by H2O or electrons by O2. Surface properties, such as crystal facets, defects, and adsorbed ions, impact charge migration, making the modification of TiO2 crystal facets a key focus.14 Moreover, TiO2 encounters limitations such as a broad band gap, low quantum yield, and swift recombination of photogenerated charges, which constrain its visible light absorption and hinder large-scale applications. In recent years, facet engineering of TiO2 crystals has gained prominence as an effective strategy to boost photocatalytic efficiency.15Various methods, including sputtering,16 aerosol pyrolysis,17 MOCVD,18 electrodeposition,19 spray pyrolysis,20 sol–gel,21 and hydrothermal synthesis,22 have been employed to fabricate TiO2 nanostructured thin films. Among these, hydrothermal synthesis is particularly favored for its simplicity, cost-effectiveness, and ability to produce well-aligned nanostructures with a high surface area. It also allows for the straightforward synthesis of single-crystalline materials.23 Hydrothermal or solvothermal techniques typically operate at temperatures below 1000 °C, offering energy efficiency compared to alternative approaches like chemical vapor deposition, which often require higher temperatures. These methods provide precise control over the morphology and crystallinity of TiO2, making them ideal for photocatalysis and sensing applications.24
For TiO2, the primary low-index facets are {101}, {001}, and {100}. Among these, the {001} facet of anatase TiO2 is particularly reactive due to its high surface energy (0.90 J m−2), which exceeds that of the {100} (0.53 J m−2) and {101} (0.44 J m−2) facets, making it a key player in photocatalytic applications.25 However, due to its thermodynamic instability, the {001} facet is rarely exposed in large quantities, with most natural and synthesized TiO2 crystals predominantly showing the {101} facet. The reactivity of the {001} facet, characterized by low atomic coordination and active surface oxygen atoms, facilitates efficient dissociative adsorption of reactant molecules, making it ideal for photocatalytic processes such as the degradation of organic pollutants including various dyes.26 Among these, Congo Red (CR) is frequently selected in photocatalytic studies due to its highly stable complex aromatic azo structure, which makes it persistent in water and resistant to natural degradation. Its metabolites, including benzidine, are toxic, exhibiting carcinogenic and mutagenic properties, and can trigger allergic reactions, increase chemical oxygen demand in water bodies, and even contribute to infertility. These characteristics make CR considerably more difficult to degrade, establishing it as a rigorous benchmark for evaluating photocatalytic performance.27,28 Recent research has focused on manipulating TiO2 crystal facets to enhance photocatalytic performance, particularly by increasing the exposure of {001} facets, which are essential for trapping holes as oxidative sites. However, some studies have also reported that {101} facets exhibit higher photocatalytic activity than {001} facets in certain applications.29
In addition to organic pollutants, the increasing levels of heavy metal contaminants have drawn significant public and scientific concern due to their toxicity, bioaccumulation, and persistence in ecosystems.30 Heavy metals such as lead (Pb2+), cadmium (Cd2+), and mercury (Hg2+) pose severe health risks, including neurological disorders, organ damage, and carcinogenic effects.31 In recent years, electrochemical sensors have gained widespread attention in chemical and biological studies due to their high sensitivity, simplicity, cost-effectiveness, and reliability in detecting trace pollutants.32 Various analytical techniques, including atomic absorption spectroscopy (AAS),33 inductively coupled plasma mass spectrometry (ICP-MS),34 and X-ray fluorescence (XRF)35 are commonly used for the detection and quantification of heavy metals. However, these methods often require expensive instrumentation, complex sample preparation, and time-consuming preconcentration procedures.36 Among electrochemical techniques, anodic stripping voltammetry (ASV) has emerged as a powerful and widely applied method for detecting heavy metal ions in water. ASV offers high sensitivity, accuracy, low cost, and the ability to detect multiple metal ions simultaneously.37 The technique involves an initial preconcentration step, where metal ions accumulate on an electrode surface, followed by a stripping step, during which the deposited metals are reoxidized, producing measurable current signals proportional to metal ion concentration.38 This makes ASV a highly efficient, rapid, and environmentally friendly approach for monitoring heavy metal contamination in water sources, offering advantages over traditional detection techniques.39
Beyond their application in photocatalysis and electrochemical sensing, TiO2 nanoparticles have also demonstrated significant antimicrobial properties. The antimicrobial mechanism primarily involves direct interaction with bacterial cell walls, leading to structural changes, increased membrane permeability, and eventual cell death.40
In this study, anatase-phase TiO2 nanoparticles with various exposed crystal facets were synthesized using inorganic modifiers such as NH4Cl, NH4SO4, and HF. The synthesized TiO2 samples were thoroughly characterized using a range of techniques, and the influence of facet modification on their photocatalytic, antimicrobial, and electrochemical properties was further investigated. The photocatalytic degradation of Congo Red (CR) dye was evaluated, alongside antimicrobial activity, to assess the material's effectiveness in environmental pollutant removal and microbial inhibition. Additionally, electrochemical sensors for the detection of Pb2+ ions were fabricated using the synthesized TiO2, targeting heavy metal sensing applications. These investigations aim to enhance the understanding of how facet modification can tune TiO2's properties, thereby improving its performance in environmental remediation and sensing applications.
Materials and methods
Materials and equipment
Tetrabutyl orthotitanate (purity 98%) was acquired from Tokyo Chemical Industry Co. Ltd, Japan. Ammonium chloride (purity 99%), hydrofluoric acid (purity 48%), sulfuric acid (purity 99%), ammonia (purity 25%) and isopropanol were acquired from Merck, Germany. Crystallographic analysis was performed using a Rigaku XRD instrument with Cu Kα X-rays (λ = 1.5406 Å) at 40 kV and 50 mA, with a cooling temperature of 23 °C. Functional groups were analyzed via FT-IR spectroscopy using an IR-Prestige 21 spectrometer, scanning from 400 to 4000 cm−1 with a resolution of 4 cm−1 and 30 scans. Thermal analysis was conducted using STA equipment in a nitrogen atmosphere, ranging from 50 °C to 800 °C. Structural and elemental characterization was done using a JEOL JSM-7610F field emission scanning electron microscope (FESEM) with integrated energy-dispersive X-ray spectroscopy (EDX) capabilities at 25 kV and SEM, EVO-18, Carl Zeiss, Germany machine.Experimental methods
For the synthesis of {001}-faceted TiO2, 8.33 mL of TBOT was mixed with 1 mL of HF in a dry Teflon-lined autoclave and heated at 180 °C for 24 hours under hydrothermal conditions. Upon the reaction's completion, the product was recovered through filtration, followed by extensive washing with water and a single wash with ethanol to remove residual impurities. To eliminate any remaining fluorine species, the product was further treated with an aqueous NaOH solution, followed by additional washing with deionized water. Finally, the purified material was dried in an oven at 105 °C, yielding {001}-faceted TiO2. Fig. S2 shows the schematic depiction of the synthesis procedure of {001}-faceted TiO2.
For the synthesis of {101}/{001} co-faceted TiO2, 2 g of TBOT was dissolved in a mixture of 15 mL deionized water and 15 mL isopropanol. Subsequently, 0.5 g (NH4)2SO4 was added to the reaction mixture, followed by stirring and ultrasonication to ensure uniform dispersion. The subsequent steps mirrored the synthesis of {101}-faceted TiO2. Fig. S3 shows the schematic depiction of the synthesis procedure of {101}/{001} co-faceted TiO2.
The (NH4)2SO4 used to prepare the {101}/{001} co-faceted TiO2 was synthesized by adding diluted H2SO4 dropwise to an NH3 solution under continuous stirring. The addition of H2SO4 was carefully monitored until the solution reached a neutral pH of 7. The resulting solution was then heated to 50 °C to facilitate water evaporation. Once crystals formed, the remaining water was removed by drying the crystals in a vacuum oven at 50 °C. The crystals were subsequently crushed using a mortar and pestle to obtain a powder.
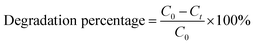 | (1) |
Scavenging experiments were carried out using 2-propanol (isopropyl alcohol) and ethylenediaminetetraacetic acid (EDTA) as scavengers for hydroxyl radicals (˙OH) and photogenerated holes (h+), respectively. In each experiment, 10 mL of the scavenger solution was added to 40 mL of 10 ppm CR dye containing 0.05 g of the catalyst, followed by halogen irradiation for 30 min. The reusability of the catalyst was evaluated under identical conditions over five consecutive cycles.42 After each cycle, the catalyst was decanted, washed three times with water, dried at 60 °C for 6 h, and reused with a fresh dye solution.
Results
Characterization
The XRD diffractogram of the synthesized TiO2 samples, shown in Fig. 1, was compared with the standard ICDD database for anatase (card no: #00-021-1272). The anatase phase, belonging to the tetragonal crystal system with the space group I41/amd (141), is characterized by prominent peaks at 2θ values of 25.28° (1 0 1), 37.80° (0 0 4), 48.05° (2 0 0), and 53.89° (1 0 5). The synthesized samples exhibited slight variations: the {101}-faceted TiO2 showed peaks at 25.35°, 37.73°, 47.84°, and 54.40°; the {001}-faceted TiO2 at 25.21°, 37.90°, 47.92°, and 53.75°; and the {101}/{001} co-faceted TiO2 at 25.30°, 37.89°, 47.89°, and 54.11°. These results demonstrate strong agreement with the standard anatase phase, confirming the successful synthesis of the desired crystal structure.An evaluation of the crystal structure was performed by determining lattice parameters, crystallite size, dislocation density, and microstrain using eqn (E1)–(E5), with outcomes tabulated in Table 1.
| Sample | Lattice parameters, Å | Size of crystallites, D (nm) | Dislocation density, δ (1015 lines per m2) | Microstrain, ε | Crystallinity index |
|---|---|---|---|---|---|
| {101} | a = b = 3.78, c = 9.53 | 6.74 | 2.2 | 0.99 | 0.70 |
| {001} | a = b = 3.79, c = 9.49 | 15.12 | 4.37 | 0.56 | 1.02 |
| {101}/{001} | a = b = 3.80, c = 9.49 | 5.47 | 3.34 | 1.01 | 1.21 |
Various crystallite parameters, such as size of the crystallite, intrinsic strain, stress, and energy density can be computed using XRD data by using different XRD models, comprising Scherrer's model, modified Williamson–Hall methods, the Monshi–Scherrer (M–S) model, the size-strain plot (SSP), and the Halder–Wagner (H–W) model. Each of these models considers different factors that affect the diffraction pattern, including peak broadening due to instrumental effects, internal strain, and stress, along with the isotropic or anisotropic nature of the crystal lattice. These factors significantly influence the XRD parameters owing to imperfections in the crystal structure, such as point defects, triple junctions, dislocations, grain boundaries, and stacking faults. These defects typically contribute to peak broadening and variations in lattice strain, which directly impact the accuracy of the crystallite size estimation. Thus, the application of multiple models allows for a more in-depth and precise calculation of the size of the crystallite and other parameters.43 In Scherrer's model, the average size of the crystallite is estimated using the equation, crystallite size, 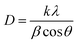 and displayed in Table 1. The parameters obtained from other models are summarized in Table 2, while the corresponding plots are shown in Fig. S4–S10, with the corresponding equations available elsewhere.44
and displayed in Table 1. The parameters obtained from other models are summarized in Table 2, while the corresponding plots are shown in Fig. S4–S10, with the corresponding equations available elsewhere.44
| Name of model | Crystallite size, D (nm); strain, ε; stress, σ (N m−2); energy density, μ (J m−3) | |||
|---|---|---|---|---|
| {101} | {001} | {101}/{001} | ||
| Williamson–Hall model | UDM | ε = 0.0032 | ε = −0.0023 | ε = 0.0018 |
| D = 7.62 | D = 5.52 | D = 5.68 | ||
| USDM | σ = 6.533 × 108 | σ = −4.633 × 108 | σ = 3.76 × 108 | |
| D = 7.62 | D = 5.52 | D = 5.68 | ||
| UDEDM | μ = 1.04 × 106 | μ = −5.22 × 106 | μ = 3.43 × 105 | |
| D = 7.62 | D = 5.52 | D = 5.68 | ||
| M–S model | D = 6.59 | D = 10.93 | D = 10.93 | |
| SSP | D = 6.25 | D = 5.35 | D = 4.9 | |
| ε = 0.0028 | ε = −0.004 | ε = 0.0014 | ||
| H–W model | D = 45.05 | D = 38.61 | D = 35.09 | |
| ε = 0.005 | ε = −0.007 | ε = 0.002 | ||
| S–S model | D = 7.70 | D = 22.36 | D = 5.78 | |
Growth preference and texture coefficient are two key parameters used to assess whether a crystalline material exhibits growth in a specific exposed plane. Growth preference provides insights into the favorable growth direction along a specific crystallographic plane and can be estimated using the normalized intensity of that plane, as calculated with eqn (E6) and (E7). Here, negative values indicate a lower preference for growth on the specific plane, whereas positive values suggest that growth on this specific plane is thermodynamically stable. In contrast, the texture coefficient (Tc) is utilized to examine the actual growth direction of the crystallographic plane. Estimation of the texture coefficient was done using the peak intensities associated with the (101), (004), (200), and (105) planes, as recorded from the XRD data, employing eqn (E8). When Tc(hkl) surpasses 1, it indicates that crystallites are more prevalently oriented in the (hkl) direction.
Table 3 presents the preferred growth values for the synthesized TiO2 samples, while Table 4 shows the texturing behavior of the synthesized TiO2 samples. For the {101}-faceted TiO2, positive preferred growth values were observed for the (004) and (105) planes, suggesting a strong tendency for growth along the {001} and {101} directions, respectively. However, the texture coefficient indicates that texturing predominantly occurs along the {101} direction, as evidenced by the higher Tc value for the (105) plane, while the (004) plane shows a significantly lower Tc value. This correlation supports the conclusion that the sample predominantly exposes the (101) crystallographic direction, confirming the alignment of crystallites along this direction. In contrast, for the {001}-faceted TiO2, a positive preferred growth value was found only for the (004) plane, and the texture coefficient for this plane exceeded 1. These findings confirm that the sample is mainly exposed along the {001} direction. Additionally, the {101}/{001}-co-faceted TiO2 exhibited positive preferred growth values for both the (004) and (105) planes, with notable Tc values for both planes, confirming that this sample exhibits growth along both the {101} and {001} directions.
| Sample | Plane | Growth preference |
|---|---|---|
| {101} | 101 | −0.38 |
| 004 | 0.46 | |
| 200 | −0.04 | |
| 105 | 0.85 | |
| {001} | 101 | −0.14 |
| 004 | 0.46 | |
| 200 | −0.08 | |
| 105 | −0.01 | |
| {101}/{100} | 101 | −0.30 |
| 004 | 1.27 | |
| 200 | −0.50 | |
| 105 | 0.66 |
| Sample | Plane (hkl) | Texture coefficient, (Tc) | Observation |
|---|---|---|---|
| {101} | 101 | 0.6500 | 101 direction texturing |
| 004 | 1.1616 | ||
| 200 | 0.7928 | ||
| 105 | 1.3956 | ||
| {001} | 101 | 0.8679 | 001 direction texturing |
| 004 | 1.3232 | ||
| 200 | 0.8571 | ||
| 105 | 0.9517 | ||
| {101}/{100} | 101 | 0.6850 | 101 and 001 direction texturing |
| 004 | 1.6152 | ||
| 200 | 0.4511 | ||
| 105 | 1.2487 |
FT-IR is a highly effective and widely employed method for analyzing materials by identifying the functional groups embedded in their structure. Fig. 2 displays the FT-IR spectra of the synthesized TiO2 samples. Absorption in the 400–700 cm−1 region, indicative of the stretching and bending vibrations of the Ti–O–Ti bond, was prominently observed in all samples, confirming its presence in the synthesized nanoparticles. Apart from this region, a peak around 1631 cm−1 was detected, attributed to the O–H bending vibrations (Ti–OH). For {001}-faceted TiO2, the absorption peaks are prominent at 423, 434, 457, 496, 556, 598, 647, and 1631 cm−1, with other samples showing close proximity to these values. Similar absorption peaks to those samples have been observed in previous literature.45,46
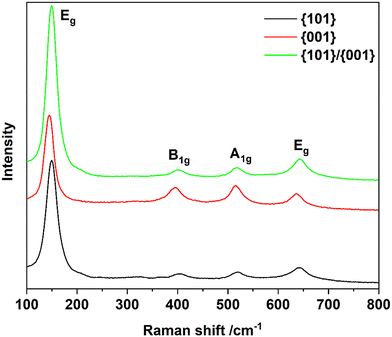
The Raman spectra, illustrated in Fig. 3, demonstrate that all samples exhibit similar spectral profiles at approximately 144, 395, 516, and 636 cm−1. The most intense peak, observed near 144 cm−1, corresponds to the Eg vibrational mode of anatase. The band at 395 cm−1 is attributed to the B1g mode, while the peak at 516 cm−1 results from the overlapping of the A1g (513 cm−1) and B1g (519 cm−1) modes of anatase. Additionally, the Eg mode at 636 cm−1 is also evident. The Eg peak results from O–Ti–O symmetric stretching, while the B1g and A1g modes arise from symmetric and asymmetric O–Ti–O bending, respectively.47 Moreover, Tian et al.48 suggested that the {001} facet percentage can be determined by analyzing the intensity of the 144 cm−1 and 516 cm−1 peaks as outlined in Table 5.
| Sample | Intensity of the 144 cm−1 peak | Intensity of the 514 cm−1 peak | Percentage of {001} | Percentage of {101} |
|---|---|---|---|---|
| {101} | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 639.96 639.96 |
1094.11 | 5.57 | 94.43 |
| {001} | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 162.15 162.15 |
6443.15 | 35.48 | 64.52 |
| {101/001} | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 353.15 353.15 |
1446.65 | 5.10 | 94.90 |
The heat response behavior of the synthesized TiO2 particles was assessed by performing thermal weight loss analysis. The thermogram (Fig. 4) for the synthesized TiO2 samples, heated from 50 °C to 800 °C under a controlled atmosphere, illustrates the weight-loss events occurring during the heating process. Both {101}-faceted and {001}-faceted TiO2 samples exhibited a similar trend in the TGA curve, with nearly identical mass losses of 6.52% and 6.74%, respectively. In contrast, {101}/{001}-co-faceted TiO2 demonstrated a slightly lower mass loss of 4.67%. The weight loss below 120 °C was attributed to the elimination of physiosorbed water molecules, while the loss above 120 °C was due to the evaporation of surface-adsorbed hydroxyl groups and the removal of unreacted residues such as F− and Cl−, which may account for the increased weight loss in {101} and {001} faceted TiO2 compared to pure TiO2. A similar weight loss pattern has been observed in previous studies.49–51
Fig. 5 illustrates the surface morphology of the synthesized {101}-faceted (Fig. 5A), {001}-faceted (Fig. 5B) and {101}/{001}-co-faceted (Fig. 5C) TiO2 samples. A large amount of aggregated nanosheets was observed at both lower and higher magnifications for the {001}-faceted TiO2. This observation verifies the formation of {001} facets, of a shape similar to those reported in previous literature.14 In contrast, the {101} faceted TiO2 particles are densely aggregated and distorted in shape. Although the structure is not clearly understood, previous studies suggest that the shape of this sample could correspond to nanowires. One such particle, appearing as a nanowire, is highlighted in the zoomed-in figure.52 Similarly, the morphology of the {101}/{001} co-faceted TiO2 samples was not clearly defined, as both low and high magnification images revealed densely aggregated, distorted, and irregular shapes. The particle sizes of the {101} and {001}-faceted TiO2 nanoparticles were found to be similar, with average dimensions of 120.106 nm and 80.833 nm, respectively, as analyzed using ImageJ for image processing and Origin for data visualization (Fig. S11). Although these two samples showed no significant size differences, the {101}/{001} co-faceted TiO2 demonstrated a substantially larger average particle size of 1027.06 nm. This notable increase is likely due to particle aggregation or enhanced growth caused by interactions between the two facets during the synthesis process.
 | ||
| Fig. 5 FESEM image of the synthesized (A) {101}-faceted, (B) {001}-faceted, and (C) {101}/{001} co-faceted TiO2 samples. | ||
The EDX spectrum analysis for the {101} and {001}-faceted TiO2 nanoparticles is shown in Fig. S12a and b. The EDX results validate the exclusive presence of titanium (Ti) and oxygen (O) in both samples, with their corresponding peaks in the spectrum at the expected locations. According to the weight percentage analysis, the {101}-faceted TiO2 contained 30.98% oxygen and 69.02% titanium, while the {001}-faceted TiO2 showed 30.05% oxygen and 69.95% titanium, reflecting similar chemical profiles. The results align well with the anticipated compositions, providing evidence of the successful synthesis of the TiO2 nanoparticles.
Photocatalytic degradation of dye
The photocatalytic performance of the TiO2 samples was assessed using CR as a model dye over a 30 min period, with the degradation percentages presented in Fig. 6(a). Both {001}-faceted and {101}/{001}-co-faceted TiO2 samples achieved complete degradation of CR (100%) within the allotted time, while the {101}-faceted TiO2 demonstrated a lower degradation efficiency of 69.36%. The enhanced photocatalytic activity of the {001}-faceted and {101}/{001}-co-faceted TiO2 can be attributed to the superior surface reactivity and larger surface area of the {001} facet.53 | ||
| Fig. 6 (a) CR degradation percentage, (b) scavenging analysis, (c) reusability, and (d) kinetics of the photocatalytic degradation of the synthesized TiO2 samples. | ||
Fig. 6(b) illustrates the degradation percentages obtained from the scavenging tests using IPA and EDTA. In the presence of isopropanol (IPA), an ˙OH radical quencher, the degradation efficiency of {001}- and {101}/{001}-faceted TiO2 decreased sharply from 100% to 12.7% and 49.88%, respectively, confirming that ˙OH radicals are the dominant oxidative species generated on the {001} surface. Likewise, addition of EDTA, a hole (h+) scavenger, reduced the degradation efficiency of {001} and {101}/{001} TiO2 to 28.81% and 20.33%, respectively.
The reusability of the photocatalysts was evaluated over five consecutive degradation cycles, as shown in Fig. 6(c). The {101}-faceted TiO2 exhibited the best performance with 79.90% degradation efficiency even after the fifth cycle, while the {001}-faceted TiO2 maintained 55.84%. In contrast, the {101}/{001}-co-faceted TiO2 initially showed the highest degradation efficiency during the first four cycles, with nearly comparable performance in each run; however, its activity declined in the last few cycles, reaching only 42.69% degradation efficiency.
Fig. 6(d) depicts the kinetics of CR dye degradation over the synthesized TiO2 samples, fitted using the Langmuir–Hinshelwood model according to the equation:54,55
| ln(C0/Ct) = Kappt |
Antimicrobial activity
The antimicrobial activity of the synthesized TiO2 samples, including {101}-faceted TiO2 (T1), {001}-faceted TiO2 (T2), and {101}/{001}-cofaceted TiO2 (T3), was assessed against two Gram-positive bacteria, two Gram-negative bacteria, and a fungal strain. Chloramphenicol served as the positive control for antibacterial activity, while Azoxystrobin was used as the positive control for antifungal activity.As shown in the results (Fig. 7), both {101} and {001}-faceted TiO2 demonstrated notable antimicrobial activity against Gram-positive bacteria but exhibited inadequate activity against Gram-negative bacteria and the fungal strain, with inhibition zones falling below the detection limit of 6 mm. Among the tested samples, {001}-faceted TiO2 (T2) exhibited the highest antibacterial activity against L. monocytogenes, with an inhibition zone of 11.5 ± 0.50 mm, followed by comparable activity against S. aureus (11.5 ± 0.50 mm). In contrast, {101}-faceted TiO2 (T1) showed slightly lower antibacterial activity, with inhibition zones of 9.2 ± 0.91 mm against S. aureus and 8.6 ± 0.40 mm against L. monocytogenes. These results suggest that both {101} and {001}-faceted TiO2 samples possess significant antimicrobial efficacy against Gram-positive bacteria under the tested conditions. However, the {101}/{001} co-faceted TiO2 (T3) failed to exhibit adequate antimicrobial activity against any of the tested microorganisms. In contrast, the positive controls (Chloramphenicol for bacteria and Azoxystrobin for fungi) displayed excellent antimicrobial activity, confirming the validity of the assay. Table 6 shows the inhibition zone diameters, summarizing the antimicrobial activity of the tested samples against the selected microbes.
 | ||
| Fig. 7 Evaluation of antimicrobial properties of the synthesized TiO2 samples against (A) S. aureus, (B) L. monocytogenes, (C) E. coli, (D) S. abony, and (E) C. albicans. | ||
| Sample name | Name of tested microorganisms, diameter of zone of inhibition (mm) | ||||
|---|---|---|---|---|---|
| Gram-positive | Gram-negative | Fungus | |||
| S. aureus | L. monocytogenes | E. coli | S. abony | C. albicans | |
| a <6 mm = no significant antimicrobial activity was observed. | |||||
| {101}-faceted TiO2 | 9.2 ± 0.91 | 8.6 ± 0.40 | <6a | <6a | <6a |
| {001}-faceted TiO2 | 11.2 ± 0.76 | 11.5 ± 0.50 | <6a | <6a | <6a |
| {101}/{001}co-faceted TiO2 | <6a | <6a | <6a | <6a | <6a |
| Positive control | 15.9 ± 2.69 | 15.2 ± 0.75 | 17.9 ± 4.25 | 17.2 ± 0.89 | 16.3 ± 1.10 |
Electrochemical analysis
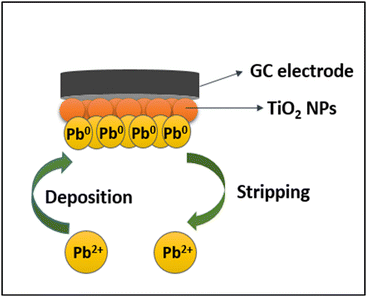
To evaluate the electrochemical performance of the {001}-modified GC electrode, cyclic voltammetry was conducted at the potential range of −0.5 to 1.0 V with a scan rate of 100 mV s−1 using 2.5 µM [Fe(CN)6]3−/4− as a redox probe containing 0.1 M NaCl as the supporting electrolyte, illustrated in Fig. 8(a). The {001}-modified electrode exhibited an anodic peak potential (Ep.a) of 0.24704 V and a cathodic peak potential (Ep.c) of 0.10115 V, resulting in a peak-to-peak separation (ΔE) of 145.89 mV. This value was significantly lower than that of the bare GC electrode [Ep.a = 0.37406 V, Ep.c = 0.08920 V, and ΔE = 284.86 mV]. The reduced ΔE suggests enhanced electron transfer kinetics. A comparable effect has been reported in the literature for the G/PANI nanocomposite-modified electrodes.56 Moreover, the {001}-modified electrode exhibited a higher anodic peak current (Ip.a = 97.28 µA cm−2) and cathodic peak current (Ip.c = 53.80 µA cm−2) compared to the bare GC electrode (Ip.a = 38.90 µA cm−2, Ip.c = 37.01 µA cm−2). This variation in both potential and current can be attributed to the surface modification of the GC electrode, which enhances its electrochemical activity and charge transfer efficiency.The effect of scan rate is crucial in the sensing process as it provides insight into the nature of mass transport at the interface.57 To study this, anodic stripping voltammetry (ASV) was conducted within a scan rate range of 10 to 120 mV s−1, using a fixed concentration of 5 ppm Pb2+, as shown in Fig. 8(b). The results show that the peak current increases with the scan rate, while the potential shifts in the positive direction, indicating the irreversibility of the reaction at the electrode surface. A linear relationship (y = 0.2351x + 0.1506) with a regression coefficient of 0.8867, obtained from the plot of peak current versus the square root of the scan rate (Fig. 8(c)), suggests that the mass transport at the electrode interface is diffusion-controlled.
The effectiveness of the fabricated sensor for detecting Pb2+ was assessed using ASV within the concentration range of 1 to 10 ppm, under the experimental conditions of a 160 s deposition time, deposition voltage of −1.2 V, scan range of −0.8 to 0.5 V, and a scan rate of 20 mV s−1 (displayed in Fig. 8(d)). ASV works by applying a higher negative potential than the reduction potential, allowing metal ions to deposit on the electrode surface. In this experiment, the reduction potential was found to be around +0.07 V, thus the deposition was performed at −1.2 V, fixed after several variations. A linear relationship (y = 0.032x + 0.6453) with a regression coefficient of 0.8519 showed good linearity between 1 and 10 ppm, with peak current increasing linearly with Pb2+ concentration (Fig. 8(e)). This behavior stems from the presence of a moiety that reacted actively with the targeted analyzed ions.58 After deposition, the reduced metals were stripped from the electrode surface within a potential range of −0.8 V to 0.5 V. The limit of detection (LOD) was calculated using the slope and standard deviation (LOD = 3 × SD/slope), yielding a value of 9.378 ppm for Pb2+ detection. The limit of quantification (LOQ) was calculated similarly (LOQ = 10 × SD/slope), with the resulting value being 31.262 ppm.
The repeatability of the electrode's response to Pb2+ ions was evaluated through multiple ASV experiments at the concentration of 0.01 ppm (shown in Fig. 8(f)). The results show consistent peak current values with no significant variation, confirming its reliability and accuracy in the detection of Pb2+ ions.
Discussion
TiO2 has garnered significant attention as one of the most extensively studied metal oxides, owing to its exceptional surface, electronic, and catalytic properties. These attributes are further enhanced when specific crystal facets are exposed, which influence its reactivity, making it a promising candidate for various applications.59 In this study, TiO2 nanoparticles were synthesized with exposed {101}, {001}, and {101}/{001} facets to explore their structural and photocatalytic properties. The successful formation of anatase-phase TiO2 was confirmed through XRD, FTIR and Raman analysis. Crystallite size calculations using various XRD models revealed that the crystallite sizes of all samples, except LSLM, fall within the range of 0–50 nm.Growth preference and texture coefficient analyses verified that the synthesized TiO2 nanoparticles were formed with specific exposed facets. For the {101}-faceted TiO2, the growth preference was observed on the (004) and (105) planes, reflecting a thermodynamically favored growth direction. However, the texture coefficient for the (004) plane was relatively low, suggesting minimal growth along the {001} direction, confirming that the {101} direction is the dominant exposed facet. On the other hand, {001}-faceted TiO2 exhibited a positive growth preference value and good texture coefficient for the (004) plane, confirming the dominance of the {001} facet in the sample. The co-faceted samples, which displayed higher texture coefficients for both the (105) and (004) planes, were characterized by the coexistence of both {101} and {001} facets. Raman analysis also confirmed growth on specific facets, as the {101}-faceted sample has 94.43% growth in the {101} direction, whereas the {001}-faceted sample has 35.48% growth in the {001} direction. However, for the {101}/{001} co-faceted sample, Raman analysis indicated growth only along the {101} direction, despite growth preference and texture coefficient analyses confirming the presence of both facets. This discrepancy may be attributed to the sample's morphology, where the particles exhibit significant aggregation, leading to cluster formation and an increase in particle size, as evidenced by the histogram analysis, which recorded a large particle size of 1027.6 nm. The larger particle size causes volume expansion, increasing the vibrational amplitude of neighboring molecules due to a rise in the mean square relative displacement. As a result, the Raman shift increases, particularly for the Eg peaks at 144 cm−1, disrupting the accurate estimation of the {001} facet percentage. A similar pattern is also seen in previous literature.60
The morphological features of the synthesized TiO2 nanoparticles were further examined using FESEM. The {001}-faceted samples displayed a nanosheet-like structure, while the {101}-faceted samples exhibited an aggregated nanowire structure, consistent with previous findings.14,52 These distinct morphologies are consistent with the crystallographic preferences observed in the XRD and texture coefficient analysis, underscoring the influence of facet engineering on the structural properties of TiO2 nanoparticles.
TiO2 nanoparticles have demonstrated versatility in a wide range of applications, including photocatalytic degradation, sensors, dye-sensitized solar cells, charge-spreading devices, photoluminescence, bactericides, optical coatings, and optoelectronic devices. In this study, the synthesized TiO2 nanoparticles were utilized for the photocatalytic degradation of CR dye, assessment of antimicrobial activity, and electrochemical sensing for Pb detection.
CR dye is a common pollutant in textile wastewater. The {001}-faceted and {101}/{001}-co-faceted TiO2 achieved complete degradation of CR dye in just 30 min, compared to 69.36% degradation by {101}-faceted TiO2 under identical conditions. This superior performance is attributed to the unique properties of the highly exposed {001} facets. The {001} facet is highly reactive due to its higher surface energy compared to the {101} facet. This is a result of the greater density of unsaturated bonds between penta-coordinated Ti (Ti5C) atoms and two-fold coordinated oxygen atoms (O2C).61 Notably, the {001} facets consist entirely of Ti5C atoms (100%), making them fully accessible for photocatalytic reactions. In contrast, the {101} facet has only 50% Ti5C atoms, which reduces its reactivity.53 Furthermore, the Ti5C–O2C bonds on the {001} facet have a larger bond length (1.95 Å), making them easier to break upon reactant adsorption. This enhances the activity of titanium and oxygen atoms on the {001} surface, promoting photocatalytic reactions.62 Furthermore, these attributes enhance the generation of reactive oxygen species (ROS) that attack dye molecules at or near the photocatalyst surface and are mainly responsible for the degradation. In particular, the {001} facets have been shown to facilitate ˙OH radical formation owing to their lower activation energy for ˙OH generation.63 This was confirmed by scavenging experiments: in the presence of isopropanol (IPA), an ˙OH radical quencher, the degradation efficiency of {001}-faceted TiO2 dropped dramatically from 100% to 12.7%, indicating that ˙OH radicals are the dominant oxidative species. In contrast, the addition of EDTA reduced the degradation efficiency of {001} and {101}/{001} TiO2 to 28.81% and 20.33%, respectively. These results demonstrate that ˙OH radicals play the primary role in the photocatalytic process over {001}-faceted TiO2, while photogenerated holes also make a significant contribution. Overall, these findings establish that the {001} facet is highly effective in facilitating ROS generation, thereby accelerating CR dye degradation.
Previous studies have also demonstrated that facet-engineered TiO2 exhibits enhanced photocatalytic activity compared to pure or commercial TiO2. For instance, Hammud et al. reported that commercial P25 (Degussa) TiO2 achieved only 60–90% Congo Red degradation after 60 min of UV irradiation at different catalyst dosages.64 Lee et al. investigated the photocatalytic degradation of methylene blue using {101}/{001}-faceted TiO2 and observed a higher activity with an apparent rate constant of 0.0527 min−1.65 Roy et al. synthesized TiO2via a green approach using amines and found that samples with ∼25% {101} and ∼75% {100}/{010} exposed facets exhibited 3.7 and 3.1-fold higher photocatalytic activity than Degussa P25 toward methyl orange and methylene blue, respectively.66 Similarly, Leu et al. demonstrated that {001}-exposed TiO2 composites showed significantly higher photocatalytic activity for methylene blue degradation under both UV and visible light irradiation,67 while Gul et al. reported that highly {001}-faceted TiO2 exhibited superior degradation of crystal violet and methyl orange compared to pure TiO2.68 In another study, John et al. employed C. odorata leaf extract for the green synthesis of N-doped {001}-faceted TiO2, achieving ∼98% degradation of methylene blue.69 Likewise, Kaur et al. synthesized E. cardamomum-wrapped TiO2, where phytochemicals in the extract preferentially stabilized the {100} facets, resulting in 97% Congo Red degradation within 150 min.70 Collectively, these findings confirm that {001}-exposed TiO2 nanoparticles, particularly those synthesized through green or modified routes, can outperform commercial P25 and represent promising candidates for advanced oxidation processes aimed at degrading persistent pollutants and improving water quality.
In addition to photocatalytic degradation, {001}-faceted TiO2 exhibited strong antimicrobial properties against Gram-positive bacteria, with inhibition zones of 11.5 ± 0.50 mm against L. monocytogenes and 11.5 ± 0.50 mm against S. aureus. In comparison, {101}-faceted TiO2 showed inhibition zones of 9.2 ± 0.91 mm against S. aureus and 8.6 ± 0.40 mm against L. monocytogenes. This enhanced antibacterial activity of {001}-faceted TiO2 is attributed to its greater reactivity, primarily due to the presence of unsaturated coordinated Ti atoms on the surface. These unsaturated Ti atoms interact readily with bacterial cell membranes, leading to membrane disruption. This disruption triggers oxidative stress, causing leakage of intracellular contents and ultimately resulting in bacterial cell death.40 {001} Facets have 100% unsaturated coordinated Ti atoms, while {101} facets have only 50%, which accounts for the superior antimicrobial properties of {001}-faceted TiO2. In contrast, the poor antimicrobial activity against Gram-negative bacteria may be due to the presence of an additional outer membrane, unlike Gram-positive bacteria, and a plasma membrane composed of both anionic and zwitterionic phospholipids, which reduces nanoparticle interaction and penetration.71 Similarly, C. albicans exhibits limited susceptibility because, as a eukaryotic organism, it has a thick cell wall and a complex, sugar-rich cell membrane that act as a protective barrier against nanoparticle entry.72
The electrochemical properties of the {001}-modified TiO2 electrode were also evaluated for Pb2+ ion detection through the fabrication of an electrochemical sensor using {001}-faceted TiO2. CV confirmed that the modified electrode exhibited superior electrochemical efficiency compared to the bare GC electrode, as evidenced by reduced potential difference and enhanced current response, indicating improved charge transfer efficiency. ASV was employed to assess the mass transport mechanism at the electrode interface, revealing that the Pb2+ detection process was governed by an irreversible, diffusion-controlled reaction. The fabricated sensor demonstrated a LOD of 9.378 ppm and a LOQ of 31.162 ppm, confirming its sensitivity for Pb2+ detection. Table 7 shows the comparison of the electrochemical sensing performance for Pb2+ between the fabricated sensor and previous studies. Although the bare GC electrode showed significant improvement and detectable sensitivity toward Pb2+ with {001}-faceted modification, the LOD and LOQ can be further enhanced using nanocomposite-based electrodes or surface functionalization. Incorporating conductive nanomaterials (graphene,73 carbon nanotubes74), metallic nanoparticles (Bi,75 Au76), or MXene-77 and MOF/COF-based78 hybrid modifiers can substantially increase the active surface area, accelerate electron transfer, and enable effective preconcentration. For example, Zhang et al.'s MWCNT/MOF (ZIF-67)-modified GCE exhibited excellent conductivity and a high density of binding sites, achieving a LOD as low as 1 nM and an extended linear range of 1.38 nM–5 µM.78 Surface functionalization with thiol or carboxyl groups can further improve selectivity for Pb2+ ions. For instance, Oliveira et al. reported ZnO nanofibers functionalized with L-cysteine for Pb2+ electrochemical sensing, achieving a LOD of 0.397 µg L−1 within a linear range of 10–140 µg L−1.79Fig. 9 illustrates the sensing mechanism for Pb2+ ion detection using the fabricated sensor. Upon applying a deposition voltage of −1.2 V, Pb2+ ions adsorbed onto the surface of the modified electrode and are reduced to Pb0. A subsequent stripping potential between −0.8 V and 0.5 V reoxidized Pb0 back to Pb2+, leading to desorption from the electrode.80 The enhanced sensing capability of the {001}-modified TiO2 electrode can be attributed to its high surface reactivity, which enables efficient interaction with Pb2+ ions. The repeatability tests further confirmed the electrode's stability and accuracy, demonstrating consistent peak current responses for multiple measurements.
| Material | Method | Linear range | Limit of detection, LOD | Limit of quantification, LOQ | Ref. |
|---|---|---|---|---|---|
| Biomass-worm like nitrogen doped-carbon | DPASV | 0.5–100 mg L−1 | 0.2 mg L−1 | — | 81 |
| TiO2@Gum Arabic | DPV | 1–10 ppm | 0.56 ppm | 1.68 ppm | 82 |
| MOF/EB-1 | Voltammetry | 0.54–1.15 ppm | 0.077 ppm | — | 83 |
| BC@TiO2NPs | DPV | 1 × 10−6–10 µM | 0.6268 × 10−6 µM | — | 84 |
| Ti/TiO2 | ASV | 0.6–13.2 µM | 140 nM | — | 85 |
| TiO2/AgCNF | SWASV | — | 40.14 nM | — | 86 |
| NiWO4/MWCNT | DPV | 50–450 µM | 0.12 µM | 87 | |
| TiON/TiO2 | SWASV | 10−5–10−1 mol L−1 | 10−5 mol L−1 | 1.0 × ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10–4 mol L−1 10–4 mol L−1 |
88 |
| {001}-faceted TiO2 | LSASV | (1–10) ppm | 9.378 ppm | 31.162 ppm | This study |
Conclusion
In this study, anatase-phase TiO2 nanoparticles with exposed {101}, {001}, and {101}/{001} facets were successfully synthesized using inorganic modifiers via a hydrothermal method. The photocatalytic degradation of CR dye under visible light irradiation demonstrated that {001}-faceted and {101}/{001} co-faceted TiO2 exhibited complete degradation within 30 min, significantly outperforming {101}-faceted TiO2. This underscores the critical role of facet engineering in optimizing TiO2 for environmental remediation applications. Furthermore, the antimicrobial studies revealed that {001}-faceted TiO2 demonstrated superior antibacterial efficacy against Gram-positive bacteria compared to {101}-faceted TiO2. Moreover, {001}-faceted TiO2 exhibited excellent electrochemical performance for Pb2+ ion detection, with a higher electroactive surface area of the fabricated sensor, facilitating efficient charge transfer and enhancing sensor performance. The sensor demonstrated a LOD of 9.378 ppm and a LOQ of 31.162 ppm. Overall, the findings demonstrate that {001}-faceted TiO2 holds great promise for multifunctional applications, including environmental pollutant degradation, antibacterial treatments, and electrochemical sensing. Future research should aim to further improve sensitivity and selectivity through the incorporation of conductive nanomaterials, metal nanoparticles, or surface functionalization with thiol/carboxyl groups. Additionally, extending the work to real wastewater samples and other emerging contaminants, such as pharmaceutical residues, will strengthen the practical applicability of these materials.Author contributions
Md. Anayet Ullah: curated and analyzed the data; wrote the manuscript. Newaz Mohammed Bahadur: visualized the whole research. Dipa Islam, Subarna Shandani dey, and Md. Farhad Ali: helped in data curation. Fataha Nur Robel and Samina Ahmed: supervised the findings of this work. Md. Sahadat Hossain: conceived and designed the study; analyzed the data; supervised the overall work and reviewed the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
Data will be made available on request.The data supporting this article have been included as part of the supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ma00760g.
Acknowledgements
The Bangladesh Council of Scientific and Industrial Research (BCSIR) is acknowledged by the authors for its financial support of research and development projects (ref. no. 39.02.0000.011.14.180.2024/1116; Date: 14.01.2025). Additionally, Md. Anayet Ullah expresses gratitude to Noakhali Science and Technology University's Department of Applied Chemistry and Chemical Engineering for supporting his MS thesis program at Noakhali, Bangladesh.References
- J. Sun, et al., Synthesis of anatase TiO2 with exposed {001} and {101} facets and photocatalytic activity, Rare Met., 2019, 38(4), 287–291, DOI:10.1007/s12598-014-0329-9.
- B. O’Regan and M. Grätzel, A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films, Nature, 1991, 353(6346), 737–740, DOI:10.1038/353737a0.
- M. Khairy and W. Zakaria, Effect of metal-doping of TiO2 nanoparticles on their photocatalytic activities toward removal of organic dyes, Egypt. J. Pet., 2014, 23(4), 419–426, DOI:10.1016/j.ejpe.2014.09.010.
- C. Zhou, S. Hong, X. Chen, Q. Li, Z. Chen and S. Yang, Screen-Assisted Self-Spreading of TiO2 Precursor Solution on FTO Substrates for High-Quality Electron Transport Layers in Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2024, 16(34), 45020–45029, DOI:10.1021/acsami.4c10820.
- X. Tian, et al., Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: a review, Nano Mater. Sci., 2021, 3(4), 390–403, DOI:10.1016/j.nanoms.2021.05.011.
- W. F. Zhang, M. S. Zhang and Z. Yin, Microstructures and Visible Photoluminescence of TiO2 Nanocrystals, Phys. Status Solidi A, 2000, 179(2), 319–327, DOI:10.1002/1521-396X(200006)179:2
![[double bond splayed right]](https://www.rsc.org/images/entities/char_e00a.gif) 319::AID-PSSA319
319::AID-PSSA319![[double bond splayed left]](https://www.rsc.org/images/entities/char_e009.gif) 3.0.CO;2-H.
3.0.CO;2-H. - H. M. Yadav, et al., Preparation and characterization of copper-doped anatase TiO2 nanoparticles with visible light photocatalytic antibacterial activity, J. Photochem. Photobiol., A, 2014, 280, 32–38 CrossRef CAS Available: https://www.sciencedirect.com/science/article/abs/pii/S1010603014000471.
- C. Euvananont, C. Junin, K. Inpor, P. Limthongkul and C. Thanachayanont, TiO2 optical coating layers for self-cleaning applications, Ceram. Int., 2008, 34(4), 1067–1071, DOI:10.1016/j.ceramint.2007.09.043.
- B. Sathyaseelan, et al., Structural, optical and morphological properties of post-growth calcined TiO2 nanopowder for opto-electronic device application: ex situ studies, J. Alloys Compd., 2016, 671, 486–492, DOI:10.1016/j.jallcom.2016.02.105.
- T. Berger, D. Monllor-Satoca, M. Jankulovska, T. Lana-Villarreal and R. Gómez, The Electrochemistry of Nanostructured Titanium Dioxide Electrodes, ChemPhysChem, 2012, 13(12), 2824–2875, DOI:10.1002/cphc.201200073.
- M. Radetić, Functionalization of textile materials with TiO2 nanoparticles, J. Photochem. Photobiol., C, 2013, 16, 62–76, DOI:10.1016/j.jphotochemrev.2013.04.002.
- G. V. Khade, M. B. Suwarnkar, N. L. Gavade and K. M. Garadkar, Green synthesis of TiO2 and its photocatalytic activity, J. Mater. Sci.: Mater. Electron., 2015, 26(5), 3309–3315, DOI:10.1007/s10854-015-2832-7.
- U. Diebold, The surface science of titanium dioxide, Surf. Sci. Rep., 2003, 48(5–8), 53–229, DOI:10.1016/S0167-5729(02)00100-0.
- M. Chen, et al., Remarkable synergistic effect between {001} facets and surface F ions promoting hole migration on anatase TiO2, Appl. Catal., B, 2017, 207, 397–403, DOI:10.1016/j.apcatb.2017.02.048.
- Z. Xiong, Z. Lei, Y. Li, L. Dong, Y. Zhao and J. Zhang, A review on modification of facet-engineered TiO2 for photocatalytic CO2 reduction, J. Photochem. Photobiol., C, 2018, 36, 24–47, DOI:10.1016/j.jphotochemrev.2018.07.002.
- H. Irie, S. Washizuka and K. Hashimoto, Hydrophilicity on carbon-doped TiO2 thin films under visible light, Thin Solid Films, 2006, 510(1–2), 21–25, DOI:10.1016/j.tsf.2005.08.374.
- L. Kavan and M. Grätzel, Highly efficient semiconducting TiO2 photoelectrodes prepared by aerosol pyrolysis, Electrochim. Acta, 1995, 40(5), 643–652, DOI:10.1016/0013-4686(95)90400-W.
- A. Monoy, et al., Surface preparation influence on the initial stages of MOCVD growth of TiO2 thin films, Thin Solid Films, 2006, 515(2), 687–690, DOI:10.1016/j.tsf.2005.12.237.
- Y. Lei, L. D. Zhang and J. C. Fan, Fabrication, characterization and Raman study of TiO2 nanowire arrays prepared by anodic oxidative hydrolysis of TiCl3, Chem. Phys. Lett., 2001, 338(4–6), 231–236, DOI:10.1016/S0009-2614(01)00263-9.
- I. Oja, A. Mere, M. Krunks, R. Nisumaa, C.-H. Solterbeck and M. Es-Souni, Structural and electrical characterization of TiO2 films grown by spray pyrolysis, Thin Solid Films, 2006, 515(2), 674–677, DOI:10.1016/j.tsf.2005.12.243.
- H. Liu, et al., Synthesis and Characterization of Titania Prepared by Using a Photoassisted Sol−Gel Method, Langmuir, 2003, 19(7), 3001–3005, DOI:10.1021/la026600o.
- X. Zhao, M. Liu and Y. Zhu, Fabrication of porous TiO2 film via hydrothermal method and its photocatalytic performances, Thin Solid Films, 2007, 515(18), 7127–7134, DOI:10.1016/j.tsf.2007.03.025.
- P. Soundarrajan, K. Sankarasubramanian, K. Sethuraman and K. Ramamurthi, Controlled (110) and (101) crystallographic plane growth of single crystalline rutile TiO2 nanorods by facile low cost chemical methods, CrystEngComm, 2014, 16(37), 8756–8768 RSC , Available: https://pubs.rsc.org/en/content/articlehtml/2014/ce/c4ce00820k.
- A. Haghighat Mamaghani, F. Haghighat and C.-S. Lee, Hydrothermal/solvothermal synthesis and treatment of TiO2 for photocatalytic degradation of air pollutants: Preparation, characterization, properties, and performance, Chemosphere, 2019, 219, 804–825, DOI:10.1016/j.chemosphere.2018.12.029.
- D. Majumder and S. Roy, Non-fluorinated synthesis of anatase TiO2 with dominant {001} facets: influence of faceted structures on formaldehyde sensitivity, New J. Chem., 2017, 41(15), 7591–7597 RSC , Available: https://pubs.rsc.org/en/content/articlehtml/2017/nj/c7nj00648a.
- H. Liu, S. Liu, Z. Zhang, X. Dong and T. Liu, Hydrothermal etching fabrication of TiO2@graphene hollow structures: mutually independent exposed {001} and {101} facets nanocrystals and its synergistic photocaltalytic effects, Sci. Rep., 2016, 6(1), 33839, DOI:10.1038/srep33839.
- A. Haleem, et al., In-Depth Photocatalytic Degradation Mechanism of the Extensively Used Dyes Malachite Green, Methylene Blue, Congo Red, and Rhodamine B via Covalent Organic Framework-Based Photocatalysts, Water, 2024, 16(11), 1588, DOI:10.3390/w16111588.
- G. E. Quintanilla-Villanueva, A. Sicardi-Segade, D. Luna-Moreno, R. E. Núñez-Salas, J. F. Villarreal-Chiu and M. M. Rodríguez-Delgado, Recent Advances in Congo Red Degradation by TiO2-Based Photocatalysts Under Visible Light, Catalysts, 2025, 15(1), 84, DOI:10.3390/catal15010084.
- L. Ye, J. Mao, J. Liu, Z. Jiang, T. Peng and L. Zan, Synthesis of anatase TiO2 nanocrystals with {101}, {001} or {010} single facets of 90% level exposure and liquid-phase photocatalytic reduction and oxidation activity orders, J. Mater. Chem. A, 2013, 1(35), 10532–10537, 10.1039/C3TA11791J.
- J. Liao, F. Yang, C.-Z. Wang and S. Lin, The crystal facet-dependent electrochemical performance of TiO2 nanocrystals for heavy metal detection: theoretical prediction and experimental proof, Sens. Actuators, B, 2018, 271, 195–202, DOI:10.1016/j.snb.2018.05.067.
- G. Yu, et al., Applications of Nanomaterials for Heavy Metal Removal from Water and Soil: A Review, Sustainability, 2021, 13(2), 713, DOI:10.3390/su13020713.
- S. Tajik, et al., Recent Developments in Polymer Nanocomposite-Based Electrochemical Sensors for Detecting Environmental Pollutants, Ind. Eng. Chem. Res., 2021, 60(3), 1112–1136, DOI:10.1021/acs.iecr.0c04952.
- M. Behbahani, N. A. G. Tapeh, M. Mahyari, A. R. Pourali, B. G. Amin and A. Shaabani, Monitoring of trace amounts of heavy metals in different food and water samples by flame atomic absorption spectrophotometer after preconcentration by amine-functionalized graphene nanosheet, Environ. Monit. Assess., 2014, 186(11), 7245–7257, DOI:10.1007/s10661-014-3924-1.
- V. L. Dressler, D. Pozebon and A. J. Curtius, Determination of heavy metals by inductively coupled plasma mass spectrometry after on-line separation and preconcentration, Spectrochim. Acta, Part B, 1998, 53(11), 1527–1539, DOI:10.1016/S0584-8547(98)00180-3.
- L. A. Hutton, G. D. O’Neil, T. L. Read, Z. J. Ayres, M. E. Newton and J. V. Macpherson, Electrochemical X-ray Fluorescence Spectroscopy for Trace Heavy Metal Analysis: Enhancing X-ray Fluorescence Detection Capabilities by Four Orders of Magnitude, Anal. Chem., 2014, 86(9), 4566–4572, DOI:10.1021/ac500608d.
- A. Fan, Y. Ling, C. Lau and J. Lu, Direct colorimetric visualization of mercury (Hg2+) based on the formation of gold nanoparticles, Talanta, 2010, 82(2), 687–692, DOI:10.1016/j.talanta.2010.05.033.
- S. Li, et al., Electrochemical microfluidics techniques for heavy metal ion detection, Analyst, 2018, 143(18), 4230–4246, 10.1039/C8AN01067F.
- C. Ariño, C. E. Banks and A. Bobrowski, Electrochemical stripping analysis, Nat. Rev. Methods Primers, 2022, 2, 62, DOI:10.1038/s43586-022-00143-5.
- A. J. Borrill, N. E. Reily and J. V. Macpherson, Addressing the practicalities of anodic stripping voltammetry for heavy metal detection: a tutorial review, Analyst, 2019, 144(23), 6834–6849, 10.1039/C9AN01437C.
- V. Stanić and S. B. Tanasković, Antibacterial activity of metal oxide nanoparticles, Nanotoxicity, Elsevier, 2020, pp. 241–274 DOI:10.1016/B978-0-12-819943-5.00011-7.
- H. Kaur, S. Kumar, R. Saini, P. P. Singh and A. Pugazhendhi, One-pot biogenic synthesis of C. limon/TiO2 with dual applications as an advance photocatalyst and antimicrobial agent, Chemosphere, 2023, 335, 139106, DOI:10.1016/j.chemosphere.2023.139106.
- H. Kaur, J. Singh, P. Rani, N. Kaur, S. Kumar and M. Rawat, A novel and one-pot synthesis of Punica granatum mediated copper oxide having flower-like morphology as an efficient visible-light driven photocatalyst for degradation of textile dyes in waste water, J. Mol. Liq., 2022, 355, 118966, DOI:10.1016/j.molliq.2022.118966.
- Ms. K. Alam, Md. S. Hossain, N. M. Bahadur and S. Ahmed, A comparative study in estimating of crystallite sizes of synthesized and natural hydroxyapatites using Scherrer Method, Williamson–Hall model, Size-Strain Plot and Halder–Wagner Method, J. Mol. Struct., 2024, 1306, 137820, DOI:10.1016/j.molstruc.2024.137820.
- M. Alam, M. Sahadat Hossain, Md. A. Ullah, N. Bahadur and S. Ahmed, Utilization of marine snails for the sustainable synthesis and crystallographic characterization of nano-crystallite hydroxyapatite, Hybrid Adv., 2024, 7, 100308, DOI:10.1016/j.hybadv.2024.100308.
- M. Rashidzadeh, Synthesis of High-Thermal Stable Titanium Dioxide Nanoparticles, Int. J. Photoenergy, 2008, 2008(1), 245981, DOI:10.1155/2008/245981.
- A. León, et al., FTIR and Raman Characterization of TiO2 Nanoparticles Coated with Polyethylene Glycol as Carrier for 2-Methoxyestradiol, Appl. Sci., 2017, 7(1), 49, DOI:10.3390/app7010049.
- Y. Yang, et al., Surface modification of (001) facets dominated TiO2 with ozone for adsorption and photocatalytic degradation of gaseous toluene, Chin. J. Chem. Phys., 2019, 32(5), 611–619, DOI:10.1063/1674-0068/cjcp1903062.
- F. Tian, Y. Zhang, J. Zhang and C. Pan, Raman Spectroscopy: A New Approach to Measure the Percentage of Anatase TiO2 Exposed (001) Facets, J. Phys. Chem. C, 2012, 116(13), 7515–7519, DOI:10.1021/jp301256h.
- T. Xu, H. Zheng, P. Zhang, W. Lin and Y. Sekiguchi, Hydrothermal preparation of nanoporous TiO2 films with exposed {001} facets and superior photocatalytic activity, J. Mater. Chem. A, 2015, 3(37), 19115–19122, 10.1039/C5TA02640G.
- G. Jeantelot, Control and Characterization of Titanium Dioxide Morphology: Applications in Surface Organometallic Chemistry, KAUST Research Repository, 2014 DOI:10.25781/KAUST-AVMKW.
- M. V. Sofianou, et al., Tuning the photocatalytic selectivity of TiO2 anatase nanoplates by altering the exposed crystal facets content, Appl. Catal., B, 2013, 142–143, 761–768, DOI:10.1016/j.apcatb.2013.06.009.
- Y. Zhou, et al., Enhanced dye-sensitized solar cells performance using anatase TiO2 mesocrystals with the Wulff construction of nearly 100% exposed {101} facets as effective light scattering layer, Dalton Trans., 2014, 43(12), 4711–4719, 10.1039/C3DT53010H.
- L. Ye, J. Mao, J. Liu, Z. Jiang, T. Peng and L. Zan, Synthesis of anatase TiO2 nanocrystals with {101}, {001} or {010} single facets of 90% level exposure and liquid-phase photocatalytic reduction and oxidation activity orders, J. Mater. Chem. A, 2013, 1(35), 10532–10537, 10.1039/C3TA11791J.
- E. Kusvuran, A. Samil, O. M. Atanur and O. Erbatur, Photocatalytic degradation kinetics of di- and tri-substituted phenolic compounds in aqueous solution by TiO2/UV, Appl. Catal., B, 2005, 58(3), 211–216, DOI:10.1016/j.apcatb.2004.11.023.
- V. Goyal, A. Singh, J. Singh, H. Kaur, S. Kumar and M. Rawat, Biogenically structural and morphological engineering of rigonella foenum-graecum mediated SnO2 nanoparticles with enhanced photocatalytic and antimicrobial activities, Mater. Chem. Phys., 2022, 282, 125946, DOI:10.1016/j.matchemphys.2022.125946.
- N. Ruecha, N. Rodthongkum, D. M. Cate, J. Volckens, O. Chailapakul and C. S. Henry, Sensitive electrochemical sensor using a graphene–polyaniline nanocomposite for simultaneous detection of Zn(II), Cd(II), and Pb(II), Anal. Chim. Acta, 2015, 874, 40–48, DOI:10.1016/j.aca.2015.02.064.
- A. Hosseini, E. Sohouli, M. Gholami, A. Sobhani-Nasab and S. A. Mirhosseini, Electrochemical Determination of Ciprofloxacin Using Glassy Carbon Electrode Modified with CoFe2O4-MWCNT, Anal. Bioanal. Electrochem., 2019, 11(8), 996–1008 CAS.
- R. Kaur, S. Rana, R. Singh, V. Kaur and P. Narula, A Schiff base modified graphene oxide film for anodic stripping voltammetric determination of arsenite, Microchim. Acta, 2019, 186(11), 741, DOI:10.1007/s00604-019-3807-9.
- D. Majumder and S. Roy, Non-fluorinated synthesis of anatase TiO2 with dominant {001} facets: influence of faceted structures on formaldehyde sensitivity, New J. Chem., 2017, 41(15), 7591–7597, 10.1039/C7NJ00648A.
- H. C. Choi, Y. M. Jung and S. B. Kim, Size effects in the Raman spectra of TiO2 nanoparticles, Vib. Spectrosc., 2005, 37(1), 33–38, DOI:10.1016/j.vibspec.2004.05.006.
- A. Junkaew, M. Ehara, L. Huang and S. Namuangruk, Facet-dependent catalytic activity of anatase TiO2 for the selective catalytic reduction of NO with NH3: a dispersion-corrected density functional theory study, Appl. Catal., A, 2021, 623, 118250, DOI:10.1016/j.apcata.2021.118250.
- A. Kumar Reddy Police, S. V. Prabhakar Vattikuti, Y.-J. Baik and B. Chan, Eco-friendly, hydrogen fluoride-free, morphology-oriented synthesis of TiO2 with exposed (001) facets, Ceram. Int., 2019, 45(2), 2178–2184, DOI:10.1016/j.ceramint.2018.10.128.
- Q. Cheng, et al., Rapid Hydroxyl Radical Generation on (001)-Facet-Exposed Ultrathin Anatase TiO2 Nanosheets for Enhanced Photocatalytic Lignocellulose-to-H2 Conversion, ACS Catal., 2022, 12(3), 2118–2125, DOI:10.1021/acscatal.1c05713.
- H. H. Hammud, H. Traboulsi, R. K. Karnati and E. M. Bakir, Photodegradation of Congo Red by Modified P25-Titanium Dioxide with Cobalt-Carbon Supported on SiO2 Matrix, DFT Studies of Chemical Reactivity, Catalysts, 2022, 12(3), 248, DOI:10.3390/catal12030248.
- T.-Y. Lee, C.-Y. Lee and H.-T. Chiu, Enhanced Photocatalysis from Truncated Octahedral Bipyramids of Anatase TiO2 with Exposed {001}/{101} Facets, ACS Omega, 2018, 3(8), 10225–10232, DOI:10.1021/acsomega.8b01251.
- N. Roy, Y. Park, Y. Sohn, K. T. Leung and D. Pradhan, Green Synthesis of Anatase TiO2 Nanocrystals with Diverse Shapes and their Exposed Facets-Dependent Photoredox Activity, ACS Appl. Mater. Interfaces, 2014, 6(19), 16498–16507, DOI:10.1021/am504084p.
- Z. Liu, et al., mpg-C3N4/anatase TiO2 with reactive {001} facets composites to enhance the photocatalytic activity of organic dye degradation, RSC Adv., 2016, 6(59), 54215–54225, 10.1039/C6RA06730A.
- A. Gul, R. Ullah, J. Sun, T. Munir and S. Bai, The fabrication of TiO2-supported clinoptilolite via F− contained hydrothermal etching and a resultant highly energetic {001} facet for the enhancement of its photocatalytic activity, RSC Adv., 2021, 11(29), 17849–17859, 10.1039/D1RA02269E.
- A. K. John and S. Palaty, Green synthesis of nitrogen-doped TiO2 nanoparticles with exposed {001} facets using Chromolaena odorata leaf extract for photodegradation of pollutants under visible light, Nano-Struct. Nano-Objects, 2024, 40, 101402, DOI:10.1016/j.nanoso.2024.101402.
- H. Kaur, S. Kumar, G. Kaur, N. Kaur, R. Badru and R. Saini, An emerging expanse: Novel and eco-friendly-biogenic synthesis of E. cardamomum-wrapped TiO2 nanoparticles for environmental and biological applications, Environ. Res., 2023, 234, 116599, DOI:10.1016/j.envres.2023.116599.
- A. Wojciechowska, A. Markowska-Szczupak and Z. Lendzion-Bieluń, TiO2-Modified Magnetic Nanoparticles (Fe3O4) with Antibacterial Properties, Materials, 2022, 15(5), 1863, DOI:10.3390/ma15051863.
- D. Mitoraj, et al., Visible light inactivation of bacteria and fungi by modified titanium dioxide, Photochem. Photobiol. Sci., 2007, 6(6), 642–648, 10.1039/b617043a.
- W. Zhou, C. Li, C. Sun and X. Yang, Simultaneously determination of trace Cd2+ and Pb2+ based on l-cysteine/graphene modified glassy carbon electrode, Food Chem., 2016, 192, 351–357, DOI:10.1016/j.foodchem.2015.07.042.
- P. Gupta, et al., Parts per trillion detection of heavy metals in as-is tap water using carbon nanotube microelectrodes, Anal. Chim. Acta, 2021, 1155, 338353, DOI:10.1016/j.aca.2021.338353.
- Y. He, Z. Wang, L. Ma, L. Zhou, Y. Jiang and J. Gao, Synthesis of bismuth nanoparticle-loaded cobalt ferrite for electrochemical detection of heavy metal ions, RSC Adv., 2020, 10(46), 27697–27705, 10.1039/D0RA02522D.
- S. Wu, K. Li, Z. Zhang and L. Chen, Synthesis of imprinted chitosan/AuNPs/graphene-coated MWCNTs/Nafion film for detection of lead ions, New J. Chem., 2020, 44(33), 14129–14135, 10.1039/D0NJ02522D.
- S. Kumar, S. Aftab, T. Singh, M. Kumar, S. Kumar and Y. Seo, Charge storage improvement in uniformly grown TiO2 on Ti3C2Tx MXene surface, J. Alloys Compd., 2023, 968, 172181, DOI:10.1016/j.jallcom.2023.172181.
- Y. Zhang, et al., Highly sensitive detection of Pb2+ and Cu2+ based on ZIF-67/MWCNT/Nafion-modified glassy carbon electrode, Anal. Chim. Acta, 2020, 1124, 166–175, DOI:10.1016/j.aca.2020.05.023.
- V. Oliveira, et al., A sensitive electrochemical sensor for Pb2+ ions based on ZnO nanofibers functionalized by L-cysteine, J. Mol. Liq., 2020, 309, 113041, DOI:10.1016/j.molliq.2020.113041.
- L. Durai and S. Badhulika, Ultra-selective, trace level detection of As3+ ions in blood samples using PANI coated BiVO4 modified SPCE via differential pulse anode stripping voltammetry, Mater. Sci. Eng., C, 2020, 111, 110806, DOI:10.1016/j.msec.2020.110806.
- C. Xu, et al., Biomass derived worm-like nitrogen-doped-carbon framework for trace determination of toxic heavy metal lead(II), Anal. Chim. Acta, 2020, 1116, 16–26, DOI:10.1016/j.aca.2020.04.033.
- S. K. Sivan, et al., Fabrication of a Greener TiO2@Gum Arabic-Carbon Paste Electrode for the Electrochemical Detection of Pb2+ Ions in Plastic Toys, ACS Omega, 2020, 5(39), 25390–25399, DOI:10.1021/acsomega.0c03781.
- J. Milikić, M. Savić, A. Janošević Ležaić, B. Šljukić and G. Ćirić-Marjanović, Electrochemical Sensing of Cadmium and Lead Ions in Water by MOF-5/PANI Composites, Polymers, 2024, 16(5), 683, DOI:10.3390/polym16050683.
- Z. Liu, et al., An Electrochemical Sensor for Detection of Lead(II) Ions Using Biochar of Spent Coffee Grounds Modified by TiO2 Nanoparticles, Molecules, 2024, 29(23), 5704, DOI:10.3390/molecules29235704.
- H. ma, et al., A study of the photodeposition over Ti/TiO2 electrode for electrochemical detection of heavy metal ions, Electrochem. Commun., 2015, 57, 18–21, DOI:10.1016/j.elecom.2015.04.015.
- V. P. Jyothilakshmi, R. Manu and S. Swaminathan, The fabrication of TiO2/AgCNF/Nafion/GC electrodes for simultaneous electrochemical detection of Cd(II), Pb(II), Cu(II), and Hg(II) by optimizing the porosity of electrospun TiO2 nanofibers, J. Appl. Electrochem., 2024, 54(10), 2377–2387, DOI:10.1007/s10800-024-02110-2.
- R. H. R. Mohammed, R. Y. A. Hassan, R. Mahmoud, A. A. Farghali and M. E. M. Hassouna, Electrochemical determination of cadmium ions in biological and environmental samples using a newly developed sensing platform made of nickel tungstate-doped multi-walled carbon nanotubes, J. Appl. Electrochem., 2024, 54(3), 657–668, DOI:10.1007/s10800-023-01976-y.
- A. M. Elsayed, A. M. Ahmed, M. T. Tammam, M. F. Eissa and A. H. Aly, Sensing of heavy metal Pb2+ ions in water utilizing the photonic structure of highly controlled hexagonal TiON/TiO2 nanotubes, Sci. Rep., 2024, 14(1), 1015, DOI:10.1038/s41598-023-50428-2.
| This journal is © The Royal Society of Chemistry 2026 |