 Open Access Article
Open Access ArticleIntegrated hierarchical Z-scheme BiOCl/NiTiO3 heterostructured photoanode and its photoelectrocatalytic application in ciprofloxacin degradation
Babatope O.
Ojo
 *a and
Nonhlangabezo
Mabuba
*a and
Nonhlangabezo
Mabuba
 *ab
*ab
aDepartment of Chemical Sciences, University of Johannesburg, South Africa. E-mail: ojobabatope86.bo@gmail.com; nmabuba@uj.ac.za
bCentre for Nanomaterials Science Research, University of Johannesburg, South Africa
First published on 26th November 2025
Abstract
In this work, we explore the application of a BiOCl/NiTiO3 heterostructured photoanode with a remarkable synergistic advantage and unique band alignment for the photoelectrocatalytic (PEC) degradation of ciprofloxacin. Combining the excellent charge transport of BiOCl and the visible-light absorption of NiTiO3, the BiOCl/NiTiO3 photoanode facilitated electro-hole charge separation and extended light absorption owing to its distinctive band gap energy and visible-light sensitivity. These attributes were confirmed by the impressive charge-transfer advantage (Rct = 157 Ω) and band gap energy (Eg) of 2.08 eV obtained from the Nyquist and Tauc plots of the photoanode. Moreover, the BiOCl/NiTiO3 heterostructured photoanode exhibited a Z-type behaviour, evident by the flow of electrons from the conduction band of BiOCl (with a higher Fermi level) to the valence band of NiTiO3 (with a lower Fermi level). This occurrence made uncombined holes and electrons in the respective valence band of BiOCl and the conduction band of NiTiO3 readily available for the generation of reactive oxygen species in the system. The BiOCl/NiTiO3 heterostructure architecture significantly aided the photoelectrocatalytic degradation of ciprofloxacin in water, achieving a remarkable 94% degradation efficiency. In addition, the heterostructured photoanode displayed outstanding stability and reusability, making it a distinct advanced material for real-time operations. Furthermore, the findings from this work demonstrate a reliable strategy for high-performance PEC design while contributing to a sustainable approach for wastewater remediation applications.
1. Introduction
Growing concerns over the health and economic impacts of environmental pollution driven by increasing levels of organic pollutants in waterbodies have intensified over the last decade.1–3 This is particularly so because research breakthroughs in the pharmaceutical industry geared toward combating various health challenges have resulted in unprecedented demand for various pharmaceutical products. Among commonly detected classes of pharmaceutical pollutants in wastewater, antibiotics remain a prevalent member often reported.4,5 Since most of these products are organic, a burgeoning level of these products has reportedly been detected in waterbodies across the globe.6 An unsettling risk of developing antibiotic-resistant pathogens may occur from long-term consumption of water polluted with antibiotics.7,8 With increasing pressure on the supply of clean, potable water to various classes of society, the capacity of many conventional water purification systems has become limited.9 The threat posed by this limitation has spurred a significant quest for robust, easily accessible and cost-effective approaches towards achieving water availability across the globe.The application of advanced materials for effective wastewater remediation has culminated in the development of research on advanced oxidation processes, particularly photoelectrocatalysis (PEC).10–12 PEC involves converting photon energy into chemical energy through photogenerated charge carriers, produced by the interaction of visible light with semiconductors. Consequently, the generated chemical energy is crucial for producing reactive species capable of degrading the organic bonds of molecules non-selectively. Interestingly, photoanode heterostructures combining two or more semiconductors with complementary properties have emerged as promising advanced materials for effective remediation of organic pollutants in wastewater. Owing to their charge-separation advantage and superior light-absorption properties, heterostructure photoanodes have effectively addressed the challenge of instability and rapid charge carrier recombination often associated with PEC.13,14 Among the numerous candidates for heterostructured photoanodes, bismuth oxychloride (BiOCl) semiconductor has garnered tremendous attention due to its distinctive photocatalytic attributes.15–17 Peculiarly, a unique bandgap range, specialized layered structure, highly reactive surface area and notable stability possessed by BiOCl favour its applications in energy and storage devices, water remediation, water splitting, and hydrogen generation. However, rapid recombination of generated charge-carrier species often impedes their photocatalytic application.18–20
Recently, nickel titanate (NiTiO3), a perovskite oxide distinct for its narrow band gap energy, excellent stability, structural robustness and scalable industrial applications, has positioned it as a complementary semiconductor for heterostructured design.21–23 These attributes confer excellent photocatalytic activity on NiTiO3-based heterojunctions, thereby promoting charge separation through interfacial electron transfer and remarkable band alignment for PEC applications.22 Harmonizing the intrinsic properties of BiOCl and NiTiO3 during heterostructured photoanode design can facilitate easy and quick reactive oxygen species (ROS) generation in photoelectrocatalysis. Moreover, a narrowed band gap often observed in heterostructured photoanodes can promote extended light absorption by reducing photocorrosion and improving stability and reusability of the photoanode over multiple cycles.
PEC applications in wastewater remediation continue to thrive as a reliable approach for recalcitrant organic pollutant mineralization in water. PEC involves the synergistic combination of photocatalytic and electrocatalytic principles to enhance ROS generation and improve charge carrier separation in water treatment applications.13,24 Applying renewable solar energy and lower bias potential than that required in electrocatalysis due to photo-activity input has broadened the scope of PEC over the years. This stems from the ease of electron flow across the electrolyte-semiconductor interface occasioned by contact initiation between the semiconductor and the electrolyte. A band bending known as a built-in electric field often ensues, creating an equilibrium potential within the system.25 This event usually enhances light absorption and charge-carrier separation at the interface of a heterostructured semiconductor. Additionally, owing to its cost-effectiveness, accessibility to photoactive advanced materials, and the application of doped and heterostructured photoanode designs, visible-light spectrum absorption has become easily attainable in PEC reactions. These advantages have also been explored for hybrid technologies, including photo-electro-Fenton,26 sono-photoelectrocatalysis27 and photo-electrocoagulation28–30 in wastewater treatment processes. Ultimately, photogenerated holes (h+) and electrons (e−), as well as directly or indirectly generated oxidative species such as hydroxyl radical (˙OH), superoxide radical (O2˙−), enjoy extended lifespans, thus amplifying the reaction kinetics of the process.
This study explores the application of a novel BiOCl/NiTiO3 heterostructured photoanode for the degradation of ciprofloxacin, a recalcitrant antibiotic commonly detected in wastewater under photoelectrocatalytic conditions. Physicochemical and electrochemical characterization were conducted to investigate the structural, morphological and electrochemical properties of the designed photoanode. Also, the synergistic advantage of combining photocatalysis and electrocatalysis in PEC was examined through reaction kinetics. The reusability and the identification of the reactive species generated in the PEC system were equally investigated. Finally, a suitable reaction scheme for the heterostructured photoanode was examined based on its electrochemical properties.
2. Materials and methods
2.1 Chemical
Reagents used in this study were sourced from Merck (South Africa) and employed for various applications without further purification. Bismuth nitrate pentahydrate (Bi(NO3)3·5H2O), sodium chloride (NaCl), nickel acetate tetrahydrate (Ni(CH3COO)2·4H2O) and titanium butoxide ((C4H9O)4Ti) were the starting materials used in synthesizing BiOCl and NiTiO3 semiconductors. Acetic acid (CH3COOH), ethanol (C2H5OH) and ethylene glycol were used as solvents during the synthetic process. Fluorine-doped tin oxide (FTO) glass (L × W × D: 50 mm × 15 mm × 2.2 mm, surface resistivity of ∼13 Ω sq−1) was used as the substrate upon which the BiOCl/NiTiO3 heterostructured photoanode was immobilized. Polyvinylidene fluoride (PVDF) and N-methyl-2-pyrrolidone (NMP) were used as binding agents to immobilize the photoanode on the FTO glass. The scavenging of reactive species during the PEC experiments was carried out in the presence of terephthalic acid (TA, 1 mmol L−1), p-benzoquinone (BQ, 1 mmol L−1), ethylenediaminetetraacetic acid (EDTA, 1 mmol L−1) and silver nitrate (AgNO3, 1 mmol L−1) as the scavenging agents for trapping hydroxyl radicals (˙OH) and superoxide radicals (O2˙− , holes (h+) and electrons (e−), respectively.2.2 Synthesis of BiOCl
BiOCl was synthesized using the hydrothermal method. Firstly, 0.015 mmol of Bi(NO3)3·5H2O was dispersed in 20 mL of ethylene glycol. The mixture was stirred on a magnetic stirrer at room temperature for 30 min before adding 0.01 mmol of NaCl. The mixture was further stirred for 2 h until a homogeneous mixture was obtained. The mixture was later transferred into a Teflon-lined autoclave and heated at 180 °C for 12 h. Upon cooling, the resulting product was washed several times with deionized water and ethanol. Subsequently, the product was dried at 60 °C for 12 h to obtain the BiOCl powder.2.3 Synthesis of NiTiO3
NiTiO3 was synthesized using a solvothermal method. Briefly, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole composition comprising Ni(CH3CO2)2·4H2O (0.05 mmol) and Ti(C4H9O)4 (0.05 mmol) was dispersed in a mixture of ethanol and acetic acid. The mixture was stirred at room temperature for 2 h until a viscous sol–gel was obtained. The obtained sol–gel was dried for 24 h at 80 °C. The obtained NiTiO3 was further subjected to hydrothermal treatment by dispersing it into 3 M KOH and transferring the mixture into a Teflon-lined autoclave. The autoclave containing the mixture was then heated for 24 h at 160 °C. Upon cooling to room temperature, the NiTiO3 was washed several times with deionized water and ethanol to remove residual carbonate and other dissolved impurities. Finally, the pure NiTiO3 was dried at 60 °C for 12 h to obtain NiTiO3 powder.
1 mole composition comprising Ni(CH3CO2)2·4H2O (0.05 mmol) and Ti(C4H9O)4 (0.05 mmol) was dispersed in a mixture of ethanol and acetic acid. The mixture was stirred at room temperature for 2 h until a viscous sol–gel was obtained. The obtained sol–gel was dried for 24 h at 80 °C. The obtained NiTiO3 was further subjected to hydrothermal treatment by dispersing it into 3 M KOH and transferring the mixture into a Teflon-lined autoclave. The autoclave containing the mixture was then heated for 24 h at 160 °C. Upon cooling to room temperature, the NiTiO3 was washed several times with deionized water and ethanol to remove residual carbonate and other dissolved impurities. Finally, the pure NiTiO3 was dried at 60 °C for 12 h to obtain NiTiO3 powder.
2.4 Synthesis of the BiOCl/NiTiO3 heterostructured photocatalyst
The BiOCl/NiTiO3 heterostructured photocatalyst was prepared by stirring 50 wt% of BiOCl and 50 wt% of NiTiO3 in ethanol for 10 min. The composite was oven-dried at 120 °C for 3 h to obtain the BiOCl/NiTiO3 powder.2.5 Characterization of photocatalysts
X-ray diffractometer (Rigaku Ultima IV, Japan) using Cu Kα radiation (k = 0.15406), coupled with a K-beta filter at 30 mA and 40 kV, was employed to study the purity and degree of crystallinity of the prepared BiOCl, NiTiO3, and BiOCl/NiTiO3 powders. X-ray photoelectron spectroscopy (Thermo, ESCAlab 250Xi, USA) was used to analyze the chemical and electronic state of the atoms in the photocatalysts. Scanning electron microscopy (SEM) (Zeiss 540 ultra, Germany), attached to an energy-dispersive X-ray spectrometer (EDS), was employed to study the morphology, elemental composition and elemental mapping of the materials. The optical property of the semiconductors was measured using UV-vis diffuse reflectance spectroscopy.2.6 Fabrication of the BiOCl/NiTiO3 heterostructured photoanode
The BiOCl/NiTiO3 heterostructured photoanode was fabricated by drop-casting a BiOCl/NiTiO3 slurry on a pre-treated FTO glass. The BiOCl/NiTiO3 slurry was prepared by mixing 50 mg of the BiOCl/NiTiO3 powder in 50 wt% NMP and 5 wt% of PVDF. This was followed by careful coating of the BiOCl/NiTiO3 slurry onto the conductive surface of the FTO glass to obtain a uniformly distributed BiOCl/NiTiO3 film on the substrate. The electrode (geometrical area: 2 cm−2) was then left to dry in an oven at 60 °C for 2 h. The same method was employed in preparing the respective BiOCl and NiTiO3 photoanodes.2.7 Electrochemical and photoelectrochemical characterization of the photoanode
Electrochemical properties of the photoanodes were investigated using an Autolab potentiostat/galvanostat (PGSTAT204, Netherlands). The prepared photoanodes were connected to the potentiostat as working electrodes, while a platinum wire and an Ag/AgCl electrode were connected as the respective counter and reference electrodes. Electrochemical impedance spectroscopy (EIS) and Mott Schottky analysis were performed in 5 mM ferri/ferrocyanide solution [Fe(CN)6]3−/4− at a scan rate of 20 mV s−1, frequency range of 100 kHz to 0.1 Hz, and bias potential of 0.22 V. Linear sweep voltammetry (LSV) was used to obtain the onset potential of the photoanodes, and chronoamperometry measurement was used to investigate the photocurrent density response of the photoanodes. These analyses were conducted in 0.1 M Na2SO4 at neutral pH, in the presence of a 150 W LED lamp as the light source. All characterizations were carried out at neutral pH.2.8 Photoelectrocatalytic degradation experiment
PEC and electrocatalytic (EC) experiments were conducted using a power source for bias current supply to the system. A 150 W LED lamp was used as the light source for photocatalytic (PC) and PEC experiments. During the PEC degradation experiment, the prepared photoanode and a platinum wire were connected to the power source as anode and cathode, respectively. The PEC reaction was performed in 50 mL of 5 mg L−1 ciprofloxacin (CPX) concentration with 0.1 M Na2SO4 as the supporting electrolyte at a known applied current density. For the EC degradation experiment, the same CPX concentration was subjected to the same operating conditions as the PEC technique, but without a light source. During the PC degradation experiment, the photoanode was exposed to the light source without applying a current density. This was equally performed using the same volume and concentration of CPX. The influence of various applied current densities and different initial concentrations of CPX on the performance of the heterostructured photoanode was also examined. Aliquots of CPX solution were collected from the reactor at time intervals of 20 min for 120 min to analyze the decay patterns during PEC, EC and PC. A UV-visible spectrophotometer was used to investigate the downward trend of CPX concentration during the experiments. The degradation efficiency of CPX was obtained using the formula in eqn (1). | (1) |
3. Results and discussions
3.1 Structural and morphological properties of the photocatalysts
The X-ray diffraction (XRD) analysis was conducted to identify the crystal structure and confirm the synthesis of the photocatalysts (Fig. 1a). The XRD pattern of NiTiO3 shows a strong similarity with its hexagonal phase (JCPDS card no. 017–0617), with notable peaks observed at 2θ ≈ 24°, 32.8°, 35.5°, 40.8°, 49.3°, 53.8°, and the twin peaks at 62.2° 63.8°. The observed peaks are indexed as diffraction planes (012), (104), (110), (113), (024), (116), (214), and (300), respectively.31 Similarly, the XRD pattern of BiOCl corresponds strongly to its tetragonal phase (JCPDS card no. 01-0600). Major peaks observed for BiOCl at 2θ ≈ 11.6°, 24.1°, 25.2°, 33.3°, and 36.4° correspond to the (001), (011), (110), (102), and (003) diffraction planes, respectively.32 The outlined peaks were observed on the XRD pattern of BiOCl/NiTiO3, demonstrating that the heterostructured photocatalyst was satisfactorily formed. However, the weak diffraction peaks of NiTiO3 observed on the XRD graph of BiOCl/NiTiO3 signify a better dispersion of BiOCl on the NiTiO3.X-ray photoelectron spectroscopy (XPS) analysis was also conducted to investigate the chemical composition and electronic states of the photocatalysts. The survey scans of BiOCl, NiTiO3, and BiOCl/NiTiO3 photocatalysts are presented in Fig. 1b, indicating the presence of bismuth (Bi), nickel (Ni), titanium (Ti), chlorine (Cl), and oxygen (O), along with their corresponding peaks. The deconvoluted spectra of the Bi 4f7/2 electronic state reveal a shift in the binding energy from 158.9 eV to 159.1 eV in BiOCl and the heterostructured BiOCl/NiTiO3, respectively (Fig. 1c). A comparable shift was also observed from 164.2 eV to 164.4 eV for the electronic state of Bi 4f5/2 as the BiOCl/NiTiO3 heterostructure photoanode was formed. The presence of Bi 4f7/2 and Bi 4f5/2 peaks demonstrates the existence of the Bi3+ oxidation state in pristine BiOCl33,34 and BiOCl/NiTiO3 heterostructured semiconductor. The deconvoluted spectra of the Cl 2p valence state (Fig. 1d) equally reveal a shift in the binding energy from 197.7 eV to 197.5 eV (Cl 2p3/2) and 199.6 eV to 199.2 eV (Cl 2p1/2) upon the formation of the BiOCl/NiTiO3 heterostructured photoanode. These observed shifts in binding energies can be attributed to electron redistribution within the BiOCl/NiTiO3 heterostructured photoanode crystal structure, a vital development for charge separation. The shifts also demonstrate electron density variation across the BiOCl/NiTiO3 interface, facilitating photogenerated charge separation through charge localization in distinctive sites.35 Moreover, the [Bi2O2]2+ and Cl− layered structure formed in BiOCl can promote charge transportation and light absorption extension in the BiOCl/NiTiO3 photoanode through internal electric field generation. Similarly, a backward shift in binding energies was observed from 855.4 eV to 854.8 eV and 873.2 eV to 872.6 eV in the respective Ni 2p3/2 and Ni 2p3/2 electronic states (Fig. 1e), further establishing the formation of the heterostructured photoanode. The two satellite peaks at 862.2 eV and 880 eV demonstrate the presence of Ni2+ oxidation state in NiTiO3.21,36 These peaks were also observed at 861.8 eV and 879.8 eV, further confirming the interfacial interaction between BiOCl and NITiO3 semiconductors. The presence of Ti 2p3/2 and Ti 2p1/2 peaks (Fig. 1f) demonstrates the existence of the Ti4+ oxidation state in pristine NiTiO3.37 Ti 2p3/2 and Ti 2p1/2 electronic states also displayed notable shifts from 458.3 eV to 459.1 eV and 464.2 eV to 465 eV, respectively, as the BiOCl/NiTiO3 heterostructured photocatalyst was formed. The O 1s peaks observed at 530.2 eV and 531.9 eV correspond to the Bi–O bond and oxygen vacancy in BiOCl,38,39 while NiTiO3 peaks at 530.1 eV and 532.9 eV correspond to Ni–O bond and physically adsorbed oxygen40 (Fig. 1g). The lattice oxygen Bi–O of BiOCl at 530.2 eV and NiTiO3 lattice oxygen Ni–O at 530.1 eV were observed to have shifted to a lower binding energy at 529.7 eV for BiOCl/NiTiO3. Similarly, the oxygen vacancy possessed by BiOCl at 531.9 eV was also observed to have shifted to a lower binding energy at 530.7 eV in the BiOCl/NiTiO3 semiconductor. This red shift is indicative of electron transfer at the interface of the BiOCl/NiTiO3 heterostructure semiconductor. Notably, the area covered by the oxygen vacancy in the BiOCl/NiTiO3 heterostructured semiconductor is significantly wider than that of pristine BiOCl. This suggests that surface modification caused by the presence of oxygen vacancies in BiOCl/NiTiO3 can enhance charge separation and water dissociation remarkably, resulting in abundant surface hydroxyl groups needed for the degradation process.35 In addition, red shifts observed in Cl 2p, Ni 2p and O 1s valence states suggest the occurrence of electron redistribution in the BiOCl/NiTiO3 semiconductor. Moreover, blue shifts observed in the Bi 4f and Ti 2p spectra can be attributed to an increase in electron density as the interfacial and ionic interactions between BiOCl and NiTiO3 were established.
The surface morphology of the pristine and heterostructure semiconductors was determined using scanning electron microscopy (SEM). BiOCl displayed irregular, interconnected, dispersed, and layered plate-like microspheres (Fig. 1h). With slight roughness on the boundaries of the particles, the linkage between the BiOCl particles allows for a suitable deposition and lacing of the other material on their surface. However, NiTiO3 exhibits a blend of grains with uneven sizes (Fig. 1i). The interactions linking these grains led to the formation of pores of varying sizes. Notably, the composition of these particles shows random dispersal of NiTiO3 particles on the BiOCl plate-like particles (Fig. 1j). The coalescence observed with the BiOCl/NiTiO3 heterostructured photocatalyst shows a healthy interaction between both pristine materials, suitable for light absorption in the heterostructure. The interfacial properties displayed by the heterostructure indicate that the BiOCl/NiTiO3 semiconductor was successfully synthesized. The EDX spectrum of the BiOCl/NiTiO3 heterostructured semiconductor shown in Fig. S1 reveals the presence of Bi, Ni, Ti, O, and Cl in the composite. The uniform distribution of atoms in the composite is shown in the EDX mapping (Fig. 1k), further establishing a successful synthesis of the heterostructure.
3.2 Electrochemical and photoelectrochemical properties of the photoanodes
Electrochemical impedance spectroscopy (EIS) was conducted to investigate the charge transport efficiency of the photoanodes. Fig. 2a presents the Nyquist plot of the fabricated photoanodes, with charge transfer resistances (Rct) of 392, 230, and 157 Ω associated with NiTiO3, BiOCl, and BiOCl/NiTiO3 photoanodes, respectively. Notably, a smaller impedance arc in the Nyquist plot corresponds to improved charge transfer efficiency.41 These results demonstrate that the BiOCl/NiTiO3 heterostructured photoanode displays a more desirable charge transfer efficiency than the individual BiOCl and NiTiO3 photoanodes. This indicates an enhanced charge transport mechanism in the BiOCl/NiTiO3 photoanode, boosting its conductivity and promoting the migration efficiency of photogenerated charge carriers. Moreover, a harmonious interfacial interaction developed between BiOCl and NiTiO3 photoanodes gave rise to band alignment in the BiOCl/NiTiO3 heterostructured photocatalyst. This interaction can propel charge separation in the semiconductor, improving the availability of charged species and reducing the difficulty of charge transfer in the system.Linear sweep voltammetry (LSV) was employed to investigate the onset potential and current density of the photoanodes at neutral pH. As presented in Fig. 2b, an onset potential of 1.39 V was obtained for the BiOCl/NiTiO3 heterostructured photoanode as compared to a delayed potential of 1.53 V and 1.57 V for BiOCl and NiTiO3 photoanodes, respectively. Since the obtained values exceed the minimum potential of 1.23 V vs. RHE required for the oxygen evolution reaction (OER) in water splitting,42,43 the BiOCl/NiTiO3 heterostructured photoanode represents a conservative potential demand for oxidation reactions compared with other pure semiconductors. Furthermore, the highest current density of 14.93 mA cm−2 for the BiOCl/NiTiO3 photoanode exceeds 2.5 times the obtained current densities for BiOCl and NiTiO3 photoanodes, suggesting that the ease of ROS generation favours the BiOCl/NiTiO3 heterostructured photoanode over the individual photoanodes. This validates the charge mobility strength inherent in the BiOCl/NiTiO3 heterojunction photoanode over the pristine semiconductors, as confirmed by the EIS results.
The transient photocurrent density was also investigated to assess the photogenerated charge separation capability of the photoanode at neutral pH. An impressive photocurrent density of 5.36 µA cm−2 was obtained for the BiOCl/NiTiO3 heterostructured photoanode compared to 2.77 µA cm−2 and 2.62 µA cm−2 for BiOCl and NiTiO3 photoanodes, respectively (Fig. 2c). This reveals that a significant suppression of photogenerated charge carrier (hole–electron) recombination was better achieved in the BiOCl/NiTiO3 heterostructured photoanode over the individual photoanodes. A linear correlation validating a mutual relationship between the transient photocurrent of semiconductors and photogenerated charge separation has been previously established.44 Consequently, improved light absorption efficiency and reduced photocorrosion phenomenon arising from improved interfacial charge transfer will be more prevalent in the BiOCl/NiTiO3 heterostructured photoanode than in the pristine photoanodes. Additionally, band alignment arising from the favourable interfacial properties of the heterostructure can lower charge recombination, thus improving the photocurrent response of the semiconductor.
3.3 Optical properties of the photocatalysts
The optical properties of the semiconductors were analyzed to determine their light absorption regions and band gap energies. As presented in Fig. 2d, NiTiO3 and BiOCl displayed a strong absorption edge around 460 nm and 395 nm, respectively. Harmoniously, the BiOCl/NiTiO3 photocatalyst equally displayed strong absorption in the visible light region with an absorption edge at 567 nm. The shift of the BiOCl/NiTiO3 absorption edge to a more visible-light region indicates a broadened light absorption capacity, arising from the conducive interfacial synergy between BiOCl and NiTiO3 photocatalysts.Moreover, the band gap energies of the photocatalysts were obtained from UV-vis diffuse reflectance spectroscopy data fitted to the Tauc equation (eqn (2)).
| αhv = β(hv − Eg)n/2 | (2) |
| ECB = X − Ec − 0.5Eg | (3) |
| EVB = ECB + Eg | (4) |
The Mott Schottky analysis was performed to determine the flat band potential and charge carrier density of the photoanodes. Using the Mott Schottky equation (eqn (5)), the Mott Schottky plots for each photoanode were obtained from the graph of 1/c2versus potential (eV vs. Ag/AgCl).
 | (5) |
The flat band potential was obtained as the X-intercept after extrapolating the linear fit of the plot. BiOCl has a negative slope (Fig. S2), indicative of a p-type behaviour, while NiTiO3 has a positive slope (Fig. S3), indicative of n-type semiconductors. Flat band potentials of 0.17 V, −0.55 V, and −0.79 V (vs. Ag/AgCl) were obtained for BiOCl, NiTiO3, and BiOCl/NiTiO3 photoanodes, respectively. Using the formula in eqn (6), the flat band potential vs. normal hydrogen electrode (NHE) values of the photoanodes were estimated as 0.37 eV and −0.36 eV for BiOCl and NiTiO3 photoanodes. These results are a product of electrolyte–semiconductor interfacial interactions, electrode geometry, electrode surface area, and smoothness of the electrode surface.45 Moreover, the negative shift obtained for the BiOCl/NiTiO3 photoanodes at −0.59 eV vs. NHE, as against −0.16 eV vs. NHE observed for the NiTiO3 photoanode, can be credited to the improved photogenerated charge separation in the BiOCl/NiTiO3 heterostructured photoanodes. Additionally, an increase in the slope of BiOCl/NiTiO3 heterostructured photoanodes (5.62 × 109) was obtained (Fig. 2f), as against the 2.48 × 1010 obtained for NiTiO3. Since the slope (obtained from eqn (7)) inversely corresponds to the charge carrier density (equivalent to the electron density), a donor density of 1.79 × 1021 cm−3 calculated for BiOCl/NiTiO3 photoanodes is about thrice the donor density obtained for NiTiO3 (4.07 × 1020 cm−3). This further confirms effective and prolonged charge separation at the heterojunction photoanode.
Efb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) vs. vs.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NHE = Efb NHE = Efb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) vs. vs.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag/AgCl + 0.194 (pH = 7) Ag/AgCl + 0.194 (pH = 7) | (6) |
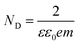 | (7) |
3.4 Photoelectrocatalytic application of the photoanodes for ciprofloxacin degradation
The photoanodes were applied for the photoelectrocatalytic degradation of 5 mg L−1 CPX under a 150 W simulated sunlight source with an optimized current density of 5 mA cm−2 at pH 7. As presented in Fig. 3a, the heterostructured BiOCl/NiTiO3 photoanode achieved an impressive 94% removal efficiency within 120 min. Its performance surpasses the individual BiOCl and NiTiO3 photoanodes, which achieved 84% and 80% degradation efficiencies, respectively. The improvement in the BiOCl/NiTiO3 heterostructured photoanode performance indicates a significant suppression in the photogenerated charge carrier recombination rate. Hence, the photo-excited heterostructure photoanode exhibits higher mobility than the individual photoanodes, allowing the generated species to attack CPX unselectively. Additionally, photoelectrocatalysis (a combination of photocatalysis and electrocatalysis) demonstrated 94% degradation efficiency compared to 18% and 86% obtained for individual photocatalysis and electrocatalysis techniques (Fig. 3b). The influence of electrochemical driving force (current density) equally facilitated charge separation in the heterostructured photoanode by initiating induced hole injection into the valence band.40 This effect enriches the availability of generated holes and electrons by drastically extending their lifespan.46,47 Consequently, the delayed charge recombination aided by enhanced charge migration across the heterostructured photoanode-electrolyte interface can accelerate ROS generation and the simultaneous degradation of the pollutant.3.5 Kinetic and synergistic studies
The photoelectrocatalytic reaction kinetics were determined for each photoanode (Fig. 3c). Also, photocatalytic and electrocatalytic reaction kinetics for the BiOCl/NiTiO3 heterostructured photoanode were determined and are presented in Fig. 3d. The reaction kinetics of all degradation techniques obtained from the plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c0/ctversus time conformed to a pseudo-first-order reaction. The kinetics were fitted into ln
c0/ctversus time conformed to a pseudo-first-order reaction. The kinetics were fitted into ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c0/ct = kt, where c0, ct, k, and t are the initial concentration (mg L−1), final concentration (mg L−1), rate constant (min−1), and time (min). Rate constant (k) value of 2.48 × 10−2 min−1 (R2 = 0.9955) was obtained for the BiOCl/NiTiO3 heterostructured photoanode, which is approximately 1.55-fold and 1.78-fold of the rate constants for BiOCl (1.62 × 10−2 min−1, R2 = 0.9894) and NiTiO3 (1.39 × 10−2 min−1, R2 = 0.9869) photoanodes (Fig. 3c). This outcome agrees with the notable suppression of photogenerated charge carrier recombination in the BiOCl/NiTiO3 photoanode, caused by the improved light absorption capacity of the heterostructure photoanode. Moreover, improved charge mobility in the BiOCl/NiTiO3 heterostructured photoanode eased the generation of reactive oxygen species, thus leading to the impressive degradation of CPX. The photoelectrocatalytic rate constant of the BiOCl/NiTiO3 heterostructured photoanode also showed a 1.66-fold and 13.55-fold increment in the respective electrocatalytic (1.70 × 10−2 min−1, R2 = 0.9952) and photocatalytic (1.83 × 10−3 min−1, R2 = 0.9761) rate constants. The heterostructured photoanode performance during the photoelectrocatalytic degradation of CPX further establishes the desirable synergy arising from combining both electrocatalysis and photocatalysis in the hybrid system.
c0/ct = kt, where c0, ct, k, and t are the initial concentration (mg L−1), final concentration (mg L−1), rate constant (min−1), and time (min). Rate constant (k) value of 2.48 × 10−2 min−1 (R2 = 0.9955) was obtained for the BiOCl/NiTiO3 heterostructured photoanode, which is approximately 1.55-fold and 1.78-fold of the rate constants for BiOCl (1.62 × 10−2 min−1, R2 = 0.9894) and NiTiO3 (1.39 × 10−2 min−1, R2 = 0.9869) photoanodes (Fig. 3c). This outcome agrees with the notable suppression of photogenerated charge carrier recombination in the BiOCl/NiTiO3 photoanode, caused by the improved light absorption capacity of the heterostructure photoanode. Moreover, improved charge mobility in the BiOCl/NiTiO3 heterostructured photoanode eased the generation of reactive oxygen species, thus leading to the impressive degradation of CPX. The photoelectrocatalytic rate constant of the BiOCl/NiTiO3 heterostructured photoanode also showed a 1.66-fold and 13.55-fold increment in the respective electrocatalytic (1.70 × 10−2 min−1, R2 = 0.9952) and photocatalytic (1.83 × 10−3 min−1, R2 = 0.9761) rate constants. The heterostructured photoanode performance during the photoelectrocatalytic degradation of CPX further establishes the desirable synergy arising from combining both electrocatalysis and photocatalysis in the hybrid system.
To further establish the clear advantage of the photoelectrocatalytic technique owing to the influence of the light source and electrochemical driving force (current density), the degree of synergy and electrochemical enhancement (E) were estimated48 using eqn (8) and (9).
 | (8) |
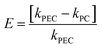 | (9) |
3.6 Influence of current density and concentration on the degradation of CPX
The influence of various electrochemical parameters on the degradation efficiency of CPX during photoelectrocatalysis was also evaluated in 0.1 M Na2SO4 electrolyte at neutral pH. The degradation efficiency of CPX grew proportionally with increased applied current density. Degradation efficiencies of 82%, 94%, and 96% were obtained upon applying 3 mA cm−2, 5 mA cm−2, and 10 mA cm−2 current densities, respectively, as shown in Fig. 3e. This further corroborates the superior influence of current density on the photoelectrocatalytic technique, which arises from improved mass transfer across the BiOCl/NiTiO3 heterostructured photoanode–electrolyte interface. Thus, enhanced migration efficiency of mobile species, including photogenerated charges and ROS, results in a more pronounced charge separation advantage gained by the BiOCl/NiTiO3 photoanode during the process. Additionally, the performance of the BiOCl/NiTiO3 heterostructured photoanode in different concentrations of CPX was evaluated. The degradation efficiencies decreased gradually from 97% to 89% as the initial concentration of CPX increased from 3 mg L−1 to 10 mg L−1 (Fig. 3f). This pattern may be attributed to the presence of more analytes competing to interact with the reactive oxygen species generated in the system as the initial concentration increases.3.7 Reusability, stability and scavenger studies
The stability of the BiOCl/NiTiO3 heterostructured photoanode was examined by subjecting it to multiple cycles of photoelectrocatalytic degradation of CPX. The photoanode exhibited remarkable stability at operating conditions involving pH 7 and 5 mA cm−2 applied current density. An average degradation efficiency of 92.5% was obtained through five cycles of photoelectrocatalytic degradation of CPX, as shown in Fig. 4a. The stability displayed by the photoanode suggests that a consistent charge separation and interfacial charge migration efficiency occurred each time the photoanode was applied. Moreover, this stability further indicates the viability of the heterostructured photoanode for real-time applications. The structural property of the BiOCl/NiTiO3 heterostructured semiconductor photoanode was examined after its use for multiple cycles of photoelectrocatalytic degradation of CPX. The structural integrity of the heterostructured semiconductor was preserved after photoelectrocatalytic application, as shown in Fig. S3.The degradation of CPX on the BiOCl/NiTiO3 heterostructured photoanode during photoelectrocatalytic degradation occurred through the influence of reactive oxygen species generated during the process. Due to their high reactivity and low stability, these species attack the bonds of the target organic pollutant non-selectively, resulting in oxidation. In addition to the photogenerated holes and electrons, influential species commonly generated in photoelectrocatalytic systems include hydroxyl radicals and superoxide radicals. Each species was trapped independently using chemical inhibition agents to quench and render them inactive during the photoelectrocatalytic process. Notably, upon inhibiting the activity of ˙OH in the system with terephthalic acid, the degradation efficiency declined by 15% (Fig. 4b), showing that ˙OH was the most predominant ROS responsible for the degradation of CPX. A 27% reduction in the degradation efficiency upon trapping h+VB with EDTA suggests that the photogenerated hole also played a significant role in the degradation process. However, drastic contractions in the degradation efficiency were obtained upon inhibiting O2˙− and e−CB to 21% and 36%, respectively, indicative of a limited influence of these species in the degradation of CPX. Eqn (10)–(14) show possible formation routes for the species in the photoelectrocatalytic system.
| BiOCl/NiTiO3 heterostructured photoanode + hv → BiOCl/NiTiO3 (h+VB, e−CB) | (10) |
| h+VB + H2O → ˙OH + H+ | (11) |
| h+VB + OH− → ˙OH | (12) |
| e−CB + O2 → O2˙− | (13) |
| (˙OH, O2˙−, h+) + ciprofloxacin → CO2 + H2O | (14) |
3.8 Proposed Z-scheme mechanism of the heterostructured photoanode
The proposed scheme illustrated in Fig. 4c shows that the BiOCl/NiTiO3 heterostructured photoanode exhibits type II behaviour. Photogenerated holes and electrons are produced in the semiconductors upon irradiation, resulting in the formation of valence and conduction bands within their interfacial structure, as illustrated by eqn (9). Before contact between the pristine semiconductors was initiated, the conduction band and valence band of NiTiO3 partially overlapped with the energy band edges of BiOCl, as obtained from the calculations of UV-vis DRS and Mott Schottky plots. Following contact initiation, Fermi level alignment is achieved within the heterostructure photoanode, resulting in a staggered alignment. Generally, heterostructure mechanisms and formation between interacting semiconductors are influenced by band bending or internal electric fields created at the interface of the participating semiconductors. Other influencing parameters include the position of their conduction and valence band edges, the semiconductor type, Fermi energy levels, and work function.14 Typical of a type II (Z-scheme) alignment, the electron from the conduction band of BiOCl recombines with the holes in the valence band of NiTiO3 upon irradiation through interfacial transmission.50,51 This spontaneous transmission leads to the formation of an internal electric field at the interface of the heterostructured photoanode, which is often observed in p–n heterostructure behavior.52 The induced internal electric field gives rise to an upward band bending and electron depletion (hole accumulation) in NiTiO3, while a downward band bending and electron accumulation in BiOCl is initiated. This results in attraction between the positively charged valence band of NiTiO3 and the negatively charged conduction band of BiOCl at the heterostructured photoanode interface, allowing electrons to migrate from the conduction band of BiOCl to the valence band of NiTiO3. Since the Fermi level of BiOCl (0.37 eV) is more positive than that of NiTiO3 (−0.36 eV), the proposed mechanism agrees with Z-scheme behaviour, which allows electron migration from the semiconductor with a higher Fermi level to the semiconductor with a lower Fermi level.53Similarly, the internal electric field formed within the BiOCl/NiTiO3 interface promoted spontaneous interfacial electron diffusion from BiOCl due to its lower work function (Wf) (range of 5.19–5.56 eV vs. NHE, pH = 754,55) to NiTiO3 with a higher work function (Wf = 5.86 eV vs. NHE, pH = 756). This migration path is triggered by the ease of electron mobility from the core to the surface of a semiconductor with a lower work function in a heterostructured semiconductor.55 Consequently, available holes at the valence band of BiOCl readily react with water to form hydroxyl radicals (˙OH), and separated electrons at the conduction band of NiTiO3 react with dissolved oxygen in the system to form superoxide radicals (O2˙−) as represented in eqn (10) and (11). The formation of O2˙− is stimulated by the more negative potential (−0.74 eV) attainable at the conduction band of NiTiO3 than O2/O2˙− = −0.33 V vs. NHE standard potential, while ˙OH formation is encouraged by a more positive potential (3.4 eV) reached by the valence band of BiOCl than the standard potential of H2O/O2 = 2.37 V vs. NHE. Additionally, the significant charge separation modelled by the BiOCl/NiTiO3 heterostructured photoanode agrees with its transient photocurrent response, which is twice the responses obtained for individual semiconductors. This accelerated photogenerated charge separation and reduced recombination arising from charge redistribution eases reactive oxygen species generation, while improving charge mobility. Consequently, the oxidation process involving the breaking of the organic bond in CPX to non-toxic H2O and CO2 occurs at the photoanode surface.
4. Conclusion
The BiOCl/NiTiO3 heterostructured semiconductor and its photoanode were successfully fabricated and applied for the photoelectrocatalytic degradation of ciprofloxacin in water. The synergistic integration gained from combining BiOCl and NiTiO3 semiconductors yielded a superior heterostructured photoanode with improved charge separation and mobility. The results from the electrochemical, optical, and photoelectrocatalytic characterization of the semiconductors showed that superior charge transfer and reduced charge recombination are easily attainable in BiOCl/NiTiO3 compared with the individual semiconductors. Moreover, the BiOCl/NiTiO3 heterostructured photoanode achieved more impressive efficiency than the individual BiOCl/NiTiO3 photoanodes when applied for the photocatalytic degradation of 5 mg L−1 of CPX. The overall photoelectrocatalytic degradation was generally performed by maintaining operating conditions of 5 mA cm−1 current density, 50 mL working solution containing 0.1 M Na2SO4 supporting electrolyte at neutral pH, and a 150 W lamp. The proposed reaction mechanism deduced from optical properties revealed that the BiOCl/NiTiO3 heterostructured photoanode exhibits Z-scheme behaviour. The calculated valence band of BiOCl exceeding the standard oxidation potential required for water oxidation (H2O/O2 = 2.37 V vs. NHE) explains the significance of h+ during CPX degradation and the ease of hydroxyl radical generation in the system. These findings underscore the importance of rational heterostructured photoanode designs in advancing photoelectrocatalytic technologies in wastewater remediation.Conflicts of interest
There are no conflicts of interest to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). The supplementary information includes the EDX spectra of BiOCl/NiTiO3 heterostructured semiconductor, Mott Schottky plot of BiOCl and NiTiO3 photoanodes and the XRD pattern of the BiOCl/NiTiO3 heterostructured semiconductor before and after use. See DOI: https://doi.org/10.1039/d5ma00827a.Acknowledgements
Financial support from the Centre of Nanomaterial Science Research, University of Johannesburg (grant no. 086342) and Global Excellence Scholarship, University of Johannesburg (grant no. 105592), is greatly appreciated.References
- R. Wu, Y. Ruan, H. Lin, C. N. T. Yuen, H. Feng and P. K. S. Lam, ACS EST Water., 2021, 1, 542–552 CrossRef CAS.
- A. B. de Souza, N. Gadi, T. van de Goor, N. C. Boelee, R. Dewil and D. Cabooter, J. Water Process Eng., 2024, 66, 105899 CrossRef.
- O. F. S. Khasawneh and P. Palaniandy, Process Saf. Environ. Prot., 2021, 150, 532–556 CrossRef CAS.
- M. Hossein and A. S. Ripanda, Toxicol. Rep., 2025, 14, 101969 CrossRef CAS PubMed.
- M. Kukreja, A. A. Kumar, N. Haq and K. A. Siddiqui, Mater. Today Commun., 2025, 45, 112403 CrossRef CAS.
- M. C. Danner, A. Robertson, V. Behrends and J. Reiss, Sci. Total Environ., 2019, 664, 793–804 CrossRef CAS.
- B. Böger, M. Surek, R. D. O. Vilhena, M. M. Fachi, A. M. Junkert, J. M. Santos, E. L. Domingos, A. D. F. Cobre, D. R. Momade and R. Pontarolo, J. Hazard. Mater., 2021, 402, 123448 CrossRef PubMed.
- M. Kang, J. Yang, S. Kim, J. Park, M. Kim and W. Park, Sci. Total Environ., 2022, 811, 152331 CrossRef CAS PubMed.
- C. F. Couto, L. C. Lange and M. C. S. Amaral, J. Water Process Eng., 2019, 32, 100927 CrossRef.
- A. Pietro, S. Murgolo, C. De Ceglie, G. Mascolo, M. Carmagnani, P. Ronco, M. Bestetti and S. Franz, Catal. Today, 2025, 450, 115205 CrossRef.
- X. Meng and Z. Zhang, Catal. Today, 2018, 315, 2–8 CrossRef CAS.
- D. Kanakaraju, B. D. Glass and M. Oelgemöller, J. Environ. Manage., 2018, 219, 189–207 CrossRef CAS PubMed.
- L. Shi, Y. Yin, L. C. Zhang, S. Wang, M. Sillanpää and H. Sun, Appl. Catal., B, 2019, 248, 405–422 CrossRef CAS.
- A. Balapure, J. Ray Dutta and R. Ganesan, RSC Appl. Interfaces, 2024, 1, 43–69 RSC.
- Z. Li, Y. Qu, K. Hu, M. Humayun, S. Chen and L. Jing, Appl. Catal., B, 2017, 203, 355–362 CrossRef CAS.
- Z. Jia, R. Lv, L. Guo, J. Zhang, R. Li, J. Liu and C. Fan, Sep. Purif. Technol., 2021, 257, 117872 CrossRef CAS.
- X. Zhou, J. Liu and J. Sun, Adv. Compos. Hybrid Mater., 2024, 7, 239 CrossRef CAS.
- L. Li, G. Liu, J. Dong, S. Cao, B. Wang, J. Zhao, M. Ji, Y. She, J. Xia and H. Li, Appl. Surf. Sci., 2025, 697, 162978 CrossRef CAS.
- Z. Xu, C. Zhang, Y. Zhang, Y. Gu and Y. An, Inorg. Chem. Commun., 2022, 138, 109277 CrossRef CAS.
- D. Sun, M. Zhang, L. Huang, Y. Qu, Y. Yu, B. Lou, H. Du, B. Xu and K. Wang, J. Alloys Compd., 2023, 969, 172433 CrossRef CAS.
- D. Peng, Y. Wang, H. Shi, W. Jiang, T. Jin, Z. Jin and Z. Chen, J. Colloid Interface Sci., 2022, 613, 194–206 CrossRef CAS PubMed.
- S. Li, S. Hu, W. Jiang, Y. Liu, Y. Zhou, Y. Liu and L. Mo, J. Colloid Interface Sci., 2018, 521, 42–49 CrossRef CAS.
- B. O. Ojo, O. A. Arotiba and N. Mabuba, Colloids Surf., A, 2022, 647, 129201 CrossRef CAS.
- T. Yao, X. An, H. Han, J. Q. Chen and C. Li, Adv. Energy Mater., 2018, 8, 1–36 Search PubMed.
- A. Vilanova, P. Dias, T. Lopes and A. Mendes, Chem. Soc. Rev., 2024, 53, 2388–2434 RSC.
- E. Brillas, Chemosphere, 2020, 250, 126198 CrossRef CAS.
- Z. Zhang, Y. Yuan, L. Liang, Y. Fang, Y. Cheng, H. Ding, G. Shi and L. Jin, Ultrason. Sonochem., 2008, 15, 370–375 CrossRef CAS PubMed.
- S. Fekadu, E. Alemayehu, R. Dewil and B. Van der Bruggen, J. Appl. Electrochem., 2021, 51, 607–618 CrossRef CAS.
- S. Fekadu, E. Alemayehu, B. Oljira, S. Tiku, D. Dadi and B. Van der Bruggen, J. Water Process Eng., 2021, 41, 102068 CrossRef.
- S. Fekadu, E. Alemayehu, P. Asaithambi and B. Van der Bruggen, Int. J. Environ. Res., 2022, 16, 1–11 CrossRef.
- M. Chettab, Q. Simon, M. Zaghrioui, C. Autret-Lambert and P. Laffez, Thin Solid Films, 2020, 714, 138384 CrossRef CAS.
- J. Wang, Y. Wei, B. Yang, B. Wang, J. Chen and H. Jing, J. Catal., 2019, 377, 209–217 CrossRef CAS.
- A. Hao, X. Ning, X. Liu, L. Zhan and X. Qiu, Chem. Eng. J., 2024, 499, 155823 CrossRef CAS.
- W. Li, S. Ao He, Z. Ying Su, W. Xu and X. Chuan Wang, Appl. Surf. Sci., 2019, 470, 707–715 CrossRef CAS.
- J. Bai, X. Zhang, C. Wang, X. Li, S. Cao, R. Zhang, X. Xiao, X. Jiang and T. Wu, Sep. Purif. Technol., 2025, 361, 131521 CrossRef CAS.
- S. Dhingra, M. Sharma, V. Krishnan and C. M. Nagaraja, J. Colloid Interface Sci., 2022, 615, 346–356 CrossRef CAS.
- D. Peng, L. Mao, J. Sun, X. Li, H. Shi and Z. Su, Int. J. Hydrogen Energy, 2025, 114, 60–70 CrossRef CAS.
- X. Ren, J. Yao, L. Cai, J. Li, X. Cao, Y. Zhang, B. Wang and Y. Wei, New J. Chem., 2019, 43, 1523–1530 RSC.
- S. Sharma, A. D. Acharya, Y. S. Thakur and Bhawna, Mater. Res. Bull., 2025, 183, 113214 CrossRef CAS.
- K. D. Jayeola, D. S. Sipuka, T. I. Sebokolodi, J. O. Babalola, M. Zhou, F. Marsken and O. A. Arotiba, ACS Appl. Mater. Interfaces, 2025, 17, 1385–1398 CrossRef CAS.
- B. O. Ojo, O. A. Arotiba and N. Mabuba, J. Environ. Chem. Eng., 2022, 10, 107224 CrossRef CAS.
- S. M. Tan and M. Pumera, ACS Nano, 2019, 13, 2681–2728 CrossRef CAS PubMed.
- Q. Wang, L. Ren, J. Zhang, X. Chen, C. Chen, F. Zhang, S. Wang, J. Chen and J. Wei, Adv. Energy Mater., 2023, 13, 2301543 CrossRef CAS.
- B. O. Orimolade and O. A. Arotiba, Sci. Rep., 2020, 1–13 Search PubMed.
- K. Sivula, ACS Energy Lett., 2021, 6, 2549–2551 CrossRef CAS.
- J. P. Velasquez-Tamayo, D. A. Torres-Ceron, S. Amaya-Roncancio, S. I. Santacruz, C. D. Acosta-Medina and E. Restrepo-Parra, Chem. Eng. J., 2023, 477, 147080 CrossRef CAS.
- X. Yang and D. Wang, ACS Appl. Energy Mater., 2018, 1, 6657–6693 CrossRef CAS.
- B. O. Orimolade, B. N. Zwane, B. A. Koiki, L. Tshwenya, G. M. Peleyeju, N. Mabuba, M. Zhou and O. A. Arotiba, J. Environ. Chem. Eng., 2020, 8, 103607 CrossRef CAS.
- A. Amedlous, M. Majdoub, E. Amaterz, Z. Anfar and A. Benlhachemi, J. Photochem. Photobiol., A, 2021, 409, 113127 CrossRef CAS.
- D. Huang, X. Yan, M. Yan, G. Zeng, C. Zhou, J. Wan, M. Cheng and W. Xue, ACS Appl. Mater. Interfaces, 2018, 10, 21035–21055 CrossRef CAS.
- Y. Wang, Z. Ma, J. Wang, J. Ben, H. Guan, P. Gu and Y. Hu, Mater. Sci. Semicond. Process., 2025, 190, 109375 CrossRef CAS.
- J. Low, J. Yu, M. Jaroniec, S. Wageh and A. A. Al-Ghamdi, Adv. Mater., 2017, 29, 1601694 CrossRef.
- T. Di, Q. Xu, W. K. Ho, H. Tang, Q. Xiang and J. Yu, ChemCatChem, 2019, 11, 1394–1411 CrossRef CAS.
- S. Gong, F. Rao, W. Zhang, Q. U. Hassan, Z. Liu, J. Gao, J. Lu, M. Hojamberdiev and G. Zhu, Chin. Chem. Lett., 2022, 33, 4385–4388 CrossRef CAS.
- L. Zhao, H. Hou, S. Wang, L. Wang, Y. Yang, C. R. Bowen, J. Wang, Z. Liao, D. Yang, R. Yan and W. Yang, Adv. Funct. Mater., 2024, 2416346, 1–16 Search PubMed.
- B. Li, W. Wang, J. Zhao, Z. Wang, B. Su, Y. Hou, Z. Ding, W. J. Ong and S. Wang, J. Mater. Chem. A, 2021, 9, 10270–10276 RSC.
| This journal is © The Royal Society of Chemistry 2026 |




