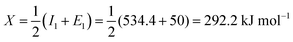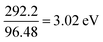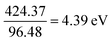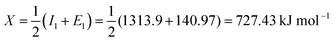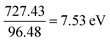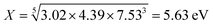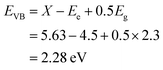Open Access Article
Open Access ArticleSynthesis and characterization of potential CeNiO3 perovskite for photoelectrochemical water splitting
Hosakote Shankar
Anusha
ab,
Vodeti
Rajeshwar
c,
Usha
Jinendra
d,
Jagadeep
Chandra S
e,
Elayaperumal
Sumitha
f,
Basavarajappa Sannappa
Hanumanthappa
g,
Vinod
Divya
a,
Mohammad
Khalid
h,
Shadma
Wahab
i,
Kotermane Mallikarjunappa
Anilkumar
*a,
Peter R.
Makgwane
j,
Honnegowdanahalli Shivabasappa Nagendra
Prasad
 k and
Harikaranahalli Puttaiah
Shivaraju
k and
Harikaranahalli Puttaiah
Shivaraju
 *abl
*abl
aDepartment of Environmental Sciences, JSS Academy of Higher Education and Research, Mysuru, 570015, India. E-mail: shivarajuenvi@gmail.com; anilkumarenvi@jssuni.edu.in; Tel: +91-8277102057
bCenter for Water, Food and Energy, GREENS TRUST, Turuvekere Taluka, Harikaranahalli, Karnataka 572215, India
cDepartment of Pharmaceutics, School of Pharmacy, Anurag University, Hyderabad, 500088, Telangana, India
dDepartment of Chemistry, The Oxford College of Engineering, Bengaluru, 560068, Karnataka, India
ePG Department of Studies and Research in Environmental Science, Kuvempu University, Shimoga, Karnataka, India
fDepartment of Microbiology, JSS Academy of Higher Education and Research, Mysuru, India
gDepartment of Biotechnology and Bioinformatics, JSS Academy of Higher Education and Research, Mysuru, India
hDepartment of Pharmacognosy, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Alkharj, Saudi Arabia
iDepartment of Pharmacognosy, College of Pharmacy, King Khalid University, Abha, 62529, Saudi Arabia
jInstitute of Catalysis and Energy Solutions (ICES), College of Science Engineering and Technology, University of South Africa, South Africa
kDepartment of Chemistry, Sri Jayachamarajendra College of Engineering, JSS Science and Technology University, Mysuru, 570 006, India
lInternational Center of Environment and Sustainability (ICESu), JSS Academy of Higher Education and Research, Mysuru, 570015, India
First published on 26th November 2025
Abstract
Photoelectrochemical (PEC) water splitting offers a sustainable pathway for hydrogen production; however, its practical implementation is often limited by the poor efficiency and stability of photoelectrodes. In this work, porous cerium nickel oxide (CeNiO3) was synthesized via a simple citrate sol–gel method coupled with a hydrothermal approach and employed as a photoanode for PEC water splitting. The structural, morphological, and optical characteristics of the material were comprehensively investigated using XRD, UV-vis spectroscopy, FESEM, EDX, XPS, PL, and FTIR analyses. The optimized CeNiO3 photoelectrode demonstrated an excellent photocurrent density of 15.14 mA cm−2 at 1.4 V vs. RHE. Electrochemical impedance spectroscopy (EIS) revealed enhanced charge transfer kinetics and suppressed recombination of photoexcited charge carriers. The superior PEC activity of CeNiO3 is attributed to its bimetallic interactions, strong solar light absorption, efficient charge separation, and rapid charge transport. These results highlight the potential of CeNiO3 as a stable and efficient photoelectrode for solar-driven hydrogen generation.
1. Introduction
The environmental concerns and interplay of energy have emerged as paramount challenges hindering the sustainable advancement of the global ecosystem. It is imperative to devise an energy reservoir that is storable, environmentally sustainable, cost-effective, and renewable to address the global energy requisites comprehensively.1 Hydrogen (H2) energy emerges as an exemplary primary energy vector for establishing a sustainable future, owing to its renewable nature, storage capability, net-zero emission characteristics, and notably high energy density of 143 KJ g−1, surpassing that of gasoline.2,3 To date, hydrogen production methods such as steam reforming, methane pyrolysis, ammonia decomposition, and solar thermochemical processes have been explored.4,5 Despite their potential, these approaches are limited by rigorous reaction conditions, high energy consumption, and challenges in separating byproducts, especially in terms of H2 storage and transportation.6 Therefore, extensive investigations have been directed towards the development of alternative hydrogen production methods that meet the increasing global hydrogen demand in an environmentally friendly and cost-effective manner. In this scenario, photo-electrochemical (PEC) water splitting, utilizing semiconductor materials, remains a subject of global interest due to its ability to directly decompose water into H2 and O2 through sunlight absorption. This approach is highly efficient and eco-friendly and holds promise for progress towards a zero-carbon future.7,8 The PEC water splitting offers a unique advantage over alternative methods due to its capability to produce H2 and O2 at distinct electrodes, mitigating issues related to gas mixing and undesirable back reactions. Moreover, the PEC system exhibits lower overpotentials when juxtaposed with electrochemical water splitting. The external voltage allows the mechanism to accelerate the reaction at the required rate by promoting charge separation and migration, resulting in high efficiency.9 In this context, most research efforts have centred on developing suitable semiconductor materials capable of serving as effective photoelectrodes for green hydrogen fuel generation. Efficient PEC water splitting requires that semiconductors have a minimal band gap of 1.23 eV to maximize light absorption, band edge alignment with water redox potentials, sustained stability, and support for effective charge carrier diffusion10.11 Additionally, semiconductor-based photoanodes or photocathodes enable lower operating potentials, facilitating high energy conversion rates in the PEC water splitting, even at low temperatures.To date, a multitude of photoelectrodes have been explored and optimized for utilization in PEC water splitting, encompassing materials such as TiO2, Fe2O3, ZnO, g-C3N4, and WO3.12,13 However, limitations such as rapid recombination, susceptibility to photo-corrosion, wide band gaps, low charge carrier mobility, and suboptimal band edge alignment restrict the applicability of these semiconductor materials as photoelectrodes.14,15 Due to their robustness, versatile composition and structure, strong visible light absorption, and adjustable band properties, perovskite oxides (ABO3) present an attractive choice for PEC water splitting photoelectrodes.16 Among the various perovskite oxides, CeNiO3 has emerged as a leading choice in photo-electro-catalysis, characterized by a narrow band gap of 1.51 eV. Its high electrocatalytic efficiency, earth-abundant nature, robust redox cycles, exceptional optical features, environmental sustainability, and the combined effects of photocatalytic and magnetic properties make it highly favourable for hydrogen evolution.17 Owing to the profusion of oxygen vacancies, CeO2 metal oxide displays n-type semiconductor properties, with impressive redox reaction capabilities caused by its spatial processes and oxygen-releasing properties.18–20 Furthermore, the incorporation of ceria (Ce) and nickel (Ni) into the A and B sites of the perovskite framework enhances its electronic and optical characteristics and improves capacitance values while maintaining robust cycling stability. This enhancement is attributed to structural alterations within the ceria crystalline matrix, primarily involving partial replacement of Ce4+ ions with Ni2+. The substitution of Ni2+ for Ce4+ in the CeNiO3 lattice induces structural modifications, which can influence defect formation, the electronic structure, and charge carrier dynamics. Specifically, the incorporation of Ni2+ can introduce oxygen vacancies and modify the local coordination environment, enhancing charge separation and transport.21,22
Research has consistently revealed that achieving a large surface area and controlling the morphology in catalysts are additional influential factors that highly influence the charge transfer mechanism and light absorption dynamics, contributing to the efficient production of hydrogen and fabrication of highly efficient photoelectrodes, achievable through suitable synthesis methodologies.23 Various methodologies have been documented for the development of perovskite materials, encompassing techniques such as electrospinning, solvothermal synthesis, co-precipitation, hydrothermal, and sol–gel methods.24,25 Among these techniques, the sol–gel approach is the most beneficial for the manufacture of CeNiO3 since it is environmentally benign and inexpensive.26 Ming Yang et al. developed a TiO2@SrTiO3@BiVO4 photoanode for hydrogen evolution, achieving an impressive photocurrent density of 3.7 mA cm−2 at 1.23 V vs. RHE under AM 1.5 G illumination, along with a peak incident photon-to-current conversion efficiency (IPCE) of 65%.27 Jin Kim and co-workers investigated PEC water splitting using Fe3O4 nanoparticles exsolved in SrTiO3, achieving a photocurrent density of 5.10 mA cm−2 at 1.23 V vs. RHE, along with excellent durability, maintaining 97% of its initial activity over 24 h.28 In their study, Ramesh Reddy and colleagues employed 3D ZnO nanostructures for PEC water splitting, achieving a photocurrent density of 0.6 mA cm−2 and showing stable performance for up to 5 h under light-on/light-off testing.29 Xie and colleagues developed a CdS nanosphere photoelectrode for PEC water splitting, achieving a photocurrent density of 5.10 mA cm−2 at 1.23 V vs. RHE.30
According to the available literature, there is a significant gap in research on the implementation of a CeNiO3 perovskite material as a photoelectrode in photoelectrochemical water splitting. This work focuses on cost-effective and stable materials, aligning with sustainable hydrogen production goals, and outlines the synthesis of a CeNiO3 perovskite material utilising a simple, eco-friendly, and economical citrate sol–gel approach. These samples are then explored as photoelectrode materials for generating hydrogen via photoelectrochemical water splitting. The manufactured CeNiO3 material was thoroughly characterised using cutting-edge equipment to discover the physicochemical attributes of the resulting material. Additionally, the photo-electro-catalytic performance of the CeNiO3 material has been examined through PEC water-splitting tests. The CeNiO3 material performed well in PEC water splitting, enabling successful generation of sustainable hydrogen fuel.
2. Experiment and methodology
2.1. Materials
Citric acid monohydrate (C6H8O7·H2O), cerium nitrate hexahydrate [Ce (NO3)3·6H2O], nickel nitrate hexahydrate [Ni (NO3)2·6H2O], ammonia water (NH3·H2O) and other solvents were sourced from Sigma-Aldrich Pvt. Ltd and chosen as precursors for the preparation of CeNiO3 catalysts. Deionized water was employed as the dissolution solvent throughout the experimental process and all the reagents used in this study were of high analytical purity.2.2. Synthesis of the CeNiO3 catalyst
The CeNiO3 catalytic material was prepared using the universally accepted citrate sol–gel technique and the synthesis techniques employed are depicted in Fig. 1. A stoichiometric volume of cerium nitrate hexahydrate, citric acid monohydrate and nickel nitrate hexahydrate (Ce![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C6H8O7·H2O
C6H8O7·H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni = 1
Ni = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was dissolved in deionized Millipore water (50 mL) and the obtained mixture solution was continuously stirred for 30–35 minutes. The pH value was adjusted between 5 and 6 approximately by adding an ammonia solution sequentially. The above mixture was kept stirring maintaining at a temperature of 90 °C until a green gel formed. The as-obtained homogenized gel was initially dried at 120 °C for 7–10 hours in order to eliminate any remaining nitrates. The ground powder was subjected to calcination at 750 °C for 5 hours to synthesize the target perovskite phase, CeNiO3.
1) was dissolved in deionized Millipore water (50 mL) and the obtained mixture solution was continuously stirred for 30–35 minutes. The pH value was adjusted between 5 and 6 approximately by adding an ammonia solution sequentially. The above mixture was kept stirring maintaining at a temperature of 90 °C until a green gel formed. The as-obtained homogenized gel was initially dried at 120 °C for 7–10 hours in order to eliminate any remaining nitrates. The ground powder was subjected to calcination at 750 °C for 5 hours to synthesize the target perovskite phase, CeNiO3.
2.3. Fabrication and preparation of the CeNiO3 photoelectrode
A working CeNiO3 photoelectrode was prepared by coating the fabricated photocatalyst as a thin film onto an FTO glass plate. A total of 5 mg of the specimen was thoroughly mixed into 500 mL of Nafion–ethanol during the first 30 minutes of ultrasonication in an effort to create a catalyst-loaded photoelectrode. Thereafter, 20 µL of the suspension was drop cast onto FTO glass measuring 1 × 1 cm2 and dried at 150 °C for 3 hours in an oven and later subjected to photoelectrochemical measurement.2.4. Characterization of CeNiO3
The designed CeNiO3 catalyst underwent a comprehensive array of advanced examinations, delving into its fundamental physicochemical and innate characteristics. Powder X-ray diffraction (XRD) characterization (Shimadzu, Japan) was employed for crystallinity and lattice configuration studies. The photoluminescence (PL) emission was investigated utilizing an FL-1039/40 fluorescence spectrometer (Horiba Jobin Yvon), specifically to examine the charge separation kinetics of the specimen. FTIR was conducted for examination of the vibrational patterns about the stretching and bending modes of the synthesized material (Bruker, ALPHA, 200619, Germany). Field emission scanning electron microscopy (FE-SEM) was carried out using a JEOL-AV JSM-7100F (Singapore) to explore the surface morphology and microstructural features of the CeNiO3 material. Energy-dispersive X-ray (EDX) assessment was carried out to discern the chemical constituents and elemental spatial distribution of the sample employing Super X (JSMIT300, JEOL, Singapore). The elemental composition of the material was elucidated through X-ray photoelectron spectroscopy (XPS) using a PHI 5000 VersaProbe III. The optical attributes of the specimen were assessed utilizing UV-visible absorption spectroscopy using a UV 2600 spectrophotometer (Shimadzu, Japan).2.5. Photo-electro-chemical characterization
Photo-electro-chemical investigations were conducted utilizing a conventional three-electrode cell configuration (CHI760E) with CeNiO3 photocatalysts serving as the working electrode, where an Ag/AgCl electrode was employed as the reference electrode and a platinum (Pt) rod functioned as the counter electrode. An aqueous solution containing 0.5 M Na2SO4 (sodium sulfate) served as the electrolytic medium, maintaining a pH of 5. The photoelectrodes underwent irradiation at an intensity of 100 mW cm−2, employing a 350 W xenon lamp equipped with an AM 1.5G filter. Linear sweep voltammetric (LSV) polarization profiles were acquired within the voltage range of 0 to 1.4 V. The potentials were referenced to the reversible hydrogen electrode (RHE) by applying the equation ERHE = EAg/AgCl + E0Ag/AgCl + 0.059 * pH. Mott–Schottky curves were generated under illumination conditions at a frequency of 1000 Hz. Electrochemical impedance spectroscopy (EIS) was explored across a frequency range of 1 to 100 kHz. The electrodes underwent durability examination via chronoamperometric (CA) studies.3. Results and discussion
3.1. XRD analysis
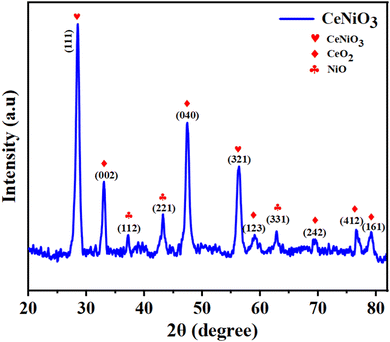
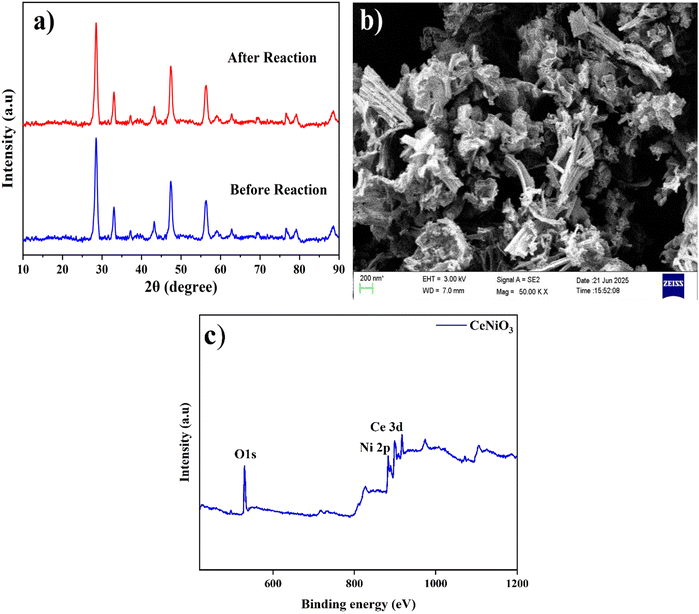
XRD assessment was conducted on perovskite CeNiO3 particles to discover their crystalline structure and phase purity. The outcomes are depicted in Fig. 2. XRD analysis of the pristine CeNiO3 catalyst has elucidated the presence of an orthorhombic phase. The XRD pattern exhibited distinct d peaks at 2θ angles of 28.45°, 33.08°, 37.16°, 43.16°, 47.48°, 56.34°, 59.12°, 62.92°, 69.51°, 76.75°, and 79.20°, which are attributed to the lattice planes of (111), (002), (112), (221), (040), (321), (123), (331), (242), (412), and (161), respectively, indicating the crystallinity of the CeNiO3 sample. These outcomes match well with the reference data (ID number: mp-776207) and previously stated research work.21 The peaks perceived at 33.08°, 47.48°, 59.12°, 69.51°, 76.75°, and 79.20° correlate to the (002), (040), (123), (242), (412) and (161) crystallographic planes of cerium dioxide (CeO2), as indicated by JCPDS No: 81-0792. The XRD pattern of cubic NiO exhibits strong signals at 2θ angles of 37.16°, 43.16°, and 62.92° analogous to the crystallographic planes (112), (221), and (331) respectively, as designated by JCPDS No: 75-0197.31 The XRD pattern, showing no additional diffraction peaks, supports the excellent crystallinity and phase homogeneity of the synthesized material.3.2. FE-SEM analysis
The surface morphologies of as-prepared CeNiO3 perovskite materials are scrutinized through the utilization of FE-SEM. Fig. 3 shows a surface morphology micrograph depicting the synthesized CeNiO3 material. These FESEM photographs indicate the formation of the CeNiO3 material with rough surface morphology. FESEM pictures of CeNiO3 unveiled a myriad of irregularly shaped pores exhibiting varying diameters and the material showcased a notably elevated surface area, highlighting its potential for enhanced catalytic activity. The porous architecture inherent in the CeNiO3 material is widely recognized for its capacity to improve the photo adsorption performance, specifically in the segregation of electron–hole pairs.32,33 | ||
| Fig. 3 (a)–(d) FE-SEM micrographs, (e) DLS results of the CeNiO3 perovskite photocatalyst and (f) and (g) TEM images. | ||
3.3 TEM analysis
The rod-like shape of the synthesised CeNiO3 nanostructures with uniform dimensions and smooth surfaces is clearly visible in the TEM image (Fig. 3(f)), indicating that a well-defined crystalline phase was successfully formed. Effective oversight throughout the synthesis process is suggested by the nanorods’ apparent well-dispersed state and lack of noticeable aggregation. The high crystallinity and purity of the prepared material are confirmed by the uniform contrast and distinct edges that were observed.Additionally, the lattice structure and elemental dispersion within the CeNiO3 matrix can be obtained from the image (Fig. 3(g)). The successful integration of Ni into the Ce lattice structure is confirmed by the distinct lattice fringes that match the interplanar spacings of the Ce and NiO3 phases. Good crystallinity and close contact between the Ce and NiO3 domains are indicated by the presence of clearly resolved fringes, which can promote effective charge transfer across the interface. These structural characteristics are anticipated to foster synergistic interactions between Ce and Ni species, improving the CeNiO3 nanocomposite's catalytic and electrochemical performance.34
3.4. EDX analysis
EDX analysis was conducted to scrutinize the degree of purity, elemental composition and distribution within the 100–300 nm range of the fabricated CeNiO3 photocatalyst. Fig. 4(a) depicts the obtained EDX outcomes. The EDX assessment verifies the existence of ceria (Ce), nickel (Ni), and oxygen (O) within the sample, providing substantial evidence for the successful formation of the CeNiO3 photocatalyst. The exceptional purity of the CeNiO3 structures synthesized via sol–gel methodologies was substantiated by the absence of discernible intense peaks corresponding to any additional constituents or impurities within the materials. DLS results of the CeNiO3 perovskite photocatalyst indicated that the particle size distribution varied in the 100–220 nm range (Fig. 3(e)). Additionally, the elemental mapping evaluation depicted in Fig. 4(b)–(e) revealed a spectrum comprising three different components, delineating the existence of Ce (red), Ni (blue), and O (yellow).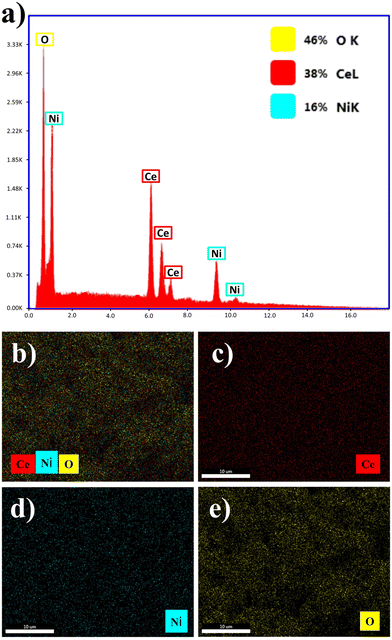 | ||
| Fig. 4 (a). EDX profiles of the CeNiO3 specimen and (b)–(e) elemental mapping analysis of the CeNiO3 perovskite photocatalyst. | ||
3.5. XPS analysis
XPS has been applied to evaluate the chemical states and elemental constituents of the CeNiO3 perovskite photocatalyst. Fig. 5(a) confirms the homogeneity of the CeNiO3 material, as evidenced by the observation of Ce 3d, Ni 2p, and O 1s signals, with no additional elemental constituents detected during the FE-SEM and EDX analyses. The determination of the binding energy of oxygen atoms provides insights into the oxygen's chemical state and enables the identification of oxygen vacancies that are induced within the material's crystalline lattice. The metal cations located at either the A-site or the B-site in the perovskite structure demonstrate ratios of An+1/An and Bn+1/Bn, respectively, showcasing their catalytic redox potential.35 The Ce 3d spectrum prominently manifests distinct peaks corresponding to Ce3+ 3d3/2 and Ce4+ 3d5/2 electron orbitals, along with the observable satellite signals (Fig. 5(b)). Binding energies for Ce3+ 3d3/2 are well recorded at 916.98 eV, 905.39 eV, and 900.15 eV. Ce4+ 3d5/2 demonstrates distinctive binding energies at 897.53 eV, 888.48 eV, and 882.77 eV, respectively.36Fig. 5(c) illustrates the Ni 2p XPS patterns, revealing spin–orbit doublets of Ni 2p3/2 and Ni 2p1/2 at 855.19 eV and 872.98 eV respectively. Additionally, there are two observable satellite spikes positioned at 861.12 eV and 883.53 eV. The dual peaks observed in the Ni 2p spectrum, positioned at 855.19 eV and 861.12 eV, are attributed to the Ni3+ oxidation state. Concurrently, the spikes positioned at 883.53 eV and 872.98 eV are assigned to the Ni2+ states.37,38 The binding energies observed in the O 1s XPS spectra, specifically the oxygen vacancies (Ov) at 531.33 eV and lattice oxygen (OL) at 528.91 eV, as demonstrated in Fig. 5(d), serve as conclusive evidence of the existence of O2− species within the crystalline framework of the CeNiO3 material.39 Higher levels of oxygen vacancies facilitate rapid ionic diffusion during the charge transfer mechanism. The XPS outcomes illustrate that the CeNiO3 material exhibits advantageous characteristics associated with two redox pairs (Ce3+/Ce4+ and Ni2+/Ni3+), along with the presence of oxygen vacancies within its structural framework. These attributes contribute to an enhanced capacity for charge storage at a rapid rate.3.6. UV-vis spectra
Band-gap energy and the light-scattering characteristics of the manufactured specimen were evaluated using UV-visible spectroscopy. Fig. 6(a) and (b) illustrate the UV-visible spectroscopic data, as well as the corresponding Tauc plot for the given experimental conditions. The CeNiO3 sample shows a red shift in its absorption onset, broadening into the visible light region, which enhances visible light absorption.40 The band-gap energy (Eg) of the fabricated CeNiO3 specimen was determined employing Tauc's equation (eqn (1)), and the outcomes are presented in Fig. 6(b).| (αhν)2 = A(hν − Eg) | (1) |
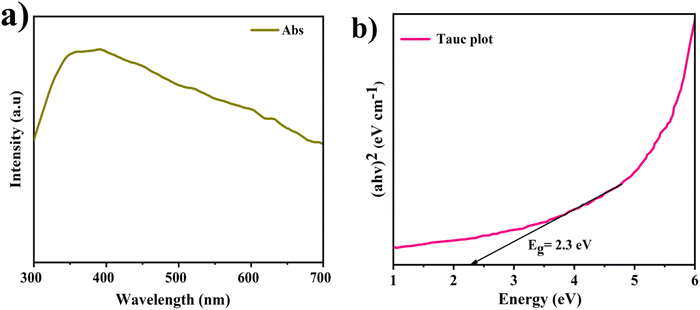 | ||
| Fig. 6 (a) UV-visible absorbance spectra of the CeNiO3 perovskite photocatalyst. (b) Calculated Tauc plot graph of the CeNiO3 perovskite photocatalyst. | ||
The CeNiO3 catalyst exhibited Eg = 2.3 eV, which implies that the material possesses advantageous optical characteristics, rendering it well-suited for engaging in photo-electro-catalytic applications using visible light.
Theoretical computation of the semiconductor's VB and conduction band CB potentials can be achieved by applying the Mulliken electronegativity and the semiconductor's bandgap through the subsequent formula.41
| EVB = X − Ee + 0.5Eg | (2) |
| ECB = EVB − Eg | (3) |
| The first ionization energy of the cerium element (Ce): I1 = 534.4 kJ mol−1 |
| The first electron affinity of the cerium element (Ce): E1 = 50 kJ mol−1 |
The absolute electronegativity of the cerium element (Ce):
Converting into eV,
For nickel (Ni): I1 = 737.1 kJ mol−1 and E1 = 111.65 kJ mol−1; therfore,
Converting into eV,
For oxygen (O): I1 = 1313.9 kJ mol−1 and E1 = 140.97 kJ mol−1; therfore,
Converting into eV,
The geometric mean of the absolute electronegativity for CeNiO3 is calculated as follows:
The band gap of CeNiO3 was obtained by the UV-vis measurements and determined to be 2.3 eV (Fig. 6b).
Therefore, the valence band of CeNiO3 is calculated as follows:
The conduction band of CeNiO3 is calculated as follows:
The computed EVB value for CeNiO3 is 2.28 eV, while its corresponding ECB value is −0.02 eV.
3.7. FTIR spectra
The functional groups and discernible chemical bonds of the synthesised CeNiO3 catalyst specimens were assessed using FTIR, and the findings are illustrated in Fig. 7(a). The fabricated specimen displayed stretching vibrations originating from crystallized water (–OH) molecules, potentially contributing to the broad transmission detected at 3440.01 cm−1 and 1773.46 cm−1. The Ni2+/Ni3+ cations, resembling the asymmetric sharp bands, were detected in the CeNiO3 sample within the spectral range of 1374.37 cm−1 to 1633.85 cm−1. The transmittance bands detected at 707.12 cm−1 are credited to the stretching of Ni–O bonds. Two discrete peaks identified at 1036.01 and 505.20 cm−1 are correlated with Ce–O stretching vibrations.21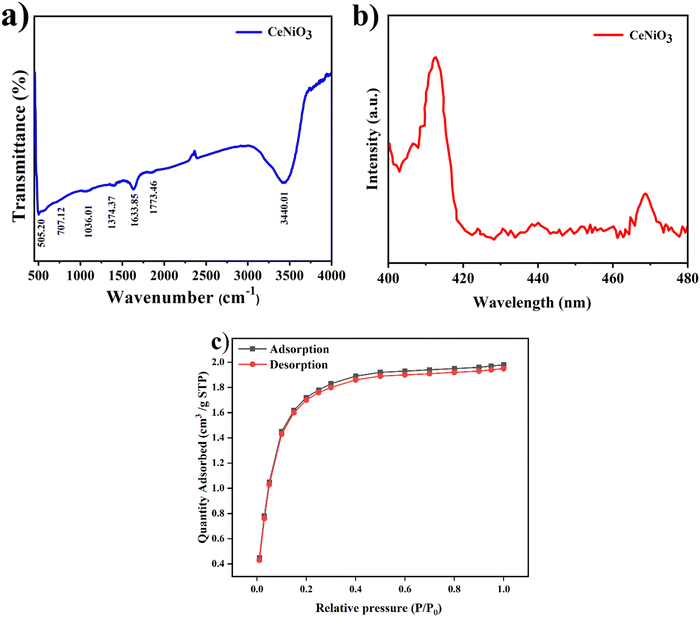 | ||
| Fig. 7 (a) FTIR spectra, (b) PL spectra of the CeNiO3 perovskite photocatalyst and (c) BET isotherm. | ||
3.8. PL analysis
To comprehend the segregation of the charges generated by light exposure during the PEC process, photoluminescence studies were performed at an excitation wavelength of 365 nm at ambient temperature. Fig. 7(b) displays the emission spectra of the CeNiO3 specimen. Generally, a decrease in PL emission intensity indicates high charge carrier separation efficacy, ultimately optimizing the photo-electro-catalytic performance of photocatalysts. The PL spectra of the CeNiO3 material disclosed dual peaks, with a lower ultraviolet-excitonic band at 412 nm and a higher green emission band at 468 nm. The UV-excitonic region is designated for charge carrier recombination, while the green emission region is attributed to the occurrence of oxygen vacancies.43 The PL spectra of CeNiO3 exhibited a considerable decline in intensity, suggesting enhanced effectiveness in transferring and separating electron and hole pairs.443.9. BET isotherm
According to IUPAC classification, the nitrogen adsorption–desorption isotherm of CeNiO3 (Fig. 7(c)) displays a Type I(a) profile, signifying a microporous structure. Adsorption inside micropores is confirmed by a plateau after a sharp uptake at a low relative pressure (P/P0 < 0.1). With a pore volume of 0.00414 cm3 g−1 and an average pore diameter of 0.9 nm, the BET surface area was found to be 18.4 m2 g−1. These values correspond to the development of a dense perovskite framework and tiny micropores. The compact configuration of metal–oxygen octahedra results in the low surface area and pore volume that are characteristic of perovskite oxides. Strong reactant interactions and effective charge transfer in catalytic or electrochemical processes can be facilitated by the confined micropores, even due to their limited porosity.3.10. Photo-electro-chemical measurements
To interrogate the photo-electro-chemical performance of the CeNiO3 perovskite photocatalyst, a three-electrode configuration was employed within a 0.5 M Na2SO4 electrolytic medium. Linear sweep voltammogram patterns of the CeNiO3 photoelectrode are illustrated in Fig. 8(a). Under dark conditions, the current density remains minimal. However, upon exposure to solar radiation, a significant augmentation in current density is observed. The elevated photocurrent density signifies the effective sensitivity of the photoelectrode materials to the solar spectrum. This pronounced generation of photocurrent signifies that the semiconductor material possesses a high degree of photoactivity.4 The resulting photocurrent densities recorded for CeNiO3 were established to be 15.14 mA cm−2 and 5.1 mA cm−2 with the influence of light and in dark mode at the specific potential of 1.4 V vs. the RHE. The exceptional functionality of CeNiO3 stems from its uniform topological characteristics, porous nature, high surface area, and electronic structure, substantially enhancing the higher light absorption with efficient charge transformation within the semiconductor material.45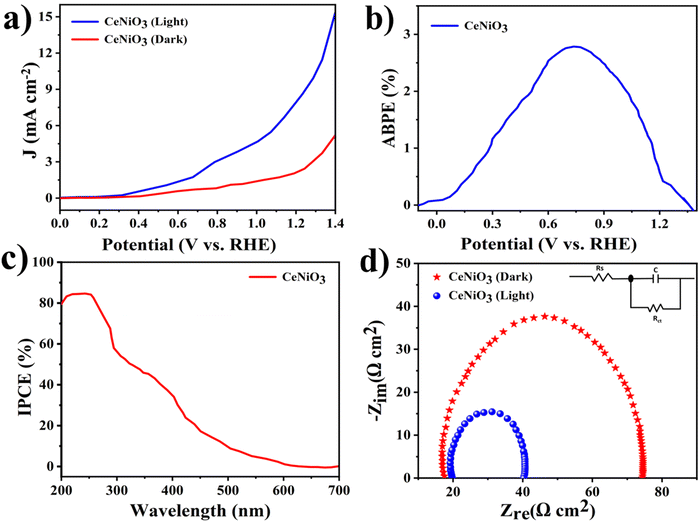 | ||
| Fig. 8 (a) LSV curves, (b) (ABPE%) plot, (c) (IPCE%) graph, and (d) EIS Nyquist plot of the CeNiO3 electrode (the inset shows an equivalent circuit model). | ||
Furthermore, achieving elevated photocurrent density under minimal applied bias poses a pivotal obstacle in improving the efficacy of water-splitting approaches. Solar-to-hydrogen (STH) conversion efficiencies (η) of the photoelectrode under the influence of an external bias potential against the RHE have been assessed using the following equation:
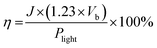 | (4) |
In this equation, J stands for the photocurrent density, and 1.23 V corresponds to the equilibrium potential that is required for splitting water into O2 and H2. Vb is the applied bias with respect to Plight, which is the power density of incident light, and RHE. Fig. 8(b) presents a plot of photoconversion efficiency (η%). The highest efficiency achieved for CeNiO3 was 2.76%, reflecting its impressive ability to convert incident light into hydrogen fuel.
Additionally, the assessment of incident photon to current conversion efficiency (IPCE) is undertaken to precisely estimate the photoelectric conversion efficacy of the photoelectrode across a spectrum of incident light wavelengths, utilizing the subsequent formula.
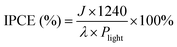 | (5) |
In this study, the photocurrent density is expressed by the variable J, the value of 1240 is a product of the speed of light, Plight depicts the luminous power density, and λ indicates the incident wavelength. As presented in Fig. 8(c), a high IPCE of 80.10% is demonstrated by the CeNiO3 photoelectrode over the 300–700 nm light spectrum. CeNiO3 demonstrates heightened photoresponsivity, potentially attributed to the provision of additional reactive sites and efficient pathways for charge carrier transmission, facilitating light capture and electron excitation.46
EIS is a method for elucidating the dynamics of charge carrier migration and assessing interfacial charge transfer resistance (Rct). Fig. 8(d) showcases the intriguing findings from the EIS analysis conducted on the CeNiO3 photoelectrode under both dark and illuminated conditions. The simulated circuit model representing the charge transfer mechanisms within photocatalysts is depicted in the inset of Fig. 8(d). The radius of the Nyquist arc collected in EIS is intricately linked to the kinetics of the photo-electro-catalytic reaction occurring on the surface of the photoelectrode. It quantifies the impedance to charge transfer at the photoelectrode–electrolyte interface. A smaller arc diameter signals higher electron transportation, which supports better segregation of light-induced charge carriers, thereby enhancing the proficiency of PEC water splitting.47 The Rct value for the CeNiO3 photoelectrode is listed in Table 1. EIS spectra (Fig. 8(d)) of CeNiO3 unveil that, in comparison with the dark mode, a smaller semicircle is noticed under irradiation, signifying faster charge carrier mobility due to its highly porous nature and dual-metal synergy observed in the CeNiO3 photoelectrode in the Na2SO4 electrolyte. Notably, there is remarkable inhibition of the charge carrier recombination.48,49
| Sample | Condition | (Rct) (Ω cm2) |
|---|---|---|
| CeNiO3 photoelectrode | Dark | 56.94 |
| CeNiO3 photoelectrode | Light | 20.13 |
To provide insight into the catalytic process, comprehensive evaluation of carrier kinetics is systematically performed. The charge separation efficiency (ηseparation) and charge injection efficiency (ηinjection) of the CeNiO3 photoelectrode were assessed employing 0.5 M Na2SO3 as a hole scavenger with Na2SO4 electrolyte, as depicted in Fig. 9(a)–(c), applying the following equation:
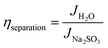 | (6) |
 | (7) |
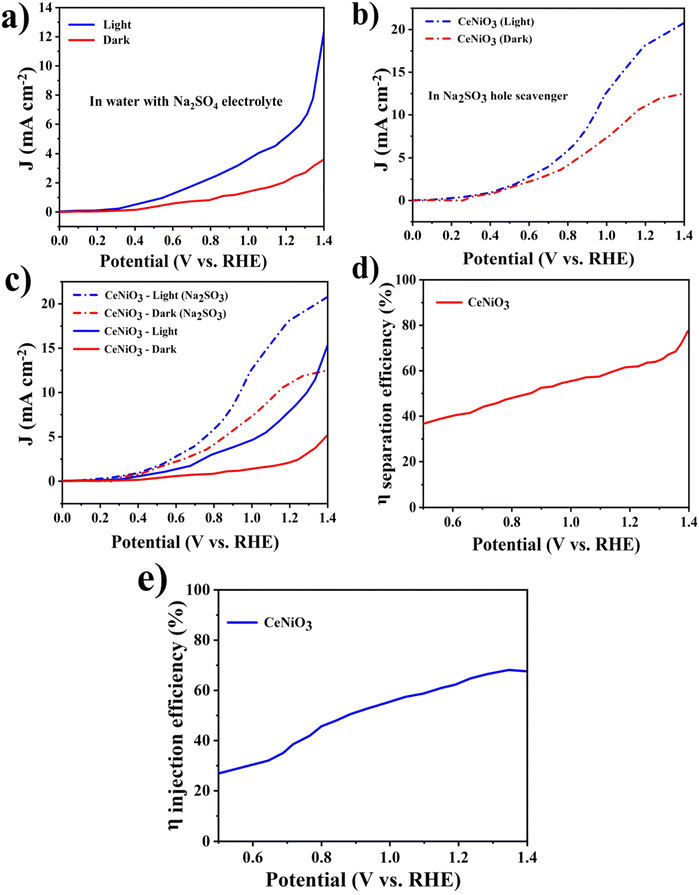
Mott–Schottky (M–S) analysis was employed to derive the flat band potential (Vfb) of the CeNiO3 material, aiding in the elucidation of its charge transfer dynamics. The following Mott–Schottky equation is exploited to derive the flat-band potential (Vfb).
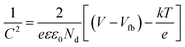 | (8) |
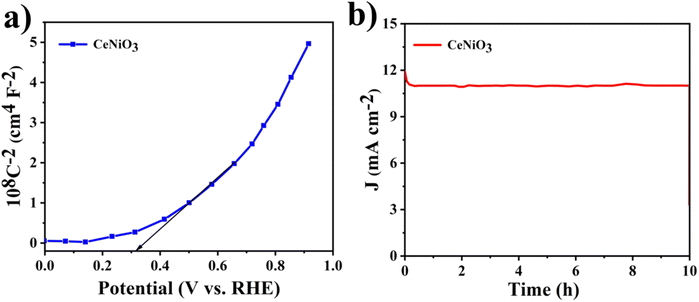
In order to ensure scalable and reliable hydrogen production, the enduring stability of the photoanode is crucial. Long-term stability of the CeNiO3 photoelectrode's photocurrent was investigated through chronoamperometric analysis. During these experiments, the photoanodes underwent continuous exposure to irradiation as presented in Fig. 10(b). The CeNiO3 photoelectrodes demonstrated sustained and durable J (mA cm−2) of 11.17 mA cm−2 at 0.8 V vs. RHE, with minimal decline in density of current observed over an extended period. Even after 10 hours of testing, the photocurrent density remains stable, demonstrating the photoelectrode's remarkable resilience against photo-induced corrosion.53 These findings underscore the photocatalyst's durability under light exposure, validating its potential for efficient use in a photoelectrochemical water-splitting system. The PEC water-splitting performance of the CeNiO3 photoelectrode was compared with that of previously reported materials, as outlined in Table 2.
The stability of the catalyst during the photoreaction was further evaluated by analyzing the XRD pattern of the used CeNiO3 sample (Fig. 11(a)). No significant changes were observed in the diffraction peaks, confirming that the crystal structure of CeNiO3 remained intact after the photoelectrocatalytic reaction. This indicates the excellent structural stability and reusability of the synthesized catalyst for practical hydrogen generation and degradation applications. The post-reaction SEM image of the CeNiO3 sample is displayed in Fig. 11(b). After the hydrogen evolution process, the morphology almost remains the same, suggesting that the catalyst's structural integrity is well maintained. The absence of any apparent particle fusion or agglomeration indicates that the CeNiO3 surface has maintained its dispersed state. Under reaction conditions, the irregularly aggregated and flake-like particles remain distinct and exhibit good morphological stability and resistance to irradiation. The XPS spectra (Fig. 11(c)) show no noticeable shift or change in peak intensities or binding energy positions upon post-analysis, suggesting that the oxidation states of Ce, Ni, and O do not significantly alter following reaction. This confirms the structural integrity and chemical stability of CeNiO3 by indicating that its surface composition and chemical environment are stable and that there has not been a noticeable shift in the electronic structure.
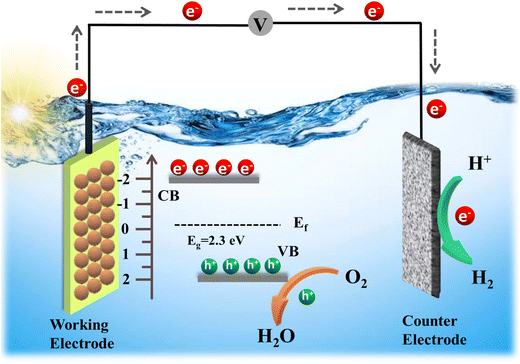
3.11. Detailed reaction mechanism of water splitting in PEC systems
The mechanism by which CeNiO3 enables water splitting encompasses a series of steps that leverage sunlight to yield H2 and O2. As illustrated in Fig. 12, this process begins with CeNiO3 absorbing photons, leading to electron–hole pair creation. These charge carriers engage in redox reactions with water adsorbed on the CeNiO3 surface, where holes oxidize water to release O2, and electrons reduce protons to form H2. CeNiO3's structure aids in efficient charge separation, positioning it as a key material in solar-driven hydrogen production. The reactions at both electrodes are summarized here.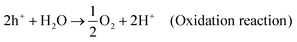 | (9) |
| 2e− + 2H+ → H2 (Reduction reaction) | (10) |
| 2H2O → 2H2 + O2 (Overall water splitting) | (11) |
4. Conclusion
To summarize, this work presents the fabrication of CeNiO3 perovskite structures using a citrate sol–gel technique, specifically designed for an enhanced hydrogen evolution reaction under a visible light driven PEC based water splitting process. Comprehensive analyses were performed on the material to investigate its crystalline arrangement, morphological features, elemental makeup, and optical properties. These assessments conclusively validated the fabrication of the CeNiO3 perovskite with the targeted structural and chemical attributes. XRD scans confirmed the orthorhombic phase in CeNiO3 and FE-SEM photographs of the specimen uncovered an uneven, porous structure. The CeNiO3 photocatalyst displayed a narrow band-gap of 2.3 eV, emerging as an ideal candidate for harnessing the power of visible light irradiation. Moreover, the VB and CB potentials of the CeNiO3 material are calculated to be 2.28 and −0.02 eV, respectively. CeNiO3 has been deployed as a photoelectrode for PEC measurements under illumination. The cerium nickel oxide photoelectrode exhibits outstanding PEC activity, with a current density of 15.14 mA cm−2 recorded at 1.4 V and an ABPE% of 2.76%. Remarkably, the CeNiO3 electrode demonstrates exceptional photoelectrochemical stability over a prolonged duration, with negligible photocurrent density deterioration. The aforementioned results illustrate the immense potential of CeNiO3 as a stable and efficient photoelectrode material for hydrogen generation. Future objectives may entail further optimisation of synthetic parameters, exploration of CeNiO3-based tandem PEC systems, and implementation into practical devices for real-world uses, thus promoting the shift towards a carbon-neutral energy economy.Author contributions
Anusha Hosakote Shankara: examination; data organization, systematic/theoretical analysis, conception, and writing – initial draft. Vinod Divya: visualization and review & editing. V. Rajeshwar, Usha Jinendra, Jagadeep Chandra S, Elayaperumal Sumitha, Basavarajappa Sannappa Hanumanthappa, Kotermane Mallikarjunappa Anilkumar, Peter R. Makgwane, and Honnegowdanahalli Shivabasappa Nagendra Prasad: review, validation, and proofreading. Mohammad Khalid and Shadma Wahab: resource and visualization. Harikaranahalli Puttaiah Shivaraju: conceptualization, methodology, supervision, writing, and proofreading – review & editing.Conflicts of interest
The authors declare that they have no conflicts of interest.Data availability
The data supporting the findings of this study are available within the article and its supplementary information (SI). Supplementary information is available. See DOI: https://doi.org/10.1039/d5ma00914f.Additional data that support the study's conclusions are available from the corresponding author upon reasonable request.
Acknowledgements
The authors would like to thank the JSS Academy of Higher Education and Research, Mysuru, India for the research facility that provided access to electronic resources, CNMS-Jain University, Bengaluru, for providing the XRD facility and electrochemical work station, and the Indian Institute of Science and Technology Roorkee, for XPS analysis. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through a large group Research Project under grant number RGP.2/274/45.References
- M. Joseph, M. Kumar, S. Haridas, C. Subrahmanyam and S. N. Remello, A review on the advancements of graphitic carbon nitride-based photoelectrodes for photoelectrochemical water splitting, Energy Adv., 2024, 3, 30–59 RSC.
- J. Zhang, H. Ma and Z. Liu, Highly efficient photocatalyst based on all oxides WO3/Cu2O heterojunction for photoelectrochemical water splitting, Appl. Catal., B, 2017, 201, 84–91 CrossRef CAS.
- J. Lin, X. Han, S. Liu, Y. Lv, X. Li and Y. Zhao, et al., Nitrogen-doped cobalt-iron oxide cocatalyst boosting photoelectrochemical water splitting of BiVO4 photoanodes, Appl. Catal., B, 2023, 320, 121947 CrossRef CAS.
- A. K. Vishwakarma, M. Hussain, S. K. Verma, V. Shukla, M. A. Shaz and O. N. Srivastava, Synthesis and characterizations of graphene/Sm doped BiFeO3 composites photoanode for efficient photo-electrochemical water splitting, Int. J. Hydrogen Energy, 2021, 46(29), 15550–15560 CrossRef CAS.
- C. Li, Y. Wang, S. Chen, W. Zhang, Z. Wang and Z. Hou, Enhanced photoelectrochemical performance based on conformal and uniform ZnO/ZnSe/CdSe heterostructures on Zn foil substrate, Int. J. Hydrogen Energy, 2020, 45(15), 8257–8272 CrossRef CAS.
- W. Wang, M. Xu, X. Xu, W. Zhou and Z. Shao, Perovskite oxide based electrodes for high-performance photoelectrochemical water splitting, Angew. Chem., Int. Ed., 2020, 59(1), 136–152 CrossRef CAS.
- R. Yukesh Kannah, S. Kavitha, Preethi, O. Parthiba Karthikeyan, G. Kumar and N. Vo Dai-Viet, et al., Techno-economic assessment of various hydrogen production methods – A review, Bioresour. Technol., 2021, 319, 124175 CrossRef CAS PubMed.
- J. W. Yoon, J. H. Kim, H. W. Jang and J. H. Lee, et al., NH2-MIL-125 (Ti)/TiO2 nanorod heterojunction photoanodes for efficient photoelectrochemical water splitting, Appl. Catal., B, 2019, 244, 511–518 CrossRef CAS.
- C. Ding, J. Shi, Z. Wang and C. Li, Photoelectrocatalytic water splitting: significance of cocatalysts, electrolyte, and interfaces, ACS Catal., 2017, 7(1), 675–688 CrossRef CAS.
- R. Li, Latest progress in hydrogen production from solar water splitting via photocatalysis, photoelectrochemical, and photovoltaic-photoelectrochemical solutions, Chin. J. Catal., 2017, 38(1), 5–12 CrossRef CAS.
- W. H. Leng, P. R. F. Barnes, M. Juozapavicius, B. C. O’Regan and J. R. Durrant, Electron diffusion length in mesoporous nanocrystalline TiO2 photoelectrodes during water oxidation, J. Phys. Chem. Lett., 2010, 1(6), 967–972 CrossRef CAS.
- Z. Liu and X. Wang, Efficient photoelectrochemical water splitting of CaBi6O10 decorated with Cu2O and NiOOH for improved photogenerated carriers, Int. J. Hydrogen Energy, 2018, 43(29), 13276–13283 CrossRef CAS.
- Z. Xie, D. Chen, J. Zhai, Y. Huang and H. Ji, Charge separation via synergy of homojunction and electrocatalyst in BiVO4 for photoelectrochemical water splitting, Appl. Catal., B, 2023, 334, 122865 CrossRef CAS.
- J. Joy, J. Mathew and S. C. George, Nanomaterials for photoelectrochemical water splitting–review, Int. J. Hydrogen Energy, 2018, 43(10), 4804–4817 CrossRef CAS.
- D. Ayodhya, Semiconductors-based Z-scheme materials for photoelectrochemical water splitting: A review, Electrochim. Acta, 2023, 448, 142118 CrossRef CAS.
- D. Yu, Z. Liu, J. Zhang, S. Li, Z. Zhao and L. Zhu, et al., Enhanced catalytic performance by multi-field coupling in KNbO3 nanostructures: Piezo-photocatalytic and ferro-photoelectrochemical effects, Nano Energy, 2019, 58, 695–705 CrossRef CAS.
- H. S. Anusha, S. Yadav, T. Tenzin, J. S. Prabagar, K. M. Anilkumar and W. Kitirote, et al., Improved CeMnO3 perovskite framework for visible-light-aided degradation of tetracycline hydrochloride antibiotic residue and methylene blue dye, Int. J. Environ. Sci. Technol., 2023, 20(12), 13519–13534 CrossRef CAS.
- N. Eswaramoorthy, L. Harikrishnan, Y. Selvaraj, A. P. Shyma, K. Lakshmanan and P. Rajesh, et al., Eco-friendly carbon quantum dots from coffee waste: Improving charge transfer in solid-state perovskite solar cells, Diamond Relat. Mater., 2025, 156, 112386 CrossRef CAS.
- S. Kuppusamy, D. Selvakumaran, P. Rajaraman, K. Lakshmanan and M. K. Bin Ahmad, Development of surface-activated La0.6Ca0.4MnO3 perovskite-type electrodes using oxygen plasma for highly stable supercapacitor application, Ceram. Int., 2024, 50(24), 52695–52706 CrossRef CAS.
- K. Lakshmanan, P. Govindasamy, P. Govindasami, S. Marappan and L. Jintae, Facile synthesis of perovskite-type CeCuO3 nanoparticles as a robust bifunctional electrocatalyst for highly stable overall water-splitting, J. Alloys Compd., 2025, 1014, 178635 CrossRef CAS.
- M. C. Maridevaru, B. Aljafari, S. Anandan and M. Ashokkumar, Synergistic impacts of sonolysis aided photocatalytic degradation of water pollutant over perovskite-type CeNiO 3 nanospheres, New J. Chem., 2022, 46(21), 10117–10127 RSC.
- M. P. Harikrishnan, P. Naveena, N. Baskaran and A. C. Bose, Fabrication of cerium nickel oxide (CeNiO3) nanoparticle on vanadium tetra sulphide (VS4) nanosheet composite materials as an enhanced electrode for supercapacitor applications, Electrochim. Acta, 2023, 462, 142729 CrossRef CAS.
- W. Yang, R. R. Prabhakar, J. Tan, S. D. Tilley and J. Moon, Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting, Chem. Soc. Rev., 2019, 48(19), 4979–5015 RSC.
- Y. Pihosh, I. Turkevych, K. Mawatari, T. Asai, T. Hisatomi and J. Uemura, et al., Nanostructured WO3/BiVO4 photoanodes for efficient photoelectrochemical water splitting, Small, 2014, 10(18), 3692–3699 CrossRef CAS PubMed.
- C. X. M. Ta, C. Akamoto, Y. Furusho and F. Amano, A macroporous-structured WO3/Mo-doped BiVO4 photoanode for vapor-fed water splitting under visible light irradiation, ACS Sustainable Chem. Eng., 2020, 8(25), 9456–9463 CrossRef CAS.
- S. S. Shenouda, T. H. AlAbdulaal, H. Y. Zahran and I. S. Yahia, Synthesis, structure identification and linear/nonlinear optics of hydrothermally grown WO3 nanostructured thin film/FTO: Novel approach, Ceram. Int., 2022, 48(6), 7663–7667 CrossRef CAS.
- M. Yang, T. Hang, C. Zhang, Y. Ma, H. Jiang and Q. Hao, et al., Engineering a Tandem S-Scheme TiO2@ SrTiO3@ BiVO4 Photoanode for Superior PEC Water Oxidation, Electrochim. Acta, 2025, 147105 CrossRef CAS.
- M. J. Kim, C. Lee, Y. R. Jo, W. G. Jung, J. S. Ha and J. H. Shim, et al., Amorphous Exsolution of Fe3O4 Nanoparticles in SrTiO3: A Path to High Activity and Stability in Photoelectrochemical Water-Splitting, Small Struct., 2025, 6(4), 2400450 CrossRef CAS.
- N. R. Reddy, A. S. Kumar, P. M. Reddy, R. R. Kakarla, J. H. Jung and T. M. Aminabhavi, et al., Efficient synthesis of 3D ZnO nanostructures on ITO surfaces for enhanced photoelectrochemical water splitting, J. Environ. Manage., 2024, 352, 120082 CrossRef CAS PubMed.
- Y. Xie, Y. Sun, R. Jiang, J. Chang, Y. Yang and L. Zhang, et al., Controllable synthesis of CdS nanospheres photoelectrode for photoelectrochemical water splitting, Resour. Chem. Mater., 2024, 3(1), 38–45 CAS.
- N. Ahmad, F. Alharthi, M. Alam, R. Wahab, S. Manoharadas and B. Alrayes, Syngas production via CO2 reforming of methane over SrNiO3 and CeNiO3 perovskites, Energies, 2021, 14(10), 2928 CrossRef CAS.
- N. Baig, I. Kammakakam, W. Falath and I. Kammakakam, Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges, Mater. Adv., 2021, 2(6), 1821–1871 RSC.
- A. K. Tomar, G. Singh and R. K. Sharma, Charge storage characteristics of mesoporous strontium titanate perovskite aqueous as well as flexible solid-state supercapacitor cell, J. Power Sources, 2019, 426, 223–232 CrossRef CAS.
- W. Gao, Z. Xia, F. Cao, J. C. Ho, Z. Jiang and Y. Qu, Comprehensive Understanding of the Spatial Configurations of CeO2 in NiO for the Electrocatalytic Oxygen Evolution Reaction: Embedded or Surface-Loaded, Adv. Funct. Mater., 2018, 28(11), 1706056, DOI:10.1002/adfm.201706056 . [cited 2025 Oct 24].
- N. Arjun, G. T. Pan and T. C. K. Yang, The exploration of Lanthanum based perovskites and their complementary electrolytes for the supercapacitor applications, Results Phys., 2017, 7, 920–926 CrossRef.
- M. Kumar, J. H. Yun, V. Bhatt, B. Singh, J. Kim and J. S. Kim, et al., Role of Ce3+ valence state and surface oxygen vacancies on enhanced electrochemical performance of single step solvothermally synthesized CeO2 nanoparticles, Electrochim. Acta, 2018, 284, 709–720 CrossRef CAS.
- S. R. Gawali, D. P. Dubal, V. G. Deonikar, S. S. Patil, S. D. Patil and P. Gomez-Romero, et al., Asymmetric supercapacitor based on nanostructured Ce-doped NiO (Ce: NiO) as positive and reduced graphene oxide (rGO) as negative electrode, ChemistrySelect, 2016, 1(13), 3471–3478 CrossRef CAS.
- M. A. Peck and M. A. Langell, Comparison of nanoscaled and bulk NiO structural and environmental characteristics by XRD, XAFS, and XPS, Chem. Mater., 2012, 24(23), 4483–4490 CrossRef CAS.
- J. Zhu, H. Li, L. Zhong, P. Xiao, X. Xu and X. Yang, et al., Perovskite oxides: preparation, characterizations, and applications in heterogeneous catalysis, ACS Catal., 2014, 4(9), 2917–2940 CrossRef CAS.
- J. S. Prabagar, T. Tenzin, S. Yadav, K. M. A. Kumar and H. P. Shivaraju, et al., Facile synthesis of NdFeO3 perovskite for photocatalytic degradation of organic dye and antibiotic, Mater. Today: Proc., 2023, 75, 15–23 Search PubMed.
- Y. Wei, J. Su, X. Wan, L. Guo and L. Vayssieres, Spontaneous photoelectric field-enhancement effect prompts the low cost hierarchical growth of highly ordered heteronanostructures for solar water splitting, Nano Res., 2016, 9, 1561–1569 CrossRef CAS.
- S. Moscow, V. Kavinkumar, M. Sriramkumar, K. Jothivenkatachalam, P. Saravanan and N. Rajamohan, et al., Impact of Erbium (Er) and Yttrium (Y) doping on BiVO4 crystal structure towards the enhancement of photoelectrochemical water splitting and photocatalytic performance, Chemosphere, 2022, 299, 134343 CrossRef CAS PubMed.
- J. Liqiang, Q. Yichun, W. Baiqi, L. Shudan, J. Baojiang and Y. Libin, et al., Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity, Sol. Energy Mater. Sol. Cells, 2006, 90(12), 1773–1787 CrossRef.
- W. Zhang, Y. Ma, X. Zhu, S. Liu, T. An and J. Bao, et al., Fabrication of Ag decorated g-C3N4/LaFeO3 Z-scheme heterojunction as highly efficient visible-light photocatalyst for degradation of methylene blue and tetracycline hydrochloride, J. Alloys Compd., 2021, 864, 158914 CrossRef CAS.
- Y. Li, Z. Hao, R. Wang, G. Wang, H. Li and C. Li, et al., Interfacial electric field optimization and co-catalyst free LaFeO3-based pp-type homojunction for efficient PEC water splitting, Chem. Eng. J., 2024, 149797 CrossRef CAS.
- R. B. Wei, P. Y. Kuang, H. Cheng, Y. B. Chen, J. Y. Long and M. Y. Zhang, et al., Plasmon-enhanced photoelectrochemical water splitting on gold nanoparticle decorated ZnO/CdS nanotube arrays, ACS Sustainable Chem. Eng., 2017, 5(5), 4249–4257 CrossRef CAS.
- S. Khoomortezaei, H. Abdizadeh and M. R. Golobostanfard, Ferro-photocatalytic enhancement of photoelectrochemical water splitting using the WO3/BiFeO3 heterojunction, Energy Fuels, 2021, 35(11), 9623–9634 CrossRef CAS.
- B. Huang, H. Wang, S. Liang, H. Qin, Y. Li and Z. Luo, et al., Two-dimensional porous cobalt–nickel tungstate thin sheets for high performance supercapattery, Energy Storage Mater., 2020, 32, 105–114 CrossRef.
- Z. H. Huang, F. F. Sun, Z. Y. Yuan, W. Sun, B. Jia and H. Li, et al., An electro-activated bimetallic zinc-nickel hydroxide cathode for supercapacitor with super-long 140,000 cycle durability, Nano Energy, 2021, 82, 105727 CrossRef CAS.
- M. P. Harikrishnan and A. C. Bose, Porous CeNiO3 with an enhanced electrochemical performance and prolonged cycle life (>50
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) via a lemon-assisted sol–gel autocombustion method, New J. Chem., 2022, 46(31), 15118–15129 RSC.
000 cycles) via a lemon-assisted sol–gel autocombustion method, New J. Chem., 2022, 46(31), 15118–15129 RSC. - H. Arandiyan, S. S. Mofarah, Y. Wang, C. Cazorla, D. Jampaiah and M. Garbrecht, et al., Impact of surface defects on LaNiO3 perovskite electrocatalysts for the oxygen evolution reaction, Chem. – Eur. J., 2021, 27(58), 14418–14426 CrossRef CAS PubMed.
- H. Khan, I. H. Lone, S. E. Lofland, K. V. Ramanujachary and T. Ahmad, Exploiting multiferroicity of TbFeO3 nanoparticles for hydrogen generation through photo/electro/photoelectro-catalytic water splitting, Int. J. Hydrogen Energy, 2023, 48(14), 5493–5505 CrossRef CAS.
- W. Bai, Y. Zhou, G. Peng, J. Wang, A. Li and P. F. X. Corvini, Engineering efficient hole transport layer Ferrihydrite-MXene on BiVO4 photoanodes for photoelectrochemical water splitting: work function and conductivity regulated, Appl. Catal., B, 2022, 315, 121606 CrossRef CAS.
- G. Liu, S. K. Karuturi, H. Chen, D. Wang, J. W. Ager and A. N. Simonov, et al., Enhancement of the photoelectrochemical water splitting by perovskite BiFeO3 via interfacial engineering, Sol. Energy, 2020, 202, 198–203 CrossRef CAS.
- D. Lee, A. Kvit and K. S. Choi, Enabling solar water oxidation by BiVO4 photoanodes in basic media, Chem. Mater., 2018, 30(14), 4704–4712 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |