Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A soft approach towards composite porous carbon/conducting polymer supercapacitors: layer-by-layer integration of pyrolyzed ZIF-8 MOF and PANI-PSS supramolecular complexes
Ana Paula
Mártire
,
Omar
Azzaroni
 ,
Waldemar
Marmisolle
,
Waldemar
Marmisolle
 * and
Matias
Rafti
* and
Matias
Rafti
 *
*
Instituto de Investigaciones Fisicoquímicas Teóricas y Aplicadas (INIFTA), Departamento de Química, Facultad de Ciencias Exactas, Universidad Nacional de La Plata-CONICET, La Plata B1904DPI, Argentina. E-mail: wmarmi@infita.unlp.edu.ar; mrafti@quimica.unlp.edu.ar
First published on 13th November 2025
Abstract
Amid the global energy crisis, the search for safe, sustainable, and high-performance energy storage is urgent. Supercapacitors—renowned for their exceptional power density and cycling stability—offer a compelling path forward, especially when engineered with eco-friendly materials and neutral-pH aqueous electrolytes. In this work, we present a simple yet powerful layer-by-layer (LbL) strategy to fabricate supercapacitor electrodes by integrating porous carbon from pyrolyzed ZIF-8 (PCZIF-8) with a polyaniline–polystyrene sulfonate (PANI-PSS) complex. The resulting films were probed via cyclic voltammetry and electrochemical quartz crystal microbalance (eQCM) to unravel their charge-storage dynamics, while galvanostatic cycling tested their long-term resilience. The composite film exhibits specific capacitances of 225 F g−1 in 0.1 M HCl and 160 F g−1 in 0.1 M KCl for 1 A g−1 charge–discharge galvanostatic curves. The specific capacitance increased by more than 1000% when a porous carbon was used instead of a non-porous one. The inclusion of PCZIF-8 delivered a substantial performance leap under mild, environmentally benign conditions—all using low-cost, readily synthesized components. This synergy between conductive polymers and porous carbons from MOFs opens a sustainable and scalable route to next-generation energy storage.
Introduction
Supercapacitors (SC) are energy storage devices known for their high power density, rapid charge/discharge rates, and extended cyclability life span.1 The capacitance can arise from two main sources, namely, the electric double-layer and a pseudocapacitive contribution from redox processes.2 While electrical double-layer capacitance (EDLC) is a consequence of charge accumulation due to migration of electrolytes under applied voltage, pseudocapacitance stems from fast reversible faradaic reactions taking place at the electrode interface. In this context, numerous combinations of carbon materials with other active components have been explored for electrode modification in supercapacitors.3–5 The inclusion of porous materials in SC through ad-hoc designed nanoarchitectures is an appealing option for performance enhancement (e.g., including porous carbon building blocks).6–9Due to the important sustainability requirement, great efforts are currently being devoted to designing SC with low environmental impact, both regarding synthetic procedures and operating conditions.10 Main challenges for such a goal are the substitution of commonly used hazardous electrolytes and highly corrosive chemicals involved in the synthesis and activation of porous materials.11 A way to tackle the above-discussed issues would be, for example, to obtain porous carbon materials using synthetic procedures that do not require harsh activation, and ideally, integrating them into electrochemical systems that could operate efficiently with aqueous neutral electrolytes.12
Thinking of possible ways to address the above challenges, metal organic frameworks (MOFs) become an interesting option. MOFs are crystalline solids composed of metal ions coordinated with organic linkers into high surface area, non-covalent porous networks that yield porous carbon particles when pyrolyzed under appropriate conditions.9,13–15 MOFs are extensively used in energy-related applications due to their tuneable pore-wall chemistry, high surface areas, and the possibility of controlling particle size and morphology.16 Most common applications use pyrolyzed MOFs for SC assembly due to their dielectric nature; however, the number of conductive MOFs reported is rapidly increasing, thus opening new paths for innovation in this regard.17,18 Pyrolysis conditions and parent MOF chosen are decisive for the resulting material and must be tuned accordingly. For example, relatively low temperatures (up to 600 °C) render incomplete carbonization and partial elimination of common carbon templates added. Although high temperature pyrolysis (up to 1000 °C) favours the appearance of additional porosity due to increased graphitization and elimination of metal atoms present, excessive and fast heating can cause the collapse of the porous network together with loss of heteroatoms from MOF linkers, which in turn reduces nanocarbon conductivity.19–21
One of the most visited MOF subclasses for this and other related applications is the so-called zeolite imidazolate frameworks (ZIFs), built from the combination of N-bidentate imidazole linkers and tetrahedrally coordinated M2+ metal ions (e.g., M = Co, Zn).22 Depending on the actual imidazole ring present, ZIF materials receive a distinctive number added to the acronym for their identification. A particularly popular member is the material composed of 2-methyl-imidazolate linkers, known as ZIF-8, which due to its biocompatibility, relatively high thermal/chemical stability, and both varied and straightforward synthesis with inexpensive chemicals and solvents, has been employed for several different applications ranging from catalysis to separations and optics23–27 Since pioneering reports from almost a decade ago, there have been multiple efforts oriented to establishing protocols for controlled production of micro/mesoporous carbon from ZIF-8 (PCZIF-8) that could be employed in the assembly of electroactive nanoarchitectures.23,28–33
One of the main issues to be addressed in the assembly of PCZIF-8 SC electrodes is ensuring mechanical stability and electrochemical connectivity when processed into films. Conducting polymers (CPs) such as poly(3,4-ethylenedioxythiophene) (PEDOT) or polyaniline (PANI) and their complexes with other polymers like poly(styrenesulfonate) (PSS) offer a compelling solution, as they can provide both adhesion and additional electrochemical activity34–36 The combination can be achieved through several approaches, e.g., by carrying surface chemical polymerization of aniline in the presence of colloidal PCZIF-8 (yielding core@shell nanoarchitectures),37via mixing with (PEDOT:PSS),36,38 or by creating porous carbon covered PANI fibres, which are then deposited on a conductive electrode substrate.39,40 Another reported method incorporates both PANI and ZIF-8 into an aerogel matrix, which is subsequently co-carbonized within the aerogel structure.41
In all the above-discussed approaches, the porous carbon nanomaterial needs to be processed using adequate solvents, deposited, and then dried before the SC can be subjected to electrochemical testing. Our contribution aims to demonstrate a sequential soft assembly of SC components, which can also perform under neutral aqueous electrolyte. For this end, we took advantage of our previous experience with electrode–electrolyte interface engineering via layer-by-layer (LbL) assembly, enabling thus precise control over film architecture by alternately depositing positively and negatively charged species, thereby promoting intimate contact in well-organized hybrid three-dimensional structures.42–45 Aqueous near-neutral conditions employed are appealing to the environmental impact of the approach; however, poses a challenge due to PANI's limited electroactivity.46,47 To overcome this limitation, we included instead (PANI:PSS) complexes obtained through ad-hoc template polymerization, improving stability and procesability.48–50 Following previously explored approaches, we propose the use of ZIF-8 MOF-derived porous carbon using commercial non-porous graphitic carbon as a benchmark.51,52
Films obtained via LbL were investigated by a combination of cyclic voltammetry and galvanostatic charge–discharge experiments. Their electrochemical capacitance and process dynamics were studied using a quartz crystal microbalance (QCM and eQCM). This work shows that the incorporation of porous carbon nanoparticles into (PANI:PSS) composite films increase the EDLC contribution to the electrode's capacitance and significantly improves its electrochemical response. This approach presents a promising pathway to develop high-performance, neutral-electrolyte supercapacitors with improved efficiency and durability.
Experimental section
Chemicals
Aniline, ammonium persulfate (APS), poly (sodium 4-styrene sulfonate) (PSS) (Mw ≈ 70 kDa), polyallylamine hydrochloride (PAH) (ca. 17.5 kDa), polyethylenimine (PEI) (50% in H2O, Mw ≈ 750 kDa), Zn(NO3)2·6H2O, 2-methyl-imidazole (2 m-Im), and (1-hexadecyl)trimethyl-ammonium bromide (99%) (CTAB) were purchased from Sigma-Aldrich. Expanded graphite was purchased from Carbone Lorraine. Methanol was obtained from Dorwil. Hydrochloric acid and potassium chloride were purchased from Anedra. All chemicals were employed as received except for aniline which was distilled and methanol which was dried and distilled as reported before.53 All solutions were prepared with deionized water (18.2 MΩ cm).ZIF-8 synthesis
ZIF-8 was obtained as reported before by mixing 250 ml of 25 mM Zn(NO3)2 and 250 ml of 100 mM 2 m-Im solutions prepared with anhydrous methanol.54 The reaction batch was stirred at 25 °C for four hours and the colorless liquid turned white. Then, most of the solvent was extracted using the rotary evaporator at 40 °C and 250 mbar for three hours. Next, the sample was collected via centrifugation (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 rpm, 10 minutes), washed twice with fresh methanol, and dried overnight at 60 °C.
400 rpm, 10 minutes), washed twice with fresh methanol, and dried overnight at 60 °C.
PCZIF-8 synthesis
ZIF-8 was pyrolyzed under flowing N2. First, the sample was dried for 2 h at 100 °C and 2 h at 200 °C. Second, the burning occurred during 4 h at 750 °C. All these temperatures were achieved with a heating rate of 10 °C min−1. Finally, a black powder was obtained indicating the presence of the ZIF-8 derived porous carbon (PCZIF-8).PCZIF-8 dispersions
The PCZIF-8 was dispersed in DI water by probe sonication in the presence of CTAB. The PCZIF-8 concentration was 1 mg mL−1. Two concentrations of CTAB were used: 10 mg ml−1 (C1) and 20 mg ml−1 (C2). The solution was ultrasonicated for 30 minutes in a beaker before being used in the LbL assembly process.GC dispersion
A graphitic carbon (GC) previously characterized was used in this work.51 Its dispersion was prepared with 10 mg ml−1 of CTAB and 1 mg mL−1 of carbon. The dispersion was ultrasonicated (probe sonication) for 30 minutes in a beaker before being used in the LbL assembly process.PANI-PSS synthesis
The PANI-PSS dispersion was prepared by chemically polymerizing aniline in the presence of PSS. Firstly, a 5 mM aniline solution in 0.5 M HCl was prepared. Then, PSS was added to reach a 5 mM concentration. After 15 minutes of stirring, APS was added to reach a 5 mM concentration, and the solution was stirred overnight. The synthesis was carried out in 40 mL falcon tubes, with a final volume of 20 mL. The preparation color was initially dark blue and then turned green.PANI-PSS dispersions
On the one hand, the batch synthesis was diluted 1/10 in 0.5 M HCl (A PANI-PSS) and used in the LbL assembly. On the other hand, the batch synthesis was neutralized with 10% KOH up to pH 7 and diluted 1/10 with DI water (N PANI-PSS) for use in the LbL assembly.Layer-by-layer assembly
The LbL assembly integrated PCZIF-8 in C1 or C2 dispersion with acid or neutral PANI-PSS dispersion in the four possible combinations. A general process starts with a previously cleaned Au or glass substrate followed by a 10-minute immersion in 1 mg mL−1 PEI solution and a 5-minute rinse in DI water. Next, the substrates were dipped in the PANI-PSS dispersion for 10 minutes, and 5 min-washed with HCl 0.5 M for A PANI-PSS or DI water for N PANI-PSS. Then, the substrates were immersed in the PCZIF-8 dispersion and rinsed for 5 minutes in DI water. This sequence yielded a single bilayer, and the n times repetitions were carried out to obtain a [PANI-PSS/PCZIF-8]n assembly, where n means the number of bilayers.Characterization/electrochemical measurements
Results and discussion
ZIF-8 pyrolysis
Firstly, ZIF-8 was synthesised to produce porous carbon. The ZIF-8 was prepared as reported before by mixing methanolic solutions of Zn(NO3)2 and 2-methylimidazole. The resulting white product was characterised using wide-angle X-ray scattering (WAXS), and the results confirm the match between calculated and experimental peaks, verifying the presence of the ZIF-8 phase (Fig. S1). Thermogravimetric analysis (TGA) was also conducted to assess thermal stability under a nitrogen atmosphere. The thermal decomposition of ZIF-8 occurred at 450 °C, consistent with previous reports (Fig. S2).22Next, the sample underwent pyrolysis at 750 C for 4 hours in a nitrogen atmosphere, resulting in a black powder identified as ZIF-8 porous carbon (PCZIF-8). This process is illustrated in Fig. 1A. The N2 adsorption isotherms for both ZIF-8 and PCZIF-8 were measured (Fig. 1B), and BET surface area analysis (Fig. S3) revealed values of 1475 m2 g−1 for fresh ZIF-8 and 875 m2 g−1 for the pyrolyzed material. The adsorption isotherm of the PCZIF-8 also revealed, in addition to the preserved microporosity, a characteristic hysteresis loop indicative of mesoporosity. Although some reduction in surface area is to be expected from pyrolysis, the value obtained is relatively lower compared to other reported protocols. This can be understood considering that, although the selected temperature is high enough to cause graphitization and conversion into amorphous carbon (see Raman spectra below), yet low enough to ensure the preservation of both N and Zn heteroatoms in the PCZIF-8. We have selected this temperature, which represents an optimal balance between the upper and lower pyrolysis limits, in order to preserve the conductivity and microporosity of the material obtained, features that contribute to the enhancement of SC performances.
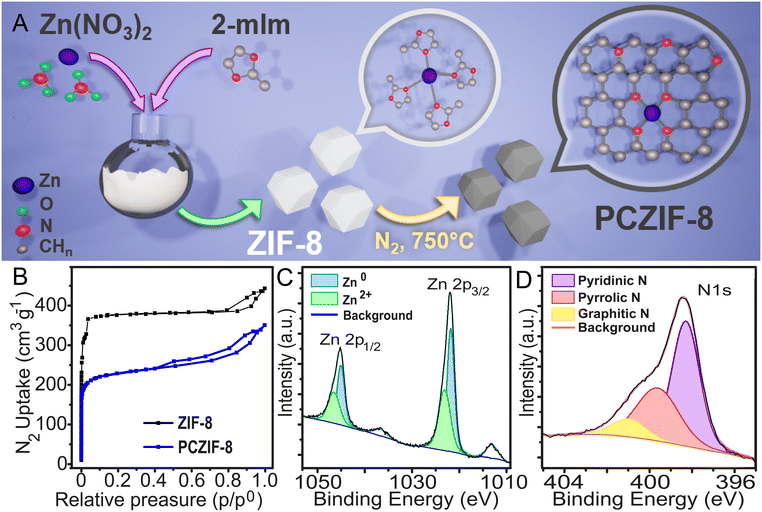 | ||
| Fig. 1 (A) Scheme of the synthesis of PCZIF-8. (B) N2 adsorption isotherm for ZIF-8 and PCZIF-8. (C) XPS Zn 2p band and (D) N 1s band for PCZIF-8. | ||
To analyse the composition of PCZIF-8, X-ray photoelectron spectroscopy (XPS) was used, focusing on the Zn (Fig. 1C) and N (1D) binding energy regions. Two oxidation states of Zn were identified: Zn2+ at 1046.22 and 1023 eV, and Zn0 at 044.75 and 1021.7 eV. Additionally, pyridinic, pyrrolic, and graphitic nitrogen were detected at 398.3, 399.67, and 400.9 eV, respectively. These results confirm that PCZIF-8 is a porous carbon material with a high surface area including nitrogen and zinc into its porous matrix.
Layer-by-layer assembly
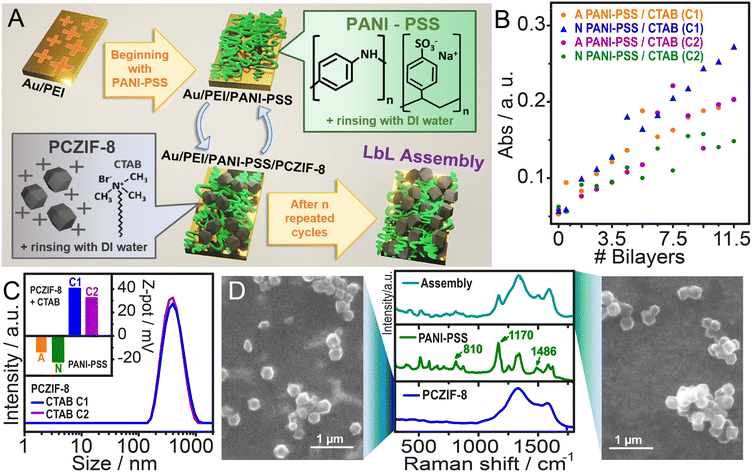
The Layer-by-Layer (LbL) technique was selected to construct hybrid films that integrate PCZIF-8 dispersions with conductive polymer complexes (PANI-PSS), as depicted in Fig. 2A. PCZIF-8 was previously dispersed in CTAB solutions by probe sonication. The LbL process begins with a clean gold substrate, onto which a layer of PEI is first deposited. Following this, alternate soaking in PANI-PSS and PCZIF-8/CTAB dispersions was performed to build the LbL assembly.To optimise the conditions for film growth, different assembly conditions were tested. PCZIF-8 was dispersed in water using two concentrations of CTAB: 10 mg ml−1 (C1) and 20 mg ml−1 (C2). The PANI-PSS dispersion was prepared in two forms: acidic (diluted in 0.5 M HCl) and neutral (neutralised with KOH and then diluted in deionized water). These variables were combined to create four different systems, which were tested by growing films on clean glass substrates and measuring absorbance at 750 nm, at which PANI-PSS shows maximum absorption (Fig. 2B). Absorbance increased with each deposition cycle for all four systems. However, the most consistent and regular growth was observed in the system combining neutral PANI-PSS with PCZIF-8 dispersed in C1. This system was selected for further experiments.
The prepared dispersions were analysed using Z-potential measurements (Fig. 2C). The PCZIF-8 dispersions had a Z-potential of +40.2 mV (C1) and, +32.2 mV (C2). In contrast, the neutral PANI-PSS dispersion showed a Z-potential of −21.6 mV and, acidic PANI-PSS -13.8 mV. These opposite surface charges confirm that electrostatic interactions make the LbL assembly feasible. Additionally, the size of the dispersed meso-microporous carbon particles was measured using dynamic light scattering (DLS), revealing a hydrodynamic size of 440 nm in diameter for both CTAB concentrations.
Next, the film produced and each component were studied by Raman spectroscopy (Fig. 2D). For the PCZIF-8 a typical graphitic carbon spectrum was observed: the G band associated with the ordered sp2 structure at 1570 cm−1 and the D band related to the defective structure at 1330 cm−1.55–57 The ratio IG/ID = 0.3 is indicative of a graphitic-type material. In the PANI-PSS sample three characteristic signals were identified: at 810 and 1170 cm−1 the C–H bending in the quinoid and benzenic ring and at 1486 cm−1 the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching.51 These five signals could also be observed in the assembly spectrum, confirming the presence of PANI-PSS and PCZIF-8 in the composite film.
N stretching.51 These five signals could also be observed in the assembly spectrum, confirming the presence of PANI-PSS and PCZIF-8 in the composite film.
To further characterise the PCZIF-8 dispersion and the assembly, microscopic images were taken with a scanning electron microscope (SEM). Fig. 2D (left), shows the PCZIF-8 dispersion deposited on a clean Au surface. The PCZIF-8 were identified as monodispersed polyhedrons with an average particle size of 290 ± 30 nm (n = 138). The same particles were observed in clusters in the LbL assembly (Fig. 2D, right) confirming the PCZIF-8 integration in the film.
Electrochemical comparison between PCZIF-8 and graphitic carbon.
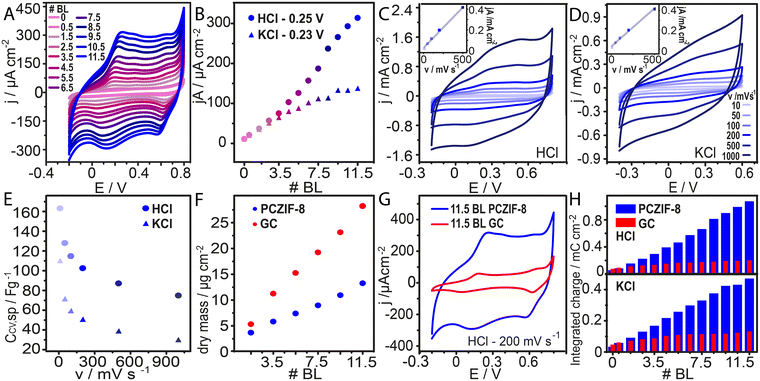
The electroactivity and electrochemical connectivity of the LbL films were evaluated through cyclic voltammetry at various stages of the construction process. For this, the assembly was built directly on the Au electrode in the Teflon electrochemical cell, and cyclic voltammograms were recorded after each PANI-PSS step deposition. Fig. 3A and S4 present cyclic voltammograms corresponding to increasing numbers of bilayers measured in HCl and KCl respectively. A steady increase in current is observed after each deposition cycle, consistent with the trend in Fig. 3B. This behaviour suggests the build-up of an electrochemically interconnected material.The electrochemical performance of the 11.5 BL assembly with PCZIF-8 was studied at different scan rates (Fig. 3C and D). The peak current showed a linear dependence on the potential scan rate, indicating a surface-confined electrochemical process with no diffusion constraints (inset Fig. 3C and D).58 For these voltammograms, eqn (1) was applied to calculate the specific voltammetric capacitance:
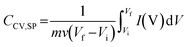 | (1) |
To evaluate the carbon porosity contribution to the film's capacitance, the PCZIF-8 assembly was compared to another assembly prepared with a commercial graphitic carbon (GC) dispersion. The dry mass growth of both films was monitored via QCM (Fig. 3F) performing the LbL process on top of a gold QCM sensor. The two systems grew linearly; however, the GC assembly reached higher mass deposition than the PCZIF-8 one.
The electrochemical growth of the GC assembly was also followed by cyclic voltammetry. Fig. S5 displays the voltammograms for this film with increasing deposition cycles. The comparison of the voltammograms of 11.5 bilayers for the two systems is presented in Fig. 3G and S6 for 0.1 M HCl and 0.1 M KCl, respectively. The performance observed was notably different; although the PCZIF-8 assembly has less deposited material, it exhibits a much higher voltammetric current response than the GC films.
Integrated charge calculations (Fig. 3H) further highlight these differences: charge increased substantially with bilayer number in PCZIF-8 films but remained nearly constant for GC assemblies.
Specific capacitance values at different scan rates for both 11.5 BL assemblies are summarized in Table S1. These results indicate that the carbon porosity enhances the electrochemical capacity, leading to an efficient performance even in lighter films.
Specific voltammetric capacitance values were also compared across different number of bilayers in Fig. S7. The disparity in electrochemical performance between the two systems becomes more pronounced when comparing specific capacitance, as GC film has a higher mass and a lower current response than the PCZIF-8 one. Furthermore, for PCZIF-8 assembly, a nearly constant specific capacitance values with increasing bilayers are observed. This trend indicates electrochemically interconnected material inclusion.
Analysis of the capacitance components
Next, to evaluate the effects contributing to the assembly's capacitance, the Trasatti and Dunn methods were employed.59,60 First, the Trasatti method considers the total capacitance (CT) as an addition of two contributions shown in eqn (2):| CT = Cc + Cd | (2) |
In this equation Cc is the electric double layer capacitance and Cd is the pseudocapacitance.4,61 The capacitance increases with decreasing scan rate; thus, the capacitance attributed to the total surface area can be estimated from eqn (3) by extrapolating to zero scan rate:
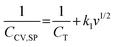 | (3) |
In this equation, v is the scan rate, k1 is a constant and CCV,SP is the specific capacitance calculated at each scan rate.
Assuming that Cc is independent of the scan rate, its value can be determined from eqn (4) by extrapolating to the infinite-scan rate limit.
| CCV,SP = Cc + k2v−1/2 | (4) |
In this equation, k2 is another constant. Fig. 4A and B show the plots from eqn (3) and (4), respectively. CCV,SP was analysed for scan rates from 10 to 500 mV s−1.
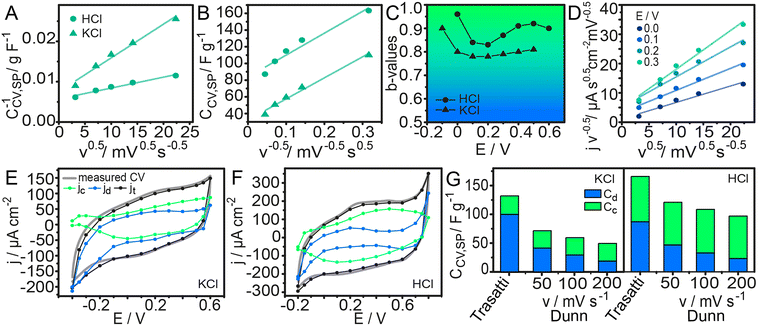 | ||
Fig. 4 (A) Dependence of the inverse of the specific capacitance with v0.5. (B) Dependence of the specific capacitance with v−0.5. (C) b-Values calculated from the slope of eqn (6). (D) j v−0.5 with v0.5 with their linear regression fitting used to obtain 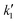 and and 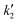 in 0.1 M HCl. Cyclic voltammograms for 100 mV s−1 with jd and jc contributions for (E) KCl and (F) HCl. (G) Specific capacitance contributions calculated by the Trasatti and Dunn methods in 0.1 M KCl and 0.1 M HCl. in 0.1 M HCl. Cyclic voltammograms for 100 mV s−1 with jd and jc contributions for (E) KCl and (F) HCl. (G) Specific capacitance contributions calculated by the Trasatti and Dunn methods in 0.1 M KCl and 0.1 M HCl. | ||
Second, Dunn method was applied, analysing the behaviour of the current density (j) with the scan rate. The dependence of j on v is expressed in eqn (5):
| j = avb | (5) |
In eqn (5), a and b are constants. Two characteristic b values are commonly recognized: b = 0.5 for diffusion-controlled processes and b = 1 for capacitive behaviour. The b value for our assemblies was determined, applying log to eqn (5):
log(j) = log(a) + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(v) log(v) | (6) |
The linear regression fitting for log(j) vs. log(v) is shown in Fig. S8. The b values obtained for our assemblies in KCl and HCl solutions are shown in Fig. 4C. All b values range between 0.78 and 0.97. Although this indicates a combination of contributions to the current density and b is not strictly equal to 1, according to previous reports, the overall behaviour of the composite film can be considered characteristic of a supercapacitor.62,63
Thus, the total current density can be expressed as the sum of two components, as shown in eqn (7):
| jT = jc + jd | (7) |
The total density current (jT) is composed by the diffusion-controlled current density (jd, b = 0.5) and the capacitive current density (jc, b = 1). Based on these b values, eqn (8) is obtained:
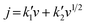 | (8) |
Here, 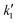 and
and 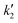 are constants. Dividing the equation by v1/2 allows expressing the relationship in linear form, as given in eqn (9).
are constants. Dividing the equation by v1/2 allows expressing the relationship in linear form, as given in eqn (9).
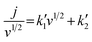 | (9) |
Fig. 4D and S9 display representative linear regressions obtained from eqn (9) using HCl and KCl as electrolyte. From the 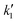 and
and 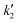 values determined along the voltammetric cycle, the diffusion-controlled (jd) and capacitive (jc) current profiles were reconstructed for each voltammogram. Results for 100 mV s−1 in KCl and HCl are shown in Fig. 4E and F, while those for 50 and 200 mV s−1 are shown in Fig. S10. Integration of each curve allowed the calculation of the specific capacitance corresponding to each contribution (Cd and Cc) at different scan rates. Fig. 4G summarizes these results along with those obtained from the Trasatti method.
values determined along the voltammetric cycle, the diffusion-controlled (jd) and capacitive (jc) current profiles were reconstructed for each voltammogram. Results for 100 mV s−1 in KCl and HCl are shown in Fig. 4E and F, while those for 50 and 200 mV s−1 are shown in Fig. S10. Integration of each curve allowed the calculation of the specific capacitance corresponding to each contribution (Cd and Cc) at different scan rates. Fig. 4G summarizes these results along with those obtained from the Trasatti method.
As shown in Fig. 4G, the component analysis indicates that, regardless of the scan rates or the specific methods used for evaluation, the differences in capacitance between acidic and neutral media are mainly attributed to the Cc component. This contribution is commonly associated with EDL processes and, in this type of system has a great influence from the PANI block, which constitutes a conducting component.64,65 Polyaniline exhibits a more pronounced electrical conductivity under acidic conditions; therefore, it is reasonable that composite materials containing PANI display a stronger EDL contribution to the overall capacitance in acidic media. On the other hand, the components that vary linearly with the square root of the scan rate are typically assigned to diffusive processes.66,67 In the materials developed here, although polyaniline contributes partially to this capacitive behaviour, the main contributors to the linear (capacitive) component are the carbon-based nanomaterials. However, no major differences in this contribution are expected when switching from acidic to neutral media.
Ionic exchange during cyclic voltammetry
During electrochemical switching, the oxidation states of the film components change, leading to the exchange of charged species and solvent captured in the polymer matrix with the electrolyte solution.68,69 To quantify this exchange, eQCM experiments were performed, combining frequency measurements with cyclic voltammetry. Fig. 5A and S11 show the current and mass change, calculated with the Sauerbrey model, in 0.1 M HCl for 50 and 200 mV s−1 scan rates. The observed trend shows a decrease in film mass during oxidation and an increase during reduction.As already reported, PANI-PSS has a negative charge excess provided by the polystyrene sulfonate (PSS) polyanion. Negative charge excess was confirmed with the z-potential measured of −21.6 mV. Then, the film is expected to exchange cations as charge carriers with the electrolyte during the redox switching.70–72 To explain the mass exchange Fig. 5B illustrates the oxidation and reduction of the film with PANI-PSS considering the cation exchange. Thus, the oxidation releases cations to the solution driving a mass loss and the reduction captures the cations from the solution leading to a mass gain. These exchanged cations are accompanied by solvent molecules, which also modifies the film hydration during redox switching.
The mass exchange was measured after different numbers of deposition cycles of the assembly procedure. Fig. 5C above and S12 represent the mass change with time during cyclic voltammetry performed at 50 and 200 mV s−1. In Fig. 5C mean mass exchange values are shown as a function of the number of deposition cycles. As expected, as the number of deposition steps increases, more conductive polymer is attached to the film and the electrochemical mass exchange increments. This result reinforces the idea that the added material is electrochemically interconnected and exchanging cations and solvent during redox switching.
Galvanostatic charge–discharge curves
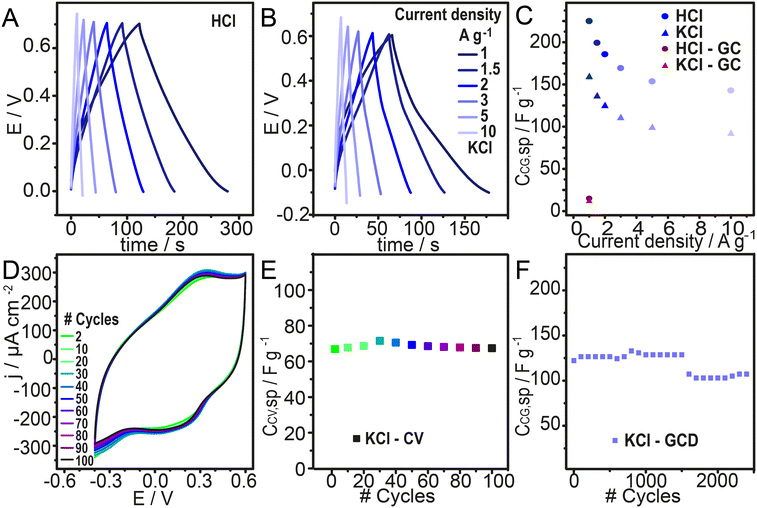
Galvanostatic charge–discharge cycles were performed at different current densities to study the energy storage in these composite films. The applied current densities were calculated by applying QCM dry mass from Fig. 3F. Charge–discharge curves are shown in Fig. 6A and B measured in HCl and KCl. Specific capacitance was calculated with eqn (10):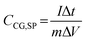 | (10) |
This enhancement is primarily attributed to the increase in the surface area provided by the PCZIF-8 inclusion. The carbon porosity significantly improves ion accessibility, facilitating efficient electrolyte penetration and maximizing the contact between the electrolyte and the conductive material. Another key factor in this improvement is the contribution of the electric double-layer formation, which plays a major role in the charge storage and calculated specific capacitance. In summary, the porous interface not only enhances ion transport but also reinforces the double-layer capacitance, making the system highly suitable for energy storage applications.
To place our results in context, Tables 1 and S2 compare the specific capacitance values from this work with those reported for PCZIF-8-based and PANI-based supercapacitors. Although the value obtained here is not the highest in the table, it must be highlighted that the other studies operate in more aggressive electrolytes and extreme pH conditions. Considering the environmental friendliness and neutral electrolytes, the capacitance values achieved here are competitive with those of the other systems. Moreover, in several cases, the current densities applied in previous works are lower than those used in these experiments. For comparison at identical current densities, Table S3 presents the results for electrodes cycled at 1 A g−1. These findings emphasized the efficiency of this composed film, which delivers competitive performance without compromising on sustainability.
| Electrodes using PCZIF-8 | Electrolyte | C CG,SP (F g−1) | Cycling stability (GCD) | Reference |
|---|---|---|---|---|
| 2 layers PCZIF-8-PANI | 1 M H2SO4 – 3 electrodes | 322–1 A g−1 | 100% - 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles - 5 mA s−1 000 cycles - 5 mA s−1 |
37 |
| Core–shell PCZIF-8@PANI | 1 M H2SO4 – 2 electrodes | 236–1 A g−1 | 86% - 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles - 5 A g−1 000 cycles - 5 A g−1 |
39 |
| Aerogel with PANI and ZIF-8 is carbonized | 2 M KOH – 3 electrodes | 338–0.5 A g−1 | 91.2% - 5000 cycles - 5 A g−1 | 41 |
| Co3O4 + PCZIF-8 and PANI polymerized outside | 3 M KOH – 3 electrodes | 1407–1 A g−1 | 87.7% - 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles - 20 A g−1 000 cycles - 20 A g−1 |
40 |
| PCZIF-8 in a Ni foam | 2 M KOH – 3 electrodes | 187–0.5 A g−1 | 100% - 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles - 5 A g−1 000 cycles - 5 A g−1 |
73 |
| ZIF-8 + phytic acid is carbonized | 6 M KOH – 3 electrodes | 219.4–1 A g−1 | 100% - 2000 cycles - 5 A g−1 | 74 |
| ChNF@ZIF-8 is carbonized | 6 M KOH – 3 electrodes | 182.5–0.2 A g−1 | 94.8% - 5000 cycles - 1 A g−1 | 75 |
| CNC@ZIF-8 is carbonized | 6 M KOH – 3 electrodes | 172–0.1 A g−1 | 94.5% - 5000 cycles - 0.1 A g−1 | 76 |
| Layer by layer PCZIF-8/PANI:PSS | 0.1 M KCl – 3 electrodes | 160–1 A g−1 | 88% - 2400 cycles - 3 A g−1 - 0.1 M KCl | This work |
| 0.1 M HCl – 3 electrodes | 225–1 A g−1 |
Film stability and cyclability
The stability in a neutral solution was tested considering the possibility of applying this material to wearable devices. Firstly, 100 cycles of cyclic voltammetry were performed. Fig. 6D shows the voltammograms, and Fig. 6E displays the integrated charge for these curves. The integrated charge did not decay during these cycles; a 100% capacity retention was observed during this test in 0.1 M KCl. Next, a more challenging experiment to study the cyclability during charge–discharge galvanostatic curves was carried out. Fig. 6F displays the specific capacitance calculated with the charge time. Then, 2400 serial cycles at 3 A g−1 were performed and the CCG,SP was calculated by applying eqn (10). A 88% capacity retention was observed after 2400 cycles in 0.1 M KCl.The prepared PANI-PSS/PCZIF-8 assembly exhibits good stability during electrochemical cycling in a neutral electrolyte, which is crucial for its practical application ensuring a long-term performance. Comparing with stability values of other electrodes shown in Table 1, the capacity retention obtained is very promising. Additionally, the ability to operate in a neutral medium is crucial for including it in wearable and portable energy storage devices. Using 0.1 M KCl makes the system more environmentally friendly, as it avoids the use of corrosive or hazardous electrolytes.
Conclusions
In this work, we successfully obtained a micro and mesoporous carbon material through the pyrolysis of ZIF-8 under environmentally friendly conditions, avoiding the use of highly corrosive chemicals or extreme pH solutions. Using this PCZIF-8, we developed hybrid electrodes via a nanoarchitectonics strategy based on the layer-by-layer (LbL) assembly method, enabling the precise integration of PCZIF-8 with a conductive polymer complex (PANI-PSS). This architecture effectively combines the advantages of both components: the high conductivity, structural stability, and electric double-layer capacitance of the carbon, together with the dual EDLC–pseudocapacitive contributions of PANI-PSS.Electrochemical analysis revealed that incorporating PCZIF-8—with its high surface area and interconnected pore network—greatly enhanced the specific capacitance of the assemblies in neutral aqueous electrolytes. The abundant porosity facilitates ion transport and maximizes the active surface available for charge storage, while the environmentally benign fabrication route avoids corrosive reagents and extreme pH conditions. These combined advantages demonstrate that the rational use of porous carbons derived from MOFs offers a straightforward, scalable, and sustainable strategy to boost the performance of supercapacitors. Such eco-friendly devices hold strong potential for secure, low-impact energy storage applications, from wearable electronics to portable power systems.
Author contributions
APM: methodology, formal analysis, visualization, writing – original draft. OA: conceptualization, resources, funding acquisition. WAM, MR: conceptualization, writing – review & editing, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: additional material characterization, control experiments and complementary results. See DOI: https://doi.org/10.1039/d5lf00286a.Acknowledgements
APM acknowledges a scholarship from CONICET. OA, WAM and MR are staff members of CONICET and acknowledge the financial support from Universidad Nacional de La Plata (PPID-X1016), CONICET (PIP 11220210100209CO), and ANPCyT (PICT2018-0780, and PICT-2021-GRFTI-00042).Notes and references
- B. E. Conway, Electrochemical supercapacitors: scientific fundamentals and technological applications, Springer, 1999 Search PubMed.
- H. Chen, T. N. Cong, W. Yang, C. Tan, Y. Li and Y. Ding, Prog. Nat. Sci., 2009, 19, 291–312 CrossRef CAS.
- A. Musa, M. M. Islam, M. A. Bin Hasan Susan and M. M. Islam, ECS J. Solid State Sci. Technol., 2023, 12, 061005 CrossRef CAS.
- A. Musa, M. A. Hasan, S. S. Aniv and M. M. Islam, J. Energy Storage, 2025, 110, 115221 CrossRef.
- M. M. Islam, M. Y. A. Mollah, M. A. B. H. Susan and M. M. Islam, RSC Adv., 2020, 10, 44884–44891 RSC.
- J. Chmiola, G. Yushin, Y. Gogotsi, C. Portet, P. Simon and P. L. Taberna, Science, 2006, 313, 1760–1763 CrossRef CAS.
- X. Liu, D. Lyu, C. Merlet, M. J. A. Leesmith, X. Hua, Z. Xu, C. P. Grey and A. C. Forse, Science, 2024, 384, 321–325 CrossRef CAS.
- M. Sevilla and A. B. Fuertes, Carbon, 2006, 44, 468–474 CrossRef CAS.
- R. R. Salunkhe, Y. V. Kaneti, J. Kim, J. H. Kim and Y. Yamauchi, Acc. Chem. Res., 2016, 49, 2796–2806 CrossRef CAS PubMed.
- Z. Chang, Y. Yang, M. Li, X. Wang and Y. Wu, J. Mater. Chem. A, 2014, 2, 10739–10755 RSC.
- S. Husien, R. M. El-taweel, A. I. Salim, I. S. Fahim, L. A. Said and A. G. Radwan, Curr. Res. Green Sustainable Chem., 2022, 5, 100325 CrossRef CAS.
- G. E. Fenoy, M. Rafti, W. A. Marmisollé and O. Azzaroni, Mater. Adv., 2021, 2, 7731–7740 RSC.
- X. Chu, F. Meng, T. Deng and W. Zhang, Nanoscale, 2021, 13, 5570–5593 RSC.
- B. Liu, H. Shioyama, T. Akita and Q. Xu, J. Am. Chem. Soc., 2008, 130, 5390–5391 CrossRef CAS PubMed.
- W. Chaikittisilp, K. Ariga and Y. Yamauchi, J. Mater. Chem. A, 2013, 1, 14–19 RSC.
- M. Arcidiácono, A. P. Mártire, J. A. Allegretto, M. Rafti, W. A. Marmisollé and O. Azzaroni, in Materials Nanoarchitectonics, Elsevier, 2024, pp. 387–428 Search PubMed.
- L. S. Xie, G. Skorupskii and M. Dincă, Chem. Rev., 2020, 120, 8536–8580 CrossRef CAS.
- L. Niu, T. Wu, M. Chen, L. Yang, J. Yang, Z. Wang, A. A. Kornyshev, H. Jiang, S. Bi and G. Feng, Adv. Mater., 2022, 34, 2200999 CrossRef CAS.
- C. Young, R. R. Salunkhe, J. Tang, C.-C. Hu, M. Shahabuddin, E. Yanmaz, M. S. A. Hossain, J. H. Kim and Y. Yamauchi, Phys. Chem. Chem. Phys., 2016, 18, 29308–29315 RSC.
- Z. Zhao, S. Liu, J. Zhu, J. Xu, L. Li, Z. Huang, C. Zhang and T. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 19871–19880 CrossRef CAS.
- W. Chaikittisilp, M. Hu, H. Wang, H. S. Huang, T. Fujita, K. C. W. Wu, L. C. Chen, Y. Yamauchi and K. Ariga, Chem. Commun., 2012, 48, 7259–7261 RSC.
- K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- J. Tang, R. R. Salunkhe, J. Liu, N. L. Torad, M. Imura, S. Furukawa and Y. Yamauchi, J. Am. Chem. Soc., 2015, 137, 1572–1580 CrossRef CAS.
- G. Lu and J. T. Hupp, J. Am. Chem. Soc., 2010, 132, 7832–7833 CrossRef CAS PubMed.
- L. D. Trino, L. G. S. Albano, D. C. Granato, A. G. Santana, D. H. S. de Camargo, C. C. Correa, C. C. Bof Bufon and A. F. Paes Leme, Chem. Mater., 2021, 33, 1293–1306 CrossRef CAS.
- Z. Jiang, H. Liu, S. A. Ahmed, S. Hanif, S. Ren, J. Xu, H. Chen, X. Xia and K. Wang, Angew. Chem., Int. Ed., 2017, 56, 4767–4771 CrossRef CAS PubMed.
- G. E. Fenoy, J. Scotto, J. Azcárate, M. Rafti, W. A. Marmisollé and O. Azzaroni, ACS Appl. Energy Mater., 2018, 1, acsaem.8b01021 CrossRef.
- S. Ma, G. A. Goenaga, A. V. Call and D. Liu, Chem. – Eur. J., 2011, 17, 2063–2067 CrossRef CAS.
- Q. Lai, Y. Zhao, Y. Liang, J. He and J. Chen, Adv. Funct. Mater., 2016, 26, 8334–8344 CrossRef CAS.
- J. Liu, D. Zhu, C. Guo, A. Vasileff and S. Qiao, Adv. Energy Mater., 2017, 7, 1700518 CrossRef.
- H. Zhong, J. Wang, Y. Zhang, W. Xu, W. Xing, D. Xu, Y. Zhang and X. Zhang, Angew. Chem., Int. Ed., 2014, 53, 14235–14239 CrossRef CAS PubMed.
- Y. Liang, J. Guo, H. Zhang, D. J. L. Brett and S. Gadipelli, Chem. Eng. J., 2024, 489, 151190 CrossRef CAS.
- N. Kumar, N. Bansal, Y. Yamauchi and R. R. Salunkhe, Chem. Mater., 2022, 34, 4946–4954 CrossRef CAS.
- K. Zhang, L. L. Zhang, X. S. Zhao and J. Wu, Chem. Mater., 2010, 22, 1392–1401 CrossRef CAS.
- S. B. Aliev, D. G. Samsonenko, E. A. Maksimovskiy, E. O. Fedorovskaya, S. A. Sapchenko and V. P. Fedin, New J. Chem., 2016, 40, 5306–5312 RSC.
- Y. Ko, Y. J. Lee, D. Kim, U.-H. Lee and J. You, ACS Appl. Electron. Mater., 2024, 6, 1045–1054 CrossRef CAS.
- A. Alameen, T. Jin, C. Xue, X. Ma, X. Du and X. Hao, J. Solid State Electrochem., 2021, 25, 777–787 CrossRef CAS.
- X. Li, Y. Liu, M. Gao, X. Zhao and K. Cai, J. Power Sources, 2023, 580, 233455 CrossRef CAS.
- R. R. Salunkhe, J. Tang, N. Kobayashi, J. Kim, Y. Ide, S. Tominaka, J. H. Kim and Y. Yamauchi, Chem. Sci., 2016, 7, 5704–5713 RSC.
- K. Chhetri, A. P. Tiwari, B. Dahal, G. P. Ojha, T. Mukhiya, M. Lee, T. Kim, S.-H. Chae, A. Muthurasu and H. Y. Kim, J. Electroanal. Chem., 2020, 856, 113670 CrossRef CAS.
- M. Shang, X. Zhang, J. Zhang, J. Sun, X. Zhao, S. Yu, X. Liu, B. Liu and X. Yi, Carbohydr. Polym., 2021, 262, 117966 CrossRef CAS.
- W. A. Marmisollé, E. Maza, S. Moya and O. Azzaroni, Electrochim. Acta, 2016, 210, 435–444 CrossRef.
- K. Ariga, J. P. Hill and Q. Ji, Phys. Chem. Chem. Phys., 2007, 9, 2319 RSC.
- R. M. Iost and F. N. Crespilho, Biosens. Bioelectron., 2012, 31, 1–10 CrossRef CAS PubMed.
- G. Decher, B. Lehr, K. Lowack, Y. Lvov and J. Schmitt, Biosens. Bioelectron., 1994, 9, 677–684 CrossRef CAS.
- A. Muzaffar, M. B. Ahamed and C. M. Hussain, Smart Supercapacitors, Elsevier, 2023, pp. 227–254 Search PubMed.
- W.-S. Huang, B. D. Humphrey and A. G. MacDiarmid, J. Chem. Soc., Faraday Trans., 1986, 82, 2385 RSC.
- G. Challa and Y. Y. Tan, Pure Appl. Chem., 1981, 53, 627–641 CrossRef CAS.
- A. M. Bonastre, M. Sosna and P. N. Bartlett, Phys. Chem. Chem. Phys., 2011, 13, 5365 RSC.
- A. P. Mártire, G. M. Segovia, O. Azzaroni, M. Rafti and W. Marmisollé, Mol. Syst. Des. Eng., 2019, 4, 893–900 RSC.
- G. E. Fenoy, B. Van der Schueren, J. Scotto, F. Boulmedais, M. R. Ceolín, S. Bégin-Colin, D. Bégin, W. A. Marmisollé and O. Azzaroni, Electrochim. Acta, 2018, 283, 1178–1187 CrossRef CAS.
- A. P. Mártire, G. E. Fenoy, O. Azzaroni, M. Rafti and W. A. Marmisollé, RSC Appl. Interfaces, 2024, 1, 511–521 RSC.
- J. A. Allegretto, M. Arcidiácono, P. Y. Steinberg, P. C. Angelomé, O. Azzaroni and M. Rafti, J. Phys. Chem. C, 2022, 126, 6724–6735 CrossRef CAS.
- J. Cravillon, R. Nayuk, S. Springer, A. Feldhoff, K. Huber and M. Wiebcke, Chem. Mater., 2011, 23, 2130–2141 CrossRef CAS.
- A. M. Rao, E. Richter, S. Bandow, B. Chase, P. C. Eklund, K. A. Williams, S. Fang, K. R. Subbaswamy, M. Menon, A. Thess, R. E. Smalley, G. Dresselhaus and M. S. Dresselhaus, Science, 1997, 275, 187–191 CrossRef CAS.
- L. G. Cançado, A. Jorio, E. H. M. Ferreira, F. Stavale, C. A. Achete, R. B. Capaz, M. V. O. Moutinho, A. Lombardo, T. S. Kulmala and A. C. Ferrari, Nano Lett., 2011, 11, 3190–3196 CrossRef.
- A. C. Ferrari and D. M. Basko, Nat. Nanotechnol., 2013, 8, 235–246 CrossRef CAS.
- A. J. Bard, L. R. Faulkner and H. S. White, Electrochemical methods: fundamentals and applications, Wiley, 3rd edn, 2022 Search PubMed.
- S. Ardizzone, G. Fregonara and S. Trasatti, Electrochim. Acta, 1990, 35, 263–267 CrossRef CAS.
- J. Wang, J. Polleux, J. Lim and B. Dunn, J. Phys. Chem. C, 2007, 111, 14925–14931 CrossRef CAS.
- N. M. Deyab, M. M. Taha and N. K. Allam, Nanoscale Adv., 2022, 4, 1387–1393 RSC.
- A. Cymann-Sachajdak, M. Graczyk-Zajac, G. Trykowski and M. Wilamowska-Zawłocka, Electrochim. Acta, 2021, 383, 138356 CrossRef CAS.
- S. Harish and P. U. Sathyakam, J. Electron. Mater., 2025, 54, 10858–10872 CrossRef CAS.
- J.-W. Jeon, S. R. Kwon and J. L. Lutkenhaus, J. Mater. Chem. A, 2015, 3, 3757–3767 RSC.
- T. Liu, L. Finn, M. Yu, H. Wang, T. Zhai, X. Lu, Y. Tong and Y. Li, Nano Lett., 2014, 14, 2522–2527 CrossRef CAS.
- O. Fasakin, K. O. Oyedotun, A. A. Mirghni, N. F. Sylla, B. A. Mahmoud and N. Manyala, Mater. Today Sustain., 2024, 28, 101028 Search PubMed.
- H. Jia, J. Zhu, L. Wang, S. Sang, W. Liu and F. Chong, J. Power Sources, 2025, 625, 235718 CrossRef CAS.
- G. Inzelt, in Electroanalytical Methods: Guide to experiments and applications, ed. F. Scholtz, Springer Berlin Heidelberg, 2010 Search PubMed.
- M. R. Deakin and D. A. Buttry, Anal. Chem., 1989, 61, 1147A–1154A CrossRef CAS.
- L. P. Bauermann and P. N. Bartlett, Electrochim. Acta, 2005, 50, 1537–1546 CrossRef CAS.
- X. Du, D. Zhang, X. Ma, W. Qiao, Z. Wang, X. Hao and G. Guan, Electrochim. Acta, 2018, 282, 384–394 CrossRef CAS.
- B. Zhang, X. Du, X. Hao, F. Gao, D. Zhang, C. Liu and G. Guan, J. Solid State Electrochem., 2018, 22, 2473–2483 CrossRef CAS.
- J. Wu, X. Zhang, F. Wei, Y. Sui and J. Qi, Mater. Lett., 2020, 258, 126761 CrossRef CAS.
- C. Guo, G. Li, Y. Wu, X. Wang, Y. Niu and J. Wu, Energies, 2023, 16, 7232 CrossRef CAS.
- Z. Shang, X. An, L. Liu, J. Yang, W. Zhang, H. Dai, H. Cao, Q. Xu, H. Liu and Y. Ni, Carbohydr. Polym., 2021, 251, 117107 CrossRef CAS.
- Y. Wang, T. Liu, X. Lin, H. Chen, S. Chen, Z. Jiang, Y. Chen, J. Liu, J. Huang and M. Liu, ACS Sustainable Chem. Eng., 2018, 6, 13932–13939 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2026 |