Oxygen-free discontinuous dewetting in a degassed mold for anisotropic colloidal hydrogel microparticle synthesis
Jiwoo
Kim
 a,
Jun Hee
Choi
a,
Jun Hee
Choi
 a,
Jeongmin
Kim
a,
Jeongmin
Kim
 b,
Jihyun
Kim
b,
Jihyun
Kim
 b,
Yoon Ho
Roh
*cd and
Ki Wan
Bong
b,
Yoon Ho
Roh
*cd and
Ki Wan
Bong
 *a
*a
aDepartment of Chemical and Biological Engineering, Korea University, Seoul, 02841, Republic of Korea. E-mail: bong98@korea.ac.kr
bDepartment of Chemical and Biological Engineering, Seoul National University, Seoul, 08826, Republic of Korea
cDepartment of Energy and Chemical Engineering, Incheon National University, Incheon, 22012, Republic of Korea
dInnovation Center for Chemical Engineering, Incheon National University, Incheon 22012, Republic of Korea
First published on 14th November 2025
Abstract
Anisotropic colloidal hydrogel microparticles offer unique advantages owing to their shape-dependent interactions, and enhanced diffusion and reaction kinetics, which is favorable in solution-based processes. However, their fabrication remains difficult, mainly due to limitations of surface energy and oxygen inhibition. In this study, we present a versatile synthesis platform for anisotropic colloidal hydrogel microparticles using oxygen-free discontinuous dewetting in a degassed mold. By a simple substitution of ambient air to inert gas, oxygen inhibition is effectively suppressed, achieving fabrication of particles with feature sizes as small as 600 nm. The colloidal nature of the synthesized hydrogel particles was verified by observing Brownian motion, and the resolution improvement was quantitatively confirmed by comparing the feature fidelity under atmospheric and oxygen-free conditions. Computational analysis revealed a sharp decrease in oxygen concentration near the mold surface under inert conditions, supporting the experimental findings. To demonstrate the applicability of the hydrogel particles, we analyzed the residual acrylate double bonds, which can act as functional moieties for straightforward post-synthetic modification. Finally, as an application of enhanced resolution of the particles, we conduct imaging flow cytometry using particles with few-micrometer dimensions and achieved an analytical throughput surpassing those of previous studies employing hundred-micrometer-scale hydrogel particles without compromising the decoding accuracy. This is made possible by the oxygen-free discontinuous dewetting in a degassed mold, which enabled the fabrication of colloidal hydrogel particles. This work provides a robust and scalable method for producing anisotropic colloidal hydrogel microparticles, enabling broader applications in microscale material systems and high-throughput analytical technologies.
1. Introduction
Anisotropic hydrogel microparticles, compared to spherical ones, interact with diverse systems according to their morphology1,2 and chemical features, making them important in fields including biological sensing,3–6 drug delivery,7,8 photonics9,10 and self-assembly.11–13 For example, in biological sensing, the anisotropic shapes of the particles encode the target molecules, allowing simple and intuitive decoding compared to conventional fluorescence coding methods.14 Although many platforms to synthesize anisotropic particles were developed, their synthesis in the colloidal size regime is yet challenging due to the surface energy tending to form a spherical shape. The synthesis of anisotropic colloidal microparticles are crucial owing to their advantage in the surface-to-volume ratio, and enhanced diffusion and reaction kinetics compared to larger particles, which is favorable in solution-based processes. One of the most general methods to synthesize colloidal particles is emulsification, but this ‘bottom up’ approach lacks freedom in tuning the structure of the particles. Also, the resulting particles are sensitive to the precursor composition and the surrounding environment, such as salt concentration and pH of the buffer solution, restraining broader applications.15,16Flow lithography, a widely adopted anisotropic hydrogel microparticle synthesis technique, relies on gas-permeable microfluidic channels to fabricate the particles.17,18 While the particle precursor liquid flows inside the channel, shaped ultraviolet (UV) light is orthogonally irradiated to the precursor, which then is polymerized into the form of the projection of the UV shape. At the same time, the ambient oxygen in the air diffuses through the device and forms a layer near the channel walls that hinder the polymer chain elongation. This inhibition effect, though essential for producing free-floating particles that do not adhere to the channel walls, significantly restricts the resolution of the resulting particles. In order to alleviate this limitation, oxygen-controlled stop flow lithography was developed.19 Nevertheless, they are technically limited in real use as the flow lithographic processes are not feasible when the oxygen is completely removed. This is because the polymerization occurs at the channel walls when the oxygen level gets low, leading to excessive particle sticking and clogging in the microfluidic channels. Also, its low productivity hampers its scalability and adoption to industrial applications.
Micromolding offers another attractive method for the synthesis of anisotropic hydrogel microparticles.20,21 Micromolding utilizes arrays of negative features, which correspond to the intended shape and dimensions of the resulting particles. Elastomeric materials such as polydimethylsiloxane (PDMS)22 and polyurethane23 are commonly used for the mold materials owing to their applicability to soft lithography, enabling reproducible and repetitious fabrication. Micromolding is more advantageous in terms of productivity compared to flow lithography, having a throughput of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 particles per second.24 It is also scalable due to its applicability to roll-to-roll or roll-to-plate processing.25 Particle replication in nonwetting templates (PRINT) is one of the representative commercialized micromolding technique.26–29 In the PRINT process, the precursor is put on a non-wetting mold such as perfluoropolyether (PFPE) and covered with another PFPE block. When pressure is applied, the excess precursor liquid is drained out due to the low surface free energy of the template, producing isolated particles with a resolution of a few ten nanometers. However, their use in particle fabrication with dimensions larger than the micrometer scale is limited due to air bubble entrapment inside the mold. Furthermore, PFPE molds are costly and require complicated manufacturing processes, limiting their accessibility for widespread use.30
000 particles per second.24 It is also scalable due to its applicability to roll-to-roll or roll-to-plate processing.25 Particle replication in nonwetting templates (PRINT) is one of the representative commercialized micromolding technique.26–29 In the PRINT process, the precursor is put on a non-wetting mold such as perfluoropolyether (PFPE) and covered with another PFPE block. When pressure is applied, the excess precursor liquid is drained out due to the low surface free energy of the template, producing isolated particles with a resolution of a few ten nanometers. However, their use in particle fabrication with dimensions larger than the micrometer scale is limited due to air bubble entrapment inside the mold. Furthermore, PFPE molds are costly and require complicated manufacturing processes, limiting their accessibility for widespread use.30
Recently, discontinuous dewetting of the liquid precursor in a degassed micromold is emerging as a promising method for fabricating polymeric microparticles.24 In this method, the gas-permeable degassed mold serves as a pump to compulsorily fill the mold cavity with the precursor. This allows various liquids to be filled in the mold cavity regardless of the precursor composition.31,32 Also, the liquid loading time is reduced 60-fold compared to the non-degassed process by simply locating the PDMS mold in the vacuum chamber for 15 min beforehand. However, a key limitation still remains: oxygen is rapidly reabsorbed into the mold within a second, forming an inhibition layer near the gas permeable mold walls that interferes with free-radical polymerization. This effect restricts resolution and hinders the reliable synthesis of particles in the few-micrometer regime. Therefore, to fabricate hydrogel particles in the few-micrometer regime, a strategy that prevents oxygen inhibition and rapid reabsorption during polymerization is essential.
In this study, we present a versatile anisotropic colloidal hydrogel microparticle synthesis platform using oxygen-free discontinuous dewetting in a degassed mold. By a simple substitution of ambient air to an inert gas, we effectively suppress the oxygen inhibition reaction during particle polymerization, achieving the production of particles with the feature size as small as 600 nm. The colloidal nature of the synthesized hydrogel particles was validated by observing their Brownian motions. Also, we demonstrate that the developed strategy significantly improves the particle resolution compared to the conventional atmospheric conditions. To elucidate the underlying mechanism, we perform computational analysis of oxygen concentration in the PDMS mold under both air and oxygen-free conditions. Furthermore, to demonstrate the utility of the synthesized hydrogel particles, we evaluate the amount of unreacted acrylate double bonds within the fabricated particles that can serve as functional moieties permitting facile functionalization of the particles. Finally, as an application of enhanced resolution and morphological accuracy of the particles, we conduct imaging flow cytometry using particles with few-micrometer dimensions. Consequently, we achieve an analytical throughput exceeding that of the previous report employing hydrogel particles of hundred-micrometer dimensions without compromising the decoding accuracy. This is made possible by the oxygen-free discontinuous dewetting in a degassed mold, which enabled the fabrication of colloidal hydrogel particles.
2. Experimental
2.1 Materials
The photoresist SU-8 was purchased from MicroChem (USA), and silicon wafer was purchased from Growth materials (Republic of Korea). The polydimethylsiloxane (PDMS) base (Sylgard A) and curing agent (Sylgard B) were purchased from Dow Corning (USA). Polyethylene glycol (PEG, MW 200 Da), polyethylene glycol diacrylate (PEGDA, MW 700 Da), 2-hydroxy-2-methylpropiophenone (Darocur 1173), Tween 20 and trichloro(1H,1H,2H,2H-perfluorooctyl)silane were purchased from Sigma Aldrich (USA). Phosphate buffered saline (PBS) tablets were purchased from Takara Bio Inc. (Japan). Tris(2-carboxyethyl)phosphine (TCEP) and streptavidin–phycoerythrin (SA–PE) were purchased from Thermo Fisher Scientific (USA). Argon gas was purchased from Dong-A Specialty Gases (Republic of Korea). Thiol–PEG–FITC (fluorescein isothiocyanate) and thiol–PEG–biotin were purchased from Biopharma PEG (USA). Methacryloxyethyl thiocarbamoyl rhodamine B was obtained from Polysciences (USA). Nucleic acid was synthesized by Integrated DNA Technologies (USA). The antibody was purchased from Bioss (USA). Norland optical adhesive 81 (NOA 81) was purchased from Norland Products Inc. (USA).2.2 Fabrication of the PDMS mold with soft lithography
Silicon wafers (master mold) were patterned with either the negative photoresist SU-8 or metal. Patterns with feature sizes above 10 μm used SU-8 and those with feature sizes below 10 μm used metal deposition. Detailed explanation of the silicon wafer patterning process using the metal deposition method is provided in the SI (Fig. S1). To obtain the PDMS mold from the master mold for particle synthesis, soft lithography was used. The PDMS base Sylgard A and the curing agent Sylgard B was mixed thoroughly in a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and poured on the patterned silicon wafer. After curing at 70 °C for 2 h, the elastomer was peeled off from the silicon wafer. The individual molds were acquired by slicing the PDMS along the pre-designed instruction line. The size of each individual mold was approximately 20 mm × 20 mm × 5 mm.
1 ratio and poured on the patterned silicon wafer. After curing at 70 °C for 2 h, the elastomer was peeled off from the silicon wafer. The individual molds were acquired by slicing the PDMS along the pre-designed instruction line. The size of each individual mold was approximately 20 mm × 20 mm × 5 mm.
2.3 Oxygen-free hydrogel microparticle synthesis procedure
The particles were synthesized in a glove box with the atmosphere substituted to argon gas. The oxygen concentration inside the glove box was maintained at 0.0%. The oxygen level was measured with an oxygen meter (G-Finder Single, GASTRON, Republic of Korea). The PDMS mold was degassed at 0.1 atm for 15 min in advance and placed inside the glove box. Then, the particle precursor of 10 μL was dropped onto the degassed PDMS mold. The precursor was composed of PEG (MW 200 Da) 75 v/v%, PEGDA (MW 700 Da) 20 v/v% and Darocur 1173 5 v/v% for all cases, unless specified. After placing a cover glass on the mold, the cover glass was moved horizontally at a steady rate leading to discontinuous dewetting of the excess precursor.24 The precursor left inside the negative pattern of the microwells was irradiated with ultraviolet light (UV) of 207 mW cm−2 for the time specified. The harvesting of the polymerized particles was carried out with dropping 200 μL of 1× PBS containing 0.05 v/v% Tween 20 (PBST) on the mold and placing at −70 °C for 20 min. The frozen PBST chunk, particles attached below, was demounted from the mold and transferred to a 5 mL tube and melted. The particles were rinsed by centrifuging and substituting 450 μL of the supernatant with PBST five times.2.4 Particle imaging and analysis
The fluorescence and bright field images of the particles were taken with an inverted microscope (Axiovert 200, Zeiss, Germany) and a scientific complementary metal-oxide-semiconductor (CMOS) camera (Prime BSI express, Teledyne Photomatics, USA). A fluorescence light source (LQ-HXP 120, Zeiss, Germany) and a filter set of λex/λem = 540/605 nm with a 565 nm dichroic mirror were used to obtain SA–PE fluorescence images of the particles. For FITC and FAM fluorescence images, a filter set of λex/λem = 480/535 nm with a 505 nm dichroic mirror were used.Scanning electron microscope (SU5000, HITACHI, Japan) images of the silicon wafer and the hydrogel microparticles were taken at magnification of 3000×. The accelerating voltage was 5.0 kV for all cases. The hydrogel particles were freeze-dried and coated with Pt before imaging.
A confocal laser scanning microscope (LSM700, Carl Zeiss, Germany) was used to acquire the fluorescence images at the central plane of the hydrogel microparticles. The z-stack images were taken with a pixel dwell of 3.15 μs and a frame size of 1024 × 1024, 16 bit. The pinhole was set at 0.50 Airy units for high resolution.
2.5 Simulation of the oxygen concentration
The numerical simulations of the oxygen concentration profiles within the PDMS mold were performed over time, following exposure to either atmospheric or oxygen-free conditions using the Transport of Diluted Species module in COMSOL Multiphysics 6.1 software (USA). The mold geometry included a cubic cavity with dimensions of 10 μm per side, embedded within a PDMS block measuring 20 mm × 20 mm × 5 mm. The initial oxygen concentration of PDMS was set to 0.102 mol m−3, reflecting the level achieved through degassing at 0.1 atm of air. The diffusion coefficient of oxygen in PDMS was set to 3.4 × 10−9 m2 s−1, and the equilibrium concentration of oxygen under ambient conditions was defined as 1.02 mol m−3.2.6 Particle tracking for Brownian motion
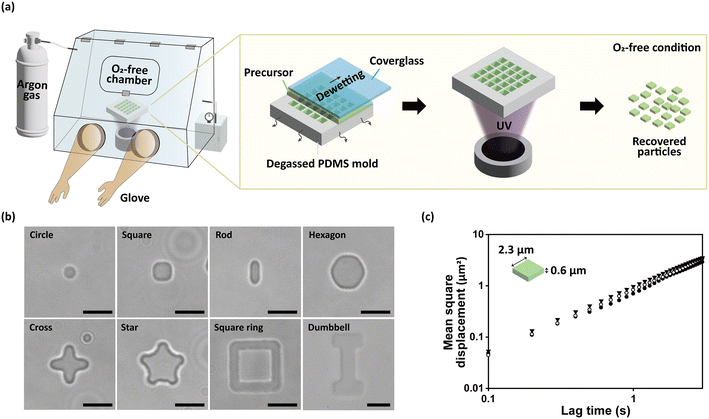
The particles utilized for demonstrating the Brownian motion of the particles were in the shape of a flat cuboid. The cuboids had dimensions of 2.3 μm × 2.3 μm × 600 nm. The precursor of the hydrogel microparticles were PEG 20 v/v%, PEGDA 65 v/v%, methacryloxyethyl thiocarbamoyl rhodamine B 10 v/v% and Darocur 1173 5 v/v%. Particles were synthesized under oxygen-free conditions, as illustrated in section 2.3. After the particles were harvested and rinsed with PBST, the buffer was substituted to deionized water. The particles suspended in deionized water were dropped on a slide glass and observed with the inverted microscope. The fluorescence images of the particles were taken continuously with 100 ms interval of time. The trajectory of the particles was analysed with ImageJ software (National Institutes of Health, USA).2.7 Functionalization of the hydrogel microparticles
After the hydrogel microparticles were synthesized, they were conjugated with the desired materials. In the case of SH–PEG–-FITC and SH–PEG–biotin, the particles were suspended in 90 μL of PBST and reacted with 10 μL of 1 mg mL−1 SH–PEG–FITC or SH–PEG–biotin at 37 °C for 48 h at 1500 rpm of shaking. As for the DNA probe, 5 μL of 1 mM DNA probe was treated with the equal amount of 0.5 mM TCEP for 1 h at the benchtop to eliminate the undesired linkage between themselves. Then, the DNA and TCEP mixture was reacted with 145 μL of the particle solution for 48 hours at 37 °C with 1500 rpm of shaking. In the case of the antibody, 5 μL of the antibody solution was reduced with 5 μL of 0.2 mM TCEP for 1 h at the benchtop and reacted with 10 μL of the hydrogel microparticles at 25 °C with 1500 rpm of shaking. For all cases, after the reaction was terminated, the particles were rinsed with PBST five times.2.8 Imaging flow cytometry
To fabricate the imaging flow channels, the negative patterns of the channel were replicated from the silicon wafer master mold by soft lithography with PDMS. After placing the peeled-off PDMS block to a new petri dish and exposing it to trichloro(1H,1H,2H,2H-perfluorooctyl)silane, PDMS of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (base
1 (base![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) curing agent) was poured and baked at 70 °C for 2 h. The PDMS block with the positive relief was peeled off and degassed for 15 min at 0.1 atm. Then, PDMS was transferred to the atmospheric conditions, and Norland optical adhesive 81 (NOA 81) was poured and cured by irradiating UV of 3.0 mW cm−2 for 3 min. The NOA channel with negative relief was peeled off, sliced into individual channels and attached to a cover glass, which was also coated with cured NOA. The width of the channel was 900 μm. The fabricated channels were affixed with fluid inlet/outlet connectors for particle solution injection and removal. The illustration of the imaging channel fabrication is provided in the SI (Fig. S2).33
curing agent) was poured and baked at 70 °C for 2 h. The PDMS block with the positive relief was peeled off and degassed for 15 min at 0.1 atm. Then, PDMS was transferred to the atmospheric conditions, and Norland optical adhesive 81 (NOA 81) was poured and cured by irradiating UV of 3.0 mW cm−2 for 3 min. The NOA channel with negative relief was peeled off, sliced into individual channels and attached to a cover glass, which was also coated with cured NOA. The width of the channel was 900 μm. The fabricated channels were affixed with fluid inlet/outlet connectors for particle solution injection and removal. The illustration of the imaging channel fabrication is provided in the SI (Fig. S2).33
The hydrogel microparticles were conjugated to SH–PEG–biotin and reacted with streptavidin–phycoerythrin (SA–PE) at 25 °C for 30 min. The fluorescence-labelled particles were rinsed five times with PBST. 1 w/v% PVA was included in 100 μL of the particle solution and vortexed thoroughly. The particles occupied approximately 1 v/v% of the total buffer solution. The particles were injected into the NOA channel inlet through a Tygon tube with argon gas by a pressure of 50 kPa. As the particles passed through the center of the imaging channel, bright field and fluorescence images were taken simultaneously with an exposure time of 10 ms. The total time to take one frame of the microparticles was approximately 1.61 s. The objective of the microscope was 4× and the camera had a CMOS sensor (a2A1920-160umBAS, Basler, Germany). The field of view of the imaging device was 1658 μm × 1037 μm. The captured images were analysed with a deep learning-based program written in Python (Fig. S3).6,34,35
3. Results and discussion
3.1 Oxygen-free discontinuous dewetting in a degassed mold
As reported by previous studies, the low wettability of the polydimethylsiloxane (PDMS) mold and the air bubble entrapment between the mold and the precursor liquid occurred by capillary repulsion hinders the complete filling of the precursor into individual microwells.36,37 To resolve this problem, discontinuous dewetting in a degassed mold was recently developed.24 Discontinuous dewetting in a degassed mold is a polymeric microparticle fabrication method that exploits negative pressure to forcibly draw the precursor into the microwells regardless of the wettability. By simply degassing the mold before the liquid loading step, the gas-permeable PDMS mold serves as a pump generating negative pressure to absorb the trapped air bubbles, facilitating the complete filling of the microwells. After the loading of the precursor, discontinuous dewetting is performed by gently sliding the cover glass placed above the PDMS mold and the precursor layer. This process is able to produce a large uniform array of the isolated particle precursor within a minute. When ultraviolet light is irradiated to the precursor array, the precursor is polymerized into individual hydrogel particles. Although this method is advantageous in fabricating highly uniform anisotropic hydrogel microparticles, it is inherently limited in synthesizing few micrometer-scale particles due to the gas permeability of PDMS. When the mold is dislocated from the desiccator after the degassing step, air permeates through the PDMS mold again within a second, hampering the polymerization of the precursor near the PDMS wall. The detailed chemical reaction equations are stated as follows: | (1) |
| Ṙ + M → RṀ | (2) |
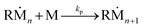 | (3) |
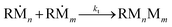 | (4) |
 | (5) |
To demonstrate our concept, we synthesized colloidal-sized hydrogel particles under oxygen-free conditions. The overall schematics of the oxygen-free discontinuous dewetting in a degassed mold for anisotropic colloidal hydrogel particle fabrication is illustrated in Fig. 1a. To rule out the effect of oxygen in the atmosphere, we substituted the air containing 21% oxygen to argon gas in a glove box. The air was substituted to argon until the oxygen level was 0.0% and kept constant. The hydrogel microparticle precursor was composed of 75 v/v% polyethylene glycol 200 (PEG 200), 20 v/v% polyethylene glycol diacrylate 700 (PEGDA 700), and 5 v/v% Darocur 1173, unless mentioned otherwise. The PDMS mold and the particle precursor were degassed for 15 min at 0.1 atm before entering the oxygen-free glove box. After the precursor was dropped on the PDMS mold, cover glass was placed on the mold instantly. Within 10 s, the microwells were completely filled with the liquid precursor without any entrapped air bubbles, identical to the conventional method.24 Following the discontinuous dewetting and the UV irradiation, particles were harvested by applying PBST on the microwells and freezing them. The microscopic images of the recovered particles are shown in Fig. 1b. The height of all the particles was 600 nm since the patterns of the silicon wafer (the master mold) had a uniform height of 600 nm. The atomic force microscopy (AFM) image of the silicon wafer is presented in Fig. S4 to show the factual height of the pattern. The smallest dimension of the synthesized particles was in the form of a cylinder with a 775 nm radius and 600 nm height, corresponding to the resolution of our platform. The SEM images of the cylinder particles are presented in Fig. S9. The length of one side of the square particles was 2.27 μm, and the length of the long side of the rod particles was 3.47 μm. As one can intuitively perceive, the length of these particles is clearly distinguishable. The smallest feature size in the in-plane direction is 1.19 μm, which corresponds to the thickness of the square ring. Furthermore, we were able to fabricate anisotropic hydrogel particles with complicated shapes of a hexagon, cross, star and dumbbell. The scanning electron microscope (SEM) images of the master mold are provided in Fig. S5. The coefficient of variation of the particle fluorescence intensity was 2.70%, verifying the chemical uniformity of our method.
The synthesized anisotropic colloidal particles can be utilized in diverse advanced applications. For example, since rod-like particles are known to have different cellular uptake according to their orientation, it can be utilized in targeted drug delivery and intracellular diagnostics.40 Star-shaped particles, characterized by their multiple protrusions, are potentially advantageous for multivalent biomolecular interactions, such as in cell–particle adhesion studies or pathogen capture, where an enhanced local surface area facilitates increased binding sites and reaction kinetics.41 Square-ring particles can be utilized as drug-loading carriers in intravenous injection by taking advantage of their hollow-centered morphologies, making them easily bend in the blood vessels like red blood cells.42 Dumbbell-shaped particles, composed of dual lobes with an anisotropic mass distribution, can serve as directional building blocks for self-assembly and can be actuated by external magnetic or acoustic fields when loaded with magnetic beads.43,44
To validate that the synthesized particles have colloidal nature, the Brownian motion of the hydrogel particles were evaluated. The colloidal particles are well known to show Brownian motion, which refers to moving randomly when suspended in fluids.45 The most widely used method to prove the Brownian motion of the particles is to calculate the mean square displacement (MSD, 〈ΔR2(τ)〉) from the particle trajectory. The formula to calculate the MSD is as follows:
| 〈ΔR2(τ)〉 = 〈(R(t + τ) − (R(t)2)〉 | (6) |
| 〈ΔR2(τ)〉 = 4Dτ | (7) |
The novelty of oxygen-free discontinuous dewetting in a degassed mold lies in its ability to bridge the gap between colloidal and microscale particle fabrication within a single platform. While there are many technologies to produce polymeric particles, they usually focus on a rather narrow range of the particle size.20,28 However, our approach offers fabrication across a broad size spectrum with precise control over particle anisotropy regardless of the precursor wettability. The detailed explanation on the synthesizable particle size range and the utilization of our method to synthesize a few hundred micrometer-scale hydrogel particles is presented in Fig. S7. The applicability of our platform to other polymers beyond hydrogels is also provided in Fig. S10. The square, cross and star-shaped particles were successfully made with Norland optical adhesive 81 (NOA 81) epoxy resin. Also, while it has been conventionally difficult to fabricate anisotropic colloidal particles with merely using common laboratory materials, our platform enables simple and straightforward synthesis of colloidal particles with diverse shapes. This versatility and scalability render oxygen-free discontinuous dewetting in a degassed mold pertinent to numerous fields such as drug delivery and biological sensors.
3.2 Enhancement in resolution of the hydrogel microparticles
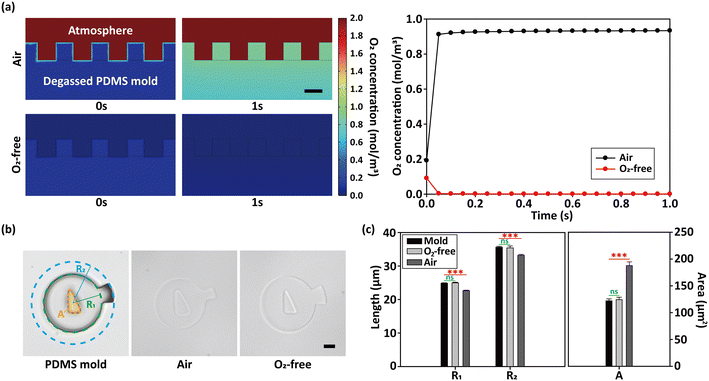
Since oxygen plays a critical role in inhibiting the polymerization of the hydrogel precursor near the PDMS interface, understanding its concentration and distribution is essential. To numerically compare the oxygen concentration profile within the PDMS mold and its surrounding environment, computational analysis was performed. The initial oxygen concentration of PDMS was set to 0.102 mol m−3 since the degassing is performed by placing the PDMS mold in the desiccator at 0.1 atm. The simulation was conducted for 1 s with 0.05 s intervals, starting immediately after the degassing is terminated. As a result, under the atmosphere conditions, the oxygen concentration of PDMS rapidly increased, recovering the ambient level within 0.1 s (Fig. 2a). In contrast, when the mold was transferred from the desiccator to the oxygen-free glove box, it decreased to 0 mol m−3 as expected, due to the lower oxygen level in the glove box compared to the desiccator.To demonstrate that the decrease in the oxygen level can actually lead to the enhancement in the hydrogel microparticle resolution compared to the conventional method, we synthesized hydrogel particles with inner and outer geometrical features by the two fabrication methods. The particles had a square protrusion and an inner right triangle hole (Fig. 2b). The body circle radius (R1), the outer circle radius (R2) and the area of the inner triangle (A) of the PDMS mold were measured to be 24.863 μm, 35.598 μm and 122.072 μm2, respectively. The particle suspension buffer composed of 75 v/v% PEGDA and 25 v/v% PEG with 0.05% Tween 20, a similar composition to the particle precursor, was used to preclude the particle swelling. The photoinitiator was not included in the buffer since it can cause further radical reactions in the particles. To statistically assess the fidelity of the particles, F-test and two-tailed t-test were performed between the mold and each case. As a result, the particles fabricated under the atmospheric conditions had a R1 of 22.666 μm , R2 of 33.216 μm of R2 and an A of 187.804 μm2 (p = 7.43 × 10−9, 6.79 × 10−7, 7.28 × 10−8; ***). These results show that the overall size of the ambient air-processed particles were fabricated smaller, but had a bigger hole compared to the PDMS mold. This is because oxygen diffuses through both near the outer frame and the triangle pillar (corresponding to the cavity of the particle) of the PDMS microwell and hinders the polymerization reaction. On the other hand, particles synthesized under oxygen-free conditiosn had a R1 of 24.987 μm, R2 of 35.415 μm and an A of 124.317 μm2, exhibiting non-significant difference with the mold. Therefore, it was validated that the oxygen-free discontinuous dewetting in a degassed mold is able to produce anisotropic hydrogel particles with higher resolution compared to the conventional method. Under atmospheric conditions, the inhibition layer thickness (δ) was calculated to be approximately 2.290 μm by subtracting the particle R1 and R2 values from those of the mold. Thus, mold patterns with the feature size smaller than around 5 μm are not able to be synthesized to hydrogel particles in the atmosphere and are only viable under the oxygen-free conditions.
3.3 Particle functionalization with post-synthesis treatment
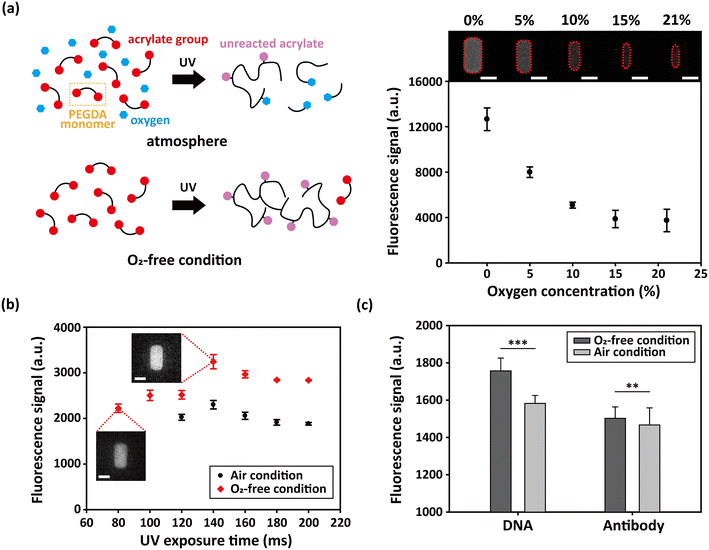
The PEG hydrogel microparticles have C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds remaining after the particle fabrication due to the unreacted acrylate groups in the PEGDA monomer.38,46 Although these residual acrylates may cause undesirable reactions due to their continued reactivity, they can also provide reactive sites for post-synthesis functionalization.14,47 For example, hydrogel microparticles can be functionalized by conjugating nucleic acids or proteins to the remnant acrylate groups of the particles to utilize them as biological sensors.48–50 Consequently, it is essential to quantify the amount of the unreacted double bonds within the hydrogel microparticles depending on their synthesis conditions. To examine the effect of oxygen concentration to the amount of unreacted acrylate double bonds (UADBs), we synthesized hydrogel particles in different oxygen concentrations and treated with thiol–PEG–FITC (fluorescein isothiocyanate). Since thiol groups react with the acrylate groups one-on-one, the fluorescence intensities of the particles indicate the amount of UADBs.51 In order to compare the developed oxygen-free discontinuous dewetting in a degassed mold with the conventional method, the feature size of the mold pattern was needed to be bigger than 5 μm, as stated in section 3.2. To exclude the effect of particle height on the fluorescence intensity, the particles were imaged with confocal microscopy. As a result, the decreased oxygen concentration led to an increased fluorescence signal (Fig. 3a), indicating a higher abundance of UADBs. This enhancement can be attributed to the reduced inhibitory effect of oxygen on radical polymerization. The presence of oxygen molecules quenched the reactive radicals, blocked the acrylate groups and reduced the efficiency of particle functionalization. In contrast, the removal of oxygen mitigated this inhibitory effect, allowing for more available acrylate groups. Additionally, under decreased oxygen conditions, the dimensions of the fabricated particles more closely matched the mold geometry (10 μm × 10 μm × 20 μm), again suggesting improved structural fidelity.
C double bonds remaining after the particle fabrication due to the unreacted acrylate groups in the PEGDA monomer.38,46 Although these residual acrylates may cause undesirable reactions due to their continued reactivity, they can also provide reactive sites for post-synthesis functionalization.14,47 For example, hydrogel microparticles can be functionalized by conjugating nucleic acids or proteins to the remnant acrylate groups of the particles to utilize them as biological sensors.48–50 Consequently, it is essential to quantify the amount of the unreacted double bonds within the hydrogel microparticles depending on their synthesis conditions. To examine the effect of oxygen concentration to the amount of unreacted acrylate double bonds (UADBs), we synthesized hydrogel particles in different oxygen concentrations and treated with thiol–PEG–FITC (fluorescein isothiocyanate). Since thiol groups react with the acrylate groups one-on-one, the fluorescence intensities of the particles indicate the amount of UADBs.51 In order to compare the developed oxygen-free discontinuous dewetting in a degassed mold with the conventional method, the feature size of the mold pattern was needed to be bigger than 5 μm, as stated in section 3.2. To exclude the effect of particle height on the fluorescence intensity, the particles were imaged with confocal microscopy. As a result, the decreased oxygen concentration led to an increased fluorescence signal (Fig. 3a), indicating a higher abundance of UADBs. This enhancement can be attributed to the reduced inhibitory effect of oxygen on radical polymerization. The presence of oxygen molecules quenched the reactive radicals, blocked the acrylate groups and reduced the efficiency of particle functionalization. In contrast, the removal of oxygen mitigated this inhibitory effect, allowing for more available acrylate groups. Additionally, under decreased oxygen conditions, the dimensions of the fabricated particles more closely matched the mold geometry (10 μm × 10 μm × 20 μm), again suggesting improved structural fidelity.
The hydrogel microparticles exhibit a higher abundance of UADBs under conditions of shorter UV exposure duration. However, if the UV exposure time is insufficient, the polymerization conversion remains too low to produce stable particles. This results in the existence of an optimal UV exposure time point at which the amount of UADBs is maximized.52 To identify this optimal point, we varied the UV exposure time from 80 ms to 200 ms in 20 ms increments. Particle synthesis was carried out using both the conventional (air) method and the oxygen-free method for comparison. The results revealed that the optimal point was 140 ms for both methods, but the signal intensity, indicative of the amount of UADBs, was 40% higher under oxygen-free conditions compared to that under air conditions (Fig. 3b). At all corresponding UV exposure times, the amount of UADBs was higher in the oxygen-free method. The particles were not able to be synthesized with an UV exposure time shorter than 120 ms under the air conditions. Also, while the peak time was the same for two cases, the signal reaches its maximum approximately 20 ms after the onset of particle formation (∼120 ms) under atmospheric conditions, whereas under oxygen-free conditions, the maximum is observed at a delayed time point. This can be explained by the differences in the amount of nascent polymer branches formed during the initial stage of the crosslinking.46 At the initial stage of the crosslinking reaction, the polymer networks are primarily composed of nascent structures—initiated monomer units that have been incorporated into polymer chains while retaining UADBs. Under atmospheric conditions, the formation of such nascent chains is limited by oxygen inhibition, and those that do form rapidly undergo further crosslinking, consuming the acrylate groups. On the other hand, in the absence of oxygen, it requires more time for the nascent structures to accumulate, thus shifting the peak time to the right. These results underscore the importance of oxygen levels in regulating the radical reaction and acrylate consumption, influencing the number of functional groups for post-synthetic particle modification.
Given that the UADBs is a key factor of post-synthetic hydrogel microparticle modification, we investigated how the differences in UADB abundance actually influence the biomolecule functionalization efficiency. Using the optimized UV exposure time, hydrogel particles were synthesized with each method and subsequently functionalized with single stranded DNA and IgG antibody. The ssDNA was modified with a thiol group at the 5′ end and labelled with 6-carboxyfluorescein (FAM) at the 3′ end. The antibodies were treated with TCEP to reduce the interchain disulfide bonds, generating two free thiol groups. They were also labelled with fluorescein isothiocyanate (FITC) for fluorescence signal generation. Thiol groups on both biomolecules enabled their simple conjugation to the hydrogel network via thiol-Michael addition click reaction. As a result, particles synthesized under oxygen-free conditions exhibited higher fluorescence intensities for both DNA and antibody conjugates (Fig. 3c), indicating increased biomolecule functionalization. Since fluorescence intensity is proportional to the amount of bound biomolecules, these results confirm that the oxygen-free method yields higher conjugation efficiency. Statistical analysis using the F-test and two-tailed t-test showed strong significance, with p-values of 3.24 × 10−11 (***) for DNA and 8.88 × 10−3 (**) for antibody conjugation. Mann–Whitney U test was also performed for further statistical reliability, acquiring p-values of 2.97 × 10−7 (***) for DNA and 5.54 × 10−4 (***) for antibody conjugation. The difference in signal intensity was more pronounced for DNA, likely due to its smaller size (∼2 nm) compared to the antibody (∼15 nm), which may facilitate more efficient diffusion into the hydrogel matrix.53 These findings demonstrate that the oxygen-free conditions enhance the functionalization efficiency of the hydrogel microparticles and improve their potential utility in biomolecular sensing and related applications.
3.4 Imaging flow cytometry with a few-micrometer scale hydrogel microparticles
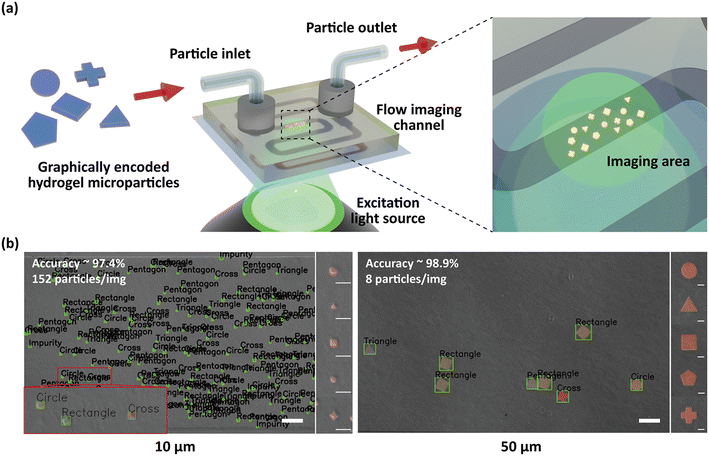
Imaging flow cytometry (IFC) integrates the high-throughput analytical capacity of conventional flow cytometry with the image-based analysis of the microscope, enabling comprehensive examination of individual analytes.54 When hydrogel microparticles are combined with the analytical performance of IFC, they can serve as a robust platform for offering detailed information of the size, shape and signal intensity of each particle. However, conventional hydrogel microparticles, typically in the order of tens or hundreds of micrometers in size, often encounter challenges in IFC due to low throughput and channel clogging.55 To overcome this limitation, we fabricated hydrogel microparticles with dimensions in the few-micrometer range and compared the analytical performance of IFC relative to the conventionally sized particles. The particles were prepared in circular, triangular, rectangular, pentagonal, and cross-like geometries with xy-plane dimensions of 10 μm and 50 μm, and vertical heights of 2 μm and 10 μm. The fabricated particles were labelled with streptavidin–phycoerythrin (SA–PE) by thiol–PEG–biotin conjugation and imaged in a NOA 81-based IFC device. The fluorescence and the bright field images were taken simultaneously using a 4× objective and a CMOS camera. The total time to take one frame of the microparticles was approximately 1.61 s. As a result, both particle groups successfully flowed through the channel, displaying clear morphological features and homogeneous fluorescence signals (Fig. 4 and S8). The decoding accuracy, defined as the ratio between the number of correctly decoded particles and the total number of the analysed particles, was 97.4% and 98.9% for 10 μm and 50 μm particles, respectively. Notably, the 10 μm particles exhibited decoding accuracy comparable to their 50 μm counterparts, owing to the high-resolution of the particles even at reduced dimensions (Fig. S3). The experimental throughput of the particles was approximately 152 particles and 8 particles per image for 10 μm and 50 μm, respectively, while the theoretical calculations suggest that a 25-fold enhancement in the throughput can be achieved. The deviation between the theoretical and experimental outcome can be explained by flow inconsistencies or random spatial distribution of the particles in suspension. By dividing the throughput with the time required, it was calculated that 94.4 particles can be analysed in a second. This represents a markedly improved throughput in IFC, exceeding that of prior studies employing hydrogel particles on the hundred-micrometer scale.55 Considering that the particle volume fraction was only 1%, there remains considerable potential to further enhance the throughput of our IFC analysis platform. These findings highlight the advantages of using few-micrometer hydrogel microparticles in IFC, offering substantial improvements in the throughput without compromising the analytical accuracy. This enhancement was made possible by the oxygen-free discontinuous dewetting in a degassed mold, which enabled the fabrication of particles with a height of 2 μm.4. Conclusions
In this study, we developed a robust platform for synthesizing anisotropic colloidal hydrogel microparticles with oxygen-free discontinuous dewetting in a degassed mold. By eliminating the oxygen inhibition effect, we successfully fabricated particles with feature sizes down to 600 nm, efficiently overcoming the longstanding resolution limitations of conventional micromolding techniques. This advancement enabled the fabrication of both colloidal and microscale particles within one platform by utilizing only common laboratory instruments. The colloidal nature of the synthesized particles was confirmed via Brownian motion analysis, and the improved morphological fidelity was validated both experimentally and computationally. Additionally, the increased amount of unreacted acrylate groups within the particle matrix offers more efficient post-synthetic functionalization compared to conventional fabrication methods. The demonstrated improvement in imaging flow cytometry throughput using few-micrometer particles highlights its practical utility in high-throughput analysis. This work not only advances the capabilities of hydrogel particle fabrication but also broadens their applicability in high-throughput analysis platforms. Future studies may expand their utilization by integrating diverse chemistries and geometries for targeted delivery and stimuli-responsive systems, further unlocking the potential of anisotropic colloidal hydrogel microparticles in fields of complex biomedical and materials science.Author contributions
Jiwoo Kim: conceptualization, methodology, validation, visualization, writing – original draft, and writing – review & editing. Jun Hee Choi: software, validation, visualization, and writing – review & editing. Jeongmin Kim: resources, methodology, and data curation. Jihyun Kim: resources, methodology, and writing – review & editing. Yoon Ho Roh: resources, supervision, and writing – review & editing. Ki Wan Bong: conceptualization, methodology, visualization, writing – original draft, writing – review & editing, supervision, project administration, and funding acquisition.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI).Supplementary information is available. See DOI: https://doi.org/10.1039/d5lc00928f.
Acknowledgements
This work was supported by the Technology Innovation Program (20018111, Development of super-fast multiplex technology for the examination of diagnosis of infectious disease and in-body response test) funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea). This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government, Ministry of Science and ICT (MSIT) (RS-2025-02263336, RS-2025-16066885).Notes and references
- Y. He and K. Park, Mol. Pharmaceutics, 2016, 13, 2164–2171 CrossRef CAS.
- C.-Y. Wu, D. Stoecklein, A. Kommajosula, J. Lin, K. Owsley, B. Ganapathysubramanian and D. Di Carlo, Microsyst. Nanoeng., 2018, 4, 21 CrossRef PubMed.
- W. Jang, S. J. Oh, E. S. Kim and K. W. Bong, Biosens. Bioelectron., 2025, 287, 117725 CrossRef CAS.
- W. Jang, E. L. Song, S. J. Mun and K. W. Bong, Biosens. Bioelectron., 2024, 261, 116465 CrossRef CAS.
- S. J. Mun, W. Jang, H.-S. Park, Y. J. Lim, T.-J. Yang and K. W. Bong, Biosens. Bioelectron., 2023, 241, 115670 CrossRef CAS PubMed.
- Y. J. Lim, J. H. Choi, S. J. Mun, J. Kim and K. W. Bong, Anal. Chem., 2024, 96, 7204–7211 CrossRef CAS PubMed.
- W. Jang, S. J. Mun, S.-Y. Kim and K. W. Bong, Colloids Surf., B, 2023, 222, 113088 CrossRef CAS PubMed.
- Q. Truong Hoang, D. Y. Kim, H. S. Park, W. Jang, T. G. Nguyen Cao, J. H. Kang, Y. T. Ko, S. J. Mun, S. H. Bhang, M. S. Shim and K. W. Bong, Adv. Funct. Mater., 2024, 34, 2306078 CrossRef CAS.
- D. S. Bykov, S. Xie, R. Zeltner, A. Machnev, G. K. L. Wong, T. G. Euser and P. S. J. Russell, Light: Sci. Appl., 2018, 7, 22 CrossRef PubMed.
- J. Y. Sim, J.-H. Choi, J.-M. Lim, S. Cho, S.-H. Kim and S.-M. Yang, Small, 2014, 10, 3979–3985 CrossRef CAS PubMed.
- C. K. Wong, X. Qiang, A. H. E. Müller and A. H. Gröschel, Prog. Polym. Sci., 2020, 102, 101211 CrossRef CAS.
- P. Panda, K. W. Bong, T. A. Hatton and P. S. Doyle, Langmuir, 2011, 27, 13428–13435 CrossRef CAS.
- W. B. Rogers, W. M. Shih and V. N. Manoharan, Nat. Rev. Mater., 2016, 1, 16008 CrossRef CAS.
- H. J. Lee, Y. H. Roh, H. U. Kim, S. M. Kim and K. W. Bong, Lab Chip, 2019, 19, 111–119 RSC.
- E. H. Lucassen-Reynders and K. A. Kuijpers, Colloids Surf., 1992, 65, 175–184 CrossRef CAS.
- F. Ravera, K. Dziza, E. Santini, L. Cristofolini and L. Liggieri, Adv. Colloid Interface Sci., 2021, 288, 102344 CrossRef CAS.
- D. Dendukuri, S. S. Gu, D. C. Pregibon, T. A. Hatton and P. S. Doyle, Lab Chip, 2007, 7, 818–828 RSC.
- H. Lee, Y. H. Roh, H. U. Kim and K. W. Bong, Biomicrofluidics, 2018, 12, 054105 CrossRef CAS PubMed.
- H. Z. An, H. B. Eral, L. Chen, M. B. Chen and P. S. Doyle, Soft Matter, 2014, 10, 7595–7605 RSC.
- H. U. Kim, Y. J. Lim, H. J. Lee, N. J. Lee and K. W. Bong, Lab Chip, 2020, 20, 74–83 RSC.
- C. L. Lewis, C.-H. Choi, Y. Lin, C.-S. Lee and H. Yi, Anal. Chem., 2010, 82, 5851–5858 CrossRef CAS.
- Y. Xia and G. M. Whitesides, Annu. Rev. Mater. Res., 1998, 28, 153–184 CrossRef CAS.
- S. Yu, H. Cho, J. P. Hong, H. Park, J. C. Jolly, H. S. Kang, J. H. Lee, J. Kim, S. H. Lee, A. S. Lee, S. M. Hong, C. Park, S. Yang and C. M. Koo, Nat. Commun., 2017, 8, 721 CrossRef.
- H. U. Kim, Y. H. Roh, S. J. Mun and K. W. Bong, ACS Appl. Mater. Interfaces, 2020, 12, 53318–53327 CrossRef CAS.
- J. G. Ok, Y. J. Shin, H. J. Park and L. J. Guo, Appl. Phys. A:Mater. Sci. Process., 2015, 121, 343–356 CrossRef CAS.
- J. Xu, D. H. C. Wong, J. D. Byrne, K. Chen, C. Bowerman and J. M. DeSimone, Angew. Chem., Int. Ed., 2013, 52, 6580–6589 CrossRef CAS PubMed.
- S. S. Dunn, S. Tian, S. Blake, J. Wang, A. L. Galloway, A. Murphy, P. D. Pohlhaus, J. P. Rolland, M. E. Napier and J. M. DeSimone, J. Am. Chem. Soc., 2012, 134, 7423–7430 CrossRef CAS.
- J. P. Rolland, B. W. Maynor, L. E. Euliss, A. E. Exner, G. M. Denison and J. M. DeSimone, J. Am. Chem. Soc., 2005, 127, 10096–10100 CrossRef CAS.
- S. E. A. Gratton, P. D. Pohlhaus, J. Lee, J. Guo, M. J. Cho and J. M. DeSimone, J. Controlled Release, 2007, 121, 10–18 CrossRef CAS PubMed.
- S. J. Mun, W. Jang, J. H. Choi, Y. J. Lim and K. W. Bong, RSC Adv., 2024, 14, 25190–25197 RSC.
- R. J. Jackman, D. C. Duffy, E. Ostuni, N. D. Willmore and G. M. Whitesides, Anal. Chem., 1998, 70, 2280–2287 CrossRef CAS PubMed.
- D. Murakami, H. Jinnai and A. Takahara, Langmuir, 2014, 30, 2061–2067 CrossRef CAS.
- J. H. Sim, H. J. Moon, Y. H. Roh, H. W. Jung and K. W. Bong, Korean J. Chem. Eng., 2017, 34, 1495–1499 CrossRef CAS.
- J. H. Choi, W. Jang, Y. J. Lim, S. J. Mun and K. W. Bong, ACS Sens., 2023, 8, 3158–3166 CrossRef CAS PubMed.
- J. H. Choi, Y. H. Kim, J. Kim, Y. J. Lim, M. J. Kim and K. W. Bong, Analyst, 2025, 150, 2118–2127 RSC.
- Y. Wang, C. E. Sims and N. L. Allbritton, Lab Chip, 2012, 12, 3036–3039 RSC.
- C. Con and B. Cui, Nanoscale Res. Lett., 2013, 8, 394 CrossRef.
- D. Dendukuri, P. Panda, R. Haghgooie, J. M. Kim, T. A. Hatton and P. S. Doyle, Macromolecules, 2008, 41, 8547–8556 CrossRef CAS.
- N. Rostami, D. Graf, L. Schranzhofer, S. Hild and T. Hanemann, Prog. Org. Coat., 2019, 130, 221–225 CrossRef CAS.
- Y. Li, M. Kröger and W. K. Liu, Nanoscale, 2015, 7, 16631–16646 RSC.
- L. Chen, H. Z. An, R. Haghgooie, A. T. Shank, J. M. Martel, M. Toner and P. S. Doyle, Small, 2016, 12, 2001–2008 CrossRef CAS PubMed.
- J. A. Champion, Y. K. Katare and S. Mitragotri, J. Controlled Release, 2007, 121, 3–9 CrossRef CAS PubMed.
- J.-G. Park, J. D. Forster and E. R. Dufresne, J. Am. Chem. Soc., 2010, 132, 5960–5961 CrossRef CAS PubMed.
- A. F. Demirörs, P. M. Johnson, C. M. van Kats, A. van Blaaderen and A. Imhof, Langmuir, 2010, 26, 14466–14471 CrossRef PubMed.
- A. Torres-Carbajal, S. Herrera-Velarde and R. Castañeda-Priego, Phys. Chem. Chem. Phys., 2015, 17, 19557–19568 RSC.
- H. J. Moon, M. Ku, Y. H. Roh, H. J. Lee, J. Yang and K. W. Bong, Langmuir, 2020, 36, 2271–2277 CrossRef CAS.
- Y. H. Roh, H. J. Lee, H. J. Moon, S. M. Kim and K. W. Bong, Anal. Chim. Acta, 2019, 1076, 110–117 CrossRef CAS.
- W. Jang, Y. J. Kim, H. K. Roh, E. L. Song and K. W. Bong, Anal. Chem., 2025, 97, 7317–7324 CrossRef CAS.
- C.-W. Song, W. Jang, J. Hong, S. Y. Lim, D. g. r. m. Moon, H. Y. Roh, K. H. Park, K. W. Bong and C.-S. Han, Sens. Actuators, B, 2025, 432, 137496 CrossRef CAS.
- Y. H. Roh, H. J. Lee, J. Y. Kim, H. U. Kim, S. M. Kim and K. W. Bong, Lab Chip, 2020, 20, 2841–2850 RSC.
- A. B. Lowe, Polym. Chem., 2014, 5, 4820–4870 RSC.
- W. Jang, J. Kim, S. J. Mun, S. M. Kim and K. W. Bong, Biomedicines, 2021, 9, 848 CrossRef CAS PubMed.
- N. W. Choi, J. Kim, S. C. Chapin, T. Duong, E. Donohue, P. Pandey, W. Broom, W. A. Hill and P. S. Doyle, Anal. Chem., 2012, 84, 9370–9378 CrossRef CAS PubMed.
- P. Rees, H. D. Summers, A. Filby, A. E. Carpenter and M. Doan, Nat. Rev. Methods Primers, 2022, 2, 86 CrossRef CAS.
- D. C. Pregibon, M. Toner and P. S. Doyle, Science, 2007, 315, 1393–1396 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |