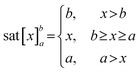High frequency acoustofluidic based controllable drug delivery system
Rui
You†
 a,
Shuting
Pan†
b,
Yuan
Ning
b,
Hongxiang
Zhang
b,
Tiechuan
Li
b,
Bo
Long
a,
Shuting
Pan†
b,
Yuan
Ning
b,
Hongxiang
Zhang
b,
Tiechuan
Li
b,
Bo
Long
 *a,
Dapeng
Ye
*a and
Xuexin
Duan
*a,
Dapeng
Ye
*a and
Xuexin
Duan
 *b
*b
aFujian Key Laboratory of Agricultural Information Sensoring Technology, College of Mechanical and Electrical Engineering, Fujian Agriculture and Forestry University, Fujian Province, China. E-mail: longbo2015@fafu.edu.cn; ydp@fafu.edu.cn
bState Key Laboratory of Precision Measuring Technology & Instruments, Tianjin University, Tianjin, China. E-mail: xduan@tju.edu.cn
First published on 12th November 2025
Abstract
Controllable drug delivery systems play an important role in personalized and precision medicine, particularly for long-term therapies. However, current release profiles, such as pulse, first-order, and zero-order releases, frequently fall short of addressing the diverse needs of patients. Herein, we introduce a novel drug delivery system utilizing the hydrodynamic method, which combines nanochannels with acoustofluidic-based modulation to achieve controllable delivery in both zero-order and first-order drug delivery systems. Employing the finite element method, we simulate the acoustic streaming field and corresponding molecular concentration field to investigate the impact of acoustic jets and shear flows on drug release. Leveraging the zero-order release of FITC-dextran through nanochannels, we integrate gigahertz (GHz) acoustofluidics into this system to demonstrate its modulation capabilities, including slow release at low power and enhanced release at high power. Furthermore, we demonstrate the GHz acoustofluidic modulation on first-order release in vitro utilizing a wirelessly powered GHz acoustofluidic-based implantable drug delivery system (GADDS). This study lays the foundation for a new generation of acoustic drug delivery platforms, paving the way for precision medicine advancements.
1 Introduction
With the rapid advancements in medical science and engineering technology, the significance of biodistribution (BD) and pharmacokinetics (PK) of drug molecules in clinical therapies has become increasingly apparent.1–4 Consequently, modern drug delivery studies are increasingly focused on developing controllable drug delivery systems (cDDSs). These cDDSs aim to replace the traditional ‘one-size-fits-all’ approach and achieve personalized and precision medicine.5–7 As a method to send therapeutic agents to targets, drug delivery systems (DDSs) play a pivotal role in clinical therapies. By regulating the drug molecule according to its characteristics, the DDS can increase the safety, portability, use ratio and patient compliance, ultimately improving clinical efficacy.8–12 Besides, developing a novel cDDS is significantly more cost-effective and time-efficient compared to creating a new chemical entity. The latter typically requires approximately $500 million and 10–12 years, whereas a novel cDDS only requires about $20–50 million and a development timeline of 3–4 years.13 This cost-performance ratio and timely development make novel cDDSs attractive and create a substantial global market.14,15 Among various cDDSs, implantable drug delivery systems (iDDSs) have garnered significant attention over the past decades, with a projected market value of nearly $155 billion by 2026. Their ability to bypass digestion and skin barriers, unlike ocular and transdermal DDSs, offers significant advantages in terms of drug utilization ratio and response time, crucial in disease treatments. Additionally, iDDSs provide long-term controllable continuous drug delivery. This can alleviate compliance issues and treatment- and pill-fatigue commonly encountered in chronic disease therapies, which account for about a half of treatment failures and cause vast numbers of deaths.16As microtechnology continues to advance, various iDDSs rooted in distinct principles have emerged, providing an extensive range of controllable drug release methods for different drugs.17–22 One such system, the controlled-release microchip actuated by chemical dissolution,23 can achieve pulsatile release of drugs to the lesion on demand, causing rapid fluctuations in plasma concentration. This pulsatile release pattern can also be achieved through micropumps leveraging water electrolysis,24,25 magnetics26–28 or shape-memory alloys.29 These systems are ideally suited for urgent medical scenarios (e.g., fatal seizures). However, they require intricate control units to achieve precise drug delivery with high dosing frequencies. For common diseases, the sustained-release featuring first-order release is commonly utilized for the technical simpleness and maturity, even though it is not the most suitable. Besides matrix-controlled DDSs,15 researchers also bring external fields to improve first-order release DDSs, such as the modulation on the on–off on the cDDS.30 As for chronic diseases (e.g., type 1 diabetes), maintaining a constant drug concentration within the therapeutic window is crucial. This necessitates a zero-order release profile, ensuring a steady drug release rate.31 Up to now, zero- and first-order releases can be both achieved through nanochannels for long-term drug delivery with minimal side effects and great efficiency.32,33 Meanwhile, with the rapid technological advancements in recent decades, people can further consider physiological variables, both the predictable and the unpredictable. This renders the traditional paradigm of constant drug delivery obsolete and potentially problematic, as it can lead to resistance and unwanted side effects. Thus, integrating a nanochannel-based iDDS with an active control method represents a promising and intriguing direction for future research.
The active control methods for iDDSs mainly include endogenous stimuli, light, ultrasound, magnetism, electricity, etc. Endogenous-stimulus iDDSs are triggered by specific internal environments. For instance, pH-responsive iDDSs often employ polymers that dissolve or swell at specific pH levels (e.g., in tumor microenvironments and gastrointestinal tracts) and provide targeted release with good biocompatibility.34 But they exhibit slow response times and cannot achieve “on–off” release.35 Light-responsive iDDSs enable spatiotemporal control through adjustable wavelength, intensity, and duration of irradiation, offering non-invasive, programmable release.36 However, their efficacy is limited by wavelength-dependent light transmittance through tissues, potential phototoxicity, and the irreversibility of many photochemical reactions. Ultrasonic-driven iDDSs use ultrasound to generate bubbles or vibrations for drug release, allowing non-invasive deep tissue penetration. Thus, their efficiency depends on ultrasound parameters and tissue properties.37 Magnetic-based iDDSs utilize external magnetic fields to control drug release, offering wireless remote control and precise dosing. However, they are susceptible to environmental interference and require strong magnetic fields.38 Electric iDDSs, such as the wireless iDDSs in ref. 39, enable precise control through electrical stimuli, facilitating closed-loop feedback for optimized release. Yet they require batteries and may cause additional reactions. Based on the above analyses, these active control methods are not suitable for nanochannel-based iDDSs.
In recent years, acoustofluidic technologies have been employed to drive liquids and manipulate particles in a wide range of miniaturized systems.40–43 Gigahertz (GHz) acoustofluidics as a hydrodynamic method has advantages of great portability (simple and small structures, low power consumption and low working voltage), high biocompatibility, a fast response time and fair extensibility. In our previous research,42 we utilized a GHz bulk acoustic wave (BAW) resonator to promote mass transfer through the jet mode and biofouling removal through the shear mode, proving its molecular diffusion modulation ability. Therefore, it may have potential to cooperate with nanochannels to meet the escalating demands of personalized medicine.
Here, we present an innovative active drug delivery system that harnesses GHz acoustic streaming and nanochannels to control drug release (Scheme 1). Leveraging microelectromechanical system (MEMS) techniques derived from the complementary metal-oxide-semiconductor (CMOS) process, we fabricated a GHz BAW resonator (Fig. S1) capable of generating efficient acoustic streaming for the modulation of drug molecule transport within nanochannels. Through finite element method (FEM) simulations, we comprehensively analyzed the flow field, pressure distribution, and molecular transport within the nanochannels at jet and shear regions. Subsequently, employing dextran molecules as a representative model, we demonstrated the in vitro active modulation capabilities of acoustic streaming in nanochannel-based zero-order release systems. Furthermore, we validated the wireless driving capacity of this hydrodynamic method by integrating it into an iDDS through an in vitro test. Overall, we establish the feasibility of a wirelessly powered GHz acoustic streaming-based controllable iDDS (GADDS), offering promising prospects for the development of iDDSs that optimize drug utilization ratios, minimize adverse effects, and ultimately enhance patient compliance, medical service quality, and therapeutic outcomes in chronic disease management.
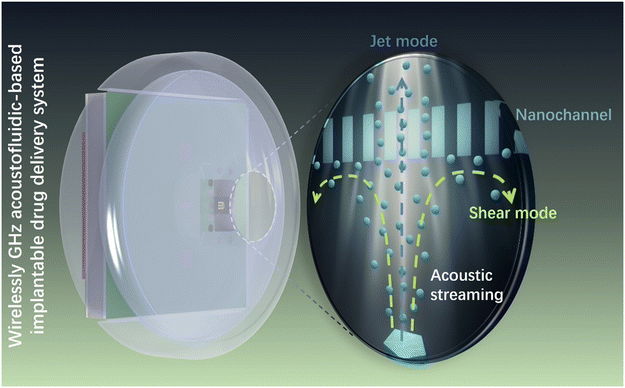 | ||
| Scheme 1 Regulation of a GADDS to confined diffusion of molecules through nanochannels using GHz acoustic streaming. | ||
2 Materials and methods
Reagents and materials
Fluorescein isothiocyanate conjugated dextran molecules (FITC-dextran) of 40, 250, and 500 kDa molecular weights (MW) were purchased from Sigma-Aldrich Inc., having a negative net charge of −1e from the fluorescein (pH = 7) and equivalent hydrodynamic diameters of 9, 26 and 34 nm, respectively.44 Standard phosphate buffer solution (PBS) of 0.01 M was purchased from Beijing Solarbio Science & Technology Co., Ltd. These reagents were used without extra purification. Anodic aluminum oxide (AAO) membranes were purchased from Hefei Pu-Yuan Nano Technology Ltd., having nanopore diameters of 20, 30 and 50 nm.Device fabrication
The BAW resonator was fabricated using CMOS compatible technology. In brief, the Bragg reflector layers were fabricated through alternately depositing aluminum nitride (AlN) and silicon dioxide (SiO2). And then thin films of molybdenum (Mo), AlN and gold (Au) were sequentially deposited on the Bragg reflector layers to form the sandwiched structure as the core piezoelectric unit. Next, a film of Au was deposited on Mo as the connected pads. The BAW resonator has a resonance frequency of 2.4GHz, which allows it to be powered by a commercial WiFi antenna (OA-W01). More detailed information could be found in Fig. S1 and our previous studies.41,42Characterization of the GHz acoustic streaming field
The flow field induced by the GHz BAWs was characterized using seeding particles and a camera. After integration with a print circuit board (PCB), the GHz BAW resonator was covered with a predesigned reservoir. The reservoir was designed for containing fluid and providing boundary situations, and fabricated using a 3D-printer (Form 3, Formlabs Inc., USA) out of photopolymer clear resin (FLGPCL04®, Formlabs Inc., USA). The seeding particles with a diameter of 10 μm were purchased from LaVision GmbH (110P8), and added into the reservoir with ultrapure (UP) water for flow field visualization. The resonator was actuated by radio frequency (RF) signals generated by a signal generator (Mini-Circuits SSG-6000RC) and amplified by a power amplifier (Mini-Circuits ZHL), and then generated acoustic streaming. The camera was positioned parallel to the reservoir and perpendicular to the acoustic jet. Through analyzing the video recording the movement of seeding particles, the GHz acoustic streaming field could be analyzed and characterized.Simulation
The simulations of the acoustic streaming-induced modulation were performed using the 2D finite element method (FEM) in COMSOL Multiphysics 5.5 (Burlington, MA, USA). Their building procedures can be split into three steps, as shown in Fig. S2. Firstly, the solid mechanics, electrostatic and pressure acoustics modules, and the piezoelectric effect and acoustic–structure boundary multiphysics modules were utilized to calculate the acoustic field in the frequency-domain study. Because the acoustic waves in the liquid are the vibrations of the liquid density and pressure at quasi-equilibria state, when we model the acoustic field, the liquid should be treated as compressible. In this step, the mechanical boundary condition at the end edges of the BAW resonator is set as a low-reflecting boundary to absorb the leakage acoustic waves. Secondly, the free and porous media flow module was employed to calculate the flow field in the stationary study according to acoustic streaming effects (i.e., eqn (5a)). The acoustic streaming effect is induced by the acoustic waves, and regarded as the second order effect. Thus, in the second step, the driven force is given by the first step. While it hardly induces the change of the liquid density, only the flow field needs to be calculated. Therefore, the liquid is treated as incompressible when we model the steady flow in the liquid part. In this step, the flow field boundary condition at side edges is set as an open boundary, and the others edges are set as walls (no slip). Thirdly, the transport of diluted species module was used to calculate the mass transfer under different driven conditions in the time-dependent study. In this step, the boundary condition at side edges and the upper edge of the sink reservoir is set as an open boundary, the boundary condition at side edges of the source reservoir is set as concentration (0.1 mol m−3) and the other edges are set as no flux. For the mesh structure, a structured quadrilateral mesh is created on domains of the BAW resonator and the jet region (maximum element size: 30 μm; minimum element size: 0.06 μm), and an unstructured triangular mesh is created on the other domains. More details of similar simulations can be found in our previous studies.40,41,45Diffusion testing system
The diffusive transport characteristics of an AAO membrane with or without a GHz acoustic streaming field were evaluated through two custom diffusion testing devices, a fluorescence spectrophotometer (Varioskan™ LUX multimode reader, Thermo Scientific™) and a UV/vis spectrophotometer (TU-1950, Beijing Puxi General Instrument Co., Ltd.). The diffusion setups were designed to have two reservoirs, the source and sink reservoirs, which were isolated through an AAO membrane. The corresponding calibration curves were determined with R2 > 0.99 before experiments. In each diffusion testing, a solution of FITC-dextran in standard PBS was loaded into the source reservoir and a solution of standard PBS was loaded into the sink reservoir. For fluorescence detection, the sample was moved to a 96/384-well plate using a pipette (sampling 20 μL for a 384-well plate and 100 μL for a 96-well plate), and the FITC-dextran concentration was determined using the multimode reader with an excitation wavelength of 494 nm and an emission wavelength of 518 nm. For absorbance detection, the FITC-dextran concentration in the sink reservoir was determined in real time using the UV/Vis spectrophotometer with an absorption wavelength of 494 nm.3 Results and discussion
Diffusion through nanochannels
The passive mass transfer of drug molecules mainly relies on molecular diffusion. In a nutshell, when a concentration gradient exists within a single-phase solution, each molecular species exhibits directional movement towards regions of lower differential concentration. This phenomenon (i.e. diffusion) is accurately characterized by Fick's law. For a binary mixture, Fick's first law can be written as:46| JA = −DAB∇cA | (1) |
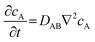 | (2) |
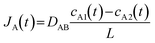 | (3) |
It has been reported that a linear release profile emerges when at least one dimension of a channel approximates the hydrodynamic diameter of the molecule traversing it.21 Consequently, in our pursuit of achieving zero-order release, we employed an AAO membrane containing nanochannels that closely resemble the size of molecules. As depicted in Fig. 1a, when the Brownian motion of the molecule is limited in two dimensions, diffusion is solely observed along the length of the nanochannel. Owing to the scale effect, surface interactions play a dominant role in the confined passage of the molecule through the nanochannel. For the vast majority of drug molecules that exhibit low charge to mass ratios, electrostatic forces are minimal and can be disregarded. Instead, physisorption, which encompasses van der Waals forces, hydrophobic interactions, and entropic considerations, dominates the confined diffusion process. Consequently, a region of low mobility emerges near the channel wall, effectively limiting the molecular transport within the channel. Macroscopically, this confinement manifests in the saturation of mass flux for concentrations exceeding a certain threshold (depicted by the green line in Fig. 1c), leading to the modification of eqn (3) as follows:46
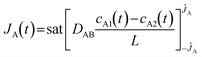 | (4) |
Subsequently, we utilized the FITC-dextran and the AAO membrane for demonstration. Dextran, a glucan with a diverse range of molecular weights, is frequently utilized as a drug carrier in numerous therapeutic applications. Solutions of 40 kDa FITC-dextran with varying concentrations were loaded into the source reservoirs. These molecules then diffused into the sink reservoir through nanochannels featuring diameters of 20 or 30 nm and lengths of 60 μm. As shown in Fig. 2a–c, the release rates exhibit a linear correlation with the concentration. Furthermore, the 30 nm nanochannel demonstrated a higher release rate compared to the 20 nm nanochannel, supporting the hypothesis that larger nanochannels facilitate the passage of more molecules and enhance free diffusion (i.e., the first-order release). Meanwhile, the diffusion would be compressed even when the nanochannel size is as large as 50 nm (Fig. 2d–f). As we analyzed above, this is because the nanochannel size approaches the hydrodynamic diameter (34 nm) of 500 kDa FITC-dextran molecules, thereby confining molecular diffusion along the length of the nanochannel. This highlights the importance of the relative size of the channel and the molecule, by which the release mode can be tuned. Notably, a larger effective diffusion area (50 nm−1: 0.5 × 0.5 mm2) has a higher release rate and a larger saturation threshold compared to a smaller diffusion area (50 nm−2: 0.3 × 0.4 mm2).
Acoustic streaming-modulated molecular transport
In our previous studies,42,47 we elucidated the different regulations of GHz acoustic streaming effects in jet and shear modes on molecular transport. This GHz acoustic streaming, being non-intrusive and compatible with a wide range of drug molecules, holds promise for various applications. The applied BAW resonator, renowned for its miniaturized footprint, energy efficiency, and CMOS compatibility, piqued our interest in exploring its potential for regulating drug release in an iDDS.When the resonator is activated by radio frequency (RF) signals, it oscillates in thickness mode and generates longitudinal BAWs to propagate through the fluid. Due to viscous dissipation, relaxation effects and turbulent fluctuations in the fluid, the GHz BAWs with a high attenuation coefficient (β ∼ ω2, ω: acoustic angular frequency) attenuate sharply as soon as they leave the resonator–fluid interface,42 giving rise to a body force (ρ0fb) on the fluid along their propagation direction. The quasi-Navier–Stokes equation that governs the acoustic streaming field is given by:
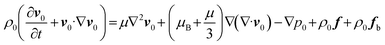 | (5a) |
| ρ0fb = −ρ0v1·∇v1 + v1∇·v1 | (5b) |
To gain insights into the impact of acoustic streaming effects on flow fields and molecular transport, we built a 2D finite element method (FEM) model using COMSOL Multiphysics. Detailed simulations of the resonator can be found in our previous studies,42,43,48 and here we employ a free and porous media flow module to simulate the diffusion within nanochannels. As shown in Fig. 3a and d, when an acoustic jet flows upward from the resonator surface, the streaming field tends to be vortical in nature, rotating around a point in each longitudinal section, constrained by the reservoir's boundaries. Notably, the membrane in jet mode is subjected to the acoustic jet, permitting a minimal flow through it, whereas in shear mode, it is subjected to the shear flow. Consequently, the influence of acoustic streaming effects on drug release comprises two parts: jet and shear parts. As illustrated in Fig. S3 & S4, the jet remains relatively constant at the same power. However, as the membrane area expands, the shear part intensifies. Taking into account the convection (i.e.eqn (5a)) induced by acoustic streaming effects in molecular transport, eqn (2) can be further rewritten as:
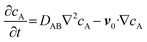 | (6) |
Later we crafted a diffusion device consisting of a main body (containing the source and sink reservoirs), an AAO membrane with 50 nm nanochannels, a BAW resonator, an evaluation board (EVB) and a subminiature version A (SMA) connector (Fig. S5). Fig. 4 illustrates the GHz acoustic streaming field visualized by 10 μm seeding particles. As the power increases, the acoustic streaming field intensifies within both the source and sink reservoirs. The flows exhibit a vortical nature, consistent with the outcomes of our simulations. These observations underscore the feasibility of integrating acoustic streaming and nanochannels in iDDSs.
Regulation of acoustic streaming effects to drug release
Drawing from the preceding analyses, it becomes evident that we can regulate the drug release rate by adjusting the applied power. To demonstrate the controlled release of drug molecules, we conducted an investigation with FITC-dextran. These zero-order releases were executed within our customized devices, sharing the same components depicted in Fig. S5. Fig. 5 illustrates the quantity of released FITC-dextran, where the applied powers were varied every 30 minutes and highlighted through distinct colors. The released quantity for each 30 minute interval was normalized, with the quantity in the initial 30 minutes serving as the baseline (set as 1).In case 1, a 1 mg mL−1 solution of 250 kDa FITC-dextran in PBS was released from a source reservoir to a sink reservoir through an AAO membrane with a diameter of 4.8 mm and 30 nm nanochannels, which had a relatively large shear part. During passive phases (0 mW), the molecules were released into the sink reservoir through nanochannels, relying on the concentration-driven diffusion. When the applied power was not high enough (200 mW), the release rate approximated that during the passive phase. Upon increasing the power to 400 mW, the intensified jet (Fig. 3f) augmented molecular transport within the nanochannels. In case 2 where the diameter of the membrane was narrowed to 2 mm to decrease the shear part, an applied power of 200 mW sufficed to enhance the molecular release, and the release rate at 400 mW increased to ∼1.5 times that of the passive phases. As previously analyzed, the influence arises from the combined action of the acoustic jet and shear flows. Thus, when the AAO membrane area is reduced, the modulation of acoustic streaming can be more efficient. In particular, comparing the release at the same applied power in cases 1 and 2, the impact of shear flow diminishes, thereby relatively amplifying the effect of the jet flow.
Based on the results from cases 1 and 2, we further conducted case 3 to demonstrate the acoustic streaming-enabled modulation on the zero-order release. Referring to the results in Fig. 2, a 10 mg mL−1 solution of 500 kDa FITC-dextran in PBS was designed to be released from the source reservoir to the sink reservoir at different powers of 100, 200 and 400 mW, traversing an AAO membrane with a release area of 0.7 × 2 mm2 and 50 nm nanochannels. When the applied power was sufficiently low (100 mW), the shear flow accounted for the most influence of the acoustic streaming field on drug release, thereby impeding molecular transport within the nanochannels and reducing the release rate to ∼0.58 times that of the passive phases (Fig. 3b and c). As the power was increased, the release rate progressively increased (∼3.8 times that of the passive phases at 400 mW), establishing a positive correlation between power intensity and release efficiency. These observations underscore the escalating significance of the acoustic jet (as the applied power is augmented) and shear flow (as the applied power is lowered), further corroborating the critical role of acoustic phenomena in drug delivery systems.
In vitro modulated release of FITC-dextran by the assembled GHz acoustofluidic-based implantable drug delivery system (GADDS)
To assess the drug release modulation potential of a GADDS, we performed an in vitro first-order release test of 40 kDa FITC-dextran through 30 nm nanochannels. The GADDS was fabricated with a diameter of 16 mm and a thickness of 4 mm (Fig. 6c), and positioned in a cuvette based custom-made setup as depicted in Fig. 6d. The GADDS hosts two compartments: one serves as a source reservoir (a shell, a nanochannel membrane, a sealing ring and a cover), and the other for fluid driving (a resonator, a PCB and an antenna) (Fig. 6a). We firstly tested the wireless driven acoustic streaming field where the receiving antenna of the compartments was immersed in water and a transmitting antenna was placed 1 cm from it. As shown in Fig. S6a, when the power is 1 W, the acoustic streaming is too weak to be observed. As the power is increased, the acoustic streaming becomes stronger and stronger as depicted in Fig. S6b and d and 6a (inset). Later this compartment was assembled with the source reservoir including a 30 nm nanochannel membrane (with a diameter of 3 mm) for the in vitro test. The GADDS was loaded with 120 μL solution of 2 mg mL−1 40 kDa FITC-dextran in PBS. In consideration of obstruction of the solution and the chamber cover, the applied power was set at 10 W. As shown in Fig. 6b, we can observe that the application of acoustic streaming led to an enhanced FITC-dextran release, which reaches 2.1 times larger than the release without acoustic streaming. In order to reduce the loss of wireless power supply in transmission, we think that the suitable application of the GADDS is the subcutaneous implantation, where the wireless charging only needs to traverse the skin. Under these conditions, the GADDS can be easily located, and subcutaneous drug refill can be achieved as well.4 Conclusions
Controlled iDDSs demonstrate significant advantages over conventional systemic administration, prompting scientists to make efforts to develop advanced active control methodologies and sophisticated iDDSs. In this study, we validate the innovative capabilities of a GADDS through nanochannel-modulated on-demand drug release and wireless power transmission using in vitro experimental models. However, substantial research and optimization remain essential prior to clinical implementation. Critical physiological factors including tissue encapsulation, immune responses, biofouling phenomena, and dynamic fluidic environments will introduce substantial complexity, potentially altering release kinetics and biocompatibility profiles. The influence of these factors should be clarified in preclinical studies in animal models. Notably, while GHz BAW resonators have demonstrated biosafety in prior applications – including the intracellular delivery system for intracellular delivery of gold nanorods (AuNRs),49 the reconfigurable acoustic streaming tweezers for nucleic acid (NA) extraction,40 and the acoustic jet micropump for ocular disease treatment41 – their long-term biocompatibility remains insufficiently established. Comprehensive long-term stability assessments spanning months to years are imperative to ensure therapeutic reproducibility and regulatory compliance before clinical deployment. And the GADDS has a low driving voltage, allowing it to receive power through only a commercial antenna with the same resonance frequency. But it is not yet mature enough. Strategic integration with energy-efficient wireless power solutions could minimize external dependencies while enhancing patient compliance for chronic therapies. Furthermore, closed-loop control architectures incorporating real-time therapeutic monitoring through integrated sensors would enable adaptive treatment regimens aligned with precision medicine principles. These critical aspects warrant thorough investigation in preclinical development phases. Ultimately, while the GADDS holds transformative potential for medical applications, its successful clinical translation necessitates rigorous in vivo validation, regulatory approvals, and patient-centric design considerations for chronic disease management.Here we presented a proof-of-concept study of a wireless driven iDDS, GADDS, that leverages nanochannel-based molecular diffusion and acoustic streaming effect-based release modulation for controllable drug delivery. Our ongoing investigations are dedicated to universality and controllability for an ideal implant. Both the AAO nanochannel and the acoustic streaming are of great biocompatibility, and independent of molecular optical and electric properties, etc. These endow the GADDS with great potential in the treatment for a vast spectrum of pathologies. Through tuning the release area and applied power, the decrease and increase to the release rate can be both performed. For high safety, this GADDS is free of batteries, and can be wirelessly driven. Besides, the GADDS has the advantages of small size, long lifespan, large drug loading ratio and good comfort. This also leaves more space and possibility for further integration and extension with other sensors that form a closed-loop to achieve smart drug delivery. Overall, although further development and improvement are needed, this proof-of-concept study sets the foundation for a generation of acoustic implants for controllable drug delivery.
Author contributions
R. You – conceptualization, data curation, formal analysis, investigation, methodology, writing – original draft, writing – review & editing; Shuting Pan – conceptualization, investigation, methodology, validation, writing – review & editing; Y. Ning – methodology, software, writing – review & editing; H. Zhang – methodology, software, writing – review & editing; T. Li – formal analysis, methodology, software, writing – review & editing; B. Long – funding acquisition, project administration, supervision, writing – review & editing; D. Ye – funding acquisition, project administration, supervision, writing – review & editing; X. Duan – conceptualization, funding acquisition, project administration, resources, supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (NSFC No. 22427807, No. 62174119), the Department of Science and Technology of Fujian Province (No. 2023J01472), and the Central Guidance for Local Science and Technology Development Special Fund Project (2024L3020). We acknowledge all members of the M/NEMS Laboratory at Tianjin University and the participants in our study for their help and assistance.References
- T. M. Allen, Science, 2004, 303, 1818–1822 CrossRef CAS PubMed.
- P. Davoodi, L. Y. Lee, Q. Xu, V. Sunil, Y. Sun, S. Soh and C.-H. Wang, Adv. Drug Delivery Rev., 2018, 132, 104–138 CrossRef CAS PubMed.
- C. Feng, Y. Wang, J. Xu, Y. Zheng, W. Zhou, Y. Wang and C. Luo, Pharmaceutics, 2024, 16, 1582 CrossRef CAS PubMed.
- Y. Lou, F. Song, M. Cheng, Y. Hu, Y. Chai, Q. Hu, Q. Wang, H. Zhou, M. Bao, J. Gu and Y. Zhang, PeerJ, 2023, 11, e15844 CrossRef.
- K. Park, J. Controlled Release, 2014, 190, 3–8 CrossRef CAS PubMed.
- A. Kumar and J. Pillai, Implantable drug delivery systems: An overview, Elsevier Inc., 2018 Search PubMed.
- A. Kar, N. Ahamad, M. Dewani, L. Awasthi, R. Patil and R. Banerjee, Biomaterials, 2022, 283, 121435 CrossRef CAS.
- Y. L. Li and B. H. Sun, Key Eng. Mater., 2019, 803, 158–166 Search PubMed.
- D. A. Domingo-Lopez, G. Lattanzi, L. H. J. Schreiber, E. J. Wallace, R. Wylie, J. O'Sullivan, E. B. Dolan and G. P. Duffy, Adv. Drug Delivery Rev., 2022, 185, 114280 CrossRef CAS.
- H. Kaji, N. Nagai, M. Nishizawa and T. Abe, Adv. Drug Delivery Rev., 2018, 128, 148–157 CrossRef CAS PubMed.
- K. P. Rajan, S. P. Thomas, A. Gopanna and M. Chavali, in Nano- and Microscale Drug Delivery Systems, Elsevier, 2017, pp. 299–319 Search PubMed.
- J. C. Imperiale, G. B. Acosta and A. Sosnik, J. Controlled Release, 2018, 285, 106–141 CrossRef CAS PubMed.
- R. K. V. Sanjay Garg, S. Garg and R. K. Verma, Pharm. Technol., 2001, 25, 1–14 Search PubMed.
- S. Capuani, G. Malgir, C. Y. X. Chua and A. Grattoni, Bioeng. Transl. Med., 2022, 7, 1–22 Search PubMed.
- C. L. Stevenson, J. T. Santini and R. Langer, Adv. Drug Delivery Rev., 2012, 64, 1590–1602 CrossRef CAS.
- N. Di Trani, A. Silvestri, A. Sizovs, Y. Wang, D. R. Erm, D. Demarchi, X. Liu and A. Grattoni, Lab Chip, 2020, 20, 1562–1576 RSC.
- H. J. Lee, N. Choi, E.-S. S. Yoon and I.-J. J. Cho, Adv. Drug Delivery Rev., 2018, 128, 132–147 CrossRef CAS PubMed.
- E. Nuxoll, Adv. Drug Delivery Rev., 2013, 65, 1611–1625 CrossRef CAS PubMed.
- D. Fitzpatrick, in Implantable Electronic Medical Devices, Elsevier, 2015, pp. 139–157 Search PubMed.
- S. H. Lee, B. H. Kim, C. G. Park, C. Lee, B. Y. Lim and Y. Bin Choy, J. Controlled Release, 2018, 286, 224–230 CrossRef CAS PubMed.
- D. Fine, A. Grattoni, S. Hosali, A. Ziemys, E. De Rosa, J. Gill, R. Medema, L. Hudson, M. Kojic, M. Milosevic, L. Brousseau III, R. Goodall, M. Ferrari and X. Liu, Lab Chip, 2010, 10, 3074 RSC.
- Y. Wang, Y. Xu, J. Song, X. Liu, S. Liu, N. Yang, L. Wang, Y. Liu, Y. Zhao, W. Zhou and Y. Zhang, Int. J. Nanomed., 2024, 19, 5837–5858 CrossRef CAS.
- J. T. Santini, M. J. Cima and R. Langer, Nature, 1999, 397, 335–338 CrossRef CAS PubMed.
- H. Joo, Y. Lee, J. Kim, J.-S. Yoo, S. Yoo, S. Kim, A. K. Arya, S. Kim, S. H. Choi, N. Lu, H. S. Lee, S. Kim, S.-T. Lee and D.-H. Kim, Sci. Adv., 2021, 7, eabd4639 CrossRef CAS PubMed.
- Y. Zhang, A. D. Mickle, P. Gutruf, L. A. McIlvried, H. Guo, Y. Wu, J. P. Golden, Y. Xue, J. G. Grajales-Reyes, X. Wang, S. Krishnan, Y. Xie, D. Peng, C.-J. Su, F. Zhang, J. T. Reeder, S. K. Vogt, Y. Huang, J. A. Rogers and R. W. Gereau, Sci. Adv., 2019, 5, 1–12 Search PubMed.
- C. Wang, S.-J. Seo, J.-S. Kim, S.-H. Lee, J.-K. Jeon, J.-W. Kim, K.-H. Kim, J.-K. Kim and J. Park, J. Controlled Release, 2018, 283, 105–112 CrossRef CAS.
- S. H. Lee, S. H. Min, Y. C. Y. M. Cho, J. H. Han, M. N. Kim, C. R. Kim, C. H. Ahn, B. H. Kim, C. Lee, Y. C. Y. M. Cho and Y. Bin Choy, J. Controlled Release, 2020, 325, 111–120 CrossRef CAS.
- S. H. Lee, Y. Bin Lee, B. H. Kim, C. Lee, Y. M. Cho, S.-N. Kim, C. G. Park, Y.-C. Cho and Y. Bin Choy, Nat. Commun., 2017, 8, 1–10 CrossRef.
- J. Fong, Z. Xiao and K. Takahata, Lab Chip, 2015, 15, 1050–1058 RSC.
- H. R. Cheong, N.-T. Nguyen, M. K. Khaw, B. Y. Teoh and P. S. Chee, Lab Chip, 2018, 18, 3207–3215 RSC.
- M. L. Laracuente, M. H. Yu and K. J. McHugh, J. Controlled Release, 2020, 327, 834–856 CrossRef CAS PubMed.
- J. M. M. Bezemer, R. Radersma, D. W. W. Grijpma, P. J. J. Dijkstra, J. Feijen and C. A. A. van Blitterswijk, J. Controlled Release, 2000, 64, 179–192 CrossRef CAS PubMed.
- F. P. Pons-Faudoa, A. Sizovs, N. Di Trani, J. Paez-Mayorga, G. Bruno, J. Rhudy, M. Manohar, K. Gwenden, C. Martini, C. Y. X. Chua, G. Varchi, M. A. Marzinke and A. Grattoni, J. Controlled Release, 2019, 306, 89–96 CrossRef CAS PubMed.
- G. Kocak, C. Tuncer and V. Bütün, Polym. Chem., 2017, 8, 144–176 RSC.
- G. He, H. Li, J. Liu, Y. Hu, Y. Liu, Z. L. Wang and P. Jiang, Adv. Mater., 2024, 36, 2312530 CrossRef CAS.
- H. A. M. Abdelmohsen, N. A. Copeland and J. G. Hardy, Drug Delivery Transl. Res., 2023, 13, 2159–2182 CrossRef.
- H. Xiao, M. Aliabouzar and M. L. Fabiilli, J. Controlled Release, 2024, 374, 205–218 CrossRef CAS.
- Y. Zheng, G. Zheng, Y. Y. Li, X. Gong, Z. Chen, L. Zhu, Y. Xu, X. Xie, S. Wu and L. Jiang, J. Controlled Release, 2023, 364, 576–588 CrossRef CAS.
- F. Del Bono, A. Bontempi, A. Dentis, N. Di Trani, D. Demarchi, A. Grattoni and P. M. Ros, IEEE Sens. J., 2023, 1 Search PubMed.
- T. Li, R. You, X. Shen, Z. Li, Y. Yang, Z. Han and X. Duan, Ultrason. Sonochem., 2025, 119, 107404 CrossRef CAS.
- R. You, Q. Fan, Z. Wang, W. Xing, Y. Wang, Y. Song, X. Duan, R. You and Y. Wang, Research, 2024, 7, 1–15 CrossRef.
- S. Pan, R. You, X. Chen, W. Pan, Q. Li, X. Chen, W. Pang and X. Duan, ACS Sens., 2023, 8, 3458–3467 CrossRef CAS.
- R. You, H. Wu, W. Pang and X. Duan, Anal. Chem., 2022, 94, 5392–5398 CrossRef CAS.
- C. E. Ioan, T. Aberle and W. Burchard, Macromolecules, 2000, 33, 5730–5739 CrossRef CAS.
- H. Zhang, Z. Tang, Z. Wang, S. Pan, Z. Han, C. Sun, M. Zhang, X. Duan and W. Pang, Phys. Rev. Appl., 2018, 9, 064011 CrossRef CAS.
- C. Cosentino, F. Amato, R. Walczak, A. Boiarski and M. Ferrari, J. Phys. Chem. B, 2005, 109, 7358–7364 CrossRef CAS PubMed.
- S. Pan, H. Zhang, W. Liu, Y. Wang, W. Pang and X. Duan, ACS Sens., 2017, 2, 1175–1183 CrossRef CAS PubMed.
- H. Wu, Z. Tang, R. You, S. Pan, W. Liu, H. Zhang, T. Li, Y. Yang, C. Sun, W. Pang and X. Duan, Nanotechnol. Precis. Eng., 2022, 5, 023001 CrossRef CAS.
- S. He, W. Pang, X. Wu, Y. Yang, W. Li, H. Qi, C. Sun, X. Duan and Y. Wang, Nanoscale, 2022, 14, 15281–15290 RSC.
Footnote |
| † R. Y. and S. P. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |