Realization of rapid diabetic retinopathy screening with lipocalin 1 in tear using enhanced immunofluorescence photonic crystal microchip
Kullaphat
Nitayachat†
a,
Dhrubajyoti
Das†
 a,
Pei-Yi
Chen
a,
Sheng-Min
Hsu
a,
Pei-Yi
Chen
a,
Sheng-Min
Hsu
 b,
Jhih-Cheng
Wang
*cde and
Han-Sheng
Chuang
b,
Jhih-Cheng
Wang
*cde and
Han-Sheng
Chuang
 *af
*af
aDepartment of Biomedical Engineering, National Cheng Kung University, Tainan 701, Taiwan. E-mail: oswaldchuang@mail.ncku.edu.tw
bDepartment of Ophthalmology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan 701, Taiwan
cDivision of Urology, Department of Surgery, Chi Mei Medical Center, Tainan, Taiwan. E-mail: tratadowang@gmail.com
dDepartment of Electrical Engineering, Southern Taiwan University of Science and Technology, Tainan, Taiwan
eSchool of Medicine, College of Medicine, National Sun Yat-sen University, Kaohsiung, Taiwan
fMedical Device Innovation Center, National Cheng Kung University, Tainan 701, Taiwan
First published on 14th November 2025
Abstract
Diabetic retinopathy (DR) is a major cause of preventable blindness, but current diagnostic tools rely on expensive imaging devices, specialized expertise, and, in some cases, invasive procedures, limiting accessibility in resource-constrained settings. There is an unmet need for rapid, non-invasive, and affordable point-of-care methods for early DR detection. We developed a portable lateral flow microfluidic chip integrated with a photonic crystal (PhC) biosensor to enhance immunofluorescence detection of lipocalin-1 (LCN-1), a biomarker associated with DR. The PhC surface provided a 2.7-fold fluorescence enhancement compared to non-PhC substrates, and the microfluidic flow was optimized using an absorbent paper design to ensure uniform fluid distribution and sufficient antigen–antibody interaction time. Analytical performance was validated using sandwiched immunoassays with carboxylate-modified particles, followed by clinical evaluation in 30 tear samples from healthy individuals, non-proliferative DR (NPDR), and proliferative DR (PDR) patients. The PhC microchip achieved a detection limit of 136 pg μL−1 and delivered quantifiable results within 15 min. In clinical testing, elevated LCN-1 levels were observed in both NPDR and PDR patients compared with healthy controls. The biosensor achieved 100% sensitivity, specificity, and accuracy in distinguishing PDR from healthy individuals, as well as 100% sensitivity with 90% specificity for NPDR versus healthy individuals, resulting in an overall diagnostic accuracy of 95%. The PhC-integrated microchip enables rapid and non-invasive detection of DR from tear samples. Its high sensitivity, specificity, and capability to differentiate between NPDR and PDR demonstrate strong potential for future adaptation into a portable point-of-care screening platform, particularly suited for low-resource settings to facilitate early intervention and reduce vision loss in diabetic patients.
Introduction
Diabetic retinopathy (DR) is a complication of diabetes that damages the blood vessels in the retina and remains one of the leading causes of irreversible vision loss worldwide.1 As the global prevalence of diabetes rises, DR is becoming an increasingly significant public health concern.2 One of the major challenges in managing DR is that it typically presents no symptoms in its early stages, making early detection and timely intervention essential to prevent vision impairment or even blindness.3,4 DR diagnosis relies mainly on imaging-based techniques such as fundus photography, fluorescein angiography (FA), and optical coherence tomography.5,6 However, these methods are limited by their reliance on expensive equipment, trained personnel, and, in the case of FA, invasive dye injections.7 Moreover, they may fail to detect subtle microvascular changes in the early disease stages, potentially delaying intervention.8–10To overcome these limitations, advanced technologies such as AI-assisted automated fundus photography and optical coherence tomography angiography (OCTA) have emerged.11,12 These systems can match expert-level performance in image interpretation and improve diagnostic efficiency.13 For instance, a 2020 study by Ting et al. demonstrated that an AI-based deep learning system could detect referable diabetic retinopathy, as well as glaucoma and age-related macular degeneration, from retinal photographs with high sensitivity and specificity, achieving performance comparable to that of retinal specialists.14 Additionally, OCTA is a non-invasive imaging technique that uses motion contrast to visualize blood flow in the retina and choroid. This technique has shown high sensitivity in detecting early microvascular changes without the need for dye injection.15 However, their accessibility remains limited in low-resource settings due to high costs and technical demands.8,16
In response, research has increasingly focused on point-of-care (POC) biosensors that utilize accessible biofluids such as tears and urine for non-invasive DR detection.17,18 Platforms such as microfluidic chips, lateral flow assays (LFA),19 and surface acoustic wave (SAW) sensors20,21 offer portability, ease of use, and rapid analysis, making them suitable for community-level screening. For example, an LFA has been developed to detect the urinary biomarker 8-hydroxy-2′-deoxyguanosine (8-OHdG), an oxidative stress marker associated with DR progression.22 The assay integrates gold nanoparticle conjugates to enhance the colorimetric signal, allowing quantitative detection via smartphone imaging within 20 min. It demonstrated 91% sensitivity and 81% specificity in distinguishing DR patients from healthy individuals. Despite its promising portability and diagnostic performance, limitations remain in detecting very low biomarker concentrations and minimizing signal interference from urine matrix components. Similarly, a detachable SAW microchip has been introduced for highly sensitive protein detection in tears. This platform enhances signal intensity by improving particle mixing and immunoassay formation in droplets. The detachable cover glass also minimizes cross-contamination during repeated use. However, both approaches face challenges in detecting very low-abundance biomarkers and require further optimization for broader POC deployment. To overcome these limitations, signal enhancement strategies such as enzymatic amplification,23 plasmonic resonance,24 and nanostructured materials25 have been explored to boost signal intensity and assay sensitivity. Among these, photonic crystals (PhCs) have gained attention for their ability to amplify fluorescence via periodic dielectric structures.26,27 PhCs are materials with a highly ordered internal arrangement that interact with light in a controlled way.28 This structure creates a photonic bandgap, enabling PhCs to reflect or confine specific wavelengths and thus enhance fluorescence signals.29 Recent reviews of photonic crystal grating resonance and interface technologies have further emphasized the growing role of photonic and fluorescence-based lab-on-a-chip systems in biomedical diagnostics. These platforms exploit enhanced light–matter interactions to achieve high-sensitivity detection in miniaturized formats, highlighting the potential of PhC-based fluorescence amplification for point-of-care applications.30–32 Building upon this direction, our laboratory has focused on improving PhC biosensor performance for tear-based DR detection. In our previous work, we developed a PhC-integrated lateral flow microchip for detecting lipocalin-1 (LCN-1) in tear samples.33 LCN-1 is a promising biomarker for early-stage DR, as its levels have been shown to increase two- to threefold in the tears of DR patients compared to healthy individuals.34,35 This system achieved a limit of detection (LoD) of 3 μg mL−1 within 15 min. However, the initial design employed gold nanoparticle (AuNP)-coated PhCs, which caused fluorescence quenching due to plasmonic interactions between the metal surface and fluorescent tags, ultimately reducing signal intensity. To overcome these limitations and advance the scientific understanding of PhC-based tear biosensors, this study introduces two major innovations. First, the photonic surface was modified with a silicon dioxide (SiO2) coating to suppress fluorescence quenching caused by plasmonic interactions in gold-coated PhCs, while maintaining strong photonic resonance. This modification enhanced light–matter coupling, improved fluorescence efficiency, and provided a more stable and biocompatible surface for biomolecule immobilization. Second, the flow-control mechanism of the microfluidic chip was optimized by redesigning the absorbent paper geometry to achieve steady and linear capillary-driven flow suitable for rapid immunoassay reactions. This adjustment enabled uniform sample distribution and consistent antigen–antibody interaction time, which were not attainable in the previous rectangular configuration. As a result of these improvements, the new SiO2-coated PhC microfluidic chip demonstrated a 2.7-fold enhancement in fluorescence intensity and an approximately 22-fold improvement in sensitivity, achieving a LoD of 136 pg μL−1 within approximately 15 min. Extending this achievement, the current study mainly focuses on the practical use of our device in a clinical trial analyzing tear samples. Clinical evaluation involves a cohort of 30 volunteers, including healthy individuals, proliferative DR (PDR), and non-proliferative DR (NPDR) patients. The platform achieved a 100% accuracy, 100% sensitivity, and 100% specificity in distinguishing PDR patients from healthy individuals. The device was also capable of distinguishing the NPDR, which is an early stage of DR, from the healthy individuals with 100% sensitivity and 90% specificity while maintaining the accuracy of 95%. While the current setup is laboratory-based, the developed platform provides a rapid, non-invasive, and accurate approach for early DR detection and stage differentiation. With further integration of compact optical readers, this system holds strong potential to evolve into a practical POC diagnostic tool for use in resource-limited settings, ultimately supporting earlier detection, timely intervention, and improved management of DR.
Methodology
Materials and reagents
Poly(methyl methacrylate) (PMMA) microspheres (250 nm; Lab261 Inc.) were used to fabricate PhC structures via a self-assembly technique. Fluorescent polystyrene nanoparticles (200 nm, yellow-green, excitation/emission, 505/515 nm; Thermo Fisher Scientific) functionalized with either carboxyl or amine groups were used as antibody-conjugated probes. Silicon dioxide (SiO2, 20 nm; Alfa Aesar) was used as a surface treatment material for PhC structures.Mouse anti-LCN-1 monoclonal IgG (11583-MM03, SinoBiological) and rabbit anti-LCN-1 polyclonal IgG (11583-RP02, SinoBiological) were employed as the capture and probe antibodies, respectively. Human full-length LCN-1 protein (ENZ-825, PROSPEC) was used as the target antigen in the immunoassay system.
Surface modification reagents included (3-aminopropyl)triethoxysilane (APTES; Sigma-Aldrich), glutaraldehyde (GA; Sigma-Aldrich), polyethyleneimine (PEI; Sigma-Aldrich), N-hydroxysulfosuccinimide (sulfo-NHS; Sigma-Aldrich), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC; Sigma-Aldrich).
Buffer systems included 50 mM MES (2-(N-morpholino)ethanesulfonic acid, pH 5.5; Sigma-Aldrich), phosphate-buffered saline (PBS; Sigma-Aldrich), and PBST (PBS with 0.01% Tween-20; Sigma-Aldrich). Non-specific binding sites were blocked using 2% bovine serum albumin (BSA; Sigma-Aldrich).
Mechanism of immunofluorescence enhancement by photonic crystals
PhCs work by controlling how light behaves when it interacts with regularly arranged materials that have different refractive indices. These repeating structures create photonic band gaps, which are specific wavelength ranges where light cannot pass through. This behavior is described by Bragg's law.36nλ = 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(θ) sin(θ) | (1) |
Microfluidic chip design and fabrication
A lateral flow microfluidic chip (Fig. 1C) integrated with a PhC biosensor to enable an on-chip sandwiched immunoassay for LCN-1 detection in a tear sample. The device was constructed from two 2 mm-thick PMMA layers: the top layer contained a sample inlet aligned with a 10 mm-diameter circular reservoir, while the bottom layer featured a 10 mm × 1 mm microchannel leading to two embedded PhC biosensors, one acting as a reaction line (test line) and the other as a control line (Fig. S1-i and ii). The PhC at the reaction line was functionalized with immobilized pAbs to specifically capture the antigen, while the control line served as a positive control, producing a consistent fluorescence signal by capturing excess probe–antibody complexes. Capillary flow was driven by absorbent paper housed in a downstream recess, which measured 40 mm in total length and comprised three consecutive sections with widths of 1 mm, 20 mm, and 25 mm (Fig. S1-iii). This stepped geometry provided gradual capillary flow control throughout the channel. This design enabled an interaction time of approximately 14 min within the detection region, allowing sufficient time for antigen–antibody binding and accurate signal readout.Microchannels and structural features were made with precision-cutting using computer numerical control milling. Surfaces were cleaned with isopropyl alcohol, detergent, and deionized water, followed by ultraviolet excimer treatment to modify the PMMA surface to enable bonding at low pressure and low temperature. The two layers were aligned, clamped, and thermally bonded at 80 °C for 8 h. Absorbent paper was laser-cut using a Venus II Desktop Laser Engraver (instant model, CO2 type) at a speed of 10%, power 8.0%, and resolution 500–1029 DPI, which produced clean edges and dimensional precision within ±50 μm. The cut paper was then inserted into the microchannel outlet. The microchip utilizes capillary force to transport samples and fluorescent particles toward the sensing area of the PhC biosensors for rapid biomarker detection.
Flowrate measurement
Flow rate tests were performed to assess the sample's reaction time with the PhC biosensor in the microfluidic chip. An 80 μL water sample containing fluorescent particles was carefully pipetted into the circular groove inlet of the microfluidic chip, which connects directly to the main channel. The channel leads to the sensing area, where the PhC biosensor is located. The sample was then absorbed by the absorbent paper at the end of the microchannel. Image sequences were recorded and analyzed using ImageJ. For each frame, the region of interest (ROI), defined as the area of tissue paper absorbing the solution, was manually selected. During this step, ImageJ tools were used to enhance contrast and perform thresholding and segmentation, enabling accurate identification of fluid boundaries. The absorbed area of the tissue paper was measured in each frame and converted into volume using a calibration factor (μL mm−2), calculated by dividing the initial sample volume (80 μL) by the chamber area in the first frame. This factor was applied across all frames to track volume changes and calculate the flow rate over time.Synthesis of self-assembled SiO2-coated PMMA PhCs
In this research, three-dimensional PhCs were fabricated using a self-assembly method with 250 nm PMMA microspheres (Fig. 2A). Colloidal particles were selected due to their cost-effectiveness and tunable optical properties, with particle size directly influencing the reflective wavelength to match biosensing targets. The PhCs naturally formed a face-centered cubic (FCC) structure, and their photonic bandgap was estimated using modified Bragg's law: | (2) |
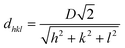 . The effective refractive index, neff is defined as:
. The effective refractive index, neff is defined as:| neff = 0.74·nPMMA + 0.26·nair | (3) |
Fabrication began by taping the edges of two glass slides to maintain a 0.12 mm spacing, achieved using two layers of 60 μm-thick adhesive tape as spacers. Both slides were then coated with 30 μL of 1% CYTOP solution (CTL-109AE, AGC Chemicals) and baked at 40 °C for 30 min to render the glass surfaces hydrophobic. Next, a 200 μL suspension of 250 nm PMMA nanoparticles was centrifuged at 13500 rpm for 8 min, and 150 μL of the supernatant was carefully removed to concentrate the colloid. The remaining 50 μL of the concentrated PMMA suspension was dropped onto one of the hydrophobically treated slides. The second slide was then placed on top and secured with binder clips, forming a uniform 120 μm gap defined by the tape thickness (Fig. S2-i). The assembly was left to dry naturally at room temperature (25 °C) for 24 h to allow the PMMA nanoparticles to self-assemble into ordered PhC structures (Fig. 2A and S2-ii). To improve the mechanical durability and ensure successful surface modification and sandwich immunoassay procedures, the PMMA PhCs were annealed at 115 °C for 10 min. During heating, the PMMA beads partially melted and fused together, which enhanced mechanical strength of the PhCs.
After that, a 20 nm SiO2 layer was deposited on the PhCs to provide hydroxyl groups for salinization and to prevent fluorescence quenching, thereby ensuring optimal optical performance for biosensing. The PhC surface was chemically modified in three steps: first, treatment with 20% APTES for 24 h to introduce amine groups via hydrolysis and condensation reactions; second, incubation with 5% GA for 1 h to form aldehyde linkers; and third, reaction with 1 mg mL−1 PEI for 16 h to increase surface amine density and improve biomolecule attachment. Following PBS washing, EDC/sulfo-NHS chemistry was used to activate the carboxyl groups of pAbs (0.01 mg mL−1), which were then conjugated to the PEI-modified surface via amide bond formation. A final blocking step with 2% BSA was applied to minimize non-specific adsorption and finally washed three times with PBS (Fig. 2B).
Sandwiched immunoassay on PhC biosensor
The sandwiched immunoassay developed in this study offers a sensitive method for detecting LCN-1 by combining antibody-based molecular recognition with optical signal enhancement from PhC substrates. In this approach, pAbs are immobilized on the SiO2-coated PhC surface as detection antibodies, enabling efficient binding to multiple epitopes on the LCN-1 antigen. The mAbs, which bind to a single specific epitope, are conjugated with fluorescent particles and serve as capture antibodies. This dual-antibody setup improves antigen binding efficiency, enhances fluorescence signal through specific detection, and increases the overall sensitivity of the assay.To prepare the detection probes, 200 nm yellow–green fluorescent nanoparticles with carboxyl and amine-functionalization were conjugated separately with mAbs via EDC/sulfo-NHS chemistry. To activate carboxyl groups in carboxylate-functionalized particles, 2.5 μL of particle solution was mixed with 25 μL of EDC and 25 μL of sulfo-NHS (10 mg mL−1) in MES buffer (pH 5.5), then incubated at 25 °C with shaking (800 rpm) for 15 min. The reaction mixture was centrifuged at 15000 g for 10 min, and the excess activation reagents in the supernatant were removed. The activated particle pellet was resuspended with 5 μL of mAb (1 mg mL−1) and 200 μL of PBS, then incubated at 4 °C for 4 h to allow the conjugation of mAbs through a stable amide bond formation. For amine-functionalized particles, mAbs were first activated under the same EDC/sulfo-NHS conditions, forming NHS esters on the antibody's carboxyl groups. These activated mAbs were then incubated with 2.5 μL of amine-modified particles at 4 °C for 4 h. After conjugation, both the particles were washed three times with PBST (0.01% Tween-20 in PBS) to remove unbound components. A blocking step with 2% BSA was performed at 25 °C for 1 h to reduce non-specific interactions. After final washing with PBST, the fluorescent mAb probes were ready for use.
Experimental setup
Sample observation and signal detection were performed using an Olympus IX71 fluorescence microscope and a QE Pro-FL spectrometer equipped with a halogen light source and optical fiber probe. Yellow–green fluorescent particles (excitation ∼500 nm) were visualized using a 500 nm dichroic mirror and captured with a DP74 camera. ImageJ software was used to quantify fluorescence intensity by isolating the green channel and analyzing ROIs. The ROI was defined by manually selecting the entire visible area of each PhC biosensor in the fluorescence image. The mean gray value (MGV) of the ROI was measured, followed by measurement of the MGV from a background region outside the sensing zone. Relative fluorescence intensity (ΔF) was calculated by subtracting the background MGV from the ROI MGV. Data were processed in excel and analyzed using GraphPad Prism for linear regression, one-way ANOVA, and t-tests. LoD was calculated using the 3-sigma method, representing the lowest signal that can be reliably distinguished from background noise.Tear sample collection
Tear samples were collected at National Cheng Kung University Hospital, Taiwan, from participants without any history of eye disease, current ocular treatment, or dry eye symptoms. All experiments were performed in accordance with the Guidelines of the Institutional Review Board (IRB agreement no. B-ER-112-052), and experiments were approved by the ethics committee at National Cheng Kung University, Taiwan. Informed consents were obtained from human participants of this study. Medical information was obtained through a pre-screening questionnaire. Tear fluid (20–30 μL) was gently and non-invasively collected using a 100 ± 1 mm glass capillary from the lower conjunctival sac before the administration of any eye drops. A trained medical technologist performed the procedure while carefully avoiding contact with the cornea and eyelids. Tear fluid was drawn into the capillary tube via natural capillary action. The collected samples were transferred into microcentrifuge tubes and stored at −30 °C to preserve protein stability. Due to the naturally high concentration of LCN-1 in tears, all samples were diluted 100-fold before analysis.Results and discussion
Characterization of SiO2-coated PMMA PhCs
The optical and structural characteristics of the fabricated PhCs were evaluated to confirm their biosensing suitability. In this work, 250 nm PMMA particles were used to develop the self-assembled PhCs. Followed by a 20 nm SiO2 coating to enable the antibody modification on the PMMA PhC. Based on modified Bragg's law eqn (2), the theoretical reflection peak for 250 nm PMMA-based PhCs was 480 nm, while spectrometer data showed a peak at 490 nm, likely due to slight size variation during self-assembly. This phenomenon is also observed in our previous study when 215 nm PMMA particles were used to develop the PhC. After SiO2 coating, a minimal blue shift to 489 nm was observed, confirming that optical resonance was retained (Fig. 3A).In our previous work,33 AuNP coatings have been used to deliver a suitable surface for antibody conjugation. However, the AuNP layer is prone to fluorescence quenching effects due to interactions between fluorescent molecules and metallic surfaces.39,43 In contrast, SiO2 coatings avoid such quenching and better preserve the reflectance enhancement properties of the PhC. AuNP-coated PhCs exhibited a reduced reflection enhancement, with fluorescence enhancement dropping to approximately 48% of its original value due to quenching effects.33 To overcome that, the AuNP coating was replaced with a SiO2 coating on the PhCs. The SiO2 coatings maintained approximately 92% of the reflectance enhancement, demonstrating their superior performance in preserving optical functionality (Fig. 3A). In addition, the SiO2 coating thickness plays a critical role in determining fluorescence quenching behavior. Previous studies have reported that quenching decreases sharply as SiO2 thickness increases from 0 to 25 nm, with strong suppression achieved at 12.5–25 nm.44 Based on this evidence, a 20 nm SiO2 layer was selected as it provides an optimal balance between minimizing quenching and maintaining photonic resonance efficiency, while also ensuring consistent and reproducible coating during fabrication.
Furthermore, the SEM images confirm the structural integrity (Fig. 3B-i) and the successful self-assembly of PMMA particles into a highly ordered FCC structure (Fig. 3B-ii). The cross-sectional SEM image in Fig. 3B-iii showed the uniformity and thickness (∼120 μm) of the SiO2-coated PMMA PhC, and confirmed successful deposition of the coating layer.
To enable antibody modification, amino groups were introduced onto the surface of the SiO2-coated PhC. These amino groups were then reacted with EDC/NHS to immobilize the target antibodies on the PhC. A functionalization protocol using APTES, GA, and PEI was employed to enhance the density and stability of amino groups on the silica layer of the PhC surface, which was confirmed by FTIR (Fig. 3C). The unmodified SiO2 surface exhibits a broad peak at 1000–1100 cm−1, corresponding to Si–O–Si stretching vibrations. After APTES-GA-PEI modification, a broad peak at 3300–3500 cm−1 corresponding to N–H stretching vibrations of primary and secondary amines. The peak in the range of 2800–3000 cm−1 indicates C–H stretching vibrations from the alkyl chains of APTES and PEI. Peaks around 1640–1650 cm−1 and 1550–1560 cm−1 are associated with N–H bending vibrations of primary amines.45–47 Additionally, comparison with the PEI reference spectrum reveals similarities in amine-related peaks, particularly the N–H stretching vibration at ∼3273 cm−1. These findings strongly confirm the successful incorporation of amine groups onto the PhC surface through APTES-GA-PEI modification.
Successful amine functionalization on the SiO2-coated PhC surface was further validated by reacting it with carboxylate-modified yellow-green fluorescent particles and measuring the fluorescence intensity. The EDC/NHS coupling chemistry was utilized to chemically bond the carboxylate group of the particles with the functionalized amino group on the PhCs. The fluorescence intensity was measured to evaluate the effectiveness of the APTES-GA-PEI modification. As shown in Fig. 3D, fluorescence intensity for the APTES-modified surface (28.38) was 7.00× higher than the PBST control (4.05), while the APTES-GA-PEI-modified surface (120.21) was 4.17× higher than APTES-only surfaces. These results demonstrate that GA and PEI substantially increased the amine group density on the SiO2-coated surface. This enhanced functionalization is critical for improving biomolecule immobilization efficiency, which directly impacts biosensor sensitivity and performance.
Fluorescence enhancement and specificity of SiO2-coated PhC
To evaluate fluorescence enhancement, the SiO2-coated PhC biosensor was compared with a non-PhC version. The PhC biosensor incorporated SiO2–PMMA PhCs, whereas the control used the same material without the PhC structure. Both platforms were evaluated through a sandwiched immunoassay using amine-modified particles and antigen concentrations of 1, 5, and 10 μg mL−1, with PBS as the negative control. For the assay, each pAb-modified PhC was incubated with 20 μL of the LCN-1 protein–mAb fluorescent particle complex at 25 °C and 400 RPM for 4 h, followed by five PBST washes. Fluorescence signals were then imaged under a microscope and quantified using ImageJ. The PhC biosensor consistently produced higher fluorescence intensities than the non-PhC version across all concentrations and controls (Fig. 4A). Quantitative analysis confirmed this enhancement, with statistical testing showing significant differences (****p < 0.0001). The results reveal an average 2.7-fold enhancement in fluorescence intensity when using the PhC compared to the non-PhC. This amplification is attributed to the unique PhC structure, which enhances fluorescence signals and improves sensitivity. In this case, the fluorophores are located at the PhC interface, where they experience partial field coupling. However, embedding the fluorophores within the periodic lattice would likely yield a much higher enhancement. These results validate the superior sensitivity of the PhC biosensor and highlight its potential for detecting low antigen concentrations. The observed linear increase in fluorescence with antigen concentration further demonstrates its reliability for diagnostic applications.To assess the specificity of the PhC biosensor, BSA and VEGF were tested alongside LCN-1. All the proteins were prepared with a concentration of 0.1 mg mL−1. No statistically significant difference was observed between the control proteins (VEGF and BSA) and the PBS group. However, a statistically significant difference (*p < 0.05) in fluorescence signal was observed between the PBS and the LCN1 sample (Fig. 4B). These results confirmed that the PhC biosensor selectively detects LCN-1 with high specificity.
Effect of functional groups on immunoassay
The functional groups play an important role in chemically bonding the target antibodies to the particles. There are amine and carboxylate modified fluorescence particles available commercially, and both of them can be used for modified with an antibody, and perform the immunoreactions. However, the modification procedure changes based on the functional groups on the particles. Therefore, to find out the functional group on the particles that provides the optimum sensitivity, here we have performed immunoassay reactions with amine and carboxylate modified fluorescence particles separately in two different experiments. Both particles were modified with monoclonal LCN-1 antibody, and a sandwiched immunoassay was performed with the PhC biosensor across antigen concentrations (0.1–100 μg mL−1). PBS was used as a negative control here. The immunoassay was performed in tubes and the fluorescence intensity was measured and evaluated to assess sensitivity and detection limits for LCN-1. An increasing trend in the fluorescence intensity with a statically significant difference between the different concentrations of LCN-1 was observed for both the particles, confirming the successful immunoassay formation (Fig. 5A and B). However, fluorescence signals at low concentrations (≤1 μg mL−1) lacked statistical significance for amine-modified particles (Fig. 5A). While comparing the sensitivity of both cases, amine-modified particles achieved a LoD of 7.55 μg mL−1 (Fig. 5C). While in the case of carboxylate-modified particles, an LoD of 80 ng mL−1 (Fig. 5D) was achieved, which is approximately 94 times higher than the amine-modified particles.The enhanced performance of carboxyl-modified particles can be attributed to differences in the reaction protocols. In the carboxyl-based method, the EDC/NHS solution was removed after activating the carboxylate groups, creating a cleaner environment for antibody conjugation. This step minimized residual reagents that could interfere with binding, reduced non-specific interactions, and promoted better antibody orientation, resulting in stronger coupling efficiency. In contrast, the amine-modified particle protocol involved direct activation of the antibody with EDC/NHS without removing the supernatant. The presence of residual chemicals could hinder the coupling process and lower efficiency. Moreover, since antibodies contain both carboxyl and amino groups, direct activation increased the risk of self-reaction between the antibodies, further reducing conjugation performance. Overall, carboxylate-modified fluorescent particles outperform amine-modified counterparts due to optimized reaction conditions that reduce chemical interference, prevent self-reaction of antibodies, and enhance immobilization efficiency. These advantages make carboxylate-modified particles suitable for immunoassay applications, and these particles were chosen further to perform the clinical studies.
Flow rate optimization of lateral flow microfluidic Chip
The flow rate measurement is important to provide maximum contact time of the samples to the PhC to enable the immunoassay. To achieve a controlled and steady flow, the absorbent paper was systematically redesigned to optimize capillary-driven fluid movement. The flow rate analysis assessed the performance of four absorbent paper designs integrated into the microchip. Four different designs, shown in Fig. 6A, varied in dimensions, with heights ranging from 35–40 mm and widths from 10–25 mm, and were used to analyze the flow rates. | ||
| Fig. 6 Absorbent paper optimization and flow performance. (A) Configurations of the four absorbent paper designs tested. (B) Comparison of flow rate profiles across the designs (design 3, n = 3). | ||
Design 1, with a simple rectangular shape, can exhibit a very rapid flow rate, with the sample volume reduced to nearly zero within 5 min. Most of the absorption occurred within the first 2 min, leading to uneven distribution and insufficient reaction time for effective biosensing. To slow the flow and improve stability, design 2 introduced a two-layer stepped structure, which extended the absorbance time and produced a more uniform profile. However, design 2 showed the slowest flow rate, extending beyond 15 min. Although this allowed prolonged reaction time, it exceeded the required limit (<15 min), making it unsuitable for rapid testing. Building on this concept, multi-layer stepped geometries (designs 3 and 4) were developed to further regulate flow and ensure uniform capillary progression across the absorbent paper. Both designs exhibited steady and consistent flow profiles compared to designs 1 and 2, which showed either excessively rapid (design 1) or slow (design 2) flow characteristics. When comparing designs 3 and 4, the main difference lies in the absorbent strip length and overall flow duration. Design 4 showed a slightly longer absorbance time of approximately 18 minutes, whereas design 3 completed the process within about 14 minutes (Video S1), as shown in Fig. 6B.
Unlike trapezoidal or triangular geometries that can cause uneven flow distribution and localized acceleration at narrow ends, the stepped design maintains a uniform cross-sectional area across each layer. This configuration provides gradual capillary resistance along the flow path, promoting a stable fluid front and sufficient absorbance time for efficient biosensing. For our intended rapid testing application, an optimal absorbance time of around 10–15 minutes was targeted to ensure efficient sample transfer while maintaining sufficient reaction time for signal development. Therefore, although both designs 3 and 4 demonstrated balanced performance, design 3 was considered more suitable as it achieved a steady flow rate within the preferred time window, ensuring test consistency without unnecessarily prolonging the overall assay duration. Hence, it was adopted to perform the clinical studies with human tear samples.
On-chip detection of LCN-1 and clinical studies with human tear samples
On the microfluidic chip, the control line was pre-conjugated with LCN-1 antigen and served as a built-in positive control, confirming proper fluid flow and successful completion of the immunoreaction in each assay. By contrast, the chip tested with PBS (no antigen) acted as the negative control in this calibration experiment, representing the baseline fluorescence signal in the absence of LCN-1. This distinction between the built-in positive control line and the PBS-based negative control ensured both assay validity and specificity. The analytical performance of the PhC microfluidic chip was evaluated through calibration with known LCN-1 concentrations (0.1, 1, 10, and 100 μg mL−1). A statistically significant difference (**p < 0.001) was observed between PBS and the LCN-1 concentration of 1 ng mL−1 (Fig. 7A). The assay also showed a strong positive correlation between LCN-1 concentration and fluorescence intensity (Fig. 7B). Control biosensors, pre-conjugated with antigen, consistently displayed higher signals, validating the sandwiched immunoassay. The calibration curve exhibited a robust linear relationship (R2 = 0.9219) between fluorescence intensity and the logarithm of antigen concentration, with a LoD of 136 pg μL−1 on-chip. The capillary-driven flow, facilitated by absorbent paper design, allowed fluid to pass through the microchannel in approximately 13–14 min, providing sufficient time for antigen–antibody binding without external pumps. Modifications in microfluidic chip design, SiO2 coating on the PhC, and use of carboxylate-modified particles enabled an approximately 22-times better sensitivity compared to our previous design.33The results from the PhC microfluidic chip were further validated by measuring the LCN-1 concentrations using a micro-volume spectrophotometer (OneDrop TOUCH Pro, Biometric Technologies). The spectrophotometer provided converted concentration values, which were plotted against the log-transformed concentrations of the antigen samples, as shown in Fig. 7C. The trend observed in these results was compared with the trend obtained from the on-chip test. The consistency between the two trends supports the accuracy and reliability of the on-chip calibration curve.
The practical utility of the PhC microfluidic chip was tested by analyzing the human tear samples. Clinical validation was performed on tear samples from 30 individuals: 10 healthy controls (cases 1–10), 10 NPDR patients (cases 11–20), and 10 PDR patients (cases 21–30). All experiments were performed in accordance with the Guidelines of the Institutional Review Board (IRB agreement no. B-ER-112-052), and experiments were approved by the ethics committee at National Cheng Kung University, Taiwan. Informed consents were obtained from human participants of this study. The study excluded individuals with any other ocular diseases, ongoing treatments, or dry eye syndrome. Before sample collection, participants completed a questionnaire, with results and medical conditions documented in supplementary tables (Table S3). Approximately 10–20 μL of tears were collected from each participant, pipetted into microcentrifuge tubes, and preserved at −30 °C to maintain sample integrity. To account for high concentrations of LCN-1 in tear samples, all samples were diluted 100-fold with 1× PBS before analysis. The final LCN-1 concentrations were determined by multiplying the measured values by the dilution factor. The quantification was conducted using an on-chip immunoassay, where each sample was measured once. To perform the immunoassay, 10 μL of the diluted tear sample was mixed with 10 μL of mAb-fluorescent particles and incubated for 1 min. Afterward, 60 μL of PBS was added to reach a total volume of 80 μL, and the solution was loaded onto the microchip. Following an incubation period of 15 min, the fluorescent intensity was measured to determine LCN-1 concentration.
The experimental results revealed distinct concentration patterns among healthy controls, NPDR patients, and PDR patients (Fig. 7D and S3). Healthy individuals (cases 1–10) showed LCN-1 levels ranging from 0.5 to 1.94 mg mL−1. Notably, case 10 presented with a relatively elevated LCN-1 level, whereas the remaining cases fell within the expected range for individuals without retinal complications.48 The higher LCN-1 level may indicate a potential risk for the future development of DR. This finding highlights the potential of the PhC microfluidic chip for detecting pre-diabetic stages. Previous studies have reported that elevated LCN-1 levels can serve as an early indicator of retinal stress or inflammation before overt DR symptoms appear.49
NPDR patients (cases 11–20) exhibited elevated LCN-1 concentrations, compared to the healthy individuals, ranging from 1.28 to 2.88 mg mL−1. Among all the NPDR patients, case 12 showed a comparatively lower concentration. A statistically significant difference (****p < 0.0001) was observed between the healthy and NPDR groups, indicating the potential of the PhC microfluidic chip for diagnosing DR at the non-proliferative stage (Fig. 7E). The receiver operating characteristic (ROC) curve revealed a sensitivity and specificity of 100% and 90%, respectively, with an area under the curve (AUC) of 0.99 (Fig. 7F and S4), achieving an overall accuracy of 95%. Based on the ROC curve, the cutoff value distinguishing healthy individuals from NPDR patients was determined to be 1.25 mg mL−1; thus, individuals with LCN-1 concentrations >1.25 mg mL−1 in tears would be classified as NPDR.
The PDR patients exhibited the highest LCN-1 levels, ranging from 2.9 to 4.8 mg mL−1. A statistically significant difference was observed between the healthy donors and the PDR (****p < 0.0001; Fig. 7E). The ROC curve demonstrated a near-perfect AUC of 1.0 and both sensitivity and specificity of 100% (Fig. 7F and S5). When comparing PDR to NPDR patients, LCN-1 levels were also significantly higher (****p < 0.0001; Fig. 7E), with sensitivity and specificity of 100% and 90%, respectively, and an AUC of 0.995 (Fig. 7F and S6). The device achieved accuracies of 100% (PDR vs. healthy) and 95% (PDR vs. NPDR). Based on the ROC curve, the cutoff value distinguishing PDR from NPDR patients was determined to be 2.85 mg mL−1; individuals with tear LCN-1 concentrations greater than 2.85 mg mL−1 were classified as having PDR. The performance of our system was compared with previously reported biosensing technologies, including photonics-based biosensors for tear analyte detection (Table S1) and other sensing platforms (Table S2). To further validate our findings, ophthalmoscope images of the participants were captured and compared with the analytical results. Images from healthy subjects showed no vascular abnormalities, whereas hemorrhages and abnormalities were evident in the retinal blood vessels of NPDR and PDR patients (Fig. S7). These observations closely matched the results obtained using the PhC microfluidic chip, confirming the accuracy of our analysis. Overall, the findings highlight the strong diagnostic potential of the PhC microfluidic chip for early DR detection and differentiation between disease stages.
Future scope and perspectives
The developed SiO2-coated PhC microfluidic chip demonstrates strong potential for early-stage DR detection from tear samples, showing that photonic fluorescence enhancement can be effectively translated into a clinically relevant assay format. However, the current prototype still relies on a laboratory fluorescence microscope for signal acquisition and quantitative analysis, which limits its immediate use as a fully portable point-of-care testing (POCT) device. To overcome this limitation, future development will focus on integrating the PhC microfluidic biosensor with compact optical readout systems. For instance, smartphone-based fluorescence readers equipped with miniature excitation sources (e.g., LEDs or laser diodes), emission filters, and image-processing algorithms could be adapted to capture and quantify the fluorescence signal directly from the PhC surface.50 By incorporating a simple optical alignment cradle or 3D-printed holder to position the chip and excitation module, the smartphone camera can act as the detector for image capture and quantitative analysis through dedicated mobile software. Similarly, portable fluorescence detection modules utilizing nanoparticle-mediated sensing could serve as an alternative low-power solution for field use.51 These integrations would transform the current system into a low-cost, user-friendly, and field-deployable diagnostic platform that eliminates the need for bulky optical instruments. These integrations would transform the current system into a compact, affordable, and user-friendly diagnostic platform that operates without bulky optical equipment, enabling the broad implementation of POCT for DR screening in resource-limited environments.Conclusion
This research presented a PhC biosensor integrated into a microfluidic chip for non-invasive, rapid, and sensitive detection of DR using tear samples. The device employed a sandwiched immunoassay targeting LCN-1 in human tear samples. For the immunoassay reaction, the carboxylate-modified fluorescent particles demonstrated superior analytical performance compared to amino-modified particles. The microfluidic chip, driven by capillary flow and optimized absorbent paper, enabled uniform sample distribution and completed the assay within 15 min. The PhC delivered an average 2.7-fold enhancement in fluorescence intensity. The device achieved a high specificity for LCN-1 over non-target proteins. On-chip calibration showed strong linearity (R2 = 0.9219) and a LoD of 136 pg μL−1. Clinical trials with tear samples from 30 individuals, including healthy and DR patients, confirmed elevated LCN-1 levels in DR cases, supporting the biosensor's clinical relevance. The device demonstrated 100% sensitivity, specificity, and accuracy in differentiating PDR from healthy individuals, and 100% sensitivity with 90% specificity for distinguishing NPDR from healthy individuals, resulting in an overall diagnostic accuracy of 95%. Overall, this platform provides a rapid, non-invasive, and accurate method for early DR detection and stage differentiation, and while the current setup remains laboratory-based, it demonstrates strong potential for future development into a portable POC screening tool.Author contributions
Kullaphat Nitayachat: conceptualization, investigation, methodology, data curation, formal analysis, writing – original draft, review & editing. Dhrubajyoti Das: conceptualization, investigation, methodology, data curation, formal analysis, writing – original draft, review & editing. Pei-Yi Chen: conceptualization, investigation, methodology, data curation, formal analysis. Sheng-Min Hsu: investigation, methodology. Jhih-Cheng Wang: project administration, supervision, funding acquisition. Han-Sheng Chuang: conceptualization, investigation, methodology, data curation, project administration, supervision, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
Data will be available on request.Supplementary information (SI): the SI includes more detailed dimensions of the microfluidic chip, photonic crystal fabrication images, fluorescence images of clinical tear samples, and calculations for sensitivity, specificity, and accuracy derived from ROC curve analyses. It also provides supporting ophthalmoscopy images of study participants, and complete patient demographic data, as well as comparison tables of tear-analyte detection technologies and a demonstration video showing fluid flow within the microfluidic chip. See DOI: https://doi.org/10.1039/d5lc00919g.
Acknowledgements
This research work was supported by the National Science and Technology Council (NSTC), Taiwan, under grant numbers: 112-2221-E-006-018-MY3, 113-2923-E-006-005-MY3, and the Chi Mei-NCKU grant.References
- Z. L. Teo, Y.-C. Tham, M. Yu, M. L. Chee, T. H. Rim, N. Cheung, M. M. Bikbov, Y. X. Wang, Y. Tang, Y. Lu, I. Y. Wong, D. S. W. Ting, G. S. W. Tan, J. B. Jonas, C. Sabanayagam, T. Y. Wong and C.-Y. Cheng, Ophthalmology, 2021, 128, 1580–1591 CrossRef PubMed.
- A. Witkin and D. Salz, Middle East Afr. J. Ophthalmol., 2015, 22, 145 CrossRef PubMed.
- T. E. Rohan, C. D. Frost and N. J. Wald, Br. Med. J., 1989, 299, 1198–1201 CrossRef CAS PubMed.
- M. Georgieva Pandova, in Visual Impairment and Blindness - What We Know and What We Have to Know, ed. G. Lo Giudice and A. Catalá, IntechOpen, 2020 Search PubMed.
- S. Kumar, Ann. Eye Sci., 2023, 8, 8–8 CrossRef.
- I. Qureshi, J. Ma and Q. Abbas, Symmetry, 2019, 11, 749 CrossRef.
- S. Vujosevic, S. J. Aldington, P. Silva, C. Hernández, P. Scanlon, T. Peto and R. Simó, Lancet Diabetes Endocrinol., 2020, 8, 337–347 CrossRef PubMed.
- H. Nouri, S.-H. Abtahi, M. Mazloumi, S. Samadikhadem, J. F. Arevalo and H. Ahmadieh, Surv. Ophthalmol., 2024, 69, 558–574 CrossRef PubMed.
- F. Yang, W. Zou, Z. Li, Y. Du, W. Gao, J. Zhang, X. Ji and J. Huang, Diabetes/Metab. Res. Rev., 2024, e3812 CrossRef CAS PubMed.
- J. M. B. De Barros Garcia, D. L. C. Isaac and M. Avila, Int. J. Retina Vitr., 2017, 3, 14 CrossRef PubMed.
- B. Pawar, S. N. Lobo, M. Joseph, S. Jegannathan and H. Jayraj, Middle East Afr. J. Ophthalmol., 2021, 28, 81–86 CrossRef.
- C.-L. Hu, Y.-C. Wang, W.-F. Wu and Y. Xi, Photodiagn. Photodyn. Ther., 2024, 49, 104331 CrossRef PubMed.
- X. Xu, M. Zhang, S. Huang, X. Li, X. Kui and J. Liu, Front. Cell Dev. Biol., 2024, 12, 1473176 CrossRef.
- V. Bellemo, Z. W. Lim, G. Lim, Q. D. Nguyen, Y. Xie, M. Y. T. Yip, H. Hamzah, J. Ho, X. Q. Lee, W. Hsu, M. L. Lee, L. Musonda, M. Chandran, G. Chipalo-Mutati, M. Muma, G. S. W. Tan, S. Sivaprasad, G. Menon, T. Y. Wong and D. S. W. Ting, Lancet Digital Health, 2019, 1, e35–e44 CrossRef PubMed.
- Q. Zhang, D. Gong, M. Huang, Z. Zhu, W. Yang and G. Ma, Front. Endocrinol., 2025, 16, 1438739 CrossRef.
- M. Prem Senthil, S. Anand, R. Chakraborty, J. E. Bordon, P. A. Constable, S. Brown, D. Al-Dasooqi and S. Simon, J. Neurol., 2024, 271, 4769–4793 CrossRef PubMed.
- F. Zhang, W. Xu, Z. Deng and J. Huang, Front. Med., 2025, 11, 1487981 CrossRef.
- T. Jamshidnejad-Tosaramandani, S. Kashanian, K. Omidfar and H. B. Schiöth, Front. Bioeng. Biotechnol., 2024, 12, 1446355 CrossRef PubMed.
- Y. Wu, Y. Hu, N. Jiang, M. W. Georgi, A. K. Yetisen and M. F. Cordeiro, Lab Chip, 2025, 25, 2291–2303 RSC.
- D. Das, H.-A. Chen, C.-L. Weng, Y.-C. Lee, S.-M. Hsu, J.-S. Kwon and H.-S. Chuang, Anal. Chim. Acta, 2024, 1325, 343117 CrossRef CAS.
- D. Das, H.-A. Chen, Y.-C. Lee, J.-S. Kwon and H.-S. Chuang, Sens. Actuators, B, 2023, 394, 134353 CrossRef CAS.
- D. P. Hainsworth, A. Gangula, S. Ghoshdastidar, R. Kannan and A. Upendran, Am. J. Ophthalmol., 2020, 213, 306–319 CrossRef CAS PubMed.
- Y. Zhang, E. Ranaei Pirmardan, H. Jiang, A. Barakat and A. Hafezi-Moghadam, Biosens. Bioelectron., 2023, 237, 115476 CrossRef CAS PubMed.
- J. T. Metternich, B. Hill, J. A. C. Wartmann, C. Ma, R. M. Kruskop, K. Neutsch, S. Herbertz and S. Kruss, Angew. Chem., Int. Ed., 2024, 63, e202316965 CrossRef CAS PubMed.
- L. Bezinge, A. Suea-Ngam, A. J. deMello and C.-J. Shih, Mol. Syst. Des. Eng., 2020, 5, 49–66 RSC.
- Y. Xiong, S. Shepherd, J. Tibbs, A. Bacon, W. Liu, L. D. Akin, T. Ayupova, S. Bhaskar and B. T. Cunningham, Micromachines, 2023, 14, 668 CrossRef.
- J. M. Thomas, Angew. Chem., Int. Ed., 2012, 51, 12946–12958 CrossRef CAS PubMed.
- M. Rybin, I. Shishkin, K. Samusev, P. Belov, Y. Kivshar, R. Kiyan, B. Chichkov and M. Limonov, Crystals, 2015, 5, 61–73 CrossRef.
- N. Ganesh, P. C. Mathias, W. Zhang and B. T. Cunningham, J. Appl. Phys., 2008, 103, 083104 CrossRef.
- S. Bhaskar, W. Wang, H. Lee, L. Liu, S. Umrao, W. Liu, A. Bacon, J. Tibbs, K. Khemtonglang, A. Tan, T. Ayupova, W.-C. Chen, X. Wang and B. T. Cunningham, Chem. Rev., 2025, 125, 6435–6540 CrossRef PubMed.
- B. T. Cunningham, M. Zhang, Y. Zhuo, L. Kwon and C. Race, IEEE Sens. J., 2016, 16, 3349–3366 CAS.
- R. Vaz, M. G. F. Sales and M. F. Frasco, TrAC, Trends Anal. Chem., 2024, 177, 117771 CrossRef CAS.
- L.-Y. Chen, S.-M. Hsu, J.-C. Wang, T.-H. Yang and H.-S. Chuang, Biomicrofluidics, 2023, 17, 044102 CrossRef CAS PubMed.
- É. Csősz, P. Boross, A. Csutak, A. Berta, F. Tóth, S. Póliska, Z. Török and J. Tőzsér, J. Proteomics, 2012, 75, 2196–2204 CrossRef PubMed.
- H.-J. Kim, P.-K. Kim, H.-S. Yoo and C.-W. Kim, Clin. Biochem., 2012, 45, 60–67 CrossRef CAS PubMed.
- C. Fenzl, T. Hirsch and O. S. Wolfbeis, Angew. Chem., Int. Ed., 2014, 53, 3318–3335 CrossRef CAS PubMed.
- B. T. Cunningham, JALA, 2010, 15, 120–135 CAS.
- Photonics crystal-enhanced fluorescence – Nanosensors Group, https://nano.ece.illinois.edu/research-smartphone-biosensors/photonic-crystal-enhanced-fluorescence/, (accessed August 6, 2025).
- Y. Xiong, Q. Huang, T. D. Canady, P. Barya, S. Liu, O. H. Arogundade, C. M. Race, C. Che, X. Wang, L. Zhou, X. Wang, M. Kohli, A. M. Smith and B. T. Cunningham, Nat. Commun., 2022, 13, 4647 CrossRef CAS.
- N. Ganesh, W. Zhang, P. C. Mathias, E. Chow, J. A. N. T. Soares, V. Malyarchuk, A. D. Smith and B. T. Cunningham, Nat. Nanotechnol., 2007, 2, 515–520 CrossRef PubMed.
- S. Bhaskar, L. Liu, W. Liu, J. Tibbs, L. D. Akin, A. Bacon and B. T. Cunningham, APL Mater., 2025, 13, 041103 CrossRef CAS.
- S. Bhaskar, W. Liu, J. Tibbs and B. T. Cunningham, Appl. Phys. Lett., 2024, 124, 161102 CrossRef CAS.
- Z. Zhang, L. Zhang, M. N. Hedhili, H. Zhang and P. Wang, Nano Lett., 2013, 13, 14–20 CrossRef CAS.
- M. M. Elsutohy, A. Selo, V. M. Chauhan, S. J. B. Tendler and J. W. Aylott, RSC Adv., 2018, 8, 35840–35848 RSC.
- E. Vilarrasa-Garcia, E. M. O. Moya, J. A. Cecilia, C. L. Cavalcante, J. Jiménez-Jiménez, D. C. S. Azevedo and E. Rodríguez-Castellón, Microporous Mesoporous Mater., 2015, 209, 172–183 CrossRef CAS.
- A. Mushtaq, H. Bin Mukhtar and A. Mohd Shariff, Res. J. Appl. Sci., Eng. Technol., 2014, 7, 1811–1820 CrossRef CAS.
- S. Nayab, H. Baig, A. Ghaffar, E. Tuncel, Z. Oluz, H. Duran and B. Yameen, RSC Adv., 2018, 8, 23963–23972 RSC.
- J.-Y. Wang, J.-S. Kwon, S.-M. Hsu and H.-S. Chuang, Lab Chip, 2020, 20, 356–362 RSC.
- J.-C. Wang, H.-Y. Ku, T.-S. Chen and H.-S. Chuang, Biosens. Bioelectron., 2017, 89, 701–709 CrossRef CAS PubMed.
- B. Wang, Y. Li, M. Zhou, Y. Han, M. Zhang, Z. Gao, Z. Liu, P. Chen, W. Du, X. Zhang, X. Feng and B.-F. Liu, Nat. Commun., 2023, 14, 1341 CrossRef CAS PubMed.
- S. Liu, J. Zhao, J. Wu, L. Wang, C. Yao, J. Hu and H. Zhang, Food Chem., 2025, 463, 141205 CrossRef CAS PubMed.
Footnote |
| † Both authors have contributed equally |
| This journal is © The Royal Society of Chemistry 2026 |






