An electrocatalytic three-component cyclization reaction: synthesis of selenium-containing cyclopentenes via intermolecular selective [3 + 2] annulation of terminal alkynes, unsaturated propionates, and diselenides
Zu-Yu
Mo†
 ,
Yi
Zhang†
,
Xin-Yu
Tang
,
Lei
Gao
,
Ying-Ming
Pan
,
Yi
Zhang†
,
Xin-Yu
Tang
,
Lei
Gao
,
Ying-Ming
Pan
 ,
Mu-Xue
He
,
Mu-Xue
He
 * and
Xian-Li
Ma
*
* and
Xian-Li
Ma
*
Guangxi Key Laboratory of Drug Discovery and Optimization, Guangxi Engineering Research Center for Pharmaceutical Molecular Screening and Druggability Evaluation, Key Laboratory of Medical and Translational Medicine, School of Pharmacy, Guilin Medical University, Guilin 541199, People's Republic of China. E-mail: mxl78@glmc.edu.cn; hemuxue@126.com; mozuyu90@163.com
First published on 4th December 2025
Abstract
Developing efficient electrochemically mediated three component cyclization reactions involving diselenides is of great significance for constructing structurally diverse organoselenium compounds. Herein, a novel electrocatalytic three-component cyclization reaction of terminal alkynes, unsaturated propionates, and diselenides has been developed, providing a simple and effective method for the synthesis of valuable selenocyclopentenes. This strategy uses inexpensive ferrocene as a redox catalyst to obtain a series of selenium-containing cyclopentene derivatives in moderate to excellent yields, characterized by mild reaction conditions, a wide substrate range, and good functional group tolerance. Preliminary mechanistic studies have been conducted, indicating that selenium radicals, selenium anions, carbon radicals, and carbocations are involved in this transformation.
Green foundation1. This study developed a novel, economical, and sustainable electrochemically mediated three-component cyclization reaction of terminal alkynes, allyl dimethyl esters, and diselenides, providing a simple and efficient method for the synthesis of valuable selenium-containing cyclopentenes. Compared with traditional electrooxidation or electroreduction processes, this method employs a paired electrolysis strategy, utilizing both anodic and cathodic reactions simultaneously to maximize energy and atom economy, offering a more practical and energy-efficient approach that aligns with green chemistry principles.2. This strategy yielded a series of selenium-cyclopentene derivatives in moderate to excellent yields under mild reaction conditions, with a broad substrate range and good functional group tolerance. Using electricity as a clean reagent and thus producing only hydrogen as a byproduct reduces energy consumption and minimizes environmental impact. Furthermore, the inexpensive ferrocene used as a redox catalyst has the potential for recycling. Moreover, compared with traditional cyclopentene construction methods, this strategy eliminates the need for substrate pre-functionalization, resulting in higher atom and step economy. 3. Future research will improve catalyst recovery and expand the substrate range for drug molecule synthesis, especially in applications related to drug molecule modification. |
Introduction
Organoselenium compounds are considered an important class of molecules with wide application in various disciplines, serving as organic catalysts,1 fluorescent probes,2 and functional organic materials.3 Additionally, they also exhibit multiple biological activities (Scheme 1a), such as antiproliferative activity, calcium channel (VDCC) blocking activity, antitumor activity, antioxidant activity, 5-LOX (lipoxygenase) inhibitory activity, and antibacterial activity.4 Among them, compound II can serve as a voltage-dependent calcium channel (VDCC) blocker, which effectively inhibits Mpro and the COVID-19 virus. Introducing selenium atoms into potential bioactive molecules can significantly enhance the natural biological activity of the substrates. Given these unique biological and chemical properties, the synthesis of organoselenium compounds has been a research hotspot for chemists.In the past decades, the synthesis of organoselenium compounds has witnessed rapid development. However, most methods generally require expensive catalysts and various transition metal catalysts,5 which has prompted people to continuously explore more economical and environmentally friendly methods for the synthesis of organoselenium compounds. Organic electrochemistry is a sustainable synthesis method with mild reaction conditions and strong controllability.6 In recent years, rapid progress has been made in the electrochemical synthesis of organoselenium compounds. Currently, there are three main types of electrochemically mediated reactions for synthesizing organoselenium compounds: (1) electrochemically mediated C–H bond selenization to construct organoselenium compounds (Scheme 1b(i));7 (2) electrochemical oxidative difunctionalization of olefins/alkynes with diselenides and nucleophiles (Scheme 1b(ii));8 and (3) electrochemically mediated two-component cyclization reactions of unsaturated hydrocarbons with diselenides (Scheme 1b(iii)).9
Although significant progress has been made in the electrochemical synthesis of organoselenium compounds, there is still limited research on electrochemically catalyzed three-component cyclization reactions involving diselenides. To date, only two cases have been reported. For example, in 2021, He's group reported an electrochemically mediated three-component reaction of diselenides with phenylhydrazine and 2,4-pentanedione to construct 4-selanylpyrazoles (Scheme 1c(i)).10 In 2023, Liang's group synthesized oxazolidine-2,4-diones through electrochemical and copper catalyzed reactions of diselenides with alkyne ketones and CO2 (Scheme 1c(ii)).11 However, both of the above reactions involve the construction of heterocycles, and there are currently no reports on the electrochemically mediated three-component cyclization of selenium to construct carbon rings. Cyclopentene is the common core of many natural products and bioactive molecules.12 Herein, we develop an electrochemical oxidative cyclization reaction of diselenides, terminal alkynes, and unsaturated propionates to synthesize selenium-containing cyclopentene derivatives, which has not been previously reported (Scheme 1d). This reaction utilizes inexpensive and easily accessible three-component raw materials to innovatively achieve electrochemical three-component [3 + 2] cyclization of carbocyclic selenides, which has the characteristics of overcoming selectivity challenges, a green and sustainable catalytic system, wide applicability, and practicality.
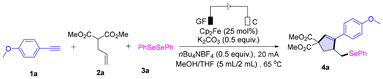
Initially, we investigated various reaction conditions for the electrochemical cyclization of 1-ethynyl-4-methoxybenzene 1a, dimethyl 2-allylmalonate 2a, and 1,2-diphenyldiselane 3a in an undivided cell (Table 1). The desired product 4a was obtained in 93% yield when graphite felt (GF) was used as the anode, a carbon rod as the cathode, Cp2Fe (25 mol%) as the catalyst, K2CO3 (0.5 equiv.) as the base, and MeOH/THF as the solvent at 65 °C and a constant current (20 mA) (Table 1, entry 1). The reaction failed when Cp2Fe was not added (entry 2). When the Cp2Fe catalyst was reduced to 15 mol%, the yield of 4a decreased to 79% (entry 3). Similarly, the presence of a base was also crucial for the reaction. When K2CO3 was not added, the yield of the desired product 4a decreased significantly (entry 4). When other bases such as Et3N and NaOAc were applied instead of K2CO3, the yield of product 4a decreased (entries 5 and 6). We also studied the effect of electrodes on the reaction, and the results showed that when graphite felt was used as the anode and the platinum sheet was used as the cathode, the yield of 4a slightly decreased (entry 7). When platinum sheets were used as electrodes, the yield of 4a dropped sharply to 23% (entry 8). Excessive or insufficient reaction temperature led to a decrease in the yield of 4a (entries 9 and 10). In addition, the decrease in constant current also led to a decrease in the yield of product 4a (entries 11 and 12). Finally, the desired product was not detected when the reaction was performed without electricity (entry 13). For more detailed optimization of conditions, see Table S1.
| Entry | Variation from standard conditions | Yieldb |
|---|---|---|
| a Reaction conditions: graphite felt (10 mm × 10 mm × 5 mm) anode, carbon rod (Φ 6 mm) cathode, constant current = 20 mA, undivided cell, 1a (0.3 mmol, 1.0 equiv.), 2a (0.6 mmol, 2.0 equiv.), 3a (0.225 mmol, 0.75 equiv.), Cp2Fe (0.075 mmol, 25 mol%), K2CO3 (0.15 mmol, 0.5 equiv.), nBu4NBF4 (0.15 mmol, 0.5 equiv.), MeOH/THF (5.0 mL/2.0 mL), 65 °C, 4 h, and 9.9 F mol−1. b Isolated yields. | ||
| 1 | None | 93 |
| 2 | No Cp2Fe | Trace |
| 3 | Cp2Fe (15 mol%) | 79 |
| 4 | Without K2CO3 | 23 |
| 5 | NaOAc instead of K2CO3 | 65 |
| 6 | Et3N instead of K2CO3 | 52 |
| 7 | GF (+)|Pt (−) | 89 |
| 8 | Pt (+)|Pt (−) | 23 |
| 9 | Reaction at 40 °C | 45 |
| 10 | Reaction at 80 °C | 61 |
| 11 | Constant current: 5 mA | 59 |
| 12 | Constant current: 10 mA | 88 |
| 13 | No electricity | 0 |
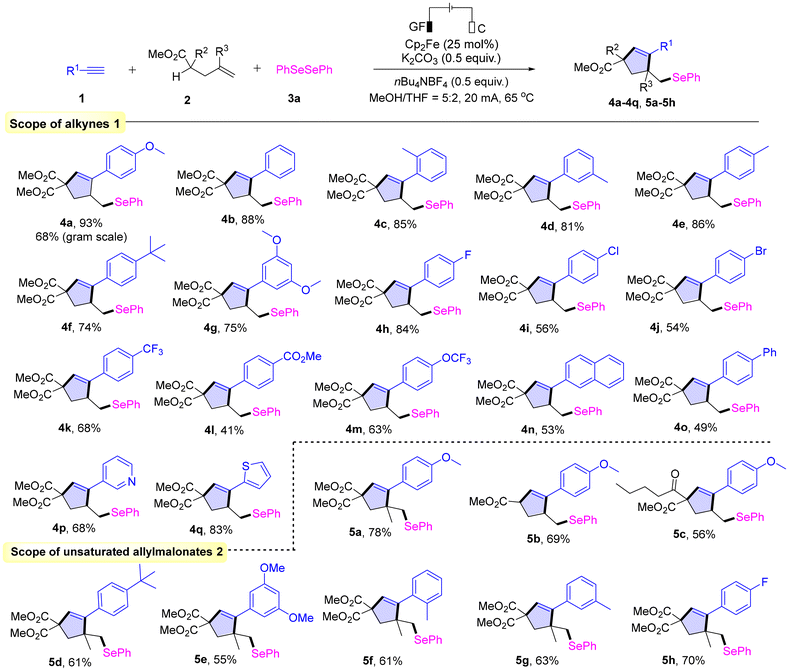
With the confirmation of optimized conditions, we next investigated the scope of alkynes 1 (Scheme 2). When there was no substituent on the benzene ring, the desired product 4b can be obtained in a yield of 88%. Substrates bearing methyl groups at the ortho-, meta- and para-positions of the phenyl ring (1c–1e) also reacted smoothly, and the corresponding desired products (4c–4e) were obtained in excellent yields (81%–86%). In addition, when other electron-donating groups such as tert-butyl or methoxy groups were present on the benzene ring (1f and 1g), the reaction also proceeded smoothly. Similarly, phenylacetylenes bearing halogen or electron-withdrawing groups (–COOMe and –OCF3) at the para-position were also suitable for this transformation and produced the corresponding products (4h–4m) in moderate to excellent yields (41%–84%). Notably, naphthalene-2-acetylene (1n) and biphenyl-4-acetylene (1o) also afforded the corresponding products 4n and 4o in 53% and 49% yields, respectively. Lastly, pyridine-3-acetylene (1p) and thiophene-2-acetylene (1q) also showed good reactivity and afforded the corresponding products 4p and 4q in 68% and 83% yields, respectively. The reaction of 1a, 2a and 3a proceeds smoothly on a gram scale, which produces product 4a in 68% yield (Fig. S2). Next, we also investigated the substrate scope of the transformation, generating the corresponding cyclopentene compound 5c in a yield of 56%. We used 2b as the substrate to examine the scope of alkynes 1. Alkynes with electron-donating groups on the benzene ring, such as 1-ethynyl-4-tert-butylbenzene and 1-ethynyl-3,5-dimethoxybenzene, were able to react with 2b and 3a to give the corresponding target products 5d and 5e in moderate yields. Furthermore, 1-ethynyl-2-methylbenzene and 1-ethynyl-3-methylbenzene could also be converted into the target products (5f and 5g) in good yields. Finally, alkynes substituted with electron-withdrawing groups, such as 1-ethynyl-4-fluorobenzene, were also successfully converted into the corresponding product 5h in a yield of 70%.
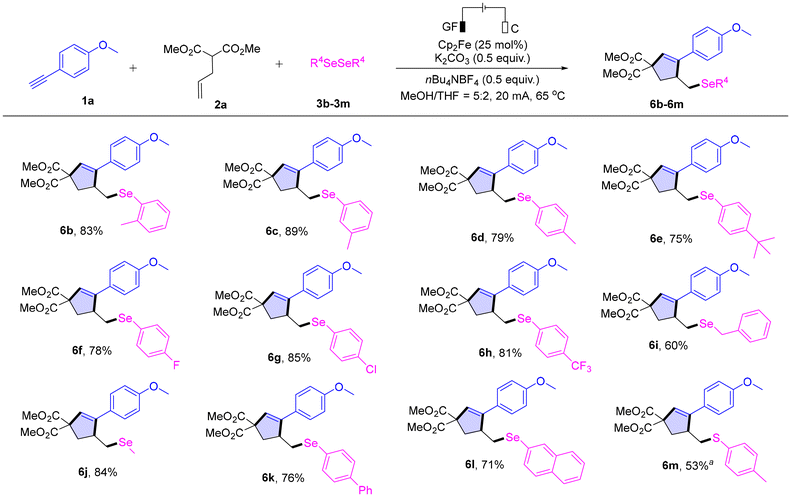
To further demonstrate the generality of this method, we investigated the generality of diselenides 3 under the standard conditions (Scheme 3). Diphenyl diselenides with methyl groups at the ortho-, meta-, and para-positions exhibited good reactivity in the reaction, yielding the target products 6b, 6c, and 6d in yields of 83%, 89%, and 79%, respectively. In addition, para-tert-butyl diphenyl diselenide also worked well, and the corresponding product 6e was isolated in 75% yield. Moreover, diphenyl diselenides bearing halogens (F and Cl) or –CF3 at the para-position on the phenyl ring both showed great reaction efficiencies and provided the corresponding products (6f–6h) in good to excellent yields (78–85%). Furthermore, dibenzyl diselenide 3i and dimethyl selenide 3j could also tolerate the electrochemical conditions well. It is worth noting that biphenyl diselenide 3k and naphthalene-2-diselenide 3l tolerated the electrochemical conditions well, giving the desired products 6k and 6l in yields of 76% and 71%, respectively. Finally, we also attempted to use 4-methylbenzenethiol 3m instead of the diphenyl diselenide as the reaction substrate and successfully obtained the desired product 6m in a yield of 53%.
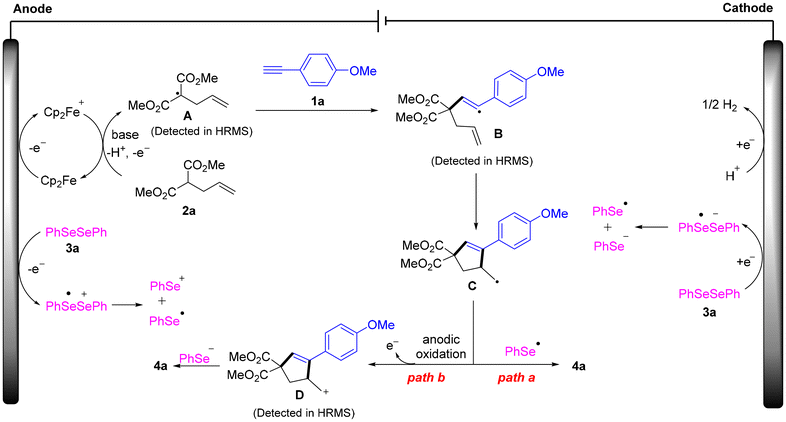
To gain a deeper mechanistic insight into this electrochemical transformation, we carried out a series of control experiments (Scheme 4a). Under standard conditions, the target product 4a was undetected when 2 equivalents of the radical scavenger 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) were added to the reaction system (Scheme 4a(i)). Meanwhile, the radical addition products 7, 8, and 9 could be detected by high-resolution mass spectrometry (HRMS). When 2 equivalents of BHT were added, 4a was completely inhibited, and the addition products 10, 11, and 12 were successfully captured by HRMS (Scheme 4a(ii)). In addition, when 1,1-diphenylethylene was added, the formation of 4a was also not observed, and the addition products 13, 14, and 15 were detected by HRMS (Scheme 4a(iii)). These experimental results indicate that the electrolysis may involve a radical process.
Cyclic voltammetry (CV) experiments were carried out to further understand the details of the oxidation process (Scheme 4b). The results showed that Cp2Fe has an oxidation peak at 0.55 V vs. Ag/AgCl (Scheme 4b(i), curve b), while no oxidation peak of 2a was detected at 0–1.5 V (Scheme 4b(i), curve a). These results indicated that Cp2Fe was preferentially oxidized under the optimized conditions. When K2CO3 was added to a mixed solution of Cp2Fe and 2a, the oxidation current increased significantly (Scheme 4b(i), curve d), suggesting that the base was critical for the electrocatalytic oxidation of 2a. In addition, diphenyl diselenide 3a showed an oxidation peak at 1.66 V vs. Ag/AgCl (Scheme 4b(ii)), corresponding to the anodic oxidation of 3a to the selenyl radical and selenide cation. Moreover, we observed a reduction peak of 3a at −1.49 V vs. Ag/AgCl (Scheme 4b(iii), red curve), indicating that 3a can undergo both anodic oxidation and cathodic reduction simultaneously.
Based on the above mechanistic studies and literature precedence,13 a plausible electrooxidative radical cyclization reaction is proposed in Scheme 5. Firstly, Cp2Fe generates Cp2Fe+via single-electron transfer (SET) at the anode. Meanwhile, dimethyl allylmalonate 2a loses a proton in the presence of base and is then oxidized by Cp2Fe+ to generate the carbon radical A. Subsequently, the carbon radical A reacts with 1a to form the alkenyl carbon radical B, which then undergoes intramolecular cyclization to form carbon radical intermediate C. Diphenyl diselenide 3a can either be oxidized at the anode to form the selenium cation and the selenium radical or be reduced at the cathode to generate the selenium radical and the selenide anion. Carbon radical intermediate C can be coupled with selenium radicals (mainly generated by cathodic reduction) to obtain the target product 4a (in path a). Furthermore, control experiments (Fig. S3d and S3e) showed that the reaction products of cationic intermediate D with NaOAc or KSCN could be detected by HRMS (Fig. S14 and S15), proving that cationic D was generated during the reaction. Therefore, carbon radical intermediate C can also undergo further anodic oxidation to form carbon radical intermediate D, which is then captured by selenide anions (generated by cathodic reduction) to generate product 4a (in path b). At the cathode, hydrogen ions gain electrons to produce hydrogen gas.
Conclusions
In summary, we have developed a novel electrochemical cyclization reaction catalyzed by Cp2Fe, which achieved the first electrochemical three-component [3 + 2] cyclization of carbocyclic selenides. Under mild electrochemical conditions, readily available and inexpensive alkynes and unsaturated propionates react with diselenides or thiophenols to synthesize a series of high-value selenium-containing cyclopentene compounds in up to 93% yields. This method features high atom economy, broad substrate applicability, and good tolerance to functional groups. Additionally, control experiments and cyclic voltammetry (CV) experiments indicate that this electrochemical transformation involves paired electrolysis processes. Further pharmacological activity research on these compounds is currently underway in our laboratory.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Experimental procedures, optimization of the reaction conditions, mechanism experiments, cyclic voltammetry tests, characterization data, and NMR spectra of all products. Supplementary information is available. See DOI: https://doi.org/10.1039/d5gc05879a.Acknowledgements
This research was financially supported by the Guangxi Science and Technology Major Program (AA24263012), the Guangxi Natural Science Foundation of China (2025GXNSFAA069025), the Guangxi Key Laboratory of Drug Discovery and Optimization (23-026-02), the National Natural Science Foundation of China (32460366), the Guilin Scientific Research and Technology Development Plan Project (20220129-2), the College Students’ Innovative Entrepreneurial Training Plan Program (S202410601129 and 202210601028), the Guangxi Key Laboratory for Pharmaceutical Molecular Discovery and Druggability Optimization (GKLDDO-2024-C01), and the Middle-aged and Young Teachers’ Basic Ability Promotion Project of Guangxi (2025KY0510).References
- Y. Kawamata, T. Hashimoto and K. Maruoka, J. Am. Chem. Soc., 2016, 138, 5206–5209 CrossRef CAS PubMed.
- Y. X. Liao, K. Li, M. Y. Wu, T. Wu and X. Q. Yu, Org. Biomol. Chem., 2014, 12, 3004–3008 RSC.
- J. Liu, X. Cai, H. Pan, A. Bandla, C. K. Chuan, S. Wang, N. Thakor, L. Liao and B. Liu, Small, 2018, 14, 1703732–1703741 CrossRef.
- (a) C. W. Nogueira, G. Zeni and J. B. Rocha, Chem. Rev., 2004, 104, 6255–6286 CrossRef CAS; (b) M. J. Parnham and H. Sies, Biochem. Pharmacol., 2013, 86, 1248–1253 CrossRef CAS PubMed; (c) P. K. Sahu, T. Umme, J. Yu, A. Nayak, G. Kim, M. Noh, J. Y. Lee, D. D. Kim and L. S. Jeong, J. Med. Chem., 2015, 58, 8734–8738 CrossRef CAS.
- (a) T. Tamai, M. Yoshikawa, S. Higashimae, A. Nomoto and A. Ogawa, J. Org. Chem., 2016, 81, 324–329 CrossRef CAS; (b) J. Chen, S. X. Su, D. C. Hu, F. H. Cui, Y. L. Xu, Y. Y. Chen, X. L. Ma, Y. M. Pan and Y. Liang, Asian J. Org. Chem., 2018, 7, 892–896 CrossRef CAS; (c) H. F. Yao, F. H. Li, J. Li, S. Y. Wang and S. J. Ji, Org. Biomol. Chem., 2020, 18, 1987–1993 RSC; (d) J. Wang, C. Wei, X. Li, P. Zhao, C. Shan, L. Wojtas, H. Chen and X. Shi, Chem. – Eur. J., 2020, 26, 5946–5950 CrossRef CAS PubMed; (e) X. Xia and Z. Wang, ACS Catal., 2022, 12, 11152–11158 CrossRef CAS.
- (a) M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117, 13230–13319 CrossRef CAS; (b) A. Ghosh, V. K. Parida and D. Banerjee, Green Chem., 2024, 26, 5770–5789 RSC; (c) E. J. Horn, B. R. Rosen and P. S. Baran, ACS Cent. Sci., 2016, 2, 302–308 CrossRef CAS PubMed; (d) C. Zhu, N. W. Ang, T. H. Meyer, Y. Qiu and L. Ackermann, ACS Cent. Sci., 2021, 7, 415–431 CrossRef CAS PubMed; (e) H. M. Lin, X. T. Li, F. H. Cui, T. Zhou, K. Li, M. R. Wang, X. J. Meng, H. T. Tang and Y. M. Pan, Sci. China Chem., 2025, 68, 5721–5732 CrossRef CAS.
- (a) J. Y. Chen, C. T. Zhong, Q. W. Gui, Y. M. Zhou, Y. Y. Fang, K. J. Liu, Y. W. Lin, Z. Cao and W. M. He, Chin. Chem. Lett., 2021, 32, 475–479 CrossRef CAS; (b) A. S. Chillal, R. T. Bhawale, S. Sharma and U. A. Kshirsagar, J. Org. Chem., 2024, 89, 14496–14504 CrossRef CAS PubMed; (c) X. Zhang, C. Wang, H. Jiang and L. Sun, Chem. Commun., 2018, 54, 8781–8784 RSC; (d) P. K. Jat, L. Yadav, A. Chouhan, K. Ucheniya and S. S. Badsara, Chem. Commun., 2023, 59, 5415–5418 RSC.
- (a) X. Kong, K. Yu, Q. Chen and B. Xu, Asian J. Org. Chem., 2020, 9, 1760–1764 CrossRef CAS; (b) L. Sun, L. Wang, H. Alhumade, H. Yi, H. Cai and A. Lei, Org. Lett., 2021, 23, 7724–7729 CrossRef CAS; (c) L. Sun, Y. Yuan, M. Yao, H. Wang, D. Wang, M. Gao, Y. H. Chen and A. Lei, Org. Lett., 2019, 21, 1297–1300 CrossRef CAS PubMed; (d) B. Hasimujiang, J. Zhu, W. Xu, H. Wang, X. Hu and Z. Ruan, Adv. Synth. Catal., 2023, 365, 2929–2935 CrossRef CAS.
- (a) X. Y. Wang, Y. F. Zhong, Z. Y. Mo, Y. L. Xu, S. H. Wu, H. T. Tang and Y. M. Pan, Adv. Synth. Catal., 2021, 363, 208–214 CrossRef CAS; (b) F. Lu, J. Xu, H. Li, K. Wang, D. Ouyang, L. Sun, M. Huang, J. Jiang, J. Hu, H. Alhumade, L. Lu and A. Lei, Green Chem., 2021, 23, 7982–7986 RSC; (c) A. B. Dapkekar and G. Satyanarayana, Chem. Commun., 2023, 59, 8719–8722 RSC; (d) J. Hua, Z. Fang, J. Xu, M. Bian, C. Liu, W. He, N. Zhu, Z. Yang and K. Guo, Green Chem., 2019, 21, 4706–4711 RSC.
- Y. Wu, J. Y. Chen, J. Ning, X. Jiang, J. Deng, Y. Deng, R. Xu and W. M. He, Green Chem., 2021, 23, 3950–3954 RSC.
- T. K. Xiong, Q. Xia, X. Q. Zhou, S. H. Li, F. H. Cui, H. T. Tang, Y. M. Pan and Y. Liang, Adv. Synth. Catal., 2023, 365, 2183–2187 CrossRef CAS.
- (a) S. Das, S. Chandrasekhar, J. S. Yadav and R. Grée, Chem. Rev., 2007, 107, 3286–3337 CrossRef CAS; (b) X. P. Zeng, Z. Y. Cao, Y. H. Wang, F. Zhou and J. Zhou, Chem. Rev., 2016, 116, 7330–7396 CrossRef CAS PubMed; (c) G. Liu, M. E. Shirley, K. N. Van, R. L. McFarlin and D. Romo, Nat. Chem., 2013, 5, 1049–1057 CrossRef CAS.
- Z. Guan, S. Zhu, Y. Ye, X. Li, Y. Liu, P. Wang, H. Zhang, Z. Huang and A. Lei, Angew. Chem., Int. Ed., 2022, 61, e202207059 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2026 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Reaction conditions: graphite felt anode, platinum plate cathode.
Reaction conditions: graphite felt anode, platinum plate cathode.